Background: Coronavirus disease 2019 (COVID-19), the disease caused by the novel severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) that originated in China in December 2019, was recently recognized as pandemic threat by the World Health Organization, with the potential of rapidly overloading health care systems and causing substantial mortality worldwide (www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020). Human-to-human transmission occurs mainly through respiratory droplets, but other routes are under investigation, because SARS-CoV-2 has been detected in several body fluids (1). So far, few data are available on ocular samples from patients with COVID-19, although conjunctivitis has been occasionally reported among COVID-19 symptoms, similar to infections caused by other human coronaviruses (2, 3). During the SARS epidemic, eye exposure to infectious fluids was associated with an increased risk for SARS-CoV transmission to health care workers (3, 4). Although SARS-CoV RNA was occasionally found in ocular specimens during the early phase of illness, its infectivity is unknown (2, 3).
With regard to COVID-19, unprotected ocular exposure was thought to be responsible for infections that occurred in the Wuhan Fever Clinic in January 2020 (3, 4); in addition, SARS-CoV-2 RNA was detected in conjunctival secretions collected from the only patient with conjunctivitis out of 30 patients with COVID-19 from a hospital in China (5). However, further studies are needed to evaluate the infectious potential of the SARS-CoV-2 RNA detected in the ocular specimens and to determine whether transmission may occur through ocular secretions (3, 4).
Objective: To present the early detection of infectious SARS-CoV-2 in ocular fluids from a patient with the first confirmed case of COVID-19 in Italy, who had been hospitalized at the National Institute for Infectious Diseases “L. Spallanzani” (INMI) in Rome.
Methods and Findings: The patient, a 65-year-old woman, travelled from Wuhan, China, to Italy on 23 January 2020 and was admitted on 29 January 2020, 1 day after symptom onset. At admission to the high isolation unit at INMI, she presented with nonproductive cough, sore throat, coryza, and bilateral conjunctivitis. She had no fever until day 4, when fever (38 °C), nausea, and vomiting began. Infection with SARS-CoV-2 was confirmed by performing real-time reverse transcription polymerase chain reaction (RT-PCR) assay on sputum samples (cycle threshold value [Ct], 16.1) on the admission day, followed by viral M gene sequencing (GenBank accession number MT008022), and virus isolation on Vero E6 cell line (2019-nCoV/Italy-INMI1). The full genome sequence was obtained from either clinical sample and or culture isolate (GISAID accession numbers EPI_ISL_410545 and EPI_ISL_410546). At admission, no other respiratory infections were detected (QIAstat-Dx® Respiratory Panel; Qiagen).
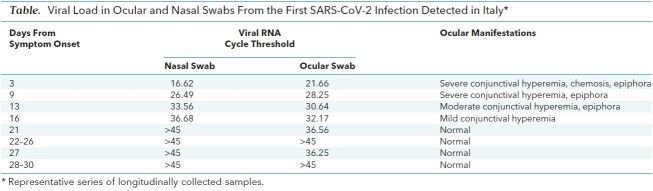
On day 3 after hospital admission, owing to the persistence of conjunctivitis, an ocular swab was collected and viral RNA was detected (Ct, 21.6). Subsequent ocular samples collected with almost daily frequency resulted positive up to day 21, with declining virus concentration (increased Ct values). Conjunctivitis greatly improved at day 15 and apparently resolved at day 20.
Five days after it became undetectable, SARS-CoV-2 RNA was detected again (Ct, 36.25) in the ocular swab sample collected at day 27 (Table). SARS-CoV-2 RNA was detected in ocular swabs days after it was undetectable in nasal swabs (Table). In addition, the Ct values detected in the late ocular samples were lower than those observed in the nasal swabs (Table 1), suggesting sustained replication in conjunctiva. With the aim of demonstrating that viral genomes detected in ocular swabs represented infectious virus, the first RNA-positive ocular sample was inoculated in Vero E6 cells, and cytopathic effect was observed 5 days postinoculum. Viral replication was confirmed by real-time RT-PCR on RNA purified from spent cell growth medium.
Table. Viral Load in Ocular and Nasal Swabs From the First SARS-CoV-2 Infection Detected in Italy*.
Discussion: We found that ocular fluids from SARS-CoV-2-infected patients may contain infectious virus, and hence may be a potential source of infection. These findings highlight the importance of control measures, such as avoiding touching the nose, mouth, and eyes and frequent hand washing. A related implication is the importance of appropriate use of personal protective equipment for ophthalmologists during clinical examination, because ocular mucosa may be not only a site of virus entry but also a source of contagion. Furthermore, we observed that ocular involvement of SARS-CoV-2 may occur early in the COVID-19 course, suggesting that measures to prevent transmission via this route must be implemented as early as possible.
Future studies are needed to define the human ocular cell types capable of supporting viral replication and the mechanisms underlying ocular tropism of SARS-CoV-2.
Biography
Note: Drs. Colavita and Lapa contributed equally.
Acknowledgment: The authors thank the Collaborators Members of the INMI COVID-19 Study Group (Appendix, available at Annals.org).
Disclosures: None. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M20-1176.
Funding: By funds to the National Institute for Infectious Diseases “Lazzaro Spallanzani” IRCCS from Ministero della Salute, Ricerca Corrente, linea1, and European Commission Horizon 2020 (EU project 101003544, CoNVat; EU project 101003551, EXSCALATE4CoV).
Reproducible Research Statement: Study protocol and data set: Available from Dr. Castilletti (e-mail, concetta.castilletti@inmi.it) through written agreement with the authors.
Corresponding Author: Concetta Castilletti, PhD, Laboratory of Virology, National Institute for Infectious Diseases “Lazzaro Spallanzani”, Via Portuense 292, 00149 Rome, Italy; e-mail, concetta.castilletti@inmi.it.
Appendix: INMI COVID-19 Study Group
Maria Alessandra Abbonizio, Chiara Agrati, Fabrizio Albarello, Gioia Amadei, Alessandra Amendola, Mario Antonini, Raffaella Barbaro, Barbara Bartolini, Martina Benigni, Nazario Bevilacqua, Licia Bordi, Veronica Bordoni, Marta Branca, Paolo Campioni, Maria Rosaria Capobianchi, Cinzia Caporale, Ilaria Caravella, Fabrizio Carletti, Concetta Castilletti, Roberta Chiappini, Carmine Ciaralli, Francesca Colavita, Angela Corpolongo, Massimo Cristofaro, Salvatore Curiale, Alessandra D'Abramo, Cristina Dantimi, Alessia De Angelis, Giada De Angelis, Rachele Di Lorenzo, Federica Di Stefano, Federica Ferraro, Lorena Fiorentini, Andrea Frustaci, Paola Gallì, Gabriele Garotto, Maria Letizia Giancola, Filippo Giansante, Emanuela Giombini, Maria Cristina Greci, Giuseppe Ippolito, Eleonora Lalle, Simone Lanini, Daniele Lapa, Luciana Lepore, Andrea Lucia, Franco Lufrani, Manuela Macchione, Alessandra Marani, Luisa Marchioni, Andrea Mariano, Maria Cristina Marini, Micaela Maritti, Giulia Matusali, Silvia Meschi, Francesco Messina, Chiara Montaldo, Silvia Murachelli, Emanuele Nicastri, Roberto Noto, Claudia Palazzolo, Emanuele Pallini, Virgilio Passeri, Federico Pelliccioni, Antonella Petrecchia, Ada Petrone, Nicola Petrosillo, Elisa Pianura, Maria Pisciotta, Silvia Pittalis, Costanza Proietti, Vincenzo Puro, Gabriele Rinonapoli, Martina Rueca, Alessandra Sacchi, Francesco Sanasi, Carmen Santagata, Silvana Scarcia, Vincenzo Schininà, Paola Scognamiglio, Laura Scorzolini, Giulia Stazi, Francesco Vaia, Francesco Vairo, Maria Beatrice Valli.
Footnotes
This article was published at Annals.org on 17 April 2020.
References
- 1. doi: 10.1001/jama.2020.3786. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 Mar 11. [Epub ahead of print] [PMID: 32159775] doi:10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed]
- 2. doi: 10.1136/bjophthalmol-2020-315994. Li JO, Lam DS, Chen Y, Ting DS. Novel coronavirus disease 2019 (COVID-19): The importance of recognising possible early ocular manifestation and using protective eyewear [Editorial]. Br J Ophthalmol. 2020;104:297-8. [PMID: 32086236] doi:10.1136/bjophthalmol-2020-315994. [DOI] [PubMed]
- 3. doi: 10.1080/09273948.2020.1738501. Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28:391-5. [PMID: 32175797] doi:10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed]
- 4. doi: 10.1016/S0140-6736(20)30313-5. Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored [Letter]. Lancet. 2020;395:e39. [PMID: 32035510] doi:10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed]
- 5. doi: 10.1002/jmv.25725. Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020 Feb 26. [Epub ahead of print] [PMID: 32100876] doi:10.1002/jmv.25725. [DOI] [PMC free article] [PubMed]