Abstract
Introduction: Breastfeeding is a protective factor for women and children. Women who smoke cigarettes during pregnancy are less likely to initiate or persist in breastfeeding. However, less is known about why this is the case.
Materials and Methods: The present study (n = 247) prospectively examined maternal/child factors that influence breastfeeding in a low-income, racially diverse at-risk sample of smoking and nonsmoking women. Pregnant women were recruited at their first prenatal appointment in an urban hospital and followed through 24-month postnatally. Women reported on the average number of cigarettes smoked/day during pregnancy, psychopathology, breastfeeding behavior, and infant reactivity.
Results: Although a greater number of cigarettes smoked/day during pregnancy was associated with a lower likelihood of initiating or persisting in breastfeeding, maternal age, education, and infant reactivity offered predictive utility above and beyond maternal smoking.
Conclusion: Smokers were less likely to initiate breastfeeding and breastfed for shorter duration than demographically similar nonsmokers; however, one of the mechanisms for reduced breastfeeding may be the psychosocial factors of younger age and lower education. Further, infant reactivity was also found to reduce the likelihood of initiating and persisting with breastfeeding.
Keywords: maternal cigarette smoking, breastfeeding initiation, breastfeeding duration, maternal and infant risk
Introduction
Breastfeeding is protective for infant and maternal health.1 Exclusive breastfeeding is recommended for the first 6 months of life and complementing foods with breastfeeding until at least 12 months.2,3 Overall rates have been increasing in the United States, with 83.2% of women initiating breastfeeding. However, rates of continued breastfeeding are lower, with breastfeeding rates of 57.6% at 6 months, and 35.9% at 12 months.4 There are also differences in rates within subgroups of women.2 Breastfeeding initiation and duration are influenced by maternal health, demographics, and infant behaviors.5 The present study examines the influence of maternal and infant factors on initiation and duration of breastfeeding in a low-income, racially diverse at-risk sample.
Smoking and breastfeeding
The American Academy of Pediatrics2 and Centers for Disease Control and Prevention (CDC)6 encourage breastfeeding women to quit smoking due to concerns over risks to infant health and development. However, the protective effects of breastfeeding are thought to outweigh potential negative consequences of nicotine exposure.7,8 Yet smokers are less likely to intend to breastfeed, to initiate breastfeeding, and breastfeed for shorter duration than nonsmoking women (for review9–11).
Little is known about specific factors that may explain why smokers are less likely to breastfeed.9 Whether the connection between smoking and breastfeeding is physiological or psychosocial is unclear10 given the many influences on breastfeeding initiation and duration. There is a dose–response relationship between number of cigarettes smoked/day and reduced duration of breastfeeding,10 but psychosocial mechanisms require further investigation. Additionally, a concern with past research has been methodological issues regarding timing and assessment of smoking.9,12 A goal of the present study is to examine a dose–response relationship of smoking on breastfeeding within the context of important psychosocial factors.
Predictors of reduced breastfeeding
Maternal risk
One possible mechanism leading to reduced initiation and duration of breastfeeding among women who smoke is higher levels of psychopathology and/or lower maternal-fetal attachment. Smoking during pregnancy is associated with depression,13 and with increased insensitivity and decreased maternal warmth in parent–child interactions.14 Heavier pregnancy smoking15 and psychopathology16 are also related to lower maternal-fetal attachment.
Demographic risk
Smoking during pregnancy is associated with social/contextual risks that could impact breastfeeding. Pregnancy smokers are more likely to be single mothers.17 Social and partner support may play a role in increasing breastfeeding,17 especially for lower income, at-risk women. Being older, more educated, and of higher income are associated with longer breastfeeding duration5 whereas women who smoke during their pregnancies, along with those who report lower maternal-fetal attachment, tend to be younger and have lower education and income.18,19 Lower income women may be aware of the benefits of breastfeeding but have more obstacles such as lack of support and difficulties with working and breastfeeding simultaneously.20 As such, women who smoke during pregnancy may be an especially vulnerable group for reduced breastfeeding.
Infant temperament and maternal behavior
Infant feeding is a dynamic, complex interaction between parent and child that is influenced by both the infant (e.g., signaling) and parent (e.g., response). In prospective studies, women who perceived their infants as having “easy” temperaments breastfed for longer21 and women who perceived their infants to be less alert and more irritable during feeding were more likely to stop breastfeeding by 6 weeks postpartum.22 The role of infant temperament is important to consider in studies of smoking and breastfeeding, given the impact of prenatal tobacco exposure on infant reactivity.23
Present study
The purpose of the study was to investigate the impact of maternal and infant factors in an at-risk sample on breastfeeding initiation and duration while accounting for the influence of other maternal and infant factors. It was hypothesized that persistent smoking would reduce the likelihood and duration of breastfeeding and that maternal characteristics associated with smoking and infant reactivity would be associated with reduced breastfeeding. Other possible factors that past research has addressed (e.g., partner status, mode of delivery24) were also examined.
Materials and Methods
Participants
Participants included 247 mother/infant dyads, with 178 infants prenatally exposed to tobacco (98 boys), and 69 not exposed (33 boys). Women were recruited at their first prenatal appointment in a local hospital. Eligibility criteria were as follows: <20 weeks gestation, single birth, ≥18 years of age, English speaking, no illicit drug use except cannabis, and alcohol use of <4 drinks/occasion or <1 drink/day after pregnancy recognition. Participating smokers were matched on maternal age and education with the closest eligible nonsmoking woman. Smokers were oversampled (2 smokers: 1 nonsmoker; Table 1).
Table 1.
Demographic Information and Descriptive Statistics
| % | M | SD | |
|---|---|---|---|
| Intended to breastfeed (third trimester) | 62.8 | ||
| Intended breastfeeding duration in months (third trimester) | 6.48 | 8.72 | |
| Planned to attend breastfeeding class | 49.7 | ||
| Initiated breastfeeding after delivery | 41.6 | ||
| Still breastfeeding at 2 months postpartum | 19.3 | ||
| Total no. of days breastfeeding | 50.75 | 104.44 | |
| Pregnancy average cigarettes/day | 3.59 | 4.54 | |
| Maternal age (years) | 24.09 | 5.00 | |
| Maternal education (years completed) | 12.31 | 1.89 | |
| Parity | 2.19 | 1.55 | |
| Maternal race (could identify as more than one race) | |||
| African American | 51 | ||
| Caucasian | 30.8 | ||
| Hispanic | 19 | ||
| Other | 8 | ||
| Married/living with partner | 45.7 | ||
| Maternal psychopathology | 0.44 | 0.13 | |
| Infant reactivity regulation (2 months) | 4.88 | 0.83 | |
| Fetal attachment | 49.42 | 10.98 |
SD, standard deviation.
Procedures and instruments
Assessments were conducted once per trimester of pregnancy and at 2, 9, 16, and 24 months of infant age. Maternal demographics including age, education, and partner status (married/living with partner, in a relationship but not living with partner, single, divorced, or widowed) were ascertained at the first prenatal appointment. At each postnatal appointment, participants were asked whether they had returned to work since giving birth, and if so, how long after delivery they returned to work. Informed consent was obtained from all participants. The study was approved by the university's Institutional Review Board.
Prenatal substance exposure
Maternal cigarette use was assessed at each prenatal and postnatal laboratory visit using the Timeline Followback Interview.25 Maternal self-reports were biologically verified via maternal saliva. Prenatal exposure was further validated through collection of infant meconium.
Breastfeeding
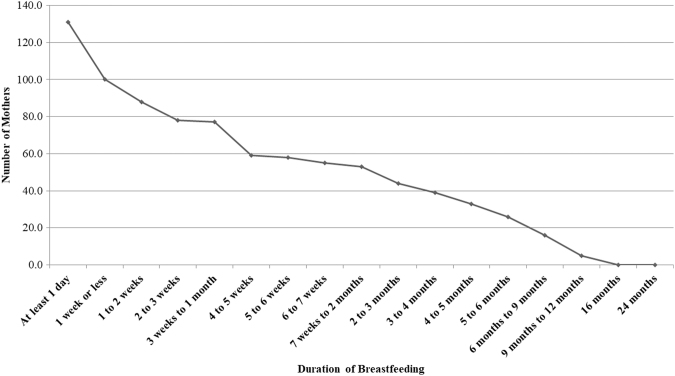
During the second trimester, women were asked whether they intended to breastfeed, and if so, for how long. At each of the four postnatal assessments, women reported whether they had breastfed their infant, if they were still currently breastfeeding, and how many days after delivery they breastfed. The duration of breastfeeding was measured as the number of days reported breastfeeding through the first 2 years of life (Fig. 1).
FIG. 1.
Duration of breastfeeding over 24 months postnatally. One hundred sixteen women did not initiate breastfeeding. Figure indicates number of women who continued to breastfeed past a given time period.
Cumulative prenatal maternal psychopathology
The prenatal cumulative risk score for maternal psychopathology was comprised of four factors: depression, stress, anger, and hostility. Correlations among these factors ranged from 0.40 to 0.68. For all items, higher scores were indicative of greater risk. The final cumulative risk score for psychopathology was created by averaging the four items with a possible maximum score of 1 (M = 0.44, SD = 0.13).
Mothers reported on their depressive symptoms using the Beck Depression Inventory26 at their second (Cronbach's α = 0.88) and third trimester (Cronbach's α = 0.86) appointments. Scores were averaged across the time points and the average score (15.45, SD = 7.71) was divided by the maximum possible score [63] to create a proportion following guidelines by Lengua et al.27 The same approach was used for stress, reported in the second (α = 0.81) and third (α = 0.83) trimesters using the Perceived Stress Scale.28 Scores were averaged (M = 25.19, SD = 7.46) and divided by the maximum possible scale score [56]. Maternal anger (α = 0.78; M = 18.96, SD = 5.94) and hostility (α = 0.83; M = 19.27, SD = 6.44) were assessed during the third trimester using the Buss Perry Questionnaire,29 and scores were divided by scale maximum scores (35).
Infant reactivity/regulation
Reactivity was measured using maternal ratings on the falling reactivity subscale of the Infant Behavior Questionnaire30 at 2 months of infant age (assessment age corrected for prematurity). The subscale included 13 items related to whether the infant had difficulties settling down to sleep, the infant's ability to soothe themselves and calm down when upset. High scores indicated higher regulation. The reliability of the subscale was α = 0.77.
Fetal attachment
Maternal-fetal attachment was measured using the 24-item Maternal-Fetal Attachment Scale31 at the third trimester assessment. Higher scores indicate higher maternal-fetal attachment. The reliability of the scale was α = 0.81.
Fetal growth
Birth weight (g), birth length (cm), and head circumference (cm) were taken by trained obstetrical nurses in the delivery room. Gestational age was calculated using dates by trained study staff. Medical chart review after delivery was used to complete the Obstetrical Complications Scale,32 designed to assess perinatal risk factors.
Data analytic strategy
Data on breastfeeding intent and actions were examined descriptively: bivariate associations were examined and group analyses compared duration and initiation of breastfeeding. Based on these analyses, two models assessing the impact of the predictors of interest (maternal, demographic, and infant risks) on breastfeeding were conducted. The first set of analyses assessed the impact of the predictors on initiation of breastfeeding as a binary outcome (i.e., presence/absence of breastfeeding) using logistic regression. The second set of analyses assessed the impact of the predictors of interest on breastfeeding duration for the women who did breastfeed using Cox regression survival analyses to determine predictors of breastfeeding desistence. In each model, average number of cigarettes smoked during pregnancy was entered first to determine the influence of smoking on breastfeeding and to subsequently determine how this relationship was impacted by other maternal/infant characteristics.
Missing data
Of the 247 participants, 242 had complete data on all variables of interest. There were no significant differences between families with complete versus missing data on maternal age, maternal education, whether they received welfare, or prenatal smoking.
Results
Descriptive data
Associations with breastfeeding duration were explored with bivariate correlations (Table 2). Potential differences in fetal growth and maternal characteristics were examined between those who did and did not breastfeed (Table 3). Intention to breastfeed led to breastfeeding initiation for 35.8% of the women, and 18.7% reported intending to breastfeed but did not subsequently do so. A smaller subgroup had indicated that they did not intend to, but subsequently breastfed (6.4%). There were no significant differences on breastfeeding duration between women who intended to (M = 22.88, SD = 19.37) and those who did not intend to breastfeed (M = 18.25, SD = 20.02). There was no significant difference in age between any of the groups (i.e., intended to breastfeed and did not breastfeed, intended to breastfeed and did breastfeed, did not intend to breastfeed and did not breastfeed, did not intend to breastfeed and did breastfeed; F(3, 183) = 0.91, n.s.) or years of education (F(3, 183) = 2.08, n.s.).
Table 2.
Correlations for Major Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Total no. of days breastfeeding | X | ||||||
| 2. Pregnancy cigarettes/day | −0.14* | X | |||||
| 3. Maternal age (years) | 0.16* | 0.18** | X | ||||
| 4. Maternal education | 0.33*** | −0.17** | 0.16* | X | |||
| 5. Maternal psychopathology (pregnancy) | −0.19** | 0.21** | −0.02 | −0.18** | X | ||
| 6. Infant reactivity regulation (2 months) | 0.14* | −0.11+ | −0.08 | 0.03 | −0.11+ | X | |
| 7. Fetal attachment | 0.04 | 0.09 | 0.15* | 0.05 | 0.06 | −0.06 | X |
p < 0.10, *p < 0.05, **p < 0.01, ***p < 0.001.
Table 3.
Group Differences by Breastfeeding Initiation and Smoking Status
| Initiated breastfeeding |
Did not initiate breastfeeding |
t | |||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Head circumference (cm) | 34.21 | 1.97 | 33.61 | 1.68 | t(224) = −2.44* |
| Birthweight (kg) | 3.27 | 0.62 | 3.20 | 0.53 | t(245) = −0.92 |
| Birth length (cm) | 50.16 | 2.84 | 50.04 | 2.72 | t(239) = −0.35 |
| Gestational age (weeks) | 39.02 | 1.66 | 38.76 | 1.99 | t(245) = −1.11 |
| Obstetrical complications | 29.76 | 6.39 | 28.10 | 7.96 | t(241) = −1.81 |
| Maternal education | 12.77 | 1.84 | 11.79 | 1.81 | t(245) = −4.20*** |
| Maternal age | 23.84 | 4.53 | 24.36 | 5.49 | t(245) = 0.82 |
| Nonsmoking (breastfed N = 48) | Persistent smoking (breastfed N = 83) | ||||
|---|---|---|---|---|---|
| Anticipated duration of breastfeeding (months) |
7.94 |
13.74 |
5.72 |
4.19 |
t(145) = 1.47 |
| Duration of breastfeeding (days) |
74.93 |
133.60 |
41.37 |
89.36 |
t(245) = 2.29* |
| Average no. of cigarettes per day during pregnancy | 0.00 | 0.00 | 4.98 | 4.65 | t(245) = −8.87*** |
p < 0.05, ***p < 0.001.
SD, standard deviation.
There were no significant differences in the number of days that the infant was breastfed when partner status (F(3, 243) = 2.53, n.s.), infant sex (t(245) = 0.56, n.s.), whether the mother returned to work by 2 months of child age (t(243) = −0.45, n.s.), whether the pregnancy was planned (t(223) = 0.13, n.s.), or whether labor was induced (t(218) = −0.72, n.s.) were examined. First-time mothers did not report any significant differences on the duration of their intention to breastfeed (M = 9.39 months, SD = 17.07; t(101) = 1.71, n.s.) or breastfeeding duration (M = 47.36 days, SD = 94.83; t(180) = −0.01, n.s.) than mothers for whom it was not their first child (M = 5.69, SD = 4.47; M = 47.56, SD = 104.28, respectively).
Smoking status group differences
Group differences between mothers who smoked versus those who did not smoke throughout their pregnancies were assessed (Table 3). Mothers who persistently smoked during pregnancy reported significantly shorter breastfeeding duration.
Models predicting breastfeeding
Regression analyses examined predictors of breastfeeding initiation and duration based on a priori preliminary analyses (Table 4), which indicated that maternal age, education, smoking, psychopathology, and infant reactivity were associated with differences in initiation or duration of breastfeeding. For both sets of regression analyses, the role of infant head circumference was explored given the significant difference in head circumference between those who did and did not initiate breastfeeding, but was not a significant predictor and did not change the pattern of findings in either model.
Table 4.
Regression Models Predicting Breastfeeding
| Logistic regression predicting presence or absence of breastfeeding | B (SE) | Wald χ2 | 95% CI for odds ratio |
|||
|---|---|---|---|---|---|---|
| Lower | Odds ratio | Upper | ||||
| No breastfeeding versus breastfeeding | ||||||
| Step 1 | ||||||
| Pregnancy average cigarettes per day | −0.07 (0.03)* | 5.96 | 0.88 | 0.93 | 0.99 | χ2(1) = 6.40, p = 0.01; Nagelkerke's R2 = 0.04 |
| Step 2 | ||||||
| Maternal age | −0.03 (0.03) | 0.92 | 0.97 | 1.03 | χ2(3) = 17.69, p = 0.001; Nagelkerke's R2 = 0.13 | |
| Maternal education | 0.32 (0.08)*** | 16.05 | 1.18 | 1.38 | 1.61 | |
| Maternal psychopathology | 0.48 (1.11) | 0.18 | 1.61 | 14.24 | ||
| Step 3 | ||||||
| Infant reactivity 2 months | 0.40 (0.17)* | 5.33 | 1.06 | 1.49 | 2.10 | χ2(1) = 5.54, p = 0.02; Nagelkerke's R2 = 0.15 |
| Cox regression predicting duration of breastfeeding | B (SE) | Wald χ2 | 95% CI for odds ratio |
|||
|---|---|---|---|---|---|---|
| Lower | Hazard ratio | Upper | ||||
| Breastfeeding duration | ||||||
| Step 1 |
|
|
|
|
|
|
| Pregnancy average cigarettes per day |
0.04 (0.02)+ |
3.29 |
1.00 |
1.04 |
1.09 |
χ2(1) = 3.31, p = 0.07 |
| Step 2 |
|
|
|
|
|
|
| Maternal age |
−0.06 (0.02)* |
6.16 |
0.90 |
0.95 |
0.99 |
χ2(3) = 22.03, p < 0.001 |
| Maternal education |
−0.17 (0.06)** |
8.13 |
0.75 |
0.85 |
0.95 |
|
| Maternal psychopathology |
0.54 (0.72) |
|
0.42 |
1.71 |
7.03 |
|
| Step 3 |
|
|
|
|
|
|
| Infant reactivity 2 months | −0.27 (0.11)* | 5.67 | 0.61 | 0.76 | 0.95 | χ2(1) = 5.60, p = 0.02 |
p < 0.10, *p < 0.05, **p < 0.01, ***p < 0.001.
CI, confidence interval; SE, standard error.
Logistic regression
A test of the model at step 1 against a constant only model was statistically significant, indicating that the predictor reliably distinguished between those who did and did not breastfeed. Prediction success overall was 57.9% (36.3% for absence of breastfeeding and 76.7% for presence of breastfeeding). Average number of cigarettes/day during pregnancy made a significant contribution to prediction. When the average number of cigarettes/day is increased by one unit (one cigarette) the odds of breastfeeding decrease by 7%.
At step 2, a test of the model at step 2 against the model at step 1 was statistically significant, indicating that the predictors as a set reliably distinguished between those who did and did not breastfeed and improved the model. Prediction success overall was 62.8% (50.4% for absence of breastfeeding and 73.6% for presence of breastfeeding). At step 2, only maternal education was a significant predictor of breastfeeding above and beyond maternal prenatal psychopathology, maternal age, and average number of cigarettes/day across pregnancy. When maternal education is increased by one unit (1 year), the odds of breastfeeding increase by a factor of 1.38.
At step 3, a test of the model at step 3 against the model at step 2 was statistically significant, indicating that the predictors as a set reliably distinguished between those who did and did not breastfeed and improved the model. Prediction success overall was 66.1% (55.8% for absence of breastfeeding and 75.2% for presence of breastfeeding). At step 3, infant reactivity was a significant predictor of breastfeeding above and beyond maternal prenatal psychopathology, maternal age, and average number of cigarettes/day across pregnancy. Maternal education remained a significant predictor at step 3. The odds of breastfeeding increase by a factor of 1.49 when infants are more regulated.
Cox regression
A test of the model at step 1 against a constant only model tended to be statistically significant, indicating that at step 1, average number of cigarettes/day during pregnancy tended to predict breastfeeding duration, such that a one cigarette/day increase prenatally increased the hazard to stop breastfeeding by 4%.
At step 2, there was a significant improvement in the model. Maternal education and age were each significantly associated with breastfeeding duration, above and beyond average cigarettes/day during pregnancy and maternal prenatal psychopathology. When maternal education is increased by 1 year the hazard to stop breastfeeding decreases by 15%. When maternal age is increased by 1 year the hazard to stop breastfeeding decreases by 5%.
At step 3, there was a significant improvement in the model. Infant reactivity was significantly associated with breastfeeding duration, above and beyond average cigarettes/day during pregnancy and maternal prenatal psychopathology. Maternal age and education remained significant. This hazard ratio indicates the hazard to stop breastfeeding decreases by 24% when infants are more regulated.
Discussion
This study examined the relation between smoking and reduced breastfeeding in the context of comorbid risks. In this study, rates of continued breastfeeding were lower than national averages.4 There was also a large discrepancy between intention to breastfeed longer and the duration of actual breastfeeding. Interestingly, there were a small number of women who did not intend to breastfeed when asked in pregnancy but did initiate and breastfed for a similar duration as women who had intended to breastfeed. Although past research has supported a women's intention to breastfeed as a powerful predictor of breastfeeding initiation and duration,33 it may be that, within high-risk samples, additional factors need to be considered. Within the present study, maternal age, education, and first-time mother status did not account for differences in intention and subsequent action. Other barriers to breastfeeding such as lack of partner and family support specific to breastfeeding may have played a role. Future research on understanding the mechanisms that lead to changes between intention and behavior could be important for targeting interventions.
Consistent with past research,9–11 women who smoked breastfed for shorter duration than demographically similar nonsmokers. For each additional cigarette smoked on average per day during pregnancy, the likelihood of initiating breastfeeding decreased and the average number of cigarettes per day predicted stopping breastfeeding more quickly. However, higher education increased the odds of initiating and continuing to breastfeed, even when controlling for maternal psychopathology and smoking. Maternal age did not account for increased likelihood of initiating but being older accounted for persisting in breastfeeding. Our findings were consistent with past research that women with less education and younger women report shorter breastfeeding durations.5 As mothers who continue to smoke during their pregnancies are often younger and less educated,19 one mechanism of risk for reduced initiation and duration of breastfeeding may be due to the demographic context of women who smoke during pregnancy. The results also highlight the potential protective role of higher education for increased likelihood of breastfeeding and for longer duration. Moreover, mothers with higher education not only smoked fewer number of cigarettes on average per day during pregnancy, but also reported fewer symptoms of psychopathology.
Although maternal psychopathology was associated on the bivariate level with reduced breastfeeding duration and smoking during pregnancy, it did not account for unique variance in initiating or persisting breastfeeding after accounting for maternal age, education, smoking, and infant reactivity. Therefore, although smoking in pregnancy often co-occurs with higher symptoms of psychopathology and these symptoms may interfere with breastfeeding,34 demographic risks associated with smoking such as lower maternal age and education and having an infant with higher levels of reactivity accounted for unique variance in reduced breastfeeding initiation and duration.
Higher infant reactivity was associated with a decreased likelihood of initiating and with desisting breastfeeding more quickly. An infant with a more difficult temperament may lead already taxed mothers to be less likely to initiate or continue to breastfeed. Further, given that prenatal exposure to nicotine has been found to have an impact on infant's reactivity and ability to regulate,23 women who smoked during their pregnancies may be at increased risk of having a more reactive infant. Therefore, smoking may act as an important marker of demographic risks, such as lower maternal age and education18,19 and higher infant reactivity,23 which were uniquely associated with decreased breastfeeding.
Among additional factors suggested by past research, fetal growth (e.g., birth weight, length), obstetrical complications, whether the pregnancy was planned, and delivery method were not associated with duration of breastfeeding. Increased smoking during pregnancy was associated with reduced fetal growth. Further, maternal-fetal attachment was not associated with smoking or breastfeeding duration; however, older age was associated with increased attachment at the bivariate level. Potential obstacles to breastfeeding, such as lack of support and returning to work, were not associated with breastfeeding in the present study. There were no significant differences in partner status and partner relationship adjustment on breastfeeding. Importantly, the CDC4 points to lack of support from health care providers, family members, and employers as potential reasons for shorter breastfeeding duration. However, it is possible that the support may need to be specific to breastfeeding, instead of general social or partner supportiveness. Therefore, future research should assess general social support and support specific to breastfeeding at work and home.
Limitations and future research
To more fully understand the differences in rates of breastfeeding among women who smoke and the interaction between physiological and psychosocial factors, future research should incorporate assessment of lactation, breast milk content, and physiology as well as other relevant influences, such as age and education. Further, past research has demonstrated differences in socioeconomic status and racial/ethnic groups on breastfeeding.2 Future research with larger samples should investigate breastfeeding considering maternal and infant physiological and psychosocial risk in the context of group differences. The present study also did not distinguish between exclusive or partial breastfeeding or assess potential differences in experiences that directly impacted decisions regarding breastfeeding, such as attendance at breastfeeding classes, hospital experiences and resources, or infant feeding difficulties.
Conclusions
Consistent with past research, smoking during pregnancy was associated with decreased likelihood of initiating and persisting with breastfeeding. Older age, more education, and less infant reactivity were associated with increased breastfeeding and offered predictive utility above and beyond maternal smoking. However, smoking in pregnancy occurs in the context of these demographic risks and also increases risk for infant reactivity and thus, may create a context of risk for reduced initiation and duration of breastfeeding. Findings support past research that indicates the complexity of the decision to breastfeed and persist with breastfeeding. Interventions targeting increasing breastfeeding initiation and duration could focus on facilitating infant reactivity and regulation. Bolstering self-efficacy, support, and intention may be particularly important for younger and less educated mothers.33 Importantly, breastfeeding may be protective against resuming preconception rates of smoking postpartum35 and increasing breastfeeding may decrease smoking behavior. Therefore, promoting breastfeeding initiation and duration with both smokers and nonsmokers could have cascading health benefits for both mothers and children.
Acknowledgments
The authors are grateful to the families who participated in the study and to Research Technicians for data collection and coding. Special thanks go to Dr. Amol Lele at Women and Children's Hospital of Buffalo for her collaboration on data collection.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
Funding Information
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health (R01DA019632).
References
- 1. U.S. Department of Health and Human Services, Office of the Surgeon General, Centers for Disease Control and Prevention, Office on Women's Health. The Surgeon General's Call to Action to Support Breastfeeding. Rockville, MD: Office of the Surgeon General, 2011 [Google Scholar]
- 2. Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk. Pediatrics 2012;129:e827–e841 [DOI] [PubMed] [Google Scholar]
- 3. Horta BL, Bahl R, Martinés JC, et al. Evidence on the Long-Term Effects of Breastfeeding: Systematic Review and Meta-analyses. Geneva, Switzerland: World Health Organization, 2007 [Google Scholar]
- 4. Centers for Disease Control and Prevention, Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion. Breastfeeding Report Card: United States, 2018. Atlanta, GA: Centers for Disease Control and Prevention, 2018 [Google Scholar]
- 5. Dieterich CM, Felice JP, O'Sullivan E, et al. Breastfeeding and health outcomes for the mother-infant dyad. Pediatr Clin North Am 2013;60:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention, Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion. Breastfeeding and Special Circumstances. Atlanta, GA: Centers for Disease Control and Prevention, 2019 [Google Scholar]
- 7. American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics 2001;108:776. [PubMed] [Google Scholar]
- 8. Woodward A, Douglas RM, Graham N, et al. Acute respiratory illness in Adelaide children: Breast feeding modifies the effect of passive smoking. J Epidemiol Community Health 1990;44:224–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amir LH. Maternal smoking and reduced duration of breastfeeding: A review of possible mechanisms. Early Hum Dev 2001;64:45–67 [DOI] [PubMed] [Google Scholar]
- 10. Amir LH, Donath SM. Does maternal smoking have a negative physiological effect on breastfeeding? The epidemiological evidence. Birth 2002;29:112–123 [DOI] [PubMed] [Google Scholar]
- 11. Horta BL, Kramer MS, Platt RW. Maternal smoking and the risk of early weaning: A meta-analysis. Am J Public Health 2001;91:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu J, Rosenberg KD, Sandoval AP. Breastfeeding duration and perinatal cigarette smoking in a population-based cohort. Am J Public Health 2006;96:309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu S-H, Valbø A. Depression and smoking during pregnancy. Addict Behav 2002;27:649–658 [DOI] [PubMed] [Google Scholar]
- 14. Schuetze P, Eiden RD, Dombkowski L. The association between cigarette smoking during pregnancy and maternal behavior during the neonatal period. Infancy 2006;10:267–288 [PMC free article] [PubMed] [Google Scholar]
- 15. Magee SR, Bublitz MH, Orazine C, et al. The relationship between maternal–fetal attachment and cigarette smoking over pregnancy. Matern Child Health J 2014;18:1017–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alhusen JL. A literature update on maternal-fetal attachment. J Obstet Gynecol Neonatal Nurs 2008;37:315–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kiernan K, Pickett KE. Marital status disparities in maternal smoking during pregnancy, breastfeeding and maternal depression. Soc Sci Med 2006;63:335–346 [DOI] [PubMed] [Google Scholar]
- 18. Cannella BL. Maternal–fetal attachment: An integrative review. J Adv Nurs 2005;50:60–68 [DOI] [PubMed] [Google Scholar]
- 19. Curtin SC, Mathews TJ. Smoking prevalence and cessation before and during pregnancy: Data from the Birth Certificate, 2014. Natl Vital Stat Rep 2016;65:1–14 [PubMed] [Google Scholar]
- 20. Guttman N, Zimmerman DR. Low-income mothers' views on breastfeeding. Soc Sci Med 2000;50:1457–1473 [DOI] [PubMed] [Google Scholar]
- 21. Vandiver TA. Relationship of mothers' perceptions and behaviors to the duration of breastfeeding. Psychol Rep 1997;80:1375–1384 [DOI] [PubMed] [Google Scholar]
- 22. Wojnar D. Maternal perceptions of early breastfeeding experiences and breastfeeding outcomes at 6 weeks. Clin Effectiveness Nurs 2004;8:93–100 [Google Scholar]
- 23. National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, 2014 [Google Scholar]
- 24. Leung GM, Lam T-H, Ho L-M. Breast-feeding and its relation to smoking and mode of delivery. Obstet Gynecol 2002;99:785–794 [DOI] [PubMed] [Google Scholar]
- 25. Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Measuring Alcohol Consumption: Psychosocial and Biochemical Methods, Litten RZ, Allen JP, et al., eds. Totowa, NJ: Humana Press, Inc., 1992, pp. 41–72 [Google Scholar]
- 26. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation, 1996 [Google Scholar]
- 27. Lengua LJ, Moran L, Zalewski M, et al. Relations of growth in effortful control to family income, cumulative risk, and adjustment in preschool-age children. J Abnormal Child Psychol 2015;43:705–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;385–396 [PubMed] [Google Scholar]
- 29. Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol 1992;63:452–459 [DOI] [PubMed] [Google Scholar]
- 30. Rothbart MK. Measurement of temperament in infancy. Child Dev 1981;52:569–578 [Google Scholar]
- 31. Cranley MS. Development of a tool for the measurement of maternal attachment during pregnancy. Nurs Res 1981;30:281–284 [PubMed] [Google Scholar]
- 32. Littman B, Parmelee A. Medical correlates of infant development. Pediatrics 1978;61:470–474 [DOI] [PubMed] [Google Scholar]
- 33. Meedya S, Fahy K, Kable A. Factors that positively influence breastfeeding duration to 6 months: A literature review. Women Birth 2010;23:135–145 [DOI] [PubMed] [Google Scholar]
- 34. Mezzacappa ES. Breastfeeding and maternal stress response and health. Nutr Rev 2004;62:261–268 [DOI] [PubMed] [Google Scholar]
- 35. Shisler S, Homish GG, Molnar DS, et al. Predictors of changes in smoking from third trimester to 9 months postpartum. Nicotine Tob Res 2015;18:84–87 [DOI] [PMC free article] [PubMed] [Google Scholar]