Abstract
Hypertrophic scars often develop following burn-related injuries. These scars can be cosmetically unappealing, but associated symptoms of pruritus, pain and restricted range of motion can impair a person’s quality of life. Laser and light therapies offer a minimally invasive, low-risk approach to treatment, with a short postoperative recovery period. As laser technology developed, studies have shown decreased scar thickness, neuropathic pain and need for surgical excision, as well as improved scar pigmentation, erythema, pliability, texture, height and pruritus. In this review, we present the evolution of laser therapy for hypertrophic burn scars, how different types of lasers work, indications, perioperative considerations and guidelines for practice management.
Keywords: Burn, Cicatrix, Hypertrophic, Contracture, Keloid, Laser therapy, Pruritus, Quality of life, Scar
Highlights.
Laser and light therapies offer a minimally invasive, low risk approach to treatment, with a short postoperative recovery period.
As laser technology developed, studies have shown decreased scar thickness, neuropathic pain, and need for surgical excision, as well as improved scar color, pliability, texture, height, and itching.
We present the evolution of laser therapy for hypertrophic burn scars, how different types of lasers work, indications, perioperative considerations, and guidelines for practice management.”
Background
The prevalence of developing a hypertrophic scar following a burn-related injury has been reported to be as high as 70% [1]. These scars can be cosmetically unappealing, with associated symptoms of pruritus, pain and restricted range of motion which can impair a person’s quality of life. Such scars are thought to develop from patterns of dysregulation of normal wound healing after trauma to the skin [2]. Surgical scar removal and contracture release remain important corrective therapies; however, there are high risks of scar recurrence. Nonsurgical interventions are often attempted prior to surgical interventions to inhibit or slow scar progression. Nonsurgical interventions include pressure garments, silicone gel sheeting, intralesional injections, cryotherapy, radiation therapy, laser and light therapy [2]. Laser and light therapies have now emerged as a minimally invasive, low-risk therapy with a short postoperative recovery period [3].
Since the first description in 1983 by Anderson and Parrish with intense pulsed light (IPL) [4] and Castro et al. with a continuous neodymium:yttrium–aluminum–garnet (Nd:YAG) laser [5], evidence has suggested that lasers could be used to treat hypertrophic scars. Anderson and Parrish introduced the principle of selective photothermolysis [4]. An appropriate wavelength, exposure time and energy level from light were focused on the target tissue. The tissue absorbs photons, resulting in either a photochemical reaction or heating. At different temperature ranges, physiologic changes occur within the tissue. From 60°C to 70°C, collagen along with other structural proteins are denatured; from 70°C to 80°C, cell membranes become permeable and nucleic acids denature; from 70°C to 100°C, coagulation necrosis results in hemostasis by closing blood vessels and denaturing plasma proteins; and above 100°C, tissue water vaporizes, resulting in physical separation or ablation. The selective nature relies on the principle that the targeted tissue has a greater optical absorption at a specific wavelength compared to the surrounding tissue. Lasers range from shorter-wavelength potassium titanium phosphate (KTP) on the visible light spectrum to longer-wavelength carbon dioxide on the infrared light spectrum [6]. Depending on the wavelength, photons are absorbed by hemoglobin, oxyhemoglobin, melanin, water, or collagen in the skin, generating heat, selectively affecting capillaries, pigmentation and scar tissue [4].
Review
Efficacy of lasers
As laser technology developed, studies have shown decreased scar thickness, neuropathic pain and need for surgical excision and improved scar pigmentation, erythema, pliability, texture, height, pruritus, heat sensitivity, contracture, function and overall quality of life, due to laser treatment [2,6–14].
Scars with variations in maturity may see improvements after the first laser session [8]. Scars can be targeted superficially to smoothen the skin, or deep to decrease tension and induce dermal remodeling of abnormal collagen. Often surgeons can tailor therapy by targeting superficial, deep, or a combination of both locations depending on the scar characteristics [7,8].
Classifying scars by dyschromia (erythema, hyper-/hypopigmentation) and scar thickness or atrophy was a way to simplify methods for determining which lasers were most appropriate depending on individual scar characteristics [10]. They are categorized as ablative or nonablative, fractional or nonfractional and vascular or nonvascular depending on their targeted mechanism of action [15]. Pulsed dye laser (PDL) therapy and ablative fractional resurfacing (AFR) laser therapy are two types of lasers that have shown the greatest efficacy when treating hypertrophic scars [8,10].
Classification of lasers
Ablative vs. nonablative lasers
Ablative lasers target the dermal and epidermal layers of the skin, resulting in damage to both layers and subsequent collagen remodeling. Pulsed CO2 lasers were the first ablative lasers that were highly efficacious; however, patients suffered from significant adverse effects [16]. The treated area during the first week would often present with edema, oozing, crusting and burning discomfort. Long-term complications were skin pigment changes, scarring and infections. Erbium:yttrium–aluminum–garnet (Er:YAG) ablative lasers were developed with a shallower absorption depth to decrease thermal damage and increase the rate of skin healing. Shallower skin penetration resulted in a lower efficacy, but a more favorable safety profile [17]. Nonablative lasers were developed to spare the epidermis and selectively damage the dermis skin layer. Damage to the dermis layer could still be achieved with a high optical penetration depth [18]. This resulted in less superficial damage compared to the ablative lasers, but less efficacy. Both ablative and nonablative lasers cause homogeneous thermal damage at their targeted depths [15].
Fractional lasers
The concept of fractional photothermolysis was first described in 2004 as a way to balance the efficacy of ablative lasers and the safety of nonablative lasers [15]. Fractional lasers maintain the selectivity of photothermolysis by targeting specific wavelengths of molecules, while creating microscopic holes or microholes. Microholes are areas of controlled widths, depths and densities surrounded by islands of healthy dermal and epidermal tissue for rapid regeneration and repair [15,19]. The surrounding unaffected skin becomes a source of viability by aiding in neocollagenesis or the creation of new healthy collagen and tissue remodeling. This decreases the thickness and improves the pliability of hypertrophic burn scars, allowing for improved range of motion across all joints [6]. Nonablative and ablative devices were modified and further categorized as either nonablative fractional resurfacing (NAFR) vascular erbium:glass lasers with wavelengths ranging from 1540 nm to 1550 nm or AFR lasers with wavelengths ranging from 10,600 nm fractional CO2 (fCO2) lasers to 2940 nm Er:YAG lasers [2].
NAFR
First introduced in 2004 [15], NAFR lasers result in selective thermolysis of the dermis layer of the skin by creating microholes. They heat the skin layer to temperatures ranging from 50°C to 70°C, resulting in irreversible coagulation of dermis proteins [6]. The epidermal layer is left unaffected and intact [3].
The lower temperatures of the NAFR laser allow for an increased safety profile but decreased efficacy compared to ablative lasers [10,20,21]. The wide safety profile allows for NAFR laser use over all areas of the body with minimal postoperative recovery time [6]. Traditionally, NAFR lasers are used for mild to moderate hypertrophic scars primarily involving the dermis and epidermis skin layers. Severe hypertrophic scars often require AFR laser tissue therapy [21].
AFR
First introduced in 2007, AFR lasers result in selective thermolysis of the dermis layer of the skin creating microholes and targeting water molecules [22]. However, temperatures rise above 100°C and vaporize the surrounding tissue. The area surrounding the thermolysis becomes a thermal coagulation zone involving the epidermis [10].
The higher temperatures achieved by the AFR lasers have achieved better efficacy profiles with better safety profiles than the traditional IPL lasers for hypertrophic scars [6]. Better safety profiles include a lower incidence of hypopigmentation and scarring [23]. AFR combines a short pulse duration with high energy to optimize efficacy by using heat to create microholes in the skin. The high temperatures induce a greater degree of dermis remodeling [10,20,21]. The surrounding undamaged tissue helps in the remodeling process, starting with a molecular cascade. Heat shock proteins, metalloproteinases and inflammatory cytokines are involved during the rapid healing response 48 hours following ablation to fill the vaporized columns with epidermal cells and restoring skin continuity [22,24]. New collagen formation and collagen remodeling results in a decrease of collagen type I and an increase in collagen type III [25]. The addition of collagen type III changes the architecture of the dermis by increasing pliability, decreasing thickness and improving molecular function [8,25,26]. This results in improved healing of unstable chronic scars [8,23,26–30]. AFR has been validated by two patient-reported outcome measures, the Vancouver Scarring Scale and the Patient and Observer Scar Assessment Score, and ultrasound by assessing scar thickness [7,8,13,25].
PDL
PDL is light device often categorized as nonablative vascular devices with wavelengths ranging from 585 to 595 nm. The mechanisms of PDL help reduce the vascularity to reduce erythema, pruritus, pigmentation, hypertrophy and neuropathic pain from hypertrophic scars. This is achieved by targeting hemoglobin, resulting in selective photothermolysis of blood vessels to decrease vascularity [12]. The pulse duration time must be shorter than the thermal relaxation time of hemoglobin to destroy capillary vasculature [31]. This selective blood vessel destruction results in tissue hypoxia and collagen catabolism. Collagen remodels and realigns to form less deposits [19,32]. PDL penetrates the skin less than AFR, limiting its use with thick hypertrophic scars greater than 1 cm. It also has limited utility treating hypertrophic scars in areas of tension. Relieving tension by small surgical scar incisions to create Z-plasties can synergistically facilitate PDL efficacy and reduce the need for larger surgical excision procedures [11]. Pruritus is thought to decrease from alterations in the release of regulatory cytokines with less scar vasculature [9]. PDL is used to treat immature thin hypertrophic scars with less than 1 cm thickness, vascular erythema, areas of minimal tension and pruritus. These lasers do not treat scar structure or result in collagen remodeling like AFR lasers [10].
PDL vs. fCO2 AFR
Although PDL lasers decrease pruritus, hypertrophy and erythema, advances in the development of fractional resurfacing lasers have added enhanced rehabilitation. Some believe they may decrease scar excision entirely, adding the additional benefit of decreased donor site morbidity [10]. AFR lasers reduce scar burden size and restore collagen architecture to a more organized array by vaporizing scars in grid structure patterns [12]. Combining AFR resurfacing with reconstructive surgical procedures has become an efficacious treatment modality [6]. AFR can manage symptoms and reduce the amount of surgical procedures. However, it does not replace the need for surgical scar release once mature scarring has formed. They have been found to reduce postoperative morbidity and improve overall outcomes [6]. The PDL mechanism works best to reduce vascularity, erythema, tenderness and pruritus for immature newer scars. PDL enhances and complements reconstructive procedures by minimizing tension and reducing scar thickness. Alternatively, the fCO2 or AFR laser mechanism serves to increase dermis pliability, improve abnormal texture, reduce thickness and rehabilitate mature older scars. Both mechanisms can be combined to synergistically treat hypertrophic burn scars [33–36].
Optimal timing for laser therapy
It was once thought that lasers should be used only after the scar had reached full maturation. Recent data suggest early initiation with a vascular device, NAFR, or AFR in the months following burn or surgical injury may decrease symptoms, decrease contractures, and increase mobility, to improve the overall rehabilitation process [10,37]. The best timing to initiate laser therapy is still unclear. Most strategies have depended on scar maturation characteristics. This takes into consideration the patient’s age, skin type, type of scar and comorbidities. Treatments can be divided into 1–9 treatments ranging from 4 weeks to 3 months between treatment intervals [6,36]. Following surgical excision, PDL, NAFR and AFR lasers have been used on the day of suture removal or weeks following suture removal with safe and effective results in preventing scar reformation [38–40]. Karmisholt et al. used a NAFR laser 1 day prior to surgery and during the early phase of wound healing. There were detectable improvements between those receiving the intervention from those not receiving lasers with scar formation. The authors concluded that early laser treatment has the potential to minimize scar formation with full-thickness wounds [41]. Initiating laser and light therapy as early as possible following surgical excision when sutures are first removed is reasonable to reduce recurrent scar formation [2].
Settings
We use the Syneron Candela PDL machine at the Johns Hopkins Burn Center. PDL utilizes a wavelength of 595 nm and we prefer a pulse width of 1.5 ms for most patients. Depending on the amount of skin pigmentation, the energy fluence or number of particles is adjusted to prevent skin trauma. Patients with Fitzpatrick I and II skin types are administered a fluence of 6 J/cm2, Fitzpatrick III and IV skin types are administered a fluence of 5 J/cm2 and Fitzpatrick V and VI skin types are administered a fluence of 4 J/cm2. The spot size is standardized to a diameter of 10 mm, with 20–30% overlap to ensure complete coverage. Each pulse of PDL is followed by sprayed cryogen to cool the targeted area, within 20–30 ms. The total surface area and number of pulses are measured and recorded for each patient.
The UltraPulse® Lumenis is an fCO2 AFR machine with three hand-piece options, ActiveFX™, DeepFX™ and SCAAR FX™. ActiveFX™ has the lowest energy and highest density. SCAAR FX™ has the highest energy and lowest density. We prefer DeepFX™ because there is a balance between energy and density. DeepFX™ is started at an energy level of 30 mJ per beam, a density of 5% and a cycle time of 300 Hz, resulting in depth of penetration of 0.5–1.5 mm. For safety, the energy level is started low at 30 mJ and titrated up to 50 mJ. It is important that the 50 mJ energy level is not exceeded. The noncollimated beam spot size is standardized to cover a diameter of 0.12 mm. If the skin blisters during application, the energy level should be reduced. For thicker scars, SCAAR FX™ may be used for deeper scar penetration. SCAAR FX™ is started at 60 mJ and 3% density and titrated to a maximum energy of 90 mJ and 1% density. The beams cover a square size of 10 mm × 10 mm or 1 cm2. The pattern should appear as a contiguous square grid with no overlap, unlike PDL. If the skin is charred, the char should be left in place, not removed. After fCO2 AFR, we apply topical triamcinolone over the treated areas as a method of laser-assisted drug delivery, to a maximum dose of 80 mg, or 1 mg/kg. Full-field CO2 is absolutely contraindicated in the treatment of burn scars because it results in a secondary burn injury that cannot re-epithelialize, except from the periphery.
Perioperative considerations
The patient’s age, scar characteristics, psychiatric conditions, neuropathic pain, chronic pain medications and views on pain and procedures should be considered. This will often determine the level of pain management and the need for either topical anesthesia or sedation under full anesthesia [6]. The PDL has the benefit of not requiring anesthesia; however, the target area must be cooled following treatment to prevent epidermal damage and new scar formation. Immature hypertrophic scars have reduced skin stability when compared to normal skin. Special caution should be taken to minimize damage to immature hypertrophic scars [42]. Lasers are usually well tolerated for treating hypertrophic scars. The most common postoperative findings following laser treatments are erythema, edema, bleeding, serous drainage and skin exfoliation lasting up to several days. It is important to choose the appropriate laser setting based on the target location of the body and Fitzpatrick skin type. A more conservative approach can minimize laser-induced damage. Laser light is transferred into heat and can cause burns if set at too high a level. Scars may also become worse if the setting is too high. It is important that only specialists with experience in treating hypertrophic burn scars and using lasers perform these procedures [6].
Fractional and nonfractional ablative lasers can also be used synergistically as a method of delivering medications into scar tissue [43–45]. Intralesional injections of corticosteroids and 5-fluorouracil (5-FU) may result in the accumulation of the medication in one location of the scar. If laser therapy is performed prior to applying topical corticosteroids or 5-FU, the uniform vaporized tissue columns allow application of an even distribution of the medications and increase the bioavailability at the scar site [46–48]. This also allows for the administration of lower doses of medications to prevent unwanted adverse effects [49]. Most laser procedures are complimented with topical medications as part of care [30,43,50].
A prospective study by Waibel et al. evaluated the efficacy of fCO2 laser therapy with either applying a topical corticosteroid or 5-FU. Topical medications were applied directly over the treated area with equal efficacy on reducing scar size and recurrence. However, patients treated with 5-FU had significantly less dermal atrophy and telangiectasia adverse effects compared to corticosteroids [45]. This evidence may support using topical 5-FU with AFR lasers prior to corticosteroids.
Immediately after treatment, a petrolatum-based ointment should be applied several times daily until the site is completely epithelialized. This takes approximately 2–3 days. Once epithelialization has taken place, a daily moisturizer should be applied for a week, containing sunscreen [6]. A day following the procedure, patients may shower and gently clean the area. Some physicians recommend applying vinegar-based compresses to the affected area. Pain is often minimal following laser treatment, lasting 1–2 days. Pruritus can last from days to weeks. Infection is uncommon, reported in < 1% of cases [10,37]. Preoperative antibiotics should be avoided unless the patient has a specific indication. Patients with a history of frequent perioral herpes infections should receive antiviral prophylaxis for head and neck laser procedures. In patients with higher Fitzpatrick skin types or more skin pigmentation, transient postinflammatory hyperpigmentation can occur from greater responses in melanin and fibroblasts [6,51,52]. Decreasing the fluence for higher Fitzpatrick skin types helps to minimize damage with PDL. Normal activity may begin following treatment. Patients should be educated to avoid exposing the area to high-intensity light early during the postoperative period. Sun protection with sun-blocking agents and compression garments can be applied after 2–3 days once the epithelial layer is restored [6]. Sunscreen should be continued indefinitely, to prevent long-term ultraviolet (UV) damage and permanent hyperpigmentation changes.
Recurrence remains a problem with pathological keloid and hypertrophic burn scars. Inflicting trauma to the skin has the potential to reinitiate the pathological process of scar formation, especially for keloid scars. Following AFR CO2 laser therapy, scar recurrence has been reported as early as 2 weeks and up to 3 years [53,54]. Following Er:YAG laser therapy, scar recurrence has been reported in 22% of scars at 8 months [43]. Following IPL Nd:YAG laser therapy, scar recurrence has been reported from 25% to 52.9% at 6 months, depending on the anatomical location of the scar [55]. Limitations in all studies relate to the lack of long-term follow-up beyond 12 months. Although these studies primarily evaluated keloid scars, recurrences have been reported following hypertrophic burn scars [25]. Long-term studies are needed for determining proper follow-up intervals.
Case experience
The following cases outline our experience treating hypertrophic burn scars using different combinations of lasers. Combining PDL and fCO2 lasers either concurrently or sequentially can synergistically increase the efficacy of treatment. Determining what laser to use depends on the characteristics of each individual scar.
Case 1
A 4-year-old boy sustained superficial partial thickness scald burns to his right buttock and thigh (Fig. 1). He was treated with debridement and xenografting, followed by autografting. Early hypervascular scars developed at the right buttock and thigh (Fig. 1a). The early hypervascular scars were first treated using PDL for 2 sessions. Upon follow-up, PDL was switched to fCO2 for 3 sessions due to scar maturation. Combining both PDL and fCO2 lasers resulted in successful scar treatment as the scar characteristics evolved over time (Fig. 1b).
Figure 1.
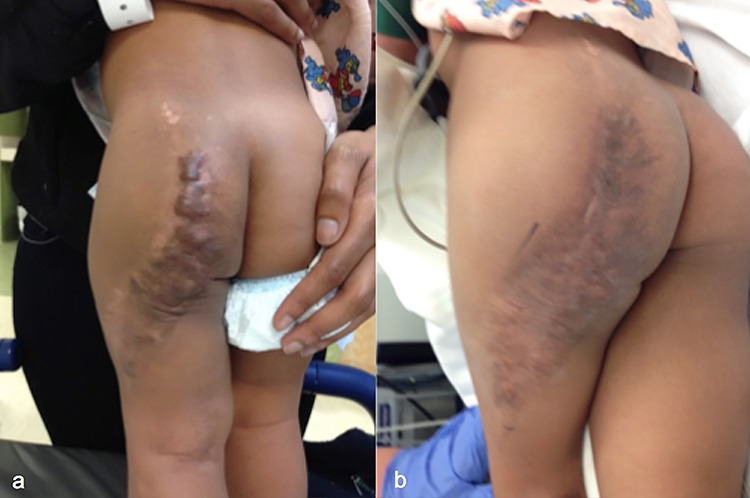
Case 1, a 4-year-old boy sustained superficial partial thickness scald burns to his right buttock and thigh, before treatment (a), and after treatment (b)
Case 2
An 8-year-old boy sustained deep partial thickness contact burns to his right forearm. He was treated with tangential excision and meshed autologous skin grafting (Fig. 2). Moderate hypertrophic scars developed at the location of the meshed graft. The early scar was treated with PDL for 4 treatment sessions. Following PDL sessions, the mature scar was successfully treated with fCO2 for 2 treatment sessions.
Figure 2.
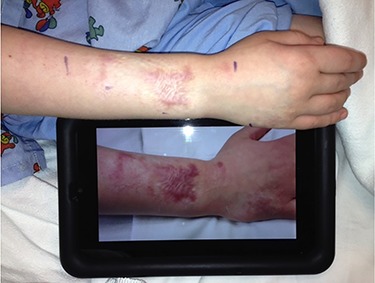
Case 2, an 8-year-old boy sustained deep partial thickness contact burns to his right forearm
Case 3
A 15-year-old girl sustained deep partial thickness grease burns to her lower extremities (Figure 3). Burns were more extensive at the location of the left leg. She was treated with tangential excision and split thickness skin grafting (Fig. 3a). Hyperemic scars developed on both lower extremities, which were successfully treated with PDL for 3 treatment sessions to the central portion of the scars and fCO2 for 3 treatment sessions to the periphery of the scars (Fig. 3b). Both PDL and fCO2 treatments were performed concurrently.
Figure 3.
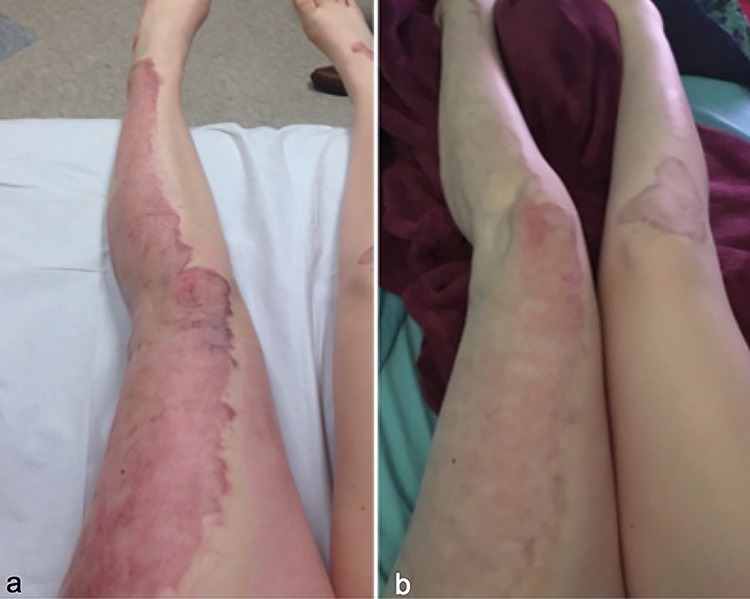
Case 3, a 15-year-old girl sustained deep partial thickness grease burns to her lower extremities, before treatment (a), after treatment (b)
Case 4
A 36-year-old female cardiac ICU nurse sustained deep partial burns to her head and neck from a house fire. The burned areas were allowed to heal by secondary re-epithelialization (Fig. 4). Hyperemic scars developed in areas of the face. She underwent PDL for 4 treatment sessions. This was followed by fCO2 for 4 treatment sessions to improve the remaining scar thickness and stiffness. Successful treatment was achieved by tailoring different laser therapies to scar characteristics (Fig. 5).
Figure 4.
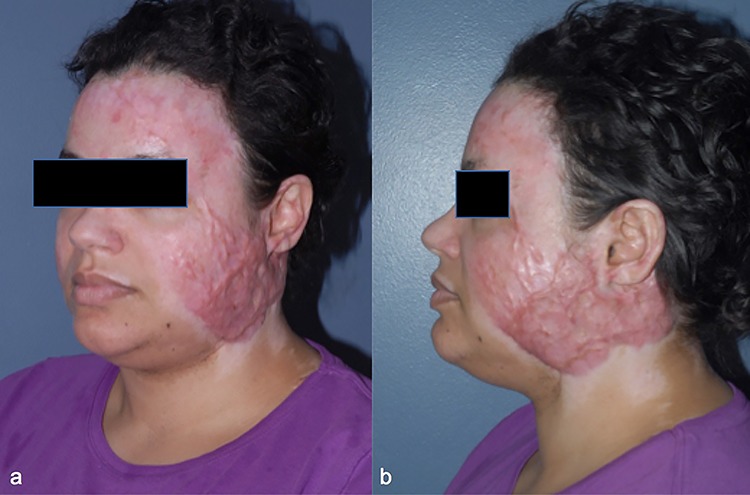
Case 4, a 36-year-old female sustained deep partial burns to her head and neck from a house fire. Oblique view (a) and lateral view (b) before treatment
Figure 5.
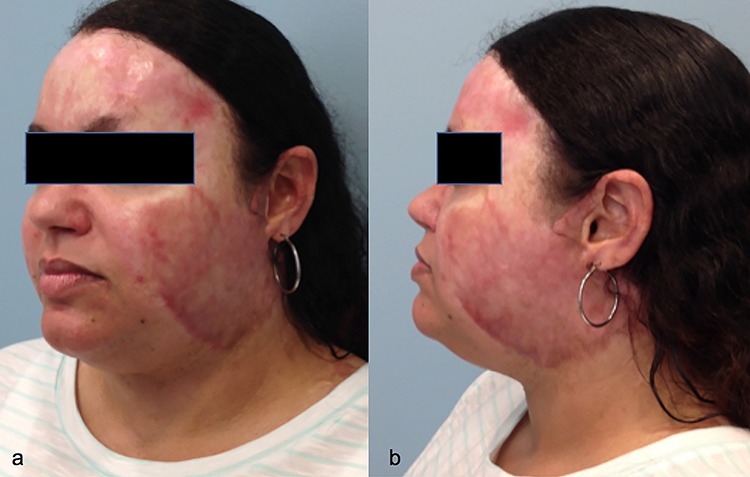
Case 4, a 36-year-old female sustained deep partial burns to her head and neck from a house fire. Oblique view (a) and lateral view (b) after treatment
Conclusions
Laser and light therapies offer a minimally invasive, low-risk approach to treatment, with a short postoperative recovery period. As laser technology developed, studies have shown decreased scar thickness, neuropathic pain and need for surgical excision, as well as improved scar pigmentation, erythema, pliability, texture, height and pruritus. In this review, we presented the evolution of laser therapy for hypertrophic burn scars, how different types of lasers work, indications, perioperative considerations and guidelines for practice management.
Acknowledgments
We thank Carrie Cox, MS, RN and Vidhi Javia, BS for their assistance with coordinating clinical research at The Johns Hopkins Burn Center.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The data used and/or analyzed during the current study are accessible online.
Authors’ contributions
KMK, MA and CSH all contributed equally to conception and design, acquisition of data, or analysis and interpretation of data. KMK and CSH drafted the manuscript. MA and CSH revised it critically for important intellectual content. All authors approved the final manuscript version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
This study was exempt from institutional review board approval.
Conflicts of interest
The authors declare that they have no competing interests.
References
- 1. Bombaro KM, Engrav LH, Carrougher GJ, Wiechman SA, Faucher L, Costa BA, et al. What is the prevalence of hypertrophic scarring following burns? Burns 2003;29:299–302. [DOI] [PubMed] [Google Scholar]
- 2. Fu X, Dong J, Wang S, Yan M, Yao M. Advances in the treatment of traumatic scars with laser, intense pulsed light, radiofrequency, and ultrasound. Burns Trauma 2019;7:1. doi: 10.1186/s41038-018-0141-0. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Willows BM, Ilyas M, Sharma A. Laser in the management of burn scars. Burns 2017;43:1379–89. [DOI] [PubMed] [Google Scholar]
- 4. Anderson RR, Parrish JA. Selective photothermolysis: Precise microsurgery by selective absorption of pulsed radiation. Science 1983;220:524–7. [DOI] [PubMed] [Google Scholar]
- 5. Castro DJ, Abergel RP, Meeker C, Dwyer RM, Lesay MA, Uitto J. Effects of the Nd:YAG laser on DNA synthesis and collagen production in human skin fibroblast cultures. Ann Plast Surg 1983;11:214–22. [PubMed] [Google Scholar]
- 6. Issler-Fisher AC, Waibel JS, Donelan MB. Laser modulation of hypertrophic scars: Technique and practice. Clin Plast Surg 2017;44:757–66. [DOI] [PubMed] [Google Scholar]
- 7. Issler-Fisher AC, Fisher OM, Smialkowski AO, Li F, van Schalkwyck CP, Haertsch P, et al. Ablative fractional CO2 laser for burn scar reconstruction: An extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns 2017;43:573–82. [DOI] [PubMed] [Google Scholar]
- 8. Hultman CS, Friedstat JS, Edkins RE, Cairns BA, Meyer AA. Laser resurfacing and remodeling of hypertrophic burn scars: The results of a large, prospective, before-after cohort study, with long-term follow-up. Ann Surg 2014;260:519–29, discussion 529–32. [DOI] [PubMed] [Google Scholar]
- 9. Allison KP, Kiernan MN, Waters RA, Clement RM. Pulsed dye laser treatment of burn scars. Alleviation or irritation? Burns 2003;29:207–13. [DOI] [PubMed] [Google Scholar]
- 10. Anderson RR, Donelan MB, Hivnor C, Greeson E, Ross EV, Shumaker PR, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: Consensus report. JAMA Dermatol 2014;150:187–93. [DOI] [PubMed] [Google Scholar]
- 11. Donelan MB, Parrett BM, Sheridan RL. Pulsed dye laser therapy and z-plasty for facial burn scars: The alternative to excision. Ann Plast Surg 2008;60:480–6. [DOI] [PubMed] [Google Scholar]
- 12. Hultman CS, Edkins RE, Lee CN, Calvert CT, Cairns BA. Shine on: Review of laser- and light-based therapies for the treatment of burn scars. Dermatol Res Pract 2012;2012:243651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khandelwal A, Yelvington M, Tang X, Brown S. Ablative fractional photothermolysis for the treatment of hypertrophic burn scars in adult and pediatric patients: A single surgeon's experience. J Burn Care Res 2014;35:455–63. [DOI] [PubMed] [Google Scholar]
- 14. Zuccaro J, Ziolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg 2017;44:767–79. [DOI] [PubMed] [Google Scholar]
- 15. Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR. Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med 2004;34:426–38. [DOI] [PubMed] [Google Scholar]
- 16. Lowe NJ, Lask G, Griffin ME, Maxwell A, Lowe P, Quilada F. Skin resurfacing with the Ultrapulse carbon dioxide laser. Observations on 100 patients. Dermatol Surg 1995 Dec; 21(12): 1025–9. [DOI] [PubMed] [Google Scholar]
- 17. Khatri KA, Ross V, Grevelink JM, Magro CM, Anderson RR. Comparison of erbium:YAG and carbon dioxide lasers in resurfacing of facial rhytides. Arch Dermatol 1999;135:391–7. [DOI] [PubMed] [Google Scholar]
- 18. Goldberg DJ, Whitworth J. Laser skin resurfacing with the Q-switched Nd:YAG laser. Dermatol Surg 1997;23: 903–6; discussion 906–7. [DOI] [PubMed] [Google Scholar]
- 19. Jin R, Huang X, Li H, Yuang Y, Li B, Cheng C, et al. Laser therapy for prevention and treatment of pathologic excessive scars. Plast Reconstr Surg 2013;132:1747–58. [DOI] [PubMed] [Google Scholar]
- 20. Oh BH, Hwang YJ, Lee YW, Choe YB, Ahn KJ. Skin characteristics after fractional photothermolysis. Ann Dermatol 2011;23:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim HS, Lee JH, Park YM, Lee YJ. Comparison of the effectiveness of nonablative fractional laser versus ablative fractional laser in thyroidectomy scar prevention: A pilot study. J Cosmet Laser Ther 2012;14:89–93. [DOI] [PubMed] [Google Scholar]
- 22. Hantash BM, Bedi VP, Kapadia B, Rahman Z, Jiang K, Tanner H, et al. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg Med 2007;39:96–107. [DOI] [PubMed] [Google Scholar]
- 23. Uebelhoer NS, Ross EV, Shumaker PR. Ablative fractional resurfacing for the treatment of traumatic scars and contractures. Semin Cutan Med Surg 2012;31:110–20. [DOI] [PubMed] [Google Scholar]
- 24. DeBruler DM, Blackstone BN, Baumann ME, McFarland KL, Wulff BC, Wilgus TA, et al. Inflammatory responses, matrix remodeling, and re-epithelialization after fractional CO2 laser treatment of scars. Lasers Surg Med 2017;49:675–85. [DOI] [PubMed] [Google Scholar]
- 25. Ozog DM, Liu A, Chaffins ML, Ormsby AH, Fincher EF, Chipps LK, et al. Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fractional carbon dioxide laser. JAMA Dermatol 2013;149:50–7. [DOI] [PubMed] [Google Scholar]
- 26. Qu L, Liu A, Zhou L, He C, Grossman PH, Moy RL, et al. Clinical and molecular effects on mature burn scars after treatment with a fractional CO(2) laser. Lasers Surg Med 2012;44:517–24. [DOI] [PubMed] [Google Scholar]
- 27. El-Zawahry BM, Sobhi RM, Bassiouny DA, Tabak SA. Ablative CO2 fractional resurfacing in treatment of thermal burn scars: An open-label controlled clinical and histopathological study. J Cosmet Dermatol 2015;14:324–31. [DOI] [PubMed] [Google Scholar]
- 28. Azzam OA, Bassiouny DA, El-Hawary MS, El Maadawi ZM, Sobhi RM, El-Mesidy MS. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: A clinical, histological, and immunohistochemical study. Lasers Med Sci 2016;31:9–18. [DOI] [PubMed] [Google Scholar]
- 29. Shumaker PR, Kwan JM, Badiavas EV, Waibel J, Davis S, Uebelhoer NS. Rapid healing of scar-associated chronic wounds after ablative fractional resurfacing. Arch Dermatol 2012;148:1289–93. [DOI] [PubMed] [Google Scholar]
- 30. Waibel J, Beer K. Ablative fractional laser resurfacing for the treatment of a third-degree burn. J Drugs Dermatol 2009;8:294–7. [PubMed] [Google Scholar]
- 31. Oliaei S, Nelson JS, Fitzpatrick R, Wong BJ. Laser treatment of scars. Facial Plast Surg 2012;28:518–24. [DOI] [PubMed] [Google Scholar]
- 32. Parrett BM, Donelan MB. Pulsed dye laser in burn scars: Current concepts and future directions. Burns 2010;36:443–9. [DOI] [PubMed] [Google Scholar]
- 33. Cheng ET, Nowak KC, Koch RJ. Effect of blended carbon dioxide and erbium:YAG laser energy on preauricular and ear lobule keloid fibroblast secretion of growth factors: A serum-free study. Arch Facial Plast Surg 2001;3:252–7. [DOI] [PubMed] [Google Scholar]
- 34. Martin MS, Collawn SS. Combination treatment of CO2 fractional laser, pulsed dye laser, and triamcinolone acetonide injection for refractory keloid scars on the upper back. J Cosmet Laser Ther 2013;15:166–70. [DOI] [PubMed] [Google Scholar]
- 35. Cohen JL, Safety GR. Efficacy evaluation of pulsed dye laser treatment, CO2 ablative fractional resurfacing, and combined treatment for surgical scar clearance. J Drugs Dermatol 2016;15:1315–9. [PubMed] [Google Scholar]
- 36. Hultman CS, Edkins RE, Cairns BA, Meyer AA. Logistics of building a laser practice for the treatment of hypertrophic burn scars. Ann Plast Surg 2013;70:581–6. [DOI] [PubMed] [Google Scholar]
- 37. Shumaker PR, Kwan JM, Landers JT, Uebelhoer NS. Functional improvements in traumatic scars and scar contractures using an ablative fractional laser protocol. J Trauma Acute Care Surg 2012;73:S116–21. [DOI] [PubMed] [Google Scholar]
- 38. Choe JH, Park YL, Kim BJ, Kim MN, Rho NK, Park NS, et al. Prevention of thyroidectomy scar using a new 1,550-nm fractional erbium-glass laser. Dermatol Surg 2009;35:1199–205. [DOI] [PubMed] [Google Scholar]
- 39. Nouri K, Jimenez GP, Harrison-Balestra C, Elgart GW. 585-nm pulsed dye laser in the treatment of surgical scars starting on the suture removal day. Dermatol Surg 2003;29:65–73, discussion 73. [DOI] [PubMed] [Google Scholar]
- 40. Sobanko JF, Vachiramon V, Rattanaumpawan P, Miller CJ. Early postoperative single treatment ablative fractional lasing of Mohs micrographic surgery facial scars: A split-scar, evaluator-blinded study. Lasers Surg Med 2015;47:1–5. [DOI] [PubMed] [Google Scholar]
- 41. Karmisholt KE, Wenande E, Thaysen-Petersen D, Phillipsen PA, Paasch U, Haedersdal M. Early intervention with non-ablative fractional laser to improve cutaneous scarring –A randomized controlled trial on the impact of intervention time and fluence levels. Lasers Surg Med 2018;50:28–36. [DOI] [PubMed] [Google Scholar]
- 42. Tong AK, Tan OT, Boll J, Parrish JA, Murphy GF.. Ultrastructure: Effects of melanin pigment on target specificity using a pulsed dye laser (577 nm). J Invest Dermatol 1987;88:747–52. [DOI] [PubMed] [Google Scholar]
- 43. Cavalie M, Sillard L, Montaudie H, Bahadoran P, Lacour JO, Passeron T. Treatment of keloids with laser-assisted topical steroid delivery: A retrospective study of 23 cases. Dermatol Ther .2015;28:74–8. [DOI] [PubMed] [Google Scholar]
- 44. Waibel JS, Wulkan AJ, Shumaker PR. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers Surg Med 2013;45:135–40. [DOI] [PubMed] [Google Scholar]
- 45. Waibel JS, Wulkan AJ, Rudnick A, Daoud A. Treatment of hypertrophic scars using laser-assisted corticosteroid versus laser-assisted 5-fluorouracil delivery. Dermatol Surg 2019;45:23–30. [DOI] [PubMed] [Google Scholar]
- 46. Forster B, Klein A, Szeimies RM, Maisch T. Penetration enhancement of two topical 5-aminolaevulinic acid formulations for photodynamic therapy by erbium:YAG laser ablation of the stratum corneum: Continuous versus fractional ablation. Exp Dermatol 2010;19:806–12. [DOI] [PubMed] [Google Scholar]
- 47. Haedersdal M, Sakamoto FH, Farinelli WA, Doukas AG, Tam J, Anderson RR. Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med 2010;42:113–22. [DOI] [PubMed] [Google Scholar]
- 48. Lee WR, Shen SC, Pai MH, Yang HH, Yuan CY, Fang JY. Fractional laser as a tool to enhance the skin permeation of 5-aminolevulinic acid with minimal skin disruption: A comparison with conventional erbium:YAG laser. J Control Release 2010;145:124–33. [DOI] [PubMed] [Google Scholar]
- 49. Ali FR, Al-Niaimi F. Laser-assisted drug delivery in dermatology: From animal models to clinical practice. Lasers Med Sci 2016;31:373–81. [DOI] [PubMed] [Google Scholar]
- 50. Issa MC, Kassuga LE, Chevrand NS, Pires MT. Topical delivery of triamcinolone via skin pretreated with ablative radiofrequency: A new method in hypertrophic scar treatment. Int J Dermatol 2013;52:367–70. [DOI] [PubMed] [Google Scholar]
- 51. Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: Evidence-based review. J Clin Aesthet Dermatol 2017;10:51–67. [PMC free article] [PubMed] [Google Scholar]
- 52. Alexis AF. Lasers and light-based therapies in ethnic skin: Treatment options and recommendations for Fitzpatrick skin types V and VI. Br J Dermatol 2013;169(Suppl 3):91–7. [DOI] [PubMed] [Google Scholar]
- 53. Conejo-Mir JS, Corbi R, Linares M. Carbon dioxide laser ablation associated with interferon alfa-2b injections reduces the recurrence of keloids. J Am Acad Dermatol 1998;39:1039–40. [DOI] [PubMed] [Google Scholar]
- 54. Garg GA, Sao PP, Khopkar US. Effect of carbon dioxide laser ablation followed by intralesional steroids on keloids. J Cutan Aesthet Surg 2011;4:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Koike S, Akaishi S, Nagashima Y, Dohi T, Hyakusoku H, Ogawa R. Nd:YAG laser treatment for keloids and hypertrophic scars: An analysis of 102 cases. Plast Reconstr Surg Glob Open 2015;2(12):e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and/or analyzed during the current study are accessible online.


