Abstract
Pore formation by membrane-active peptides, naturally encountered in innate immunity and infection, could have important medical and technological applications. Recently, the well-studied lytic peptide melittin has formed the basis for the development of combinatorial libraries from which potent pore-forming peptides have been derived, optimized to work under different conditions. We investigate three such peptides, macrolittin70, which is most active at neutral pH; pHD15, which is active only at low pH; and MelP5_Δ6, which was rationally designed to be active at low pH but formed only small pores. There are three, six, and six acidic residues in macrolittin70, pHD15, and MelP5_Δ6, respectively. We perform multi-microsecond simulations in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) of hexamers of these peptides starting from transmembrane orientations at neutral pH (all residues at standard protonation), low pH (acidic residues and His protonated), and highly acidic environments in which C-termini are also protonated. Previous simulations of the parent peptides melittin and MelP5 in 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) are repeated in POPC. We find that the most potent pore-forming peptides exhibit strong interpeptide interactions, including salt bridges, H-bonds, and polar interactions. Protonation of the C-terminus promotes helicity and pore size. The proximity of the peptides allows fewer lipid headgroups to line the pores than in previous simulations, making the pores intermediate between barrel stave and toroidal. Based on these structures and geometrical arguments, we attempt to rationalize the factors that under different conditions can increase or decrease pore stability and propose mutations that could be tested experimentally.
Significance
Many peptides are able to form pores in biological membranes, and this process is of great biological, pharmaceutical, and technological interest. Here, we use computer simulations of peptides whose pore-forming ability has been experimentally characterized to understand which molecular features are responsible for their potency or lack thereof. We find that strong interactions between adjacent peptides are favorable for pore formation. In contrast, interactions between nonadjacent peptides, often involving a charged C-terminus, hinder the formation of a large pore. We propose a number of sequence changes that should, according to our results, improve or impair pore formation. If verified by experiment, this work could lead to better rational design of pore-forming peptides.
Introduction
Pore formation in lipid bilayers by membrane-active peptides has attracted interest from the viewpoint of both medicine (1,2) and technology (3,4). Such peptides include the naturally produced antimicrobial peptides (AMPs) as well as synthetic analogs. In their native form, AMPs have seen limited clinical use (5). Nonetheless, they provide an attractive template for membrane-targeted peptide-based therapy (2), and their cationic charge makes them inherently more attracted to bacteria (6).
Melittin from the venom of Apis melifera is one of the best-studied membrane-active peptides. It is a lytic peptide targeting both mammalian and bacterial membranes, but it has many common features with the AMPs that target preferentially bacterial membranes. It has been the subject of an immense number of clinical, biophysical, electrophysiological, and computational studies (7, 8, 9, 10, 11, 12), and its pore formation mechanism has been studied with a variety of methods (13, 14, 15, 16, 17). Under usual conditions, melittin makes only transient pores (18).
Over the past few years, Wimley and co-workers pioneered the use of combinatorial libraries to discover membrane-active peptides with higher pore-forming ability. The first effort based on melittin led to the MelP5 family, which can make stable, large pores at a much lower peptide concentration (19,20). Subsequently, MelP5 has served as the basis for discovering second-generation peptides that form large pores either at low pH (pHD) (21) or at neutral pH (macrolittins) (22). Notably, efforts at rational design of low-pH active peptides were less successful (23). Analysis of the dependence of the pHD peptide action on pH and peptide concentration showed that pore formation is solely dependent on the bound peptide-to-lipid ratio (24). AFM studies offered direct evidence of pore formation by these peptides (24,25).
Previous work from our group on pore-forming peptides included both implicit and explicit solvent simulations. For example, implicit solvent modeling found that AMPs bind more strongly to pores than to the flat membrane (26,27). A number of microsecond-scale explicit solvent simulations were carried out on the Anton supercomputer. A tetramer of melittin starting from a transmembrane orientation relaxed in 9 μs into a well-defined toroidal pore in DMPC (28), whereas peptides of the magainin family created thin water defects in a funnel-like arrangement (29). A hexamer of MelP5 remained around a sizable aqueous pore, whereas a hexamer of melittin broke apart (30). Barrel structures of protegrin with a positive shear were stable, but those with zero shear were unstable (31).
Here, we investigate some of the aforementioned potent pore-forming peptides discovered by combinatorial library screening: one macrolittin, which is most active at neutral pH (22); one pHD peptide, which is active only at low pH (21); and one rationally designed pH-sensitive analog of MelP5 that failed to form large pores (23). Table 1 shows the sequence of these peptides along with their progenitors. MelP5 essentially removes positive charge from the C-terminus (Ct) of melittin. Macrolittin70 (mac70) adds three E residues and changes Gly12 to Leu and Ile17 to Gln. pHD15 adds three more acidic residues and changes two Lys residues to His. MelP5_Δ6 has the same number of acidic residues as pHD15, in partly different locations, but lacks completely basic residues. The comparison of melittin and MelP5 in DMPC was the subject of a previous publication (30). Here, we focus on the last three peptides and ask the following questions: 1) Why are mac70 and, at low pH, pHD15, better pore formers than MelP5? And 2) why does MelP5_Δ6 make smaller pores than pHD15 under the same conditions?
Table 1.
Sequence of Peptides Related to This Study
| Peptide: | Sequence: |
|---|---|
| Melittin | GIGAVLKVLT TGLPALISWI KRKRQQ |
| MelP5 | ---------A ---------- -AAQ-L |
| mac70 | ---E---E-A -L--E-Q--- -AAQ-L |
| pHD15 | ---E--HE-A DD--D-QE-- HAAQ-L |
| MelP5_Δ6 | ------EE-A DD-----D-- EAAQ-L |
Only the differences of each peptide from the sequence of melittin are marked with the symbol of the newly introduced amino acid.
Methods
Hexameric starting structures were prepared as previously described for MelP5 (29,30). That is, six peptides (named A through F) in ideal helical conformation and in parallel, transmembrane orientation were placed on a circle of radius ∼6 Å, with the hydrophobic face oriented toward the lipids (see Fig. S6). The choice of parallel hexamers needs to be explained. These peptides likely form heterogeneous pores with varying oligomeric states. In addition, some of them generate macromolecule-size pores, likely involving many more than six peptides. Ideally, a variety of oligomers and initial configurations should be considered, but this was not feasible with the available computational resources. Hexamers were chosen because they are small enough to be easily simulated and large enough to exhibit an interesting difference in behavior between melittin and MelP5 (30). Parallel helix arrangements were chosen because they are more consistent with experiment for melittin and MelP5 (30), although a theoretical proof of their higher stability is lacking.
Low pH was emulated by protonating the His, Glu, and Asp residues and, in some simulations, also the Ct. The pKa values of these titratable groups in small peptides are 6.6, 4.3, 3.9, and 3.7, respectively (32), but could be raised by interactions between such residues in our systems. In any case, Asp, Glu, and Ct may not be fully protonated at pH 5, at which most experiments are done. Ideally, a constant-pH simulation (33) would dynamically select the correct protonation state depending on configuration and pH value. Because such simulations are not feasible on the timescale desired here and on the computer platform used, we performed two sets of simulations: one (“low pH”) with all His, Glu, and Asp protonated but the Ct unprotonated and another (“very low pH”) with the Ct also protonated, emulating a highly acidic environment. The intrinsic pKa of the Ct is not much lower than that of Asp, but its relatively large distance from the other negative charges might make its pKa less affected by the surroundings. Lys and Arg residues are taken to be fully charged under the conditions considered here. Although Lys residues could deprotonate in a hydrophobic environment at neutral pH (34, 35, 36), in our simulations they are always fully solvated by water (see Table S10) and thus unlikely to experience large pKa shifts from solution.
Systems with balancing counter ions were built using the CHARMM-GUI server (37) using 180 POPC lipids and a water slab at least 17.5 Å thick (Table 2). Thus, the P:L ratio was 1:30, which is high enough for pore formation by all studied peptides, including melittin (20). Subsequently, 10- to 20-μs simulations were performed on the Anton 2 special-purpose computer (38) in the NPT ensemble using the CHARMM 36 force field (39) and TIP3P water (40). The equations of motion were integrated using the multigrator method (41). Bond constraints implemented in Anton (42) allowed a 2.5-fs timestep. A temperature of 310 K and a pressure of 1 bar were maintained using the Nose-Hoover thermostat (43) and the Martyna, Tobias, and Klein (MTK) barostat (44), respectively. Cutoff distances for nonbonded interactions were determined automatically by Anton.
Table 2.
Systems Simulated
| System | Atoms | Waters | Ions | Duration (μs) | Dimensions: x, y, z (Å) |
|---|---|---|---|---|---|
| Melittin | 47,655 | 6967 | 30 Cl− | 10 | 85.0, 85.0, 74.0 |
| MelP5 | 49,170 | 7548 | 12 Cl− | 10 | 84.2, 84.2, 77.0 |
| pHD15, pH 7 | 49,110 | 7518 | 36 K+ | 10 | 84.2, 84.2, 77.2 |
| pHD15, low pH | 49,131 | 7517 | 12 Cl− | 10 | 84.2, 84.2, 77.2 |
| pHD15, very low pH | 48,963 | 7457 | 18 Cl− | 20 | 86.0, 86.0, 77.1 |
| mac70, pH 7 | 49,257 | 7541 | 6 K+ | 10 | 84.8, 84.8, 77 |
| mac70, low pH | 49,227 | 7523 | 12 Cl− | 10 | 84.8, 84.8, 77 |
| mac70, very low pH | 48,903 | 7411 | 18 Cl− | 10 | 86.5, 86.5, 76.1 |
| MelP5_Δ6, low pH | 48,792 | 7436 | 0 | 10 | 85.5, 85.5, 77.6 |
| MelP5_Δ6, very low pH | 48,783 | 7429 | 6 Cl− | 20 | 86.1, 86.1, 76.9 |
All contain six peptides and 180 POPC lipids.
It would have been ideal to perform multiple simulations of each system to assess the statistical reliability of our observations. However, this was unfeasible for all the systems considered with the available computational resources. Needing the long timescale to ensure at least partial equilibration of the pore structures, we opted to cover more peptides and protonation state conditions rather than ensure statistical reliability for fewer peptides under fewer conditions. The goal of this initial exploration was to obtain qualitative pointers for further experimentation rather than converged quantitative results.
Pore radii as a function of z were estimated by calculating the volume occupied by water in 1-Å-thick slabs parallel to the membrane using the COOR SEARCH command of the CHARMM program (45). An effective radius was extracted assuming that each slab is circular. Although this approach does not ensure that the waters counted are contiguous, we verified visually that this indeed is the case in our simulations. The minimal value of radius along z is taken to be the pore radius (29,30). Helix tilt angles were computed using the COOR HELIX command in CHARMM, which implements an algorithm by Aqvist (46), and helicity was computed using CPPTRAJ (47), which implements the DSSP algorithm (48). We calculate average peptide-peptide, peptide-lipid, and all residue-residue pair interaction energies over the last 2 μs of the simulations using the CHARMM INTE command. Only short-range interactions are included in this calculation.
Results
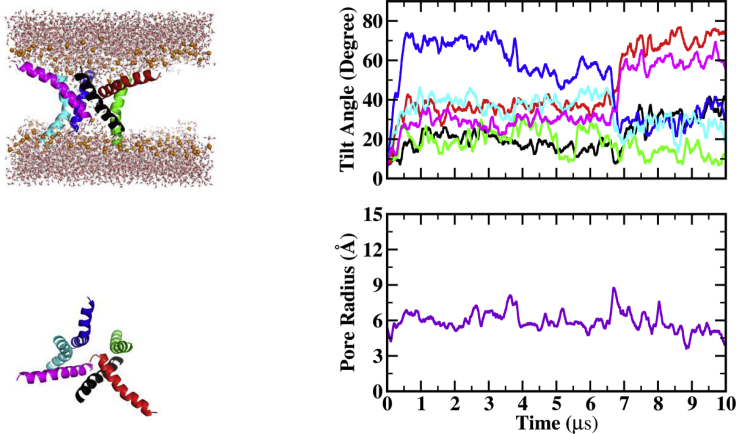
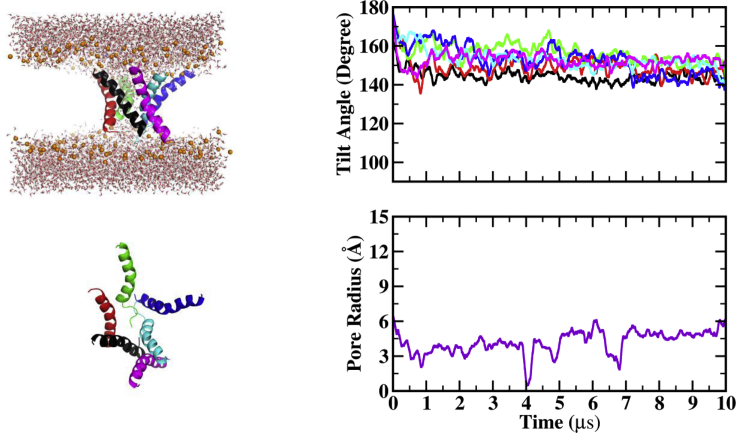
Whereas previous work from our group was done in DMPC lipids (28, 29, 30), all simulations in this work were carried out in thicker POPC membranes because this is the lipid used in the relevant experiments (21,22). To assess the effect of the lipid and provide a proper reference for comparison, previous simulations of melittin and MelP5 in DMPC are repeated here in POPC. Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 show the last snapshot and plots of the pore radius and peptide tilt angles for each of the systems studied. In these figures, the following colors are used: A, black; B, red; C, green; D, blue; E, cyan; and F, magenta. In side snapshots, phosphorus atoms are shown as orange spheres. There are six peptides in each simulation, so 15 protein pair interactions can occur, with six of them between adjacent peptides (i.e., A-B, B-C, C-D, D-E, E-F, and A-F) and the rest between nonadjacent peptides. Tables S1–S9 show the first seven or eight important interactions averaged over the last 2 μs of each simulation. For each of them, the first row shows peptide pairs, and the next three rows show the three most important residue pair interactions for the corresponding peptide pair. The first letter in the name of each residue represents the peptide’s name, and the other characters show amino acid letter and sequence number (the 26th residue includes the Ct). The state of each peptide can be defined according to its tilt angle: inserted state or I-state (0–30°/150–180°), tilted state or T-state (30–60°/120–150°), and surface state or S-state (60–120°) (29). Table 3 shows results for pore radius, peptide tilt angle, number of water molecules in the pore, number of lipid headgroups in the pore, and average helicity over the last 2 μs.
Figure 1.
Last frame, pore radius and tilt angle for melittin hexamer. To see this figure in color, go online.
Figure 2.
Last frame, pore radius and tilt angle for MelP5 hexamer. To see this figure in color, go online.
Figure 3.
Last frame for pHD15 hexamer at neutral pH. To see this figure in color, go online.
Figure 4.
Last frame, pore radius and tilt angle for pHD15 hexamer at low pH with C-terminus deprotonated. To see this figure in color, go online.
Figure 5.
Last frame, pore radius and tilt angle for pHD15 hexamer at highly acidic conditions with C-terminus protonated. To see this figure in color, go online.
Figure 6.
Last frame, pore radius and tilt angle for mac70 hexamer at neutral pH. To see this figure in color, go online.
Figure 7.
Last frame, pore radius and tilt angle for mac70 hexamer at low pH with C-terminus deprotonated. To see this figure in color, go online.
Figure 8.
Last frame, pore radius and tilt angle for mac70 hexamer at highly acidic conditions with C-terminus protonated. To see this figure in color, go online.
Figure 9.
Last frame, pore radius and tilt angle for MelP5_Δ6 hexamer at low pH with C-terminus deprotonated. To see this figure in color, go online.
Figure 10.
Last frame, pore radius and tilt angle for MelP5_Δ6 hexamer at highly acidic conditions with C-terminus protonated. To see this figure in color, go online.
Table 3.
Pore Radius (Å), Tilt Angles (Degrees), Number of Waters inside the Pore, Number of Lipid Headgroups (Phosphorus) inside the Pore, and Average Helicity (%) in Different Systems over the Last 2 μs
| Quantity | Melittin | MelP5 | pHD15, Low pH | pHD15, Very Low pH | ||
|---|---|---|---|---|---|---|
| Radius (Å) | 4 ± 2 | 5 ± 1 | 4 ± 1 | 9 ± 1 | – | |
| Tilt angles (degrees) | A | 17 ± 7 | 33 ± 6 | 163 ± 5 | 130 ± 10 | |
| B | 33 ± 6 | 71 ± 6 | 165 ± 5 | 138 ± 9 | – | |
| C | 19 ± 8 | 13 ± 6 | 151 ± 6 | 133 ± 6 | – | |
| D | 12 ± 6 | 32 ± 7 | 145 ± 6 | 129 ± 8 | – | |
| E | 14 ± 6 | 27 ± 8 | 156 ± 6 | 137 ± 7 | – | |
| F | 13 ± 7 | 60 ± 6 | 138 ± 5 | 130 ± 10 | – | |
| All | 18 ± 10 | 39 ± 21 | 153 ± 11 | 133 ± 9 | – | |
| Water | 117 ± 29 | 146 ± 29 | 173 ± 22 | 308 ± 55 | – | |
| Phosphorus | 5 ± 2 | 4 ± 2 | 5 ± 2 | 8 ± 2 | – | |
| Helicity (%) | 79 | 83 | 74 | 85 | – | |
| mac70, pH 7 | mac70, Low pH | mac70, Very Low pH | MelP5_Δ6, Low pH | MelP5_Δ6, Very Low pH | ||
| Radius (Å) | 7 ± 1 | 5 ± 1 | 10 ± 1 | 6 ± 1 | 4 ± 1 | |
| Tilt angles (degrees) | A | 131 ± 7 | 144 ± 5 | 134 ± 7 | 40 ± 11 | 59 ± 5 |
| B | 140 ± 7 | 146 ± 6 | 140 ± 7 | 89 ± 7 | 45 ± 5 | |
| C | 131 ± 5 | 151 ± 5 | 140 ± 6 | 27 ± 8 | 28 ± 6 | |
| D | 145 ± 8 | 144 ± 6 | 118 ± 8 | 51 ± 8 | 19 ± 5 | |
| E | 132 ± 10 | 150 ± 5 | 135 ± 7 | 37 ± 7 | 50 ± 5 | |
| F | 138 ± 7 | 153 ± 4 | 133 ± 8 | 41 ± 10 | 28 ± 5 | |
| All | 136 ± 9 | 148 ± 6 | 133 ± 10 | 47 ± 22 | 38 ± 15 | |
| Water | 268 ± 37 | 149 ± 25 | 354 ± 52 | 166 ± 32 | 177 ± 22 | |
| Phosphorus | 4 ± 2 | 4 ± 1 | 8 ± 2 | 7 ± 2 | 6 ± 2 | |
| Helicity (%) | 84 | 80 | 88 | 80 | 81 | |
Melittin hexamer
Fig. 1 shows results from the simulation of the melittin hexamer in POPC. The initial pore closes gradually within 1 μs but then grows and reaches a maximum of 11 Å at 4 μs. This large pore has five members with one peptide (F) located behind A and having no contact with the pore water. Then, one peptide (D) starts leaving the pore to stay alone in the membrane. As a result, the pore radius decreases gradually to 6 Å at 7 μs. This pore with four members remains stable until 9 μs. Then, the pore dries out in 0.5 μs and does not change until the end of the simulation. Peptide-peptide interaction energies of the four pore members between 7–9 and 9.5–10 μs are −27 ± 44 and −20 ± 34 kcal/mol, respectively. The closing of the pore may be temporary and due to fluctuations in interaction energies and attraction between residues close to Ct of B and E, which are nonadjacent. The range of tilt angles for the four peptides around the pore is 5–35°.
Compared with melittin hexamer in DMPC (30), we observe some differences. At 10 μs, the pore remains wet in DMPC but dries up in POPC. Pore closing dynamics is slower in POPC than DMPC: the initial closing of the pore takes 100 ns in DMPC, i.e., 1/10th of the time in POPC. For pore peptides in DMPC, the range of tilt angles is 20–50°, and peptide-peptide interaction energy is −11 ± 10 kcal/mol. So, melittins in POPC are closer to I-state and closer to each other with stronger interactions. The pore radius in DMPC is almost two times larger than in POPC. The stable pore has three (30) or four (28) members in DMPC and four members in POPC.
As in DMPC, the main interactions in POPC occur between residues close to the Cts of the peptides. Because melittins have no NH2 group in our simulations, strong salt bridges form between Q26 and K21/R22/K23/R24 (Table S1). The Ct of two peptides (C and A) is substantially unfolded.
MelP5 hexamer
Fig. 2 shows the results for the MelP5 hexamer. The pore radius is stable at ∼6 Å during the 10 μs of the simulation. All six peptides contribute to pore formation and can be found in the T-state, the S-state (e.g., D between 0.5 and 3.5 μs with tilt angle of 69 ± 7° or B between 7 and 10 μs with tilt angle of 70 ± 7°), or the I-state (e.g., A and C in most of the simulation). The average interaction energies between peptide pairs over the last 2 μs of the simulation has a range of −5 ± 5 to −63 ± 20 kcal/mol. Table S2 shows that interpeptide interactions between nonadjacent peptides occur between residues close to the Ct, such as K21-L26, K21-Q25, and K21-Q24. On the other hand, interactions between adjacent peptides usually occur between one polar or positively charged residue close to Ct (e.g., K21, Q24, Q25, and L26) of one peptide and one polar residue in the middle of the sequence (e.g., T11, S18, or W19) of another peptide (Table S2).
In the case of MelP5 in DMPC, all six peptides are members of the pore, but their tilt angles are mainly in the T-state, and the range of average interaction energies is −2.5 ± 3.5 to −9 ± 8 kcal/mol. The pore radius is two times larger than in POPC. MelP5 molecules in POPC stay tighter to each other and interact more strongly than in DMPC, and this results in a smaller pore.
pHD15 at neutral pH
We simulated mac70 and pHD15 hexamers at neutral, low, and very low pH (see Methods for explanation). The peptides remained stably inserted into the membrane in all but the pHD15 neutral-pH simulation (Fig. 3). In that simulation, several peptides started to unfold and/or move to the membrane surface. A substantial water column remained in the membrane, lined by lipid headgroups. Peptides remained adsorbed near the pore edge, probably biased by the initial conditions. We deem that the pore is likely to dissipate upon continuation of the simulation. The instability of this oligomer is probably due to electrostatic repulsion. With a charge of −4 at neutral pH, intramolecular i-i+3/4 repulsions between acidic residues (E4–E8, E8–D12, and D11–D15) tend to unfold the helix, whereas intermolecular repulsions push the peptides to the surface. Experimentally, pHD peptides are random coil and are not fully adsorbed on the membrane surface at neutral pH (21). Presumably, 10 μs is too short a time for the peptides to completely unfold and dissociate from the membrane. This simulation is not analyzed and discussed any further. It is expected that MelP5_Δ6 would show similar behavior at neutral pH due to electrostatic repulsions of E7-E11 and E8-D12; therefore, this system was not simulated.
pHD15 in acidic environment
At low pH with His, Asp, and Glu protonated (Fig. 4), the pore radius decreases slightly and then increases to a maximum of 10 Å at 3.2 μs, with the tilt angles of peptides D and F reaching a minimum at 117°. After that, the pore radius has two plateaus at 6 ± 1 Å (4–5.5 μs) and 4 ± 1 Å (6.5–10 μs). The Ct of three peptides becomes substantially unfolded. Three protein-protein interactions between nonadjacent pairs affect pore radius: A-D, B-D, and D-F. Fig. S1 shows that the radius is maximal when the absolute value of the sum of these three interactions is minimal, and the small pore during the last 3 μs is a result of strong attractions between these pairs, especially A-D. Table S3 shows that L26/Ct participates in many of these interactions. The range of tilt angles over the last 4 μs is 135–170°, and the average helicity over the last 2 μs is 74%.
In a highly acidic environment in which Ct is also protonated (Fig. 5), the first 3 μs proceeds similarly as with deprotonated Ct, but then the pore radius is always greater. A protonated Ct has a lower propensity to unfold, bringing the average helicity up to 88%. The range of tilt angles is 115–145° over the last 2 μs. As a result, L26 cannot make strong interactions across the pore (Table S4). There is a minimum in the pore radius around 10 μs when E is located behind D and F and has lower contribution to pore formation. After 15 μs, E gradually enters the pore, and the radius increases. Fig. S2 shows that the minimal pore radius is a result of a strong D-F interaction, which pushes E away from the pore. In contrast, strong interactions of D-E and E-F after 15 μs open the pore. Table S4 shows that Q17 of D or E participates in most of these strong interactions.
mac70
At neutral pH, there is a wide and stable pore during the simulation of mac70 (Fig. 6). Tilt angles are mostly in the T-state, especially in the second half of the simulation (range 125–150°), and the average helicity is rather high at 88%. Table S5 shows that strong protein-protein interactions occur between adjacent peptides, and salt bridges between Lys (K21 and K7) and Glu (E15) have high contribution in these interactions.
At low pH in which Glu residues are protonated, there is a small pore that closes frequently (Fig. 7). Compared with neutral pH, tilt angles are closer to I-state (range 140–155°), and the average helicity is 80%. Fig. S3 shows that there are nonadjacent protein-protein interactions between A-C, C-E, and A-E during the simulation. These interactions cause B, D, and F to have lower contact with pore water, especially at the Ct. The main component of these interactions is between K21 and L26 (Table S6).
When the Ct is also protonated, pore radius is similar to the neutral pH case (Fig. 8). The range of tilt angles is 120–145°, and the average helicity is again 88%. Table S7 shows that protein-protein interactions between adjacent peptides are stronger than nonadjacent ones. However, these interactions are not as strong as in the two previous cases because there is no negatively charged residue and no salt bridge can form. Thus, polar interactions between polar/acidic/positively charged residues are important components of these interactions.
MelP5_Δ6 in acidic environment
For MelP5_Δ6 at low pH (Fig. 9), some peptides are located close to upper or lower membrane surfaces. For instance, the tilt angle for D between 1 and 6 μs is 61 ± 9°, and the tilt angle for B between 3 and 7 μs is 75 ± 6°. The pore radius has minimal values between 5 and 6 μs when these two peptides are in the S-state simultaneously. Over the last 2 μs, B comes to the surface with a tilt angle of 89 ± 3° and abandons the pore. The range of tilt angles is broad, covering all three states. This behavior is due to the lack of positively charged residues in the sequence. The only positive charge is at the N-terminus, and the only negative charge is Leu at Ct. So, peptides may come to S-state and form strong interactions between the termini of nonadjacent peptides (Table S8).
When the Ct is also protonated, there is no negative charge in the sequence for strong interaction with the N-terminus. So, no peptide comes to the surface. As can be seen in Fig. 10, the pore radius decreases gradually from ∼6 to ∼4 Å, and a small pore remains to the end of the simulation. Fig. S4 shows that after 1.5 μs, a strong interaction of A-C occurs, which pushes B away from the pore, especially at the Ct. Similarly, the interaction of A-E forces F to have less contact with the pore water. In other words, the pore becomes small because A enters the pore to have interactions with C and E, and interactions A-B and A-F are not strong enough to push back A and reopen the pore. Table S9 shows that Q24 and Q25 have important contribution in nonadjacent peptide interactions.
Discussion
The molecular dynamics simulations presented here point to three simple geometric rules that determine pore size. First, (obviously) more peptides make a larger pore. Second, when a peptide spans the membrane, higher tilt leads to a larger pore because the projection of the peptide on the xy plane is longer. Third, interactions between nonadjacent peptides block the pore, decreasing its radius. Based on these rules, we can analyze our results and distinguish favorable from unfavorable interactions for pore formation. We start by making five comparisons between the systems simulated here and in earlier work, and then we discuss the nature of the pore, point out the limitations of our approach, and finally propose changes that could improve or impair pore quality. Table 4 summarizes the findings from the simulations together with what is known for each peptide from the experiment.
Table 4.
Summary of Experimental and Simulation Findings for Each Peptide
| Peptide | Experiment | Simulation |
|---|---|---|
| Melittin | Transient pores at high peptide concentration (e.g., P:L ≥ 1:50) (18,20) | Very small and unstable pore with four peptides. Two peptides leave the pore. |
| MelP5 | Active at lower peptide concentrations than melittin (e.g., P:L = 1:500) (20). Requires higher peptide concentrations than pHD15 at low pH and mac70 (22). | Small but stable pore |
| pHD15, pH 7 | Peptide is random coil and does not bind the membrane (21,24). | Helices unfold and come to the surface. Water pore seems unstable. |
| pHD15, pH 5 | Potent pore former at very low peptide concentrations (21) | A large pore forms when Ct is protonated as well as Glu, Asp, and His residues. |
| mac70, neutral and low pH | Potent pore former at low peptide concentrations (22) | Large pores at neutral pH and low pH where Glu residues and Ct are protonated |
| MelP5_Δ6 | Designed to be active at low pH, but needs high concentrations of peptide and only makes small pores (23) | Small, defect-like pore at low pH |
DMPC versus POPC
Melittin and MelP5 hexamers were simulated in DMPC in a previous study (30) and in POPC in this work. For both peptides, pore radius is twofold larger in DMPC than POPC. The headgroups of DMPC and POPC are the same, but the tail group of POPC is longer than DMPC, and there is a double bond in one POPC tail. For the last 2 μs of the two simulations, we have calculated average interaction energies between each peptide at the pore with lipid tails. Because all six MelP5 molecules are present in the pore, the average is calculated over the hexamer. For melittin, the average is calculated over B, C, and E that line the pore, excluding D, F, and A from the calculation because the first two have no contact with the pore, and A is covered partially by F. The average interaction energies in POPC are −69 ± 19 and −76 ± 21 kcal/mol for melittin and MelP5, respectively. In DMPC, these interaction energies are −70 ± 10 and −77 ± 9 kcal/mol for melittin and MelP5, respectively. The difference between these numbers is negligible and in the range of statistical error. So, the double bond in POPC does not influence peptide-lipid interactions. The difference of pore sizes in DMPC and POPC is most likely due to membrane thickness. It is harder to form a pore in thicker membranes (49). Equivalently, the pore line tension is estimated to be higher in POPC than in DMPC (50). Melittin and MelP5 need to be more perpendicular to span POPC than DMPC. Thus, tilt angles are more likely to be found in I-state, and peptides are closer to each other. As a result, protein-protein interactions are stronger, and pores are smaller.
MelP5 versus mac70 at neutral pH
Mac70 peptides at neutral pH are more tilted than MelP5 and make a larger pore. Both peptides have one negatively charged residue at the Ct, but mac70 has three additional negative charges due to Glu residues. So, mac70 has a higher potential for making salt bridges and consequently stronger protein-protein interactions (Tables S2 and S5). In mac70, adjacent peptides interact very strongly, whereas in MelP5, adjacent peptide interactions are similar to interactions between nonadjacent peptides (e.g., D-F, A-C, and B-E), which are not beneficial for pore formation. These unfavorable interactions occur between residues close to the Ct (residue 21–26). Because these residues are identical in both sequences, there should be proper residues before I20 for strong interactions between adjacent peptides. Table S5 shows that salt bridges K21-E15 and K7-E15 are present in mac70, which makes a large pore. On the other hand, polar residues in MelP5, such as T11 and S18, and W19 cannot make strong interactions to overcome the effect of bonds between Ct (Table S2).
mac70 at neutral versus low pH
Experimental studies show that mac70 is slightly less potent at low pH than at neutral pH (22). Thus, it is interesting to compare mac70 in neutral pH and low pH. With the Ct deprotonated, the pore radius is smaller and the peptides more perpendicular at low pH. When Glu residues are protonated at low pH, a salt bridge can only form between K21-Ct. This interaction usually occurs between nonadjacent peptides (e.g., A-E, C-E, and A-C), reducing pore radius. However, when the Ct is also protonated, no salt bridge can form between nonadjacent peptides. In addition, Ct is less hydrophilic and less likely to be unfolded. So, helicity increases, and the peptide can span the membrane more easily. As a result, the pore radius and tilt angles are similar in neutral and highly acidic conditions (Figs. 6 and 8). Polar interactions stabilize the pore at low pH, and among polar residues (T11, E15, Q17, S18), Q17 has the highest contribution in these interactions (Table S7). S18 is more important than T11 due to its position and lower steric effect for the hydroxyl group. It is worth noting that K7, which is very important in making salt bridges at neutral pH, is not so at low pH. Interaction energy results could provide some rationalization for the lower potency of mac70 at low pH. At neutral pH (Table S5), the difference between average interaction energies of adjacent and nonadjacent peptides is more than 40 kcal/mol, whereas at low pH (Table S7), this difference is a few kcal/mol. So, at low pH, nonadjacent peptides are more likely to interact and reduce pore radius.
mac70 versus pHD15
At low pH with deprotonated Ct, although pHD15 makes a large pore at 3.2 μs, both peptides eventually make a small pore with radius ∼5 Å. When Ct is protonated, however, the pore radius of both peptides is ∼9 Å. So, both peptides appear to have similar potency at low pH. Table S4 shows that D11 and D12 of pHD15 do not have important contributions at low pH. Their effect is to inactivate pHD15 at neutral pH, as found experimentally (22). His residues in pHD15 have a similar role as Lys residues in mac70. It can be inferred from Tables S4 and S7 that E15 of mac70 is more important in interactions than D15 of pHD15. This has been observed experimentally (21) and is probably because of the longer side chain of Glu, which makes it more flexible in interactions. It is also interesting that in both peptides, Q17 is the most important residue in interactions between adjacent peptides.
MelP5_Δ6 versus pHD15
Both peptides cannot make a pore at neutral pH because of several i-i+3/4 electrostatic repulsions. However, under acidic conditions in which Glu, Asp, His, and the Ct are protonated, pHD15 makes a large pore, but the pore by MelP5_Δ6 is small. This finding is consistent with experiment (21,23). In the pHD15 hexamer, when interactions between nonadjacent molecules occur and one peptide enters the pore and reduces pore size, interactions between adjacent peptides can bring out the entered peptide and reopen the pore. In the case of MelP5_Δ6, however, interactions between adjacent peptides are not strong enough to move the entered peptide (Fig. S5). Comparing Tables S4 and S9, it can be seen that Q17 in pHD15 has a large contribution to adjacent interactions. The 17th residue in MelP5_Δ6 is Ile, which cannot make strong polar interactions. Table S9 shows that Q24 and Q25 of nonadjacent peptides interact, which is expected because of their distances. The other difference between pHD15 and MelP5_Δ6 is that there are two His residues in pHD15 (H7 and H21) that are replaced by Glu in MelP5_Δ6. Our results do not allow a judgment about this difference because His residues in pHD15 interact with Q17 or D15, which are not present in MelP5_Δ6.
Toroidal versus barrel-stave pore
Toroidal pores are lined by lipid headgroups, whereas in barrel-stave pores, the lipid headgroups are on the membrane surface. Table 3 shows the number of lipid headgroups with |z| < 7 Å in each system. This number is quite low (4–8) for the systems studied here and tends to correlate with pore radius. For comparison, the same number for melittin in DMPC is 15 ± 3 and for MelP5 in DMPC is 16 ± 2 (30). This is a result of the tight interactions between the peptides in these systems. Thus, the pores here appear to be intermediates between classical toroidal and barrel-stave pores.
Limitations
As insightful as they may be, our simulations have a number of inherent limitations that should be noted. First, albeit long by current standards, 10–20 μs may not be sufficient for certain slow processes. For example, we still observe a pore in the neutral pH simulation of pHD15, when experiment suggests there should not be one. Second, simulations are stochastic. Multiple simulations of each system and extraction of average trends would have been ideal, but that was beyond our present computational capabilities. We opted to perform a single, long simulation for several peptides under a variety of conditions rather than multiple simulations for fewer peptides under fewer conditions. Third, we only simulate a fixed and small number of peptides in a small unit cell. Thus, we cannot reproduce the experimental finding of macromolecule-size pores (21,22). We are thus forced to extrapolate from the small pores we observe to the large pores that have been substantiated by experiments. Fourth, most of the analysis in this work is based solely on peptide-peptide interaction energies, which neglect solvation effects and entropy. Thus, electrostatic interactions are probably overemphasized, and hydrophobic interactions are underestimated. Peptide-lipid and peptide-water interactions can be equally important, as shown in previous work (30). Finally, only a parallel initial arrangement of the helices has been considered here. In other peptides, the arrangement was found to have a substantial impact on the pore properties (29).
Proposed rational modifications
The molecular dynamics simulations provide plausible structures for the pores and a list of interactions that appear important in stabilizing them. They could, thus, provide the basis of rational design. This is not trivial, however, because even a single mutation could have unpredictable effects. Ideally, one should also simulate each proposed mutant, but that would demand further resources. In addition, pore formation involves multiple steps, i.e., folding and partitioning at the membrane interface, membrane insertion, aggregation, and wetting. A mutation that appears favorable in the pore state might unfavorably affect one of the other steps. Nevertheless, we venture a few tentative suggestions, which based on our results, would increase or reduce pore-forming potency:
-
1)
Because many interactions detrimental to pore formation involve the charged Ct, it is better to remove the negative charge from Ct to prevent salt bridges with nonadjacent peptides. This can be done by patching the Ct with different groups such as NH2, CH3, and NH-CH3. Several experimental studies suggested that amidation of the Ct increases helical propensity and improves leakage and biological activity of a number of AMPs (51, 52, 53, 54, 55), consistent with our findings. It should be pointed out here that natural melittin is amidated, whereas in this and previous work, we studied the nonamidated version.
-
2)
Q17 in mac70 and pHD15 has the highest contribution to interactions between adjacent peptides at low pH. So, we predict that changing I17 in MelP5 and MelP5_Δ6 to Q would improve pore-forming potency. Or, conversely, removing Q17 from mac70 and pHD15 would reduce potency.
-
3)
Among polar residues, such as Ser, Thr, Asn, and Gln, the amide side chain can make stronger interactions. So, it might be useful to replace Ser or Thr by Asn or Gln.
-
4)
According to the interaction tables for mac70 and pHD15, the 15th residue is important for interactions between adjacent peptides. Thus, mutation of A15 in MelP5 and MelP5_Δ6 by a polar or acidic residue might be advantageous for a large pore.
-
5)
It seems that residues with longer side chains (e.g., Asp versus Glu or Asn versus Gln) are more flexible and have higher potential for a more potent pore former.
-
6)
Our results show that residues close to the N-terminus (i.e., residues 1–6) in mac70 and pHD15 have a negligible effect at neutral and low pH. So, mutation of E4 to Ala, as in MelP5, would not be detrimental. However, this inference is based only on interpeptide interactions. If E4 has an unfavorable interaction with lipid headgroups, it might stabilize the pore by forcing the peptide to stay near the aqueous pore (30).
-
7)
According to the results for DMPC and POPC, a longer peptide is required to make a larger pore in a thicker membrane. Because residues close to N-terminus do not usually contribute in pore formation interactions, lengthening the peptide by adding nonpolar residues close to N-terminus might improve the pore-forming potential.
Conclusions
In this work, we examined the pore-forming potency of several 26-residue melittin-like peptides at neutral and low pH using long molecular dynamics simulations. The initial configurations and the lipids were the same, so any differences in pore structure at the same pH are due to differences in sequence. The main finding is that strong interactions between adjacent peptides (such as the E15-K7/K21 salt bridges in mac70) and higher tilt angles are beneficial for the formation of a larger pore. On the contrary, interactions between nonadjacent peptides tend to reduce pore size. In these simulations, most interactions between nonadjacent peptides occur between residues closer to the Ct (residues 21–26). So, there should be interaction sites before residue 21 such that Ct residues and middle residues of two adjacent peptides can interact strongly and force each other to be more tilted, making a larger pore. At low pH, in which negatively charged residues are protonated and salt bridge formation is impossible, polar interactions are critical for pore formation. So, polar residues should be present in the middle of the sequence. We found that polar residues with amide side chains, such as Q17, have the highest contribution in neighboring peptides’ interactions. We also examined the effect of lipid tails on pore formation and found that the smaller pores made by melittin and MelP5 in POPC than in DMPC are due to the thickness of the membranes, not peptide interactions with lipid tails. Based on these findings, we proposed several sequence changes that should improve or impair pore formation. Experimental testing of these predictions, as well as computer simulations of the proposed mutants, are straightforward. In the future, the coupling of simulation and experiment could provide a validated molecular picture of peptide-stabilized membrane pores and the potential to engineer pores with desired properties.
Author Contributions
A.S., L.P., and A.P.-A. performed the simulations and the analysis. T.L. designed and supervised the project. A.S. and T.L. wrote the manuscript with a contribution from L.P.
Acknowledgments
This work was supported by National Institutes of Health (NIH) (GM117146), and infrastructure support was provided in part by Research Centers in Minority Institutions grant no. 8G12MD007603 from the NIH. Anton computer time was provided by the Pittsburgh Supercomputing Center through grant R01GM116961 from the NIH. The Anton machine at Pittsburgh Supercomputing Center was generously made available by D.E. Shaw Research, and computer time was provided by the National Center for Multiscale Modeling of Biological Systems through grant no. P41GM103712-S1 from the NIH and Pittsburgh Supercomputing Center.
Editor: Markus Deserno.
Footnotes
Almudena Pino-Angeles’s present address is School of Pharmacy, Queen’s University Belfast, Belfast, UK.
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2020.02.024.
Supporting Material
References
- 1.Yeung A.T., Gellatly S.L., Hancock R.E. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. 2011;68:2161–2176. doi: 10.1007/s00018-011-0710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohner K. Membrane-active antimicrobial peptides as template structures for novel antibiotic agents. Curr. Top. Med. Chem. 2017;17:508–519. [PubMed] [Google Scholar]
- 3.Bayley H., Cremer P.S. Stochastic sensors inspired by biology. Nature. 2001;413:226–230. doi: 10.1038/35093038. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan R.S., Satheesan R., Mahendran K.R. Autonomously assembled synthetic transmembrane peptide pore. J. Am. Chem. Soc. 2019;141:2949–2959. doi: 10.1021/jacs.8b09973. [DOI] [PubMed] [Google Scholar]
- 5.Bradshaw J. Cationic antimicrobial peptides : issues for potential clinical use. BioDrugs. 2003;17:233–240. doi: 10.2165/00063030-200317040-00002. [DOI] [PubMed] [Google Scholar]
- 6.Matsuzaki K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta. 2009;1788:1687–1692. doi: 10.1016/j.bbamem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey C.E. The actions of melittin on membranes. Biochim. Biophys. Acta. 1990;1031:143–161. doi: 10.1016/0304-4157(90)90006-x. [DOI] [PubMed] [Google Scholar]
- 8.Raghuraman H., Chattopadhyay A. Melittin: a membrane-active peptide with diverse functions. Biosci. Rep. 2007;27:189–223. doi: 10.1007/s10540-006-9030-z. [DOI] [PubMed] [Google Scholar]
- 9.Gajski G., Garaj-Vrhovac V. Melittin: a lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 2013;36:697–705. doi: 10.1016/j.etap.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T., Nomura F., Takiguchi K. Multiple membrane interactions and versatile vesicle deformations elicited by melittin. Toxins (Basel) 2013;5:637–664. doi: 10.3390/toxins5040637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alia O., Laila M., Antonious A.-D. Antimicrobial effect of melittin isolated from Syrian honeybee (apismellifera) venom and its wound healing potential. Int. J. Pharm. Sci. Rev. Res. 2013;21:318–324. [Google Scholar]
- 12.Jamasbi E., Mularski A., Separovic F. Model membrane and cell studies of antimicrobial activity of melittin analogues. Curr. Top. Med. Chem. 2016;16:40–45. doi: 10.2174/1568026615666150703115919. [DOI] [PubMed] [Google Scholar]
- 13.Tosteson M.T., Tosteson D.C. Activation and inactivation of melittin channels. Biophys. J. 1984;45:112–114. doi: 10.1016/S0006-3495(84)84130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz G., Zong R.T., Popescu T. Kinetics of melittin induced pore formation in the membrane of lipid vesicles. Biochim. Biophys. Acta. 1992;1110:97–104. doi: 10.1016/0005-2736(92)90299-2. [DOI] [PubMed] [Google Scholar]
- 15.Ladokhin A.S., Selsted M.E., White S.H. Sizing membrane pores in lipid vesicles by leakage of co-encapsulated markers: pore formation by melittin. Biophys. J. 1997;72:1762–1766. doi: 10.1016/S0006-3495(97)78822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L., Harroun T.A., Huang H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001;81:1475–1485. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M.-T., Sun T.-L., Huang H.W. Process of inducing pores in membranes by melittin. Proc. Natl. Acad. Sci. USA. 2013;110:14243–14248. doi: 10.1073/pnas.1307010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiedman G., Herman K., Hristova K. The electrical response of bilayers to the bee venom toxin melittin: evidence for transient bilayer permeabilization. Biochim. Biophys. Acta. 2013;1828:1357–1364. doi: 10.1016/j.bbamem.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krauson A.J., He J., Wimley W.C. Gain-of-function analogues of the pore-forming peptide melittin selected by orthogonal high-throughput screening. J. Am. Chem. Soc. 2012;134:12732–12741. doi: 10.1021/ja3042004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiedman G., Fuselier T., Wimley W.C. Highly efficient macromolecule-sized poration of lipid bilayers by a synthetically evolved peptide. J. Am. Chem. Soc. 2014;136:4724–4731. doi: 10.1021/ja500462s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiedman G., Kim S.Y., Hristova K. pH-triggered, macromolecule-sized poration of lipid bilayers by synthetically evolved peptides. J. Am. Chem. Soc. 2017;139:937–945. doi: 10.1021/jacs.6b11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S., Kim S.Y., Hristova K. Potent macromolecule-sized poration of lipid bilayers by the macrolittins, a synthetically evolved family of pore-forming peptides. J. Am. Chem. Soc. 2018;140:6441–6447. doi: 10.1021/jacs.8b03026. [DOI] [PubMed] [Google Scholar]
- 23.Wiedman G., Wimley W.C., Hristova K. Testing the limits of rational design by engineering pH sensitivity into membrane-active peptides. Biochim. Biophys. Acta. 2015;1848:951–957. doi: 10.1016/j.bbamem.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S.Y., Pittman A.E., Hristova K. Mechanism of action of peptides that cause the pH-triggered macromolecular poration of lipid bilayers. J. Am. Chem. Soc. 2019;141:6706–6718. doi: 10.1021/jacs.9b01970. [DOI] [PubMed] [Google Scholar]
- 25.Pittman A.E., Marsh B.P., King G.M. Conformations and dynamic transitions of a melittin derivative that forms macromolecule-sized pores in lipid bilayers. Langmuir. 2018;34:8393–8399. doi: 10.1021/acs.langmuir.8b00804. [DOI] [PubMed] [Google Scholar]
- 26.Mihajlovic M., Lazaridis T. Antimicrobial peptides bind more strongly to membrane pores. Biochim. Biophys. Acta. 2010;1798:1494–1502. doi: 10.1016/j.bbamem.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Y., Lazaridis T. Activity determinants of helical antimicrobial peptides: a large-scale computational study. PLoS One. 2013;8:e66440. doi: 10.1371/journal.pone.0066440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leveritt J.M., III, Pino-Angeles A., Lazaridis T. The structure of a melittin-stabilized pore. Biophys. J. 2015;108:2424–2426. doi: 10.1016/j.bpj.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pino-Angeles A., Leveritt J.M., III, Lazaridis T. Pore structure and synergy in antimicrobial peptides of the magainin family. PLoS Comput. Biol. 2016;12:e1004570. doi: 10.1371/journal.pcbi.1004570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pino-Angeles A., Lazaridis T. Effects of peptide charge, orientation, and concentration on melittin transmembrane pores. Biophys. J. 2018;114:2865–2874. doi: 10.1016/j.bpj.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipkin R., Pino-Angeles A., Lazaridis T. Transmembrane pore structures of β-hairpin antimicrobial peptides by all-atom simulations. J. Phys. Chem. B. 2017;121:9126–9140. doi: 10.1021/acs.jpcb.7b06591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimsley G.R., Scholtz J.M., Pace C.N. A summary of the measured pK values of the ionizable groups in folded proteins. Protein Sci. 2009;18:247–251. doi: 10.1002/pro.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen W., Morrow B.H., Shen J.K. Recent development and application of constant pH molecular dynamics. Mol. Simul. 2014;40:830–838. doi: 10.1080/08927022.2014.907492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L., Vorobyov I., Allen T.W. The different interactions of lysine and arginine side chains with lipid membranes. J. Phys. Chem. B. 2013;117:11906–11920. doi: 10.1021/jp405418y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gleason N.J., Vostrikov V.V., Koeppe R.E., II Buried lysine, but not arginine, titrates and alters transmembrane helix tilt. Proc. Natl. Acad. Sci. USA. 2013;110:1692–1695. doi: 10.1073/pnas.1215400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panahi A., Brooks C.L., III Membrane environment modulates the pKa values of transmembrane helices. J. Phys. Chem. B. 2015;119:4601–4607. doi: 10.1021/acs.jpcb.5b00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jo S., Kim T., Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- 38.Shaw D.E., Grossman J.P., Young C. Anton 2: raising the bar for performance and programmability in a special-purpose molecular dynamics supercomputer. In International Conference for High Performance Computing. Networking, Storage and Analysis. 2014:41–53. [Google Scholar]
- 39.Huang J., MacKerell A.D., Jr. CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 2013;34:2135–2145. doi: 10.1002/jcc.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jorgensen W.L., Chandrasekhar J., Klein M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983;79:926–935. [Google Scholar]
- 41.Lippert R.A., Predescu C., Shaw D.E. Accurate and efficient integration for molecular dynamics simulations at constant temperature and pressure. J. Chem. Phys. 2013;139:164106. doi: 10.1063/1.4825247. [DOI] [PubMed] [Google Scholar]
- 42.Kuskin J.S., Young C., Shaw D.E. Incorporating flexibility in Anton, a specialized machine for molecular dynamics simulation. 2008. Incorporating flexibility in Anton, a specialized machine for molecular dynamics simulation; pp. 343–354. [Google Scholar]
- 43.Hoover W.G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A Gen. Phys. 1985;31:1695–1697. doi: 10.1103/physreva.31.1695. [DOI] [PubMed] [Google Scholar]
- 44.Martyna G.J., Tobias D.J., Klein M.L. Constant pressure MD algorithms. J. Chem. Phys. 1994;101:4177–4189. [Google Scholar]
- 45.Brooks B.R., Brooks C.L., III, Karplus M. CHARMM: the biomolecular simulation program. J. Comput. Chem. 2009;30:1545–1614. doi: 10.1002/jcc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Åqvist J. A simple way to calculate the axis of an α-helix. Comput. Chem. 1986;10:97–99. [Google Scholar]
- 47.Roe D.R., Cheatham T.E., III PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013;9:3084–3095. doi: 10.1021/ct400341p. [DOI] [PubMed] [Google Scholar]
- 48.Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 49.Bennett W.F., Hong C.K., Tieleman D.P. Antimicrobial peptide simulations and the influence of force field on the free energy for Pore Formation in lipid bilayers. J. Chem. Theory Comput. 2016;12:4524–4533. doi: 10.1021/acs.jctc.6b00265. [DOI] [PubMed] [Google Scholar]
- 50.West A., Ma K., Kindt J.T. Simulation studies of structure and edge tension of lipid bilayer edges: effects of tail structure and force-field. J. Phys. Chem. A. 2013;117:7114–7123. doi: 10.1021/jp400371k. [DOI] [PubMed] [Google Scholar]
- 51.Strandberg E., Tiltak D., Ulrich A.S. Influence of C-terminal amidation on the antimicrobial and hemolytic activities of cationic α-helical peptides. Pure Appl. Chem. 2007;79:717–728. [Google Scholar]
- 52.Won A., Khan M., Ianoul A. Investigating the effects of L- to D-amino acid substitution and deamidation on the activity and membrane interactions of antimicrobial peptide anoplin. Biochim. Biophys. Acta. 2011;1808:1592–1600. doi: 10.1016/j.bbamem.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 53.Dennison S.R., Phoenix D.A. Influence of C-terminal amidation on the efficacy of modelin-5. Biochemistry. 2011;50:1514–1523. doi: 10.1021/bi101687t. [DOI] [PubMed] [Google Scholar]
- 54.da Silva A.V., De Souza B.M., Palma M.S. The effects of the C-terminal amidation of mastoparans on their biological actions and interactions with membrane-mimetic systems. Biochim. Biophys. Acta. 2014;1838:2357–2368. doi: 10.1016/j.bbamem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Mura M., Wang J., Phoenix D.A. The effect of amidation on the behaviour of antimicrobial peptides. Eur. Biophys. J. 2016;45:195–207. doi: 10.1007/s00249-015-1094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.