Abstract
Traumatic peripheral nerve injury is a worldwide clinical issue with high morbidity. The severity of peripheral nerve injury can be classified as neurapraxia, axonotmesis or neurotmesis, according to Seddon’s classification, or five different degrees according to Sunderland’s classification. Patients with neurotmesis suffer from a complete transection of peripheral nerve stumps and are often in need of surgical repair of nerve defects. The applications of autologous nerve grafts as the golden standard for peripheral nerve transplantation meet some difficulties, including donor nerve sacrifice and nerve mismatch. Attempts have been made to construct tissue-engineered nerve grafts as supplements or even substitutes for autologous nerve grafts to bridge peripheral nerve defects. The incorporation of stem cells as seed cells into the biomaterial-based scaffolds increases the effectiveness of tissue-engineered nerve grafts and largely boosts the regenerative process. Numerous stem cells, including embryonic stem cells, neural stem cells, bone marrow mesenchymal stem cells, adipose stem cells, skin-derived precursor stem cells and induced pluripotent stem cells, have been used in neural tissue engineering. In the current review, recent trials of stem cell-based tissue-engineered nerve grafts have been summarized; potential concerns and perspectives of stem cell therapeutics have also been contemplated.
Keywords: Peripheral nerve injury, Tissue-engineered nerve grafts, Seed cells, Stem cells, Peripheral nerve regeneration
Highlights.
Traumatic peripheral nerve injury is a common clinical issue that leads to nerve dysfunction and chronic pain.
Tissue-engineered nerve grafts containing bio-scaffolds, seed cells, and neurotrophic factors can be used as alternatives to substitute autologous nerve grafts for repairing peripheral nerve injury.
Stem cells can differentiate into Schwann-like cells, provide a suitable microenvironment for nerve regeneration, and thus are considered as ideal seed cells.
Background
Peripheral nerve injury is a universal clinical issue with an estimated incidence of 13.9–23 per 100,000 persons per year [1,2]. Unlike nerves in the central nervous system, nerves in the peripheral nervous system obtain certain spontaneous regenerative abilities following nerve injury. Accordingly, the severities and consequences of peripheral nerve injury-induced neuropathies are generally flexible [3]. Patients with mild peripheral nerve injuries may recover, while patients with severe peripheral nerve injuries and long nerve defects often suffer from impaired motor, sensory and autonomic nerve functions and are in need of peripheral nerve repair surgeries. According to the National Center for Health Statistics based on Classification of Diseases, 9th Revision, Clinical Modification for the following categories: ICD-9 CM Code: 04.3, 04.5, 04.6, 04.7, more than 50,000 peripheral nerve repair procedures were performed in the year of 1995 [4]. It is reported that, in the recent year, more than 200,000 patients received peripheral nerve repair procedures, causing an excessive burden on economy and society [5,6].
Review
Anatomy and classification of peripheral nerve injury
Peripheral nerve injury, especially traumatic peripheral nerve injury, is categorized according to a variety of classes or grades by neuroscientists and surgeons to aid in prognosis and treatment. In 1943, Sir Herbert Seddon introduced a classification system and described three classes of peripheral nerve injury—neurapraxia, axonotmesis and neurotmesis (Figure 1)—based on the severity of nerve injury, the recovery time and the prognosis [7].
Figure 1.
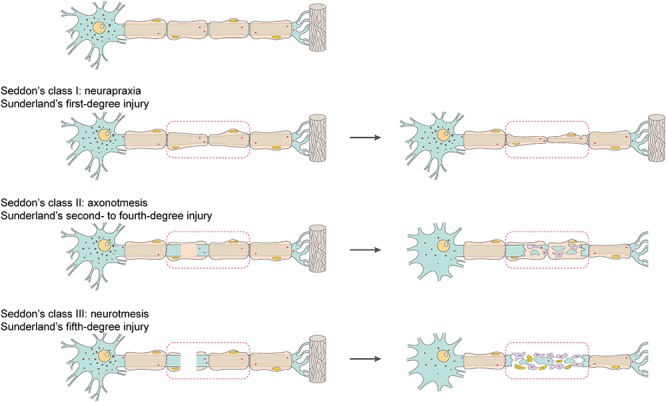
Schematic representation of the classification of peripheral nerve injury. The severity of peripheral nerve injury is classified as class I (neurapraxia), class II (axonotmesis) or class III (neurotmesis) by Seddon and first- to fifth-degree by Sunderland
Neurapraxia (class I), a nerve injury commonly induced by focal demyelination and/or ischemia, is the mildest type of peripheral nerve injury [8]. In neurapraxia, the conduction of nerve impulses is blocked in the injured area, motor and sensory connection is lost, but all morphological structures of the nerve stump, including the endoneurium, perineurium and epineurium, remain intact. Since the axon is not separated from the soma in neurapraxia, Wallerian degeneration does not occur. The injured peripheral nerve generally achieves full recovery of nerve conduction and function, although the recovery process may be highly variable, from hours and days to weeks or even a few months [8]. Axonotmesis (class II) is a comparatively more severe type of peripheral nerve injury and is normally caused by crush, stretch or percussion [8]. In axonotmesis, the epineurium is intact, while the perineurium and endoneurium may be disrupted [8]. The axon is separated from the soma and the axon and the myelin sheath are disrupted; Wallerian degeneration occurs in the axon stump distal to the injury site within 24–36 hours after peripheral nerve injury. There are sensory and motor deficits, as well as nerve conduction failure distal to the injury site in this degree of nerve injury. Remaining surrounding stroma benefits axonal elongation along the intact tissue framework. Functional recovery can be expected if the injured nerve stump retains a certain level of integrity of the physiological structure and organization. The prognosis of axonotmesis also largely depends on the distance of the site of lesion to the target organ. However, for most cases, self-regeneration is extremely limited and appropriate surgical intervention is required. Neurotmesis (class III) is caused by nerve transection or neurotoxins and is the most severe degree of peripheral nerve injury. In neurotmesis, the entire nerve stump, including the endoneurium, perineurium and epineurium, is completely severed. Neurotmesis leads to the rupture of axon, myelin sheath and connective tissues and, correspondingly, results in poor prognosis.
Sunderland, in 1951, expanded Seddon’s classification, especially regarding axonotmesis, to five degrees [9]. Sunderland’s first-degree injury indicates the lowest degree of nerve injury and is equal to Seddon’s neurapraxia. Sunderland’s second-, third- and fourth-degree injuries are equal to Seddon’s axonotmesis. In Sunderland’s second-degree injury, the endoneurial tubes, perineurium and epineurium remain intact, although the axon is disrupted. In Sunderland’s third-degree injury, besides the disruption of axon at the injured site, the continuities of the endoneurium and perineurium are also lost. In Sunderland’s fourth-degree injury, only the integrity of the epinurium is left—the continuities of the axon, endoneurium and perineurium are impaired [10]. Sunderland’s fifth-degree injury corresponds to the definition of neurotmesis in Seddon’s classification and represents the highest degree of nerve injury, with a complete nerve defect. To avoid excess tension, these severe peripheral nerve injuries with long nerve gaps require nerve graft implantation for surgical intervention instead of neurorrhaphy [11].
Tissue-engineered nerve graft-based treatment of peripheral nerve injury
The transplantation of autologous nerve graft is the golden standard for the treatment of severe peripheral nerve injury. Nevertheless, the application of autologous nerve graft has several critical and insurmountable disadvantages, including the sacrifice of a healthy donor nerve, limited sources of donor nerves, donor site morbidity and donor nerve mismatch [12]. The transplantation of xenogeneic or allogeneic nerve grafts may solve the issue of limited sources, but may induce severe immunological problems [13]. Under the circumstances, tissue-engineered nerve grafts have emerged as an effective treatment of severe peripheral nerve injury.
A tissue-engineered nerve graft is an artificial nerve graft constructed of a biomaterial-based scaffold, seed cells and neurotrophic factors [14,15]. The biomaterial-based scaffold offers a physical structural support for the growth and elongation of injured nerves. Incorporated seed cells and neurotrophic factors further enhance the therapeutic effect of the biomaterial-based scaffold [16,14]. A variety of materials, including synthetic materials such as polyglycolic acid and poly(lactin-co-glycolic acid), as well as natural materials, such as chitosan, silk fibroin, extracellular matrix components, polysaccharides and metallic materials, have been used to construct neural scaffolds [15]. Many neurotrophic factors, such as nerve growth factor, brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor, ciliary neurotrophic factor and neurotrophin-3, are commonly applied [14]. Schwann cells, as the main glial cells in the peripheral nervous system and the main structural and functional cells in peripheral nerve regeneration, are utilized as natural seed cells [17].
After peripheral nerve injury, Schwann cells adaptively respond to axonal interruption, switching from a highly myelinated state to a de-differentiated state. De-differentiated Schwann cells engulf axon and myelin debris and form a regeneration path for axon growth [18,19]. Moreover, activated Schwann cells secrete a group of cytokines, including tumor necrosis factor α, interleukin-1α and leukemia inhibitory factor, to recruit macrophages and facilitate debris digestion. Schwann cells also secrete a group of neurotrophic factors, including nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor, to encourage neuron survival and axon elongation [20,21,18]. For peripheral nerve injuries with a long nerve defect (longer than 4–5 cm in human patients), the bio-scaffold alone may not achieve satisfactory repair effects and the application of Schwann cells or Schwann-like cells, combined with a nerve graft, is essential [16,22]. For instance, it is reported that hollow autogenous venous nerve conduit can only repair a peripheral nerve defect of up to 3 cm in length in rabbit or human, while a Schwann cells-added nerve conduit can repair a 6 cm nerve gap [23–25]. Schwann cells have been also used as excellent seed cells to promote the regeneration of other types of tissues, such as skin [26]. Nevertheless, the use of Schwann cells in tissue-engineered nerve grafts has some significantly distinct drawbacks, including the surgical need to collect autologous Schwann cells and the difficulty in culturing and expanding Schwann cells to adequate amount. Although the method for culturing Schwann cells has improved and the required culture time has been shortened, it can still take 2 weeks to obtain enough Schwann cells, with a high degree of purity, for transplantation [27,28]. The use of xenogeneic or allogeneic Schwann cells, on the other hand, may induce immunological rejection similar as the use of xenogeneic or allogeneic nerve grafts [29]. The effect of allogeneic Schwann cells has been examined by transplanting nerve conduits filled with allogeneic genetically labeled Schwann cells. Allogeneic Schwann cells are rejected 6 weeks after in vivo transplantation without immunosuppressive therapy [30].
Compared with Schwann cells, undifferentiated stem cells have a strong expansion capacity. Stem cells can differentiate to numerous specialized cell types, including Schwann cells. In addition, a variety of types of stem cells, such as stem cells taken from umbilical cord blood after birth, bone marrow stem cells and adipose stem cells, can be collected from an autograft to reduce immunogenicity. Therefore, stem cells exhibit great clinical potentials and may be used as seed cells for the construction of cell-based tissue-engineered nerve grafts.
Applications of stem cells in neural tissue engineering
For the generation of stem cell-based tissue-engineered nerve grafts, stem cells are generally isolated, cultured, expanded and incorporated into a biomaterial-based scaffold in vitro. The constructed tissue-engineered nerve graft is then sutured to the injured site to bridge the peripheral nerve defect. Following nerve graft transplantation, stem cells differentiate into Schwann-like cells, secrete proteins that can accelerate axon growth, such as neurotrophic factors and extracellular matrix components, provide a favorable microenvironment and promote myelin formation and nerve regeneration (Figure 2). Stem cells from many different sources have been applied in neural tissue engineering, and some extensively used stem cells are introduced in Table 1.
Figure 2.
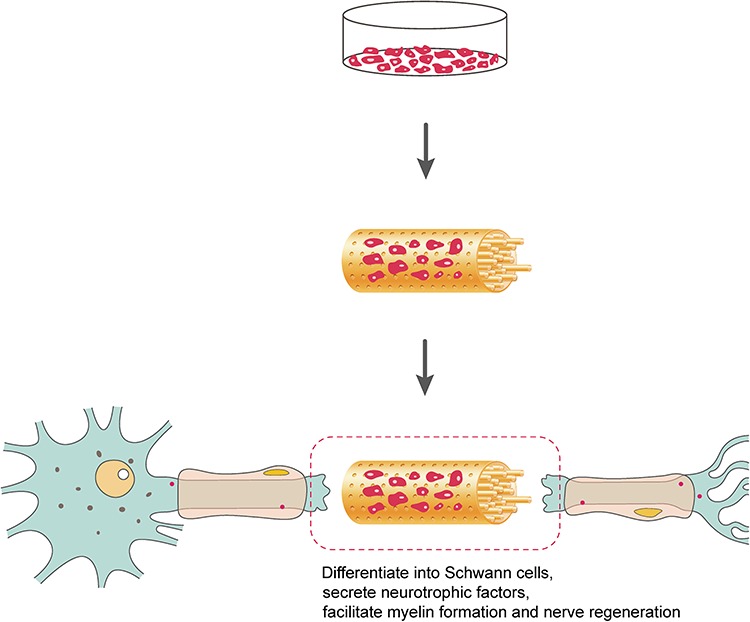
Schematic representation of the repair of peripheral nerve damage with a stem cell-based tissue-engineered nerve graft. Stem cells are isolated, cultured, expanded and incorporated into a neural scaffold containing an outer porous nerve conduit and numerous inner luminal fillers to construct a stem cell-based tissue-engineered nerve graft. Implanted stem cells differentiate into Schwann cells, secrete neurotrophic factors and promote peripheral nerve regeneration
Table 1.
Effects of stem cell-based tissue-engineered nerve grafts
| Cell | Scaffold | Effect | Reference |
| Neural crest cells derived from human embryonic stem cells | Tubular conduit manufactured from trimethylene carbonate ε-caprolactone block-copolymer | Stimulate sciatic nerve regeneration and the expression of repair-related genes | [34] |
| Neural stem cells | Neurotrophin-3-incorporated hyaluronic acid–collagen conduit | Facilitate re-innervations of damaged facial nerve | [40] |
| Neural stem cells | Nerve growth factor-incorporated chitosan/collagen conduit | Increase BrdU-positive cells in bridge grafting, promote nerve repair | [41] |
| Bone marrow mesenchymal stem cells | Silk fibroin-based scaffold | Accelerate axonal growth, increase gene expressions of S100, brain-derived neurotrophic factor, ciliary neurotrophic factor and basic fibroblast growth factor | [49] |
| Bone marrow stromal cells | Silicone tube | Improve walking behavior, reduce loss of gastrocnemius muscle weight and electromyographic magnitude, increase the number of regenerating axons within the tube | [53] |
| Bone marrow stromal cell- derived Schwann cells | Trans-permeable tube filled with three-dimensional collagen | Is safe and effective for accelerating the regeneration of transected axons and for functional recovery of injured nerves | [56] |
| Autologous bone marrow mesenchymal stem cells | Chitosan/poly(lactic-co-glycolic acid) scaffold | Exhibit more efficient nerve recovery in locomotive activity observation, electrophysiological assessments and FluoroGold retrograde tracing tests | [57] |
| Undifferentiated and differentiated adipose-derived stem cells | Silicone conduit containing type I collagen gel | Exhibit functional recovery of facial nerve regeneration close to that in autologous nerve graft positive controls | [58] |
| Schwann cell-like differentiated adipose-derived stem cells | Fibrin conduit | Improve axonal and fiber diameter, reduce muscle atrophy, evoke potentials at the level of the gastrocnemius muscle and regeneration of motor neurons | [63] |
| Induced pluripotent stem cells | Poly l-lactide and poly ε-caprolactone composed, two-layered bioabsorbable polymer tube | Show more vigorous axonal regeneration, faster recovery of motor function, assessed by the print length factor, and faster recovery of sensory function assessed by the time of foot withdrawal reflex | [79] |
| Human induced pluripotent stem cell-derived neural crest-like cells | Silicone tube | Enhance myelination and angiogenesis, promote axonal regrowth and motor functional recovery | [82] |
Embryonic stem cells
Embryonic stem cells are pluripotent stem cells that can differentiate to all three embryonic germ layers and form all types of cells or tissues of the body, except for fetal cells. Neurospheres derived from human embryonic stem cells can differentiate to cells with morphological and molecular features of Schwann cells and physical interactions with axons [31]. Schwann cells differentiated from human embryonic stem cells can not only express Schwann cell markers, glial fibrillary acidic protein, S100 and p75, but also induce the myelination of dorsal root ganglia neurons [32]. The direct microinjection of mouse embryonic stem cell-derived neural progenitor cells into the surrounding epineurium as a natural conduit after a 1 cm rat sciatic nerve transection leads to extensive morphological and functional recovery. Injected stem cells survive and differentiate to myelin-forming cells up to 3 months after cell transplantation [33]. Neural crest cells derived from human embryonic stem cells produce a range of bioactive trophic factors, stimulate the outgrowth of neurites when co-cultured with neurons in vitro and promote the regeneration of injured rat sciatic nerves when seeded into a biodegradable nerve conduit to bridge peripheral nerve gaps in vivo [34]. Besides embryonic stem cells, many other fetal-derived stem cells, including amniotic tissue-derived stem cells, umbilical cord-derived mesenchymal stem cells and Wharton’s Jelly mesenchymal stem cells, are also applied in stem cell-based nerve regeneration therapies [35].
However, embryonic stem cells have tumorigenic properties and may induce the formation of teratomas [36,37]. In addition, the usage of embryonic stem cells poses ethical uncertainty. Adult stem cells, on the contrary, generally do not trigger ethical controversy and are considered as suitable seed cells in tissue engineering and regenerative medicine.
Neural stem cells
Neural stem cells, as the primordial cells in the nervous system, are an essential cell source of neurons and glial cells and an important cell source for nerve regeneration [38]. Transplanted neural stem cells in injured peripheral nerves can differentiate into neurons and Schwann-like cells; secrete many critical neurotrophic factors, such as brain-derived neurotrophic factor, fibroblast growth factor, nerve growth factor, insulin-like growth factor and hepatocyte growth factor; and encourage angiogenesis, nerve growth and myelin formation [39]. Neural stem cells can be embedded and expanded in a neurotrophin-3 composited hyaluronic acid–collagen conduit. The transplantation of the neural stem cell-based nerve conduit to a transected rabbit facial nerve increases the voltage amplitude of electromyography and facilitates facial nerve repair [40]. A comparison study shows that neural stem cell-combined nerve conduits exhibit a similar regenerative effect as nerve autografts and a better regenerative effect than nerve conduits without seed cells when repairing a 10 mm rabbit facial nerve defect [41]. Engineered neural stem cells that over-express glial cell line-derived neurotrophic factor, as compared with normal neural stem cells, exhibit even better regenerative abilities in repairing both acute and chronic peripheral nerve injury [42,43]. A mechanism study showed that implanted neural stem cells increase the abundance of IL12p80, which stimulates Schwann cell differentiation and promotes the functional recovery of injured peripheral nerves [44]. In spite of the encouraging repairing effects of neural stem cells, the clinical use of neural stem cells may be limited by the difficulty in collecting them and the possibility of tumor formation [45].
Bone marrow mesenchymal stem cells
Mesenchymal stem cells are multipotent adult stem cells that can be found in many tissues, such as bone marrow, umbilical cord blood, peripheral blood, fallopian tube and lung. Bone marrow mesenchymal stem cells can be easily collected through the aspiration of the bone marrow in a standardized method and then expanded on a large scale for subsequent applications. Moreover, cultured bone marrow mesenchymal stem cells lack immune recognition, have immunosuppressive action and can be allogenically transplanted without inducing immune rejection [46,47]. Bone marrow mesenchymal stem cells have been reported as one of the most widely used cell sources for nerve regeneration.
Bone marrow mesenchymal stem cells can differentiate to Schwann-like cells and boost neurite outgrowth when co-cultured with neurons [48]. Yang et al. showed that seeding bone marrow mesenchymal stem cells as supporting cells into a silk fibronin-based nerve conduit increases the expression of Schwann cell marker S100, elevates the secretion of many growth factors, including brain-derived neurotrophic factor, ciliary neurotrophic factor and basic fibroblast growth factor, and supports the histological and functional recovery of rats with sciatic nerve injury [49]. Zhao et al. also demonstrate that, compared with the plain nerve graft, the acellular nerve graft supplemented with bone marrow mesenchymal stem cells exhibits better repairing effects in axon growth, target muscle preservation and walking track when bridging a 10 mm sciatic nerve defect in mice [50].
The promoting effects of bone marrow mesenchymal stem cells on peripheral nerve regeneration may be complex and may not only depend on their differentiation into Schwann-like cells [16]. Elevated immunostaining of vascular endothelial growth factor is detected after the application of Schwann-like cells induced from bone marrow mesenchymal stem cells, suggesting that bone marrow mesenchymal stem cells may also contribute to angiogenesis [51]. Bromodeoxyuridine (BrdU) labeling of injected bone marrow mesenchymal stem cells reflects that 5% of BrdU cells express Schwann cell marker [52]. Moreover, Chen et al. found that bone marrow mesenchymal stem cells obtained from rat bilateral femurs and tibias grow to fibroblast-shaped cells instead of Schwann-like cells. Still, bone marrow mesenchymal stem cells secrete many neurotrophic factors, such as nerve growth factor, brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor, and ciliary neurotrophic factor, as well as many extracellular matrix components, such as collagen, fibronectin and laminin. The transplantation of a silicone tube containing bone marrow mesenchymal stem cells to a rat sciatic nerve gap improves rat walking behavior, reduces muscle atrophy and stimulates axon regeneration [53].
Besides rodents, bone marrow mesenchymal stem cells have been applied to repair long nerve gaps in larger animals, such as dogs [54] and goats [55]. Bone marrow mesenchymal stem cells are also used to treat peripheral nerve injuries in primates. Schwann cells induced from bone marrow mesenchymal stem cells are filled into a biodegradable conduit filled with collagen sponge to bridge a 20 mm cynomolgus monkey median nerve defect. Transplanted cells accelerate the growth of axons and the recovery of electrophysiological parameters without massive cell proliferation or any observable abnormalities [56]. Similarly, another study revealed that the repairing effect of a chitosan/poly(lactic-co-glycolic acid)-based autologous bone marrow mesenchymal stem cell-containing tissue-engineered nerve graft is much better than the plain nerve conduit in repairing a 50 mm median nerve defect in rhesus monkeys. Implanted autologous marrow mesenchymal stem cells do not induce any detectable abnormalities in blood tests or histopathological examination [57].
Adipose stem cells
Although the benefits of bone marrow mesenchymal stem cells in peripheral nerve regeneration have been well demonstrated, the use of bone marrow mesenchymal stem cells can be invasive, limited by tissue source and ethically controversial [58]. Compared with other types of stem cells, adipose stem cells can be collected by a less invasive liposure procedure and therefore exhibit great clinical potential [58]. The proliferation and differentiation capabilities of adipose stem cells are much higher than many other adult stem cells as well [59]. Moreover, adipose stem cells can be induced into spindle shaped cells that express Schwann cell markers, secrete neurotrophic factors, stimulate neurite outgrowth and form myelin sheaths [60,61]. These advantages of adipose stem cells make them a good cell source for transplantation strategies.
For instance, adipose stem cells can be collected from visceral fat encasing the stomach and intestines and seeded into a fibrin nerve conduit to construct an adipose stem cell-based tissue-engineered nerve graft. Morphological and biochemical studies demonstrate that implanted adipose stem cells differentiate into a Schwann cell phenotype. Functional studies demonstrate that implanted adipose stem cells improve the mean amplitudes of compound muscle action potential, the atrophy of target muscle and axonal and fiber diameter after transplantation to bridge a 1 cm rat sciatic nerve gap. The regenerative effect of an adipose-derived stem cell-seeded fibrin conduit is similar to the autograft but much better than a primary Schwann cell-seeded fibrin conduit or a Schwann cell-like differentiated bone marrow-derived mesenchymal stem cell-seeded fibrin conduit [62,63]. In another study, undifferentiated adipose stem cells or adipose stem cells that are differentiated into a Schwann cell phenotype are incorporated into silicone nerve conduits containing type I collagen gel to bridge rat facial nerve defects of 7 mm. Both the applications of undifferentiated and differentiated adipose stem cells increase myelinated fiber numbers and myelin thickness and bring functional improvement of facial palsy without neuroma formation [58]. Adipose stem cells are also applied by embedding adipose stem cells in fibrin glue and covering the injured nerve with cell-containing fibrin glue. Fibrin glue provides additional extracellular support, while adipose stem cells not only encourage the restoration of blood supply and motor function, but also retrogradely protect the survival of dorsal root ganglion sensory neurons [64]. It is worth noting that several factors, such as donor age and harvest site/layer, may affect and even limit the growth properties of adipose stem cells [65–67]. Therefore, to maximize the regenerative abilities of adipose stem cells, the quality of applied adipose stem cells should be strictly controlled.
Skin-derived precursor stem cells
Skin-derived precursor cells, similar as adipose stem cells, are easily accessible adult stem cells. Skin-derived precursor cells are stem cells derived from the dermis. They can differentiate into various cell types, including Schwann cells [68,69]. The in vitro co-culture of skin-derived precursor cell-derived Schwann cells and dorsal root ganglia neurons induces myelination, while the in vivo transplantation of skin-derived precursor cells or skin-derived precursor cell-derived Schwann cells to the injured mice sciatic nerve improves dysmyelinating disorder [70]. To visualize the in vivo status of implanted skin-derived precursor cells, skin-derived precursor cells are labeled with Green fluorescent protein (GFP) prior to seeding into a nerve conduit to bridge a 16 mm rat sciatic nerve gap. The expressions of Schwann cell markers, such as S100 and glial fibrillary acidic protein, are detected in some GFP-positive cells around regenerating nerve fibers, suggesting that skin-derived precursor cells differentiate into Schwann cells and contribute to a faster recovery rate of injured peripheral nerves [71]. Another study used tracking dye to label porcine skin-derived mesenchymal stem cells. Labeled autologous porcine skin-derived mesenchymal stem cells can be well-preserved at 2 and 4 weeks after seeding to neural scaffolds and transplanting to the injured sites of miniature pigs [72]. The application of skin-derived precursor cells to a 12 mm rat sciatic nerve defect increases histomorphometrical and electrophysiological parameters, reaching a comparable result as those seeded with Schwann cells [73]. Moreover, skin-derived precursor cells can also treat delayed nerve repair and improve chronic denervation [74,75].
Induced pluripotent stem cells
Recently, the development of induced pluripotent stem cell technology expands cell source for cell therapies and largely pushes the progress of regenerative medicine [76,77]. Undifferentiated induced pluripotent stem cells can differentiate to neural crest stem cells or even Schwann cells with myelinating abilities [32,78]. The transplantation of a bio-absorbable nerve conduit seeded with neurospheres derived from induced pluripotent stem cells to a 5 mm mice sciatic nerve gap significantly boosts the growth of axons and the functional recovery of motor and sensory nerve functions at 4, 8 and 12 weeks after surgery [79]. A long-term follow-up study shows that induced pluripotent stem cells enhance axonal regeneration and myelination without inducing teratomas at 24 and 48 weeks after surgery [80]. Induced pluripotent stem cell-based nerve conduits, when combined with basic fibroblast growth factor, exhibit even better regenerative effects [81]. Ouchi T et al. generated low-affinity nerve growth factor receptor (LNGFR)- and thymocyte antigen-1 (THY-1)-positive neural crest-like cells (LT-NCLCs) from human induced pluripotent stem cells [82]. Kimura H et al. further investigated the biological effects of these cells by filling cells into a silcone tube and transplant the tube to bridge a 6 mm interstump gap in NOD-SCID mice [83]. Neural crest-like cells promote axon elongation, advance nerve remyelination and largely enhance motor function recovery, achieving a similar effect to that in the autograft group [82,83]. However, it was reported by another study that human induced pluripotent stem cell-derived Schwann cell precursors may not be able to form a myelin sheath, although they possess similar engraftment and migration characteristics as Schwann cells [84].
The downsides of induced pluripotent stem cells are that they exhibit some similar characteristics to embryonic stem cells, including malignant potential [77,85]. The generation technology of qualified induced pluripotent stem cells lacks reliability as well [86]. These realistic barriers limit the clinical application of induced pluripotent stem cells in tissue-engineered nerve grafts as seed cells.
Challenges and perspectives
A growing literature base of pre-clinical trials of stem cell-based tissue-engineered nerve grafts illuminates the promising future of the application of stem cells. The effectiveness of stem cells in clinical trials is also satisfying, although the number of reported clinical cases is not much. For instance, a 23-year-old female patient with median and ulnar nerve injury received a transplantation of NeuraGen® guides filled with autologous skin-derived precursor cells. Examinational results from pinch gauge test, static two-point discrimination, touch test with monofilaments, electrophysiological test and MRI demonstrate that the biological functions of injured median and ulnar nerves are recovered during a 3-year follow-up period [87]. In another study, a total of 22 patients with median or ulnar nerve injuries received application of autologous bone marrow mononuclear cells into silicone tubes. Compared with another 22 patients treated with empty silicone tubes, patients treated with silicone tubes filled with autologous bone marrow mononuclear cells have better motor function, sensation and the effect of pain on function at 1 year after surgery [88].
In spite of the excellent prospects, there still exists some troubling details affecting the safety and efficiency of stem cell therapy. For cell-based therapies, applied cells should be collected and cultured in advance, expanded to a large population and cryopreserved prior to transplantation [10]. Many attempts can be exploited to advance the clinical application of stem cells. For instance, the tumorigenicity of stem cells should be noted, although malignancy does not normally occur after stem cell administrations [10]. Cell banks can be constructed and preserved to ensure the quantity and quality of stem cells [35]. The mobilization, homing and migration, as well as the delivery methods, of stem cells should be further improved to increase the viability of applied stem cells [17,89]. To maintain phenotypic stability, the heterogeneity of many stem cells—for example, mesenchymal stem cells—should also be taken into consideration since the in vitro culture and expansion of cells may further exacerbate the heterogeneity of stem cell populations.
On the other hand, it is worth noting that severe and prolonged peripheral nerve injury often leads to muscle atrophy. Therefore, stem cells can be expanded from injecting to the injured nerve sites to target muscles. For instance, it has been demonstrated that the application of motor neurons derived from murine embryonic stem cells to the gastrocnemius muscles after mice tibial nerve transection stimulates motor function recovery and ameliorates denervation atrophy [90]. Similarly, the injection of adipose stem cells into the gastrocnemius muscle improves muscle atrophy and nerve function [91].
Conclusion
Over the last few years, the technology involved in the construction of tissue-engineered nerve grafts has seen great progress. The incorporation of stem cells, such as embryonic stem cells, neural stem cells, bone marrow mesenchymal stem cells, adipose stem cells, skin-derived precursor stem cells and induced pluripotent stem cells has enhanced the therapeutic effects of tissue-engineered nerve grafts. The extensive effectiveness of stem cells enlightens the promising future of the large-scale clinical use of stem cells.
Funding
This work was supported by the National Major Project of Research and Development [grant numbers 2017YFA0104700 and 2016YFC1101603], the National Natural Science Foundation of China [grant numbers 31730031 and 31700926], Jiangsu Provincial Key Medical Center and the Priority Academic Program Development of Jiangsu Higher Education Institutions of China [PAPD].
Authors’ contributions
SY and YZ contributed equally to this work.
Conflicts of interest
None declared.
References
- 1. Asplund M, Nilsson M, Jacobsson A, von Holst H. Incidence of traumatic peripheral nerve injuries and amputations in Sweden between 1998 and 2006. Neuroepidemiology. 2009;32:217–28. [DOI] [PubMed] [Google Scholar]
- 2. Li R, Liu Z, Pan Y, Chen L, Zhang Z, Lu L. Peripheral nerve injuries treatment: A systematic review. Cell Biochem Biophys. 2014;68:449–54. [DOI] [PubMed] [Google Scholar]
- 3. Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evans GR. Peripheral nerve injury: A review and approach to tissue engineered constructs. Anat Rec. 2001;263:396–404. [DOI] [PubMed] [Google Scholar]
- 5. Tian L, Prabhakaran MP, Ramakrishna S. Strategies for regeneration of components of nervous system: Scaffolds, cells and biomolecules. Regen Biomater. 2015;2:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt CE, Leach JB. Neural tissue engineering: Strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. [DOI] [PubMed] [Google Scholar]
- 7. Kaya Y, Sarikcioglu L. Sir Herbert Seddon (1903–1977) and his classification scheme for peripheral nerve injury. Childs Nerv Syst. 2015;31:177–80. [DOI] [PubMed] [Google Scholar]
- 8. Robinson LR. Traumatic injury to peripheral nerves. Muscle Nerve. 2000;23:863–73. [DOI] [PubMed] [Google Scholar]
- 9. Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491–516. [DOI] [PubMed] [Google Scholar]
- 10. Sullivan R, Dailey T, Duncan K, Abel N, Borlongan CV. Peripheral nerve injury: Stem cell therapy and peripheral nerve transfer. Int J Mol Sci. 2016;17:2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caillaud M, Richard L, Vallat JM, Desmouliere A, Billet F. Peripheral nerve regeneration and intraneural revascularization. Neural Regen Res. 2019;14:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gu X, Ding F, Yang Y, Liu J. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog in Neurobiol. 2011;93:204–30. [DOI] [PubMed] [Google Scholar]
- 13. Wong ML, Griffiths LG. Immunogenicity in xenogeneic scaffold generation: Antigen removal vs. decellularization. Acta Biomater. 2014;10:1806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gu X, Ding F, Williams DF. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials. 2014;35:6143–56. [DOI] [PubMed] [Google Scholar]
- 15. Yi S, Xu L, Gu X. Scaffolds for peripheral nerve repair and reconstruction. Exp Neurol. 2019;319:112761. [DOI] [PubMed] [Google Scholar]
- 16. Jones S, Eisenberg HM, Jia X. Advances and future applications of augmented peripheral nerve regeneration. Int J Mol Sci. 2016;17:E1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ren Z, Wang Y, Peng J, Zhao Q, Lu S. Role of stem cells in the regeneration and repair of peripheral nerves. Rev Neurosci. 2012;23:135–43. [DOI] [PubMed] [Google Scholar]
- 18. Jessen KR, Mirsky R, Lloyd AC. Schwann cells: Development and role in nerve repair. Cold Spring Harb Perspect Biol. 2015;7:a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Painter MW. Aging Schwann cells: Mechanisms, implications, future directions. Curr Opin Neurobiol. 2017;47:203–8. [DOI] [PubMed] [Google Scholar]
- 20. Brushart TM, Aspalter M, Griffin JW, Redett R, Hameed H, Zhou C, et al. . Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Exp Neurol. 2013;247:272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madduri S, Gander B. Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J Peripher Nerv Syst. 2010;15:93–103. [DOI] [PubMed] [Google Scholar]
- 22. Kornfeld T, Vogt PM, Bucan V, Peck CT, Reimers K, Radtke C. Characterization and Schwann cell seeding of up to 15.0 cm long spider silk nerve conduits for reconstruction of peripheral nerve defects. J Funct Biomater. 2016;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strauch B, Ferder M, Lovelle-Allen S, Moore K, Kim DJ, Llena J. Determining the maximal length of a vein conduit used as an interposition graft for nerve regeneration. J Reconstructive Microsurg. 1996;12:521–7. [DOI] [PubMed] [Google Scholar]
- 24. Chiu DT, Strauch B. A prospective clinical evaluation of autogenous vein grafts used as a nerve conduit for distal sensory nerve defects of 3 cm or less. Plast Reconstr Surg. 1990;86:928–34. [DOI] [PubMed] [Google Scholar]
- 25. Strauch B, Rodriguez DM, Diaz J, Yu HL, Kaplan G, Weinstein DE. Autologous Schwann cells drive regeneration through a 6-cm autogenous venous nerve conduit. J Reconstructive Microsurg. 2001;17:589–95discussion 96-7. [DOI] [PubMed] [Google Scholar]
- 26. Carr MJ, Johnston AP. Schwann cells as drivers of tissue repair and regeneration. Curr Opin Neurobiol. 2017;47:52–7. [DOI] [PubMed] [Google Scholar]
- 27. Dilwali S, Patel PB, Roberts DS, Basinsky GM, Harris GJ, Emerick KS, et al. . Primary culture of human Schwann and schwannoma cells: Improved and simplified protocol. Hear Res. 2014;315:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Casella GT, Bunge RP, Wood PM. Improved method for harvesting human Schwann cells from mature peripheral nerve and expansion in vitro. Glia. 1996;17:327–38. [DOI] [PubMed] [Google Scholar]
- 29. Zhu C, Huang J, Xue C, Wang Y, Wang S, Bao S, et al. . Skin derived precursor Schwann cell-generated acellular matrix modified chitosan/silk scaffolds for bridging rat sciatic nerve gap. Neurosci Res. 2018;135:21–31. [DOI] [PubMed] [Google Scholar]
- 30. Mosahebi A, Fuller P, Wiberg M, Terenghi G. Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp Neurol. 2002;173:213–23. [DOI] [PubMed] [Google Scholar]
- 31. Ziegler L, Grigoryan S, Yang IH, Thakor NV, Goldstein RS. Efficient generation of schwann cells from human embryonic stem cell-derived neurospheres. Stem Cell Rev Rep. 2011;7:394–403. [DOI] [PubMed] [Google Scholar]
- 32. Liu Q, Spusta SC, Mi R, Lassiter RN, Stark MR, Hoke A, et al. . Human neural crest stem cells derived from human ESCs and induced pluripotent stem cells: Induction, maintenance, and differentiation into functional schwann cells. Stem Cells Transl Med. 2012;1:266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cui L, Jiang J, Wei L, Zhou X, Fraser JL, Snider BJ, et al. . Transplantation of embryonic stem cells improves nerve repair and functional recovery after severe sciatic nerve axotomy in rats. Stem Cells. 2008;26:1356–65. [DOI] [PubMed] [Google Scholar]
- 34. Jones I, Novikova LN, Novikov LN, Renardy M, Ullrich A, Wiberg M, et al. . Regenerative effects of human embryonic stem cell-derived neural crest cells for treatment of peripheral nerve injury. J Tissue Eng Regen Med. 2018;12:e2099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang L, Jones S, Jia X. Stem cell transplantation for peripheral nerve regeneration: Current options and opportunities. Int J Mol Sci. 2017;18:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rippon HJ, Bishop AE. Embryonic stem cells. Cell Prolif. 2004;37:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee G, Chambers SM, Tomishima MJ, Studer L. Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc. 2010;5:688–701. [DOI] [PubMed] [Google Scholar]
- 38. Gage FH, Temple S. Neural stem cells: Generating and regenerating the brain. Neuron. 2013;80:588–601. [DOI] [PubMed] [Google Scholar]
- 39. Wang C, Lu CF, Peng J, Hu CD, Wang Y. Roles of neural stem cells in the repair of peripheral nerve injury. Neural Regen Res. 2017;12:2106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang H, Wei YT, Tsang KS, Sun CR, Li J, Huang H, et al. . Implantation of neural stem cells embedded in hyaluronic acid and collagen composite conduit promotes regeneration in a rabbit facial nerve injury model. J Transl Med. 2008;6:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guo BF, Dong MM. Application of neural stem cells in tissue-engineered artificial nerve. Otolaryngol Head Neck Surg. 2009;140:159–64. [DOI] [PubMed] [Google Scholar]
- 42. Heine W, Conant K, Griffin JW, Hoke A. Transplanted neural stem cells promote axonal regeneration through chronically denervated peripheral nerves. Exp Neurol. 2004;189:231–40. [DOI] [PubMed] [Google Scholar]
- 43. Shi Y, Zhou L, Tian J, Wang Y. Transplantation of neural stem cells overexpressing glia-derived neurotrophic factor promotes facial nerve regeneration. Acta Otolaryngol. 2009;129:906–14. [DOI] [PubMed] [Google Scholar]
- 44. Lee DC, Chen JH, Hsu TY, Chang LH, Chang H, Chi YH, et al. . Neural stem cells promote nerve regeneration through IL12-induced Schwann cell differentiation. Mol Cell Neurosci. 2017;79:1–11. [DOI] [PubMed] [Google Scholar]
- 45. Johnson TS, O'Neill AC, Motarjem PM, Nazzal J, Randolph M, Winograd JM. Tumor formation following murine neural precursor cell transplantation in a rat peripheral nerve injury model. J Reconstr Microsurg. 2008;24:545–50. [DOI] [PubMed] [Google Scholar]
- 46. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. [DOI] [PubMed] [Google Scholar]
- 47. Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells 2019;8:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Caddick J, Kingham PJ, Gardiner NJ, Wiberg M, Terenghi G. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia. 2006;54:840–9. [DOI] [PubMed] [Google Scholar]
- 49. Yang Y, Yuan X, Ding F, Yao D, Gu Y, Liu J, et al. . Repair of rat sciatic nerve gap by a silk fibroin-based scaffold added with bone marrow mesenchymal stem cells. Tissue Eng Part A. 2011;17:2231–44. [DOI] [PubMed] [Google Scholar]
- 50. Zhao Z, Wang Y, Peng J, Ren Z, Zhan S, Liu Y, et al. . Repair of nerve defect with acellular nerve graft supplemented by bone marrow stromal cells in mice. Microsurgery. 2011;31:388–94. [DOI] [PubMed] [Google Scholar]
- 51. Fan L, Yu Z, Li J, Dang X, Wang K. Schwann-like cells seeded in acellular nerve grafts improve nerve regeneration. BMC Musculoskelet Disord. 2014;15:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cuevas P, Carceller F, Dujovny M, Garcia-Gomez I, Cuevas B, Gonzalez-Corrochano R, et al. . Peripheral nerve regeneration by bone marrow stromal cells. Neurol Res. 2002;24:634–8. [DOI] [PubMed] [Google Scholar]
- 53. Chen CJ, Ou YC, Liao SL, Chen WY, Chen SY, Wu CW, et al. . Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007;204:443–53. [DOI] [PubMed] [Google Scholar]
- 54. Ding F, Wu J, Yang Y, Hu W, Zhu Q, Tang X, et al. . Use of tissue-engineered nerve grafts consisting of a chitosan/poly(lactic-co-glycolic acid)-based scaffold included with bone marrow mesenchymal cells for bridging 50-mm dog sciatic nerve gaps. Tissue Eng Part A. 2010;16:3779–90. [DOI] [PubMed] [Google Scholar]
- 55. Muheremu A, Chen L, Wang X, Wei Y, Gong K, Ao Q. Chitosan nerve conduits seeded with autologous bone marrow mononuclear cells for 30 mm goat peroneal nerve defect. Sci Rep. 2017;7:44002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wakao S, Hayashi T, Kitada M, Kohama M, Matsue D, Teramoto N, et al. . Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Exp Neurol. 2010;223:537–47. [DOI] [PubMed] [Google Scholar]
- 57. Hu N, Wu H, Xue C, Gong Y, Wu J, Xiao Z, et al. . Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts. Biomaterials. 2013;34:100–11. [DOI] [PubMed] [Google Scholar]
- 58. Watanabe Y, Sasaki R, Matsumine H, Yamato M, Okano T. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. J Tissue Eng Regen Med. 2017;11:362–74. [DOI] [PubMed] [Google Scholar]
- 59. Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, et al. . Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–41. [DOI] [PubMed] [Google Scholar]
- 60. Xie S, Lu F, Han J, Tao K, Wang H, Simental A, et al. . Efficient generation of functional Schwann cells from adipose-derived stem cells in defined conditions. Cell Cycle. 2017;16:841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: Implications for cell-based transplantation therapy. Neuroscience. 2013;236:55–65. [DOI] [PubMed] [Google Scholar]
- 62. Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207:267–74. [DOI] [PubMed] [Google Scholar]
- 63. di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ, Terenghi G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience. 2011;181:278–91. [DOI] [PubMed] [Google Scholar]
- 64. Masgutov R, Masgutova G, Mullakhmetova A, Zhuravleva M, Shulman A, Rogozhin A, et al. . Adipose-derived mesenchymal stem cells applied in fibrin glue stimulate peripheral nerve regeneration. Front Med (Lausanne). 2019;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Engels PE, Tremp M, Kingham PJ, di Summa PG, Largo RD, Schaefer DJ, et al. . Harvest site influences the growth properties of adipose derived stem cells. Cytotechnology. 2013;65:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sowa Y, Imura T, Numajiri T, Nishino K, Fushiki S. Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: Influence of age and anatomic site of origin. Stem Cells Dev. 2012;21:1852–62. [DOI] [PubMed] [Google Scholar]
- 67. Tremp M, Meyer Zu Schwabedissen M, Kappos EA, Engels PE, Fischmann A, Scherberich A, et al. . The regeneration potential after human and autologous stem cell transplantation in a rat sciatic nerve injury model can be monitored by MRI. Cell Transplant. 2015;24:203–11. [DOI] [PubMed] [Google Scholar]
- 68. Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabe-Heider F, et al. . A dermal niche for multipotent adult skin-derived precursor cells. Nature Cell Biol. 2004;6:1082–93. [DOI] [PubMed] [Google Scholar]
- 69. Biernaskie JA, McKenzie IA, Toma JG, Miller FD. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat Protoc. 2006;1:2803–12. [DOI] [PubMed] [Google Scholar]
- 70. McKenzie IA, Biernaskie J, Toma JG, Midha R, Miller FD. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci. 2006;26:6651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Marchesi C, Pluderi M, Colleoni F, Belicchi M, Meregalli M, Farini A, et al. . Skin-derived stem cells transplanted into resorbable guides provide functional nerve regeneration after sciatic nerve resection. Glia. 2007;55:425–38. [DOI] [PubMed] [Google Scholar]
- 72. Park BW, Kang DH, Kang EJ, Byun JH, Lee JS, Maeng GH, et al. . Peripheral nerve regeneration using autologous porcine skin-derived mesenchymal stem cells. J Tissue Eng Regen Med. 2012;6:113–24. [DOI] [PubMed] [Google Scholar]
- 73. Walsh S, Biernaskie J, Kemp SW, Midha R. Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience. 2009;164:1097–107. [DOI] [PubMed] [Google Scholar]
- 74. Walsh SK, Gordon T, Addas BM, Kemp SW, Midha R. Skin-derived precursor cells enhance peripheral nerve regeneration following chronic denervation. Exp Neurol. 2010;223:221–8. [DOI] [PubMed] [Google Scholar]
- 75. Khuong HT, Kumar R, Senjaya F, Grochmal J, Ivanovic A, Shakhbazau A, et al. . Skin derived precursor Schwann cells improve behavioral recovery for acute and delayed nerve repair. Exp Neurol. 2014;254:168–79. [DOI] [PubMed] [Google Scholar]
- 76. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. [DOI] [PubMed] [Google Scholar]
- 77. Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. [DOI] [PubMed] [Google Scholar]
- 78. Wang A, Tang Z, Park IH, Zhu Y, Patel S, Daley GQ, et al. . Induced pluripotent stem cells for neural tissue engineering. Biomaterials. 2011;32:5023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Uemura T, Takamatsu K, Ikeda M, Okada M, Kazuki K, Ikada Y, et al. . Transplantation of induced pluripotent stem cell-derived neurospheres for peripheral nerve repair. Biochem Biophys Res Commun. 2012;419:130–5. [DOI] [PubMed] [Google Scholar]
- 80. Uemura T, Ikeda M, Takamatsu K, Yokoi T, Okada M, Nakamura H. Long-term efficacy and safety outcomes of transplantation of induced pluripotent stem cell-derived neurospheres with bioabsorbable nerve conduits for peripheral nerve regeneration in mice. Cells Tissues Organs. 2014;200:78–91. [DOI] [PubMed] [Google Scholar]
- 81. Ikeda M, Uemura T, Takamatsu K, Okada M, Kazuki K, Tabata Y, et al. . Acceleration of peripheral nerve regeneration using nerve conduits in combination with induced pluripotent stem cell technology and a basic fibroblast growth factor drug delivery system. J Biomed Mater Res A. 2014;102:1370–8. [DOI] [PubMed] [Google Scholar]
- 82. Ouchi T, Morikawa S, Shibata S, Fukuda K, Okuno H, Fujimura T, et al. . LNGFR(+)THY-1(+) human pluripotent stem cell-derived neural crest-like cells have the potential to develop into mesenchymal stem cells. Differentiation. 2016;92:270–80. [DOI] [PubMed] [Google Scholar]
- 83. Kimura H, Ouchi T, Shibata S, Amemiya T, Nagoshi N, Nakagawa T, et al. . Stem cells purified from human induced pluripotent stem cell-derived neural crest-like cells promote peripheral nerve regeneration. Sci Rep. 2018;8:10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Muhammad A, Kim K, Epifantseva I, Aghamaleky-Sarvestany A, Simpkinson ME, Carmona S, et al. . Cell transplantation strategies for acquired and inherited disorders of peripheral myelin. Ann Clin Transl Neurol. 2018;5:186–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. . Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–6. [DOI] [PubMed] [Google Scholar]
- 86. Okada M, Yoneda Y. The timing of retroviral silencing correlates with the quality of induced pluripotent stem cell lines. Biochim Biophys Acta. 2011;1810:226–35. [DOI] [PubMed] [Google Scholar]
- 87. Grimoldi N, Colleoni F, Tiberio F, Vetrano IG, Cappellari A, Costa A, et al. . Stem cell salvage of injured peripheral nerve. Cell Transplant. 2015;24:213–22. [DOI] [PubMed] [Google Scholar]
- 88. Braga-Silva J, Gehlen D, Padoin AV, Machado DC, Garicochea B, Costa da Costa J. Can local supply of bone marrow mononuclear cells improve the outcome from late tubular repair of human median and ulnar nerves? J Hand Surg Eur Vol. 2008;33:488–93. [DOI] [PubMed] [Google Scholar]
- 89. Lehmann HC, Hoke A Use of engineered Schwann cells in peripheral neuropathy: Hopes and hazards. Brain Res. 2016;1638:97–104. [DOI] [PubMed] [Google Scholar]
- 90. Kubo T, Randolph MA, Groger A, Winograd JM. Embryonic stem cell-derived motor neurons form neuromuscular junctions in vitro and enhance motor functional recovery in vivo. Plast Reconstr Surg. 2009;123:139S–48S. [DOI] [PubMed] [Google Scholar]
- 91. Schaakxs D, Kalbermatten DF, Raffoul W, Wiberg M, Kingham PJ. Regenerative cell injection in denervated muscle reduces atrophy and enhances recovery following nerve repair. Muscle Nerve. 2013;47:691–701. [DOI] [PubMed] [Google Scholar]


