Abstract
Background
Whilst the pharmacological profiles and mechanisms of antidepressants are varied, there are common reasons why they might help people to stop smoking tobacco. Firstly, nicotine withdrawal may produce depressive symptoms and antidepressants may relieve these. Additionally, some antidepressants may have a specific effect on neural pathways or receptors that underlie nicotine addiction.
Objectives
To assess the evidence for the efficacy, safety and tolerability of medications with antidepressant properties in assisting long‐term tobacco smoking cessation in people who smoke cigarettes.
Search methods
We searched the Cochrane Tobacco Addiction Specialized Register, which includes reports of trials indexed in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and PsycINFO, clinicaltrials.gov, the ICTRP, and other reviews and meeting abstracts, in May 2019.
Selection criteria
We included randomized controlled trials (RCTs) that recruited smokers, and compared antidepressant medications with placebo or no treatment, an alternative pharmacotherapy, or the same medication used in a different way. We excluded trials with less than six months follow‐up from efficacy analyses. We included trials with any follow‐up length in safety analyses.
Data collection and analysis
We extracted data and assessed risk of bias using standard Cochrane methods. We also used GRADE to assess the certainty of the evidence.
The primary outcome measure was smoking cessation after at least six months follow‐up, expressed as a risk ratio (RR) and 95% confidence intervals (CIs). We used the most rigorous definition of abstinence available in each trial, and biochemically validated rates if available. Where appropriate, we performed meta‐analysis using a fixed‐effect model.
Similarly, we presented incidence of safety and tolerance outcomes, including adverse events (AEs), serious adverse events (SAEs), psychiatric AEs, seizures, overdoses, suicide attempts, death by suicide, all‐cause mortality, and trial dropout due to drug, as RRs (95% CIs).
Main results
We included 115 studies (33 new to this update) in this review; most recruited adult participants from the community or from smoking cessation clinics. We judged 28 of the studies to be at high risk of bias; however, restricting analyses only to studies at low or unclear risk did not change clinical interpretation of the results. There was high‐certainty evidence that bupropion increased long‐term smoking cessation rates (RR 1.64, 95% CI 1.52 to 1.77; I2 = 15%; 45 studies, 17,866 participants). There was insufficient evidence to establish whether participants taking bupropion were more likely to report SAEs compared to those taking placebo. Results were imprecise and CIs encompassed no difference (RR 1.16, 95% CI 0.90 to 1.48; I2 = 0%; 21 studies, 10,625 participants; moderate‐certainty evidence, downgraded one level due to imprecision). We found high‐certainty evidence that use of bupropion resulted in more trial dropouts due to adverse events of the drug than placebo (RR 1.37, 95% CI 1.21 to 1.56; I2 = 19%; 25 studies, 12,340 participants). Participants randomized to bupropion were also more likely to report psychiatric AEs compared with those randomized to placebo (RR 1.25, 95% CI 1.15 to 1.37; I2 = 15%; 6 studies, 4439 participants).
We also looked at the safety and efficacy of bupropion when combined with other non‐antidepressant smoking cessation therapies. There was insufficient evidence to establish whether combination bupropion and nicotine replacement therapy (NRT) resulted in superior quit rates to NRT alone (RR 1.19, 95% CI 0.94 to 1.51; I2 = 52%; 12 studies, 3487 participants), or whether combination bupropion and varenicline resulted in superior quit rates to varenicline alone (RR 1.21, 95% CI 0.95 to 1.55; I2 = 15%; 3 studies, 1057 participants). We judged the certainty of evidence to be low and moderate, respectively; in both cases due to imprecision, and also due to inconsistency in the former. Safety data were sparse for these comparisons, making it difficult to draw clear conclusions.
A meta‐analysis of six studies provided evidence that bupropion resulted in inferior smoking cessation rates to varenicline (RR 0.71, 95% CI 0.64 to 0.79; I2 = 0%; 6 studies, 6286 participants), whilst there was no evidence of a difference in efficacy between bupropion and NRT (RR 0.99, 95% CI 0.91 to 1.09; I2 = 18%; 10 studies, 8230 participants).
We also found some evidence that nortriptyline aided smoking cessation when compared with placebo (RR 2.03, 95% CI 1.48 to 2.78; I2 = 16%; 6 studies, 975 participants), whilst there was insufficient evidence to determine whether bupropion or nortriptyline were more effective when compared with one another (RR 1.30 (favouring bupropion), 95% CI 0.93 to 1.82; I2 = 0%; 3 studies, 417 participants). There was no evidence that any of the other antidepressants tested (including St John's Wort, selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs)) had a beneficial effect on smoking cessation. Findings were sparse and inconsistent as to whether antidepressants, primarily bupropion and nortriptyline, had a particular benefit for people with current or previous depression.
Authors' conclusions
There is high‐certainty evidence that bupropion can aid long‐term smoking cessation. However, bupropion also increases the number of adverse events, including psychiatric AEs, and there is high‐certainty evidence that people taking bupropion are more likely to discontinue treatment compared with placebo. However, there is no clear evidence to suggest whether people taking bupropion experience more or fewer SAEs than those taking placebo (moderate certainty). Nortriptyline also appears to have a beneficial effect on smoking quit rates relative to placebo. Evidence suggests that bupropion may be as successful as NRT and nortriptyline in helping people to quit smoking, but that it is less effective than varenicline. There is insufficient evidence to determine whether the other antidepressants tested, such as SSRIs, aid smoking cessation, and when looking at safety and tolerance outcomes, in most cases, paucity of data made it difficult to draw conclusions. Due to the high‐certainty evidence, further studies investigating the efficacy of bupropion versus placebo are unlikely to change our interpretation of the effect, providing no clear justification for pursuing bupropion for smoking cessation over front‐line smoking cessation aids already available. However, it is important that where studies of antidepressants for smoking cessation are carried out they measure and report safety and tolerability clearly.
Plain language summary
Do medicines used to treat depression help people to quit smoking?
Background and review questions
Some medicines and supplements that have been used to treat depression (antidepressants) have also been tested to see whether they can help people to stop smoking. Two of these treatments ‐ bupropion (sometimes called Zyban) and nortriptyline ‐ are sometimes given to help people quit smoking. This review looks at whether using antidepressants actually helps people to stop smoking (for six months or longer), and also looks at the safety of using these medicines.
Study characteristics
This review includes 115 studies looking at how helpful and safe different antidepressants are when used to quit smoking. Most of the studies were conducted in adults. We included studies of any length when looking at safety, but studies needed to be at least six months long when assessing whether people had managed to quit smoking. The evidence is up to date to May 2019.
Key results
Using the antidepressant, bupropion, makes it 52% to 77% more likely that a person will successfully stop smoking, which is equal to five to seven more people successfully quitting for six months or more for every one hundred people who try to quit. There is evidence that people who use the antidepressant, nortriptyline, to quit smoking also improve their chances of success. There is not enough evidence to determine whether other antidepressants help people to quit smoking.
There is evidence that bupropion increases unwanted effects, particularly those relating to mental health, and that unwanted effects may increase the chance that people stop using the medicine. However, the evidence does not suggest that bupropion is more likely to result in death, hospitalization, or life‐threatening events, like seizures. There is not enough information to draw clear conclusions about the safety of nortriptyline for stopping smoking.
The evidence does not suggest that taking bupropion at the same time as other stop‐smoking medicines, like varenicline (sometimes known as Champix or Chantix) or nicotine replacement therapy makes people more likely to quit smoking. People are as likely to quit smoking when using bupropion as when using nortriptyline or nicotine replacement therapy, however people using varenicline are more likely to quit than those using bupropion.
Certainty of evidence
There is high‐certainty evidence that bupropion helps people to quit smoking, meaning further research is very unlikely to change this conclusion. However, there is also high‐certainty evidence to suggest that people using bupropion are more likely to stop taking the medicine because of unpleasant effects than those taking a pill without medication (a placebo). The certainty of the evidence was moderate, low or very low for the other key questions we looked at. This means that the findings of those questions may change when more research is carried out. In most cases this was because there were not enough studies or studies were too small.
Summary of findings
Background
Description of the condition
Tobacco use is one of the leading causes of preventable illness and death worldwide, accounting for over eight million deaths annually (GBD RFC 2017). Extrapolation based on current smoking trends, suggests that without widespread quitting, approximately 400 million tobacco‐related deaths will occur between 2010 and 2050, mostly among current smokers (Jha 2011). Most smokers would like to stop (CDC 2017); however, quitting tobacco use is difficult. This is because users develop both a psychological and physiological dependence on smoking. The physiological dependence is caused by a component of tobacco, called nicotine (McNeill 2017).
Description of the intervention
Whilst antidepressant medications are primarily used for the treatment of depression and disorders of negative affect, they have also been used to help individuals stop smoking. They offer an alternative to other frontline smoking cessation therapies, such as nicotine replacement therapy (NRT), and nicotine agonists, such as varenicline.
The following medications and substances, regarded as having antidepressant properties, have been investigated for their effect on smoking cessation in at least one study.
Tricyclic antidepressants (TCAs): doxepin, imipramine and nortriptyline
Monoamine oxidase inhibitors (MAOIs): moclobemide, selegiline, lazabemide, and EVT302
Selective serotonin reuptake inhibitors (SSRIs): fluoxetine, paroxetine, sertraline, citalopram, and zimeledine
Atypical antidepressants: bupropion, tryptophan, venlafaxine
Extracts of St. John's wort (Hypericum perforatum L)
Dietary supplement: S‐Adenosyl‐L‐Methionine (SAMe)
Of the antidepressant medications indicated for smoking cessation, the most commonly used is bupropion. It has both dopaminergic and adrenergic actions, and appears to be an antagonist at the nicotinic acetylcholinergic receptor (Fryer 1999). It has been licensed as a prescription aid to smoking cessation in many countries. The usual dose for smoking cessation is 150 mg once a day for three days, increasing to 150 mg twice a day continued for 7 to 12 weeks, and quit attempts are generally initiated one week after starting pharmacotherapy.
Following bupropion, the second most commonly tested medication for smoking cessation is the TCA, nortriptyline. It enhances noradrenergic and serotonergic activity by blocking reuptake of these neurotransmitters (Benowitz 2000). It is licensed for smoking cessation in New Zealand. The recommended regimen is 10 to 28 days of titration before the quit attempt, followed by a 12‐week dose of 75 mg to 100 mg daily (Cahill 2013).
No other antidepressants are currently licensed for use as smoking cessation aids, although others have been tested for possible use.
How the intervention might work
Multiple observations have provided a rationale for studying the effects of antidepressant medications for smoking cessation: a history of depression is found more frequently amongst smokers than nonsmokers, nicotine may have antidepressant effects, and antidepressants influence the neurotransmitters and receptors involved in nicotine addiction (Benowitz 2000; Kotlyar 2001). It has also been hypothesized that cessation may precipitate depression, however evidence suggests that this is unlikely to be the case, and that cessation may actually reduce the likelihood of depression (Taylor 2014).
The diverse pharmacological targets of antidepressants means their mechanisms of action are varied. Evidence suggests bupropion may aid smoking cessation by blocking nicotine effects, relieving withdrawal (Cryan 2003; West 2008), and reducing depressed mood (Lerman 2002a). Monoamine oxidase‐A (MOA‐A) inhibitors may aid smoking cessation by substituting the ability of smoking to act as a MOA inhibitor (Lewis 2007). It has been hypothesized that SSRIs might be helpful because they increase serotonin, which is also associated with improving negative affect (Benowitz 2000). The mechanisms of other antidepressants for smoking cessation remain unstudied.
Although there is an evident relationship between alleviating negative affect and antidepressant pharmacology, it is unclear whether antidepressants work mostly due to reducing negative affect, reducing urges to smoke or withdrawal symptoms, or by acting as nicotine blockers.
Why it is important to do this review
The ongoing impact of smoking on global morbidity and mortality necessitates effective and safe treatments to aid smoking cessation. Since the last update of this review was published in 2014 (Hughes 2014), a substantial amount of new evidence has emerged to assess antidepressants as smoking cessation aids. This has the potential to change or strengthen our conclusions regarding the efficacy of some of these antidepressants when compared with no treatment, whilst also strengthening the evidence regarding the safety of those antidepressant currently being used to help people quit smoking (bupropion and nortriptyline). Further evidence on safety outcomes may help to clarify the potential interaction between bupropion and seizures, as well as psychiatric adverse events. Multiple trials and observational studies have previously associated bupropion with increasing the risk of medically important adverse events, including seizures, anxiety, depression, and insomnia (Aubin 2012). New evidence may also help us to directly compare the safety and efficacy of antidepressants with other front‐line smoking cessation medications, providing a further aid to decision making when helping people to quit tobacco smoking.
Objectives
To assess the evidence for the efficacy, safety and tolerability of medications with antidepressant properties in assisting long‐term tobacco smoking cessation in people who smoke cigarettes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and cluster‐RCTs.
Types of participants
We included tobacco smokers of any age, with or without a history of mental illness. We did not include pregnant women, as these smokers are covered in a separate Cochrane Review (Coleman 2015).
Types of interventions
We included trials studying pharmacotherapies with antidepressant properties for smoking cessation. We included trials assessing different doses, durations and schedules of antidepressants.
We excluded trials where an additional, uncontrolled non‐antidepressant intervention component was used in only one of the trial arms. This is because the confounding effects of this intervention would have made it difficult to determine whether any change in outcome was related to the antidepressant or the confounding intervention component. Additionally, we excluded trials investigating antidepressant use for smoking harm reduction or relapse prevention, as they are covered elsewhere (Lindson‐Hawley 2016 and Livingstone‐Banks 2019, respectively).
Comparators
The following comparators were eligible for assessing safety, efficacy and tolerability: placebo, no pharmacotherapy, alternative therapeutic control, or different dosages/treatment regimes of the same antidepressant.
Types of outcome measures
Primary outcomes
Efficacy, measured as smoking cessation
For this outcome we only included studies that set out to report smoking cessation rates at least six months after baseline, in line with the standard methods of Cochrane Tobacco Addiction. Where cessation was assessed at multiple intervals, we report only the longest follow‐up data. Additionally, where multiple definitions of abstinence are assessed, we report the strictest of these definitions (e.g. continuous/prolonged abstinence over point prevalence abstinence). We also report biochemical validation of abstinence over self‐reported abstinence (but it was not necessary for abstinence to have been biochemically validated for a study to be included).
Secondary outcomes
-
Safety, measured as:
number of people experiencing adverse events (AEs) of any severity (e.g. abnormal test findings, clinically significant symptoms and signs, changes in physical examination findings, hypersensitivity, and progression or worsening of underlying disease)
number of people experiencing psychiatric AEs (e.g. adverse events relating to mental health)
number of people experiencing serious adverse events (SAEs), i.e. events that result in death, are life‐threatening (immediate risk of death), require inpatient hospitalization or prolongation of existing hospitalization, result in persistent or significant disability or incapacity, and/or result in congenital anomaly or birth defect (e.g. seizures, overdoses, suicide attempts, death by suicide, all‐cause mortality).
We also recorded the following SAEs specifically, as these have previously been associated with the use of antidepressants for smoking cessation.
Number of people experiencing seizures
Number of people experiencing overdoses
Number of people experiencing suicide attempts
Number of people experiencing death by suicide
Number of people experiencing all‐cause mortality
Tolerability, measured as the number of participants who dropped out of the trial due to adverse events
For all safety and tolerability outcomes, we considered studies with follow‐up of any length.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Tobacco Addiction Specialized Register. At the time of the updated search in May 2019, the Register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL; 2019, Issue 4); MEDLINE (via OVID) to update April 2019; Embase (via OVID) to April 2019; PsycINFO (via OVID) to update April 2019; US National Library of Medicine to April 2019. See the Cochrane Tobacco Addiction website for full search strategies and a list of other resources searched to populate the Register. We searched the Register for reports of studies evaluating bupropion, nortriptyline or any other pharmacotherapy classified as having an antidepressant effect. Search terms included relevant individual drug names or antidepressant* or antidepressive*. See Appendix 1 for the Register search strategy.
Searching other resources
We searched ClinicalTrials.gov (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform (ICTRP) through the Cochrane Tobacco Addiction Specialized Register.
Data collection and analysis
Selection of studies
Two review authors (of JHB, JLB, NL, SH) independently screened titles and abstracts resulting from our searches for relevance, and obtained full‐text records of reports of eligible or possibly eligible studies. Two review authors (of JHB, JLB, NL, SH) then independently screened each full‐text record for eligibility. Any disagreements were resolved through discussion with a third review author. For conference abstracts or trial registry entries where the record contained insufficient evidence for us to determine the eligibility of the study, we attempted to contact study investigators to obtain any additional data needed to make a final decision. We recorded all screening decisions made and presented the flow of studies and references through the reviewing process using a PRISMA flow diagram (Moher 2009).
Data extraction and management
Two review authors (of BH, JHB, JLB, NL, SH) independently extracted the following study data and compared the findings. Any discrepancies were resolved by mutual consent.
Type of antidepressant
Country and setting
Recruitment method
Definition of smoker used
Participant demographics (i.e. average age, gender, average cigarettes per day)
Intervention and control description (including dose, schedule, and behavioural support common to all arms)
Efficacy outcome(s) used in meta‐analysis, including length of follow‐up, definition of abstinence, and biochemical validation of smoking cessation
Any analysis investigating the interaction between efficacy and participants' depression status
Safety and tolerability outcomes, including AEs, psychiatric AEs, SAEs, types of SAEs, withdrawals due to treatment
Sources of funding and declarations of interest
Assessment of risk of bias in included studies
We assessed included studies for risks of selection bias (method of random sequence generation and allocation concealment), bias due to an absence of blinding (taking into account both performance and detection bias in a single domain), attrition bias (levels and reporting of loss to follow‐up), and any other threats to study validity, using the Cochrane 'Risk of bias' tool (Higgins 2011). For each new study in this update, two review authors (of JHB, JLB, NL, SH) independently assessed each study for each domain, in accordance with 'Risk of bias' guidance developed by Cochrane Tobacco Addiction to assess smoking cessation studies. Where there was any disagreement on the assessment, it was resolved through discussion with a third review author.
We considered studies at high risk of performance and detection bias where there was no blinding of participants or personnel or where there was evidence of unblinding; at unclear risk if insufficient information was available with which to judge; and at low risk if the study reported blinding of participants and personnel in detail and there was no evidence of unblinding. We considered studies to be at low risk of attrition bias where over half of the participants were followed up at the longest follow‐up and where numbers followed up were similar across arms (difference < 20%).
Measures of treatment effect
Smoking cessation
We calculated cessation rates for all studies that reported cessation at least six months following baseline. For each study, we used the strictest available criteria to define cessation as described above.
Where data were available, we expressed cessation as a risk ratio (RR) for each study. We calculated this as follows: (quitters in treatment group/total randomized to treatment group)/(quitters in control group/total randomized to control group), alongside 95% confidence intervals (CIs). A RR > 1 indicates increased likelihood of quitting in the intervention group than in the control condition.
Adverse events (AEs) and serious adverse events (SAEs)
We calculated AE rates for all studies that reported adequate data, regardless of study length. Where numerical data were available, we expressed safety and tolerability data as RRs (95% CI). We calculated this as follows: (number of participants reporting (S)AEs in treatment group/total randomized to treatment group)/(number of participants reporting (S)AEs in control group/total randomized to control group). A RR > 1 indicates an increased likelihood of experiencing an AE or SAE in the intervention group than in the control condition.
In addition to overall AEs and overall SAEs, we calculated RRs (95% CI) for the following safety and tolerability outcomes, where data were available.
Psychiatric AEs
Seizures
Overdoses
Suicide attempts
Death by suicide
All‐cause mortality
Dropout due to adverse events
Insomnia
Anxiety
Unit of analysis issues
We only judged one cluster‐RCT to be eligible for inclusion (Siddiqi 2013). This study was not pooled in any meta‐analysis due to substantial heterogeneity of programme effects across clusters.
Dealing with missing data
As far as possible, we used an intention‐to‐treat (ITT) analysis with people who dropped out or were lost to follow‐up treated as continuing smokers. Where participants appeared to have been randomized, but were not included in the data presented by the authors (and we were unable to obtain these), we noted this in the study description (see Characteristics of included studies). We extracted numbers lost to follow‐up from study reports and used these to assess the risk of attrition bias.
Assessment of heterogeneity
Before pooling studies, we considered both methodological and clinical variance between studies. Where pooling was deemed appropriate we investigated statistical heterogeneity using the I2 statistic (Higgins 2003). This describes the percentage variability in effect estimates that is due to heterogeneity rather than sampling error (chance).
Assessment of reporting biases
Where a comparison included a sufficient number of studies (≥ 10), we generated funnel plots to analyse and report on potential publication bias as advised by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
We therefore generated funnel plots for the following comparisons.
Bupropion versus placebo/control ‐ smoking cessation
Bupropion versus placebo/control ‐ AEs
Bupropion versus placebo/control ‐ SAEs
Bupropion versus placebo/control ‐ seizures
Bupropion versus placebo/control ‐ suicide attempts
Bupropion versus placebo/control ‐ death by suicide
Bupropion versus placebo/control ‐ all‐cause mortality
Bupropion versus placebo/control ‐ dropout due to drug
Bupropion versus placebo/control ‐ anxiety
Bupropion versus placebo/control ‐ insomnia
Bupropion and nicotine replacement therapy (NRT) versus NRT alone ‐ smoking cessation
Bupropion versus NRT ‐ smoking cessation
Data synthesis
For each type of medication and comparison where more than one eligible trial was identified, we performed separate meta‐analyses of cessation and safety outcomes using Mantel‐Haenszel fixed‐effect methods. We pooled RRs and 95% CIs from individual study estimates to estimate pooled RRs (95% CIs). Where studies contributed more than one intervention arm to a pooled analysis, we split the control arm to avoid double‐counting.
We also carried out post hoc, exploratory analyses to inform our approach to safety and tolerance for the next update of this review. We combined the following comparisons when evaluating AEs, psychiatric AEs, SAEs, and dropouts due to adverse effects.
Bupropion compared to placebo/control
Bupropion plus NRT compared to NRT alone
Bupropion plus varenicline compared to varenicline alone
The rationale for this was that these studies all tested the additional effect of bupropion, and there was no evidence of an interaction for safety and tolerability outcomes (whereas there may be for effectiveness). We subgrouped studies by their comparison type, though acknowledge that these subgroups may currently be underpowered to detect differences between groups.
Subgroup analysis and investigation of heterogeneity
For comparisons where we had sufficient data, we separated participant data into the following subgroups to determine whether antidepressants had differential effects on the relevant population or intervention groups.
Split by mental health diagnoses: mental health diagnoses versus no mental health diagnoses
Split by level of behavioural support: multisession group support versus multisession individual counselling versus low intensity support versus not specified. To be identified as low intensity, support had to be regarded as part of the provision of routine care, i.e. time spent with smoker (including assessment for the trial) less than 30 minutes at the initial consultation, with no more than two further assessment and reinforcement visits.
Where reported, we also extracted data from analyses evaluating a potential interaction between current depression or past history of depression and quit rates. We relied upon the definition of depression used by study authors, which included both formal diagnoses and scores on validated depression scales. This interaction is investigated in more detail in van der Meer 2013.
Sensitivity analysis
We carried out the following sensitivity analyses.
We excluded studies from meta‐analyses that were judged to be at high risk of bias for any of the assessed bias domains. We judged whether this exclusion notably altered the pooled RRs (95% CI).
We excluded studies from meta‐analyses with industry support. We did this in two stages: 1) we excluded studies that were funded by the pharmaceutical industry; 2) we excluded studies that were funded by the pharmaceutical industry or where the study medication was provided by the pharmaceutical industry. We judged whether this exclusion notably altered the pooled RRs (95% CI).
Summary of findings and assessment of the certainty of the evidence
We created 'Summary of findings' tables using standard Cochrane methodology (Higgins 2019), for the following comparisons, which we judged to be most clinically relevant.
Bupropion compared to placebo/control
Bupropion plus NRT compared to NRT alone
Bupropion plus varenicline compared to varenicline alone
We judged these comparisons to be of most relevance because bupropion is currently the only antidepressant used as a front‐line therapy for smoking cessation worldwide.
Following standard Cochrane methodology (Higgins 2019), we used GRADEpro GDT software and the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for smoking cessation, SAEs, and dropout due to adverse events of the drug, and to draw conclusions about the certainty of the evidence within the text of the review (Schünemann 2013). We chose these outcomes as they are important factors to consider regarding pharmaceutical efficacy, safety and tolerability, and are therefore useful to both clinicians and patients when deciding whether to provide or use a smoking cessation pharmacotherapy.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies
Results of the search
The most recent literature search for this update generated 397 records. After duplicates were removed, 362 records remained for title and abstract screening. We ruled out 213 records at this stage, leaving 149 records for full‐text screening. At this stage we identified 33 new, included studies (reported across 85 records in total) and three ongoing studies. See Figure 1 for full details of record/study flow information for the most recent updated search.
1.
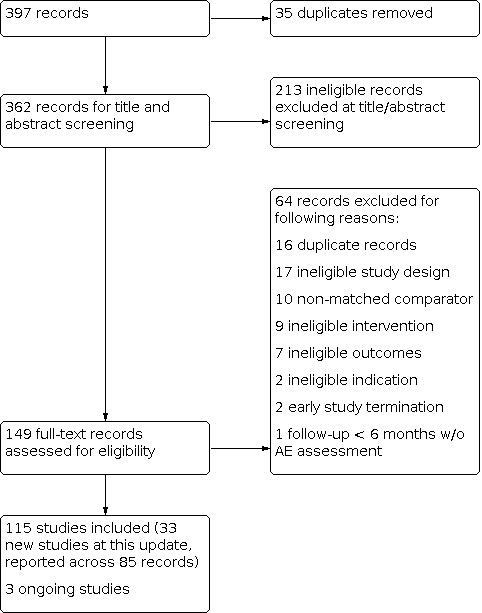
Flow diagram for 2019 search update only
Included studies
We identified 33 additional eligible trials at this update, yielding a total of 115 included trials. The new trials studied:
bupropion: Anthenelli 2016; Benli 2017; Cinciripini 2018; CTRI/2013/07/003830; Ebbert 2014; Elsasser 2002; Fatemi 2013; Gilbert 2019; Gray 2011; Gray 2012; Johns 2017; Karam‐Hage 2011; Moreno‐Coutino 2015; NCT00132821; NCT00308763; NCT00495352; NCT00593099; NCT01406223; Perkins 2013; Rose 2014; Rose 2017; Sheng 2013; Singh 2010; Tidey 2011; Urdapilleta‐Herrera 2013; Weiner 2012; White 2005; Zincir 2013
EVT302: Berlin 2012
fluoxetine: Minami 2014; NCT00578669
lazabemide: Berlin 2002
St John’s wort: Barnes 2006
Further details of these newly included, as well as previously included studies, including dosing schedules, are recorded in the Characteristics of included studies tables.
Bupropion
Overall, we included 87 studies of bupropion. Outcomes for four of these studies are based only on conference abstracts or pharmaceutical company data (Ferry 1992; Ferry 1994; Selby 2003; SMK20001).
The majority of trials were conducted in North America, but we also included studies from Australia (Myles 2004); Brazil (Haggsträm 2006); China (Sheng 2013); Europe (Aubin 2004; Dalsgarð 2004; Fossati 2007; Górecka 2003; Rovina 2009; Stapleton 2013; Wagena 2005; Wittchen 2011; Zellweger 2005); India (CTRI/2013/07/003830; Johns 2017; Singh 2010); Israel (Planer 2011); New Zealand (Holt 2005); Pakistan (Siddiqi 2013); Taiwan (NCT00495352); and Turkey (Benli 2017; Uyar 2007; Zincir 2013). Three studies were carried out across multiple continents (Anthenelli 2016; Tonnesen 2003; Tonstad 2003).
A number of trials specifically recruited cohorts of participants with health conditions, including:
alcoholism (Grant 2007; Hays 2009; Karam‐Hage 2011)
bipolar disorder (NCT00593099)
cancer (Schnoll 2010)
cardiovascular disease (Eisenberg 2013; Planer 2011; Rigotti 2006; Tonstad 2003)
chronic obstructive pulmonary disease (Górecka 2003; Tashkin 2001; Wagena 2005)
mild depression (Moreno‐Coutino 2015)
psychiatric conditions (Anthenelli 2016)
schizophrenia (Evins 2001; Evins 2005; Evins 2007; Fatemi 2013; George 2002; George 2008; NCT00495352; Weiner 2012)
post‐traumatic stress disorder (Hertzberg 2001)
tuberculosis or suspected tuberculosis (Siddiqi 2013; CTRI/2013/07/003830)
Three of the studies in people with cardiovascular disease, and one other, enrolled hospital inpatients (Eisenberg 2013; Planer 2011; Rigotti 2006; Simon 2009).
Trials also studied specific populations of:
adolescents (Gray 2011; Gray 2012; Killen 2004; Muramoto 2007)
African‐Americans (Ahluwalia 2002; Cox 2012)
healthcare workers (Zellweger 2005)
hospital staff (Dalsgarð 2004)
low‐income and minority (NCT00308763)
Maori (Holt 2005)
males (Rose 2017)
smokers awaiting surgery (Myles 2004)
smokers who had previously failed to quit smoking using bupropion (Gonzales 2001; Selby 2003)
smokers who had just failed to quit using nicotine replacement therapy (NRT) (Hurt 2003; Rose 2013; Rose 2014).
More than half the bupropion studies followed participants for at least 12 months from the start of treatment or the target quit day. Twenty‐nine studies followed up participants for six months. The duration of follow‐up was below six months for 12 of the included studies, was of unknown duration for six studies, and one study measured number of days abstinent rather than numbers abstinent at a particular time point (Perkins 2013). However, these studies did measure safety outcomes; therefore they contributed data to our meta‐analyses of adverse events data, but not smoking cessation data.
In those studies which met or exceeded the six‐month follow‐up threshold, the majority reported an outcome of sustained (prolonged) abstinence. However, in 25 (33%) studies, only point prevalence rates were given, or the definition of abstinence was unclear.
Forty‐six trials evaluated bupropion for smoking cessation as a single pharmacotherapy versus placebo/non‐pharmacotherapeutic control, and three studies compared different doses of bupropion (Hurt 1997; Muramoto 2007; Swan 2003). Both Muramoto 2007 and Swan 2003 compared a 150 mg dose per day with a 300 mg dose per day, whereas Hurt 1997 looked at 100 mg per day versus 150 mg per day versus 300 mg per day. We pooled studies in which bupropion was used in combination with another pharmacotherapy or versus another pharmacotherapy in separate comparisons, as listed below.
Bupropion as an adjunct to NRT versus NRT alone (16 trials)
Bupropion as an adjunct to varenicline versus varenicline alone (6 trials)
Bupropion versus NRT (10 trials)
Bupropion versus varenicline (10 trials)
Bupropion versus nortriptyline (3 trials)
Bupropion versus gabapentin (1 trial)
Nortriptyline
We included 10 studies of the tricyclic antidepressant, nortriptyline in this review. Hall and colleagues conducted three trials (Hall 1998; Hall 2002; Hall 2004), and Prochazka and colleagues two (Prochazka 1998; Prochazka 2004), with all these trials conducted in the USA. One study was conducted in Australia (Richmond 2013), two in Brazil (Da Costa 2002; Haggsträm 2006), one in the Netherlands (Wagena 2005), and one in the UK (Aveyard 2008).
Richmond 2013 was the only study to be conducted in a specialist population, recruiting male prisoners who had been incarcerated for at least one month and had at least six months remaining of their sentences.
All studies were placebo controlled. They used nortriptyline doses of 75 mg/day to 100 mg/day or titrated doses to serum levels recommended for depression during the week prior to the quit date.
Treatment duration ranged from 12 to 14 weeks. Nearly all studies used a definition of cessation based on a sustained period of abstinence. Aveyard 2008, Hall 1998, Hall 2002, Hall 2004, and Richmond 2013 reported outcomes at ≥ 12 months of follow‐up and the other six studies had a maximum follow‐up of six months.
The three studies by Hall and colleagues used factorial designs to test nortriptyline versus placebo crossed with different intensities of behavioural support (Hall 1998; Hall 2002; Hall 2004). Conversely, the remaining studies provided a set amount of behavioural support to all participants, ranging from brief behavioural counselling to repeated group and individual sessions.
Six studies tested nortriptyline as a monotherapy, and four studies tested nortriptyline as an adjunct to NRT.
Selective serotonin reuptake inhibitors (SSRIs)
Fluoxetine
Seven studies of fluoxetine have been included in this review, with two of these studies identified for inclusion in the current update (Minami 2014; NCT00578669).
The majority of these trials took place in the USA (Brown 2014; Minami 2014; NCT00578669; Niaura 2002; Saules 2004; Spring 2007), and one in Iceland (Blondal 1999). Participants were recruited from clinics (Blondal 1999; Brown 2014; Niaura 2002; Saules 2004; Spring 2007), the community (Minami 2014), or through an unknown recruitment method (NCT00578669).
Brown 2014 was the only study to be conducted in a specialist population, recruiting smokers with elevated depressive symptoms.
Six of these studies conducted follow‐up to at least six months for cessation outcomes. Minami 2014 had a follow‐up duration of fewer than six months, so we only evaluated adverse events data for this study.
Four studies used varying doses of fluoxetine as a single pharmacotherapy: Niaura 2002 compared a 30 mg daily dose, a 60 mg daily dose, or placebo for 10 weeks; Spring 2007 used 60 mg or placebo for 12 weeks; NCT00578669 compared 20 mg daily for eight weeks preceding and following the target quit date to placebo. Minami 2014 also compared fluoxetine as a monotherapy (20 mg daily for 8 weeks prior to and following the target quit date) to placebo only.
The remaining three trials investigated fluoxetine as an adjunct to NRT, and used similar doses of fluoxetine: Blondal 1999 used 20 mg/day or placebo for three months as an adjunct to nicotine inhaler; Saules 2004 used 20 mg/day or 40 mg/day or placebo for 10 weeks as an adjunct to nicotine patch; and Brown 2014 compared 10 weeks of 20 mg daily fluoxetine, 16 weeks of 20 mg daily fluoxetine, or no additional treatment in participants using nicotine patch for eight weeks.
Paroxetine
One trial assessed paroxetine (20 mg, 40 mg or placebo) for nine weeks as an adjunct to nicotine patch (Killen 2000). It was conducted in the USA, with participants recruited from the community. It measured smoking cessation (defined as 7‐day point prevalence) at six months follow‐up.
Sertraline
One trial with six‐month follow‐up assessed sertraline (200 mg/day) for 11 weeks versus placebo in conjunction with six individual counselling sessions. All participants had a past history of major depression (Covey 2002).
Monoamine oxidase inhibitors
Moclobemide
Moclobemide was tested for smoking cessation in one placebo‐controlled trial, carried out in France (Berlin 1995). Participants were recruited using advertisements in community healthcare settings. Treatment with 400 mg/day began one week before quit day and continued for two months, reducing to 200 mg/day for a further month. No behavioural counselling was provided. Final follow‐up for smoking cessation (defined as prolonged abstinence) was at 12 months.
Selegiline
Five long‐term trials testing selegiline are included in this review, carried out in the USA (George 2003; Kahn 2012; Killen 2010; Weinberger 2010), and Israel (Biberman 2003). All studies recruited participants from the community.
Almost all studies delivered selegiline as a monotherapy compared to placebo, excluding Biberman 2003, which used a combination therapy of selegiline and nicotine patch compared to placebo.
Three studies used 10 mg/day of oral treatment (Biberman 2003; George 2003; Weinberger 2010), and two used 6 mg/day of patch treatment (Kahn 2012; Killen 2010). The nicotine patches also used in Biberman 2003 delivered 21 mg/day of nicotine for eight weeks. Three studies had treatment durations of nine weeks (George 2003; Kahn 2012; Weinberger 2010), one had a treatment duration of eight weeks (Killen 2010), and one continued therapy for 26 weeks (Biberman 2003). Three of the studies completed follow‐up at six months (George 2003; Kahn 2012; Killen 2010), and two continued follow‐up to 12 months (Biberman 2003; Weinberger 2010).
Lazabemide
Berlin 2002 is the only study of lazabemide included in this review. Due to its nature as a dose‐finding, exploratory study, its follow‐up period for smoking cessation was only eight weeks. Therefore, we only consider its safety data within this review.
The study was conducted in both France and Belgium; however, the method of participant recruitment is not reported. Participants were given either 50 mg lazabemide, 100 mg lazabemide or placebo. It was halted early due to liver toxicity observed in trials of the medication for other indications.
EVT302
Berlin 2012 is the only study of EVT302 included in this review. Its follow‐up for smoking cessation is only eight weeks, therefore we only consider its safety data within this review.
The study was conducted in Germany, with participants recruited through media advertisements. It compared EVT302 monotherapy (5 mg/day for 1 week preceding and 7 weeks following the target quit date) with placebo. It additionally compared EVT302 combination therapy with nicotine patch (21 mg/day for 7 weeks post‐target quit date) versus placebo EVT302 and nicotine patch.
Venlafaxine
Cinciripini 2005 is the only study of venflaxine included in this review. It recruited from the community and compared venlafaxine at a dose of up to 225 mg/day with placebo. All participants also received nicotine patches and nine brief individual counselling sessions; follow‐up was for 12 months.
Hypericum (St John’s wort)
Three studies of hypericum are included (Barnes 2006; Parsons 2009; Sood 2010), with Barnes 2006 newly included at this update. These studies took place in the USA (Sood 2010) and the UK (Barnes 2006; Parsons 2009). Participants were recruited from the community (Barnes 2006; Sood 2010) and stop‐smoking clinics (Parsons 2009).
All three studies reported prolonged abstinence at six months. Barnes 2006 compared 300 mg/day to 600 mg/day, starting one week prior to the target quit date and continuing for 12 weeks thereafter; Parsons 2009 compared 14 weeks of 900 mg/day St John's wort to placebo, starting two weeks prior to target quit date and continuing for 12 weeks thereafter; Sood 2010 compared 900 mg/day, 1800 mg/day, and placebo for 12 weeks.
S‐Adenosyl‐L‐Methionine (SAMe)
Sood 2012 is the only study of SAMe included in this review. It compared 1600 mg/day or 800 mg/day SAMe to placebo for eight weeks, with smoking cessation follow‐up at six months.
Excluded studies
For studies that were potentially relevant, but that we excluded, we have provided our reasons for exclusion in Characteristics of excluded studies. Reasons that records were excluded at full‐text stage for this update specifically, are also summarized in Figure 1.
As part of this update to the review, we have excluded studies investigating the use of antidepressants for smoking relapse prevention and harm reduction, as these studies are included in other reviews (Lindson‐Hawley 2016; Livingstone‐Banks 2019). Therefore, we have now excluded seven studies of relapse prevention (Covey 2007; Croghan 2007; Hall 2011; Hays 2001; Hays 2009; Hurt 2003; Killen 2006), and one of harm reduction (Hatsukami 2004), which were included in the previous update (Hughes 2014).
We identified the following three ongoing studies as part of our search which are likely to be relevant for inclusion when complete.
NCT03326128: compares two high doses of bupropion (300 mg/day to 450 mg/day, starting 4 weeks prior to and following target quit date).
NCT03342027: a factorial trial comparing bupropion to placebo, as well as an eight‐session tailored behavioural intervention.
Zawertailo 2018: compares bupropion (150 mg/day for the first 3 days, then twice daily for the remainder of the 12 weeks, starting 7 days prior to target quit day) and varenicline (0.5 mg once daily for first 3 days, then 0.5 mg twice daily for next 4 days, then 1 mg twice daily for the remainder of the 12 weeks, starting 7 days prior to target quit day).
Further details of these ongoing studies are summarized in the Characteristics of ongoing studies table.
Risk of bias in included studies
Overall, we judged 12 studies to be at low risk of bias (low risk of bias across all domains), 28 at high risk of bias (high risk of bias in at least 1 domain), and the remaining 75 at unclear risk of bias. Reasons for the judgements made below are detailed in the Characteristics of included studies table, and a summary illustration of the 'Risk of bias' profile across studies is shown in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed selection bias through investigating methods of random sequence generation and allocation concealment for each study. We rated 46 studies at low risk for random sequence generation, 68 at unclear risk and one at high risk (Moreno‐Coutino 2015). We judged 31 studies to be at low risk for allocation concealment, 79 at unclear risk and five at high risk. When assessing both random sequence generation and allocation concealment, we assessed studies to be at unclear risk where there was insufficient methodological information available to be sure whether adequate measures had been taken to avoid selection bias.
Blinding
We assessed any risk of bias linked to blinding as one domain. However, we took into account both performance and detection bias when making this judgement. We judged 32 studies to be at low risk of bias for this domain, 64 at unclear risk and 19 at high risk. Where studies stated that they were "double‐blind" only, with no explicit clarification of who was blinded, we judged this to be unclear risk.
Incomplete outcome data
We judged studies to be at a low risk of attrition bias where the numbers of participants lost to follow‐up were clearly reported and the overall number lost to follow‐up was not more than 50%, and the difference in loss to follow‐up between groups was no greater than 20%. This is in accordance with 'Risk of bias' guidance produced by Cochrane Tobacco Addiction for assessing smoking cessation studies. We judged 69 of the studies to be at low risk of bias, 34 at unclear risk and 12 at high risk.
Other potential sources of bias
We found three studies with other sources of potential bias beyond those domains detailed previously. Siddiqi 2013 demonstrated substantial heterogeneity of programme effects across the different clusters of their cluster‐RCT. Twenty per cent of participants in the control arm smoked only hookah (no cigarettes) compared with 4% in the intervention arm. We judged that this put the study at high risk of bias. Weiner 2012 details that there was insufficient study drug available to meet demand. It is unclear how this was dealt with and whether it is accounted for in the dropouts reported. We judged this to be an unclear risk of bias. Finally, Zincir 2013 details that there were no adverse events recorded during their study. This seems highly unlikely according to the common definition of adverse events, and there is no detail given of how adverse events were measured in the study. We have therefore judged this to put the study at high risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Bupropion compared to placebo/no pharmacotherapy control for smoking cessation.
| Bupropion compared to placebo/control for smoking cessation | ||||||
| Population: people who smoke Setting: any; studies conducted in Asia, Australasia, Europe, USA Intervention: bupropion Comparison: placebo/control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo/control | Risk with bupropion | |||||
| Smoking cessation (at least six months follow‐up) | Study population | RR 1.64 (1.52 to 1.77) | 17,866 (46 RCTs) | ⊕⊕⊕⊕ High | ||
| 11 per 100 | 18 per 100 (17 to 20) | |||||
| Serious adverse events | Study population | RR 1.16 (0.90 to 1.48) | 10,625 (21 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 2 per 100 | 3 per 100 (2 to 3) | |||||
| Dropouts due to adverse events of the drug | Study population | RR 1.37 (1.21 to 1.56) | 12,340 (25 RCTs) | ⊕⊕⊕⊕ High | ||
| 7 per 100 | 9 per 100 (8 to 10) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to imprecision. Confidence interval encompasses no difference as well as clinically significant increase. Total number of events less than 300.
Summary of findings 2. Bupropion plus NRT compared to NRT alone for smoking cessation.
| Bupropion plus NRT compared to NRT alone for smoking cessation | ||||||
| Population: people who smoke Setting: any; studies conducted in UK, USA Intervention: bupropion and NRT Comparison: NRT alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with NRT alone | Risk with bupropion and NRT | |||||
| Smoking cessation (at least six months follow‐up) | Study population | RR 1.19 (0.94 to 1.51) | 3487 (12 RCTs) | ⊕⊕⊝⊝ Lowa,b | ||
| 19 per 100 | 22 per 100 (17 to 28) | |||||
| Serious adverse events | Study population | RR 1.52 (0.26 to 8.89) | 607 (3 RCTs) | ⊕⊝⊝⊝ Very lowc,d | ||
| 1 per 100 | 1 per 100 (0 to 6) | |||||
| Dropouts due to adverse events of the drug | Study population | RR 1.67 (0.95 to 2.92) | 538 (2 RCTs) | ⊕⊕⊝⊝ Lowd | ||
| 7 per 100 | 11 per 100 (6 to 19) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NRT: nicotine replacement therapy; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to inconsistency. Unexplained statistical heterogeneity (I2 = 52%). bDowngraded one level due to imprecision. Confidence interval encompasses no difference as well as clinically significant benefit. cDowngraded one level due to risk of bias. One of the three included studies judged to be at high risk of bias. Removing this study reduced the point estimate to 1.00. dDowngraded two levels due to imprecision. Fewer than 100 events.
Summary of findings 3. Bupropion plus varenicline compared to varenicline alone for smoking cessation.
| Bupropion plus varenicline compared to varenicline alone for smoking cessation | ||||||
| Population: people who smoke Setting: any; studies conducted in USA Intervention: bupropion and varenicline Comparison: varenicline alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with varenicline alone | Risk with bupropion and varenicline | |||||
| Smoking cessation (at least six months follow‐up) | Study population | RR 1.21 (0.95 to 1.55) | 1057 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 21 per 100 | 26 per 100 (20 to 33) | |||||
| Serious adverse events | Study population | RR 1.23 (0.63 to 2.42) | 1268 (5 RCTs) | ⊕⊕⊝⊝ Lowb | ||
| 2 per 100 | 3 per 100 (1 to 6) | |||||
| Dropouts due to adverse events of the drug | Study population | RR 0.80 (0.45 to 1.45) | 1230 (4 RCTs) | ⊕⊕⊝⊝ Lowb | ||
| 4 per 100 | 3 per 100 (2 to 6) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to imprecision. Fewer than 300 events overall. Confidence intervals encompass clinically significant benefit as well as no difference. bDowngraded two levels due to imprecision. Fewer than 100 events overall. Confidence intervals encompass clinically significant harm as well as clinically significant benefit.
Bupropion versus placebo/no pharmacotherapy control
Smoking cessation
There was evidence to suggest that bupropion was effective when compared to placebo or a non‐pharmacotherapeutic control to assist smoking cessation. Our meta‐analysis included 46 trials in which bupropion was the sole pharmacotherapy, with 17,866 participants: pooled risk ratio (RR) 1.64, 95% confidence interval (CI) 1.52 to 1.77; I2 = 15%; high‐certainty evidence; Analysis 1.1; Table 1). The results were not sensitive to the exclusion of studies judged to be at high or unclear risk of bias overall. We excluded one cluster‐RCT of bupropion versus no pharmacotherapy from our meta‐analysis due to substantial heterogeneity of programme effects across clusters. This trial detected no evidence of a difference between bupropion and no pharmacotherapy (both groups received behavioural support) for smoking cessation at any follow‐up point (adjusted RR at 6‐month follow‐up: 1.1, 95% CI 0.5 to 2.3; 1299 participants) (Siddiqi 2013). Sensitivity analyses excluding studies with industry support did not indicate that our findings were sensitive to the inclusion of these studies (see Table 4).
1.1. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 1: Smoking cessation
1. Sensitivity analyses excluding industry‐supported studies.
| Comparison and outcome | RR and CI excluding industry funded studies | RR and CI excluding studies with funding or medication provided by industry |
| Analysis 1.1 | 1.49 (1.33 to 1.66); studies = 26 | 1.48 (1.26 to 1.74); studies = 14 |
| Analysis 1.4 | 1.24 (1.14 to 1.35); studies = 9 | 1.24 (1.09 to 1.41); studies = 7 |
| Analysis 1.5 | 0.85 (0.60 to 1.21); studies = 11 | 0.88 (0.61 to 1.26); studies = 10 |
| Analysis 1.6 | 1.19 (0.72 to 1.94); studies = 4 | 1.19 (0.72 to 1.94); studies = 4 |
| Analysis 1.7 | 2.04 (0.23 to 17.84); studies = 7 | 2.61 (0.11 to 60.51); studies = 6 |
| Analysis 1.8 | Not estimable | Not estimable |
| Analysis 1.9 | Not estimable | Not estimable |
| Analysis 1.10 | Not estimable | Not estimable |
| Analysis 1.11 | 1.51 (0.44 to 5.27); studies = 6 | 1.51 (0.44 to 5.27); studies = 6 |
| Analysis 1.12 | 2.08 (0.93 to 4.64); studies = 4 | 2.27 (0.46 to 11.17); studies = 2 |
| Analysis 1.13 | 1.85 (1.55 to 2.20); studies = 11 | 1.85 (1.55 to 2.20); studies = 7 |
| Analysis 1.14 | 1.32 (0.98 to 1.77); studies = 11 | 1.11 (0.77 to 1.58); studies = 8 |
| Analysis 2.1 | 1.09 (0.91 to 1.32); studies = 11 | 0.78 (0.46 to 1.32); studies = 4 |
| Analysis 2.2 | 1.21 (1.02 to 1.43); studies = 2 | 1.24 (0.98 to 1.56); studies = 1 |
| Analysis 2.3 | 2.06 (0.20 to 21.67); studies = 2 | Not estimable |
| Analysis 2.4 | 2.93 (0.12 to 72.31); studies = 1 | 2.93 (0.12 to 72.31); studies = 1 |
| Analysis 2.5 | Not estimable | Not estimable |
| Analysis 2.6 | Not estimable | Not estimable |
| Analysis 2.7 | 0.68 (0.12 to 3.98); studies = 1 | 0.68 (0.12 to 3.98); studies = 1 |
| Analysis 2.10 | 1.04 (0.16 to 6.83); studies = 1 | Not estimable |
| Analysis 2.8 | 1.26 (0.60 to 2.65); studies = 1 | Not estimable |
| Analysis 2.9 | 1.62 (0.72 to 3.65); studies = 2 | Not estimable |
| Analysis 3.1 | 1.14 (0.85 to 1.51); studies = 2 | Not estimable |
| Analysis 3.2 | 1.05 (0.98 to 1.12); studies = 3 | 1.80 (0.53 to 6.16); studies = 1 |
| Analysis 3.3 | 1.31 (0.60 to 2.84); studies = 3 | Not estimable |
| Analysis 3.4 | 1.15 (1.03 to 1.30); studies = 2 | Not estimable |
| Analysis 3.5 | Not estimable | Not estimable |
| Analysis 3.6 | 0.34 (0.01 to 8.27); studies = 2 | Not estimable |
| Analysis 3.7 | 0.34 (0.04 to 3.27); studies = 2 | Not estimable |
| Analysis 3.8 | Not estimable | Not estimable |
| Analysis 3.9 | 0.34 (0.01 to 8.40); studies = 1 | Not estimable |
| Analysis 3.12 | 0.72 (0.37 to 1.40); studies = 4 | Not estimable |
| Analysis 3.10 | 1.49 (0.95 to 2.33); studies = 1 | Not estimable |
| Analysis 3.11 | 1.48 (1.15 to 1.89) | Not estimable |
CI: confidence interval; RR: risk ratio
We found no evidence suggesting that the effect of bupropion on smoking cessation depended upon the level of behavioural support offered to people stopping smoking. Three trials directly compared bupropion and placebo in factorial designs varying the behavioural support. There was no evidence from any of the three trials that the efficacy of bupropion differed between the lower and higher levels of behavioural support (Hall 2002; McCarthy 2008), or by the type of counselling approach used (Schmitz 2007). We also carried out a between‐study subgroup analysis of the possible interaction with behavioural support. We did this by classifying studies into low and high intensities of behavioural support (further split into delivery to a group or to individuals), using the criteria set in the Cochrane Review of NRT versus control (Hartmann‐Boyce 2018). Low‐intensity support consisted of less than 30 minutes at the initial consultation, with no more than two further assessment and reinforcement visits. Only one small trial met this criteria (Myles 2004). We found no evidence of a difference between subgroups (I2 = 0%; Analysis 1.2).
1.2. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 2: Smoking cessation ‐ subgroup by level of behavioural support
One trial directly compared bupropion and placebo in a cohort of participants with mental health disorders to a cohort without (Anthenelli 2016). There was no evidence indicating that the effect of bupropion depended upon whether people had or did not have a psychiatric disorder. We also carried out a between studies subgroup analysis to assess the potential interaction between cessation rates and mental health disorders. We did this by pooling studies (or subgroups of studies) into groups depending upon whether the participants were recruited specifically because they had a mental health disorder or they represented the general population (including some studies that excluded people with current mental health disorders). Some of these groups included people with serious mental health disorders, such as people with schizophrenia (Evins 2001; Evins 2005; George 2002), or other disorders including post‐traumatic stress disorder (PTSD; Hertzberg 2001), and a mix of mental health disorders (Anthenelli 2016). We found no evidence of a differential effect of bupropion on cessation between subgroups (Analysis 1.3; I2 = 15%).
1.3. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 3: Smoking cessation ‐ subgroup by mental health disorders
Depression
Four studies comparing bupropion to placebo/control analysed whether there was any interaction between depression and smoking quit rates (Anthenelli 2016 (analysis reported in West 2018); Aubin 2004; Cinciripini 2018; Kalman 2011). We did not find any evidence of this (Table 5).
2. Depression as a moderator of the relationship between antidepressants and smoking cessation.
| Study ID | Antidepressant | Direction of relationship | Evidence for interaction |
| Anthenelli 2016 | Bupropion | None | "Varenicline, bupropion and NRT were all effective in smokers with mental health problems (assessed with a number of variables, e.g. diagnostic history, HADS, use of psychotropic medication), and their relative efficacy was similar to that in smokers without a psychiatric history." |
| Aubin 2004 | Bupropion | None | "A similar subgroup analysis performed according to previous history of depression (evaluated by the MINI questionnaire) also failed to reveal an interaction with bupropion treatment." |
| Aveyard 2008 | Nortriptyline | None | "Participants randomised to nortriptyline plus nicotine replacement therapy for smoking cessation experienced less depression (OR 0.15) and anxiety early in the quit attempt when the risk of return to smoking is at its highest than those randomised to placebo plus nicotine replacement therapy. Contrary to expectations, no evidence was found that this led to greater abstinence." |
| Cinciripini 2018 | Bupropion | None | "Several measures failed to demonstrate significant effects as a function of time, treatment, or the interaction of treatment and time. For example, CES‐D scales including Depressive Affect, Interpersonal Relations, Positive Affect, and Somatic Symptoms, failed to demonstrate any effects of treatment or any treatment by time interactions." |
| Da Costa 2002 | Nortriptyline | Negative | "The best results were obtained with educational intervention, in those patients having no personal history of depression, who received the active drug. A negative history of depression was, however, the most important factor for the success of the treatment." |
| George 2003 | Selegiline | None (history), negative (current) | “There was no significant influence of a past history of major depression on smoking cessation outcomes (B = ‐0.49, SE = 0.90, Wald Statistic = 0.29, df = 1, p = .59), and when past history of major depression was entered into the logistic regression model as a covariate, it did not predict treatment failure with selegiline study medication (medication past history of depression status interaction: B = ‐0.02, SE = 1.03, Wald statistic = 0.00, df = 1, p = .98)." and "Furthermore, bivariate logistic regression analysis confirmed that having depressive symptoms at baseline negatively predicted smoking cessation outcomes with SEL on this continuous abstinence measure (B = 18.9, SE = 0.58, Wald statistic = 1048.9, df = 1, P < .01)." |
| Hall 2002 | Bupropion, nortripyline | Positive (for bupropion) | “There were higher abstinence rates for bupropion than nortriptyline for participants with a history of depressive disorder" |
| Kahn 2012 | Selegiline | None | "At the final HAM‐D assessment, the selegiline group (n = 90) reported a mean increase of 0.41 points and the placebo group (n = 85) reported a mean increase of 0.21 points. The difference between treatment groups was not statistically significant (t test, p = .65)." |
| Kalman 2011 | Bupropion | None | “Interaction effects between medication and tobacco dependence and medication and depressive symptoms were also nonsignificant.” |
| Killen 2000 | Paroxetine | None | "A stepwise logistic regression analysis was used to examine the association of abstinence at Week 26 with the variables [including depression scores] listed in Table 1. None of these variables were prospectively associated with abstinence." |
| Saules 2004 | Fluoxetine | None | “Examination of pre‐specified subgroups (i.e., gender, race, and history of major depressive disorder) did not reveal significant differences in smoking cessation by group” |
| Spring 2007 | Fluoxetine | None | Fluoxetine initially enhanced cessation for smokers with a history of major depression (P = .02) but subsequently impaired cessation regardless of depressive history. |
| Stapleton 2013 | Bupropion | Positive | “There was some evidence that the relative effectiveness of bupropion and NRT differed according to depression (χ2 = 2.86, P = 0.091), with bupropion appearing more beneficial than NRT in those with a history of depression (29.8 versus 18.5%)." |
| Wagena 2005 | Bupropion, nortriptyline | Positive (for bupropion) | “Results indicated that bupropion SR [sustained release] treatment was efficacious in helping smokers who were classified as depressed in achieving prolonged abstinence from smoking throughout the 26‐week period. The number of depressed participants from the nortriptyline‐treated group was considered too low to study this relationship." |
CES‐D: Center for Epidemiologic Studies Depression; df: degrees of freedom; HADS: Hospital Anxiety and Depression scale; HAM‐D: Hamilton Depression Rating Scale; MINI: Mini‐International Neuropsychiatric Interview; NRT: nicotine replacement therapy; OR: odds ratio; SE: standard error
Safety
There was evidence to suggest that taking bupropion increased the incidence of adverse events (AEs) relative to placebo or non‐pharmacotherapeutic control (RR 1.14, 95% CI 1.11 to 1.18; 19 studies, 10,893 participants; Analysis 1.4). However, a moderate degree of heterogeneity was detected between studies (I2 = 63%). Meta‐analysis of 21 studies did not provide clear evidence that the use of bupropion increased the likelihood of serious adverse events (SAEs) (RR 1.16, 95% CI 0.90 to 1.48; I2 = 0%; 21 studies, 10,625 participants; moderate‐certainty evidence; Analysis 1.5; Table 1), however the CIs encompassed both no difference as well as a clinically significant increase.
1.4. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 4: Adverse events
1.5. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 5: Serious adverse events
There was also evidence to suggest bupropion increased the likelihood of developing psychiatric AEs. We meta‐analysed six studies (RR 1.25, 95% CI 1.15 to 1.36; 6 studies, 4439 participants; Analysis 1.6). This effect is largely driven by Anthenelli 2016 (with an overall weighting of 96.9%), however as we judged this study to be at low risk of bias, and the effects are consistent with those detected by the other studies included in the analysis (I2 = 15%), this is not deemed to be problematic.
1.6. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 6: Psychiatric adverse events
There was insufficient evidence to determine whether bupropion use was associated with the likelihood of seizures (RR 2.93, 95% CI 0.64 to 13.37; I2 = 0%; 13 studies, 7344 participants; Analysis 1.7), risk of overdose (RR 2.15, 95% CI 0.23 to 19.86; I2 = 0%; 5 studies, 5585 participants; Analysis 1.8), suicide attempts (RR 1.62, 95% CI 0.29 to 8.92; I2 = 0%; 10 studies, 6484 participants; Analysis 1.9), risk of death by suicide (RR 0.34, 95% CI 0.01 to 8.26; I2 = n/a; 14 studies, 8822 participants; Analysis 1.10), or all‐cause mortality risk (RR 0.89, 95% CI 0.42 to 1.87; I2 = 0%; 21 studies, 11,403 participants; Analysis 1.11). In all cases the number of events reported were very low, which resulted in substantial imprecision and CIs encompassing both clinically significant benefit and harm.
1.7. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 7: Seizures
1.8. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 8: Overdoses
1.9. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 9: Suicide attempts
1.10. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 10: Death by suicide
1.11. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 11: All‐cause mortality
However, there was evidence that those randomized to receive bupropion were more likely to report symptoms of anxiety (RR 1.42, 95% CI 1.21 to 1.67; I2 = 40%; 11 studies, 7406 participants; Analysis 1.12) and insomnia (RR 1.78, 95% CI 1.62 to 1.96; I2 = 12%; 22 studies, 11,077 participants; Analysis 1.13) at follow‐up.
1.12. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 12: Anxiety
1.13. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 13: Insomnia
Tolerability
There was evidence that the risk of dropout due to AEs of the drug was higher in groups receiving bupropion relative to placebo or no pharmaceutical treatment (RR 1.37, 95% CI 1.21 to 1.56; I2 = 19%; 25 studies, 12,340 participants; high certainty evidence; Analysis 1.14; Table 1). Our point estimate suggests that participants taking bupropion had a 21% to 56% increased risk of dropping out relative to control.
1.14. Analysis.

Comparison 1: Bupropion versus placebo/no pharmacotherapy control, Outcome 14: Dropouts due to drug
We carried out sensitivity analyses (not shown) for all of the above safety and tolerability analyses, removing studies at overall high risk of bias, where this was relevant. In no cases did this change the interpretation of the effect. Additional sensitivity analyses, excluding studies with industry support, did not indicate that our findings were sensitive to the inclusion of these studies (see Table 4).
Bupropion plus nicotine replacement therapy (NRT) versus NRT alone
Smoking cessation
There was moderate statistical heterogeneity in the results of 12 studies comparing bupropion plus nicotine replacement therapy (NRT) to NRT alone for smoking cessation (RR 1.19, 95% CI 0.94 to 1.51; I2 = 52%; 12 studies, 3487 participants; low certainty evidence; Analysis 2.1; Table 2). The analysis thus found no clear evidence of a benefit of using bupropion plus NRT over using NRT alone. Nine of the 12 studies used nicotine patch, but two studies provided participants with nicotine lozenge (Piper 2009; Smith 2009), and one offered a choice of NRT (Stapleton 2013). However, splitting the analysis into these subgroups did not explain the heterogeneity detected (I2 = 0% for subgroup differences), nor did the exclusion of studies that did not use a bupropion placebo in the control arm (Smith 2009; Stapleton 2013). Removing the three studies deemed to be at an overall high risk of bias did not change the interpretation of the pooled effect estimate (Rose 2013; Smith 2009; Stapleton 2013). Sensitivity analyses excluding studies with industry support did not indicate that our findings were sensitive to the inclusion of these studies (see Table 4). Although the direction of the effect estimate changed when studies funded by the pharmaceutical industry, or where the medication was supplied by the pharmaceutical industry, were excluded; 95% CIs still encompassed evidence of benefit as well as harm.
2.1. Analysis.

Comparison 2: Bupropion plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 1: Smoking cessation
Depression
None of the relevant included studies investigated depression as a moderator of smoking quit rates.
Safety
There was evidence to indicate an increased risk of AEs when using combination bupropion and NRT relative to taking NRT alone (RR 1.21, 95% CI 1.02 to 1.43; I2 = 0%; 2 studies, 313 participants; Analysis 2.2); however the number of events was low (n = 192), and when one study at high risk of bias was removed the outcome become more imprecise and the CI spanned one (RR 1.24, 95% CI 0.98 to 1.56). There was insufficient evidence for the other safety outcomes we analysed for this comparison (SAEs, seizures, suicide attempts, death by suicide, all‐cause mortality). Very few studies had relevant data, and those that did recorded few events. In the case of the SAEs outcome, the removal of one study deemed to be at high risk of bias changed the effect estimate from RR = 1.52 (95% CI 0.26 to 8.89; I2 = 0%; 3 studies, 607 participants; very low certainty evidence; Analysis 2.3; Table 2) to RR = 1.00 (95% CI 0.06 to 15.83; I2 = n/a; 2 studies, 538 participants). Although this did not change the clinical interpretation of the result it does demonstrate that the effect estimate was highly dependent on this potentially biased study.
2.2. Analysis.

Comparison 2: Bupropion plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 2: Adverse events
2.3. Analysis.

Comparison 2: Bupropion plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 3: Serious adverse events
There was some evidence that bupropion plus NRT led to increased reporting of insomnia in comparison to NRT alone (RR 1.55, 95% CI 1.24 to 1.93; I2 = 0%; 2 studies, 556 participants; Analysis 2.8); however there was no clear evidence of an increase in anxiety in the bupropion plus NRT groups (RR 1.58, 95% CI 0.97 to 2.56; I2 = 47%; 3 studies, 1218 participants; Analysis 2.9). In both cases the results were based on a small number of studies and event rates were low (< 300).
2.8. Analysis.

Comparison 2: Bupropion plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 8: Insomnia
2.9. Analysis.

Comparison 2: Bupropion plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 9: Anxiety
Tolerability
Only two studies measured dropout due to AEs of the drug, providing insufficient information to draw conclusions and an imprecise pooled effect estimate (RR 1.67, 95% CI 0.95 to 2.92; I2 = 0%; 2 studies, 538 participants; low certainty evidence; Analysis 2.10; Table 2).
2.10. Analysis.

Comparison 2: Bupropion plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 10: Dropouts due to drug
Removing studies judged to be at high risk of bias from safety and tolerability analyses did not affect the interpretation of these effects, and sensitivity analyses, excluding studies with industry support, did not indicate that our findings were sensitive to the inclusion of these studies (see Table 4).
Bupropion plus varenicline versus varenicline alone
Smoking cessation
Our analysis did not find evidence that combination bupropion and varenicline resulted in higher smoking cessation rates than varenicline alone (RR 1.21, 95% CI 0.95 to 1.55; I2 = 15%; 3 studies, 1057 participants; moderate certainty evidence; Analysis 3.1; Table 3). Confidence intervals encompassed the possibility of no clinically significant difference in quit rates as well as a clinically significant benefit of bupropion combined with varenicline. We did not carry out a sensitivity analysis to account for risk of bias as we did not judge any of the studies in the analysis to be at high risk. A sensitivity analysis excluding studies with industry support did not indicate that our findings were sensitive to the inclusion of these studies (see Table 4).
3.1. Analysis.

Comparison 3: Bupropion plus varenicline versus varenicline alone, Outcome 1: Smoking cessation
Depression
None of the relevant included studies investigated a potential link between depression and quit rates.
Safety
There was evidence to indicate an increased risk of AEs, as well as psychiatric AEs, when taking combination bupropion and varenicline compared to varenicline alone (AEs: RR 1.09, 95% CI 1.02 to 1.17; I2 = 78%; 4 studies, 1043 participants; Analysis 3.2) (psychiatric AEs: RR 1.15, 95% CI 1.03 to 1.30; I2 = 75%; 2 studies, 835 participants; Analysis 3.4). However, in both cases we observed substantial heterogeneity, meaning these pooled estimates should be treated with caution.
3.2. Analysis.

Comparison 3: Bupropion plus varenicline versus varenicline alone, Outcome 2: Adverse events
3.4. Analysis.

Comparison 3: Bupropion plus varenicline versus varenicline alone, Outcome 4: Psychiatric adverse events
We did not find evidence to suggest an increased likelihood of SAEs (low certainty evidence; Table 3), overdoses, seizures, suicide attempts, death by suicide, or all‐cause mortality in the combination bupropion and varenicline trial arms in comparison to varenicline alone. However, there were few studies and events for these outcomes. In all cases (apart from those outcomes with no events; Analysis 3.5; Analysis 3.8) CIs encompassed one (Analysis 3.3; Analysis 3.6; Analysis 3.7; Analysis 3.9).
3.5. Analysis.

Comparison 3: Bupropion plus varenicline versus varenicline alone, Outcome 5: Seizures
3.8. Analysis.

Comparison 3: Bupropion plus varenicline versus varenicline alone, Outcome 8: Death by suicide
3.3. Analysis.

Comparison 3: Bupropion plus varenicline versus varenicline alone, Outcome 3: Serious adverse events
3.6. Analysis.

Comparison 3: Bupropion plus varenicline versus varenicline alone, Outcome 6: Overdoses
3.7. Analysis.

Comparison 3: Bupropion plus varenicline versus varenicline alone, Outcome 7: Suicide attempts
3.9. Analysis.

Comparison 3: Bupropion plus varenicline versus varenicline alone, Outcome 9: All‐cause mortality
There was some evidence that randomization to combination bupropion and varenicline resulted in greater reporting of anxiety (RR 1.55, 95% CI 1.01 to 2.38; I2 = 0%; 2 studies, 499 participants; Analysis 3.10) and insomnia (RR 1.45, 95% CI 1.14 to 1.84; I2 = 0%; 2 studies, 499 participants; Analysis 3.11) than varenicline alone at follow‐up. However, these results should be treated with caution as there were a low number of events in both analyses (< 200).
3.10. Analysis.

Comparison 3: Bupropion plus varenicline versus varenicline alone, Outcome 10: Anxiety
3.11. Analysis.

Comparison 3: Bupropion plus varenicline versus varenicline alone, Outcome 11: Insomnia
Tolerability
We did not find evidence to suggest an increased likelihood of dropout due to drug in the combination bupropion and varenicline trial arms in comparison to varenicline alone. However, results were imprecise and CIs encompassed one (low certainty evidence; Analysis 3.12; Table 3).
3.12. Analysis.

Comparison 3: Bupropion plus varenicline versus varenicline alone, Outcome 12: Dropouts due to drug
Where it was relevant to carry out sensitivity analyses removing studies judged to be at high risk of bias for the above safety and tolerability analyses, we found no evidence of a change in the clinical interpretation of results. Further sensitivity analyses excluding studies with industry support did not indicate that our findings were sensitive to the inclusion of these studies (see Table 4).
Exploratary safety and tolerability analyses combining comparisons
We carried out exploratory post hoc analyses combining the AEs (Analysis 4.1), psychiatric AEs (Analysis 4.2), SAEs (Analysis 4.3), and dropout due to adverse events (Analysis 4.4) outcomes across the above three comparisons (i.e. bupropion versus control; bupropion plus NRT versus NRT; bupropion plus varenicline versus varenicline). We subgrouped by the original comparison to test for any potential interactions. Significant subgroup differences were not detected for any of the outcomes; however these results should be treated with caution as some of the subgroups were underpowered. Overall pooled effects indicated that AEs (RR 1.14, 95% CI 1.11 to 1.17; I2 = 70%; subgroup differences I2 = 3%; 25 studies, 12,249 participants; Analysis 4.1), psychiatric AEs (RR 1.24, 95% CI 1.15 to 1.33; I2 = 45%; subgroup differences I2 = 23%; 8 studies, 5274 participants; Analysis 4.2) and dropouts due to AEs of the drug (RR 1.35, 95% CI 1.20 to 1.52; I2 = 12%; subgroup differences I2 = 44%; 30 studies, 14,108 participants; Analysis 4.4) were all increased by bupropion. However, there was substantial overall heterogeneity for the adverse event outcome, and some moderate heterogeneity between subgroups for the dropout due to AEs outcome ‐ although the latter did not reach statistical significance (P = 0.17). There was still no clear evidence of an increased risk of SAEs when using bupropion. However, despite combining more studies there was still some imprecision in this result, meaning that 95% CIs still incorporated a potential increase in SAEs when using bupropion, as well as no increase (RR 1.17, 95% CI 0.93 to 1.47; I2 = 0%; subgroup differences I2 = 0%; 28 studies, 12,500 participants; Analysis 4.3).
4.1. Analysis.

Comparison 4: Exploratory safety analysis: effects of bupropion only across comparisons, Outcome 1: Adverse events
4.2. Analysis.

Comparison 4: Exploratory safety analysis: effects of bupropion only across comparisons, Outcome 2: Psychiatric adverse events
4.3. Analysis.

Comparison 4: Exploratory safety analysis: effects of bupropion only across comparisons, Outcome 3: Serious adverse events
4.4. Analysis.

Comparison 4: Exploratory safety analysis: effects of bupropion only across comparisons, Outcome 4: Dropouts due to drug
Bupropion versus front‐line smoking cessation monotherapies
Smoking cessation
We found evidence to suggest bupropion was less effective than varenicline for smoking cessation (RR 0.71, 95% CI 0.64 to 0.79; I2 = 0%; 6 studies, 6286 participants; Analysis 5.1), whereas there was no clear evidence that bupropion resulted in better cessation rates than NRT (RR 0.99, 95% CI 0.91 to 1.09; I2 = 18%; 10 studies, 8230 participants; Analysis 6.1). This was based upon our meta‐analyses of 10 relevant studies, in which we pooled studies investigating all forms of NRT (patch, lozenge, or a choice). When comparing the results of our analyses across separate subgroups of NRT (including combination patch and lozenge) we found that there was no strong evidence of significant subgroup differences (I2 = 47.8%; P = 0.12). In neither case did removing studies deemed to be at an overall high risk of bias change the interpretation of the effect estimates.
5.1. Analysis.

Comparison 5: Bupropion versus varenicline, Outcome 1: Smoking cessation
6.1. Analysis.

Comparison 6: Bupropion versus nicotine replacement therapy (NRT), Outcome 1: Smoking cessation
Depression
One post hoc analysis found that bupropion was more effective than NRT in those with a history of depression (Stapleton 2013). See Table 5.
Safety
There was evidence that randomization to bupropion resulted in minimal difference in reporting of AEs when compared to both varenicline and NRT (Analysis 5.2; Analysis 6.2). The same was true of SAEs, however there were much fewer events in these analyses, meaning they were underpowered and we can have less certainty in their results (Analysis 5.3; Analysis 6.3). When focusing on psychiatric AEs only there was no evidence of a difference when comparing bupropion to varenicline (Analysis 5.4); and heterogeneity was so high when comparing bupropion to NRT that it was deemed inappropriate to present a pooled estimate (I2 = 92%; Analysis 6.4). There was insufficient evidence to indicate whether bupropion increased the risk of many of the other safety outcomes assessed (seizures, overdoses, suicide attempts, death by suicide and all‐cause mortality) when compared to varenicline and NRT due to a paucity of relevant data, meaning that when estimates could be calculated these were extremely imprecise with CIs encompassing both potential benefit and harm of the intervention.
5.2. Analysis.

Comparison 5: Bupropion versus varenicline, Outcome 2: Adverse events
6.2. Analysis.

Comparison 6: Bupropion versus nicotine replacement therapy (NRT), Outcome 2: Adverse events
5.3. Analysis.

Comparison 5: Bupropion versus varenicline, Outcome 3: Serious adverse events
6.3. Analysis.

Comparison 6: Bupropion versus nicotine replacement therapy (NRT), Outcome 3: Serious adverse events
5.4. Analysis.

Comparison 5: Bupropion versus varenicline, Outcome 4: Psychiatric adverse events
6.4. Analysis.

Comparison 6: Bupropion versus nicotine replacement therapy (NRT), Outcome 4: Psychiatric adverse events
We also found evidence that participants in the bupropion groups experienced more insomnia and anxiety than people in the varenicline groups (insomnia: RR 1.40, 95% CI 1.22 to 1.60; I2 = 9%; 3 studies, 5208 participants; Analysis 5.10; anxiety: RR 1.28, 95% CI 1.07 to 1.53; I2 = 0%; 2 studies, 4705 participants; Analysis 5.11) and NRT groups (insomnia: RR 1.31, 95% CI 1.10 to 1.55; I2 = 47%; 2 studies, 4128 participants; Analysis 6.10; anxiety: RR 1.31, 95% CI 1.06 to 1.62; I2 = 67%; 2 studies, 4855 participants; Analysis 6.11) at follow‐up. However, we detected moderate heterogeneity for both the insomnia and anxiety outcomes for the comparison to NRT, and when we carried out a sensitivity analysis, removing the study judged to be at high risk of bias, for the anxiety outcome (Analysis 6.11) the 95% CIs shifted to incorporate no between‐group difference in anxiety (RR 1.23, 95% CI 0.99 to 1.53; I2 = 13%; 1 study, 4028 participants).
5.10. Analysis.

Comparison 5: Bupropion versus varenicline, Outcome 10: Insomnia
5.11. Analysis.

Comparison 5: Bupropion versus varenicline, Outcome 11: Anxiety
6.10. Analysis.

Comparison 6: Bupropion versus nicotine replacement therapy (NRT), Outcome 10: Insomnia
6.11. Analysis.

Comparison 6: Bupropion versus nicotine replacement therapy (NRT), Outcome 11: Anxiety
Tolerability
Compared to both varenicline (RR 1.12, 95% CI 0.96 to 1.31; I2 = 23%; 6 studies, 6103 participants; Analysis 5.12) and NRT (RR 1.14, 95% CI 0.95 to 1.38; I2 = 33%; 4 studies, 4825 participants; Analysis 6.12) there was no clear evidence that bupropion led to an increase in trial dropouts due to AEs; however in both cases the CIs encompassed fewer dropouts in the comparator as well as no difference.
5.12. Analysis.

Comparison 5: Bupropion versus varenicline, Outcome 12: Dropouts due to drug
6.12. Analysis.

Comparison 6: Bupropion versus nicotine replacement therapy (NRT), Outcome 12: Dropouts due to drug
We carried out sensitivity analyses for all of the safety and tolerability analyses, removing studies judged to be at high risk of bias, where appropriate. None of these analyses resulted in a difference in the clinical interpretation of effects.
Bupropion versus other pharmacotherapies
Smoking cessation
There was no clear evidence that bupropion was more effective than nortriptyline in aiding smoking cessation (RR 1.30, 95% CI 0.93 to 1.82; I2 = 0%; 3 studies, 417 participants; Analysis 7.1), although event rates were low (101 participants), and the result imprecise. This result was similar when one study judged to be at high risk of bias (Hall 2002), was removed from the analysis.
7.1. Analysis.

Comparison 7: Bupropion versus nortriptyline, Outcome 1: Smoking cessation
Depression
Only two trials examined the interaction between depression and quit rates for bupropion and nortriptyline (Hall 2002; Wagena 2005). Both of the within‐study analyses found that participants classified as depressed were more likely to quit using bupropion than nortriptyline (Table 5).
Safety
There was insufficient evidence to determine whether bupropion increased the risk of any of the safety outcomes included in this review when compared to nortriptyline (Analysis 7.2; Analysis 7.3), or gabapentin (Analysis 8.1). Where data were available, it was sparse and resulted in imprecise pooled estimates encompassing one. In one instance (bupropion versus nortriptyline; insomnia outcome) heterogeneity was so high that it was not appropriate to present a pooled estimate (I2 = 90%; Analysis 7.3).
7.2. Analysis.

Comparison 7: Bupropion versus nortriptyline, Outcome 2: Serious adverse events
7.3. Analysis.

Comparison 7: Bupropion versus nortriptyline, Outcome 3: Insomnia
8.1. Analysis.

Comparison 8: Bupropion versus gabapentin, Outcome 1: Serious adverse events
Tolerability
There was also insufficient evidence to determine whether bupropion increased the risk of trial dropouts due to adverse events when compared to both nortriptyline (Analysis 7.4) and gabapentin (Analysis 8.2), with imprecise estimates in both cases.
7.4. Analysis.

Comparison 7: Bupropion versus nortriptyline, Outcome 4: Dropouts due to drug
8.2. Analysis.

Comparison 8: Bupropion versus gabapentin, Outcome 2: Dropouts due to drug
Where possible, for the above safety and tolerability outcomes, we carried out sensitivity analyses removing studies judged to be at high risk of bias; however in the rare cases where this was appropriate there was no appreciable change in the interpretation of the effect estimates.
Bupropion at different doses
Smoking cessation
There was no clear evidence to indicate a differential effect between a 150 mg or 300 mg dose of bupropion on the likelihood of smoking cessation. Whilst the pooled estimate was 1.08 in favour of a 300 mg dose, the 95% CI encompassed a potential benefit of either dose (RR 1.08, 95% CI 0.93 to 1.26; I2 = 49%; 3 studies, 2042 participants; Analysis 9.1).
9.1. Analysis.

Comparison 9: Bupropion (different doses), Outcome 1: Smoking cessation
Depression
None of the relevant included studies investigated a potential link between depression and quit rates.
Safety
We were unable to draw conclusions about any of the safety outcomes for this comparison. Analyses that could be carried out (SAEs Analysis 9.2; overdoses Analysis 9.3; suicide attempts Analysis 9.4; death by suicide Analysis 9.5; all‐cause mortality Analysis 9.6; insomnia Analysis 9.7; anxiety Analysis 9.8), suffered from substantial imprecision due to a low number of events (ranging from 0 to 99), and in all cases 95% CIs encompassed one.
9.2. Analysis.

Comparison 9: Bupropion (different doses), Outcome 2: Serious adverse events
9.3. Analysis.

Comparison 9: Bupropion (different doses), Outcome 3: Overdoses
9.4. Analysis.

Comparison 9: Bupropion (different doses), Outcome 4: Suicide attempts
9.5. Analysis.

Comparison 9: Bupropion (different doses), Outcome 5: Death by suicide
9.6. Analysis.

Comparison 9: Bupropion (different doses), Outcome 6: All‐cause mortality
9.7. Analysis.

Comparison 9: Bupropion (different doses), Outcome 7: Insomnia
9.8. Analysis.

Comparison 9: Bupropion (different doses), Outcome 8: Anxiety
Tolerability
Our analysis of dropouts due to adverse event data also suffered from imprecision (Analysis 9.9), and we were unable to draw conclusions.
9.9. Analysis.

Comparison 9: Bupropion (different doses), Outcome 9: Dropouts due to drug
A sensitivity analysis removing studies judged to be at high risk of bias was only appropriate for the cessation outcome; removing the one study deemed to be at high risk of bias did not alter the clinical interpretation of the result.
Other antidepressant monotherapies versus control
Smoking cessation
Pooling six trials comparing nortriptyline to placebo showed evidence of benefit of nortriptyline over placebo (RR 2.03, 95% CI 1.48 to 2.78; I2 = 16%; 6 studies, 975 participants; Analysis 10.1) for smoking cessation. Removing two studies judged to be at high risk of bias did not influence the result (Hall 2002; Prochazka 1998).
10.1. Analysis.

Comparison 10: Nortriptyline versus placebo, Outcome 1: Smoking cessation
We did not find clear evidence to indicate that selective serotonin reuptake inhibitors (SSRIs) increased the likelihood of smoking cessation relative to control (RR 0.93, 95% CI 0.71 to 1.22; I2 = 0%; 4 studies, 1594 participants; Analysis 11.1); however there was a low number of events across studies (193 participants) and this should be taken into account. We subgrouped our meta‐analysis by the type of SSRI used in the trial (2 fluoxetine: Niaura 2002; Spring 2007; 1 paroxetine: Killen 2000; 1 sertraline: Covey 2002), and found no evidence of a subgroup difference (I2 = 0%).
11.1. Analysis.

Comparison 11: Selective serotonin reuptake inhibitors (SSRIs) versus placebo, Outcome 1: Smoking cessation
We also found no clear evidence that monoamine oxidase inhibitors (MAOIs) increased the likelihood of smoking cessation relative to control (RR 1.29, 95% CI 0.93 to 1.79; I2 = 0%; 6 studies, 827 participants; Analysis 12.1); however, again event rates were low, resulting in imprecision (193 participants). There was no effect of removing the one study deemed to be at high risk of bias (George 2003). Our meta‐analysis included one trial of moclobemide (Berlin 1995) and five of selegiline (Biberman 2003; George 2003; Kahn 2012; Killen 2010; Weinberger 2010), and we subgrouped these accordingly. We did not identify any evidence of a subgroup difference (I2 = 0%).
12.1. Analysis.

Comparison 12: Monoamine oxidase inhibitor (MAOI) versus placebo, Outcome 1: Smoking cessation
One trial of venlafaxine (Cinciripini 2005) showed no evidence of increased smoking cessation compared to placebo (RR 1.22, 95% CI 0.64 to 2.32; I2 = n/a; 1 study, 147 participants; Analysis 13.1). However, the effect estimate was imprecise and CIs encompassed both potential benefit and harm.
13.1. Analysis.

Comparison 13: Venlafaxine versus placebo, Outcome 1: Smoking cessation
Two small trials comparing St John’s wort to placebo (Parsons 2009; Sood 2010), provided no clear evidence to suggest it was a better smoking cessation aid than placebo when pooled (RR 0.81, 95% CI 0.26 to 2.53; I2 = 29%; 2 studies, 261 participants; Analysis 14.1); however there was substantial imprecision.
14.1. Analysis.

Comparison 14: Hypericum (St John's wort) versus placebo, Outcome 1: Smoking cessation
The one trial assessing S‐Adenosyl‐L‐Methionine (SAMe) compared to placebo provided no evidence of benefit for smoking cessation (RR 0.70; CI 0.24 to 2.07; I2 = n/a; 1 study; 120 participants; Analysis 15.1); however, the number of included participants and the number of events were small.
15.1. Analysis.

Comparison 15: S‐Adenosyl‐L‐Methionine (SAMe) versus placebo, Outcome 1: Smoking cessation
Depression
One within‐study comparison found that a past history of depression did not appear to moderate the efficacy of nortriptyline, but subgroup numbers were small (Hall 1998). However, another within‐study analysis found that the most effective factor for ensuring the efficacy of nortriptyline was a negative history of depression (Da Costa 2002).
Of the three studies conducting post hoc analyses of fluoxetine (Saules 2004; Spring 2007) and paroxetine (Killen 2000) to assess the interaction between depression and antidepressant quit rates, none provided evidence to support this interaction (George 2003; Kahn 2012).
The two studies conducting post hoc analyses of selegiline to assess the interaction between depression and antidepressant quit rates also did not provide evidence to support this interaction (George 2003; Kahn 2012).
Safety
One trial investigated the likelihood of SAEs when randomized to receive nortriptyline in comparison to placebo (Haggsträm 2006). No SAEs were reported in either trial arm (Analysis 10.2). The insomnia and anxiety outcomes provided insufficient evidence of any effect for this comparison (Analysis 10.3; Analysis 10.4).
10.2. Analysis.

Comparison 10: Nortriptyline versus placebo, Outcome 2: Serious adverse events
10.3. Analysis.

Comparison 10: Nortriptyline versus placebo, Outcome 3: Insomnia
10.4. Analysis.

Comparison 10: Nortriptyline versus placebo, Outcome 4: Anxiety
There was insufficient evidence to indicate whether SSRIs increased the risk of AEs relative to placebo. Only one small trial of fluoxetine investigated this (NCT00578669; Analysis 11.2).
11.2. Analysis.

Comparison 11: Selective serotonin reuptake inhibitors (SSRIs) versus placebo, Outcome 2: Adverse events
For the comparison of MAOIs relative to placebo, there was no evidence of increased risk of experiencing either AEs (Analysis 12.2), psychiatric AEs (Analysis 12.3), or SAEs (Analysis 12.4); however the latter two analyses suffered from substantial imprecision and should be treated with caution. Substantial imprecision and heterogeneity also meant that we were unable to draw conclusions regarding insomnia and anxiety (Analysis 12.5; Analysis 12.6).
12.2. Analysis.

Comparison 12: Monoamine oxidase inhibitor (MAOI) versus placebo, Outcome 2: Adverse events
12.3. Analysis.

Comparison 12: Monoamine oxidase inhibitor (MAOI) versus placebo, Outcome 3: Psychiatric adverse events
12.4. Analysis.

Comparison 12: Monoamine oxidase inhibitor (MAOI) versus placebo, Outcome 4: Serious adverse events
12.5. Analysis.

Comparison 12: Monoamine oxidase inhibitor (MAOI) versus placebo, Outcome 5: Insomnia
12.6. Analysis.

Comparison 12: Monoamine oxidase inhibitor (MAOI) versus placebo, Outcome 6: Anxiety
The one study assessing safety outcomes for St John's wort versus placebo (Parsons 2009), did not provide sufficient evidence to assess whether it increased the likelihood of SAEs or all‐cause mortality specifically (Analysis 14.2; Analysis 14.3), and a study of SAMe versus placebo (Sood 2012), did not provide sufficient evidence on AEs or insomnia (Analysis 15.2; Analysis 15.3).
14.2. Analysis.

Comparison 14: Hypericum (St John's wort) versus placebo, Outcome 2: Serious adverse events
14.3. Analysis.

Comparison 14: Hypericum (St John's wort) versus placebo, Outcome 3: All‐cause mortality
15.2. Analysis.

Comparison 15: S‐Adenosyl‐L‐Methionine (SAMe) versus placebo, Outcome 2: Adverse events
15.3. Analysis.

Comparison 15: S‐Adenosyl‐L‐Methionine (SAMe) versus placebo, Outcome 3: Insomnia
Tolerability
Our meta‐analysis including four studies comparing nortriptyline to placebo found evidence that dropout due to treatment was approximately twice as likely when randomized to nortriptyline (RR 1.99, 95% CI 1.18 to 3.36; I2 = 23%; 4 studies, 537 participants; Analysis 10.5). This result should be treated with caution due to imprecision; however the removal of two studies judged to be at high risk of bias did not change the interpretation of the result (Hall 2004; Prochazka 1998).
10.5. Analysis.

Comparison 10: Nortriptyline versus placebo, Outcome 5: Dropouts due to drug
There was also evidence to suggest SSRIs may increase the likelihood of dropout due to drug (RR 2.59, 95% CI 1.70 to 3.94; I2 = 0%; 3 studies, 1270 participants; Analysis 11.3). When the four included studies were subgrouped into two of fluoxetine (Niaura 2002; Spring 2007), and one of sertraline (Covey 2002), there was no evidence of a subgroup difference (I2 = 0%).
11.3. Analysis.

Comparison 11: Selective serotonin reuptake inhibitors (SSRIs) versus placebo, Outcome 3: Dropouts due to drug
There was some evidence that there may be an increased risk of drug discontinuation in the MAOI groups, and this persisted when we removed one study judged to be at high risk of bias. However, there was substantial imprecision in this analysis (Analysis 12.7).
12.7. Analysis.

Comparison 12: Monoamine oxidase inhibitor (MAOI) versus placebo, Outcome 7: Dropouts due to drug
One study each assessed dropout due to drug for St John's wort versus placebo (Parsons 2009; Analysis 14.4), venflaxine versus placebo (Cinciripini 2005; Analysis 13.2), and SAMe versus placebo (Sood 2012; Analysis 15.4). These studies did not provide sufficient evidence to draw clear conclusions.
14.4. Analysis.

Comparison 14: Hypericum (St John's wort) versus placebo, Outcome 4: Dropouts due to drug
13.2. Analysis.

Comparison 13: Venlafaxine versus placebo, Outcome 2: Dropouts due to drug
15.4. Analysis.

Comparison 15: S‐Adenosyl‐L‐Methionine (SAMe) versus placebo, Outcome 4: Dropouts due to drug
Other antidepressant combination therapies versus control
Smoking cessation
Pooling four trials using nortriptyline as an adjunct to nicotine patch therapy (Aveyard 2008; Hall 2004; Prochazka 2004; Richmond 2013), did not provide evidence of a benefit of combination nortriptyline and NRT for smoking cessation relative to NRT alone (RR 1.21, 95% CI 0.94 to 1.55; I2 = 26%; 4 studies, 1644 participants; Analysis 16.1); however there was imprecision around the effect estimate, with the CIs encompassing both no difference and a clinically significant benefit. The interpretation of the result remained the same when we removed studies judged to be at an overall high risk of bias.
16.1. Analysis.

Comparison 16: Nortriptyline plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 1: Smoking cessation
Three trials evaluated fluoxetine as an adjunct to NRT (Blondal 1999; Brown 2014; Saules 2004), but also did not provide evidence of an increased likelihood of smoking cessation relative to NRT alone when pooled (RR 0.70, 95% CI 0.48 to 1.03; I2 = 0%; 3 studies, 466 participants; Analysis 17.1). Again, interpretation did not change when we removed studies judged to be at risk of bias, and there was evidence of imprecision. However, in this instance CIs encompassed the possibility of no difference and a clinically significant harm.
17.1. Analysis.

Comparison 17: Selective serotonin reuptake inhibitor (SSRI) plus NRT versus NRT alone, Outcome 1: Smoking cessation
Depression
One study comparing nortriptyline plus NRT to NRT alone found no evidence supporting depression as a moderator of abstinence in either the combination nortriptyline and NRT or the placebo arms of the trial (Aveyard 2008).
Safety
One trial investigated the effect of combination nortriptyline and NRT on the likelihood of insomnia when compared to NRT alone (Prochazka 2004). However the study only reported a very small number of events across trial arms, resulting in substantial imprecision, and making it impossible to draw any conclusions (Analysis 16.2).
16.2. Analysis.

Comparison 16: Nortriptyline plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 2: Insomnia
There was also insufficient evidence from one study investigating the effect of selegiline plus NRT versus NRT alone on SAEs (Biberman 2003; Analysis 18.1). Similarly Berlin 2012 alone provides insufficient data to assess the effects of EVT302 plus NRT versus NRT alone on AEs (Analysis 19.1) and SAEs (Analysis 19.2).
18.1. Analysis.

Comparison 18: Selegeline plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 1: Serious adverse events
19.1. Analysis.

Comparison 19: EVT302 plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 1: Adverse events
19.2. Analysis.

Comparison 19: EVT302 plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 2: Serious adverse events
Tolerability
One trial investigated the effect of combination nortriptyline and NRT on the likelihood of trial discontinuation (Prochazka 2004); however events were low, making it impossible to draw conclusions (Analysis 16.3). There was insufficient information available from the one study comparing dropout due to AEs between selegiline plus NRT versus NRT alone (Biberman 2003; Analysis 18.2), and the one study comparing EVT302 plus NRT versus NRT alone (Berlin 2012; Analysis 19.3) to draw conclusions.
16.3. Analysis.

Comparison 16: Nortriptyline plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 3: Dropouts due to drug
18.2. Analysis.

Comparison 18: Selegeline plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 2: Dropouts due to drug
19.3. Analysis.

Comparison 19: EVT302 plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 3: Dropouts due to drug
Other antidepressants at different doses
Smoking cessation
We are unable to evaluate the efficacy of 300 mg versus 600 mg St John's wort, as the one trial comparing these differences (Barnes 2006) had too small a sample size (28 participants), with no individuals abstinent from smoking at 12 months follow‐up. One study compared the efficacy of 30 mg versus 60 mg of fluoxetine, and found the same quit rates in both groups (RR 1.00, 95% CI 0.63 to 1.59; I2 = n/a; 656 participants; Analysis 20.1); however this result should be treated with caution due to imprecision.
20.1. Analysis.

Comparison 20: Fluoxetine (30 mg versus 60 mg), Outcome 1: Smoking cessation
Depression
These studies did not investigate depression as a moderator of smoking quit rates.
Safety
Berlin 2002 only followed up participants to eight weeks and therefore we did not use efficacy data from this study; however they reported safety data. The study compared 100 mg with 200 mg daily doses of lazabemide. No SAEs were recorded during the trial (Analysis 21.1). There was insufficient evidence to conclude whether participants randomized to the higher dose were more likely to suffer from symptoms of insomnia (Analysis 21.2) or anxiety (Analysis 21.3).
21.1. Analysis.

Comparison 21: Lazabemide (100 mg versus 200 mg), Outcome 1: Serious adverse events
21.2. Analysis.

Comparison 21: Lazabemide (100 mg versus 200 mg), Outcome 2: Insomnia
21.3. Analysis.

Comparison 21: Lazabemide (100 mg versus 200 mg), Outcome 3: Anxiety
Due to the very small sample size of Barnes 2006, there was insufficient evidence to assess the likelihood of AEs in participants receiving a 300 mg daily dose of St. John's wort versus a 600 mg dose (28 participants; Analysis 22.2). Similarly, there was insufficient evidence investigating the effect of a 800 mg daily dose of SAMe versus a 1600 mg daily dose on the risk of AEs (Sood 2012; Analysis 23.1).
22.2. Analysis.

Comparison 22: Hypericum (St John's wort) (300 mg versus 600 mg), Outcome 2: Adverse events
23.1. Analysis.

Comparison 23: S‐Adenosyl‐L‐Methionine (SAMe) (800 mg versus 1600 mg), Outcome 1: Adverse events
Tolerabiliy
Niaura 2002 found some evidence that a 60 mg daily dose of fluoxetine compared to a 30 mg dose daily increased the likelihood of trial discontinuation due to drug treatment (RR 0.64, 95% CI 0.46 to 0.87; I2 = n/a; 1 study, 656 participants; Analysis 20.2).
20.2. Analysis.

Comparison 20: Fluoxetine (30 mg versus 60 mg), Outcome 2: Dropouts due to drug
However, there was insufficient evidence to conclude whether participants randomized to the higher 200 mg dose of lazabemide were more likely to drop out of the trial due to the medication than participants randomized to the lower 100 mg dose (Berlin 2002; Analysis 21.4), or whether participants randomized to a 1600 mg dose of SAMe were more likely to drop out than those randomized to a 800 mg dose (Sood 2012; Analysis 23.2).
21.4. Analysis.

Comparison 21: Lazabemide (100 mg versus 200 mg), Outcome 4: Dropouts due to drug
23.2. Analysis.

Comparison 23: S‐Adenosyl‐L‐Methionine (SAMe) (800 mg versus 1600 mg), Outcome 2: Dropouts due to drug
Discussion
Summary of main results
This review summarizes and evaluates the evidence investigating the efficacy, safety and tolerability of different types of antidepressant for smoking cessation. This review includes 115 studies in total. Forty‐six trials of 17,866 participants provide a large, high‐certainty evidence base confirming the benefit of bupropion used as a single pharmacotherapy for smoking cessation (Table 1). The pooled estimate suggests that bupropion increased long‐term quitting success by 52% to 77% when compared with placebo. Treatment effects appeared to be comparable across the range of populations, settings and types of behavioural support studied, including those with and without a past history of depression. There is evidence to suggest that bupropion increases the risk of adverse events (AEs), including psychiatric AEs, and the likelihood that users will discontinue treatment; however evidence does not currently suggest that bupropion increases the risk of serious adverse events (SAEs).
Our review finds no evidence of an additional benefit of adding bupropion to nicotine replacement therapy (NRT) (low‐certainty evidence; Table 2) or varenicline treatment (moderate‐certainty evidence; Table 3) when compared to NRT or varenicline alone, respectively. There was insufficient evidence to draw conclusions on safety outcomes when using bupropion as an adjunct to NRT or varenicline. This is due to a lack of studies assessing these outcomes, and few events recorded for those studies that did (very low‐certainty and low‐certainty evidence, respectively).
The evidence does not suggest a difference in the efficacy of bupropion plus NRT, or nortriptyline, for smoking cessation. However, participants taking bupropion may be between 21% and 36% less likely to quit than those treated with varenicline, based on evidence from 6286 participants. The evidence relating to the safety of treatment is inconclusive when comparing bupropion to NRT, varenicline and nortriptyline due to a paucity of studies, overall participants, and events.
We found evidence that nortriptyline is also an effective agent to aid smoking cessation when compared with placebo, based on a meta‐analysis of six studies, including 975 participants. However, there is no clear evidence that other antidepressants, including selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), venflaxine, St John's wort, and S‐Adenosyl‐L‐Methionine (SAMe), are effective as cessation aids. Therefore, despite SSRIs being commonly used to treat depression there does not seem to be any justification for continuing to pursue their use for smoking cessation, where other more clearly effective options exist.
Few studies examined whether current or previous depression moderated the effectiveness of antidepressants to aid smoking cessation. Those comparing bupropion to placebo found no evidence of an interaction between depression and use of bupropion. Studies contributing to other comparisons found varied but uncertain results.
Overall completeness and applicability of evidence
The searches conducted for this study were broad and identified any studies where a drug was described as being an 'antidepressant' or 'antidepressive'. In cases where we were unsure of whether a medication was classed as an antidepressant, we conducted a brief literature search to clarify whether they had been used in other research as antidepressants, so as to ensure we included all relevant medications. We also searched trial registers to identify any ongoing or completed but unpublished, registered studies assessing the efficacy and safety of antidepressants for smoking cessation.
Studies included in this review recruited adult smokers who were typically motivated to quit. Of the study populations included in our review, the lowest mean cigarettes smoked per day was 10, and the highest was 44, meaning that most studies included participants with significant tobacco addiction. These results may not apply to populations with few symptoms of tobacco addiction. In addition, the minority of studies specifically recruited participants with mental health disorders. Of these five studies, one was weighted particularly heavily in the meta‐analysis (Anthenelli 2016). Anthenelli 2016 recruited a subset of participants with mental health disorders, who were described as 'clinically stable', suggesting that they may not be entirely representative of the wider population diagnosed with a mental health disorder. Further studies are needed among those with depression to provide greater confidence in our findings, which suggest that bupropion is as effective for smoking cessation in people with a mental health diagnosis as those without.
Certainty of the evidence
Of the 115 studies included in this review, we judged 12 to be at low risk of bias for all domains, and 28 to be at high risk in one or more domains. We judged the remaining 75 studies to be at an unclear risk due to a lack of reporting of key information. In these cases it is impossible to know whether these studies were at any risk of bias or whether the information was simply not reported. To investigate the potential impact of studies that we judged to be at high risk of bias on results, we carried out sensitivity analyses, removing studies judged to be at high risk from analyses and observing the effects on results (where this was possible). In most cases this had no effect on the clinical interpretation of the analyses.
We assessed the certainty of the evidence by creating 'Summary of findings' tables and carrying out GRADE ratings (Schünemann 2013) for three of the comparisons (bupropion versus placebo/no pharmacotherapy control (Table 1); bupropion plus NRT versus NRT alone (Table 2); bupropion plus varenicline versus varenicline alone (Table 3). The efficacy of bupropion versus placebo/pharmacotherapy control for smoking cessation generated high‐certainty evidence. We judged combination bupropion and varenicline to be of moderate certainty, whilst we judged the combination bupropion and NRT to be of low‐certainty evidence. We judged the safety outcomes for bupropion versus placebo/pharmacotherapy control of SAEs and dropout due to drug to have moderate‐ and high‐certainty evidence, respectively. However, for the bupropion combination therapy with NRT or varenicline comparisons, we judged the evidence for these safety outcomes to be of very low‐ and low‐certainty, respectively. The main reason for downgrading the evidence was imprecision (low overall numbers of participants and events), as well as risk of bias in one case (judgements of high risk that may affect the result), and inconsistency (moderate heterogeneity in analysis detected) in another case.
Potential biases in the review process
We consider the review process used to be robust, and do not believe we have introduced any biases. For outcome assessment, we followed the standard methods used for Cochrane Tobacco Addiction cessation reviews. Our search of the Cochrane Tobacco Addiction Specialized Register, allowed us to capture three ongoing studies. However, there may be unpublished data that our searches did not uncover.
We generated and interpreted funnel plots for all analyses that included 10 or more studies. Four of these were for outcomes summarized in our 'Summary of findings' tables (smoking cessation, SAEs and dropout due to adverse events of the drug) and contributed to our GRADE ratings for the following comparisons: bupropion versus placebo/pharmacotherapy control (Figure 3, Figure 4, Figure 5, respectively), and smoking cessation for bupropion plus NRT versus NRT alone (Figure 6). None of these plots appeared to demonstrate evidence of publication bias. However, only 12 studies contributed to the funnel plot for bupropion plus NRT versus NRT alone (a relatively small number), so this should be interpreted with caution. We also tested whether the inclusion of studies funded by the pharmaceutical industry, or where a pharmaceutical company had supplied the medication for the study, was impacting on the pooled results of our analyses. In no case did there appear to be any clear evidence of this (Table 4).
3.

Funnel plot of comparison: 1 Bupropion versus placebo/control, outcome: 1.1 Smoking cessation.
4.

Funnel plot of comparison: 1 Bupropion versus placebo/control, outcome: 1.5 Serious adverse events.
5.

Funnel plot of comparison: 1 Bupropion versus placebo/control, outcome: 1.14 Dropouts due to drug.
6.

Funnel plot of comparison: 2 Bupropion and NRT versus NRT alone, outcome: 2.1 Smoking cessation.
We considered participants lost to follow‐up as smokers, which is current best practice in this field (West 2005). The Cochrane Tobacco Addiction policy is to present effect estimates as risk ratios (RRs), as these are easier to interpret than odds ratios (ORs), but this means that where there are no events measured in both comparison groups RRs cannot be calculated, and therefore do not contribute to the meta‐analysis. We considered alternative statistical approaches to deal with this, but concluded that other approaches would be more difficult to interpret and that overall conclusions would not change as a result.
Agreements and disagreements with other studies or reviews
The findings of this review are in agreement with the conclusions of other reviews and guidelines in a variety of populations (Cahill 2013; Hughes 2005; McRobbie 2005; Mills 2006, Tsoi 2010). USA smoking cessation guidelines continue to recommend bupropion as a first‐line therapy (Fiore 2008), and recommend nortriptyline as a second‐line therapy due to possible AEs. Open uncontrolled trials and observational studies of bupropion have shown real‐life quit rates comparable to those found in the clinical trials included in this review (Paluck 2006; Wilkes 2005). In addition, our findings regarding the beneficial effect of bupropion for smoking cessation, specifically in smokers with mental illness, are consistent with a subset from a separate Cochrane Review evaluating smoking cessation treatments exclusively in populations with current or past depression (van der Meer 2013).
However, our findings on the effectiveness of bupropion as an adjunct to NRT differ from the results of the United States Public Health Service (USPHS) clinical practice guideline (Fiore 2008). Whereas we did not detect a significant difference in efficacy when bupropion and NRT were used together compared to NRT alone, the USA guideline reported an OR of 1.30 (95% confidence interval (CI) 1.0 to 1.80) favouring combination therapy (Fiore 2008, Table 6.28). The difference in meta‐analytic outcomes may be because our analysis included several studies of hard‐to‐treat populations not included in the USPHS analysis. Also, it could be because our analysis was a collation of 12 direct, within‐study randomized comparisons, whereas the USPHS carried out an indirect across‐study comparison of the results from the combination arms of three trials and the patch‐alone arms of 32 studies.
Cahill 2013 used both direct and indirect statistical comparisons to compare the efficacy of bupropion to NRT and varenicline, using network meta‐analysis. The effect estimates generated resulted in similar conclusions to the ones drawn here, i.e. bupropion and single‐form NRT resulted in similar quit rates and varenicline resulted in higher quit rates than bupropion. However, indirect comparisons made by Cahill 2013 also suggested that combination NRT was more effective than bupropion, whereas our subgroup analysis did not provide clear evidence of this.
Similar to our findings, other studies and systematic reviews looking at the SAE profile of bupropion remain inconclusive (Cahill 2013; Grandi 2011; Wightman 2010). Whilst our review did not find that bupropion significantly increased the incidence of seizures (RR 1.64, 95% CI 0.08 to 33.95), with substantial imprecision detected, the point estimate does indicate a rate of approximately 1.5 events per 1000 people taking bupropion, compared to a rate of 1 per 1000 cases presented elsewhere (Cahill 2013).
In contrast to the findings of the very large‐scale EAGLES trial, we have concluded that bupropion significantly increases psychiatric AEs (Anthenelli 2016). Whilst Anthenelli 2016 contributes over 95% of psychiatric AE data to our meta‐analysis, it concluded that bupropion does not significantly increase the incidence of psychiatric AEs. This discrepancy may be the result of including psychiatric AEs of any severity in our relevant meta‐analysis, whereas Anthenelli 2016 used a composite measure of only moderate and severe intensity psychiatric events for their primary analysis. It is not possible for us to corroborate whether we would find the same if we were only to include moderate and severe psychiatric events, as study reporting does not allow us to discriminate between these events according to severity.
Taking into account the combined evidence from this review and Cahill 2013, suggesting that varenicline is more efficacious than bupropion, and evidence from Cahill 2016, suggesting that psychiatric AEs are not increased by varenicline; varenicline may be a more suitable option for people who wish to take a prescription medication to quit smoking, especially those with mental health disorders.
Authors' conclusions
Implications for practice.
Bupropion and nortriptyline are effective pharmacological aids for smoking cessation. There is no good evidence that one is superior to the other. Bupropion increases the rate of long‐term quitting by approximately 52% to 77%, and this effect appears to be stable regardless of the amount of behavioural support provided, and whether participants have current or a history of mental health disorders.
Bupropion may cause an increase in adverse events (AEs), and specifically psychiatric AEs, leading to discontinuation of drug use in some users (approximately 9%). However, estimates of serious adverse events (SAEs, i.e. events that result in hospitalization, disability or death) include the possibility of no difference as well as a potential 1% increase when compared to placebo.
There is no evidence of higher quit rates when combining bupropion with either nicotine replacement therapy (NRT) or varenicline relative to each drug taken alone.
Bupropion appears to be as effective as NRT for smoking cessation; however varenicline may result in somewhere between 27% to 56% higher quit rates than bupropion.
There is a paucity of data investigating the efficacy and safety of antidepressants other than bupropion for smoking cessation, but there is sufficient data to show that, in the light of the effectiveness of other medications, selective serotonin reuptake inhibitors (SSRIs) offer no worthwhile increase in smoking cessation rates.
The evidence is insufficient to draw conclusions about whether existing depression modifies the efficacy of antidepressants for smoking cessation.
Implications for research.
There is high‐certainty evidence that bupropion increases quit rates at six months or longer in adults motivated to quit. We consider that further research is highly unlikely to change our confidence in the efficacy of bupropion in this population. However, further studies could increase our confidence in the likelihood of SAEs and any future studies comparing bupropion to placebo should ensure these are recorded and reported in detail.
More studies assessing the efficacy and safety of different doses of bupropion, as well as doses higher than 300 mg would clarify the most effective bupropion dosing strategy.
More high‐certainty studies are needed to assess the efficacy of bupropion when combined with varenicline treatment or NRT treatment.
More high‐certainty studies are needed to assess whether bupropion is particularly efficacious for supporting smoking cessation in people with depression.
New studies of any antidepressant used as a treatment for smoking cessation should ensure that they measure and report on the number of participants experiencing SAEs and AEs, as well as reporting on the number of dropouts due to treatment. These numbers should be reported separately by study arm, as well as overall. Specifically, studies of bupropion should report on numbers of psychiatric AEs and provide more detail on the severity of these events.
What's new
| Date | Event | Description |
|---|---|---|
| 4 May 2021 | Amended | Minor corrections to figures in Summary of Findings tables (no changes to interpretation) |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 3, 1997
| Date | Event | Description |
|---|---|---|
| 24 January 2020 | New search has been performed | 33 new included studies identified and study data added to existing comparators |
| 24 January 2020 | New citation required but conclusions have not changed | 33 new included studies; additional safety analyses added. Main conclusions remain unchanged |
| 14 June 2016 | Amended | Corrected typographical error in Abstract results. Risk ratio for buproprion + NRT (12 trials) changed from 1.9 to 1.19. Now matches meta‐analysis 1.5 |
| 8 October 2013 | New search has been performed | Updated with 24 new included studies. Studies of S‐Adenosyl‐L‐Methionine and St John's wort included for the first time. Meta‐analyses of serious adverse events added |
| 8 October 2013 | New citation required but conclusions have not changed | Conclusions largely unchanged. Efficacy findings unchanged |
| 22 June 2011 | Amended | Additional table converted to appendix to correct pdf format |
| 5 October 2009 | Amended | Correction to excluded studies table, detail added to Carrão 2007 |
| 30 July 2009 | New search has been performed | Updated with 13 new included trials including 3 of selegiline, not previously covered. No substantial change to effects; main conclusions not altered |
| 17 June 2008 | Amended | Converted to new review format |
| 11 October 2006 | New citation required but conclusions have not changed | Seventeen new trials were added to the review for Issue 1, 2007. There were no major changes to the reviewers' conclusions. |
| 16 July 2004 | New citation required but conclusions have not changed | New trials of bupropion, nortriptyline and fluoxetine were added for Issue 4, 2004, and additional information on adverse effects was included. There were no major changes to the reviewers' conclusions. |
| 8 January 2003 | New citation required but conclusions have not changed | New trials of bupropion and nortriptyline were added to the review in Issue 2, 2003. There were no major changes to the reviewers' conclusions. |
| 19 September 2001 | New citation required but conclusions have not changed | Four new studies on bupropion, and one each on nortriptyline and paroxetine were added to the review in Issue 1, 2002. In press data from a trial of fluoxetine are included which differ from unpublished data previously used. The reviewers' conclusions about the efficacy of bupropion and nortriptyline were not changed substantively. |
| 28 August 2000 | New citation required and conclusions have changed | Updates the earlier Cochrane Review 'Anxiolytics and antidepressants for smoking cessation'. Anxiolytics are evaluated in a separate review. |
Notes
This review was first published as part of the review 'Anxiolytics and antidepressants for smoking cessation.' From Issue 4, 2000 the classes of drugs are reviewed separately.
Acknowledgements
Our thanks to all of the previous authors of this review (John Hughes, Lindsay Stead, Tim Lancaster, Kate Cahill). We also thank Drs Niaura, Borrelli, Spring, Fiore, Hurt, Mizes, Ferry, Schuh, Cinciripini, Hays, Prochazka, Ahluwalia, Mayo, Collins, Novotny, Brown, David, Evins, Le Foll, Glover, McClernon, Le Foll and Piper for assistance with additional information or data on studies.
We thank Ryan J Courtney (University of New South Wales, National Drug and Alcohol Research Centre) and Dr Emily Peckham (University of York) for their peer review comments, Sandra Wilcox for consumer review comments and Professor Jamie Brown for editorial review, on this update.
This review was authored by employees of Cochrane Tobacco Addiction, which receives infrastructure funding from the National Institute for Health Research (NIHR). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health. This particular publication arises from research funded by Research England's Strategic Priorities Fund (SPF) QR allocation. JHB was funded in part by the NIHR Oxford Biomedical Research Centre (BRC).
Appendices
Appendix 1. Specialized Register search strategy
Searched using CRS web
#1 (bupropion or zyban):TI,AB,MH,EMT,KY,XKY
#2 nortriptyline:TI,AB,MH,EMT,KY,XKY
#3 (monoamine oxidase inhib*):TI,AB,MH,EMT,KY,XKY
#4 (moclobemide or selegiline or lazabemide):TI,AB,MH,EMT,KY,XKY
#5 (SSRI* or (selective serotonin re?uptake inhibitor*)):TI,AB,MH,EMT,KY,XKY
#6 (fluoxetine or sertraline or paroxetine or zimelidine):TI,AB,MH,EMT,KY,XKY
#7 (doxepin or imipramine or tryptophan or venlafaxine):TI,AB,MH,EMT,KY,XKY
#8 ((john?s wort) or hypericum):TI,AB,MH,EMT,KY,XKY
#9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
(MH, EMT, KY and XKY are keyword fields)
Data and analyses
Comparison 1. Bupropion versus placebo/no pharmacotherapy control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Smoking cessation | 46 | 17866 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.52, 1.77] |
| 1.2 Smoking cessation ‐ subgroup by level of behavioural support | 46 | 17866 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.52, 1.77] |
| 1.2.1 Multisession group behavioural support | 10 | 2001 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.44, 2.16] |
| 1.2.2 Multisession individual counselling | 31 | 15033 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.50, 1.77] |
| 1.2.3 Low‐intensity support | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [0.32, 25.68] |
| 1.2.4 Not specified | 4 | 785 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.00, 2.00] |
| 1.3 Smoking cessation ‐ subgroup by mental health disorders | 46 | 17866 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.52, 1.77] |
| 1.3.1 Psychiatric conditions | 5 | 2180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.30, 2.15] |
| 1.3.2 Non‐psychiatric | 42 | 15686 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.51, 1.77] |
| 1.4 Adverse events | 19 | 10893 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.11, 1.18] |
| 1.5 Serious adverse events | 21 | 10625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.90, 1.48] |
| 1.6 Psychiatric adverse events | 6 | 4439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.15, 1.37] |
| 1.7 Seizures | 13 | 7344 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.64, 13.37] |
| 1.8 Overdoses | 5 | 5585 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.23, 19.86] |
| 1.9 Suicide attempts | 10 | 6484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.29, 8.92] |
| 1.10 Death by suicide | 14 | 8822 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.26] |
| 1.11 All‐cause mortality | 21 | 11403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.42, 1.87] |
| 1.12 Anxiety | 11 | 7406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.21, 1.67] |
| 1.13 Insomnia | 22 | 11077 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.62, 1.96] |
| 1.14 Dropouts due to drug | 25 | 12340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.21, 1.56] |
Comparison 2. Bupropion plus nicotine replacement therapy (NRT) versus NRT alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Smoking cessation | 12 | 3487 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.94, 1.51] |
| 2.1.1 Patch alone | 9 | 1774 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.84, 1.84] |
| 2.1.2 Lozenge alone | 2 | 1051 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.81, 1.81] |
| 2.1.3 Choice of NRT | 1 | 662 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.73, 1.28] |
| 2.2 Adverse events | 2 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.02, 1.43] |
| 2.3 Serious adverse events | 3 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.26, 8.89] |
| 2.4 Seizures | 1 | 527 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 72.31] |
| 2.5 Suicide attempts | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.6 Death by suicide | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.7 All‐cause mortality | 2 | 731 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.12, 3.98] |
| 2.8 Insomnia | 2 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.24, 1.93] |
| 2.9 Anxiety | 3 | 1218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.97, 2.56] |
| 2.10 Dropouts due to drug | 2 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.92] |
2.4. Analysis.

Comparison 2: Bupropion plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 4: Seizures
2.5. Analysis.

Comparison 2: Bupropion plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 5: Suicide attempts
2.6. Analysis.

Comparison 2: Bupropion plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 6: Death by suicide
2.7. Analysis.

Comparison 2: Bupropion plus nicotine replacement therapy (NRT) versus NRT alone, Outcome 7: All‐cause mortality
Comparison 3. Bupropion plus varenicline versus varenicline alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Smoking cessation | 3 | 1057 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.95, 1.55] |
| 3.2 Adverse events | 4 | 1043 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.02, 1.17] |
| 3.3 Serious adverse events | 5 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.63, 2.42] |
| 3.4 Psychiatric adverse events | 2 | 835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.03, 1.30] |
| 3.5 Seizures | 1 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.6 Overdoses | 2 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.27] |
| 3.7 Suicide attempts | 3 | 1056 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.27] |
| 3.8 Death by suicide | 2 | 727 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.9 All‐cause mortality | 2 | 727 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.40] |
| 3.10 Anxiety | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.01, 2.38] |
| 3.11 Insomnia | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.14, 1.84] |
| 3.12 Dropouts due to drug | 4 | 1230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.45, 1.45] |
Comparison 4. Exploratory safety analysis: effects of bupropion only across comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Adverse events | 25 | 12249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.11, 1.17] |
| 4.1.1 Bupropion versus control | 19 | 10893 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.11, 1.18] |
| 4.1.2 Bupropion + NRT versus NRT | 2 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.02, 1.43] |
| 4.1.3 Bupropion + varenicline versus varenicline | 4 | 1043 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.02, 1.17] |
| 4.2 Psychiatric adverse events | 8 | 5274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.15, 1.33] |
| 4.2.1 Bupropion versus control | 6 | 4439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.15, 1.37] |
| 4.2.2 Bupropion + varenicline versus varenicline | 2 | 835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.03, 1.30] |
| 4.3 Serious adverse events | 28 | 12500 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.93, 1.47] |
| 4.3.1 Bupropion versus control | 21 | 10625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.90, 1.48] |
| 4.3.2 Bupropion + NRT versus NRT | 3 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.26, 8.89] |
| 4.3.3 Bupropion + varenicline versus varenicline | 5 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.63, 2.42] |
| 4.4 Dropouts due to drug | 30 | 14108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.20, 1.52] |
| 4.4.1 Bupropion versus control | 25 | 12340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.21, 1.56] |
| 4.4.2 Bupropion + NRT versus NRT | 2 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.92] |
| 4.4.3 Bupropion + varenicline versus varenicline | 4 | 1230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.45, 1.45] |
Comparison 5. Bupropion versus varenicline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Smoking cessation | 6 | 6286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.64, 0.79] |
| 5.2 Adverse events | 5 | 5780 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.95, 1.00] |
| 5.3 Serious adverse events | 4 | 4742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.94, 2.04] |
| 5.4 Psychiatric adverse events | 2 | 4051 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.99, 1.16] |
| 5.5 Seizures | 4 | 5389 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.16 [0.92, 55.42] |
| 5.6 Overdoses | 2 | 4210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.14, 6.25] |
| 5.7 Suicide attempts | 3 | 4239 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [0.31, 28.96] |
| 5.8 Death by suicide | 5 | 5600 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.9 All‐cause mortality | 5 | 6074 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [0.31, 28.96] |
| 5.10 Insomnia | 3 | 5208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.22, 1.60] |
| 5.11 Anxiety | 2 | 4705 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.07, 1.53] |
| 5.12 Dropouts due to drug | 6 | 6103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.96, 1.31] |
5.5. Analysis.

Comparison 5: Bupropion versus varenicline, Outcome 5: Seizures
5.6. Analysis.

Comparison 5: Bupropion versus varenicline, Outcome 6: Overdoses
5.7. Analysis.

Comparison 5: Bupropion versus varenicline, Outcome 7: Suicide attempts
5.8. Analysis.

Comparison 5: Bupropion versus varenicline, Outcome 8: Death by suicide
5.9. Analysis.

Comparison 5: Bupropion versus varenicline, Outcome 9: All‐cause mortality
Comparison 6. Bupropion versus nicotine replacement therapy (NRT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Smoking cessation | 10 | 8230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.09] |
| 6.1.1 Patch | 8 | 5778 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.16] |
| 6.1.2 Lozenge | 2 | 694 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.67, 1.22] |
| 6.1.3 Patch + lozenge | 2 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.55, 0.98] |
| 6.1.4 Choice of NRT | 2 | 1038 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.87, 1.33] |
| 6.2 Adverse events | 2 | 4097 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.98, 1.06] |
| 6.3 Serious adverse events | 5 | 5624 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.83, 1.80] |
| 6.4 Psychiatric adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.5 Seizures | 1 | 4028 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.24] |
| 6.6 Overdoses | 1 | 4028 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.12, 74.19] |
| 6.7 Suicide attempts | 2 | 4514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.22, 12.75] |
| 6.8 Death by suicide | 2 | 4514 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 6.9 All‐cause mortality | 3 | 5313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.38, 4.84] |
| 6.10 Insomnia | 2 | 4128 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.10, 1.55] |
| 6.11 Anxiety | 2 | 4855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.06, 1.62] |
| 6.12 Dropouts due to drug | 4 | 4825 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.95, 1.38] |
6.5. Analysis.

Comparison 6: Bupropion versus nicotine replacement therapy (NRT), Outcome 5: Seizures
6.6. Analysis.

Comparison 6: Bupropion versus nicotine replacement therapy (NRT), Outcome 6: Overdoses
6.7. Analysis.

Comparison 6: Bupropion versus nicotine replacement therapy (NRT), Outcome 7: Suicide attempts
6.8. Analysis.

Comparison 6: Bupropion versus nicotine replacement therapy (NRT), Outcome 8: Death by suicide
6.9. Analysis.

Comparison 6: Bupropion versus nicotine replacement therapy (NRT), Outcome 9: All‐cause mortality
Comparison 7. Bupropion versus nortriptyline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Smoking cessation | 3 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.93, 1.82] |
| 7.2 Serious adverse events | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 7.3 Insomnia | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.4 Dropouts due to drug | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.47, 1.44] |
Comparison 8. Bupropion versus gabapentin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Serious adverse events | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 8.2 Dropouts due to drug | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.50, 10.06] |
Comparison 9. Bupropion (different doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Smoking cessation | 3 | 2042 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.93, 1.26] |
| 9.2 Serious adverse events | 2 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.30, 5.94] |
| 9.3 Overdoses | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.17] |
| 9.4 Suicide attempts | 2 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.17] |
| 9.5 Death by suicide | 2 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 9.6 All‐cause mortality | 2 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [0.12, 71.68] |
| 9.7 Insomnia | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.85, 1.63] |
| 9.8 Anxiety | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.35, 2.20] |
| 9.9 Dropouts due to drug | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.75, 4.44] |
Comparison 10. Nortriptyline versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Smoking cessation | 6 | 975 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.48, 2.78] |
| 10.2 Serious adverse events | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 10.3 Insomnia | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.28, 1.21] |
| 10.4 Anxiety | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.34, 1.20] |
| 10.5 Dropouts due to drug | 4 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.18, 3.36] |
Comparison 11. Selective serotonin reuptake inhibitors (SSRIs) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Smoking cessation | 4 | 1594 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.71, 1.22] |
| 11.1.1 Fluoxetine | 2 | 1236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.30] |
| 11.1.2 Paroxetine | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.64, 1.82] |
| 11.1.3 Sertraline | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.30, 1.64] |
| 11.2 Adverse events | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [0.11, 67.40] |
| 11.2.1 Fluoxetine | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [0.11, 67.40] |
| 11.3 Dropouts due to drug | 3 | 1270 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [1.70, 3.94] |
| 11.3.1 Fluoxetine | 2 | 1136 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [1.75, 4.23] |
| 11.3.2 Sertraline | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.30, 5.56] |
Comparison 12. Monoamine oxidase inhibitor (MAOI) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Smoking cessation | 6 | 827 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.93, 1.79] |
| 12.1.1 Moclobemide | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.67, 3.68] |
| 12.1.2 Selegiline | 5 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.88, 1.78] |
| 12.2 Adverse events | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.12] |
| 12.2.1 Selegeline | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.16] |
| 12.2.2 EVT302 | 1 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.15] |
| 12.3 Psychiatric adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.3.1 Selegeline | 1 | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.02, 3.74] |
| 12.4 Serious adverse events | 4 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.37, 3.68] |
| 12.4.1 Moclobemide | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 12.4.2 Selegeline | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 12.4.3 Lazabemide | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.12, 2.32] |
| 12.4.4 EVT302 | 1 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.00 [0.36, 134.32] |
| 12.5 Insomnia | 5 | 752 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.15, 1.97] |
| 12.5.1 Moclobemide | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.21 [1.64, 16.61] |
| 12.5.2 Selegeline | 3 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.91, 1.60] |
| 12.5.3 Lazabemide | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [0.78, 9.00] |
| 12.6 Anxiety | 2 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.48, 2.22] |
| 12.6.1 Selegeline | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.42, 2.27] |
| 12.6.2 Lazabemide | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.19, 8.32] |
| 12.7 Dropouts due to drug | 5 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.07, 2.86] |
| 12.7.1 Moclobemide | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.38, 10.12] |
| 12.7.2 Selegeline | 2 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.94, 3.85] |
| 12.7.3 Lazabemide | 1 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.69, 3.62] |
| 12.7.4 EVT302 | 1 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.25, 8.84] |
Comparison 13. Venlafaxine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Smoking cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.2 Dropouts due to drug | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.33, 28.95] |
Comparison 14. Hypericum (St John's wort) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Smoking cessation | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.26, 2.53] |
| 14.2 Serious adverse events | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.36, 15.57] |
| 14.3 All‐cause mortality | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 73.24] |
| 14.4 Dropouts due to drug | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.36, 3.96] |
Comparison 15. S‐Adenosyl‐L‐Methionine (SAMe) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Smoking cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.2 Adverse events | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.69, 3.65] |
| 15.3 Insomnia | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.07, 36.11] |
| 15.4 Dropouts due to drug | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.24, 17.76] |
Comparison 16. Nortriptyline plus nicotine replacement therapy (NRT) versus NRT alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Smoking cessation | 4 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.94, 1.55] |
| 16.2 Insomnia | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.30, 3.32] |
| 16.3 Dropouts due to drug | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.00 [1.31, 76.28] |
Comparison 17. Selective serotonin reuptake inhibitor (SSRI) plus NRT versus NRT alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Smoking cessation | 3 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.48, 1.03] |
| 17.1.1 Fluoxetine | 3 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.48, 1.03] |
Comparison 18. Selegeline plus nicotine replacement therapy (NRT) versus NRT alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Serious adverse events | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 18.2 Dropouts due to drug | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.42, 4.75] |
Comparison 19. EVT302 plus nicotine replacement therapy (NRT) versus NRT alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Adverse events | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.25] |
| 19.2 Serious adverse events | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.12, 72.23] |
| 19.3 Dropouts due to drug | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.12, 72.23] |
Comparison 20. Fluoxetine (30 mg versus 60 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 20.1 Smoking cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.2 Dropouts due to drug | 1 | 656 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.87] |
Comparison 21. Lazabemide (100 mg versus 200 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 21.1 Serious adverse events | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 21.2 Insomnia | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.43, 3.01] |
| 21.3 Anxiety | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.38] |
| 21.4 Dropouts due to drug | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.25 [1.48, 12.22] |
Comparison 22. Hypericum (St John's wort) (300 mg versus 600 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 22.1 Smoking cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.2 Adverse events | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.63, 2.67] |
22.1. Analysis.

Comparison 22: Hypericum (St John's wort) (300 mg versus 600 mg), Outcome 1: Smoking cessation
Comparison 23. S‐Adenosyl‐L‐Methionine (SAMe) (800 mg versus 1600 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 23.1 Adverse events | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.26, 1.33] |
| 23.2 Dropouts due to drug | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.19, 21.18] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahluwalia 2002.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: community‐based healthcare centre Recruitment method: community volunteers |
|
| Participants | 600 African American smokers randomized; 70% female, average age 44; average cigarettes per day 17; 27% had possible clinical depression CES‐D > 16 | |
| Interventions |
Common components: 8 sessions of in‐person or telephone counselling and self‐help guide |
|
| Outcomes |
|
|
| Funding Source | National Cancer Institute. GlaxoSmithKline provided study medication. | |
| Author conflicts of interest | Dr Ahluwalia has served as a consultant for GlaxoSmith2Kline and Pharmacia Consumer. GlaxonSmithKline provided study medication but played no role in the design, conduct of the study, or interpretation and analysis of the data. | |
| Notes | Continuous abstinence rates shown in Figure 3 of paper. Figures obtained from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization codes were generated in blocks of 50 and sent to the pharmaceutical company..." |
| Allocation concealment (selection bias) | Low risk | Quote: " ... [the pharmaceutical company]... packaged the treatment and then shipped the blinded drug to the investigator." Shows blinded drugs were provided to investigator |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Blinding was successful. At the end of treatment, 58% (150/259) of participants correctly guessed that they received bupropion SR [sustained release], and 41% (104/253) correctly guessed they received placebo." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Approximately 32% lost to follow‐up in each group; included as smokers |
Anthenelli 2016.
| Study characteristics | ||
| Methods | Study design: RCT Countries: USA, Australia, Canada, Denmark, Finland, Germany, New Zealand, South Africa, Spain, Bulgaria, Russian Federation, Slovakia, Argentina, Brazil, Chile, and Mexico Setting: clinical trial centres, academic centres, and outpatient clinics treating patients with and without psychiatric disorders Recruitment method: from the investigators' own clinics; through newspaper, radio, and television advertising; fliers and posters |
|
| Participants | 8144 participants; 56% female; average age 46.5; average cigarettes per day 21, mean FTND 5.8 Specialist population: participants were made up of two cohorts (a psychiatric cohort (N = 4074) and a non‐psychiatric cohort (N = 3984)). Participants were included in the psychiatric cohort if they met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR) diagnostic criteria for mood disorders including major depressive disorder or bipolar disorder; anxiety disorders including panic disorder, with or without agoraphobia, post‐traumatic stress disorder, obsessive‐compulsive disorder, social phobia, and generalized anxiety disorder; psychotic disorders including schizophrenia and schizoaffective disorders; or borderline personality disorder. Participants in the non‐psychiatric cohort had no confirmed history of DSM‐IV‐TR Axis I or II disorders. |
|
| Interventions |
Common components: smoking cessation counselling consisting of 10 minute sessions at each of the 15 clinic visits, totalling 2 hours and 30 minutes |
|
| Outcomes |
|
|
| Funding Source | Pfizer and GlaxoSmithKline | |
| Author conflicts of interest | RMA reports receiving grants from Pfizer and Alkermes, and providing consulting and advisory board services to Pfizer, Arena Pharmaceuticals, and Cerecor. RMA's writing of this manuscript was supported, in part, by National Institute on Alcohol Abuse and Alcoholism grant numbers U01 AA013641 and R01 AA019720; National Institute on Drug Abuse/Veterans Affairs Co‐operative Studies numbers 1031 and 1032; and Veterans Affairs Merit Award number NEUA‐003‐08S. NLB reports providing consulting and advisory board services to Pfizer and GlaxoSmithKline, and having been a paid expert witness in litigation against tobacco companies. RW reports receiving grants from Pfizer, Johnson & Johnson, and GlaxoSmithKline, and receiving personal fees for advisory board services from Pfizer and GlaxoSmithKline. RW's salary is funded by Cancer Research UK. AEE reports receiving grants from Pfizer and Forum Pharmaceuticals, and receiving personal fees for advisory board services from Pfizer and Reckitt Benckiser. AEE's writing of the manuscript was supported by a National Institute on Drug Abuse Career Award in Patient‐Oriented Research, number K24 DA030443. LSA, TM, DL, and CR are employees and stockholders of Pfizer. JA is an employee of GlaxoSmithKline and stockholder of that company. AK is a PAREXEL employee working on behalf of GlaxoSmithKline. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation administrator, independent from the clinical study team, prepared the computer‐generated randomisation schedule used to assign participants to treatment using a block size of 8 (1:1:1:1 ratio) for each of the 20 diagnosis by region combinations." |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators obtained participant identification numbers via a web‐based or telephone call‐in drug management system. Study product kit codes did not allow deciphering of randomised treatment or block size. As such, participants, investigators, and research personnel were masked to treatment assignments." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “The triple dummy design feature required participants to take study medications as masked tablets dispensed in separate varenicline and bupropion pill bottles each with matching placebo along with either applying active or placebo patches on a daily basis.” Quote: “Investigators obtained participant identification numbers via a web‐based or telephone call‐in drug management system. Study product kit codes did not allow deciphering of randomised treatment or block size. As such, participants, investigators, and research personnel were masked to treatment assignments.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 439/2037 (21.6%) of the varenicline group, 448/2034 (22.0%) of the bupropion group, 481/2038 (23.6%) of the patch group and 483/2035 (23.7%) of the placebo group were lost to follow‐up. Therefore, loss to follow‐up was less than 50% and similar across study arms. |
Aubin 2004.
| Study characteristics | ||
| Methods | Study design: RCT
Country: France Setting: 74 cessation outpatient clinics Recruitment: volunteers |
|
| Participants | 504 participants randomized: 56% female, average age 41, average cigarettes per day: not stated | |
| Interventions |
Common components: motivational support at clinic visits at baseline, w3, w7, w12 and 3 phone calls TQD, 2 to 3 days later, w5, w18 |
|
| Outcomes | Abstinence at w26 (continuous from w4) Validation: CO < 10 ppm | |
| Funding Source | GlaxoSmithKline | |
| Author conflicts of interest | The lead author (H J Aubin) is a paid consultant of GSK | |
| Notes | First included as Lebargy 2003 based on abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The computerized randomization schedule, prepared by the sponsor, was inaccessible to the investigator who was provided with a specific set of sequential treatment numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "The computerized randomization schedule, prepared by the sponsor, was inaccessible to the investigator who was provided with a specific set of sequential treatment numbers." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double‐blind" "Blinding was assured by matching the placebo to the bupropion tablets..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26% of the placebo and 27% of the bupropion groups lost; included as smokers |
Aveyard 2008.
| Study characteristics | ||
| Methods | Study design: RCT Country: UK Setting: National Health Service stop smoking clinics Recruitment: people attending clinics |
|
| Participants | 901 smokers, ≥ 10/day; 46% female, average age 43, average cigarettes per day 21 | |
| Interventions |
All participants received free NRT and had behavioural support, the majority attending group sessions run by cessation specialists |
|
| Outcomes |
|
|
| Funding Source | Cancer Research UK and National Institute for Health Research. Medication provided by King Pharmaceuticals | |
| Author conflicts of interest | PA has done consultancy work for the pharmaceutical and biotechnology industry that has led to payments to him and his institution. This includes work for companies providing smoking cessation treatment, including NRT. MM has received consultancy income from the European Network for Smoking Prevention and has provided scientific consultancy services through the University of Oxford ISIS Innovation to the National Audit Office and G‐Nostics. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician generated the randomisation schedule in Stata. We used block randomisation, with randomly ordered block sizes of two, four, and six, stratified by stop smoking adviser." |
| Allocation concealment (selection bias) | Low risk | Study nurses recruited participants, and the study administrator (who had not met the participants) allocated participants in sequence against the list for each adviser. Only the administrator and the pharmacist knew the allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Advisers, participants, and study staff...were blind to allocation... tablets were encapsulated, and identical powder filled capsules provided the placebos." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% intervention, 17% control lost at 12 months, included as smokers. Authors note that majority of losses were already smoking. |
Barnes 2006.
| Study characteristics | ||
| Methods | Study design: RCT Country: UK Setting: private consulting room in a community pharmacy Recruitment method: advertisements were placed in newspapers regional to the pharmacy; information leaflets were placed in the pharmacy, along with a window display on smoking cessation which mentioned the study; local radio interviews were given | |
| Participants | 28 participants randomized; 17% female; average age 42.8; average cigarettes per day 15.5; FTND: 26 ppts < 8 and 2 ppts ≥ 8 | |
| Interventions |
Common components: one hour of general smoking cessation advice and motivational support |
|
| Outcomes |
|
|
| Funding Source | Lichtwer Pharma (UK) Ltd | |
| Author conflicts of interest | Lead author received funding by fellowship from Lichtwer Pharma UK Ltd | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomisation list of random treatment assignments ('A' or 'B', corresponding to lower and higher dosages of SJW, respectively) in blocks of 4 without stratification was prepared in advance." |
| Allocation concealment (selection bias) | High risk | Quote: “Participants enrolled into the study were assigned to the next consecutive treatment.” As the pharmacist was unblinded, they would therefore have been aware of the allocation of the participants. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “This was a prospective, open, uncontrolled, pharmacy‐based, pilot study.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/15 in once daily arm + 10/13 in twice daily arm were lost to follow‐up. Therefore, loss to follow‐up is greater than 50% in each trial arm. |
Benli 2017.
| Study characteristics | ||
| Methods | Study design: RCT Country: Turkey Setting: a smoking cessation clinic Recruitment method: participants applied to the smoking cessation clinic directly by calling the Turkish Ministry of Health's 'stop smoking' helpline and making an appointment. |
|
| Participants | An unspecified number of participants were randomised. 405 participants were analysed. 17.5% female; average age 35.2; average age 35.2; average cigarettes per day 23; mean FTND 6.3 | |
| Interventions |
Common components: behavioural therapy support with a biopsychosocial approach |
|
| Outcomes |
|
|
| Funding Source | No funding | |
| Author conflicts of interest | The authors declare that they have no competing interests | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients who were to receive the medication were randomly determined by the medication support center in order to provide a constant distribution rate of varenicline and bupropion and so that physicians would not be aware of the medication distribution.” Comment: no further detail is provided. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients who were to receive the medication were randomly determined by the medication support center in order to provide a constant distribution rate of varenicline and bupropion and so that physicians would not be aware of the medication distribution.” Comment: no further detail is provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: “Patients who were to receive the medication were randomly determined by the medication support center in order to provide a constant distribution rate of varenicline and bupropion and so that physicians would not be aware of the medication distribution” Comment: some attempt appears to have been made to blind physicians to group assignment, however no further detail is given, so it is unclear whether participants and outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only those followed up at 12 months are included in analysis |
Berlin 1995.
| Study characteristics | ||
| Methods | Study design: RCT Country: France Setting: clinic Recruitment: by adverts in general practices or from occupational medicine departments |
|
| Participants | 88 smokers randomized; no current major depression or anxiety disorders; 57% had history of MDD | |
| Interventions |
No behavioural intervention or counselling |
|
| Outcomes |
|
|
| Funding Source | Roche | |
| Author conflicts of interest | None specified | |
| Notes | There were no serious adverse reactions. Insomnia was more common in drug (36%) than placebo (7%) groups. There were 4 dropouts for adverse effects/relapse in drug and 2 in placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind, but blinding at allocation not explicit |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind" but further detail not provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Relapses and subjects lost from follow‐up were considered treatment failures." Number lost to follow‐up not reported |
Berlin 2002.
| Study characteristics | ||
| Methods | Study design: RCT Countries: France and Belgium Setting: General practices and anti‐smoking clinics |
|
| Participants | 330 participants randomized; 43.9% female; average age 39.9; average cigarettes per day 24.7; mean FTND 6.2 | |
| Interventions |
Common components: brief cognitive behavioral intervention at each visit, totalling 2 hours |
|
| Outcomes |
|
|
| Funding Source | F Hoffmann‐La Roche | |
| Author conflicts of interest | None detailed | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “subjects were assigned a treatment number according to the computer‐generated randomization table” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Eligible subjects were assigned a treatment number according to the computer‐generated randomization table.” Comment: no further information is provided, therefore who was blinded and how is unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | “This was a randomized, placebo‐controlled, double‐blind, parallel‐group, multicenter proof‐of‐concept study” Comment: no further information is provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 60% in placebo (68/114); 62% 100 mg/day lazabemide (67/108); and 54% 200 mg/day lazabemide (58/108) were lost to follow‐up. Therefore, loss to follow‐up is above 50% in all groups. |
Berlin 2012.
| Study characteristics | ||
| Methods | Study design: RCT Country: Germany Setting: investigation centres Recruitment method: media advertisements |
|
| Participants | 412 participants randomized; 37.4% female; average age 35; average cigarettes per day 19; mean FTND 5.4 | |
| Interventions |
Common components: educational booklet on smoking cessation and a 10‐minute counselling session at each visit, totalling 1 hour and 50 minutes |
|
| Outcomes |
|
|
| Funding Source | Evotec NeuroSciences GmbH | |
| Author conflicts of interest | Ivan Berlin has received consultancy payments and travel funding from Pfizer Ltd and Sanofi Aventis in the last 5 years. He received a consultancy payment from Evotec Ltd for preparing the current study's research protocol. Ian M Hunneyball, Doris Greiling, Stephen Jones and Hermann Fuder were employees of Evotec. Hans‐Detlev Stahl is an employee of ClinPharm International GmbH Prufzentrum Leipzig. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomisation was performed by an independent statistician. A block size of 20 was used with each block containing medication assignments in a 7:7:3:3 ratio for EVT302 5 mg/day or placebo and EVT302 5 mg/day or placebo on top of NP [nicotine pill]. No stratification was used. Medication numbers were generated for a total of 25 blocks. The randomisation list was uploaded into the [interactive voice recognition system (IVRS)] allowing the centralised use of randomisation.” No detail of how sequences were generated |
| Allocation concealment (selection bias) | Low risk | Quote: "A central randomisation with an interactive voice recognition system (IVRS) was used which indicated the treatment to deliver upon the investigators’ call." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study stated as being double‐blinded, although no further information is given beyond this. Nicotine pill is unblinded, however |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates are all below 50% ‐ EVT302: 16 (10%); placebo: 14 (11%); EVT302 + nicotine pill: 5 (8%); placebo + nicotine pill: 2 (3%) |
Biberman 2003.
| Study characteristics | ||
| Methods | Study design: RCT Country: Israel Setting: 3 community‐based clinics Recruitment: mailing to members of public health service provider |
|
| Participants | 109 smokers randomised; 38% females, average age 42, average cigarettes per day 27 to 30 | |
| Interventions |
Common components: behavioural support from trained family physician; weekly then fortnightly visits for 12 weeks |
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | No serious AEs, no significant differences in AEs, 2 selegiline discontinuations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Four hundred dice‐throwing generated a randomized sequence code; 199 containers prepacked with selegiline and 201 containers prepacked with placebo were numbered accordingly." Comment: judged adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "The code was sealed, kept secretly and was revealed for the first time when the last participant finished the 12 months of follow‐up. The first participant who joined the trial after the initial visit run‐in phase received the first bottle from the container set number 001, the second
participant from set number 002 and so on. The trial coordinator arranged participant’s allocation." Comment: judged adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double‐blind" (see above) "No discontinuation difference for selegiline or placebo was observed among the groups, which implies masking success." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19 lost to follow‐up, included as smokers in meta‐analysis |
Blondal 1999.
| Study characteristics | ||
| Methods | Study design: RCT Country: Iceland Setting: cessation clinic Recruitment: community volunteers |
|
| Participants | 100 smokers randomized; 62% female; average age 41; average cigarettes per day 28 | |
| Interventions |
Common components: 5 x 1 hr group behaviour therapy. Advised to use 6 to 12 inhalers/day for up to 6 months |
|
| Outcomes |
|
|
| Funding Source | Oddur Olafsson Fund, Pharmacia and Upjohn Consumer Health Care. Delta Pharmaceutical Company provided fluoxetine and placebo and fluoxetine analyses. Helsingborg, Sweden provided a grant, nicotine inhalers and nicotine analyses. | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization; part of the randomization procedure was performed by the manufacturer at another location where the code was also kept until it was broken in May 1997. |
| Allocation concealment (selection bias) | Low risk | Randomization codes applied to pill boxes which were then allocated sequentially. "This part of the randomization procedure was performed by the manufacturer at another location where the code was also kept." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double‐blind." "...pill boxes, with either fluoxetine or an identical appearing placebo containing the same ingredients except fluoxetine, were labelled with numbers ranging from 100 to 210." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low numbers lost to follow‐up but reported results exclude 5 withdrawals; 3 from fluoxetine group due to adverse effects ‐ nervousness and anxiety, 1 from fluoxetine due to pregnancy, 1 from placebo who had purchased fluoxetine |
Brown 2007.
| Study characteristics | ||
| Methods | Study design: 2x2 factorial RCT Country: USA Setting: 2 clinical sites (Butler Hospital, Miriam Hospital) Recruitment: community volunteers |
|
| Participants | 524 participants randomised; 48% female; average age 44; average cigarettes per day 25 | |
| Interventions |
2 x 2 factorial design. Alternative psychosocial treatments were standard cessation therapy or plus CBT for depression. Both had 12 x 90 min groups twice weekly/weekly/monthly for 12 weeks. TQD 5th session. Collapsed in this analysis |
|
| Outcomes |
|
|
| Funding Source | National Institutes of Health | |
| Author conflicts of interest | None specified | |
| Notes | First included as Brown 2006, part unpublished data. Some genotyping studies combine these participants with those reported in Collins 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned to one of two treatment sites, where they were to receive one of two manualized group treatments ... Participants were then randomly assigned to receive one of two medication conditions, bupropion or placebo, using the urn randomization technique." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Whereas we were able to balance the drug and placebo conditions on an individual basis, behavioral treatments were randomized by group and thus were more susceptible to fluctuations in recruitment and to the availability at both sites of pairings of a senior and a junior therapist trained in CBTD". Knowledge of behavioural assignment was probably not concealed but seems unlikely to have led to individual selection bias. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double‐blind." Psychological condition unlikely to be blinded but unlikely to affect comparisons included in this review. "All participants and study staff were blind to medication condition." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 81% provided complete outcome data at all follow‐ups, not related to treatment condition. All participants included in ITT analyses |
Brown 2014.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: clinic Recruitment: via newspaper, radio, and television advertisements |
|
| Participants | 216 smokers with elevated depressive symptoms (CES‐D score ≥ 6) randomized; 38.4% female, average age 45.9; average cigarettes per day 21; mean FTND 5.6 | |
| Interventions |
Common components: nicotine patch for 8 weeks starting on TQD (21 mg/day for 4 weeks, 14 mg/day for 2 weeks, 7 mg/day for last 2 weeks), 5 sessions of brief behavioural smoking cessation treatment (in person and phone over 4 weeks, 20 to 30 mins each), totalling 140 minutes |
|
| Outcomes |
|
|
| Funding Source | American Cancer Society | |
| Author conflicts of interest | LHP reports receiving grant/research support from Medtronic, Neuronetics, HRSA, and NeoSync; serving on an advisory panel for Abbott; and serving as a consultant for Wiley, Springer, Qatar National Research Fund, and Abbott | |
| Notes | New for 2013 Significantly higher abstinence in 16 week arm than in 10‐week arm, results presented separately in meta‐analysis with control divided. N abstinent not reported, extrapolated from percentages provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomly assigned to one of the three treatment conditions using urn randomization” |
| Allocation concealment (selection bias) | High risk | Quote: "Open‐label" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over 90% followed up at 12 months. Similar rates across arms |
Cinciripini 2005.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: clinic Recruitment: community volunteers |
|
| Participants | 135 smokers randomized; 50% female, average age 46, average cigarettes per day 27 | |
| Interventions |
Common components: 6 weeks, 22 mg nicotine patch, and 9 x 15‐min behavioural counselling |
|
| Outcomes |
|
|
| Funding Source | National Institutes for Health and National Institute for Drug Abuse. Medication provided free of charge by Wyeth Ayerst Laboratories. | |
| Author conflicts of interest | None specified | |
| Notes | First included as Cinciripini 1999 based on abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. Stratification by depression history |
| Allocation concealment (selection bias) | Low risk | Randomization by pharmacy, all study personnel with direct patient contact blind |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double‐blind... Blinding of the study staff to the medication was maintained using prenumbered pill containers, assigned to each participant at randomization by the pharmacy. All study personnel with direct patient contact were blind to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Majority of participants followed up (65 intervention; 63 control), participants lost to follow‐up counted as smokers |
Cinciripini 2013.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: clinic Recruitment: community volunteers |
|
| Participants | 294 participants randomized; 39% female; average age 44; average cigarettes per day 20; mean FTND 4.5 | |
| Interventions |
Common components: 10 individual counselling sessions (6 in person, 4 via phone, 240 mins total) |
|
| Outcomes |
|
|
| Funding Source | National Institute on Drug Abuse, National Cancer Institute | |
| Author conflicts of interest | Dr Cinciripini served on the scientific advisory board of Pfizer, conducted educational talks sponsored by Pfizer on smoking cessation (2006‐2008), and has received grant support from Pfizer. | |
| Notes | New for 2013. In less than 1% of the total cases, participants who did not attend a follow‐up were coded as abstinent because they were abstinent at the following data point. All other losses to follow‐up counted as smokers. Author provided further detail on AE measurements via email. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Adaptive randomization,” no further detail provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: “Blinded” but no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 73% followed up at 6 months, similar rates across arms, all lost to follow‐up known to be smokers |
Cinciripini 2018.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: hospital‐based out‐patient clinic specializing in cancer prevention Recruitment method: paid and unpaid media advertising |
|
| Participants | 385 participants randomised; 41.5% female; average age 49.0; average cigarettes per day 19.7; mean FTND 2.1 | |
| Interventions |
Common components: in‐person and phone counselling, totalling 215 minutes |
|
| Outcomes |
|
|
| Funding Source | The project was supported by the United States National Institutes of Health (NIH) grant R01DA024709 (Principle Investigator PMC) and by The University of Texas MD Anderson’s Cancer Center Support Grant CA016672, funded by the National Cancer Institute (NCI). Pfizer (New York, NY) provided the active and matching placebo varenicline capsules | |
| Author conflicts of interest | PMC served on the scientific advisory board of Pfizer Pharmaceuticals, conducted educational talks sponsored by Pfizer on smoking cessation (2006–08) and has received grant support and medication support from Pfizer. MKH participated in two multisite Pfizer‐funded trials and received varenicline from Pfizer to conduct four NIH‐funded trials. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “an algorithm developed and managed by study data managers, whose role was limited to data quality and integrity management”. |
| Allocation concealment (selection bias) | Unclear risk | No details as to how randomly‐generated sequence was transferred and implemented to staff delivering medication to participants. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “Participants, medical and research staff who interacted with participants and the study investigators were blinded to group assignment.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates are as follows: 20/56 placebo; 48/166 varenicline; 38/163 combination. Dropout rates are below 50% in each arm |
Collins 2004.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: 2 clinical research sites Recruitment: community volunteers |
|
| Participants | 555 participants randomized; excluding history of psychiatric disorder including MDD; 57% female, average age 46, average cigarettes per day 21 | |
| Interventions |
Common components: 7 sessions group behavioural counselling |
|
| Outcomes |
|
|
| Funding Source | National Cancer Institute, National Institute on Drug Abuse, National Center for Research Resources. Treatment provided free of charge by GlaxoSmithKline. | |
| Author conflicts of interest | None specified | |
| Notes | Replaces Lerman 2002 which reported subset of data. Denominators supplied by 1st author, excludes 114 who withdrew before intervention. Some study details from Lerman 2006. Some genotyping studies combine these participants with those reported in Brown 2007. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was determined by a computer‐generated randomization scheme operated by a senior data manager; stratification was carried out by study site" (Lerman 2006) |
| Allocation concealment (selection bias) | Low risk | Centrally generated and allocation concealed from counsellors and assessors |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Placebo used but blinding procedure not described in detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% lost to follow‐up at 6‐month follow‐up; included as smokers |
Covey 2002.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: clinic Recruitment: volunteers |
|
| Participants | 134 smokers with a history of past MDD were randomized; 65% female; average age 44.5 | |
| Interventions |
Common components: 9 x 45 min individual counselling sessions at clinic visits |
|
| Outcomes |
|
|
| Funding Source | Pfizer, Inc and National Institute on Drug Abuse | |
| Author conflicts of interest | "Pfizer, Inc., provided support for conducting the study.” | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" "Medications were provided in prepared bottles that were numbered according to the randomization schedule and dispensed at each visit. All study staff at the clinic site were blinded to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The subjects lost to follow‐up after random assignment were considered treatment failures." Total participants lost to follw‐up at 6 months not reported |
Cox 2012.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: urban community‐based clinic Recruitment: volunteers, via healthcare settings and via community |
|
| Participants | 540 African American light smokers (≤ 10 cigarettes per day for ≥ 2 years, smoked on ≥25 days in past month); 66% female; average age 47; average cigarettes per day 8; average FTND 3.2 | |
| Interventions |
Common components: up to 6 one‐to‐one 15‐20 minute individual counselling sessions, self‐help guide at start |
|
| Outcomes |
|
|
| Funding Source | National Cancer Institute, National Institutes of Health, National Institute for Minority Health and Disparities | |
| Author conflicts of interest | Dr JS Ahluwalia serves as a consultant to Pfizer Pharmaceuticals, Inc; Dr NL Benowitz serves as a consultant to Pfizer Pharmaceuticals, Inc, and has been a paid expert witness in litigation against tobacco companies; Dr RF Tyndale holds shares in Nicogen Research, Inc, a company that is focused on novel smoking cessation treatment approaches | |
| Notes | New for 2013 update. SAEs only reported at week 3 (none reported), not included in SAE analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Both participants and investigators were blinded to the pharmacotherapy condition." No further information provided, unclear if counsellors blinded to treatment condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30% lost to follow‐up at 6 months, no difference between groups |
CTRI/2013/07/003830.
| Study characteristics | ||
| Methods | Study design: RCT Country: India Setting: 2 primary health centres |
|
| Participants | Current smokers currently undergoing treatment for tuberculosis | |
| Interventions |
No information given as to whether the trial was placebo‐controlled All participants given standard counselling, totalling 30 minutes |
|
| Outcomes |
|
|
| Funding Source | United States Agency for International Developement through World Health Organization | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Permuted block randomization, fixed”. This however may not be computer generated, and therefore not truly random |
| Allocation concealment (selection bias) | High risk | Study is open‐label, so both participants and researchers are aware of drug allocation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study is open‐label, so both participants and researchers are aware of drug allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not specified |
Da Costa 2002.
| Study characteristics | ||
| Methods | Study design: RCT Country: Brazil Setting: cessation clinic Recruitment: volunteers to a smokers' support group |
|
| Participants | 144 smokers randomized; "predominantly female"; age, cigarettes per day not described | |
| Interventions |
Common components: 6‐weekly group CBT |
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each patient chose a blind number from a box ...' Comment: probably adequate |
| Allocation concealment (selection bias) | Unclear risk | Quote: "... with each number corresponding to a “medication kit” that was externally undistinguishable. Patients and professionals participating in this study were blindfolded for this distribution." Comment: potentially adequate but difference in numbers in each group not accounted for |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind" but insufficient detail provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost in each group not clear |
Dalsgarð 2004.
| Study characteristics | ||
| Methods | Study design: RCT Country: Denmark Setting: 5 hospitals Recruitment: hospital staff |
|
| Participants | 335 smokers including physicians, nurses, other hospital service and admin staff; 75% female; average age 43; average cigarettes per day 19 | |
| Interventions |
Common components: motivational support around TQD, at weeks 3 and 7, and at 12‐week follow‐up |
|
| Outcomes |
|
|
| Funding Source | GlaxoSmithKline | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was computer generated and blinded |
| Allocation concealment (selection bias) | Low risk | Allocation was double‐blinded and bupropion and placebo tablets were identical in form and number. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind" Comment: clear that participants were blinded but unclear if all staff were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32% of the bupropion group and 43% the placebo group discontinued treatment, included in analysis |
Ebbert 2014.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: Mayo Clinic in Rochester, Minnesota and University of Minnesota |
|
| Participants | 506 participants randomized; 47% female; average age 42.0; average cigarettes per day 19.6; mean FTND 5.3 | |
| Interventions |
Common components: brief behavioral counselling at each clinic visit, totalling 110 minutes |
|
| Outcomes |
|
|
| Funding Source | The clinical trial was supported by National Institutes of Health (NIH) grant CA 138417 (primary investigator, Dr Ebbert). Medication (varenicline) was provided by Pfizer | |
| Author conflicts of interest | All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Ebbert reports serving as an investigator for clinical trials funded by Pfizer, receipt of consultancy fees from GlaxoSmithKline, research support from Pfizer, and research support from Orexigen and JHP Pharmaceuticals outside of the current study. Dr Hatsukami reports receipt of research support from Nabi Biopharmaceuticals outside of the current study. Dr Hays reports serving as an investigator for clinical trials funded by Pfizer. Dr Hurt reports receipt of consulting fees from Pfizer, an unrestricted grant from Pfizer Medical Education Group, and provision of expert testimony in Florida tobacco litigation cases. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “computer‐generated randomization sequence with variable‐sized blocks ranging from 2 to 8 stratified by study site”. |
| Allocation concealment (selection bias) | Low risk | Central pharmacy was used to allocate interventions |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “Study medication was labeled and dispensed according to participant identification, ensuring that treatment assignment remained concealed from the participant, investigators, and all study personnel having participant contact.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates are as follows: 40/249 varenicline + bupropion; 42/257 varenicline + placebo Dropout rate is below 50% in all trial arms |
Eisenberg 2013.
| Study characteristics | ||
| Methods | Study design: RCT Country: Canada Setting: 38 hospitals Recruitment: hospital patients with acute myocardial infarction |
|
| Participants | 392 smokers of at least 10 cigarettes per day, hospitalized with enzyme positive acute myocardial infarction. 16% female; average age 54; average cigarettes per day 23; average FTND not specified | |
| Interventions |
Common components: 7 one‐to‐one counselling sessions by research nurses at baseline and all follow‐ups of < 20 mins (average 5) – mix of phone and in‐person |
|
| Outcomes |
|
|
| Funding Source | Canadian Institutes of Health Research and Heart and Stroke Foundation of Quebec | |
| Author conflicts of interest | Drs Eisenberg and Gervais reported that they served as paid consultants for Pfizer Canada Inc.'s Varenicline Advisory Board. Dr Gervais reported that he received funds from Pfizer Canada Inc., for lectures including service on speaker bureaus, development of educational presentations, and travel/accommodations/meeting ex‐ penses. Dr Eisenberg received funding from Pfizer Canada Inc., to perform the Evaluation of Varenicline (Champix) in Smoking Cessation for Patients Post‐Acute Coronary Syndrome [EVITA] Trial; NCT00794573). | |
| Notes | New for 2013 update Patients not allowed to smoke whilst hospitalized. SAEs reported over 12 months, so not included in analysis. No quit extracted from percentages provided; denominators do not include 9 deaths in bupropion and 6 deaths in placebo group, all deemed not to be related to study medication. Adherence to treatment: 72.3% bupropion, 82% placebo took at least 1 pill per day |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done via an internet website using random blocks of 2 and 4 and was stratified by center to ensure that similar numbers of patients were randomized to the 2 arms of the study at each study center" |
| Allocation concealment (selection bias) | Low risk | Allocation performed centrally, see above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind." "All clinical end points were adjudicated by members of the Endpoints Evaluation Committee who were blinded to treatment assignment." Comment: no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 77% followed up at 12 months, similar across arms |
Elsasser 2002.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: community‐based Recruitment method: recruited from the community |
|
| Participants | 17 participants randomized; 41.2% female; average age 16.5; average cigarettes per day not specified; mean FTND not specified All participants were between 14‐19 years old |
|
| Interventions |
All participants recieved an unkown number and duration of behavioural modification sessions. |
|
| Outcomes |
|
|
| Funding Source | Funding receieved from GlaxoWellcome | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “randomized, double‐blind, placebo‐controlled trial” Comment: no further information given |
| Allocation concealment (selection bias) | Unclear risk | Quote: “randomized, double‐blind, placebo‐controlled trial” Comment: no further information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: “randomized, double‐blind, placebo‐controlled trial” Comment: no further information given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rates are as follows: 2/9 (22.2%) in the placebo; 4/8 (50%) of the bupropion group. Therefore dropout was higher than 20% between the two groups. |
Evins 2001.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: outpatient clinic Recruitment: volunteers |
|
| Participants | 18 smokers with stable schizophrenia (excluding 1 dropout prior to medication); 39% female; average age 45.5/42.7; average cigarettes per day 34 | |
| Interventions |
Common components: 9 x 1 hour weekly group CBT |
|
| Outcomes |
|
|
| Funding Source | National Association for Research on Schizophrenia and Affective Disorders. Medication provided by Glaxo Wellcome Inc | |
| Author conflicts of interest | None specified | |
| Notes | 2‐year follow‐up also reported. 3 additional quitters, not used in meta‐analysis since additional therapy used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Subjects were randomly assigned to 12 weeks of double‐blind bupropion SR, 150 mg/day, or an identical appearing placebo tablet added to their usual medication regimen." Comment: unclear if all staff members were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Nineteen subjects were enrolled and 18 subjects completed the 6‐month smoking cessation trial." |
Evins 2005.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: clinic Recruitment: volunteers |
|
| Participants | 56 smokers with schizophrenia (excluding 6 dropouts prior to medication); 27% female; average age 45, average cigarettes per day 37/26 | |
| Interventions |
Common components: 12 session group CBT |
|
| Outcomes |
|
|
| Funding Source | National Association for Research on Schizophrenia and Affective Disorders. Medication provided by GlaxoSmithKline | |
| Author conflicts of interest | None specified | |
| Notes | There was a significant treatment effect at EOT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind" with "identical placebo tablets." No further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only people taking at least one dose of study medication included in analyses in paper. 5 in each group lost to follow‐up and included as smokers |
Evins 2007.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: community mental health centre Recruitment: outpatients |
|
| Participants | 51 smokers (≥10 cigarettes per day) with schizophrenia; average age 44; average cigarettes per day 28/25 | |
| Interventions |
Common components: 12 session group CBT, TQD week 4 |
|
| Outcomes |
|
|
| Funding Source | Massachusetts Department of Mental Health. Medication provided by GlaxoSmithKline | |
| Author conflicts of interest | None specified | |
| Notes | Used in bupropion + NRT versus NRT comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Participants and investigators remained blind to the treatment condition (bupropion or placebo) throughout the follow‐up period." "Assessment of treatment assignment was at the level of chance for both participants and staff at Weeks 4 and 12 for both treatment assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20% of the bupropion group and 18% of the placebo group were lost to follow‐up at week 12; included as smokers. All other participants followed up at 12 months |
Fatemi 2013.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: not specified Recruitment method: not specified |
|
| Participants | 24 participants randomized; percentage female unspecified; average age not specified; average cigarettes per day not specified, mean FTND not specified All participants had been diagnosed with schizophrenia or schizoaffective disorder. |
|
| Interventions |
Common components: 20 minutes of antismoking counselling at each visit, totalling 80 minutes |
|
| Outcomes |
|
|
| Funding Source | Grant support recieved from the National Institute on Drug Abuse (grant # R01DA024674‐01A1) to SHF. Pfizer provided free samples of varenicline and placebo and had no role in design or conduct of this study. Watson Laboratories provided free samples of Bupropion SR. | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information provided |
| Allocation concealment (selection bias) | Unclear risk | No relevant information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No relevant information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate is 41%, but difference between groups not detailed |
Ferry 1992.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: clinic Recruitment: not specified |
|
| Participants | 42 male smokers | |
| Interventions |
Common components: group smoking cessation and relapse prevention counselling |
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | Abstract with no further details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind," no further detail provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given |
Ferry 1994.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: Veterans Medical Centre Recruitment: not specified |
|
| Participants | 190 smokers | |
| Interventions |
Common components: group smoking cessation and relapse prevention counselling; TQD within first 4 weeks |
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | Abstract with long‐term abstinence data supplied by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind," no further detail provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 72% followed‐up intervention, 61% followed‐up control. "The most conservative approach to analysis would reclassify all of these individuals as smokers due to protocol violation." |
Fossati 2007.
| Study characteristics | ||
| Methods | Study design: RCT Country: Italy Setting: primary care clinics Recruitment: patients of 71 general practitioners |
|
| Participants | 593 smokers randomised; 40% female; average age 49; average cigarettes per day 22 | |
| Interventions |
Common components: GP visits at enrolment and 4, 7, 26 & 52 weeks, phone calls 1 day pre‐TQD, 3 days post‐TQD, 10 weeks post‐enrolment. Classified as low intensity |
|
| Outcomes |
|
|
| Funding Source | Mario Negri Institute and GlaxoSmithKline | |
| Author conflicts of interest | Dr Apolone has received consulting and lecture fees from GlaxoSmithKline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Stated to be double‐blind, but not explicit that GPs blind to randomization code. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind", further detail not provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15% Bupropion and 17% placebo did not attend 12‐month follow‐up, included as smokers |
Gariti 2009.
| Study characteristics | ||
| Methods | Study design: 2x2 factorial RCT Country: USA Setting: university Recruitment: self‐referral from community |
|
| Participants | 260 light smokers (6‐15 cigarettes per day) motivated to quit; 57% female, average age 54; average cigarettes per day 11; average FTND 4 | |
| Interventions |
|
|
| Outcomes |
|
|
| Funding Source | National Institute on Drug Abuse | |
| Author conflicts of interest | None specified | |
| Notes | New for 2013 update Used in direct comparison of bupropion and NRT only, pooling 1+2 versus 3+4 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized ‘urn randomization’ |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind, double‐dummy" for medication component. "Neither the nurses nor the participants knew which of the two formulations contained the active formulation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data included as smokers. Similar losses to follow‐up across both groups |
George 2002.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: mental health clinic Recruitment: outpatients |
|
| Participants | 32 smokers with schizophrenia motivated to quit; 44% female; average age 41/45; average cigarettes per day 24 | |
| Interventions |
Common components: 10 x 60‐minute weekly group therapy |
|
| Outcomes |
|
|
| Funding Source | National institute on Drug Abuse, US Department of Veterans Affairs, National Alliance for Research on Schizophrenia and Depression. Medication provided by GlaxoSmithKline | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Both subjects and research staff were blinded to study medication assignment. Study medications were prepared by research pharmacists at CMHC, using encapsulation of SR bupropion tablets with blue 00 opaque capsules; placebo capsules contained only a dextrose matrix." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Subjects who were lost during the trial or at 6‐month follow‐up were counted as smokers." Number followed‐up at 6 months not reported |
George 2003.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: outpatient smoking research clinic Recruitment: community volunteers |
|
| Participants | 40 smokers; 63% female; average age 49; average cigarettes per day 23 | |
| Interventions |
|
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | "The main side effects of SEL were anorexia, gastrointestinal symptoms, and insomnia. None of the differences in adverse event ratings were significant in the SEL compared with the PLA group, and the drug was well tolerated compared with the placebo group. Reports of anxiety/agitation in both the SEL and PLA groups during the trial were high." Funding: National Institute on Drug Abuse, US Department of Veteran Affairs, National Alliance for Research on Schizophrenia and Depression |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, adequacy of blinding tested in research staff; results suggested blinding was adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 29/40 not assessed at 6 months. Greater loss to follow‐up in placebo, exact data not reported |
George 2008.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: mental health centre Recruitment: outpatients |
|
| Participants | 58 smokers with schizophrenia or schizoaffective disorder (excludes 1 receiving no study medication); 40% female; average age 40; average cigarettes per day ~23 | |
| Interventions |
Common components: nicotine patch (21 mg/24 hrs) for 8 weeks from TQD and group behaviour therapy 10‐weekly sessions |
|
| Outcomes |
|
|
| Funding Source | National Institute on Drug Abuse, National Alliance for Research on Schizophrenia and Depression | |
| Author conflicts of interest | None specified | |
| Notes | Bupropion as adjunct to NRT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double blind" but no additional details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/29 intervention and 10/29 control did not complete trial, included as smokers |
Gilbert 2019.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: from the community Recruitment method: newspaper ads and community and university postings |
|
| Participants | 105 participants randomised; 42% female; average age 26.4; average cigarettes per day 17.9, mean FTND 4.2 | |
| Interventions |
Common components: an abbreviated form of the American Lung Association smoking cessation program |
|
| Outcomes |
|
|
| Funding Source | Supported by the National Institute on Drug Abuse (NIDA) Grant R01 DA012289 awarded to David G Gilbert | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: randomized by “urn technique without replacement approach via a 28:28:28:16 ratio to one of four groups.” |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “Researchers and participants in the quit groups were blind to pill and patch type.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates: 0/34 – bupropion; 0/38 – nicotine patch; 0/35 – placebo |
Gonzales 2001.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: 16 clinical trial centres Recruitment: volunteers who had previously failed to quit using bupropion |
|
| Participants | 450 smokers who had previously used bupropion for at least 2 weeks without adverse effects and failed to quit; 55% female in placebo arm, 48% female in bupropion arm; average age 45; average cigarettes per day not specified | |
| Interventions |
Common components: brief individual counselling at visits weeks 1‐7, 9, 12, + telephone counselling at 4 months and 5 months |
|
| Outcomes |
Adverse events: measured for unspecified duration |
|
| Funding Source | GlaxoWellcome Inc | |
| Author conflicts of interest | None specified | |
| Notes | 6‐month data published. 12‐month data presented in a poster used since 2003 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants who satisfied the inclusion criteria were randomized to the treatment phase and received either bupropion SR ... or matching placebo. Eligible participants were assigned a protocol‐specific treatment number on the basis of a randomization code provided by GlaxoWellcome." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Even though participants and the site staff were blinded to the drug assignments and the site staff did not encourage participants to speculate on their assignments, the lower placebo abstinence rates in the current study may be attributable to the previous experiences of participants with bupropion in their previous cessation attempts." However, little difference in completion between two arms, suggesting blinding may have been successful. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "...all participants who stopped participating in the study during the treatment phase were considered to be smokers." Number of participants followed‐up at 12 months unclear |
Gonzales 2006.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: 19 clinical trial centres Recruitment: community volunteers |
|
| Participants | 673 participants, with prior exposure to bupropion excluded; 46% female; average age 42; average cigarettes per day 21 | |
| Interventions |
Common components: brief (< 10‐minute) standardized individual counselling at 12 weekly visits during drug phase and 11 clinic/phone visits during follow‐up, problem solving and relapse prevention |
|
| Outcomes |
|
|
| Funding Source | Pfizer, Inc | |
| Author conflicts of interest | Dr Gonzales reports having received research contracts from Pfizer, Sanofi‐Aventis, GlaxoSmithKline, and Nabi Biopharmaceuticals; consulting fees and honoraria from Pfizer, Sanofi‐Aventis, and GlaxoSmithKline; and owning 5 shares of Pfizer stock. Dr Rennard reports having had or currently having a number of relationships with companies who provide product and/or services relevant to outpatient management of COPD. These relationships include serving as a consultant for Adams, Almirall, Altana, Array Bio‐ pharma, AstraZeneca, Aventis, Biolipox, Centocor, Dey, Critical Therapeutics, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Ono Pharma, Otsuka, RJ Reynolds, Roche, Sankyo, Schering‐Plough, Scios, and Wyeth; advising regarding clinical trials for Altana, Astra‐ Zeneca, Aventis, Centocor, GlaxoSmithKline, Novartis, Pfizer, and Philip Morris; and speaking at continuing medical education programs and performing funded research both at basic and clinical levels for Altana, Astra‐Zeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis. Dr Nides reports having received research grants, consulting fees, and honoraria from Pfizer, Sanofi‐Aventis, and GlaxoSmithKline. Dr Oncken reports having received research grants, consulting fees, and honoraria from Pfizer; receiving, at no cost, nicotine replacement and placebo products from GlaxoSmith‐ Kline for smoking cessation studies; and receiving honoraria from Pri‐Med. Drs Azoulay, Watsky, Gong, Williams, and Reeves and Mr Billing report owning Pfizer stock or having stock options in Pfizer. | |
| Notes | Bupropion was an active control for varenicline. Bupropion versus placebo and bupropion versus varenicline comparisons contribute to review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "predefined ... computer‐generated randomization sequence", 1:1:1, using block size of 6, stratified by centre |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Participants and investigators were blinded to drug treatment assignments[, and] ... were not encouraged to guess their treatment assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up similar across conditions; 44% bupropion, 39.5% varenicline, 46% placebo, all included in analyses |
Górecka 2003.
| Study characteristics | ||
| Methods | Study design: RCT Country: Poland Setting: Smokers' clinic Recruitment: smokers with a diagnosis of COPD and failure to stop smoking with advice alone |
|
| Participants | 70 smokers with COPD 43% female; average age 56; average cigarettes per day 24 |
|
| Interventions |
Common components: support at clinic visits at baseline, 2 weeks, EOT |
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not described but presumably no blinding, as participants will have known assignment based on patch versus pill |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
Grant 2007.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: 2 substance use disorder clinics Recruitment: alcoholics in residential or outpatient treatment programmes |
|
| Participants | 58 alcoholic smokers; 16% female; average age 40; average cigarettes per day 25 | |
| Interventions |
Common components: 1‐hour cessation group (and 4‐weekly assessment visits) |
|
| Outcomes |
|
|
| Funding Source | National Institute on Alcohol Abuse and Alocholism | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind" but unclear who was blinded, no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Higher loss in bupropion (40%) than placebo (21%) but still within 20% range of each other. ITT analysis |
Gray 2011.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: university research clinic or high school health clinic Recruitment method: through local secondary schools, colleges, universities, and community media advertisements |
|
| Participants | All participants were between 12 and 21 years 134 participants randomized; 41.8% female; average age 18.5; average cigarettes per day 10.8; mean FTND 4.2 |
|
| Interventions |
All participants received smoking cessation booklets and were eligible for a weekly bonus payment of USD 5 throughout active treatment for completion of study materials, including daily smoking diaries. In addition, all participants received USD 30 for completing the initial assessment visit, USD 20 for completing the initial medication management visit, and USD 20 for completing the final post‐treatment follow‐up visit. |
|
| Outcomes |
|
|
| Funding Source | Funding was provided by the National Institute on DrugAbuse, Grants R01 DA17460 (HPU, KMG), K12DA000357 (KMG), K23 DA020482 (MJC), and R25DA020537 (ALL); by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant K12HD055885 (KJH); and by the US Public Health Service, Grant M01 RR01070 (Medical University of South Carolina Clinical and Translational Research Center) | |
| Author conflicts of interest | Dr Gray has received research support from Pfizer, Inc. (medication and placebo supply for research funded by the National Institute on Drug Abuse). Dr Hartwell has received grant support through Global Research Awards for Nicotine Dependence, an independent competitive grants program supported by Pfizer Inc. Dr Hiott is a past speakers' bureau member of Bristol‐Myers Squibb and Abbott Labs. Dr Deas has been an advisory board and speakers' bureau member of Eli Lilly and Company. Dr Upadhyaya is a past consultant and/or advisory board member of Eli Lilly and Company and Shire Pharmaceuticals. Dr Upadhyaya is an ex‐stockholder of New River Pharmaceutical Company, is a past speakers' bureau member of Shire Pharmaceuticals and Pfizer, Inc., and has received research support from Cephalon, Inc., Eli Lilly and Company, and Pfizer Inc. Dr Upadhyaya recently became an employee of, and is a holder of stock in, EliLilly and Company. The other investigators deny any potential conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: “double blinded; encapsulated by the university Investigational Drug Service so that the active and placebo medication appeared identical”. No further information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates: 12/37 (32.4%) in bupropion and contingnecy management; 13/36 (36.1%) in bupropion and non‐contingnecy management; 14/29 (48.3%) in placebo % contingnecy management; 10/32 (31.3%) in placebo and non‐contingnecy management Loss to follow‐up was less than 50% and similar across groups. |
Gray 2012.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: community Recruitment method: community media advertisement (e.g. flyers, newspapers, advertisements) |
|
| Participants | 29 participants randomized; 51.8% female; average age 18.9; average cigarettes per day 15.6; mean FTND 6.7 Adolescent smokers, aged 15–20 |
|
| Interventions |
All participants recieved quit smoking brochures and brief individual cessation counselling, totalling 90 minutes. |
|
| Outcomes |
|
|
| Funding Source | Medical University of South Carolina Hollings Cancer Center Pilot Research Program and the National Institutes of Health (K12DA000357, K23DA020482, R25DA020537, and UL1RR029882) | |
| Author conflicts of interest | Dr Upadhyaya is an employee and stockholder of Eli Lilly and Company. The other authors do not have potential conflicts to declare. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “The university investigational drug service encased medications in identical‐appearing capsules and dispensed them in weekly blister packs with specific instructions on day/ time for each dose.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The paper gives study retention figures, but does not specify whether they are lost to followup. |
Haggsträm 2006.
| Study characteristics | ||
| Methods | Study design: RCT Country: Brazil Setting: smoking cessation clinic Recruitment: community volunteers |
|
| Participants | 156 smokers; FTND > 4; 70% female in placebo and nortriptyline arms, 59% in bupropion arm; average age 44; average cigarettes per day not specified | |
| Interventions |
Common components: 6 x 15‐min individual CBT, weekly then bi‐weekly |
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy. "Both investigators and patients were blind to the treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers lost to follow‐up not reported, all included as smokers |
Hall 1998.
| Study characteristics | ||
| Methods | Study design: 2x2 factorial RCT Country: USA Setting: clinic Recruitment: community volunteers |
|
| Participants | 199 smokers, 33% had history of MDD; 55% female; average age 40; average cigarettes per day 21‐25 | |
| Interventions |
2 x 2 factorial design. Alternative psychological Rxs were 10 sessions of CBT or 5 sessions of health education control. Collapsed in this analysis |
|
| Outcomes |
|
|
| Funding Source | National Instutute on Drug Abuse and Veterans Administration | |
| Author conflicts of interest | None specified | |
| Notes | There were no significant main or intervention effects for MDD category, so these are pooled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomization, after stratification on history of MDD and number of cigarettes smoked |
| Allocation concealment (selection bias) | Low risk | Allocation generated at enrolment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Medication was placebo controlled and double blind. Placebo and active drug were identical in appearance." However, no detail on who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30% did not complete treatment in placebo and 17% in active groups. Analyses with missing = smoking given |
Hall 2002.
| Study characteristics | ||
| Methods | Study design: 3 x 2 factorial RCT Country: USA Setting: cessation research centre Recruitment: community volunteers |
|
| Participants | 220 smokers; 40% to 47% female; average age 37‐43; average cigarettes per day 20‐23 | |
| Interventions |
3 x 2 factorial design. Alternative psychological interventions were Medical Management (MM, physician advice, S‐H, 10 mins to 20 mins 1st visit, 5 minds at 2, 6, 11 weeks) or Psychosocial Intervention (PI, as MM plus 5 x 90‐min group sessions at 4, 5, 7, 11 weeks) |
|
| Outcomes |
Adverse events: measured for unspecified period |
|
| Funding Source | National Institute on Drug Abuse, National Cancer Institute | |
| Author conflicts of interest | None specified | |
| Notes | No significant interaction between pharmacotherapy and behaviour therapy, so behavioural therapy arms collapsed in main analysis. Bupropion and nortriptyline compared to placebo and head‐to‐head. Levels of support compared for bupropion only, ppa rates used. Not included in behavioural support subgroup. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were stratified by number of cigarettes smoked, sex and history of depression vs no history, and randomly assigned to 1 of the 6 experimental cells." |
| Allocation concealment (selection bias) | Low risk | Quote: "We encapsulated both drugs to maintain the patency of the bupropion formulation and to provide a blinded drug. All participants received capsules that were identical in number and appearance" but blinding of allocation not explicit. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Double‐blind but participants informed about adverse effects of each drug and 87% of participants taking active drug guessed that they were (compared to 67% placebo group). Bupropion participants no more likely than nortriptyline participants to correctly identify which drug they had received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19% lost to follow‐up at 52 weeks. No significant difference across conditions. Included as smokers in analyses |
Hall 2004.
| Study characteristics | ||
| Methods | Study design: 2 x 2 factorial RCT Country: USA Setting: clinic Recruitment: community volunteers |
|
| Participants | 160 smokers; 41% female; average age ~38; average cigarettes per day ~19 | |
| Interventions |
2 x 2 factorial design. Nortriptyline versus placebo and brief versus extended treatment. Brief treatment: nicotine patch for 8 weeks from quit date, and 5 group counselling sessions, total 7.5 hrs Extended treatment: first 12 weeks as for brief treatment, then same dose continued to week 52 then tapered. Individual counselling every 4 weeks, total 3 hours to 4.5 hours. Phone counselling, total 40 mins to 80 mins |
|
| Outcomes |
|
|
| Funding Source | National Institute on Drug Abuse | |
| Author conflicts of interest | None specified | |
| Notes | Factorial design, brief and extended treatment entered in meta‐analysis separately. In the active extended treatment arm, participants were still receiving nortriptyline at the time of final follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization stratified on cigarettes per day, prior NRT use, MDD history; method not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "participants given active drug were more likely to guess that they had received active drug (63%) than the placebo participants were to believe they were taking active drug (37%)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% lost at week 52, included as smokers |
Hertzberg 2001.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: Veterans Affairs Medical Centre (VAMC) Recruitment: VAMC outpatient volunteers |
|
| Participants | 15 male veterans with post‐traumatic stress disorder; average age 50; average cigarettes per day 33 | |
| Interventions |
Common components: individual counselling pre‐quit, weeks 1, 2, 4, 8, 12 |
|
| Outcomes |
|
|
| Funding Source | Glaxo Wellcome Inc, National Cancer Institute | |
| Author conflicts of interest | None specified | |
| Notes | 2 of the successful quitters were taking bupropion at 6 months, prescribed after end of study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind, no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Uneven attrition between arms; very high percentage lost to follow‐up in placebo group. 30% of the participants receiving bupropion SR did not complete the full 12‐week trial; 80% of the placebo group failed to complete the trial and were considered to have resumed smoking. |
Holt 2005.
| Study characteristics | ||
| Methods | Study design: RCT Country: New Zealand Setting: cessation clinic Recruitment: Maori community volunteers aged 16‐70 |
|
| Participants | 134 smokers; 72% female; average age 42/38 | |
| Interventions |
Common components: counselling at 3 clinic visits during medication and 3 monthly follow‐ups, motivational phone call 1 day before and 2 days after TQD |
|
| Outcomes |
|
|
| Funding Source | GlaxoSmithKline | |
| Author conflicts of interest | P3 Research, the Wellington School of Medicine and Health Sciences, and the Medical Research Institute of New Zealand have all received research grants from GlaxoSmithKline and Novartis. SH and RB have received fees for consulting and reimbursement for attending symposia from GlaxoSmithKline and Novartis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization using a computer generated code |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Neither the study team nor the participant was aware of which treatment had been allocated until the end of the 12 month study period." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High and uneven loss to follow‐up, with less than half of placebo group followed up at 12 months. 36% lost in bupropion group and 52% in placebo at 12 months. "Participants who were lost to follow up were categorised as smokers ... often this was confirmed by family members or friends." |
Hurt 1997.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: multicentre Recruitment: community volunteers |
|
| Participants | 615 smokers; 55% female; average age 44; average cigarettes per day 27 | |
| Interventions |
Common components: physician advice, S‐H materials, and brief individual counselling by study assistant at each visit |
|
| Outcomes | Smoking cessation: prolonged abstinence at 12 months (starting from day 22). Validated by CO ≤ 10 ppm Adverse events: measured for 52 weeks |
|
| Funding Source | Glaxo Wellcome | |
| Author conflicts of interest | None specified | |
| Notes | 300 mg compared with placebo in main analysis There was no evidence that history of major depression or alcoholism interacted with treatment condition or was associated with poorer outcomes. Prolonged abstinence rates at 12 months as supplied by Glaxo Wellcome: 300 mg 21; 150 mg 23; Placebo 15 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, stratified by site, method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind" but no detail given on who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Subjects who missed a follow‐up visit were considered to be smoking.... The rate of completion of the study increased with the dose and was 57 percent, 65 percent, 64 percent, and 71 percent for the placebo, 100‐mg, 150‐mg, and 300‐mg groups, respectively..." |
Johns 2017.
| Study characteristics | ||
| Methods | Study design: RCT Country: India Setting and recruitment method not specified |
|
| Participants | 300 participants randomized | |
| Interventions |
|
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States trial was randomized, no further detail given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States only that the study was ‘double‐blind’, no further detail given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No relevant information given |
Jorenby 1999.
| Study characteristics | ||
| Methods | Study design: 2 x 2 factorial RCT Country: USA Setting: multicentre clinical trial units Recruitment: community volunteers |
|
| Participants | 893 smokers; 52% female; average age 43; average cigarettes per day 25 | |
| Interventions |
Common components: brief (< 15 min) individual counselling session at each weekly assessment. One telephone call 3 days after quit day |
|
| Outcomes |
|
|
| Funding Source | Glaxo Wellcome | |
| Author conflicts of interest | Dr Jorenby has organized medical education presentations sponsored by Glaxo Wellcome and SmithKline Beecham. Dr Leischow has served as a consultant for McNeil Consumer Products, Pharmacia and Upjohn, and Glaxo Wellcome and has organized medical education presentations sponsored by Glaxo Wellcome. Dr Nides has served as a consultant for Glaxo Wellcome, Novartis, and SmithKline Beecham and has organized medical education presentations sponsored by Glaxo Wellcome. Dr Rennard has served as a consultant for Glaxo Wellcome, Novartis, and SmithKline Beecham and has organized medical education presentations sponsored by Glaxo Wellcome. Dr Muramoto has organized medical education presentations sponsored by Glaxo Wellcome. Mr Daughton has served as a consultant for SmithKline Beecham and Hoechst Marion Roussel and has organized medical education presentations sponsored by Glaxo Wellcome and Hoechst Marion Roussel. Dr Fiore has served as a consultant for Novartis, Glaxo Wellcome, SmithKline Beecham, and McNeil Consumer Products and has organized medical education presentations sponsored by Novartis, Elan Pharma, Lederle Laboratories, Glaxo Wellcome, McNeil Consumer Products, and SmithKline Beecham. Dr Baker has served as a consultant for SmithKline Beecham and has organized medical education presentations sponsored by Elan Pharma and Glaxo Wellcome. | |
| Notes | Primary outcome for study was PP abstinence; this analysis uses continuous abstinence since quit day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were randomly assigned to one of four treatments with use of an unequal‐cell design...[but] Randomization was not balanced within sites." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind" but no further detail provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All subjects who discontinued treatment early or who were lost to follow‐up were classified as smokers." Approximately 20% left the study and provided no additional information. 15% stopped taking medication but participated in follow‐up assessments. |
Jorenby 2006.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: multicentre clinical trial units Recruitment: community volunteers |
|
| Participants | 683 smokers (in relevant arms), with prior exposure to bupropion excluded; 41% female; average age 42; average cigarettes per day 22 | |
| Interventions |
Common components: brief (< 10 min) individual counselling at each weekly assessment for 12 weeks and 5 follow‐up visits. One telephone call 3 days after quit day |
|
| Outcomes |
|
|
| Funding Source | Pfizer Inc | |
| Author conflicts of interest | Dr Jorenby reported receiving research support from Pfizer, Nabi Biopharmaceutical, Sanofi‐Aventis and consulting fees from Nabi Biopharmaceutical. Dr Hays reported receiving a research grant from Pfizer. Dr Rigotti reported receiving research grant funding and consulting fees from GlaxoSmithKline, which markets smoking cessation medications, and Pfizer and Sanofi‐Aventis, which are developing smoking cessation medications. Dr Rigotti also reported receiving consulting fees from Merck, which is developing smoking cessation medications. | |
| Notes | Bupropion was an active control for varenicline. Bupropion versus placebo and bupropion versus varenicline comparisons contribute to the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was completed centrally by using a computer‐generated list and sites used an electronic system to assign participants to treatment." |
| Allocation concealment (selection bias) | Low risk | Quote: "Folders [containing medication or placebo] for all participants (regardless of treatment assignment) were identical throughout the treatment phase including a period of dose titration (week 1) and treatment at the target dose (weeks 2‐12)." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "in a double‐blind manner," no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over the period of treatment and follow‐up 14% of those receiving varenicline were lost to follow‐up; 14% randomized to bupropion lost to follow‐up; 16% of the placebo group were lost to follow‐up. "Participants whose smoking status was unknown or whose carbon monoxide level was higher than 10 ppm were classified as smoking during both the treatment phase and follow‐up." |
Kahn 2012.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: clinics Recruitment: community |
|
| Participants | 246 smokers; 49% female; average age 46; average cigarettes per day 22 | |
| Interventions |
Common components: 9 weekly individual counselling sessions of approximately 10 mins each |
|
| Outcomes |
|
|
| Funding Source | National Institutes of Health, National Institute on Drug Abuse | |
| Author conflicts of interest | None specified | |
| Notes | New for 2013 update Some additional information on study characteristics provided by author. Mean compliance rates 91.6% and 91.3% for the selegiline and placebo groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Adaptive randomization," method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind," no further details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 70% placebo and 74% STS followed up at 12 months |
Kalman 2011.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: not specified Recruitment: Veterans Administration Medical Center |
|
| Participants | 143 smokers with 2 to 12 months alcohol abstinence, with history of alcohol abuse or dependence; mean age 49; 17% female; average cigarettes per day 20.8; mean FTND 5.9 | |
| Interventions |
Common components: nicotine patch (7 weeks starting on TQD; 21 mg weeks 1‐4, 14 mg weeks 5‐6, 7 mg week 7) and 8 weekly counselling sessions starting 1 week before TQD (one‐to‐one sessions based on CBT and MI) |
|
| Outcomes |
|
|
| Funding Source | National Institute of Drug Abuse, National Institute on Alcohol Abuse and Alocholism | |
| Author conflicts of interest | None specified | |
| Notes | New for 2013 update N quit calculated from percentages provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Urn randomization," no further details provided |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind" but no detail on who was blinded in terms of study staff, including counsellors. "Both medication groups performed at the chance level in judging medication assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 participants who dropped out prior to receiving medication, not included in denominators. Further 18% intervention and 14% control lost at 24 weeks, counted as smoking in analyses. |
Karam‐Hage 2011.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: University of Michigan outpatient addictions clinic Recruitment method: patients admitted to the outpatient intensive treatment programme |
|
| Participants | Alcohol‐ and nicotine‐dependent patients 11 participants randomized; 55% female; average age 19.7; average cigarettes per day 1 pack; mean FTND 4.8 |
|
| Interventions |
Common components: minimal smoking cessation counselling and booklet “You Can Quit Smoking” |
|
| Outcomes |
|
|
| Funding Source | University of Michigan's General Clinical Research Center (GCRC) Grant # MO1 RR00042 | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No relevant information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates are as follows: 1/5 placebo; 1/6 bupropion |
Killen 2000.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: clinic Recruitment: advertisements |
|
| Participants | 224 smokers; 46% female; average age 46; average cigarettes per day 26 | |
| Interventions |
Common components: self‐help manual and 15 min behavioural counselling at weeks 1 and 4 |
|
| Outcomes |
|
|
| Funding Source | University of California Tobacco‐Related Disease Research Program, SmithKline Beecham | |
| Author conflicts of interest | None specified | |
| Notes | 40 mg and 20 mg dose pooled in meta‐analysis from 2009. 20/75 quit on 40 mg, 15/75 on 20 mg | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind" but unclear who exactly was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Those failing to provide confirmation [of smoking status] were reclassified as smokers." Number lost to follow‐up not reported |
Killen 2004.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: continuation high schools Recruitment: adolescents at schools |
|
| Participants | 211 adolescent smokers, at least 1 failed quit attempt; 31% female; average age 17; average cigarettes per day 15 | |
| Interventions |
Common components: weekly 45‐min group sessions, skills training |
|
| Outcomes |
|
|
| Funding Source | National Cancer Institute. GlaxoSmithKline provided medication | |
| Author conflicts of interest | None specified | |
| Notes | Low compliance with both bupropion and patch therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double‐blind." Though further details not provided, assessment of blind suggests it was successful (30% placebo and 31% bupropion correctly guessed assignment) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 38% bupropion and 35% placebo lost at 6 months, included in analysis |
Killen 2010.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: community Recruitment: radio, newspapers, community website and notices distributed via local organizations |
|
| Participants | 243 smokers, 18‐65 years old. 30% female; average age 45; average cigarettes per day 19 | |
| Interventions |
Common components: 9 sessions of individual counselling to develop cognitive and behavioural skills to resist urges to smoke |
|
| Outcomes |
|
|
| Funding Source | National Institute on Drug Abuse. Medication and matching placebo provided by Somerset Pharmaceuticals, Inc. | |
| Author conflicts of interest | None specified | |
| Notes | New for 2013 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Low risk | Participant assigned sequential ID numbers corresponding with drug “pre‐packaged and labelled by ID only at an off‐site location by an individual who had no association with the participants.” |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “Treatment assignment was concealed from staff and both research staff and participants were blind to week 52.” Assessment of blinding in participants and study staff suggests it was successful |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 87% followed up at 12 months, same in both arms. Missing counted as smokers |
Levine 2010.
| Study characteristics | ||
| Methods | Study design: 2 x 2 factorial RCT Country: USA Setting: not specified Recruitment: community volunteers |
|
| Participants | 349 weight‐concerned women smokers; average age 42; average cigarettes per day 21; mean FTND 5.2 | |
| Interventions |
Counselling conditions
Common components: 12 x 90‐minute group counselling sessions delivered over 3 months |
|
| Outcomes |
|
|
| Funding Source | National Institute on Drug Abuse. Medication supplied by GlaxoSmithKline | |
| Author conflicts of interest | Dr Marcus has served as a consultant to GlaxoSmithKline and Sanofi‐Aventis. Dr Perkins has served as a consultant for GlaxoSmithKline | |
| Notes | New for 2013 update Counselling arms collapsed in analyses (same intensity, just differed in content). N abstinent calculated from percentages given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blocked randomization, method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind," no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over half lost to follow‐up at 12 months. 48% followed up overall, similar rates between groups |
McCarthy 2008.
| Study characteristics | ||
| Methods | Study design: 2 x 2 factorial RCT Country: USA Setting: cessation clinic Recruitment: community volunteers |
|
| Participants | 463 smokers; 50% female; average age 36‐41; average cigarettes per day 22 | |
| Interventions |
Counselling conditions
|
|
| Outcomes |
|
|
| Funding Source | National Cancer Institute, National Instutute on Drug Abuse. GlaxoSmithKline provided placebo medication | |
| Author conflicts of interest | Douglas E Jorenby has received research support from Nabi Biopharmaceutical and Pfizer, Inc. and consulting fees from Nabi Biopharmaceutical. Saul Shiffman serves as consultant to GlaxoSmithKline Consumer Healthcare on an exclusive basis regarding over‐the‐counter smoking cessation products and also is a partner in a company that is developing a new nicotine medication. He is a cofounder of invivodata, inc., which provides electronic diary services for clinical research. In 1998 the University of Wisconsin appointed Dr Fiore to a named Chair, made possible by an unrestricted gift to the university from GlaxoWellcome. GlaxoSmithKline provided complimentary active and placebo medication used in this study | |
| Notes | Counselling conditions collapsed in main analysis, entered separately in subgroup analysis by intensity. Psychoeducation arms placed in multisession individual counselling subgroup due to high level of contact received, though not classified as counselling in paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Staff who screened and enrolled participants were unaware of the experimental condition to be assigned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind (for medication). "Research staff who interacted with participants were blind to participants' medication condition assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 171 (37%) failed to attend quit date visit or lost to follow‐up, similar across groups, included in ITT analysis |
Minami 2014.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: community. Recruitment method: "recruited from local community" |
|
| Participants | Patient with elevated depressive symptoms, as indicated by a Center for Epidemiologic Studies Depression Scale (CES‐D) score > 6 206 participants randomized; 48% female; average age 43; average cigarettes per day 21; mean FTND 5.5 |
|
| Interventions |
Common components: 8‐week supply of nicotine patches and brief counselling, totalling a maximum of 150 minutes |
|
| Outcomes |
|
|
| Funding Source | NIDA | |
| Author conflicts of interest | Dr Price reports receiving grant/research support from Medtronic, Neuronetics, NIH, HRSA, and NeoSync; serving on an advisory panel for Abbott; and serving as a consultant for Wiley, Springer, Qatar National Research Fund, and Abbott. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "urn randomization to balance the groups on gender, depressive symptoms (CES‐D ≥16), and nicotine dependence (FTND ≥ 7)." |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “assignment was double‐blind, such that neither participants nor study staff (including physicians, research assistants, and counselors) were aware of whether the participant was taking fluoxetine or placebo." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No relevant information given |
Moreno‐Coutino 2015.
| Study characteristics | ||
| Methods | Study design: RCT Country: Mexico Setting: smoking cessation clinic Recruitment method: people seeking smoking cessation treatment at clinic |
|
| Participants | Heavy smokers with minimal/mild depressive symptomatology. 60 participants randomized; 38% female; average age 45; average cigarettes per day 18.2; mean FTND 4.7 |
|
| Interventions |
Common components: 4 individual in‐person CBT sessions (over 4 weeks, 2 pre‐quit and 2 post‐quit), plus 0.1 mg low nicotine cigarettes |
|
| Outcomes |
|
|
| Funding Source | Mexican National University Macro‐project in Addictions | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Those who agreed to continue in the study, entered the raffle (three different color balls in a dark box) to assign a treatment setting, and were evaluated." |
| Allocation concealment (selection bias) | High risk | Quote: “Those who agreed to continue in the study, entered the raffle (three different color balls in a dark box) to assign a treatment setting, and were evaluated." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “evaluations and treatments were conducted by clinical psychologists who were not blind to the study." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate from each group (> 50%). Significantly more dropouts from NRT only arm |
Muramoto 2007.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: research clinic Recruitment: adolescent community volunteers |
|
| Participants | 312 adolescents (14 to 17); 46% females; median age 16; median cigarettes per day 11 | |
| Interventions |
Common components: brief (10‐20 mins) individual counselling session pre‐quit and at each weekly assessment |
|
| Outcomes |
|
|
| Funding Source | National Cancer Institute, The Robert Wood Johnson Foundation, GlaxoSmithKline | |
| Author conflicts of interest | Dr Muramoto has received research contracts from GlaxoSmithKline, Pfizer, and Sanofi‐Aventis and is a speaker for Pfizer. Dr Leischow is a speaker and consultant for Pfizer, and at the time this study was conducted he was receiving research support from GlaxoSmithKline. | |
| Notes | 300 mg arm contributes to main analysis. 2/105 quit in 150 mg group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Active study medication and identical‐appearing placebo were prepackaged into 3 sets of identical‐appearing blister cards in accordance with a computer‐generated randomization list." |
| Allocation concealment (selection bias) | Low risk | Quote: "... a research assistant assigned the subject the next treatment number (and associated blister cards) in sequence." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Study subjects and researchers remained blind to treatment group assignment throughout the study." "9.6% in the 300 mg group accurately guessed their treatment assignment. Across all treatment groups, there were no significant differences in the proportion of subjects who accurately guessed their treatment group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Slightly higher lost to follow‐up/declined further participation in placebo group (30%) than active arms (18%). ITT analysis |
Myles 2004.
| Study characteristics | ||
| Methods | Study design: RCT Country: Australia Setting: preoperative clinic Recruitment: smokers awaiting surgery |
|
| Participants | 47 smokers expected to undergo surgery within 8‐14 weeks; 34% female; average age 45/40; 49% smoked 21‐30 cigarettes per day | |
| Interventions |
Common components: advice at baseline, 1 phone call 2‐4 days after TQD. Low intensity |
|
| Outcomes |
|
|
| Funding Source | Alfred Hospital Research Trust, Glaxo Wellcome | |
| Author conflicts of interest | None specified | |
| Notes | More dropouts in placebo group. Only 20 had surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated from a table of random numbers into one of two groups: active (bupropion) or placebo (identical appearance) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind," no further detail provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17% lost to follow‐up in the bupropion group; 9% lost to follow‐up in the placebo group. "Patients lost to follow‐up were assumed to still be smoking." |
NCT00132821.
| Study characteristics | ||
| Methods | Study design: 2 x 2 factorial RCT Country: USA Setting: sleep clinic Recruitment: not specified |
|
| Participants | 59 participants enrolled; smoking at least 20 cigarettes per day. No further patient characteristics given | |
| Interventions | Starting 1 week prior to quit day
Added on quit day
|
|
| Outcomes |
|
|
| Funding Source | National Institute on Drug Abuse | |
| Author conflicts of interest | None specified | |
| Notes | Study detailed in trials registry only and results not reported. Attempt to contact the investigator for further information was unsuccessful | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No relevant information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No relevant information given |
NCT00308763.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: not specified Recruitment: not specified |
|
| Participants | 594 younger, low‐income, and minority smokers enrolled. No further patient characteristics given | |
| Interventions |
|
|
| Outcomes |
|
|
| Funding Source | NIH (R01HL066025) | |
| Author conflicts of interest | None specified | |
| Notes | Study detailed in trials registry only and results not reported. Attempt to contact the investigator for further information was unsuccessful | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No relevant information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No relevant information given |
NCT00495352.
| Study characteristics | ||
| Methods | Study design: RCT Country: Taiwan Setting: not specified Recruitment: not specified |
|
| Participants | 360 motivated psychiatric outpatients with schizophrenia enrolled. No further patient characteristics detailed | |
| Interventions |
|
|
| Outcomes |
|
|
| Funding Source | Yu‐Li Hospital; Department Of Health, Executive Yuan, ROC (Taiwan); National Health Research Institutes, Taiwan | |
| Author conflicts of interest | None specified | |
| Notes | Study detailed in trials registry only and results not reported. Attempt to contact the investigator for further information was unsuccessful | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No relevant information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No relevant information given |
NCT00578669.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting and recuirment method not specified |
|
| Participants | Participants had elevated depression symptoms. 206 participants randomized; 48% female; average age 44; average cigarettes per day 21; mean FTND 5.7 |
|
| Interventions |
Common components: nicotine patch as well as "standard smoking cessation treatment" |
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Placebo controlled, but no further information on blinding provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Clinical trial registry implies 100%, but not explicitly, so we concluded there was not sufficient information given |
NCT00593099.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Recruitment method: outpatient mental health clinics Setting: not specified |
|
| Participants | Participants were clinically stable outpatients with DSM‐IV diagnoses of bipolar I disorder. 5 participants randomized; 60% female; average age 57; average cigarettes per day 20; mean FTND 6.4 |
|
| Interventions |
Common components: weekly sessions of manualized group behavioral therapy |
|
| Outcomes |
|
|
| Funding Source | NIDA; National Alliance for Research in Schizophrenia and Depression | |
| Author conflicts of interest | Dr Weinberger reports receiving grant support from Sepracor, Inc. and the National Alliance for Research on Schizophrenia and Depression (NARSAD). Dr George reports that he received grant support from the National Institute on Drug Abuse (NIDA), NARSAD, The Donaghue Medical Research Foundation, Sanofi‐Aventis, Targacept, and Sepracor. Inc. He is on Advisory Boards and a consultant to Pfizer, Inc. Eli Lilly, Janssen, and Evotec. Dr Chengappa reports that he received grant support from Janssen‐Ortho, Inc, Stanley Medical Research Institute, NIDA, NARSAD. He is on Advisory Boards for Astra Zeneca and Lilly. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Paper states that the trial was placebo controlled, but no further information is given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates are as follows: 1/3 in placebo; 1/2 in bupropion. Therefore loss to follow‐up was less than 50% and similar between groups. |
NCT01406223.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting and recruitment method not specified |
|
| Participants | 76 participants randomized; 53% female; average age 38.8 | |
| Interventions |
|
|
| Outcomes |
|
|
| Funding Source | Not specified | |
| Author conflicts of interest | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Stated that the study is placebo controlled and there is “double masking”, but no further detail is given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rates are as follows: 12/18 varenicline; 18/20 varenicline |
Niaura 2002.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: 16 clinical trial centres Recruitment: community volunteers |
|
| Participants | 989 non‐depressed smokers; 61% female; average age 42; average cigarettes per day 28 | |
| Interventions |
Common components: 9 sessions (60‐90 mins) individual CBT. Included coping skills, stimulus control techniques and relapse prevention |
|
| Outcomes |
|
|
| Funding Source | Eli Lilly and Company | |
| Author conflicts of interest | None specified | |
| Notes | Originally based on abstract and data from authors. From 2002 based on full report. Numbers quit derived from rounded quit rates (10% quit in each group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind but further detail not provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data in treatment phase addressed, but unclear whether missing data in follow‐up phase addressed. At 12 months, 42% missing data, similar across all arms; missing data counted as smokers in our analyses. |
Nides 2006.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: 5 clinical sites Recruitment: volunteers (phase II study) |
|
| Participants | 638 smokers (255 in relevant arms, including 2 bupropion and 4 placebo who did not start medication); 51% female; average age 41; average cigarettes per day 20 | |
| Interventions |
Common components: up to 10 mins counselling at 7 weekly clinic visits, 12 weeks and 24 weeks |
|
| Outcomes |
|
|
| Funding Source | Pfizer | |
| Author conflicts of interest | Dr Nides has received research grants, consulting fees, and honoraria from Pfizer, Sanofi‐Aventis, and GlaxoSmithKline. Dr Oncken has received research grants, consulting fees, and honoraria from Pfizer; received, at no cost, nicotine replacement and placebo products from GlaxoSmithKline for smoking cessation studies; and received honoraria from Pri‐Med. Dr Gonzales reports having received research contracts from Pfizer, Sanofi‐Aventis, GlaxoSmithKline and Nabi Biopharmaceuticals; consulting fees and honoraria from Pfizer, Sanofi‐Aventis, and GlaxoSmithKline; and owning 5 shares of Pfizer stock. Dr Rennard has had or currently has a number of relationships with companies that provide product and/or services relevant to outpatient management of chronic obstructive pulmonary disease. These relationships include serving as a consultant (Adams, Almirall, Altana, Array Biopharma, AstraZeneca, Aventis, Biolipox, Centocor, Dey, Critical Therapeutics, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Ono Pharma, Otsuka, RJ Reynolds, Roche, Sankyo, Schering‐Plough, Scios, and Wyeth); advising regarding clinical trials (Altana, AstraZeneca, Aventis, Centocor, GlaxoSmithKline, Novartis, Pfizer, and Philip Morris); speaking at continuing medical education programmes; and performing funded research at both basic and clinical levels (Altana, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis). He owns no stock in any pharmaceutical companies. Drs Watsky and Reeves and Mr Anziano are employees of Pfizer and own Pfizer stock or have stock options. | |
| Notes | Bupropion was an active control for varenicline. Bupropion versus placebo and bupropion versus 2 mg varenicline comparisons contribute to review. Inclusion of 6 pretreatment dropouts has minimal effect on risk ratio | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...a randomization list was computer generated using a method of randomly permuted blocks and a pseudorandom number generator." |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators assigned medication to subjects in numerical order of acceptance into the study." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double‐blind", "to preserve treatment blinding," no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Subjects who dropped out for any reason were considered to be smokers at all subsequent time points." 9.5% of varenicline tartrate 0.3 mg, once daily; 7% of varenicline tartrate 1.0 mg, once daily; 11 % of varenicline tartrate 1.0 mg, twice daily; 6% of bupropion hydrochloride 150 mg, twice daily and 13% of the placebo group were lost to follow‐up. |
Parsons 2009.
| Study characteristics | ||
| Methods | Study design: 2 x 2 factorial RCT Country: UK Setting: smoking cessation clinic Recruitment: direct mail from general practitioner (GP), stop smoking service, newspaper advertisements |
|
| Participants | 143 adult smokers; 62% female; average age 46; average cigarettes per day 21; mean FTND 5.5 | |
| Interventions |
Common components: 7 weekly individual behavioural support sessions in clinic |
|
| Outcomes |
|
|
| Funding Source | Cancer Research UK | |
| Author conflicts of interest | None specified | |
| Notes | New for 2013 Factorial trial ‐ also tested the use of chromium versus placebo for weight loss. Arms collapsed for analysis; no difference detected |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Via computer program |
| Allocation concealment (selection bias) | Low risk | Independent statistician sent randomization codes to medication packing company, medication allocated in sequence. Researchers blind to allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “Participants, therapists, and outcome assessors were blind to the treatment allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over 90% followed up at 6 months, similar between groups |
Perkins 2013.
| Study characteristics | ||
| Methods | Study design: cross‐over trial Country: USA Setting: university research centre Recruitment method: "recruitment notices” were used |
|
| Participants | 45 participants randomized; 60% female; average age 36; average cigarettes per day 16; mean FTND 4.6 | |
| Interventions |
|
|
| Outcomes |
|
|
| Funding Source | Funded by National Institutes of Health | |
| Author conflicts of interest | Dr Perkins has served as a consultant for Embera Neurotherapeutics, which is unrelated to the current study. Dr Lerman has served as a consultant for GlaxoSmithKline, Pfizer and Astra Zeneca. She has received research funding, unrelated to the current study, from Pfizer and Astra Zeneca. Dr Chengappa has research funding from Pfizer that is unrelated to the current study. Dr Sparks, Mr Karelitz and Ms Jao have no potential conflicts of interest or disclosures to report. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “assigned randomly”, but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Analysis of participants knowledge of drug allocation revealed no significant differences between trial arms: “The respective number (percentage) of subjects identifying the medication as bupropion, modafinil, placebo or do not know were four, two, five and 34 (8.9, 4.4, 11.1 and 75.6%) of 45 during the bupropion condition; seven, three, four and 31 (15.6, 6.7, 8.9 and 68.9%) of 45 during the modafinil condition; and four, three, eight and 30 (9.1, 6.8, 18.2 and 67.9%) of 44 (1 subject with missing data) during the placebo condition. None of these values differed by medication condition, indicating successful blinding.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No relevant information given |
Piper 2007.
| Study characteristics | ||
| Methods | Study design: RCT
Setting: none specified Country: USA Recruitment: volunteers |
|
| Participants | 608 smokers; 58% female; average age 42; average cigarettes per day 22 | |
| Interventions |
Common components: three 10‐min counselling sessions over 3 weeks |
|
| Outcomes |
|
|
| Funding Source | National Institutes for Health | |
| Author conflicts of interest | In 1998 the University of Wisconsin appointed Dr Fiore to a named chair, made possible by an unrestricted gift to the university from GlaxoWellcome. Dr Baker has received monies to conduct clinical trials from pharmaceutical companies (Nabi, Glaxo, Pfizer, Sanofi) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was conducted in double‐blind fashion using blocked randomization within each of the 10 [orientation session] cohorts." No further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind, but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32% of bupropion and 36% of placebo groups lost at 12 months. "Participants who could not be reached at follow‐up were considered to be smoking for the purposes of follow‐up analyses." |
Piper 2009.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: community Recruitment: volunteers |
|
| Participants | 1504 smokers; 58% female; average age 45; average cigarettes per day 21.4 | |
| Interventions |
Common components: 7 one‐to‐one 10 to 20‐min counselling sessions |
|
| Outcomes |
|
|
| Funding Source | Majority of funding from National Institute on Drug Abuse and National Center for Research Resources. Medication provided to participants at no extra cost by GlaxoSmithKline. | |
| Author conflicts of interest | The authors report the following potential conflicts of interest for the last 5 years: Dr Smith has received research support from Elan Corporation. Dr Baker has served as an investigator on research projects sponsored by pharmaceutical companies, including Sanofi‐Synthelabo, Pfizer Inc, and Nabi Biopharmaceuticals. Dr Jorenby has received research support from the National Institute on Drug Abuse, the National Cancer Institute, Pfizer Inc, Sanofi‐Synthelabo, and Nabi Biopharmaceuticals. He has received support for educational activities from the National Institute on Drug Abuse and the Veterans Administration and consulting fees from Nabi Biopharmaceuticals. Dr Fiore has received honoraria from Pfizer. He has served as an investigator on research studies at the University of Wisconsin that were funded by Pfizer, Sanofi‐Synthelabo, GlaxoSmithKlein, and Nabi Biopharmaceuticals. In 1998, the University of Wisconsin appointed Dr Fiore to a named chair funded by an unrestricted gift to University of Wisconsin from Glaxo Wellcome. | |
| Notes | New for 2013 update Placebo outcomes reported as a whole in published report, author provided data for individual groups. 1 versus 6 in Analyses 1.1, 1.2 and 1.3. 2 versus 3 included in Analysis 1.5. 1 versus 4 in Analysis 1.7.1, 1 versus 3 in Analysis 1.7.2 and 1 versus 5 in Analysis 1.7.3 (intervention arm split in three to avoid triple counting) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified. "Randomization was double‐blind and used a block randomization scheme with sex and self‐reported race as the blocking variables." |
| Allocation concealment (selection bias) | Low risk | "Staff did not know to which type(s) of medication a participant would be assigned until the moment of randomization, and study staff were blinded to whether the medication was active or placebo." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double blind" but no further detail provided. "Study staff were blinded to whether the medication was active or placebo" Comment: type of medication (i.e. patch, gum, pill) would have been apparent to both groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90 dropouts (out of 1504). Analyses conducted using ITT. Individuals with missing data considered to be smoking |
Planer 2011.
| Study characteristics | ||
| Methods | Study design: RCT Country: Israel Setting: hospitals, Jersulem Recruitment: patients hospitalized for acute coronary syndrome in 2 separate campuses in Jerusalem |
|
| Participants | 151 smokers with diagnosis of acute coronary syndrome, motivated to quit; average age 51.9; 20.1% female; average cigarettes per day 31 | |
| Interventions |
Common components: counselling (at least 15 min of motivational support) during hospitalization and continued after discharge (at least 2 visits with physician and nurse at 1 month and 2 months and weekly telephone call by nurse during first and second month, then monthly telephone calls during rest of the year) |
|
| Outcomes |
|
|
| Funding Source | GlaxoSmithKline | |
| Author conflicts of interest | None specified | |
| Notes | New for 2013 update Study stopped early after interim analysis indicated no benefit OR adjusted for age, sex, invasive procedure, risk factors, Fagerstrom score, cigarettes per day: 0.90 (95% confidence interval (CI) 0.39 to 2.09) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized," method not specified |
| Allocation concealment (selection bias) | Unclear risk | Method not specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and staff blind to treatment assignment, "Numbered study bottles were supplied by the study co‐ordinator and remained concealed from the patients and medical staff." Comment: no biochemical validation but participants blind to condition so differential misreport unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 lost to follow‐up in each group |
Prochazka 1998.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: VAMC and Army Medical Centre Recruitment: outpatient clinics and campus advertisements |
|
| Participants | 214 smokers (excludes 29 early dropouts); 38% female; average age 47 | |
| Interventions |
Common components: 2 behavioural group sessions prior to drug therapy. During treatment individual support was provided by the study nurse. |
|
| Outcomes |
|
|
| Funding Source | Department of Veterans Affairs, US Department of Defense | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "An unblinded research pharmacist recommended dosage reductions for those above the therapeutic range and dosage increases for those who were subtherapeutic. To maintain blinding, dose reductions and increases on an equal number of randomly selected placebo‐treated subjects were also recommended...our blinding was only partially effective. Because of the high frequency of dry mouth, the nurse and subjects were often able to identify the active drug." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 75% dropout rate in placebo, 61% in drug group, majority classified as ineffective therapy |
Prochazka 2004.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: clinic Recruitment: outpatient clinic and community volunteers |
|
| Participants | 158 smokers; 54% female; average cigarettes per day 22 | |
| Interventions |
Common components: brief counselling from nurse at weekly visits |
|
| Outcomes |
|
|
| Funding Source | Department of Veterans Affairs | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were stratified by history of previous major depression and randomized by means of a computer‐generated random number list that was held by the Research Pharmacy Service of the Denver Veterans Affairs Medical Center." |
| Allocation concealment (selection bias) | Low risk | Quote: "Once a patient was enrolled, the Research Pharmacy Service randomized the subject according to the randomization list." Judged adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "...our blinding was only partially effective. Because of the high frequency of dry mouth, the study nurse was often able to identify the active drug." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Subjects who dropped out were counted as smokers." Number of dropouts not given |
Richmond 2013.
| Study characteristics | ||
| Methods | Study design: RCT Country: Australia Setting: 18 prisons Recruitment: referral from clinic staff, flyers and posters in prisons |
|
| Participants | 425 male prisoners aged > 18, incarcerated for ≥ 1 month with ≥ 6 months of current sentence remaining; FTND ≥ 5; average age 34; average cigarettes per day 23; 83% FTND ≥ 6 | |
| Interventions |
Common components: two x 30‐minute counselling sessions with CBT. Self‐help materials, access to quitline. 10 weeks NRT patch started on TQD; 21 mg weeks 1‐6, 14 mg/day weeks 7‐8, 7 mg/day weeks 9‐10 |
|
| Outcomes |
|
|
| Funding Source | National Health and Medical Research Council, NSW Department of Health, Queensland Department of Health. NRT provided free of charge by GlaxoSmithKline. | |
| Author conflicts of interest | Tony Butler is supported by an ARC future Fellowship | |
| Notes | New for 2013 update N quit extrapolated from percentages provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization algorithm,” no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: “Follow‐up assessments were conducted… by a prison nurse research assistant who was blind to group allocation.” Identical placebo. No further information on blinding provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 80% followed up at 12 months, similar in both groups |
Rigotti 2006.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: hospitals Recruitment: volunteers |
|
| Participants | 248 smokers hospitalized with cardiovascular disease (excludes 3/3 dropped prior to treatment and 2 placebo deaths during follow‐up); 31% female; average age 56; average cigarettes per day 23/21 | |
| Interventions |
Common components: multicomponent CBT cessation and relapse prevention programme, motivational interviewing approach. Begun in hospital, 30‐45 mins, 5 x 10 min post‐discharge contacts (2 days, 1 week, 3 weeks, 8 weeks, 12 weeks), self‐help, chart prompt for physician. Total time 80‐95 mins |
|
| Outcomes |
|
|
| Funding Source | National Heart, Lung and Blood Institute, National Institutes of Health General Clinical Research Centers Program, GlaxoSmithKline | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computer program, the study statistician generated a sequence of randomly‐permuted blocks of 4 within strata formed by study site and daily cigarette consumption (10 vs 10)." |
| Allocation concealment (selection bias) | Low risk | Quote: "The study pharmacist used this sequence, concealed from enrolment staff, to assign participants to study arm. Subjects and study personnel, except the statistician and pharmacist, were blind to treatment assignment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Subjects and study personnel, except the statistician and pharmacist, were blind to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Subjects were considered smokers if they were lost to follow‐up..."; 23% lost to follow‐up in the bupropion group and 23% in the placebo group |
Rose 2013.
| Study characteristics | ||
| Methods | Study design: RCT Country; USA Setting: clinic Recruitment: community volunteers |
|
| Participants | 440 smokers who did not respond successfully to cessation treatment with NRT (phase 1 = 335 participants whose smoking did not decrease by > 50% after 1 week NRT (prior to TQD); phase 2 = 105 participants who lapsed within one week after TQD); 50% female; average age 43; average cigarettes per day 22; mean FTND 5.8 | |
| Interventions |
Common components: cessation programme with nicotine patch (discontinued after 1 week in Phase 1 varenicline arm) and 4 to 6 brief (< 15 mins) counselling sessions |
|
| Outcomes |
|
|
| Funding Source | Supported by grant to Duke University from Philip Morris USA. NRT donated by GlaxoSmithKline | |
| Author conflicts of interest | None specified | |
| Notes | New for 2013 update Phase 1 and Phase 2 combined in meta‐analysis. Sensitivity analyses including both separately did not detect any significant effect on the pooled result. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind," no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | < 50% followed up at 6 months in both phases, similar rates of dropout across all arms. 27 participants censored from reported analyses, mainly for protocol violations, included as smoking here. |
Rose 2014.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: university Recruitment method: newspaper, radio, and television advertisements |
|
| Participants | Participants were nicotine patch non‐responders (failed to show a reduction of more than 50% in smoking after 1 week of nicotine patch treatment) 222 participants randomized; 54.5% female; average age 44.1; average cigarettes per day 20.7; mean FTND 6.1 |
|
| Interventions |
Common components: brief support at each study session, totalling 1 hour and 45 minutes |
|
| Outcomes |
|
|
| Funding Source | National Institute on Drug Abuse grant 1P50 DA027840 and a grant from Philip Morris USA. The sponsors had no role in the planning or execution of the study, data analysis, or publication of results. Active bupropion sustained‐release and placebo tablets were supplied by Murty Pharmaceuticals, under contract from the National Institute on Drug Abuse. | |
| Author conflicts of interest | The authors have consulting and patent purchase agreements with Philip Morris International for nicotine inhalation technology and consulting agreements with Targacept and Novartis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: “The study was a double‐blind, parallel‐arm adaptive treatment trial." Placebo tablets were used. No further information provided regarding who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates are as follows: 41/113 (36.3%) for varenicline and bupropion; 38/109 (34.9%) for varenicline and placebo |
Rose 2017.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: research centre (Duke Center for Smoking Cessation) Recruitment method: not specified |
|
| Participants | All participants were male 174 participants randomized; 0% female; average age 44.0; average cigarettes per day 20.0; mean FTND 5.5 |
|
| Interventions |
Common components: precessation patches for 1 week prior to pharmacological treatments above, and brief support was provided at each session, totalling 1 hour and 30 minutes |
|
| Outcomes |
|
|
| Funding Source | Grant 1P50 DA027840 from the National Institute on Drug Abuse and a grant from Philip Morris, USA | |
| Author conflicts of interest | The authors disclose consulting and patent purchase agreements with Philip Morris International relating to reduced risk tobacco products. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: “double‐blind, placebo‐controlled, parallel‐arm trial”. No further information provided regarding who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates are as follows: (13.1%) in the bupropion and varenicline arm; 13/90 (14.4%) in the placebo and varenicline arm; 11/84. Therefore dropout was low and similar between groups. |
Rovina 2009.
| Study characteristics | ||
| Methods | Study design: RCT Country: Greece Setting: cessation clinic Recruitment: clinic attenders invited to participate |
|
| Participants | 205 smokers; 40% female; average age 45; average cigarettes per day 37 | |
| Interventions |
|
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | All the authors of this paper declare that they have no financial or other potential conflicts of interest concerning the subject of this manuscript. | |
| Notes | New for 2013 update 3 versus 4 used analyses, 1 and 2 not included in any analyses (effect of different counselling would confound effect of bupropion) Authors do not report n abstinent, numbers included in meta‐analysis extrapolated from applying percentage to overall n randomized |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not stated, 3:1:1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label, participants and staff aware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 90% followed up at 12 months, but authors do not specify percentage per group and do not specify how participants lost to follow‐up were treated. Authors only provide percentages abstinent, so n abstinent in this review may be inflated. |
Saules 2004.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: cessation clinic Recruitment: volunteers |
|
| Participants | 150 smokers; 55% female; average age 40 | |
| Interventions |
Common components: TQD end of week 4, CBT 6 sessions starting 2 weeks before TQD, 11 clinic visits |
|
| Outcomes |
|
|
| Funding Source | National Institute on Drug Abuse, State of Michigan. Nicotine patch provided by McNeil Consumer Healthcare | |
| Author conflicts of interest | None specified | |
| Notes | Authors provided quit numbers by treatment group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind" but no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers lost to follow‐up not provided by study arm but high: at six months, only 58 of 150 subjects were followed up. Subjects who dropped out of the study or lost to follow‐up were considered to be smoking again. |
Schmitz 2007.
| Study characteristics | ||
| Methods | Study design: 2 x 2 factorial RCT Country: USA Setting: research clinic Recruitment: community volunteers |
|
| Participants | 154 women smokers; average age 48; average cigarettes per day 21 | |
| Interventions |
Common components: either CBT based on relapse prevention model, or group support therapy, both 7 weekly 60‐min meetings, TQD morning of 1st session, 10 days after start of medications |
|
| Outcomes |
|
|
| Funding Source | National Institute on Drug Abuse. Bupropion provided by GlaxoSmithKline. | |
| Author conflicts of interest | None specified | |
| Notes | Group therapy variants collapsed in main analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn procedure, balancing on a range of outcome‐related variables |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators and research staff were blind to the randomization codes, which were kept by a faculty member independent of the research and treatment team." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind," further information not provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 'enrolment failures' who did not receive any treatment are excluded from analyses. Other non‐completers and losses to follow‐up included in ITT analysis |
Schnoll 2010.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: not specified (presumably clinic) Recruitment: patient lists from physicians treating people with cancer |
|
| Participants | 246 cancer patients smoking ≥ 2 cigarettes per day; 48% female; average age 54.8; average cigarettes per day 17.5; mean FTND 3.2; 32% had tobacco‐related tumours | |
| Interventions |
Common componenets: 8 weeks nicotine patches and 5 sessions of behavioural counselling (3 in person, 2 over phone) |
|
| Outcomes |
|
|
| Funding Source | National Cancer Institute. NRT provided free of charge from GlaxoSmithKline. | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified by depression status. Method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double‐blind," no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 65% intervention and 72% control followed up at 6 months |
Selby 2003.
| Study characteristics | ||
| Methods | Study design: RCT Country: Canada Setting: 15 clinical centres Recruitment: community volunteers |
|
| Participants | 284 smokers previously exposed to bupropion for at least 2weeks, not quit for more than 24 hours in previous month | |
| Interventions |
Behavioural support not described |
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | Based on abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given, unclear how participants lost to follow‐up treated in outcome data. 70% intervention group and 50% control group completed study |
Sheng 2013.
| Study characteristics | ||
| Methods | Study design: RCT Country: China Setting: hospital outpatient centres Recruitment method: newspaper advertisements and by word of mouth |
|
| Participants | Participants were mainly male 257 participants randomized; 5.5% female; average age 39.1; average cigarettes per day 22.5; mean FTND 5.6 |
|
| Interventions |
All participants were given the same brief education and counselling was administered to both groups by research staff. Counselling topics included motivation, identification of smoking triggers, coping responses, weight management, and use of the medications. The total duration of conselling was 1 hour and 30 minutes. |
|
| Outcomes |
|
|
| Funding Source | Zhejiang Jinxin Pharmaceutical Co, Ltd | |
| Author conflicts of interest | L‐XS, Z‐NJ, and G‐ZX declare that they have undertaken research and consultancy for, and received honoraria for speaking at meetings for, the manufacturers of smoking cessation medications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Participants were assigned to one of two study arms using a computer algorithm to generate a random list of treatment assignments." |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “Participants in the control arm received placebo pills identical in appearance. All study personnel were blinded to treatment assignment. The same brief education and counseling were administered to both groups by a research staff.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates are as follows: 14/127 (11.0%) in the bupropion arm; 18/130 (13.9%) in the placebo. Therefore, dropout rates are low and similar between groups. |
Siddiqi 2013.
| Study characteristics | ||
| Methods | Study design: cluster‐RCT Country: Pakistan Setting: 33 health centres Recruitment: patients from participating health centres with suspected pulmonary tuberculosis |
|
| Participants | 1955 adult smokers with suspected tuberculosis (1299 included in arms relevant to this review), smoking ≥ 1 cigarettes per day or smoking hookah on a daily basis; 5% female; average age 41; average cigarettes per day 19 (where one hookah counts as 2 cigarettes) | |
| Interventions |
Common components: 2 sessions of brief, in‐person behavioural support (Note, third arm received usual care only, not included in this review) |
|
| Outcomes | Smoking cessation: continuous abstinence at 6 months. Validated by CO ≤ 9 ppm | |
| Funding Source | International Development Research Centre | |
| Author conflicts of interest | Link provided to list of declarations of interest, but link does not give access to active webpage | |
| Notes | New for 2013 Reported narratively only due to substantial heterogeneity of program effects across clusters. 275/659 quit intervention versus 254/640 control, adjusted risk ratio 1.1 (0.5 to 2.3) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Quote: “A researcher who was blinded to center identity” allocated conditions |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No clinics dropped out post‐randomization. Over 90% of participants followed up at 6 months |
| Other bias | High risk | Substantial heterogeneity of programme effects across clusters. 20% of participants in control arm smoked only hookah (no cigarettes) compared to 4% in intervention arm |
Simon 2004.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: VAMC outpatient units Recruitment: outpatients |
|
| Participants | 244 smokers, 79% veterans; 5% female; average age 50; average cigarettes per day 24 | |
| Interventions |
Common components: 3 months CBT counselling, self‐help materials and telephone follow‐up counselling |
|
| Outcomes |
|
|
| Funding Source | California Tobacco‐Related Disease Research Program | |
| Author conflicts of interest | None specified | |
| Notes | Used in bupropion + NRT versus NRT comparison 2 placebo and 3 bupropion deaths excluded from denominators Originally based on abstract, now uses published data and sustained quitting outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We assigned participants to the 2 study arms by using a computer algorithm to generate a random list of treatment assignments." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "All study personnel engaged in providing interventions to participants were blinded to treatment assignment." "Blinding appeared to be effective in our study; an approximately equal number of participants were able to guess what their treatment had been at the end of the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 244 participants enrolled, 3 (1%) were lost to follow‐up (all randomized to the placebo arm).... Participants lost to follow‐up were considered smokers." |
Simon 2009.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: VAMC hospital Recruitment: hospitalised volunteers |
|
| Participants | 83 inpatients smoking at least 5 cigarettes per day in previous year, smoking in week before admission, in contemplation or preparation stage of change | |
| Interventions |
Common components: individual CBT 30‐60 min during hospital stay + 5 phone calls at week 1, week 3, week 5, week 8, week 12, recycling encouraged |
|
| Outcomes |
|
|
| Funding Source | California Tobacco‐Related Disease Research Program | |
| Author conflicts of interest | None specified | |
| Notes | 1 death in bupropion, 1 in placebo excluded from analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer algorithm to generate a random list of treatment assignments." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All study personnel engaged in providing interventions to participants were blinded to treatment assignment." "A significant percentage of participants were able to guess correctly whether they were taking active bupropion or placebo" but as results did not favour intervention group, authors suggest this unblinding did not bias the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 withdrawals, 1 lost to follow‐up, 1 death in placebo, 2 withdrawals, 1 lost, 1 death in bupropion. All except deaths included in meta‐analysis |
Singh 2010.
| Study characteristics | ||
| Methods | Study design: RCT Country: India Setting: anti‐smoking clinic of Vallabhbhai Patel Chest Institute Recruitment method: not clearly specified |
|
| Participants | Participants almost solely men 30 participants randomized; 3.3% female; average age 43.1; average cigarettes per day 18.8; mean FTND 5.6 |
|
| Interventions |
Common components: physician advice based on National Cancer Institute's 5 A's i.e. ASK, ADVICE, ASSESS, ASSIST and ARRANGE. Brief face‐to‐face personalized anti‐smoking advice was given at each of the 11 visits. |
|
| Outcomes |
|
|
| Funding Source | Quote: “nil” | |
| Author conflicts of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “At the baseline, subjects were randomly assigned to two groups” Comment: no further information is given |
| Allocation concealment (selection bias) | Unclear risk | Quote: “At the baseline, subjects were randomly assigned to two groups” Comment: no further information is given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “It was a single blind placebo control study." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No relevant information given |
Smith 2009.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: 12 primary care clinics Recruitment: volunteers from primary care clinics |
|
| Participants | 1346 smokers; 56% female; average age 44; average cigarettes per day 20.3 | |
| Interventions |
Common components: quitline counselling (state provided). All participants received initial session, then could elect to receive up to 4 additional calls + could call for additional support if required. |
|
| Outcomes |
|
|
| Funding Source | Majority of funding from National Institutes of Health, National Institute on Drug Abuse, and National Cancer Institute. Medication provided to participants at no cost by GlaxoSmithKline | |
| Author conflicts of interest | Dr Smith has received research support from Elan Corporation plc. Dr Jorenby has received research support from Pfizer Inc, Sanofi‐Synthelabo, and Nabi Biopharmaceuticals and has received consulting fees from Nabi Biopharmaceuticals. Dr Fiore has received honoraria from Pfizer Inc and has served as an investigator on research studies at the University of Wisconsin that were funded by Pfizer Inc, Sanofi‐Synthelabo, and Nabi Biopharmaceuticals. In 1998, the University of Wisconsin (UW) appointed Dr Fiore to a named Chair funded by an unrestricted gift to UW from Glaxo Wellcome. Dr Baker has served as an investigator on research projects sponsored by pharmaceutical companies including Sanofi‐Synthelabo, Pfizer Inc, and Nabi Biopharmaceuticals. | |
| Notes | New for 2013 update No control so does not contribute to primary analysis. 4 versus 2 used in Analysis 1.5. 1 versus 3 used in Analysis 1.7.1, 1 versus 2 used in Analysis 1.7.2, and 1 versus 5 used in Analysis 1.7.3 (n in 1 divided equally between subgroups to avoid triple counting) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Smokers were randomized to the 5 treatment conditions within each clinic with blocking on sex and self‐identified race." Insufficient detail with which to judge. |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 158 individuals who did not pick up study medication at first point not included in analyses; 122 withdrawals and 9 deaths considered to be smoking |
SMK20001.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: 6 clinical trial centres Recruitment: volunteers for phase II trial |
|
| Participants | 286 smokers; 48% female; average age 42; average cigarettes per day not soecified | |
| Interventions |
No information about behavioural support |
|
| Outcomes | Smoking cessation: continuous abstinence at 12 months. Validated by CO ≤ 10 ppm | |
| Funding Source | GlaxoSmithKline | |
| Author conflicts of interest | None specified | |
| Notes | Identified from GSK trials website. Also included a novel cessation aid | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind but methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 34% lost in bupropion, 29% placebo, included as smokers |
Sood 2010.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: Community Recruitment: press releases and local advertising |
|
| Participants | 118 adult smokers; 82% female; average age 38; average cigarettes per day 20; mean FTND 5.0 | |
| Interventions |
Common components: 12‐week behavioural intervention using Mayo Clinic ‘Smoke Free and Living It’ manual (type and number of sessions not stated) |
|
| Outcomes |
|
|
| Funding Source | National Cancer Institute | |
| Author conflicts of interest | None specified | |
| Notes | New for 2013 update Groups 1 and 2 combined in meta‐analysis; no significant difference between the two (at 24 weeks, 1/39 abstinent intervention 1, 2/40 abstinent intervention 2) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated centrally by Mayo Clinic Division of Biostatistics |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: “Blinded” with matched placebo, no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 43% dropped out within first 12 weeks, unclear how many dropped out by 24 weeks. Not given by arm |
Sood 2012.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: clinic Recruitment: community volunteers |
|
| Participants | 120 smokers; 47% female; average age 40; average cigarettes per day 20; mean FTND 5.2 | |
| Interventions |
Common components: behavioural counselling using “Smoke Free and Living It” manual at every clinic visit (approx. 7) |
|
| Outcomes |
|
|
| Funding Source | National Institutes of Health | |
| Author conflicts of interest | None specified | |
| Notes | New for 2013 update SAMe is a dietary supplement used to treat depression No difference between arms 1 and 2, hence combined in meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Blinded," no further detail provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 57% followed up overall, similar rates between groups |
Spring 2007.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: clinic Recruitment: community volunteers |
|
| Participants | 247 smokers; 54% female; average age 44; average cigarettes per day 23 | |
| Interventions |
Common components: group behavioural counselling, 9 meetings over 12 weeks |
|
| Outcomes |
|
|
| Funding Source | National Institutes of Health, Veterans Affairs. Medication provided by Eli Lilly and Company. | |
| Author conflicts of interest | None specified | |
| Notes | First included as Spring 2004 with unpublished data. Full publication reports sustained abstinence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study pharmacist stratified participants by depression history and used computer‐generated random numbers to assign them to drug or placebo." |
| Allocation concealment (selection bias) | Unclear risk | Allocated by unblinded pharmacist, method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Research staff and participants were blinded to medication status." "Drug assignment was guessed correctly by 59.8% of placebo and 64.6% of fluoxetine participants. Facilitators guessed correctly for 65.3% of placebo and 55.6% of fluoxetine participants." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals/lost to follow‐up 40% for fluoxetine, 48% placebo. Authors report similar results from missing assumed smoking and generalized estimating equation (GEE) analyses. All participants included in meta‐analysis |
Stapleton 2013.
| Study characteristics | ||
| Methods | Study design: RCT Country: UK Setting: smoking cessation clinics Recruitment: people attending smoking cessation clinics |
|
| Participants | 1071 daily smokers; 53% female; average age 41; average cigarettes per day 20 | |
| Interventions |
Common components: 7 weekly behavioural support sessions as per standard service protocol. Mainly group, 60‐90 mins each |
|
| Outcomes |
|
|
| Funding Source | Department of Health for England. Study medication provided free of charge by Pfizer UK, GSK UK and Novartis UK. | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization and packaging was organized by an independent statistician at the host site.” |
| Allocation concealment (selection bias) | Low risk | Quote: “On enrolment, participants selected their envelope from a large batch and signed it before breaking the seal to reveal their allocation.” |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label, no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 61.5% followed up at both 1 month and 6 months, no significant difference between groups. Prolonged abstinence only imputed for 16% of total |
Swan 2003.
| Study characteristics | ||
| Methods | Study design: 2 x 2 factorial RCT Country: USA Setting: HMO Recruitment: volunteers from Group Health Co‐op membership |
|
| Participants | 1524 smokers; 57% female; average age 45; average cigarettes per day 23 | |
| Interventions | Factorial design crossing 2 drug doses with 2 intensities of behavioural counselling:
|
|
| Outcomes |
|
|
| Funding Source | National Cancer Institute | |
| Author conflicts of interest | None specified | |
| Notes | Based on published data from 2004 No dose/behavioural treatment interaction at 12 months so arms collapsed to compare 300 mg vs 150 mg Effects differed at 3 months and 12 months. Effect of higher dose disappeared and additional support aided recycling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Open‐label randomized trial...The computer code for the procedure calculated probabilities of group assignment that were dynamically modified based on the number of members in each group so that final group sizes were equal. No restrictions such as stratification or blocking were used as part of the randomization process." |
| Allocation concealment (selection bias) | Low risk | Procedure built into study database |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar percentage lost to follow‐up across all groups (approx 15%) Nonresponders treated as smoking |
Tashkin 2001.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: multicentre Recruitment: advertisements for volunteers |
|
| Participants | 404 smokers with mild to moderate COPD (excludes 7 early dropouts who did not take any study medication); 45% female; average age 53‐54; average cigarettes per day 28 | |
| Interventions |
Common components: brief face‐to‐face counselling at each clinic visit (weeks 1‐7, 10, 12), telephone counselling 3 days after TQD |
|
| Outcomes |
|
|
| Funding Source | Glaxo Wellcome Inc | |
| Author conflicts of interest | None specified | |
| Notes | ITT population defined as those taking at least one dose of study medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomised as per code provided by Glaxo Wellcome, using block sizes of four stratified by centre. Within each block of four, two participants were assigned placebo and two bupropion SR. The randomisation codes were kept at the study sites during the trial and we instructed investigators to break the code only for a medical emergency." |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind study, but further detail not provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 64% intervention and 73% control followed up at 6 months. "All participants who withdrew from the study were taken to be smokers thereafter." |
Tidey 2011.
| Study characteristics | ||
| Methods | Study design: factorial trial Country: USA Setting: Providence Veterans Affairs Medical Center and the Brown University Center for Alcohol and Addiction Studies Recruitment method: advertisements posted in the surrounding community and at an outpatient clinic at a local VA medical centre |
|
| Participants | Participants diagnosed with schizophrenia or schizoaffective disorder as confirmed by the Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders 57 participants randomized; 29% female; average age 45.1; average cigarettes per day 27; mean FTND 7.1 |
|
| Interventions |
As this is a factorial trial, all participants were randomized to contingency management or none. |
|
| Outcomes |
|
|
| Funding Source | NIH grant R01‐DA17566 to the first author and a Senior Research Career Scientist Award from the Department of Veterans Affairs to the second author | |
| Author conflicts of interest | None detailed | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomized by coin toss." |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States "double‐blind", but no further information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 94% follow‐up in all groups, with no between‐group differences |
Tonnesen 2003.
| Study characteristics | ||
| Methods | Study design: RCT Country: 8 European countries, Australia, New Zealand Setting: 28 clinical trial centres Recruitment: community volunteers |
|
| Participants | 710 smokers; 51% female; average age 42; median cigarettes per day 20 | |
| Interventions |
Common components: brief motivational support at weekly clinic visits and telephone support during follow‐up. 11 clinic visits and 10 phone calls scheduled |
|
| Outcomes |
|
|
| Funding Source | GlaxoSmithKline | |
| Author conflicts of interest | S Tonstad has received honoraria from Glaxo‐SmithKline for lectures on smoking cessation. R Sweet is a former employee of GlaxoSmithKline. A Hider and J Townsend are currently employees of GlaxoSmithKline. For A Hjalmarsson, PI VanSpiegel, P Tonnesen: no conflict of interest was declared | |
| Notes | First included 2003 as Tonstad 2001 ITT population defined as those taking at least one dose of study medication excludes 3 randomized participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "GlaxoSmithKline created a randomization schedule in a 3:1 bupropion: placebo ratio. Each centre received a list with treatment numbers and subjects were consecutively assigned a treatment number at the baseline visit." |
| Allocation concealment (selection bias) | Low risk | Quote: "GlaxoSmithKline supplied bupropion SR 150 mg and placebo‐to‐match tablets for oral administration as white, film‐coated tablets." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind but methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% of bupropion SR and 12% placebo were lost to follow‐up |
Tonstad 2003.
| Study characteristics | ||
| Methods | Study design: RCT Country: 10 countries including European countries, Australia, and NZ Setting: 28 clinical trial centres Recruitment: volunteers with CVD |
|
| Participants | 629 smokers with stable CVD; 23% female; average age 55; average cigarettes per day 25; 49% had history of MI | |
| Interventions |
Common components: brief motivational support at weekly clinic visits and telephone support during follow‐up. 9 clinic visits and 10 phone calls scheduled |
|
| Outcomes |
|
|
| Funding Source | GlaxoSmithKline | |
| Author conflicts of interest | None specified | |
| Notes | First included 2003 as McRobbie 2003. ITT population = 626 defined as those taking at least one dose of study medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind, but no further detail provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number missing follow‐up in each group not provided. At 12 months, 38% bupropion and 50% placebo had prematurely discontinued treatment. "Subjects with missing investigator assessments were assumed to be smokers at that visit." |
Urdapilleta‐Herrera 2013.
| Study characteristics | ||
| Methods | Study design: RCT Country: Mexico Setting: not specified Recruitment: not specified |
|
| Participants | 94 "chronic smokers" randomised; average age 48; average pack per year 25 | |
| Interventions |
Common components: CBT |
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | Only limited information available as study is only reported as a conference abstract. Outcome data is insufficient to include in meta‐analysis as it is unclear whether percentages reported were calculated using all participants randomized or only those followed up as the denominator. Attempt to contact the authors was unsuccessful. Results are summarized narratively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double‐blind" although no information given regarding who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No relevant information given |
Uyar 2007.
| Study characteristics | ||
| Methods | Study design: RCT Country: Turkey Setting: cessation clinic Recruitment: cessation clinic patients |
|
| Participants | 131 smokers; 19% female; average age 36 | |
| Interventions |
Common components: brief counselling on consequences of smoking with follow‐up for 24 weeks ‐ more than low intensity |
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | First included based on abstract. Contributes to bupropion versus control and bupropion versus nicotine patch | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly allocated", method not described, unclear why fewer in control condition |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of any losses to follow‐up |
Wagena 2005.
| Study characteristics | ||
| Methods | Study design: RCT Country: Netherlands Setting: university medical centre Recruitment: community volunteers |
|
| Participants | 255 smokers with or at risk of COPD; 51% female; average age 51; average cigarettes per day 23 | |
| Interventions |
Common components: individual counselling 10‐20 mins at baseline, 1 week and 3 weeks post‐TQD (TQD typically day 11). Telephone support TQD, 2 weeks, 4 weeks, 6 weeks, 8 weeks, 11 weeks |
|
| Outcomes |
|
|
| Funding Source | Netherlands Asthma Foundation, Netherlands Organization for Health Research and Development. Lundbeck BV provided nortriptyline free of charge | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated by pharmacist, stratified by COPD severity, block size 33 |
| Allocation concealment (selection bias) | Low risk | Research staff blinded throughout study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind but "at both time points, participants receiving active drug compared with those receiving placebo were more likely to guess that they had received bupropion SR and nortriptyline treatment (72% vs 43%, P.01; and 62% vs 37%; P=.001; respectively)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 (12%) bupropion, 13 (16%) nortriptyline, 12 (13%) lost or withdrawn. All included in ITT analysis |
Weinberger 2010.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: clinics Recruitment: community volunteers |
|
| Participants | 101 smokers (excludes 2 taking no medication); 50% femalel; average age 47; average cigarettes per day 22 | |
| Interventions |
Common components: brief weekly counselling |
|
| Outcomes |
|
|
| Funding Source | National Institute of Drug Abuse, Veteran's Administration, Women's Health Research at Yale, NIH, University of Toronto | |
| Author conflicts of interest | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Both participants and research staff were blinded to study medication assignment," Comment: assessments of staff and participants suggest blinding was adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 27.5% selegiline, 42% placebo lost at 6 months. Including all participants is less conservative |
Weiner 2012.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Setting: Maryland Psychiatric Research Center Recruitment method: clinically stable outpatients from the Maryland Psychiatric Research Center volunteered to participate |
|
| Participants | Participants had a DSM‐IV diagnosis of schizophrenia or schizoaffective disorder made through a best estimate diagnostic approach. 46 participants randomised; 19.6% female; average age 49; average cigarettes per day 44.0; mean FTND 5.8 |
|
| Interventions |
All participants had a 9‐week group support programme led by staff trained in the education model of the American Cancer Society Fresh Start Program modified for people with schizophrenia. Each session was structured and incorporated relation exercises with practice “homework”. The first group sessions were designed to increase awareness of specific smoking habits and to develop a ‘Quit Plan’. A Quit Day Ceremony was held at the fifth group session. Subsequent sessions focused on reworking the Quit Plan. Later groups focused on strategies for participants minimizing weight gain, managing high risk situations, and imagining themselves as non‐smokers. |
|
| Outcomes |
|
|
| Funding Source | Veterans Affairs Capitol Network (VISN 5) Mental Illness Research, Education, and Clinical Center. National Institute of Mental Health Grant (MH068580‐01), Advance Center for Intervention Services Research | |
| Author conflicts of interest | Ms Ball has served as a consultant to ePharmaSolutions and Pfizer; Dr Gold has served as a consultant to Merck, AstraZeneca, Solvay, Pfizer, and GlaxoSmithKline. Dr Evins has served as a consultant to Pfizer, Boehringer, and Schering Plough and has received grant/research support from GlaxoSmithKline and Pfizer. Dr Buchanan has served as a consultant to Abbott and ClaxoSmithKline; has received grant/research support from Novartis and Janssen; has served on advisory boards for AstraZeneca, Wyeth, Schering Plough, Solvay and Pfizer, and has received other material or financial support from Bristol‐Myers Squibb, Otsuka, Pfizer and Cephalon. Drs Weiner and McMahon and Ms Buchholz report no financial or other relationship relevant to the subject of this article. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Random assignments made by the statistician." Comment: no further information given |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Random assignments made by the statistician." Comment: no further information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: “double‐blind, placebo‐controlled clinical trial." Comment: no further information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates are as follows: 8/24 (33.3%) in the bupropion group; 6/22 (27.3%) in the placebo group. Therefore overall dropout was less than 50% and similar between groups. |
| Other bias | Unclear risk | “While the target completion number was 40 there was insufficient study drug available to meet this goal." It is unclear how this was dealt with and whether it is accounted for in the dropouts reported in the flow diagram. However loss to follow‐up was similar between arms. |
White 2005.
| Study characteristics | ||
| Methods | Study design: RCT Country: Canada Setting: university Recruitment method: local media |
|
| Participants | 36 participants randomized; 61.1% female; average age 41.9; average cigarettes per day 24.0; mean FTND 7.2 | |
| Interventions |
All participants each week received 15‐minute one‐to‐one smoking cessation counselling with a study investigator, using the Mayo Clinic workbook ‘"Smoke‐Free and Living It" for a total of 1 hour and 30 minutes |
|
| Outcomes |
|
|
| Funding Source | Calgary Centre for Advancement of Health. Gabapentin (Neurontin) samples were donated through an informal arrangement with a local representative of Pfizer Canada Inc. | |
| Author conflicts of interest | None detailed | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “we conducted a randomized, open‐label pilot trial." Comment: no further information given |
| Allocation concealment (selection bias) | Unclear risk | Quote: “we conducted a randomized, open‐label pilot trial." Comment: no further information given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “we conducted a randomized, open‐label pilot trial” Comment: open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates are as follows: 9/19 (47.4%) in the bupropion group; 6/17 (35.3%) in the gabapentin group. Therefore overall attrition was less than 50% and similar between arms. |
Wittchen 2011.
| Study characteristics | ||
| Methods | Study design: RCT Country: Germany Setting: 167 primary care clinics Recruitment: patients at participating primary care clinics |
|
| Participants | 467 "current regular smokers"; 52% female; average age 43; average cigarettes per day 20 | |
| Interventions |
|
|
| Outcomes |
|
|
| Funding Source | Patients covered all costs for pharmaceutical treatments. Sponsored by the Federal Ministry of Education and Research; additional support provided by GlaxoSmithKline GmbH & Co and Pharmacia GmbH | |
| Author conflicts of interest | None specified | |
| Notes | New for 2013 update 3 versus 2 included in primary analyses. 2 versus 4 included in Analysis 1.7 comparison of NRT with bupropion. 1 not used as results versus bupropion would be confounded with CBT |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Generated by the study center"; used to put 4 different coloured questionnaires in random order |
| Allocation concealment (selection bias) | High risk | Quote: "questionnaires were distributed consecutively to all attending patients on the target days by nurses. Thus, the assignment of patients was entirely dependent on the consecutive attendance of patients and the random assignment of a color. Doctors were not allowed to interfere with this study procedure." But numbers allocated to groups very uneven and discussion states: "Random checks of this procedure [randomization] and quality assurance tests by study monitors revealed that in some cases in the latter part of the study treatment was based on patient and physician preferences." Comment: therefore no concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither participants nor providers were blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar number of dropouts between groups; participants lost to follow‐up considered smokers for meta‐analysis |
Zellweger 2005.
| Study characteristics | ||
| Methods | Study design: RCT Countries: 12 European countries Setting: 26 clinical trial centres Recruitment: volunteers, healthcare professionals (qualified practising physician or nurse) |
|
| Participants | 667 smokers (excludes 1 centre enrolling 20 people, and 3 people who took no medication); 64% female; average age 40; average cigarettes per day 23; 32% doctor, 68% nurse | |
| Interventions |
Common components: Brief (10‐15 min) motivational support at weekly clinic visits and telephone support one day before TQD, 3 days after TQD, monthly during follow‐up |
|
| Outcomes |
|
|
| Funding Source | GlaxoSmithKline | |
| Author conflicts of interest | None specified | |
| Notes | Continuous abstinence rates and information on adverse events from GlaxoSmithKline data. One centre excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind but further detail not provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost to follow‐up not stated. Participants with missing assessments or dropouts considered to be smoking |
Zincir 2013.
| Study characteristics | ||
| Methods | Study design: naturalistic clinical follow‐up study Country: Turkey Setting: outpatient smoking cessation clinic in a hospital Recruitment method: patients who presented at the smoking cessation outpatient clinic were included in the study on a voluntary basis |
|
| Participants | 300 participants randomized; average age: 45.8 in those who stopped smoking and 40.8 in those who continued smoking; average boxes of cigarettes per year: 23.62 in those who stopped smoking and 23.26 in those who continued smoking; mean FTND: 5.9 in those who stopped smoking and 6.7 in those who continued smoking | |
| Interventions |
|
|
| Outcomes |
|
|
| Funding Source | None specified | |
| Author conflicts of interest | None detailed | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “…they were randomized to the pharmacological therapy groups” Comment: No further information given |
| Allocation concealment (selection bias) | Unclear risk | Quote: “…they were randomized to the pharmacological therapy groups" Comment: no further information given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “This was a naturalistic clinical follow‐up study." Comment: those involved in the study were therefore unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 300 participants were randomized and 251 completed the study. Therefore 49/300 (16.3%) were lost to follow‐up overall. However, it is impossible to establish the number lost to follow‐up by group. |
| Other bias | High risk | Quote: “no adverse event was reported during the study”. This is highly unlikely to be correct. Additionally, there is no explanation of how adverse events were assessed. |
AE: adverse event CBGT: cognitive behavioral group therapy CBT: cognitive behavioural therapy CES‐D: Center for Epidemiologic Studies Depression Scale CO: carbon monoxide (in exhaled breath) COPD: chronic obstructive pulmonary disease CVD: cardiovascular disease EOT: end of treatment FTND: Fagerstrom Test for Nicotine Dependence FTQ: Fagerstrom Tolerance Questionnaire ITT: intention‐to‐treat MDD: major depressive disorder MI: myocardial infarction mins: minutes NRT: nicotine replacement therapy ppa: point prevalence abstinence ppm: parts per million RCT: randomized controlled trial RP: relapse prevention Rx: treatment SAE: serious adverse event SAMe: S‐Adenosyl‐L‐Methionine S‐H: self‐help SR: sustained release TQD: target quit date VAMC: Veterans Affairs Medical Center
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akbarpour 2010 | Bupropion ‐ short follow‐up |
| Aryanpur 2016 | Arms not matched ‐ different behavioural interventions in each |
| Banham 2010 | Not RCT ‐ review of smoking cessation treatment for people with severe mental illness |
| Becker 2003 | St John's wort ‐ short follow‐up (1 month) |
| Berlin 2005 | Befloxatone (reversible monoamine oxidase‐B inhibitor) ‐ data not published, treatment reported to have had no effect on abstinence rates |
| Bloch 2010 | Bupropion ‐ trial in people with schizophrenia, short follow‐up and cessation not reported |
| Bowen 1991 | Tryptophan ‐ short follow‐up Tryptophan 50 mg/kg/day, with high carbohydrate low protein diet (7/1 ratio), versus placebo and low carbohydrate high protein diet (1/1 ratio) for two weeks |
| Brauer 2000 | Selegiline ‐ only preliminary short‐term results available. Six month follow‐up planned |
| Breitling 2008 | Trial of practitioner education and financial incentives, or cessation drug costs reimbursement |
| Brody 2013 | Ineligible outcomes ‐ less than six months follow‐up and no safety data reported |
| Carrão 2007 | Sertraline ‐ combined with buspirone so effect of sertraline could not be isolated |
| Chan 2005 | Bupropion ‐ case control study in pregnant women |
| Chandrashekar 2015 | Short‐term follow‐up and no safety assessment |
| Christenhusz 2012 | Not randomized to treatments, only treatment strategies |
| Cornelius 1997 | Fluoxetine ‐ cessation not an outcome. Fluoxetine reduced the amount smoked by depressed alcoholic smokers |
| Cornelius 1999 | Fluoxetine ‐ short‐term outcome in a study of depressed alcoholic participants not attempting to quit |
| Covey 2007 | Previously included. Relapse prevention study. See Livingstone‐Banks 2019 |
| Croghan 2007 | Previously included. Relapse prevention study. See Livingstone‐Banks 2019 |
| Cropsey 2015 | Randomization to treatment strategy, not actual treatment |
| Dalack 1995 | Fluoxetine ‐ refers to, but does not report on a cessation study |
| Dale 2002 | Bupropion ‐ used for smokeless tobacco cessation, not smoking cessation |
| Dale 2007 | Bupropion ‐ for smokeless tobacco cessation, see Ebbert 2011 |
| Daniela 2008 | Sertraline and buspirone ‐ effect of antidepressant confounded with that of anxiolytic |
| Edwards 1989 | Doxepin ‐ short follow‐up (2 months) |
| EUCTR2005‐006189‐32‐AT | Arms not matched |
| Evins 2008 | Bupropion ‐ long‐term results not presented due to high loss to follow‐up |
| Fatemi 2005 | Bupropion ‐ short‐term cross‐over trial |
| Frederick 1997 | Venlafaxine ‐ short follow‐up (8 weeks) |
| Gawin 1989 | Buspirone ‐ open trial |
| Gifford 2011 | Bupropion ‐ test of behavioural therapy, all participants received bupropion |
| Glover 2002 | Bupropion ‐ used for smokeless tobacco cessation, not smoking cessation |
| Gold 2002 | Bupropion ‐ non‐random assignment, participant preference |
| Grandi 2011 | Bupropion ‐ not RCT, review of bupropion use in patients with CVD |
| Grassi 2009 | Not a RCT, pre‐post study of influence of smoking ban on people's selection of smoking cessation treatment |
| Hall 2009 | Bupropion ‐ all participants received bupropion for quitting, test of extended CBT or NRT |
| Hall 2011 | Previously included. Relapse prevention study. See Livingstone‐Banks 2019 |
| Hatsukami 2004 | Previously included. Harm reduction study. See Lindson‐Hawley 2016 |
| Hawk 2008 | Bupropion ‐ short follow‐up (12 weeks). Compares 1 week to 4 week pre‐quit use |
| Hawk 2015 | Interventions not matched ‐ same intervention post‐quit date |
| Hays 2001 | Previously included. Relapse prevention study. See Livingstone‐Banks 2019 |
| Hays 2009 | Previously included. Relapse prevention study. See Livingstone‐Banks 2019 |
| Hilberink 2005 | Bupropion ‐ test of NRT + counselling, one cluster received bupropion but is not a test of bupropion |
| Hitsman 1999 | Fluoxetine ‐ the majority of participants in this study were also part of the multicentre trial reported in Niaura 2002 |
| Houtsmuller 2002 | Selegiline ‐ short‐term laboratory study |
| Hurt 2003 | Previously included. Relapse prevention study. See Livingstone‐Banks 2019 |
| Hussain 2010 | Bupropion ‐ short follow‐up, trial in unmotivated smokers |
| Isgro 2015 | Topiramate not an antidepressant |
| Jacobs 1971 | Imipramine ‐ short follow‐up. Outcome was reduction in smoking to less than 10% of baseline |
| Kalman 2004 | Bupropion ‐ short follow‐up (12 weeks) |
| Khunrong 2016 | Ineligible outcomes |
| Killen 2006 | Previously included. Relapse prevention study. See Livingstone‐Banks 2019 |
| Kotz 2009 | Nortriptyline ‐ pharmacotherapy was confounded with additional counselling from nurse (control group 1), compared to usual care |
| Kras 2010 | St John's wort ‐ short follow‐up |
| Lawvere 2006 | St John's wort ‐ uncontrolled study |
| Li 2009 | Bupropion ‐ short follow‐up |
| Miller 2003 | Bupropion ‐ short follow‐up (8 weeks) |
| Monuteaux 2007 | Bupropion ‐ participants were adolescent non‐smokers, not for cessation |
| Mooney 2008 | Bupropion ‐ short follow‐up, bupropion for opioid and tobacco dependence |
| Mooney 2016 | Bupropion same in both arms |
| Naranjo 1990 | Fluoxetine ‐ study of short‐term smoking behaviour |
| NCT00032084 | Trial terminated before completion |
| NCT00119210 | Trial terminated before completion |
| NCT00136747 | Smoking cessation not measured |
| NCT00136786 | Smoking cessation not measured |
| NCT00158171 | Cessation not measured ‐ harm reduction study |
| NCT00248118 | Bupropion ‐ trial was terminated prior to completion |
| NCT00320697 | Pharmacotherpaies not matched |
| NCT00390923 | Selegiline ‐ study terminated early due to lack of efficacy, results available at 9 weeks only |
| NCT00484692 | Bupropion ‐ used as an active control to a psychosocial intervention, cannot estimate pharmacotherapy effect |
| NCT00580853 | Does not measure smoking cessation ‐ ability to resist smoking |
| NCT00670904 | No randomization ‐ participants chose their medication |
| NCT00936299 | Bupropion ‐ no abstinence outcome reported and follow‐up only 16 weeks |
| NCT01850589 | Behavioural intervention and pharmacotherapy is different between arms |
| NCT01965405 | All participants in all arms receive the same bupropion treatment |
| NCT02736474 | Both naltrexone and bupropion given together in same arm |
| NCT03471767 | Bupropion given in both arms |
| NCT03920319 | Wrong outcomes |
| Neumann 2000 | Bupropion ‐ smokers randomized to 1 or 2 months of medication (300 mg/day). 91/165 randomized were not included in the analysis, including some 1‐month group participants who requested further medication. |
| Neumann 2002 | Bupropion ‐ short‐term follow‐up. Comparison of 300 mg and 150 mg doses |
| Niederhofer 2004 | Participants are required to be abstinent for at least five days prior to enrolment to trial |
| Olmstead 1999 | Bupropion ‐ all participants received bupropion. Short‐term follow‐up |
| Paluck 2006 | Bupropion ‐ uncontrolled prospective observational study |
| Pomerleau 1991 | Fluoxetine ‐ no cessation data reported |
| Raynor 2005 | Bupropion ‐ short (90 day) follow‐up. Substudy within a larger trial with long‐term follow‐up, not yet published |
| Robinson 1991 | Buspirone ‐ case series |
| Rovina 2003 | Bupropion ‐ abstract only, trial report not available. Insufficient information to determine inclusion |
| Schepis 2006 | Bupropion ‐ abstract only, trial report not available. Insufficient information to determine inclusion |
| Sellers 1987 | Zimelidine or citalopram (SSRIs) ‐ placebo‐controlled cross‐over design study of smoking behaviour and alcohol use in non‐depressed heavy drinkers |
| Sherman 2008 | Bupropion ‐ trial of NRT as adjunct to bupropion |
| Shiffman 2000 | Bupropion ‐ placebo‐controlled short‐term study of effects on craving and withdrawal in participants not wanting to quit smoking permanently |
| Shoptaw 2008 | Bupropion ‐ tested for methamphetamine dependence. Reduction in smoking was a secondary outcome. Only 48/73 participants smoked, quitting not reported. |
| Sittipunt 2007 | Nortriptyline ‐ only 3‐month follow‐up |
| Sonntag 2003 | Bupropion ‐ abstract only, trial report not available. Insufficient information to determine inclusion |
| Spring 1995 | Fluoxetine ‐ 6‐month cessation not reported. Primarily a study of post‐cessation weight gain |
| Stein 1993 | Fluoxetine ‐ does not report outcomes from a double‐blind study |
| Steinberg 2009 | Bupropion ‐ confounded with nicotine inhaler and treatment duration in comparison with nicotine patch alone |
| Strayer 2004 | Bupropion ‐ all participants prescribed bupropion. Test of behavioural interventions, not bupropion. Adverse event data from author used |
| Swanson 2003 | Bupropion +/‐ nicotine patch. Unable to confirm correct denominators |
| Tidey 2009 | Bupropion ‐ laboratory study, outcomes included urge to smoke, not cessation |
| Toll 2007 | Bupropion ‐ all participants had same pharmacotherapy |
| Weinberger 2008 | Bupropion for people with bipolar disorder. Short follow‐up (8 weeks). Only 5 participants |
| Weiner 2001 | Bupropion ‐ no control group |
| Winhusen 2012 | Bupropion confounded by other agents |
| Zernig 2008 | Bupropion ‐ used as an active control to a psychosocial intervention, cannot estimate pharmacotherapy effect |
| ZYB30011 | Bupropion ‐ follow‐up only to end of treatment (7 weeks) |
CBT: cognitive behavioural therapy; NRT: nicotine replacement therapy; CVD: cardiovascular disease; RCT: randomized controlled trial; SSRI: selective serotonin reuptake inhibitor
Characteristics of ongoing studies [ordered by study ID]
NCT03326128.
| Study name | High dose bupropion for smoking cessation |
| Methods | Triple‐blind randomized trial |
| Participants | 300 heavy smokers who also experience psychiatric symptoms |
| Interventions |
Common components: standard smoking cessation counselling for 8 weeks |
| Outcomes |
|
| Starting date | May 2019 |
| Contact information | Lauren Whitted, 323‐442‐1197, lwhitted@usc.edulwhitted@usc.edu |
| Notes |
NCT03342027.
| Study name | Smoking cessation interventions for people living with HIV in Nairobi, Kenya |
| Methods | 2 x 2 factorial, double‐blind randomized controlled trial |
| Participants | 300 participants people living with HIV, who smoke and who are receiving care in a methadone maintenance program will be randomized |
| Interventions |
|
| Outcomes | Smoking cessation: 7‐day point prevalence abstinence at 36 weeks. Validated by expired CO < 7 ppm |
| Starting date | 20 August 2019 |
| Contact information | Wendy Potts, (410) 706‐2490, wpotts@som.umaryland.edu |
| Notes |
Zawertailo 2018.
| Study name | The Medication Aids for Tobacco Cessation and Health (MATCH) Study |
| Methods | Randomized, open‐label, parallel‐arm trial |
| Participants | 968 smokers motivated to quit |
| Interventions |
Common components: weekly motivational emails |
| Outcomes | Smoking cessation: continuous abstinence at 52 weeks. Validated by saliva cotinine |
| Starting date | 1 May 2014 |
| Contact information | Laurie Zawertailo, 1 416 535 8501 ext 77422, laurie.zawertailo@camh.ca |
| Notes |
TQD: target quit date
Differences between protocol and review
The changes below were made for the 2020 update.
We no longer include harm reduction and relapse prevention studies as these are covered in other reviews (Lindson‐Hawley 2016; Livingstone‐Banks 2019).
We changed the wording of the primary outcome from smoking abstinence to smoking cessation. These terms measure the same thing however we feel that the latter term makes it clearer that we are measuring the act of quitting smoking.
We have specified exactly which safety and tolerability outcomes were assessed as follows: 1) adverse events (AEs), 2) serious adverse events (SAEs), and 3) dropouts due to AEs, and also collected information on the following specific SAEs: seizures; overdoses; suicide attempts; death by suicide; and all‐cause mortality. We went back to all previously included studies to check that these were extracted uniformly across studies.
We explicitly state that we investigated whether studies had investigated depression status as a modifier of efficacy. This has been extracted uniformly across studies and the information is now summarized in Table 5.
Any observational studies are now excluded from this review. There were previously included to assess safety outcomes.
We have explicitly stated that "We excluded trials where an additional, uncontrolled non‐antidepressant intervention component was used in only one of the trial arms" and checked that included studies conform to this requirement.
We no longer assess the outcome: reduction in smoking, as this is not deemed to be a clinically‐relevant outcome ‐ there is no evidence that it results in health benefits, and studies that aim specifically to reduce smoking are covered in our harm reduction review (Lindson‐Hawley 2016).
We restructured our 'Summary of findings' tables to include the most clinically‐relevant comparators and to include safety outcomes as well as efficacy.
We carry out sensitivity analyses, excluding studies from meta‐analyses with industry funding, or where the medication was supplied by the pharmaceutical industry. We judged whether this exclusion notably altered the pooled risk ratios (RRs) (95% confidence interval (CI)) and summarized the results in Table 4.
We carried out a post hoc, exploratory analysis merging the following safety and tolerability outcome data: AEs, psychiatric AEs, SAEs and dropouts due to drug, across three comparisons, that effectively all compared bupropion to no bupropion treatment (1) bupropion versus placebo/no pharmacotherapy control; 2) bupropion plus nicotine replacement therapy (NRT) versus NRT; 3) bupropion plus varenicline versus varenicline). We carried out a subgroup analysis to test for any interactions between comparisons.
Contributions of authors
For the most recent update NL, JHB and SH decided on changes to analyses and the presentation of the review. SH updated the text of the review and all other authors commented. SH, NL, JHB, JLB screened and extracted study data, and BH also extracted study data.
Sources of support
Internal sources
-
Nuffiled Department of Primary Care Health Sciences, University of Oxford, UK
Editorial base for Cochrane Tobacco Addiction
External sources
-
National Institute for Health Research, UK
Infrastructure funding for Cochrane Tobacco Addiction
-
Research England’s Strategic Priorities Fund (SPF), UK
Funding to carry out this particular Cochrane Tobacco Addiction Review
Declarations of interest
SH: none reported
JHB: none reported
JLB: none reported
BH: none reported
NL: none reported
Edited (no change to conclusions)
References
References to studies included in this review
Ahluwalia 2002 {published data only}
- Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA 2002;288:468-74. [DOI] [PubMed] [Google Scholar]
- Boardman T, Catley D, Mayo MS, Ahluwalia JS. Self-efficacy and motivation to quit during participation in a smoking cessation program. International Journal of Behavioral Medicine 2005;12:266-72. [DOI] [PubMed] [Google Scholar]
- Catley D, Harris KJ, Okuyemi KS, Mayo MS, Pankey E, Ahluwalia JS. The influence of depressive symptoms on smoking cessation among African Americans in a randomized trial of bupropion. Nicotine & Tobacco Research 2005;7(6):859-70. [DOI] [PubMed] [Google Scholar]
- Harris KJ, Ahluwalia JS, Catley D, Okuyemi KS, Mayo MS, Resnicow K. Successful recruitment of minorities into clinical trials: the Kick It at Swope project. Nicotine & Tobacco Research 2003;5:575-84. [DOI] [PubMed] [Google Scholar]
- Harris KJ, Okuyemi KS, Catley D, Mayo MS, Ge B, Ahluwalia JS. Predictors of smoking cessation among African-Americans enrolled in a randomized controlled trial of bupropion. Preventive Medicine 2004;38:498-502. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Ahluwalia JS, Ebersole Robinson M, Catley D, Mayo MS, Resnicow K. Does menthol attenuate the effect of bupropion among African American smokers? Addiction 2003;98:1387-93. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Guo H, Lynam IM, Powell JN, Okuyemi KS, Bronars CA, et al. The impact of perceived treatment assignment on smoking cessation outcomes among African-American smokers. Journal of General Internal Medicine 2008;23(9):1361-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AZ, Zhou Q, Cox LS, David SP, Ahluwalia JS, Benowitz NL, et al. Association of CHRNA5-A3-B4 SNP rs2036527 with smoking cessation therapy response in African-American smokers. Clinical Pharmacology and Therapeutics 2014;96(2):256-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anthenelli 2016 {published data only}
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387(10037):2507-20. [DOI] [PubMed] [Google Scholar]
- Anthenelli RM, Gaffney M, Benowitz NL, West R, McRae T, Russ C, et al. Predictors of neuropsychiatric adverse events with smoking cessation medications in the randomized controlled EAGLES Trial. Journal of General Internal Medicine 2019;34(6):862-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CL, Pietri G. A cost-effectiveness analysis of varenicline for smoking cessation using data from the EAGLES trial. ClinicoEconomics and Outcomes Research 2018;10:67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Pipe A, West R, Hays JT, Tonstad S, McRae T, et al. Cardiovascular safety of varenicline, bupropion, and nicotine patch in smokers: a randomized clinical trial. JAMA Internal Medicine 2018;178(5):622-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EudraCT 2010-022914-15. A phase 4, randomized, double-blind, active and placebo -controlled, multicenter study evaluating the neuropsychiatric safety and efficacy of 12 weeks varenicline tartrate 1mg bid and bupropion hydrochloride 150mg bid for smoking cessation in subjects with and without a history of psychiatric disorders. clinicaltrialsregister.eu/ctr-search/search?query=2010-022914-15 (first received 14 October 2011).
- Evins AE, Benowitz NL, West R, Russ C, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with psychotic, anxiety, and mood disorders in the EAGLES Trial. Journal of Clinical Psychopharmacology 2019;39(2):108-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01456936. Study evaluating the safety and efficacy of varenicline and bupropion for smoking cessation in subjects with and without a history of psychiatric disorders. clinicaltrials.gov/ct2/show/NCT01330030 (first received 21 October 2011).
- NCT01574703. Study to evaluate cardiac assessments following different treatments of smoking cessation medications in subjects with and without psychiatric disorders. clinicaltrials.gov/ct2/show/NCT01574703 (first received 10 April 2012).
- West R, Evins AE, Benowitz NL, Russ C, McRae T, Lawrence D, et al. Factors associated with the efficacy of smoking cessation treatments and predictors of smoking abstinence in EAGLES. Addiction 2018;113(8):1507-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C, Oskooilar N, Guevarra K, Linh Tong M, Grosz D, Morrissey J, et al. A double-blind, active-and placebo-controlled evaluation of the neuropsychiatric safety and efficacy of varenicline and bupropion for smoking cessation in subjects with (pre-existing) psychiatric disorders: an objective blinded analysis. Neuropsychopharmacology 2015;40:S106-271. [Google Scholar]
Aubin 2004 {published data only}
- Aubin HJ, Lebargy F, Berlin I, Bidaut-Mazel C, Chemali-Hudry J, Lagrue G. Efficacy of bupropion and predictors of successful outcome in a sample of French smokers: a randomized placebo-controlled trial. Addiction 2004;99:1206-18. [DOI] [PubMed] [Google Scholar]
- Lebargy F, Aubin HJ, Lagrue G, Bidaut-Mazel C, Chemali-Hudry J, Poulain L. A placebo-controlled, double-blind study of Zyban LP: An effective and well-tolerated aid to smoking cessation - preliminary results (POS4-69). In: Society for Research on Nicotine and Tobacco 9th Annual Meeting; 2003 February 19-22; New Orleans (LA). 2003.
Aveyard 2008 {published data only}
- Aveyard P, Johnson C, Fillingham S, Parsons A, Murphy M. Nortriptyline plus nicotine replacement versus placebo plus nicotine replacement for smoking cessation: pragmatic randomised controlled trial. BMJ 2008;336(7655):1223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveyard P, Johnson C, Murphy M, Johnstone E, Walton R, Fillingham S, et al. A pragmatic randomised controlled trial to test the efficacy of nortriptyline plus nicotine replacement therapy (NRT) versus a placebo plus NRT in helping smokers to stop and testing the role of noradrenergic and dopaminergic genetic variants in smoking cessation [PI-TS-02]. In: Society for Research on Nicotine and Tobacco 8th European Meeting; 2006 September 23-26; Kusadasi, Turkey. 2006.
Barnes 2006 {published data only}
- Barnes J, Barber N, Wheatley D, Williamson EM. A pilot randomised, open, uncontrolled, clinical study of two dosages of St John's wort (Hypericum perforatum) herb extract (LI-160) as an aid to motivational/behavioural support in smoking cessation. Planta Medica 2006;72(4):378-82. [DOI] [PubMed] [Google Scholar]
Benli 2017 {published data only}
- Benli AR, Erturhan S, Oruc MA, Kalpakci P, Sunay D, Demirel Y. A comparison of the efficacy of varenicline and bupropion and an evaluation of the effect of the medications in the context of the smoking cessation programme. Tobacco Induced Diseases 2017;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berlin 1995 {published data only}
- Berlin I, Said S, Spreux Varoquaux O, Launay JM, Olivares R, Millet V, et al. A reversible monoamine oxidase A inhibitor (moclobemide) facilitates smoking cessation and abstinence in heavy, dependent smokers. Clinical Pharmacology and Therapeutics 1995;58:444-52. [DOI] [PubMed] [Google Scholar]
- Berlin I, Spreux Varoquaux O, Said S, Launay JM. Effects of past history of major depression on smoking characteristics, monoamine oxidase-A and -B activities and withdrawal symptoms in dependent smokers. Drug and Alcohol Dependence 1997;45:31-7. [DOI] [PubMed] [Google Scholar]
Berlin 2002 {published data only}
- Berlin I, Aubin HJ, Pedarriosse AM, Rames A, Lancrenon S, Lagrue G. Lazabemide, a selective, reversible monoamine oxidase B inhibitor, as an aid to smoking cessation. Addiction 2002;97:1347-54. [DOI] [PubMed] [Google Scholar]
Berlin 2012 {published data only}
- Berlin I, Hunneyball IM, Greiling D, Jones SP, Fuder H, Stahl HD. A selective reversible monoamine oxidase B inhibitor in smoking cessation: effects on its own and in association with transdermal nicotine patch. Psychopharmacology 2012;223(1):89-98. 9400123000015756 [DOI] [PubMed] [Google Scholar]
Biberman 2003 {published data only}
- Biberman R, Neumann R, Katzir I, Gerber Y. A randomized controlled trial of oral selegiline plus nicotine skin patch compared with placebo plus nicotine skin patch for smoking cessation. Addiction 2003;98:1403-7. [DOI] [PubMed] [Google Scholar]
Blondal 1999 {published data only}
- Blondal T, Gudmundsson LJ, Tomasson K, Jonsdottir D, Hilmarsdottir H, Kristjansson F, et al. The effects of fluoxetine combined with nicotine inhalers in smoking cessation - a randomized trial. Addiction 1999;94:1007-15. [DOI] [PubMed] [Google Scholar]
Brown 2007 {published data only}
- Abrantes AM, Strong DR, Lloyd-Richardson EE, Niaura R, Kahler CW, Brown RA. Regular exercise as a protective factor in relapse following smoking cessation treatment. American Journal on Addictions 2009;18(1):100-1. [DOI] [PubMed] [Google Scholar]
- Brown RA, Niaura R, Lloyd-Richardson EE, Strong DR, Kahler CW, Abrantes AM, et al. Bupropion and cognitive-behavioral treatment for depression in smoking cessation. Nicotine & Tobacco Research 2007;9:721-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Brown RA, Papandonatos GD, Kahler CW, Lloyd-Richardson EE, Munafo MR, et al. Pharmacogenetic clinical trial of sustained-release bupropion for smoking cessation. Nicotine & Tobacco Research 2007;9:821-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Niaura R, Papandonatos GD, Shadel WG, Burkholder GJ, Britt DM, et al. Does the DRD2-Taq1 A polymorphism influence treatment response to bupropion hydrochloride for reduction of the nicotine withdrawal syndrome? Nicotine & Tobacco Research 2003;5:935-42. [DOI] [PubMed] [Google Scholar]
- David SP, Strong DR, Leventhal AM, Lancaster MA, McGeary JE, Munafo MR, et al. Influence of a dopamine pathway additive genetic efficacy score on smoking cessation: results from two randomized clinical trials of bupropion. Addiction 2013;108(12):2202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Strong DR, Munafo MR, Brown RA, Lloyd-Richardson EE, Wileyto PE, et al. Bupropion efficacy for smoking cessation is influenced by the DRD2 Taq1A polymorphism: Analysis of pooled data from two clinical trials. Nicotine & Tobacco Research 2007;9(12):1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, David SP, Brightman M, Strong D, McGeary JE, Brown RA, et al. Dopamine D4 receptor gene variation moderates the efficacy of bupropion for smoking cessation. Pharmacogenomics Journal 2012;12(1):86-92. 9400123000012694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wileyto EP, Heitjan DF. Modeling smoking cessation data with alternating states and a cure fraction using frailty models. Statistics in Medicine 2010;29(6):627-38. 9400123000005712 [DOI] [PMC free article] [PubMed]
- Okun ML, Levine MD, Houck P, Perkins KA, Marcus MD. Subjective sleep disturbance during a smoking cessation program: associations with relapse. Addictive Behaviors 2011;36(8):861-4. 9400123000011571 [9400123000011571] [DOI] [PMC free article] [PubMed]
- Strong DR, Kahler CW, Leventhal AM, Abrantes AM, Lloyd-Richardson E, Niaura R, et al. Impact of bupropion and cognitive-behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine & Tobacco Research 2009;11(10):1142-53. 9400123000005451 [DOI] [PMC free article] [PubMed]
- Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE, et al. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Archives of General Psychiatry 2008;65(6):683-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2014 {published data only}
- Brown RA, Abrantes AM, Strong DR, Niaura R, Kahler CW, Miller IW, et al. Efficacy of sequential use of fluoxetine for smoking cessation in elevated depressive symptom smokers. Nicotine & Tobacco Research 2014;16(2):197-207. [DOI] [PubMed] [Google Scholar]
- Brown RA, Strong DR, Abrantes AM, Miller IW, Kahler CW, Niaura R, et al. Efficacy of sequential use of fluoxetine for smoking cessation in elevated depressive symptom smokers. Society for Research on Nicotine & Tobacco 17th Annual Meeting; 2011 February 16-19; Toronto 2011:28S. 9400123000006076 [DOI] [PubMed]
- Brown RA, Strong DR, Miller IW, Kahler CW, Niaura R, Price LH. Efficacy of sequential vs. concurrent use of fluoxetine in smoking cessation for elevated depressive symptom smokers (SYM8D). In: Society for Research on Nicotine and Tobacco 13th Annual Meeting; 2007 February 21-24, Austin (TX). 2007.
- Minami H, Kahler CW, Bloom EL, Strong DR, Abrantes AM, Zywiak WH, et al. Effects of depression history and sex on the efficacy of sequential versus standard fluoxetine for smoking cessation in elevated depressive symptom smokers. Addictive Disorders & Their Treatment 2015;14(1):29-39. [Google Scholar]
Cinciripini 2005 {published data only}
- Cinciripini PM, Tsoh JY, Friedman K, Wetter D, Cinciripini LG, Skaar KL. A placebo controlled evaluation of venlafaxine for smoking cessation: preliminary findings [Abstract A18]. In: Society for Research on Nicotine and Tobacco Annual Meeting; 1998 Mar 27-29; New Orleans (LA). 1998.
- Cinciripini PM, Tsoh JY, Wetter DW, Lam C, Moor C, Cinciripini L, et al. Combined effects of venlafaxine, nicotine replacement, and brief counseling on smoking cessation. Experimental and Clinical Psychopharmacology 2005;13:282-92. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Wetter D, Minna J, Tsoh JY, Gritz ER, Baile WF. The effects of brief counseling, transdermal nicotine replacement and antidepressant therapy on smoking cessation among smokers carrying the DRD2 A1 allele (PA3A). In: Society for Research on Nicotine and Tobacco Fifth Annual Meeting; Mar 5-7 1999; San Diego, California. 1999.
- Cinciripini PM, Wetter DW, Tomlinson GE, Tsoh JY, Moor CA, Cinciripini LG, et al. The effects of the DRD2 polymorphism on smoking cessation and negative affect: Evidence for a pharmacogenetic effect on mood. Nicotine & Tobacco Research 2004;6:229-39. [DOI] [PubMed] [Google Scholar]
Cinciripini 2013 {published data only}
- Cinciripini PM, Green CE, Robinson JD, Karam-Hage M, Engelmann JM, Minnix JA, et al. Benefits of varenicline vs. bupropion for smoking cessation: a Bayesian analysis of the interaction of reward sensitivity and treatment. Psychopharmacology 2017;234(11):1769-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, et al. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry 2013;70(5):522-33. 9400107000000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Engelmann JM, Xian J, Minnix JA, Lam CY, Karam-Hage M, et al. Pharmacological intervention and abstinence in smokers undergoing cessation treatment: a psychophysiological study. International Journal of Psychophysiology 2018;123:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Stevens EM, Robinson JD, Cui Y, Deweese MM, Engelmann JM, et al. Brain responses to cigarette-related and emotional images in smokers during smoking cessation: no effect of varenicline or bupropion on the late positive potential. Nicotine & Tobacco Research 2017;21(2):234-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cinciripini 2018 {published data only}
- Cinciripini PM, Minnix JA, Green CE, Robinson JD, Engelmann JM, Versace F, et al. An RCT with the combination of varenicline and bupropion for smoking cessation: clinical implications for front line use. Addiction 2018;113:1673-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2004 {published data only}
- Bergen AW, Javitz HS, Su L, He Y, Conti DV, Benowitz NL, et al. The DRD4 Exon III VNTR, Bupropion, and Associations With Prospective Abstinence. Nicotine & Tobacco Research 2013;15(7):1190-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini WH, Wileyto EP, Epstein L, Restine S, Hawk L, Shields P, et al. Catechol-O-methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biological Psychiatry 2007;61(1):111-8. [DOI] [PubMed] [Google Scholar]
- Collins B, Wileyto P, Patterson F, Rukstalis M, Audrain-McGovern J, Kaufmann V, et al. Gender differences in smoking cessation in a placebo-controlled trial of bupropion with behavioral counseling. Nicotine & Tobacco Research 2004;6:27-37. [DOI] [PubMed] [Google Scholar]
- Conti DV, Lee W, Li D, Liu J, Van Den BD, Thomas PD, et al. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems-based candidate gene study of smoking cessation. Human Molecular Genetics 2008;17(18):2834-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Strong DR, Leventhal AM, Lancaster MA, McGeary JE, Munafo MR, et al. Influence of a dopamine pathway additive genetic efficacy score on smoking cessation: results from two randomized clinical trials of bupropion. Addiction 2013;108(12):2202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Strong DR, Munafo MR, Brown RA, Lloyd-Richardson EE, Wileyto PE, et al. Bupropion efficacy for smoking cessation is influenced by the DRD2 Taq1A polymorphism: Analysis of pooled data from two clinical trials. Nicotine & Tobacco Research 2007;9(12):1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AB, Wileyto EP, Jepson C, Lori A, Cubells JF, Lerman C. Galanin receptor 1 (GALR1) SNP is associated with craving and smoking relapse. Neuropsychopharmacology 2011;36:S378. 9400123000012457 [9400123000012457]
- Gold AB, Wileyto EP, Lori A, Conti D, Cubells JF, Lerman C. Pharmacogenetic association of the galanin receptor (GALR1) SNP rs2717162 with smoking cessation. Neuropsychopharmacology 2012;37(7):1683-8. 9400123000014652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Redden DT, Berrettini WH, Shields PG, Restine SL, Pinto A, et al. No evidence for a major role of polymorphisms during bupropion treatment. Obesity 2006;14(11):1863-7. [DOI] [PubMed] [Google Scholar]
- Javitz HS, Swan GE, Lerman C. The dynamics of the urge-to-smoke following smoking cessation via pharmacotherapy. Addiction 2011;106(10):1835-45. 9400123000011869 [DOI] [PubMed]
- Lee AM, Jepson C, Hoffmann E, Epstein L, Hawk LW, Lerman C, et al. CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biological Psychiatry 2007;62(6):635-41. [DOI] [PubMed] [Google Scholar]
- Lee W, Bergen AW, Swan GE, Li D, Liu J, Thomas P, et al. Gender-stratified gene and gene-treatment interactions in smoking cessation. Pharmacogenomics Journal 2012;12(6):521-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology 2006;31:231-42. [DOI] [PubMed] [Google Scholar]
- Lerman C, Niaura R, Collins BN, Wileyto P, Audrain MJ, Pinto A, et al. Effect of bupropion on depression symptoms in a smoking cessation clinical trial. Psychology of Addictive Behaviors 2004;18:362-6. [DOI] [PubMed] [Google Scholar]
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu AY, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug and Alcohol Dependence 2002;67:219-23. [DOI] [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH Jr, Pinto A, et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychology 2003;22:541-8. [DOI] [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Pinto A, Hawk L, et al. Pharmacogenetic investigation of smoking cessation treatment. Pharmacogenetics 2002;12:627-34. [DOI] [PubMed] [Google Scholar]
- Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clinical Pharmacology & Therapeutics 2008;84:320-5. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Epstein L, Audrain J, Niaura R, Hawk L, Shields PG, et al. Can the blind see? Participant guess about treatment arm assignment may influence outcome in a clinical trial of bupropion for smoking cessation. Journal of Substance Abuse Treatment 2008;34(2):234-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE, et al. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Archives of General Psychiatry 2008;65(6):683-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileyto EP, Patterson F, Niaura R, Epstein LH, Brown RA, Audrain-McGovern J, et al. Recurrent event analysis of lapse and recovery in a smoking cessation clinical trial using bupropion. Nicotine & Tobacco Research 2005;7:257-68. [DOI] [PubMed] [Google Scholar]
- Wileyto P, Patterson F, Niaura R, Epstein L, Brown R, Audrain-McGovern J, et al. Do small lapses predict relapse to smoking behavior under bupropion treatment? Nicotine & Tobacco Research 2004;6:357-66. [DOI] [PubMed] [Google Scholar]
Covey 2002 {published data only}
- Berlin I, Chen H, Covey LS. Depressive mood, suicide ideation and anxiety in smokers who do and smokers who do not manage to stop smoking after a target quit date. Addiction 2010;105(12):2209-16. 9400123000005900 [DOI] [PubMed]
- Covey LS, Glassman AH, Stetner F, Rivelli S, Stage K. A randomized trial of sertraline as a cessation aid for smokers with a history of major depression. American Journal of Psychiatry 2002;159:1731-7. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F, Rivelli S. A trial of sertraline for smokers with past major depression. Society for Research on Nicotine and Tobacco Meeting. Arlington, VA (www.srnt.org/events/abstracts99/index.htm) 2000.
- NCT00063323. Maintenance treatment for abstinent smokers. clinicaltrials.gov/ct2/show/NCT00063323 (first received 1 July 2003).
Cox 2012 {published data only}
- Berg CJ, Cox LS, Choi WS, Mayo MS, Krebill R, Bronars CA, et al. Assessment of depression among African American light smokers. Journal of Health Psychology 2012;17(2):197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TS, Cox LS, Nollen NL, Thomas JL, Berg CJ, Mayo MS, et al. Perceived treatment assignment and smoking cessation in a clinical trial of bupropion. Cancer Epidemiology, Biomarkers & Prevention 2011;20(4):721. 9400123000011648 [Google Scholar]
- Buchanan TS, Sanderson Cox L, Thomas JL, Nollen NL, Berg CJ, Mayo MS, et al. Perceived treatment assignment and smoking cessation in a clinical trial of bupropion versus placebo. Nicotine & Tobacco Research 2013;15(2):567-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Faseru B, Mayo MS, Krebill R, Snow TS, Bronars CA, et al. Design, baseline characteristics, and retention of African American light smokers into a randomized trial involving biological data. Trials 2011;12:22. 9400123000005878 [DOI] [PMC free article] [PubMed]
- Cox LS, Nollen NL, Mayo MS, Choi WS, Faseru B, Benowitz NL, et al. Bupropion for smoking cessation in African American light smokers: a randomized controlled trial. Journal of the National Cancer Institute 2012;104(4):290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faseru B, Choi WS, Krebill R, Mayo MS, Nollen NL, Okuyemi KS, et al. Factors associated with smoking menthol cigarettes among treatment-seeking African American light smokers. Addictive Behaviors 2011;36(12):1321-4. 9400123000011663 [DOI] [PMC free article] [PubMed]
- Faseru B, Nollen NL, Mayo MS, Krebill R, Choi WS, Benowitz NL, et al. Predictors of cessation in African American light smokers enrolled in a bupropion clinical trial. Addictive Behaviors 2013;38(3):1796-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen NL, Mayo MS, Ahluwalia JS, Tyndale RF, Benowitz NL, Faseru B, et al. Factors associated with discontinuation of bupropion and counseling among African American light smokers in a randomized clinical trial. Annals of Behavioral Medicine 2013;46(3):336-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AZ, Cox LS, Nollen N, Faseru B, Okuyemi KS, Ahluwalia JS, et al. CYP2B6 and bupropion's smoking-cessation pharmacology: the role of hydroxybupropion. Clinical Pharmacology and Therapeutics 2012;92(6):771-7. 9400123000017581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AZ, Zhou Q, Cox LS, David SP, Ahluwalia JS, Benowitz NL, et al. Association of CHRNA5-A3-B4 SNP rs2036527 with smoking cessation therapy response in African-American smokers. Clinical Pharmacology and Therapeutics 2014;96(2):256-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTRI/2013/07/003830 {published data only}
- CTRI/2013/07/003830. A study to evaluate different strategies-(medicine, enhanced counselling, standard counselling) for stopping smoking in TB patients in TB program in India. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2013/07/003830 (first received 23 July 2013).
Da Costa 2002 {published data only}
- da Costa C, Younes R, Lourenco M. Smoking cessation: A randomized double-blind study comparing nortriptyline to placebo [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A354. [Google Scholar]
- da Costa CL, Younes RN, Lourenco MT-C. Stopping smoking: a prospective, randomized, double-blind study comparing nortriptyline to placebo. Chest 2002;122:403-8. [DOI] [PubMed] [Google Scholar]
Dalsgarð 2004 {published data only}
- Dalsgarð OJ, Hansen NC, Søes-Petersen U, Evald T, Høegholm A, Barber J, et al. A multicenter, randomized, double-blind, placebo-controlled, 6-month trial of bupropion hydrochloride sustained-release tablets as an aid to smoking cessation in hospital employees. Nicotine & Tobacco Research 2004;6:55-61. [DOI] [PubMed] [Google Scholar]
- Dalsgarð OJ, Vestbo J. A multicenter, randomised, double-blind, placebo-controlled 6 month trial to evaluate efficacy and tolerability of bupropion hydrochloride sustained release (SR) tablets as treatment for nicotine dependency in healthcare workers and as an aid to smoking cessation (ZYB30009). Poster and oral presentation. In: European Congress on Tobacco or Health; 2002 June 20-22; Warsaw, Poland. 2002.
Ebbert 2014 {published data only}
- Ebbert JO, Hatsukami DK, Croghan IT, Schroeder DR, Allen SS, Hays JT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA 2014;311(2):155-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong AS, Elrashidi MY, Schroeder DR, Ebbert JO. Depressive symptoms among patients receiving varenicline and bupropion for smoking cessation. Journal of Substance Abuse Treatment 2015;52:78-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenberg 2013 {published data only}
- Benowitz NL, Prochaska JJ. Smoking cessation after acute myocardial infarction. Journal of the American College of Cardiology 2013;61(5):533-5. 9400123000017824 [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Grandi SM, Gervais A, Joseph L, O'Loughlin J, Paradis G, et al. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: A randomized, placebo-controlled trial. Canadian Journal of Cardiology 2011;27(5 Suppl 1):S344. 9400123000012454 [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Grandi SM, Gervais A, O'Loughlin J, Paradis G, Rinfret S, et al. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: A randomized, placebo-controlled trial. Journal of the American College of Cardiology 2013;61(5):524-32. 9400123000017546 [DOI] [PubMed] [Google Scholar]
- Filion KB, Grandi SM, Joseph L, O'Loughlin J, Paradis G, Pilote L, et al. The effect of bupropion on symptoms of depression among patients attempting to quit smoking post-myocardial infarction: the zesca trial. Circulation 2012;125 Suppl 10:P090. [Google Scholar]
- Grandi S, Filion K, Gervais A, Joseph L, O'Loughlin J, Paradis G, et al. The effect of adherence to treatment on smoking abstinence in patients post-acute myocardial infarction. In: 61th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, ACC.12; 2012 March 24-27; Chicago (IL). 2012:E1749.
- Grandi SM, Eisenberg MJ, Joseph L, O'Loughlin J, Paradis G, Filion KB. Cessation treatment adherence and smoking abstinence in patients after acute myocardial infarction. American Heart Journal 2016;173:35-40. [DOI] [PubMed] [Google Scholar]
- Grandi SM, Filion KB, Gervais A, Joseph L, O'Loughlin J, Paradis G, et al. The effect of smoking cessation on weight at 12 months in patients post-myocardial infarction [abstract]. In: Circulation. Vol. 10 Suppl. 2012:P090. 9400123000018293
- Grandi SM, Filion KB, Gervais A, Joseph L, O'Loughlin J, Paradis G, et al. The effect of treatment adherence on smoking abstinence in patients post-acute myocardial infarction. Journal of Population Therapeutics and Clinical Pharmacology 2012;19(2):123-4. [Google Scholar]
- Grandi SM, Filion KB, Gervais A, Joseph L, O'Loughlin J, Paradis G, et al. Weight change in patients attempting to quit smoking post-myocardial infarction. American Journal of Medicine 2014;127(7):641-9. [DOI] [PubMed] [Google Scholar]
- Grandi SM, Filion KB, Joseph L, O'Loughlin J, Pilote L, Eisenberg MJ. Baseline predictors of relapse to smoking at 12 months in patients post-myocardial infarction. Canadian Journal of Cardiology 2013;29(10 Suppl 1):S257. [Google Scholar]
- Shimony A, Grandi SM, Pilote L, Joseph L, O'Loughlin J, Paradis G, et al. Utilization of evidence-based therapy for acute coronary syndrome in high-income and low/middle-income countries. American Journal of Cardiology 2014;113(5):793-7. [DOI] [PubMed] [Google Scholar]
- Windle SB, Grandi S, Shimony A, Gervais A, Joseph L, O'Loughlin J, et al. Use of medical therapy in patients 12 months post-acute myocardial infarction. Journal of the American College of Cardiology 2012;59(13 Suppl 1):E364. [Google Scholar]
- Zhang DD, Eisenberg M, Grandi SM, Joseph L, Pilote L, Filion K. Bupropion, smoking cessation, and health-related quality of life following an acute myocardial infarction. Canadian Journal of Cardiology 2013;29(10 Suppl 1):S290-1. [PubMed] [Google Scholar]
- Zhang DD, Eisenberg MJ, Grandi SM, Joseph L, O'Loughlin J, Paradis G, et al. Bupropion, smoking cessation, and health-related quality of life following an acute myocardial infarction. Journal of Population Therapeutics and Clinical Pharmacology 2014;21(3):e346-56. [PubMed] [Google Scholar]
Elsasser 2002 {published data only}
- Elsasser GN, Guck TP, Destache CJ, Daher PM, Frey DR, Jones J, et al. Sustained release bupropion in the treatment of nicotine addiction among teenage smokers (RP-32). In: Rapid Communication Poster Abstracts. Society for Research on Nicotine and Tobacco 8th Annual Meeting; 2002 February 20-23; Savannah, Georgia. 2002.
Evins 2001 {published data only}
- Evins AE, Cather C, Rigotti NA, Freudenreich O, Henderson DC, Olm Shipman CM, et al. Two-year follow-up of a smoking cessation trial in patients with schizophrenia: increased rates of smoking cessation and reduction. Journal of Clinical Psychiatry 2004;65:307-11. [DOI] [PubMed] [Google Scholar]
- Evins AE, Mays VK, Rigotti NA, Tisdale T, Cather C, Goff DC. A pilot trial of bupropion added to cognitive behavioral therapy for smoking cessation in schizophrenia. Nicotine & Tobacco Research 2001;3:397-403. [DOI] [PubMed] [Google Scholar]
Evins 2005 {published data only}
- Evins AE, Cather C, Culhane M, Freudenreich O, Rigotti NA, Goff DC. Smoking cessation in schizophrenia: A double blind placebo controlled trial of bupropion SR added to cognitive behavioral therapy. Biological Psychiatry 2004;55:806. [Google Scholar]
- Evins AE, Cather C, Deckersbach T, Freudenreich O, Culhane MA, Olm-Shipman CM, et al. A double-blind placebo-controlled trial of bupropion sustained-release for smoking cessation in schizophrenia. Journal of Clinical Psychopharmacology 2005;25:218-25. [DOI] [PubMed] [Google Scholar]
- Evins AE, Deckersbach T, Cather C, Freudenreich O, Culhane MA, Henderson DC, et al. Independent effects of tobacco abstinence and bupropion on cognitive function in schizophrenia. Journal of Clinical Psychiatry 2005;66:1184-90. [DOI] [PubMed] [Google Scholar]
Evins 2007 {published data only}
- Evins AE, Cather C, Culhane M, Birnbaum AS, Horowitz J, Hsieh E, et al. A placebo-controlled study of bupropion SR added to high dose nicotine replacement therapy for smoking cessation or reduction in schizophrenia (POS2-104). In: Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 February 15-18; Orlando (FL). 2006.
- Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, et al. A 12-week double-blind, placebo-controlled study of bupropion SR added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. Journal of Clinical Psychopharmacology 2007;27:380-6. [DOI] [PubMed] [Google Scholar]
- NCT00307203. Safety and effectiveness of sustained release bupropion in treating individuals with schizophrenia who smoke. clinicaltrials.gov/ct2/show/NCT00307203 (first received 27 March 2006).
Fatemi 2013 {published data only}
- Fatemi SH, Yousefi MK, Kneeland RE, Liesch SB, Folsom TD, Thuras PD. Antismoking and potential antipsychotic effects of varenicline in subjects with schizophrenia or schizoaffective disorder: a double-blind placebo and bupropion-controlled study. Schizophrenia Research 2013;146(1-3):376-8. [DOI] [PubMed] [Google Scholar]
Ferry 1992 {published and unpublished data}
- Ferry LH, Robbins AS, Scariati PD, Masterson A, Abbey DE, Burchette RJ. Enhancement of smoking cessation using the antidepressant bupropion hydrochloride. Circulation 1992;86(4 Suppl 1):I-671. [Google Scholar]
Ferry 1994 {published and unpublished data}
- Ferry LH, Burchette RJ. Efficacy of bupropion for smoking cessation in non depressed smokers [Abstract]. Journal of Addictive Diseases 1994;13(4):249. [Google Scholar]
Fossati 2007 {published data only}
- Ferketich AK, Fossati R, Apolone G. An evaluation of the Italian version of the Fagerstrom Test for Nicotine Dependence. Psychological Reports 2008;102:687-94. [DOI] [PubMed] [Google Scholar]
- Fossati R, Apolone G, Negri E, Compagnoni A, La Vecchia C, Mangano S, et al. A double-blind, placebo-controlled, randomized trial of bupropion for smoking cessation in primary care. Archives of Internal Medicine 2007;167:1791-7. [DOI] [PubMed] [Google Scholar]
Gariti 2009 {published data only}
- Gariti P, Lynch K, Alterman A, Kampman K, Xie H, Varillo K. Comparing smoking treatment programs for lighter smokers with and without a history of heavier smoking. Journal of Substance Abuse Treatment 2009;37(3):247-55. 9400123000005384 [DOI] [PMC free article] [PubMed]
George 2002 {published data only}
- George TP, Vessicchio JC, Termine A, Bregartner TA, Feingold A, Rounsaville BJ, et al. A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biological Psychiatry 2002;52:53-61. [9400123000002832] [DOI] [PubMed] [Google Scholar]
George 2003 {published data only}
- George TP, Vessicchio JC, Termine A, Jatlow PI, Kosten TR, O'Malley SS. A preliminary placebo-controlled trial of selegiline hydrochloride for smoking cessation. Biological Psychiatry 2003;53(2):136-43. [DOI] [PubMed] [Google Scholar]
- Lara XD, Vessicchio JC, Termine A, Kosten TR, O'Malley SS, George TP. Selegiline versus placebo for smoking cessation in nicotine dependent refractory smokers (PO2 02). In: Society for Research on Nicotine and Tobacco 7th Annual Meeting; 2001 March 23-23; Seattle (WA). 2001:53. 9400123000002571
George 2008 {published data only}
- George TP, Vessicchio JC, Sacco KA, Weinberger AH, Dudas MM, Allen TM, et al. A placebo-controlled trial of bupropion combined with nicotine patch for smoking cessation in schizophrenia. Biological Psychiatry 2008;63(11):1092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Weinberger AH, Sacco KA. Sustained-release bupropion combined with transdermal nicotine patch for smoking cessation in schizophrenia (SYM11C). In: Society for Research on Nicotine and Tobacco 13th Annual Meeting; 2007 February 21-24; Austin (TX). 2007.
- Moss TG, Sacco KA, Allen TM, Weinberger AH, Vessicchio JC, George TP. Prefrontal cognitive dysfunction is associated with tobacco dependence treatment failure in smokers with schizophrenia. Drug and Alcohol Dependence 2009;104(1-2):94-9. 9400123000005367 [DOI] [PMC free article] [PubMed]
Gilbert 2019 {published data only}
- Gilbert DG, Rabinovich NE, Gilbert-Matuskowitz EA, Klein KP, Pergadia ML. Smoking cessation symptoms across 67 days compared with randomized controls-moderation by nicotine replacement therapy, bupropion, and negative-affect traits. Experimental and Clinical Psychopharmacology 2019;27(6):536-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzales 2001 {published data only}
- Gonzales D, Nides M, Ferry LH, Segall N, Herrero L, Modell J, et al. Retreatment with bupropion SR: results from 12-month follow-up (RP-83). In: Rapid Communication Poster Abstracts. Society for Research on Nicotine and Tobacco 8th Annual Meeting; 2002 February 20-23; Savannah (GA). 2002.
- Gonzales DH, Nides MA, Ferry LH, Kustra RP, Jamerson BD, Segall N, et al. Bupropion SR as an aid to smoking cessation in smokers treated previously with bupropion: A randomized placebo-controlled study. Clinical Pharmacology and Therapeutics 2001;69:438-44. [DOI] [PubMed] [Google Scholar]
Gonzales 2006 {published data only}
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006;296:47-55. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M. Varenicline effective for smoking cessation. Journal of Family Practice 2006;55(10):848-9. [PubMed] [Google Scholar]
- Hays JT, Croghan IT, Baker CL, Cappelleri JC, Bushmakin AG. Changes in health-related quality of life with smoking cessation treatment. European Journal of Public Health 2012;22(2):224-9. [DOI] [PubMed] [Google Scholar]
- Hays JT, Leischow SJ, Lawrence D, Lee TC. Adherence to treatment for tobacco dependence: association with smoking abstinence and predictors of adherence. Nicotine & Tobacco Research 2010;12(6):574-81. 9400123000005608 [DOI] [PubMed]
- Jackson KC, Nahoopii R, Said Q, Dirani R, Brixner D. An employer-based cost-benefit analysis of a novel pharmacotherapy agent for smoking cessation. Journal of Occupational & Environmental Medicine 2007;49(4):453-60. [DOI] [PubMed] [Google Scholar]
- King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology 2012;37(3):641-50. 9400123000012713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prignot J. Care for adherence to treatment for tobacco dependence. Breathe 2011;7(3):291. 9400123000011702
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology 2008;197(3):371-7. [DOI] [PubMed] [Google Scholar]
Górecka 2003 {published data only}
- Górecka D, Bednarek M, Nowinski A, Puscinska E, Goljan-Geremek A, Zielinski J. Effect of treatment for nicotine dependence in patients with COPD [Wyniki leczenia uzaleznienia od nikotyny chorych na przewlekla obturacyjna chorobe pluc]. Pneumonologia i Alergologia Polska 2003;71:411-7. [PubMed] [Google Scholar]
Grant 2007 {published data only}
- Grant KM, Kelley SS, Smith LM, Agrawal S, Meyer JR, Romberger DJ. Bupropion and nicotine patch as smoking cessation aids in alcoholics. Alcohol 2007;41(5):381-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00044434. Bupropion as a smoking cessation aid in alcoholics. clinicaltrials.gov/ct2/show/NCT00044434 (first received 30 August 2002).
Gray 2011 {published data only}
- Carpenter MJ, Baker NL, Gray KM, Upadhyaya HP. Assessment of nicotine dependence among adolescent and young adult smokers: A comparison of measures. Addictive Behaviors 2010;35(11):977-82. 9400123000005998 [DOI] [PMC free article] [PubMed]
- Gray KM, Carpenter MJ, Baker NL, Hartwell KJ, Lewis AL, Hiott DW, et al. Bupropion SR and contingency management for adolescent smoking cessation. Journal of Substance Abuse Treatment 2011;40(1):77-86. 9400123000005880 [DOI] [PMC free article] [PubMed]
- Gray KM, Carpenter MJ, Baker NL, Klintworth EM, Leinbach AS, Upadhyaya HP, et al. Bupropion SR and contingency management in adolescent smokers: main findings. In: College on Problems of Drug Dependence 71st Annual Meeting; 2009 June 20-25; Reno/Sparks (NV). Reno/Sparks, Nevada, 2009:74.
Gray 2012 {published data only}
- Gray KM, Carpenter MJ, Lewis AL, Klintworth EM, Upadhyaya HP. Varenicline versus bupropion XL for smoking cessation in older adolescents: a randomized, double-blind pilot trial. Nicotine & Tobacco Research 2012;14(2):235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haggsträm 2006 {published data only}
- Haggsträm FM, Chatkin JM, Sussenbach-Vaz E, Cesari DH, Fam CF, Fritscher CC. A controlled trial of nortriptyline, sustained-release bupropion and placebo for smoking cessation: preliminary results. Pulmonary Pharmacology & Therapeutics 2006;19:205-9. [DOI] [PubMed] [Google Scholar]
Hall 1998 {published data only}
- Haas AL, Munoz RF, Humfleet GL, Reus VI, Hall SM. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. Journal of Consulting & Clinical Psychology 2004;72:563-70. [DOI] [PubMed] [Google Scholar]
- Hall SM, Gorecki JA, Reus VI, Humfleet GL, Munoz RF. Belief about drug assignment and abstinence in treatment of cigarette smoking using nortriptyline. Nicotine & Tobacco Research 2007;9(4):467-71. [DOI] [PubMed] [Google Scholar]
- Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet G, Hartz DT, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Archives of General Psychiatry 1998;55:683-90. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Reus VI, Gorecki J, Hall SM, Humfleet GL, Munoz RF, et al. Therapeutic drug monitoring of nortriptyline in smoking cessation: a multistudy analysis. Clinical Pharmacology & Therapeutics 2008;83:436-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2002 {published data only}
- Hall SM, Gorecki JA, Reus VI, Humfleet GL, Munoz RF. Belief about drug assignment and abstinence in treatment of cigarette smoking using nortriptyline. Nicotine & Tobacco Research 2007;9(4):467-71. [DOI] [PubMed] [Google Scholar]
- Hall SM, Humfleet G, Maude-Griffin R, Reus VI, Munoz R, Hartz DT. Nortriptyline versus bupropion and medical management versus psychological intervention in smoking treatment (PA 5A). In: Society for Research on Nicotine and Tobacco 7th Annual Meeting; 2001 March 23-23; Seattle (WA). 2001:31.
- Hall SM, Humfleet GL, Reus VI, Munoz RF, Hartz DT, Maude-Griffin R. Psychological intervention and antidepressant treatment in smoking cessation. Archives of General Psychiatry 2002;59:930-6. [DOI] [PubMed] [Google Scholar]
- Hall SM, Lightwood JM, Humfleet GL, Bostrom A, Reus VI, Munoz R. Cost-effectiveness of bupropion, nortriptyline, and psychological intervention in smoking cessation. Journal of Behavioral Health Services & Research 2005;32:381-92. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Reus VI, Gorecki J, Hall SM, Humfleet GL, Munoz RF, et al. Therapeutic drug monitoring of nortriptyline in smoking cessation: a multistudy analysis. Clinical Pharmacology & Therapeutics 2008;83:436-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2004 {published data only}
- Hall SM, Humfleet GL, Reus VI, Munoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. American Journal of Psychiatry 2004;161:2100-7. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Reus VI, Gorecki J, Hall SM, Humfleet GL, Munoz RF, et al. Therapeutic drug monitoring of nortriptyline in smoking cessation: a multistudy analysis. Clinical Pharmacology & Therapeutics 2008;83:436-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hertzberg 2001 {published data only}
- Hertzberg MA, Moore SD, Feldman ME, Beckham JC. A preliminary study of bupropion sustained-release for smoking cessation in patients with chronic posttraumatic stress disorder. Journal of Clinical Psychopharmacology 2001;21:94-8. [DOI] [PubMed] [Google Scholar]
Holt 2005 {published data only}
- Holt S, Timu-Parata C, Ryder-Lewis S, Weatherall M, Beasley R. Efficacy of bupropion in the indigenous Maori population in New Zealand. Thorax 2005;60:120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurt 1997 {published and unpublished data}
- Dale LC, Glover ED, Sachs DP, Schroeder DR, Offord KP, Croghan IT, et al. Bupropion for smoking cessation: predictors of successful outcome. Chest 2001;119:1357-64. [DOI] [PubMed] [Google Scholar]
- Glaxo Wellcome. Presentation for FDA approval of Bupropion sustained release for smoking cessation (10 December 1996). Dr J Andrew Johnston.
- Hayford KE, Patten CA, Rummans TA, Schroeder DR, Offord KP, Croghan IT, et al. Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. British Journal of Psychiatry 1999;174:173-8. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Glover ED, Sachs DPL, Dale LC, Schroeder DR. Bupropion for smoking cessation: A double-blind, placebo-controlled dose response trial. Journal of Addictive Diseases 1996;15:137. [Google Scholar]
- Hurt RD, Sachs DPL, Glover ED, Offord KP, Johnston JA, Dale LC, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. New England Journal of Medicine 1997;337:1195-202. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Fiedler-Kelly J, Glover ED, Sachs DP, Grasela TH, DeVeaugh-Geiss J. Relationship between drug exposure and the efficacy and safety of bupropion sustained release for smoking cessation. Nicotine & Tobacco Research 2001;3:131-40. [DOI] [PubMed] [Google Scholar]
Johns 2017 {published data only}
- Johns DA. The efficacy of combination therapy with varenicline and bupropion for smoking cessation. Annals of Oncology 2017;28 Suppl 2:6-8. [Google Scholar]
Jorenby 1999 {published data only}
- Durcan MJ, White J, Jorenby DE, Fiore MC, Rennard SI, Leischow SJ, et al. Impact of prior nicotine replacement therapy on smoking cessation efficacy. American Journal of Health Behavior 2002;26:213-20. [DOI] [PubMed] [Google Scholar]
- Jamerson BD, Nides M, Jorenby DE, Donahue R, Garrett P, Johnston JA, et al. Late-term smoking cessation despite initial failure: an evaluation of bupropion sustained release, nicotine patch, combination therapy, and placebo. Clinical Therapeutics 2001;23:744-52. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine 1999;340:685-91. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Fiore MC. Cost-benefit analysis of sustained-release bupropion, nicotine patch, or both for smoking cessation. Preventive Medicine 2000;30:209-16. [DOI] [PubMed] [Google Scholar]
- Smith SS, Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston AJ, et al. Targeting smokers at increased risk for relapse: treating women and those with a history of depression. Nicotine & Tobacco Research 2003;5:99-109. [DOI] [PubMed] [Google Scholar]
Jorenby 2006 {published data only}
- Hays JT, Croghan IT, Baker CL, Cappelleri JC, Bushmakin AG. Changes in health-related quality of life with smoking cessation treatment. European Journal of Public Health 2012;22(2):224-9. [DOI] [PubMed] [Google Scholar]
- Hays JT, Leischow SJ, Lawrence D, Lee TC. Adherence to treatment for tobacco dependence: association with smoking abstinence and predictors of adherence. Nicotine & Tobacco Research 2010;12(6):574-81. 9400123000005608 [DOI] [PubMed]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006;296:56-63. [DOI] [PubMed] [Google Scholar]
- King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology 2012;37(3):641-50. 9400123000012713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology 2008;197(3):371-7. [DOI] [PubMed] [Google Scholar]
Kahn 2012 {published data only}
- Kahn R, Gorgon L, Jones K, McSherry F, Glover ED, Anthenelli RM, et al. Selegiline transdermal system (STS) as an aid for smoking cessation. Nicotine & Tobacco Research 2012;14(3):377-82. 9400123000014198 [DOI] [PMC free article] [PubMed]
- NCT00439413. Selegiline for smoking cessation - 1. clinicaltrials.gov/ct2/show/NCT00439413 (first received 23 February 2007).
Kalman 2011 {published data only}
- Kalman D, Herz L, Monti P, Kahler CW, Mooney M, Rodrigues S, et al. Incremental efficacy of adding bupropion to the nicotine patch for smoking cessation in smokers with a recent history of alcohol dependence: results from a randomized, double-blind, placebo-controlled study. Drug and Alcohol Dependence 2011;118(2-3):111-8. 9400123000011678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Kalman D. Effects of bupropion on simulated demand for cigarettes and the subjective effects of smoking. Nicotine & Tobacco Research 2010;12(4):416-22. 9400123000005651 [DOI] [PMC free article] [PubMed]
- McGeary J. Predictors of relapse in a bupropion trial for smoking cessation in recently-abstinent alcoholics: Preliminary results using an aggregate genetic risk score. Behavior Genetics 2011;41(6):923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeary JE, Knopik VS, Hayes JE, Palmer RH, Monti PM, Kalman D. Predictors of relapse in a bupropion trial for smoking cessation in recently-abstinent alcoholics: Preliminary results using an aggregate genetic risk score. Substance Abuse: Research and Treatment 2012;6(1):107-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00304707. Effectiveness of bupropion for smokers recovering from alcohol dependence. clinicaltrials.gov/ct2/show/NCT00304707 (first received 20 March 2017).
Karam‐Hage 2011 {published data only}
- Karam-Hage M, Strobbe S, Robinson JD, Brower KJ. Bupropion-SR for smoking cessation in early recovery from alcohol dependence: a placebo-controlled, double-blind pilot study. American Journal of Drug and Alcohol Abuse 2011;37(6):487-90. 9400123000011679 [DOI] [PMC free article] [PubMed] [Google Scholar]
Killen 2000 {published data only}
- Killen JD, Fortmann SP, Schatzberg A, Hayward C, Varady A. Onset of major depression during treatment for nicotine dependence. Addictive Behaviors 2003;28:461-70. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Schatzberg AF, Hayward C, Sussman L, Rothman M, et al. Nicotine patch and paroxetine for smoking cessation. Journal of Consulting and Clinical Psychology 2000;68:883-9. [PubMed] [Google Scholar]
Killen 2004 {published data only}
- Killen JD, Robinson TN, Ammerman S, Hayward C, Rogers J, Samuels D. Major depression among adolescent smokers undergoing treatment for nicotine dependence. Addictive Behaviors 2004;29:1517-26. [DOI] [PubMed] [Google Scholar]
- Killen JD, Robinson TN, Ammerman S, Hayward C, Rogers J, Stone C, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. Journal of Consulting and Clinical Psychology 2004;72:729-35. [DOI] [PubMed] [Google Scholar]
Killen 2010 {published data only}
- Killen JD, Fortmann SP, Murphy GMJ, Hayward C, Fong D, Lowenthal K, et al. Failure to improve cigarette smoking abstinence with transdermal selegiline + cognitive behavior therapy. Addiction 2010;105(9):1660-8. 9400123000005871 [DOI] [PMC free article] [PubMed]
- NCT01330030. Selegiline patch for treatment of nicotine dependence. clinicaltrials.gov/ct2/show/NCT01330030 (first received 6 April 2011).
- Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Ameli N, et al. Response to transdermal selegiline smoking cessation therapy and markers in the 15q24 chromosomal region. Nicotine & Tobacco Research 2015;17(9):1126-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 2010 {published data only}
- Creswell KG, Cheng Y, Levine MD. A test of the stress-buffering model of social support in smoking cessation: is the relationship between social support and time to relapse mediated by reduced withdrawal symptoms? Nicotine & Tobacco Research 2015;17(5):566-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MD, Cheng Y, Kalarchian MA, Perkins KA, Marcus MD. Dietary intake after smoking cessation among weight-concerned women smokers. Psychology of Addictive Behaviors 2012;26(4):969-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MD, Perkins KA, Kalarchian MA, Cheng Y, Houck PR, Slane JD, et al. Bupropion and cognitive behavioural therapy for weight-concerned women smokers. Archives of Internal Medicine 2010;170(6):543-50. 9400123000005577 [DOI] [PMC free article] [PubMed]
- Okun ML, Levine MD, Houck P, Perkins KA, Marcus MD. Subjective sleep disturbance during a smoking cessation program: associations with relapse. Addictive Behaviors 2011;36(8):861-4. 9400123000011571 [DOI] [PMC free article] [PubMed]
McCarthy 2008 {published data only}
- McCarthy DE, Piasecki TM, Jorenby DE, Lawrence DL, Shiffman S, Baker TB. A multi-level analysis of non-significant counseling effects in a randomized smoking cessation trial. Addiction 2010;105(12):2195-208. 9400123000005902 [DOI] [PMC free article] [PubMed]
- McCarthy DE, Piasecki TM, Lawrence DL, Fiore MC, Baker TB. Efficacy of bupropion SR and individual counseling among adults attempting to quit smoking (POS1-041). In: Society for Research on Nicotine and Tobacco 10th Annual Meeting; 2004 February 18-21; Phoenix (AZ). 2004.
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Baker TB. Psychological mediators of bupropion sustained-release treatment for smoking cessation. Addiction 2008;103(9):1521-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine & Tobacco Research 2008;10:717-29. [DOI] [PubMed] [Google Scholar]
- McCarthy DE. Mechanisms of tobacco cessation treatment: Self-report mediators of counseling and bupropion sustained release treatment. Dissertation Abstracts International: Section B: The Sciences and Engineering 2007;67(9-B):5414. [Google Scholar]
- Minami H, Tran LT, McCarthy DE. Using ecological measures of smoking trigger exposure to predict smoking cessation milestones. Psychology of Addictive Behaviors 2015;29(1):122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01621009. Bupropion SR plus counseling for smoking cessation. clinicaltrials.gov/ct2/show/NCT01621009 (first received 15 June 2012).
Minami 2014 {published data only}
- Minami H, Kahler CW, Bloom EL, Prince MA, Abrantes AM, Strong DR, et al. Effects of sequential fluoxetine and gender on prequit depressive symptoms, affect, craving, and quit day abstinence in smokers with elevated depressive symptoms: a growth curve modeling approach. Experimental and Clinical Psychopharmacology 2014;22(5):392-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreno‐Coutino 2015 {published data only}
- Moreno-Coutiño A, García-Anguiano F, Ruiz-Velasco S, Medina-Mora ME. Assessment of depressive symptoms in severe smokers with minimal-mild depressive symptomatology receiving pre-smoking abstinence for integrated treatment: a randomized clinical trial. Salud Mental 2015;38(6):433-9. [Google Scholar]
- Moreno-Coutino A, Perez-Lopez A, Gallegos LV. Predictors of retention in a multicomponent treatment for smokers. Revista de Psiquiatria Clinica 2016;43(6):134-8. [Google Scholar]
Muramoto 2007 {published data only}
- Best D. Bupropion assists with tobacco cessation in adolescents but relapse is high. Journal of Pediatrics 2008;152(5):738-9. 9400123000011733 [DOI] [PubMed]
- Floden L, Taren DL, Muramoto ML, Leischow SJ. BMI changes in adolescents treated with bupropion SR for smoking cessation. Obesity 2016;24(1):26-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leischow SJ, Muramoto ML, Matthews E, Floden LL, Grana RA. Adolescent smoking cessation with bupropion: the role of adherence. Nicotine & Tobacco Research 2016;18(5):1202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto ML, Leischow SJ, Sherrill D, Matthews E, Strayer LJ. Randomized, double-blind, placebo-controlled trial of 2 dosages of sustained-release bupropion for adolescent smoking cessation. Archives of Pediatrics & Adolescent Medicine 2007;161:1068-74. [DOI] [PubMed] [Google Scholar]
- Taren D, Fankem S, Muramoto M. Weight loss in adolescents who quit smoking with bupropion [RP-071]. In: Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005 March 20-23; Prague, Czech Republic. Rapid Communications posters. 2005.
Myles 2004 {published data only}
- Myles PS, Leslie K, Angliss M, Mezzavia P, Lee L. Effectiveness of bupropion as an aid to stopping smoking before elective surgery: a randomised controlled trial. Anaesthesia 2004;59:1053-8. [DOI] [PubMed] [Google Scholar]
NCT00132821 {published data only}
- NCT00132821. Impact of smoking cessation on sleep - 5. clinicaltrials.gov/ct2/show/NCT00132821 (first received 22 August 2005).
NCT00308763 {published data only}
- NCT00308763. Nicotine patch and bupropion to reduce smoking rates in younger, low-income, and minority individuals. clinicaltrials.gov/ct2/show/NCT00308763 (first received 30 March 2006).
NCT00495352 {published data only}
- NCT00495352. The pharmacogenetic study, readiness to change, and pharmacological intervention for smoking cessation in schizophrenia. clinicaltrials.gov/ct2/show/NCT00495352 (first received 3 July 2007).
NCT00578669 {published data only}
- NCT00578669. Sequential use of fluoxetine for smokers With elevated depressive symptoms. clinicaltrials.gov/ct2/show/NCT00578669 (first received 21 December 2007).
NCT00593099 {published data only}
- NCT00593099. A preliminary study of sustained-release bupropion for smoking cessation in bipolar affective disorder. www.clinicaltrials.gov/ct2/show/NCT00593099 (first received 14 January 2008).
NCT01406223 {published data only}
- NCT01406223. Mechanistic evaluations of pre-cessation therapies for smoking cessation. clinicaltrials.gov/ct2/show/NCT01406223 (first recieved 1 August 2011).
Niaura 2002 {published data only}
- Borrelli B, Niaura R, Keuthen NJ, Goldstein MG, Depue JD, Murphy C, et al. Development of major depressive disorder during smoking-cessation treatment. Journal of Clinical Psychiatry 1996;57:534-8. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Papandonatos G, Spring B, Hitsman B, Niaura R. Experimenter-defined quit dates for smoking cessation: adherence improves outcomes for women but not for men. Addiction 2004;99:378-85. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Spring B, Niaura R, Hitsman B, Papandonatos G. Influences of gender and weight gain on short-term relapse to smoking in a cessation trial. Journal of Consulting and Clinical Psychology 2001;69:511-15. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Spring B, Niaura R, Kristeller J, Ockene JK, Keuthen NJ. Weight suppression and weight rebound in ex-smokers treated with fluoxetine. Journal of Consulting and Clinical Psychiatry 1999;67:124-31. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue DE, Borrelli B, Hitsman B, Niaura R, et al. Influence of fluoxetine on positive and negative affect in a clinic-based smoking cessation trial. Psychopharmacology 2004;173:153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Spring B, Borrelli B, McChargue D, Hitsman B, Niaura R, et al. Elevated positive mood: a mixed blessing for abstinence. Psychology of Addictive Behaviors 2006;20:36-43. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Spring B, Borrelli B, Niaura R, Papandonatos GD. Influence of antidepressant pharmacotherapy on behavioral treatment adherence and smoking cessation outcome in a combined treatment involving fluoxetine. Experimental & Clinical Psychopharmacology 2001;9:355-62. [DOI] [PubMed] [Google Scholar]
- Mizes JS, Sloan DM, Segraves K, Spring B, Pingatore R, Kristeller J. Fluoxetine and weight-gain in smoking cessation - examination of actual weight-gain and fear of weight-gain [abstract]. Psychopharmacology Bulletin 1996;32:491. [Google Scholar]
- Niaura R, Goldstein M, Spring B, Keuthen N, Kristeller J, DePue J, et al. Fluoxetine for smoking cessation: A multicenter randomized double blind dose response study. Society for Behavioral Medicine Annual Meeting; 1997 April 18; San Francisco (CA).
- Niaura R, Spring B, Borrelli B, Hedeker D, Goldstein MG, Keuthen N, et al. Multicenter trial of fluoxetine as an adjunct to behavioral smoking cessation treatment. Journal of Consulting and Clinical Psychology 2002;70:887-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Niaura R, Borrelli B, Spring B. Subgroups of smokers with different success rates after treatment with fluoxetine for smoking cessation [abstract]. Nicotine & Tobacco Research 1999;1:281. [Google Scholar]
Nides 2006 {published data only}
- NCT00150241. A seven-week dose-ranging study of CP-526,555 compared with placebo and zyban for smoking cessation. clinicaltrials.gov/ct2/show/NCT00150241 (first received 8 September 2005).
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: Results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Archives of Internal Medicine 2006;166:1561-8. [DOI] [PubMed] [Google Scholar]
- Oncken C, Watsky E, Reeves K, Anziano R. Varenicline is efficacious and well tolerated in promoting smoking cessation: results from a 7-week, randomized, placebo- and bupropion-controlled trial. In: Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005 March 20-23; Prague, Czech Republic. 2005.
Parsons 2009 {published data only}
- EudraCT 2005-000662-39. A 2x2 phase II randomized controlled trial to investigate the efficacy of NRT plus St John's wort versus NRT plus placebo in smoking cessation and to examine the efficacy of chromium nicotinate versus placebo in preventing weight gain while stopping smoking. - SJW and chromium in smoking cessation. clinicaltrialsregister.eu/ctr-search/search?query=2005-000662-39 (first received 4 October 2005).
- ISRCTN31302738. A 2 x 2 phase II randomised controlled trial to investigate the efficacy of St John's wort versus placebo in smoking cessation and the efficacy of chromium intake in preventing weight gain. isrctn.com/ISRCTN31302738 (first received 8 February 2006).
- Parsons A, Ingram J, Inglis J, Aveyard P, Johnstone E, Brown K, et al. A proof of concept randomised placebo controlled factorial trial to examine the efficacy of St John's wort for smoking cessation and chromium to prevent weight gain on smoking cessation. Drug and Alcohol Dependence 2009;102(1-3):116-22. 9400123000005315 [DOI] [PubMed]
Perkins 2013 {published data only}
- Perkins KA, Lerman C, Karelitz JL, Jao NC, Chengappa KN, Sparks GM. Sensitivity and specificity of a procedure for early human screening of novel smoking cessation medications. Addiction 2013;108(11):1962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piper 2007 {published data only}
- Bekiroglu K, Russell MA, Lagoa CM, Lanza ST, Piper ME. Evaluating the effect of smoking cessation treatment on a complex dynamical system. Drug and Alcohol Dependence 2017;180:215-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Efficacy of bupropion alone and in combination with nicotine gum. Nicotine & Tobacco Research 2007;9:947-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Mediators of bupropion treatment effects (SYM 2C). Society for Research on Nicotine and Tobacco 14th Annual Meeting; 2008 February 26 - March 1; Portland (OR) 2008:31. 9400123000005187 [9400123000005187]
- Piper ME, Federman EB, Smith SS, Fiore MC, Baker TB. Efficacy of bupropion SR alone and combined with 4-mg gum (PA2-2). In: Society for Research on Nicotine and Tobacco 10th Annual Meeting; 2004 February 18-21, Phoenix (AZ). 2004:18.
- Piper ME, Federmen EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. Journal of Abnormal Psychology 2008;117:94-105. [DOI] [PubMed] [Google Scholar]
- Piper ME. Bupropion alone and in combination with nicotine gum: Efficacy, mediation and moderation. Dissertation Abstracts International: Section B: The Sciences and Engineering 2007;67(9-B):5418. [Google Scholar]
Piper 2009 {published and unpublished data}
- Asthana A, Johnson HM, Piper ME, Fiore MC, Baker TB, Stein JH. Effects of smoking intensity and cessation on inflammatory markers in a large cohort of active smokers. American Heart Journal 2010;160(3):458-63. 9400123000005744 [DOI] [PMC free article] [PubMed]
- Berg KM, Piper M, Fiore M, Baker T, Jorenby DE. Alcohol use after tobacco cessation: immediate consequences. Journal of General Internal Medicine 2012;27(Suppl 2):99-574. [Google Scholar]
- Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving suppression. Journal of Consulting and Clinical Psychology 2012;80(1):54-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Bloom AJ, Baker TB, Smith SS, Piper ME, Martinez M, et al. Pharmacotherapy effects on smoking cessation vary with nicotine metabolism gene (CYP2A6). Addiction 2014;109(1):128-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Lanza ST, Chu W, Baker TB, Piper ME. Anhedonia: its dynamic relations with craving, negative affect, and treatment during a quit smoking attempt. Nicotine & Tobacco Research 2017;19(6):703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culverhouse RC, Chen LS, Saccone NL, Ma Y, Piper ME, Baker TB, et al. Variants in the CHRNA5-CHRNA3-CHRNB4 region of chromosome 15 predict gastrointestinal adverse events in the TTURC smoking cessation trial. Nicotine & Tobacco Research 2019;22(2):248-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. American Heart Journal 2011;161(1):145-51. 9400123000005846 [DOI] [PMC free article] [PubMed]
- Japuntich SJ, Leventhal AM, Piper ME, Bolt DM, Roberts LJ, Fiore MC, et al. Smoker characteristics and smoking-cessation milestones. American Journal of Preventive Medicine 2011;40(3):286-94. 9400123000006012 [DOI] [PMC free article] [PubMed]
- Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, Baker TB. The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. Journal of Consulting and Clinical Psychology 2011;79(1):34-42. 9400123000006131 [DOI] [PMC free article] [PubMed]
- Loh WY, Piper ME, Schlam TR, Fiore MC, Smith SS, Jorenby DE, et al. Should all smokers use combination smoking cessation pharmacotherapy? Using novel analytic methods to detect differential treatment effects over 8 weeks of pharmacotherapy. Nicotine & Tobacco Research 2012;14(2):131-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Ebssa L, Witkiewitz K, Shiffman S. Paths to tobacco abstinence: A repeated-measures latent class analysis. Journal of Consulting and Clinical Psychology 2015;83(4):696-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction 2011;106(2):418-27. 9400123000005906 [DOI] [PMC free article] [PubMed]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine & Tobacco Research 2010;12(6):647-57. 9400123000005794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Kenford S, Fiore MC, Baker TB. Smoking cessation and quality of life: changes in life satisfaction over 3 years following a quit attempt. Annals of Behavioral Medicine 2012;43(2):262-70. 9400123000012954 [DOI] [PMC free article] [PubMed]
- Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh WY, et al. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology 2011;216(4):569-78. 9400123000011701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Archives of General Psychiatry 2009;66(11):1253-62. 9400123000005399 [DOI] [PMC free article] [PubMed]
- Smith SS, Fiore MC, Baker TB. Smoking cessation in smokers who smoke menthol and non-menthol cigarettes. Addiction 2014;109(12):2107-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Planer 2011 {published data only}
Prochazka 1998 {published data only}
- Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D. A randomized trial of nortriptyline for smoking cessation. Archives of Internal Medicine 1998;158:2035-9. [DOI] [PubMed] [Google Scholar]
Prochazka 2004 {published data only}
- NCT00018148. Combined nortriptyline and transdermal nicotine for smoking cessation. clinicaltrials.gov/ct2/show/NCT00018148 (first received 5 July 2001).
- Prochazka AV, Kick S, Steinbrunn C, Miyoshi T, Fryer GE. A randomized trial of nortriptyline combined with transdermal nicotine for smoking cessation. Archives of Internal Medicine 2004;164:2229-33. [DOI] [PubMed] [Google Scholar]
- Prochazka AV, Reyes R, Steinbrunn C, Miyoshi T. Randomized trial of nortriptyline combined with transdermal nicotine for smoking cessation (PO3 26). In: Society for Research on Nicotine and Tobacco 7th Annual Meeting; 2001 March 23-23; Seattle (WA). 2001:73. [DOI] [PubMed]
Richmond 2013 {published data only}
- Richmond R, Indig D, Butler T, Wilhelm K, Archer V, Wodak A. A randomized controlled trial of a smoking cessation intervention conducted among prisoners. Addiction 2013;108(5):966-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rigotti 2006 {published data only}
- Rigotti N, Thorndike A, Regan S, Pasternak R, Chang Y, McKool K, et al. Safety and efficacy of bupropion for smokers hospitalized with acute cardiovascular disease [abstract]. Nicotine & Tobacco Research 2005;7:682. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Thorndike AN, Regan S, McKool K, Pasternak RC, Chang Y, et al. Bupropion for smokers hospitalized with acute cardiovascular disease. American Journal of Medicine 2006;119:1080-7. [DOI] [PubMed] [Google Scholar]
- Thorndike AN, Regan S, McKool K, Pasternak RC, Swartz S, Torres-Finnerty N, et al. Depressive symptoms and smoking cessation after hospitalization for cardiovascular disease. Archives of Internal Medicine 2008;168(2):186-91. [DOI] [PubMed] [Google Scholar]
Rose 2013 {published data only}
- Rose JE, Behm FM. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. American Journal of Psychiatry 2013;170:860-7. 9400107000001313 [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 2014 {published data only}
- Rose JE, Behm FM. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. American Journal of Psychiatry 2014;171(11):1199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Combination varenicline/bupropion treatment benefits male NRT-nonresponders. In: Society for Research on Nicotine and Tobacco 19th Annual Meeting; 2013 March 13-16 Boston (MA). 2013:261.
Rose 2017 {published data only}
- NCT01806779. Combination bupropion/varenicline for smoking cessation in male smokers. clinicaltrials.gov/ct2/show/NCT01806779 (first received 7 March 2013).
- Rose JE, Behm FM. Combination varenicline/bupropion treatment benefits highly dependent smokers in an adaptive smoking cessation paradigm. Nicotine & Tobacco Research 2017;19(8):999-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rovina 2009 {published data only}
Saules 2004 {published and unpublished data}
- Saules KK, Schuh LM, Arfken CL, Reed K, Kilbey MM, Schuster CR. Double-blind placebo-controlled trial of fluoxetine in smoking cessation treatment including nicotine patch and cognitive-behavioral group therapy. American Journal on Addictions 2004;13:438-46. [DOI] [PubMed] [Google Scholar]
- Schuh LM, Downey KK, Hopper JA, Tancer M, Schuster CR. Fluoxetine in smoking cessation treatment. College on Problems of Drug Dependence 62nd Annual Meeting; 2000 June 17-20; San Juan, Puerto Rico 2000.
Schmitz 2007 {published data only}
- Schmitz JM, Stotts AL, Mooney ME, Delaune KA, Moeller GF. Bupropion and cognitive-behavioral therapy for smoking cessation in women.[erratum appears in Nicotine Tob Res. 2007 Jul;9(7):785]. Nicotine & Tobacco Research 2007;9(6):699-709. [DOI] [PubMed] [Google Scholar]
Schnoll 2010 {published data only}
- Martinez E, Tatum KL, Weber DM, Kuzla N, Pendley A, Campbell K, et al. Issues related to implementing a smoking cessation clinical trial for cancer patients. Cancer Causes & Control 2009;20(1):97-104. 9400123000005109 [DOI] [PMC free article] [PubMed]
- Schnoll R, Lazev A, Sobel M, Tatum K, Butler D, Lerman C. Preliminary results from a randomized trial of bupropion for smoking cessation among cancer patients. In: Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005 March 20-23; Prague, Czech Republic. 2005.
- Schnoll RA, Martinez E, Langer C, Miyamoto C, Leone F. Predictors of smoking cessation among cancer patients enrolled in a smoking cessation program. Acta Oncologica 2011;50(5):678-84. 9400123000006110 [DOI] [PubMed]
- Schnoll RA, Martinez E, Tatum KL, Weber DM, Kuzla N, Glass M, et al. A bupropion smoking cessation clinical trial for cancer patients. Cancer Causes & Control 2010;21(6):811-20. 9400123000005737 [DOI] [PubMed]
Selby 2003 {published and unpublished data}
- GlaxoSmithKline Clinical Trials Register. Study No: ZYB40001. A randomized, double-blind, placebo-controlled, 12-week smoking cessation trial of Zyban (150 mg bid) in adult smokers previously treated with Zyban. ctr.glaxowellcome.co.uk-Summary-bupropion-IV_ZYB40001.pdf (accessed 23 August 2006).
- Selby P, Ainslie M, Stepner N, Roberts J. Sustained-release bupropion (Zybanr) is effective in the re-treatment of relapsed adult smokers. American Journal of Respiratory and Critical Care Medicine 2003;167(7):A47. [Google Scholar]
- Selby P, Brands B, Stepner N. Retreatment with ZYban SR: 52 week follow-up of a Canadian Multicentre trial (POS3-63). In: Society for Research on Nicotine and Tobacco 9th Annual Meeting; 2003 February 19-22; New Orleans. 2003.
- Selby P, Brosky G, Baker R, Lertzman M, Dakin P, Roberts J. Zyban is effective in the retreatment of relapsed adult smokers (PO4 68). In: Society for Research on Nicotine and Tobacco 7th Annual Meeting; 2001 March 23-23; Seattle (WA). 2001:114.
Sheng 2013 {published data only}
- Sheng LX, Tang YL, Jiang ZN, Yao CH, Gao JY, Xu GZ, et al. Sustained-release bupropion for smoking cessation in a Chinese sample: a double-blind, placebo-controlled, randomized trial. Nicotine & Tobacco Research 2013;15(2):320-5. 9400123000018059 [DOI] [PubMed] [Google Scholar]
Siddiqi 2013 {published data only}
- Dogar O, Jawad M, Shah SK, Newell JN, Kanaan M, Khan MA, et al. Effect of cessation interventions on hookah smoking: post-hoc analysis of a cluster-randomized controlled trial. Nicotine & Tobacco Research 2014;16(6):682-8. [DOI] [PubMed] [Google Scholar]
- Siddiqi K, Khan A, Ahmad M, Dogar O, Kanaan M, Newell JN, et al. Action to stop smoking in suspected tuberculosis (assist) in Pakistan: A cluster randomized, controlled trial. Annals of Internal Medicine 2013;158(9):667-75. 9400107000000417 [DOI] [PubMed] [Google Scholar]
- Siddiqi K, Khan A, Ahmad M, Shafiq-ur-Rehman. An intervention to stop smoking among patients suspected of TB--evaluation of an integrated approach. BMC Public Health 2010;10:160. 9400123000011594 [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2004 {published data only}
- Caplan BJ. The "bupropion for smoking cessation" trial from a family practice perspective. Archives of Internal Medicine 2005;165:470. [DOI] [PubMed] [Google Scholar]
- Simon JA, Duncan C, Carmody TP, Hudes ES. Bupropion for smoking cessation: a randomized trial. Archives of Internal Medicine 2004;164:1797-803. [DOI] [PubMed] [Google Scholar]
- Simon JA, Duncan C, Carmody TP, Hudes ES. Bupropion plus nicotine replacement no better than replacement alone. Journal of Family Practice 2004;53:953-4. [Google Scholar]
Simon 2009 {published data only}
- Simon JA, Duncan C, Huggins J, Solkowitz S, Carmody TP. Sustained-release bupropion for hospital-based smoking cessation: a randomized trial. Nicotine & Tobacco Research 2009;11(6):663-9. [DOI] [PubMed] [Google Scholar]
Singh 2010 {published data only}
- Singh P, Kumar R. Assessment of the effectiveness of sustained release Bupropion and intensive physician advice in smoking cessation. Lung India 2010;27(1):11-8. 9400123000006043 [DOI] [PMC free article] [PubMed]
Smith 2009 {published data only}
- Loh WY, Piper ME, Schlam TR, Fiore MC, Smith SS, Jorenby DE, et al. Should all smokers use combination smoking cessation pharmacotherapy? Using novel analytic methods to detect differential treatment effects over 8 weeks of pharmacotherapy. Nicotine & Tobacco Research 2012;14(2):131-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine & Tobacco Research 2010;12(6):647-57. 9400123000005794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, McCarthy DE, Japuntich SJ, Christiansen B, Piper ME, Jorenby DE, et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Archives of Internal Medicine 2009;169(22):2148-55. 9400123000005493 [DOI] [PMC free article] [PubMed]
SMK20001 {unpublished data only}
- SMK 20001. A multi-center, double-blind, double-dummy, placebo-controlled, randomized, parallel group, dose response evaluation of a new chemical entity (NCE) and Zyban (bupropion hydrochloride) sustained release (300mg/day) versus placebo as aids to smoking cessation. gsk-studyregister.com/en/trial-details/?id=SMK20001 (accessed 2 March 2020).
Sood 2010 {published data only}
- NCT00405912. St. John's wort for tobacco cessation. clinicaltrials.gov/ct2/show/NCT00405912 (first received 30 November 2006).
- Sood A, Ebbert JO, Prasad K, Croghan IT, Bauer B, Schroeder DR. A randomized clinical trial of St. John's wort for smoking cessation. Journal of Alternative and Complementary Medicine 2010;16(7):761-7. 9400123000005770 [DOI] [PMC free article] [PubMed]
Sood 2012 {published data only}
- Sood A, Prasad K, Croghan IT, Schroeder DR, Ehlers SL, Ebbert JO. S-Adenosyl-L-methionine (SAMe) for smoking abstinence: A randomized clinical trial. Journal of Alternative and Complementary Medicine 2012;18(9):854-9. 9400123000015738 [DOI] [PMC free article] [PubMed] [Google Scholar]
Spring 2007 {published data only}
- Carrington A, Doran N, Spring B. Fluoxetine moderates the association between trait-anxiety and smoking status following behavioral treatment for smoking cessation (POS4-81). In: Society for Research on Nicotine and Tobacco 9th Annual Meeting; 2003 February 19-22; New Orleans (LA). 2003.
- NCT00113737. Fluoxetine as a quit smoking aid for depression prone. clinicaltrials.gov/ct2/show/NCT00113737 (first received 10 June 2005).
- Spring B, Doran N, Pagoto S, McChargue D, Cook JW, Bailey K, et al. Fluoxetine, smoking, and history of major depression: A randomized controlled trial. Journal of Consulting & Clinical Psychology 2007;75:85-94. [DOI] [PubMed] [Google Scholar]
- Spring B, Doran N, Pagoto S, McChargue DE, Cook JW, Bailey K, et al. Reduced abstinence for smokers previously treated with fluoxetine (PA1-1). In: Society for Research on Nicotine and Tobacco 10th Annual Meeting; 2004 February 18-21; Phoenix (AZ). 2004.
Stapleton 2013 {published data only}91464711
- Stapleton J, West R, Hajek P, Wheeler J, Vangeli E, Abdi Z, et al. Randomized trial of NRT, bupropion, and NRT plus bupropion for smoking cessation: effectiveness in clinical practice. Addiction 2013;108(12):2193-201. [DOI: 10.1111/add.12304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swan 2003 {published data only}
- Catz S, Jack LM, Swan GE, McClure J. Adherence to bupropion SR in a smoking cessation effectiveness trial (POS2-77). In: Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 February 15-18; Orlando (FL). 2006.
- Jack LM, Swan GE, Thompson E, Curry SJ, McAfee T, Dacey S, et al. Bupropion SR and smoking cessation in actual practice: methods for recruitment, screening, and exclusion for a field trial in a managed-care setting. Preventive Medicine 2003;36:585-93. [DOI] [PubMed] [Google Scholar]
- Javitz HS, Swan GE, Zbikowski SM, Curry SJ, McAfee TA, Decker DL, et al. Cost-effectiveness of different combinations of bupropion SR dose and behavioral treatment for smoking cessation: a societal perspective. American Journal of Managed Care 2004;10:217-26. [PubMed] [Google Scholar]
- McAfee T, Zbikowski SM, Bush T, McClure J, Swan G, Jack LM, et al. The effectiveness of bupropion SR and phone counseling for light and heavy smokers (PA2-1). In: Society for Research on Nicotine and Tobacco 10th Annual Meeting; 2004 February 18-21; Phoenix (AZ). 2004.
- Swan GE, Jack LM, Curry S, Chorost M, Javitz H, McAfee T, et al. Bupropion SR and counseling for smoking cessation in actual practice: Predictors of outcome. Nicotine & Tobacco Research 2003;5:911-21. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Javitz HS, McAfee T, McClure JB. Predictors of 12-month outcome in smokers who received bupropion sustained-release for smoking cessation. Central Nervous System Drugs 2008;22(3):239-56. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Valdes AM, Ring HZ, Ton CC, Curry SJ, et al. Joint effect of dopaminergic genes on likelihood of smoking following treatment with bupropion SR. Health Psychology 2007;26:361-8. [DOI] [PubMed] [Google Scholar]
- Swan GE, Javitz HS, Jack LM, Curry SJ, McAfee T. Heterogeneity in 12-month outcome among female and male smokers. Addiction 2004;99:237-50. [DOI] [PubMed] [Google Scholar]
- Swan GE, McAfee T, Curry SJ, Jack LM, Javitz H, Dacey S, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Archives of Internal Medicine 2003;163:2337-44. [DOI] [PubMed] [Google Scholar]
- Swan GE, Valdes AM, Ring HZ, Khroyan TV, Jack LM, Ton CC, et al. Dopamine receptor DRD2 genotype and smoking cessation outcome following treatment with bupropion SR. Pharmacogenomics Journal 2005;5:21-9. [DOI] [PubMed] [Google Scholar]
Tashkin 2001 {published data only}
- Patel MK, Tashkin DP, Kanner RE, Bailey WC, Buist A, Anderson PJ, et al. A multicenter evaluation of the effects of bupropion hydrochloride sustained release tablets (Bup SR) versus placebo in a population of smokers with chronic obstructive pulmonary disease (PO130). In: 11th World Conference on Tobacco or Health; 2000 Aug 6-11; Chicago (IL). Vol. 1. 2000:118.
- Tashkin D, Kanner R, Bailey W, Buist S, Anderson P, Nides M, et al. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet 2001;357:1571-5. [DOI] [PubMed] [Google Scholar]
Tidey 2011 {published data only}
- NCT00136760. Contingent incentives plus bupropion for smoking in people with schizophrenia. clinicaltrials.gov/ct2/show/NCT00136760 (first received 29 August 2005).
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Reid N. Effects of contingency management and bupropion on cigarette smoking in smokers with schizophrenia. Psychopharmacology 2011;217(2):279-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tonnesen 2003 {published data only}
- Bollinger CT, Gilljam H, Lebargy F, Spiegel PI, Edwards J, Hider A, et al. Bupropion hydrochloride (Zyban) is effective and well tolerated as an aid to smoking cessation - a multicountry study. Abstract and presentation at 11th Annual meeting of European Respiratory Society, Berlin, September 22-26 2001. European Respiratory Journal 2001;18(Suppl 33):12s. [Google Scholar]
- Tonnesen P, Tonstad S, Hjalmarson A, Lebargy F, Spiegel PI, Hider A, et al. A multicentre, randomized, double-blind, placebo-controlled, 1-year study of bupropion SR for smoking cessation. Journal of Internal Medicine 2003;254:184-92. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Aaserud E, Hjalmarson A, Peiffer G, Molen T, Hider A, et al. Zyban is an effective and well tolerated aid to smoking cessation in a general smoking population - a multi-country study. Society for Research on Nicotine and Tobacco 3rd Europe Conference; 2001 September 19-22; Paris, France 2001:46.
Tonstad 2003 {published data only}
- McRobbie H, Brath H, Astbury C, Hider A, Sweet R. Bupropion hydrochloride sustained release (SR) is an effective and well tolerated aid to smoking cessation in smokers with cardiovascular disease 12 month follow-up phase data (ZYB40014). In: Abstract and Presentation at European Respiratory Society meeting; 2002 September 14-18; Stockholm, Sweden. 2002.
- Tonstad S, Farsang C, Klaene G, Lewis K, Manolis A, Perruchoud AP, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. European Heart Journal 2003;24:946-55. [DOI] [PubMed] [Google Scholar]
- Spiegel PI, Lewis K, Seinost G, Astbury C, Hider A, Sweet R. Bupropion hydrochloride (Zyban) is an effective and well tolerated aid to smoking cessation in smokers with cardiovascular disease - a multicountry study. Abstract and presentation at 11th Annual meeting of European Respiratory Society, Berlin, September 22-26. European Respiratory Journal 2001;18(Suppl 33):13s. [Google Scholar]
Urdapilleta‐Herrera 2013 {published data only}
- Urdapilleta-Herrera E, Pina-Rosales MF, Vargas-Rojas MI, Ramirez-Venegas A, Sansores-Martinez R. Bupropion together with cognitive-conductual therapy (CBT) is an alternative for a long-term abstinence of smoking. European Respiratory Journal 2013;42(57):3347. [Google Scholar]
Uyar 2007 {published data only}
- Uyar M, Bayram N, Filiz A, Elbek O, Topçu A, Dikensoy O, et al. Comparison of nicotine patch and bupropion in treating tobacco dependence. European Respiratory Journal 2005;26(Suppl 49):388s. [Google Scholar]
- Uyar M, Filiz A, Bayram N, Elbek O, Herken H, Topcu A, et al. A randomized trial of smoking cessation. Medication versus motivation. Saudi Medical Journal 2007;28(6):922-6. [PubMed] [Google Scholar]
Wagena 2005 {published data only}
- Quaak M, Schayck Constant P, Postma Dirkje S, Wagena Edwin J, Schooten Frederik J. Genetic variants in the serotonin transporter influence the efficacy of bupropion and nortriptyline in smoking cessation. Addiction 2012;107(1):178-87. 9400123000013216 [DOI] [PubMed]
- Schayck CP, Kaper J, Wagena EJ, Wouters EF, Severens JL. The cost-effectiveness of antidepressants for smoking cessation in chronic obstructive pulmonary disease (COPD) patients. Addiction 2009;104(12):2110-7. 9400123000005573 [DOI] [PubMed]
- Wagena EJ, Knipschild PG, Huibers MJ, Wouters EF, Schayck CP. Efficacy of bupropion and nortriptyline for smoking cessation among people who are at risk for or have chronic obstructive pulmonary disease: Results from a randomized, placebo-controlled trial. Nicotine & Tobacco Research 2005;7(4):683-4. 9400123000004372 [Google Scholar]
Weinberger 2010 {published and unpublished data}
- Weinberger AH, George TP, Mckee SA. Differences in smoking expectancies in smokers with and without a history of major depression. Addictive Behaviors 2011;36(4):434-7. 9400123000005996 [DOI] [PMC free article] [PubMed]
- Weinberger AH, Mckee SA, George TP. Changes in smoking expectancies in abstinent, reducing, and non-abstinent participants during a pharmacological trial for smoking cessation. Nicotine & Tobacco Research 2010;12(9):937-43. 9400123000005825 [DOI] [PMC free article] [PubMed]
- Weinberger AH, Reutenauer EL, Jatlow PI, O'Malley SS, Potenza MN, George TP. A double-blind, placebo-controlled, randomized clinical trial of oral selegiline hydrochloride for smoking cessation in nicotine-dependent cigarette smokers. Drug and Alcohol Dependence 2010;107(2-3):188-95. 9400123000005586 [DOI] [PMC free article] [PubMed]
- Weinberger AH, Reutenauer EL, O'Malley SS, Potenza MN, George TP. A randomized placebo-controlled clinical trial of selegiline for smoking cessation: Preliminary results (POS5-29). Society for Research on Nicotine and Tobacco 15th Annual Meeting; 2009 April 27-30; Dublin, Ireland.
- Weinberger AH, Reutenauer EL, Solorzano M, O'Malley SS, Potenza MN, George TP. A randomized placebo controlled clinical trial of selegiline for smoking cessation (abstract 653). CPDD 71st Annual Meeting; 2009 June 20-25; Reno (NV).
Weiner 2012 {published data only}
- Mann-Wrobel MC, Bennett ME, Weiner EE, Buchanan RW, Ball MP. Smoking history and motivation to quit in smokers with schizophrenia in a smoking cessation program. Schizophrenia Research 2011;126(1-3):277-83. 9400123000006008 [DOI] [PubMed]
- Weiner E, Ball MP, Buchholz AS, Gold JM, Evins AE, McMahon RP, et al. Bupropion sustained release added to group support for smoking cessation in schizophrenia: a new randomized trial and a meta-analysis. Journal of Clinical Psychiatry 2012;73(1):95-102. 9400123000011967 [DOI] [PubMed]
White 2005 {published data only}
- White WD, Crockford D, Patten S, el Guebaly N. A randomized, open-label pilot comparison of gabapentin and bupropion SR for smoking cessation. Nicotine & Tobacco Research 2005;7(5):809-13. [DOI] [PubMed] [Google Scholar]
Wittchen 2011 {published data only}
- Wittchen HU, Hoch E, Klotsche J, Muehlig S. Smoking cessation in primary care - a randomized controlled trial of bupropion, nicotine replacements, CBT and a minimal intervention. International Journal of Methods in Psychiatric Research 2011;20(1):28-39. 9400123000009843 [DOI] [PMC free article] [PubMed] [Google Scholar]
Zellweger 2005 {published data only}
- Puska P, Brath H, Astbury C, Hider AE. Zyban is an effective and well tolerated aid to smoking cessation in a healthcare professionals population - a multi-country study. In: Society for Research on Nicotine and Tobacco 3rd European Conference; 2001 September 19-22; Paris, France. 2001:45.
- Zellweger JP, Blaziene A, Astbury C, Hider A, Hogue S. Bupropion hydrochloride sustained release is an effective and well tolerated aid to smoking cessation in a healthcare professionals population - a multicountry study. Abstract and presentation at 11th Annual meeting of European Respiratory Society, Berlin, September 22-26 2001. European Respiratory Journal 2001;18(Suppl 33):166s. [Google Scholar]
- Zellweger JP, Boelcskei PL, Carrozzi L, Sepper R, Sweet R, Hider AZ. Bupropion SR vs placebo for smoking cessation in health care professionals. American Journal of Health Behavior 2005;29:240-9. [DOI] [PubMed] [Google Scholar]
Zincir 2013 {published data only}
- Zincir SB, Kaymak E, Sunbul EA, Zincir N. Comparison of the effects of varenicline, bupropion and nicotine replacement therapy on smoking cessation program [Vareniklin, bupropion ve nikotin yerine koyma tedavilerinin sigara birakma programini surdurme uzerine etkilerinin karsilastirilmasi]. Bulletin of Clinical Psychopharmacology 2012;22 Suppl 1:S35. [Google Scholar]
- Zincir SB, Zincir S, Kaymak E, Sunbul EA. Comparison of the effectiveness of varenicline, extended-release bupropion and nicotine replacement therapy on the success and the maintenance of a smoking cessation program. Klinik Psikofarmakoloji Bulteni 2013;23(3):224-30. [Google Scholar]
References to studies excluded from this review
Akbarpour 2010 {published data only}
- Akbarpour F, Rezaei O, Khodaie-Ardakani MR, Sheikhvatan M, Goodarzi H, Dolatshahi B. A double-blind placebo-controlled trial of bupropion for smoking abstinence and cognition improvement in schizophrenia. Minerva Psichiatrica 2010;51(4):263-9. 9400123000016548
Aryanpur 2016 {published data only}
- Aryanpur M, Hosseini M, Masjedi MR, Mortaz E, Tabarsi P, Soori H, et al. A randomized controlled trial of smoking cessation methods in patients newly-diagnosed with pulmonary tuberculosis. BMC Infectious Diseases 2016;16:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banham 2010 {published data only}
Becker 2003 {unpublished data only}
- Becker B, Bock B, Carmona-Barros R. St. John's Wort oral spray reduces withdrawal symptoms during quitting smoking (POS4-82). In: Society for Research on Nicotine and Tobacco 9th Annual Meeting; 2003 February 19-22; New Orleans (LA). 2003.
Berlin 2005 {published data only}
- Berlin I, Covey LS, Jiang HP, Hamer D. Lack of effect of D2 dopamine receptor TaqI A polymorphism on smoking cessation. Nicotine & Tobacco Research 2005;7:725-8. [DOI] [PubMed] [Google Scholar]
Bloch 2010 {published data only}
Bowen 1991 {published data only}
- Bowen DJ, Spring B, Fox E. Tryptophan and high-carbohydrate diets as adjuncts to smoking cessation therapy. Journal of Behavioral Medicine 1991;14(2):97-110. [DOI] [PubMed] [Google Scholar]
Brauer 2000 {unpublished data only}
- Brauer LH, Paxton DA, Stock CT, Rose JE. Selegiline and transdermal nicotine for smoking cessation. Society for Research on Nicotine and Tobacco Annual Conference; 2000 Feb 18-20; Arlington (VA) 2000.
Breitling 2008 {published data only}
- Breitling LP, Twardella D, Brenner H. High effectiveness of short treatment with bupropion for smoking cessation in general care. Thorax 2008;63:476-7. [DOI] [PubMed] [Google Scholar]
Brody 2013 {published data only}
- Brody AL, Mukhin AG, Stephanie Shulenberger, Mamoun MS, Kozman M, Phuong J, et al. Treatment for tobacco dependence: effect on brain nicotinic acetylcholine receptor density. Neuropsychopharmacology 2013;38(8):1548-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrão 2007 {published data only}
- Carrao JL, Moreira LB, Fuchs FD. The efficacy of the combination of sertraline with buspirone for smoking cessation. A randomized clinical trial in nondepressed smokers. European Archives of Psychiatry & Clinical Neuroscience 2007;257:383-8. [DOI] [PubMed] [Google Scholar]
Chan 2005 {published data only}
- Chan B, Einarson A, Koren G. Effectiveness of bupropion for smoking cessation during pregnancy. Journal of Addictive Diseases 2005;24(2):19-23. [DOI] [PubMed] [Google Scholar]
Chandrashekar 2015 {published data only}
- Chandrashekar M, Sattar FA, Bondade S, Kumar KK. A comparative study of different modalities of treatment in nicotine dependence syndrome. Asian Journal of Psychiatry 2015;17:29-35. [DOI] [PubMed] [Google Scholar]
Christenhusz 2012 {published data only}
- Christenhusz LC, Prenger R, Pieterse ME, Seydel ER, Palen J. Cost-effectiveness of an intensive smoking cessation intervention for COPD outpatients. Nicotine & Tobacco Research 2012;14(6):657-63. [DOI] [PubMed] [Google Scholar]
Cornelius 1997 {published data only}
- Cornelius JR, Salloum IM, Ehler JG, Jarrett PJ, Cornelius MD, Black A, et al. Double-blind fluoxetine in depressed alcoholic smokers. Psychopharmacology Bulletin 1997;33:165-70. [PubMed] [Google Scholar]
Cornelius 1999 {published data only}
- Cornelius JR, Perkins KA, Salloum IM, Thase ME, Moss HB. Fluoxetine versus placebo to decrease the smoking of depressed alcoholic patients [letter]. Journal of Clinical Psychopharmacology 1999;19:183-4. [DOI] [PubMed] [Google Scholar]
Covey 2007 {published data only}
- Covey LS, Botello-Harbaum M, Glassman AH, Masmela J, LoDuca C, Salzman V, et al. Smokers' response to combination bupropion, nicotine patch, and counseling treatment by race/ethnicity. Ethnicity & Disease 2008;18:59-64. [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Jiang H, Fried J, Masmela J, LoDuca C, et al. A randomized trial of bupropion and/or nicotine gum as maintenance treatment for preventing smoking relapse. Addiction 2007;102:1292-302. [DOI] [PubMed] [Google Scholar]
Croghan 2007 {published data only}
- Clark MM, Hurt RD, Croghan IT, Patten CA, Novotny P, Sloan JA, et al. The prevalence of weight concerns in a smoking abstinence clinical trial. Addictive Behaviors 2006;31:1144-52. [DOI] [PubMed] [Google Scholar]
- Croghan IT, Hurt RD, Croghan GA, Sloan JA. Comparing nicotine inhaler, bupropion and nicotine inhaler plus bupropion in treating tobacco dependence [abstract]. Nicotine & Tobacco Research 2005;7:680-1. [Google Scholar]
- Croghan IT, Hurt RD, Dakhil SR, Croghan GA, Sloan JA, Novotny PJ, et al. Randomized comparison of a nicotine inhaler and bupropion for smoking cessation and relapse prevention. Mayo Clinic Proceedings 2007;82:186-95. [DOI] [PubMed] [Google Scholar]
- Croghan IT, Hurt RD, Ebbert JO, Croghan GA, Polk Jr OD, Stella PJ, et al. Racial differences in smoking abstinence rates in a multicenter, randomized, open-label trial in the United States. Journal of Public Health 2010;18(1):59-68. 9400123000006023 [DOI] [PMC free article] [PubMed]
Cropsey 2015 {published data only}
- Cropsey KL, Jardin BF, Burkholder GA, Clark CB, Raper JL, Saag MS. An algorithm approach to determining smoking cessation treatment for persons living with HIV/AIDS: results of a pilot trial. Journal of Acquired Immune Deficiency Syndromes 2015;69(3):291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalack 1995 {published data only}
- Dalack GW, Glassman AH, Rivelli S, Covey LS, Stetner F. Mood, major depression, and fluoxetine response in cigarette smokers. American Journal of Psychiatry 1995;152:398-403. [DOI] [PubMed] [Google Scholar]
Dale 2002 {published data only}
- Dale LC, Ebbert JO, Schroeder DR, Croghan IT, Rasmussen DF, Trautman JA, et al. Bupropion for the treatment of nicotine dependence in spit tobacco users: a pilot study. Nicotine Tobacco Research 2002;4(3):267-74. [DOI] [PubMed] [Google Scholar]
Dale 2007 {published data only}
- Dale LC, Ebbert JO, Glover ED, Croghan IT, Schroeder DR, Severson HH, et al. Bupropion SR for the treatment of smokeless tobacco use. Drug & Alcohol Dependence 2007;90(1):56-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JL, Ebbert JO, Patten CA, Dale LC, Bronars CA, Schroeder DR. Measuring nicotine dependence among smokeless tobacco users. Addictive Behaviors 2006;31(9):1511-21. [DOI] [PubMed] [Google Scholar]
Daniela 2008 {published data only}
- Daniela I, Carmen C. The combination of sertraline with buspirone for smoking cessation process - The effectiveness and adverse events. Toxicology Letters 2008;180(Suppl 1):S130. [Google Scholar]
Edwards 1989 {published data only}
- Edwards NB, Murphy JK, Downs AD, Ackerman BJ, Rosenthal TL. Doxepin as an adjunct to smoking cessation: a double-blind pilot study. American Journal of Psychiatry 1989;146(3):373-6. [DOI] [PubMed] [Google Scholar]
- Murphy JK, Edwards NB, Downs AD, Ackerman BJ, Rosenthal TL. Effects of doxepin on withdrawal symptoms in smoking cessation. American Journal of Psychiatry 1990;147(10):1353-7. [DOI] [PubMed] [Google Scholar]
EUCTR2005‐006189‐32‐AT {published data only}
- EU Clinical Trials Register. Clinical evaluation study: "Bupropion versus Psychodynamic mental training according to Dr. Grohs for smoke withdrawal" [Klinische Evaluierungsstudie: "Bupropion versus Psychodynamisches Mentaltraining nach Dr. Grohs zur Raucherentwohnung]. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2005-006189-32-AT (first received 1 February 2006).
Evins 2008 {published data only}
- Evins AE, Alpert JE, Pava J, Petersen TJ, Farabaugh AH, Fava M. A double blind placebo controlled trial of bupropion added to nicotine patch and cognitive behavioral therapy in smokers with current or past unipolar depressive disorder. Neuropsychopharmacology 2005;30 Suppl 1:S91. [Google Scholar]
- Evins AE, Culhane MA, Alpert JE, Pava J, Liese BS, Farabaugh A, et al. A controlled trial of bupropion added to nicotine patch and behavioral therapy for smoking cessation in adults with unipolar depressive disorders. Journal of Clinical Psychopharmacology 2008;28:660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fatemi 2005 {published data only}
- Fatemi SH, Stary JM, Hatsukami DK, Murphy SE. A double-blind placebo-controlled cross over trial of bupropion in smoking reduction in schizophrenia. Schizophrenia Research 2005;76(2-3):353-6. [DOI] [PubMed] [Google Scholar]
Frederick 1997 {published data only}
- Frederick SL, Hall SM, Sees KL. The effect of venlafaxine on smoking cessation in subjects with and without a history of depression. NIDA Research Monograph 1997;174:208. [Google Scholar]
Gawin 1989 {published data only}
- Gawin F, Compton M, Byck R. Buspirone reduces smoking [letter]. Archives of General Psychiatry 1989;46(3):288-9. [DOI] [PubMed] [Google Scholar]
Gifford 2011 {published data only}
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, et al. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior Therapy 2011;42(4):700-15. 9400123000012398 [DOI] [PubMed]
Glover 2002 {published data only}
- Glover ED, Glover PN, Sullivan CR, Cerullo CL, Hobbs G. A comparison of sustained-release bupropion and placebo for smokeless tobacco cessation. American Journal of Health Behavior 2002;26(5):386-93. [DOI] [PubMed] [Google Scholar]
Gold 2002 {published data only}
- Gold PB, Rubey RN, Harvey RT. Naturalistic, self-assignment comparative trial of bupropion SR, a nicotine patch, or both for smoking cessation treatment in primary care. American Journal of Addiction 2002;11(4):315-31. [DOI] [PubMed] [Google Scholar]
Grandi 2011 {published data only}
- Grandi S, Shimony A, Eisenberg MJ. The efficacy and safety of bupropion started in-hospital for smoking cessation in patients with cardiovascular disease: A systematic review and meta-analysis. Circulation 2011;124(21 Suppl 1). 9400123000012459 [9400123000012459]
Grassi 2009 {published data only}
Hall 2009 {published data only}
- Hall SM, Humfleet GL, Muñoz RF, Reus VI, Robbins JA, Prochaska JJ. Extended treatment of older cigarette smokers. Addiction 2009;104(6):1043-52. 9400123000005370 [DOI] [PMC free article] [PubMed]
Hall 2011 {published data only}
- Hall SM, Humfleet GL, Munoz RF, Reus VI, Prochaska JJ, Robbins JA. Using extended cognitive behavioral treatment and medication to treat dependent smokers. American Journal of Public Health 2011;101(12):2349-56. 9400123000011870 [DOI] [PMC free article] [PubMed]
- Prochaska JJ, Hall SM, Humfleet G, Munoz RF, Reus V, Gorecki J, et al. Physical activity as a strategy for maintaining tobacco abstinence: a randomized trial. Preventive Medicine 2008;47(2):215-20. 9400123000004887 [DOI] [PMC free article] [PubMed]
Hatsukami 2004 {published data only}
- Hatsukami DK, Rennard S, Patel MK, Kotlyar M, Malcolm R, Nides MA, et al. Effects of sustained-release bupropion among persons interested in reducing but not quitting smoking. American Journal of Medicine 2004;116:151-7. [DOI] [PubMed] [Google Scholar]
- Rennard S, Hatsukami D, Malcolm RE, Patel MK, Jamerson BD, Dozier G. Zyban (bupropion HCL SR) vs placebo as an aid to smoking reduction among smokers unwilling and unable to quit smoking (PO4 77). In: Society for Research on Nicotine and Tobacco 7th Annual Meeting; 2001 March 23-23; Seattle (WA). 2001:117.
Hawk 2008 {unpublished data only}
- Hawk LW, Mahoney MC, Ashare RL, Rhodes JD, Oliver JA, Cummings KM, et al. Preliminary evidence of extinction of smoking behavior with bupropion (PA9-4). In: Society for Research on Nicotine and Tobacco 14th Annual Meeting; 2008 February 26-March 1; Portland (OR). 2008.
Hawk 2015 {published data only}
- Hawk LW Jr, Ashare RL, Rhodes JD, Oliver JA, Cummings KM, Mahoney M. Does extended pre quit bupropion aid in extinguishing smoking behavior? Nicotine & Tobacco Research 2015;17(11):1377-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hays 2001 {published data only}
- Abel GA, Hays JT, Decker PA, Croghan GA, Kuter DJ, Rigotti NA. Effects of biochemically confirmed smoking cessation on white blood cell count. Mayo Clinical Proceedings 2005;80:1022-8. [DOI] [PubMed] [Google Scholar]
- Cox LS, Patten CA, Niaura RS, Decker PA, Rigotti N, Sachs DP, et al. Efficacy of bupropion for relapse prevention in smokers with and without a past history of major depression. Journal of General Internal Medicine 2004;19:828-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcan MJ, Deener G, White J, Johnston JA, Gonzales D, Niaura R, et al. The effect of bupropion sustained-release on cigarette craving after smoking cessation. Clinical Therapeutics 2002;24:540-51. [DOI] [PubMed] [Google Scholar]
- Durcan MJ, Johnston JA, White J, Gonzales D, Sachs DP, Rigotti N, et al. Bupropion SR for relapse prevention: a "slips-allowed" analysis. American Journal of Health Behavior 2004;28(5):456-63. [PubMed] [Google Scholar]
- Gonzales D, Bjornson W, Durcan MJ, White JD, Johnston JA, Buist AS, et al. Effects of gender on relapse prevention in smokers treated with bupropion SR. American Journal of Preventive Medicine 2002;22:234-39. [DOI] [PubMed] [Google Scholar]
- Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. A randomized, controlled trial. Annals of Internal Medicine 2001;135:423-33. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Wolter TD, Rigotti N, Hays JT, Niaura R, Durcan MJ, et al. Bupropion for pharmacologic relapse prevention to smoking - Predictors of outcome. Addictive Behaviors 2002;27(4):493-507. [DOI] [PubMed] [Google Scholar]
- Rigotti N, Thorndike AN, Durcan MJ, White JD, Johnston AJ, Niaura R, et al. Attenuation of post-cessation weight gain in smokers taking bupropion: The effect of gender. In: Abstract Book. Society for Research on Nicotine and Tobacco 6th Annual Meeting; 2000 Feb 18-20; Arlington (VA). 2000.
Hays 2009 {published data only}
- Hays JT, Hurt RD, Decker PA, Croghan IT, Offord KP, Patten CA. A randomized, controlled trial of bupropion sustained-release for preventing tobacco relapse in recovering alcoholics. Nicotine & Tobacco Research 2009;11(7):859-67. 9400123000005366 [DOI] [PMC free article] [PubMed]
Hilberink 2005 {published data only}
Hitsman 1999 {published data only}
- Hitsman B, Pingitore R, Spring B, Mahableshwarkar A, Mizes JS, Segraves KA, et al. Antidepressant pharmacotherapy helps some cigarette smokers more than others. Journal of Consulting and Clinical Psychiatry 1999;67:547-54. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Spring B, Borrelli B, Niaura R, Papandonatos G. Adherence to medication versus behavioral therapy as predictors of smoking cessation in combined treatment involving fluoxetine [abstract]. Society for Research on Nicotine and Tobacco Annual Conference; 2000 Feb 18-20; Arlington (VA) 2000.
Houtsmuller 2002 {published data only}
- Houtsmuller EJ, Stitzer ML. Selegiline effects on smoking and abstinence [abstract]. CPDD Annual Meeting; 1998 June 12-17; Scottsdale (AZ) 1998.
- Houtsmuller EJ, Thornton JA, Stitzer ML. Effects of selegiline (l-deprenyl) during smoking and short-term abstinence. Psychopharmacology (Berl) 2002;163:213-20. [DOI] [PubMed] [Google Scholar]
Hurt 2003 {published and unpublished data}
- Hurt RD, Croghan GA, Sloan JA, Krook JE, Silberstein PT. Bupropion for relapse prevention after nicotine patch therapy [PA 5B abstract ]. In: Society for Research on Nicotine and Tobacco 7th Annual Meeting; 2001 March 23-23; Seattle (WA). 2001:32.
- Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. Journal of Clinical Oncology 2003;21:914-20. [DOI] [PubMed] [Google Scholar]
Hussain 2010 {published data only}
Isgro 2015 {published data only}
- Isgro M, Doran N, Heffner JL, Bekman N, Wong E, Tibbs J. Type a/type b alcoholism predicts differential response to topiramate in a smoking cessation trial in dually-diagnosed men. Alcoholism: Clinical and Experimental Research 2015;39(Suppl 1):76A. [Google Scholar]
Jacobs 1971 {published data only}
- Jacobs MA, Spilken AZ, Norman MM, Wohlberg GW, Knapp PH. Interaction of personality and treatment conditions associated with success in a smoking control program. Psychosomatic Medicine 1971;33(6):545-56. [DOI] [PubMed] [Google Scholar]
Kalman 2004 {unpublished data only}
- Kalman D, Engler P, Monti P. Preliminary findings from a pilot treatment study of smokers in early alcohol recovery (POS1-072). In: Society for Research on Nicotine and Tobacco 10th Annual Meeting; 2004 February 18-21, Phoenix (AZ). 2004.
Khunrong 2016 {published data only}
- Khunrong P, Sittipunt C. Comparison of efficacy of varenicline and nortriptyline - short-term smoking cessation in outpatient setting. European Respiratory Journal 2016;48(60):4601. [Google Scholar]
Killen 2006 {published data only}
- Killen JD, Fortmann SP, Murphy GM Jr, Hayward C, Arredondo C, Cromp D, et al. Extended treatment with Bupropion SR for cigarette smoking cessation. Journal of Consulting and Clinical Psychology 2006;74(2):286-94. [DOI] [PubMed] [Google Scholar]
- Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Schatzberg AF, et al. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics 2011;156(3):275-84. 9400123000006100 [DOI] [PubMed]
Kotz 2009 {published data only}
- Kotz D, Wesseling G, Huibers MJ, Schayck OC. Efficacy of confrontational counselling for smoking cessation in smokers with previously undiagnosed mild to moderate airflow limitation: study protocol of a randomized controlled trial. BMC Public Health 2007;7:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz D, Wesseling G, Huibers MJ, Schayck OC. Efficacy of confronting smokers with airflow limitation for smoking cessation. European Respiratory Journal 2009;33:754-62. [DOI] [PubMed] [Google Scholar]
Kras 2010 {published data only}
- Kras M, Stough C, Scholey A, Kure C, Camfield D. Hypericum perforatum, nicotine patches and combination hypericum perforatum/nicotine patches for smoking cessation. European Neuropsychopharmacology 2010;20:S608-9. 9400123000006088
Lawvere 2006 {published data only}
- Lawvere S, Mahoney MC, Cummings KM, Hyland AJ. St John's Wort for smoking cessation: twelve months post cessation. In: Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005 March 20-23; Prague, Czech Republic. 2005.
- Lawvere S, Mahoney MC, Cummings KM, Kepner JL, Hyland A, Lawrence DD, et al. A Phase II study of St. John's Wort for smoking cessation. Complementary Therapies in Medicine 2006;14(3):175-84. [DOI] [PubMed] [Google Scholar]
Li 2009 {published data only}
- Li J, Zhang T, Wang B, Li X. An efficacy analysis of bupropion for smoking cessation in schizophrenia. Zhongguo Xinyao Yu Linchuang Zazhi [Chinese Journal of New Drugs and Clinical Remedies] 2009;28(3):231-4. 9400123000016534
Miller 2003 {published data only}
- Miller H, Ranger-Moore J, Hingten M. Bupropion SR for smoking cessation in pregnancy: a pilot study [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6):S133. [Google Scholar]
Monuteaux 2007 {published data only}
- Monuteaux MC, Spencer TJ, Faraone SV, Wilson AM, Biederman J. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry 2007;68(7):1094-101. [DOI] [PubMed] [Google Scholar]
Mooney 2008 {published data only}
- Mooney ME, Poling J, Gonzalez G, Gonsai K, Kosten T, Sofuoglu M. Preliminary study of buprenorphine and bupropion for opioid-dependent smokers. American Journal on Addictions 2008;17(4):287-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M, Gonzalez G, Gonsai K, Poling J, Kosten T. Buprenorphine and bupropion combination for opioid-dependent smokers. In: 68th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2006 June 17-22; Scottsdale (AZ). 2006.
Mooney 2016 {published data only}
- Mooney ME, Schmitz JM, Allen S, Grabowski J, Pentel P, Oliver A. Bupropion and naltrexone for smoking cessation: a double-blind randomized placebo-controlled clinical trial. Clinical Pharmacology and Therapeutics 2016;100(4):344-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naranjo 1990 {published data only}
- Naranjo CA, Kadlec KE, Sanhueza P, Woodley Remus D, Sellers EM. Fluoxetine differentially alters alcohol intake and other consummatory behaviors in problem drinkers. Clinical Pharmacology and Therapeutics 1990;47:490-8. [DOI] [PubMed] [Google Scholar]
NCT00032084 {published data only}
- NCT00032084. S0002 - a program to quit smoking with or without bupropion in treating patients with stage I or II non-small cell lung cancer who have undergone surgery. clinicaltrials.gov/show/NCT00032084 (first received 27 January 2003).
NCT00119210 {published data only}
- NCT00119210. Pilot study of bupropion for smoking cessation in postpartum non-breastfeeding women. clinicaltrials.gov/show/NCT00119210 (accessed 13 July 2005).
NCT00136747 {published data only}
- NCT00136747. The effects of memantine and bupropion on acute, reinforcing, and conditioned effects of cigarettes - 1. clinicaltrials.gov/show/NCT00136747 (accessed 29 August 2005).
NCT00136786 {published data only}
- NCT00136786. Effect of memantine versus bupropion on smoking relapse in nicotine-dependent individuals - 3. clinicaltrials.gov/show/NCT00136786 (first received 29 August 2005).
NCT00158171 {published data only}
- NCT00158171. Effectiveness of various smoking cessation therapies in reducing smoking in adolescents - 1. clinicaltrials.gov/show/NCT00158171 (first received 29 August 2005).
NCT00248118 {published data only}
- NCT00248118. Efficacy and safety of bupropion for treatment of adolescent smoking. clinicaltrials.gov/ct2/show/NCT00248118 (first received 3 November 2005).
NCT00320697 {published data only}
- NCT00320697. Smoking relapse prevention in schizophrenia. clinicaltrials.gov/show/NCT00320697 (first received 3 May 2006).
NCT00390923 {published data only (unpublished sought but not used)}
- NCT00390923. Testing a full substitution therapy approach as treatment of tobacco dependence. clinicaltrials.gov/ct2/show/NCT00390923 (first received 23 October 2006).
NCT00484692 {published data only}
- NCT00484692. Randomized trial of ultrashort psychotherapy vs sustained-release bupropion for smoking cessation. clinicaltrials.gov/show/NCT00484692 (first received 3 May 2006).
NCT00580853 {published data only}
- NCT00580853. The effect of varenicline (Chantix) and bupropion (Zyban) on smoking lapse behavior. clinicaltrials.gov/show/nct00580853 (first received 27 December 2007).
NCT00670904 {published data only}
- NCT00670904. Randomized trial assessing the effectiveness of a pharmacist-delivered program for smoking cessation. clinicaltrials.gov/show/NCT00670904 (first received 2 May 2008). [DOI] [PubMed]
NCT00936299 {published data only}
- NCT00936299. Bupropion for ADHD in adolescents with substance use disorder. clinicaltrials.gov/ct2/show/NCT00936299 (first received 10 July 2009).
NCT01850589 {published data only}
- NCT01850589. Comparison of conservative and aggressive smoking cessation treatment strategies in a vascular surgery office practice. clinicaltrials.gov/show/NCT01850589 (first received 10 July 2009).
NCT01965405 {published data only}
- NCT01965405. Smoking cessation for people living with HIV/AIDS. clinicaltrials.gov/show/nct01965405 (first received 18 October 2013).
NCT02736474 {published data only}
- NCT02736474. Naltrexone and bupropion combination on obese, smoking patients with schizophrenia. clinicaltrials.gov/show/NCT02736474 (first received 13 April 2016).
NCT03471767 {published data only}
- NCT03471767. AXS-05 phase II trial on smoking behavior. clinicaltrials.gov/show/NCT03471767 (first received 21 March 2018).
NCT03920319 {published data only}
- NCT03920319. Bupropion and cigarette-related cues in smokers. clinicaltrials.gov/show/nct03920319 (first received 18 April 2019).
Neumann 2000 {published and unpublished data}
- Neumann JK, Peeples B, East J, Ellis AR. Nicotine reduction: effectiveness of bupropion. British Journal of Psychiatry 2000;177:87-8. [DOI] [PubMed] [Google Scholar]
Neumann 2002 {published data only}
- Neumann JK, Peeples B, Seneker A. Nicotine reduction and bupropion. Chest 2002;121:1378. [DOI] [PubMed] [Google Scholar]
Niederhofer 2004 {published data only}
- Niederhofer H, Huber M. Bupropion may support psychosocial treatment of nicotine-dependent adolescents: preliminary results. Pharmacotherapy 2004;24(11):1524-8. [DOI] [PubMed] [Google Scholar]
Olmstead 1999 {published data only}
- Olmstead R, Kelly J, Chin C, Iwamoto-Schaap PN, Madsen DC, Huerta L, et al. Combined bupropion and mecamylamine treatment for smoking cessation: a pilot trial. In: Society for Research on Nicotine and Tobacco Fifth Annual Meeting; 1999 March 5-7; San Diego (CA). 1999.
Paluck 2006 {published data only}
- Paluck EC, McCormack JP, Ensom MH, Levine M, Soon JA, Fielding DW. Outcomes of bupropion therapy for smoking cessation during routine clinical use. Annals of Pharmacotherapy 2996;40(2):185-90. [DOI] [PubMed] [Google Scholar]
Pomerleau 1991 {published data only}
- Pomerleau OF, Pomerleau CS, Morrell EM, Lowenbergh JM. Effects of fluoxetine on weight gain and food intake in smokers who reduce nicotine intake. Psychoneuroendocrinology 1991;16:433-40. [DOI] [PubMed] [Google Scholar]
Raynor 2005 {published data only}
- Raynor DA. Adherence to pharmacological smoking cessation treatment among weight-concerned women. Dissertation Abstracts International: Section B: The Sciences and Engineering 2005;65(8-B):4301. [Google Scholar]
Robinson 1991 {published data only}
- Robinson MD, Smith WA, Cederstrom EA, Sutherland DE. Buspirone effect on tobacco withdrawal symptoms: a pilot study. Journal of the American Board of Family Practice 1991;4(2):89-94. [PubMed] [Google Scholar]
Rovina 2003 {unpublished data only}
- Gratziou C, Rovina N, Athanassa Z, Francis K, Evangelou E, Chiotis D, et al. Evaluation of prolonged bupropion treatment as an aid in smoking cessation [abstract]. European Respiratory Journal 2002;20 Suppl 38:611s. [Google Scholar]
- Rovina N, Gratziou C, Nikoloutsou I, Athanassa Z, Francis K, Roussos C. Ideal duration of therapy with bupropion HCL: Comparison between short and long treatment. In: Abstract book: Society for Research on Nicotine and Tobacco 5th European Meeting; 2003 November 20-22; Padua. 2003.
- Rovina N, Gratziou C, Nikoloutsou I, Athanassa Z, Francis K, Roussos C. Short or prolonged treatment with bupropion HCL in smoking cessation therapy. European Respiratory Journal 2003;22 Suppl 45:165s. [Google Scholar]
Schepis 2006 {unpublished data only}
- Schepis TS, Warren KA, Rao U. Evaluation of a cognitive-behavioral smoking cessation treatment for adolescents and young adults (POS2-53). In: Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 February 15-18; Orlando (FL). 2006.
Sellers 1987 {published data only}
- Sellers EM, Naranjo CA, Kadlec K. Do serotonin uptake inhibitors decrease smoking? Observations in a group of heavy drinkers. Journal of Clinical Psychopharmacology 1987;7:417-20. [PubMed] [Google Scholar]
Sherman 2008 {published data only}
- Sherman SE, Aldana I, Estrada M, York L. Comparing the tolerability and effectiveness of two treatment regimens in a smoking clinic. Military Medicine 2008;173(6):550-4. [DOI] [PubMed] [Google Scholar]
Shiffman 2000 {published data only}
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology 2000;148:33-40. [DOI] [PubMed] [Google Scholar]
Shoptaw 2008 {published data only}
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug and Alcohol Dependence 2008;96(3):222-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sittipunt 2007 {published data only}
- Sittipunt C, Kawkitinarong K, Wongtim S, Udompanich V. The effectiveness of nortriptyline plus brief motivation counseling for the treatment of smoking cessation in Thai active smokers [Abstract]. Respirology 2007;12(Suppl 4):A223. [Google Scholar]
Sonntag 2003 {published data only}
- Hoch E, Wittchen HU. Population health perspective on smoking cessation: A randomized controlled trial of different methods in primary health care (RPOS 3-71). In: Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 February 15-18; Orlando (FL). 2006.
- Sonntag H, Hoch E, Jahn B, Spiegel B, Pfister H, Wittchen HU. Smoking cessation in primary care: implementation effectiveness and optimized allocation. Suchtmedizin in Forschung und Praxis 2003;5:137-41. [Google Scholar]
Spring 1995 {published data only}
- Spring B, Wurtman J, Wurtman R, el Khoury A, Goldberg H, McDermott J, et al. Efficacies of dexfenfluramine and fluoxetine in preventing weight gain after smoking cessation. American Journal of Clinical Nutrition 1995;62(6):1181-7. [DOI] [PubMed] [Google Scholar]
Stein 1993 {published data only}
- Stein RA, Jarvik ME, Gorelick DA. Impairment of memory by fluoxetine in smokers. Experimental and Clinical Psychopharmacology 1993;1:188-93. [Google Scholar]
Steinberg 2009 {published data only}
- Steinberg MB, Greenhaus S, Schmelzer AC, Bover MT, Foulds J, Hoover DR, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Annals of Internal Medicine 2009;150(7):447-54. [DOI] [PubMed] [Google Scholar]
Strayer 2004 {unpublished data only}
- Strayer SM, Flusche A, Hodge J, Martindale JR. Effectiveness trial of Zyban for smoking cessation in the outpatient setting (POS1-044). In: Abstract Book. Society for Research on Nicotine and Tobacco 10th Annual Meeting; 2004 February 18-21; Phoenix (AZ). 2004:45.
Swanson 2003 {published data only}
- Swanson NA, Burroughs CC, Long MA, Lee RW. Controlled trial for smoking cessation in a Navy shipboard population using nicotine patch, sustained-release bupropion, or both. Military Medicine 2003;168:830-4. [PubMed] [Google Scholar]
Tidey 2009 {published data only}
- Tidey JW, Rohsenow DJ. Intention to quit moderates the effect of bupropion on smoking urge. Nicotine & Tobacco Research 2009;11(3):308-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toll 2007 {published data only}
- Jatlow P, Toll BA, Leary V, Krishnan-Sarin S, O'Malley SS. Comparison of expired carbon monoxide and plasma cotinine as markers of cigarette abstinence. Drug and Alcohol Dependence 2008;98(3):203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Mckee SA, Toll BA, Krishnan-Sarin S, Cooney JL, Makuch RW, et al. Risk factors for treatment failure in smokers: Relationship to alcohol use and to lifetime history of an alcohol use disorder. Nicotine & Tobacco Research 2008;10(12):1793-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, O'Malley SS, Katulak NA, Wu R, Dubin JA, Latimer A, et al. Comparing gain- and loss-framed messages for smoking cessation with sustained-release bupropion: a randomized controlled trial. Psychology of Addictive Behaviors 2007;21(4):534-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, Salovey P, O'Malley SS, Mazure CM, Latimer A, Mckee SA. Message framing for smoking cessation: the interaction of risk perceptions and gender. Nicotine & Tobacco Research 2008;10(1):195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinberger 2008 {published data only}
- Weinberger AH, Vessicchio JC, Sacco KA, Creeden CL, Chengappa KN, George TP. A preliminary study of sustained-release bupropion for smoking cessation in bipolar disorder. Journal of Clinical Psychopharmacology 2008;28(5):584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiner 2001 {published data only}
- Weiner E, Ball MP, Summerfelt A, Gold J, Buchanan RW. Effects of sustained-release bupropion and supportive group therapy on cigarette consumption in patients with schizophrenia. American Journal of Psychiatry 2001;158(4):635-7. [DOI] [PubMed] [Google Scholar]
Winhusen 2012 {published data only}
- Winhusen T, Stitzer M, Woody G, Brigham G, Kropp F, Ghitza U et al. Design considerations for a study to evaluate the impact of smoking cessation treatment on stimulant use outcomes in stimulant-dependent individuals. Contemporary Clinical Trials 2012;33(1):197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zernig 2008 {published data only}
- Zernig G, Wallner R, Grohs U, Kriechbaum N, Kemmler G, Saria A. A randomized trial of short psychotherapy versus sustained-release bupropion for smoking cessation. Addiction 2008;103(12):2024-31. [DOI] [PubMed] [Google Scholar]
ZYB30011 {unpublished data only}
- ZYB 30011. A multicentre, randomised, double- blind, placebo controlled study to evaluate the efficacy and tolerability of bupropion hydrochloride (SR) sustained release (2 x 150mg per day) versus placebo as an aid to smoking cessation in smokers with at least one cardiovascular (CV) risk factor. gsk-studyregister.com/en/trial-details/?id=ZYB%2030011 (accessed 4 August 2009).
References to ongoing studies
NCT03326128 {published data only}
- NCT03326128. High dose bupropion for smoking cessation. clinicaltrials.gov/ct2/show/NCT03326128 (first received 31 October 2017).
NCT03342027 {published data only}
- NCT03342027. Smoking cessation interventions for people living with HIV in Nairobi, Kenya. clinicaltrials.gov/ct2/show/NCT03342027 (first received 14 November 2017).
Zawertailo 2018 {published data only}
- NCT02146911. The MATCH (Medication Aids for Tobacco Cessation and Health) Study. clinicaltrials.gov/ct2/show/NCT02146911 (first received 26 May 2014).
- Zawertailo L, Mansoursadeghi-Gilan T, Zhang H, Hussain S, Le Foll B, Selby P. Varenicline and bupropion for long-term smoking cessation (the MATCH Study): protocol for a real-world, pragmatic, randomized controlled trial. JMIR Research Protocols 2018;7(10):e10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aubin 2012
- HJ Aubin. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs 2002;62:45-52. [DOI] [PubMed] [Google Scholar]
Benowitz 2000
- Benowitz NL, Peng MW. Non-nicotine pharmacotherapy for smoking cessation. CNS Drugs 2000;13:265-85. [Google Scholar]
Cahill 2013
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD009329. [DOI: 10.1002/14651858.CD009329.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cahill 2016
- Cahill K, Lindson‐Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD006103. [DOI: 10.1002/14651858.CD006103.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2017
- Centers for Disease Control and Prevention. Quitting Smoking Among Adults—United States, 2000–2015. Morbidity and Mortality Weekly Report 2017;52:1457-64. [DOI] [PubMed] [Google Scholar]
Coleman 2015
- Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD010078. [DOI: 10.1002/14651858.CD010078.pub2] [DOI] [PubMed] [Google Scholar]
Cryan 2003
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology 2003;168:347-58. [DOI] [PubMed] [Google Scholar]
Ebbert 2011
- Ebbert J, Montori VM, Erwin PJ, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No: CD004306. [DOI: 10.1002/14651858.CD004306.pub4] [DOI] [PubMed] [Google Scholar]
Fiore 2008
- Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. AHRQ publication No. 00-0032. Rockville, MD: US Dept of Health and Human Services. Public Health Services, 2008. [Google Scholar]
Fryer 1999
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. Journal of Pharmacology and Experimental Therapeutics 1999;288:88-92. [PubMed] [Google Scholar]
GBD RFC 2017
- GBD 2017 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1923-94. [DOI: 10.1016/S0140-6736(18)32225-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 13 January 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Hartmann‐Boyce 2018
- Hartmann‐Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD000146. [DOI: 10.1002/14651858.CD000146.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). The Cochrane Collaboration, 2019. Available from www.training.cochrane.org/handbook 2019.
Hughes 2005
- Hughes JR, Stead LF, Lancaster T. Nortriptyline for smoking cessation: A review. Nicotine & Tobacco Research 2005;7:491-9. [DOI] [PubMed] [Google Scholar]
Jha 2011
- Jha P. Avoidable deaths from smoking: a global perspective. Public Health Reviews 2011;33(2):569-600. [Google Scholar]
Kotlyar 2001
- Kotlyar M, Golding M, Hatsukami DK, Jamerson BD. Effect of nonnicotine pharmacotherapy on smoking behavior. Pharmacotherapy 2001;21:1530-48. [DOI] [PubMed] [Google Scholar]
Lerman 2002a
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu AY, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug and Alcohol Dependence 2002;67:219-23. [DOI] [PubMed] [Google Scholar]
Lewis 2007
- A Lewis, JH Miller, RA Lea. Monoamine oxidase and tobacco dependence. NeuroToxicology 2007;28(1):182-95. [DOI] [PubMed] [Google Scholar]
Lindson‐Hawley 2016
- Lindson-Hawley N, Hartmann‐Boyce J, Fanshawe TR, Begh R, Farley A, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD005231. [DOI: 10.1002/14651858.CD005231.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Livingstone‐Banks 2019
- Livingstone‐Banks J, Norris E, Hartmann‐Boyce J, West R, Jarvis M, Chubb E, et al. Relapse prevention interventions for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD003999. [DOI: 10.1002/14651858.CD003999.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
McNeill 2017
- McNeill A, Robson D. A man before his time: Russell's insights into nicotine, smoking, treatment and curbing the smoking problem. Addiction 2017;113(4):759-63. [DOI: 10.1111/add.14043] [DOI] [PubMed] [Google Scholar]
McRobbie 2005
- McRobbie H, Lee M, Juniper Z. Non-nicotine pharmacotherapies for smoking cessation. Respiratory Medicine 2005;99:1202-12. [DOI] [PubMed] [Google Scholar]
Mills 2006
- Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Annals of Medicine 2012;44(6):588-97. [DOI: 10.3109/07853890.2012.705016] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264-9. [DOI: 10.7326/0003-4819-151-4-200908180-00135] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Taylor 2014
- Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ 2014;348:g1151. [DOI: 10.1136/bmj.g1151] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsoi 2010
- Tsoi DT, Porwal M, Webster AC. Efficacy and safety of bupropion for smoking cessation and reduction in schizophrenia: systematic review and meta-analysis. British Journal of Psychiatry 2010;196(5):346-53. [DOI: 10.1192/bjp.bp.109.066019] [DOI] [PubMed] [Google Scholar]
van der Meer 2013
- Meer RM, Willemsen MC, Smit F, Cuijpers P. Smoking cessation interventions for smokers with current or past depression. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD006102. [DOI: 10.1002/14651858.CD006102.pub2] [DOI] [PubMed] [Google Scholar]
West 2005
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100(3):299-303. [DOI: 10.1111/j.1360-0443.2004.00995.x] [DOI] [PubMed] [Google Scholar]
West 2008
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology 2008;197(3):371-7. [DOI] [PubMed] [Google Scholar]
West 2018
- West R, Evins AE, Benowitz NL, Russ C, McRae T, Lawrence D, et al. Factors associated with the efficacy of smoking cessation treatments and predictors of smoking abstinence in EAGLES. Addiction 2018;113(8):1507-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wightman 2010
- Wightman DS, Foster VJ, Krishen A, Richard NE, Modell JG. Meta-analysis of suicidality in placebo-controlled clinical trials of adults taking bupropion. Primary Care Companion to the Journal of Clinical Psychiatry 2010;12(5):e1-8. 9400123000006102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkes 2005
- Wilkes S, Evans A, Henderson M, Gibson J. Pragmatic, observational study of bupropion treatment for smoking cessation in general practice. Postgraduate Medical Journal 2005;81:719-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hughes 1994
- Hughes JR. Non-nicotine pharmacotherapies for smoking cessation. Journal of Drug Development 1994;6:197-203. [Google Scholar]
Hughes 2000
- Hughes JR, Stead LF, Lancaster T. Anxiolytics and antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI] [PubMed] [Google Scholar]
Hughes 2002
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI] [PubMed] [Google Scholar]
Hughes 2003
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No: CD000031. [DOI: 10.1002/14651858.CD000031] [DOI] [PubMed] [Google Scholar]
Hughes 2004
- Hughes J, Stead L, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No: CD000031. [DOI: 10.1002/14651858.CD000031.pub2] [DOI] [PubMed] [Google Scholar]
Hughes 2007a
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD000031. [DOI: 10.1002/14651858.CD000031.pub3] [DOI] [PubMed] [Google Scholar]
Hughes 2014
- Hughes JR, Stead LF, Hartmann‐Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD000031. [DOI: 10.1002/14651858.CD000031.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


