Abstract
Background
A previous systematic review found that giving neoadjuvant chemotherapy before surgery improved survival compared with radiotherapy. However, the role of neoadjuvant chemotherapy followed by surgery versus surgery alone is still unclear.
Objectives
To assess the role of neoadjuvant chemotherapy in women with early or locally‐advanced cervical cancer.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library) (to Issue 8, 2012), MEDLINE (OVID) (to Aug 2012), LILACS (to Aug 2012), Physician's Data Query (PDQ) (to Aug 2012). We sought both published and unpublished trials and undertook systematic searches of a number of trial sources with no restrictions.
Selection criteria
Randomised trials comparing neoadjuvant chemotherapy with surgery in women with early or locally‐advanced cervical cancer who had not undergone any prior treatment likely to interfere with the treatment comparison. Trials giving radical radiotherapy for inoperable tumours and/or post‐operative radiotherapy were also eligible. The primary outcome was overall survival (OS). Secondary outcomes were progression‐free survival (PFS), local and distant recurrence, rates of resection and surgical morbidity.
Data collection and analysis
Two authors independently extracted and checked data from trial reports, Depending on the type of outcome, trial hazard ratios (HRs) and odds ratios (ORs) were obtained or estimated from trial reports, or sought from trial investigators.
Main results
Six trials (1078 women) were identified for inclusion in this updated review. All six trials provided data on OS (1071 women) and PFS (1027 women). Data on resection rates and pathological response were only available for five trials (908 to 940 women) and data on recurrence were only available for four trials (737 women). Both OS (HR 0.77, 95% confidence interval (CI) 0.62 to 0.96, P = 0.02) and PFS (HR 0.75, 95% CI 0.61 to 0.93, P = 0.008) were significantly improved with neoadjuvant chemotherapy. The estimate for local recurrence was in favour of neoadjuvant chemotherapy (OR 0.67, 95% CI 0.45 to 0.99, P = 0.04), although heterogeneity was observed. The result was no longer significant when the random‐effects model was used (OR 0.60, 95% CI 0.32 to 1.12, P = 0.11). Whilst not significant, estimates for distant recurrence (OR 0.72, 95% CI 0.45 to 1.14, P = 0.16) and rates of resection (OR 1.55, 95% CI 0.96 to 2.50, P = 0.07) tended to favour neoadjuvant chemotherapy, although heterogeneity was observed. Exploratory analyses of pathological response showed a significant decrease in adverse pathological findings with neoadjuvant chemotherapy (OR 0.54, 95% CI 0.40 to 0.73, P = < 0.0001 for lymph node status; OR 0.58, 95% CI 0.41 to 0.82, P = 0.002 for parametrial infiltration) which, despite substantial heterogeneity, was still significant when the random‐effects model was used. There were also no differences in the effect of neoadjuvant chemotherapy on survival according to total cisplatin dose, chemotherapy cycle length or by cervical cancer stage.
Authors' conclusions
Both OS and PFS were improved with neoadjuvant chemotherapy. Although the effects were less clear on all other pre‐specified outcomes, they all tended to be in favour of neoadjuvant chemotherapy. Whilst these results appear to indicate that neoadjuvant chemotherapy may offer a benefit over surgery alone for women with early‐stage or locally‐advanced cervical cancer, the evidence is based on only a small number of trials, and further research may be warranted.
Keywords: Female; Humans; Chemotherapy, Adjuvant; Chemotherapy, Adjuvant/methods; Disease‐Free Survival; Neoadjuvant Therapy; Neoadjuvant Therapy/methods; Randomized Controlled Trials as Topic; Treatment Outcome; Uterine Cervical Neoplasms; Uterine Cervical Neoplasms/drug therapy; Uterine Cervical Neoplasms/pathology; Uterine Cervical Neoplasms/surgery
Plain language summary
Chemotherapy given before surgery, compared with surgery alone for women with cervical cancer
Around the world, cervical cancer is the second most common cancer in women. In some countries, screening (with smear tests) has reduced the number of women with cervical cancer, but large numbers of women still die from the disease every year.
Where the cancer has not spread outside the cervix (early‐stage disease) women may have an operation to remove it by taking out the cervix, womb, the fallopian tubes, and maybe other nearby tissues (radical surgery). Or they might have treatment with x‐rays (radical radiotherapy). Both of these treatments have been shown to be as good as each other. If the tumour is bigger, or has spread to tissues around the cervix (locally‐advanced disease) women may also receive chemotherapy (drug treatment) at the same time as radiotherapy (chemoradiation).
Giving chemotherapy before radical surgery (neoadjuvant chemotherapy) might shrink the tumour. This may make surgery easier and help to remove any tiny tumours that cannot be easily seen. A previous review found that women getting chemotherapy before radical surgery lived longer than women who got radical radiotherapy. However, we do not know whether giving chemotherapy before radical surgery is better than radical surgery on its own.
This review found six trials that included 1078 women. Using information from the trials, we found that giving chemotherapy before surgery helped women to live longer and also to live longer without cancer. It was not clear whether chemotherapy made radical surgery easier or helped to stop the cancer from coming back. The type of drugs used, and how they were given, did not affect the results. Also, results were similar in women with both early stage and more advanced stages of disease.
In one trial, all of the women also had radiotherapy after surgery (post‐operative radiotherapy). In the other trials, up to two thirds of women got this post‐operative radiotherapy. We are not sure how this extra treatment affects the results. It may also give women more side‐effects.
Although neoadjuvant chemotherapy seems to help women with cervical cancer live for longer and also to live for longer without disease, the results are based on only a small number of trials. If new drugs or new combinations of drugs show promising results, it may be worth doing more trials with these new treatments of neoadjuvant chemotherapy before surgery.
Background
Description of the condition
Globally, cervical cancer is the third most common female cancer with over 500,000 new cases diagnosed every year (WHO 2006; Jemal 2011). More than 85% of these cases and deaths occur in economically developing and medically under‐served countries, largely in sub‐Saharan Africa, South America and South‐Central Asia where it is often the second most common female cancer (Jemal 2011). Whilst there has been a decline in cases seen in North America, some European countries, Australia and New Zealand (Sasieni 1995; Arbyn 2007; Howlader 2011; Jemal 2011), mainly as a result of successful screening programs (Devesa 1995), there are still almost 300,000 deaths from this disease recorded annually (Jemal 2011).
Description of the intervention
Historically, the standard treatment for earlier‐stage patients (defined as FIGO stage IB1 and usually with tumours less than 4cm) has been radical surgery in patients with operable disease, or radical radiotherapy. Both of these treatments have been shown to be equally effective, with 5‐year survival in the region of 80% to 90% (Landoni 1997; Benedet 1998) and 20‐year survival in the region of 72 to 77% (Maneo 2011). Patients with locally‐advanced disease (defined as FIGO stage IB2 and usually with tumours greater than 4cm, IIB, III and IVA) were usually treated with radical radiotherapy, which consisted of external beam radiotherapy and internal brachytherapy. However, the discovery that cervical cancer tumours were sensitive to chemotherapy (Friedlander 1983) led to the initiation of studies looking at adding chemotherapy to both radiotherapy and surgery. Following a National Cancer Institute (NCI) alert in 1999 (NCI 1999), chemoradiotherapy became standard care for women with locally‐advanced cervical cancer. However, surgery is still a valid treatment option, especially in earlier stage disease.
How the intervention might work
Possible advantages to giving neoadjuvant chemotherapy before surgery, especially in light of the advances in surgical techniques in recent years, include the potential for reducing tumour volume, increasing resectability (Sardi 1990) and helping to control micrometastatic disease (Thigpen 1981). Neoadjuvant chemotherapy may also have the potential to provide a viable alternative to chemoradiotherapy when access to radiotherapy is poor or if there are unavoidable delays in delivering radiotherapeutic treatment (Basile 2006).
Why it is important to do this review
The results of a previous review found a benefit of giving neoadjuvant chemotherapy prior to surgery when compared with radical radiotherapy (NACCCMA 2003). However, the best way to assess what chemotherapy adds to surgery is to compare it directly with surgery alone. A recent review of chemotherapy for cervical cancer (Tierney 2008) looked at trials that compared adding neoadjuvant chemotherapy to surgery with surgery alone, but results from individual trials were conflicting which suggested the need for a comprehensive and systematic review of all relevant trials addressing this question.
We first published this review early in 2010 (Rydzewska 2010) and update it here with recent searches and additional data.
Objectives
The primary aim of this review was to assess the effects of neoadjuvant chemotherapy in women with early or locally‐advanced cervical cancer compared with planned surgery alone. A secondary aim was to assess whether any trial characteristics had any impact on the effects of neoadjuvant chemotherapy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Women (of any age) with early‐stage or locally‐advanced cervical cancer who had not undergone any form of prior chemotherapy likely to interfere with the treatment comparison.
Types of interventions
Neoadjuvant chemotherapy followed by radical surgery versus radical surgery. Trials that included women found to have inoperable tumours and who received radical radiotherapy instead of surgery were included, provided that the same criteria were used across both arms of the trial. Also, trials that gave post‐operative radiotherapy were included as long as the post‐operative treatment was given in both arms.
Types of outcome measures
Overall survival (OS) was the primary outcome and defined as the time to death. Secondary outcomes were progression‐free survival (PFS) (defined as the time to progression or death), local and distant recurrence, rates of radical resection and surgical morbidity. After searches were completed it was found that most of the included trials reported pathological findings (associated with a high risk of progression or recurrence). Therefore, although pathological response was not an outcome measure that was pre‐specified in the protocol (Rydzewska 2008), we also conducted additional post‐hoc analyses to investigate whether rates of adverse pathological findings within individual trials had any influence on the overall results.
Search methods for identification of studies
To avoid publication bias, we sought both published and unpublished trials and undertook systematic searches of a number of sources, with no restriction on the language of publication. Search strategies were tailored to individual databases to maximise their potential for identifying relevant trials.
Electronic searches
We searched the following databases electronically:
MEDLINE (OVID) (1966 to Aug 2012)
LILACS (1982 to Aug 2012)
We also searched the following trial registers electronically (to identify any potentially eligible unpublished trials):
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library) (1966 to Issue 8, 2012)
Physician's Data Query (PDQ) (all online records up to Aug 2012)
We also searched the following conference proceedings electronically (or by handsearching, if the electronic version was unavailable):
American Society of Clinical Oncology (ASCO) annual meeting abstracts (1995 to 2012)
International Journal of Gynecological Cancer Society (IGCS) biennial meeting abstracts (2003 to 2010)
European Society of Gynaecological Oncology (ESGO) biennial meeting abstracts (2003 to 2011)
We searched MEDLINE using the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Higgins 2011) together with MeSH and free text terms specific to the review (Appendix 1). This search strategy was also amended to search the CENTRAL (Appendix 2) and LILACS (Manriquez 2008; Appendix 3) databases.
We searched ASCO abstracts using "cervi$" as a keyword, and PDQ using "cervical cancer", "surgery", and "chemotherapy" as keywords.
Searching other resources
We handsearched reference lists of relevant publications and reviews to identify any further potentially eligible trial reports.
We contacted authors of relevant trials to ask if they could provide further summary data that had not been reported in the trial publication.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts, identified by both electronic searching and by handsearching of conference proceedings and reference lists, to a reference management database and removed any duplicates. Where potentially relevant abstracts were identified, we obtained full publications and these were assessed independently by two review authors.
Data extraction and management
We extracted data on patient characteristics, interventions and outcomes and two review authors (LR, CV) independently checked these, with any disagreements resolved by consensus with a third author when necessary (JT). We sought further information from trial authors where papers did not contain information on all outcomes stated in the protocol, and to allow for the inclusion of updated follow‐up, where available.
Assessment of risk of bias in included studies
The methodological quality of included studies was independently assessed by two review authors (LR, CV), using the risk of bias tool (Table 1) from the Cochrane Handbook (Higgins 2011) and any disagreements were resolved by consensus with a third review author as necessary (JT). Trials were individually assessed for risk of bias on the basis of adequate sequence generation, allocation concealment, blinding, whether incomplete outcome data was presented or if there was evidence of selective outcome reporting. Trials considered to be free of substantial biases that might affect the results were included in the meta‐analysis, with any potential sources of bias clearly highlighted (Risk of bias in included studies).
1. Table 01 Risk of Bias Tool.
| Sequence generation. | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. | Was the allocation sequence adequately generated? |
| Allocation concealment. | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. | Was allocation adequately concealed? |
| Blinding of participants, personnel and outcome assessors Assessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Was knowledge of the allocated intervention adequately prevented during the study? |
| Incomplete outcome data Assessments should be made for each main outcome (or class of outcomes). | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. | Were incomplete outcome data adequately addressed? |
| Selective outcome reporting. | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. | Are reports of the study free of suggestion of selective outcome reporting? |
| Other sources of bias. | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre‐specified in the review’s protocol, responses should be provided for each question/entry. |
Was the study apparently free of other problems that could put it at a high risk of bias? |
Measures of treatment effect
For meta‐analyses of time‐to‐event outcomes such as OS and PFS, the most appropriate statistic is the hazard ratio (HR). Where available the HR and associated statistics were either extracted directly from the trial report or provided directly by the trialists. When the HR was not available, it was estimated indirectly from Kaplan‐Meier curves or other summary statistics using published methods (Parmar 1998; Williamson 2002; Tierney 2007). Where possible, we used a number of methods to indirectly estimate the HR to check its reliability. For dichotomous outcomes such as rates of local and distant recurrence and rates of radical resection, we calculated an odds ratio (OR).
Data synthesis
We combined the HRs or ORs from each of the individual eligible trials in a meta‐analysis to give a pooled HR or OR, using the fixed‐effect model. We also used the random‐effects model to test the robustness of the results to the choice of model.
Subgroup analysis and investigation of heterogeneity
We assessed heterogeneity using the I2 statistic and Chi2. Analyses were carried out in pre‐specified trial subgroups categorised by chemotherapy dose intensity (greater than 25mg/m2/week and 25mg/m2or less per week) and cycle length (greater than14 days and 14 days or less) to explore potential differences in treatment effect using the Chi2 test for interaction and any potential causes of heterogeneity. Further subgroup analyses examined whether there were any differences in the treatment effect between groups of trials when categorised by cervical cancer stage (IB only and IB to IIIB). We planned pre‐specified trial and subgroup analyses only on the primary outcome of OS.
Results
Description of studies
See: Characteristics of included studies.
Results of the search
Initial searches retrieved 4919 references, 12 of which were identified as potentially eligible randomised trials. However, six of these were later found to be duplicate citations of the same studies, leaving six potentially eligible trials. Search updates in 2012 retrieved a further 6098 references, 2 of which were identified as new, potentially eligible randomised trials. One of these trials was subsequently found to be ineligible and the other could not be included due to a lack of available data (see Excluded studies).
Included studies
We included six trials (Sardi 1997; Napolitano 2003; Cai 2006; Katsumata 2006; Eddy 2007; Chen 2008). Included studies randomised between 107 to 291 women with FIGO stages IB to IIIB from 1987 to 2005. One trial (Eddy 2007) included only women with FIGO IB2 (bulky) disease. Two trials (Sardi 1997; Cai 2006) recruited women with both IB1 and IB2 disease, although in both of these trials the proportion of women with IB2 disease was higher (57% (Sardi 1997 ) and 64% (Cai 2006)). Of the remaining three trials, two (Katsumata 2006; Chen 2008) randomised women with stage IB2 to IIB disease, where most were classed as stage IB to IIA (66%), and one (Napolitano 2003) randomised women with stage IB to IIIB disease. Further details are given in Characteristics of included studies.
All trials compared the addition of neoadjuvant chemotherapy to surgery with surgery. Cisplatin‐based chemotherapy was used in all trials, although there was some variation in the treatment regimens. Four trials used regimens based on cisplatin and vincristine with or without bleomycin and/or mitomycin (Sardi 1997; Napolitano 2003; Katsumata 2006; Eddy 2007) and two trials used cisplatin and 5 FU with or without mitomycin (Cai 2006; Chen 2008). The total cisplatin dose ranged from 140mg/m2 to 300mg/m2 given in 2 to 4 cycles at 10 to 21 day intervals and cisplatin dose intensity varied from 17mg/m2 per week to 50mg/m2per week. Further details are given in Characteristics of included studies.
The type of surgery used in six trials was comparable to a Type III Piver radical hysterectomy. Pelvic lymphadenectomy was performed in three trials (Napolitano 2003; Cai 2006; Chen 2008) and both pelvic and para‐aortic lymphadenectomy in a further two trials (Sardi 1997; Eddy 2007). No further information was available for one trial (Katsumata 2006) as to whether lymphadenectomy was carried out in addition to the radical surgery performed.
Two trials gave radical radiotherapy to those patients with inoperable tumours (Sardi 1997; Napolitano 2003). Many women in each of the individual trials also received post‐operative radiotherapy. In four trials (Napolitano 2003; Cai 2006; Eddy 2007; Chen 2008) between 36% and 61% of women who underwent radical hysterectomy also received post‐operative radiotherapy. No information was available for one trial (Katsumata 2006) about how many women received post‐operative radiotherapy. All five of these trials gave post‐operative radiotherapy (with or without brachytherapy) to resected patients because of risk factors for recurrence found at the time of surgery. In one trial (Sardi 1997), 100% of women who underwent radical hysterectomy also received post‐operative radiotherapy, regardless of risk factors. Four trials (Sardi 1997; Napolitano 2003; Cai 2006; Eddy 2007) gave total external beam radiotherapy doses ranging from 45 to 60 Gy in 1.7 to 2.0 Gy fractions, and three of these (Sardi 1997; Napolitano 2003; Eddy 2007) also gave brachytherapy in doses ranging from 25 to 60 Gy. Information available from the trial report for one further trial (Chen 2008) stated that post‐operative pelvic radiotherapy consisted of 3 Gy to the entire pelvis and an additional 2 Gy for parametrial tissue, but it was unclear whether this referred to total dose or dose per fraction given. In one trial (Eddy 2007), radical radiotherapy was given to patients both on and off protocol and furthermore, patients whose disease had progressed beyond the cervix during neoadjuvant chemotherapy were treated with standard chemoradiotherapy. Within individual trials, similar proportions of women on both treatment arms received radiotherapy. Further details of radiotherapy and surgery are given in Characteristics of included studies.
Three of the trials were stopped early. One (Katsumata 2006) was stopped on the recommendation of the data and safety monitoring committee, following an interim analysis which showed that OS in the neoadjuvant arm was inferior to that in the surgery‐only arm, and that the predictive probability of significant superiority of the neoadjuvant arm was extremely low. One (Eddy 2007) closed early due to poor accrual and the extensive use of off‐protocol radiotherapy, and a further trial (Sardi 1997) was ended after successive interim analyses demonstrated statistically‐significant differences between treatment arms.
Data on survival and PFS were available for all six trials. Hazard ratios for OS and PFS were obtained directly from the trial publication for one trial (Eddy 2007) and indirectly using either the P‐values and number of events or the Kaplan‐Meier curves for two further trials (Napolitano 2003; Cai 2006). For the remaining three trials, we obtained HRs using summary data (Katsumata 2006; Chen 2008) or individual patient data (Sardi 1997). Local and distant recurrence rate data were available directly from the publications for three trials (Sardi 1997, Cai 2006, Eddy 2007) and from summary data supplied by the trialist for one further trial (Chen 2008). Sufficient information on both rates of radical resection and pathological response was provided directly in five trial publications (Sardi 1997, Napolitano 2003, Cai 2006, Eddy 2007; Chen 2008) to calculate odds ratios (ORs). Four trials reported only qualitative information on surgical morbidity (Katsumata 2006; Cai 2006, Eddy 2007; Chen 2008).
Excluded studies
One eligible trial identified when searches were updated in 2012 (Mossa 2010) was found to be an update of a trial already included in the original review (Napolitano 2003). However, insufficient data were presented in the publication to use the updated trial results in this review, and the trialist was unable to provide us with updated summary data. We excluded another trial (Wen 2012) from the review because chemotherapy was allowed on both arms (any patients not amenable to surgery received chemoradiotherapy instead).
Risk of bias in included studies
We assessed the methodological quality of the included studies (see Characteristics of included studies) using the risk of bias tool (Table 1).
Allocation
All included trials provided information on baseline characteristics and arms appeared to be well‐balanced within individual trials. All trials were described as being 'randomised' or referred to the 'random assignment' of patients. However, actual methods of sequence randomisation were only stated for three trials (Sardi 1997; Napolitano 2003; Cai 2006) and only one trial referred to a method of allocation concealment (Cai 2006). This may be a source of bias.
Blinding
Blinding was not possible for the trials included in this review due to the differences in the interventions between treatment arms, but this could not affect the primary outcome (OS). However, the lack of blinding may have had an impact on the more subjective outcomes, for example pathological findings.
Incomplete outcome data
Two trials appeared to carry out intention‐to‐treat analyses (Sardi 1997; Katsumata 2006). One trial (Chen 2008) excluded only three patients (2%) from survival analyses along with a further eight patients (5%) from analyses of recurrence. Another trial (Napolitano 2003) excluded 20 stage III patients (10%) from statistical analyses of PFS. Furthermore, this same trial also analysed four patients who had crossed over from the treatment to the control arm according to the treatment given rather than by randomisation arm. A further two trials excluded one patient (1%) (Cai 2006) and 3 patients (1%) (Eddy 2007) respectively. However, despite these exclusions, data for the outcomes of OS and PFS were still available for between 95% to 99% of patients from all of the eligible trials, and therefore the risk of bias associated with these exclusions overall is likely to be low.
Selective reporting
The protocol (Rydzewska 2008) stated that formal methods (as described by Egger et al in the Cochrane Handbook (Higgins 2011)) would be used to investigate the presence of reporting bias if enough eligible trials were included in the meta‐analysis. Searches yielded only six trials, which would be insufficient for meaningful investigation of potential reporting bias. However, we sought to minimise any potential reporting bias by comprehensively searching a variety of sources. All trials appeared to be free of selective outcome reporting bias except for one (Chen 2008) that reported PFS but not OS. However, summary data for this outcome were successfully obtained directly from the trialist. Therefore, the potential risk of bias associated with selective reporting in this trial is low.
Recurrence data were only available for four trials (Sardi 1997; Cai 2006; Eddy 2007; Chen 2008) and data on rates of resection were available for five trials (Sardi 1997; Napolitano 2003; Cai 2006; Eddy 2007; Chen 2008) corresponding to 68% and 88% of patients from eligible trials respectively. Therefore, there is the possibility of an increased risk of bias associated with these two outcomes. Although data on surgical morbidity were available for three trials, insufficient data were available to allow for quantitative analysis.
Other potential sources of bias
Of the three trials that closed prematurely (Sardi 1997; Katsumata 2006; Eddy 2007), only two (Sardi 1997; Katsumata 2006) were terminated for outcome‐related reasons. However, as formal stopping rules were used in both of these trials, it is unlikely that there is a risk of bias associated with their early closure.
Effects of interventions
Overall survival
Data on OS were available for 1071 of the 1078 patients from all 6 trials. We obtained the HR directly for four trials (Sardi 1997; Katsumata 2006; Eddy 2007; Chen 2008) and indirectly for two trials (Napolitano 2003; Cai 2006;). Overall, the estimate was in favour of neoadjuvant chemotherapy (HR 0.77, 95% CI 0.62 to 0.96, P = 0.02 (Analysis 1.1)). Although there was some variation between trials (Chi2 = 6.87, df = 5, P = 0.23, I2 = 27%), the result was similar when the random‐effects model was applied (HR 0.76, 95% CI 0.59 to 0.99, P = 0.04 (Analysis 1.2)).
1.1. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 1 Overall survival (fixed‐effect analysis).
1.2. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 2 Overall survival (random‐effects analysis).
Progression‐free survival
Data on PFS were available for 1027 of the 1078 patients from all 6 trials. We obtained the HR directly for four trials (Sardi 1997; Katsumata 2006, Eddy 2007; Chen 2008) and indirectly for two trials (Napolitano 2003, Cai 2006). Overall, there was a significant benefit of neoadjuvant chemotherapy (HR 0.75, 95% CI 0.61 to 0.93, P = 0.008 (Analysis 1.3)). Again, whilst there was some evidence that the size of the effect varied between trials (Chi2 = 7.90, df = 5, P = 0.16, I2 = 37%), the results were similar when the random‐effects model was applied (HR 0.72, 95% CI 0.55 to 0.95, P = 0.02 (Analysis 1.4)).
1.3. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 3 Progression‐free survival (fixed‐effect analysis).
1.4. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 4 Progression‐free survival (random‐effects analysis).
Local and distant recurrence rates
Data on local and distant recurrence rates were only available for 737 of the 752 patients from 4 trials (Sardi 1997; Cai 2006; Eddy 2007; Chen 2008). There was a significant benefit of neoadjuvant chemotherapy (OR 0.67, 95% CI 0.45 to 0.99, P = 0.04 (Analysis 1.5)). However, there was clear evidence of differences in the effect between trials (Chi2 = 7.22, df = 3, P = 0.07, I2 = 58%), such that when the random‐effects model was used, the result was no longer significant (OR 0.60, 95% CI 0.32 to 1.12, P = 0.11 (Analysis 1.6)). For distant recurrence, the estimate was also in favour of neoadjuvant chemotherapy (OR 0.72, 95% CI 0.45 to 1.14, P = 0.16 (Analysis 1.7)), however, the confidence intervals were wide and the result was not significant. There was also some evidence that the size of the effect varied between trials (Chi2 = 4.79, df =3, P = 0.19, I2 = 37% (Analysis 1.8)).
1.5. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 5 Rates of local recurrence (fixed‐effect analysis).
1.6. Analysis.
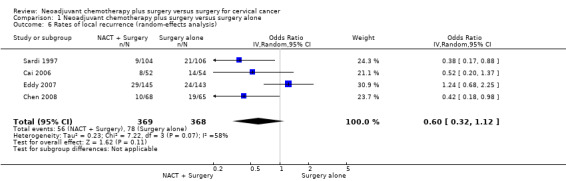
Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 6 Rates of local recurrence (random‐effects analysis).
1.7. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 7 Rates of distant recurrence (fixed‐effect analysis).
1.8. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 8 Rates of distant recurrence (random‐effects analysis).
Radical resection rates
Data on rates of radical resection (i.e. the proportion of women in each arm who underwent radical hysterectomy) were available for 940 out of 944 patients from 5 trials (Sardi 1997; Napolitano 2003; Cai 2006; Eddy 2007; Chen 2008). However, the results differed substantially between trials (Chi2 = 11.21, df = 2, P = 0.004, I2 = 82%). In two of the trials (Sardi 1997; Napolitano 2003), there were marked increases in radical resection rates with neoadjuvant chemotherapy, whereas no difference was seen for the other three trials (Cai 2006, Eddy 2007; Chen 2008). Overall there was no significant benefit of neoadjuvant chemotherapy when either the fixed‐effect (OR 1.55, 95% CI 0.96 to 2.50, P = 0.07 (Analysis 1.9)) or random‐effects (OR 2.87, 95% CI 0.69 to 11.90, P = 0.15 (Analysis 1.10)) models were applied.
1.9. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 9 Rates of radical resection (fixed‐effect analysis).
1.10. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 10 Rates of radical resection (random‐effects analysis).
Surgical morbidity
Although surgical morbidity was pre‐specified as a secondary outcome in the protocol (Rydzewska 2008) there were insufficient data available from included trials to allow a quantitative analysis. Three trials (Cai 2006; Katsumata 2006; Chen 2008) stated that surgical morbidity was similar across both the neoadjuvant and control groups. A further trial (Eddy 2007) suggested that a lower frequency of urological events on the neoadjuvant arm might be a reflection of increased operability, and therefore hints at a potential decrease in surgical morbidity for the neoadjuvant patients. The remaining two trials (Sardi 1997; Napolitano 2003) did not report on the incidence of surgical morbidity.
Pathological response
Most trials reported pathological findings even though the type and extent of findings reported varied across trials. Five trials reported on both lymph node status and parametrial infiltration (Sardi 1997; Napolitano 2003; Cai 2006; Eddy 2007; Chen 2008); two trials reported on those patients with vascular space involvement (Sardi 1997; Cai 2006); and two trials gave information on numbers of patients with positive surgical margins (Napolitano 2003; Eddy 2007). As there was insufficient information available to look at the effect of neoadjuvant chemotherapy on either vascular space involvement or positive surgical margins, we only analysed lymph node status and parametrial infiltration. Data were available for 908 of the 944 patients from 5 trials, and although these were post‐hoc, exploratory analyses, both of these outcomes showed a significant benefit of neoadjuvant chemotherapy (OR 0.54, 95% CI 0.40 to 0.73, P = < 0.0001 for lymph node status (Analysis 1.11); OR 0.58, 95% CI 0.41 to 0.82, P = 0.002 for parametrial infiltration (Analysis 1.13)). However, although there was evidence of statistical heterogeneity (Chi2 = 11.22, df = 4, P = 0.02, I2 = 64% for lymph node status; Chi2 = 8.89, df = 4, P = 0.06, I2 = 55% for parametrial infiltration) results were still significant when the random‐effects model was applied (OR 0.47, 95% CI 0.27 to 0.81, P = 0.006 for lymph node status (Analysis 1.12); OR 0.52, 95% CI 0.30 to 0.91, P = 0.02 for parametrial infiltration (Analysis 1.14)).
1.11. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 11 Rates of pathological findings ‐ lymph node metastases or positive lymph nodes (fixed‐effect analysis).
1.13. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 13 Rates of pathological findings ‐ parametrial infiltration (fixed‐effect analysis).
1.12. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 12 Rates of pathological findings ‐ lymph node metastases or positive lymph nodes (random‐effects analysis).
1.14. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 14 Rates of pathological findings ‐ parametrial infiltration (random‐effects analysis).
Subgroup analyses
Three trials used 'high' (>25mg/m2/week) dose‐intensity cisplatin (35mg/m2 (Sardi 1997; Eddy 2007); 50mg/m2 (Chen 2008)) whereas the other three trials used 'low' (≤25mg/m2/week) dose intensity cisplatin (17mg/m2 (Napolitano 2003); 23mg/m2 (Katsumata 2006); 25mg/m2 (Cai 2006)). There was no evidence of a difference in the effect of neoadjuvant chemotherapy on survival by dose intensity (Chi2 = 0.31, P = 0.58 (Analysis 1.15)). Grouping the trials by chemotherapy cycle length produces the same subsets of trials, and therefore the same results as for the dose intensity analysis (Analysis 1.16). Grouping trials according to whether they included only stage IB patients (Sardi 1997; Cai 2006; Eddy 2007) or whether they also included women with more advanced stages of disease (IB to IIIB patients (Napolitano 2003); and IB2 to IIB patients (Katsumata 2006; Chen 2008)) showed there was no evidence of any difference in effect of neoadjuvant chemotherapy on OS by stage (Chi2 = 0.00, P = 0.99 (Analysis 1.17)).
1.15. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 15 Overall survival by dose intensity.
1.16. Analysis.
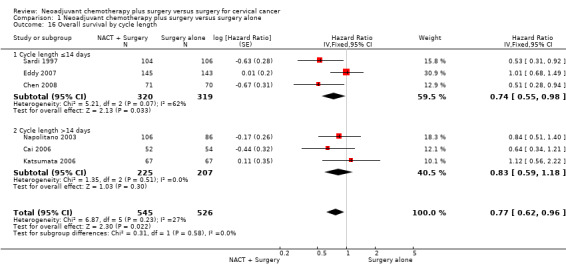
Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 16 Overall survival by cycle length.
1.17. Analysis.

Comparison 1 Neoadjuvant chemotherapy plus surgery versus surgery alone, Outcome 17 Overall survival by stage.
Discussion
This updated review aimed to determine whether neoadjuvant chemotherapy given prior to surgery can improve outcomes in women with cervical cancer. In the original review (Rydzewska 2010), although overall the results tended towards showing a benefit of neoadjuvant chemotherapy, there were inconsistencies both by outcome and by trial, and the only statistically significant result was for progression‐free survival (PFS). As the majority of recurrences and deaths from cervical cancer take place within the first three years after treatment, we might have expected the results for overall survival (OS) and PFS to be broadly similar, which was not the case. Potentially important to note was that one trial (Napolitano 2003) excluded poorer prognosis patients from the analysis of PFS but not from that of OS, which may have contributed to the more favourable result for PFS. Furthermore, at the time of the original review, we did not have data on OS for another of the eligible trials (Chen 2008), which may also have contributed to the discrepancy between the findings for these outcomes at the time.
For this update, although no new, eligible trials were identified, we were able to obtain additional summary data from one of the trialists (Chen 2008) for the outcomes of survival, local recurrence and distant recurrence. With the inclusion of these supplementary data, we now find greater consistency between the outcomes of survival and PFS, with significant improvements for both with neoadjuvant chemotherapy. Amongst the remainder of the pre‐specified analyses, the results still all tend towards a benefit of neoadjuvant chemotherapy. Inclusion of the additional data has led to the result for local recurrence becoming significant. However, it should be noted that this result is still only based on 68% of all known randomised patients.
Whilst the results for OS and PFS are now broadly similar, as we might expect, and the included studies are fairly similar in terms of design and chemotherapy regimen employed, there is still some variation between the results of the individual studies. Pre‐specified trial group analyses by chemotherapy cycle length (or dose intensity) were unable to explain these differences. There was also no evidence that the different stages of cervical cancer included influenced the effect of neoadjuvant chemotherapy, although it is not possible to investigate this more thoroughly without the collection and re‐analysis of individual patient data from all randomised trials.
Post‐operative radiotherapy was used in all the trials and was fairly similar and balanced between treatment arms. However, large differences in the proportion of patients within each of the individual trials that received this post‐operative treatment may be contributing to the variation in the individual trial results. A further consideration may be that for patients receiving chemotherapy, surgery and radiotherapy as primary treatment, there is not only the potential for increased side effects, but also reduced opportunity for effective salvage therapy should they develop isolated pelvic recurrences.
In contrast to radiotherapy which is classed as a local treatment, chemotherapy is a systemic treatment, so therefore we might have anticipated an overall reduction in distant recurrences. However, that was not the case for this review where a significant benefit was observed in terms of reduced local recurrence rates, but not for distant recurrence. It is not possible to attribute this reduction in local recurrences solely to the administration of neoadjuvant chemotherapy, particularly since a proportion of patients in all trials also received post‐operative radiotherapy, albeit that the proportions were similar on each arm. Interestingly, the most marked effects of treatment were observed in the Sardi trial (Sardi 1997), in which all patients received post‐operative radiotherapy in addition to neoadjuvant chemotherapy and surgery.
Due to the potentially confounding effect of post‐operative radiotherapy, the only pre‐specified outcome that might be influenced solely by neoadjuvant chemotherapy is the rate of resection. However, for this outcome no difference in resectability was observed between the trial arms overall, and the results also varied substantially between individual trials.
Two phase II trials (Buda 2005; Lissoni 2009) have assessed the prognostic value of pathological response on survival in patients with cervical cancer. They reported that optimal pathological tumour response is a significant prognostic factor and could be used as a surrogate outcome for survival. Therefore, we also undertook post‐hoc, exploratory analyses looking at the rates of pathological high‐risk factors, based on lymph node status and parametrial infiltration. Based on the available data, our results suggest a significant benefit of neoadjuvant chemotherapy for decreasing adverse pathological findings. In some trials this seems to lead to better local and distant control and a benefit in OS and PFS, but this pattern does not hold across all trials. If survival benefit is linked to the level of pathological response then it should follow that more effective neoadjuvant chemotherapy schedules would improve outcomes for women with cervical cancer. In a published phase II trial in stage IB2 to IVA patients (Lissoni 2009), the authors concluded that three neoadjuvant cycles of paclitaxel, ifosfamide and cisplatin (TIP) at three‐weekly intervals followed by surgery, was a valid alternative to chemoradiation, but that whilst pathological response was favourable when compared to paclitaxel and cisplatin (TP), the grade 3 or 4 haematological toxicity was considerable (TIP 78% vs TP 29%). It is also noteworthy that the women in this trial are younger (median age 45 years for TIP and 42 years for TP) and with better performance status than the general population of women with cervical cancer, and so this regimen may not be tolerated by older, less fit women.
Although chemoradiation is the current standard of treatment, potential delays to definitive treatment or lack of access to radiotherapy, especially in the developing world, mean that there is continued interest in the use of neoadjuvant chemotherapy. A number of other phase II trials are also looking at alternative neoadjuvant chemotherapy regimens in locally‐advanced cervical cancer. Carboplatin is considered to have similar effectiveness to cisplatin but with easier administration and less associated toxicity, and is being evaluated in combination with paclitaxel as a dose‐dense, weekly neoadjuvant regimen prior to chemoradiation. Results from this trial, presented atthe American Society of Clinical Oncology (McCormack 2009), show a high response rate with limited grade 3 or 4 toxicity (13%). Also, because raised levels of epidermal growth factor receptor (EGFR) (Kersemaekers 1999; Kim 2004) and vascular endothelial growth factor (VEGF) (Loncaster 2000) are considered to be independent prognostic factors, there is also increasing interest in the use of newer biological agents that act as EGFR and VEGF inhibitors. Thus, two further phase II trials were initiated, looking at giving either cetuximab as single‐agent neoadjuvant chemotherapy prior to chemoradiation (NCT00292955) or carboplatin in combination with bevacizumab (NCT00600210). The former trial is still ongoing but the latter has been terminated early for poor accrual with the accrual status currently unknown. It remains to be seen whether the results will indicate a feasible alternative to current neoadjuvant chemotherapy regimens in the surgical setting. In addition to these phase II studies, in 2002, a large multi‐centre phase III trial was initiated by the European Organization for the Research and Treatment of Cancer (EORTC), comparing cisplatin‐based neoadjuvant chemotherapy, prior to surgery, with the current standard of cisplatin‐based chemoradiotherapy (EORTC 55994). Another two trials comparing standard platinum‐based chemoradiation with neoadjuvant chemotherapy regimens comprised of either paclitaxel plus carboplatin (NCT00193739) or gemcitabine plus cisplatin (NCT01000415), followed by surgery are also ongoing in India and Thailand respectively. The results of these three trials are keenly anticipated, and will be important in determining whether neoadjuvant chemotherapy prior to surgery is a valid alternative to chemoradiation.
Authors' conclusions
Implications for practice.
Both overall survival and progression‐free survival were significantly improved with neoadjuvant chemotherapy. However, there was some variation in the results of individual studies and the meta‐analysis is based on only a small number of trials and randomised patients. Therefore, adding neoadjuvant chemotherapy to surgery cannot be recommended outside the context of clinical trials.
Implications for research.
If results from current, ongoing trials show that neoadjuvant chemotherapy prior to surgery is a valid alternative to chemoradiation, and if new neoadjuvant chemotherapy regimens and targeted biological agents perform well prior to chemoradiation, further trials of these agents in the surgical setting may be warranted.
What's new
| Date | Event | Description |
|---|---|---|
| 11 February 2015 | Amended | Contact details updated. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 27 March 2014 | Amended | Contact details updated. |
| 24 August 2012 | New citation required but conclusions have not changed | Supplementary data provided from one trial (Chen 2008) for outcomes of survival, local recurrence and distant recurrence. |
| 24 August 2012 | New search has been performed | Searches updated. |
Acknowledgements
We are grateful to the UK Medical Research Council for funding this work.
The authors also thank Huijen Chen (Zhongnan Hospital of Wuhan University, Wuhan, China), Gary Eddy (Mercer School of Medicine, Macon, Georgia, USA), Shamshad Ali (Roswell Park Cancer Institute, Buffalo, New York, USA) and Noriyuki Katsumata (National Cancer Center Hospital, Tokyo, Japan) for providing us with supplementary data for their trials.
Appendices
Appendix 1. Search strategy for MEDLINE (OVID)
"randomized controlled trial".pt.
controlled clinical trial.pt.
"randomized".ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
humans.sh.
9 and 10
(cervi* adj3 canc*).ab,ti.
(cervi* adj3 carcinoma*).ab,ti.
(cervi* adj3 tumor*).ab,ti.
(cervi* adj3 tumour*).ab,ti.
(cervi* adj3 neoplas*).ab,ti.
exp uterine cervical neoplasms/
12 or 13 or or 14 or 15 or 16 or 17
drug therapy.fs.
chemotherapy.ab,ti.
19 or 20
hysterectomy.ab,ti.
surgery.ab,ti.
22 or 23
11 and 18 and 21 and 24
Appendix 2. Search Strategy for CENTRAL
#1 MeSH descriptor Uterine Cervical Neoplasms explode all trees #2 (cervi* near canc*):ti,ab,kw #3 (cervi* near carcinoma*):ti,ab,kw #4 (cervi* near neoplasm*):ti,ab,kw #5 (cervi* near tumour*):ti,ab,kw #6 (cervi* near tumor*):ti,ab,kw #7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) #8 MeSH descriptor Drug Therapy explode all trees #9 (chemotherapy):ti,ab,kw #10 (#8 OR #9) #11 (hysterectomy):ti,ab,kw #12 (surgery):ti,ab,kw #13 (#11 OR #12) #14 (#7 AND #10 AND #13)
Appendix 3. Search strategy for LILACS
(Tw estud$ OR Tw clinic$ OR AB grupo$ OR CT COMPARATIVE STUDY OR Tw placebo$ OR Tw random$ OR Ti compara$ OR Ti tratamiento OR Tw control$ OR MH/dt) AND NOT ((CT ANIMALS FEMALE OR CT ANIMALS MALE OR CT CATS OR CT CATTLE OR CT CHICK EMBRYO OR CT DOGS OR CT GUINEA PIGS OR CT IN VITRO OR CT MICE OR CT RABBITS OR CT RATS) OR (MH Prevalence OR MH Practice Guidelines OR MH Diagnosis, Differential OR MH Cross‐Sectional Studies OR MH predictive value of tests) OR (Ti clinical AND case OR Ti updat$ OR Ti Epidemiol$ OR Ti clinical$ AND case$ OR Ti caso AND clinico OR Ti review OR Ti diagno$ AND treatment OR Ti descrip$ OR Ti consenso OR Ti caso$ AND control$ OR Ti analisis AND critico) OR (AB retrospectiv$ and stud$ OR AB estudio AND retrospectivo OR AB revis$ AND ficha$ OR AB revision AND bibliograf$ OR AB estud$ AND descript$ OR AB presenta AND caso OR AB describe AND caso OR AB serie AND clinica OR AB puesta AND al AND dia OR AB tratamiento AND diagnostic$ AND revis$ OR AB experien$ AND caso$ OR AB analisis AND critico) OR (PT case reports OR PT review) AND NOT (Tw estud$ OR AB grupo$ OR Tw control$ OR Tw random$)) and (Mh uterine cervical neoplasms/) or (tw Cerv$ AND (Tw carcinoma$ or Tw cancer$)) and (Mh Drug therapy/) or (Tw chemotherapy) or (Tw surg$)
Updated 2012:
"UTERINE CERVICAL NEOPLASMS" and ("CHEMOTHERAPY" or "CHEMOTHERAPY, adjuvant") and "SURGERY" [Subject descriptor] or (cervi$ and (cancer$ or tumor$ or tumour$ or malignan$ or carcinoma$ or neoplas$) and chemotherap$ and surg$) [Words]
Data and analyses
Comparison 1. Neoadjuvant chemotherapy plus surgery versus surgery alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival (fixed‐effect analysis) | 6 | 1071 | Hazard Ratio (Fixed, 95% CI) | 0.77 [0.62, 0.96] |
| 2 Overall survival (random‐effects analysis) | 6 | 1071 | Hazard Ratio (Random, 95% CI) | 0.76 [0.59, 0.99] |
| 3 Progression‐free survival (fixed‐effect analysis) | 6 | 1027 | Hazard Ratio (Fixed, 95% CI) | 0.75 [0.61, 0.93] |
| 4 Progression‐free survival (random‐effects analysis) | 6 | 1027 | Hazard Ratio (Random, 95% CI) | 0.72 [0.55, 0.95] |
| 5 Rates of local recurrence (fixed‐effect analysis) | 4 | 737 | Odds Ratio (IV, Fixed, 95% CI) | 0.67 [0.45, 0.99] |
| 6 Rates of local recurrence (random‐effects analysis) | 4 | 737 | Odds Ratio (IV, Random, 95% CI) | 0.60 [0.32, 1.12] |
| 7 Rates of distant recurrence (fixed‐effect analysis) | 4 | 737 | Odds Ratio (IV, Fixed, 95% CI) | 0.72 [0.45, 1.14] |
| 8 Rates of distant recurrence (random‐effects analysis) | 4 | 737 | Odds Ratio (IV, Random, 95% CI) | 0.67 [0.35, 1.27] |
| 9 Rates of radical resection (fixed‐effect analysis) | 5 | 940 | Odds Ratio (IV, Fixed, 95% CI) | 1.55 [0.96, 2.50] |
| 10 Rates of radical resection (random‐effects analysis) | 5 | 940 | Odds Ratio (IV, Random, 95% CI) | 2.87 [0.69, 11.90] |
| 11 Rates of pathological findings ‐ lymph node metastases or positive lymph nodes (fixed‐effect analysis) | 5 | 908 | Odds Ratio (IV, Fixed, 95% CI) | 0.54 [0.40, 0.73] |
| 12 Rates of pathological findings ‐ lymph node metastases or positive lymph nodes (random‐effects analysis) | 5 | 908 | Odds Ratio (IV, Random, 95% CI) | 0.47 [0.27, 0.81] |
| 13 Rates of pathological findings ‐ parametrial infiltration (fixed‐effect analysis) | 5 | 908 | Odds Ratio (IV, Fixed, 95% CI) | 0.58 [0.41, 0.82] |
| 14 Rates of pathological findings ‐ parametrial infiltration (random‐effects analysis) | 5 | 908 | Odds Ratio (IV, Random, 95% CI) | 0.52 [0.30, 0.91] |
| 15 Overall survival by dose intensity | 6 | 1071 | Hazard Ratio (Fixed, 95% CI) | 0.77 [0.62, 0.96] |
| 15.1 Cisplatin dose intensity >25mg/m2/week | 3 | 639 | Hazard Ratio (Fixed, 95% CI) | 0.74 [0.55, 0.98] |
| 15.2 Cisplatin dose intensity ≤25mg/m2/week | 3 | 432 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.59, 1.18] |
| 16 Overall survival by cycle length | 6 | 1071 | Hazard Ratio (Fixed, 95% CI) | 0.77 [0.62, 0.96] |
| 16.1 Cycle length ≤14 days | 3 | 639 | Hazard Ratio (Fixed, 95% CI) | 0.74 [0.55, 0.98] |
| 16.2 Cycle length >14 days | 3 | 432 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.59, 1.18] |
| 17 Overall survival by stage | 6 | 1071 | Hazard Ratio (Fixed, 95% CI) | 0.77 [0.62, 0.96] |
| 17.1 Stage IB only | 3 | 604 | Hazard Ratio (Fixed, 95% CI) | 0.78 [0.58, 1.03] |
| 17.2 Stage IB‐IIIB | 3 | 467 | Hazard Ratio (Fixed, 95% CI) | 0.77 [0.55, 1.09] |
Characteristics of studies
Characteristics of included studies [author‐defined order]
Sardi 1997.
| Methods | 1987‐1992 RCT | |
| Participants | 210 patients randomised (Neoadjuvant chemotherapy: 104; Surgery alone: 106) 210 patients analysed 5 patients excluded from trial report reinstated in this analysis using previously collected individual patient data, with permission from the trialist Stage IB1‐IB2; squamous |
|
| Interventions |
Comparison: Neoadjuvant chemotherapy + surgery (or radiotherapy) vs Surgery (or radiotherapy) Chemotherapy : Cisplatin 50 mg/m2 IV in 15mins Vincristine 1mg/m2 in push Bleomycin 25mg/m2 continuous infusion over 6hrs (day 1‐3)
Surgery:
Radiotherapy : All unresectable patients
Post‐operative radiotherapy: All resectable patients
|
|
| Outcomes | Survival: measured from day of diagnosis to death Disease‐free survival: measured from day of diagnosis to time of progression Also; toxicity, clinical response and pathological findings |
|
| Notes | Authors state that neoadjuvant chemotherapy can improve survival because of increased operability with free surgical margins and a decrease in pathological risk factors in unselected, bulky (>4cm diameter) stage 1B patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Patients were randomised according to aleatoric tables of admission numbers |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Chemotherapy administered intravenously, therefore blinding not possible. The lack of blinding could not affect the primary outcome of survival and is also unlikely to affect progression‐free survival and although it may affect the more subjective outcomes, for example pathological findings, the overall risk of bias is likely to be low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Three patients from the control group and two patients from the neoadjuvant group who did not complete the treatment regimen and were excluded from the study were reinstated using the individual patient data supplied |
| Selective reporting (reporting bias) | Low risk | Comment: Individual patient data available for all review outcomes |
| Other bias | Low risk | Quote: In three consecutive years, interim analysis demonstrated statistically significant differences between both treatments; therefore, in 1992, we decided to end the trial Comment: Trial stopped early for benefit but formal stopping rule used, therefore unlikely to be biased |
Napolitano 2003.
| Methods | 1986‐1995 RCT | |
| Participants | 192 patients randomised (Neoadjuvant chemotherapy: 106; Surgery alone: 86) 192 patients analysed for overall survival 156 patients analysed for progression‐free survival as trial report excluded 20 patients (who did not receive surgery) from analyses (Neoadjuvant chemotherapy: 86; Surgery alone: 70) Stage IB‐IIIB; squamous |
|
| Interventions |
Comparison: Neoadjuvant chemotherapy + surgery (or radiotherapy) vs Surgery (or radiotherapy) Chemotherapy : Cisplatin 50mg/m2 (day 1) Vincristine 1mg/m2 (day 1), Bleomycin 25mg/m2 (days 1 & 3)
Surgery:
Radiotherapy : All unresectable patients
Post‐operative radiotherapy: All resectable patients with parametrial infiltration, lymph node positivity or positive surgical margins
|
|
| Outcomes | Survival: measured from time from initial diagnosis to last follow‐up Disease‐free survival: local and distant recurrence defined as recurrence of disease after a disease‐free period between surgical operation and last follow up Also; morbidity (after surgery or radiotherapy), toxicity |
|
| Notes | Authors note that responsiveness of cervical cancer to neoadjuvant chemotherapy allows surgical treatment in a larger number of patients and results in longer overall survival and disease‐free survival | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Randomisation was done by means of an algorithm that divided the patients into two homogenous arms according to the parameters considered: age, tumour size, FIGO stage and radiological state of the lymph nodes. In order to assign more patients to the presumably favourable arm we decided to allocate 55% of patients to the neoadjuvant chemotherapy arm and 45% to the CO arm |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Chemotherapy administered intravenously, therefore blinding not possible. The lack of blinding could not affect the primary outcome of survival and is also unlikely to affect progression‐free survival, and although it may affect the more subjective outcomes, for example pathological findings, the overall risk of bias is likely to be low |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: (re progression‐free survival) No surgical operation was performed on four patients classified as stage III not responsive to the chemotherapy of the NACT arm and 16 patients of the control arm, at the same time and stage, and they were therefore these 20 patients were excluded from statistical evaluation Comment: Not an intention‐to treat analysis of progression‐free survival. For this trial, both overall and progression‐free survival were analysed by stage. For progression‐free survival only stage III patients who had undergone surgery were analysed. However, all of these patients were from only one arm of the trial (NACT). Therefore, together with the 20 patients that had been excluded from statistical analyses for not responding to chemotherapy, 36 patients in total (n = 20 NACT arm and n = 16 control arm) were not available for inclusion in the analysis of progression‐free survival in this review |
| Selective reporting (reporting bias) | Low risk | Quote: All main outcomes reported |
| Other bias | Low risk | Comment: Study appears to be free of other sources of bias |
Cai 2006.
| Methods | 1999‐2001 RCT | |
| Participants | 107 patients randomised (Neoadjuvant chemotherapy: 53; Surgery alone: 54) 106 patients analysed (Neoadjuvant chemotherapy: 52; Surgery alone: 54) 1 protocol violation (patient refused any treatment) excluded from ITT analysis Stage IB1‐IB2; squamous and adenocarcinoma |
|
| Interventions |
Comparison: Neoadjuvant chemotherapy + surgery (with or without radiotherapy) vs Surgery (with or without radiotherapy) Chemotherapy: Cisplatin 75mg/m2 (day 1) 5‐FU 24mg/kg/d (day 1 to 5)
Surgery:
Post‐operative radiotherapy: All patients with deep cervical invasion, parametrial extension or positive lymph nodes
|
|
| Outcomes |
Primary Survival: 5‐year overall survival (not defined more fully) Secondary Progression‐free survival: recurrence of disease (not defined more fully) Also; clinical response and pathological findings |
|
| Notes | Authors concluded that neoadjuvant chemotherapy can effectively eliminate the pathological risk factors and improve long‐term survival in patients with locally‐advanced cervical cancer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Assigned by block randomisation from a computer generated table created before the start of the study |
| Allocation concealment (selection bias) | Low risk | Quote: Treatments in the table were coded so that no one could discover treatment allocation before randomisation Quote: Codes were revealed after we had obtained the patient's informed consent |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Chemotherapy administered intravenously, therefore blinding was not possible. The lack of blinding could not affect the primary outcome of survival and is also unlikely to affect progression‐free survival and although it may affect the more subjective outcomes, for example pathological findings, the overall risk of bias is likely to be low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: There was 1 protocol violation in the NAC (refusal of any therapy), so we excluded this case from the analysis Comment: Although report states that intention‐to‐treat analysis carried out, the exclusion of one patient means this was not the case. However, with only one patient excluded, the risk of bias is minimal |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes described in the methods were reported |
| Other bias | Low risk | Comment: Study appears to be free from other sources of biases |
Katsumata 2006.
| Methods | 2001‐2005 RCT | |
| Participants | 134 patients randomised (Neoadjuvant chemotherapy: 67; Surgery alone: 67) 134 patients analysed Only 108 patients analysed in trial report but summary data on all randomised patients provided directly by trialist Stage IB2‐IIB (bulky); squamous |
|
| Interventions |
Comparison: Neoadjuvant chemotherapy + surgery (with or without radiotherapy) vs Surgery (with or without radiotherapy) Chemotherapy: Bleomycin 7mg day 1‐5; Vincristine 0.7mg/m2 day 5; Mitomycin 7mg/m2 day 5; Cisplatin 14mg/m2 day 1‐5
Surgery:
(no further information given in abstract) Post‐operative radiotherapy: All patients with positive surgical margins, metastatic nodes, infiltration to parametrium and/or deep myometrial invasion (no further information given in abstract) |
|
| Outcomes |
Primary Survival: not defined more fully (no further information given in abstract) Secondary Progression‐free survival: not defined more fully (no further information given in abstract) Also; clinical response, pathological findings |
|
| Notes | Authors note that neoadjuvant chemotherapy did not demonstrate clinical benefit and conventional radical hysterectomy still remains as a standard treatment option for bulky stage I/II cervical cancer Data monitoring committee recommended termination of the study due to inferior overall survival in neoadjuvant arm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: Randomly assigned Comment: Only abstract available: no detailed information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: Only abstract available: no detailed information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Chemotherapy administered intravenously, therefore blinding not possible. The lack of blinding could not affect the primary outcome of survival and is also unlikely to affect progression‐free survival and although it may affect the more subjective outcomes, for example pathological findings, the overall risk of bias is likely to be low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Only abstract available: no detailed information provided Abstract provided only information on interim analysis of 108 patients; However, summary data provided by trialists based on all randomised patients (n = 134) |
| Selective reporting (reporting bias) | Low risk | Comment: Summary data provided by trialists for all outcomes requested by review authors |
| Other bias | Low risk | Quote: The first planned interim analysis was performed in July 2005 using data from 108 patients registered as of 11/04. Data and Safety Monitoring Committee recommended to terminate the study because overall survival in NAC arm was inferior to that in RH arm (HR 2.11, multiplicity adjusted 99% CI 0.34 to 13.2) and the predictive probability of significant superiority using Spiegelhalter's method of NAC arm was extremely low (6.4%). Comment: Trial stopped early for detriment but formal stopping rule used therefore unlikely to be biased |
Eddy 2007.
| Methods | 1996‐2001 RCT | |
| Participants | 291 patients randomised (Neoadjuvant chemotherapy: 147; Surgery alone: 144) 288 patients analysed (Neoadjuvant chemotherapy: 145; Surgery alone: 143) 3 patients reported as ineligible (Neoadjuvant chemotherapy: 2; Surgery alone: 1) 2 patients (one in each group) with the wrong primary tumour and 1 patient in the neoadjuvant chemotherapy group with the wrong cell type Stage IB (bulky); squamous, adenosquamous, adenocarcinoma |
|
| Interventions |
Comparison: Neoadjuvant chemotherapy + surgery (with or without radiotherapy or chemoradiotherapy) vs Surgery (with or without radiotherapy) Chemotherapy: Cisplatin 50mg/m2 Vincristine 1mg/m2
Surgery:
Post‐operative radiotherapy: All surgery alone patients with positive pelvic nodes, parametrial margins and para‐aortic nodes
Post‐operative chemoradiotherapy: All neoadjuvant chemotherapy patients with disease progression beyond the cervix
|
|
| Outcomes | Overall survival: length of life from entry onto the study to death or to the date of last contact Progression‐free survival: date from protocol registration to date of reappearance of disease, clinical progression of existing disease or death whichever comes first. Clinical progression defined as a 50% or greater increase in the cross product of the tumour compared with the baseline product
Also; clinical response, pathological findings, surgical outcome, toxicity |
|
| Notes | Authors state that although not definitive, results of this study have led GOG to recommend against adding neoadjuvant chemotherapy to RHPPL in future randomised trials of stage 1B2 cervical cancer patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: Prospective random allocation Comment: No method of randomisation stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Chemotherapy administered intravenously, therefore blinding not possible. The lack of blinding could not affect the primary outcome of survival and is also unlikely to affect progression‐free survival and although it may affect the more subjective outcomes, for example pathological findings, the overall risk of bias is likely to be low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3 patients excluded from analysis post randomisation. 2 patients (one in each group) with the wrong primary tumour, 1 patient (neoadjuvant chemotherapy) with the wrong cell type. Not strictly an intention‐to‐treat analysis but it appears that patients were excluded prior to outcome data being seen so unlikely to introduce bias |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes stated in the methods reported in results |
| Other bias | Low risk | Quote: Trial was stopped early due to poor accrual and the chronic use of off‐protocol radiotherapy Comment: The early stopping of the trial was unrelated to outcome therefore unlikely to introduce bias |
Chen 2008.
| Methods | Jan 1999 ‐ Apr 2004 RCT | |
| Participants | 144 patients randomised (Neoadjuvant chemotherapy: 72; Surgery alone: 72) 141 patients analysed (Neoadjuvant chemotherapy: 71; Surgery alone: 70)
For progression‐free survival: 133 patients analysed (Neoadjuvant chemotherapy: 68; Surgery alone: 65)
Stage IB2‐IIB; Squamous, adenocarcinoma or adenosquamous |
|
| Interventions |
Comparison: Neoadjuvant chemotherapy + surgery (with or without radiotherapy) vs Surgery (with or without radiotherapy) Chemotherapy: Cisplatin 50mg/m2 (day 1) Mitomycin 4mg/m2 (day 1‐5) 5‐FU 24mg/kg/day (day 1‐5)
Surgery:
Post‐operative radiotherapy: All patients with lymph node metastasis, parametrial infiltration, vascular space involvement, vaginal invasion and ovarian metastasis
|
|
| Outcomes | Survival: defined as the period from initial treatment until cervical cancer‐related death Progression‐free survival: defined as the period from initial treatment until recurrence or date of last follow‐up Also; clinical response, surgical morbidity, pathological findings |
|
| Notes | Authors state that the modified preoperative NAC is well tolerated and beneficial in reducing tumour size, eliminating pathological risk factors, and improving prognosis for responders. It also avoids the delay of effective treatment for patients that did not respond to neoadjuvant chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: Patients were randomly assigned Comment: No method of randomisation stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Chemotherapy administered intravenously, therefore blinding not possible. The lack of blinding could not affect the primary outcome of survival and is also unlikely to affect progression‐free survival and although it may affect the more subjective outcomes, for example pathological findings, the overall risk of bias is likely to be low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: Two patients were excluded because of their refusal of further treatment after surgery. Quote: One patient in the NAC group died from another disease. We thus was excluded them from the recurrence analysis. Comment: This was not an intention to treat analysis. Two patients (control arm) were excluded because of their refusal of further treatment after surgery and one further patient (NACT arm) was excluded from statistical analyses because she died from another disease (based on supplementary information provided by the trialist). Furthermore, another 8 patients were excluded from the analysis of progression‐free survival as they had uncontrollable disease. However, with only three patients excluded from the survival analysis, the risk of bias is minimal and only 8 out of 144 randomised patients were excluded from the analysis of progression‐free survival, the arms were still fairly well balanced (68:65) |
| Selective reporting (reporting bias) | Low risk | Comment: Although overall survival and progression‐free survival were reported in the methods, only progression‐free survival was reported in the results. However, results for survival were obtained as summary data directly from the trialist, so this should minimise the risk of bias |
| Other bias | Low risk | Comment: Study appears to be free from other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Wen 2012 | Patients not amenable to surgery (in both arms) received chemoradiation. Therefore this trial did not satisfy the pre‐specified eligibility criteria for inclusion in this systematic review. |
Differences between protocol and review
Additional post‐hoc exploratory analyses of pathological response.
Contributions of authors
Original review:
Larysa Rydzewska designed the protocol, identified RCTs for inclusion, extracted data from the trial publications, performed the analyses and wrote the final review. Jayne Tierney helped to design the protocol, cross‐checked the extracted data, advised on interpretation of results and commented on the review. Claire Vale cross‐checked the extracted data, advised on the interpretation of results and commented on the review. Paul Symonds advised on the interpretation of results and commented on the review.
Review update:
Larysa Rydzewska appraised the results of updated searches to identify any new, potentially eligible trials, collaborated with trialists to obtain additional, unpublished, summary data for inclusion in the updated review, performed the final analyses and updated the text of the review. Claire Vale and Paul Symonds both advised on the interpretation of the updated results and commented on the updated review.
Sources of support
Internal sources
Medical Research Council, UK.
University of Leicester, UK.
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Cai 2006 {published data only}
- Cai HB, Chen HZ, Yin HH. Randomized study of preoperative chemotherapy versus primary surgery for stage IB cervical cancer. The Journal of Obstetrics and Gynaecology Research 2006;32(3):315‐23. [PUBMED: 16764623] [DOI] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen H, Liang C, Zhang L, Huang S, Wu X. Clinical efficacy of modified preoperative neoadjuvant chemotherapy in the treatment of locally advanced (stage IB2 to IIB) cervical cancer: randomized study. Gynecologic Oncology 2008;110(3):308‐15. [PUBMED: 18606439] [DOI] [PubMed] [Google Scholar]
Eddy 2007 {published data only}
- Eddy GL, Bundy BN, Creasman WT, Spirtos NM, Mannel RS, Hannigan E, et al. Treatment of ("bulky") stage IB cervical cancer with or without neoadjuvant vincristine and cisplatin prior to radical hysterectomy and pelvic/para‐aortic lymphadenectomy: a phase III trial of the gynecologic oncology group. Gynecologic Oncology 2007;106(2):362‐9. [PUBMED: 17493669] [DOI] [PubMed] [Google Scholar]
Katsumata 2006 {published data only}
- Katsumata N, Yoshikawa H, Hirakawa T, Saito T, Kuzuya K, Fujii T, et al. Phase III randomized trial of neoadjuvant chemotherapy (NAC) followed by radical hysterectomy (RH) versus RH for bulky stage I/II cervical cancer (JCOG 0102). Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings (Post‐Meeting Edition) 2006;24(18S):5013. [Google Scholar]
Napolitano 2003 {published data only}
- Napolitano U, Imperato F, Mossa B, Framarino ML, Marziani R, Marzetti L. The role of neoadjuvant chemotherapy for squamous cell cervical cancer (Ib‐IIIb): a long‐term randomized trial. European Journal of Gynaecological Oncology 2003;24(1):51‐9. [PUBMED: 12691318] [PubMed] [Google Scholar]
Sardi 1997 {published data only}
- Sardi JE, Giaroli A, Sananes C, Ferreira M, Soderini A, Bermudez A, et al. Long‐term follow‐up of the first randomized trial using neoadjuvant chemotherapy in stage Ib squamous carcinoma of the cervix: the final results. Gynecologic Oncology 1997;67(1):61‐9. [PUBMED: 9345358] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Wen 2012 {published data only}
- Wen H, Wu X, Li Z, Wang H, Zang R, Sun M, et al. A prospective randomized controlled study on multiple neoadjuvant treatments for patients with stage IB2 to IIA cervical cancer. International Journal of Gynecologic Cancer 2012;22(2):296‐302. [PUBMED: 22274319 ] [DOI] [PubMed] [Google Scholar]
Additional references
Arbyn 2007
- Arbyn M, Raifu AO, Autier P, Ferlay J. Burden of cervical cancer in Europe: estimates for 2004. Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO 2007;18(10):1708‐15. [PUBMED: 17369600] [DOI] [PubMed] [Google Scholar]
Basile 2006
- Basile S, Angioli R, Manci N, Palaia I, Plotti F, Benedetti‐Panici P. Gynecological cancers in developing countries: the challenge of chemotherapy in low‐resources setting. International Journal of Gynecological Cancer 2006;16:1491‐7. [PUBMED: 16884356 ] [DOI] [PubMed] [Google Scholar]
Benedet 1998
- Benedet JL, Odicino F, Maisonneuve P, Severi G, Creasman WT, Shepherd J, et al. Carcinoma of the cervix uteri. Journal of Epidemiology and Biostatistics 1998;6(1):5‐34. [PUBMED: 11385777] [PubMed] [Google Scholar]
Buda 2005
- Buda A, Fossati R, Colombo N, Fei F, Floriani I, Gueli Alletti D, et al. Randomized trial of neoadjuvant chemotherapy comparing paclitaxel, ifosfamide, and cisplatin with ifosfamide and cisplatin followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the SNAP01 (Studio Neo‐Adjuvante Portio) Italian Collaborative Study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 2005;23(18):4137‐45. [PUBMED: 15961761] [DOI] [PubMed] [Google Scholar]
Devesa 1995
- Devesa SS, Blot WJ, Stone BJ, Miller BA, Tarone RE, Fraumeni JF Jr. Recent cancer trends in the United States. Journal of the National Cancer Institute 1995;87(3):175‐82. [PUBMED: 7707404] [DOI] [PubMed] [Google Scholar]
Friedlander 1983
- Friedlander M, Kaye SB, Sullivan A, Atkinson K, Elliott P, Coppleson M, et al. Cervical carcinoma: a drug‐responsive tumor ‐ experience with combined cisplatin, vinblastine, and bleomycin therapy. Gynecologic Oncology 1983;16(2):275‐81. [PUBMED: 6195052] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions; version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Howlader 2011
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al (eds). SEER Cancer Statistics Review, 1975‐2008. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011.
Jemal 2011
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics, 2011. CA: A Cancer Journal for Clinicians 2011;61:69‐90. [DOI: 10.3322/caac.20107; PUBMED: 21296855] [DOI] [PubMed] [Google Scholar]
Kersemaekers 1999
- Kersemaekers AM, Fleuren GJ, Kenter GG, Broek LJ, Uljee SM, Hermans J, et al. Oncogene alterations in carcinomas of the uterine cervix: overexpression of the epidermal growth factor receptor is associated with poor prognosis. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research 1999;5(3):577‐86. [PUBMED: 10100709] [PubMed] [Google Scholar]
Kim 2004
- Kim GE, Kim YB, Cho NH, Chung HC, Pyo HR, Lee JD, et al. Synchronous coexpression of epidermal growth factor receptor and cyclooxygenase‐2 in carcinomas of the uterine cervix: a potential predictor of poor survival. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research 2004;10(4):1366‐74. [PUBMED: 14977838] [DOI] [PubMed] [Google Scholar]
Landoni 1997
- Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib‐IIa cervical cancer. Lancet 1997;350(9077):535‐40. [PUBMED: 9284774] [DOI] [PubMed] [Google Scholar]
Lissoni 2009
- Lissoni AA, Colombo N, Pellegrino A, Parma G, Zola P, Katsaros D, et al. A phase II, randomized trial of neo‐adjuvant chemotherapy comparing a three‐drug combination of paclitaxel, ifosfamide, and cisplatin (TIP) versus paclitaxel and cisplatin (TP) followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the Snap‐02 Italian Collaborative Study. Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO 2009;20(4):660‐5. [PUBMED: 19181826] [DOI] [PubMed] [Google Scholar]
Loncaster 2000
- Loncaster JA, Cooper RA, Logue JP, Davidson SE, Hunter RD, West CM. Vascular endothelial growth factor (VEGF) expression is a prognostic factor for radiotherapy outcome in advanced carcinoma of the cervix. British Journal of Cancer 2000;83(5):620‐5. [PUBMED: 10944602] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maneo 2011
- Maneo A, Colombo A, Mangioni C, Landoni F. Randomized study between radical surgery and radiotherapy for the treatment of stage IB‐IIA cervical cancer. 20‐year update. Conference proceedings from 17th International Meeting of the European Society of Gynaecological Oncology (ESGO) in International Journal of Gynecological Cancer 2011;21(Supplement 3):S25. [Google Scholar]
Manriquez 2008
- Manriquez JJ. A highly sensitive search strategy for clinical trials in Literatura Latino Americana e do Caribe em Ciencias da Saude (LILACS) was developed. Journal of Clinical Epidemiology 2008;61(4):407‐11. [PUBMED: 18313567] [DOI] [PubMed] [Google Scholar]
McCormack 2009
- McCormack M, Ledermann JA, Hall‐Craggs MA, Symonds RP, Warwick H, Simonds I, et al. A phase II study of weekly neoadjuvant chemotherapy followed by radical chemoradiation for locally advanced cervical cancer. Journal of Clinical Oncology, 2009 ASCO Annual Meeting Proceedings (Post‐Meeting Edition) 2009;27(15S):5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mossa 2010
- Mossa B, Mossa S, Corosu L, Marziani R. Follow‐up in a long‐term randomized trial with neoadjuvant chemotherapy for squamous cell cervical carcinoma. European Journal of Gynaecological 0ncology 2010;31(5):497‐503. [PUBMED: 21061788] [PubMed] [Google Scholar]
NACCCMA 2003
- Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Meta‐analysis Collaboration (NACCCMA). Neoadjuvant chemotherapy for locally advanced cervical cancer: a systematic review and meta‐analysis of individual patient data from 21 randomised trials. European Journal of Cancer 2003;39(17):2470‐86. [PUBMED: 14602133] [DOI] [PubMed] [Google Scholar]
NCI 1999
- National Cancer Institute. NCI Issues Clinical Announcement on Cervical Cancer: Chemotherapy plus Radiation Improves Survival [Web Page]. 1999. http://www.cancer.gov/newscenter/cervicalcancer.
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;24:2815‐2834. [PUBMED: 9921604] [DOI] [PubMed] [Google Scholar]
Sardi 1990
- Sardi J, Sananes C, Giaroli A, Maya G, Paola G. Neoadjuvant chemotherapy in locally advanced carcinoma of the cervix uteri. Gynecologic Oncology 1990;38(3):486‐93. [PUBMED: 1699851] [DOI] [PubMed] [Google Scholar]
Sasieni 1995
Thigpen 1981
- Thigpen T, Shingleton H, Homesley H, Lagasse L, Blessing J. Cis‐platinum in treatment of advanced or recurrent squamous cell carcinoma of the cervix: a phase II study of the Gynecologic Oncology Group. Cancer 1981;48(4):899‐903. [PUBMED: 7196794] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8(1):16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2008
- Tierney JF, Vale C, Symonds P. Concomitant and neoadjuvant chemotherapy for cervical cancer. Clinical Oncology 2008;20(6):401‐416. [PUBMED: 18571391] [DOI] [PubMed] [Google Scholar]
WHO 2006
- World Health Organisation. Comprehensive cervical cancer control: a guide to essential practice. Integrating health care for sexual reproductive health and chronic diseases 2006; Vol. http://www.who.int/reproductive‐health/publications/cervical_cancer_gep/index.htm.
Williamson 2002
- Williamson PR, Tudor Smith C, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;21:3337‐51. [PUBMED: 12407676 ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Rydzewska 2008
- Rydzewska L, Tierney J, Burdett S, Symonds PRP. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007406] [DOI] [Google Scholar]
Rydzewska 2010
- Rydzewska L, Tierney J, Vale CL, Symonds PR. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007406.pub2] [DOI] [PubMed] [Google Scholar]


