Abstract
Background
Inverted urothelial papilloma (IUP) of the upper urinary tract is an uncommon benign tumour that occasionally presents as a polypoid mass causing urinary obstruction. Histologically, IUP is characterised by a proliferating urothelium arranged in cords and trabeculae, in continuity with overlying intact epithelium, and extending into the lamina propria in a non-invasive, endophytic manner. Cytological atypia is minimal or absent. Top differential diagnoses include urothelial carcinoma with inverted growth pattern and florid ureteritis cystica. Although urothelial carcinomas of the upper urinary tract with prominent inverted growth pattern commonly harbour microsatellite instability, the role of the mutator phenotype pathway in IUP development is still unclear. The aim of this study was to describe two additional cases of IUP of the upper urinary tract, along with an extensive literature review.
Case presentation
We observed two polypoid tumours originating in the renal pelvis and the distal ureter, respectively. Both patients, a 76-year-old woman and a 56-year-old man, underwent surgery because of the increased likelihood of malignancy. Histology was consistent with IUP and patients are alive and asymptomatic after long-term follow-up (6 years for the renal pelvis lesion and 5 years for the ureter lesion). The tumours retained the expression of the mismatch-repair protein MLH1, MSH2, and PMS2 whereas loss of MSH6 was found in both cases.
Conclusions
When completely resected, IUP does not require rigorous surveillance protocols, such as those for urothelial carcinoma and exophytic urothelial papilloma. It is therefore important for the surgical pathologist to be aware of this rare entity in order to ensure correct patient management.
Keywords: Inverted urothelial papilloma, Upper urinary tract, Molecular markers, Microsatellite instability
Background
Inverted urothelial papilloma (IUP) is a rare lesion, histologically similar to inverted papilloma of the nasal cavity and paranasal sinuses. First reported in 1927 by Paschkis as “polypoid adenoma of the bladder” [1], it was later described in 1963 by Potts and Hirst as a distinct tumour entity of the urinary bladder [2]. IUP accounts for approximately 2% of all urothelial neoplasms. It usually occurs at the bladder neck, trigone or prostatic urethra, but is rare in the upper urinary tract. To the best of our knowledge, 68 IUP cases of the renal pelvis and ureter have been described in the English literature (Tables 1 and 2) [3–52].
Table 1.
IUP of the renal pelvis (RP) previously reported in the English Literature (NS = Not Stated; NA = Not Assessed)
| Reference | Age | Sex | Presentation | Site | Gross/Maximum Diameter (cm) | Associated Urothelial Lesions | Treatment | Recurrence (Follow-Up) |
|---|---|---|---|---|---|---|---|---|
| Matz et al. (1974) [3] | 68 | M | Haematuria, flank pain | Left RP | Nodule/1.5 | None | Nephroureterectomy | None (2 ys) |
| Assor (1976) [4] | 79 | M | Haematuria, flank discomfort | Right RP | Sessile polyp/1.5 | None | Partial resection | NS |
| Cameron et al. (1976) [5] | 58 | F | NS | RP (side NS) | NS/3 | None | Nephroureterectomy | Patient died of carcinoma of the endometrium four years later |
| Di Cello et al. (1980) [6] | 53 | M | Asymptomatic | Left uretero-pelvic junction | Sessile polyp/3 | None | Nephroureterectomy | NS |
| Theoret et al. (1980) [7] | 89 | M | Asymptomatic (autopsy finding) | RP (side NS) | NS | None | NA | NA |
| Uyama et al. (1981) [8] | 73 | M | Haematuria | Left RP | NS/2.5 | None | Nephroureterectomy, radiation and chemotherapy | None (5 ys) |
| Anderström et al. (1982) [9] | 62 | M | Asymptomatic | Left RP | Nodule/3 | Synchronous grade 2 transitional cell carcinoma of contralateral RP and non-invasive grade 2 transitional cell carcinoma of the bladder; history of recurrent grade 2 transitional cell carcinoma of the bladder | Extracorporeal resection of ureter and RP and autotransplant of kidney to bladder | Patient died of metastatic poorly differentiated squamous cell carcinoma of the bladder three years later; no recurrence in the kidney where IUP was diagnosed |
| Anderström et al. (1982) [9] | 49 | M | Ureteral colic | Right RP | Nodule/NS | None | NS | NS |
| Watters et al. (1983) [10] | 65 | M | Haematuria | Left RP | Pedunculated polyp/1 | None | Nephroureterectomy | NS |
| Lausten et al. (1984) [11] | 63 | M | NS | Right RP | Pedunculated polyp/1 | Grade 3 invasive polypoid transitional cell carcinoma in the contralateral RP after 8 years | Nephrectomy | None (8.5 ys) |
| Taylor et al. (1986) [12] | 65 | M | Haematuria | Right RP | Sessile polyp/NS | None | Nephroureterectomy | None (2 ys) |
| Schulze et al. (1986) [13] | 53 | M | Haematuria | Right RP | Sessile polyp/2.5 | None | Nephroureterectomy | NS |
| Schulze et al. (1986) [13] | 55 | M | Haematuria | Left RP and ureter | Not apparent at gross examination | None | Nephrectomy | NS |
| Romanelli (1986) [14] | 52 | M | Haematuria, renal colic, | Right RP | Sessile polyp/2.1 | None | Nephroureterectomy | NS |
| Yamaguchi et al. (1988) [15] | 73 | M | Haematuria | Left RP | Pedunculated polyp/0.6 | Synchronous low grade transitional cell carcinoma of the bladder (ureteral orifice) | Nephrectomy | None (1 y) |
| Schultz et al. (1988) [16] | 58 | M | Haematuria | Left RP | NS | Synchronous superficial grade 2 transitional cell carcinoma of the contralateral ureter (nephroureterectomy with excision of the bladder cuff) | Pyelotomy and endoscopic resection | IUP of the bladder 1 y later |
| Aubert et al. (1988) [17] | 34 | M | Haematuria | Left RP | NS | None | Nephroureterectomy | None (18 months) |
| Kyriakos et al. (1989) [18] | 73 | F | Asymptomatic | Multiple lesions: Junction between a upper pole major calyx and right RP (I); right calix (II); distal right ureter (III and IV) | Polyp/2.6 (I); slightly elevated nodule/1 (II); polyp/0.5 (III); polyp/1.2 (IV) | None | Nephroureterectomy | None (11 months) |
| Bagley et al. (1990) [19] | 64 | M | Haematuria | Right RP | Nodule/1 | Recurrent transitional cell carcinoma of the bladder | Ureteropyeloscopy with endoscopic resection | None (6 months) |
| Bassi et al. (1991) [20] | 51 | M | Haematuria, flank pain | Left RP | Sessile polyp/0.5 | None | Partial resection | NS |
| Vlassopopulos et al. (1992) [21] | 59 | M | Haematuria, flank pain | Left RP | Sessile polyp/2 | None | Nephroureterectomy | None (12 months) |
| Ueda T et al. (1992) [22] | 71 | M | Asymptomatic | Right RP | Nodule/4 | None | Nephrectomy | Synchronous clear cell carcinoma of the homolateral kidney, treated with surgery and anticancer drugs. No recurrence from IUP (21 months) |
| Spevack et al. (1995) [23] | 64 | M | Haematuria | Right RP | Pedunculated polyp/2.5 | None | Partial resection | None (42 months) |
| Chiura et al. (1998) [24] | 63 | M | Haematuria | Right RP | NS/3 | Transitional cell carcinoma of the left distal ureter three years later, treated with surgery, radiation therapy and chemotherapy | Nephroureterectomy | None (1 y after surgery for carcinoma) |
| Chiura et al. (1998) [24] | 53 | M | Haematuria | RP | NS | Pyelitis cystica | Nephroureterectomy | NS |
| Chiura et al. (1998) [24] | 64 | M | Asymptomatic | Right RP | NS | Recurrent transitional cell carcinoma of the bladder (previous and subsequent to IUP diagnosis) | Ureteroscopy and biopsy | Transitional cell carcinoma in the homolateral kidney and ureter 9 ys later |
| Darras et al. (2005) [25] | 52 | M | Haematuria, occasional discomfort in the lower abdomen | Left RP | Polyp/NS | Synchronous IUP of the bladder | Partial resection | None (NS) |
| Luo et al. (2012) [26] | 62 | M | Asymptomatic | Right RP | Pedunculated polyp | None | Nephroureterectomy | None (NS) |
| Luo et al. (2012) [26] | 66 | M | Haematuria | Left RP | Pedunculated polyp | None | Nephroureterectomy | None (NS) |
| Luo et al. (2012) [26] | 64 | M | Haematuria | Left RP | Pedunculated polyp | None | Nephroureterectomy | None (NS) |
| Luo et al. (2012) [26] | 73 | F | Flank pain | Right RP | Pedunculated polyp | None | Nephroureterectomy | None (NS) |
Table 2.
IUP of the ureter (U) previously reported in the English Literature (NS = Not Stated; NA = Not Assessed)
| Reference | Age | Sex | Presentation | Site | Gross/Maximum Diameter (cm) | Associated Urothelial Lesions | Treatment | Rrecurrence (Follow-Up) |
|---|---|---|---|---|---|---|---|---|
| Geisler et al. (1980) [27] | 77 | M | Flank pain | Left middle U | Pedunculated/2.5 | None | NephroUectomy | NS |
| Silverstein et al. (1981) [28] | 65 | M | Asymptomatic | Left middle U | Pedunculated/2.5 | None | Partial resection | NS |
| Silverstein et al. (1981) [28] | 68 | M | Haematuria | Right middle U | Polypoid/2.5 | None | NephroUectomy | NS |
| Fromowitz et al. (1981) [29] | 75 | M | Haematuria | Right U, at junction of proximal and middle thirds | Flat, polypoid/1.8 | None | NephroUectomy | NS |
| Fromowitz et al. (1981) [29] | 56 | M | Asymptomatic | Right distal U | Raised/1.1 | Adenocarcinoma of the bladder 7 months later with three recurrences during next 2 ys | NephroUectomy | None (2 ys) |
| Ajrawat et al. (1982) [30] | 86 | F | Flank pain | Right distal U | Lobulated mass/1.5 | None | Partial resection | NS |
| Naito et al. (1983) [31] | 68 | M | Haematuria | Right distal U | Pedunculated/1.5 | None | NephroUectomy | None (2 ys) |
| Jacobellis et al. (1983) [32] | 59 | F | Haematuria, flank pain | Left lumbar U | Sessile/3 | Synchronous conventional papilloma of homolateral lower calix | NephroUectomy | NS |
| Embon et al. (1984) [33] | 69 | M | Haematuria | Right distal U | Polypoid/3 | None | Partial resection | None (9 months) |
| Lausten et al. (1984) [11] | 60 | M | Asymptomatic | Right proximal U | Sessile tumour/ 0.3 | Grade 2 non-invasive transitional cell papilloma located above the homolateral Uic orifice 1 and half years earlier | Cranial heminephroUectomy | None (19 months) |
| Lausten et al. (1984) [11] | 71 | M | Flank pain (prostatism) | Right proximal U | Pedunculated tumour/ 1 | None | Partial U resection | None (18 months) |
| Perrin et al. (1984) [34] | 63 | M | Haematuria, renal colic | Left middle U | Polypoid/NS | None | Partial resection | Dead after 2 ys of cirrhosis; no recurrence of Ual lesion |
| Mottola et al. (1984) [35] | 56 | M | Haematuria, flank pain | Right lumbar U | NS | None | Partial resection | None (12 months) |
| Palvio (1985) [36] | 50 | M | Haematuria | Distal portion of the left U (above the Ual orifice) | Pedunculated tumour/ NS | After 8 ys from the first diagnosis of IUP of the distal U, the patient underwent nephroUectomy for two lesions at the Uopelvic junction and in the distal part of the U (IUP with areas of non-invasive transitional cell carcinoma, grade 2) | TUR | Yes, after 3 ys |
| Moss et al. (1987) [37] | 79 | M | Asymptomatic | Right U | NS/1 | None | U resection during hemicolectomy | None (3 months) |
| Corkill et al. (1987) [38] | 62 | M | Haematuria | Left distal U | Polypoid/0.8 | None | Partial resection | None (7ys) |
| Duchek et al. (1987) [39] | 24 | M | Haematuria, renal colic | Right middle U | Pedunculated lesion/NS | None | Local resection | None (5 ys) |
| Abulafi A et al. (1987) [40] | 62 | M | Haematuria | Right proximal U | Pedunculated lesion/NS | None | Local resection | NS |
| Villani U et al. (1987) [41] | 56 | M | Haematuria | Left pelvic U | NS | Synchronous grade 2 papillary transitional cell carcinoma of the bladder | Local resection | None (1y) |
| Kostakopolulos et al. (1988) [42] | 66 | M | Haematuria, renal colic | Left U | NS | None | Partial resection | None (6 months) |
| Garritano et al. (1988) [43] | 49 | M | Haematuria | Left middle U | Pedunculated lobulated tumour/3 | None | Local resection | None (5 ys) |
| Aubert et al. (1988) [17] | 71 | M | Haematuria, flank pain | Right lower U | NS | None | Partial resection | None (5 ys) |
| Page et al. (1991) [44] | 56 | M | Haematuria | Distal U, bilateral | Multiple sessile lesions/right side lesion: 3 cm; 2 lesions of the left side: 2 cm each) | None | Right side: partial Uectomy; Left side: complete Uectomy | NS |
| Kunimi et al. (1994) [45] | 42 | M | Flank pain | Left middle U | Pedunculated polyp/ 2.7 | Superficial transitional cell carcinoma grade 2 of the bladder (23 months later) | NephroUectomy | None (20 months after the diagnosis of carcinoma) |
| de Knijff et al. (1997) [46] | 63 | M | Urinary frequency and urge | Right distal U | NS/2 | Invasive bladder tumour six years later, treated with cystoprostatectomy | Local resection | None |
| Hoekx et al. (1998) [47] | 71 | M | Haematuria, flank pain | Left distal U and right distal U | Smooth surface/NS | Synchronous grade 2 transitional cell carcinoma of the bladder (T1N0M0) | Left partial resection and right nephroUectomy | Multiple recurrences of urinary badder carcinoma (duration of follow-up NS) |
| Lyon et al. (2006) [48] | 59 | M | Haematuria | Left proximal U | Sessile lesion/2.5 | None | Local resection | None (1 y) |
| Kilciler et al. (2008) [49] | 62 | M | Haematuria, flank pain | Middle U (side NS) | NS/2 | None | NephroUectomy | None (NS) |
| Mertziotis et al. (2012) [50] | 62 | M | Haematuria, flank pain | Right upper U | Exophytic lesion/4 | None | Nephrouretectomy | None (14 months) |
| Murtaza et al. (2012) [51] | 35 | M | Flank pain | Left distal U | Multiple small to large polypoid lesions | None | Local resection | None (6 months) |
| Lopez-Fontana et al. (2012) [52] | 30 | M | Haematuria | Right distal U | Polypoid lesion/1.6 | None | Partial Uectomy | None (4 months) |
| Luo et al. (2012) [26] | 70 | M | Haematuria | Right U | Pedunculated | None | NephroUectomy | None (NS) |
| Luo et al. (2012) [26] | 61 | M | Flank Pain | Left U | Pedunculated | Not specified | Partial Uctomy | None (NS) |
| Luo et al. (2012) [26] | 67 | M | Asymptomatic | Left U | Multiple lesions/ Pedunculated | None | NephroUectomy | None (NS) |
| Luo et al. (2012) [26] | 67 | M | Haematuria | Left U | Multiple lesions/ Pedunculated | None | Local resection | None (NS) |
| Luo et al. (2012) [26] | 73 | M | Haematuria | Left U | Pedunculated | Not specified | Partial Uctomy | None (NS) |
| Luo et al. (2012) [26] | 68 | M | Haematuria | Left U | Pedunculated | Not specified | Partial Uctomy | None (NS) |
Histological diagnosis of IUP can be difficult and several pathological conditions may enter differential diagnosis, including other urothelial neoplasms with endophytic growth patterns (i.e. papillary urothelial neoplasm of low malignant potential, low- and high-grade urothelial carcinoma), nested urothelial carcinoma, paraganglioma, carcinoid tumour, florid von Brunn nest proliferation and cystitis cystica et glandularis. Most of the investigated immunohistochemical markers are of little use in routine practice, and microscopic assessment remains the current gold standard. IUPs are benign tumours and can be successfully treated by conservative surgery. While specific molecular alterations are well described for papillary urothelial neoplasms, only few studies have been conducted on inverted lesions, suggesting a correlation between inverted growth and mismatch repair deficiency in urothelial carcinoma of the upper urinary tract [53].
Two additional cases of polypoid IUP of the renal pelvis and the ureter are herein presented with a systematic review of the literature.
Clinical cases
Case 1
A 76-year-old woman was admitted with persistent right flank pain and macroscopic haematuria. A computed tomography (CT) scan revealed a 2-cm polypoid lesion in the right renal pelvis, causing mild proximal hydronephrosis. The patient was otherwise in good health and advised to undergo nephroureterectomy due to the high likelihood of malignancy. Postoperative course was unremarkable, and the patient was discharged eight days after admission. She is alive and free of disease six years after treatment.
Case 2
A 56-year-old man presented with gross haematuria. A CT scan demonstrated a filling defect in the lower third of the right ureter with no evidence of lithiasis. Owing to the distal location of the lesion, segmentary ureterectomy was performed. The patient is asymptomatic five years after complete excision of the tumour.
Pathological findings
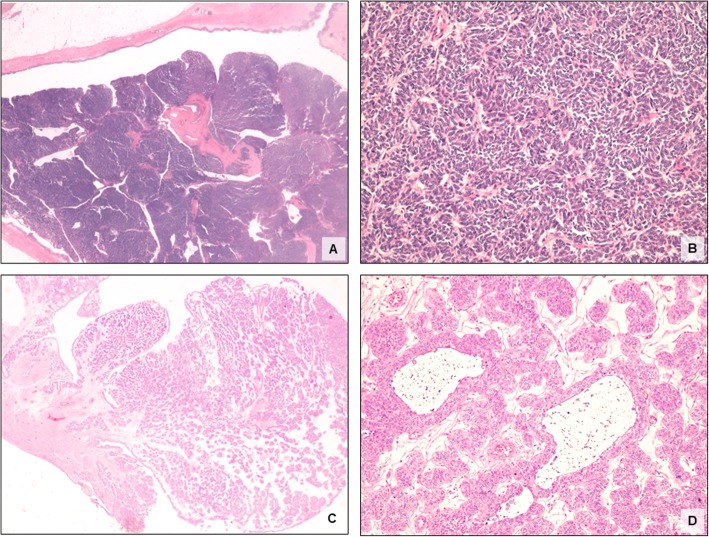
Both cases displayed similar gross and histological features. In case 1, a sessile polypoid tumour measured 2 cm in greatest diameter. Case 2 presented as a 1.4-cm polypoid mass with a thin stalk. Microscopically, both lesions consisted of anastomosing trabeculae and cords growing downward into the lamina propria and lacked any true exophytic papillary component. Prominent peripheral palisading was seen in the trabeculae. There was no evidence of significant nuclear atypia and less than 1/10 high-power field mitotic figures were found. Hyalinised collagenous stroma was seen in case 1. Microcyst formation and foci of squamous metaplasia were occasionally observed in case 2. Histology was consistent with IUP (Fig. 1).
Fig. 1.
Histological features of two cases of IUP of the upper urinary tract. Sessile polypoid tumour of the renal pelvis consisting of anastomosing trabeculae and cords growing downward into the lamina propria, with prominent peripheral palisading in the trabeculae (Case 1: a, b). Pedunculated polypoid IUP of the distal ureter characterized by microcyst formation and foci of squamous metaplasia (Case 2: c, d)
Representative sections of the lesions were selected for immunohistochemical analysis. As primary antibodies, we used rabbit monoclonal Ki-67 (clone 30.9, ready to use; Ventana, Tucson, AZ), rabbit monoclonal CK20 (clone SP33, ready to use; Ventana), mouse monoclonal PMS2 (clone A16–4, ready to use; Ventana), mouse monoclonal MLH1 (clone M1, ready to use; Ventana), mouse monoclonal MSH2 (clone G219–1129, ready to use; Ventana) and rabbit monoclonal MSH6 (clone SP93, ready to use; Ventana). Sections were stained on a Ventana BenchMark ULTRA immunostainer (Ventana Medical Systems). The procedure involved pretreatment with Cell Conditioning 1 followed by antibody incubation. The signal was then developed with ultraView Universal DAB Detection Kit for antibodies against Ki-67 and CK20. OptiView DAB IHC Detection Kit was employed for all other antibodies.
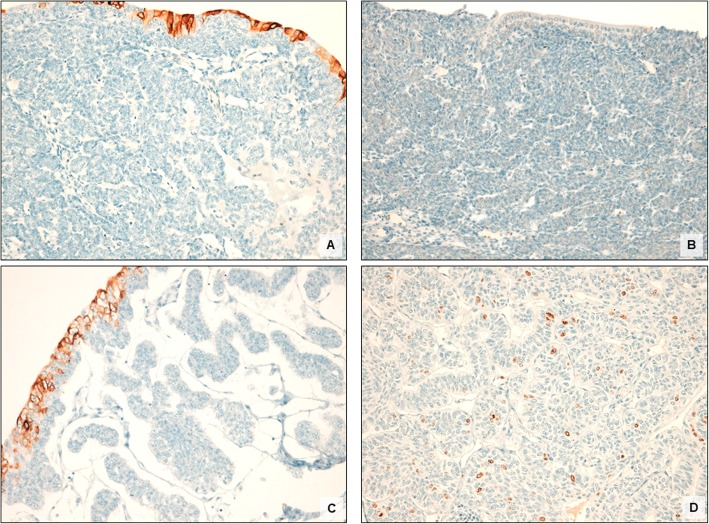
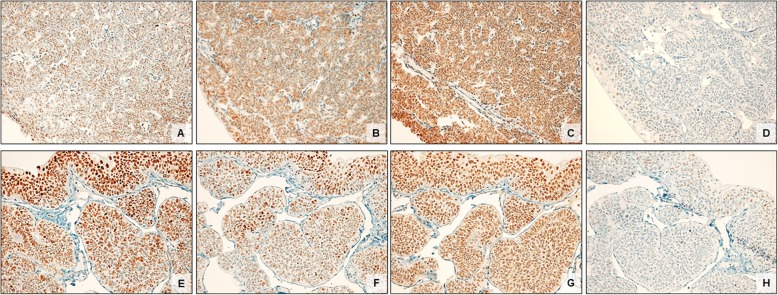
Both lesions were negative for CK20 and exhibited uniformly low Ki-67 (< 1%) (Fig. 2). Expression of the mismatch-repair protein was considered positive if at least 10% of neoplastic cells showed nuclear staining [54]. Loss of MSH6 was seen in both cases, alongside with retention of MLH1, MSH2, and PMS2 expression (Fig. 3).
Fig. 2.
Immunohistochemical results in two cases of IUP pf the upper urinary tract. Both cases were negative for CK20 immunostaining (Case 1: a; Case 2: c) and showed low Ki-67 labelling index (< 1%) (Case 2: b; Case 2: d)
Fig. 3.
Expression of the mismatch-repair proteins in two cases of IUP of the upper urinary tract (Case 1: a-d; Case 2: e-h). Nuclear staining for MLH1 (a, e), MSH2 (b, f), PMS2 (c, g) was observed in both cases, whereas the tumours showed loss of MSH6 expression (d, h)
Discussion
IUP of the upper urinary tract is a benign tumour with 68 cases described to date in the English literature. It usually manifests in middle-aged adults within the 6th or 7th decade of life, and males are more commonly affected than females [26].
The most frequent presenting symptoms are haematuria, macroscopic or microscopic, and renal colic. Irritative symptoms, as well as urinary tract obstruction, have also been reported [55]. In a high percentage of cases, however, tumours are asymptomatic and detected during unrelated clinical investigations.
Preoperative diagnosis of IUP is difficult. Imaging studies may reveal non-specific findings such as filling defects of obstructive masses, often associated with hydronephrosis, hydroureter or renal stones [56]. Cytological morphology falls within the range of normal or mild atypia since IUP is covered by a normal and intact mucosal layer. Accurate preoperative diagnosis requires biopsy and visualisation through endoscopic examination. These procedures also provide therapeutic indications, thus avoiding unnecessary nephroureterectomy [26]. Due to the high likelihood of malignancy, preoperative biopsies were not carried out in our cases and patients underwent radical surgery.
Grossly, IUP presents as a solid or polypoid mass with smooth mucosal, non-papillary covering surface. Most tumours measure less than 3 cm in diameter but can reach up to 8 cm or more. They usually occur as solitary lesions, although 3.6–6% are bilateral or multicentric [55].
Histologically, IUP is characterised by endophytic growth of epithelial elements arranged in nests and cords, growing down from the surface urothelium into the lamina propria with expansible borders. Cystic areas and foci of squamous metaplasia are common. Neither fibrovascular cores nor desmoplasia are seen in IUP and stromal inflammation is minimal. Necrosis and mitotic activity are absent. Distinction between inverted papilloma and urothelial carcinoma with an endophytic growth pattern can be challenging. Contrary to IUP, urothelial carcinoma with inverted configuration shows cytological atypia, mitoses, nuclear pleomorphism and often displays an exophytic papillary component. In addition, invasion into the muscularis propria may occur in urothelial carcinoma but not in IUP. When biopsies are of small size or morphological artefacts and tangential sectioning obscure the lesion, differentiating between these biologically different entities becomes increasingly difficult [57].
Recently, Wobker et al. described 13 cases of a unique urothelial tumour occurring exclusively in the renal pelvis and ureter, named polypoid urothelial proliferation with inverted growth pattern (PUTIP). Morphologically, PUTIP exhibits hybrid features between a totally inverted PUNLMP, IUP and florid proliferation of von Brunn nests [58]. PUTIP may show a distinct inverted papilloma–like component with densely hyalinised collagenous stroma, but lacks the thin anastomosing cords typical of IUP.
In the present study, we observed low Ki-67 proliferation index and negativity for CK20 in both cases. A number of immunohistochemical markers have been shown to be frequently expressed in urothelial carcinomas, including the proliferation marker Ki-67 and CK20 [59]. IUP may be aneuploid and demonstrate high proliferative activity, although these features do not necessarily correlate with malignant behaviour [60, 61].
Our cases showed loss of MSH6 by immunohistochemistry, whereas expression of MSH2, MLH1 and PMS2 was retained. The molecular genetic abnormalities of IUP appear to differ from those of urothelial carcinoma, suggesting that these two neoplasms are unrelated [62]. Inverted-type urothelial carcinomas of the renal pelvis can be associated with MSI. Hartmann and co-authors examined 132 urothelial carcinomas of the upper urinary tract exhibiting some degree of inverted growth, and found that 35 (26.5%) were microsatellite unstable by polymerase chain reaction analysis [53]. Similar results were obtained by Harper in 214 patients with upper tract urothelial carcinoma tested for mismatch repair protein loss by immunohistochemistry [63]. In a multicentric study conducted on 62 IUPs of the urinary bladder Eiber and co-authors demonstrated aberrant immunostaining for MSH2 (5.8%), MLH1 (11.8%) and MSH6 (3.8%) [62]. As previously described, cellular loss of one MMR protein is not sufficient to cause detectable microsatellite defects [64]. Therefore, our observation may be spurious and unrelated to microsatellite instability, and should be confirmed in a larger series of IUPs of the upper urinary tract. In addition, our patients did not show any stigmata of Lynch syndrome or HNPCC-associated background.
Regarding treatment options, nephroureterectomy, local resection or partial ureterectomy with preservation of the kidney, and endoscopic surgery may be of use [65]. After excision, some authors recommend a follow-up protocol (endoscopy and radiographical studies) similar to that used in patients with low-grade urothelial carcinoma [26], while others do not advocate this rigorous and long-term follow-up due to the low risk of recurrence and favourable prognosis of IUP [66].
In conclusion, IUP of the upper urinary tract is an extremely rare tumour characterised by an inverted pattern of growth and constituted by normal to minimally atypical proliferating urothelium. The absence of progression of IUP on long-term follow-up argues against the need of patients’ continuous surveillance when strict diagnostic criteria are followed, a complete resection can be ascertained and no history of previous or concurrent urothelial malignancies is recorded.
Acknowledgements
None.
Abbreviations
- IUP
Inverted Urothelial Papilloma
- CT
Computed Tomography
- PUTIP
Polypoid Urothelial Proliferation with Inverted Growth Pattern
- RP
Renal Pelvis
- NS
Not Stated
- NA
Not Applicable
Authors’ contributions
RS conceived and designed the study, evaluated histological slides and contributed in writing the manuscript. ICG contributed in evaluating histological slides, collecting the data and writing the manuscript. JIL contributed with a case and participated to the design and implementation of the research. GN contributed to the design and implementation of the research, to the analysis of the results and in writing the manuscript. VC contributed in writing the manuscript. The author(s) read and approved the final manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article.
Compliance with ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Consent for publication
All authors have agreed with the submission in its present form.
Competing interests
No conflict of interest has been declared.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paschkis R. Uber Adenoma der Harnblase. Z Urol Chir. 1927;21:315–325. [Google Scholar]
- 2.Potts IF, Hirst E. Inverted papilloma of the bladder. J Urol. 1963;90:175–179. doi: 10.1016/S0022-5347(17)64384-2. [DOI] [PubMed] [Google Scholar]
- 3.Matz LR, Wishart VA, Goodman MA. Inverted urothelial papilloma. Pathology. 1974;6:37–44. doi: 10.3109/00313027409077154. [DOI] [PubMed] [Google Scholar]
- 4.Assor D. Inverted papilloma of the renal pelvis. J Urol. 1976;116:354. doi: 10.1016/S0022-5347(17)58951-X. [DOI] [PubMed] [Google Scholar]
- 5.Cameron MK, Lupton CH. Inverted papilloma of the lower urinary tract. Br J Urol. 1976;48:567–577. doi: 10.1111/j.1464-410X.1976.tb06703.x. [DOI] [PubMed] [Google Scholar]
- 6.Di Cello V, Brischi G, Durval A, Mincione GP. Inverted papilloma of the ureteropelvic junction. J Urol. 1980;123:110. doi: 10.1016/S0022-5347(17)55806-1. [DOI] [PubMed] [Google Scholar]
- 7.Theoret G, Paquin F, Schick E, Martel A. Inverted papilloma of urinary tract. Urology. 1980;16:149–151. doi: 10.1016/0090-4295(80)90069-2. [DOI] [PubMed] [Google Scholar]
- 8.Uyama T, Moriwaki S. Inverted papilloma with malignant change of renal pelvis. Urology. 1981;17:200–201. doi: 10.1016/0090-4295(81)90242-9. [DOI] [PubMed] [Google Scholar]
- 9.Anderstrom C, Johansson S, Pettersson S. Inverted papilloma of the urinary tract. J Urol. 1982;127:1132–1134. doi: 10.1016/S0022-5347(17)54266-4. [DOI] [PubMed] [Google Scholar]
- 10.Watters G, Grant A, Wiles S, Kneale K, Mitterdorfer A. Inverted papilloma of the upper urinary tract. Br J Urol. 1983;55:176–179. doi: 10.1111/j.1464-410X.1983.tb06549.x. [DOI] [PubMed] [Google Scholar]
- 11.Lausten GS, Anagnostaki L, OF T. Inverted papilloma of the upper urinary tract. Eur Urol. 1984;10:67–70. doi: 10.1159/000463516. [DOI] [PubMed] [Google Scholar]
- 12.Taylor FM, Arroyo JG. Inverted papilloma of the renal pelvis. Cytologic features of ureteral washings. Acta Cytol. 1986;30:166–168. [PubMed] [Google Scholar]
- 13.Schulze S, Holm-Nielsen A, Ravn V. Inverted papilloma of upper urinary tract. Urology. 1986;28:58–61. doi: 10.1016/0090-4295(86)90186-X. [DOI] [PubMed] [Google Scholar]
- 14.Romanelli R. Inverted urothelial papilloma. Report of five cases and review of the literature. Pathologica. 1986;78:89–97. [PubMed] [Google Scholar]
- 15.Yamaguchi K, Kitagawa N, Zama S, Yanagi S, Ito H, Matsuzaki O, Nagao K. Inverted papilloma of renal pelvis associated with transitional cell carcinoma of the bladder. Urol Int. 1988;43:302–304. doi: 10.1159/000281361. [DOI] [PubMed] [Google Scholar]
- 16.Schultz RE, Boyle DE. Inverted papilloma of renal pelvis associated with contralateral ureteral malignancy and bladder recurrence. J Urol. 1988;139:111–113. doi: 10.1016/S0022-5347(17)42310-X. [DOI] [PubMed] [Google Scholar]
- 17.Aubert J, Dore B, Villemonteix P, Touchard G. Inverted papilloma of upper urinary tract. Four case reports. Eur Urol. 1988;15:150–152. doi: 10.1159/000473418. [DOI] [PubMed] [Google Scholar]
- 18.Kyriakos M, Royce RK. Multiple simultaneous inverted papillomas of the upper urinary tract. A case report with a review of ureteral and renal pelvic inverted papillomas. Cancer. 1989;63:368–380. doi: 10.1002/1097-0142(19890115)63:2<368::AID-CNCR2820630229>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Bagley DH, McCue P, Blackstone SA. Inverted papilloma of renal pelvis: flexible ureteroscopic diagnosis and treatment. Urology. 1990;36:336–338. doi: 10.1016/0090-4295(90)80242-F. [DOI] [PubMed] [Google Scholar]
- 20.Bassi P, Piazza R, Milani C, Aragona F, Oliva G, Dalla PP. Inverted papilloma of the renal pelvis. Urol Int. 1991;46:73–76. doi: 10.1159/000281781. [DOI] [PubMed] [Google Scholar]
- 21.Vlassopoulos G, Sakkas G, Legaki S, Sofras F, Karagiannis A. Inverted papilloma of the renal pelvis. Int Urol Nephrol. 1992;24:345–346. doi: 10.1007/BF02550624. [DOI] [PubMed] [Google Scholar]
- 22.Ueda T, Akimoto S, Shimazaki J, Matsuzaki M, Nagao K. Inverted papilloma of the renal pelvis associated with renal cell carcinoma: a case report. Hinyokika Kiyo. 1992;38:561–563. [PubMed] [Google Scholar]
- 23.Spevack L, Herschorn S, Srigley J. Inverted papilloma of the urinary tract. J Urol. 1995;153:1202–1204. doi: 10.1016/S0022-5347(01)67552-9. [DOI] [PubMed] [Google Scholar]
- 24.Chiura AN, Wirtschafter A, Baglew DH. Upper urinary tract inverted papillomas. Urology. 1998;52:514–516. doi: 10.1016/S0090-4295(98)00225-8. [DOI] [PubMed] [Google Scholar]
- 25.Darras J, Inderadjaja N, Vossaert P. Synchronous inverted papilloma of bladder and renal pelvis. Urology. 2005;65:798.e25–798.e28. doi: 10.1016/j.urology.2004.10.066. [DOI] [PubMed] [Google Scholar]
- 26.Luo JD, Wang P, Chen J, Liu B, Wang S, Shen BH, Xie LP. Upper urinary tract inverted papillomas: report of 10 cases. Oncol Lett. 2012;4:71–74. doi: 10.3892/ol.2012.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geisler CH, Mori K, Leiter E. Lobulated inverted papilloma of the ureter. J Urol. 1980;123:270–271. doi: 10.1016/S0022-5347(17)55894-2. [DOI] [PubMed] [Google Scholar]
- 28.Silverstein SV, Carlton CE., Jr Inverted papilloma of ureter. Urology. 1981;17:160–162. doi: 10.1016/0090-4295(81)90227-2. [DOI] [PubMed] [Google Scholar]
- 29.Fromowitz FB, Steinbook ML, Lautin EM, Friedman AC, Kahan N, Bennett MJ, Koss LG. Inverted papilloma of the ureter. J Urol. 1981;126:113–116. doi: 10.1016/S0022-5347(17)54404-3. [DOI] [PubMed] [Google Scholar]
- 30.Ajrawat HS, Skogg DP, Asirwatham JE, Gonder MJ. Lobulated inverted papilloma of ureter. Urology. 1982;20:290–292. doi: 10.1016/0090-4295(82)90641-0. [DOI] [PubMed] [Google Scholar]
- 31.Naito S, Minoda M, Hirata H. Inverted papilloma of ureter. Urology. 1983;22:290–291. doi: 10.1016/S0090-4295(83)80018-1. [DOI] [PubMed] [Google Scholar]
- 32.Jacobellis U, Resta L, Ruotolo G. Inverted papilloma of the ureter. Eur Urol. 1983;9:370–371. doi: 10.1159/000474129. [DOI] [PubMed] [Google Scholar]
- 33.Embon OM, Saghi N, Bechar L. Inverted papilloma of ureter. Eur Urol. 1984;10:139–140. doi: 10.1159/000463772. [DOI] [PubMed] [Google Scholar]
- 34.Perrin P, Dutrieux N, Durand L. Inverted papilloma of the ureter. Br J Urol. 1984;56:223. doi: 10.1111/j.1464-410X.1984.tb05368.x. [DOI] [PubMed] [Google Scholar]
- 35.Mottola A, Selli C, Carini M. Inverted papilloma of the ureter. Ital J Surg Sci. 1984;14:341–344. [PubMed] [Google Scholar]
- 36.Palvio DH. Inverted papillomas of the urinary tract. A case of multiple, recurring inverted papillomas of the renal pelvis, ureter and bladder associated with malignant change. Scand J Urol Nephrol. 1985;19:299–302. doi: 10.3109/00365598509180275. [DOI] [PubMed] [Google Scholar]
- 37.Moss JG, Gunn AA. Inverted papilloma of the ureter lying within an ileal carcinoid. Br J Urol. 1987;60:272. doi: 10.1111/j.1464-410X.1987.tb05501.x. [DOI] [PubMed] [Google Scholar]
- 38.Corkill M, Srigley J, Graham R, Herschorn S. Inverted papilloma: an uncommon benign cause of a ureteral filling defect. Urol Radiol. 1987;9:164–167. doi: 10.1007/BF02932652. [DOI] [PubMed] [Google Scholar]
- 39.Duchek M, Hallmans G, Hietala SO, Ljungberg B, Thore J. Inverted papilloma with intussusception of the ureter. Case report. Scand J Urol Nephrol. 1987;21:147–149. doi: 10.3109/00365598709180312. [DOI] [PubMed] [Google Scholar]
- 40.Abulafi A, Leese T, Osborn DE. Inverted papilloma of the ureter. Br J Urol. 1987;59:480. doi: 10.1111/j.1464-410X.1987.tb04852.x. [DOI] [PubMed] [Google Scholar]
- 41.Villani U, Leoni S, Casolari E. Inverted papilloma of the ureter: two cases of conservative therapy. Eur Urol. 1987;13:125–127. doi: 10.1159/000472750. [DOI] [PubMed] [Google Scholar]
- 42.Kostakopoulos A, Delakas D, Legaki S, Sofras F. Inverted papilloma of the ureter. Acta Urol Belg. 1988;56:471–474. [PubMed] [Google Scholar]
- 43.Garritano A, Vecchioli Scaldazza C, Morosetti C. Inverted papilloma of the ureter. Eur Urol. 1988;14:249–250. doi: 10.1159/000472949. [DOI] [PubMed] [Google Scholar]
- 44.Page CM, Nelson JH, Drago JR. Multifocal, synchronous inverted papillomas involving the ureter. J Urol. 1991;145:357–358. doi: 10.1016/S0022-5347(17)38339-8. [DOI] [PubMed] [Google Scholar]
- 45.Kunimi K, Uchibayashi T, Egawa M. A case of inverted papilloma of the ureter: is the DNA ploidy pattern associated with occurrence of transitional cell carcinoma of the bladder? Int Urol Nephrol. 1994;26:17–22. doi: 10.1007/BF02768239. [DOI] [PubMed] [Google Scholar]
- 46.de Knijff DW, Theunissen PH, Delaere KP. Inverted papilloma of the ureter with subsequent invasive bladder cancer. Acta Urol Belg. 1997;65:45–46. [PubMed] [Google Scholar]
- 47.Hoekx L, Wyndaele JJ. Bilateral ureteral inverted papilloma with synchronous transitional cell tumor of the bladder. Acta Urol Belg. 1998;66:17–19. [PubMed] [Google Scholar]
- 48.Lyon MB, Zorn KC, Orvieto MA, Rapp DE, Gerber GS, Shalhav AL. Case report: laparoscopic resection of ureteral inverted papilloma. J Endourol. 2006;20:399–401. doi: 10.1089/end.2006.20.399. [DOI] [PubMed] [Google Scholar]
- 49.Kilciler M, Bedir S, Erdemir F, Ors O, Kibar Y, Dayanc M. Evaluation of urinary inverted papillomas: a report of 13 cases and literature review. Kaohsiung J Med Sci. 2008;24:25–30. doi: 10.1016/S1607-551X(08)70069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mertziotis N, Kozyrakis D, Petrolekas A, Terzi M, Kapranos N. Inverted papilloma of the ureter: study of a rare case with emphasis on clinicopathologic implications. Can Urol Assoc J. 2012;6:e274–e276. doi: 10.5489/cuaj.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murtaza B, Akmal M, Niaz WA, Ahmad H, Mahmood A. Inverted papilloma of ureter: a rare cause of hydronephosis. J Coll Physicians Surg Pak. 2012;22:542–544. [PubMed] [Google Scholar]
- 52.Lopez-Fontana G, Alvarez-Ossorio JL, Ramos JI, Castineiras J, Moyano JL, Castillo OA. Ureteral inverted papilloma: laparoscopic distal ureterectomy and Boari flap. Arch Esp Urol. 2012;65:759–761. [PubMed] [Google Scholar]
- 53.Hartmann A, Dietmaier W, HofstadterF BLJ, Cheville JC, Blaszyk H. Urothelial carcinoma of the upper urinary tract: inverted growth pattern is predictive of microsatellite instability. Hum Pathol. 2003;34:222–227. doi: 10.1053/hupa.2003.22. [DOI] [PubMed] [Google Scholar]
- 54.Sarode VR, Robinson L. Screening for lynch syndrome by immunohistochemistry of mismatch repair proteins. Significance of indeterminate result and correlation with mutational studies. Arch Pathol Lab Med. 2019;143:1225–1233. doi: 10.5858/arpa.2018-0201-OA. [DOI] [PubMed] [Google Scholar]
- 55.Jorgensen PH, Vainer B, Hermann GG. A clinical and molecular review of inverted papilloma of the urinary tract: how to handle? APMIS. 2015;123:920–929. doi: 10.1111/apm.12456. [DOI] [PubMed] [Google Scholar]
- 56.Gupta R, Paner GP, Amin MB. Neoplasms of the upper urinary tract: a review with focus on urothelial carcinoma of the pelvicalyceal system and aspects related to its diagnosis and reporting. Adv Anat Pathol. 2008;15:127–139. doi: 10.1097/PAP.0b013e31817145a9. [DOI] [PubMed] [Google Scholar]
- 57.Amin M, Gomez J, Young R. Urothelial transitional cell carcinoma with endophytic growth patterns: a discussion of patterns of invasion and problems associated with assessment of invasion in 18 cases. Am J Surg Pathol. 1997;21:1057–1068. doi: 10.1097/00000478-199709000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Wobker SE, Zhong M, Epstein JI. Polypoid urothelial tumor with inverted growth pattern in the renal pelvis: morphologic and molecular characteristics of a unique diagnostic entity. Hum Pathol. 2017;59:26–33. doi: 10.1016/j.humpath.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 59.Jones TD, Zhang S, Lopez-Beltran A, Eble JN, Sung MT, MacLennan GT, Montironi R, Tan PH, Zheng S, Baldridge LA, Cheng L. Urothelial carcinoma with an inverted growth pattern can be distinguished from inverted papilloma by fluorescence in situ hybridization, immunohistochemistry, and morphologic analysis. Am J Surg Pathol. 2007;31:1861–1867. doi: 10.1097/PAS.0b013e318060cb9d. [DOI] [PubMed] [Google Scholar]
- 60.Cheville JC, Wu K, Sebo TJ, Cheng L, Riehle D, Lohse CM, Shane V. Inverted urothelial papilloma: is ploidy, MIB-1 proliferative activity, or p53 protein accumulation predictive of urothelial carcinoma. Cancer. 2000;88:632–636. doi: 10.1002/(SICI)1097-0142(20000201)88:3<632::AID-CNCR21>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 61.Broussard JN, Tan HP, Epstein JI. Atypia in inverted urothelial papillomas: pathology and prognostic significance. Hum Pathol. 2004;351:499–504. doi: 10.1016/j.humpath.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 62.Eiber M, van Oers JM, Zwarthoff EC, van der Kwast TH, Ulrich O, Helpap B, Stoerkel S, Blaszyk H, Cheville J, Sauter G, Wild PJ, Stoehr R, Hofstaedter F, Hartmann A. Low frequency of molecular changes and tumor recurrence in inverted papillomas of the urinary tract. Am J Surg Pathol. 2007;31:938–946. doi: 10.1097/01.pas.0000249448.13466.75. [DOI] [PubMed] [Google Scholar]
- 63.Harper HL, McKenney JK, Heald B, Stephenson A, Campbell SC, Plesec T, Magi-Galluzzi C. Upper tract urothelial carcinomas: frequency of association with mismatch repair protein loss and lynch syndrome. Mod Pathol. 2017;30:146–156. doi: 10.1038/modpathol.2016.171. [DOI] [PubMed] [Google Scholar]
- 64.Giedl J, Schneckenpointner R, Filbeck T, Ruemmele P, Hofstaedter F, Burger M, Hartmann A, Stoehr R. Low frequency of HNPCC-associated microsatellite instability and aberrant MMR protein expression in early-onset bladder cancer. Am J Clin Pathol. 2014;142:634–639. doi: 10.1309/AJCPVTCJ4VU5HKVZ. [DOI] [PubMed] [Google Scholar]
- 65.Picozzi S, Casellato S, Bozzini G, Ratti D, Macchi A, Rubino B, Pace G, Carmignani L. Inverted papilloma of the bladder: a review and an analysis of the recent literature of 365 patients. Urol Oncol. 2013;31:1584–1590. doi: 10.1016/j.urolonc.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Patel P, Reikie BA, Maxwell JP, Yilmaz A, Gotto GT, Trpkov K. Long-term clinical outcome of inverted urothelial papilloma including cases with focal papillary pattern: is continuous surveillance necessary? Urology. 2013;82:857–860. doi: 10.1016/j.urology.2013.06.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.