Abstract
Traumatic brain injury (TBI) is known to cause short- and long-term synaptic changes in the brain, possibly underlying downstream cognitive impairments. Neuronal levels of neurogranin, a calcium-sensitive calmodulin-binding protein essential for synaptic plasticity and postsynaptic signaling, are correlated with cognitive function. This study aims to understand the effect of TBI on neurogranin by characterizing changes in protein expression at various time points after injury. Adult, male rats were subjected to either controlled cortical impact (CCI) or control surgery. Expression of neurogranin and post-synaptic density 95 (PSD-95) were evaluated by Western blot in the cortex and hippocampus at 24 h and 1, 2, and 4 weeks post-injury. We hypothesized that CCI reduces neurogranin levels in the cortex and hippocampus, and demonstrate different expression patterns from PSD-95. Neurogranin levels were reduced in the ipsilateral cortex and hippocampus up to 2 weeks after injury but recovered to sham levels by 4 weeks. The contralateral cortex and hippocampus were relatively resistant to changes in neurogranin expression post-injury. Qualitative immunohistochemical assessment corroborated the immunoblot findings. Particularly, the pericontusional cortex and ipsilateral Cornu Ammonis (CA)3 region showed marked reduction in immunoreactivity. PSD-95 demonstrated similar expression patterns to neurogranin in the cortex; however, in the hippocampus, protein expression was increased compared with sham at the 2 and 4 week time points. Our results indicate that CCI lowers neurogranin expression with temporal and regional specificity and that this occurs independently of dendritic loss. Further understanding of the role of neurogranin in synaptic biology after TBI will elucidate pathological mechanisms contributing to cognitive dysfunction.
Keywords: CCI, cognitive dysfunction, neurogranin, PSD-95, synaptic plasticity
Introduction
Traumatic brain injury (TBI) is a pervasive neurological disorder that has short- and long-term pathological consequences. Changes in mood, memory, and cognition are paired with disruptions in neuronal activity, which increase the risk for neurodegenerative diseases later in life.1,2 Pre-clinical injury models have shown significant changes in axonal architecture, synaptic structure, dendritic morphology, and spine density as a result of diffuse axonal injury and synaptic loss.3,4 Axonal and synaptic regeneration processes contribute to recovery; however, pre-injury levels were not reached, and performance on learning and memory tasks, such as the Morris Water Maze, did not completely recover to pre-injury performance levels.4–6 In addition to neuronal structure, synaptic plasticity, particularly in the hippocampus, is impaired after injury. Electrophysiological studies have demonstrated deficits in induction, maintenance, and strength of long-term potentiation processes both immediately and weeks after injury.7–11 Current rehabilitation efforts suggest that neuroplasticity changes, as evidenced by environmental enrichment therapies, for example, underlie observed improvements in cognitive function.12–14 Therefore, understanding underlying mechanisms of synaptic dysfunction after injury is imperative for advancing toward potential means of recovery.
Neurogranin is a small, 7.5 kD protein that is highly abundant in post-synaptic dendritic spines within the rat cerebral cortex, hippocampus, amygdala, and striatum.15,16 Through its calcium-dependent interactions with calmodulin and tightly regulated phosphorylation and oxidation, it has been shown to play an essential role in synaptic plasticity and post-synaptic signaling.17,18 Knockout animals present deficits in spatial learning tasks and aberrant electrophysiological properties,19–21 whereas overexpression studies have shown improved contextual memory encoding, fear-conditioned extinction learning, and enhanced long-term potentiation.22–24 Within the last decade, neurogranin has been extensively investigated in biomarker studies of Alzheimer's disease (AD) as a correlate of synaptic loss and memory impairment. Although there are reductions in neuronal mRNA levels as well as in derived exosomes, neurogranin is elevated in cerebrospinal fluid compared with healthy controls.25–33 More recently, researchers have attempted to evaluate the potential of neurogranin as an acute biomarker of TBI, and have found similarly elevated levels compared with uninjured controls.34,35
Our study sought to characterize cortical and hippocampal expression of neurogranin in a controlled cortical impact (CCI) model of TBI. Using immunoblotting and immunohistochemical techniques, we assessed protein expression and localization at 24 h and at 1, 2, and 4 weeks after injury. We compared the expression pattern of neurogranin to post-synaptic density 95 (PSD-95), another post-synaptic protein, to verify that these changes are independent of synaptic degeneration after injury. Changes in PSD-95 expression have been tightly linked to synaptic spine development and remodeling.36,37 Overexpression of PSD-95 led to increased spine density and maturation, whereas reductions in PSD-95 caused by RNA interference or modulation of upstream modulatory proteins, such as synaptosomal nerve-associated protein 25 (SNAP-25) or serum-inducible kinase (SNK), decreased spine numbers and improper spine development.38–40 Further, PSD-95 expression has been previously characterized in pre-clinical models of TBI and is indicative of changes in dendritic integrity.41–46 We hypothesized that injury induced significant decreases in neurogranin expression across both the cortex and hippocampus, and that these changes were sustained up to 4 weeks post-injury.
Methods
Animals and CCI
All experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee in accordance with the guidelines established by the National Institutes of Health in the Guide for the Care and Use of Laboratory Animals. Animals were housed up to two rats per cage in the University of Pittsburgh vivarium with a 12:12 light/dark photoperiod (lights on at 7:00 a.m.) and provided food and water ad libitum. Animals were also monitored daily by veterinary technicians.
A total of 96 adult male Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing 275–350 g were used in this study and were randomly assigned to receive either sham or CCI injury (n = 6 per group). Half of the animals were processed for immunoblotting and the other half for iummunohistochemistry. Rats were anesthetized using 4% isoflurane with a 2:1 N2O/O2 mixture in a ventilated anesthesia chamber. Following endotracheal intubation, the rats were mechanically ventilated with a 2% isoflurane mixture. Animals were placed in a stereotaxic frame and body temperature was monitored by rectal thermistor probe and maintained at 37°C with a heating pad. Following a midline incision, the soft tissues were reflected and a 7 mm craniectomy was performed over the right parietal cortex, between bregma and lambda, and centered 5 mm lateral of the sagittal suture to expose the dura mater. Control sham injury animals were subjected to anesthesia and surgical procedures but did not receive a TBI. The CCI injury device is a small bore (1.975 cm) double-acting stroked-constrained pneumatic cylinder with a 5.0 cm stroke. An impactor tip (6 mm in diameter, flat tip) was set to produce a tissue deformation of 2.8 mm at a velocity of 4 m/sec with a dwell time of 150 msec. After each sham or CCI injury, the scalp was sutured, gas anesthetics were turned off, and righting time was monitored. Once ambulatory, the animals were returned to their home cages.
Immunoblotting
At 24 h or 1, 2, or 4 weeks post-injury, animals received an overdose of sodium pentobarbital (intraperitoneally, 100 mg/kg Fatal-plus, Vortech Pharmaceuticals, Dearborn, MI) and were rapidly decapitated. Ipsilateral and contralateral cortex and hippocampus were dissected on a chilled ice plate and immediately frozen in liquid nitrogen and stored at -80°C. Samples were homogenized by sonication in lysis buffer (0.1 M NaCl, 0.01 M Tris-Cl, 0.001 M ethylenediaminetetraacetic acid [EDTA], pH 7.6) with protease and phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO) The homogenized whole cell samples were centrifuged at 13,000g at 4 °C for 30 min, and the supernatants collected. Total protein concentration was determined by a BCA protein assay kit (Thermo Scientific, Pittsburgh, PA) using a 96-well microplate reader (Biotek, Winooski, VT).
To assess neurogranin and PSD-95 expression, whole cell lysates were boiled for 10 min prior to blotting. 15 μg of cortical and 10 μg of hippocampal protein samples and molecular weight markers (Bio-Rad, Hercules, CA) were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The resolved proteins were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Invitrogen, Carlsbad, CA). The blots were blocked in 5% nonfat dry milk in tris-buffered saline (TBS) for 1 h. Rabbit anti-neurogranin (Abcam, Cambridge, MA) primary antibody was diluted (1:1000) in blocking solution and incubated overnight at 4°C. The following day, the membranes were washed with 1 × TBS buffer, incubated in blocking solution containing horseradish peroxidase secondary antibodies for 1 h. Proteins were visualized using a chemiluminescence detection system (Supersignal, Pierce). The membranes were stripped and probed with mouse anti-β-actin antibody (diluted 1:5000, Sigma-Aldrich) then stripped again and probed with rabbit anti-PSD-95 antibody (diluted, 1:1000, Abcam, Cambridge, MA). Blots were imaged with the Chemidoc Imager (BioRad). Optical density of neurogranin and PSD-95 were measured using ImageJ (National Institutes of Health) and normalized to β-actin levels. One hemisphere from a single brain region for both sham and CCI-injured animals were loaded on one gel to directly compare injury effect. Values are presented as the ratio of optical densities of samples as a percentage of sham (100%) for each time point. Data are expressed as the group means ± standard error of the mean (SEM).
Immunohistochemistry
Animals received an overdose of sodium pentobarbital (intraperitoneally, 100 mg/kg Fatal-plus) at 24 h or 1, 2, or 4 weeks after sham or CCI injury. Animals were transcardially perfused with saline, followed by a mixture of 10% neutral buffered formalin (Fisher Scientific, Waltham, MA). The brains were post-fixed for an additional 24 h in 10% neutral buffered formalin, and cryoprotected in 30% sucrose in 0.1 M phosphate buffered saline (PBS) for 48 h at 4°C. The brains were frozen in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA) and cut into 35 μm thick coronal sections on a cryostat (Leica Microsystems Inc., Buffalo Grove, IL). Sections at -3.0 to -3.7mm post-bregma were selected and processed for immunofluorescence staining.
Immunohistochemical staining was completed on free-floating tissue sections. One section per animal, at the level of the anterior hippocampus, was rinsed with 0.1 M TBS buffer and blocked with 10% normal goat serum and 0.1% Triton X-100 in 0.1 M TBS for 1 h. Sections were incubated overnight at 4°C with the same rabbit anti-Neurogranin (Abcam), an antibody utilized for immunoblot analysis. Following incubation of horseradish peroxidase secondary antibody, sections were washed, and diaminobenzidine (DAB) was used to visualize the reaction product. Sections were mounted on Superfrost Plus slides (Fisher Scientific), cover-slipped using Permount medium (Fisher Scientific), and qualitatively assessed by light microscopy using a Nikon 90i microscope (Melville, NY).
Statistical analysis
Data are presented as mean ± SEM. Immunoblot data were compared using a non-parametric, Mann–Whitney, t test for each time point and a p value <0.05 was considered statistically significant for all tests. Statistical tests were completed using GraphPad Prism (GraphPad, La Jolla, CA).
Results
Ipsilateral hemisphere showed reduced cortical expression of neurogranin in after injury
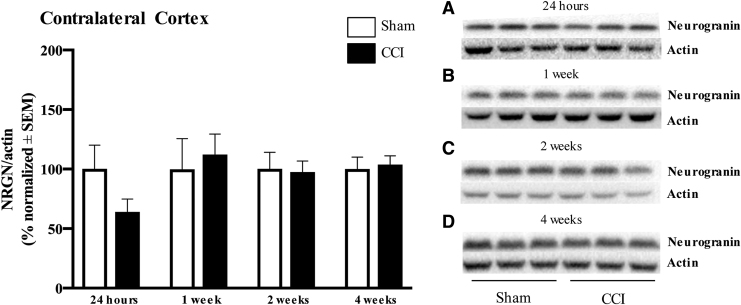
Cortical neurogranin protein expression after CCI was assessed by Western blot. Neurogranin expression in the ipsilateral cortex was not significantly different from the control group 24 h after injury (p > 0.05; Fig. 1A). However, at 1 and 2 weeks after injury, neurogranin expression was significantly reduced in CCI injured animals compared with sham (1 week: 45.0 ± 4.0% vs. 100.0 ± 14.0%, p = 0.0022; 2 weeks: 53.7 ± 6.0% vs. 100.0 ± 22.0%, p = 0.0411; Fig. 1B and C). At 4 weeks post-injury, neurogranin protein expression recovered to control levels (p > 0.05; Fig. 1D). In the contralateral cortex, all time points assessed from 24 h to 4 weeks post-injury showed no change in neurogranin expression from the control group level (p > 0.05; Fig. 2A–D).
FIG. 1.
The ipsilateral cortex shows reduced levels of neurogranin 1 and 2 weeks after injury. (A) Assessment of ipsilateral cortical neurogranin (band at 15 kDa) levels at 24 h after injury revealed no significant difference between sham and controlled cortical impact (CCI) injuries. (B, C) Assessment of ipsilateral cortical neurogranin 1 and 2 weeks after injury showed significant reduction in protein levels in CCI compared with sham-injured animals (1 week: p = 0.0022; 2 weeks: p = 0.0411). (D) Assessment of neurogranin expression at 4 weeks shows no difference between sham and CCI injuries. Neurogranin expression is normalized to actin (band at 42 kD). *p < 0.05, **p < 0.01; n = 6 per group, representative n = 3 per group shown.
FIG. 2.
There is no significant effect of injury on neurogranin expression in the contralateral cortex. (A–D) Assessment of contralateral cortical neurogranin (band at 15 kDa) levels at 24 h to 4 weeks after injury revealed no difference between sham and controlled cortical impact (CCI) animals. Neurogranin expression was normalized to actin (band at 42 kDa). n = 6 per group, representative n = 3 per group shown.
Ipsilateral hippocampus neurogranin levels remained lower for longer than levels in the contralateral hippocampus
At 24 h post-injury, the hippocampus in both the ipsilateral and contralateral hemispheres showed significant reductions in neurogranin abundance compared with the control group (ipsilateral: 39.0 ± 8.5% vs. 100.0 ± 17.1%, p = 0.0022; contralateral: 40.5 ± 10.1% vs. 100.0 ± 14.3%, p = 0.0087; Figs. 3A and 4A). The contralateral hippocampus recovered to control levels by 1 week post-injury and remained stable through 4 weeks post-injury (p > 0.05; Fig. 4B–D). The ipsilateral hippocampus maintained lower neurogranin levels 1 and 2 weeks after injury (1 week: 36.0 ± 3.1% vs. 100.0 ± 20.6%, p = 0.0043, 2 weeks: 41.3 ± 15.5 vs. 100.0 ± 7.6%, p = 0.0260; Fig. 3B and C). By the 4 week time point, protein levels returned to control levels (p > 0.05; Fig. 3D).
FIG. 3.
Neurogranin levels are reduced in the ipsilateral hippocampus up to 2 weeks post-injury. (A–C) Assessment of ipsilateral hippocampal neurogranin (band at 15 kDa) levels at 24 h, 1 week, and 2 weeks after injury revealed significant reduction in protein levels in controlled cortical impact (CCI) injured animals compared with controls (24 h: p = 0.0095; 1 week: p = 0.0087; 2 weeks: p = 0.0260). (D) Assessment of neurogranin expression in the ipsilateral hippocampus 4 weeks after injury shows no difference between sham and CCI. Neurogranin expression was normalized to actin (band at 42 kDa). *p < 0.05, **p < 0.01; n = 6 per group, representative n = 3 per group shown.
FIG. 4.
Reductions in contralateral hippocampal neurogranin recover after 24 h post-injury. (A) Assessment of contralateral hippocampal neurogranin (band at 15 kDa) levels at 24 h post-injury revealed significant reductions in protein levels in controlled cortical impact (CCI) injured animals compared with controls (p = 0.0087). (B–D) Assessment of neurogranin expression in the contralateral hippocampus 1–4 weeks after injury shows no significant difference between sham and CCI. Neurogranin expression was normalized to actin (band at 42 kDa). **p < 0.01; n = 6 per group, representative n = 3 per group shown.
PSD-95 demonstrated initial increase followed by subsequent reduction in protein levels in the cortex
To assess whether these decreases in neurogranin expression were independent of dendritic loss, we compared it with another post-synaptic protein, PSD-95. Contrary to a non-significant reduction in ipsilateral cortical neurogranin expression, at 24 h after injury, injured animals showed a trend for higher PSD-95 expression in this brain region, but it did not reach significance (162.5 ± 20.1% vs. 100.0 ± 21.3%, p = 0.0931). PSD-95 expression in the ipsilateral cortex pattern shifted to being significantly lower than sham levels 1 week after injury (44.0 ± 6.2% vs. 100.0 ± 13.9%, p = 0.0260; Fig. 5B). However, PSD-95 protein expression returned to control levels by 2 and 4 weeks after injury (p > 0.05; Fig. 5C and D). Contralateral cortex PSD-95 expression was similar to controls at all time points. (p > 0.05; Fig. 6A–D).
FIG. 5.
Post-synaptic density 95 (PSD-95) protein expression is only decreased 1 week after injury in the ipsilateral cortex. (A) Assessment of ipsilateral cortical PSD-95 (band at 80 kDa) levels at 24 h after injury revealed a trend of increased protein expression in controlled cortical impact (CCI) compared with sham-injured groups (p = 0.0589). (B) Evaluation of PSD-95 levels 1 week after injury shows a significant decrease in protein expression in CCI-injured animals compared with sham (p = 0.0260) (C, D) Evaluation of PSD-95 levels in the ipsilateral cortex 2 and 4 weeks after injury showed no significant changes in expression between and sham and CCI injured groups. PSD-95 expression was normalized to actin (band at 42 kDa). *p < 0.05; n = 6 per group, representative n = 3 per group shown.
FIG. 6.
There is no significant effect of injury on post-synaptic density 95 (PSD-95) protein expression in the contralateral cortex. (A–D) Assessment of contralateral cortical PSD-95 (band at 80 kDa) levels at 24 h to 4 weeks after injury revealed no significant difference between sham and controlled cortical injury (CCI) injured animals. PSD-95 expression was normalized to actin (band at 42 kDa). n = 6 per group, representative n = 3 per group shown.
PSD-95 initially showed lower expression in the hippocampus but was increased at later time points
Similar to neurogranin expression in the ipsilateral hippocampus, injured animals showed significantly decreased PSD-95 expression at 24 h and 1 week post-injury compared with sham controls (24 h: 44.5 ± 4.9% vs. 100.0 ± 12.0%, p = 0.0022; 1 week: 35.2 ± 5.7% vs. 100.0 ± 22.4%, p = 0.0087; Fig. 7A and B). Two weeks after injury, there was no difference in PSD-95 expression in CCI injured animals compared with sham (p > 0.05; Fig. 7C). Interestingly, 4 weeks after injury, there was significantly increased PSD-95 expression in the ipsilateral hippocampus, which is distinct from neurogranin expression (160.7 ± 19.0% vs. 100.0 ± 12.0%, p = 0.0303; Fig. 7D). The contralateral hippocampus showed no change in PSD-95 expression in the injured group compared with sham at the 24 h and 1 week time-points (p > 0.05; Fig. 8A and B). However, at 2 weeks, there was also higher protein expression in CCI injured animals than in sham (162.3 ± 8.3% vs. 100.0 ± 11.1%, p = 0.0043; Fig. 8C) and a modest increased trend at 4 weeks, but this did not reach significance (211.2 ± 46.2% vs. 100.0 ± 25.6%, p = 0.0823; Fig. 8D).
FIG. 7.
Post-synaptic density 95 (PSD-95) levels recover by 2 weeks post-injury in the ipsilateral hippocampus. (A, B) Assessment of ipsilateral hippocampal PSD-95 (band at 80 kDa) levels at 24 h and 1 week after injury significant reductions in protein expression in controlled cortical impact (CCI) compared with sham- injured animals (24 h: p = 0.0022; 1 week: p = 0.0087). (C) Assessment of PSD-95 in the ipsilateral hippocampus 2 weeks after injury showed no significant change in protein expression between sham and CCI groups. (D) Four weeks after injury, we observe significant increases in PSD-95 protein levels in the ipsilateral hippocampus in CCI injured animals compared with sham (p = 0.0303). PSD-95 expression was normalized to actin (band at 42 kDa). *p < 0.05, **p < 0.01; n = 6 per group, representative n = 3 per group shown.
FIG. 8.
Contralateral hippocampal post-synaptic density 95 (PSD-95) levels are significantly increased 2 weeks after injury. (A, B) Assessment of PSD-95 (band at 80 kDa) levels at 24 h and 1 week after injury revealed no significant difference between sham and controlled cortical impact (CCI). (C) Evaluation of PSD-95 levels at 2 weeks post-injury shows a significant increase in CCI injured animals compared with control (p = 0.0043). (D) At 4 weeks post-injury, there was a trend of increased protein expression of PSD-95 (p = 0.0823). PSD-95 expression was normalized to actin (band at 42 kDa). **p < 0.01; n = 6 per group, representative n = 3 per group shown.
Qualitative immunohistochemical analysis showed trends similar to immunoblotting
To corroborate our Western blot data, and determine regional neurogranin expression changes, we conducted immunohistochemistry on rat tissue 24 h and 1, 2, and 4 weeks after injury. At all time points after injury, the contralateral cortex showed no apparent change in staining from the sham animals, similar to the Western blot results. At 24 h, the ipsilateral and contralateral hippocampus showed decreased immunoreactivity, particularly in the pyramidal cell layer of Cornu Ammonis (CA)1 and CA3 and in the stratum radiatum layer (Fig. 9A). Additionally, the granular layer of the dentate gyrus exhibited reduced neurogranin immunoreactivity in the contralateral hippocampus. At later time points, the contralateral hippocampus demonstrated recovery in immunoreactivity, paralleling the Western blot data. The ipsilateral hippocampus showed continued decreases in neurogranin immunoreactivity, especially in CA1, CA3, and the stratum radiatum layer at 1 and 2 weeks after injury (Fig. 9B and C). At 4 weeks, immunoreactivity was restored (Fig. 9D).
FIG. 9.
Immunohistochemical analysis of neurogranin expression shows similar pattern to Western blot data. Sham and controlled cortical impact (CCI) injured animal tissue was stained for neurogranin to assess regional changes in protein expression. The white square highlights the Cornu Ammonis (CA)3 region of the ipsilateral hippocampus, which demonstrated noticeable changes in immunoreactivity compared with sham. (A) At 24 h after injury, neurogranin expression was reduced in the superior cortex, near the injury site, and in the ipsilateral and contralateral hippocampus compared with sham. (B, C) At 1 and 2 weeks post-injury the ipsilateral cortex had degenerated; however, the ipsilateral hippocampus demonstrates continued reduction in neurogranin expression compared with sham. (D) Neurogranin protein levels were restored to sham levels 4 weeks post-injury. Whole section images were acquired at 4 × magnification, and CA3 images were acquired at 10 × magnification. Scale bar indicates 1 mm on whole section and 500 μm on the CA3 image.
The ipsilateral cortex was the only region that showed different expression patterns between the histology and the Western blotting. At 24 h after injury, decreased neurogranin expression was observed in the pericontusional, somatosensory area, throughout all cortical layers (Fig. 9A). Although the images shown in Figure 9 represent the median change in neurogranin expression, there was a degree of variability in the amount of neurogranin loss among the animals, with some animals showing more reductions in immunoreactivity than others compared with sham. The Western blot data showed no significant difference in neurogranin expression from control animals at this time point.
Discussion
Neurogranin mediates cognitive processes through its interactions with calmodulin. By shuttling calmodulin to the dendritic spine, neurogranin fine-tunes downstream calcium and calmodulin-dependent signaling, most importantly long-term potentiation.47 Reduced levels of the protein have been correlated with dysfunctional synaptic plasticity and learning and memory deficits.19–21 Further, elevated levels of this protein have been observed in cerebrospinal fluid of patients with AD,25,27–33 and more recently, increased amounts were detected in serum acutely after TBI.34,35 The mechanism for this observation in plasma after TBI is unknown; however, it is hypothesized that the combination of neurogranin's small size and injury-induced cellular degeneration and blood–brain barrier breakdown explain its leakiness and subsequent detection so acutely after the injury event.34 Post-mortem analysis of neurogranin processing, = showed a significantly increased ratio of peptides to full-length neurogranin in brain tissue of AD patients compared with controls,31 indicative of synaptic degeneration and perhaps another potential mechanism for observed increased levels in in the blood after TBI. Given the biomarker data as well as the intersection of neurogranin's role in cognition and subsequent deficits after TBI, we hypothesized that CCI would decrease neurogranin protein expression. Our study demonstrates that CCI reduces neurogranin protein levels; however, the expression shifts across time and across brain regions. Additionally, PSD-95 protein expression is altered after CCI, demonstrating distinct expression patterns from neurogranin particularly in the hippocampus, at later time points.
Twenty-four hours after CCI injury, the contralateral and ipsilateral hippocampus showed significant reductions in neurogranin protein levels. Given its role in regulated long-term potentiation, neurogranin is primarily located in post-synaptic dendritic spines.15 Previous studies have shown decreases in dendritic spines and total dendrite number hours to days after injury, primarily in the ipsilateral hippocampus,3,42 as well as in the dentate gyrus of both hemispheres.33 Interestingly, the cortex was resistant to changes in neurogranin expression compared with sham injury. The observed decrease in expression compared with sham was not significant, even though a similar decrease in immunoreactivity was observed in using histological methods. This could be caused by the surrounding cortical tissue sample collected during dissection diluting the effect of injury at the epicenter. Additionally, not all post-synaptic proteins show immediate decreases following TBI. Ansari and coworkers showed that although markers associated with oxidative stress were reduced within hours to days after injury, synaptic associated protein 97 (SAP-97), demonstrated a 96 h delay in reductions in the hippocampus.41 Additionally, other studies of calcium-sensitive proteins such as calcineurin, a calcium-sensitive phosphatase, showed peak increases in the ipsilateral cortex 1 week post-injury,43,48 in addition to the decreased expression of α-calcium calmodulin kinase II at this time point.49 Perhaps there is a delay in the pathogenesis of calcium-dependent proteins in secondary biochemical processes in the cortex.
At 1 and 2 weeks after CCI injury, the ipsilateral hippocampus continued to show significantly decreased levels of neurogranin. The ipsilateral cortex demonstrated a delayed, lowered expression compared with sham animals. At these time points, previous investigations showed recovery in neuronal number and re-innervation of synaptic connections, whereas others demonstrate sustained deficits in synaptic plasticity and transmission. One week after fluid percussion impact, mice were unable to induce long-term potentiation (LTP) in the CA1 of the hippocampus.49 Further, rats showed diminished maintenance of LTP 1 and 8 weeks after mild to severe injury, which correlated to worsened performance at Morris Water Maze tasks.10 Number of synaptic connections, as measured by ultrastructural microscopy, after CCI decreased by 60% just 2 days after injury.5 At the 10 day time point, there was evidence of re-innervation; however, even at 60 days post-injury, the synaptic number did not reach control levels.5 A follow-up study found that although pre-synaptic innervations recover 1 week post-injury, hippocampal synaptic strength remains somewhat impaired through 2 weeks.9 Additionally, previous work from our group demonstrated impairments in synaptic transmission and vesicle release at the 1 and 2 week time points, with recovery by 4 weeks.50 Discerning the relationship between pre- and post-synaptic signaling and feedback processes would be interesting to investigate in the context of the pathophysiology of cognitive dysfunction after injury.
Our immunohistochemical evaluation of neurogranin expression closely corroborated our Western blot results and provided regional resolution of neurogranin expression across brain areas. We found that the ipsilateral hemisphere showed the most significant changes in neurogranin immunoreactivity, specifically in the pericontusional cortex, CA3, and stratum radiatum. A reduction in neurogranin expression, especially in the hippocampus, suggests that LTP and subsequent encoding of learning and memory behaviors is reliant on regulated protein expression.22,51 This pathology is similar to that observed in neurogranin knockout or knockdown models, where LTP is significantly reduced and long-term depression (LTD) is enhanced.19,21 Together, our Western blot and histological data indicate that reduction in neurogranin plays a role in the deficits in synaptic plasticity after TBI.
Although neurogranin levels recovered to baseline in both brain regions and hemispheres by 4 weeks after injury, neurogranin still may contribute to the disruption of synaptic plasticity. Neurogranin's interaction with calmodulin is finely regulated by post-translational modifications. Neurogranin is transiently phosphorylated by protein kinase C (PKC) in response to synaptic activation, impairing its ability to bind to calmodulin17,52–54 and it has been shown that levels of the phosphorylated form increase after induction of LTP and conversely decrease after LTD.55,56 Further, oxidation of neurogranin has the same effect, by changing its confirmation and prohibiting binding to calmodulin.57 Interestingly, N-methyl-d-aspartate (NMDA) receptor activation leads to transient increases in both phosphorylation and oxidation of neurogranin.18 A hallmark feature of TBI is glutamate excitotoxicity.58 This leads to overactivation of NMDA receptors and release of intracellular calcium stores, and disrupts multiple downstream processes.59 Chronic cognitive deficits of these acute insults demonstrate the subtle, long-term consequences in synaptic plasticity that are beyond overt changes in expression. Further studies understanding enzymatic regulation of neurogranin, subsequent interaction with calmodulin, and its role in downstream signaling cascades may implicate neurogranin in continued pathological processes once protein expression is restored.
PSD-95 is a scaffolding protein involved in organizing and stabilizing post-synaptic complexes. Although it is not involved in LTP directly, it influences dynamics of glutamate receptors.59 We report that PSD-95 showed protein expression patterns similar to those of neurogranin in the cortex and at early time points in the hippocampus. There are no changes in protein expression in the contralateral cortex for either neurogranin or PSD-95. In the ipsilateral cortex, PSD-95 is significantly reduced at 1 week post-injury, however it is recovered by 2 weeks post-injury. Neurogranin remained significantly lower at the 2 week time point. Interestingly, in the hippocampus, there was a noticeable difference in post-injury protein expression between neurogranin and PSD-95, specifically at 2 and 4 weeks post-injury. Initially, we observed similar decreases of neurogranin and PSD-95 levels in the ipsilateral hippocampus at 24 h and 1 week post-injury, which can most likely be attributed to dendritic loss. However, although neurogranin levels recovered to baseline at 4 weeks post-injury, we observed a significant increase in PSD-95 expression at that time point compared with controls. Similarly, contralateral hippocampal PSD-95 expression appeared to be increased at the 2 and 4 week time points.
Previous studies of post-synaptic changes after TBI utilize PSD-95 as a marker of synaptic integrity, as its expression is reported to be tightly associated with the formation and maturation of dendritic spines. Specifically, overexpression of PSD-95 advances spine maturation and increases overall number, whereas decreased levels impair proper growth and reduce density.36–40 Studies examining the time course of oxidative stress markers acutely after moderate CCI in rats showed decreased PSD-95 expression 24–96 h in the ipsilateral cortex and 48–96 h in the hippocampus.41,46 This time point also closely associates to observed loss in dendritic spine number and mushroom morphology at 3 days post-moderate CCI in mice.3 An investigation examining dendritic density in a fluid percussion injury model in rats showed acute loss of spines at 24 h after injury followed by recovery above control levels in some regions at 1 week post-injury.42 A partner study using the same injury model reported acute reductions in PSD-95 levels as early as 18 h after fluid percussion injury in rats in both hemispheres of the cortex and hippocampus with the most persistent loss in the ipsilateral hippocampus.43 Further, Wang and coworkers utilized transgenic mice to evaluate hypothermic conditions on dendritic branching and spine density 1 and 7 days after severe CCI demonstrating a tight correlation of injury-induced PSD-95 and spine number loss and subsequent recovery of both measures with treatment.45
There are, however, some contradictory reports that make this relationship less transparent. In cluster of differentiation 1 (CD1) mice, a moderate CCI injury caused a delayed loss of PSD-95 protein in the hippocampus; only noting reduced levels at the 1 week time point. Conversely, Patel and coworkers reported increased PSD-95 expression after moderate CCI in rats.60 Beyond spine density, some have shown an increase in average individual spine area and head width for remaining spines, particularly at 1 week post-CCI and fluid percussion injury in mice and rats.11,61 How PSD-95 is explicitly involved in the relationship between spine densities versus area warrants further investigation. Our data at 2 and 4 weeks demonstrate a potential compensatory mechanism by which increased PSD-95 expression parallels increased spine area.
Variations in reports of PSD-95 levels after injury and correlation to dendritic integrity allude to not only heterogeneity of injury pathology among rodent species and injury paradigms but also perhaps a diversity of secondary injury cascade mechanisms at play. Although we cannot conclude definitively whether changes neurogranin are distinct from loss of dendritic spines, we demonstrate, to a degree, a unique post-injury pathology of neurogranin that is independent of PSD-95-mediated synaptic degeneration. Further, the distinct and prolonged deficits of neurogranin warrant further investigation into the capacity in which the protein contributes to post-injury sequelae.
Cognitive dysfunction is a predominant symptom of clinical and pre-clinical TBI.62–65 This study is the first to measure brain neurogranin expression after TBI. Given neurogranin's direct role synaptic biology, further work is needed to elucidate its role in contributing to the cognitive and behavioral dysfunction after TBI.
Acknowledgments
We thank Tara Byrne and all members of the Dixon laboratory for their help in the completion of this manuscript.
Funding Information
This work was supported by the National Institutes of Health grant R01NS106925, the Veterans Affairs Merit Award I01RX001127, and the Pennsylvania Department of Health, PA Consortium on TBI (PACT) grant 4100077083.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Cristofori I., and Levin H.S. (2015). Traumatic brain injury and cognition. Handb. Clin. Neurol. 128, 579–611 [DOI] [PubMed] [Google Scholar]
- 2. Wilson L., Stewart W., Dams-O'Connor K., Diaz-Arrastia R., Horton L., Menon D.K., and Polinder S. (2017). The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 16, 813–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao X., Deng P., Xu Z.C., and Chen J. (2011). Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus. PLoS One 6, e24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park K., and Biederer T. (2013). Neuronal adhesion and synapse organization in recovery after brain injury. Future Neurol. 8, 555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheff S. W., Price D. A., Hicks R. R., Baldwin S. A., Robinson S., and Brackney C. (2005). Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J. Neurotrauma 22, 719–732 [DOI] [PubMed] [Google Scholar]
- 6. Semchenko V.V., Bogolepov N.N., Stepanov S.S., Maksimishin S.V., and Khizhnyak A.S. (2006). Synaptic plasticity of the neocortex of white rats with diffuse-focal brain injuries. Neurosci. Behav. Physiol. 36, 613–618 [DOI] [PubMed] [Google Scholar]
- 7. D'Ambrosio R., Maris D.O., Grady M.S., Winn H.R., and Janigro D. (1998). Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Research 786, 64–79 [DOI] [PubMed] [Google Scholar]
- 8. Miyazaki S., Katayama Y., Lyeth B.G., Jenkins L.W., DeWitt D.S., Goldberg S.J., Newlon P.G., and Hayes R.L. (1992). Enduring suppression of hippocampal long-term potentiation following traumatic brain injury in rat. Brain Res 585, 335–339 [DOI] [PubMed] [Google Scholar]
- 9. Norris C.M., and Scheff S.W. (2009). Recovery of afferent function and synaptic strength in hippocampal CA1 following traumatic brain injury. J. Neurotrauma 26, 2269–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanders M.J., Sick T.J., Perez-Pinzon M.A., Dietrich W.D., and Green E.J. (2000). Chronic failure in the maintenance of long-term potentiation following fluid percussion injury in the rat. Brain Res. 861, 69–76 [DOI] [PubMed] [Google Scholar]
- 11. Schwarzbach E., Bonislawski D.P., Xiong G., and Cohen A.S. (2006). Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus 16, 541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kempermann G., Kuhn H.G., and Gage F.H. (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493. [DOI] [PubMed] [Google Scholar]
- 13. Greenough W.T., and Volkmar F.R. (1973). Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp. Neurol. 40, 491–504 [DOI] [PubMed] [Google Scholar]
- 14. Turner A.M., and Greenough W.T. (1985). Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 329, 195–203 [DOI] [PubMed] [Google Scholar]
- 15. Alvarez-Bolado G., Rodríguez-Sánchez P., Tejero-Díez P., Fairén A., and Díez-Guerra F.J. (1996). Neurogranin in the development of the rat telencephalon. Neuroscience 73, 565–580 [DOI] [PubMed] [Google Scholar]
- 16. Represa A., Deloulme J.C., Sensenbrenner M., Ben-Ari Y., and Baudier J. (1990). Neurogranin: immunocytochemical localization of a brain-specific protein kinase C substrate. J. Neurosci. 10, 3782–3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen S.-J., Sweatt J.D., and Klann E. (1997). Enhanced phosphorylation of the postsynaptic protein kinase C substrate RC3/neurogranin during long-term potentiation. Brain Res. 749, 181–187 [DOI] [PubMed] [Google Scholar]
- 18. Wu J., Huang K.-P., and Huang F.L. (2003). Participation of NMDA-mediated phosphorylation and oxidation of neurogranin in the regulation of Ca2+- and Ca2+/calmodulin-dependent neuronal signaling in the hippocampus. J. Neurochem. 86, 1524–1533 [DOI] [PubMed] [Google Scholar]
- 19. Huang K.-P., Huang F.L., Jäger T., Li J., Reymann K.G., and Balschun D. (2004). Neurogranin/RC3 enhances long-term potentiation and learning by promoting calcium-mediated signaling. J. Neurosci. 24, 10,660–10,669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miyakawa T., Yared E., Pak J.H., Huang F.L., Huang K.-P., and Crawley J.N. (2001). Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus 11, 763–775 [DOI] [PubMed] [Google Scholar]
- 21. Pak J.H., Huang F.L., Li J., Balschun D., Reymann K.G., Chiang C., Westphal H., and Huang K.-P. (2000). Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: a study with knockout mice. PNAS 97, 11,232–11,237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones K.J., Templet S., Zemoura K., Kuzniewska B., Pena F.X., Hwang H., Lei D.J., Haensgen H., Nguyen S., Saenz C., Lewis M., Dziembowska M., and Xu W. (2018). Rapid, experience-dependent translation of neurogranin enables memory encoding. Proc. Natl. Acad. Sci. U.S.A. 115, E5805–E5814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhong L., Brown J., Kramer A., Kaleka K., Petersen A., Krueger J.N., Florence M., Muelbl M.J., Battle M., Murphy G.G., Olsen C.M., and Gerges N.Z. (2015). Increased prefrontal cortex neurogranin enhances plasticity and extinction learning. J. Neurosci. 35, 7503–7508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhong L., Cherry T., Bies C.E., Florence M.A., and Gerges N.Z. (2009). Neurogranin enhances synaptic strength through its interaction with calmodulin. EMBO J. 28, 3027–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Casaletto K.B., Elahi F.M., Bettcher B.M., Neuhaus J., Bendlin B.B., Asthana S., Johnson S.C., Yaffe K., Carlsson C., Blennow K., Zetterberg H., and Kramer J.H. (2017). Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers. Neurology 89, 1782–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang J.W., Schumacher E., Coulter P.M., Vinters H.V., and Watson J.B. (1997). Dendritic translocation of RC3/neurogranin mRNA in normal aging, Alzheimer disease and fronto-temporal dementia. J. Neuropathol. Exp. Neurol. 56, 1105–1118 [DOI] [PubMed] [Google Scholar]
- 27. De Vos A., Jacobs D., Struyfs H., Fransen E., Andersson K., Portelius E., Andreasson U., De Surgeloose D., Hernalsteen D., Sleegers K., Robberecht C., Van Broeckhoven C., Zetterberg H., Blennow K., Engelborghs S., and Vanmechelen E. (2015). C-terminal neurogranin is increased in cerebrospinal fluid but unchanged in plasma in Alzheimer's disease. Alzheimers Dement. 11, 1461–1469 [DOI] [PubMed] [Google Scholar]
- 28. De Vos A., Struyfs H., Jacobs D., Fransen E., Klewansky T., De Roeck E., Robberecht C., Van Broeckhoven C., Duyckaerts C., Engelborghs S., and Vanmechelen E. (2016). The cerebrospinal fluid neurogranin/BACE1 ratio is a potential correlate of cognitive decline in Alzheimer's disease. J. Alzheimers Dis. 53, 1523–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hellwig K., Kvartsberg H., Portelius E., Andreasson U., Oberstein T.J., Lewczuk P., Blennow K., Kornhuber J., Maler J.M., Zetterberg H., and Spitzer P. (2015). Neurogranin and YKL-40: independent markers of synaptic degeneration and neuroinflammation in Alzheimer's disease. Alzheimers Res. Ther. 7, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kester M.I., Teunissen C.E., Crimmins D.L., Herries E.M., Ladenson J.H., Scheltens P., Flier W.M. van der, Morris J.C., Holtzman D.M., and Fagan A.M. (2015). Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol. 72, 1275–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kvartsberg H., Portelius E., Andreasson U., Brinkmalm G., Hellwig K., Lelental N., Kornhuber J., Hansson O., Minthon L., Spitzer P., Maler J.M., Zetterberg H., Blennow K., and Lewczuk P. (2015). Characterization of the postsynaptic protein neurogranin in paired cerebrospinal fluid and plasma samples from Alzheimer's disease patients and healthy controls. Alzheimers Res. Ther. 7, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thorsell A., Bjerke M., Gobom J., Brunhage E., Vanmechelen E., Andreasen N., Hansson O., Minthon L., Zetterberg H., and Blennow K. (2010). Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer's disease. Brain Res. 1362, 13–22 [DOI] [PubMed] [Google Scholar]
- 33. Winston C.N., Goetzl E.J., Akers J.C., Carter B.S., Rockenstein E.M., Galasko D., Masliah E., and Rissman R.A. (2016). Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement. 3, 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peacock W.F., Van Meter T.E., Mirshahi N., Ferber K., Gerwien R., Rao V., Sair H.I., Diaz-Arrastia R., and Korley F.K. (2017). Derivation of a three biomarker panel to improve diagnosis in patients with mild traumatic brain injury. Front. Neurol. 8, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang J., Korley F.K., Dai M., and Everett A.D. (2015). Serum neurogranin measurement as a biomarker of acute traumatic brain injury. Clin. Biochem. 48, 843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pak D.T.S., and Sheng M. (2003). Targeted protein degradation and synapse remodeling by an inducible protein kinase. Science 302, 1368–1373 [DOI] [PubMed] [Google Scholar]
- 37. Gerrow K., Romorini S., Nabi S.M., Colicos M.A., Sala C., and El-Husseini A. (2006). A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron 49, 547–562 [DOI] [PubMed] [Google Scholar]
- 38. El-Husseini A.E.-D., Schnell E., Chetkovich D.M., Nicoll R.A., and Bredt D.S. (2000). PSD-95 involvement in maturation of excitatory synapses. Science 290, 1364–1368 [PubMed] [Google Scholar]
- 39. Fossati G., Morini R., Corradini I., Antonucci F., Trepte P., Edry E., Sharma V., Papale A., Pozzi D., Defilippi P., Meier J.C., Brambilla R., Turco E., Rosenblum K., Wanker E.E., Ziv N.E., Menna E., and Matteoli M. (2015). Reduced SNAP-25 increases PSD-95 mobility and impairs spine morphogenesis. Cell Death Differ. 22, 1425–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ehrlich I., Klein M., Rumpel S., and Malinow R. (2007). PSD-95 is required for activity-driven synapse stabilization. Proc. Natl. Acad. Sci. U. S. A. 104, 4176–4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ansari M.A., Roberts K.N., and Scheff S.W. (2008). A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J. Neurotrauma 25, 513–526 [DOI] [PubMed] [Google Scholar]
- 42. Campbell J.N., Register D., and Churn S.B. (2011). Traumatic brain injury causes an FK506-sensitive loss and an overgrowth of dendritic spines in rat forebrain. J. Neurotrauma 29, 201–217 [DOI] [PubMed] [Google Scholar]
- 43. Campbell J.N., Low B., Kurz J.E., Patel S.S., Young M.T., and Churn S.B. (2012). Mechanisms of dendritic spine remodeling in a rat model of traumatic brain injury. J. Neurotrauma 29, 218–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wakade C., Sangeetha S.R., Laird M.D., Dhandapani K.M., and Vender J.R. (2010). Delayed reduction in hippocampal post-synaptic density protein-95 expression temporally correlates with cognitive dysfunction following controlled cortical impact in mice. J. Neurosurg. 113, 1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang C., Zhao C., Jiang G., Gu X., Feng J., and Jiang J. (2016). The role of posttraumatic hypothermia in preventing dendrite degeneration and spine loss after severe traumatic brain injury. Sci. Rep. 6, 37063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ansari M.A., Roberts K.N., and Scheff S.W. (2008). Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic. Biol. Med. 45, 443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kubota Y., Putkey J.A., and Waxham M.N. (2007). Neurogranin controls the spatiotemporal pattern of postsynaptic Ca2+/CaM signaling. Biophys. J. 93, 3848–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kurz J.E., Parsons J.T., Rana A., Gibson C.J., Hamm R.J., and Churn S.B. (2005). A significant increase in both basal and maximal calcineurin activity following fluid percussion injury in the rat. J. Neurotrauma 22, 476–490 [DOI] [PubMed] [Google Scholar]
- 49. Schwarzbach E., Bonislawski D.P., Xiong G., and Cohen A.S. (2006). Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus 16, 541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carlson S.W., Yan H., Ma M., Li Y., Henchir J., and Dixon C.E. (2016). Traumatic brain injury impairs soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex formation and alters synaptic vesicle distribution in the hippocampus. J. Neurotrauma 33, 113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang K.-P., Huang F.L., and Shetty P.K. (2011). Stimulation-mediated translocation of calmodulin and neurogranin from soma to dendrites of mouse hippocampal CA1 pyramidal neurons. Neuroscience 178, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baudier J., Deloulme J.C., Van Dorsselaer A., Black D., and Matthes H.W. (1991). Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17). Identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J. Biol. Chem. 266, 229–237 [PubMed] [Google Scholar]
- 53. Huang K.P., Huang F.L., and Chen H.C. (1993). Characterization of a 7.5-kDa protein kinase C substrate (RC3 protein, neurogranin) from rat brain. Arch. Biochem. Biophys. 305, 570–580 [DOI] [PubMed] [Google Scholar]
- 54. Ramakers G.M.J., Graan P.N.E.D., Urban I.J.A., Kraay D., Tang T., Pasinelli P., Oestreicher A.B., and Gispen W.H. (1995). Temporal differences in the phosphorylation state of pre- and postsynaptic protein kinase C substrates B-50/GAP-43 and neurogranin during long term potentiation. J. Biol. Chem. 270, 13,892–13,898 [DOI] [PubMed] [Google Scholar]
- 55. Ramakers G.M.J., Heinen K., Gispen W.-H., and Graan P.N.E de. (2000). Long term depression in the CA1 field is associated with a transient decrease in pre- and postsynaptic PKC substrate phosphorylation. J. Biol. Chem. 275, 28,682–28,687 [DOI] [PubMed] [Google Scholar]
- 56. van Dam E.J.M., Ruiter B., Kamal A., Ramakers G.M.J., Gispen W.H., and de Graan P.N.E. (2002). N-methyl-d-aspartate-induced long-term depression is associated with a decrease in postsynaptic protein kinase C substrate phosphorylation in rat hippocampal slices. Neurosci. Lett. 320, 129–132 [DOI] [PubMed] [Google Scholar]
- 57. Mahoney C.W., Pak J.H., and Huang K.-P. (1996). Nitric oxide modification of rat brain neurogranin identification of the cysteine residues involved in intramolecular disulfide bridge formation using site-directed mutagenesis. J. Biol. Chem. 271, 28,798–28,804 [DOI] [PubMed] [Google Scholar]
- 58. Palmer A.M., Marion D.W., Botscheller M.L., Swedlow P.E., Styren S.D., and DeKosky S.T. (1993). Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J. Neurochem. 61, 2015–2024 [DOI] [PubMed] [Google Scholar]
- 59. Luo P., Fei F., Zhang L., Qu Y., and Fei Z. (2011). The role of glutamate receptors in traumatic brain injury: Implications for postsynaptic density in pathophysiology. Brain Res. Bull. 85, 313–320 [DOI] [PubMed] [Google Scholar]
- 60. Patel M.V., Sewell E., Dickson S., Kim H., Meaney D.F., and Firestein B.L. (2019). A role for postsynaptic density 95 and its binding partners in models of traumatic brain injury. J. Neurotrauma 36, 2129–2138 [DOI] [PubMed] [Google Scholar]
- 61. Pijet B., Stefaniuk M., and Kaczmarek L. (2019). MMP-9 contributes to dendritic spine remodeling following traumatic brain injury. Neural Plast. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dixon C. E., Kochanek P. M., Yan H. Q., Schiding J. K., Griffith R. G., Baum E., Marion D. W., and Dekosky S. T. (1999). One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J. Neurotrauma 16, 109–122 [DOI] [PubMed] [Google Scholar]
- 63. Pierce J.E.S., Smith D.H., Trojanowski J.Q., and McIntosh T.K. (1998). Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience 87, 359–369 [DOI] [PubMed] [Google Scholar]
- 64. Piot-Grosjean O., Wahl F., Gobbo O., and Stutzmann J.-M. (2001). Assessment of sensorimotor and cognitive deficits induced by a moderate traumatic injury in the right parietal cortex of the rat. Neurobiol. Dis. 8, 1082–1093 [DOI] [PubMed] [Google Scholar]
- 65. Fujimoto S.T., Longhi L., Saatman K.E., and McIntosh T.K. (2004). Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 28, 365–378 [DOI] [PubMed] [Google Scholar]