Abstract
Purpose:
To describe the study design and characteristics at first visit of participants in the longitudinal Scotopic Microperimetric Assessment of Rod Function in Stargardt Disease (SMART) study.
Methods:
Scotopic microperimetry (sMP) was performed in one designated study eye in a subset of participants with molecularly proven ABCA4-associated Stargardt disease (STGD1) enrolled in a multicenter natural history study (ProgStar). Study visits were every 6 months over a period ranging from 6 to 24 months, and also included fundus autofluorescence (FAF).
Results:
SMART enrolled 118 participants (118 eyes). At the first visit of SMART, the mean sensitivity in mesopic microperimetry was 11.48 (±5.05; range 0.00–19.88) dB and in sMP 11.25 (±5.26; 0–19.25) dB. For FAF, all eyes had a lesion of decreased autofluorescence (mean lesion size 3.62 [±3.48; 0.10–21.46] mm2), and a total of 76 eyes (65.5%) had a lesion of definitely decreased autofluorescence with a mean lesion size of 3.46 (±3.60; 0.21–21.46) mm2.
Conclusions:
Rod function is impaired in STGD1 and can be assessed by sMP. Testing rod function may serve as a potential outcome measure for future clinical treatment trials. This is evaluated in the SMART study.
Keywords: Scotopic microperimetry, Mesopic microperimetry, Stargardt disease, Endpoints, Clinical trials
Introduction
Stargardt disease or Stargardt macular dystrophy was initially described by the German ophthalmologist Karl Stargardt in 1909 [1] which is now related to at least three different genetic mutations, Stargardt disease 1 (STGD1; OMIM #248200 [ABCA4]), Stargardt disease 3 (STGD3; OMIM #600110 [ELOV4]), and Stargardt disease 4 (STGD4, OMIM #603786 [PROM1]). STDG 1 is an auto-somal recessively inherited disorder caused by mutations in the ABCA4 gene [2]. With an estimated incidence of 10–12.5 per 100,000, it is the most common juvenile macular degeneration [3]. STGD1 is both genotypically and phenotypically a very heterogeneous disease with significant variation in the age at onset and rate of progression [4].
Although there are at present no approved treatments, several therapeutic options are in preclinical or early clinical phases [3, 5, 6]. The Natural History of the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) studies were launched to characterize the natural history of disease progression using a variety of structural and functional measures, including fundus autofluorescence (FAF; primary endpoint), spectral-domain optical coherence tomography (SD-OCT, using a Heidelberg Engineering [Heidelberg, Germany] device, respectively) and mesopic microperimetry (MP-1; Nidek Technologies, Padova, Italy). The purpose is not only to gain a better understanding of the natural course, but also to determine possible clinical or imaging markers of progression that may evaluate the safety and efficacy of treatments [4].
First reports of ABCA4 expression found its presence in rod photoreceptors [7], and subsequently in foveal cones by immunofluorescence microscopy and Western blot analysis [8], hence demonstrating that both cones and rods express the defective gene. Additionally, it has been shown that the spectrum of clinically defined ABCA4-related disease is notably broad, ranging from “typical” STGD1 to cone-rod dystrophy and rod-cone dystrophies/retinitis pigmentosa [9]. It has recently been shown that eyes with STGD 1 and generalized cone dysfunction on full-field electroretinography progress to also develop rod dysfunction. Those eyes with initial rod involvement were also more prone to develop clinically significant electrophysiological deterioration compared to those with normal ERGs [10]. However, it is not fully understood why and how cones and rods are affected in different ways during the natural course of STGD1. Further-more, characterizing the time course and magnitude of clinical/subclinical rod and cone involvement over time may play an important role in possible therapeutic targeting, clinical trial design and outcome interpretation.
Crossland et al. [11] modified a Nidek MP-1 microperimeter (Nidek) to measure scotopic retinal sensitivity by adding a short-wavelength and neutral density filter.
After the multicenter prospective ProgStar study had been launched, the decision was made to also test scotopic function in the “Scotopic Microperimetric Assessment of Rod Function in Stargardt Disease” (SMART) study. The SMART study allows, for the first time, the evaluation of rod function and its correlation with morphological damage in the natural history of STGD1 in a large and well-defined cohort of participants. Ultimately, the understanding of the differential impairment of the cone and rod photoreceptors, through microperimetry (MP) findings, may establish consistent and reliable parameters to monitor participants and investigate potential treatments for STGD1, which thus far, has not been available for participants with ABCA4-related retinal disease. Herein, we describe the study design and baseline characteristics of participants participating in SMART.
Methods
Study Design and Eligibility Criteria
The SMART study was an ancillary study of the prospective ProgStar study, a longitudinal cohort study with standardized visits every 6 months ± 5 weeks (up to a total of 5 visits over 24 months ± 5 weeks) [4]. Participant data in ProgStar are derived from clinical examinations and central reading center (RC) grading of retinal imaging (FAF and SD-OCT) and mesopic MP using standardized protocols across all sites.
The primary objective of the SMART study was:
To assess the yearly rate of progression of STGD1 using macular sensitivity under scotopic testing conditions.
The secondary objectives were:
To correlate scotopic microperimetric changes with functional measures such as mesopic microperimetric changes, visual acuity and anatomical status/progression as determined by, ERG, SD-OCT and FAF.
To determine the earliest functional deficits in STGD1.
To establish the best scotopic microperimetric parameters to monitor patients with STGD1.
Inclusion Criteria
A mandatory inclusion criterion for SMART study was being a participant of the ProgStar Study. In ProgStar, the study participants were required to meet the following inclusion criteria, as previously published [4]: (1) presence on FAF of at least 1 well-demarcated area of atrophy with a minimum diameter of 300 μm, and the total area of all atrophic lesions being 12 mm2 or less (equivalent to no more than 5 standard disc areas in a least 1 eye) as certified by the site principal investigator (PI); (2) best-corrected visual acuity (BCVA) of 20 early treatment diabetic retinopathy (ETDRS) letters at 1 m (Snellen equivalent, 20/400) or better in the study eye(s); (3) presence of at least 2 likely disease-causing variants in ABCA4 or 1 likely disease-causing variant with at least 1 eye with flecks at the level of the retinal pigment epithelium typical for STGD1; (4) clear ocular media and adequate pupillary dilation to permit good-quality FAF and SD-OCT imaging; (5) age of at least 6 years; (6) ability to cooperate during examinations; and (7) participant’s ability and willingness to undergo ocular examinations once every 6 months for up to 24 months.
Exclusion Criteria
Exclusion criteria were: (1) presence of ocular disease, such as choroidal neovascularization, glaucoma, or diabetic retinopathy, in either eye, that may confound assessment of the retina morphologically and functionally as determined by the site PI; (2) intra-ocular surgery in the primary study eye(s) within 90 days before any eligible visit; (3) current or previous participation in a clinical trial to treat Stargardt disease; (4) current participation in, or participation within the last 6 months in any drug trial; (5) diagnosis of any systemic disease with a limited survival prognosis; (6) any condition that would interfere with attending regular follow-up visits every 6 months for up to 24 months; and (7) sound medical reason for nonenrollment in the opinion of the PI.
Clinical Sites and Study Organization
The ProgStar organizational structure has been described in detail previously [4]. The SMART study was performed at six participating centers (Wilmer Eye Institute, Johns Hopkins University, Baltimore, MD, USA; Hoover Low Vision Rehabilitation Services, Greater Baltimore Medical Center, Baltimore, MD, USA; Retina Foundation of the Southwest, Dallas, TX, USA; Moorfields Eye Hospital NHS Foundation, London, UK; Institut de la Vision, Centre Hospitalier National d’Ophtalmologie (CHNO) des Quinze-Vingts, Paris, France; Center for Ophthalmology, Eberhard-Karls University Hospital, Tübingen, Germany). The members of the SMART study group are provided in the online supplementary material (for all online suppl. material, see www.karger.com/doi/10.1159/000488711). The RC (located at the Doheny Imaging Reading Center, Doheny Eye Institute, David Geffen School of Medicine at University of California, Los Angeles (Los Angeles, CA, USA) was responsible for certifying clinical center staff on the acquisition of scotopic MP (sMP). Beyond the manual of procedures, an instructional movie was created by one of the authors (M.G.B.) available for participating staff via a locked website (http://www.progstar.org). Periodic quality assurance visits by the DCC to the clinical centers were carried out to monitor the accuracy of data entry and the completeness of records, in addition to ensuring that the clinical centers had standardized procedures for the collection of prospective data. The RC was responsible for grading sMP results. Two RC-certified graders independently reviewed images. At least one of the graders was a senior-level grader. Discordant initial assessments underwent adjudication. If consensus could not be reached between 2 adjudicating graders, an RC investigator determined the final assessment. After being processed and analyzed by the RC, all data derived from grading were transferred electronically from the RC to the DCC. All data were entered into a custom-built database in REDCap (available at http://www.project-redcap.org/cite.php) and checked for completeness and consistency by the DCC.
Study Procedures: Assessment of Rod Function
The ProgStar and SMART studies collected data every 6 months over 24 months. Therefore, up to 5 visits took place during the study (baseline, months 6, 12, 18, and 24). If enrollment and prospective evaluation of an eligible participant in the prospective ProgStar study started significantly earlier than the start of the SMART study, then only 6, 12, or 18 months of follow-up were available. The last visit of the prospective ProgStar study was also the last SMART study visit. At each visit, all participants underwent microperimetric examination under scotopic condition in addition to the procedures included in the ProgStar Study protocol (i.e., BCVA, complete ophthalmic exam [including dilated fundoscopy], FAF imaging, mesopic MP and SD-OCT. Full-field electroretinogram according to the International Society for Clinical Electrophysiology of Vision (ISCEV) standards was performed at the baseline visit (or within the previous 5 years) [12]. sMP was performed after mesopic MP in the designated study eye prior to image acquisition SD-OCT and FAF images. However, only at the first examination were OCT scans used to center the grid pattern onto the anatomical fovea. The acquisition protocols for FAF, SD-OCT and mesopic MP in ProgStar have been published previously in detail [4]. Briefly, FAF images were obtained using a Heidelberg Engineering device such as Spectralis™ OCT (with or without BluePeak), Spectralis™ OCT Plus (with or without BluePeak), Spectralis™ FA + OCT, Spectralis HRA + OCT, Spectralis™ HRA). During the acquisition process, the concept of short-wavelength reduced-illuminance autofluorescence imaging was applied during FAF acquisition by reducing the laser beam to 25% of its conventional setting and only higher powers if necessary [4]. For this purpose, a special software tool was developed and provided by Heidelberg Engineering to all participating sites in the ProgStar study setting [4, 13].
Acquisition protocols are provided for both mesopic and scotopic MP in the online supplementary material. Figure 1 illustrates the different grid patterns for mesopic and scotopic MP. Both utilized spot size 3. SMART assessed the scotopic macular sensitivity driven by rod cells using controlled fundus perimetry under dark-adapted conditions. Only one eye per participant was enrolled in the SMART study and was tested. The choice of the study eye was based on the following recommended selection criteria, although the final decision was at the PI’s discretion:
The eye that had the smaller lesion within the range defined in the prospective ProgStar study protocol.
The eye that had the better vision in terms of BCVA.
The eye that had the better fixation stability, which was defined by the smaller bivariate contour ellipse area (BCEA) in mesopic MP.
Fig. 1.
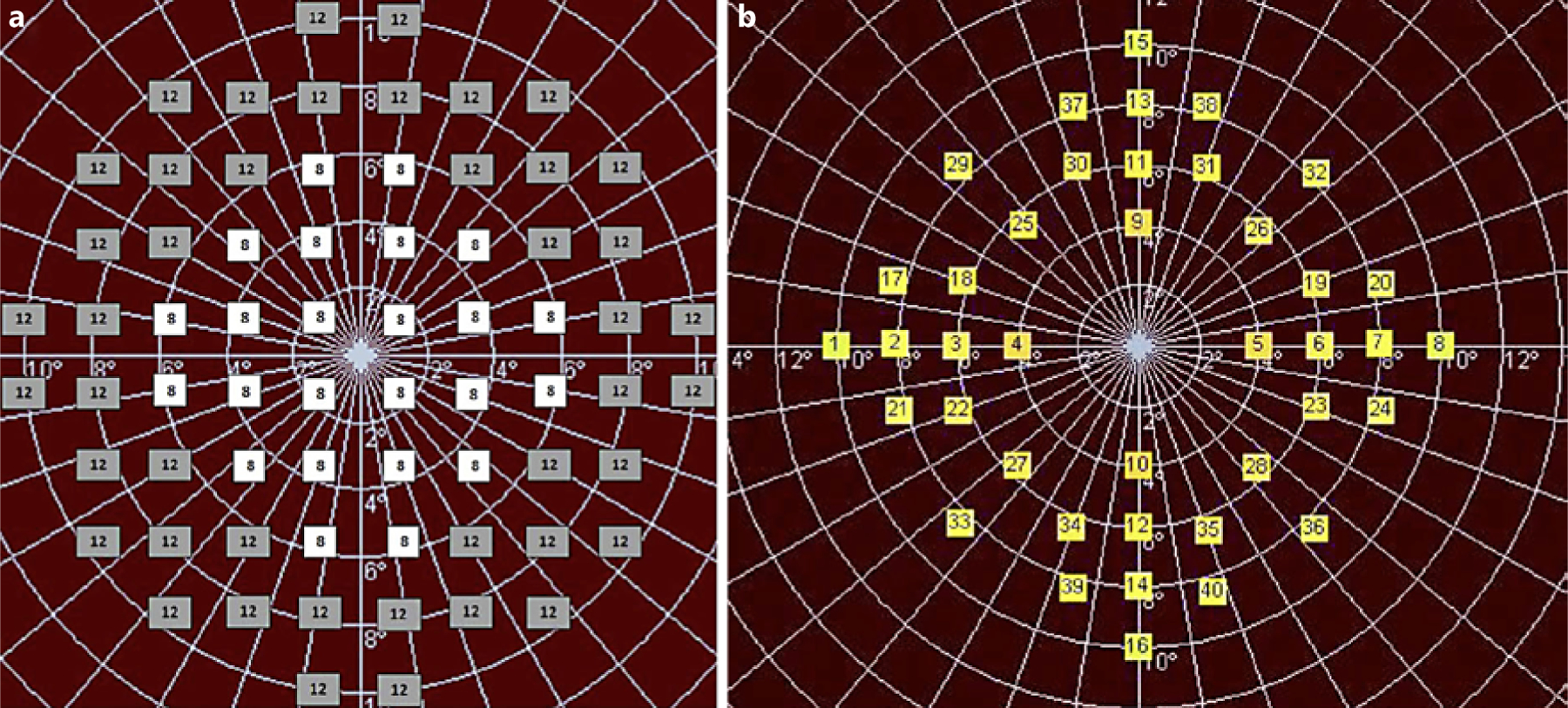
Grid pattern for mesopic (a) and scotopic (b) microperimetry exam. Retinal sensitivity at each location was determined by iteratively adjusting the light intensity until the dimmest visible stimulus for each point was found. The scotopic exam was the last microperimetric exam during the standardized ProgStar study visit. Therefore, the scotopic grid was designed to minimize the effects of exhaustion with a reduced number of test locations. Its pattern also considers the foveal rod-free region.
The study eye was dark-adapted for 30 min after the mesopic MP by covering it with light-tight eye padding (while dark-adapting, the fellow eye was tested with mesopic MP if also enrolled into the prospective ProgStar study). Exact times of covering and uncovering the eye were recorded. It was assured that no light leaked through the pad borders during dark adaptation. No light was allowed in the testing room during sMP, except by the light from the operator’s monitor, which was covered by a red filter; room luminance was <0.1 lux as assessed during the site certification process prior to enrollment of the first participant.
A blue filter + 1 neutral density filter was inserted in the MP-1 device prior to sMP. Forty retinal locations were assessed by iteratively adjusting the light intensity until the dimmest visible stimulus was found. At the baseline visit, the grid was centered on the participant’s anatomical fovea regardless of the her/his fixation location. Examiners identified the anatomical fovea on a previously-obtained SD-OCT image, and used that positional information to guide the manual placement of the grid. In cases of foveal atrophy, the graders looked for the point of maximal inner retinal layer convergence and used the adjoining B-scans immediately superior and inferior to the approximate foveal center in order to determine the center as precisely as possible. From May 18, 2015, an alignment (“Fovea on OCT”) tool provided by Nidek® was applied for the import of a participant’s SD-OCT image (acquired with a Heidelberg Engineering® SD-OCT device) for the automatic centration of the MP test pattern grid onto the anatomical fovea.
Grading of FAF and MP Exams
Fundus Autofluorescence
Atrophic lesions on FAF images were graded by RC using a semiautomated software tool (RegionFinder; Heidelberg Engineering) according to previously established grading protocols [13, 14]. Two distinct types of areas of decreased autofluorescence were quantified. Herein, the level of darkness was used to define an area of decreased autofluorescence qualitatively as being definitely or questionably reduced. Blood vessels and the optic nerve head served as the reference point for 100% level of darkness. Definitely decreased autofluorescence (DDAF) describes areas in which the level of darkness was close to 100% (at least 90%; Fig. 2). Regions with levels approximately between 50 and 90% darkness were defined as questionably decreased autofluorescence (QDAF; Fig. 2) [15].
Fig. 2.
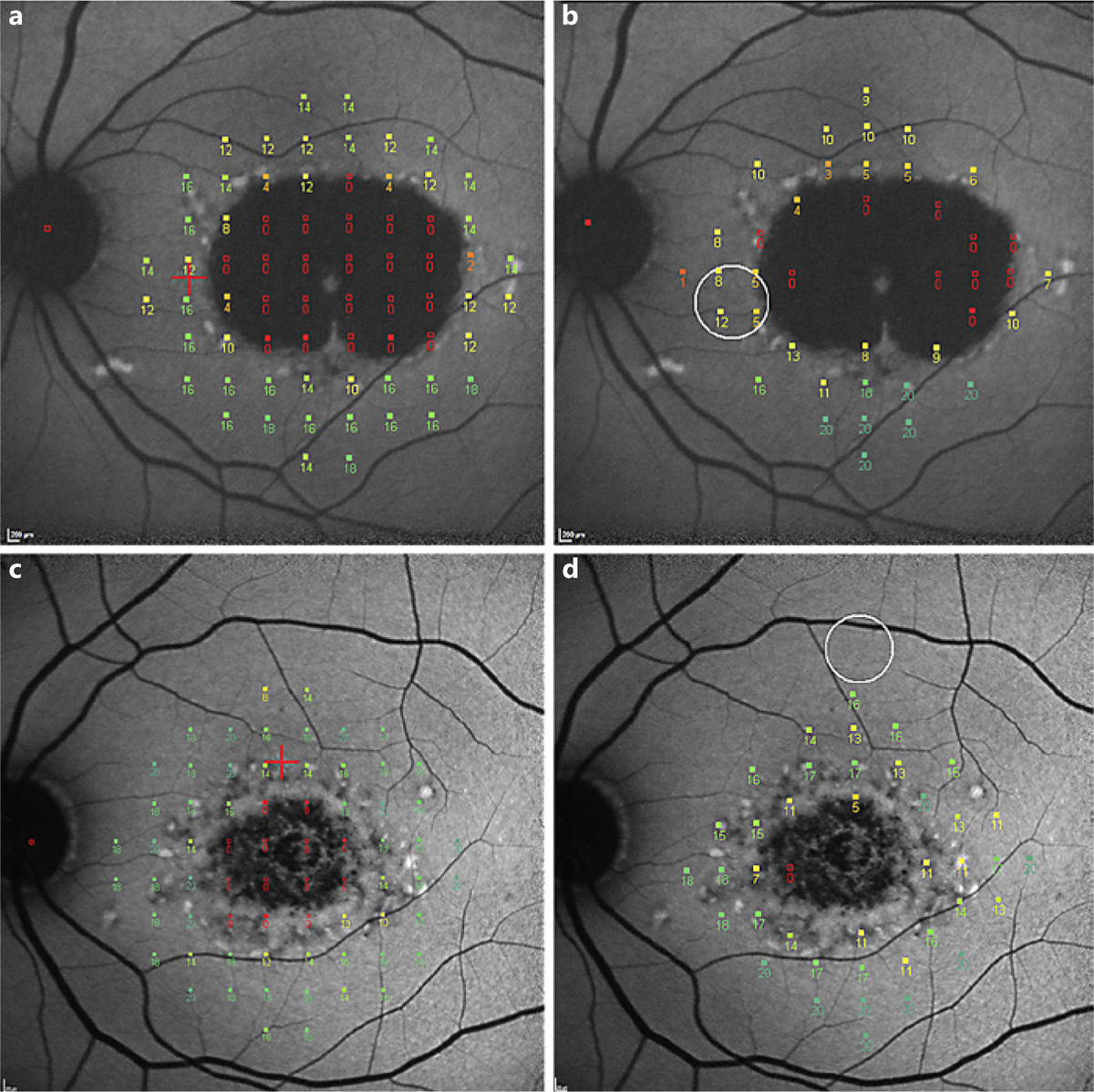
Results from mesopic (a, c) and scotopic (b, d) microperimetric exams super-imposed onto the corresponding fundus autofluorescence images of corresponding eyes. The eye shown in a and b shows a lesion of definitely decreased autofluorescence (this type of lesion has at least 90% darkness level compared to the optic nerve head [OHN]). The eye in c and d shows a lesion of questionably decreased autofluorescence (this lesion has darkness levels between 50– and 90% compared to OHN). Sensitivity values for the individual locations (range 0–20 dB) are shown.
Microperimetry
A scale of 0–20 dB served to determine the sensitivity for each test location. The term “deep scotoma” was defined for test locations with 0 dB (i.e., retinal locations where only the brightest stimulus was detected or no stimulus at all was detected), and the term “relative scotoma” for test locations with sensitivity better than 0 dB but less than 12 dB [16]. Mean sensitivity across all tested locations, and number of absolute and relative scotoma were calculated.
Fixation stability was recorded (dynamic testing), which created a cloud of fixation events for each test session [17]. Fixation stability was then quantified as a continuous variable, the BCEA. The BCEA offers clear advantages over categorical biomarkers of fixation stability in STGD1 [18]. The global BCEAs for 1, 2 and 3 standard deviations were calculated using the following equation:
σH and σV are the standard deviations of horizontal and vertical eye movements, ρ is the Pearson product-moment correlation co-efficient of fixation positions in the horizontal and the vertical meridian, k is a constant dependent on the chosen probability area which is given by the equation:
P is the probability area and e is the base of the natural logarithm. P is the chosen probability for the SD that the BCEA is based on, and the equation is solved for k [16–18]:
Ethics
The studies were conducted according to the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP) Guidelines, the applicable regulatory requirements, and the current Declaration of Helsinki and are in compliance with the Health Insurance Portability and Accountability Act. Ethics committee approval was granted by the local institutional review boards before enrollment of the first participant. The studies were registered at www.clinicaltrials.gov (identifier, NCT01977846). All participants gave written informed consent prior to enrollment.
Results
A total of 259 participants (489 eyes) were enrolled between October 21, 2013, and January 30, 2015 into the ProgStar study, 230 participants (88.8%) with both eyes. During ProgStar, 118 eyes of 118 participants were enrolled into SMART: in 41 (30.8%) participants, the first SMART visit was also at the time of ProgStar study baseline visit, 75 (56.4%) were enrolled into SMART during the 6-month follow-up visit of ProgStar, and 17 (12.8%) during the 12-month visit.
The characteristics of the participants at their respective first visit in SMART are summarized in Table 1. All eyes had according to the inclusion criteria a lesion of at least QDAF. Of the 118 eyes enrolled into SMART, 76 (65.5%) eyes had a lesion of DDAF at the first visit. The mean lesion size of DDAF was 3.46 (±3.60; range 0.21–21.46) mm2. The mean total lesion size (QDAF and/or DDAF) was 3.62 (±3.48; 0.10–21.46) mm2.
Table 1.
Demographic characteristics at baseline visit of participants enrolled in the SMART study at the first visit (n = 118)
| Age | |
| At first visit, mean ± SD, years | 34.5±15.1 |
| Younger than 18 years | 19 (16.1%) |
| 18–29 years | 29 (24.6%) |
| 30–39 years | 32 (27.1%) |
| 40–49 years | 18 (15.3%) |
| 50–59 years | 12 (10.1%) |
| Older than 60 years | 8 (6.8%) |
| Gender | |
| Female | 62 (53%) |
| Male | 56 (47%) |
| Race | |
| White/Middle Eastern | 98 (83%) |
| Black | 11 (9%) |
| Asian/Indian | 6 (5%) |
| Other/multiracial | 1 (1%) |
| Unknown | 2 (2%) |
| Latino | |
| Yes | 3 (2.5%) |
| No | 114 (96.6%) |
| Could not be determined | 1 (0.9%) |
| Smoking historya | |
| Never smoker | 85 (72.6%) |
| Former smoker | 20 (17.1%) |
| Current smoker | 12 (10.3%) |
| Vitamin A supplementationa | |
| Yes | 15 (12.8%) |
| No | 102 (87.2%) |
Information missing for 1 participant.
Mean sensitivity in mesopic MP at the first visit was 11.48 (±5.05; 0.00–19.88) dB and in sMP 11.25 (±5.26; 0–19.25) dB; further parameters derived from mesopic and scotopic MP at first visit of SMART are summarized in Table 2.
Table 2.
Baseline mesopic and scotopic microperimetry data of eyes enrolled in the SMART study at the first visit
| Mesopic microperimetry | Scotopic microperimetry | |
|---|---|---|
| Sensitivity, dB | 11.48±5.05 (0.00–19.88) | 11.25±5.26 (0–19.25) |
| Percentage of points with normal sensitivity absolute scotomaa | 64±32 (0–100) | 54±33 (0–100) |
| Percentage of points with relative absolute scotomaa | 15±15 (0–70) | 29±19 (0–85) |
| Percentage of points with absolute scotomaa | 20±25 (0–100) | 17±25 (0–100) |
| Distance of preferred retinal location to foveal center, degrees | 5.57±4.70 (0.00–26.00) | 5.84±4.36 (0.00–22.00) |
| Bivariate contour ellipse area - 2 standard deviations, deg2 | 30.34±37.07 (0.37–266.90) | 33.41±33.38 (1.81–234.89) |
Data are presented as mean ± standard deviation (range).
Percentage of points with normal function, relative and absolute scotoma are provided because the absolute numbers of tested points differed between mesopic (n = 68) and scotopic (n = 40) microperimetry.
In the electrophysiological assessment using full-field clinical electroretinography according to ISCEV standards (obtained either at first visit of SMART, or at the baseline visit of ProgStar, or within the past 5 years), 86 participants had a normal and 26 an abnormal ERG (missing for 6 participants).
Discussion
Several STGD1 treatment approaches including pharmacological, gene augmentation and stem cell-therapy are in early clinical phases, although there are currently no FDA-approved therapies [5, 6]. The success of clinical trials evaluating efficacy is largely dependent on sensitive and reproducible outcome measures. Visual acuity is unlikely to be an appropriate outcome measure for STGD1 treatment trials with 1-year duration given its slow and also inconsistent progression [19, 20]. ProgStar was therefore designed to evaluate different imaging modalities (FAF, SD-OCT) and psychophysical tests (mesopic MP) that may serve as surrogate endpoints. Testing rod function by dark-adapted MP may open additional pathways towards possible new visual function biomarkers.
From at least the beginning of the 4th decade of life, there is a progressive decline in the number of rods, at approximately 2 rods/mm2 every day [21]. Previous studies in age-related macular degeneration (AMD) found a pre-dilection for parafoveal loss of rods over cones in the early, nonexudative form of the disease with rod loss preceding and being more severe than cone loss both in histological examinations and psychophysical results [22]. Indeed, prominent dark-adapted dysfunction can be attributed to the rod system at relatively early stages of AMD, with greater magnitude of rod dysfunction than cone dysfunction [23]. Visual function measures can predict the risk of future VA loss in patients with GA and good baseline VA; hence, they may allow the identification of the highest risk group for VA loss and a more efficient design of clinical trials [24]. Recent clinical studies have shown that in AMD the sensitivity of the rods decreases more rapidly than the sensitivity of the cones [21]. Although AMD and STGD1 are two different pathophysiological entities, they may share some common pathogenic features such as the photo-oxidative processes initiated by retinal pigment epithelium bisretinoids which could explain suggested links to light exposure in both STGD1 and AMD [6]. Hence, testing scotopic visual function may be indeed a promising additional outcome measure that may start to progress at a different stage of the disease, at different rates, or in a different pattern compared to mesopic MP. Specific therapies, such as gene therapy, may have selective effects on the rod versus cone system given their different metabolism [22].
A limitation of our study is that comparison between mesopic and scotopic function of individual retinal locations is in general not possible based on the difference in patterns used in ProgStar and SMART: these allude both to the numbers of locations tested (40 vs. 64 of the standard) and the retinal locations themselves. This difference was partly to try to limit participant fatigue and increase cooperation/reliability; but also it is of note that under fully dark-adapted conditions, a central scotopic scotoma exists in all people with healthy retinas, corresponding to the foveal rod-free region (measuring about 0.35 mm and subtends approximately 1.25°) [25].
Testing scotopic function may serve as an additional potential outcome measure in clinical trials of STGD1. The baseline findings of SMART identify rod involvement in STGD1, and longitudinal serial testing of rod function with sMP in SMART, associated with comparison with the structural testing and mesopic MP in the ProgStar study will further enhance our understanding and inform clinical trial design.
Supplementary Material
Acknowledgments
The authors thank Melissa Kasilian for assistance with editing the figures of this article.
Disclosure Statement
Dr. Hendrik Scholl is supported by the Foundation Fighting Blindness Clinical Research Institute (FFB CRI); Shulsky Foundation, New York, NY; National Centre of Competence in Research (NCCR) Molecular Systems Engineering (University of Basel and ETH Zürich), Swiss National Science Foundation. Dr. Scholl is a paid consultant of the following entities: Boehringer Ingelheim Pharma GmbH & Co. KG; Gerson Lehrman Group; and Guide-point. Dr. Scholl is member of the Scientific Advisory Board of the Astellas Institute for Regenerative Medicine; ReNeuron Group Plc/Ora Inc.; and Vision Medicines, Inc. Dr. Scholl is member of the Data Monitoring and Safety Board/Committee of the following entities: Genentech Inc./F. Hoffmann-La Roche Ltd; and ReNeuron Group Plc/Ora Inc. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Johns Hopkins University and Bayer Pharma AG have an active research collaboration and option agreement. These arrangements have also been reviewed and approved by the University of Basel (Universitätsspital Basel, USB) in accordance with its conflict of interest policies. Dr. Hendrik Scholl is principal investigator of grants at the USB sponsored by the following entity: Acucela Inc.; NightstaRx Ltd.; Ophthotech Corporation; Grants at USB are negotiated and administered by the institution (USB) which receives them on its proper accounts. Individual investigators who participate in the sponsored project(s) are not directly compensated by the sponsor but may receive salary or other support from the institution to support their effort on the project(s).
David G. Birch is a consultant for AGTC (Alachua, FL, USA), Ionis (Carlsbad, CA, USA), Genentech (South San Francisco, CA, USA), Nightstar (London, UK), Nacuity (Ft. Worth, TX, USA). Dr. Birch is supported by the Foundation Fighting Blindness Clinical Research Institute (FFB CRI).
Janet Sunness is a consultant for Genentech, on the scientific advisory board of Acucela and Apellis, and on the data safety and monitoring committee for Cell Cure’s OpRegen study.
Dr. West is a scientific technical advisory committee member for the Alcon Research Institute, and for Research to Prevent Blindness.
Michael Ip is a consultant of Allergan, Thrombogenics, Omeros, Genentech, Quark, Alimera, and Boehringer Ingelheim.
None of the authors has a commercial conflict of interest related to the content of this article.
Funding Sources
This study was supported by the Foundation Fighting Blindness Clinical Research Institute. Rupert W. Strauss is supported by the Austrian Science Fund (FWF; Project number: J 3383-B23) and Foundation Fighting Blindness Clinical Research Institute. Etienne M. Schönbach is supported by the German National Academy of Sciences Leopoldina, Grant Number LPDS 2015-14. Michel Michaelides is supported by an FFB Career Development Award, the Macular Society, Fight for Sight, Retinitis Pigmentosa Fighting Blindness, and the National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology.
Footnotes
Some material from this article has been presented at the annual meeting of the Association for Research in Vision and Ophthalmology (ARVO), Denver, May 7–11, 2017.
References
- 1.Stargardt K: Ueber familiaere, progressive Degeneration in der Makulagegend des Auges. Graefes Arch Clin Exp Ophthalmol 1909; 71:534–549. [Google Scholar]
- 2.Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR: A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 1997;15:236–246. [DOI] [PubMed] [Google Scholar]
- 3.Tanna P, Strauss RW, Fujinami K, Michaelides M: Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol 2017; 101:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strauss RW, Ho A, Munoz B, Cideciyan AV, Sahel JA, Sunness JS, Birch DG, Bernstein PS, Michaelides M, Traboulsi EI, Zrenner E, Sadda S, Ervin AM, West S, Scholl HP: The natural history of the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) Studies: design and baseline characteristics: ProgStar Report No. 1. Ophthalmology 2016; 123:817–828. [DOI] [PubMed] [Google Scholar]
- 5.Scholl HP, Strauss RW, Singh MS, Dalkara D, Roska B, Picaud S, Sahel JA: Emerging therapies for inherited retinal degeneration. Sci Transl Med 2016;8:368rv366. [DOI] [PubMed] [Google Scholar]
- 6.Sears AE, Bernstein PS, Cideciyan AV, Hoyng C, Charbel Issa P, Palczewski K, Rosenfeld PJ, Sadda S, Schraermeyer U, Sparrow JR, Washington I, Scholl HPN: Towards treatment of Stargardt disease: workshop organized and sponsored by the Foundation Fighting Blindness. Transl Vis Sci Technol 2017;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun H, Nathans J: Stargardt’s ABCR is localized to the disc membrane of retinal rod outer segments. Nat Genet 1997;17:15–16. [DOI] [PubMed] [Google Scholar]
- 8.Molday LL, Rabin AR, Molday RS: ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat Genet 2000;25:257–258. [DOI] [PubMed] [Google Scholar]
- 9.Simonelli F, Testa F, Zernant J, Nesti A, Rossi S, Rinaldi E, Allikmets R: Association of a homozygous nonsense mutation in the ABCA4 (ABCR) gene with cone-rod dystrophy phenotype in an Italian family. Ophthalmic Res 2004;36:82–88. [DOI] [PubMed] [Google Scholar]
- 10.Fujinami K, Lois N, Davidson AE, Mackay DS, Hogg CR, Stone EM, Tsunoda K, Tsubota K, Bunce C, Robson AG, Moore AT, Webster AR, Holder GE, Michaelides M: A longitudinal study of Stargardt disease: clinical and electrophysiologic assessment, progression, and genotype correlations. Am J Ophthalmol 2013;155:1075–1088 e1013. [DOI] [PubMed] [Google Scholar]
- 11.Crossland MD, Luong VA, Rubin GS, Fitzke FW: Retinal specific measurement of dark-adapted visual function: validation of a modified microperimeter. BMC Ophthalmol 2011; 11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M: ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 2015;130:1–12. [DOI] [PubMed] [Google Scholar]
- 13.Strauss RW, Munoz B, Jha A, Ho A, Cideciyan AV, Kasilian ML, Wolfson Y, Sadda S, West S, Scholl HP, Michaelides M: Comparison of short-wavelength reduced-illuminance and conventional autofluorescence imaging in Stargardt macular dystrophy. Am J Ophthalmol 2016;168:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuehlewein L, Hariri AH, Ho A, Dustin L, Wolfson Y, Strauss RW, Scholl HP, Sadda SR: Comparison of manual and semiautomated fundus autofluorescence analysis of macular atrophy in Stargardt disease phenotype. Retina 2016;36:1216–1221. [DOI] [PubMed] [Google Scholar]
- 15.Strauss RW, Munoz B, Ho A, Jha A, Michaelides M, Mohand-Said S, Cideciyan AV, Birch D, Hariri AH, Nittala MG, Sadda S, Scholl HPN; ProgStar Study Group: Incidence of atrophic lesions in Stargardt disease in the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) Study: report No. 5. JAMA Ophthalmol 2017;135:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schonbach EM, Wolfson Y, Strauss RW, Ibrahim MA, Kong X, Munoz B, Birch DG, Cideciyan AV, Hahn GA, Nittala M, Sunness JS, Sadda SR, West SK, Scholl HPN; ProgStar Study Group: Macular sensitivity measured with microperimetry in Stargardt disease in the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) Study: report No. 7. JAMA Ophthalmol 2017;135:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schonbach EM, Ibrahim MA, Kong X, Strauss RW, Munoz B, Birch DG, Sunness JS, West SK, Scholl HPN: Metrics and acquisition modes for fixation stability as a visual function biomarker. Invest Ophthalmol Vis Sci 2017;58:BIO268–BIO276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schönbach EM, Ibrahim MA, Strauss RW, Birch DG, Cideciyan AV, Hahn GA, Ho A, Kong X, Nasser F, Sunness JS, Zrenner E, Sadda SR, West SK, Scholl HPN: Fixation location and stability using the MP-1 microperimeter in Stargardt disease ProgStar report No. 3. Ophthlamol Retina 2016;1:68–76. [DOI] [PubMed] [Google Scholar]
- 19.Kong X, Strauss RW, Michaelides M, Cideciyan AV, Sahel JA, Munoz B, West S, Scholl HP: Visual acuity loss and associated risk factors in the retrospective Progression of Stargardt Disease Study (ProgStar report No. 2). Ophthalmology 2016;123:1887–1897. [DOI] [PubMed] [Google Scholar]
- 20.Kong X, Strauss RW, Cideciyan AV, Michaelides M, Sahel JA, Munoz B, Ahmed M, Ervin AM, West SK, Cheetham JK, Scholl HPN, ProgStar Study Group: Visual acuity change over 12 months in the prospective Progression of Atrophy Secondary to Stargardt Disease (ProgStar) Study: ProgStar report number 6. Ophthalmology 2017;124: 1640–1651. [DOI] [PubMed] [Google Scholar]
- 21.Nebbioso M, Barbato A, Pescosolido N: Scotopic microperimetry in the early diagnosis of age-related macular degeneration: preliminary study. Biomed Res Int 2014;2014: 671529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curcio CA, Medeiros NE, Millican CL: Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci 1996;37: 1236–1249. [PubMed] [Google Scholar]
- 23.Owsley C, Jackson GR, Cideciyan AV, Huang Y, Fine SL, Ho AC, Maguire MG, Lolley V, Jacobson SG: Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci 2000;41: 267–273. [PubMed] [Google Scholar]
- 24.Sunness JS, Rubin GS, Broman A, Applegate CA, Bressler NM, Hawkins BS: Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology 2008;115:1480–1488.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curcio CA, Allen KA: Topography of ganglion cells in human retina. J Comp Neurol 1990;300:5–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


