Abstract
Background
In December 2019, a pneumonia caused by a novel coronavirus (SARS-CoV-2) emerged in Wuhan, China and has rapidly spread around the world since then.
Aim
This study aims to understand the research gaps related to COVID-19 and propose recommendations for future research.
Methods
We undertook a scoping review of COVID-19, comprehensively searching databases and other sources to identify literature on COVID-19 between 1 December 2019 and 6 February 2020. We analysed the sources, publication date, type and topic of the retrieved articles/studies.
Results
We included 249 articles in this scoping review. More than half (59.0%) were conducted in China. Guidance/guidelines and consensuses statements (n = 56; 22.5%) were the most common. Most (n = 192; 77.1%) articles were published in peer-reviewed journals, 35 (14.1%) on preprint servers and 22 (8.8%) posted online. Ten genetic studies (4.0%) focused on the origin of SARS-CoV-2 while the topics of molecular studies varied. Nine of 22 epidemiological studies focused on estimating the basic reproduction number of COVID-19 infection (R0). Of all identified guidance/guidelines (n = 35), only ten fulfilled the strict principles of evidence-based practice. The number of articles published per day increased rapidly until the end of January.
Conclusion
The number of articles on COVID-19 steadily increased before 6 February 2020. However, they lack diversity and are almost non-existent in some study fields, such as clinical research. The findings suggest that evidence for the development of clinical practice guidelines and public health policies will be improved when more results from clinical research becomes available.
Keywords: COVID-19, SARS-CoV-2, scoping review, communicable diseases, pandemics, coronavirus infections, global health emergency
Introduction
A new type of coronavirus (severe acute respiratory syndrome coronavirus 2; SARS-CoV-2) that began in Wuhan, China in late 2019 has spread across the world since then. The virus has caused an outbreak of viral pneumonia, which has been named Coronavirus disease (COVID-19). As of 24:00 on 6 February 2020, over 31,000 cases and 636 deaths had been confirmed in China [1]. Furthermore, more than 1,770,000 cases had been diagnosed in 213 countries, areas or territories as at 13 April 2020 [2]. On 23 January 2020, Chinese authorities imposed a lockdown of Wuhan [3]. On 30 January 2020, the World Health Organization (WHO) declared the outbreak a Public Health Emergency of International Concern (PHEIC) [4] and on 11 March 2020, a pandemic [5].
The WHO [6-9], the United States (US) Centers for Disease Control and Prevention (CDC) [10,11], the European Centre for Disease Prevention and Control (ECDC) [12,13] as well as Chinese researchers have issued several guidance documents or guidelines to help address the outbreaks. Meanwhile, many scientific journals have rapidly published a number of articles, comments, editorials and perspectives related to COVID-19. It may however be challenging for the global research community to find all the available evidence: many of the first studies on COVID-19 were published in Chinese, and because of the rapidly developing situation, the latest studies are often available on websites or preprint servers only [14].
Scoping reviews are regarded as a valid tool to map the available evidence on a given topic, to clarify the characteristics of body of literature, to organise the key concepts and their relationship and to analyse knowledge gaps [15]. The methodology continues to be developed, and a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRSIMA) extension for Scoping Reviews (PRISMA-SCR) including reporting guidance was published in 2018 [16]. Given the urgency of the COVID-19 epidemic and the need to understand and access information about it, a scoping review was considered suitable for the situation. We therefore conducted this scoping review to help identify research gaps related to this new viral disease and propose recommendations for future research on COVID-19.
Methods
Search strategy
We performed a systematic search of MEDLINE via PubMed, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang Data and China Biology Medicine (CBM) on 27 February 2020 with the terms “COVID-19” OR “SARS-CoV-2” OR “2019 novel coronavirus” OR “2019-nCoV” OR “Wuhan coronavirus” OR “novel coronavirus” OR “Wuhan seafood market pneumonia virus” OR “Wuhan virus”, published between 1 December 2019 and 6 February 2020 (see Supplement S1 for details of search strategies). Because of potential delays in indexing of databases, we also searched selected infectious disease journals (Supplementary Table S1). We also searched Google Scholar; the official websites of WHO (https://www.who.int/), US CDC (https://www.cdc.gov/), ECDC (https://www.ecdc.europa.eu/en), Public Health England (PHE) (https://www.gov.uk/government/organisations/public-health-england); some preprint servers, including BioRxiv (https://www.biorxiv.org/), ChemRxiv (https://chemrxiv.org/), medRxiv (https://www.medrxiv.org/) and SSRN (https://www.ssrn.com/index.cfm/en/); and reference lists of the identified articles to find reports of additional studies.
Inclusion and exclusion criteria
We included all literature related to COVID-19 published in English and Chinese between 1 December 2019 and 6 February 2020 without restrictions, including guidance/guidelines, reviews, clinical studies, basic research, epidemiological studies and comments. Documents and guidance/guidelines posted by international organisations, government institutions, associations and societies were also included. We excluded news reports that were not published in scientific journals, and articles where we failed to access full text despite contacting the authors.
Article selection and data extraction
Two reviewers (ML and XL) screened all titles, abstracts and full texts independently and solved disagreements by consensus or consultation with a third reviewer. Then the following information was extracted: (i) title, (ii) first author, (iii) whether peer-reviewed or not, (iv) journal, (v) publication or posted date, (vi) first author’s country (or international organisation), (vii) type of article/study and (viii) topic. The details are shown in Supplementary Table S2.
Data analysis
We conducted a descriptive analysis of the characteristics of the included literature. We described the source where we found the article, publication date, type of article/study, and topic of article/study or guidance/guideline on COVID-19 to examine the existing gaps in research. We categorised the literature into guidance/guidelines and consensus statements, reviews, clinical studies (including randomised controlled trials and observational studies), basic research, epidemiological studies, editorial comments on COVID-19 and other categories if identified. We conducted this scoping review in accordance with the PRISMA-ScR Checklist [16] (Supplementary Table S3).
Results
Search results
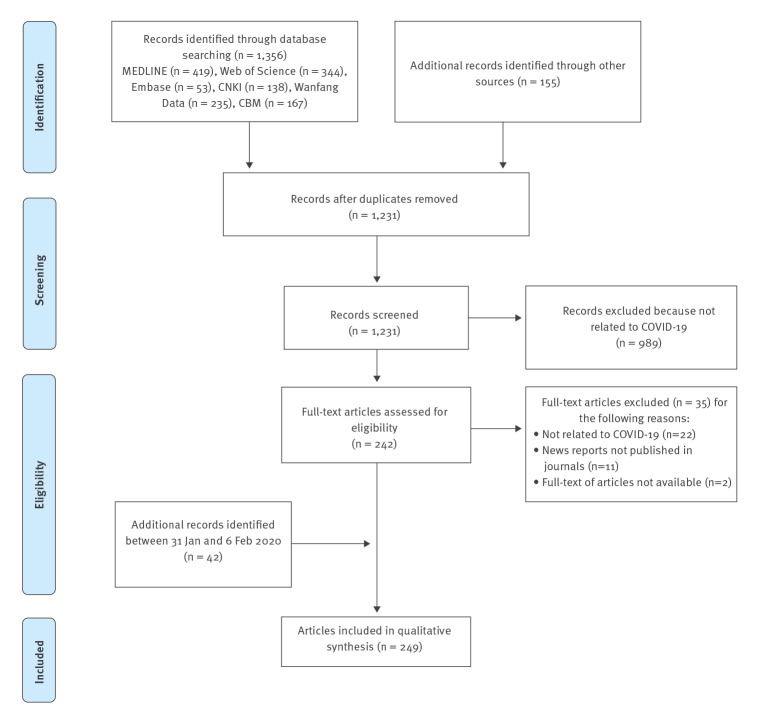
We identified 1,511 records, 280 of which were excluded as duplicates. Title and abstract screening were conducted for the remaining 1,231 articles, 989 of which were excluded because of being unrelated to COVID-19. For two articles, we failed to access the full text after contacting the authors. We retrieved the full texts of the 242 remaining articles. After further screening and supplementary searching of articles published or posted between 31 January 2020 and 6 February 2020, we identified an additional 42 articles and a total of 249 articles were included in the review (Figure 1).
Figure 1.
Flowchart of selection process for the scoping review of coronavirus disease (COVID-19) articles/studies and results, 1 December 2019–6 February 2020
CBM: China Biology Medicine; CNKI: China National Knowledge Infrastructure.
Characteristics of included articles/studies
Of the 249 included articles/studies, 147 (59.0%) were from China. The article/study type varied vastly, which we broadly characterised into 11 types (Table 1). Of these, guidance/guidelines and consensuses statements were the most common (n = 56; 22.5%).
Table 1. Characteristics of the coronavirus disease (COVID-19) articles/studies included in the scoping review, 10 January–6 February 2020 (n = 249).
| Characteristic | Number of articles/studies | Percentage (%) | |
|---|---|---|---|
| Publication platform | Journal | 192 | 77.1 |
| Other than journala | 57 | 22.9 | |
| Journal (n = 192) | The Lancet | 13 | 6.8 |
| Journal of Medical Virology | 12 | 6.3 | |
| New Medicine | 9 | 4.7 | |
| The New England Journal of Medicine | 9 | 4.7 | |
| Eurosurveillance | 8 | 4.2 | |
| Journal of Traditional Chinese Medicine | 7 | 3.6 | |
| British Medical Journal (BMJ) | 7 | 3.6 | |
| Radiology | 5 | 2.6 | |
| Travel Medicine and Infectious Disease | 5 | 2.6 | |
| Chinese Nursing Research | 5 | 2.6 | |
| Chinese Journal of Tuberculosis and Respiration | 4 | 2.1 | |
| Nature | 4 | 2.1 | |
| Chinese Journal of Contemporary Paediatrics | 3 | 1.6 | |
| Emerging Microbes and Infections | 3 | 1.6 | |
| The Journal of the American Medical Association (JAMA) | 3 | 1.6 | |
| Journal of Hospital Infection | 3 | 1.6 | |
| Journal of Travel Medicine | 3 | 1.6 | |
| Herald of Medicine | 3 | 1.6 | |
| Chinese Journal of Emergency Medicine | 3 | 1.6 | |
| Chinese Journal of Paediatrics | 3 | 1.6 | |
| Other | 80 | 41.7 | |
| First author’s country or international organisation | China | 147 | 59.0 |
| United States | 33 | 13.3 | |
| United Kingdom | 16 | 6.4 | |
| WHO | 10 | 4.0 | |
| Canada | 7 | 2.8 | |
| Germany | 6 | 2.4 | |
| Other | 30 | 12.1 | |
| Publication or posted date | 10–15 Jan | 6 | 2.4 |
| 16–20 Jan | 7 | 2.8 | |
| 21–25 Jan | 38 | 15.3 | |
| 26–31 Jan | 93 | 37.3 | |
| 1–6 Feb | 105 | 42.2 | |
| Type of article/study | Guidance/guideline or consensus statement | 56 | 22.6 |
| Review | 39 | 15.7 | |
| Basic research | 35 | 14.1 | |
| Letter | 25 | 10.0 | |
| Epidemiological studyb | 22 | 8.8 | |
| Editorial | 20 | 8.0 | |
| Comments | 11 | 4.4 | |
| News item | 9 | 3.6 | |
| Case report | 9 | 3.6 | |
| Cross-sectional study | 7 | 2.8 | |
| Case series | 5 | 2.0 | |
| Other | 11 | 4.4 | |
| Topic | Prevention and control | 33 | 13.3 |
| Outbreak reporting | 30 | 12.0 | |
| Genetics | 22 | 8.8 | |
| Transmissibility | 22 | 8.8 | |
| Clinical features | 21 | 8.4 | |
| Diagnosis and treatment | 19 | 7.6 | |
| Molecular biology | 15 | 6.0 | |
| Management | 14 | 5.6 | |
| Characteristics of SARS-CoV-2c | 11 | 4.4 | |
| Drug-relatedd | 8 | 3.2 | |
| Traditional Chinese medicine | 8 | 3.2 | |
| Lessons and challenges | 7 | 2.8 | |
| Transmission pattern | 7 | 2.8 | |
| Surveillance and screening | 5 | 2.0 | |
| Mental health | 4 | 1.6 | |
| Other | 23 | 9.2 | |
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; WHO: World Health Organization.
a Includes the websites of WHO, United States Centers for Disease Control and Prevention (US CDC), European Centre for Disease Prevention and Control (ECDC) and Public Heath England (PHE), and preprint servers.
b Other than cross-sectional studies.
c Includes reviews and correspondence that discussed the characteristics of the virus in general.
d Other than traditional Chinese medicine.
Sources of articles/studies
Of all included articles/studies, 192 (77.1%) were published in peer-reviewed journals, 35 (14.1%) were posted on preprint servers and 22 (8.8%) were published on the official websites of public health organisations. The journal with the highest number of articles was The Lancet, with 13 (6.8%) published articles. Of preprint articles, most (n = 28) were posted on BioRxiv. Articles published on official websites were mainly COVID-19 guidance/guidelines, including 10 WHO interim guidance documents, nine US CDC interim guidelines/guidance documents, two ECDC guidance documents and one Communicable Diseases Network Australia (CNDA) guideline.
Publication date
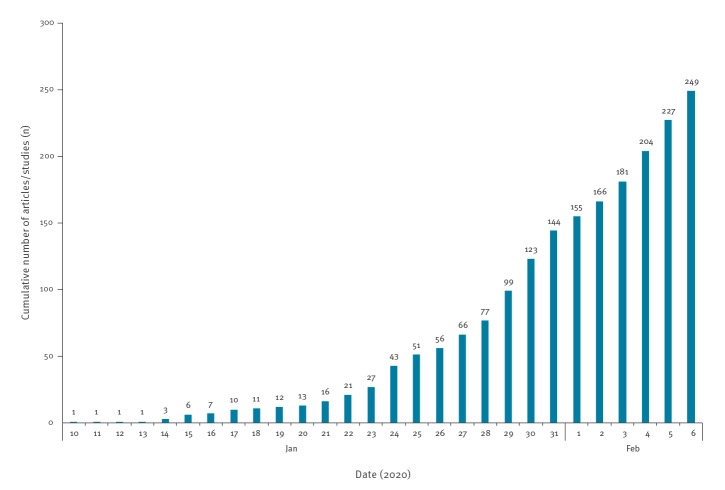
Figure 2 shows the cumulative number of articles published daily between 10 January 2020 and 6 February 2020. As at 6 February 2020, the number of articles on COVID-19 had been steadily increasing. Of the 192 articles that were published in peer-reviewed journals, the highest number of journal publications on a single day was on 30 January, with 24 articles (12.5%). For the 35 preprints, the number posted per day rose steadily from 19 January 2020 to 6 February 2020.
Figure 2.
Cumulative number of coronavirus disease (COVID-19)-related articles/studies included in the scoping review, 10 January–6 February 2020 (n = 249)
Type of article/study
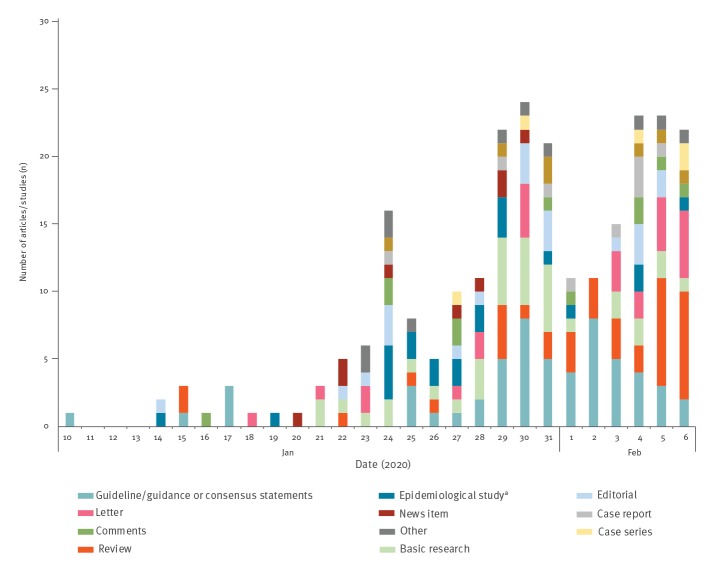
The types of articles/studies published on each day are shown in Figure 3. The daily number of guidance/guidelines peaked between 29 January and 3 February whereas the number of published reviews showed an increasing trend since 29 January 2020. Only one systematic review was identified [17]. We found no randomised controlled studies or cohort studies.
Figure 3.
Number of coronavirus disease (COVID-19)-related articles/studies published per day according to type, 10 January–6 February 2020 (n = 249)
a Including cross-sectional studies.
Topics
The different types of articles/studies focused on different topics. The basic research could be divided broadly into two categories: 21 genetic studies and 12 molecular biology studies. Ten genetic studies traced the origin of SARS-CoV-2 and tried to determine the possible virus reservoir. Among these, most suggested that SARS-CoV-2 evolved from a bat-CoV, namely bat-SL-CoVZC45, bat-SL-CoVZXC21, bat-SL-CoVZX45 and bat-CoV-RaTG13 as potential candidates [18-26]. However, Ji et al. [18] found snakes to be the most probable reservoir for SARS-CoV-2 while Guo et al. [26] suggested mink could be a candidate reservoir. Of the molecular studies, five [27-31] showed that the key receptor of SARS-CoV-2 is angiotensin converting enzyme 2 (ACE2), which is highly expressed in lung type II alveolar cells (AT2) [27], positive cholangiocytes [29], upper oesophagus, stratified epithelial cells and absorptive enterocytes from ileum and colon [30]. The other studies included an assessment of the cross-reactivity of anti-SARS-CoV antibodies with SARS-CoV-2 spike protein [32], and SARS-CoV-2 main proteases [33,34].
The main topic of epidemiological studies was the estimation of the transmissibility of COVID-19. The value of the basic reproduction number (R0) varied across studies [35-43], however, all estimated it to be higher than one, which indicates the potential for sustained human-to-human transmission. According to the nine articles [35-43], R0 ranges between 2.2 and 3.9. Some studies showed that the transmissibility of SARS-CoV-2 is comparable to [37,44] or even higher [39] than SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV). In addition, studies focused on the disease burden associated with COVID-19 [45] and the global patterns of disease dispersion [46,47].
Most reviews on COVID-19 gave a brief summary of the clinical features [48-51] and the characteristics of SARS-CoV-2 [52-54], as well as recommendations on how to prevent and control [55-60] this novel pneumonia. A systematic review [17] explored the possibility of using lopinavir/ritonavir (LPV/r) to treat COVID-19, with the results supporting the use of LPV/r as a part of an experimental regimen for COVID-19 pneumonia treatment. Clinical features were reported in 21 studies [48-51,61-77]. The main symptoms of patients with COVID-19 at onset were found to be fever and cough, with a reduced lymphocyte count, which is similar to previous beta coronavirus infections [78,79].
Seventeen of the 56 editorials, comments and letters [80-96] were first reports or comments on the situation of the COVID-19 epidemic. Some [97-101] also briefly introduced the general information and characteristics of the new virus. The mapping of article/study type and topics, as well as associated gaps, is shown in Table 2.
Table 2. Mapping of coronavirus disease (COVID-19) article/study types and topics, 10 January–6 February 2020 (n = 249).
| Topic | Article type | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Guidance/guideline or consensus statement (n) | Review (n) | Basic research (n) | Letter (n) | Epidemiological studya (n) | Editorial (n) | Comments (n) | News item (n) | Case report (n) | Cross-sectional study (n) | Case series (n) | Otherb (n) | |
| Prevention and control | 23 | 6 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Outbreak reporting | 0 | 0 | 0 | 3 | 0 | 10 | 4 | 9 | 0 | 0 | 0 | 4 |
| Genetics | 0 | 1 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Transmissibility | 0 | 1 | 0 | 4 | 13 | 3 | 0 | 0 | 0 | 0 | 1 | 0 |
| Clinical features | 0 | 4 | 0 | 2 | 0 | 0 | 2 | 0 | 5 | 2 | 4 | 2 |
| Diagnosis and treatment | 11 | 3 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 |
| Molecular biology | 0 | 2 | 12 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Management | 12 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Characteristics of SARS-CoV-2 | 0 | 4 | 0 | 1 | 0 | 3 | 1 | 0 | 1 | 0 | 0 | 1 |
| Drug-relatedc | 0 | 2 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Traditional Chinese medicine | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Lessons and challenges | 0 | 3 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Transmission pattern | 0 | 0 | 0 | 2 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Surveillance and screening | 2 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mental health | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 |
| Otherd | 8 | 3 | 0 | 1 | 3 | 3 | 0 | 0 | 0 | 3 | 0 | 2 |
a Other than cross-sectional studies.
b Includes perspectives, case-control study and investigation protocols.
c Other than traditional Chinese medicine.
d Guidance/guideline or consensus statement: guidance for laboratory biosafety, caring and travellers, and national capacity review tools; review: reviews on human resources of healthcare, the causes and counter-measures of Wuhan ‘stigma’, and public health; letter: outbreak assessment; epidemiology study: studies on disease burden, the number of unreported cases, and infection fatality; editorial: journal’s opinion on matters related to COVID-19, and incidence rate estimation; cross-sectional study: hazard vulnerability analyses, epidemiology reports, and studies on public attitudes and perception; other: investigation protocol.
Guidance/guidelines and consensus statements
Of the 56 published guidance/guidelines and consensuses statements, 35 were guidance/guidelines. Nine of the 35 addressed the treatment and management of COVID-19 infection, eight addressed prevention and five addressed diagnostics. Ten of the guidance/guidelines were interim guidance documents issued by the WHO, including those on COVID-19 prevention, surveillance, assessment, care, management and mask use [6-9,102-107]. The US CDC published nine interim guidance/guidelines documents for evaluating, preventing and managing the new coronavirus [10,11,108-114]. In addition, ECDC published two guidance documents about COVID-19 patient care and the management of persons having had contact with SARS-CoV-2 cases [12,13]. Chinese researches also published 14 rapid-advice guidance/guidelines documents on diagnosis, prevention and management of COVID-19, all of which were interim guidance/guidelines documents developed by hospitals [115-128].
Only eight of the guidance documents/guidelines formed a guideline development group (GDG) [129]; the recommendations of 15 guidance documents/guidelines, including six developed by the WHO, were difficult to distinguish. Only ten guidance/guidelines fulfilled the strict principles of evidence-based practice and cited reference documents, which were mainly epidemic reports, government documents, and indirect evidence related to SARS-CoV or MERS-CoV [6,7,105,116-118,120,122,125,126]. Only two guidelines, both developed by Chinese researchers, were graded using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [116,117]. Among the 35 guidance/guidelines, one [115] was completely on Traditional Chinese medicine and one [116] covered Chinese medicine. One Australian guideline [130] was adapted from SARS-CoV guidelines.
Discussion
Our scoping review shows that while the number of articles on COVID-19 has been constantly increasing, as at 6 February, there were still clear gaps in several study types and research fields. We identified that some study types, in particular randomised controlled trials and cohort studies, were still non-existent before 6 February. According to a preliminary search of the Cochrane Network database up to 10 April 2020, the number of randomised controlled trials (RCTs) (n = 8) and observational studies (n = 42) still remains low [131].
We also found that there were only a few studies on clinical practice, making it difficult to develop clinical practice guidelines and health policies. The reason for the gaps in this area may be the rapid development of the outbreak and limited understanding of the new virus and the disease caused by it. Moreover, it takes time to conduct clinical research. When facing a public health emergency with a previously unknown cause, researchers should conduct studies on whether some clinical practice and public health interventions from other public health emergencies can be used as indirect evidence. However, we identified no such studies in our review.
We found that 14% of the studies related to COVID-19 were posted on preprint servers. This approach of sharing research as quickly as possible is very reasonable, especially in the case of such public health emergency. Previous studies have shown that preprints can accelerate progress in handling outbreaks of infectious disease [132,133].
The research topics in different types of articles/studies had both similarities and differences. Basic research was mostly focused on exploring the origin and reservoirs of the new virus, while epidemiological studies mainly focused on its transmissibility. Reviews and reports provided more general information of the virus and the outbreak, while guidance/guidelines included recommendations on how to prevent and control it.
Clinical practice guidelines are statements that include recommendations intended to optimise patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options [134]. Clinical practice guidelines can inform healthcare workers' actions [134], and, especially when public health emergencies occur, rapid advice guidelines can guide clinicians in terms of how to perform related work [135]. After the outbreak of COVID-19, the WHO, US CDC and ECDC released guidance/guidelines as soon as possible, as did several Chinese institutions. However, most of these documents did not establish formal guideline development groups, and they did not fulfil the strict principles of evidence-based practice. For example, most guidance/guidelines did not grade the quality of evidence and strength of recommendations, and thus owed to the emerging crisis, such guidance/guidelines need to be considered with these limitations in mind. In 2007, the WHO published guidance about the process of developing rapid advice guidelines [129], stating that when a public health emergency occurs, a rapid review is needed and the development time should not exceed 6 months [135]. However, considering the limited time to set up panels, this could be a challenge for guidance/guideline developers. Nonetheless, we still expect guidance/guideline developers to establish formal development groups and fulfil the evidence-based practice principles.
Our scoping review can help researchers identify research gaps so as to conduct research to fill these gaps. For example, in the current situation, a systematic review to estimate the incubation period or research on new drugs or treatments, would be of great importance. This scoping review has several strengths. We performed a systematic search of a comprehensive set of sources, including databases, preprint servers, and official websites of international organisations and associations at the early stage of the pandemic. Furthermore, our large sample size is sufficient to illustrate the state of research and identify research gaps related to COVID-19 at the onset of the pandemic.
This study also has some limitations. Because of the delay in indexing, some articles published as at 6 February 2020 may not have been identified. Also, because our retrieval time was only until this date, articles published or posted after this date, of which there have been many, have not been included in the analysis. As some preprints, guidance/guidelines and disease control plans are constantly updated, the publication date we extracted may not be the time of their first publication time. Also, we did not assess the quality of the included literature because of diversity of the types of included articles. Another limitation of our study was that it only included articles published in English and Chinese, which could introduce publication bias. However, as the epidemic was most heavily affecting China until early February, it is reasonable to expect that literature published in English and Chinese up until this point in time covered the majority of the available knowledge. Finally, we were unable to access the full texts of two articles despite contacting the authors. However, compared with the total number of articles included in the review, we anticipate that the exclusion of these two articles is unlikely to have a major impact.
Conclusion
This scoping review shows the state of literature published or posted online related to COVID-19 as at 6 February 2020. The number of articles in this field has steadily increased since the outbreak became evident. However, the types of studies lacked diversity, especially clinical studies. More clinical research is needed, but in the rapidly evolving global pandemic, we encourage researchers to continuously review the latest literature, to take into account the latest available evidence and avoid overlapping work, and to improve evidence for the development of clinical practice guidelines and public health policies.
Acknowledgements
Funding statement: 2020 Key R & D project of Gansu Province; Special funding for prevention and control of emergency of COVID-19 from Key Laboratory of Evidence Based Medicine and Knowledge Translation of Gansu Province (No. GSEBMKT-2020YJ01).
The members of the COVID-19 evidence and recommendations working group: Xiao Liu (Evidence-based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China); Nan Yang (Evidence-based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China); Shuya Lu (Sichuan Provincial People’s hospital, Chengdu, China ); Peipei Du ( School of Public Health, Chengdu Medical College, Chengdu, China); Yanfang Ma (Evidence-based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China); Zijun Wang (Evidence-based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China); Qianling Shi (The First School of Clinical Medicine, Lanzhou University, Lanzhou, China); Hairong Zhang (Evidence-based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China); Qiangqiang Guo (School of Public Health, ShanXi Medical University, Taiyuan,China); Yuting Yang (Children's Hospital of Chongqing Medical University, Chongqing, China); Bo Yang (Children's Hospital of Chongqing Medical University, Chongqing, China); Shouyuan Wu (School of Public Health, Lanzhou University, Lanzhou, China); Xiaoqin Wang (Michael G. DeGroote Institute for Pain Research and Care, McMaster University, Hamilton, Ontario, Canada).
Supplementary Data
Supplementary Data
Supplementary Data
Supplementary Data
Conflict of interest: None declared.
Authors’ contributions: All authors have read and agree to the published version of the manuscript. Conceptualisation, YC and XW; methodology, ML, XL and JE; software, YL, MR and JW; data extraction, QW, SZ, MR, XZ, LW, QZ and SY; formal analysis, XL and ML; resources, ML and WL; writing—original draft preparation, ML, XL, WM and XQ; writing—review and editing, YX, XY, YC, XW, SY, XF, WM, JE, EL and XQ; visualisation, ML and XL; supervision, YC and XW; project administration, YC; funding acquisition, YC.
References
- 1.National Health Commission of the People’s Republic of China (NHC PRC). Feb 7: Daily briefing on novel coronavirus cases in China. Beijing: NHC PRC; 7 Feb 2020. Available from: http://en.nhc.gov.cn/2020-02/07/c_76323.htm
- 2.World Health Organization (WHO). Coronavirus disease (COVID-19) outbreak situation. Geneva: WHO. [Accessed 14 April 2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 3.National Health Commission of the People’s Republic of China (NHC PRC). Timeline of China's fight against the novel coronavirus. Beijing: NHC PRC; 24 Jan 2020. Available from: http://en.nhc.gov.cn/2020-03/20/c_78021.htm
- 4.World Health Organization (WHO). Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). Geneva: WHO; 30 Jan 2020. Available from: https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)
- 5.World Health Organization (WHO). WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Geneva: 11 Mar 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 6.World Health Organization (WHO). Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected. Interim guidance. Geneva: WHO. [Accessed 25 Jan 2020]. Available from: https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125
- 7.World Health Organization (WHO). Home care for patients with suspected novel coronavirus (nCoV) infection presenting with mild symptoms and management of contacts. Interim guidance. Version 1. Geneva: WHO; 20 Jan 2020. Available from: https://reliefweb.int/sites/reliefweb.int/files/resources/20200120-ncov-home-care-infected-patients.pdf
- 8.World Health Organization (WHO). Risk communication and community engagement (RCCE) readiness and initial response for novel coronaviruses (nCoV): Interim guidance. Geneva: WHO; 26 Jan 2020. Available from: https://apps.who.int/iris/handle/10665/330377
- 9.World Health Organization (WHO). Laboratory testing of 2019 novel coronavirus (2019-nCoV) in suspected human cases. Interim guidance. Geneva: WHO; 17 Jan 2020. Available from: https://www.healthynewbornnetwork.org/hnn-content/uploads/Interim-laboratory-eng.pdf
- 10.United States Centers for Disease Control and Prevention (US CDC). Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed 2019 Coronavirus Disease 2019 (COVID-19)in Healthcare Settings. Atlanta: US CDC. [Accessed 6 Feb 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html
- 11.United States Centers for Disease Control and Prevention (US CDC). Interim Guidance for Implementing Home Care of People Not Requiring Hospitalization for Coronavirus Disease 2019 (COVID-19). Atlanta: US CDC. [Accessed 6 Feb 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-home-care.html
- 12.European Centre for Disease Prevention and Control (ECDC). Contact tracing: Public health management of persons, including healthcare workers, having had contact with COVID-19 cases in the European Union. Stockholm: ECDC. [Accessed 6 Feb 2020]. Available from: https://www.ecdc.europa.eu/en/publications-data/public-health-management-persons-having-had-contact-novel-coronavirus-cases
- 13.European Centre for Disease Prevention and Control (ECDC). Infection prevention and control for the care of patients with 2019-nCoV in healthcare settings. Stockholm: ECDC; 2 Feb 2020. Available from: https://www.ecdc.europa.eu/en/publications-data/infection-prevention-and-control-care-patients-2019-ncov-healthcare-settings
- 14. Johansson MA, Reich NG, Meyers LA, Lipsitch M. Preprints: An underutilized mechanism to accelerate outbreak science. PLoS Med. 2018;15(4):e1002549. 10.1371/journal.pmed.1002549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467-73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 17.Jiang H, Deng HF, Wang Y, Liu Z, Sun MW, Zhou P, et al. [The possibility of using Lopinave/Litonawe (LPV/r) as treatment for novel coronavirus 2019-nCov pneumonia: a quick systematic review based on earlier coronavirus clinical studies]. Chin J Emerg Med. 2020;29(2):182-6. Chinese. Available from: http://rs.yiigle.com/yufabiao/1179572.htm
- 18. Ji W, Wang W, Zhao X, Zai J, Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 2020;92(4):433-40. 10.1002/jmv.25682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JY, Shi JS, Qiu DA, Liu C, Li X, Zhao Q, et al. [Bioinformatics analysis of the 2019 novel coronavirus genome]. J Bioinform. 2020;01(01):1-10. Chinese. Available from: http://kns.cnki.net/kcms/detail/23.1513.Q.20200120.0839.002.html
- 20. Wu F, Zhao S, Yu B, Chen YM, Wang W, Hu Y, et al. Complete genome characterisation of a novel coronavirus associated with severe human respiratory disease in Wuhan, China. bioRxiv. 2020;919183: (Preprint). Available from: 10.1101/2020.01.24.919183 [DOI] [Google Scholar]
- 21. Dong N, Yang X, Ye L, Chen K, Chan EWC, Yang M, et al. Genomic and protein structure modelling analysis depicts the origin and infectivity of 2019-nCoV, a new coronavirus which caused a pneumonia outbreak in Wuhan, China. bioRxiv. 2020;913368: (Preprint). Available from: 10.1101/2020.01.20.913368 [DOI] [Google Scholar]
- 22. Paraskevis D, Kostaki EG, Magiorkinis G, Panayiotakopoulos G, Sourvinos G, Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect Genet Evol. 2020;79:104212. 10.1016/j.meegid.2020.104212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stoermer M. Homology Models of Wuhan Coronavirus 3CLpro Protease. ChemRxiv. 2020;11637294: (Preprint). Available from: 10.26434/chemrxiv.11637294.v1 [DOI] [Google Scholar]
- 24. Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221-36. 10.1080/22221751.2020.1719902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramaiah A, Arumugaswami V. Insights into Cross-species Evolution of Novel Human Coronavirus 2019-nCoV and Defining Immune Determinants for Vaccine Development. bioRxiv. 2020;925867: (Preprint). Available from: 10.1101/2020.01.29.925867 [DOI] [Google Scholar]
- 26. Guo Q, Li M, Wang CH, Wang PH, Fang ZC, Tan J, et al. Host and infectivity prediction of Wuhan 2019 novel coronavirus using deep learning algorithm. bioRxiv. 2020;914044: (Preprint). Available from: 10.1101/2020.01.21.914044 [DOI] [Google Scholar]
- 27. Zhao Y, Zhao ZX, Wang YJ, Zhou YQ, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv. 2020;919985: (Preprint). Available from: 10.1101/2020.01.26.919985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127-20. 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chai XQ, Hu LF, Zhang Y, Han WY, Lu Z, Ke A, et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv. 2020;931766: (Preprint). Available from: 10.1101/2020.02.03.931766 [DOI] [Google Scholar]
- 30. Zhang H, Kang ZJ, Gong HY, Xu D, Wang J, Li ZF, et al. The digestive system is a potential route of 2019-nCov infection a bioinformatics analysis based on single-cell transcriptomes. bioRxiv. 2020;927806: (Preprint). Available from: 10.1101/2020.01.30.927806 [DOI] [Google Scholar]
- 31. Yang M, Zhao JM, Zhang Z. More Than Pneumonia, The Potential Occurrence of Multiple Organ Failure in 2019 Novel Coronavirus Infection. SSRN. 2020;3532272: (Preprint). Available from: 10.2139/ssrn.3532272 [DOI] [Google Scholar]
- 32. Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9(1):382-5. 10.1080/22221751.2020.1729069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu ZJ, Peng C, Shi YL, Zhu ZD, Mu KJ, Wang XY, et al. Nelfinavir was predicted to be a potential inhibitor of 2019-nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation. bioRxiv. 2020;921627: (Preprint). Available from: 10.1101/2020.01.27.921627 [DOI] [Google Scholar]
- 34. Li Y, Zhang JY, Wang N, Li HO, Shi Y, Guo G, et al. Therapeutic Drugs Targeting 2019-nCoV Main Protease by High-Throughput Screening. bioRxiv. 2020;922922: (Preprint). Available from: 10.1101/2020.01.28.922922 [DOI] [Google Scholar]
- 35.Zhou T, Liu QH, Yang ZM, Liao JY, Yang KX, Bai W, et al. [Preliminary Prediction of the basic reproduction number of the Wuhan novel coronavirus 2019‐nCoV]. J Evid Based Med. 2020;20(3): 359-364. Chinese. Available from: 10.7507/1672-2531.202001118 [DOI] [PMC free article] [PubMed]
- 36. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689-97. 10.1016/S0140-6736(20)30260-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen MW, Peng ZH, Xiao YN, Zhang L. Modelling the epidemic trend of the 2019 novel coronavirus outbreak in China. bioRxiv. 2020;916726: (Preprint). Available from: 10.1101/2020.01.23.916726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Riou J, Althaus CL. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020;25(4):2000058. 10.2807/1560-7917.ES.2020.25.4.2000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Read J M, Bridgen J R, Cummings D A, Ho A, Jewell C P. Novel coronavirus 2019-nCoV: early estimation of epidemiological parameters and epidemic predictions. medRxiv. 2020;20018549: (Preprint). Available from: 10.1101/2020.01.23.20018549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Majumder MS, Mandl KD. Early Transmissibility Assessment of a Novel Coronavirus in Wuhan, China. SSRN. 2020;3524675: (Preprint). Available from: 10.2139/ssrn.3524675 [DOI] [Google Scholar]
- 41. Liu T, Hu JX, Kang M, Li LF, Zhong HJ, Xiao JP, et al. Transmission Dynamics of 2019 Novel Coronavirus (2019-nCoV). SSRN. 2020;3526307: (Preprint). Available from: 10.2139/ssrn.3526307 [DOI] [Google Scholar]
- 42. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199-207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang C, Wang M. Origin time and epidemic dynamics of the 2019 novel coronavirus. bioRxiv. 2020;919688: (Preprint). Available from: 10.1101/2020.01.25.919688v3 [DOI] [Google Scholar]
- 44. Zhang X, Wu K, Yue X, Zhu Y, Wu J. Inhibition of SARS-CoV gene expression by adenovirus-delivered small hairpin RNA. Intervirology. 2007;50(2):63-70. 10.1159/000097391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ming W, Huang J, Zhang CJP. Breaking down of healthcare system: Mathematical modelling for controlling the novel coronavirus (2019-nCoV) outbreak in Wuhan, China. bioRxiv. 2020;922443: (Preprint). Available from: 10.1101/2020.01.27.922443v1 [DOI] [Google Scholar]
- 46. Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K. Potential for global spread of a novel coronavirus from China. J Travel Med. 2020;27(2):taaa011. 10.1093/jtm/taaa011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J Travel Med. 2020;27(2):taaa008. 10.1093/jtm/taaa008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Habibzadeh P, Stoneman EK. The novel coronavirus: a bird’s eye view. Int J Occup Environ Med. 2020;11(2):65-71. 10.15171/ijoem.2020.1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi HS, Han XY, Fan YQ, Liang B, Yang F, Han P, et al. [Radiologic Features of Patients with 2019-nCoV Infection]. Journal of Clinical Radiology. 2020;02(06):1-8. Chinese. Available from: 10.13437/j.cnki.jcr.20200206.002 [DOI]
- 50. Fang F, Luo XP. [Facing the pandemic of 2019 novel coronavirus infections: the pediatric perspectives]. Zhonghua Er Ke Za Zhi. 2020;58(2):81-5. [DOI] [PubMed] [Google Scholar]
- 51.Li L, Liu JJ, Jin RH, Li HJ. [The legal class B infectious disease - the 2019 novel coronavirus (2019-nCoV) infected pneumonia in Wuhan, China: a review]. New Medicine. 2020;30(1):14-21. Chinese. Available from: 10.12173/j.issn.1004-5511.2020.01.06 [DOI]
- 52. Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418-23. 10.1002/jmv.25681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen YM. [Pathogenicity, prevention and control of coronaviruses]. Journal of Microbes And Infections. 2020;15(1):5-12. Chinese. Available from: http://jmi.fudan.edu.cn/CN/abstract/abstract816.shtml
- 54. Ralph R, Lew J, Zeng T, Francis M, Xue B, Roux M, et al. 2019-nCoV (Wuhan virus), a novel Coronavirus: human-to-human transmission, travel-related cases, and vaccine readiness. J Infect Dev Ctries. 2020;14(1):3-17. 10.3855/jidc.12425 [DOI] [PubMed] [Google Scholar]
- 55.Zhang WF, He JM, Tie JF, Su YX, Ren Z. [Resistance and disinfection of Coronavirus]. Chinese Journal of Disinfection. 2020;37(1):63-67. Chinese. Available from: http://kns.cnki.net/KCMS/detail/11.2672.R.20200130.1723.002.html
- 56. Liao X, Wang B, Kang Y. Novel coronavirus infection during the 2019-2020 epidemic: preparing intensive care units-the experience in Sichuan Province, China. Intensive Care Med. 2020;46(2):357-60. 10.1007/s00134-020-05954-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li D. [Community Pevention Guidance for Noval Coronavirus Pneumonia]. Herald of Medicine. 2020;39(03):315-318. Chinese. Available from: http://kns.cnki.net/kcms/detail/42.1293.R.20200203.1403.004.html
- 58.Shen Y, Lu H. [Improving the understanding of diagnosis and treatment of novel coronavirus infection]. Chin J Infect Dis. 2020;38(01):6-8. Chinese. Available from: http://rs.yiigle.com/CN311365202001/1183535.htm
- 59.Gong RR, Zhen Y, Wang YF, Tong F, Bai ZG, Campbell China Network. [The public perceptions of non-pharmaceutical interventions for reducing transmission of respiratory infection: an evidence policy brief for social collaborative control of the new coronavirus (2019-nCoV) infected pneumonia]. New Medicine. 2020;30(02):89-93. Chinese. Available from: 10.12173/j.issn.1004-5511.2020.02.02 [DOI]
- 60.Yang Y, Cai HH, Geng Y. [Application of self-made protective face shield in protection of novel coronavirus pneumonia]. Infection, Inflammation, Repair. 2020; 02(02): 1-2. Chinese. Available from: http://kns.cnki.net/kcms/detail/11.5225.R.20200202.1841.002.html
- 61. Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill. 2020;25(5). 10.2807/1560-7917.ES.2020.25.5.2000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ryu S, Chun BC, Korean Society of Epidemiology 2019-nCoV Task Force Team An interim review of the epidemiological characteristics of 2019 novel coronavirus. Epidemiol Health. 2020;42:e2020006. 10.4178/epih.e2020006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lei J, Li J, Li X, Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(1):18. 10.1148/radiol.2020200236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kanne JP. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020;295(1):16-7. 10.1148/radiol.2020200241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514-23. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li L, Ren M, Zhang Y, Li W, Zhao H, Liang L, et al. [Lung CT image of a confirmed case of the 2019 novel coronavirus (2019-nCoV) infected pneumonia (With differential diagnosis of the SARS)]. New Medicine. 2020;30(1):4-6. Chinese. Available from: 10.12173/j.issn.1004-5511.2020.01.03 [DOI]
- 67.Cai J, Wang X, Ge Y, Xia A, Chang H, Tian H, et al. [First case of 2019 novel coronavirus infection in children in Shanghai]. Chin J Pediatr. 2020;58(02):86-87. Chinese. Available from: 10.3760/cma.j.issn.0578-1310.2020.02.002 [DOI] [PubMed]
- 68. Liu P, Tan XZ. 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295(1):19. 10.1148/radiol.2020200257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pan Y, Guan H. Imaging changes in patients with 2019-nCov. Eur Radiol. 2020. 10.1007/s00330-020-06713-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology. 2020;295(1):202-7. 10.1148/radiol.2020200230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang W, Hu H, Song L, Gong X, Qu Y, Lu Z. [Image of pulmonary and diagnosis of atypical novel coronavirus (2019-nCoV) infected pneumonia: case series of 14 patients]. New Medicine. 2020;30(1):7-9. Chinese. Available from: 10.12173/j.issn.1004-5511.2020.01.04 [DOI]
- 74. Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, et al. Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295(1):210-7. 10.1148/radiol.2020200274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. An P, Chen X, Jiang X, Su J, Xiao Y, Ding Y, et al. Clinical Features of 2019 Novel Coronavirus Pneumonia Presented Gastrointestinal Symptoms But Without Fever Onset. SSRN. 2020;3532530: (Preprint). Available from: 10.2139/ssrn.3532530 [DOI] [Google Scholar]
- 76. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929-36. 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hui DSI, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264-6. 10.1016/j.ijid.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu SL, Saif L. Emerging viruses without borders: The Wuhan coronavirus. Viruses. 2020;12(2):130. 10.3390/v12020130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen P, Mao L, Nassis GP, Harmer P, Ainsworth BE, Li F. Coronavirus disease (COVID-19): The need to maintain regular physical activity while taking precautions. J Sport Health Sci. 2020;9(2):103-4. 10.1016/j.jshs.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liu SL. New virus in China requires international control effort. Nature. 2020;577(7791):472. 10.1038/d41586-020-00135-z [DOI] [PubMed] [Google Scholar]
- 84. Gralinski LE, Menachery VD. Return of the Coronavirus: 2019-nCoV. Viruses. 2020;12(2):135. 10.3390/v12020135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lippi G, Plebani M. The novel coronavirus (2019-nCoV) outbreak: think the unthinkable and be prepared to face the challenge. Diagnosis (Berl). 2020. [Epub ahead of print]. 10.1515/dx-2020-0015 [DOI] [PubMed] [Google Scholar]
- 86. Long JB, Ehrenfeld JM. The role of augmented intelligence (AI) in detecting and preventing the spread of novel coronavirus. J Med Syst. 2020;44(3):59. 10.1007/s10916-020-1536-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nkengasong J. China’s response to a novel coronavirus stands in stark contrast to the 2002 SARS outbreak response. Nat Med. 2020;26(3):310-1. 10.1038/s41591-020-0771-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jiang S, Xia S, Ying T, Lu L. A novel coronavirus (2019-nCoV) causing pneumonia-associated respiratory syndrome. Cell Mol Immunol. 2020. 10.1038/s41423-020-0372-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: Challenges for fighting the storm. Eur J Clin Invest. 2020;50(3):e13209. 10.1111/eci.13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yoo JH. The Fight against the 2019-nCoV Outbreak: an Arduous March Has Just Begun. J Korean Med Sci. 2020;35(4):e56. 10.3346/jkms.2020.35.e56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kickbusch I, Leung G. Response to the emerging novel coronavirus outbreak. BMJ. 2020;368:m406. 10.1136/bmj.m406 [DOI] [PubMed] [Google Scholar]
- 92. Eurosurveillance Editorial Team Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Euro Surveill. 2020;25(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. The Lancet Emerging understandings of 2019-nCoV. Lancet. 2020;395(10221):311. 10.1016/S0140-6736(20)30186-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tang JW, Tambyah PA, Hui DSC. Emergence of a novel coronavirus causing respiratory illness from Wuhan, China. J Infect. 2020;80(3):350-71. 10.1016/j.jinf.2020.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470-3. 10.1016/S0140-6736(20)30185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kelvin DJ, Rubino S. Fear of the novel coronavirus. J Infect Dev Ctries. 2020;14(1):1-2. 10.3855/jidc.12496 [DOI] [PubMed] [Google Scholar]
- 97. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92(4):401-2. 10.1002/jmv.25678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382(8):760-2. 10.1056/NEJMe2001126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cheng VCC, Wong SC, To KKW, Ho PL, Yuen KY. Preparedness and proactive infection control measures against the emerging novel coronavirus in China. J Hosp Infect. 2020;104(3):254-5. 10.1016/j.jhin.2020.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Duarte R, Furtado I, Sousa L, Carvalho CFA. The 2019 Novel Coronavirus (2019-nCoV): Novel Virus, Old Challenges. Acta Med Port. 2020;33(3):155. 10.20344/amp.13547 [DOI] [PubMed] [Google Scholar]
- 101.Zhang WH. [Identification of a novel coronavirus and the landscape of prevention and control of emerging infectious diseases]. Chin J Infect Dis. 2020,38:Epub ahead of print. Chinese. Available from: 10.3760/cma.j.issn.1000-6680.2020.01.002 [DOI]
- 102.World Health Organization (WHO). Surveillance case definitions for human infection with novel coronavirus (nCoV). Interim guidance v2. Geneva: WHO; 15 Jan 2020. Available from: https://www.who.int/who-documents-detail/surveillance-case-definitions-for-human-infection-withnovel-coronavirus-(ncov)
- 103.World Health Organization (WHO). Global Surveillance for human infection with novel coronavirus (2019-nCoV). Interim guidance. Geneva: WHO; 21 Jan 2020. Available from: https://www.ephi.gov.et/images/20200121-global-surveillance-for-2019-ncov.pdf
- 104.World Health Organization (WHO). Advice on the use of masks the community, during home care and in health care settings in the context of the novel coronavirus (2019-nCoV) outbreak. Interim guidance. Geneva: WHO; 29 Jan 2020. Available from: https://www.who.int/docs/default-source/documents/advice-on-the-use-of-masks-2019-ncov.pdf
- 105.World Health Organization (WHO). Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected. Interim guidance. Geneva: WHO; 28 Jan 2020. Available from: https://apps.who.int/iris/handle/10665/330893
- 106.World Health Organization (WHO). National capacities review tool for a novel coronavirus (nCoV). Geneva: WHO; 10 Jan 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/national-capacities-review-tool-for-a-novel-coronavirus-ncov.pdf
- 107.World Health Organization (WHO). Household transmission investigation protocol for 2019-novel coronavirus (2019-nCoV) infection. Geneva: WHO; 25 Jan 2020. Available from: https://www.who.int/publications-detail/household-transmission-investigation-protocol-for-2019-novel-coronavirus-(2019-ncov)-infection
- 108.United States Centers for Disease Control and Prevention (US CDC). Update and Interim Guidance on Outbreak of 2019 Novel Coronavirus (2019-nCoV) in Wuhan, China. Atlanta: US CDC; 17 Jan 2020. Available from: https://emergency.cdc.gov/han/han00426.asp
- 109.United States Centers for Disease Control and Prevention (US CDC). Update and Interim Guidance on Outbreak of 2019 Novel Coronavirus (2019-nCoV). Atlanta: US CDC; 1 Feb 2020. Available from: https://emergency.cdc.gov/han/han00427.asp
- 110.United States Centers for Disease Control and Prevention (US CDC). Lab Update: Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with 2019 Novel Coronavirus (2019-nCoV). Atlanta: US CDC. [Accessed 6 Feb 2020]. Available from: https://www.cdc.gov/csels/dls/locs/2020/interim_lab_biosafety_guidelines_for_handling_and_processing_specimens_associated_with_2019_novel_coronavirus.html
- 111.United States Centers for Disease Control and Prevention (US CDC). Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19). Atlanta: US CDC. [Accessed 6 Feb 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html
- 112.United States Centers for Disease Control and Prevention (US CDC). Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19). Atlanta: US CDC. [Accessed 6 Feb 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html
- 113.United States Centers for Disease Control and Prevention (US CDC). Interim Guidance for Ships on Managing Suspected Coronavirus Disease 2019. Atlanta: US CDC. [Accessed 6 Feb 2020]. Available from: https://www.cdc.gov/quarantine/maritime/recommendations-for-ships.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Ftravelers%2Frecommendations-for-ships.html
- 114.United States Centers for Disease Control and Prevention (US CDC). Interim Clinical Guidance for Management of Patients with Confirmed 2019 Coronavirus Disease (COVID-19). Atlanta: US CDC. [Accessed 6 Feb 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
- 115.Wang YG, Qi WS, Ma JJ, Ruan LG, Lu YR, Li XC, et al. [Clinical characteristics and syndrome differentiation of new coronavirus (2019-nCoV) pneumonia in traditional Chinese medicine]. J Tradit Chin Med. 2020,61(04),281-285. Chinese. Available from: http://kns.cnki.net/KCMS/detail/11.2166.R.20200129.1258.002.html.
- 116.Jin YH, Lin C, Cheng ZS, Cheng H, Deng T, Fan YP, et al. [A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (Standard version)]. Medical Journal of Chinese People's Liberation Army. 2020;45(1):1-21. Chinese. Available from: http://www.cnki.com.cn/Article/CJFDTotal-JFJY202001002.htm. [DOI] [PMC free article] [PubMed]
- 117.Li SY, Huang WZ, Liao XL, Li DD, Du LY, Song JJ, et al. [Disease control of 2019-novel coronavirus infection in hospital: West China urgent recommendation]. Chinese Journal of Evidence-Based Medicine. 2020;20(2):125-133. Chinese. Available from: http://www.cjebm.com/article/10.7507/1672-2531.202001121
- 118.Li TS, Cao W, Weng L, Fan HW. Shi JH. [Peking Union Medical College Hospital's proposal for the diagnosis and treatment of novel coronavirus-infected pneumonia (V2.0)]. Medical Journal of Peking Union Medical College Hospital. 2020;01(01):1-5. Chinese. Available from: https://kns.cnki.net/KCMS/detail/11.5882.r.20200130.1430.002.html
- 119.Ni Z, Luo FM, Wang JM, Liu TT, Zhang TX, Yang W, et al. [Recommendations for nebulization therapy in patients with novel coronavirus pneumonia]. Chinese Journal of Respiratory and Critical Care Medicine. 2020;19(02):120-124. Chinese. Available from: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDAUTO&filename=ZGHW202002009&v=MjM5ODU3cWZZK1pyRnlubVVyckFQeXJEZWJHNEhOSE1yWTlGYllSOGVYMUx1eFlTN0RoMVQzcVRyV00xRnJDVVI=
- 120.Zhao DC, Jin RM, Liu ZS, Yin W. [Recommendation for the diagnosis and treatment of novel coronavirus infection in children in Hubei (trial first version)]. Chin J Contemp Pediatr. 2020;22(02):96-9. Chinese. Available from: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDAUTO&filename=DDKZ202002004&v=MDE1NzQ5RllJUjhlWDFMdXhZUzdEaDFUM3FUcldNMUZyQ1VSN3FmWStackZ5bm1WTHpCSVNuQWRMRzRITkhNclk= [DOI] [PMC free article] [PubMed]
- 121.Wang JH, Bao L, Shi Y. [NICU emergency prepardness plan during the epidemic novel coronavirus infection]. Chin J Contemp Pediatr. 2020;22(02):91-5. Chinese. Available from: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDAUTO&filename=DDKZ202002002&v=MTI2MjIxTHV4WVM3RGgxVDNxVHJXTTFGckNVUjdxZlkrWnJGeW5uVXI3TElTbkFkTEc0SE5ITXJZOUZab1I4ZVg=
- 122.Shi Y, Fu JH, Wang LS, Yang J, Du LZ, Zhou WH. [Novel Coronavirus Infection Prevention and Control Plan for Newborns in Perinatal Period (First Edition)]. Chin J Contemp Pediatr. 2020;22(02):87-90. Chinese. Available from: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDAUTO&filename=DDKZ202002001&v=MTkyMjdTN0RoMVQzcVRyV00xRnJDVVI3cWZZK1pyRnlublZiekpJU25BZExHNEhOSE1yWTlGWllSOGVYMUx1eFk=
- 123.Peng X, Hu Y, Yao JH, Liu W, Li C, Zhang X, et al. [Emergency nursing management for the prevention of novel coronavirus infection in oral and maxillofacial surgery]. Nursing Research. 2020;34(03):365-367. Chinese. Available from: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDAUTO&filename=SXHZ202003002&v=MTM5OTNJOUZab1I4ZVgxTHV4WVM3RGgxVDNxVHJXTTFGckNVUjdxZlkrWnJGeW5nVTd6T05qWERkTEc0SE5ITXI=
- 124.Chen T, Chen G, Guo W, Xie M, Ma K, Yan L, et al. [A quick guide to diagnosis and treatment of pneumonia with novel coronavirus infections (Third Edition)]. Herald of Medicine. 2020;39(03):305-307. Chinese. Available from: http://kns.cnki.net/kcms/detail/42.1293.r.20200130.1803.002.html
- 125. Chen ZM, Fu JF, Shu Q, Chen YH, Hua CZ, Li FB, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020;5. 10.1007/s12519-020-00345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang XY, Wu J, Lu XH, Zhang RF, Zhou Y, Li LY, et al. [Recommended strategies for maternal management of novel coronavirus (2019-nCoV) infection in Henan Province]. Herald of Medicine. 2020;55(02):200-202. Chinese. Available from: https://kns.cnki.net/KCMS/detail/41.1340.r.20200328.1323.012.html
- 127.Fudan University. [Quick screening and management of children with suspected or confirmed COVID-19: A rapid advice guideline]. Herald of Medicine. 2020;15(01):1-4. Chinese. Available from: http://www.cjebp.net/CN/Y2020/V15/I1/1
- 128.Wang YT, Huang WZ, Song JJ, Li SY, Wang YN, Du LY, et al. [Graded personal protection plan for prevention of novel coronavirus pneumonia in medial personnel in West China Hospital]. Chinese Journal of Evidence-Based Medicine. 2020;20(03):369-372. Chinese. Available from: http://www.cjebm.com/article/10.7507/1672-2531.202001120
- 129. Schünemann HJ, Hill SR, Kakad M, Vist GE, Bellamy R, Stockman L, et al. Transparent development of the WHO rapid advice guidelines. PLoS Med. 2007;4(5):e119. 10.1371/journal.pmed.0040119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Communicable Diseases Network Australia (CDNA). Coronavirus Disease 2019 (COVID-19). CDNA National Guidelines for Public Health Units. Canberra: CDNA. [Accessed 7 Feb 2020]. Available from: https://www1.health.gov.au/internet/main/publishing.nsf/Content/cdna-song-novel-coronavirus.htm
- 131.Cochrane Network. Living mapping and living network meta-analysis of Covid-19 studies. [Accessed 14 Apr 2020]. Available from: https://covid-nma.com/
- 132. Rawlinson C, Bloom T. New preprint server for medical research. BMJ. 2019;365:l2301. 10.1136/bmj.l2301 [DOI] [PubMed] [Google Scholar]
- 133. Johansson MA, Reich NG, Meyers LA, Lipsitch M. Preprints: An underutilized mechanism to accelerate outbreak science. PLoS Med. 2018;15(4):e1002549. 10.1371/journal.pmed.1002549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Committee on Standards for Developing Trustworthy Clinical Practice Guidelines/Board on Health Care Services/Institute of Medicine. Clinical practice guidelines we can trust. Washington: The National Academies Press; 2011. Available from: https://www.nap.edu/catalog/13058/clinical-practice-guidelines-we-can-trust
- 135. Garritty CM, Norris SL, Moher D. Developing WHO rapid advice guidelines in the setting of a public health emergency. J Clin Epidemiol. 2017;82:47-60. 10.1016/j.jclinepi.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.