Summary
Pinpoint control over endogenous gene expression in vivo has long been a fevered dream for clinicians and researchers alike. With the recent repurposing of programmable, RNA-guided DNA endonucleases from the CRISPR bacterial immune system, this dream is becoming a powerful reality. Engineered CRISPR/Cas9 based transcriptional regulators and epigenome editors have enabled researchers to perturb endogenous gene expression in vivo, allowing for the therapeutic reprogramming of cell and tissue behavior. For this technology to be of maximal use, a variety of technological hurdles still need to be addressed. Better understanding of the design principle controlling gene expression together with technologies that enable spatiotemporal control of transcriptional engineering are fundamental for rational design, improved efficacy and ultimately safe translation to humans._In this review, we will discuss recent advances and integrative strategies that can help pave the path towards a new class of transcriptional therapeutics.
Keywords: Synthetic Transcription Factor, CRISPR, Cas9, Epigenome Editing, Synthetic Gene Circuits, In vivo cellular programming
Introduction
The coordination of gene expression lies at the heart of cell identity and tissue function, giving rise to hundreds of different cell types capable of executing highly specific metabolic, developmental, structural, and immunologic functions. Regulation of gene expression is somewhat of a circuitous process mediated by transcription factor activity, concentration, chromatin architecture, and nuclear positioning ultimately modulating a promoter’s ability to recruit RNA polymerases. Transcription factor availability and activity is controlled both by the chromatin structure surrounding a transcription factor coding sequence as well as transmitted extracellular signaling cues (Venkatesh and Workman, 2015). Additionally, under certain conditions, sequence changes in cis-regulatory elements (e.g. transcription factor binding sites, enhancer/silencer sequences, insulator boundaries) can result in profound changes in the behavior of gene regulatory networks (GRNs) and the gene expression profiles they control, occasionally generating disease phenotypes (Schuijers et al., 2018; van der Harst and Verweij, 2018).
Synthetic control of transcription would provide the ability to program cell fate, collective cellular behaviors and tissue function in vivo potentially resulting in the reestablishment of healthy tissue GRNs during disease states. It is instrumental especially when it comes to the management of complex conditions ranging from obesity to regenerative diseases (Majewski and Pastinen, 2011; Subramanian et al., 2005). However, for many years transcriptional control processes have widely been viewed as “undruggable”. Recently, a variety of approaches have been developed to satisfy this goal ranging from small molecules that control chromatin modifiers (epi-drugs) to synthetic transcription factors derived from designer DNA binding elements such as transcriptional activator-like elements (TALEs) (Cong et al., 2012; Kundakovic et al., 2009; Lanzillotta et al., 2013). Initially, pioneering works developed engineered zinc finger transcriptional regulators and TALE proteins as powerful tools to enable transcriptional modulation (Beerli et al., 1998; Jouvenot et al., 2003; Miller et al., 2011; Snowden et al., 2002; Zhang et al., 2011). Both of these systems rely on a fusion protein consisting of a transcriptional modulator fused to a synthetic protein built from a modular array of DNA binding peptide sequences that can specifically recognize either individual bases or short nucleic acid sequences. When these proteins are designed to recognize a nucleic acid sequence within the promoter region of a target gene of interest, a specific change in transcription can be initiated. Engineered zinc finger and TALE-mediated transcriptional modulators have been used in a variety of potentially therapeutic applications ranging from cell fate reprogramming efforts to the rescue of muscle function in Duchenne’s Muscular Dystrophy mouse models and more recently in clinical trials (Eguchi et al., 2016; Jouvenot et al., 2003; Lu et al., 2008, https://www.sangamo.com/collaborations). While effective at modulating transcription, these systems suffer from an ease of engineering standpoint and are cumbersome to use when trying to modulate gene expression at multiple loci. Nevertheless, these studies have set the stage for the field of transcriptional therapeutics.
The recent repurposing of programmable, RNA binding, DNA targeting Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) proteins for transcriptional control has provided a modular transcriptional engineering platform that is relatively simple to use, multiplexable, highly scalable, and significantly easier to program when compared to TALEs and synthetic zinc finger transcriptional activators. Evolving from genome editing, several epigenomic modifications can now be made at a target genomic locus using catalytically dead Cas9 (dCas9) proteins that are either fused to, or capable of recruiting epigenomic effector domains (Table 1). In brief, CRISPR based synthetic transcription factors function by recruiting a modified Cas protein to a target locus through the use of a short guide RNA (gRNA) sequence. Upon a recognition and hybridization event between the gRNA and the complementary target DNA sequence, the effector is localized to the target region, resulting in an event such as a change to local DNA CpG methylation, histone tail modifications, or rate of RNA polymerase recruitment to the locus. Ultimately, the final outcome of such events is transcriptional modulation.
Table 1.
List of CRISPR based transcription factors and epigenomic modifiers along with a brief description of how they function, and previously described use cases.
| Effector Delivered | Function: | Example Target Gene(s) | Reference |
|---|---|---|---|
| dCas9-VP64 | virion protein 64 or VP64 ; 4x fusion of VP16 transcriptional activation protein derived from Herpes Simplex Virus. Used to achieve moderate increases in transcriptional output, may be most useful when only a small change in transcription is desired. | Some targets include NANOG, MYOD1, ILRN1, and VEGFA | (Perez-Pinera et al., 2013) |
| dCas9-SunTag- VP64 | Peptide based scaffold recruitment system capable of localizing up to 24 repeats of VP64 domains through a GCN4-single chain variable fragment interaction. Thus, it recruits multiple copies of an antibody-fusion protein. | Ascl1, Neurog2 and Neurod1; CXCR and CDKN1B | (Tanenbaum et al., 2014; Zhou et al., 2018) |
| MS2-P65-HSF1 /dCas9- VP64 | Heat Shock Factor1 (HSF1) involves in activating genes important for cellular stress response. P65 is nuclear factor NF-kappa-B p65 subunit that is responsible for initiating transcription. MS2-P65-HSF1, is fusion protein consisting of activator domains of P65 and HSF1 tagged to an MS2 phage capsid. The complex is recruited to gRNA through MS2 aptamers. VP64 together with MS2-P65-HSF1 exert strong transcriptional activator function. | Up to ten targets including MYOD1, ASCL1, and KLF4 and lincRNA transcripts | (Konermann et al., 2015) |
| dCas9-p300 | Histone acetylase core subunit p300 fused to dCas9. Acetylation of histone tails weakens interaction between DNA and histone proteins, allowing for increased access of a genomic locus by host transcriptional machinery. | ILRN1, Oct4, MYOD1 | (Hilton et al., 2015) |
| dCas9-VPR | Tripartite transcriptional activator consisting of VP64, P65, and Rta. Replication and transcription activator (RTA) is a potent immediate early transcription activator | Targeted MIAT, TTN, and ASCL1 | (Chavez et al., 2015) |
| dCas9-LSD1 | Lysine specific demethylase removes methyl groups from histone tails specifically at lysine residues. Lysine demethylation is associated with transcriptional repression. Kearns et al utilized dCas9- LSD1 to study role of enhancers in the maintenance of Oct4 and Sox2 expression in mouse ESC’s. | Oct4 | (Kearns et al., 2015) |
| dCas-Dnmt3a | dCas9 fused to DNA methyltransferase, capable of local deposition of methyl groups at target genomic loci. It can be used to repress expression of target genes. Has been used at CTCF loci, capable of reorganizing local chromatin loop architecture. | CTCF Loop Domain | (Liu et al., 2016) |
| dCas9-Tet1 | Cytosine demethylase Ten-eleven translocation methylcytosine dioxygenase 1 (Tet1) fused directly to dCas9. Can be used to remove methyl groups from 5-methylcytosine (5-mC) residues, promoting transcription. | BDNF and npas4 | (Liu et al., 2016) |
| dCas9-HDAC3 | Histone Deacetylation through the fusion of dCas9 to full length human Histone deacetylase3 (HDAC3). Primarily used to deacetylate histones yielding a tighter binding interaction between histones and DNA. Typically associated with the repression of local gene expression. | MeCP2 and Smn1 | (Kwon et al., 2017) |
| dCas9-CLOUD | Chromatin loop reorganization using CRISPR-dCas9 (CLOuD9) induce synthetic looping for targeted chromatin loop reorganization. Can be used to change the location of distal regulatory elements which may result in the modulation of endogenous gene expression. Re-established beta globin expression in vitro through formation of a long- range chromatin loop. | Beta-globin and Oct4 | (Morgan et al., 2017) |
| CRISPR-GO | CRISPR-genome organization (CRISPR-GO) modulates gene expression through a chemically inducible, two component system that can reversibly change the 3D location of gRNA targeted loci. In vitro silencing of XIST and CXCR4 were achieved through positioning of loci near nuclear lamina. | XIST, Pten, and CXCR4 | (Wang et al., 2018) |
| dCas9-KRAB- MeCp2 | Bipartite Transcriptional Repressor. Fusion protein consisting of KRAB transcriptional repressor subunit fused to MeCp2. Enhanced repressive activity when targeted to promoters of target genes compared to dCas9-KRAB alone. KRAB domain is Kruppel- associated box domain, a 75-amino acid transcriptional repressor found in a subset of zinc finger proteins and can result in formation of H3K9me3 mark. MeCp2 is Methyl- CpG-binding protein 2 and can also induce transcriptional repressor function. | Targets included CXCR4, BRCA1, and MAPK3 | (Yeo et al., 2018) |
| dCas9-DamN132a | Dcas9 fused to a mutant DNA adenine methyltransferase that is capable of depositing unique, N6 methyladenine (m6a) marks to adenine residues. Used along with synthetic, m6a readers to orthogonally activate or repress elements in synthetic gene circuits. | Only targeted synthetic reporter constructs, not endogenous loci | (Park et al., 2019) |
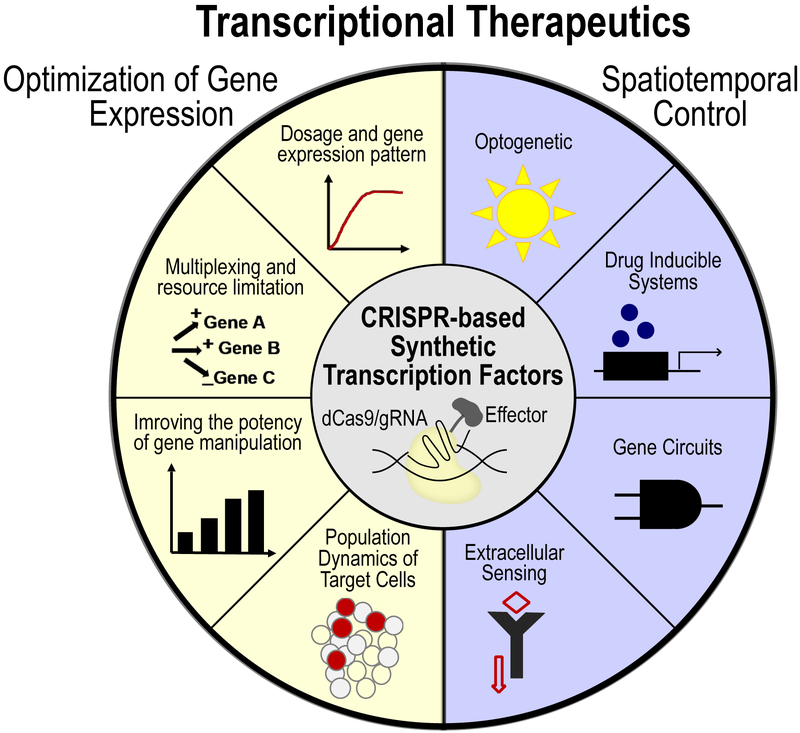
Despite recent progress, a wide range of engineering challenges and basic biological questions still need to be addressed, before CRISPR-mediated transcriptional engineering can be of maximal use in the clinic. Here, we have focused on recent studies where the technology has been used in vivo and highlight related design principles. We will also discuss a subset of key elements that are important to consider during in vivo transcriptional modulation as well as recent advances in synthetic biology that can be employed to facilitate a new generation of in vivo transcriptional therapeutics (Figure 1).
Figure 1.
CRISPR based synthetic transcription factors for transcriptional therapeutics. Schematic shows potential challenges and areas for improvement (left) as well as tools and technologies for designing more controllable systems in future (right).
In vivo Transcriptional Modulation with CRISPR Synthetic Transcription Factors
Over the last half decade, a great amount of progress has been made retrofitting the CRISPR/Cas9 system to orthogonally control the expression of endogenous genes. Several challenges surrounding delivery methodologies and effector potency have become major roadblocks when it comes applying transcriptional modifiers in vivo. In this line, progress in decoding epigenetic regulation of gene expression and transcriptional dynamics has resulted in clever designs and the construction of a wide range of transcriptional effectors.
Recombinant Adeno Associated Virus (rAAV) serotypes are currently considered among the gold standards for nucleic acid-based therapy given their proven safety profiles in clinical trials, low immunogenicity, high transduction efficiency, and ability to specifically transduce a subset of tissues through the use of recombinant serotypes. The primary challenge with using rAAV vectors to deliver transcriptional modulation components is the fact that these vectors have a rather small genomic payload capacity (roughly 4.7kb). dCas9 from Streptococcus pyogenes, when fused to synthetic transcription factors, exceeds the packaging capacity of a single vector. As a result, for efficient delivery a minimum of two separate AAV vectors are needed to deliver dCas9 based transcription factors. This limitation requires the engineering of split dCas9 architectures (Moreno et al., 2018; Chew et al., 2016), or alternatively, the use of protein binding aptamer modified gRNAs that can recruit synthetic effector domains.
Engineering Gene Activation In vivo
A growing body of work has shown therapeutic in vivo modulation of target gene expression using synthetic CRISPR transcription factors (See Table 2 for additional information on design, delivery and practical points) (Chew et al., 2016; Liao et al., 2017; Matharu et al., 2018; Moreno et al., 2018; Thakore et al., 2018). Initial proof of concept studies aimed to demonstrate that Cas9 based transcriptional modulators could specifically modulate the expression of target genes. In the first application of such systems, Chew and colleagues were able to use Cas9-VP64-p65-Rta (VPR) to modestly upregulate target genes (Chew et al., 2016). Shortly afterward, Liao and colleagues utilized Cas9 based transcription factors to efficiently upregulate target genes in vivo for several therapeutic use cases including Type I diabetes, Duchenne’s Muscular Dystrophy, and drug-induced renal failure (Liao et al., 2017). These therapeutic demonstrations were made possible by the development of a dual vector AAV delivery system, a modification to the synergistic activation mediator (SAM) system to reduce transgene payload, and rational engineering of gRNA secondary structures (Table 2). SAM system uses dCas9-VP64, gRNA with two hairpin aptamers with MS2 domains that recruit MS2-p65-HSF1. Through their optimization, Liao et al. could remove VP64 from the system without notable decrease in gene activation. Therefore, they used Cas9 with truncated gRNA and MS2-p65-HSF1 in vivo.
Table 2.
Overview of studies used CRISPR based synthetic transcription factors in vivo.
| Effector Delivered |
Design Principle and Delivery | Practical Notes and Applications | Reference |
|---|---|---|---|
| dCas9-VP64- p65-Rta (VPR) | Split Cas9-VPR architecture and truncated gRNAs delivered by AAV9 to mice through intraperitoneal injection. Truncated gRNAs enable further decrease the size but also provide usage of Cas9 nuclease competent protein without DNA cleavage. | Of note measurable immune responses to Cas9 were observed but did not cause major tissue damage. When targeted Pd-11 and CD47 researchers were able to achieve modest upregulation ranging from 2 to 3fold. | (Chew et al., 2016) |
| spCas9 and MS2-p65-HSF1 | To work around AAV packaging limit, Liao and colleagues used truncated gRNA and importantly RNA aptamer-based recruitment of effectors to Cas9 to activate target genes in vivo. To improve activation efficiency in vivo, researchers removed repetitive sequences and optimized GC content of gRNAs to improve effector recruitment. spCas9 expression cassette and effector/gRNA were packaged separately into AAV9 vectors. | The study shows Pdx1 mediated reprogramming of hepatic tissues into pancreatic like beta cells to rescue insulin production in a Type-I diabetic mouse model. Renal function was maintained by upregulation of IL-2 to protect against cisplatin induced kidney damage and upregulation of utrophin was utilized to alleviate Duchenne Muscular Dystrophy symptoms. | (Liao et al., 2017) |
| dCas9-SunTag Scaffold (10x GCN4) and scFv-p65-HSF1 | Generated a transgenic mouse model carrying both a constitutively expressed dCas9-SunTag scaffold and a constitutively expressed, scFv-p65-HSF1 transcriptional activator (SPH). Researchers decided to use the SunTag scaffold with p65-HSF1 to improve activation efficiency of target genes. SPH activators showed 2 to 3-fold superior activation when compared with SAM, SunTag or VPR. | gRNAs targeting Ascl1, Neurog2, and Neurod1 were delivered in a single AAV2 vector. Roughly a 34% reprogramming efficiency was achieved. Reprogrammed neurons demonstrated sustained action potentials, post synaptic currents, and exhibited neural cell like morphology. | (Zhou et al., 2018) |
| Cas12a-VPR | By altering the length of gRNA simultaneous activation and editing was achieved. Sleeping Beauty transposon plasmids and hydrodynamic tail injection were used. | Using Cas12a-VPR they simultaneously generated indels in TP53 and induced the expression of a different target genes (transgenic GFP). | (Breinig et al., 2019) |
| dCas9-VP64 | dCas9-VP64 was chosen specifically for its modest ability to activate target genes in order to restore SIM1 to a physiologically relevant level of expression. dCas9-VP64 and gRNAs were separately packaged into AAV-DJ serotype and delivered directly to the hypothalamus through stereotaxic injection. Using SaCas9-VP64 (4.3 kb) vs SpCas9- vp64 (5.4 kb) could improve packaging efficiency. | By targeting the remaining functional SIM1 or Mc4r allele in haploinsufficient mediated obese mouse models, researchers were able to restore gene expression resulting in significant weight loss and reduced feeding. | (Matharu et al., 2018) |
| CRISPReader: SpCas9-VP64 and SpCas9- MS2-VPR | To overcome the low payload capacity of traditional AAV vectors, the authors created a positive feedback expression loop composed of a promoterless CRISPR-dCas9 system dubbed CRISPReader. dCas9- VP64 was expressed using a minimal TATA promoter; its own transcription was then further promoted by the binding of gRNA upstream of the TATA box, along with the target gene. | This paper targeted Apoal to reduce the cholesterol serum levels in hypercholesterolemic mice. Higher expression of Apoal and high-density lipoproteins and lower levels of total cholesterol were achieved as compared with conventional dual-AAV systems. | (Zhan et al., 2019) |
| dSpCas9-TET3 | Due to lentiviral delivery mode “all in one” vectors were used. Additionally, authors tested high-fidelity dCas9 variant with reduced non-specific DNA contacts. | Authors achieved effective demethylation of Rasal1 and Kloth promoters CpG islands, reactivation of target genes and significant reduction in kidney fibrosis. | (Xu et al., 2018) |
| dSaCas9- Kruppel Associated Box Protein (KRAB) | dSaCas9-KRAB and gRNAs targeting Pcks9 for repression were delivered via an AAV8 serotype which has a high tropism for mouse hepatic tissues. dSaCas9 was chosen over the more commonly used spdCas9 because it has a significantly smaller coding sequence, making it easier to package into AAV vectors. | They employed CRISPR-based transcriptional repression for Pcks9 to reduce circulatory cholesterol levels in adult mice. High doses of AAV-dSaCas9- KRAB expression generated an immune response, liver injury and ineffective repression in longer term. | (Thakore et al., 2018) |
| dCas9-KRAB | Sequences coding for dCas9-KRAB and gRNAs targeting Syt1 for repression were packaged into lentiviral vectors and sterotaxically injected into the dentate gyrus of mice. The study showed multiplex repression and control using cell type specific promoters. | Repression of Syt1 expression in the dentate gyrus revealed that it may have a role in memory and cognitive function. This paper also demonstrated that dCas9-KRAB is more efficient at target gene knockdown compared to RNAi mediated repression. | (Zheng et al., 2018) |
| dCas9-KRAB | To work around the low payload capacity of traditional AAV vectors, researchers split dCas9-KRAB into two lobes and tagged each respective lobe with an intein peptide sequence that is capable of a dimerization and recombination event that can reconstitute the functional activity of split dCas9-KRAB. One vector also contained a gRNA expression cassette carrying a gRNA sequence targeting Nrl for repression. | By targeting Nrl, a master transcription factor that is responsible for the regulation of rod versus cone photoreceptor cell fate in the retina, researchers were able reprogram rod-like photoreceptors into cone-like photoreceptors, preventing further retinal degradation in a Retinitis Pigmentosa mouse model. | (Moreno et al., 2018) |
dCas9 based transcription factors (with full length gRNAs) have also been used to modulate the transcription of multiple genes in neural tissues in vivo. In an attempt to improve activation efficiency of CRISPR based transcription factors in vivo, Zhou and colleagues borrowed favorable properties from two previously described CRISPR based activation platforms. In vitro experiments revealed that by appending the CRISPR/SunTag system (Zhou et al., 2018), with the second-generation activator, SAM, the enhanced number of effectors localized to a target gene significantly improved transcriptional output. By creating a Cre-dependent mouse model carrying expression cassettes for their improved activation platform, Zhou and colleagues were able to simultaneously upregulate multiple genes and reprogram astrocytes into functional neurons with sustained action potentials, in vivo (Table 2). Additionally, the feasibility of targeting up to 10 genes simultaneously in this mouse model was evaluated in both hepatic and neural tissues. Follow-up RNA sequencing data suggests that multiplex activation at this scale is possible, though it is not clear whether activation of every single target is fully achieved in each single cell or if the expression is more of a mosaic pattern, meaning each cell is only capable of activating a subset of target genes. While this study offers a proof of concept for a CRISPR-based transcriptional modulation platform in vivo, it is vital to validate the findings following dCas9 delivery to better capture in vivo therapeutic conditions.
More recently, researchers utilized CRISPR-based transcriptional activation to rescue obesity phenotypes in happloinsufficient mouse models by increasing the transcriptional output from a remaining, functional allele. Matharu and colleagues attempted to deliver AAV packaged dCas9-VP64 through stereotaxic hypothalamus injections to restore normal body weight in both Sim1 and Mc4r happloinsufficient mouse models (Matharu et al., 2018). Targeting either a distal, cis-regulatory enhancer element or the promoter of Sim1 in vivo resulted in upregulation of Sim1, significant weight reduction, and reduced feeding activity that was phenotypically stable even after 9 months (Matharu et al., 2019). These data suggest dCas9 based transcriptional modulation may be a meaningful therapeutic intervention for haploinsufficiency-induced obesity. It is important to note in some cases even modest alteration of the target gene can have a phenotypically meaningful effect (e.g. Sim1 activation). Hence, it is imperative that the fold change in target gene is examined in parallel with downstream GRNs and phenotypic outcomes (Xu et al., 2018).
While these studies shape a first wave of CRISPR-based transcriptional activation in vivo, the field is still evolving rapidly. For instance, a recent study showed a proof of concept for usage of multifunctional CRISPR systems in vivo. Breinig and colleagues used CRISPR/Cas12a to demonstrate orthogonal gene editing and activation in the same cell using a single CRISPR system while only altering gRNA length (Breinig et al., 2019). Another study by Zhan and colleagues showed all-in-one delivery of dCas9-VP64 in AAV by eliminating the use of a promoter, instead using a minimal TATA box. A positive feedback expression loop was implemented through preliminary transcription initiation relying on the TATA box and subsequent promotion of transcription of dCas9-VPR and the target gene using the TATA box and binding of the gRNA upstream of the minimal promoter. Gene expression is then amplified at the level of translation through use of RNA activators which recruit ribosomes to specific mRNA (Zhan et al., 2019).
Engineering Gene Repression In vivo
Although with lower frequency, studies focusing on in vivo transcriptional repression have recently been published as well. Using a dual vector, AAV8 delivery system in adult mice, one carrying a Pcks9 targeted gRNA and the other carrying Staphylococcus aureus dCas9- Kruppel-associated box domain (KRAB), researchers were able to significantly reduce liver Pcks9 gene expression levels ultimately yielding a reduction in circulating low density lipoprotein (LDL) concentration that was durable for at least 24 weeks post injection (Thakore et al., 2018). Cas9 based transcriptional repressors have also been applied in brain tissues to study the effect of target gene expression on neurotransmitter activity. Using dCas9-KRAB and gRNAs targeting Syt1, lentivirally delivered through stereotactic injection to the dentate gyrus, Zheng and colleagues were able to significantly reduce Syt1 expression (Zheng et al., 2018). Syt1 knockdown mice showed reduced cognitive and memory functions compared to controls. However, the study also provides helpful insights for future therapeutic strategies. First, it demonstrated that CRISPR-based repression is superior to RNAi-mediated knockdown in post mitotic neurons. It provides a protocol for multiplexed silencing of up to five genes, in vivo. Additionally, it demonstrated that silencing by dCas9-KRAB can be controlled in a cell type specific fashion using cell type specific promoters. Finally, the researchers showed that even slight mismatches between gRNA and target locus sequence can disrupt dCas9 binding activity at a target locus, suggesting that dCas9 based repression has a high targeting specificity. Moreno and colleagues recently used CRISPR-based transcriptional repression in an attempt to correct retinitis pigmentosa phenotypes in vivo. The observed variability in retinitis pigmentosa disease causing mutations makes it difficult to generate and validate genome editing strategies at a patient specific level. In an attempt to work around this roadblock, researchers attempted to create a broadly useful direct reprogramming strategy to convert rod-like photoreceptors to cone-like photoreceptors in order to prevent further vision loss in the patients through the repression of Nrl, a master regulator of rod versus cone photoreceptor cell fate. This study used a split Cas9 architecture in order to work around AAV vector payload limitations. Using a retinitis pigmentosa mouse model, they demonstrated a therapeutic benefit for dCas9-KRAB mediated Nrl repression in retina when delivered postnatally to day seven mouse pups (Moreno et al., 2018). Following mice until postnatal day 50, treated mice demonstrated improved visual acuity compare to diseased controls, suggesting that CRISPR based synthetic transcriptional repression may prevent further loss of vision in retinitis pigmentosa patients.
The studies described above have been proof of concepts that broaden the therapeutic applications of CRISPR-based synthetic transcription factors in vivo. Interestingly, several other unique applications might now be within the realm of possibility. For instance, it may be possible to engineer host resistance prior to exposure to select pathogenic agents through targeted upregulation of host genes that confer protective coverage following exposure and may be of great value to healthcare providers working in high risk environments. As an example, using a genome wide, dCas9 based transcriptional activation screen, Heaton and colleagues identified that upregulation of B4GALNT2 conferred resistance to infection against a number of different avian influenza strains when tested in vitro (Heaton et al., 2017). Programming solid tumor cells and their microenvironments also offers a potentially valuable therapeutic strategy as this technology develops. However, there are several gaps that need to be addressed in order to achieve a rapid and safe translation to the clinic. In the next sections we will review a subset of these challenges and discuss potential engineering strategies aimed at solving them (Figure 1).
Optimization of Gene Expression for Transcriptional Therapeutics
Component Dosage and Gene Expression Dynamics
The dynamics of a gene’s expression in a given cell can follow distinct kinetic models depending on gene function, cell type and potentially, the homeostatic needs of the tissue. Some genes need to have a sharp on/off response when a stimulus threshold is reached (digital control) while others may utilize a more graded response where gene expression is proportional to the initial stimulus (analog control) (Lorberbaum and Barolo, 2013) (Figure 2A). As an example, for stress response genes like NF-κB, a transcription factor involved in the control of inflammatory and immune responses, an analog and graded response is favorable in order to appropriately harmonize both inflammatory cues and cell responses (Giorgetti et al., 2010). Digital transcriptional responses on the other hand, may trigger the sharp pattern boundaries observed during developmental morphogenesis. The engineering of synthetic transcription factors offers a promising strategy to modulate or mimic such processes and may help develop therapeutics that offer a high degree of similarity to normal physiological processes. High cooperativity between transcription factors or stronger binding affinity for DNA sequences favors characteristics of digital regulation for a gene circuit (Bradley et al., 2016; Purcell and Lu, 2014). Accordingly, manipulation of gRNA binding strength, CRISPR dosage, and the behavior of engineered CRISPR systems (e.g. On:Off ratio) provide attractive strategies for directing such patterns of gene expression for both discovery and therapeutic purposes. For instance, engineering chromatin remodeling repression domain Mxi1 bestow stronger repression to dCas9 protein and improved digital-like gene regulation in synthetic gene circuits (Gander et al., 2017). However, this notion is less studied when controlling natural gene expression in mammalian cells. How changes in digital versus analogue natural expression patterns in a target gene influences the cell and tissue phenotypic outcome is not yet fully clear, particularly during disease states. To systematically address this question improved understanding of chromatin architecture and transcriptional regulation at the target locus will enable a rational engineering approach. Future studies on understanding the dynamic behavior of a target gene, quantifying and optimizing the relationship between target gene transcript level, its natural expression pattern, the amount of gRNA and dCas9, and the type of transcriptional effector are likely critical steps to answer this question.
Figure 2.
Optimization of gene expression in CRISPR-based transcriptional modulation and the associated intricacies. A. Digital versus. analogue regulation of a gene circuit determined by the cooperativity between transcription factors and binding affinity. B. Multiplexing ability derived through use of RNA binding domains which orthogonally recruit binding proteins and their effectors. C. Resource Allocation of finite cellular transcriptional machinery which determines activity of synthetic circuits through sharing those resources with the demand from the host cell D. Potent transcription controls through use of a variety of effectors fused to dCas9 (blue-activators; grayscale- repressors) E. Cellular dynamics following genetic manipulation by synthetic circuits, showing population heterogeneity after transcriptional modulation.
Multiplexing and Resource Limitation
Our genes function within interconnected networks, and as the network responds to stimuli, different genes with the network are activated and repressed. In order to better model and treat disease, simultaneous activation and repression of multiple genes (gene network engineering) may be of therapeutic interest, especially as our understanding on how gene expression is modulated through distal regulatory elements and chromatin conformation improves. Enhancing in vivo delivery payload capacity and developing size-efficient gRNA expression cassettes are necessary to satisfy these requirements for polygenic disease phenotypes (Kiani et al., 2015). Cas9 proteins cannot discriminate between different gRNAs and this becomes a major challenge when one wishes to deliver different effectors at two or more target loci. An elegant workaround to this challenge can be found through the use of protein binding RNA aptamers. dCas9 gRNA can tolerate base extensions on two of its structural stem loops or its 3’ end, allowing for the addition of peptide recruiting aptamers (Mali et al., 2013). This allows for the recruitment of different transcriptional modulators based solely on aptamer choice, which frees dCas9 to act as a multifunctional epigenomic modulator solely based on gRNA input. Zalatan and colleagues tested the insertion of multiple protein binding aptamer pairs including MS2, PP7, and COM into gRNA scaffolds and their data suggests that different aptamer-peptide interactions are highly orthogonal to one another and are capable of recruiting similar levels of transcriptional modulators (Zalatan et al., 2015) (Figure 2B).
A fair amount of research has gone into the construction of gRNA transcripts that can be expressed using RNA polymerase II as it can offer an avenue for an improved spatiotemporal regulation (Kiani et al., 2014). RNA polymerase II driven RNA transcripts undergo additional processing for maturation, which may affect the functionality of produced gRNAs. Moreover, in the case of multiplexing from single promoters, gRNAs need be separated from one another. This can be achieved by inserting self-cleaving ribozymes between gRNAs which automatically fold and cleave themselves from a primary transcript following transcription or the inclusion of an orthogonal RNA processing endonuclease (e.g. Csy4) target sites in between the primary gRNA transcripts (Menn et al., 2018; Xu et al., 2017).
As we move towards more sophisticated multi-layered genetic programming with CRISPR, the competition for finite cellular resources (transcription factors, RNA polymerases, and free ribosomes) may become a major challenge (Figure 2C). This issue influences both the modularity and composability of the engineered gene circuits and may affect the basal behavior of host gene regulatory networks. Here the field can benefit from recent studies in the field of synthetic biology where the robust and predictable design of large-scale genetic circuits has been an important objective (Del Vecchio, 2015). The application of systems and control theory has been employed for maintaining robustness and improving system operation in the context of synthetic gene circuits (Del Vecchio et al., 2016). Engineering feedback control is a common proposed practice to improve the reliability of synthetic gene circuits. This is especially important for therapeutic objectives like stable cell fate programming or gaining control over biochemical, homeostatic functions such as insulin production and glucose control (Del Vecchio et al., 2017). Using cell type specific promoters can decrease the interference between multiple transcriptional units; however, it can come with a price. For example, when using cell type specific RNA polymerase II promoters to induce expression of gRNAs or CRISPR components, a lower dosage of gRNA or Cas9 can be achieved. The decrease in dosage may negatively influence transcriptional modulation, increase operational noise, and decrease the strength of downstream phenotype. Again, the application of synthetic biology-based practices such as model driven optimization of Polymerase II driven gRNA expression level or the use of self-replicating gRNAs may provide potential solutions (Kiani et al., 2014; Menn et al., 2018; Wagner et al., 2018).
Improving the Potency of Gene Manipulation
Improving our understanding of GRNs controlling target genes of interest can provide additional targets for modulation within a cell type that may improve the potency and permissiveness to transcriptional engineering. A comprehensive understanding of transcriptional and epigenomic regulation at a given locus will also be critical when it comes to selecting the effectors to manipulate existing chromatin marks. It should be noted that depending on the target gene, its function, and cellular context, different fold changes in expression may be required to achieve desired phenotypes. In other words, just because it is possible to increase the expression of a target gene by one thousand-fold, does not mean that it is useful or necessary.
Initial dCas9 based transcriptional modulation platforms started out with the fusion of relatively simple activation mediators derived from tetramer repeats of the herpes simplex virus transcriptional activator, VP16. First generation activators provided modest increases in transcriptional output typically offering less than 100-fold increases of target gene mRNA output (Perez-Pinera et al., 2013). Combinatorial screenings of transcriptional activation domains have resulted in the construction of increasingly potent activators leading to the use of bi/tripartite activators like VPR and SAM, as well as peptide scaffold-based activators like SunTag-VP64 (which can deliver up to 24 tandem repeats of VP64). These second generation activators provide significantly higher levels of activation (>1000 fold in some cases) compared to first generation activators, likely through their ability to recruit an increased number and variety of cofactors that can more efficiently recruit RNA polymerase and better stabilize transcription initiation complexes (Chavez et al., 2015; Kiani et al., 2015; Tanenbaum et al., 2014) (Figure 2D).
Similar advances in synthetic repressor constructs have been made in recent years as well. The standard dCas9-KRAB repressor, which has demonstrated a fair amount of variability in its ability to repress target genes has recently been improved upon through creation and screening of several bipartite repressors consisting of KRAB and a secondary repressor domain. Data from Yeo and colleagues’ recent study demonstrates a consistent, synergistic, and enhanced repressive effect against a variety of endogenous targets (coding and noncoding) when using dCas9-KRAB-MeCP2 construct (Yeo et al., 2018) (Figure 2D). With the number of choices available to modulate transcription- either activation or repression- the question now becomes: What tool is right for the job? Until we develop multiscale models of gene regulation considering cell type, target gene, and microenvironmental context, this is less clear and, in most cases, needs to be empirically determined. It will be attractive to understand how the complex, robust, and more physiological setting of in vivo (e.g. multiple cell types, physiological levels of growth factors, oxygen and physical cues) influences the outcome, dynamic, and duration of gene activation/repression. We already know from in vivo transcription factor-based reprogramming that native in vivo environment can facilitate reprogramming outcome (Srivastava and DeWitt, 2008).
Population Dynamics of Engineered Cells
When studying gene regulation in tissues and multicellular systems, studying bulk cell populations can only quantify the average expression level of a given gene whereas the expression may be highly heterogenous between the cells within the population (Figure 2D). Additionally, due to the discrete cellular state defined by both cell type and tissue microenvironment, heterotypic cells of a particular tissue or different organs in the body can show different magnitude of response to a same transcriptional regulator . Bintu and colleagues used a single cell population in vitro with time lapse imaging of single cells in an attempt to gain a more comprehensive understanding of the temporal dynamics of gene repression through the comparison of different chromatin regulators (Bintu et al., 2016). The authors described a duration-dependent fractional silencing and memory in the cell population. Their findings suggest that the repression of a target gene often exhibits an “all-or-none response” pattern in a given cell. For example, when using DNMT3B to represses target genes, cells developed a slowly formed repressed state that is stably maintained. HDAC4 treated cells on the other hand, demonstrated an increased rate in both the acquisition and loss of the repressive state. This setting provides a well-controlled system for studying the dynamics of gene expression at a single cell resolution. However, it remains to be investigated whether the function of a given CRISPR based epigenome editor can follow similar dynamics when targeting distinct loci across different mammalian cells in multicellular tissues. It is possible that other existing parameters such as the type of chromatin mark, 3D genome structure surrounding a target gene, interaction with other transcription factors, and the local nuclear microenvironment may impact how the same chromatin regulator behaves at different loci. While this study uses a simple reverse Tetracycline repressor (rTetR) to recruit a chromatin modulator upstream of a reporter on a human artificial chromosome, it sets the stage for future efforts aimed at gathering a more dynamic assessment of CRISPR-based synthetic transcription factors. Advances in spatiotemporal analysis of gene expression in multicellular tissues will eventually enable multiscale studies (different loci of a cell and different cell types in a tissue) to decode design principle of transcriptional engineering in vivo (Pichon et al., 2018; Wang et al., 2018).
Engineering Control Over CRISPR-based Synthetic Transcription Factors
During organism development and stem cell differentiation, an intricate dance of gene expression is executed as development continues. This process of cell fate control and intercellular communication eventually shapes global tissue homeostasis and function. For example, during liver bud development from stem cells, a series of rapid changes in transcription factor expression are executed over time in order to direct the differentiation and organization of cell types, as they develop, into the hepatic liver lobules that make up a functional liver (Camp et al., 2017). Similar biological designs are present in tissue responses to injury, immune defense, and regeneration of organs. Thus, when thinking about the therapeutic applications of CRISPR, mimicking these biological processes through the design of multi input, spatiotemporally regulated CRISPR or layered circuit designs may provide an opportunity for the generation of more efficient transcriptional therapeutics. When it comes to controlling the expression of target genes, the methods of external control are rather limited. Most commonly, tetracycline responsive promoters are used to induce the expression or repression of a target gene. Tetracycline responsive promoter architecture is quite orthogonal, dose responsive, and capable of efficiently exerting control over the expression of the downstream gene but is not very modular. One cannot control a target gene at one time point, and another target gene at a downstream time point using tetracycline alone (Gossen and Bujard, 1992). This presents a challenge if coordinated, temporal control of different genes is required in vivo. Here we will review select technologies that enable researchers to engineer when, where and how CRISPR based synthetic transcription factors work in vivo.
Applying Optogenetic Systems to Control CRISPR
Recently, several groups have developed gene expression systems that rely on the input of light, a field dubbed optogenetics. Optogenetic control of gene expression is accomplished by using a two component, light inducible system composed of a dCas9-CIB1 protein fusion and a CRY2-Effector Domain fusion, which dimerizes upon exposure to a 450 nm light source (Nihongaki et al., 2015; Polstein and Gersbach, 2015) (Figure 3A). This system has also been used for neuronal cell fate programming or the inhibition of bladder cancer cell growth upon the activation of P53 (Lin et al., 2016; Nihongaki et al., 2017). Light induced, two component, gene expression systems demonstrate quick induction kinetics, comparable to tetracycline-controlled circuits. Optogenetic systems have been developed that rely on a different wavelength of light for effector domain recruitment (Levskaya et al., 2009), broadening the range of effectors that can be orthogonally recruited to dCas9. The ability to spatiotemporally pattern the expression of multiple genes simultaneously, mediated by gRNA input, allows for several applications including in vivo neural control, modulation of developmental processes, and guided vascular regeneration (Hibberd et al., 2018; Putri and Chen, 2018; Sako et al., 2016).
Figure 3.
Novel approaches to engineer control in CRISPR based transcriptional modulation. A. Optogenetic control of CRISPR-mediated gene expression through excitation with blue (450nm wavelength) light which initiates heterodimerization of CRY2 with CIB1 (fused to dCas9) and recruitment of the effector. B. (Left) Multilayered transcriptional control in which expression of gRNA from Layer1 modulates expression of gRNA from Layer2 which regulates transcriptional modulation. C. Utilization of recombinases for transcriptional regulation of a gene mediated through excision of recombinases and ligation of the remaining nucleotides (left) or inversion of the sequence (right) to result in a functional domain. D. Extracellular signal regulation of CRISPR activity through the use of receptors which, upon ligand binding, release engineered dCas9.
Drug Inducible Systems
Expanding off of light inducible systems, a series of new chemically induced, two component, dCas9 mediated gene expression systems have also been recently developed including both abscisic and gibberellic acid induced, two-component systems. (Gao et al., 2016). Similar to the optogenetic systems described above, these chemically induced systems rely on dCas9 protein fusions that recruit a correspondingly tagged effector domain following exposure to a specific small molecule reagent, like gibberellic acid (Gao et al., 2016). However, gibberellic acid has been implicated as a hepatotoxic reagent (Troudi et al., 2010). While the work by Gao et al. shows a big step forward, development of small molecules that are both orthogonal and compatible with human biology are of great value.
Programmable Synthetic Gene Circuits
Another set of methods for controlling CRISPR-based transcription lies in the development of programmable, synthetic gene circuits. These include methods such as layered gene circuits in which one gRNA controls expression of another gRNA or methods for recombinase-based logic gates (Kiani et al., 2014; Nissim et al., 2014; Weinberg et al., 2017) (Figure 3B, C). Recombinases are a class of sequence specific, DNA binding enzymes that are capable of initiating a variety of site-specific recombination events. Upon binding of a pair of recognition sequences, a recombinase can initiate one of three reactions: an excision and ligation event, an integration event, or an inversion event. A variety of recombinases are already well characterized, most of which are highly orthogonal to one another, making them a very attractive means of control over synthetic gene circuit components. Another trait that makes recombinases useful mediators of gene circuits is the fact that depending on the orientation of a pair of recognition sites, a single recombinase can execute different types of reactions (typically an excision or inversion event). Recently, Weinberg and colleagues created the BLADE system (Boolean Logic and Arithmetic through DNA Excision), a scalable and modular synthetic gene circuit system that relies solely on recombinase inputs to execute changes in synthetic cellular logic. A large number of logic circuits were constructed including a two input, four output circuit and a six input AND gate. Their two input, four output gate relies on the use of two recombinases (no recombinase present, recombinase “A”, recombinase “B”, or recombinase “A” and “B” present) that control the structure of a primary transcript that can be edited to one of four independent states based on recombinase input. This type of circuit allows for iterative changes in transcript expression based on recombinase input. However, these changes in transcript expression are mediated by irreversible excision events, meaning that any single computation can be executed only once. This same group of researchers interfaced their two input four output circuit design with dCas9-VP64 to control endogenous gene expression. Four gRNAs were stored within one gene expression cassette that could be selectively accessed and transcribed depending on recombinase input (Weinberg et al., 2017).
Extracellular Sensing
Our cells are constantly being exposed to signaling cues from their neighbors and their environments. Platforms integrating extracellular signaling cues to selectively change gene expression provide an elegant solution for the enhanced modulation of cell fate and function in multicellular settings. A large variety of membrane proteins ranging from Notch receptors to G-Protein Coupled Receptors (GPCR) are present on all mammalian cells, allowing them to sense extracellular inputs and subsequently transmit extracellular signals into changes in gene expression. Recently, synthetic ligands have been developed to orthogonally translate defined extracellular cues (ligands on neighboring cells, soluble cues, and small molecule drugs) directly into changes in gene expression (Figure 3D). In an effort to expand the number of functional inputs available to control endogenous gene expression, Daringer and colleagues developed the MESA system (Modular Extracellular Sensor Architecture) which relies on a dimerization event between two recombinant transmembrane proteins, each carrying half of a ligand binding domain, that are brought together upon partial binding of rapamycin, a small molecule drug (Daringer et al., 2014). Each receptor carries two intracellular domains, one being an effector domain (typically a transcription factor) and the other being a sequence specific protease. When the two intracellular domains are brought into close proximity of one another, the protease domain cleaves a linker peptide sequence domain holding the transcription factor close the membrane, freeing it to translocate to the nucleus to execute a targeted change in transcription. Building off their work, Schwarz and colleagues extended the range of extracellular cues the MESA system was able to respond to by replacing the rapamycin binding domains with a split, single chain variable fragment receptor architecture. The split antibody receptor architecture dramatically expands the range of extracellular signals that can be used to selectively change the behavior of a designed gene circuit (Schwarz et al., 2017). Integrating the MESA and BLADE systems simply by replacing receptor bound transcription factors with recombinases may allow for a highly dynamic and scalable synthetic gene circuit platform that allows for tight control over developmental processes in vitro.
By combining a split dCas9-VP64 system with a similar transmembrane architecture to the one described in the MESA system, Baeumler and colleagues were able to create synthetic signaling routines based on extracellular cues and gRNA input. These engineered receptors, dubbed dCas9-SynR’s create AND gate logic and respond to extracellular cues in a dose dependent manner (Baeumler et al., 2017). In a recent effort, cell sensing and response were engineered by coupling dCas9 with GPCRs. This design could provide dose-dependent, reversible, and multiplexed control of gene expression (Kipniss et al., 2017) (Figure 3D).
Improving Cell and Context Specificity
In order to control the expression of CRISPR circuit elements, several strategies have been developed including the use of riboswitches, tissue specific miRNAs, and tissue specific or context dependent promoters. Placing CRISPR components under the control of RNA polymerase II driven tissue specific promoters (e.g. MHCK7 promoter for cardiac tissue, Albumin promoter for hepatocytes, etc.) can help spatially restrict genome engineering efforts only to target tissues (Gorski et al., 1986; Xu et al., 2017). Using an alternative approach, Nissim and colleagues employed miRNAs to repress the expression of effector components and suggest that integrating binding sites for tissue specific miRNAs from undesirable cells into expression cassettes helps differentially regulate gene circuit function (Nissim et al., 2014). For a more comprehensive review we refer readers to the review by Pineda and colleagues (Pineda et al., 2017). Additionally, combining cell and tissue specific regulatory components with high tropism AAV vectors (AAV8 for hepatocytes, as an example) will greatly help localize the expression of effector components even further and provide an enhanced layer of safety to in vivo transcriptional therapeutics.
Concluding Remarks
Transcriptional control over endogenous gene expression has been the holy grail of many scientific fields for decades. With the advent of RNA guided targeting of endogenous loci mediated by the CRISPR system, transcriptional control of endogenous genes has become significantly easier and more accessible to the scientific community. However, as it stands today, we have an incomplete picture of how exactly epigenomic and transcriptomic changes mediate phenotypic effects. There is simply not enough information available for a researcher to be able to pull down a gene of interest and reliably predict what functional regulatory elements influence its expression upon perturbations in a given human cell type or a specific context. Plus, our quantitative understanding of some of the kinetic parameters that are involved in chromatin regulation, maintenance, and the dynamics of CRISPR-DNA interaction in native environments remain in their infancy. This lack of predictive capacity makes it difficult to engineer the transcription of a target locus with complete assurance. Gaining comprehensive insights into molecular kinetic parameters and interactions can help in deciding which epigenetic modifiers, when, where, and to what degree should be used in order to mediate a desired phenotypic change.
From a clinical standpoint, CRISPR-mediated transcriptional control may provide a platform for powerful and deeply personalized therapeutics. Early work with dCas9 based synthetic transcription factors have shown great promise in preclinical models for treating diseases including Duchenne’s Muscular Dystrophy, Type 1 diabetes, and retinitis pigmentosa. While exciting and relatively simple to use, through this review we have covered several challenges that still need to be addressed before this technology reaches its full clinical potential. The advent of modified and Cas proteins along with the development of advanced delivery tools will likely provide an easier path to target human tissues. Engineering control in vivo over timing of action, tissue distribution, safety, and immunogenicity are still key objectives in this field. Controlling the dosage of AAV or CRISPR will be important for a sustained transcriptional modulation perhaps via avoiding aggressive patient immune responses (Chew et al., 2016). Recently, studies have reported existing humoral and cellular immune responses in a subset of human blood samples (Ferdosi et al., 2019; Vaidyanathan et al., 2018). However, it was shown that Cas9 protein is amenable to rational engineering while preserving its editing or transcriptional function (Ferdosi et al., 2019). It will be important to test these less immunogenic Cas9 variants in vivo for transcriptional modulation. Additionally, other approaches such as transient immunosuppression or personalized use of a type of CRISPR system based on immunoprofiling of a given patient can help for in vivo translation.
While the field is growing, integration with advances in other scientific areas such as synthetic biology will be critical to overcome challenges. Whereas animal studies are undoubtedly valuable, there is still a gap between these studies and human trials. Thus, the integration of human based in vitro micro-physiological systems including organoids, Organ-on-a-Chip technology, and multi-organ microphysiological platforms with CRISPR tools is critical to broaden our understanding of human specific effects that provides an accelerated and safer translation to patient populations. Such integration will contribute to more reliable assessments of safety, efficacy and host immune responses. Despite these remaining technological hurdles, CRISPR-based synthetic transcription factors are likely here to stay, as they provide a very promising, easy to use platform for investigating gene function, regulatory elements, and (perhaps one day) the management of complex human diseases.
Acknowledgments:
The authors wish to thank Dr. Samira Kiani for comments and critical discussions during the preparation of the manuscript. ME is supported by R01 EB024562 from NIH-NIBIB, R01HL141805 from NIH-NHLBI and Arizona Biomedical Research Council New Investigator Award (ADHS16-162402).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: The authors declare no competing interests.
References:
- Baeumler TA, Ahmed AA, and Fulga TA (2017). Engineering Synthetic Signaling Pathways with Programmable dCas9-Based Chimeric Receptors. Cell Rep 20, 2639–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerli R, Segal D, Dreier B, and Barbas C (1998). Toward controlling gene expression at will: Specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. PNAS 95, 14628–14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bintu L, Yong J, Antebi YE, McCue K, Kazuki Y, Uno N, Oshimura M, and Elowitz MB (2016). Dynamics of epigenetic regulation at the single-cell level. Science 351, 720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R, Buck M, and Wang B (2016). Recognizing and engineering digital-like logic gates and switches in gene regulatory networks. Current Opinion in Microbiology 33, 74–82. [DOI] [PubMed] [Google Scholar]
- Breinig M, Schweitzer AY, Herianto AM, Revia S, Schaefer L, Wendler L, Cobos Galvez A, and Tschaharganeh DF (2019). Multiplexed orthogonal genome editing and transcriptional activation by Cas12a. Nat Methods 16, 51–54. [DOI] [PubMed] [Google Scholar]
- Camp JG, Sekine K, Gerber T, Loeffler-Wirth H, Binder H, Gac M, Kanton S, Kageyama J, Damm G, Seehofer D, et al. (2017). Multilineage communication regulates human liver bud development from pluripotency. Nature 546, 533–538. [DOI] [PubMed] [Google Scholar]
- Chavez A, Scheiman J, Vora S, Pruitt BW, Tuttle M, E PRI, Lin S, Kiani S, Guzman CD, Wiegand DJ, et al. (2015). Highly efficient Cas9-mediated transcriptional programming. Nat Methods 12, 326–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew WL, Tabebordbar M, Cheng JK, Mali P, Wu EY, Ng AH, Zhu K, Wagers AJ, and Church GM (2016). A multifunctional AAV-CRISPR-Cas9 and its host response. Nat Methods 13, 868–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Zhou R, Kuo YC, Cunniff M, and Zhang F (2012). Comprehensive interrogation of natural TALE DNA-binding modules and transcriptional repressor domains. Nat Commun 3, 968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daringer NM, Dudek RM, Schwarz KA, and Leonard JN (2014). Modular extracellular sensor architecture for engineering mammalian cell-based devices. ACs Synth Biol 3, 892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio D (2015). Modularity, context-dependence, and insulation in engineered biological circuits. Trends Biotechnol 33, 111–119. [DOI] [PubMed] [Google Scholar]
- Del Vecchio D, Abdallah H, Qian Y, and Collins JJ (2017). A Blueprint for a Synthetic Genetic Feedback Controller to Reprogram Cell Fate. Cell Syst 4, 109–120 e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio D, Dy AJ, and Qian Y (2016). Control theory meets synthetic biology. J R Soc Interface 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi A, Wleklinski M, Spurgat M, Heiderscheit E, Kropornicka A, Vu C, Bhimsaria D, Swanson S, Stewart R, Ramanathan P, et al. (2016). Reprogramming cell fate with a genome-scale library of artificial transcription factors. PNAS 113, E8257–E8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdosi SR, Ewaisha R, Moghadam F, Krishna S, Park JG, Ebrahimkhani MR, Kiani S, and Anderson KS (2019). Multifunctional CRISPR-Cas9 with engineered immunosilenced human T cell epitopes. Nat Commun 10, 1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gander M, Vrana J, Voje W, Carothers J, and Klavins E (2017). Digital logic circuits in yeast with CRISPR-dCas9 NOR gates. Nature Communications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Xiong X, Wong S, Charles EJ, Lim WA, and Qi LS (2016). Complex transcriptional modulation with orthogonal and inducible dCas9 regulators. Nat Methods 13, 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti L, Siggers T, Tiana G, Caprara G, Notarbartolo S, Corona T, Pasparakis M, Milani P, Bulyk ML, and Natoli G (2010). Noncooperative interactions between transcription factors and clustered DNA binding sites enable graded transcriptional responses to environmental inputs. Mol Cell 37, 418–428. [DOI] [PubMed] [Google Scholar]
- Gorski K, Carneiro M, and Schibler U (1986). Tissue-specific in vitro transcription from the mouse albumin promoter. Cell 47, 767–776. [DOI] [PubMed] [Google Scholar]
- Gossen M, and Bujard H (1992). Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A 89, 5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton BE, Kennedy EM, Dumm RE, Harding AT, Sacco MT, Sachs D, and Heaton NS (2017). A CRISPR Activation Screen Identifies a Pan-avian Influenza Virus Inhibitory Host Factor. Cell Rep 20, 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd TJ, Feng J, Luo J, Yang P, Samineni VK, Gereau R.W.t., Kelley N, Hu H, and Spencer NJ (2018). Optogenetic Induction of Colonic Motility in Mice. Gastroenterology 155, 514–528 e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton IB, D'Ippolito AM, Vockley CM, Thakore PI, Crawford GE, Reddy TE, and Gersbach CA (2015). Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol 33, 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenot Y, V. G, Zhang L, Liu P-Q, Oshimura M, Feinberg AP, Wolffe AP, Ohlsson R, and Gregory PD (2003). Targeted regulation of imprinted genes by synthetic zinc-finger transcription factors. Gene Therapy 10, 513–522. [DOI] [PubMed] [Google Scholar]
- Kearns NA, Pham H, Tabak B, Genga RM, Silverstein NJ, Garber M, and Maehr R (2015). Functional annotation of native enhancers with a Cas9-histone demethylase fusion. Nat Methods 12, 401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani S, Beal J, Ebrahimkhani MR, Huh J, Hall RN, Xie Z, Li Y, and Weiss R (2014). CRISPR transcriptional repression devices and layered circuits in mammalian cells. Nat Methods 11, 723–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani S, Chavez A, Tuttle M, Hall RN, Chari R, Ter-Ovanesyan D, Qian J, Pruitt BW, Beal J, Vora S, et al. (2015). Cas9 gRNA engineering for genome editing, activation and repression. Nat Methods 12, 1051–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipniss NH, Dingal P, Abbott TR, Gao Y, Wang H, Dominguez AA, Labanieh L, and Qi LS (2017). Engineering cell sensing and responses using a GPCR-coupled CRISPR-Cas system. Nat Commun 8, 2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, Nishimasu H, et al. (2015). Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517, 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Chen Y, Guidotti A, and Grayson DR (2009). The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Mol Pharmacol 75, 342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon DY, Zhao YT, Lamonica JM, and Zhou Z (2017). Locus-specific histone deacetylation using a synthetic CRISPR-Cas9-based HDAC. Nat Commun 8, 15315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzillotta A, Pignataro G, Branca C, Cuomo O, Sarnico I, Benarese M, Annunziato L, Spano P, and Pizzi M (2013). Targeted acetylation of NF-kappaB/RelA and histones by epigenetic drugs reduces post-ischemic brain injury in mice with an extended therapeutic window. Neurobiol Dis 49, 177–189. [DOI] [PubMed] [Google Scholar]
- Levskaya A, Weiner OD, Lim WA, and Voigt CA (2009). Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461, 997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HK, Hatanaka F, Araoka T, Reddy P, Wu MZ, Sui Y, Yamauchi T, Sakurai M, O'Keefe DD, Nunez-Delicado E, et al. (2017). In Vivo Target Gene Activation via CRISPR/Cas9-Mediated Trans-epigenetic Modulation. Cell 171, 1495–1507 e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Dong L, Wang W, Liu Y, Huang W, and Cai Z (2016). An Efficient Light-Inducible P53 Expression System for Inhibiting Proliferation of Bladder Cancer Cell. Int J Biol Sci 12, 1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S, Shu J, Dadon D, Young RA, and Jaenisch R (2016). Editing DNA Methylation in the Mammalian Genome. Cell 167, 233–247 e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberbaum DS, and Barolo S (2013). Gene regulation: when analog beats digital. Curr Biol 23, R1054–1056. [DOI] [PubMed] [Google Scholar]
- Lu Y, Tian C, Danialou G, Gilbert R, Petrof BJ, Karpati G, and Nalbantoglu J (2008). Targeting artificial transcription factors to the utrophin A promoter: effects on dystrophic pathology and muscle function. J Biol Chem 283, 34720–34727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski J, and Pastinen T (2011). The study of eQTL variations by RNA-seq: from SNPs to phenotypes. Trends Genet 27, 72–79. [DOI] [PubMed] [Google Scholar]
- Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, and Church GM (2013). CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol 31, 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matharu N, Rattanasopha S, Tamura S, Maliskova L, Wang Y, Bernard A, Hardin A, Eckalbar WL, Vaisse C, and Ahituv N (2018). CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matharu N, Rattanasopha S, Tamura S, Maliskova L, Wang Y, Bernard A, Hardin A, Eckalbar WL, Vaisse C, and Ahituv N (2019). CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn DJ, Pradhan S, Kiani S, and Wang X (2018). Fluorescent Guide RNAs Facilitate Development of Layered Pol II-Driven CRISPR Circuits. ACS Synth Biol 7, 1929–1936. [DOI] [PubMed] [Google Scholar]
- Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, et al. (2011). A TALE nuclease architecture for efficient genome editing. Nat Biotechnol 29, 143–148. [DOI] [PubMed] [Google Scholar]
- Moreno AM, Fu X, Zhu J, Katrekar D, Shih YV, Marlett J, Cabotaje J, Tat J, Naughton J, Lisowski L, et al. (2018). In Situ Gene Therapy via AAV-CRISPR-Cas9-Mediated Targeted Gene Regulation. Mol Ther 26, 1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SL, Mariano NC, Bermudez A, Arruda NL, Wu F, Luo Y, Shankar G, Jia L, Chen H, Hu JF, et al. (2017). Manipulation of nuclear architecture through CRISPR-mediated chromosomal looping. Nat Commun 8, 15993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihongaki Y, Furuhata Y, Otabe T, Hasegawa S, Yoshimoto K, and Sato M (2017). CRISPR-Cas9-based photoactivatable transcription systems to induce neuronal differentiation. Nat Methods 14, 963–966. [DOI] [PubMed] [Google Scholar]
- Nihongaki Y, Yamamoto S, Kawano F, Suzuki H, and Sato M (2015). CRISPR-Cas9-based photoactivatable transcription system. Chem Biol 22, 169–174. [DOI] [PubMed] [Google Scholar]
- Nissim L, Perli SD, Fridkin A, Perez-Pinera P, and Lu TK (2014). Multiplexed and programmable regulation of gene networks with an integrated RNA and CRISPR/Cas toolkit in human cells. Mol Cell 54, 698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Patel N, Keung AJ, and Khalil AS (2019). Engineering Epigenetic Regulation Using Synthetic Read-Write Modules. Cell 176(1-2):227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pinera P, Kocak DD, Vockley CM, Adler AF, Kabadi AM, Polstein LR, Thakore PI, Glass KA, Ousterout DG, Leong KW, et al. (2013). RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat Methods 10, 973–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichon X, Lagha M, Mueller F, and Bertrand E (2018). A Growing Toolbox to Image Gene Expression in Single Cells: Sensitive Approaches for Demanding Challenges. Mol Cell 71, 468–480. [DOI] [PubMed] [Google Scholar]
- Pineda M, Moghadam F, Ebrahimkhani MR, and Kiani S (2017). Engineered CRISPR Systems for Next Generation Gene Therapies. ACS Synth Biol 6, 1614–1626. [DOI] [PubMed] [Google Scholar]
- Polstein LR, and Gersbach CA (2015). A light-inducible CRISPR-Cas9 system for control of endogenous gene activation. Nat Chem Biol 11, 198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell O, and Lu T (2014). Synthetic analog and digital circuits for cellular computation and memory. Current Opinion in Microbiology 29, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putri RR, and Chen L (2018). Spatiotemporal control of zebrafish (Danio rerio) gene expression using a light-activated CRISPR activation system. Gene 677, 273–279. [DOI] [PubMed] [Google Scholar]
- Sako K, Pradhan SJ, Barone V, Ingles-Prieto A, Muller P, Ruprecht V, Capek D, Galande S, Janovjak H, and Heisenberg CP (2016). Optogenetic Control of Nodal Signaling Reveals a Temporal Pattern of Nodal Signaling Regulating Cell Fate Specification during Gastrulation. Cell Rep 16, 866–877. [DOI] [PubMed] [Google Scholar]
- Schuijers J, Manteiga JC, Weintraub AS, Day DS, Zamudio AV, Hnisz D, Lee TI, and Young RA (2018). Transcriptional Dysregulation of MYC Reveals Common Enhancer-Docking Mechanism. Cell Rep 23, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz KA, Daringer NM, Dolberg TB, and Leonard JN (2017). Rewiring human cellular input-output using modular extracellular sensors. Nat Chem Biol 13, 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden A, Gregory P, Case C, and Pabo C (2002). December 23, 2002. Current Biology 12, 2159–2166. [DOI] [PubMed] [Google Scholar]
- Srivastava D, and DeWitt N (2008). In Vivo Cellular Reprogramming: The Next Generation Cell 166, 1386–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum ME, Gilbert LA, Qi LS, Weissman JS, and Vale RD (2014). A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 159, 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakore PI, Kwon JB, Nelson CE, Rouse DC, Gemberling MP, Oliver ML, and Gersbach CA (2018). RNA-guided transcriptional silencing in vivo with S. aureus CRISPR-Cas9 repressors. Nat Commun 9, 1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troudi A, Mahjoubi Samet A, and Zeghal N (2010). Hepatotoxicity induced by gibberellic acid in adult rats and their progeny. Exp Toxicol Pathol 62, 637–642. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan S, Azizian K, Haque AKM, Henderson J, Hendel A, Shore S, Antony J, Hogrefe R, Kormann M, Porteus M, et al. (2018). Uridine Depletion and Chemical Modification Increase Cas9 mRNA Activity and Reduce Immunogenicity without HPLC Purification. Mol Ther Nucleic Acids 12:530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Harst P, and Verweij N (2018). Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ Res 122(3):433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh S, and Workman JL (2015). Histone exchange, chromatin structure and the regulation of transcription. Nat Rev Mol Cell Biol 16, 178–189. [DOI] [PubMed] [Google Scholar]
- Wagner TE, Becraft JR, Bodner K, Teague B, Zhang X, Woo A, Porter E, Alburquerque B, Dobosh B, Andries O, et al. (2018). Small-molecule-based regulation of RNA-delivered circuits in mammalian cells. Nat Chem Biol 14, 1043–1050. [DOI] [PubMed] [Google Scholar]
- Wang H, Xu X, Nguyen CM, Liu Y, Gao Y, Lin X, Daley T, Kipniss NH, La Russa M, and Qi LS (2018). CRISPR-Mediated Programmable 3D Genome Positioning and Nuclear Organization. Cell 175, 1405–1417 e1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Allen WE, Wright MA, Sylwestrak EL, Samusik N, Vesuna S, Evans K, Liu C, Ramakrishnan C, Liu J, et al. (2018). Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg BH, Pham NTH, Caraballo LD, Lozanoski T, Engel A, Bhatia S, and Wong WW (2017). Large-scale design of robust genetic circuits with multiple inputs and outputs for mammalian cells. Nat Biotechnol 35, 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zhao L, Gao Y, Xu J, and Han R (2017). Empower multiplex cell and tissue-specific CRISPR-mediated gene manipulation with self-cleaving ribozymes and tRNA. Nucleic Acids Res 45, e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Tan X, Tampe B, Wilhelmi T, Hulshoff M, Saito S, Moser T, Kalluri R, Hasenfuss G, Zeisberg E, et al. (2018). High-fidelity CRISPR/Cas9- based gene-specific hydroxymethylation rescues gene expression and attenuates renal fibrosis. Nature Communications 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo NC, Chavez A, Lance-Byrne A, Chan Y, Menn D, Milanova D, Kuo CC, Guo X, Sharma S, Tung A, et al. (2018). An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat Methods 15, 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalatan JG, Lee ME, Almeida R, Gilbert LA, Whitehead EH, La Russa M, Tsai JC, Weissman JS, Dueber JE, Qi LS, et al. (2015). Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell 160, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan H, Zhou Q, Gao Q, Li J, Huang W, and Liu Y (2019). Multiplexed promoterless gene expression with CRISPReader. Genome Biol 20, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Cong L, Lodato S, Kosuri S, Church GM, and Arlotta P (2011). Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat Biotechnol 29, 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Shen W, Zhang J, Yang B, Liu YN, Qi H, Yu X, Lu SY, Chen Y, Xu YZ, et al. (2018). CRISPR interference-based specific and efficient gene inactivation in the brain. Nat Neurosci 21, 447–454. [DOI] [PubMed] [Google Scholar]
- Zhou H, Liu J, Zhou C, Gao N, Rao Z, Li H, Hu X, Li C, Yao X, Shen X, et al. (2018). In vivo simultaneous transcriptional activation of multiple genes in the brain using CRISPR-dCas9-activator transgenic mice. Nat Neurosci 21, 440–446. [DOI] [PubMed] [Google Scholar]