Abstract
Background:
The myocardial contraction fraction (MCF: stroke volume to myocardial volume) is a volumetric measure of left ventricular myocardial shortening. We examined the relationship of MCF, measured by cardiac magnetic resonance imaging (cMRI), to incident cardiovascular (CV) events within the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods:
Participants (n=5,000, aged 45-84 years) underwent cMRI. Primary outcome: CVD events (myocardial infarction, resuscitated cardiac arrest, stroke, coronary heart disease: CHD death, and stroke death). Secondary outcomes: CHD and heart failure (HF) events. Cox proportional hazards regression was used to estimate the hazard ratio (HR) and 95% confidence intervals (CI) for outcomes.
Results:
There were 299 incident CVD, 188 CHD, and 151 HF events over 10.2 years. The lowest MCF quartile was associated with an increased risk for incident CVD [HR 2.42, CI: 1.58-3.72], CHD [HR 2.32, CI: 1.36-3.96] and HF events [HR 1.99, CI: 1.15-3.44]. In a model adjusted for demographics, CV risk factors, antihypertensive and lipid-lowering medication use, each standard deviation decrease in MCF was associated with incident CVD [HR 1.42, CI: 1.23-1.64)], CHD [HR 1.40, CI: 1.17-1.67] and HF [HR 1.58, CI: 1.30-1.94]. In a subgroup analysis of participants with preserved ejection fraction and without left ventricular hypertrophy, the lowest MCF quartile and each standard deviation decrease in MCF was also associated with an increased risk for incident CVD in fully-adjusted analyses.
Conclusions:
MCF is a novel measure that can be measured using cMRI. In this multi-ethnic cohort, MCF is a measure that can be used to predict incident CVD events.
Keywords: myocardial contraction fraction, cardiovascular disease, epidemiology
Introduction
Left ventricular (LV) ejection fraction (EF) is a commonly used measure to assess LV chamber function. Reduced LVEF is a strong predictor for cardiovascular (CV) morbidity and mortality including incident heart failure (HF).1,2 Although LVEF does not need to be indexed to body size, age or gender, it has numerous disadvantages. LVEF cannot differentiate physiologic from pathologic hypertrophy, and is preserved in more than half of patients with HF, thus it is unable to predict CV disease (CVD) outcomes3 and incident HF among individuals with preserved EF (HFpEF). 4,5 These limitations have led to an interest in developing alternative measures that can provide a comprehensive assessment of LV myocardial performance, integrating structure and function, and provide risk stratification for individuals at highest risk for incident CVD events.6–10 Identification of those at highest risk for incident CVD events is important as these individuals may benefit from early therapeutic interventions which may delay or prevent substantial CV morbidity and mortality.
Using 3D echocardiography, King et al.11 described a novel unitless volumetric index: “the myocardial contraction fraction (MCF)”, defined as the ratio of stroke volume (SV) to myocardial volume (MV), calculated as the left ventricular mass (LVM) divided by the specific gravity of the myocardium. MCF has been evaluated as a prognostic marker in individuals with amyloid and non-ischemic dilated cardiomyopathies.12‘14 It is unknown if reduced MCF levels predict incident CVD events and HF events across different racial and ethnic groups.
We investigated whether MCF, assessed using cardiac magnetic resonance imaging (cMRI), predicts incident CVD outcomes and separately, HF events in participants from the Multi-Ethnic Study of Atherosclerosis (MESA), a large population-based multi-ethnic cohort. Additionally, we assessed the factors associated with lower MCF levels.
Methods
Study Population
As previously described,15,16 MESA is a population-based cohort study initiated to further the understanding of the pathogenesis of CVD, determine the predictors of subclinical CVD, and to determine the factors associated with progression to clinical heart disease and CVD outcomes among a racially/ethnically diverse population. The study included 6,814 adults (aged 45-84 years) who were asymptomatic at time of enrollment during the baseline exam (2000-2002). Participants who self-identified as White, African American, Hispanic, or Chinese-American were recruited from 6 US sites (New York, New York; Baltimore, Maryland; Forsyth County, North Carolina; Chicago, Illinois; St Paul, Minnesota; and Los Angeles, California).15 Each field center recruited participants from ≥2 race/ethnic groups. Multiple measures were collected at baseline exam including imaging tests such as cMRI. cMRI was performed on 5,004 participants. Participants have been followed since 2000 and follow up data is available for 5,000 participants. The current analysis was restricted to 5,004 participants with complete cMRI measures at baseline examination (Exam 1: July 2000 to August 2002). Participants with no follow-up data on CV outcomes (n=4) were excluded, leaving a final sample size of 5,000 participants for the current analyses. In a subgroup analysis, participants with no follow-up data on CV outcomes (n=4), an LVEF <50% (n=85), and LV hypertrophy (LVH: LVM indexed to body surface area [BSA]: n=522), were excluded, leaving a final sample size of 4,393 participants. To address the effect of body size on LVH, we also repeated the subgroup analysis using LVH indexed to height2.7 instead of BSA. The final sample size for that subgroup analysis was 3,802. MESA was approved by the institutional review boards of each study site and all participants provided informed consent.
Data Collection & Clinical Covariates
Detailed description of data collection and processing procedures from Exam 1 can be found in the eMethods (Supplement).15 Clinic blood pressure measurements, anthropometric, laboratory data, and standardized questionnaires were obtained. Participants were invited to complete cMRI after Exam 1.
Cardiac MRI
As previously described, cMRI was performed with 1.5-T magnets.17 Details of the cMRI protocol can be found in the eMethods. eTable 1 provides the definitions of the cMRI parameters used within the present analyses. For the present analyses, LVM was indexed (LVMI) to BSA. BSA was calculated as 0.007184 x height (m) 0.725 x weight (kg)0.425. LVH was defined as LVM indexed to BSA (≥ 84.6 g/m2 in females, ≥ 106.2 g/m2 in males) and separately, defined as LVM indexed to indexed to height2.7 (≥ 38 g/m2.7 in females, ≥ 45.1 g/m2.7 in males) using previously published cut points for LVH in the MESA cohort.18 LV end-diastolic volume (EDV) and LV end-systolic volume (ESV) were calculated using Simpson’s rule (the summation of areas on each separate slice multiplied by the sum of slice thickness and image gap). LV SV was defined as LVEDV-LVESV. LVEF was defined as [(SV/LVEDV) x 100], MV was calculated as (LVM / 1.05 g/ml). MCF was calculated as (SV / MV). As previously described,17 interobserver variability was measured in 79 cardiac MRI readings selected at random (3% of the entire cohort at the baseline exam) by comparing the original MRI volumes and mass readings with results from a second review performed between 3 and 6 months later. Intraobserver variability was also assessed at the baseline exam in 75 MRI readings performed in the same manner. Reviewers were blinded to the results of the initial reading at the time of the second and third readings. High reproducibility was observed for each component of MCF (LVM and SV). Interobserver variability: intraclass correlation coefficient 0.949 and 0.976 for LVM and SV, respectively. Intraobserver variability: intraclass correlation coefficient 0.967 and 0.965 for LVM and SV, respectively.
Cardiovascular Events
The primary outcome measure for this study was incident “hard CVD” events defined as myocardial infarction: MI, resuscitated cardiac arrest, stroke (not including transient ischemic attack), coronary heart disease (CHD) death, and stroke death. As a secondary outcome, we examined “hard CHD” events (MI, resuscitated cardiac arrest, and CHD death) as well as incident HF. Event adjudication and follow-up procedures can be found in the eMethods.19
Statistical Analyses
The study population was divided into quartiles based on the distribution of MCF, with the highest quartile (Q4) serving as a referent group. Descriptive analyses of participant characteristics for each MCF quartile were performed. Unadjusted cumulative incidence rates for hard CVD events per 1,000 person-years were calculated as the total number of incident hard CVD events in each MCF quartile divided by the total number of follow-up time in each quartile. Cox proportional hazards regression was used to estimate the hazard ratio (HR) and 95% confidence intervals (CI) for incident hard CVD events in each MCF quartile versus the referent. Model 1 adjusted for age, sex, and race/ethnicity. Model 2 included the variables in Model 1 plus body mass index (BMI), education level, blood pressure (systolic and diastolic), heart rate, low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol, c-reactive protein (CRP), diabetes, smoking, estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73m2, alcohol use, moderate and vigorous physical activity, antihypertensive medication use, and lipid-lowering medication use. Log-transformation was used for skewed variables including CRP. Linear trends across quartiles were assessed by including quartile-specific median MCF values as a continuous variable. Cox proportional hazards regression was used to estimate the HR and 95% CIs for incident hard CVD events associated with each standard deviation decrease in MCF. Two adjusted models were estimated using the covariables in Models 1 and 2. Subgroup analyses were performed examining the interactions with age (<65 years vs. ≥ 65 years), sex, race/ethnicity, prevalent hypertension, and diabetes for incident hard CVD, hard CHD, and HF events. The analyses were repeated separately using incident hard CHD events and incident HF events as an outcome and EF quartiles as the predictor. In a separate subgroup analysis, we repeated the analyses for the primary outcome of hard CVD events and limited the sample to participants with preserved LVEF (≥50%) and without LVH. In secondary analyses, logistic regression was used to assess the associations of each covariable in Models 1 and 2 with the risk of being in the lowest MCF quartile (Q1). Each covariable was first tested separately in a univariate model and thereafter, a multivariable model was used adjusting for all variables simultaneously. Receiver operating characteristic curves (ROC) were constructed to evaluate the area under the curve (AUC), sensitivity, and specificity of MCF for each outcome. A Youden index, the sum of sensitivity and specificity minus one, was calculated for each ROC to determine the optimal cut-off threshold for MCF.
Statistical analyses were performed using SPSS v.23 (IBM SPSS Statistics for Windows, Version 23.0; IBM, Armonk, NY) and MedCalc Statistical Software version 17.11.5 (MedCalc Software Bvba, Ostend, Belgium. P values of <0.05 were considered statistically significant.
Results
Participant Characteristics
The mean ± SD age of participants in the current analysis was 61.5 ± 10.1 years; 52.4% were female, 39.1% were White, 25.7% were African American, 22.2% were Hispanic, and 13.1% were Chinese-American. MCF was symmetrically distributed within the sample (eFigure 1). Mean ± SD MCF was 0.65 ± 0.14. During a median follow-up of 10.2 ± 2.4 years, there were 299 incident cases of hard CVD events, 188 cases of hard CHD events, and 151 cases of HF.
Table 1 shows the baseline characteristics of the sample across MCF quartiles (lowest MCF quartile, Q1: MCF ≤0.55; highest MCF quartile, Q4: MCF >0.74). The percentage of males, African Americans, participants with diabetes, albuminuria, and hypertension decreased from Q1 to Q4 (p<0.001). Age, BMI, systolic/diastolic blood pressure, and heart rate also decreased significantly across MCF quartiles (p<0.001). There was a significant trend of decreasing LVM, LVMI, and MV (p<0.001) and a significant trend for increasing SV and LVEF (p<0.001) from Q1 to Q4.
Table 1.
Characteristics of the analytic cohort of MESA participants by MCF quartiles at Exam 1
| Quartile 4 (n=1250) |
Quartile 3 (n=1250) |
Quartile 2 (n=1250) |
Quartile 1 (n=1250) |
P-trend | |
|---|---|---|---|---|---|
| Levels of MCF | MCF >0.74 | 0.64<MCF≤0.74 | 0.55<MCF≤0.64 | MCF ≤0.55 | |
| Demographic Characteristics | |||||
| Mean (SD) age, years | 59.8 ± 9.9 | 61.2 ± 9.9 | 61.6 ± 10.4 | 63.5 ± 9.9 | <0.001 |
| Female sex, (%) | 963 (77.0) | 774 (61.9) | 548 (43.8) | 335 (26.8) | <0.001 |
| Race | <0.001 | ||||
| White, (%) | 547 (43.8) | 500 (40.0) | 480 (38.4) | 428 (34.2) | |
| Asian, (%) | 235 (18.8) | 176 (14.1) | 160 (12.8) | 82 (6.6) | |
| African-American, (%) | 198 (15.8) | 291 (23.3) | 345 (27.6) | 450 (36.0) | |
| Hispanic, (%) | 270 (21.6) | 283 (22.6) | 265 (21.2) | 290 (23.2) | |
| Education < HS, (%) | 198 (15.8) | 206 (16.5) | 186 (14.9) | 211 (16.9) | 0.716 |
| Clinical Characteristics | |||||
| Mean (SD) Body mass index (kg/m2) | 26.7 ± 5.1 | 27.7 ± 5.1 | 27.9 ± 4.7 | 28.6 ± 4.7 | <0.001 |
| Diabetes, (%) | 82 (6.6) | 114 (9.1) | 126 (10.1) | 194 (15.5) | <0.001 |
| Mean (SD) Fasting glucose, mg/dL | 90.4 ± 18.9 | 94.1 ± 25.6 | 97.0 ± 28.8 | 103.8 ± 38.7 | <0.001 |
| Mean (SD) Total cholesterol, mg/dL | 197.8 ± 35.4 | 194.7 ± 35.3 | 193.2 ± 34.1 | 191.5 ± 36.4 | <0.001 |
| Mean (SD) LDL, mg/dL | 117.5 ± 31.2 | 116.7 ± 31.5 | 117.2 ± 30.5 | 117.4 ± 32.1 | 0.986 |
| Mean (SD) HDL, mg/dL | 56.1 ± 16.0 | 51.7 ± 14.9 | 50.0 ± 14.3 | 47.1 ± 13.1 | <0.001 |
| eGFR <60 ml/min/1.73 m2, (%) | 105 (8.4) | 109 (8.7) | 105 (8.4) | 134 (10.7) | 0.071 |
| Albuminuria (urinary albumin to creatinine ratio ≥17 mg/g for men and ≥25 mg/g for women), (%) | 68 (5.5) | 124 (9.9) | 163 (13.0) | 256 (20.5) | <0.001 |
| Median (25- 75th percentile) C-reactive protein, mg/L | 1.54 (0.69-4.10) | 1.92 (0.82-4.43) | 1.71 (0.76-3.59) | 1.95 (0.87-4.07) | 0.001 |
| Mean (SD) clinic SBP, mmHg | 119.4 ± 20.1 | 124.7 ± 21.2 | 126.2 ± 20.1 | 131.4 ± 22.0 | <0.001 |
| Mean (SD) clinic DBP, mmHg | 68.0 ± 9.4 | 70.7 ± 9.9 | 72.7 ± 9.7 | 75.9 ± 10.5 | <0.001 |
| Mean (SD) heart rate, bpm | 62.0 ± 9.0 | 62.4 ± 9.0 | 62.3 ± 8.9 | 64.7 ± 10.6 | <0.001 |
| Antihypertensive medication use, (%) | 325 (26.0) | 453 (36.2) | 449 (35.9) | 538 (43.0) | <0.001 |
| Lipid lowering medications, (%) | 175 (14.0) | 188 (15.0) | 220 (17.6) | 211 (16.9) | 0.015 |
| Prevalent hypertension status, (%) | 390 (31.2) | 530 (42.4) | 518 (41.4) | 680 (54.4) | <0.001 |
| Health Behaviors | |||||
| Alcohol consumption, (%) | <0.001 | ||||
| None | 327 (26.2) | 285 (22.8) | 230 (18.4) | 183 (14.6) | |
| Former | 229 (18.3) | 276 (22.1) | 291 (23.3) | 366 (29.3) | |
| Current | 684 (55.5) | 689 (55.1) | 729 (58.3) | 701 (56.1) | |
| Cigarette smoking, (%) | <0.001 | ||||
| None | 767 (61.4) | 680 (54.3) | 620 (49.6) | 506 (40.5) | |
| Former | 399 (31.9) | 435 (34.8) | 439 (35.1) | 514 (41.1) | |
| Current | 84 (6.7) | 135 (10.8) | 191 (15.3) | 226 (18.1) | |
| Mean (SD) Moderate + Vigorous physical activity, MET-min/week, (%) | 0.005 | ||||
| Quartile1 [0-2040 MET-min/week] | 313 (25.0) | 328 (26.2) | 296 (23.7) | 316 (25.3) | |
| Quartile 2 [2040-4095 MET-min/week] | 331 (26.5) | 345 (27.6) | 277 (22.2) | 292 (23.4) | |
| Quartile 3 [4095-7545 MET-min/week] | 320 (25.6) | 313 (25.0) | 319 (25.5) | 300 (24.0) | |
| Quartile 4 [>7545 MET-min/week] | 286 (22.9) | 264 (21.1) | 358 (28.6) | 342 (27.4) | |
| Cardiac MRI measures | |||||
| Left ventricular mass, g | 117.3 ± 25.0 | 136.3 ± 30.3 | 152.3 ± 33.7 | 175.1 ± 41.9 | <0.001 |
| Left ventricular mass index, g/m2 | 66.2 ± 10.1 | 74.4 ± 11.9 | 80.9 ± 13.6 | 90.2 ± 18.1 | <0.001 |
| Left ventricular myocardial volume, mL | 111.7 ± 23.8 | 129.8 ± 28.9 | 145.0 ± 32.1 | 166.8 ± 39.9 | <0.001 |
| Left ventricular stroke volume, mL | 92.6 ± 18.8 | 89.1 ± 19.5 | 86.6 ± 18.9 | 77.4 ± 18.8 | <0.001 |
| Left ventricular ejection fraction, % | 73.2 ± 5.3 | 70.9 ± 5.8 | 68.4 ± 6.1 | 63.6 ± 8.4 | <0.001 |
Numbers in the table are percentages or mean ± standard deviation. To convert glucose to mmol/L, multiply values by 0.0556; to convert cholesterol to mmol/L, multiply values by 0.0259.
HS: high school, SBP: systolic blood pressure, DBP: diastolic blood pressure, BPM: beats per minute, LDL: low-density lipoprotein cholesterol, HDL: high-density lipoprotein cholesterol, eGFR: estimated glomerular filtration rate, MCF: myocardial contraction fraction, MESA: Multi-Ethnic Study of Atherosclerosis, MET: metabolic equivalent, Min: minutes, mmHg: millimeters of mercury, SD: standard deviation
Correlates of the lowest MCF quartile among participants
After full multivariable adjustment, older age, male sex, African-American race/ethnicity, higher BMI (per 1 kg/m2 increase), former and current smoking, albuminuria, higher diastolic blood pressure (per 5mmHg increase), and increased heart rate (per 10 beats per minute increase) were all associated with an increased odds of being in the lowest MCF quartile (eTable 2). Asian race/ethnicity and higher HDL levels (per 10mg/dL increase) were associated with a decreased odds of being in the lowest MCF quartile.
Primary Outcome: Association of MCF with hard CVD events
Participants in Q1 had the highest incidence rate of hard CVD events (Table 2). When compared to Q4 (referent), Q1, Q2, and Q3 were each associated with an increased risk of hard CVD events in unadjusted models (Table 2). Participants in Q1 had a 5-fold increased risk of hard CVD events (HR 4.98, 95% CI 3.37-7.36, Table 2). In a model adjusting for age, sex, race, and CV risk factors, the HRs of incident CVD events from the highest to lowest MCF quartile were 1.00 (referent); 1.43 (95% CI 0.91-2.23); 1.70 (95% CI 1.10-2.62); and 2.42 (95% CI 1.58-3.72) (p-trend <0.001, Model 2, Table 2). When expressed as a continuous variable, MCF (per 0.14 decrease) was associated with an increased hazard of incident hard CVD events in univariate and multivariable-adjusted models (p<0.001, Table 2). When the sample size was restricted to individuals with preserved LVEF and without LVH, the lowest MCF quartiles and each standard deviation decrease in MCF was also associated with an increased hazard of incident hard CVD events in univariate and multivariable-adjusted models (Table 3). The association was similar when LVH was indexed to BSA and height2.7.
Table 2.
Hazard ratio for hard CVD events associated with MCF quartiles
| Q4 | Q3 | Q2 | Q1 | P-trend | |
|---|---|---|---|---|---|
| Characteristics | |||||
| Levels of MCF | MCF >0.74 | 0.64<MCF≤0.74 | 0.55<MCF≤0.64 | MCF ≤0.55 | |
| Number of events | 31 | 58 | 73 | 137 | |
| Number at risk | 1250 | 1250 | 1250 | 1250 | |
| Incidence rate per 1,000 person-years | 2.53 | 4.87 | 6.20 | 12.60 | |
| Model | Hazard ratio (95% CI) | ||||
| Unadjusted | 1.0 (ref) | 1.92 (1.24-2.97) | 2.45 (1.61-3.72) | 4.98 (3.37-7.36) | <0.001 |
| Model 1 | 1.0 (ref) | 1.74 (1.12-2.70) | 2.11 (1.37-3.23) | 3.75 (2.48-5.67) | <0.001 |
| Model 2 | 1.0 (ref) | 1.43 (0.91-2.23) | 1.70 (1.10-2.62) | 2.42 (1.58-3.72) | <0.001 |
| MCF as a continuous measure (per one SD = 0.14 decrease) | |||||
| Unadjusted | 1.83 (1.63-2.09) | <0.001 | |||
| Model 1 | 1.66 (1.45-1.89) | <0.001 | |||
| Model 2 | 1.42 (1.23-1.64) | <0.001 | |||
Model 1 includes adjustment for age, sex, and race/ethnicity
Model 2 includes Model 1 variables + additional adjustment for body mass index, education level <HS, systolic blood pressure, diastolic blood pressure, heart rate, LDL cholesterol, HDL cholesterol, Log2 C-reactive protein, diabetes, smoking (none, former, current), estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73m2, alcohol use (none, former, current), moderate + vigorous quartiles of physical activity, antihypertensive medication use, and lipid-lowering medication use.
CI: confidence interval, CVD: cardiovascular disease, HS: high school, HDL: high density lipoprotein cholesterol, LDL: low density lipoprotein cholesterol, MCF: myocardial contraction fraction, Q: quartile, SD: standard deviation
Table 3.
Hazard ratio for hard CVD events associated with MCF quartiles among participants with preserved left ventricular ejection fraction and without left ventricular hypertrophy.
| Panel A. Results when left ventricular hypertrophy defined as left ventricular mass indexed to body surface area (n=4,393) | |||||
|---|---|---|---|---|---|
| Q4 | Q3 | Q2 | Q1 | P-trend | |
| Characteristics | |||||
| Levels of MCF | MCF >0. 75 | 0.66<MCF≤0.75 | 0.57<MCF≤0.66 | MCF ≤0.57 | |
| Number of events | 25 | 48 | 59 | 92 | |
| Number at risk | 1097 | 1099 | 1099 | 1098 | |
| Incidence rate per 1,000 person-years | 2.32 | 4.57 | 5.68 | 9.26 | |
| Model | Hazard ratio (95% CI) | ||||
| Unadjusted | 1.0 (ref) | 1.97 (1.22-3.20) | 2.45 (1.53-3.91) | 4.00 (2.56-6.22) | <0.001 |
| Model 1 | 1.0 (ref) | 1.79 (1.10-2.92) | 2.06 (1.28-3.32) | 3.00 (1.87-4.80) | <0.001 |
| Model 2 | 1.0 (ref) | 1.44 (0.87-2.36) | 1.70 (1.05-2.77) | 2.07 (1.27-3.37) | 0.002 |
| MCF as a continuous measure (per one SD = 0.14 decrease) | |||||
| Unadjusted | 1.69(1.47-1.95) | <0.001 | |||
| Model 1 | 1.50 (1.29-1.75) | <0.001 | |||
| Model 2 | 1.33 (1.13-1.57) | 0.001 | |||
| Panel B. Results when left ventricular hypertrophy defined as left ventricular mass indexed to height2.7 (n=3,802) | |||||
| Q4 | Q3 | Q2 | Q1 | P-trend | |
| Characteristics | |||||
| Levels of MCF | MCF >0.76 | 0.66<MCF≤0.76 | 0.58<MCF≤0.66 | MCF ≤0.58 | |
| Number of events | 17 | 40 | 54 | 73 | |
| Number at risk | 950 | 951 | 951 | 950 | |
| Incidence rate per 1,000 person-years | 1.82 | 4.38 | 6.02 | 8.42 | |
| Model | Hazard ratio (95% CI) | ||||
| Unadjusted | 1.0 (ref) | 2.41 (1.37-4.26) | 3.31 (1.92-5.72) | 4.65 (2.74-7.89) | <0.001 |
| Model 1 | 1.0 (ref) | 2.19 (1.23-3.87) | 2.69 (1.54-4.70) | 3.34 (1.90-5.86) | <0.001 |
| Model 2 | 1.0 (ref) | 1.75 (0.98-3.14) | 2.27 (1.29-4.01) | 2.43 (1.36-4.33) | 0.002 |
| MCF as a continuous measure (per one SD = 0.14 decrease) | |||||
| Unadjusted | 1.71 (1.47-2.00) | <0.001 | |||
| Model 1 | 1.50 (1.27-1.78) | <0.001 | |||
| Model 2 | 1.37 (1.14-1.64) | 0.001 | |||
Model 1 includes adjustment for age, sex, and race/ethnicity
Model 2 includes Model 1 variables + additional adjustment for body mass index, education level <HS, systolic blood pressure, diastolic blood pressure, heart rate, LDL cholesterol, HDL cholesterol, Log2 C-reactive protein, diabetes, smoking (none, former, current), estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73m2, alcohol use (none, former, current), moderate + vigorous quartiles of physical activity, antihypertensive medication use, and lipid-lowering medication use.
CI: confidence interval, CVD: cardiovascular disease, HS: high school, HDL: high density lipoprotein cholesterol, LDL: low density lipoprotein cholesterol, MCF: myocardial contraction fraction, Q: quartile, SD: standard deviation
Secondary Outcomes: Association of MCF with hard CHD events and HF
The incidence rates for hard CHD events decreased with increasing MCF quartiles (eTable 3). After full adjustment for all covariates, Q1 was associated with a 2.3-fold increased risk of hard CHD events when compared to Q4 (HR 2.32, 95% CI 1.36-3.96, Model 2, eTable 3). Similar results were observed with MCF modeled as a continuous variable (HR 1.40, 95% CI 1.17-1.67, per 0.14 decrease).
The incidence rate for HF events was 7.73/1000 person-years for participants in Q1 compared to 1.63/1000 person-years for participants in Q4 (eTable 4). In a model adjusting for CV risk factors, Q1 was associated with a 2-fold increased risk for HF events (HR1.99, 95% CI 1.15-3.44, Model 2, eTable 4). When expressed as a continuous variable, MCF was associated with HF in multivariable models adjusting for all covariates (HR 1.58, 95% CI 1.30-1.94, per 0.14 decrease).
There were no significant interactions by age, sex, race, prevalent hypertension status, or diabetes status for incident hard CVD or incident hard CHD events. However, age modified the association of MCF with incident HF. For any decrease in MCF level, there was a greater risk of HF among participants < 65 years old (HR 1.94, 95% CI 1.35-2.71 compared to participants ≥ 65 years old (HR 1.43, 95% CI 1.13-1.80, pinteraction: 0.026).
Association of EF with hard CVD, hard CHD, and HF events
LVEF was not associated with an increased risk of incident hard CVD or hard CHD events in unadjusted and fully adjusted models (eTables 5 and 6). In contrast, the lowest LVEF quartile had a 2-fold increased risk for HF in both unadjusted and multivariable-adjusted models (linear trend p<0.001, eTable 7). LVEF as a continuous variable was significantly associated with HF in multivariable models (HR 1.61, 95% CI 1.42-1.84, per 7.40 decrease).
AUC and cut-off threshold for MCF associated with hard CVD, hard CHD, and HF events
Figure 1 and eTable 8 depict the AUC and optimal cut-off thresholds for MCF associated with hard CVD, hard CHD, and HF events. The AUC for hard CVD, hard CHD, and HF events was 0.663 (95% CI 0.649-0.676, p<0.0001), 0.665 (95% CI 0.652-0.678, p<0.0001), and 0.695 (95% CI 0.682-0.708, p<0.0001), respectively. The optimal cut-off threshold for MCF in predicting hard CVD and CHD events was 0.60 (sensitivity/specificity: 62.8%/62.3% for hard CVD events and sensitivity/specificity: 63.3%/61.7% for hard CHD events, respectively). The optimal cut-off threshold for predicting HF events was 0.52 (sensitivity/specificity: 51,7%/81.6% respectively).
Figure 1:
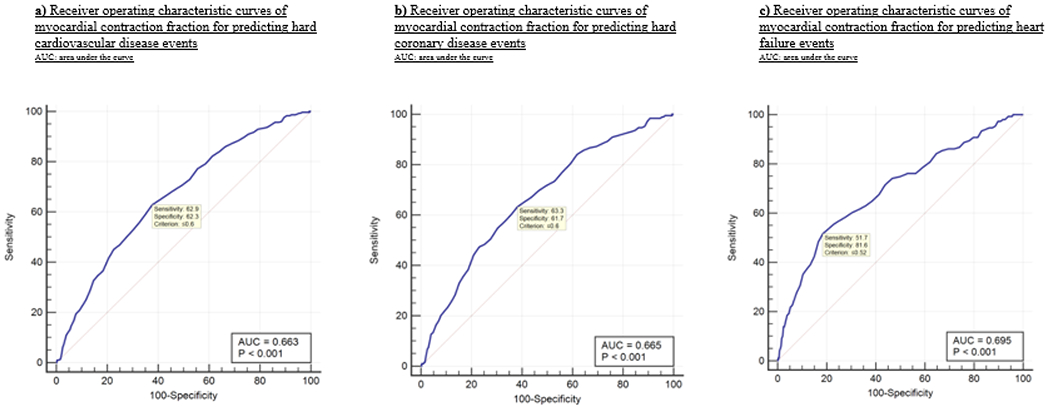
Receiver operating characteristic curves of myocardial contraction fraction for predicting hard cardiovascular disease, hard coronary disease, and heart failure events
Discussion
In this large multi-ethnic population-based cohort of individuals, MCF was associated with an increased risk of incident hard CVD, hard CHD, and HF events. Over a median follow-up of 10.2 years, participants within the lowest MCF quartile had a 2.4 increased risk of incident hard CVD events, a 2.3 increased risk of hard CHD events, and a 2-fold increased risk of HF events compared to those within the highest MCF quartile in models adjusting for CV risk factors. Lower levels of MCF were also significantly associated with incident CVD, CHD, and HF events when adjusted for CV risk factors.
Few data have evaluated the association of reduced MCF and CVD outcomes. 3,12–14,20 Our results are consistent with data from the Framingham Heart Study Offspring cohort.3 Among White participants without clinical CVD in the Framingham Heart Study Offspring cohort, the lowest MCF quartile was associated with a seven-fold increased risk for CVD events (HR: 7.11, 95% CI 1.60-31.59, p=0.010) when compared to the highest MCF quartile, in a model adjusting for gender and the Framingham Risk Score. This study was limited by small sample size (n=304), lack of minority representation, and was underpowered to examine different types of CV endpoints. Arenja et al.13 also demonstrated that a depressed MCF level was associated with a higher risk of the combined outcome of cardiac death, heart transplantation, sudden cardiac death aborted by appropriate implantable cardioverter defibrillator discharge due to ventricular tachycardia or fibrillation, and hospitalization due to congestive heart failure among individuals with non-ischemic dilated cardiomyopathy. Our study extends both of these findings and further suggests that within a large multi-ethnic cohort, MCF is an independent predictor of several types of CVD events. Among 66 individuals with cardiac amyloid and preserved LVEF, 14 MCF <30, as assessed by 2D echocardiography, was associated with an approximately three-fold increased risk of death (HR 2.841, 95% CI 1.214-6.648). Similarly, Maurer et al.20 demonstrated that reduced MCF (per 10% decrease, assessed by 2D echocardiography) was associated with a higher incidence of a composite of atherosclerotic cardiovascular disease, HF, and all-cause mortality among elderly participants in the Cardiovascular Health Study. Limitations of both studies include the inability to examine subtypes of CVD events and the use of 2D echocardiography M-mode dimensions for estimating LVM and LV volume. Assessment of LVM and LV volume by cMRI is a gold standard and has been shown to be more reproducible when compared to 2D echocardiography. 21–24 Whether the estimation of MCF by 2D echocardiography is similar to that estimated by cMRI is currently unknown.
Although LVEF is the most common tool used to identify abnormal LV function, it can remain preserved in many disease states, cannot distinguish pathologic from physiologic remodeling or hypertrophy, and can mask significant underlying regional dysfunction.10 Additionally, results from prior population-based studies 3,8 demonstrate that with the exception of incident HF, preserved LVEF is not predictive of incident CVD events, a finding which our study also confirms. Similarly, other studies have also explored the utility of myocardial strain imaging.9,10,25–28 Although tagged MRI is a common cMRI method to assess regional strain,10 very few studies have examined the predictive power of tagged MRI with incident CVD events.21,29 In a recent study, regional LV diastolic strain, as quantified by tagged cMRI, was related to incident HF over an 8-year follow up period among participants within the MESA cohort (HR 2.25, 95% CI 1.30-3.89).29 Although tagged MRI is a promising new measure, it is expensive, requires additional imaging and processing, and is currently limited to only research settings.25 In contrast, MCF does not require additional imaging, is simple to calculate from routine cMRI measures, and is sensitive to changes in myocardial dysfunction.
The possible mechanisms by which MCF is predictive of incident CVD and HF events may be understood from a pathophysiological perspective. MV and myocardial muscle mass remain constant throughout the cardiac cycle. 11,30 However during systole, the ventricular myocardium simultaneously shortens longitudinally and circumferentially and thickens radially 9 and reduces its contained volume by the amount of the SV. Therefore, SV is a measure of the amount by which the myocardium contracts (i.e. shortens) during systole relative to the total MV. 11 Thus, MCF (defined as SV/MV) represents an index of the fractional shortening of the myocardium in volumetric terms.11 Consequently, a decrease in MCF, which is highly correlated with global longitudinal strain on echocardiography,20,31 indicates abnormal myocardial shortening and is reflective of abnormalities in myocardial properties induced by hypertrophy, inflammation, microvascular dysfunction, and alterations of the interstitium. These abnormalities can lead to regional myocardial dysfunction and may reflect underlying fibrosis or ischemia due to macro or microvascular disease.32 Because MCF is a ratio of SV to MV, it can also be interpreted as a measure that incorporates information on both LV structure and function. LV parameters that combine information on structure and function are sensitive to varying physiologic and pathologic conditions.6 Moreover, they have recently emerged as a promising new investigative area of research.6,8
In our study, older age, male sex, higher BMI, smoking, albuminuria, higher diastolic blood pressure, and elevated heart rate were all significant predictors of reduced MCF. These correlates have been previously shown to be associated with alterations in SV or LVM, the 2 main parameters within MCF.33–38. Additionally, African American race/ethnicity was also associated with reduced MCF levels. Although the underlying pathophysiological mechanism underlying this observation is unclear and may be related to differences in the prevalence and control of comorbid conditions such as hypertension and diabetes, some studies have demonstrated that African Americans have higher LVM39 and worse baseline contractile function when compared to other racial groups. 40,41 Conversely, Asian race/ethnicity was associated with a decreased risk of reduced MCF levels. Lastly, while our study demonstrated that an optimal cut-off threshold of 0.6 is associated with hard CVD and CHD events, this finding needs to be investigated further in future studies.
There are some strengths and limitations of our study. We used data from a prospective and large multi-ethnic population-based cohort of participants. The long duration of follow-up provided sufficient statistical power to examine the association of MCF across subtypes of CVD events. Additionally, cMRI is an accurate and reproducible technique for assessing LV morphology and function 42 and thus our results may be less susceptible to significant measurement error. However, although the MESA cohort attempted to primarily recruit participants who were asymptomatic at baseline, our main results did include individuals with systolic dysfunction and LVH. To address this, we conducted subgroup analyses to exclude participants with an LVEF <50% and without LVH however our study may still be affected by selection and survival biases. Additionally, given the small number of participants with reduced LVEF, we did not have sufficient power to determine whether MCF is predictive of incident CVD events among participants with reduced LVEF at baseline exam. Echocardiography was not performed at baseline exam among participants included in our study. As such, we could not correlate MCF with diastolic function. Further, data using left atrial size calculations, feature tracking cMRI, and late gadolinium enhancement are not available from the baseline MESA study and we could not include these parameters in our risk models.
Conclusions
MCF is a volumetric measure of myocardial shortening that can assess chamber performance and is associated with incident hard CVD, hard CHD, and HF events after full multivariable adjustment for CV risk factors. In contrast LVEF did not have predictive power for incident hard CVD and hard CHD events. The results of our study suggest that MCF may be used among a multi-ethnic population to identify those at highest risk for incident CVD events.
Supplementary Material
Highlights.
Myocardial contraction fraction (MCF) is a measure of left ventricular myocardial shortening.
MCF (stroke volume to myocardial volume) can be measured using cardiac magnetic resonance imaging.
Lower MCF levels are associated with a 2-fold increased risk for incident cardiovascular disease.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI) and by grants UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources, Bethesda, MD. This work was also supported by the National Institutes of Health (KL2TR001874), by the NHLBI (HL047540, HL117323-02S2, K24-HL125704) and the National Institute of Aging (K24-AG036778). Dr. Abdalla receives support through 18AMFDP34380732 from the American Heart Association and from K23 HL141682-01A1 from NHLBI.
Abbreviations and Acronyms
- CHD
coronary heart disease
- CVD
cardiovascular disease(s)
- EF
ejection fraction
- HF
heart failure
- LV
left ventricular
- MCF
myocardial contraction fraction
- cMRI
cardiac magnetic resonance imaging
- MV
myocardial volume
- SV
stroke volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
There are no potential conflicts of interest.
References:
- 1.Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation. 2006;113(24):2851–2860. [DOI] [PubMed] [Google Scholar]
- 2.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108(8):977–982. [DOI] [PubMed] [Google Scholar]
- 3.Chuang ML, Gona P, Salton CJ, et al. Usefulness of the left ventricular myocardial contraction fraction in healthy men and women to predict cardiovascular morbidity and mortality. Am J Cardiol. 2012;109(10):1454–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43(3):317–327. [DOI] [PubMed] [Google Scholar]
- 5.Vasan RS, Benjamin EJ. Diastolic heart failure--no time to relax. N Engl J Med. 2001;344(1):56–59. [DOI] [PubMed] [Google Scholar]
- 6.Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol. 2011;58(17):1733–1740. [DOI] [PubMed] [Google Scholar]
- 7.Khouri MG, Peshock RM, Ayers CR, de Lemos JA, Drazner MH. A 4-tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas heart study. Circ Cardiovasc Imaging. 2010;3(2):164–171. [DOI] [PubMed] [Google Scholar]
- 8.Mewton N, Opdahl A, Choi EY, et al. Left ventricular global function index by magnetic resonance imaging--a novel marker for assessment of cardiac performance for the prediction of cardiovascular events: the multi-ethnic study of atherosclerosis. Hypertension. 2013;61(4):770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah AM, Solomon SD. Myocardial deformation imaging: current status and future directions. Circulation. 2012;125(2):e244–248. [DOI] [PubMed] [Google Scholar]
- 10.Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009; 11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King DL, El-Khoury Coffin L, Maurer MS. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol. 2002;40(2):325–329. [DOI] [PubMed] [Google Scholar]
- 12.Arenja N, Fritz T, Andre F, et al. Myocardial contraction fraction derived from cardiovascular magnetic resonance cine images-reference values and performance in patients with heart failure and left ventricular hypertrophy. Eur Heart J Cardiovasc Imaging. 2017;18(12):1414–1422. [DOI] [PubMed] [Google Scholar]
- 13.Arenja N, Riffel JH, Fritz T, et al. Diagnostic and Prognostic Value of Long-Axis Strain and Myocardial Contraction Fraction Using Standard Cardiovascular MR Imaging in Patients with Nonischemic Dilated Cardiomyopathies. Radiology. 2017;283(3):681–691. [DOI] [PubMed] [Google Scholar]
- 14.Tendler A, Helmke S, Teruya S, Alvarez J, Maurer MS. The myocardial contraction fraction is superior to ejection fraction in predicting survival in patients with AL cardiac amyloidosis. Amyloid. 2015;22(1):61–66. [DOI] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 16.Olson JL, Bild DE, Kronmal RA, Burke GL. Legacy of MESA. Glob Heart. 2016;11(3):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186(6 Suppl 2):S357–365. [DOI] [PubMed] [Google Scholar]
- 18.Brumback LC, Kronmal R, Heckbert SR, et al. Body size adjustments for left ventricular mass by cardiovascular magnetic resonance and their impact on left ventricular hypertrophy classification. Int J Cardiovasc Imaging. 2010;26(4):459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. JAm Coll Cardiol. 2008;52(25):2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurer MS, Koh WJ, Bartz TM, et al. Relation of the Myocardial Contraction Fraction, as Calculated from M-Mode Echocardiography, With Incident Heart Failure, Atherosclerotic Cardiovascular Disease and Mortality (Results from the Cardiovascular Health Study). Am J Cardiol. 2017;119(6):923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arai AE. New Insights from Major Prospective Cohort Studies with Cardiovascular Magnetic Resonance (CMR). Curr Cardiol Rep. 2015;17(6):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopal AS, Keller AM, Rigling R, King DL, Jr., King DL. Left ventricular volume and endocardial surface area by three-dimensional echocardiography: comparison with two-dimensional echocardiography and nuclear magnetic resonance imaging in normal subjects. J Am Coll Cardiol. 1993;22(1):258–270. [DOI] [PubMed] [Google Scholar]
- 23.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90(1):29–34. [DOI] [PubMed] [Google Scholar]
- 24.Rademakers FE. Magnetic resonance imaging in cardiology. Lancet. 2003;361(9355):359–360. [DOI] [PubMed] [Google Scholar]
- 25.Tee M, Noble JA, Bluemke DA. Imaging techniques for cardiac strain and deformation: comparison of echocardiography, cardiac magnetic resonance and cardiac computed tomography. Expert Rev Cardiovasc Ther. 2013;11(2):221–231. [DOI] [PubMed] [Google Scholar]
- 26.Yan RT, Bluemke D, Gomes A, et al. Regional left ventricular myocardial dysfunction as a predictor of incident cardiovascular events MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. 2011;57(17):1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100(21):1673–1680. [DOI] [PubMed] [Google Scholar]
- 28.Sengelov M, Jorgensen PG, Jensen JS, et al. Global Longitudinal Strain Is a Superior Predictor of All-Cause Mortality in Heart Failure With Reduced Ejection Fraction. JACC Cardiovasc Imaging. 2015;8(12):1351–1359. [DOI] [PubMed] [Google Scholar]
- 29.Ambale-Venkatesh B, Armstrong AC, Liu CY, et al. Diastolic function assessed from tagged MRI predicts heart failure and atrial fibrillation over an 8-year follow-up period: the multi-ethnic study of atherosclerosis. Eur Heart J Cardiovasc Imaging. 2014;15(4):442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King DL, Coffin Lel K, Maurer MS. Noncompressibility of myocardium during systole with freehand three-dimensional echocardiography. J Am Soc Echocardiogr. 2002;15(12):1503–1506. [DOI] [PubMed] [Google Scholar]
- 31.Matthews SD, Rubin J, Cohen LP, Maurer MS. Myocardial Contraction Fraction: A Volumetric Measure of Myocardial Shortening Analogous to Strain. J Am Coll Cardiol. 2018;71(2):255–256. [DOI] [PubMed] [Google Scholar]
- 32.Choi EY, Rosen BD, Fernandes VR, et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2013;34(30):2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heckbert SR, Post W, Pearson GD, et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48(11):2285–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luczak ED, Leinwand LA. Sex-based cardiac physiology. Annu Rev Physiol. 2009;71:1–18. [DOI] [PubMed] [Google Scholar]
- 35.Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens. 2004;18(6):453–459. [DOI] [PubMed] [Google Scholar]
- 36.Opdahl A, Ambale Venkatesh B, Fernandes VRS, et al. Resting heart rate as predictor for left ventricular dysfunction and heart failure: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2014;63(12): 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turkbey EB, McClelland RL, Kronmal RA, et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging. 2010;3(3):266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2(3):191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kizer JR, Arnett DK, Bella JN, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension. 2004;43(6):1182–1188. [DOI] [PubMed] [Google Scholar]
- 40.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandes VR, Polak JF, Edvardsen T, et al. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol. 2006;47(12):2420–2428. [DOI] [PubMed] [Google Scholar]
- 42.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21(16):1387–1396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


