Abstract
The controlled activation of dormant primordial follicles is important for the maintenance of periodic ovulation. Previous reports have clearly identified the signaling pathway in granulosa cells and oocytes that controls the activation of primordial follicles; however, the exact cue for the in vivo activation of dormant primordial follicles is yet to be elucidated. In this study, we found that almost all activated primordial follicles made contact with blood vessels. Based on this result, we speculated that the contact between primordial follicles and blood vessels may provide a cue for the activation of dormant primordial follicles. To confirm this hypothesis, we attempted to activate dormant primordial follicles within the ovaries by inducing angiogenesis through the use of biodegradable gels containing recombinant vascular endothelial growth factor and in cultured ovarian tissues by increasing the serum concentration within the culture medium. The activation of dormant primordial follicles was promoted in both experiments, and our results indicated that an increase in the supply of the serum component, from new blood vessels formed via angiogenesis, to the dormant primordial follicles is the cue for their in vivo activation. In the ovaries, angiogenesis often occurs during every estrous cycle, and it is therefore likely that angiogenesis is the crucial event that influences the activation of primordial follicles.
Keywords: Angiogenesis, Oogenesin 1, Ovary, Primordial follicle
Several studies have reported the factors and the signaling pathways in oocytes and the surrounding granulosa cells that activate dormant primordial follicles [1,2,3,4,5,6,7,8,9,10,11,12]. In oocytes, the phosphatidylinositol 3-kinase (PI3K)-AKT-forkhead box O3 (FOXO3) signaling pathway regulates the activation of dormant primordial follicles, and phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibits this pathway and suppresses the activation of dormant primordial follicles [13, 14]. Then, KIT ligand (KITL)-KIT signaling acts as the upstream PI3K-AKT-FOXO3 signaling pathway in oocytes [1]. Granulosa cells surrounding an oocyte express KITL, and the expression of KITL is up-regulated through the activation of the mechanistic target of rapamycin complex 1 (mTORC1) in granulosa cells [1, 11]. Thus, the regulatory mechanism from granulosa cells to an oocyte has been clarified; however, the cue that activates the granulosa cells surrounding the dormant primordial follicles remains unknown.
In this study, we focused on the relationship between dormant primordial follicles and blood vessels because we found that almost all activated primordial follicles made contact with blood vessels in adult mouse ovaries. Within the ovaries, angiogenesis occurs in every estrus cycle and is essential for follicle development and corpus luteum formation. The detailed relationship between primordial follicles and blood vessels, however, remains unknown. Based on this, we conducted two experiments that included an in vivo study using biodegradable gels containing recombinant vascular endothelial growth factor (VEGF) and an in vitro study examining cultured mouse ovaries to clarify the role of blood vessels in the activation of primordial follicles. For the in vivo experiment, we transplanted a biodegradable gel containing recombinant VEGF into the bursa of a mouse ovary and induced angiogenesis. In the in vitro experiment, we reproduced the condition where dormant primordial follicles come into contact with blood vessels under culture conditions by controlling the concentration of fetal bovine serum (FBS) within the medium. From these experiments, we demonstrated that increased supply from new blood vessels formed by angiogenesis provides the cue for the activation of dormant primordial follicles in mouse ovaries.
Materials and Methods
Mice
All mice used in our experiments were housed in an environmentally controlled room maintained at 23 ± 1°C with a 12 h light/12 h dark cycle. Animal care and experiments were conducted in accordance with the Guidelines for Animal Experimentation of Aichi Medical University. Experiments in this study were approved by the Animal Care and Use Committee of Aichi Medical University. In this article, two types of transgenic mice were used. Oog1pro3.9 mice were provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan (Accession No. BRC06134), and R26-H2B-mCherry mice were provided by the RIKEB Centre for Life Science Technologies (Accession No. CDB.0239K, http://www.clst.riken.jp/arg/reporter_mice.html). Polymerase chain reaction genotyping of each transgenic mouse was performed as reported previously [15, 16]. For the analysis of the relationship between primordial follicles and blood vessels and to determine the effect of transplantation of biodegradable gels, 4- and 10-week-old female ICR mice (Japan SLC, Shizuoka, Japan) were used, respectively.
Transplantation of biodegradable gels
Biodegradable gels (MedGel, Tokyo, Japan) that were cut into 2 × 2 mm pieces were immersed in 10 μl of 0.1 × phosphate buffered saline (PBS), recombinant mouse VEGF (100 ng/ml, 1 μg/ml, and 10 μg/ml; R&D Systems, Minneapolis, MN, USA), recombinant mouse basic fibroblast growth factor (bFGF; 100 ng/ml, 1 μg/ml, and 10 μg/ml; R&D Systems), or recombinant mouse hepatocyte growth factor (HGF; 100 ng/ml, 1 μg/ml, and 10 μg/ml; R&D Systems) and then stored in an incubator for 1 h at 37°C. After incubation, biodegradable gels were transplanted into the bursa of the ovaries of 10-week-old female ICR mice under anesthesia achieved using a mixed anesthetic reagent that contained 60 μg/ml medetomidine, 800 μg/ml midazolam, and 100 μg/ml butorphanol [17]. The mixed anesthetic reagent was administered at 50 μl/10 g body weight. A biodegradable gel containing 0.1 × PBS was transplanted into the bursa of the left ovary, and a gel containing recombinant VEGF, bFGF, or HGF was transplanted into the right ovary. The surgical procedure was performed according to a previous report [18]. Ovaries from non-treated 10-week-old female ICR mice were used as controls. Ovaries were removed 5 days after transplantation and fixed with SUPER FIX (KURABO, Osaka, Japan) at 4°C overnight. Then, fixed ovaries were embedded in paraffin and were sliced into 5-μm-thick serial sections.
Ovarian tissue culture
On postnatal day 0 (P0), Oog1pro3.9/R26-H2B-mCherry female mouse ovaries were used for ovarian tissue culture experiments. Whole P0 mouse ovaries were cultured in a 30 mm cell culture insert (Merck Millipore, Darmstadt, Germany). The culture conditions were maintained and detailed methods were performed as reported previously [19].
Analysis of the activation of dormant primordial follicles in cultured P0 mouse ovaries
Images of cultured P0 Oog1pro3.9/R26-H2B-mCherry mouse ovaries were captured at 24 h intervals for 10 days using a confocal microscope (LSM 710, Carl Zeiss Microimaging, Oberkochen, Germany). The Z-step size was 10 μm, and the Z-stack thickness was 100–150 μm. Information regarding the imaging methods for cultured ovaries is detailed in a previous report [19]. First, the number of AcGFP1-positive follicles was counted on culture day 10. Next, the area of the cultured ovaries was measured using ImageJ software (http://rsbweb.nih.gov/ij/). The detailed measurement procedure has been previously reported [19]. The area was then multiplied by the thickness of the Z-step (10 μm), and the sum of the volumes of each slice was determined. Finally, the density of the AcGFP1-positive follicles was calculated. To evaluate the effect of 0%, 5%, and 10% FBS on the activation and growth of primordial follicles, ovaries were cultured at each FBS concentration.
To evaluate the effect of recombinant VEGF (R&D System) on the activation of dormant primordial follicles, the left ovaries were cultured as controls and the right ovaries were cultured in medium containing recombinant VEGF (50, 100, and 200 ng/ml).
Measurement of the AcGFP1 signal in cultured P0 Oog1pro3.9/R26-H2B-mCherry mouse ovaries
The signal intensity of AcGFP1 in oocytes and follicle areas in cultured P0 Oog1pro3.9/R26-H2B-mCherry mouse ovaries were measured using ImageJ, and their interrelationship was analyzed. The area of primordial and primary follicles was measured by tracing the outline of follicles that were visualized according to mCherry signal in granulosa cells. The conditions for image capture for visualizing the primordial to primary follicle transition were performed using cultured P4 Oog1pro3.9/R26-H2B-mCherry mouse ovaries. The detailed information is provided in our previous report [19]. We then measured 115 follicles in five P0 Oog1pro3.9/R26-H2B-mCherry mouse ovaries.
Immunohistochemistry
The deparaffinized sections were boiled in the antigen activator (immunosavor, Nissin EM, Tokyo, Japan) for 45 min. Then, endogenous peroxidase activity was blocked by treatment with 0.6% hydrogen peroxide that was diluted in PBS at room temperature for 30 min. The sections were then treated with anti-FOXO3a antibody (1:2000 dilution; product no. D19A7; Cell Signaling Technology, Danvers, MA, USA), anti-CD31 antibody (1:100 dilution; ab28364; Abcam, Cambridge, UK), or anti-KITL antibody (1:400 dilution; ab64677; Abcam) and subjected to immunoperoxidase staining using the VECTASTAIN ABC Kit (Vector Laboratories, Burlingame, CA, USA) in accordance with the standard protocol. All sections were counterstained with hematoxylin (Muto Pure Chemicals, Tokyo, Japan).
To investigate the relationship between primordial follicles and blood vessels, all serial sections of 4-week-old mouse ovaries (346 sections in total) were stained alternately with anti-FOXO3a antibody and anti-CD31 antibody. Then, primordial follicles were classified into activated and dormant primordial follicles, where FOXO3a localized in the nucleus of oocytes in dormant primordial follicles and FOXO3a localized in the cytosol of oocytes in activated primordial follicles. In this study, primordial follicles are defined as follicles where an oocyte is surrounded in a layer of flat granulosa cells. Next, each primordial follicle was divided into two groups according to their contact with blood vessels as assessed by observation of the neighboring slides stained with anti-CD31 antibody. In this experiment, two ovaries that were removed from different mice were used.
Sections of the ovaries where a biodegradable gel containing each solution was transplanted were stained with anti-FOXO3a antibody every 10th section. To evaluate the effect of angiogenesis on the activation of dormant primordial follicles, the ratio of activated primordial follicles in the right ovary treated with recombinant VEGF, bFGF, or HGF was divided by the ratio in the left ovary treated with 0.1 × PBS.
To evaluate the effect of recombinant VEGF, bFGF, and HGF on angiogenesis, sections of ovaries in which the biodegradable gel was transplanted were stained with anti-CD31 antibody every 40th section. First, the area in the range of 100 μm from the ovarian surface was measured using ImageJ. Then, the number of CD31-positive blood vessels localized within 100 μm of the ovarian surface was counted, as almost all primordial follicles were localized within 100 μm of the ovarian surface (data not shown). Finally, the density of the blood vessels was calculated.
To evaluate the effect of transplanted biodegradable gels containing 10 μg/mL recombinant VEGF on the expression of KITL in primordial follicles, the number of primordial follicles that expressed KITL was counted every 10th section. Then, the ratio of primordial follicles that expressed KITL in the right ovary was divided by the ratio in the left ovary. The ovaries used in this experiment were the same ovaries in which the number of activated primordial follicles was counted by staining with anti-FOXO3a antibody.
To investigate the relationship between the number of AcGFP1-positive follicles and the number of blood vessels in cultured P0 mouse ovaries, the number of CD31-positive blood vessels localized within 50 μm of the ovarian surface was counted every second section. Then, the density of the blood vessels was calculated. The preparation for the paraffin sections of cultured P0 mouse ovaries was performed in the same manner as that used for the ovaries in which a biodegradable gel was transplanted. The sections for counting the number of blood vessels were created from P0 mouse ovaries that were cultured for 48 h because AcGFP1-positive primary follicles were observed after culture day 2. The sections were also stained by anti-KITL antibody every second section, and the number of primordial follicles that expressed KITL was counted to analyze the effect of serum on the activation of primordial follicles.
Statistical analyses
Statistical analyses were performed using the software R (http://www.r-project.org/). First, the normality of the data was evaluated using the Shapiro-Wilk normality test. Then, the data were analyzed using one-way analysis of variance or with two-sample t-tests.
Results
Relationship between activated primordial follicles and blood vessels
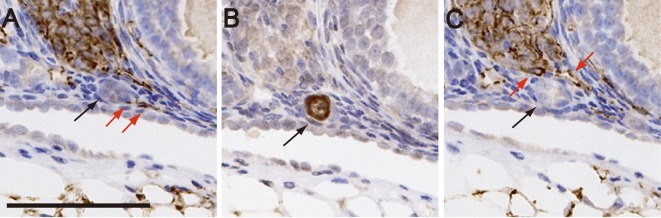
Serial sections of 4-week-old ICR mouse ovaries were stained alternately with anti-FOXO3a antibody and anti-CD31 antibody, and the number of activated primordial follicles that were in contact with blood vessels was counted (Fig. 1). The number of activated primordial follicles derived from both the ovaries of the tested mice was 335, while the number derived from each ovary was 210 and 125, respectively. Among the total number of activated primordial follicles, the number of activated primordial follicles that contacted blood vessels was 310 (92.5%; 310/335), with the number for each ovary being 191/210 and 119/125, respectively. In contrast, the number of primordial follicles that did not contact blood vessels was 1414, and almost all of them were in the dormant state (1389/1414) with the number for each ovary being 707/726 and 682/688, respectively.
Fig. 1.
Relationship between primordial follicles and blood vessels in the ovaries of 4-week-old female ICR mice. (A and C) Ovarian serial sections stained with anti-CD31 antibody. (B) Ovarian serial sections stained with anti- FOXO3a antibody. Black arrows in A, B, and C indicate the same activated primordial follicle. Red arrows indicate blood vessels that contact the activated primordial follicle. Scale bar, 100 μm.
Induction of angiogenesis in mouse ovaries using a biodegradable gel containing recombinant VEGF, bFGF, or HGF
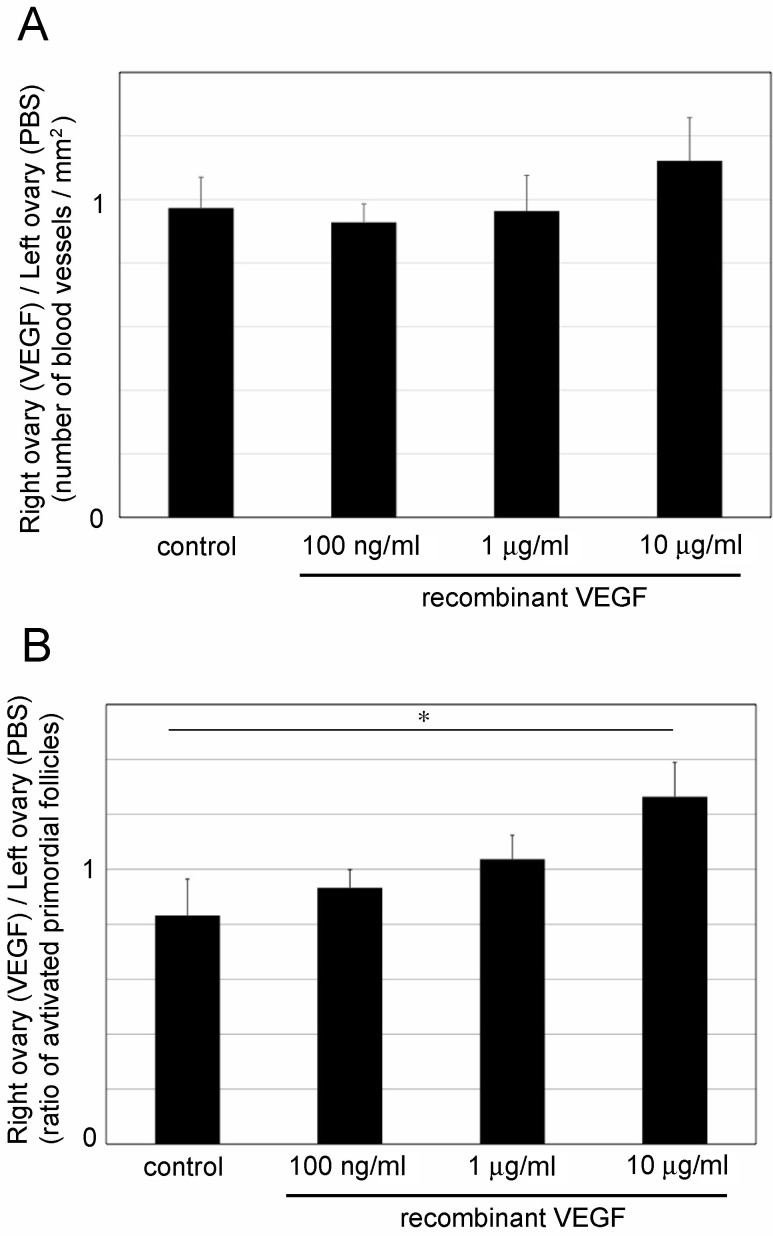
Transplanted biodegradable gels were completely digested at 5 to 10 days post-transplantation. Based on this, the ovaries were removed on day 5 after transplantation, and activated primordial follicles were counted. In this experiment, the condition of the primordial follicles was distinguished by the localization of FOXO3a in oocytes, and the ratios of activated primordial follicles in the right and left ovaries were compared to evaluate the effect of angiogenesis (Fig. 2 and Table 1). The number of blood vessels tended to increase in the ovaries that were transplanted with a biodegradable gel containing recombinant VEGF (Fig. 2A; 10 μg/ml VEGF: P = 0.068). In control mice, the ratio of activated primordial follicles in the left ovary tended to be higher than that in the right ovary (P = 0.09; Table 1). These results indicated that the number of activated primordial follicles was significantly increased by the explant of biodegradable gels containing 10 μg/ml of recombinant VEGF (Fig. 2B; control: 0.83 ± 0.13 and 10 μg/ml VEGF: 1.23 ± 0.13). This increase correlated with an increase of the number of blood vessels.
Fig. 2.
Effect of transplantation of biodegradable gels containing recombinant vascular endothelial growth factor (VEGF) in 10-week-old ICR mouse ovaries. (A) Number of blood vessels in the ovaries transplanted with a biodegradable gel containing recombinant VEGF. (B) Ratio of activated primordial follicles in the ovaries in which a biodegradable gel containing recombinant VEGF was transplanted. Mean ± SD (n = 3 for 10 μg/ml VEGF, n = 4 for 100 ng/ml and 1 μg/ml VEGF, and n = 5 for control). * P < 0.05.
Table 1. Number of activated and dormant follicles on day 5 after the transplantation of biodegradable gels.
| No. of mouse - L/R | Number of primordial follicles | R/L (ratio of activated primordial follicles) | ||
|---|---|---|---|---|
| active (/total) | dormant | |||
| Control | ||||
| 1-L | 81 (0.27) | 223 | 0.62 | |
| 1-R | 50 (0.17) | 251 | ||
| 2-L | 116 (0.20) | 471 | 0.97 | |
| 2-R | 112 (0.19) | 474 | ||
| 3-L | 63 (0.24) | 200 | 0.76 | |
| 3-R | 49 (0.18) | 219 | ||
| 4-L | 75 (0.17) | 372 | 0.83 | |
| 4-R | 45 (0.14) | 278 | ||
| 5-L | 59 (0.18) | 265 | 0.98 | |
| 5-R | 56 (0.18) | 259 | ||
| VEGF 100 ng/ml | ||||
| 1-L | 122 (0.25) | 359 | 0.82 | |
| 1-R | 112 (0.21) | 428 | ||
| 2-L | 86 (0.17) | 359 | 0.95 | |
| 2-R | 81 (0.16) | 434 | ||
| 3-L | 46 (0.20) | 187 | 0.97 | |
| 3-R | 57 (0.19) | 242 | ||
| 4-L | 73 (0.31) | 166 | 0.76 | |
| 4-R | 57 (0.23) | 188 | ||
| VEGF 1 μg/ml | ||||
| 1-L | 38 (0.18) | 172 | 1.00 | |
| 1-R | 37 (0.18) | 167 | ||
| 2-L | 37 (0.17) | 178 | 1.14 | |
| 2-R | 49 (0.20) | 200 | ||
| 3-L | 79 (0.27) | 214 | 1.08 | |
| 3-R | 77 (0.29) | 187 | ||
| 4-L | 54 (0.29) | 133 | 0.92 | |
| 4-R | 68 (0.26) | 189 | ||
| VEGF 10 μg/ml | ||||
| 1-L | 49 (0.16) | 252 | 1.22 | |
| 1-R | 65 (0.20) | 262 | ||
| 2-L | 47 (0.16) | 253 | 1.07 | |
| 2-R | 42 (0.17) | 208 | ||
| 3-L | 11 (0.20) | 45 | 1.40 | |
| 3-R | 19 (0.28) | 50 | ||
The averages of R/L for each group are shown in Fig. 2B. L, left ovary; R, right ovary. VEGF, vascular endothelial growth factor.
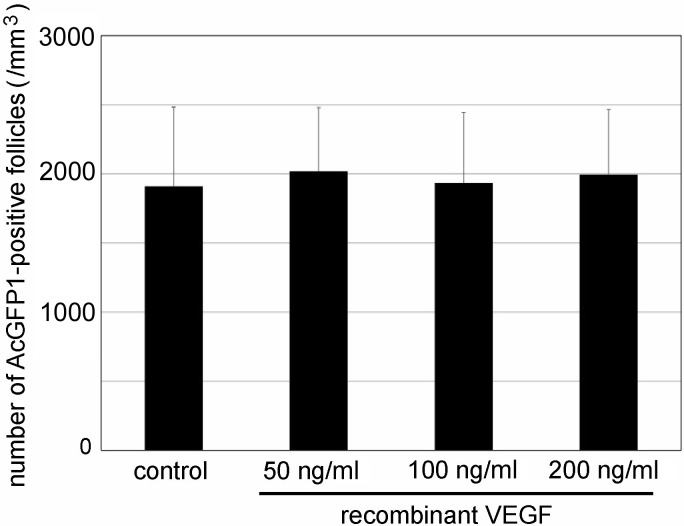
Recombinant VEGF was unable to directly promote the activation of dormant primordial follicles (Fig. 3). These results indicated that angiogenesis can promote the activation of dormant primordial follicles in mouse ovaries.
Fig. 3.
Effect of recombinant vascular endothelial growth factor (VEGF) on the activation of primordial follicles in cultured P0 Oog1pro3.9/R26-H2B-mCherry mouse ovaries. Mean ± SD (n = 3 for 200 ng/ml, n = 4 for 50 ng /ml, n = 5 for 100 ng /ml, and n = 12 for control.
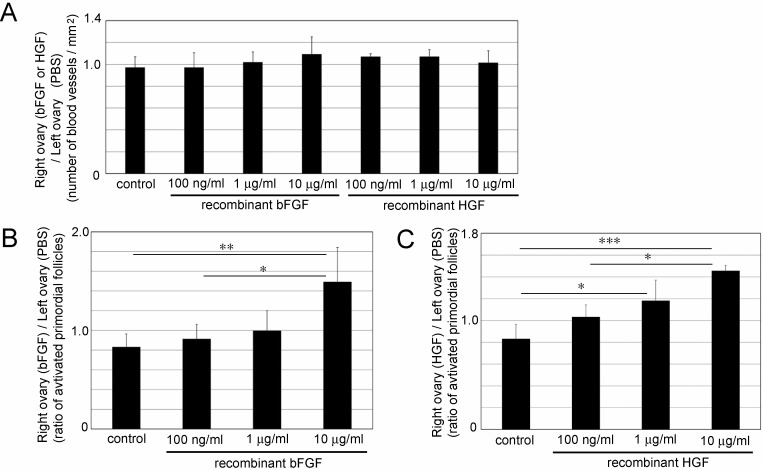
bFGF and HGF did not significantly increase the number of blood vessels (Fig. 4A); however, the number of activated primordial follicles was significantly increased by the transplantation of biodegradable gels containing 10 μg/ml recombinant bFGF (Fig. 4B; 1.49 ± 0.35) or 1 μg/ml and 10 μg/ml recombinant HGF (Fig. 4C; 1 μg/ml: 1.18 ± 0.19 and 10 μg/ml: 1.46 ± 0.05).
Fig. 4.
Effect of transplantation of a biodegradable gel containing recombinant basic fibroblast growth factor (bFGF) or hepatocyte growth factor (HGF) in 10-week-old ICR mouse ovaries. (A) Number of blood vessels in the ovaries transplanted with a biodegradable gel containing recombinant bFGF or HGF. (B) Ratio of activated primordial follicles in the ovaries in which a biodegradable gel containing recombinant bFGF was transplanted. (C) Ratio of activated primordial follicles in the ovaries in which a biodegradable gel containing recombinant HGF was transplanted. Mean ± SD (n = 4 for 100 ng/ml, 1 μg/ml and 10 μg/ml bFGF and HGF, and n = 5 for control). * P < 0.05, ** P < 0.01 and *** P < 0.005.
Visualization of the primordial to primary follicle transition under culture conditions
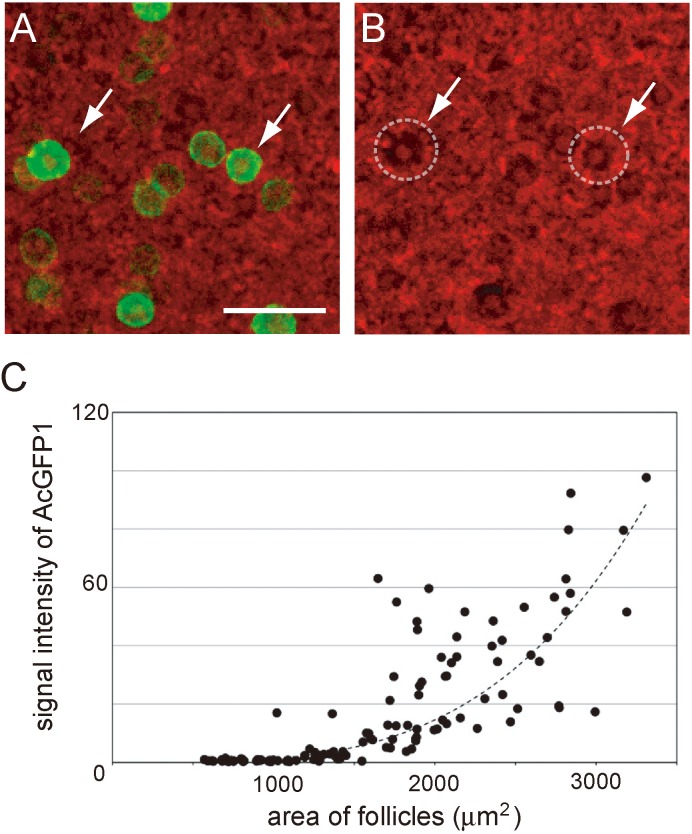
To visualize the primordial to primary follicle transition, we used transgenic mouse ovaries containing two transgenes (Oog1pro3.9 and ROSA26-H2B-mCherry). Oog1pro3.9 is the transgene that facilitates AcGFP1 gene binding to Oogenesin 1 (Oog1) promoter. Oog1 is an oocyte-specific gene expressed from the pachytene stage of the first meiosis [16, 20]. The expression gradually increases as with follicle growth, and we therefore captured cultured P0 mouse ovaries at 24 h intervals for 10 days using a confocal microscope that could visualize the AcGFP1 signal in primary follicles (Fig. 5A). The results indicated that the signal for AcGFP1 in almost all primordial follicles was too low to detect under this capturing condition. Then, the AcGFP1 signal gradually increased with follicle growth (Fig. 5B). In a previous report, we demonstrated that the area of the primordial follicles was < 1000 μm2 and that of primary follicles ranged from 1000 to 3000 μm2 [21]. Based on this, follicles in cultured P0 mouse ovaries were classified on the basis of the follicular area. As indicated by our results, the signal of AcGFP1 in almost all primary follicles was detectable, and AcGFP1 in all primordial follicles was negative under this capturing condition (Fig. 5B). Based on this, AcGFP1-positive follicles in P0 mouse ovaries that were cultured for 10 days were defined as primary follicles and AcGFP1-negative follicles were defined as primordial follicles. All images of P0 Oog1pro3.9/R26-H2B-mCherry mouse ovaries in this article were captured using these confocal microscopy conditions.
Fig. 5.
Relationship between follicle growth and AcGFP1 intensity. (A) Cultured P0 Oog1pro3.9/R26-H2B-mCherry mouse ovaries on culture day 5. Green, AcGFP1; red, mCherry. (B) Measurement of the follicle area by tracing the mCherry signal in the granulosa cells. White dashed lines represent the outline of each follicle. Panel A and B present the same area of the cultured P0 mouse ovary, and white arrows indicate the same follicles in A and B. (C) Relationship between the area of the follicles and AcGFP1 intensity. The dashed line represents the approximate curve, y = 0.1165e0.0023x (R2 = 0.764). Scale bar, 100 μm.
Effect of serum on the activation of dormant primordial follicles
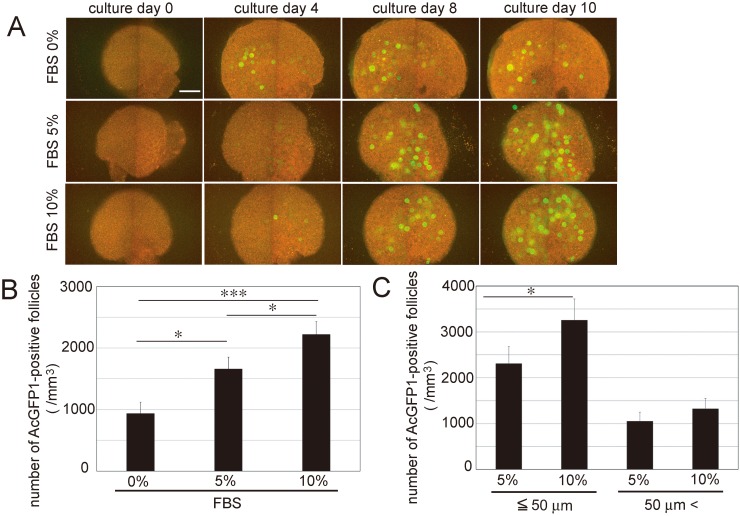
The ovaries of P0 transgenic mice were cultured in culture medium that contained 0%, 5%, or 10% FBS for 10 days, and the number of AcGFP1-positive primary follicles was counted on culture day 10. Their number increased according to the concentration of FBS (Fig. 6A and B; 0% FBS: 935.0 ± 183.7, 5% FBS: 1658.3 ± 192.3, and 10% FBS: 2220.6 ± 210.7). There was no significant difference between the number at 10% and 20% FBS (20% FBS: 2093.7 ± 243.4).
Fig. 6.
Culture of P0 Oog1pro3.9/R26-H2B-mCherry mouse ovaries. (A) Cultured P0 Oog1pro3.9/R26-H2B-mCherry mouse ovaries. (B) Density of AcGFP1-positive follicles in cultured P0 mouse ovaries on culture day 10. (C) Density of AcGFP1-positive follicles greater than or less than 50 μm from the ovarian surface of cultured P0 mouse ovaries on culture day 10. Mean ± SD (n = 3). Scale bar, 100 μm. * P < 0.05, *** P < 0.005.
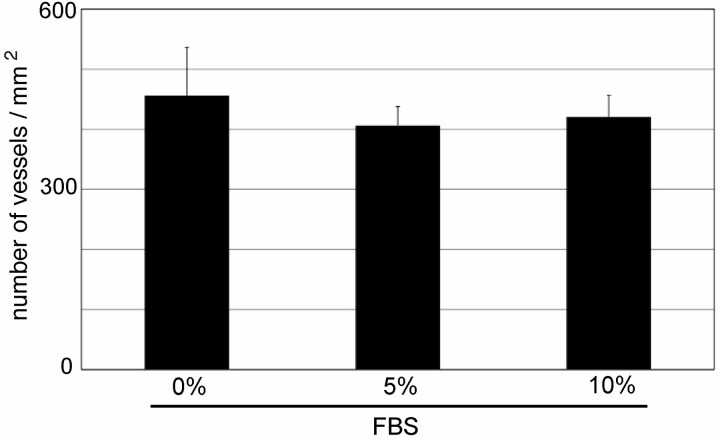
In addition to the number of AcGFP1-positive primary follicles, we investigated their localization in cultured ovaries on culture day 10. We found that the number of AcGFP1-positive primary follicles primarily increased within 50 μm of the ovarian surface (Fig. 6C). In the area within 50 μm of the ovarian surface, the number of blood vessels was not different among each group (Fig. 7). These results indicated that primordial follicles in the cultured ovaries were activated by the supply of serum components from the culture medium.
Fig. 7.
Number of blood vessels within 50 μm of the surface of the cultured P0 mouse ovaries. Mean ± SD (n = 3 for 10% fetal bovine serum (FBS), n = 4 for 5% FBS and n = 7 for 0% FBS).
Expression of KITL in primordial follicles
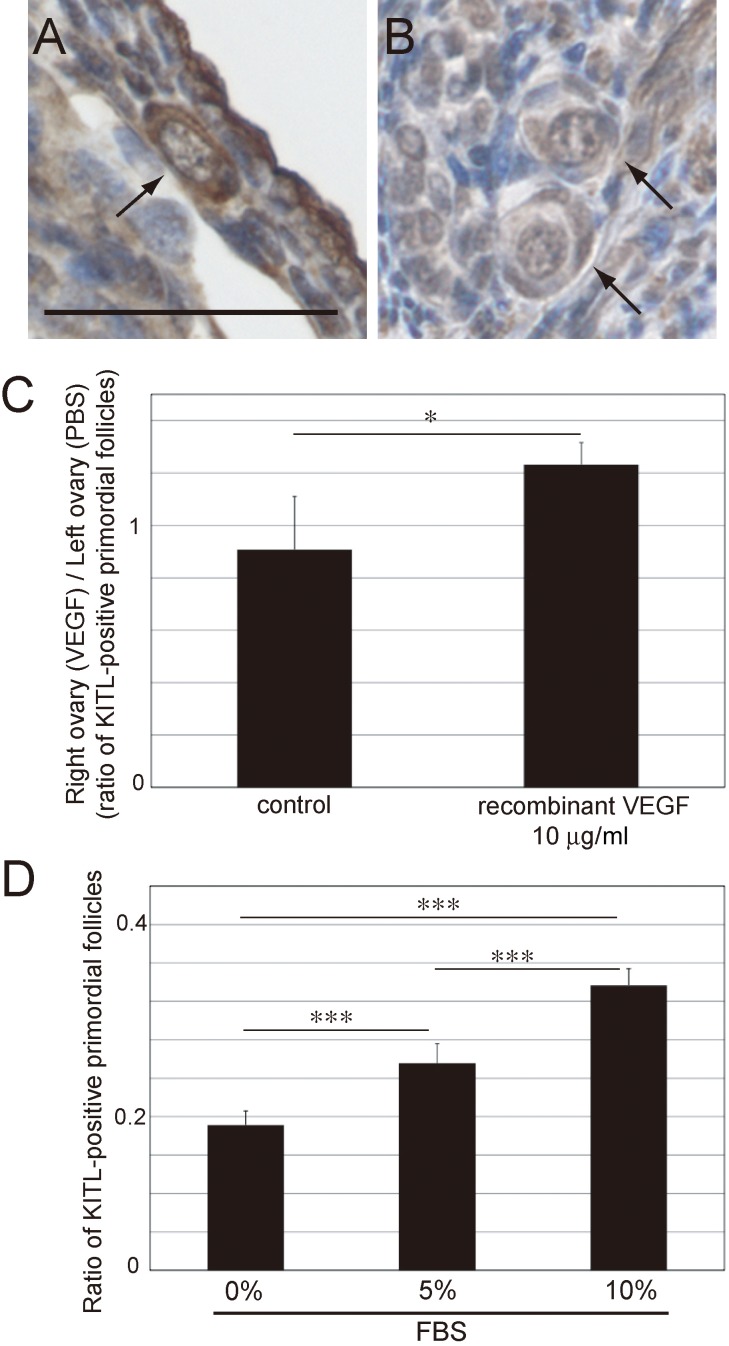
To confirm if angiogenesis and FBS increased the expression of KITL in primordial follicles, we counted the number of primordial follicles that were positive for KITL expression (Fig. 8A–C). After its expression in granulosa cells, KITL binds to KIT on the oocyte, and the KIT-KITL complex moves into the cytoplasm of oocytes [22]. Based on this, the number of primordial follicles that expressed KITL in granulosa cells or oocytes was counted in this experiment (Fig. 8A). As indicated by our results, the expression of KITL increased in the ovaries that were transplanted with biodegradable gel containing 10 μg/ml recombinant VEGF (Fig. 8C). Furthermore, we confirmed the expression of KITL in cultured P0 mouse ovaries on culture day 2. Our results indicated that the expression of KITL increased as FBS concentration was increased (Fig. 8D). These results indicated that the supply of serum components from blood vessels promoted the activation of dormant primordial follicles via the KITL-KIT signaling pathway.
Fig. 8.
Expression of KIT ligand (KITL) in 10-week-old ICR mouse ovaries in which a biodegradable gel containing vascular endothelial growth factor (VEGF) was transplanted and in cultured P0 mouse ovaries. (A) A primordial follicle that expressed KITL (KITL-positive primordial follicles) in a 10-week-old ICR mouse ovary. (B) Primordial follicles that did not express KITL in a 10-week-old ICR mouse ovary. (C) Ratio of KITL-positive primordial follicles in the ovaries in which a biodegradable gel containing 10 μg/ml recombinant VEGF was transplanted. (D) Ratio of KITL-positive primordial follicles in the cultured P0 mouse ovaries. Arrows indicate primordial follicles. Mean ± SD (n = 3 for 10 μg/ml recombinant VEGF, n = 5 for control; n = 3 for 10% fetal bovine serum (FBS), n = 4 for 5% FBS and n = 7 for 0% FBS). Scale bar: 50 μm. * P < 0.05, *** P < 0.005.
Discussion
Antral follicles possess a vascular network within the thecal layer, and in contrast, pre-antral follicles have no surrounding vascular network and rely on oxygen and nutrients that are supplied by stromal blood vessels [23]. In this study, we demonstrated that activated primordial follicles contacted blood vessels and that primordial follicles lacking contact with blood vessels were dormant (Fig. 1). This finding presented two possibilities. The first is that angiogenesis is induced by activated primordial follicles and new blood vessels make contact with them, and the second is that the contact with blood vessels activates dormant primordial follicles. The results in Fig. 6C indicated that primordial follicles near the ovarian surface in the cultured ovary were vigorously activated by the addition of 10% FBS. The number of blood vessels in this area was not different among the cultured P0 mouse ovaries; however, the expression of KITL was promoted according to the increased FBS concentration (Figs. 7 and 8). These results suggest that the serum component must be responsible for the activation of dormant primordial follicles. Furthermore, the induction of angiogenesis caused by the transplantation of biodegradable gels containing recombinant VEGF promoted the formation of blood vessels and the activation of dormant primordial follicles (Fig. 2). These results revealed that the activation of dormant primordial follicles was promoted by the supply of serum components from new blood vessels formed by angiogenesis in the ovaries. Serum contains many substances, and it is therefore difficult to identify the most important factor in serum that induces the activation of dormant primordial follicles. Nutrients and growth factors that circulate in the blood do enable the activation of mTOR signaling, and activated mTOR signaling in the granulosa cells of the primordial follicles promotes the expression of KITL [1, 11, 24]. Therefore, we considered that the supply of serum components from the blood vessels activates mTOR signaling in the granulosa cells and promotes the expression of KITL. Given this, it is likely that primordial follicles were activated thorough the PI3K-AKT-FOXO3 signaling pathway that was activated by KIT-KITL signaling. To confirm this hypothesis, we analyzed the expression of KITL in the ovaries that were transplanted with a biodegradable gel containing 10 μg/mL recombinant VEGF and in cultured P0 mouse ovaries (Fig. 8). These results indicated that the increased number of blood vessels and the supply of serum components promoted the expression of KITL in primordial follicles (Fig. 8C and D). Remodeling of the vascular network and angiogenesis occurred in the ovary during every estrous cycle, and it is therefore likely that the periodic activation of dormant primordial follicles is induced by angiogenesis.
A mouse ovary is enveloped by a bursa, and based on this, the transplanted biodegradable gel can remain in the bursa and release recombinant VEGF to the ovary for long periods of time. Additionally, most of primordial follicles localize near the ovarian surface in adult mice. Based on this, our experiment involving the transplantation of biodegradable gels is ideal to evaluate the relationship between angiogenesis and the activation of dormant primordial follicles. We observed that the transplantation of a biodegradable gel containing recombinant VEGF induced angiogenesis and promoted the activation of dormant primordial follicles in vivo (Figs. 2 and 8). When we removed the ovaries 5 days after transplantation, ovulated oocytes were present in some mice, indicating that the transplantation of biodegradable gel did not disturb the ovarian physiology. In addition to recombinant VEGF, we transplanted biodegradable gels containing recombinant bFGF and HGF. These factors are known to induce angiogenesis [25]. Both factors promoted the activation of dormant primordial follicles in the same manner as did VEGF; however, they did not increase the number of blood vessels in the ovaries (Fig. 4). During the 5 days following the transplantation of biodegradable gels, ovulation and other physiological phenomena occurred normally in the ovaries, and it is possible that these phenomena may affect the number of blood vessels. Although VEGF did not directly activate dormant primordial follicles (Fig. 3), it was previously reported that bFGF and HGF directly activated dormant primordial follicles [26, 27]. Therefore, their effect on the activation of dormant primordial follicles was stronger than that of recombinant VEGF (Figs. 2B, 4B and 4C).
Interestingly, the ratio of activated primordial follicles in the left ovaries of control mice was higher than that in the right ovaries (Fig. 2 and Table 1). We speculated that the high ratio of activated primordial follicles in the left ovaries is related to the anatomical feature of the left ovarian vein. The right gonadal vein directly drains into the inferior vena cava, while the left gonadal vein drains into the left renal vein. Due to this anatomical difference, the hemodynamics in the left testicular vein are weaker than those in the right testicular. It is considered that these weak hemodynamics cause left varicocele [28]. We confirmed that the left ovarian vein of a mouse also drains into the left renal vein (data not shown), and based on this, the anatomical difference may affect the activation efficiency of dormant primordial follicles in the ovaries.
The form and number of the granulosa cell layer is the criteria for the classification of follicle stage in almost all studies; however, this classification is not appropriate to observe the process of follicle growth in cultured ovaries in real time. In particular, it is difficult to distinguish between primordial and early primary follicles in the cultured ovary. In this study, we used ovaries from Oog1pro3.9 mice. Oog1 expresses in the oocytes of all follicle stages, and the expression is gradually increased according to follicle development (Fig. 5). Therefore, it is possible to observe the primordial to primary transition by the change in signal intensity of AcGFP1 in the cultured ovaries of Oog1pro3.9 mice. The localization of FOXO3a indicates the condition of primordial follicles, but it is not clear if activated primordial follicles directly grow into primary follicles. We considered that activated primordial follicles may revert back to a dormant state before growing into primary follicles, as there are dormant primordial follicles of various sizes in mouse ovaries. Therefore, it is important to thoroughly observe the growing process from the primordial to primary follicle stage to clearly determine the effect of angiogenesis and FBS on the growth of primordial follicles. The culture method using Oog1pro3.9 mice revealed that FBS can promote not only the activation of dormant primordial follicles but also the primordial to primary transition (Figs. 6 and 8).
Our results demonstrated that the supply of serum substances from blood vessels provides the cue for the activation of dormant primordial follicles in mouse ovaries. Serum in the culture medium activated only a portion of dormant primordial follicles, however, so there must be some multi-regulatory system that controls the activation and the growth of primordial follicles in the ovary. To control the growth of primordial follicles artificially, we need to understand this multi-regulatory system that functions in the gonadotropin-independent stage in more detail.
Acknowledgments
We thank Naojiro Minami for providing Oog1pro3.9 mice. This research was supported by JSPS (KAKENHI # JP18K06881).
References
- 1.Zhang H, Risal S, Gorre N, Busayavalasa K, Li X, Shen Y, Bosbach B, Brännström M, Liu K. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol 2014; 24: 2501–2508. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson EE, Skinner MK. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod 2003; 69: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson EE, Skinner MK. Kit ligand and basic fibroblast growth factor interactions in the induction of ovarian primordial to primary follicle transition. Mol Cell Endocrinol 2004; 214: 19–25. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol 2002; 188: 65–73. [DOI] [PubMed] [Google Scholar]
- 5.Kezele PR, Nilsson EE, Skinner MK. Insulin but not insulin-like growth factor-1 promotes the primordial to primary follicle transition. Mol Cell Endocrinol 2002; 192: 37–43. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of Anti-Müllerian Hormone (AMH) on ovarian primordial follicle assembly. PLoS One 2011; 6: e20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2003; 301: 215–218. [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development 2007; 134: 199–209. [DOI] [PubMed] [Google Scholar]
- 9.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S, Sugishita Y, Morimoto Y, Hosoi Y, Yoshioka N, Ishizuka B, Hsueh AJ. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA 2013; 110: 17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adhikari D, Flohr G, Gorre N, Shen Y, Yang H, Lundin E, Lan Z, Gambello MJ, Liu K. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod 2009; 15: 765–770. [DOI] [PubMed] [Google Scholar]
- 11.Hsueh AJ. Fertility: the role of mTOR signaling and KIT ligand. Curr Biol 2014; 24: R1040–R1042. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan EK, Hsueh AJ. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA 2010; 107: 10280–10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol 2008; 321: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008; 319: 611–613. [DOI] [PubMed] [Google Scholar]
- 15.Abe T, Kiyonari H, Shioi G, Inoue K, Nakao K, Aizawa S, Fujimori T. Establishment of conditional reporter mouse lines at ROSA26 locus for live cell imaging. Genesis 2011; 49: 579–590. [DOI] [PubMed] [Google Scholar]
- 16.Ishida M, Okazaki E, Tsukamoto S, Kimura K, Aizawa A, Kito S, Imai H, Minami N. The promoter of the oocyte-specific gene, Oog1, functions in both male and female meiotic germ cells in transgenic mice. PLoS One 2013; 8: e68686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai S, Takagi Y, Kaneko S, Kurosawa T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp Anim 2011; 60: 481–487. [DOI] [PubMed] [Google Scholar]
- 18.Danforth DR, Arbogast LK, Ghosh S, Dickerman A, Rofagha R, Friedman CI. Vascular endothelial growth factor stimulates preantral follicle growth in the rat ovary. Biol Reprod 2003; 68: 1736–1741. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu K, Iwase A, Murase T, Masubuchi S. Ovarian tissue culture to visualize phenomena in mouse ovary. J Vis Exp 2018. doi: 10.3791/57794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minami N, Aizawa A, Ihara R, Miyamoto M, Ohashi A, Imai H. Oogenesin is a novel mouse protein expressed in oocytes and early cleavage-stage embryos. Biol Reprod 2003; 69: 1736–1742. [DOI] [PubMed] [Google Scholar]
- 21.Komatsu K, Koya T, Wang J, Yamashita M, Kikkawa F, Iwase A. Analysis of the effect of leukemia inhibitory factor on follicular growth in cultured murine ovarian tissue. Biol Reprod 2015; 93: 18. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Zhang Y, Li J, Zheng N, Xu X, Yang J, Xia G, Zhang M. MAPK3/1 participates in the activation of primordial follicles through mTORC1-KITL signaling. J Cell Physiol 2018; 233: 226–237. [DOI] [PubMed] [Google Scholar]
- 23.Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol 2006; 4: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149: 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaga T, Kawano H, Sakaguchi M, Nakazawa T, Taniyama Y, Morishita R. Hepatocyte growth factor stimulated angiogenesis without inflammation: differential actions between hepatocyte growth factor, vascular endothelial growth factor and basic fibroblast growth factor. Vascul Pharmacol 2012; 57: 3–9. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol 2001; 175: 123–130. [DOI] [PubMed] [Google Scholar]
- 27.Guglielmo MC, Ricci G, Catizone A, Barberi M, Galdieri M, Stefanini M, Canipari R. The effect of hepatocyte growth factor on the initial stages of mouse follicle development. J Cell Physiol 2011; 226: 520–529. [DOI] [PubMed] [Google Scholar]
- 28.Itoh M, Moriyama H, Tokunaga Y, Miyamoto K, Nagata W, Satriotomo I, Shimada K, Takeuchi Y. Embryological consideration of drainage of the left testicular vein into the ipsilateral renal vein: analysis of cases of a double inferior vena cava. Int J Androl 2001; 24: 142–152. [DOI] [PubMed] [Google Scholar]