Abstract
Classical resistance classifications (multidrug resistance [MDR], extensive drug resistance [XDR], pan-drug resistance [PDR]) are very useful for epidemiological purposes, however, they may not correlate well with clinical outcomes, therefore, several novel classification criteria (e.g., usual drug resistance [UDR], difficult-to-treat resistance [DTR]) were introduced for Gram-negative bacteria in recent years. Microbiological and resistance data was collected for urinary tract infections (UTIs) retrospectively, corresponding to the 2008.01.01–2017.12.31. period. Isolates were classified into various resistance categories (wild type/susceptible, UDR, MDR, XDR, DTR and PDR), in addition, two new indicators (modified DTR; mDTR and mcDTR) and a predictive composite score (pMAR) were introduced. Results: n = 16,240 (76.8%) outpatient and n = 13,386 (69.3%) inpatient UTI isolates were relevant to our analysis. Citrobacter-Enterobacter-Serratia had the highest level of UDR isolates (88.9%), the Proteus-Providencia-Morganella group had the highest mDTR levels. MDR levels were highest in Acinetobacter spp. (9.7%) and Proteus-Providencia-Morganella (9.1%). XDR- and DTR-levels were higher in non-fermenters (XDR: 1.7%–4.7%. DTR: 7.3%–7.9%) than in Enterobacterales isolates (XDR: 0%–0.1%. DTR: 0.02%–1.5%). Conclusions: The introduction of DTR (and its’ modifications detailed in this study) to the bedside and in clinical practice will definitely lead to substantial benefits in the assessment of the significance of bacterial resistance in human therapeutics.
Keywords: clinical microbiology, indicators, urinary tract infection, Gram-negative, drug resistance, usual drug resistance, difficult-to-treat resistance, UDR, DTR, MDR, XDR, PDR
1. Introduction
The emergence and worldwide spread of antibiotic-resistant bacterial pathogens is one of the most serious concerns for modern medicine and a major public health issue, which requires participation of all the relevant stakeholders on a global scale [1,2,3,4,5]. Infections caused by drug resistant bacteria are associated with decreased quality of life (QoL) in the affected patients, increased costs for the healthcare infrastructure, the necessity to use antibiotics that are more expensive or have a disadvantageous toxicity profile and an increased mortality rate overall [6,7,8]. To make matters worse, the pipeline in the field of antibiotic discovery and the development of novel agents is plagued by a strong disincentive for drug developers, as the return-on-investment for these drugs pales in comparison to other drugs for the therapy of chronic non-communicable diseases or cancer [9,10]. Although in recent years, so-called public-private partnerships (including the 10 × 20 Initiative of the US Food and Drug Administration and the New Drugs 4 Bad Bugs [ND4BB] programme of the European Medicines Agency) have tried to make the field of antimicrobial discovery an attractive field of investment for pharmaceutical companies, it is a far cry from the golden age of antibiotic discovery (1960–1980), when new drugs continuously appeared on the market [11,12,13]. Some reports have gone as far as to suggesting that mortality due to drug resistant pathogens will be one of the leading causes of death by 2050, surpassing the mortality rate of malignant illnesses [14].
If overall mortality and their economic impact is taken into consideration, the group of “ESKAPE” pathogens, namely E: Enterococcus faecium, S: Staphylococcus aureus or recently Stenotrophomonas maltophilia, K: Klebsiella pneumoniae or recently C: Clostridioides difficile, A: Acinetobacter baumannii, P: Pseudomonas aeruginosa, E: Enterobacter spp., or recently Enterobacteriaceae) present the most clinical challenges [15,16]. A plethora of resistance mechanisms has been described in various bacterial species; some of these resistance mechanisms are plasmid-mediated, which allows for their widespread dissemination and outbreak-formation (particularly in Gram-negative bacteria; e.g., transmission of carbapenemase genes), while some bacteria possess intrinsic resistance mechanisms present in all species (e.g., resistance to tetracyclines, nitrofurantoin and polymyxin B in Proteus, Providencia and Morganella species) [17,18,19,20]. During susceptibility-testing and choosing the appropriate therapy, both clinicians and clinical microbiologists need to be aware of intrinsic resistance and local developments in acquired resistance levels [21].
As the clinical problem of bacterial drug resistance has emerged, several methods were developed to classify bacterial species into resistance categories; however, due to differences in these classifications, notable inconsistencies occurred in the reporting of epidemiological and clinical results [22]. The first global consensus criteria for the classification of drug resistance levels in bacteria were outlined by the collaborative efforts of the US Centers for Disease Control and Prevention (CDC) and the European Centre for Disease Control and Prevention (ECDC), namely the definitions for multidrug resistance (MDR), extensive drug resistance (XDR) and pan-drug resistance (PDR) [23]. These resistance categories are useful in reporting epidemiological data for the assessment of the resistance situation is a given geographical region. However, this categorization of resistance does not take into account the differences in the clinical utility and pharmacological features of the individual drugs tested; these characteristics may significantly alter the clinical outcomes [24]. This was highlighted by several reports, where they concluded that an infection with an MDR pathogen alone (without taking into consideration the drugs used for therapy) is not associated with an increased mortality rate, while the use of antibiotics with pronounced toxicities (e.g., colistin in monotherapy or combination therapy) carries a risk of excess mortality [25,26,27]. In the recent years, several novel classification criteria for bacterial resistance were published, with the aim of improving the correlation between resistance data and clinical outcomes: multiple antibiotic resistance (MAR) index [28,29], usual drug resistance (UDR; a definition created by McDonell et al. in 2016, defining resistance which may still be effectively treated with first-line drugs; first used in the context of clinical trials involving novel antibiotics [30,31]) and difficult-to-treat resistance (DTR; a definition created by Kadri et al. in 2018, highlighting resistance to first-line antibiotics with low toxicity; first used in the context of Gram-negative bloodstream infections [24]).
From the context of human medicine, urinary tract infections (UTIs) are the second most common infections in developed countries, representing an important factor of morbidity and mortality, both among outpatients and hospitalized patients (corresponding to 20%–60% of infections overall) [32,33]. In addition, UTIs are a sizable economic burden for healthcare institutions and national economies: their substantial economic impact is attributable to their pharmacological therapy, hospital costs and lost working days due to recovery; the economic losses caused by UTIs have been estimated to be around three billion US dollars [34]. The most common causes of UTIs in both community and nosocomial settings are Gram-negative bacteria; members of the Enterobacterales order are the most prevalent (E. coli is the considered the as principal etiological agent, however, the relevance of other members of the order should not be underestimated) causative pathogens, nevertheless, there is an increasing appreciation for the pathogenic role of non-fermenting Gram-negative bacteria (NFGNB; with Acinetobacter spp. and P. aeruginosa having the highest relevance) in UTIs, especially in patients with predisposing factors for development of complicated UTIs [35,36].
Even though the mortality rate associated with UTIs caused by Gram-negative bacteria falls below the mortality of bloodstream infections caused by the same pathogens, due to their high prevalence and constant developments in resistance levels, these infections may be considered as an important target for antibiotic resistance surveillance and stewardship interventions [37]. The epidemiology and patient characteristics of UTIs in our local setting (Albert Szent-Györgyi Clinical Center; Szeged, Hungary) has been extensively characterized previously [20,38,39,40,41]. The aim of the present study was to report on the resistance levels in Gram-negative UTI pathogens in the same healthcare setting retrospectively, using existing and novel classifications of bacterial resistance (which were not previously used in UTIs), over a 10-year study period (2008–2017). In addition, the study aims to introduce novel indicators of resistance, which may be useful in the characterization of resistance in UTIs in the future.
2. Materials and Methods
2.1. Study Design, Data Collection
In the present study, microbiological and resistance data was collected retrospectively, corresponding to the time period between 1 January 2008 and 31 December 2017 (10 years) at the Institute of Clinical Microbiology, University of Szeged. The Institute is the primary diagnostic laboratory of the Albert Szent-Györgyi Clinical Center, which is a primary- and tertiary-care teaching hospital in the Southern Great Plain of Hungary (serving as a specialized healthcare center to about 600,000 people, based on the most recent census data) [42]. The collection of the data used in this study was performed manually, in the records of the Institute’s laboratory information system (LIS), corresponding to urine samples positive for Gram-negative pathogens included in this study. Samples where bacterial colony counts were deemed clinically significant (>105 CFU/mL; however, this was subject to interpretation by the senior clinical microbiologists at the time of isolation, based on the information provided on the clinical request forms and international guidelines for the diagnosis of UTIs) and that were positive for the nitrite and leukocyte-esterase tests were included in the data analysis [39]. Only the first isolate per patient was included in the study; however, isolates with different antibiotic-susceptibility patterns from the same patient were considered as different individual isolates. For the purpose of the statistical comparisons, the origin (inpatient vs. outpatient) of the positive samples were also recorded. The study was deemed exempt from ethics review by the Institutional Review Board as no patient data was recorded and data anonymity was maintained.
2.2. Identification of Isolates during the Study Period
Ten microliters of each uncentrifuged urine sample was cultured on UriSelect chromogenic agar (Bio-Rad, Berkeley, CA, USA), blood agar (bioMérieux, Marcy-l’Étoile, Lyon, France) and eosin-methylene blue agar (EMB; Bio-Rad, Berkeley, CA, USA) plates with a calibrated loop, according to the manufacturer’s instructions; the plates were incubated at 37 °C for 24–48 h, aerobically [39]. In the first part of the study period (2008–2012), presumptive, biochemical reaction-based methods and VITEK 2 Compact ID/AST (bioMérieux, Marcy-l’Étoile, France) were used for bacterial identification. Starting from 2013, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was introduced to the workflow of the Institute of Clinical Microbiology. Mass spectrometry was performed by the microFlex LT MALDI Biotyper (Bruker Daltonics, Bremen, Germany) instrument, using the MALDI Biotyper RTC 3.1 software (Bruker Daltonics, Bremen, Germany) and MALDI Biotyper Library 3.1 for the spectrum analysis. The sample preparation procedure, methodology, and the technical details of the MALDI-TOF MS measurements were described elsewhere [39].
2.3. Susceptibility Testing of Relevant Isolates
Antimicrobial susceptibility testing for the relevant Gram-negative bacterial isolates were performed based on the methodological recommendations and standards of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) valid at the time of the interpretation; the Kirby–Bauer disk diffusion method (Liofilchem, Abruzzo, Italy), and E-test strips (in case of fosfomycin susceptibility testing; Liofilchem, Abruzzo, Italy) on Mueller–Hinton agar (MHA) plates, in addition, broth microdilution in a cation-adjusted Mueller–Hinton broth (in case of colistin susceptibility testing; MERLIN Diagnostik) was also used. For the verification of discrepant results, the VITEK 2 Compact ID/AST (bioMérieux, Marcy-l’Étoile, France) was used. Susceptibility testing included all relevant antibiotics for the respective bacteria, to allow for their classification into resistance categories based on the criteria detailed in Section 2.4; an antibiotic was not tested and was excluded from the analysis if intrinsic resistance was present, based on the EUCAST Expert Rules on Intrinsic Resistance and Exceptional Phenotypes [43]. To allow for easier data analysis, the bacterial isolates were grouped in six separate groups as follows, based on their similarities in intrinsic resistance: E. coli, Klebsiella spp., Citrobacter-Enterobacter-Serratia spp., Proteus-Providencia-Morganella spp., Pseudomonas aeruginosa and Acinetobacter spp., respectively [20,38,39,40,41]. The interpretation of susceptibility results was performed based on EUCAST breakpoints valid at the time of the interpretation. Intermediate results were grouped with and reported as resistant. The following strains were used as quality control (QC): S. aureus ATCC 29213, E. faecalis ATCC 29212, P. mirabilis ATCC 35659, E. coli ATCC 25922, P. aeruginosa ATCC 27853, A. baumannii ATCC 19606 and S. maltophilia ATCC 13637.
2.4. Classification of Isolates into Resistance Categories
During data analysis, respective isolates were classified into various resistance categories. Isolates were considered Wt/susceptible if they were susceptible to all tested antibiotics, excluding those where intrinsic non-susceptibility is present. Isolates were classified in the usual drug resistance (UDR; defined by McDonell et al. [30]) category, if they were resistant to at least one tested antibiotic outside of their realm of intrinsic non-susceptibility. Classification of the isolates as multidrug resistant (MDR; defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories), extensively drug resistant (XDR; bacterial isolates remain susceptible to only one or two antibiotic categories) and pandrug-resistant (PDR; non-susceptibility to all agents in all antimicrobial categories) was based on the CDC/ECDC recommendations [23]. The designation of difficult-to-treat resistance (DTR; defined by Kadri et al. [24]) was used if an isolate showed resistance to carbapenems (imipenem, meropenem and ertapenem/doripenem), extended-spectrum cephalosporins (the ones relevant for respective pathogens) and fluoroquinolones (ciprofloxacin, levofloxacin and moxifloxacin) [24]. Modified difficult-to-treat resistance (mDTR; introduced in this study, relevant in all bacterial categories, except for P. aeruginosa) was used if an isolate showed resistance to extended-spectrum cephalosporins (the ones relevant for respective pathogens) and fluoroquinolones (ciprofloxacin, levofloxacin and moxifloxacin), fosfomycin (for Enterobacterales isolates) and sulfamethoxazole-trimethoprim (for Enterobacterales and Acinetobacter spp.). For E. coli only, an additional category (mcDTR; modified difficult-to-treat resistance in E. coli) was also introduced, which includes susceptibility data for nitrofurantoin as well.
As a part of this study, a predictive composite score was also introduced (pMAR), which is an augmented version of the multiple antibiotic resistance (MAR) index described previously [28,29], based on the formula (1) below, where nMDR is the number of MDR isolates in the respective category (e.g., E. coli, Citrobacter-Enterobacter-Serratia), ABMDR is the minimum number of antibiotics needed for the respective bacterial group to become MDR, nall is the number of all isolates in the respective group and ABall is the number of all antibiotics tested. The pMAR value corresponds to the percentage chance (0%–100%) that the isolated pathogen in the present clinical situation will not be treatable by first-line antibiotics, based on local epidemiological characteristics.
| (1) |
2.5. Statistical Analyses
Statistical analyses, including the descriptive analysis and statistical tests (Student’s t-test and Mann–Whitney U test) were performed with the SPSS software version 24 (IBM SPSS Statistics for Windows 24.0, IBM Corp., Armonk, NY, USA). The normality of variables was tested using Shapiro–Wilk tests. A correlation analysis was also performed with the aim of assessing the temporal nature of changes in the ratio of UDR isolates during the study period; the coefficient of determination (R2) was also calculated. These analyses were performed using the Past 3.16 statistical software (Paleontological Museum, University of Oslo; Oslo, Norway). p values <0.05 were considered statistically significant.
3. Results
During the 10-year study period (1 January 2008–31 December 2017), the study site has received 21,150 urine samples from outpatient clinics and 19,325 samples from inpatient departments that turned out to be positive for a significant urinary pathogen. Out of these isolates, n = 16,240 (76.8%) of outpatient isolates and n = 13,386 (69.3%) of inpatient isolates were Gram-negative bacteria relevant to our analyses (p = 0.038). The distribution of isolates is presented in Table 1.
Table 1.
Distribution of pathogens from urinary tract infections in our local setting (Albert Szent-Györgyi Clinical Center; Szeged, Hungary) between 2008 and 2017.
| Bacterial Isolates | Outpatients | Inpatients |
|---|---|---|
| Citrobacter-Enterobacter-Serratia | 2.6% (n = 554) | 3.0% (n = 578) |
| Acinetobacter spp. | 0.7% (n = 143) | 0.7% (n = 133) |
| Pseudomonas aeruginosa | 2.8% (n = 588) | 5.7% (n = 1096) |
| Proteus-Providencia-Morganella | 5.0% (n = 1058) | 7.2% (n = 1392) |
| Klebsiella spp. | 8.9% (n = 1895) | 13.4% (n = 2592) |
| Gram-positive cocci | 20.7% | 20.7% |
| Escherichia coli | 56.8% (n = 12002) | 42.3% (n = 8173) |
| Candida spp. | 0.4% | 6.0% |
| Other | 2.1% | 1.0% |
Values in boldface represent isolates included in our analysis.
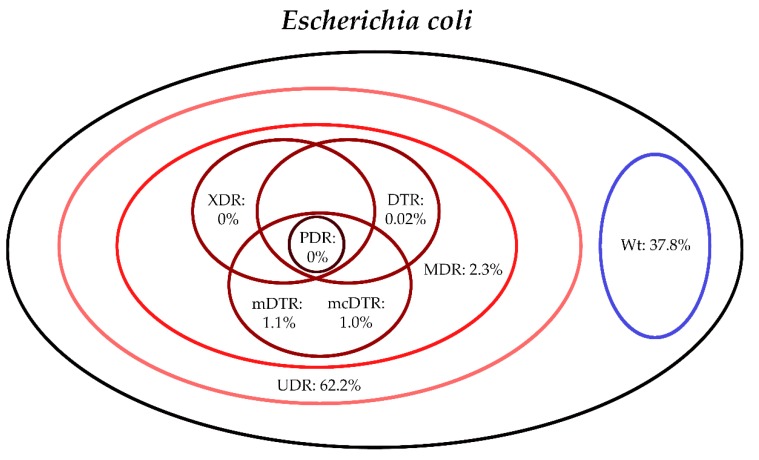
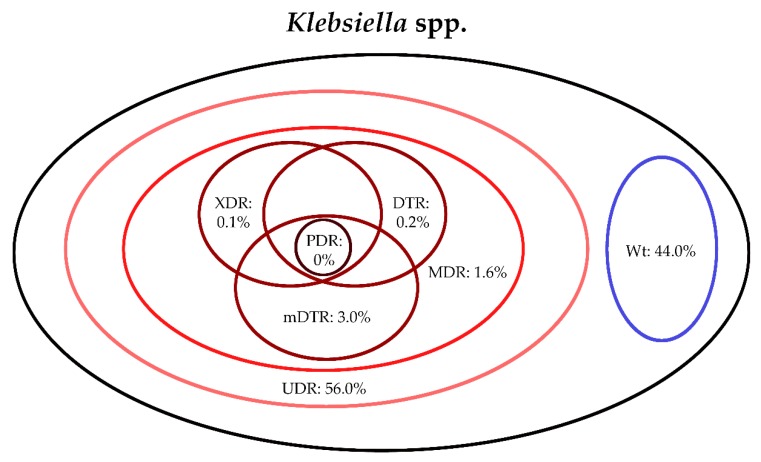
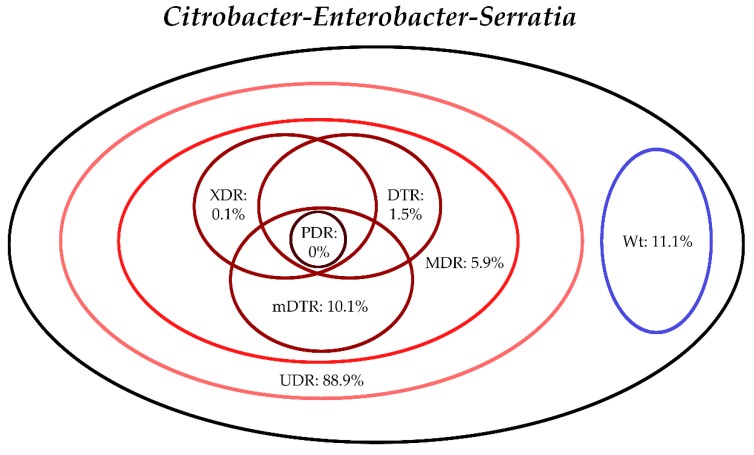
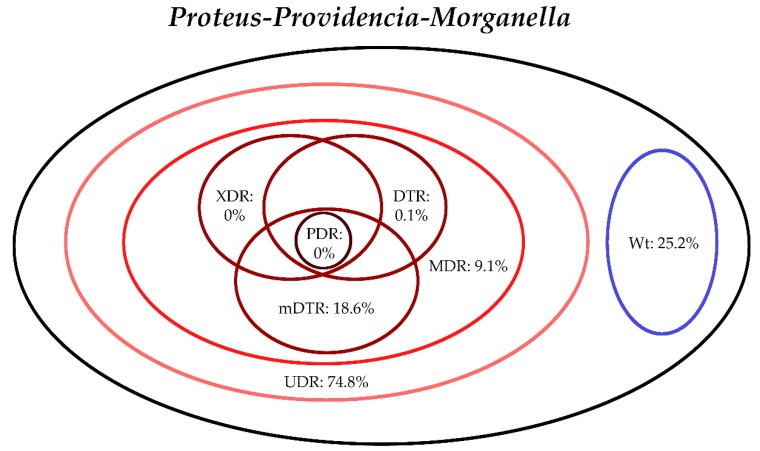
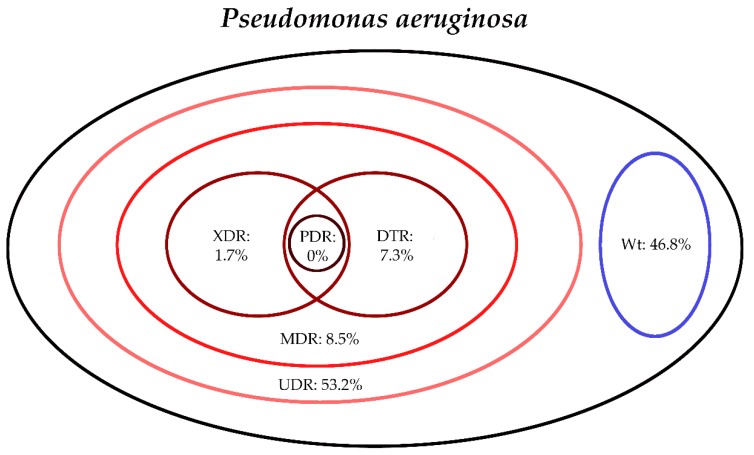
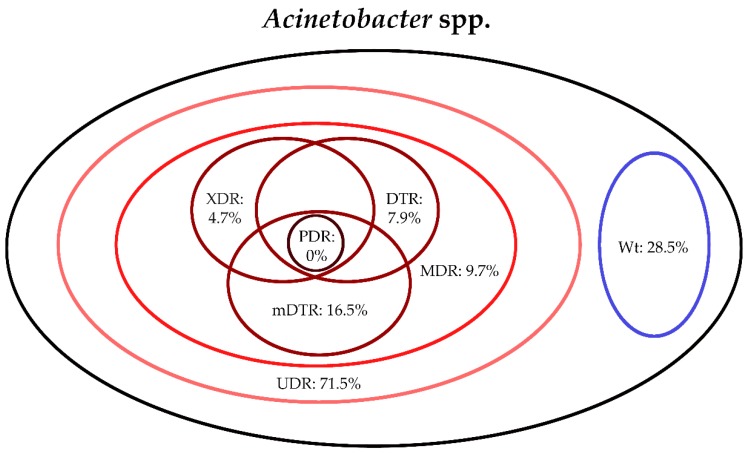
The classification of respective Gram-negative urinary isolates into the resistance categories defined in Section 4 is presented in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6 (the differences in resistance categories among inpatient and outpatient isolates are presented in Supplementary Tables S1–S6), while the temporal trends associated with the prevalence of UDR isolates over the 10-year study period is presented in Figure 7, Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12, respectively. Out of the Enterobacterales isolates groups surveyed, members of the Citrobacter-Enterobacter-Serratia (CES) had the highest level of UDR isolates (88.9%; and consequently, the lowest number of wild-type isolates; Figure 3 and Figure 9), while among NFGNB, Acinetobacter spp. had high levels of UDR (71.5%; Figure 6 and Figure 12). mDTR levels were highest in the Proteus-Providencia-Morganella group (18.6%; Figure 4); the comparison between Acinetobacter (16.9%) and P. aeruginosa is not possible, as mDTR was not interpreted in that group. MDR levels in Acinetobacter spp. and P. aeruginosa species were the highest (9.7% and 8.5%, respectively) overall, while among Enterobacterales, high levels of MDR were seen in Proteus-Providencia-Morganella (9.1%) and Citrobacter-Enterobacter-Serratia (5.9%) (Figure 3, Figure 4, Figure 5 and Figure 6.). Levels of DTR were 5-850 times higher in NFGNB than other Gram-negative isolates (7.3% and 16.9% vs. 0.02%–1.5%, respectively). XDR-levels were very low for E. coli, Proteus-Providencia-Morganella, Klebsiella spp., and CES isolates (0%, 0%, 0.1% and 0.1%, respectively; Figure 1, Figure 2, Figure 3 and Figure 4.). Very few isolates were resistant to colistin (n = 0 in Enterobacterales, n = 24 for Acinetobacter spp. and n = 27 for P. aeruginosa) throughout the 10-year study period, however, all of these isolates were susceptible to at least one other agent, therefore, no PDR isolates were detected in any bacterial groups overall (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6.).
Figure 1.
Classification of E. coli isolates from UTIs into resistance categories (2008–2017). Variation between inpatient and outpatient isolates is presented in Supplementary Table S1.
Figure 2.
Classification of Klebsiella spp. isolates from UTIs into resistance categories (2008–2017). Variation between inpatient and outpatient isolates is presented in Supplementary Table S2.
Figure 3.
Classification of Citrobacter-Enterobacter-Serratia spp. isolates from UTIs into resistance categories (2008–2017). Variation between inpatient and outpatient isolates is presented in Supplementary Table S3.
Figure 4.
Classification of Proteus-Providencia-Morganella spp. isolates from UTIs into resistance categories (2008–2017). Variation between inpatient and outpatient isolates is presented in Supplementary Table S4.
Figure 5.
Classification of Pseudomonas aeruginosa isolates from UTIs into resistance categories (2008–2017). Variation between inpatient and outpatient isolates is presented in Supplementary Table S5.
Figure 6.
Classification of Acinetobacter spp. isolates from UTIs into resistance categories (2008–2017). Variation between inpatient and outpatient isolates is presented in Supplementary Table S6.
Figure 7.
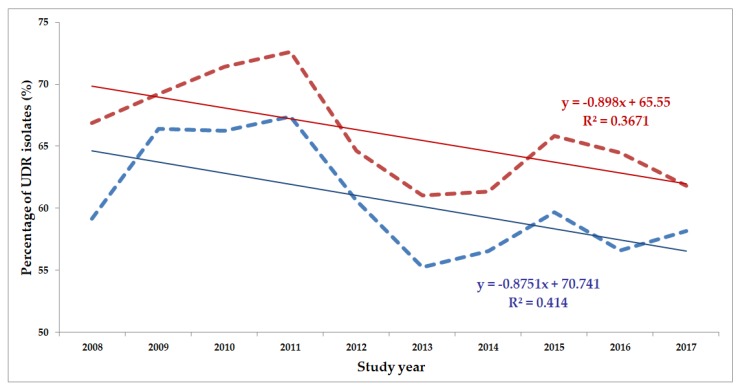
Changing trends of E. coli UDR isolates over the 10-year study period (2008–2017). Blue dashed line indicates isolates from outpatients, while red dashed line isolates from inpatients; R2: coefficient of determination; R2outpatients: 0.3671 (36.71%; not significant), R2inpatients: 0.414 (41.40%; p = 0.049).
Figure 8.
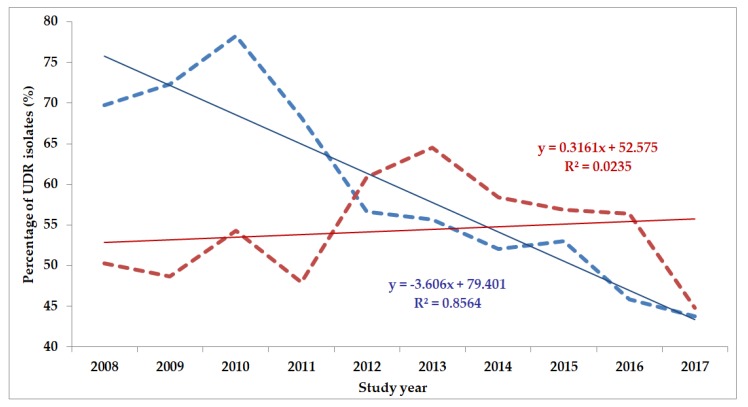
Changing trends of Klebsiella spp. UDR isolates over the 10-year study period (2008–2017). blue dashed line indicates isolates from outpatients, while red dashed line isolates from inpatients; R2: coefficient of determination. R2outpatients: 0.8564 (85.64%; p=0.012), R2inpatients: 0.0235 (2.35%; not significant).
Figure 9.
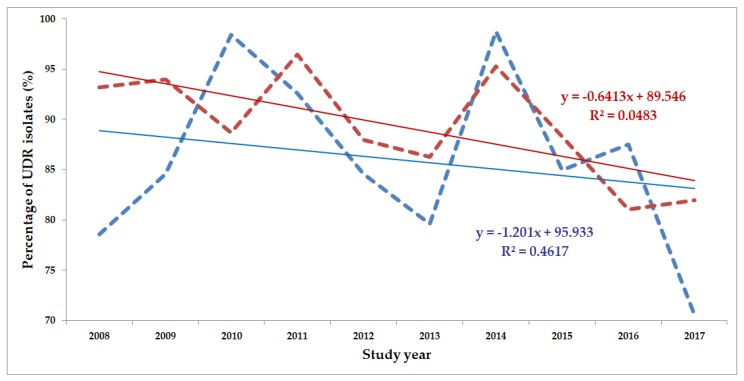
Changing trends of Citrobacter-Enterobacter-Serratia UDR isolates over the 10-year study period (2008–2017). Blue dashed line indicates isolates from outpatients, while red dashed line isolates from inpatients; R2: coefficient of determination. R2outpatients: 0.4617 (46.17%; p = 0.043), R2inpatients: 0.0483 (4.83%; not significant).
Figure 10.
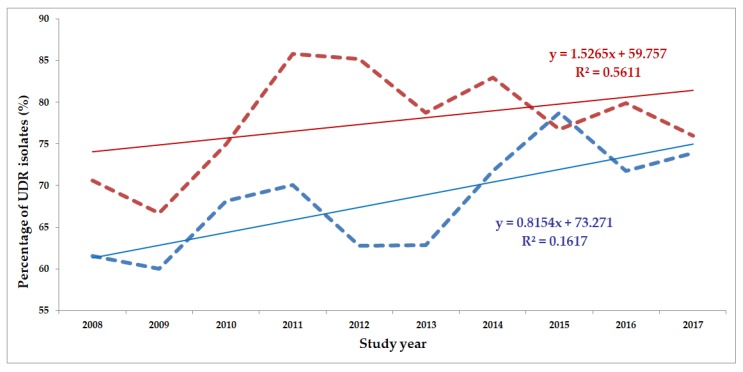
Changing trends of Proteus-Providencia-Morganella UDR isolates over the 10-year study period (2008–2017). Blue dashed line indicates isolates from outpatients, while red dashed line isolates from inpatients; R2: coefficient of determination. R2outpatients: 0.1617 (16.17%; not significant), R2inpatients: 0.5611 (56.11%; p = 0.036).
Figure 11.
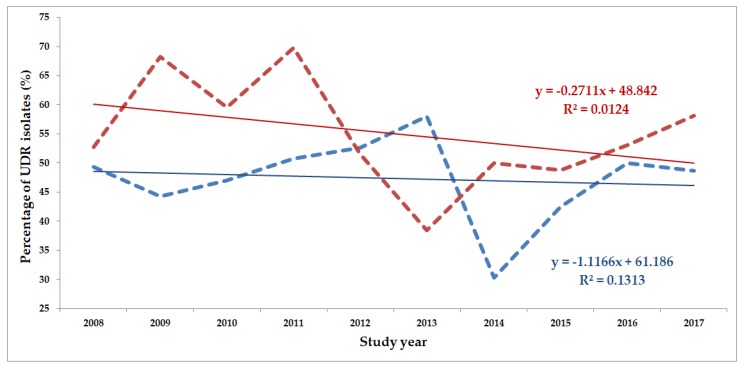
Changing trends of Pseudomonas aeruginosa UDR isolates over the 10-year study period (2008–2017). Blue dashed line indicates isolates from outpatients, while red dashed line isolates from inpatients; R2: coefficient of determination. R2outpatients: 0.1313 (13.13%; not significant), R2inpatients: 0.0124 (1.24%; not significant).
Figure 12.
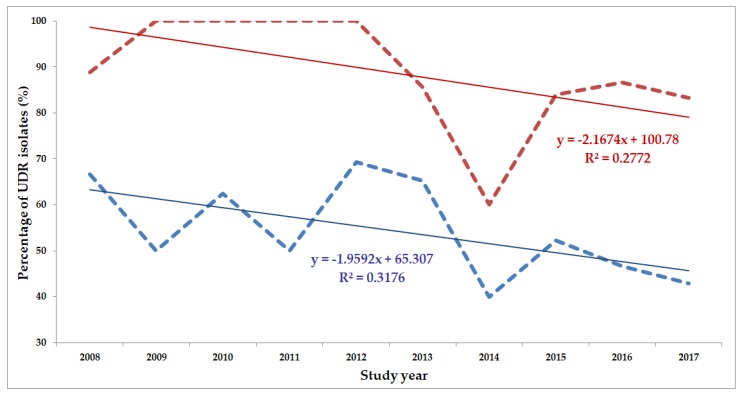
Changing trends of Acinetobacter spp. UDR isolates over the 10-year study period (2008–2017). Blue dashed line indicates isolates from outpatients, while red dashed line isolates from inpatients; R2: coefficient of determination. R2outpatients: 0.3176 (31.76%; not significant), R2inpatients: 0.2772 (27.72%; not significant).
The prevalence of UDR isolates for E. coli was lowest in 2013 (Figure 7), for Klebsiella spp. in 2017 (Figure 8), for Citrobacter-Enterobacter-Serratia in 2017 (Figure 9), for Proteus-Providencia-Morganella in 2009 (Figure 10), for P. aeruginosa in 2013 and 2014 (Figure 11) and for Acinetobacter spp. in 2014 (Figure 12). Varying levels of decreasing tendencies in the levels of UDR isolates were seen over time in almost all isolate groups, except in case of inpatient samples of Klebsiella spp. and inpatient/outpatient samples of Proteus-Providencia-Morganella, where an increasing tendency was seen. However, statistically significant correlation over time was only observed in inpatient samples of E. coli (p = 0.049), inpatient samples of Proteus-Providencia-Morganella (p = 0.036) and outpatient samples of CES (p = 0.043).
Based on the previous analysis of susceptibility data, pMAR values were calculated, suggesting the percentage chance that the isolates from the bacterial group will be resistant to first-line antibiotics (Table 2); values were ranging between 18.9% and 44.5%, respectively. The highest pMAR value was recorded for Proteus-Providencia-Morganella isolates (40.3%), while the lowest was seen for P. aeruginosa isolates (20.2%) overall.
Table 2.
pMAR values corresponding to respective urinary pathogens in the study period (2008–2017).
| Bacterial Group | Outpatients | Inpatients | Overall |
|---|---|---|---|
| Citrobacter-Enterobacter-Serratia | 37.9% | 38.2% | 38.1% |
| Acinetobacter spp. | 27.1% | 44.5% | 35.7% |
| P. aeruginosa | 18.9% | 21.3% | 20.2% |
| Proteus-Providencia-Morganella | 37.6% | 42.6% | 40.3% |
| Klebsiella spp. | 30.3% | 29.5% | 29.9% |
| E. coli | 25.3% | 27.7% | 26.1% |
4. Discussion
In the present study, 10-years’ worth of microbiological and resistance data for Gram-negative UTIs in Hungary were assessed, in light of existing and novel resistance classifications [24,28,30]. The surveillance of the etiology and susceptibility patterns in UTIs allow for targeted stewardship interventions in various healthcare facilities [37,44,45,46]. Resistance in Gram-negative bacteria has become one of the main concerns of clinicians (especially since the 2000s) [6]; in addition, plasmid-mediated resistance to carbapenems and colistin in Enterobacterales and non-fermenters may lead to widespread dissemination of pathogens with very few treatment options left [17,47,48,49]. Although the severity and mortality rate of UTIs (compared to invasive infections) is less pronounced, the economic burden of drug resistant urinary tract infections is significant, especially if these patients need to be hospitalized [32]. Therefore, the data presented herein may be useful for experts in clinical microbiology, epidemiology and public health, both in Hungary and on the International scene to highlight that growing levels of resistance is a severe issue in the context of UTIs, in outpatients and inpatients alike. Besides the “classical” drug resistance classifications (MDR-XDR-PDR), novel resistance categories found in the literature, and not used before in the context of UTIs (namely UDR and DTR) were also included [24,29,30]. In addition to these, our aim was to further the number of these resistance criteria with additional choices (mDTR, mcDTR and a predictive pMAR score) relevant in the classification of UTI pathogens.
In the report by Kadri et al. [24], the analysis of 46,521 isolates from bloodstream infections (originating from 173 US hospitals) were performed, pertaining to a four-year study period (2009–2013): in their study, n = 471 (1.0%) of isolates were DTR overall, with significant variation among different species (E. coli: 0.04%, Klebsiella spp.: 1.7%, Enterobacter spp.: 0.6%, P. aeruginosa: 2.3% and A. baumannii: 18.3%). In our study, UDR isolates were the most common in most cases; this classification describes isolates that are not wild-type (i.e., fully susceptible) strains, but their therapy is manageable with standard agents. The case behind DTR (non-susceptibility to all first-line agents, based on Kadri et al. [24]) was that in case of these isolates, resistance is present against all agents with high efficacy, safety and low-toxicity (namely broad-spectrum cephalosporins, fluoroquinolones and carbapenems) and clinicians are forced to use other antibiotics with more disadvantageous or limited pharmacological properties, e.g., aminoglycosides (nephrotoxicity, ototoxicity, neurotoxicity, poor penetration into several anatomical sites), tetracyclines (especially tigecycline in case of drug resistant infections; low serum levels and black-box warning for increased mortality levels), colistin (nephrotoxicity, neurotoxicity, difficulties with formulations and dosing, poor penetration into several anatomical sites) [6,24,50,51]. Nonetheless, resistance to these “reserve” antibiotics should also be monitored to ascertain their clinical utility; for example, based on our previous reports, the levels of aminoglycoside resistance (gentamicin) in UTIs for E. coli was between 5% and 15%, 18% and 25% for Klebsiella spp., 8% and 25% for CES pathogens, 17% and 20% in Proteus-Providencia-Morganella, 31% and 47% for P. aeruginosa and 7% and 59% for Acinetobacter spp. [20,38,39,40,41], while colistin resistance was <1% in all respective groups, demonstrated in this study. The introduction of mDTR and mcDTR was also indicated, because in many institutions, carbapenem-sparing antimicrobial stewardship regimens are enforced to ensure low levels of resistance against these agents, when they are truly needed [52]. Thus, instead of carbapenems, sulfamethoxazole-trimethoprim and fosfomycin resistance (in addition to nitrofurantoin in case of mcDTR) were taken into consideration, as these are all drugs used as first-line antibiotics in the therapy of urinary tract infections [53,54].
Classical resistance classifications (MDR-XDR-PDR) are very useful for epidemiological purposes; however, they may not correlate well with clinical outcomes [24,25,26,27,30,31]. Although several international bodies have published estimates on the excess mortality of MDR infections in Europe [55] and the US [56], these projections have been made based on predictive mathematical models, the relevance of which have been questioned by several reports [57,58]. Likewise, reports published on PDR infections have highlighted that clinical outcomes in their cases was not necessarily associated with the PDR status of the pathogens [59,60]. The category of MDR may be a source of discrepancies; for example, an extended-spectrum β-lactamase-producing and fluoroquinolone-resistant E. coli isolate may still be readily treated with fosfomycin. Similarly, ampicillin/sulbactam may still be effective highly resistant A. baumannii isolate; both of the mentioned agents are safe and effective alternative [24,39,40]. Another difficulty may stem from the large number of agents (may be as high as 15–17) that need to be tested to verify the XDR/PDR status of an isolate, which is usually not performed in most clinical laboratories [43]; this may lead to the reporting of erroneous results [24]. The latter is further complicated by the introduction of novel antibiotics (e.g., ceftolozane/tazobactam, ceftazidime/avibactam, eravacycline, delafloxacin-meglumine) into the routine susceptibility-testing platforms [16,24]. For the reasons mentioned above, in addition to its clinically-centered philosophy, introduction of DTR (and its modifications detailed in this study) to the bedside and in the clinical practice will definitely lead to substantial benefits in the assessment of the significance of bacterial resistance in human therapeutics [24]. The more frequent application of DTR (and m(c)DTR) by more healthcare-providers and in more published studies (preferably in correlation with clinical data) will lead to its wider recognition.
Acknowledgments
The authors would like to thank the staff of the Institute of Clinical Microbiology for the excellent laboratory assistance during the routine diagnostic work. M.G. was supported by the ESCMID Mentorship and Observership Programme.
Abbreviations
CDC: Centers for Disease Control and Prevention; CES: Citrobacter-Enterobacter-Serratia; CFU: colony-forming units; DTR: difficult-to-treat resistance; ECDC: European Centers for Disease Control and Prevention; EMB: eosine-methylene blue; EUCAST: European Committee on Antimicrobial Susceptibility Testing; ID: identification; LIS: laboratory information system; MALDI-TOF MS: matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; MAR: Multiple antibiotic resistance index; mDTR: modified difficult-to-treat resistance; mcDTR: modified difficult-to-treat resistance for Escherichia coli; MDR: multidrug-resistance; MHA: Mueller–Hinton agar; n.s.: not significant; ND4BB: New Drugs 4 Bad Bugs; NFGNB: non-fermenting Gram-negative bacteria; pMAR: a predictive composite score; PDR: pandrug resistance; R2: coefficient of determination; QC: quality control; QoL: quality of life; SXT: sulfamethoxazole-trimethoprim; UDR: usual drug resistance; UTI: urinary tract infection; Wt: wild-type; XDR: extensive drug resistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-1729/10/2/16/s1: Supplementary Table S1. Distribution of Escherichia coli isolates among different resistance categories, 2008–2017; Supplementary Table S2. Distribution of Klebsiella spp. isolates among different resistance categories, 2008–2017; Supplementary Table S3. Distribution of Citrobacter-Enterobacter-Serratia (CES) isolates among different resistance categories, 2008–2017; Supplementary Table S4. Distribution of Proteus-Providencia-Morganella isolates among different resistance categories, 2008–2017; Supplementary Table S5. Distribution of Pseudomonas aeruginosa isolates among different resistance categories, 2008–2017; Supplementary Table S6. Distribution of Acinetobacter spp. isolates among different resistance categories, 2008–2017.
Author Contributions
M.G. conceived and designed the study, performed data collection and analysis, wrote and revised the full paper. M.Á., A.L. were the senior microbiologists, performed the identification of the bacterial isolates and interpreted susceptibility-testing results during the study period. Z.B. performed statistical data analysis and prepared the figures. K.B. wrote and revised the full paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest, monetary or otherwise.
References
- 1.Shallcross L.J., Howard S.J., Fowler T., Davies S.C. Tackling the threat of antimicrobial resistance: From policy to sustainable action. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015;370:20140082. doi: 10.1098/rstb.2014.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laxminarayan R., Duse A., Wattal C., Zaidi A.K.M., Wertheim H.F.L., Sumpradit N., Vlieghe E., Hara G.L., Gould I.M., Goossens H., et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organisation . Antimicrobial Resistance: Global Report on Surveillance. WHO; Geneva, Switzerland: 2014. [(accessed on 12 January 2020)]. pp. 1–256. Available online: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1. [Google Scholar]
- 4.Dyar O.J., Huttner B., Schouten J., Pulcini C. What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017;23:793–798. doi: 10.1016/j.cmi.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Gajdács M., Paulik E., Szabó A. Knowledge, attitude and practice of community pharmacists regarding antibiotic use and infectious diseases: A cross-sectional survey in Hungary (KAPPhA-HU) Antibiotics. 2020;9:41. doi: 10.3390/antibiotics9020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajdács M. The concept of an ideal antibiotic: Implications for drug design. Molecules. 2019;24:892. doi: 10.3390/molecules24050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassini A., Högberg D.L., Plachouras D., Quattrocchi A., Hoxha A., Simonsen G.N., Colomb-Cotinat M., Kretzschmar M.E., Devleesschauwer B., Cecchini M., et al. Burden of the AMR Collaborative Group. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019;19:55–56. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munita J.M., Arias C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016;4:481–511. doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darrow J.J., Kesselheim A.S. Drug development and FDA approval, 1938–2013. N. Engl. J. Med. 2014;370:e39. doi: 10.1056/NEJMp1402114. [DOI] [PubMed] [Google Scholar]
- 10.Lyddiard D., Jones G.L., Greatrex B.W. Keeping it simple: Lessons from the golden era of antibiotic discovery. FEMS Microbiol. Lett. 2016;363:84. doi: 10.1093/femsle/fnw084. [DOI] [PubMed] [Google Scholar]
- 11.Infectious Diseases Society of America The 10 × 20 Initiative: Pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin. Infect. Dis. 2010;50:1081–1083. doi: 10.1086/652237. [DOI] [PubMed] [Google Scholar]
- 12.Hughes D., Karlén A. Discovery and preclinical development of new antibiotics. Ups. J. Med. Sci. 2014;119:162–169. doi: 10.3109/03009734.2014.896437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rex J.H. ND4BB: Addressing the antimicrobial resistance crisis. Nat. Rev. Microbiol. 2014;12:231–232. doi: 10.1038/nrmicro3245. [DOI] [Google Scholar]
- 14.O’Neill J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. [(accessed on 12 January 2020)]; Available online: https://amr-review.org/sites/default/files/AMRReviewPaper-Tacklingacrisisforthehealthandwealthofnations_1.pdf.
- 15.Rice L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2009;197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 16.Gajdács M., Albericio F. Antibiotic resistance: From the bench to patients. Antibiotics. 2019;8:e129. doi: 10.3390/antibiotics8030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santajit S., Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed. Res. Int. 2016;2016:2475067. doi: 10.1155/2016/2475067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medina E., Pieper D.H. Tackling Threats and future problems of multidrug-resistant bacteria. Curr. Top. Microbiol. Immunol. 2016;398:3–33. doi: 10.1007/82_2016_492. [DOI] [PubMed] [Google Scholar]
- 19.Cui X., Zhang H., Du H. Carbapenemases in enterobacteriaceae: Detection and antimicrobial therapy. Front Microbiol. 2019;10:1823. doi: 10.3389/fmicb.2019.01823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gajdács M., Urbán E. Comparative epidemiology and resistance trends of proteae in urinary tract infections of inpatients and outpatients: A 10-year retrospective study. Antibiotics. 2019;8:91. doi: 10.3390/antibiotics8030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ha D.R., Haste N.M., Gluckstein D.P. The role of antibiotic stewardship in promoting appropriate antibiotic use. Am. J. Lifestyle Med. 2017;13:376–383. doi: 10.1177/1559827617700824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fridkin S.K., Cleveland A.A., See I., Lynfield R. Emerging infections program as surveillance for antimicrobial drug resistance. Emerg. Infect. Dis. 2015;21:1578–1581. doi: 10.3201/eid2109.150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Paterson D.L. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 24.Kadri S.S., Adjemian J., Lai Y.L., Spaulding A.B., Ricotta E., Prevots D.R., Powers J.H., III Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: Retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin. Infect. Dis. 2018;67:1803–1814. doi: 10.1093/cid/ciy378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul M., Daikos G.L., Durante-Mangoni E., Yahav D., Carmeli Y., Benattar Y.D., Zusman O. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018;18:391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 26.Zak-Doron Y., Dishon Benattar Y., Pfeffer I., Daikos G.L., Skiada A., Antoniadou A., Yahav D. The association between empirical antibiotic treatment and mortality in severe infections caused by carbapenem-resistant gram-negative bacteria: A prospective study. Clin. Infect. Dis. 2018;67:1815–1823. doi: 10.1093/cid/ciy371. [DOI] [PubMed] [Google Scholar]
- 27.Burnham J.P., Lane M.A., Kollef M.H. Impact of sepsis classification and multi-drug-resistance status on outcome among patients treated with appropriate ther-apy. Crit. Care Med. 2015;43:1580–1586. doi: 10.1097/CCM.0000000000001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Dulaimi M.M.K., Mutalib S.A., Ghani M.A., Zaini N.A.M., Ariffin A.A. Multiple antibiotic resistance (MAR), plasmid profiles, and DNA polymorphisms among vibrio vulnificus isolates. Antibiotics. 2019;8:e68. doi: 10.3390/antibiotics8020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gajdács M. Epidemiology and resistance levels of enterobacteriaceaeisolates from urinary tract infections expressed as multiple antibiotic resistance (MAR) indices. J. Pharm. Res. Int. 2019;29:1–7. doi: 10.9734/jpri/2019/v29i330238. [DOI] [Google Scholar]
- 30.McDonnell A., Rex J.H., Goosens H., Bonten M., Fowler V.G., Dane A. Efficient Delivery of Investigational Antibacterial Agents via Sustainable Clinical Trial Networks. Clin. Infect. Dis. 2016;63:S57–S59. doi: 10.1093/cid/ciw244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rex J.H., Talbot G.H., Goldberger M.J., Eisenstein B.I., Echols R.M., Tomayko J.F., Dudley M.N., Dane A. Progress in the fight against multidrug-resistant bacteria 2005–2016: Modern noninferiority trial designs enable antibiotic development in advance of epidemic bacterial resistance. Clin. Infect. Dis. 2017;65:141–146. doi: 10.1093/cid/cix246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hooton T.M., Bradley S.F., Cardenas D.D., Colgan R., Geerlings S.E., Rice J.C., Saint S., Schaeffer A.J., Tambayh P.A., Tenke P., et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the infectious diseases society of America. Clin. Infect. Dis. 2010;50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 33.Wiedemann B., Heisig A., Heisig P. Uncomplicated urinary tract infections and antibiotic resistance-epidemiological and mechanistic aspects. Antibiotics. 2014;3:341–352. doi: 10.3390/antibiotics3030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmering J.E., Tang F., Cavanaugh J.E., Polgreen L.A., Polgreen P.M. The Increase in hospitalizations for urinary tract infections and the associated costs in the United States, 1998–2011. Open Forum. Infect. Dis. 2017;4:ofw281. doi: 10.1093/ofid/ofw281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calzi A., Grignolo S., Caviglia I., Calevo M.G., Losurdo G., Piaggio G., Bandettini R., Castagnola E. Resistance to oral antibiotics in 4569 Gram-negative rods isolated from urinary tract infection in children. Eur. J. Pediatr. 2016;175:1219–1225. doi: 10.1007/s00431-016-2763-1. [DOI] [PubMed] [Google Scholar]
- 36.Stefaniuk E., Suchocka U., Bosacka K., Hryniewicz W. Etiology and antibiotic susceptibility of bacterial pathogens responsible for community-acquired urinary tract infections in Poland. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35:1363–1369. doi: 10.1007/s10096-016-2673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbo L.M., Hooton T.M. Antimicrobial stewardship and urinary tract infections. Antibiotics. 2014;3:174–192. doi: 10.3390/antibiotics3020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gajdács M., Urbán E. Resistance trends and epidemiology of citrobacter-enterobacter-serratia in urinary tract infections of inpatients and outpatients (RECESUTI): A 10-year survey. Medicina. 2019;55:285. doi: 10.3390/medicina55060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gajdács M., Ábrók M., Lázár A., Burián K. Comparative Epidemiology and resistance trends of common urinary pathogens in a tertiary-care hospital: A 10-year surveillance study. Medicina. 2019;55:356. doi: 10.3390/medicina55070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gajdács M., Burián K., Terhes G. Resistance levels and epidemiology of non-fermenting gram-negative bacteria in urinary tract infections of inpatients and outpatients (RENFUTI): A 10-year epidemiological snapshot. Antibiotics. 2019;8:143. doi: 10.3390/antibiotics8030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gajdács M., Dóczi I., Ábrók M., Lázár A., Burián K. Epidemiology of candiduria and Candida urinary tract infections in inpatients and outpatients: Results from a 10-year retrospective survey. Cent. Eur. J. Urol. 2019;72:209–214. doi: 10.5173/ceju.2019.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hospital Bed Count and Patient Turnover Report 2017. National Health Insurance Fund of Hungary. [(accessed on 12 January 2020)]; Available online: http://www.neak.gov.hu/felso_menu/szakmai_oldalak/publikus_forgalmi_adatok/gyogyito_megelozo_forgalmi_adat/fekvobeteg_szakellatas/korhazi_agyszam.html.
- 43.Leclercq R., Cantón R., Brown D.F.J., Giske C.G., Heisig P., MacGowan A.P., Mouton J.W., Nordmann P., Rodloff A.C., Rossolini G.M., et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2013;19:141–160. doi: 10.1111/j.1469-0691.2011.03703.x. [DOI] [PubMed] [Google Scholar]
- 44.Issakhanian L., Behzadi P. Antimicrobial agents and urinary tract infections. Curr. Pharm. Des. 2019;25:1409–1423. doi: 10.2174/1381612825999190619130216. [DOI] [PubMed] [Google Scholar]
- 45.Jahandeh N., Ranjbar R., Behzadi P., Behzadi E. Uropathogenic Escherichia coli virulence genes: Invaluable approaches for designing DNA microarray probes. Cent. Eur. J. Urol. 2015;68:452–458. doi: 10.5173/ceju.2015.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gajdács M., Szabó A. Physicians’ opinions towards antibiotic use and resistance in the southeastern region of Hungary. Orv. Hetil. 2019 doi: 10.1556/650.2019.31598. accepted. [DOI] [PubMed] [Google Scholar]
- 47.Poole K. Pseudomonas Aeruginosa: Resistance to the max. Front. Microbiol. 2011;2:65. doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iredell J., Brown J., Tagg K. Antibiotic resistance in enterobacteriaceae: Mechanisms and clinical implications. BMJ. 2016;352:h6420. doi: 10.1136/bmj.h6420. [DOI] [PubMed] [Google Scholar]
- 49.Björn B. Acquired resistance to colistin via chromosomal and plasmid-mediated mechanisms in klebsiella pneumoniae. Infect. Microb. Dis. 2019;1:10–19. [Google Scholar]
- 50.Levison M.E., Levison J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 2009;23:791–799. doi: 10.1016/j.idc.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nation R.L., Garonzik S.M., Thamlikitkul V., Giamarellos-Bourboulis E.J., Forrest A., Paterson D.L., Li J., Silveira F.P. Dosing guidance for intravenous colistin in critically Ill patients. Clin. Infect. Dis. 2017;64:565–571. doi: 10.1093/cid/ciw839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corcione S., Lupia T., Maraolo A.E., Mornese Pinna S., Gentile S., De Rosa F.G. Carbapenem-sparing strategy: Carbapenemase, treatment, and stewardship. Curr. Opin. Infect. Dis. 2019;32:663–673. doi: 10.1097/QCO.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 53.Gajdács M., Ábrók M., Lázár A., Burián K. Susceptibility patterns of extended-spectrum beta-lactamase-producing (ESBL) urinary pathogens: Single-center experience. Gyógyszerészet. 2019;63:405–411. [Google Scholar]
- 54.Gajdács M., Ábrók M., Lázár A., Burián K. Microbiology of urine samples obtained through suprapubic bladder aspiration: A 10-year epidemiological snapshot. Dev. Health Sci. 2019;2:76–78. doi: 10.1556/2066.2.2019.012. [DOI] [Google Scholar]
- 55.European Antimicrobial Resistance Surveillance Network (EARS-Net) [(accessed on 12 January 2020)]; Available online: https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/ears-net.
- 56.CDC Antibiotic/Antimicrobial Resistance (AR/AMR) [(accessed on 12 January 2020)]; Available online: https://www.cdc.gov/drugresistance/biggest_threats.html.
- 57.Abat C., Rolain J.M., Dubourg G., Fournier P.E., Chaudet H., Raoult D. Evaluating the clinical burden and mortality attributable to antibiotic resistance: The disparity of empirical data and simple model estimations. Clin. Infect. Dis. 2017;65:S58–S63. doi: 10.1093/cid/cix346. [DOI] [PubMed] [Google Scholar]
- 58.Abat C., Fournier P.E., Jimeno M.T., Rolain J.M., Raoult D. Extremely and pandrug-resistant bacteria extra-deaths: Myth or reality? Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:1687–1697. doi: 10.1007/s10096-018-3300-0. [DOI] [PubMed] [Google Scholar]
- 59.Falagas M.E., Rafailidis P.I., Matthaiou D.K., Virtzili S., Nikita D., Michalopoulos A. Pandrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii infections: Characteristics and outcome in a series of 28 patients. Int. J. Antimicrob. Agents. 2008;32:450–454. doi: 10.1016/j.ijantimicag.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Elemam A., Rahimian J., Mandell W. Infection with panresistant Klebsiella pneumoniae: A report of 2 cases and a brief review of the literature. Clin. Infect. Dis. 2009;49:271–274. doi: 10.1086/600042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.