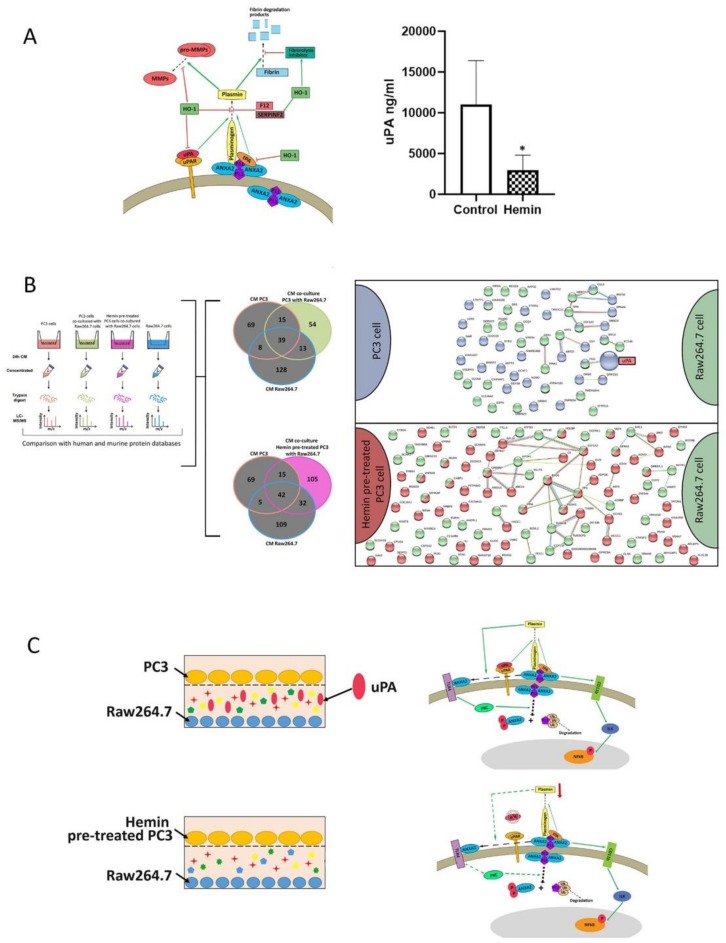
Figure 3.
Secretome analyses of conditioned media (CM) from the co-culture transwell system between PC3 and Raw246.t cells. Effect of HO-1 induction. (A) Model of plasmin regulation by cell surface annexin A2 and HO-1. Annexin A2 forms a heterotetrameric complex, consisting of two molecules of annexin A2 and one copy of the S100A10P11 (P11) dimer, which binds to the tissue-plasminogen activator tPA and plasminogen. The urokinase-plasminogen activator (uPA) is bound to its receptor (uPAR) and forms the uPA/uPAR complex that co-localizes with the annexin A2-S100A10 complex. This causes plasminogen to cleave into plasmin, which activates pro-MMPs (matrix metallo-proteases) into active MMPs and also degrades fibrin. High expression of HO-1 decreases the extracellular matrix degradation by inhibiting the uPA/uPAR complex and inactivating pro-MMPs. It can also inhibit the cleavage of plasminogen into plasmin, directly or by activating SERPINF2, which together with F12 inactivates plasmin formation. Further, high HO-1 expression causes activation of fibrinolysis inhibitors, inhibiting fibrin degradation (left panel). uPA concentration, expressed as ng/mL, in conditioned media of PC3 cells treated with hemin (50 µM, 24 h) or PBS as control. One of three independent experiments is shown (*p < 0.05) (right panel). (B) Schematic workflow of sample obtaining and processing of conditioned media (CM) for analysis by mass spectrometry. Exclusion/inclusion criteria to form differential protein lists represented as Venn diagrams, where the areas colored with green or pink correspond to the differential proteins of CMs from the co-culture of PC3 with Raw264.7 or Hemin pre-treated PC3 with Raw264.7, respectively. Gray areas correspond to shared proteins, excluded from the differential lists. Protein–protein interactions between the differential proteins of CMs from the co-culture of PC3 with Raw264.7 or Hemin pre-treated PC3 with Raw264.7. (C) Schematic representation of the proposed model for proteins associated with prostate cancer (PCa) and bone metastasis affected by the interaction of soluble factors between PC3 and Raw264.7 cells.