Abstract
Background
Itch is an unpleasant sensation that can be debilitating, especially if it is chronic and of non-histaminergic origin, as treatment options are limited. Endothelin-1 (ET-1) is a potent endogenous vasoconstrictor that also has the ability to induce a burning, non-histaminergic pruritus when exogenously administered, by activating the endothelin A receptor (ETAR) on primary afferents. ET-1 is released endogenously by several cell-types found in the skin, including macrophages and keratinocytes. Mast cells express ETARs and can thereby be degranulated by ET-1, and mast cell proteases chymase and carboxypeptidase A3 (CPA3) are known to either generate or degrade ET-1, respectively, suggesting a role for mast cell proteases in the regulation of ET-1-induced itch. The mouse mast cell proteases (mMCPs) mMCP4 (chymase), mMCP6 (tryptase), and CPA3 are found in connective tissue type mast cells and are the closest functional homologs to human mast cell proteases, but little is known about their role in endothelin-induced itch.
Methods
In this study, we evaluated the effects of mast cell protease deficiency on scratching behavior induced by ET-1. To investigate this, mMCP knock-out and transgenic mice were injected intradermally with ET-1 and their scratching behavior was recorded and analyzed.
Results
CPA3-deficient mice and mice lacking all three proteases demonstrated highly elevated levels of scratching behavior compared with wild-type controls. A modest increase in the number of scratching bouts was also seen in mMCP6-deficient mice, while mMCP4-deficiency did not have any effect.
Conclusion
Altogether, these findings identify a prominent role for the mast cell proteases, in particular CPA3, in the protection against itch induced by ET-1.
Keywords: Itch, Chymase, Carboxypeptidase A3, Tryptase, Mice
Background
Itch is an unpleasant sensation that elicits the desire to scratch, and is most commonly caused either by light touch or by substances (pruritogens) that activate itch receptors on sensory neurons, either directly or indirectly. Histamine is the most extensively studied pruritogen, but a large and heterogeneous group of other pruritogens is also capable of inducing (histamine-independent) itch. Such histamine-independent itch can be challenging to treat effectively, especially in chronic pruritus [1]. Endothelin-1 (ET-1), a 21-amino acid peptide [2], is the most potent endogenous vasoconstrictor in the human cardiovascular system [3]. ET-1 can also induce pain when exogenously administered, and when lower doses are injected into the skin it can act as a powerful non-histaminergic pruritogen [4]. Two other ET isoforms have been identified, endothelin-2 (ET-2), and endothelin-3 (ET-3) [5], but ET-1 is the most widely expressed of these [6, 7]. ET-1 is mostly synthesized and released from vascular endothelial cells [2, 3] but is also produced by a variety of other cells, such as keratinocytes [8], vascular smooth muscle cells [9], dorsal root ganglia and spinal cord neurons [10, 11], macrophages, and mast cells [12, 13]. Endothelins bind to two subtypes of G protein-coupled receptors (GPCRs), the endothelin A receptor (ETAR) [14] and endothelin B receptor (ETBR) [15]. In vertebrates, the endothelin receptors are widely expressed throughout the body, and studies on both mice and humans have revealed that ETAR has the highest expression within the cardiovascular system and the lung, while ETBR is the predominant endothelin receptor in the brain ([16]; summarized in [3]). Due to its vasoconstriction properties and high expression within the cardiovascular system, ET-1 has an important role in regulating blood pressure [3] but ET-1 can also activate ETARs on primary afferents (ETAR/Ednra is expressed in 2-6% of primary afferents [17, 18]) and can transmit and potentiate both pain and itch [19–23]. Furthermore, ETARs are found on mast cells and ET-1 is capable of inducing mast cell degranulation [24, 25].
Mast cells are a part of the immune system and have an important role in host defense. When activated, they can degranulate and release a variety of different active mediators, many of which have pro-inflammatory or protective functions. Some of these substances, such as histamine, serotonin, leukotriene C4, and tryptase, can act directly on receptors on primary afferents as pruritogens [26, 27] and it has been suggested that mast cells are capable of producing ET-1 [12, 13]. Furthermore, mast cells can release proteases that affect ET-1 and its production, such as carboxypeptidase A3 (CPA3) that degrades ET-1 [28, 29] and chymase and Cathepsin E [30] that can convert the inactive precursor big-ET-1 into active ET-1 [31, 32]. It has previously been shown that mast cells are necessary for protection against otherwise fatal ET-1-induced toxicity in mice [33].
In the current study, we investigated the possible involvement of connective tissue mast cell proteases in ET-1-induced itch, induced by intradermal ET-1 administration at low (sub-lethal) doses. Our data reveal that CPA3 and, to a lesser extent, tryptase, play important roles in the protection against ET-1-induced scratching.
Methods
Generation of transgenic animals
Mice deficient in the mast cell proteases mMCP4 (Mcpt4-/- [34]), mMCP6 (Mcpt6-/- [35]) and CPA3 (Cpa3-/- [36]) were generated as previously described. Mice deficient in all three proteases (Mcpt4-/-Mcpt6-/-Cpa3-/-) were then generated by intercrossing the above mentioned strains together on a C57BL/6 J genetic background [37]. The transgenic Cpa3Y356L,E378A mouse strain, where the protease is rendered inactive by mutating the active sites of the enzyme, was generated as previously described [29]. The accuracy of the transgenic lines has been evaluated in previous analyses, where each mutation was shown to result in absence or inactivation of the respective protein [34–36, 38]. Mice were genotyped by PCR using the following primer combinations: mMCP-4 gene (Mcpt4) (forward primer, 59-CAA GGT CCA ACTAAC TCC CTT TGT GCT CC-39, wild-type [WT] reverse, 59- GGT GAT CTC CAG ATG GGC CAT GTA AGG GCG-39, knock-out [KO] reverse, 59-GGG CCA GCT CAT TCC TCC CAC TCA TGA TCT-39), mMCP-6 gene (Tpsb2) (forward primer, 59-TTT AGC TGG ACT CAG GCT GTG CTC CTC ACT-39, WT reverse, 59-CTC CTG AAT TGG AGC TAA CCC TGG GAT TCT-39, KO reverse, 59-GAC CAT GTG ATC GCG CTT CT-39), and CPA3 gene (Cpa3) (forward primer, 59-GGA CTG TTC ATC CCC AGG AAC C-39, reverse 1, 59-CTG GCG TGC TTT TCATTC TGG-39, reverse 2, 59-GTC CGG ACA CGC TGA ACT TG-39). Wild-type mice were on a C57BL/6 J genetic background.
Behavior
All behavioral tests were performed on adult (> 7 weeks old) female and male mice. Control mice were gender- and age-matched wild-type mice (C57BL/6 J) housed in the same animal room. All behavior analyses were performed during the day (light) part of a 12 h day/night cycle, in a controlled environment of 20–24 °C and 45–65% humidity and by the same female investigator. All animal procedures were approved by the local ethical committee in Uppsala and followed the Directive 2010/63/EU of the European Parliament and of the Council, The Swedish Animal Welfare Act (SFS 1988:534), The Swedish Animal Welfare Ordinance (SFS 1988:539), and the provisions regarding the use of animals for scientific purposes: DFS 2004:15 and SJVFS 2012:26.
Itch studies
50 μl of vehicle (0.9% saline, Fresenius Kabi) or endothelin-1 (20 pmol, Sigma-Aldrich) were injected intradermally in the back of the neck. Where the ETAR antagonist BQ-123 was used, 10 pmol of endothelin-1 were injected, with or without 10 nmol of BQ-123 (Sigma-Aldrich) in one intradermal injection. Each mouse was then placed in a cage with wooden chips and recorded for 1 h using a digital video camera. Afterwards, AniTracker® v1.2 was used to score the videos for the duration and frequency of grooming and scratching behavior. A scratching episode was defined as a bout of scratching by either hind paw; from the time point it was lifted until placed back on the ground. The videos were scored by observers blinded to the genotype and treatment given. The results for each group are expressed as the mean frequency of scratching episodes/60 min, the mean duration of scratching episodes/60 min, and, where relevant, as the mean length of scratching episodes (total duration/total frequency) and as the mean frequency of scratching episodes per each 10 min interval. Results are presented as mean ± SEM.
Statistics
For all sets of data, Gaussian distribution was assessed by a Shapiro-Wilks test. Student’s t test, two-tailed, was used to compare two groups. When the groups were more than two, 1-way ANOVA and either Dunnett’s or Bonferroni multiple comparison post hoc tests were used for parametric data, for non-parametric data Kruskal-Wallis and Dunn’s multiple comparison post hoc test were used. Two-way repeated measurement ANOVA with Bonferroni multiple comparison post hoc test was used to analyze the development of scratching frequency over time. Grubbs’ test was used to identify significant outliers (https://www.graphpad.com/quickcalcs/Grubbs1.cfm, GraphPad Software, Inc.). All other calculations were performed using Prism version 5.04 (GraphPad Software, Inc., San Diego, CA). Results are presented as mean ± S.E.M. P values of < 0.05 were considered significant. In the figures, * refers to P ≤ 0.05, ** refers to P ≤ 0.01, *** refers to P ≤ 0.001, **** refers to P ≤ 0.0001 and n.s. stands for not significant. Lower case “n” indicates the number of animals used in a set of experiments.
Results
Mcpt6-/- mice scratch more frequently than controls in response to vehicle injection
First, Mcpt4-/-, Mcpt6-/-, Cpa3Y356L,E378A, Mcpt4-/-Mcpt6-/-Cpa3-/- mice and C57BL/6 J controls were injected with vehicle (0.9% sterile saline) to evaluate the basal scratching behavior resulting from handling and injection (Supplementary Fig. 1). The protease-deficient mice did not differ from controls in response to saline, with the exception of Mcpt6-/- mice that scratched slightly more frequently than controls (Supplementary Fig. 1a, P < 0.05), but no differences were observed with regard to scratching duration in 60 min or mean length of scratching episodes (Supplementary Fig. 1b-1c). This indicates that Mcpt6-/- mice may in general be more sensitive to intradermal injections, irrespective of substance injected.
Blocking the ETA receptor significantly decreases ET-1-induced scratching
ET-1 transmits itch by binding to and activating ETARs on primary afferents. To exclude possible off-target effects by ET-1, we administrated the peptide in wild-type mice with or without the selective ETAR antagonist BQ-123 [39] and evaluated the scratching behavior. Various different dosage combinations of ET-1 and BQ-123 have been reported, and we decided to follow the example of Trentin et al. [20], where 10 pmol of ET-1 were injected together with 10 nmol of BQ-123. The antagonist was effective in reducing the total scratching, with regards to both the frequency of scratching bouts (Fig. 1a, P < 0.001) and scratching duration (Fig. 1b, P < 0.001). For reference, the scratching levels of both treatment groups were compared with vehicle-injected wild-type controls (data from Supplementary Fig. 1a-1b). No statistical difference was found between antagonist-treated and vehicle-injected animals (Fig. 1a, b; P > 0.05) but a significant difference between ET-1-treated and vehicle-injected mice was seen (Fig. 1a, b; P < 0.001). Analysis of scratching frequency over time and in 10-min intervals with the antagonist treatment as a factor revealed that scratching was almost completely abolished within the first 40 min in BQ-123-treated mice (Fig. 1c). Post hoc analysis showed that in the time interval between 10 and 20 min, the difference in scratching frequency between treatment groups became significant (Fig. 1c, P < 0.05). Overall analysis on scratching frequency with time showed that BQ-123 was effective in reducing scratching (Fig. 1c, P < 0.05). Taken together, the above results show that BQ-123 blocks ET-1-induced scratching behavior and that ET-1-induced scratching depends specifically on ETAR activation. In the group that only received ET-1 (10 pmol), half of the animals showed only a modest increase in scratching behavior (Fig. 1a, b) and therefore it was decided to increase the dose to 20 pmol in subsequent experiments.
Fig. 1.
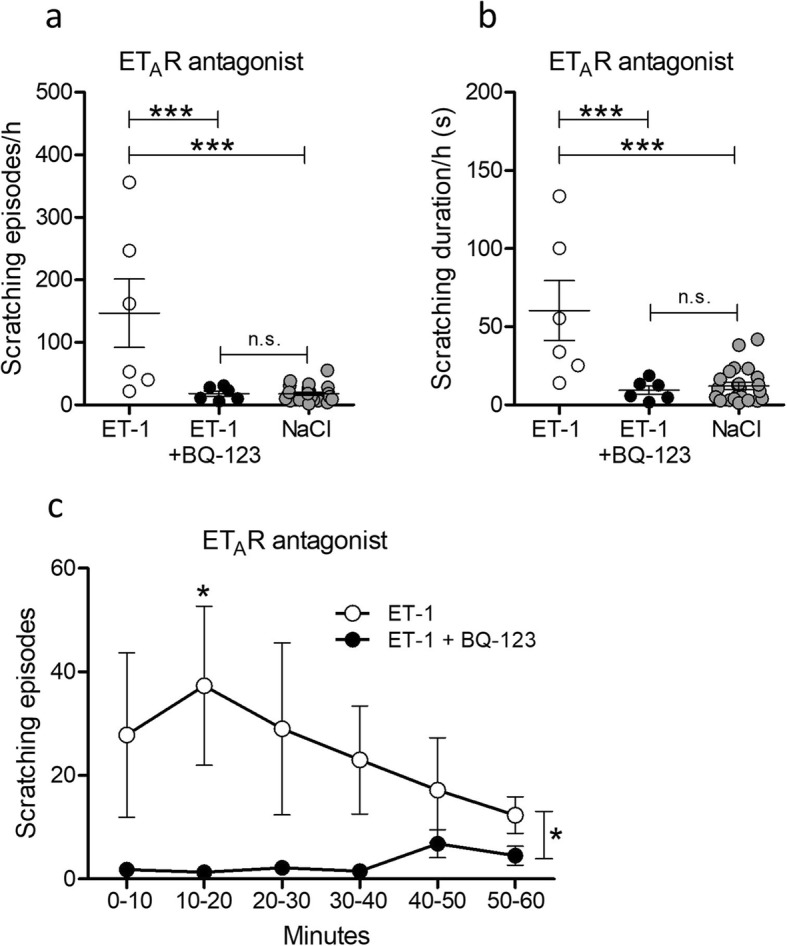
ET-1-induced scratching is effectively abolished by antagonizing the ETA receptor. Wild-type (C57BL/6 J) mice were injected intradermally in the nape with either ET-1 (10 pmol, n = 6) or ET-1 together with ETAR antagonist BQ-123 (10 pmol + 10 nmol, n = 6). a, b BQ-123 was very effective in reducing the total scratching frequency and duration and the scratching behavior was similar to vehicle-injected (saline) animals (n = 24, data from Supplementary Figure 1). c When the frequency results from (a) were analyzed over time with the BQ-123 treatment as a factor, it was seen that antagonist-treated mice scratched significantly less overall (indicated by significance bar on the far right), and post hoc analysis showed significant difference between treatment groups in the interval between 10 and 20 min. Results are presented as mean ± SEM. *P ≤ 0.05; ***P ≤ 0.001; n.s., not significant; 1-way ANOVA with Bonferroni multiple comparison (a, b); 2-way ANOVA with Bonferroni multiple comparison (c). Vehicle data in (a, b) is the same data as presented in Supplementary Figure 1a-1b
Mcpt4-/-Mcpt6-/-Cpa3-/- mice show profoundly enhanced scratching behavior when provoked with ET-1
Mouse connective tissue mast cells express mMCP4, mMCP5, mMCP6, mMCP7, and CPA3 [40] and to investigate how the loss of connective tissue mast cell-specific proteases affects ET-1-induced scratch behavior, we tested the Mcpt4-/-Mcpt6-/-Cpa3-/- mouse line by injecting ET-1 intradermally in the nape and compared them with wild-type controls (Fig. 2a, d). The Mcpt4-/-Mcpt6-/-Cpa3-/- line lacks the protease mMCP5 as the protein depends on CPA3 for storage [36], and mMCP7 is only expressed in low levels in mature mast cells [37], rendering a line that is almost devoid of connective tissue mast cell-specific proteases. In the Mcpt4-/-Mcpt6-/-Cpa3-/- mice, a large increase in scratching was seen; in 1 h the protease-deficient mice scratched on average 1214.0 ± 66 times while the controls had 492.0 ± 70 scratching episodes during the same time period (Fig. 2a, P < 0.0001). The Mcpt4-/-Mcpt6-/-Cpa3-/- animal displaying the most exaggerated scratching behavior had more than 2200 scratching episodes during the 60 min; this animal was found to be a significant outlier according to Grubb’s test. However, including or excluding that animal did not affect the statistical significance of the result (P < 0.0001 in both instances, the animal is indicated with a § in the respective figure). When total scratching duration was analyzed (Fig. 2b), the results were similar to those seen for scratching frequency; the protease-deficient mice spent significantly more time scratching than controls (P < 0.0001). The Mcpt4-/-Mcpt6-/-Cpa3-/- animals also had shorter scratching episodes on average (Fig. 2c, P = 0.0025). When the scratching episode frequency was analyzed over time and in 10-min intervals (Fig. 2d), it was seen that the Mcpt4-/-Mcpt6-/-Cpa3-/- knockouts scratched more over time than the controls overall (P < 0.0001) and post hoc testing revealed that the difference was statistically significant during the first 40 min of the test (0-10 min: P < 0.01, 10-40 min: P < 0.001). The above results indicate that the mast cell-specific proteases play an important protective role in ET-1-induced scratch behavior. A video demonstrating the typical scratching behavior 10 min. after ET-1 injection in a wild-type control and a Mcpt4-/-Mcpt6-/-Cpa3-/- mouse can be found in online Supplementary Materials (Movie S1).
Fig. 2.
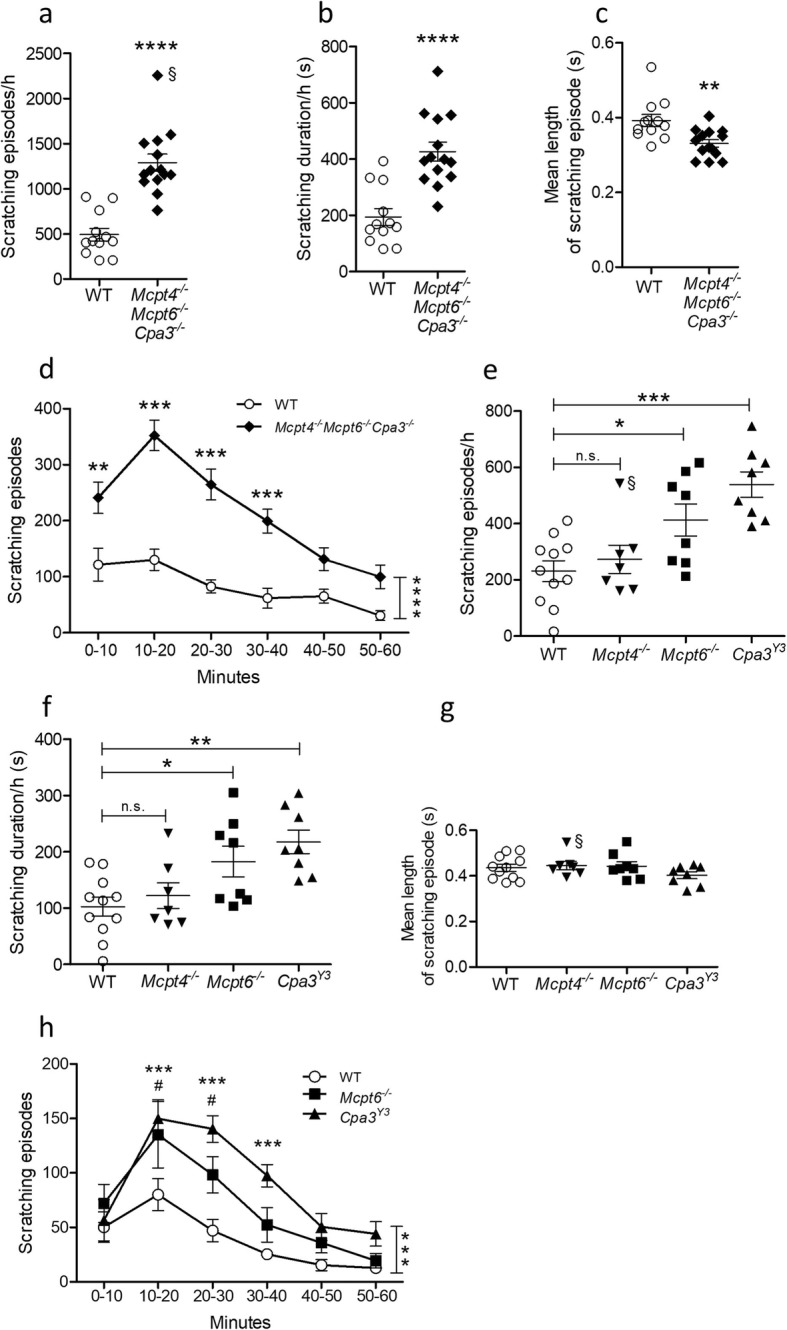
The mast cell protease-deficient mouse lines Mcpt4-/-Mcpt6-/-Cpa3-/-, Cpa3Y356L,E378A, and Mcpt6-/- scratch more than wild-type controls when injected with ET-1. a When injected with ET-1 (20 pmol), Mcpt4-/-Mcpt6-/-Cpa3-/- mice (n = 14) had a much higher number of scratching episodes in 60 min than wild-type controls (WT, n = 12). b Mcpt4-/-Mcpt6-/-Cpa3-/- mice spent more total time scratching during the 60 min test. c The Mcpt4-/-Mcpt6-/-Cpa3-/- mice also had shorter scratching episodes on average than controls. d The results from (a) analyzed over time. The Mcpt4-/-Mcpt6-/-Cpa3-/- mice scratched more frequently than controls overall (vertical significance bar), and post hoc analysis showed that they scratched significantly more in the first four intervals of the test. e Both Mcpt6-/- (n = 8) and Cpa3Y356L,E378A mice (Cpa3Y3, n = 8) scratched more frequently in 60 min than wild-type controls (n = 11) when injected with ET-1. Mcpt4-/- mice (n = 7) did not differ from controls in their scratching behavior. fMcpt6-/- and Cpa3Y356L,E378A mice also spent more time scratching after ET-1 injection than controls. g But no difference was seen in the mean length of scratching episodes. h The results from (e) analyzed over time. Both Mcpt6-/- and Cpa3Y356L,E378A mice scratched more than controls over the duration of the test (indicated by vertical significance bar). Further analysis showed that Cpa3Y356L,E378A mice scratched more than controls in the intervals between 10 and 40 min (indicated by stars), and Mcpt6-/- mice scratched significantly more between 10 and 30 min (indicated by #). Results are presented as mean ± SEM. #/*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; n.s, not significant; Student’s t test (a-c); 2-way ANOVA with Bonferroni multiple comparison (d, h);
1-way ANOVA (e-g). Statistical outliers as identified by Grubb’s test are indicated with the symbol §
Additional file 2: Movie S1.
The Mcpt6-/- and Cpa3Y356L,E378A mouse lines have an exaggerated scratching response to ET-1
Next, we aimed to identify which of the mast cell proteases was/were responsible for the protective effect against ET-1-induced scratching. We therefore evaluated the pruritic effects of an intradermal injection of ET-1 on the single mast cell protease knock-out lines Mcpt4-/- and Mcpt6-/-, as well as the transgenic line with enzymatically inactive CPA3 (Cpa3Y356L,E378A) (Fig. 2e, h).
Cpa3Y356L,E378A mice were initially used in this study instead of Cpa3-/- mice, since it has been shown that Cpa3-/- mice also lack mouse mast cell protease 5 (mMCP5) [36] and are thus functionally double-deficient in enzyme activity. CPA3 has been shown to protect against ET-1 toxicity in vivo [29]. Here, we noted that Cpa3Y356L,E378A mice scratched more frequently than controls during the 60 min test (Fig. 2e, P < 0.001). When scratching duration was analyzed, the findings were similar (Fig. 2f); the Cpa3Y356L,E378A animals spent more total time scratching than controls (P < 0.01). No difference was observed between groups in the mean length of scratching episodes (Fig. 2g, P > 0.05).
mMCP4 is a chymase, and it has been demonstrated that mast cell chymase can cleave the inflammatory neuropeptides substance P and vasoactive intestinal peptide (VIP [41, 42]), both of which can induce itch and degranulate mast cells [43–45]. Despite these potential abilities of mMCP4 to protect against itch, we saw no difference in scratching frequency, scratching duration or mean episode length in Mcpt4-/- mice compared with controls (Fig. 2e, g; P > 0.05), indicating that mMCP4 is not involved in regulating ET-1-induced scratching. One Mcpt4-/- animal was a significant outlier in scratching frequency (Fig. 2e) and another was an outlier in the mean length of scratching episodes (Fig. 2g). However, including or excluding these individuals did not have any effects on the results of the statistical comparison with controls, i.e., no differences were observed (P > 0.05, the animals are indicated with a § in the respective figures).
Finally, we investigated the role of the tryptase mMCP6 in ET-1-induced scratching. The analysis showed that Mcpt6-/- mice scratched more frequently and spent more time scratching than controls (Fig. 2e, f; P < 0.05), but the statistical significance was lower than seen between Cpa3Y356L,E378A mice and controls.
The development of the scratching behavior for all single protease transgenic lines was also analyzed over time in 10-min intervals (Fig. 2 h). The analysis revealed that both Cpa3Y356L,E378A and Mcpt6-/- animals had more frequent scratching episodes than controls overall during the test (P = 0.0002), but no difference was observed in Mcpt4-/- animals (data not shown). Post hoc testing on the individual intervals showed that the scratching episode frequency of Cpa3Y356L,E378A mice was elevated compared with controls in the intervals between 10 and 40 min (Fig. 2h, P < 0.001). Mcpt6-/- mice had elevated scratching frequency compared with controls in the intervals between 10-30 min (Fig. 2h, P < 0.05).
Taken together, the above data indicate that the mast cell proteases CPA3, and to a smaller extent, mMCP6, play a role in attenuating ET-1-induced scratching, while mMCP4 does not.
mMCP5 deficiency does not have an effect on ET-1-induced scratch behavior
It has previously been shown that CPA3 catalyzes the breakdown of ET-1 in vitro [29] and is important in reducing the toxic effects of ET-1 administered intraperitoneally [29, 33], and our results indicate that CPA3 is also involved in attenuating the pruritic effects of ET-1 administered intradermally. The question still remained if the extensive scratching behavior demonstrated by the Mcpt4-/-Mcpt6-/-Cpa3-/- mouse line was exclusively the result of lacking CPA3 and mMCP6. As mentioned above, when CPA3 is removed from the mast cell, mMCP5 (elastase) is lost as well (at the protein but not mRNA level) [36]. The Cpa3Y356L,E378A mouse line expresses CPA3 that is devoid of enzymatic activity and possesses functional mMCP5, while the Mcpt4-/-Mcpt6-/-Cpa3-/- mouse line has neither CPA3 nor mMCP5. This prompted the question if lacking mMCP5 had any effect on ET-1-induced scratching. To test this possibility, we performed the ET-1 test on the Cpa3-/- mouse line, which lacks both CPA3 and mMCP5 (Fig. 3a, d). The Cpa3-/- mice had a higher frequency of scratching bouts than their wild-type counterparts (Fig. 3a, P = 0.0094), and their scratching duration was elevated vs. controls (Fig. 3b, P = 0.022). There was no difference between Cpa3-/- mice and controls in the mean length of scratching episodes (Fig. 3c, P = 0.904). When the scratching frequency was analyzed over time and in 10-min intervals (Fig. 3d), it was seen that the Cpa3-/- mice scratched more than controls overall (P = 0.0094). Post hoc analysis on the intervals revealed that Cpa3-/- mice displayed more scratching episodes than controls in the interval between 20 and 30 min (P < 0.001).
Fig. 3.
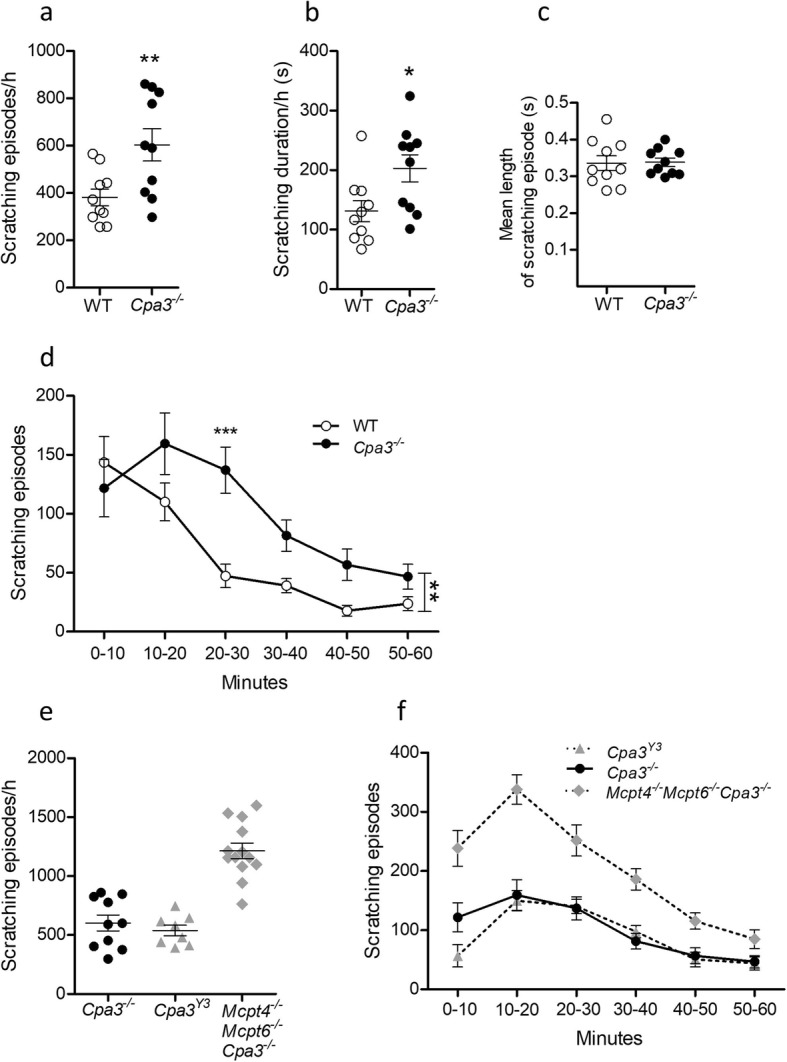
ET-1-induced scratching is not affected by mMCP5 deficiency. aCpa3-/- mice (n = 10) are deficient in both CPA3 and mMCP5, and had more frequent scratching bouts in 60 min than wild-type controls (WT, n = 10) when injected with ET-1 (20 pmol). bCpa3-/- mice also spent more time scratching during the 60 min. c No difference was seen between genotypes in the mean length of scratching episodes. d When the frequency results from (a) were analyzed over time, it was seen that Cpa3-/- mice scratched significantly more than controls overall (indicated by vertical significance bar), and post hoc analysis showed a significant difference in the interval between 20 and 30 min. e, f Visual comparison of the total frequency of scratching episodes and scratching frequency development over time, between Cpa3-/- mice (data from (a)), Cpa3Y356L,E378A mice (Cpa3Y3, data from Fig. 2e) and Mcpt4-/-Mcpt6-/-Cpa3-/- mice (data from Fig. 2a). The two CPA3-deficient mouse lines follow a similar scratching pattern and magnitude, while the Mcpt4-/-Mcpt6-/-Cpa3-/- mice demonstrate more exaggerated behavior. Results are presented as mean ± SEM. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, Student’s t test (a-c), 2-way ANOVA with Bonferroni multiple comparison (d)
Direct statistical comparison between groups tested at different time points, using different batches of ET-1, is not feasible since ET-1 is a very potent peptide that degrades with time despite freezer storage. This was confirmed by comparing the wild-type controls that were used in three sets of experiments using different batches of ET-1, and small but significant differences were found between the groups (data not shown). However, a visual comparison of the total scratching frequency in Cpa3-/- mice, Cpa3Y356L,E378A mice and Mcpt4-/-Mcpt6-/-Cpa3-/- mice (Fig. 3e), and how the scratching frequency develops over time (Fig. 3f), suggests that Cpa3Y356L,E378A and Cpa3-/- animals have a similar pattern of scratching behavior and magnitude, while the Mcpt4-/-Mcpt6-/-Cpa3-/- mice show more exaggerated behavior. Together, these findings indicate that mMCP5 does not contribute to the protection against the scratching behavior seen in the Mcpt4-/-Mcpt6-/-Cpa3-/- mice.
Discussion
Here, we investigated the possible involvement of mouse connective tissue mast cell-specific proteases in the scratching behavior induced by ET-1. Both mMCP6 and CPA3 deficiency, as well as the combined loss of mMCP4, mMCP6 and CPA3, resulted in significantly increased scratching behavior compared with wild-type controls, while mMCP4-deficiency alone did not have an effect. We could also conclude that mMCP5 is not involved in ET-1-induced scratching behavior.
ET-1 induces itch and pain through the ETA receptor on primary afferents
ET-1 binds to and activates the G protein-coupled receptors ETAR and ETBR [46], and in humans it can cause pain, hyperalgesia, and intense pruritus when exogenously administered, depending on the dose and the route of administration [22, 23, 47]. In the periphery, it has been reported that ETAR is mainly responsible for pain and itch transmission, while ETBR has generally been coupled to analgesic and antipruritic effects [20, 48, 49]. Similar to what has been seen with other algogens, central administration of ET-1 induces analgesia, which is mediated through both ETAR and ETBR [50]. Since animals cannot describe their sensations, a useful behavioral model to distinguish between algogens and pruritogens has been developed in mice: the mouse cheek model [51]. In this model, a substance is injected intradermally in the cheek and if the substance is an algogen the mouse responds to the pain by wiping the face with its forepaws, while pruritogens will elicit scratches by the hind paws. When ET-1 was tested in the cheek test, the mice exhibited both pain and itch behaviors [21], indicating that ET-1 is a substance that can induce both sensations. This suggests that the particular sensation experienced upon injection of ET-1 has certain painful properties experienced by the animals, a feeling that human volunteers have described as “burning itch” [22]. Furthermore, ET-1 is capable of degranulating mast cells through ETAR, causing release of pruritogens such as histamine and leukotriene C4 [24, 25, 52]. However, ET-1-induced itch is generally considered to be non-histaminergic. It depends upon other neuronal signaling mechanisms than histamine [49, 53] and histamine 1 receptor antagonists have little effect on the scratching behavior [20, 49]. Degranulation also releases large amounts of mast cell proteases, which have been reported to have both protective and inflammatory properties [40, 54, 55].
Multiple mast cell protease deficiency and ET-1-induced scratching
Mcpt4-/-Mcpt6-/-Cpa3-/- mice lack all connective tissue type mast cell-specific proteases, and this results in abnormal mast cell secretory granule composition and morphology, as well as reduction in heparin content [37]. It is therefore not surprising that these animals have abnormal physiological, and consequently, behavioral, responses to provocation. In our study, we saw greatly increased scratching behavior upon ET-1 injection in Mcpt4-/-Mcpt6-/-Cpa3-/- mice compared with controls, indicating that mast cell-specific proteases serve an important function in attenuating the itch induced by the peptide. In order to evaluate the contribution from the individual proteases, however, we also assessed mice with single deficiency of the respective proteases.
These analyses revealed that both Cpa3Y356L,E378A and Mcpt6-/- animals scratched significantly more than controls in response to ET-1, but the Mcpt4-/-Mcpt6-/-Cpa3-/- mouse line exhibited scratching that was of an even greater magnitude. Since the Mcpt4-/-Mcpt6-/-Cpa3-/- mouse line is lacking both mMCP6 and CPA3, a certain additive effect was not unexpected but this prompted the question if the intense scratching behavior was only due to the lack of these two proteases, or if mMCP5-deficiency also played a role. mMCP5 is a mast cell elastase which has not been extensively studied and no connection to itch has been reported, but it has been shown to have a role in mediating epidermal burn injuries in mice [56]. Since Mcpt4-/-Mcpt6-/-Cpa3-/- mice lack CPA3, they also lose the ability to store the elastase mMCP5. To investigate the potential properties of mMCP5, the ET-1 test was also performed on Cpa3-/- mice (that lack both CPA3 and mMCP5), to see if their scratching behavior would follow a similar pattern and magnitude as seen in Cpa3Y356L,E378A mice (that possess mMCP5 but enzymatically non-functional CPA3) or Mcpt4-/-Mcpt6-/-Cpa3-/- mice. However, the results from the Cpa3-/- mice did not resemble the greatly enhanced scratching seen in the multiple protease-deficient mice. This indicates that mMCP5 does not have an important role in the protection against ET-1-induced scratching.
CPA3 and ET-1-induced scratch behavior
CPA3 is not as well studied as mMCP4 and mMCP6, and has mainly received attention for its role in host defense by cleaving a particular type of toxins, sarafotoxins, that can be found in snake and bee venom [29, 57]. Sarafotoxins belong to the endothelin family and it has been demonstrated that CPA3 cleaves both sarafotoxin S6b and ET-1 at the C-terminal, which results in inactive and non-toxic products [29]. Mast cell chymase also cleaves those substrates, but at an internal location, which is not sufficient to reduce the toxicity [29]. CPA3 is necessary to protect against otherwise fatal ET-1 toxicity when it is injected intraperitoneally in doses between 1 and 3 nmols [29, 33]. In the current study, a considerably lower dose of ET-1 (20 pmol) and another route of administration (intradermal) were used to investigate ET-1-induced itch instead of toxicity, and our results show that CPA3-deficiency results in significantly exaggerated scratching behavior compared with controls. This result was seen in all the mouse lines that lack functional CPA3: Cpa3Y356L,E378A, Cpa3-/-, and Mcpt4-/-Mcpt6-/-Cpa3-/-. This suggests that CPA3 has an important role in protection against exogenously administered ET-1 with regard to pruritus.
mMCP4 and ET-1-induced scratch behavior
Chymase has the capability to produce ET-1 by cleaving the precursor peptide Big-ET-1 in vivo [58]. Chymase has also been reported to cleave known itch mediators such as interleukins 6, 13, and 33 (IL-6, IL-13, IL-33 [59, 60]) as well as substance P and VIP [41–43]. Despite these properties, there is little data that links mMCP4 directly to pruritus, but studies have shown that chymase inhibition is successful in attenuating itch and skin inflammation in a mouse AD model [61, 62]. In our study, no effects of chymase on ET-1-induced scratching behavior were observed. This is in accordance with a previous study showing that mMCP4 knockouts have the same resistance to ET-1-like toxicity (induced by sarafotoxins) as wild-type controls, while the toxicity was fatal in mast cell-deficient mice [57].
mMCP6 and ET-1-induced scratch behavior
Mcpt6-/- mice demonstrated higher levels of scratching behavior than controls in response to ET-1. This could potentially be explained by direct ET-1 inactivation catalyzed by mMCP6. Alternatively, mMCP6 could affect signaling events downstream of the binding of ET-1 to its receptor. Human tryptase has been reported to cleave CGRP in vitro in an efficient manner [63] and ET-1 induces the release of CGRP and glutamate from primary afferents through ETAR activation [64]. Since CGRP has been implicated in itch modulation [65, 66], mMCP6 may have an effect on ET-1-induced scratch behavior indirectly, through the degradation of CGRP. However, the Mcpt6-/- mice may also be more sensitive to the injection of an inert vehicle (Supplementary Fig. 1a), which further complicates the interpretation of their scratching behavior.
Conclusions
Here, we have investigated the effects of connective tissue mast cell protease deficiency on ET-1-induced scratching behavior. We found that the mast cell carboxypeptidase CPA3 was critical for attenuating ET-1-induced scratching, and our data also indicate that mast cell tryptase can contribute to such protection. Importantly, the protease expression profile of mouse and human skin mast cells is highly analogous and the substrate specificities of the corresponding mouse and human mast cell proteases are closely related [40, 55], and it is therefore likely that human mast cell proteases have a similar impact on itch as seen in the approach adopted here. Altogether, these findings establish a novel principle for how itch responses can be regulated. Intriguingly, since mast cells are also strongly implicated in promoting itch signaling (e.g., by secreting histamine and serotonin), our present investigations introduce the notion that mast cells can both promote and attenuate itch.
Supplementary information
Additional file 1: Figure S1.mMCP6-deficient mice potentially scratch more than controls in response to vehicle injection.a)Mcpt6-/- mice (n = 6) had slightly more frequent scratching bouts in 60 minutes than wild-type controls (WT, n = 24) when injected intradermally with vehicle (0.9% saline, 50 μL), while Mcpt4-/- (n = 9), Cpa3Y356L,E378A (Cpa3Y3, n = 8) and Mcpt4-/-Mcpt6-/-Cpa3-/- mice (n = 10) did not. b) No difference was seen between groups in scratching duration after vehicle injection, c) or in mean length of scratching episodes. Vehicle data for controls are pooled from three different experiments. Results are presented as mean ± SEM. *P ≤ 0.05, Kruskal-Wallis. Statistical outliers as identified by Grubb’s test are indicated with the symbol §.
Acknowledgements
We acknowledge Hans-Reimer Rodewald and Thorsten Feyerabend for the contribution of transgenic lines.
Authors’ contributions
EIM and JB performed the behavioral experiments. MG performed genotyping and handled the mouse colony. GP contributed the transgenic mouse lines. EIM, GP, and MCL designed the study. All authors contributed to the writing of the manuscript. All authors have approved the manuscript.
Funding
This work was supported by grants from the Swedish Research Council (2015-02442; 2016-00851), Uppsala University, The Brain foundation and the foundations of Ragnar Söderberg. MCL is a Ragnar Söderberg Fellow in Medicine. Open access funding provided by Uppsala University.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files) and available from the corresponding author on reasonable request.
Ethics approval and consent to participate
We have obtained ethical approval for the animal studies included (please see the “Methods” section). The study does not contain human participants/material.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12974-020-01795-4.
References
- 1.Pereira MP, Steinke S, Bruland P, Stander HF, Dugas M, Augustin M, et al. Management of chronic pruritus: from the dermatological office to the specialized itch center: a review. Itch (Phila). 2017;2(2):e6. doi: 10.1097/itx.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 3.Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, et al. Endothelin. Pharmacol Rev. 2016;68(2):357–418. doi: 10.1124/pr.115.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenzel RR, Zbinden S, Noll G, Meier B, Luscher TF. Endothelin-1 induces vasodilation in human skin by nociceptor fibres and release of nitric oxide. Br J Clin Pharmacol. 1998;45(5):441–446. doi: 10.1046/j.1365-2125.1998.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, et al. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokolovsky M. Endothelins and sarafotoxins: physiological regulation, receptor subtypes and transmembrane signaling. Pharmacol Ther. 1992;54(2):129–149. doi: 10.1016/0163-7258(92)90030-4. [DOI] [PubMed] [Google Scholar]
- 7.Masaki T. Endothelins: homeostatic and compensatory actions in the circulatory and endocrine systems. Endocr Rev. 1993;14(3):256–268. doi: 10.1210/edrv-14-3-256. [DOI] [PubMed] [Google Scholar]
- 8.Yohn JJ, Morelli JG, Walchak SJ, Rundell KB, Norris DA, Zamora MR. Cultured human keratinocytes synthesize and secrete endothelin-1. J Invest Dermatol. 1993;100(1):23–26. doi: 10.1111/1523-1747.ep12349932. [DOI] [PubMed] [Google Scholar]
- 9.Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991;325(14):997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- 10.Giaid A, Gibson SJ, Ibrahim BN, Legon S, Bloom SR, Yanagisawa M, et al. Endothelin 1, an endothelium-derived peptide, is expressed in neurons of the human spinal cord and dorsal root ganglia. Proc Natl Acad Sci U S A. 1989;86(19):7634–7638. doi: 10.1073/pnas.86.19.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasue F, Kuwaki T, Kisanuki YY, Yanagisawa M, Moriya H, Fukuda Y, et al. Increased sensitivity to acute and persistent pain in neuron-specific endothelin-1 knockout mice. Neuroscience. 2005;130(2):349–358. doi: 10.1016/j.neuroscience.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 12.Ehrenreich H, Burd PR, Rottem M, Hultner L, Hylton JB, Garfield M, et al. Endothelins belong to the assortment of mast cell-derived and mast cell-bound cytokines. New Biol. 1992;4(2):147–156. [PubMed] [Google Scholar]
- 13.Liu Y, Yamada H, Ochi J. Immunocytochemical studies on endothelin in mast cells and macrophages in the rat gastrointestinal tract. Histochem Cell Biol. 1998;109(4):301–307. doi: 10.1007/s004180050230. [DOI] [PubMed] [Google Scholar]
- 14.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, et al. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348(6303):732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 16.Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135(3):561–571. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18(1):145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 18.Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, et al. Molecular architecture of the mouse nervous system. Cell. 2018;174(4):999–1014. doi: 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piovezan AP, D'Orleans-Juste P, Tonussi CR, Rae GA. Endothelins potentiate formalin-induced nociception and paw edema in mice. Can J Physiol Pharmacol. 1997;75(6):596–600. doi: 10.1139/y97-057. [DOI] [PubMed] [Google Scholar]
- 20.Trentin PG, Fernandes MB, D'Orleans-Juste P, Rae GA. Endothelin-1 causes pruritus in mice. Exp Biol Med (Maywood). 2006;231(6):1146–1151. [PubMed] [Google Scholar]
- 21.Gomes LO, Hara DB, Rae GA. Endothelin-1 induces itch and pain in the mouse cheek model. Life Sci. 2012;91(13-14):628–633. doi: 10.1016/j.lfs.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira SH, Romitelli M, de Nucci G. Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol. 1989;13(Suppl 5):S220–S222. doi: 10.1097/00005344-198900135-00065. [DOI] [PubMed] [Google Scholar]
- 23.Dahlof B, Gustafsson D, Hedner T, Jern S, Hansson L. Regional haemodynamic effects of endothelin-1 in rat and man: unexpected adverse reaction. J Hypertens. 1990;8(9):811–817. doi: 10.1097/00004872-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Yamamura H, Nabe T, Kohno S, Ohata K. Endothelin-1, one of the most potent histamine releasers in mouse peritoneal mast cells. Eur J Pharmacol. 1994;265(1-2):9–15. doi: 10.1016/0014-2999(94)90217-8. [DOI] [PubMed] [Google Scholar]
- 25.Matsushima H, Yamada N, Matsue H, Shimada S. The effects of endothelin-1 on degranulation, cytokine, and growth factor production by skin-derived mast cells. Eur J Immunol. 2004;34(7):1910–1919. doi: 10.1002/eji.200424912. [DOI] [PubMed] [Google Scholar]
- 26.Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol. 2006;530(1-2):172–178. doi: 10.1016/j.ejphar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Solinski HJ, Kriegbaum MC, Tseng PY, Earnest TW, Gu X, Barik A, et al. Nppb neurons are sensors of mast cell-induced itch. Cell Rep. 2019;26(13):3561–3573. doi: 10.1016/j.celrep.2019.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metsarinne KP, Vehmaan-Kreula P, Kovanen PT, Saijonmaa O, Baumann M, Wang Y, et al. Activated mast cells increase the level of endothelin-1 mRNA in cocultured endothelial cells and degrade the secreted peptide. Arterioscler Thromb Vasc Biol. 2002;22(2):268–273. doi: 10.1161/hq0202.103994. [DOI] [PubMed] [Google Scholar]
- 29.Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR. Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J Exp Med. 2007;204(11):2629–2639. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henningsson F, Yamamoto K, Saftig P, Reinheckel T, Peters C, Knight SD, et al. A role for cathepsin E in the processing of mast-cell carboxypeptidase A. J Cell Sci. 2005;118(Pt 9):2035–2042. doi: 10.1242/jcs.02333. [DOI] [PubMed] [Google Scholar]
- 31.Lees WE, Kalinka S, Meech J, Capper SJ, Cook ND, Kay J. Generation of human endothelin by cathepsin E. FEBS Lett. 1990;273(1-2):99–102. doi: 10.1016/0014-5793(90)81060-2. [DOI] [PubMed] [Google Scholar]
- 32.Semaan W, Desbiens L, Houde M, Labonte J, Gagnon H, Yamamoto D, et al. Chymase inhibitor-sensitive synthesis of endothelin-1 (1-31) by recombinant mouse mast cell protease 4 and human chymase. Biochem Pharmacol. 2015;94(2):91–100. doi: 10.1016/j.bcp.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432(7016):512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 34.Tchougounova E, Pejler G, Abrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J Exp Med. 2003;198(3):423–431. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, et al. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol. 2008;180(7):4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feyerabend TB, Hausser H, Tietz A, Blum C, Hellman L, Straus AH, et al. Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol Cell Biol. 2005;25(14):6199–6210. doi: 10.1128/MCB.25.14.6199-6210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grujic M, Calounova G, Eriksson I, Feyerabend T, Rodewald HR, Tchougounova E, et al. Distorted secretory granule composition in mast cells with multiple protease deficiency. J Immunol. 2013;191(7):3931–3938. doi: 10.4049/jimmunol.1301441. [DOI] [PubMed] [Google Scholar]
- 38.Waern I, Karlsson I, Thorpe M, Schlenner SM, Feyerabend TB, Rodewald HR, et al. Mast cells limit extracellular levels of IL-13 via a serglycin proteoglycan-serine protease axis. Biol Chem. 2012;393(12):1555–1567. doi: 10.1515/hsz-2012-0189. [DOI] [PubMed] [Google Scholar]
- 39.Ihara M, Ishikawa K, Fukuroda T, Saeki T, Funabashi K, Fukami T, et al. In vitro biological profile of a highly potent novel endothelin (ET) antagonist BQ-123 selective for the ETA receptor. J Cardiovasc Pharmacol. 1992;20(Suppl 12):S11–S14. doi: 10.1097/00005344-199204002-00005. [DOI] [PubMed] [Google Scholar]
- 40.Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol. 2007;95:167–255. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- 41.Caughey GH, Leidig F, Viro NF, Nadel JA. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. J Pharmacol Exp Ther. 1988;244(1):133–137. [PubMed] [Google Scholar]
- 42.Akahoshi M, Song CH, Piliponsky AM, Metz M, Guzzetta A, Abrink M, et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J Clin Invest. 2011;121(10):4180–4191. doi: 10.1172/JCI46139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azimi E, Reddy VB, Pereira PJS, Talbot S, Woolf CJ, Lerner EA. Substance P activates Mas-related G protein-coupled receptors to induce itch. J Allergy Clin Immunol. 2017;140(2):447–453. doi: 10.1016/j.jaci.2016.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rukwied R, Heyer G. Cutaneous reactions and sensations after intracutaneous injection of vasoactive intestinal polypeptide and acetylcholine in atopic eczema patients and healthy controls. Arch Dermatol Res. 1998;290(4):198–204. doi: 10.1007/s004030050290. [DOI] [PubMed] [Google Scholar]
- 45.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 47.Namer B, Hilliges M, Orstavik K, Schmidt R, Weidner C, Torebjork E, et al. Endothelin 1 activates and sensitizes human C-nociceptors. Pain. 2008;137(1):41–49. doi: 10.1016/j.pain.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, et al. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9(8):1055–1061. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 49.Liang J, Kawamata T, Ji W. Molecular signaling of pruritus induced by endothelin-1 in mice. Exp Biol Med (Maywood). 2010;235(11):1300–1305. doi: 10.1258/ebm.2010.010121. [DOI] [PubMed] [Google Scholar]
- 50.D’Amico M, Di Filippo C, Rossi F. Selective and non-selective ET antagonists reveal an ET(A)/ET(B) receptor mediated ET-1-induced antinociceptive effect in PAG area of mice. Life Sci. 1997;61(25):PL 397–PL 401. doi: 10.1016/s0024-3205(97)00988-0. [DOI] [PubMed] [Google Scholar]
- 51.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139(3):681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamura H, Nabe T, Kohno S, Ohata K. Endothelin-1 induces release of histamine and leukotriene C4 from mouse bone marrow-derived mast cells. Eur J Pharmacol. 1994;257(3):235–242. doi: 10.1016/0014-2999(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 53.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106(27):11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caughey GH. Mast cell proteases as protective and inflammatory mediators. Adv Exp Med Biol. 2011;716:212–234. doi: 10.1007/978-1-4419-9533-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014;14(7):478–494. doi: 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- 56.Bankova LG, Lezcano C, Pejler G, Stevens RL, Murphy GF, Austen KF, et al. Mouse mast cell proteases 4 and 5 mediate epidermal injury through disruption of tight junctions. J Immunol. 2014;192(6):2812–2820. doi: 10.4049/jimmunol.1301794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313(5786):526–530. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 58.Houde M, Jamain MD, Labonte J, Desbiens L, Pejler G, Gurish M, et al. Pivotal role of mouse mast cell protease 4 in the conversion and pressor properties of big-endothelin-1. J Pharmacol Exp Ther. 2013;346(1):31–37. doi: 10.1124/jpet.112.202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao W, Oskeritzian CA, Pozez AL, Schwartz LB. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J Immunol. 2005;175(4):2635–2642. doi: 10.4049/jimmunol.175.4.2635. [DOI] [PubMed] [Google Scholar]
- 60.Roy A, Ganesh G, Sippola H, Bolin S, Sawesi O, Dagalv A, et al. Mast cell chymase degrades the alarmins heat shock protein 70, biglycan, HMGB1, and interleukin-33 (IL-33) and limits danger-induced inflammation. J Biol Chem. 2014;289(1):237–250. doi: 10.1074/jbc.M112.435156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imada T, Komorita N, Kobayashi F, Naito K, Yoshikawa T, Miyazaki M, et al. Therapeutic potential of a specific chymase inhibitor in atopic dermatitis. Jpn J Pharmacol. 2002;90(3):214–217. doi: 10.1254/jjp.90.214. [DOI] [PubMed] [Google Scholar]
- 62.Terakawa M, Fujieda Y, Tomimori Y, Muto T, Tanaka T, Maruoka H, et al. Oral chymase inhibitor SUN13834 ameliorates skin inflammation as well as pruritus in mouse model for atopic dermatitis. Eur J Pharmacol. 2008;601(1-3):186–191. doi: 10.1016/j.ejphar.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 63.Tam EK, Caughey GH. Degradation of airway neuropeptides by human lung tryptase. Am J Respir Cell Mol Biol. 1990;3(1):27–32. doi: 10.1165/ajrcmb/3.1.27. [DOI] [PubMed] [Google Scholar]
- 64.Khodorova A, Richter J, Vasko MR, Strichartz G. Early and late contributions of glutamate and CGRP to mechanical sensitization by endothelin-1. J Pain. 2009;10(7):740–749. doi: 10.1016/j.jpain.2009.01.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, Zylka MJ. Peptidergic CGRPalpha primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron. 2013;78(1):138-51. [DOI] [PMC free article] [PubMed]
- 66.Rogoz K, Andersen HH, Lagerstrom MC, Kullander K. Multimodal use of calcitonin gene-related peptide and substance P in itch and acute pain uncovered by the elimination of vesicular glutamate transporter 2 from transient receptor potential cation channel subfamily V member 1 neurons. J Neurosci. 2014;34(42):14055–14068. doi: 10.1523/JNEUROSCI.1722-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1.mMCP6-deficient mice potentially scratch more than controls in response to vehicle injection.a)Mcpt6-/- mice (n = 6) had slightly more frequent scratching bouts in 60 minutes than wild-type controls (WT, n = 24) when injected intradermally with vehicle (0.9% saline, 50 μL), while Mcpt4-/- (n = 9), Cpa3Y356L,E378A (Cpa3Y3, n = 8) and Mcpt4-/-Mcpt6-/-Cpa3-/- mice (n = 10) did not. b) No difference was seen between groups in scratching duration after vehicle injection, c) or in mean length of scratching episodes. Vehicle data for controls are pooled from three different experiments. Results are presented as mean ± SEM. *P ≤ 0.05, Kruskal-Wallis. Statistical outliers as identified by Grubb’s test are indicated with the symbol §.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files) and available from the corresponding author on reasonable request.


