Abstract
Objectives:
To determine if mortality differs between roller and centrifugal pumps utilized during extracorporeal membrane oxygenation (ECMO) in infants weighing less than 10kg.
Design:
Retrospective propensity matched cohort study
Setting:
All ECMO centers reporting to the Extracorporeal Life Support Organization (ELSO)
Patients:
All patients <10kg supported on ECMO during 2011–2016 within ELSO Registry
Interventions:
Centrifugal and roller pump recipients were propensity matched (1:1) based on predicted probability of receiving a centrifugal pump using demographic variables, indication for ECMO, central vs peripheral cannulation, and pre-ECMO patient management.
Measurements and Main Results:
12,890 patients <10kg were supported with ECMO within the ELSO registry during 2011–2016. Patients were propensity matched into a cohort of 8,366. Veno-arterial and Veno-venous ECMO runs were propensity matched separately. The propensity matched cohorts were similar except earlier year of ECMO (standardized mean difference = 0.49) in the roller pump group. Within the propensity matched cohort, survival to discharge was lower in the centrifugal pump group (57% vs 59%, OR 0.91, 95% CI 0.83–0.99, p=0.04). Hemolytic, infectious, limb injury, mechanical, metabolic, neurologic, pulmonary, and renal complications were more frequent in the centrifugal pump group. Hemorrhagic complications were similar between groups. Hemolysis mediated the relationship between centrifugal pumps and mortality (indirect effect=0.023, p<0.001).
Conclusions:
In this propensity-score matched cohort study of 8,366 ECMO recipients weighing <10kg, those supported with centrifugal pumps had increased mortality and ECMO complications. Hemolysis was evaluated as a potential mediator of the relationship between centrifugal pump use and mortality and met criteria for full mediation.
Keywords: Extracorporeal Membrane Oxygenation, Pump, Hemolysis, Complications, Survival, Children
Introduction
Extracorporeal membrane oxygenation (ECMO) has an established role in the management of children with cardiopulmonary failure, but this support is associated with considerable morbidity and mortality. ECMO circuits incorporate either roller or centrifugal pumps to achieve circuit flow and support cardiac output. Roller pumps deliver a fixed output utilizing sequential compression of the circuit tubing, while centrifugal pumps deliver flow in an afterload dependent manner utilizing centrifugal forces generated by a rotating impeller.(1) Controversy over the ideal pump type remains. Centrifugal pumps have several advantages including elimination of direct and repeated compression on circuit tubing, prevention of over-pressurization of the circuit, and the ability to configure smaller and mobile circuits. However, previous analyses of pediatric patients comparing centrifugal and roller pump circuits have shown variable effects on patient outcomes. Some studies have demonstrated no difference in complication or mortality rates according to pump type.(2) Other studies have shown improved outcomes with centrifugal pumps.(3–5) However, many studies of small children have demonstrated higher rates of complications, including hemolysis, hyperbilirubinemia and renal injury in those supported with centrifugal pumps compared to roller pumps.(6–9)
Utilization of centrifugal pumps in small children supported on ECMO has increased with the introduction of magnetically supported rotor centrifugal pumps.(10) Newer centrifugal pumps reduce shear stress and the localized heat generation associated with previous centrifugal pump model design.(11, 12) However, the use of smaller cannula and lower flow rates required for infants in combination with centrifugal pumps may lead to increase rates of hemolysis, circuit thrombosis, or inadequate support.
In this study, we aimed to evaluate in-hospital mortality difference between centrifugal and roller blood pumps used to configure ECMO circuits for children <10 kg from 2011 through 2016. We hypothesized that centrifugal pumps would be associated with higher mortality compared with roller pumps. Secondarily we hypothesized that hemolysis is a mediator of the mortality difference by pump type.
Materials and Methods
Study Population
A de-identified dataset was obtained from the Extracorporeal Life Support Organization (ELSO) Registry including ECMO supported patients from 310 contributing US and international centers. Standardized forms were used by the member centers for data reporting. Data are reported to the registry after approval by the local institutional review boards. A data use agreement between ELSO and member centers facilitates release of limited de-identified datasets to investigators from member centers for purposes of quality improvement, presentation, and publication. Boston Children’s Hospital Institutional Review Board waived the need for research oversight. With estimated low risk mortality of 50%, high risk mortality of 55%, and significance set at p<0.05, a study population of 2,100 patients per group was needed to obtain 90% power.
The study population included children weighing less than 10kg reported to the ELSO Registry during the years 2011–2016. Patients with missing information related to pump type, mode of ECMO, indication for ECMO, or survival to hospital discharge were excluded. In order to analyze current support strategies, only magnetically stabilized centrifugal pumps and hollow fiber oxygenators were included in the analysis. Extracted data included demographic, diagnostic and procedural information, pre-ECMO support, ECMO circuit details, ECMO complications (supplemental Table 1), and survival to hospital discharge.(10, 13)
Data Categorization
Indication for ECMO was classified as respiratory, cardiac, or extracorporeal cardiopulmonary resuscitation (ECPR) by the contributing center. Cannulation was ‘central’ if the right (or common) atrium or aorta were listed as cannulation sites, or ‘peripheral’ if neither were listed as cannulation sites. Cardiothoracic and congenital diaphragmatic hernia procedures were identified by submitted Current Procedural Terminology (CPT) codes. Risk Adjustment for Congenital Heart Surgery 1 (RACHS-1) category was similarly identified based on CPT codes. The RACHS-1 consensus categorization of congenital heart surgeries was based on perceived risk of in hospital death, and subsequently validated in two large multi-institutional datasets.(14) ELSO registry complication codes were used to categorize complications reported by the member centers according to a previously defined method.(15)
Propensity Matching
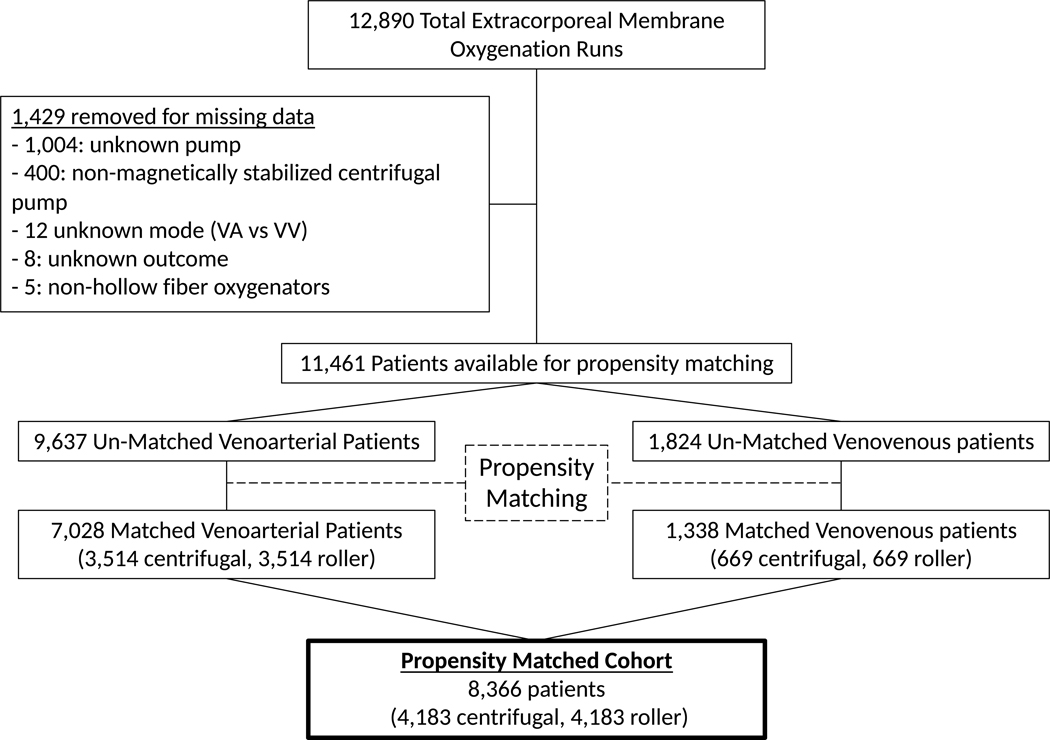
In order to reduce confounders, a propensity matched cohort was created based on the predicted probability of receiving a centrifugal pump. Variables incorporated into logistic regression were pre-ECMO variables available in the ELSO registry, including age, weight, race, gender, indication for ECMO, central cannulation, use of selected pre-ECMO supports (mechanical circulatory support, vasopressors, vasodilators, pulmonary hypertension therapies, surfactant, use of bicarbonate or tromethamine [THAM]), mode of mechanical ventilation, blood gas values, cardiac surgery prior to ECMO, and congenital diaphragmatic hernia surgery prior to ECMO. Missing data in specific numerical variables (pre-ECMO pH, pCO2, HCO3) was imputed using predictive mean matching to allow for inclusion of variables in propensity matching. Due to fundamental differences between veno-arterial (VA) and veno-venous (VV) ECMO, propensity matching was done independently for VA and VV ECMO patients, with the resulting propensity matched cohorts combined for analysis (Figure 1). This approach was utilized to maximize retention of study subjects within the final propensity matched cohort, while at the same time attempting to minimize bias created by forcing matches between inherently different populations. Within the VA ECMO group, logistic regression was used to estimate the predicted probability of receiving a centrifugal pump. Centrifugal pump recipients were matched 1:1 using greedy nearest neighbor matching with caliper of 0.1 without replacement to roller pump recipients with a similar propensity to be a centrifugal pump recipient. The VV ECMO group was propensity matched using the same technique, however because roller pumps were more common in the VV ECMO group, roller pump users were matched 1:1 with centrifugal pump users who had a similar propensity to be a roller pump recipient. Subgroups analyses utilized novel propensity matched cohorts for each subgroup, rather than subgroups of the initial propensity matched cohort. All subgroups were propensity matched using the same variables listed above, with the exception of the cardiac subgroup in which RACHS-1 category was added to account for variation in cardiac surgical complexity.
Fig 1:
Schematic diagram of the propensity matching process.
Statistical Analysis for comparing centrifugal and roller pumps
The primary study outcome was survival to hospital discharge, with important ECMO complications included as secondary outcomes. Descriptive statistics were presented for continuous variables using medians with interquartile range (IQR) and for categorical variables using frequencies with percentages. Differences in baseline characteristics between roller and centrifugal recipients in the un-matched and match cohorts were compared using standardized differences, with values <0.1 (10%) considered to be acceptable.(16, 17) For group-wise comparison, discrete variables were compared using Fisher exact tests and medians of continuous variables were compared using Mann-Whitney U tests, as appropriate. Mediation analysis was conducted by first establishing zero-order relationships between predictor, mediator, and outcome, as well as conducting logistic regression of the effect the predictor and mediator had on the outcome. The indirect effect was tested using a bootstrap estimation approach. Statistical significance was set at a p-value <0.05. All data were analyzed using RStudio (Version 1.0.143 – © 2009–2016 RStudio, Inc.).
Results
Study Population
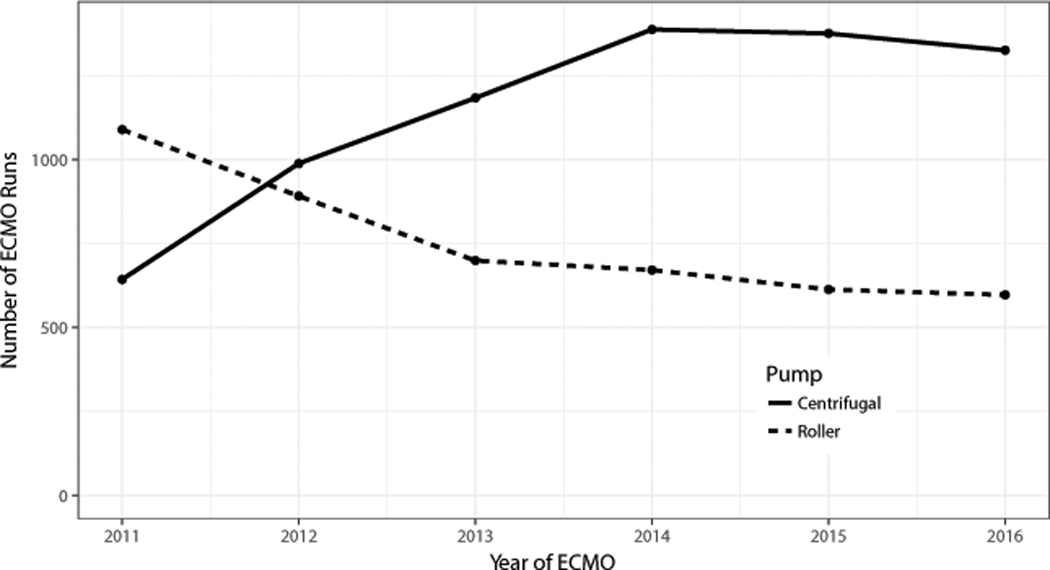
There were 12,890 total ECMO runs available for analysis. Use of centrifugal pumps exceeded use of roller pumps in children weighing <10kg during the year 2012 and remained the predominant pump type reported to ELSO for the remainder of the study period (Figure 2). After removing 1,429 runs for missing data, 11,461 runs were available for propensity matching (Figure 1). Pre-ECMO characteristics of centrifugal and roller pump users are compared in Table 1. Prior to propensity matching roller pump recipients were younger (median age 5 vs 12 days, standardized mean difference [SMD] = 0.19) and smaller (median weight 3.4 vs 3.5 kg, SMD=0.21) compared to the centrifugal pump group. Roller pumps were used more frequently for respiratory indications and less frequently for cardiac indications or ECPR (SMD=0.34). Roller pumps less frequently supported VA ECMO circuits (78 vs 88%, SMD=0.29) and were less frequently paired with central cannulation (30 vs 41%, SMD=0.24). Difference also existed with regards to race, gender, pre-ECMO support, pre-ECMO blood gas values, pre-ECMO surgeries, and ventilation strategy. Blood gas values were missing or incomplete in 1819 (16%) subjects and ECMO duration was missing in 164 (1%) subjects, these were imputed prior to propensity matching.
Fig 2:
Total number of Extracorporeal Membrane Oxygenation (ECMO) runs in pediatric patients <10kg by pump type and year
Table 1:
Pre-ECMO characteristics of the cohort, before and after propensity matching
| Characteristic | Unmatched | Matched | ||||
|---|---|---|---|---|---|---|
| Centrifugal | Roller | Standardized difference | Centrifugal | Roller | Standardized Difference | |
| n | 6901 | 4560 | 4183 | 4183 | ||
| Year of ECMO | 0.47 | 0.49 | ||||
| 2011 | 643 (9.3) | 1089 (23.9) | 367 (8.8) | 1001 (23.9) | ||
| 2012 | 988 (14.3) | 891 (19.5) | 588 (14.1) | 822 (19.7) | ||
| 2013 | 1183 (17.1) | 699 (15.3) | 724 (17.3) | 633 (15.1) | ||
| 2014 | 1387 (20.1) | 671 (14.7) | 835 (20.0) | 614 (14.7) | ||
| 2015 | 1375 (19.9) | 613 (13.4) | 864 (20.7) | 560 (13.4) | ||
| 2016 | 1325 (19.2) | 597 (13.1) | 805 (19.2) | 553 (13.2) | ||
| Age (Days, median[IQR]) | 12 [2, 94] | 5 [1, 40] | 0.19 | 7 [1, 55] | 6 [1, 46] | 0.02 |
| Weight (kg, median[IQR]) | 3.5 [3.0, 4.6] | 3.4 [2.9, 4.0] | 0.21 | 3.4 [2.9, 4.1] | 3.4 [2.9, 4.0] | 0.02 |
| Race (%) | 0.26 | 0.03 | ||||
| White | 3719 (53.9) | 2399 (52.6) | 2263 (54.1) | 2235 (53.4) | ||
| Black | 986 (14.3) | 917 (20.1) | 782 (18.7) | 776 (18.6) | ||
| Hispanic | 859 (12.4) | 703 (15.4) | 621 (14.8) | 659 (15.8) | ||
| Asian | 432 (6.3) | 141 (3.1) | 123 (2.9) | 131 (3.1) | ||
| Multiple | 373 (5.4) | 179 (3.9) | 158 (3.8) | 163 (3.9) | ||
| Other | 519 (7.5) | 219 (4.8) | 234 (5.6) | 217 (5.2) | ||
| Unknown | 13 (0.2) | 2 (0.0) | 2 (0.0) | 2 (0.0) | ||
| Male Sex (%) | 3733 (54.1) | 2543 (55.8) | 0.06 | 2325 (55.6) | 2345 (56.1) | 0.01 |
| Indication (%) | 0.34 | 0.03 | ||||
| Respiratory | 2908 (42.1) | 2687 (58.9) | 2263 (54.1) | 2324 (55.6) | ||
| Cardiac | 2753 (39.9) | 1311 (28.7) | 1329 (31.8) | 1297 (31.0) | ||
| ECPR | 1240 (18.0) | 562 (12.3) | 591 (14.1) | 562 (13.4) | ||
| Venoarterial (%) | 6102 (88.4) | 3535 (77.5) | 0.29 | 3514 (84.0) | 3514 (84.0) | <0.01 |
| Central Cannulation (%) | 2776 (41.2) | 1304 (29.7) | 0.24 | 1290 (30.8) | 1292 (30.9) | <0.01 |
| Pre-ECMO support | ||||||
| Vasopressors | 5916 (90.1) | 3875 (89.3) | 0.03 | 3511 (83.9) | 3543 (84.7) | 0.02 |
| Pulmonary hypertension therapies | 3139 (47.8) | 2756 (63.5) | 0.32 | 2324 (55.6) | 2390 (57.1) | 0.03 |
| Vasodilators | 2467 (37.6) | 1349 (31.1) | 0.14 | 1254 (30.0) | 1246 (29.8) | <0.01 |
| Alkalization agent | 1418 (21.6) | 1064 (24.5) | 0.07 | 926 (22.1) | 945 (22.6) | 0.01 |
| Mechanical Cardiac Support | 966 (14.7) | 493 (11.4) | 0.10 | 507 (12.1) | 492 (11.8) | 0.01 |
| Surfactant | 538 (8.2) | 701 (16.2) | 0.24 | 458 (10.9) | 507 (12.1) | 0.04 |
| Pre-ECMO Blood Gas | ||||||
| pH (mean (sd)) | 7.17 (0.19) | 7.19 (0.18) | 0.10 | 7.18 (0.18) | 7.18 (0.18) | <0.01 |
| pCO2 (mmHg, mean (sd)) | 61.52 (28.42) | 62.46 (26.69) | 0.03 | 63.00 (27.27) | 62.96 (26.19) | <0.01 |
| HCO3- (mmol/L mean (sd)) | 21.54 (7.20) | 22.77 (6.58) | 0.18 | 22.52 (7.02) | 22.54 (6.52) | <0.01 |
| Pre-ECMO1 Surgery | ||||||
| Cardiothoracic (%) | 2544 (71.2) | 1227 (58.1) | 0.28 | 1228 (29.4) | 1210 (28.9) | 0.01 |
| Diaphragm (%) | 73 (2.0) | 78 (3.7) | 0.10 | 59 (1.4) | 72 (1.7) | 0.03 |
| Ventilator Type | 0.21 | 0.01 | ||||
| Conventional | 2945 (42.7) | 1883 (41.3) | 1709 (40.9) | 1708 (40.8) | ||
| High Frequency Ventilation | 2089 (30.3) | 1781 (39.1) | 1573 (37.6) | 1596 (38.2) | ||
| Unknown | 1867 (27.1) | 896 (19.6) | 901 (21.5) | 879 (21.0) | ||
ECMO: Extracorporeal membrane oxygenation; ECPR: Extracorporeal cardiopulmonary resuscitation
Propensity Matching
11,461 patients were propensity matched into a cohort of 8,366 (4,183 in each pump type group). VA and VV ECMO groups were propensity matched separately. Distributions of propensity scores are shown in supplemental figures 1A and 1B. After propensity matching, the groups had similar baseline characteristics, with the exception of earlier year of ECMO (SMD = 0.49) in the roller pump group (Table 1).
Outcomes within the Propensity Matched Cohort
Within the propensity matched cohort, survival to discharge was lower in those patients who were supported with centrifugal pumps (57% vs 59%, Odds Ratio = 0.91, 95% Confidence Interval 0.83–0.99, p=0.035, Table 2). ECMO flow rates at 4 and 24 hours were lower in the roller pump group (110 vs 113, p=0.002 and 111 vs 116 ml/kg/min, p<0.001 respectively, Table 2). The rate of hemorrhagic complications was similar between groups, but rates of all other reported complications were higher in the centrifugal pump group compared to the roller pump group (Table 2).
Table 2:
Outcomes within the propensity matched cohort
| Outcome | Centrifugal (n=4183) | Roller (n=4183) | Odds Ratio (95% CI)* | p value |
|---|---|---|---|---|
| n | 4183 | 4183 | ||
| Discharged Alive (%) | 2383 (57.0) | 2479 (59.3) | 0.91 (0.83, 0.99) | 0.035 |
| Hours on ECMO1 | 132 [75, 238] | 145 [89, 252] | - | <0.001 |
| ECMO Flow at 4 hours (ml/kg/min) | 113 [97, 135] | 110 [97, 131] | - | 0.002 |
| ECMO Flow at 24 hours (ml/kg/min) | 116 [100, 138] | 111 [97, 133] | - | <0.001 |
| Complications | ||||
| Cardiovascular (%) | 2367 (56.6) | 2004 (47.9) | 1.42 (1.30, 1.54) | <0.001 |
| Inotropic medications during ECMO** (%) | 1823 (43.6) | 1563 (37.4) | 1.29 (1.19, 1.41) | <0.001 |
| Mechanical (%) | 1645 (39.3) | 1336 (31.9) | 1.38 (1.26, 1.51) | <0.001 |
| Renal (%) | 1528 (36.5) | 1250 (29.9) | 1.35 (1.23, 1.48) | <0.001 |
| Hemorrhagic (%) | 1159 (27.7) | 1182 (28.3) | 0.97 (0.88, 1.07) | 0.59 |
| Metabolic (%) | 1068 (25.5) | 695 (16.6) | 1.72 (1.55, 1.92) | <0.001 |
| Neurologic (%) | 837 (20.0) | 739 (17.7) | 1.17 (1.04, 1.30) | 0.007 |
| Hemolysis (%) | 744 (17.8) | 171 (4.1) | 5.08 (4.28, 6.05) | <0.001 |
| Pulmonary (%) | 403 (9.6) | 318 (7.6) | 1.30 (1.11, 1.51) | 0.001 |
| Infectious (%) | 361 (8.6) | 228 (5.5) | 1.64 (1.38, 1.95) | <0.001 |
| Limb (%) | 33 (0.8) | 6 (0.1) | 5.54 (2.49, 14.68) | <0.001 |
Odds ratios utilize roller pump as reference, CI – Confidence Interval
Included within Cardiovascular complications
ECMO: Extracorporeal membrane oxygenation
Mediation analysis evaluated the hypothesis that hemolysis mediated the effect of pump type on survival. Pump type was a significant predictor of hemolysis (OR=5.1, 95% CI: 4.3–6.0, p=<0.001) and mortality (OR=1.1, 95% CI 1.0–1.2, p=0.03), hemolysis was a significant predictor of mortality (OR=2.0, 95% CI: 1.7–2.3, p<0.001) supporting the mediation hypothesis. Pump type was no longer a significant predictor of mortality after controlling for hemolysis (OR=1.0, 95% CI: 0.9–1.1, p=0.98) consistent with full mediation. The indirect effect was tested using a bootstrap estimation approach which yielded a statistically significant indirect coefficient (B=0.023, p<0.001) which indicated that use of centrifugal pumps is associated with 2.3% increased probability of mortality as mediated through hemolysis.
Subgroup analyses utilized individually created propensity matched cohorts for subgroups of VV ECMO versus VA ECMO; ECMO indication; and neonate versus non-neonatal patient (Table 3). These analyses demonstrated a survival advantage for roller pumps in ECMO for respiratory indications (68% vs 65%, p=0.038), and ECMO for neonates (61% vs 58%, p<0.026).
Table 3:
Sub-group analyses of survival by pump type
| Stratification variable | Group | n** | Discharged Alive (%) | Odds Ratio (95% CI) * | p value | |
|---|---|---|---|---|---|---|
| Centrifugal | Roller | |||||
| All | 4183 | 2383 (57.0) | 2479 (59.3) | 0.91 (0.83, 0.99) | 0.035 | |
| Mode | ||||||
| Veno-Arterial ECMO1 | 3514 | 1907 (54.3) | 1981 (56.4) | 0.92 (0.84, 1.01) | 0.080 | |
| Veno-Venous ECMO1 | 669 | 476 (71.2) | 498 (74.4) | 0.85 (0.67, 1.08) | 0.197 | |
| Indication | ||||||
| Cardiac | 1289 | 628 (48.7) | 654 (50.7) | 0.92 (0.79, 1.08) | 0.325 | |
| Respiratory | 2265 | 1467 (64.8) | 1534 (67.7) | 0.87 (0.77, 0.99) | 0.038 | |
| ECPR2 | 549 | 262 (47.7) | 267 (48.6) | 0.96 (0.76, 1.22) | 0.809 | |
| Age | ||||||
| Neonates (≤28 days) | 2900 | 1695 (58.4) | 1779 (61.3) | 0.88 (0.80, 0.98) | 0.026 | |
| Non-Neonates (>28 days) | 1266 | 695 (54.9) | 699 (55.2) | 0.99 (0.84, 1.15) | 0.905 | |
Odds ratios utilize roller pump as reference CI – Confidence Interval
number per group
ECMO: Extracorporeal membrane oxygenation; ECPR: Extracorporeal cardiopulmonary resuscitation
Discussion
Extracorporeal membrane oxygenation (ECMO) circuits utilize either centrifugal or roller pumps for circuit flow and to augment cardiac output. There is institutional variability in the choice of pump. Centrifugal pump use in children <10kg increased over the study period to become the predominant type reported to the ELSO Registry. In this propensity-score matched cohort study of 8,366 children <10kg supported with ECMO, centrifugal pump users had an increased rate of in-hospital death. Important complications including hemolysis, renal, cardiovascular and neurological complications were more frequent in those who were supported with centrifugal pumps. Hemolysis appeared to be a mediator of the mortality difference by pump type, though this must be interpreted in light of inconsistent reporting of hemolysis to the ELSO registry. Subgroup analysis utilizing novel propensity matched cohorts demonstrated centrifugal pumps to be associated with higher mortality in the neonatal population, and for those with respiratory indications.
This is the largest targeted multicenter analysis of the type of ECMO pump used in small children and the first study to demonstrate a survival difference by pump type. We included small children, informed by previous studies demonstrating higher rates of hemolysis with centrifugal pumps in this age group.(6, 8, 9, 18, 19) The previously demonstrated difference in hemolysis rates by age may be due to lower flow rates, smaller cannula resulting in increased shear stress to red cells, intrinsic differences in neonatal red blood cell fragility, and/or larger burden of transfused cells in younger children.(18, 20) Therefore, we focused on hemolysis as a potential mediator of the observed mortality difference, and indeed found that hemolysis met criteria for mediation. Controversy remains over the role of hemolysis in important outcomes, such as survival to hospital discharge. Many studies have shown no association between hemolysis and mortality, even in young children.(6–9, 19) Other reports demonstrate an association between increased hemolysis and mortality in otherwise equivalent populations.(18, 21) Hemolysis releases free heme-proteins which are directly nephrotoxic, leading to acute kidney injury, which is associated with mortality.(22, 23, 23–26) Additionally, free hemoglobin scavenges nitric oxide, adenosine, and ADAMTS13 causing platelet thrombi and microvascular obstruction which leads to multi-organ injury.(27) Our analysis, which included only magnetically levitated centrifugal pumps, supports the hypothesis that the increased mortality observed in patients supported with centrifugal pumps was related to increased rates of hemolysis.
In addition to hemolysis, rates of cardiovascular, mechanical, renal, metabolic, neurologic, pulmonary, infectious, and limb complications were higher among centrifugal pump recipients. Shearing forces from the centrifugal pump mechanism of action have been associated with increased hemolysis, hyperbilirubinemia, as well as platelet dysfunction from acquired von Willebrand factor deficiency.(6–9, 28–30) Neurological injury mediated by critical illness and deranged cerebral blood flow may be exacerbated in the centrifugal pump supported population due to platelet dysregulation and acquired bleeding tendency.(28–33) Pump type does not readily explain many of the other increases in complication rates, such as infections and metabolic complications. However, if hemolysis and subsequent renal and neurologic injury lead to increased risk of death, multiorgan dysfunction and therefore occurrence of other complications might be expected. Inotropic medications were use more frequently in the centrifugal pump group compared to the roller pump group. An alternative hypothesis stemming from this observation is that the increased morbidity and mortality among centrifugal pump recipients may be related to inadequate ECMO flow to support cardiac output provided by centrifugal compared to roller pumps. Centrifugal pumps, in contrast to roller pumps, are afterload dependent, and this characteristic may lead to sustained or transient low flow states. However, this hypothesis is not supported by observed ECMO flows recorded 4 and 24 hours after cannulation, which were higher among centrifugal pump recipients.
We used data from the ELSO Registry, an international collaboration with more than 300 contributing centers. The size of the dataset and inclusion of all ECMO indications support the concept that generalizable conclusions may be drawn. Our study was, however, a retrospective analysis of registry data, with patients subjected to differences in institutional procedures and protocols which ultimately contribute to differences in patient care. Our dataset did not include anonymous hospital identifiers, so institutional differences in volume and clinical care, which are likely important in ECMO outcomes, could not be assessed. This may be particularly relevant to institutional experience and the learning curve associated with transitioning to centrifugal pumps. We additionally are unable to identify the centers that are primarily neonatal, however we did achieve matching in propensity cohorts on age and pre-ECMO support variables, such as surfactant and pulmonary hypertension therapies, that would correlate with neonatal respiratory ECMO. Additionally, other circuit components aside from type of pump were not investigated in detail, as they were outside the scope of this study. We utilized a propensity score matched cohort approach to reduce confounding by indication within our cohort, however, significant differences in year of ECMO remained between our roller and centrifugal pump cohorts. The accuracy of diagnostic data and presence of complications was not independently confirmed. For example, hemolysis is defined in the ELSO Registry as plasma free hemoglobin of >50mg/dL, therefore lack of reported hemolysis may be due failure to test for plasma free hemoglobin, hemolysis below the defined threshold, or absence of hemolysis.(21) We are unable to restrict our analysis to patients managed at institutions which measure plasma free hemoglobin routinely due to lack of anonymous center variable in our dataset. Our mediation analysis should be viewed in light of this significant limitation. In the future, more standardized testing for hemolysis should be encouraged among ELSO contributing centers. In-hospital mortality, the primary outcome of our study is a core element of the ELSO Registry dataset, unlikely to be affected by the issues associated with diagnosis of hemolysis. Subgroup analyses were not powered for small, but possibly clinically significant differences in mortality in the smaller populations, hence we have focused on those subgroups where the identified mortality difference is maintained, rather than suggesting that certain populations are spared. We tested our patient characteristic and pump physiology-based hypothesis on children <10kg who received all types of ECMO support, for any indication. As such, our results are generalizable to any small child undergoing this therapy.
Further investigation is required to explore and validate the results of this study. Though a randomized controlled trial is theoretically desirable, it is unlikely to be feasible due to issues of cost and experience. In a randomized study, centers without experience using roller pumps would be asked to support patients using roller pumps, or vice versa, which would introduce confounding into the study. Granular, multi-institutional data collated with specific attention to cannulation strategy, cannula dimensions, other circuit components, ECMO flows, and rates of hemolysis, using standardized data collection strategies should be conducted to better characterize the interaction between pump type and outcomes in small children. Additionally, the results of this study emphasize the importance of investigating how new technology may interact differently with small children, compared to adults. This problem is not unique to pediatric ECMO pumps, indeed significant attention has been given to the development of pediatric specific ventricular assist devices.(34–36) It is possible that outcomes of children managed with centrifugal pumps could be improved if small patient-optimized equipment were available.
Conclusions
Use of centrifugal pumps in children weighing <10kg is increasing with technological advances and ease of use of these devices. In this propensity-score matched cohort study of 8,366 pediatric ECMO recipients weighing <10kg, centrifugal pump use was associated with increased in-patient mortality as well as increased rates of cardiovascular, neurological, renal, pulmonary, mechanical, hemolytic, infectious, and limb complications. Hemolysis may mediate the observed increase in mortality. These data support an ongoing role for roller pumps in small children receiving ECMO, however, future prospective studies with more granular detail regarding institutional factors, and ECMO circuit components in addition to pump type are required to improve outcomes for this vulnerable population.
Supplementary Material
Acknowledgments
Supported, in part, by the Rochelle E. Rose Cardiac Intensive Care Unit Research Funds.
Footnotes
Reprints will not be ordered
Copyright form disclosure: Dr. Thiagarajan’s institution received funding form Pfizer and Bristol Myers Squibb. Dr. Barbaro disclosed that he is the Extracorporeal Life Support Organization (ELSO) Registry Chair, and he received support for article research from National Institutes of Health (NIH) and K12 HL138039 Training to Advance Care Through Implementation science in Cardiac And Lung illnesses (TACTICAL). Dr. Alexander’s institution received funding from Tenax Therapeutics (supplied levosimendan for an expanded access protocol clinical trial) and Novartis, and he disclosed off-label product use of ECMO. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Green TP, Kriesmer P, Steinhorn RH, et al. : Comparison of pressure-volume-flow relationships in centrifugal and roller pump extracorporeal membrane oxygenation systems for neonates. ASAIO Trans 1991; 37:572–6 [PubMed] [Google Scholar]
- 2.Dalton HJ, Reeder R, Garcia-Filion P, et al. : Factors Associated with Bleeding and Thrombosis in Children Receiving Extracorporeal Membrane Oxygenation (ECMO). Am J Respir Crit Care Med 2017; 196:rccm.201609–1945OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan IS, Codispoti M, Sanger K, et al. : Superiority of centrifugal pump over roller pump in paediatric cardiac surgery: prospective randomised trial. Eur J Cardiothorac Surg 1998; 13:526–32 [DOI] [PubMed] [Google Scholar]
- 4.Meyer AD, Wiles AA, Rivera O, et al. : Hemolytic and thrombocytopathic characteristics of extracorporeal membrane oxygenation systems at simulated flow rate for neonates. Pediatr Crit Care Med 2012; 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luciani GB, Hoxha S, Torre S, et al. : Improved Outcome of Cardiac Extracorporeal Membrane Oxygenation in Infants and Children Using Magnetic Levitation Centrifugal Pumps. Artif Organs 2016; 40:27–33 [DOI] [PubMed] [Google Scholar]
- 6.Barrett CS, Jaggers JJ, Cook EF, et al. : Pediatric ECMO outcomes: Comparison of centrifugal versus roller blood pumps using propensity score matching. ASAIO J 2013; 59:145–151 [DOI] [PubMed] [Google Scholar]
- 7.Delaplain PT, Zhang L, Nguyen DV., et al. : Effect of pump type on outcomes in neonates with congenital diaphragmatic hernia requiring ECMO. Perfus (United Kingdom) 2018; 33:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien C, Monteagudo J, Schad C, et al. : Centrifugal pumps and hemolysis in pediatric extracorporeal membrane oxygenation (ECMO) patients: An analysis of Extracorporeal Life Support Organization (ELSO) registry data. J Pediatr Surg 2017; 52:975–978 [DOI] [PubMed] [Google Scholar]
- 9.Barrett CS, Jaggers JJ, Cook EF, et al. : Outcomes of neonates undergoing extracorporeal membrane oxygenation support using centrifugal versus roller blood pumps. Ann Thorac Surg 2012; 94:1635–1641 [DOI] [PubMed] [Google Scholar]
- 10.Barbaro RP, Paden ML, Guner YS, et al. : Pediatric Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017; 63:456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehle K, Philipp A, Zeman F, et al. : Technical-Induced Hemolysis in Patients with Respiratory Failure Supported with Veno-Venous ECMO - Prevalence and Risk Factors. PLoS One 2015; 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawson DS, Lawson AF, Walczak R, et al. : North American neonatal extracorporeal membrane oxygenation (ECMO) devices and team roles: 2008 survey results of Extracorporeal Life Support Organization (ELSO) centers. J Extra Corpor Technol 2008; 40:166–74 [PMC free article] [PubMed] [Google Scholar]
- 13.Thiagarajan RR, Barbaro RP, Rycus PT, et al. : Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017; 63:60–67 [DOI] [PubMed] [Google Scholar]
- 14.Jenkins KJ, Gauvreau K, Newburger JW, et al. : Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 2002; 123:110–118 [DOI] [PubMed] [Google Scholar]
- 15.Thiagarajan RR, Laussen PC, Rycus PT, et al. : Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation 2007; 116:1693–1700 [DOI] [PubMed] [Google Scholar]
- 16.Leisman DE: Ten Pearls and Pitfalls of Propensity Scores in Critical Care Research: A Guide for Clinicians and Researchers. Crit Care Med 2019; 47:176–185 [DOI] [PubMed] [Google Scholar]
- 17.Austin PC, Grootendorst P, Anderson GM: A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007; 26:734–53 [DOI] [PubMed] [Google Scholar]
- 18.Okochi S, Cheung EW, Barton S, et al. : An Analysis of Risk Factors for Hemolysis in Children on Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med 2018; [DOI] [PubMed] [Google Scholar]
- 19.Dalton HJ, Cashen K, Reeder RW, et al. : Hemolysis During Pediatric Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med 2018; 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Halloran CP, Alexander PMA, Andren KG, et al. : RBC Exposure in Pediatric Extracorporeal Membrane Oxygenation: Epidemiology and Factors Associated With Large Blood Transfusion Volume. Pediatr Crit care Med 2018; 19:767–774 [DOI] [PubMed] [Google Scholar]
- 21.Lou S, MacLaren G, Best D, et al. : Hemolysis in pediatric patients receiving centrifugal-pump extracorporeal membrane oxygenation: Prevalence, risk factors, and outcomes. Crit Care Med 2014; 42:1213–1220 [DOI] [PubMed] [Google Scholar]
- 22.Rother RP, Bell L, Hillmen P, et al. : The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 2005; 293:1653–62 [DOI] [PubMed] [Google Scholar]
- 23.Qian Q, Nath KA, Wu Y, et al. : Hemolysis and Acute Kidney Failure. Am J Kidney Dis 2010; 56:780–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilburn DJ, Shekar K, Fraser JF: The Complex Relationship of Extracorporeal Membrane Oxygenation and Acute Kidney Injury: Causation or Association? Biomed Res Int 2016; 2016:1094296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schumacher DJ, Spector ND, Calaman S, et al. : Putting the Pediatrics Milestones Into Practice: A Consensus Roadmap and Resource Analysis. Pediatrics 2014; 133:898–906 [DOI] [PubMed] [Google Scholar]
- 26.Askenazi DJ, Ambalavanan N, Hamilton K, et al. : Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr Crit Care Med 2011; 12:e1–6 [DOI] [PubMed] [Google Scholar]
- 27.Carcillo JA: Multiple Organ System Extracorporeal Support in Critically Ill Children. Pediatr Clin North Am 2008; 55:617–646 [DOI] [PubMed] [Google Scholar]
- 28.Linneweber J, Chow TW, Kawamura M, et al. : In vitro comparison of blood pump induced platelet microaggregates between a centrifugal and roller pump during cardiopulmonary bypass. Int J Artif Organs 2002; 25:549–55 [DOI] [PubMed] [Google Scholar]
- 29.Meyer AL, Malehsa D, Budde U, et al. : Acquired von Willebrand Syndrome in Patients With a Centrifugal or Axial Continuous Flow Left Ventricular Assist Device. JACC Hear Fail 2014; 2:141–145 [DOI] [PubMed] [Google Scholar]
- 30.Halaweish I, Cole A, Cooley E, et al. : Roller and Centrifugal Pumps: A Retrospective Comparison of Bleeding Complications in Extracorporeal Membrane. ASAIO J 2015; 61:496–501 [DOI] [PubMed] [Google Scholar]
- 31.O’Brien NF, Hall MW: Extracorporeal membrane oxygenation and cerebral blood flow velocity in children. Pediatr Crit Care Med 2013; 14:e126–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rilinger JF, Smith CM, DeRegnier RAO, et al. : Transcranial Doppler Identification of Neurologic Injury during Pediatric Extracorporeal Membrane Oxygenation Therapy. J Stroke Cerebrovasc Dis 2017; 26:2336–2345 [DOI] [PubMed] [Google Scholar]
- 33.Parolari A, Alamanni F, Naliato M, et al. : Adult cardiac surgery outcomes: role of the pump type. Eur J Cardiothorac Surg 2000; 18:575–82 [DOI] [PubMed] [Google Scholar]
- 34.Adachi I: Current status and future perspectives of the PumpKIN trial. Transl Pediatr 2018; 7:162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldwin JT, Borovetz HS, Duncan BW, et al. : The National Heart, Lung, and Blood Institute Pediatric Circulatory Support Program. Circulation 2006; 113:147–155 [DOI] [PubMed] [Google Scholar]
- 36.Addonizio LJ: Pediatric Ventricular Assist Devices — First Steps for Babies. N Engl J Med 2012; 367:567–568 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.