Abstract
Complex facial trauma requires complex repair and solutions. This process is challenging for the surgeon who seeks to manage the expectations of the patient and family while achieving the best possible result. Historically, the use of pedicled flaps, and then free tissue transfer, were the primary techniques utilized. Advancements in soft-tissue reconstruction, such as perforator flaps and pre-expanded and prefabricated flaps, allow refinement of the soft-tissue reconstruction process to create the best initial soft-tissue coverage. The advent of contemporary technologies, such as virtual surgical planning, stereolithography and customized implants and plates, facilitates a tailored approach to the patient’s reconstructive needs for precise bony reconstruction. When surgical and technological techniques are combined in complementary multistage reconstructions, better reconstructive and aesthetic outcomes are achievable than ever before. In this review, the authors present a summary of the management of complex facial trauma based on the senior author’s broad experience. Initial management and contemporary reconstructive techniques and technology to provide optimal outcomes are reviewed. A case series of complex facial traumas and their reconstructive process is also presented to demonstrate how complementary staged procedures can yield an optimal result. We believe the reconstructive surgeon managing complex facial trauma should strive to incorporate contemporary technologies and techniques into their armamentarium to provide the best patient care.
Keywords: Facial trauma, Facial reconstruction, Soft-tissue reconstruction, Facial skeletal reconstruction
Background
Reconstruction after extensive maxillofacial trauma is challenging for a multitude of reasons, as the experienced maxillofacial or reconstructive surgeon can attest. Loss of native soft tissue and bone, bony comminution and destruction of the facial buttresses makes reconstruction technically difficult with the loss of the building blocks of the maxillofacial skeleton. Delicate native soft tissues with intricate muscular, cartilaginous and ligamentous structures with unique personal characteristics cannot be replicated. Consequently, successful functional and aesthetic reconstruction requires expertise and technical excellence. Many surgeons lack the experience to become an expert, owing to the triage of such cases to centers of excellence and sub-specialization of surgical services. Without extensive, longitudinal experience with complex facial trauma, expertise is unattainable. Unfortunately, even with extensive experience, achieving a satisfactory result is difficult when delicate native structures are destroyed.
While surgical reconstruction is technically difficult, management of patient and family expectations may be just as challenging. The emotional burden of trauma is difficult for the patient and family, with broad implications for the patient and society [1]. Disfigurement can lead to difficulty with interpersonal relationships and social withdrawal, difficulty with psychosocial adjustment and reduced quality of life [2]. Acute trauma and difficulties with psychosocial adjustment complicates managing expectations for the patient and their family, all of whom may expect a more ‘normal’ result than contemporary reconstruction can provide. Complex facial trauma involves staged operations—coping with the long process is an emotional challenge for patient and family.
Management of other traumatic injuries and their sequelae may dictate surgical options as well as timing. With multiple consulting teams, surgical management priorities often conflict. This necessitates close communication and cooperation with all teams involved, as well as hospital support staff and services, if optimal patient care is to be provided. In this review, we intend to provide the reader a framework for achieving an excellent functional and cosmetic result when reconstructing the patient with severe facial trauma.
Review
Preoperative evaluation
In the absence of exsanguination requiring immediate intervention, evaluation begins once the patient is medically stabilized with a secured airway and cleared by the trauma service of other life-threatening injuries. Cervical spine injuries are commonly associated with maxillofacial trauma, occurring in 1–7% of patients, and must be identified and managed appropriately [3–5].
Facial height, width, projection and symmetry should be evaluated by palpation. Functional considerations to assess include occlusion, dentition, patency of the nasal airway and orbital volume. Visual acuity should be checked and, in the event of orbital trauma, ophthalmology consultation should be obtained.
In addition to thorough physical examination, contemporary practice mandates a facial bone computed tomography (CT) scan. Where available, three-dimensional (3D) reconstruction of the CT should be performed, as this enhances appreciation for displaced or missing skeletal components. For more advanced planning, facial skeletal models can be 3D printed based on the 3D CT scan, or virtual surgical planning (VSP) can be performed utilizing 3D CT technology. These technologies are particularly helpful for planning free osseous or osteocutaneous tissue transfer, preoperatively generating precisely designed plates and cutting guides. Intraoperative CT or real-time image-guided surgery may also be of assistance with complex facial reconstruction, particularly around the orbit [6].
Microvascular free tissue transfer plays an essential role in soft-tissue coverage for facial trauma, often superseding options lower on the ‘reconstructive ladder’. By taking the ‘reconstructive elevator’ to free tissue transfer the surgeon can often achieve a better reconstructive outcome, particularly with free tissue transfer success rates in the head and neck region being >95%. In some circumstances, free tissue transfer may be the only reconstructive option, such as when bone is needed. Multiple free tissue transfers may be needed for extensive trauma, which can be done with a >95% success rate [7]. If this is to be done in a single operation, multiple surgical teams may be warranted.
Principles of reconstruction
Reconstruction begins with thorough initial wound debridement of all devitalized tissue and any foreign bodies. Devitalized tissues are likely to declare by 36–48 hours after the incident trauma. Soft tissues that can be closed primarily tension-free should be closed at this time. Drains may be left in any dead space or contaminated wound to reduce risk of infection. After wound debridement, maxillofacial skeletal structure must be restored first. Open reduction internal fixation (ORIF) with titanium plates is performed first where there are no large bony gaps. One then may proceed with temporary plating as a bridge if definitive bony reconstruction is not feasible. Stabilization of the maxillofacial skeleton allows for reduction of pain and establishes a framework for planning definitive reconstruction.
The mandible must be reconstructed and rigidly stabilized to restore a normal appearing lower-third of the face and allow masticatory function. If bone is missing, either free vascularized bone or non-vascularized bone graft may be required. Traditionally, if the bone defect is greater than 5–6 cm, a free vascularized bone graft will be required [8]. Midfacial defects involving the maxilla, orbits, palate, nasal and paranasal tissues can be quite complex to reconstruct. The nasal and oral cavities must be reconstructed and separated, the orbits supported and dead space obliterated. Bone may be required for support of the soft tissues and restoration of dentition via osseointegrated implants. It is essential to reconstruct the vertical and horizontal facial buttresses to restore proper facial height, width and projection and prevent soft-tissue collapse [9]. Bone graft may be required to achieve an optimal result. Upper-third (frontal) defects require recontouring of the frontal bone and nasoorbitoethmoid (NOE) complex. If the nasofrontal duct is not patent, frontal sinus obliteration or cranialization is required. Modern endoscopic techniques may allow the patency of the nasofrontal ducts to be assessed and repaired in a delayed, more conservative approach than traditional methods [10].
A good quality soft-tissue reconstruction is essential to cover exposed bony structures or plates and achieve wound healing. This may need to be performed after initial skeletal stabilization prior to further osseous reconstruction if there is soft-tissue loss with exposed structures. Careful surgical planning and flap selection allows for combination of reconstructive and cosmetic procedures to yield an optimal outcome.
Flap selection for soft-tissue and bony reconstructions
Well established options for facial soft-tissue reconstruction include the radial forearm free flap (RFFF), anterolateral thigh (ALT) free flap, ‘free-style’ perforator flaps and the pedicled supraclavicular artery (SCA) fasciocutaneous flap. The RFFF has a long-established role in head and neck reconstruction. The flap is reliable, pliable and thin and has a long pedicle that is a good size match for vessels in the head and neck [11–13]. There is known donor-site morbidity with use of this flap compared to other options, notably a large unaesthetic scar [14]. The ALT fasciocutaneous free flap has low donor-site morbidity with a reliably long, good-caliber pedicle. It is a workhorse free flap for head and neck reconstruction when a large area of skin and soft tissue is needed [15, 16]. The patient’s body habitus should be closely assessed preoperatively as the ALT flap may have significant subcutaneous tissue, particularly in Western cultures. ‘Free-style’ perforator flaps allow the surgeon to obtain a flap with appropriate size, color, thickness and texture while minimizing donor-site morbidity [17, 18]. They allow the surgeon to work around aberrant anatomy but are highly technique-based with a learning curve. The surgeon should be highly skilled at super-microsurgery and able to adapt to the use of a potentially short pedicle [19]. However, once well versed in these flaps, the surgeon’s options are increased for distant soft-tissue coverage with low morbidity. The SCA flap is a reliable local flap for head and neck reconstruction. Often forgotten about in the age of free tissue transfer, the flap provides thin and pliable soft tissue for coverage of the lower-third of the face or the neck [20]. This flap confers the advantages of decreased operative time and postoperative monitoring without limiting future reconstructive options.
For bony reconstruction, a vascularized fibula free flap is the workhorse in maxillofacial reconstruction and the senior author’s flap of choice. The bone is of good quality and tolerates segmental osteotomies for contouring. The peroneal artery pedicle is reliable and of sufficient length. If bone and soft tissue are needed, the fibula can be harvested as an osteoseptocutaneous free flap [12, 21]. The fibula free flap offers the advantage of relatively low donor-site morbidity with a large segment of bicortical bone that is thick enough for osseointegrated implants. Other options for bony reconstruction include scapular flaps, the RFFF and deep circumflex iliac flaps [22]. The deep circumflex iliac flap can be harvested as an osseous, osteocutaneous or osteomyocutaneous flap. The osseous flap provides a large amount of bone but entails significant donor-site morbidity with violation of the abdominal oblique muscles. If harvested as an osteocutaneous flap, the tissue contains a significant subcutaneous component. Perforators must be identified preoperatively, and tedious dissection may be required [23]. The osteocutaneous RFFF is reliable with a long pedicle but has limited donor bone and is associated with high donor-site morbidity: unaesthetic donor site, risk of radius fracture, chronic pain, wound healing complications and, reportedly, impaired wrist function [24]. Consequently, this flap is not recommended when other bony donor sites are available. The scapular flap may have the lowest donor-site morbidity of these options and can be harvested as a chimeric flap with a large amount of bone and soft tissue, but requires an intraoperative position change [25]. The pedicle is often relatively short as well. Knowledge of each of these flap options is important to accommodate the patient’s needs and preferences for the traumatic defect at hand.
Case examples
Three case examples are included to demonstrate how multiple staged procedures using contemporary methods are required to produce a functional and cosmetic facial reconstruction with the best possible result.
Case 1
A 35-year-old male fell from a ladder while roofing, sustaining LeFort 1 and paramedian palatal fracture; right NOE fracture; left zygomaticomaxillary complex (ZMC) fracture; fractures of the bilateral supraorbital rims, orbital floors and lateral walls and nasal bones; and comminuted fractures of the anterior and posterior walls of the frontal sinus (Fig. 1a, b). He underwent creation of a submental airway and was placed in maxillomandibular fixation (Fig. 1c). ORIF was performed to his NOE, bilateral supraorbital rims, left lateral orbital rim and ZMC and right orbital floor with hybrid Medpore titanium implant. ORIF of frontal sinus fractures was performed with plate and mesh (Fig. 1d, e). Closed reduction was performed on his nose. Ten months later he required fat grafting to the temporal fossa (7 cc to each side) for temporal wasting and revision of a left eyelid scar (Fig. 1f). Fourteen months following initial trauma he underwent rhinoplasty and inferior turbinectomy for traumatic nasal deformity and nasal airway (Fig. 1g). Fig 1h shows the final result at 10 months after his last nasal surgery.
Figure 1.

A 35-year-old male with multiple facial fracture (a and b). The maxillomandibular fixation was placed (c). Open reduction internal fixation (ORIF) of the frontal sinus fractures with plate and mesh (d and e). Fat grafting was performed to correct his temporal fossa depression on both sides at 10 months later (f), 14 months later before rhinoplasty (g), and the final result at 10 months after his last nasal surgery (h)
Case 2
A 46-year-old male had a gunshot shot to the face and suffered from comminuted fractures of his left middle and lower face. The patient underwent initial external fixator placement for mandibular stabilization prior to transfer to our care (Fig. 2a). He subsequently underwent ORIF of complex bilateral mandible fractures with reconstruction plates and ORIF of left communized midface fractures with multiple miniplates (Fig. 2b, c). A left SCA flap was raised and transferred inside the mouth to provide intraoral soft-tissue coverage and restore intraoral lining (Fig. 2d, e, f). He subsequently underwent two debulking procedures of the intraoral supraclavicular flap at 4 and 36 months, respectively, and had a good reconstructive result at a 3-year follow-up (Fig. 2g, h).
Figure 2.

A 46-year-old male underwent initial external fixator placement for mandibular stabilization after gunshot wound to his left face (a). He underwent open reduction internal fixation (ORIF) of complex bilateral mandible fractures with reconstruction plates and ORIF of left communized midface fractures with multiple miniplates (b and c). A left supraclavicular artery (SCA) flap was raised and transferred inside the mouth to provide intraoral soft-tissue coverage and restore intraoral lining (d, e and f), with a good reconstructive result at a 3-year follow-up (g and h)
Case 3
An 18-year-old male suffered a degloving injury of the left face and temporal scalp in an unhelmeted motorcycle accident (Fig. 3a). His bony injuries included loss of the left zygomatic arch and a portion of the zygomatic body and lateral orbital wall (Fig. 3b). After initial wound debridement and reconstruction of the zygomatic arch with a plate and the zygomatic body and lateral orbital wall with Medpore implants (Fig. 3c, d), his extensive facial soft-tissue coverage was achieved with a free ALT perforator flap from the left thigh (Fig. 3e). After allowing for soft-tissue swelling to subside, he presented several months later in need of resuspension of the ALT flap and lateral canthopexy for ectropion (Fig. 3f). After further soft-tissue atrophy of the ALT free flap he underwent flap debulking, mid-facelift with Medpore malar implant placement for augmentation of the deficient ZMC and even lateral canthoplasty. To restore a natural temporal hairline, the patient underwent tissue expander placement to the parietal scalp with subsequent advancement of the hairline and facial scar revision. Finally, an epidermal split-thickness skin graft from the scalp was applied to the face for better color-match of his well-healed ALT flap. During a 25-month follow-up, he has had a good result after the above multiple procedures (Fig. 3g, h).
Figure 3.
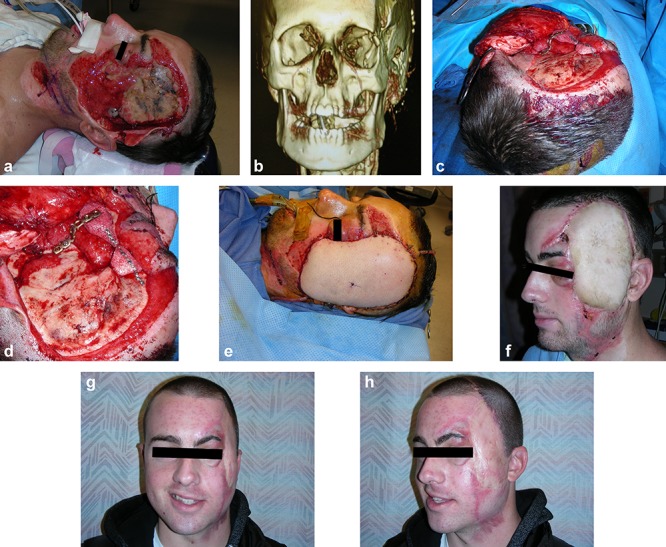
An 18-year-old male sustained a degloving injury to the left face and temporal scalp (a), with loss of the left zygomatic arch and the portion of the zygomatic body and lateral orbital wall (b). The zygomatic body and lateral orbital wall were reconstructed with Medpore implants (c and d), his extensive facial soft-tissue coverage was achieved with a free anterolateral thigh perforator flap from the left thigh (e). The appearance several months later after initial reconstruction (f) and has had a good result after above multiple procedures during a 25-month follow-up (g and h)
Discussion
As demonstrated in the case examples above, contemporary reconstruction techniques yield excellent results when carefully planned. Such contemporary reconstructions are characterized by precise bony reconstruction and more advanced tissue transfer techniques which are facilitated by technological and surgical innovations, resulting in optimal function and cosmesis.
Several technological developments have facilitated advancements in bony reconstruction. Most notably, 3D CT scanning and processing software which allows for preoperative planning with enhanced 3D diagnostics. This software enables measurement; manipulation or surgical simulation; stereolithographic modeling; customized plating systems, splints and cutting guides; customized prefabricated implants; and real-time intraoperative surgical naviga-tion [26–30].
Stereolithographic models based on the preoperative imaging can be used for surgical planning and preoperative plate adaptation for maxillofacial reconstruction [31]. This process, known as VSP, allows for patient-specific plate and splint creation based on virtual models, thereby decreasing the time of operative planning and execution [32–34]. This process can be applied to osteotomies of the craniofacial skeleton, or when performing vascularized bone transfer from other parts of the body, such as the fibula [35]. Time savings are quite notable when compared to traditional dental modeling for orthognathic surgery [36], and some literature suggests a substantial cost saving by reduced operative time, while others promote improved time efficiency [33, 37]. It should be noted that VSP and some preoperative planning technologies are not cheap. Whether time savings translate to long-term cost reductions remains a topic of debate and requires further analysis [38]. The greatest clinical caveat to VSP is that the surgeon must be aware of the inability of the software to account for soft-tissue considerations, such as pedicle location and length, tissue size requirements and perforator location [35, 39]. In the properly selected patient, preoperative VSP for modeling of prefabricated plates, splints or cutting guides is helpful for maximizing the surgical result.
The introduction of prefabricated custom-made polyetheretherketone (PEEK) implants has revolutionized craniomaxillofacial reconstruction of traumatic and oncologic defects. PEEK implants are excellent for correcting both cosmetic and functional defects when autologous tissue is unavailable, of insufficient quality or requires unacceptable donor-site morbidity [30]. Prefabricated implants may be particularly helpful in regions requiring fine contouring, which may be quite difficult with traditional materials like titanium mesh and polymethylmethacrylate [29]. However, the surgeon must be conscious of the traditional drawbacks of alloplastic material—primarily, a risk of infection and, rarely, extrusion. Presently, the PEEK complication profile appears comparable to that of autologous tissue. Intraoperative navigational surgery uses imaging data to guide the surgeon’s location or anatomic structures and tool placement to accurately implement the virtual plan. Customized plating systems or navigational surgery are not indicated for uncomplicated operations but may be particularly helpful in areas where exposure is difficult, such as orbital reconstruction, reconstruction of complex craniofacial or orthognathic defects, head and neck tumor resection and foreign body removal [28].
Real-time surgical navigation systems are based on the patient’s preoperative imaging. Surgical image guidance systems allow surgeons to see instrument position in real-time, relative to the patient’s imaging. Instrument position is visualized in relation to anatomic landmarks identified on imaging. The purpose of these technologies is to improve surgical accuracy, predictability and efficiency in difficult-to-visualize areas and reconstructions that rely on highly precise reductions [40, 41]. For management of facial trauma, applications are most helpful for operations on the orbit and paranasal sinuses, ZMC and, potentially, in orthognathic surgeries [42, 43]. Explanation of the various technologies for real-time surgical navigation are beyond the scope of this article but can be found in several nicely written publications [6, 28, 44]. We anticipate this technology will continue to be refined and its use expanded in facial reconstruction.
When considering soft-tissue reconstructions, the introduction of perforator flaps and super-microsurgery has provided surgeons new options for maxillofacial reconstruction. Pre-expanded and prefabricated perforator flaps yield innovative reconstructive options, and the SCA flap has seen a resurgence in popularity due to excellent skin color, texture and thickness match to facial skin.
Among the newest innovations in microsurgical reconstruction is the advent of pre-expanded perforator flaps. Mostly commonly used in Asia, pre-expanded perforator flaps have been used for reconstruction of head and neck [45–47], hand and upper extremity [48–50], axilla [51], abdominal wall [52] and perineum for reconstruction of genitalia [53]. This technique combines targeted tissue expansion with perforator flaps, thereby creating more soft tissue on a given pedicle with a robust fabricated blood supply due to the expansion process [54]. The reconstructive surgeon can therefore gain more tissue from ideal donor sites in order to replace ‘like with like’ and replicate the regional anatomy of the face. Appropriate perforators supplying the selected donor site are identified preoperatively, and a tissue expander inserted into the choke zone vascular territory between perforators. After completion of the expansion process, the flap is designed on the selected perforator and its pedicle transferred as planned [55]. Pre-expanded perforator flaps can be designed to be of any thickness, but most commonly are designed to be thin and easily contoured to be used as free or local flaps for recontouring of large soft-tissue defects [54–56]. This is particularly pertinent for reconstruction of facial trauma, where thin, pliable tissue is needed to most closely replicate facial soft tissues. Importantly, donor-site morbidity is minimized, decreasing need for skin grafting of the donor site or requiring local flap closure of the donor site [54].
Like pre-expanded perforator flaps, prefabricated flaps allow for another unique method of facial reconstruction. The prefabricated flap, first described by Shen in 1982, allows transfer of neovascularized skin following microvascular free tissue transfer of a selected flap to the selected donor vessel, thereby creating an axially supplied donor site for the face [57–59]. Prefabrication of the blood supply to the neovascularized tissues is thought to improve flap survival. A prefabricated flap can subsequently be expanded to increase the volume of tissue transferred, thereby creating a pre-expanded prefabricated flap [60, 61]. When used for facial reconstruction, prefabricated flaps are most frequently created in the supraclavicular or cervicothoracic regions due to proximity and similarity in color, texture and thickness to facial skin [60–62].
Pre-expanded and prefabricated flap techniques have yielded many innovative options for facial reconstruction; however, they are not without downsides. Multiple operations are required: one for prefabrication (if applicable), with or without tissue expander placement, and one for final flap creation. In the event of tissue expander infection or extrusion, additional patient morbidity is experienced, further delaying reconstruction. Additionally, preoperative identification of appropriate perforators about which to base the reconstruction requires additional time, effort and cost [56]. In the carefully selected patients, these two techniques are an excellent choice for facial soft-tissue reconstruction.
‘Free-style’ perforator flaps, first introduced by Mardini and Wei in 2003, are a relatively new innovation in microsurgical reconstruction [63]. Free-style flaps allow the surgeon to obtain a flap with appropriate size, color, thickness and texture while minimizing donor-site morbidity, based on a single perforator [17–19]. With this technique, Doppler identification of a perforator vessel may permit elevation of a flap from any part of the body; this is particularly useful when encountering aberrant anatomy while harvesting another flap. Having identified a useful perforator, retrograde dissection allows the surgeon to obtain a pedicle of appropriate length and size without knowing the source vessel. A recent meta-analysis found free-style perforator flaps to be reliable, with a complication rate similar to that of traditional free flaps; the same authors argue pedicled free-style flaps could be considered before free flaps for head and neck reconstructions, where free-style flap efficacy has been demonstrated [17, 64].
Recent years have shown a resurgence in the popularity of the SCA flap for head and neck reconstruction since its first description by Lamberty in 1979 [20, 65–67]. This fasciocutaneous flap has come into favor for multiple reasons. The tissue is thin and pliable, and provides an excellent color and texture match to facial skin. Anatomic proximity to the face and reliable vascular anatomy allows for use as a pedicle flap with less donor-site morbidity than many free flaps, thereby preserving free flap donor sites and recipient vessels [20]. Dissection and inset can be performed more quickly than free tissue transfer with shorter ICU stays and postoperative monitoring, making it ideal for patients who are poor candidates for free tissue transfer, in low resource settings or for salvage soft-tissue coverage after failed free tissue transfer [65]. Importantly, multiple clinical studies [20, 68–71] and recent systematic reviews [65, 72] demonstrate similar complication profiles between the SCA flap and free tissue transfer. The versatility of the SCA flap has been expanded with the development of pre-expanded and prefabricated perforator flaps. Multiple authors describe the use of the SCA donor skin for reconstruction of facial soft-tissue defects, citing the defining characteristics of this flap, notably the reliable vascular pedicle and similarity to facial soft tissue [45, 57, 59, 61]. Using these techniques, the optimal functional and cosmetic reconstruction can be provided to the patient.
Finally, a discussion of contemporary facial soft-tissue reconstruction is incomplete without mention of ALT fasciocutaneous free flaps. The ALT has become a workhorse for microvascular head and neck reconstruction when large areas of skin and soft tissue are needed. The ALT is known for low donor-site morbidity with a reliably long pedicle of 8–16 cm with good caliber, usually >2 mm, facilitating easier inset and microvascular anastomosis [15, 16]. The flap is designed as a fasciocutaneous flap, fascial flap or myocutaneous flap, with inclusion of vastus lateralis for bulk, or a chimeric flap with inclusion of the fascia lata all pedicled on the lateral circumflex femoral artery. The skin is pliable and thickness of the flap can be decreased with a suprafascial dissection or trimmed to the subdermal fat level [15, 73]. Few, if any, other donor sites can provide the same amount of skin, fat, muscle and fascia with minimal donor-site morbidity. Importantly, the ALT flap is pliable enough to be folded, tubed or packed into cavities, which is frequently encountered when reconstructing traumatic soft-tissue facial resurfacing and defects of the mandible, maxilla, scalp or oral cavity [74, 75]. Due to the pliable nature of this flap and reliability of the pedicle, the ALT free flap has become a favorite of the senior author for durable soft-tissue coverage of traumatic facial defects, as demonstrated in in the case reports.
For more complex and extensive blast injury to the face, even with the surgeon’s best efforts, restoring the face to ‘normal’ may be impossible. Thus, the surgeon’s task is to provide the optimal functional and aesthetic outcome contemporary surgical techniques allow. Innovations such as face transplantation, the highest rung of the reconstructive ladder, may be the patient’s only hope of regaining a ‘normal’ appearance. While adept surgical skill is important, it is not the only crucial component to obtaining an optimal result. In order to provide the best patient care for complex maxillofacial trauma, several sources of hospital support must be in order. This includes the surgeon’s team, the resident and/or fellow team, skillful anesthesia and operating room teams, the team that will provide postoperative care and the support of other medical or surgical services. Lapse in performance or judgement of any of these support services can have catastrophic consequences for the patients, particularly in the perioperative period of free tissue transfer. All hospital support services are essential and the importance of their role in patient care should not be overlooked. When all resources are organized and available, the best care can be delivered as surgeons address challenging reconstructions.
Conclusions
Modern surgical and technological advancement allows surgeons to push reconstructive boundaries. Embracing a multi-staged approach to complex reconstructions will facilitate optimal outcomes. Utilizing an approach that embraces contemporary techniques—technological advancements such as VSP in concert with customized plates or PEEK implants and reliable, carefully planned soft-tissue flaps—effective reconstructions can be performed that are both cosmetically and functionally acceptable to the patient and surgeon after severe native tissue loss. In order to provide the best patient care, reconstructive surgeons must continuously expand their reconstructive armamentarium with contemporary techniques and technologies to provide patients optimal cosmetic and functional results.
Abbreviations
3D, Three-dimensional; ALT, Anterolateral thigh; CT, Computed tomography; NOE, Nasoorbitoethmoid; ORIF, Open reduction internal fixation; PEEK, Prefabricated custom-made polyetheretherketone; RFFF, Radial forearm free flap; SCA, Supraclavicular artery; VSP, Virtual surgical planning; ZMC, Zygomaticomaxillary complex.
Conflicts of interest
The authors declare that they have no competing interests.
References
- 1. Levine E, Degutis L, Pruzinsky T, Shin J, Persing JA. Quality of life and facial trauma: Psychological and body image effects. Ann Plast Surg. 2005;54:502–10. [DOI] [PubMed] [Google Scholar]
- 2. Elegbede A, Mermulla S, Diaconu SC, McNichols C, Liang Y, Liang F, et al. Patient-reported outcomes in facial reconstruction: Assessment of FACE-Q scales and predictors of satisfaction. Plast Reconstr Surg Glob Open. 2018;6:e2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hackl W, Fink C, Hausberger K, Ulmer H, Gassner R. The incidence of combined facial and cervical spine injuries. J Trauma. 2001;50:41–5. [DOI] [PubMed] [Google Scholar]
- 4. Mulligan RP, Friedman JA, Mahabir RC. A nationwide review of the associations among cervical spine injuries, head injuries, and facial fractures. J Trauma. 2010;68:587–92. [DOI] [PubMed] [Google Scholar]
- 5. Sinclair D, Schwartz M, Gruss J, McLellan B. A retrospective review of the relationship between facial fractures, head injuries, and cervical spine injuries. J Emerg Med. 1988;6:109–12. [DOI] [PubMed] [Google Scholar]
- 6. Kaduk WM, Podmelle F, Louis PJ. Surgical navigation in reconstruction. Oral Maxillofac Surg Clin North Am. 2013;25:313–33. [DOI] [PubMed] [Google Scholar]
- 7. Hanasono MM, Corbitt CA, Yu P, Skoracki RJ. Success of sequential free flaps in head and neck reconstruction. J Plast Reconstr Aesthet Surg. 2014;67:1186–93. [DOI] [PubMed] [Google Scholar]
- 8. Foster RD, Anthony JP, Sharma A, Pogrel MA. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: An outcome analysis of primary bony union and endosseous implant success. Head Neck. 1999;21:66–71. [DOI] [PubMed] [Google Scholar]
- 9. Gruss JS, Mackinnon SE. Complex maxillary fractures: Role of buttress reconstruction and immediate bone grafts. Plast Reconstr Surg. 1986;78:9–22. [PubMed] [Google Scholar]
- 10. Louis PJ, Morlandt AB. Advancements in maxillofacial trauma: A historical perspective. J Oral Maxillofac Surg. 2018;76:2256–70. [DOI] [PubMed] [Google Scholar]
- 11. Wong CH, Wei FC. Microsurgical free flap in head and neck reconstruction. Head Neck. 2010;32:1236–45. [DOI] [PubMed] [Google Scholar]
- 12. Hurvitz KA, Kobayashi M, Evans GR. Current options in head and neck reconstruction. Plast Reconstr Surg. 2006;118:122e–33e. [DOI] [PubMed] [Google Scholar]
- 13. Santamaria E, Granados M, Barrera-Franco JL. Radial forearm free tissue transfer for head and neck reconstruction: Versatility and reliability of a single donor site. Microsurgery. 2000;20:195–201. [DOI] [PubMed] [Google Scholar]
- 14. Swanson E, Boyd JB, Manktelow RT. The radial forearm flap: Reconstructive applications and donor-site defects in 35 consecutive patients. Plast Reconstr Surg. 1990;85:258–66. [PubMed] [Google Scholar]
- 15. Wei FC, Jain V, Celik N, Chen HC, Chuang DC, Lin CH. Have we found an ideal soft-tissue flap? An experience with 672 anterolateral thigh flaps. Plast Reconstr Surg. 2002;109:2219–26. [DOI] [PubMed] [Google Scholar]
- 16. Lin DT, Coppit GL, Burkey BB. Use of the anterolateral thigh flap for reconstruction of the head and neck. Curr Opin Otolaryngol Head Neck Surg. 2004;12:300–4. [DOI] [PubMed] [Google Scholar]
- 17. Qian Y, Li G, Zang H, Cao S, Liu Y, Yang K, et al. A systematic review and meta-analysis of free-style flaps: Risk analysis of complications. Plast Reconstr Surg Glob Open. 2018;6:e1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei FC, Mardini S. Free-style free flaps. Plast Reconstr Surg. 2004;114:910–6. [DOI] [PubMed] [Google Scholar]
- 19. Wallace CG, Kao HK, Jeng SF, Wei FC. Free-style flaps: A further step forward for perforator flap surgery. Plast Reconstr Surg. 2009;124:e419–26. [DOI] [PubMed] [Google Scholar]
- 20. Granzow JW, Suliman A, Roostaeian J, Perry A, Boyd JB. Supraclavicular artery island flap (SCAIF) vs free fasciocutaneous flaps for head and neck reconstruction. Otolaryngol Head Neck Surg. 2013;148:941–8. [DOI] [PubMed] [Google Scholar]
- 21. Wei FC, Chen HC, Chuang CC, Noordhoff MS. Fibular osteoseptocutaneous flap: Anatomic study and clinical application. Plast Reconstr Surg. 1986;78:191–200. [DOI] [PubMed] [Google Scholar]
- 22. Likhterov I, Roche AM, Urken ML. Contemporary osseous reconstruction of the mandible and the maxilla. Oral Maxillofac Surg Clin North Am. 2019;31:101–16. [DOI] [PubMed] [Google Scholar]
- 23. Kimata Y, Uchiyama K, Sakuraba M, Ebihara S, Hayashi R, Asakage T, et al. Deep circumflex iliac perforator flap with iliac crest for mandibular reconstruction. Br J Plast Surg. 2001;54:487–90. [DOI] [PubMed] [Google Scholar]
- 24. Kearns M, Ermogenous P, Myers S, Ghanem AM. Osteocutaneous flaps for head and neck reconstruction: A focused evaluation of donor site morbidity and patient reported outcome measures in different reconstruction options. Arch Plast Surg. 2018;45:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eskander A, Kang SY, Teknos TN, Old MO. Advances in midface reconstruction: Beyond the reconstructive ladder. Curr Opin Otolaryngol Head Neck Surg. 2017;25:422–30. [DOI] [PubMed] [Google Scholar]
- 26. Kantar RS, Ceradini DJ, Gelb BE, Levine JP, Staffenberg DA, Saadeh PB, et al. Facial transplantation for an irreparable central and lower face injury: A modernized approach to a classic challenge. Plast Reconstr Surg. 2019;144:264e–83e. [DOI] [PubMed] [Google Scholar]
- 27. Edwards SP. Computer-assisted craniomaxillofacial surgery. Oral Maxillofac Surg Clin North Am. 2010;22:117–34. [DOI] [PubMed] [Google Scholar]
- 28. Bell RB. Computer planning and intraoperative navigation in cranio-maxillofacial surgery. Oral Maxillofac Surg Clin North Am. 2010;22:135–56. [DOI] [PubMed] [Google Scholar]
- 29. Jalbert F, Boetto S, Nadon F, Lauwers F, Schmidt E, Lopez R. One-step primary reconstruction for complex craniofacial resection with PEEK custom-made implants. J Craniomaxillofac Surg. 2014;42:141–8. [DOI] [PubMed] [Google Scholar]
- 30. Alonso-Rodriguez E, Cebrian JL, Nieto MJ, Del Castillo JL, Hernandez-Godoy J, Burgueno M. Polyetheretherketone custom-made implants for craniofacial defects: Report of 14 cases and review of the literature. J Craniomaxillofac Surg. 2015;43:1232–8. [DOI] [PubMed] [Google Scholar]
- 31. Cohen A, Laviv A, Berman P, Nashef R, Abu-Tair J. Mandibular reconstruction using stereolithographic 3-dimensional printing modeling technology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:661–6. [DOI] [PubMed] [Google Scholar]
- 32. He W, Tian K, Xie X, Wang X, Li Y, Wang X, et al. Individualized surgical templates and titanium microplates for Le fort I osteotomy by computer-aided design and computer-aided manufacturing. J Craniofac Surg. 2015;26:1877–81. [DOI] [PubMed] [Google Scholar]
- 33. Resnick CM, Inverso G, Wrzosek M, Padwa BL, Kaban LB, Peacock ZS. Is there a difference in cost between standard and virtual surgical planning for Orthognathic surgery? J Oral Maxillofac Surg. 2016;74:1827–33. [DOI] [PubMed] [Google Scholar]
- 34. Suojanen J, Leikola J, Stoor P. The use of patient-specific implants in orthognathic surgery: A series of 32 maxillary osteotomy patients. J Craniomaxillofac Surg. 2016;44:1913–6. [DOI] [PubMed] [Google Scholar]
- 35. Ettinger KS, Alexander AE, Arce K. Computed tomographic angiography perforator localization for virtual surgical planning of Osteocutaneous fibular free flaps in head and neck reconstruction. J Oral Maxillofac Surg. 2018;76:2220–30. [DOI] [PubMed] [Google Scholar]
- 36. Schwartz HC. Does computer-aided surgical simulation improve efficiency in bimaxillary orthognathic surgery? Int J Oral Maxillofac Surg. 2014;43:572–6. [DOI] [PubMed] [Google Scholar]
- 37. Toto JM, Chang EI, Agag R, Devarajan K, Patel SA, Topham NS. Improved operative efficiency of free fibula flap mandible reconstruction with patient-specific, computer-guided preoperative planning. Head Neck. 2015;37:1660–4. [DOI] [PubMed] [Google Scholar]
- 38. Fatima A, Hackman TG, Wood JS. Cost-effectiveness analysis of virtual surgical planning in mandibular reconstruction. Plast Reconstr Surg. 2019;143:1185–94. [DOI] [PubMed] [Google Scholar]
- 39. Deek NF, Wei FC. Computer-assisted surgery for segmental mandibular reconstruction with the osteoseptocutaneous fibula flap: Can we instigate ideological and technological reforms? Plast Reconstr Surg. 2016;137:963–70. [DOI] [PubMed] [Google Scholar]
- 40. Dubois L, Schreurs R, Jansen J, Maal TJ, Essig H, Gooris PJ, et al. Predictability in orbital reconstruction: A human cadaver study. Part II: Navigation-assisted orbital reconstruction. J Craniomaxillofac Surg. 2015;43:2042–9. [DOI] [PubMed] [Google Scholar]
- 41. Andrews BT, Surek CC, Tanna N, Bradley JP. Utilization of computed tomography image-guided navigation in orbit fracture repair. Laryngoscope. 2013;123:1389–93. [DOI] [PubMed] [Google Scholar]
- 42. Collyer J. Stereotactic navigation in oral and maxillofacial surgery. Br J Oral Maxillofac Surg. 2010;48:79–83. [DOI] [PubMed] [Google Scholar]
- 43. Azarmehr I, Stokbro K, Bell RB, Thygesen T. Surgical navigation: A systematic review of indications, treatments, and outcomes in oral and maxillofacial surgery. J Oral Maxillofac Surg. 2017;75:1987–2005. [DOI] [PubMed] [Google Scholar]
- 44. Bobek SL. Applications of navigation for orthognathic surgery. Oral Maxillofac Surg Clin North Am. 2014;26:587–98. [DOI] [PubMed] [Google Scholar]
- 45. Pallua N, Kim BS. Pre-expanded supraclavicular artery perforator flap. Clin Plast Surg. 2017;44:49–63. [DOI] [PubMed] [Google Scholar]
- 46. Wang AW, Zhang WF, Liang F, Li JY, Zhang XF, Niu XT. Pre-expanded thoracodorsal artery perforator-based flaps for repair of severe scarring in cervicofacial regions. J Reconstr Microsurg. 2014;30:539–46. [DOI] [PubMed] [Google Scholar]
- 47. Wang C, Zhang J, Yang S, Hyakusoku H, Song P, Pu LL. The clinical application of preexpanded and prefabricated super-thin skin perforator flap for reconstruction of post-burn neck contracture. Ann Plast Surg. 2016;77:S49–52. [DOI] [PubMed] [Google Scholar]
- 48. Wang C, Zhang J, Yang S, Song P, Yang L, Pu LL. Pre-expanded and prefabricated abdominal superthin skin perforator flap for total hand resurfacing. Clin Plast Surg. 2017;44:171–7. [DOI] [PubMed] [Google Scholar]
- 49. Hocaoglu E, Arinci A, Berkoz O, Ozkan T. Free pre-expanded lateral circumflex femoral artery perforator flap for extensive resurfacing and reconstruction of the hand. J Plast Reconstr Aesthet Surg. 2013;66:1788–91. [DOI] [PubMed] [Google Scholar]
- 50. Zang M, Zhu S, Song B, Jin J, Liu D, Ding Q, et al. Reconstruction of extensive upper extremity defects using pre-expanded oblique perforator-based paraumbilical flaps. Burns. 2012;38:917–23. [DOI] [PubMed] [Google Scholar]
- 51. Kulahci Y, Sever C, Uygur F, Oksuz S, Sahin C, Duman H. Pre-expanded pedicled thoracodorsal artery perforator flap for postburn axillary contracture reconstruction. Microsurgery. 2011;31:26–31. [DOI] [PubMed] [Google Scholar]
- 52. Cheng A, Saint-Cyr M. Use of a pre-expanded “propeller” deep inferior epigastric perforator (DIEP) flap for a large abdominal wall defect. J Plast Reconstr Aesthet Surg. 2013;66:851–4. [DOI] [PubMed] [Google Scholar]
- 53. Dong L, Dong Y, He L, Liu C, Zhang Z, Xiao B, et al. Penile reconstruction by preexpanded free scapular flap in severely burned patient. Ann Plast Surg. 2014;73:S27–30. [DOI] [PubMed] [Google Scholar]
- 54. Pu LL, Wang C. Future perspectives of Pre-expanded perforator flaps. Clin Plast Surg. 2017;44:179–83. [DOI] [PubMed] [Google Scholar]
- 55. Wang C, Zhang J, Hyakusoku H, Song P, Pu LL. An overview of pre-expanded perforator flaps: Part 2, clinical applications. Clin Plast Surg. 2017;44:13–20. [DOI] [PubMed] [Google Scholar]
- 56. Wang C, Yang S, Pu LL. Pre-expanded super-thin skin perforator flaps. Clin Plast Surg. 2017;44:31–40. [DOI] [PubMed] [Google Scholar]
- 57. Pribaz JJ, Fine N, Orgill DP. Flap prefabrication in the head and neck: A 10-year experience. Plast Reconstr Surg. 1999;103:808–20. [DOI] [PubMed] [Google Scholar]
- 58. Yao ST. Microvascular transplantation of prefabricated free thigh flap. Plast Reconstr Surg. 1982;69:568. [DOI] [PubMed] [Google Scholar]
- 59. Margulis A, Agam K, Icekson M, Dotan L, Yanko-Arzi R, Neuman R. The expanded supraclavicular flap, prefabricated with thoracoacromial vessels, for reconstruction of postburn anterior cervical contractures. Plast Reconstr Surg. 2007;119:2072–7. [DOI] [PubMed] [Google Scholar]
- 60. Topalan M, Guven E, Demirtas Y. Hemifacial resurfacing with prefabricated induced expanded supraclavicular skin flap. Plast Reconstr Surg. 2010;125:1429–38. [DOI] [PubMed] [Google Scholar]
- 61. Yang DP, Zhang P. Facial resurfacing with prefabricated induced expanded skin flap. J Craniofac Surg. 2019;30:1131–4. [DOI] [PubMed] [Google Scholar]
- 62. Li Q, Zan T, Gu B, Liu K, Shen G, Xie Y, et al. Face resurfacing using a cervicothoracic skin flap prefabricated by lateral thigh fascial flap and tissue expander. Microsurgery. 2009;29:515–23. [DOI] [PubMed] [Google Scholar]
- 63. Mardini S, Tsai FC, Wei FC. The thigh as a model for free style free flaps. Clin Plast Surg. 2003;30:473–80. [DOI] [PubMed] [Google Scholar]
- 64. Kokkoli E, Shih HS, Spyropoulou GA, Jeng SF. Local free-style perforator flaps in head and neck reconstruction: An update and a useful classification. Plast Reconstr Surg. 2016;137:1863–74. [DOI] [PubMed] [Google Scholar]
- 65. Nthumba PM. The supraclavicular artery flap: A versatile flap for neck and orofacial reconstruction. J Oral Maxillofac Surg. 2012;70:1997–2004. [DOI] [PubMed] [Google Scholar]
- 66. Lamberty BG. The supra-clavicular axial patterned flap. Br J Plast Surg. 1979;32:207–12. [DOI] [PubMed] [Google Scholar]
- 67. Chiu ES, Liu PH, Friedlander PL. Supraclavicular artery island flap for head and neck oncologic reconstruction: Indications, complications, and outcomes. Plast Reconstr Surg. 2009;124:115–23. [DOI] [PubMed] [Google Scholar]
- 68. Kozin ED, Sethi RK, Herr M, Shrime MG, Rocco JW, Lin D, et al. Comparison of perioperative outcomes between the supraclavicular Artery Island flap and Fasciocutaneous free flap. Otolaryngol Head Neck Surg. 2016;154:66–72. [DOI] [PubMed] [Google Scholar]
- 69. Epps MT, Cannon CL, Wright MJ, Chaffin AE, Newsome RE, Friedlander PL, et al. Aesthetic restoration of parotidectomy contour deformity using the supraclavicular artery island flap. Plast Reconstr Surg. 2011;127:1925–31. [DOI] [PubMed] [Google Scholar]
- 70. Vinh VQ, Ogawa R, Van Anh T, Hyakusoku H. Reconstruction of neck scar contractures using supraclavicular flaps: Retrospective study of 30 cases. Plast Reconstr Surg. 2007;119:130–5. [DOI] [PubMed] [Google Scholar]
- 71. Alves HRN, Ishida LC, Ishida LH, Besteiro JM, Gemperli R, Faria JC, et al. A clinical experience of the supraclavicular flap used to reconstruct head and neck defects in late-stage cancer patients. J Plast Reconstr Aesthet Surg. 2012;65:1350–6. [DOI] [PubMed] [Google Scholar]
- 72. Sukato DC, Timashpolsky A, Ferzli G, Rosenfeld RM, Gordin EA. Systematic review of supraclavicular artery island flap vs free flap in head and neck reconstruction. Otolaryngol Head Neck Surg. 2019;160:215–22. [DOI] [PubMed] [Google Scholar]
- 73. Chen YC, Scaglioni MF, Carrillo Jimenez LE, Yang JC, Huang EY, Lin TS. Suprafascial anterolateral thigh flap harvest: A better way to minimize donor-site morbidity in head and neck reconstruction. Plast Reconstr Surg. 2016;138:689–98. [DOI] [PubMed] [Google Scholar]
- 74. Ali RS, Bluebond-Langner R, Rodriguez ED, Cheng MH. The versatility of the anterolateral thigh flap. Plast Reconstr Surg. 2009;124:e395–407. [DOI] [PubMed] [Google Scholar]
- 75. Jaiswal R, Pu LL. Reconstruction after complex facial trauma: Achieving optimal outcome through multiple contemporary surgeries. Ann Plast Surg. 2013;70:406–9. [DOI] [PubMed] [Google Scholar]


