Abstract
The identification of proteins that bind selectively to nucleic acid sequences is an ongoing challenge. We previously synthesized nucleobase amino acids designed to replace proteinogenic amino acids; these were incorporated into proteins to bind specific nucleic acids predictably. An early example involved selective cell free binding of the hnRNP LL RRM1 domain to its i-motif DNA target via Watson-Crick-like H-bonding interactions. In this study, we employ the X-ray crystal structure of transcriptional regulator Rob bound to its micF promoter, which occurred without DNA distortion. Rob proteins modified in vivo with nucleobase amino acids at position 40 exhibited altered DNA promoter binding, as predicted on the basis of their Watson-Crick-like H-bonding interactions with promoter DNA A-box residue Gua-6. Rob protein expression ultimately controls phenotypic changes, including resistance to antibiotics. Although Rob proteins with nucleobase amino acids were expressed in Escherichia coli at levels estimated to be only a fraction of that of the wild-type Rob protein, those modified proteins that bound to the micF promoter more avidly than the wild type in vitro also produced greater resistance to macrolide antibiotics roxithromycin and clarithromycin in vivo, as well as the β-lactam antibiotic ampicillin. Also demonstrated is the statistical significance of altered DNA binding and antibiotic resistance for key Rob analogues. These preliminary findings suggest the ultimate utility of nucleobase amino acids in altering and controlling preferred nucleic acid target sequences by proteins, for probing molecular interactions critical to protein function, and for enhancing phenotypic changes in vivo by regulatory protein analogues.
Bacterial transcriptional regulator Rob belongs to the AraC/XylS family, structurally homologous with family members MarA and SoxS.1–6 These contain similar helix-turn-helix motifs that might mediate specific DNA binding7 using similar sequences. The best studied sequence is the Mar/Sox/Rob box,8 present in the promoter regions of many genes encoding proteins. These proteins are responsible for preventing potentially threatening conditions, including oxidative stress, antibiotic inhibition, heavy metals, and acidic pH.2–6 They often exhibit strongly overlapping functions, but differentiated mechanisms have been detected.6–11 Thus, differences in environmental activating signals have been observed.12–14 In some bacteria, each regulates the same genes and facilitates cellular processes, including efflux pump upregulation and porin synthesis downstream regulation,10,15–18 but the Rob mechanism is sometimes unique. Only Rob, e.g., is expressed constitutively,3,19 and >104 Rob copies can be present in a single bacterium.4 Additionally, MarA and SoxS (15.2 and 12.9 kDa, respectively) are much smaller than Rob (33.1 kDa) and lack the Rob C-terminal domain. Some crosstalk between Rob and regulators MarA and SoxS has been found and depends on the environment.4
The crystal structure of Rob complexed to the micF promoter showed that two helix-turn-helix (HTH) motifs of the N-terminal domain were involved in DNA binding;20 the mechanism was similar to those of regulators SoxS and MarA. All three bind to an ~ 20 bp DNA sequence, part of the promoter region of the regulated gene.20 These binding sites represent two conserved sequences, box A and box B. In the crystal structure, Trp36 has a van der Waals contact with Cyt-7, whereas Arg40 can form a hydrogen bond with the Gua-6 O6 atom. The interactions between Rob and the A-box are in good agreement with data found during the crystal structure study of MarA in complex with its promoter.21 MicF RNA, a small inhibitory RNA, regulates synthesis of the outer membrane porin OmpF, which is involved in a multidrug resistance mechanism.22,23 A number of multidrug efflux pumps are found in Gram-negative bacteria,24 but macrolide efflux in Gram-negative bacteria such as Escherichia coli mainly involves Acr-AB-TolC, an ABC transporter.17,25,26 TolCP3, a promoter for the acrAB operon regulating porin OmpC synthesis, has a similar two-box sequence.27
No Rob-tolCP3 crystal structure has been reported, but on the basis of structural similarities to micF, it seems likely that Rob binds both promoters analogously. We have employed proteins containing nucleobase amino acids (e.g., 1–5) to study such interactions, enabling nucleic acid recognition using familiar interactions such as base pairing. Previously, in a quite different cell free system, this approach provided inferences regarding the interaction of a nucleic acid binding motif (RRM1) of transcriptional factor hnRNP LL with its i-motif DNA substrate.28 Here, the nucleobase-containing Rob proteins have been produced in vivo; their functions were studied both in vitro and in vivo. The same four nucleobases were purposefully used in addition to 5; the crystal structure employed here for experimental design potentially enables validation of both studies.
In this work, we prepared Rob constructs based on the structure of a micF–Rob complex.20 Key amino acid Arg40 (first HTH motif), predicted to be H-bonded to the O6 atom of Gua-6 of the micF A-box, was replaced with other amino acids, including nucleobase amino acids (1–5) (Figure 1) as well as phenylalanine and leucine. All Rob samples were purified by Strep-Tactin chromatography and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Figure S1) and mass spectrometry of two modified Rob proteins (Table S1).
Figure 1.

Structures of nucleobase amino acids (1–5) as well as phenylalanine and leucine incorporated at position 40 of Rob in cellulo by nonsense codon suppression.
Our modeling using measurements from Protein Data Bank entry 1D5Y20 suggested that the substitution of amino acids 1 and 2 in lieu of Arg40, whose side chain is not fully extended in the crystal structure, would orient the exocyclic N4 atom of their side chains in about the same position relative to O6 of Gua-6 as the guanidine moiety of Arg40, thus enabling H-bonding. Rob samples with amino acids 3 and 4 would not have access to this H-bonding mode, nor would the Leu and Phe side chains; these were studied as controls. All modified Rob samples were analyzed using an electrophoretic mobility shift assay (EMSA) in the presence of one of two promoter DNA substrates, i.e., micF and tolCP3 (Table 1). First, the DNA binding ability of wild-type Rob for both promoters was compared from 0 to 250 nM Rob (Figure 2). Wild-type Rob (Rob-wt) bound to both DNA substrates, but its affinity for micF was ~ 4 times greater than for tolCP3 (Table 1 and Figure 2). The DNA binding affinities of the five Rob proteins having nucleobase amino acids at position 40 were then compared with that of wild-type Rob (Table 1 and Figures S2–S4). Not surprisingly, Rob40–1 with cytosine base amino acid 1 in position 40 had the strongest affinity, possibly because full Watson–Crick base pairing can be involved in this observed strong interaction (BC50 values of 42 and 190 nM for micF and tolCP3 DNA, respectively). Its stronger affinity (apparent in Table 1) was reinforced by the EMSA data showing DNA binding at lower concentrations than Rob-wt (Figure S2). Rob40–2 protein also exhibited reasonable binding affinity for the two DNA promoter regions (BC50 values of 53 and 200 nM, respectively). Amino acid 2 has an exocyclic amino group in the same position as 1, enabling H-bond formation with the Gua-6 O6 atom, and H-bond interactions between the ring N atoms of Gua-6 and amino acid 2. Rob40–3, which has a uracil base amino acid, can plausibly also form a H-bond with Gua-6 by wobble base pairing (Figure S5), but this binding is ~ 2–3 times weaker than for Rob40–1 [BC50 values of 104 and 410 nM for micF and tolCP3 DNA, respectively (Table 1)].
Table 1.
Comparison of Binding of Different Rob Samples with Mutations at Position 40 with micF and tolCP3 DNA Substratesa
| DNA binding, BC50 (nM) | ||
|---|---|---|
| Rob sample | micF | tolCP3 |
| Rob-wt | 63 ± 11 | 240 ± 50 |
| Rob40-1 | 42 ± 5 | 190 ± 40 |
| Rob40-2 | 53 ± 8 | 200 ± 30 |
| Rob40-3 | 104 ± 13 | 410 ± 30 |
| Rob40-4 | 250 ± SO | >500 |
| Rob40-5 | 206 ± 4 | |
| Rob40-Phe | 410 ± 15 | |
| Rob40-Leu | >400 | |
Rob40-1 vs Rob-wt: p < 0.05. Rob40-2 vs Rob-wt: p > 0.05.
Figure 2.

EMSA analysis of the binding of wild-type Rob to micF and tolCP3 DNA promoters. The sequences shown are the strands of the promoter duplexes putatively recognized by Rob.
To demonstrate that Gua-6 of the A-box contributes to the strong interaction with Arg40 of Rob, the properties of a micF mutant, micFmutA, in which the GC sequence at micF DNA positions 6 and 7 was changed to AT were studied by an EMSA (Figure 3). The promoter analogue containing AT in lieu of GC greatly diminished wild-type Rob affinity (BC50 of 2500 nM vs a BC50 of 63 nM for unmodified micF). Significantly, for Rob40-3, binding activity with micFmutA DNA was recovered ~ 5-fold due to AT binding [BC50 of ~ 450 nM vs a BC50 of 2500 nM for Rob-wt (Figure 3)]. This finding underscores the importance of DNA promoter nucleoside-6 in the interaction of micF with Rob, as suggested by the structure of the Rob-micF complex.20
Figure 3.

EMSA comparative analysis of binding of Rob-wt and Rob40–3 to mutant micFmutA DNA.
Amino acid 4 at Rob position 40 has a p-nitro substituent on an aromatic nucleus, which should preclude strong Watson-Crick H-bonding to Gua-6, but this was apparent for only tolCP3 DNA. No affinity for tolCP3 DNA was found for this Rob mutant (Figure S4). Comparatively, for micF promoter DNA, Arg40 substitution by 4 diminished binding affinity, but only ~ 4-fold (Table 1). To obtain additional insights into the apparent affinity of Rob40–4 for micF promoter DNA, we prepared Rob40 protein constructs containing phenylalanine and leucine. While substitution of Arg40 with Leu completely eliminated any detectable affinity for micF DNA, supporting the importance of position 40 for DNA binding, the Rob40–Phe mutant exhibited weak binding [BC50 = 410 nM (Table 1)]. One plausible explanation for the apparent affinity of Rob40–4 and Rob40–Phe for micF DNA may involve weak stacking interactions. These may be the first observations of stacking of an aromatic residue in a nucleobase amino acid-containing protein with a DNA nucleobase, but this stacking is weak at best relative to the binding observed for Rob40–1 and Rob40–2. Also studied was Rob40–5, which retained some affinity for micF DNA, due either to H-bonding to the DNA in spite of the altered nucleobase connectivity or to weak stacking. Minimal base stacking by 5 supports the interpretation that this process is not involved in the enhanced binding of Rob containing 1 or 2.
Rob regulation of porins OmpF and OmpC synthesis has been demonstrated25–27,29 and is linked to the appearance of an antibiotic-resistant bacterial phenotype. Many publications connect tolC expression with bacterial resistance to macro-lides.27,29 The assembly of TolC into efflux complexes, stimulated by acidic pH, could explain the increased sensitivity of Gram-negative bacteria to macrolides at pH >7.29
One effect of nucleobase amino acids at Rob position 40 was studied in vivo in a medium containing the macrolide antibiotic roxithromycin (Rox). A set of cultures, overexpressing modified Rob proteins, including those having amino acids 1–4 at position 40, was prepared and compared with control cultures having Arg (wt), Leu, and Phe at the same position. E. coli cultures were prepared in strain BL-21(DE-3)-Pyl, expressing the PylRS-tRNAPyl orthogonal pair from plasmid pTECH-Pyl-OP, by transformation with pETRob-wt, pETRob40-Leu, and pETRob40-TAG plasmids. These were grown in the absence (for pETRob-wt and pETRob40-Leu) and presence of 2 mM (nucleobase) amino acids (Phe and 1–4). Culture BL-21(DE-3)-Pyl-pET28b, without Rob expression, was the negative control. Roxithromycin sensitivity was determined in LB medium (pH 8.9); under this condition, E. coli cells without Rob overexpression demonstrated the greatest sensitivity to Rox (Figure S6). All cultures were tested under the same conditions with different antibiotic concentrations (1.56–25 μg/mL) affording inhibitor IC50 values (Figure 4).
Figure 4.
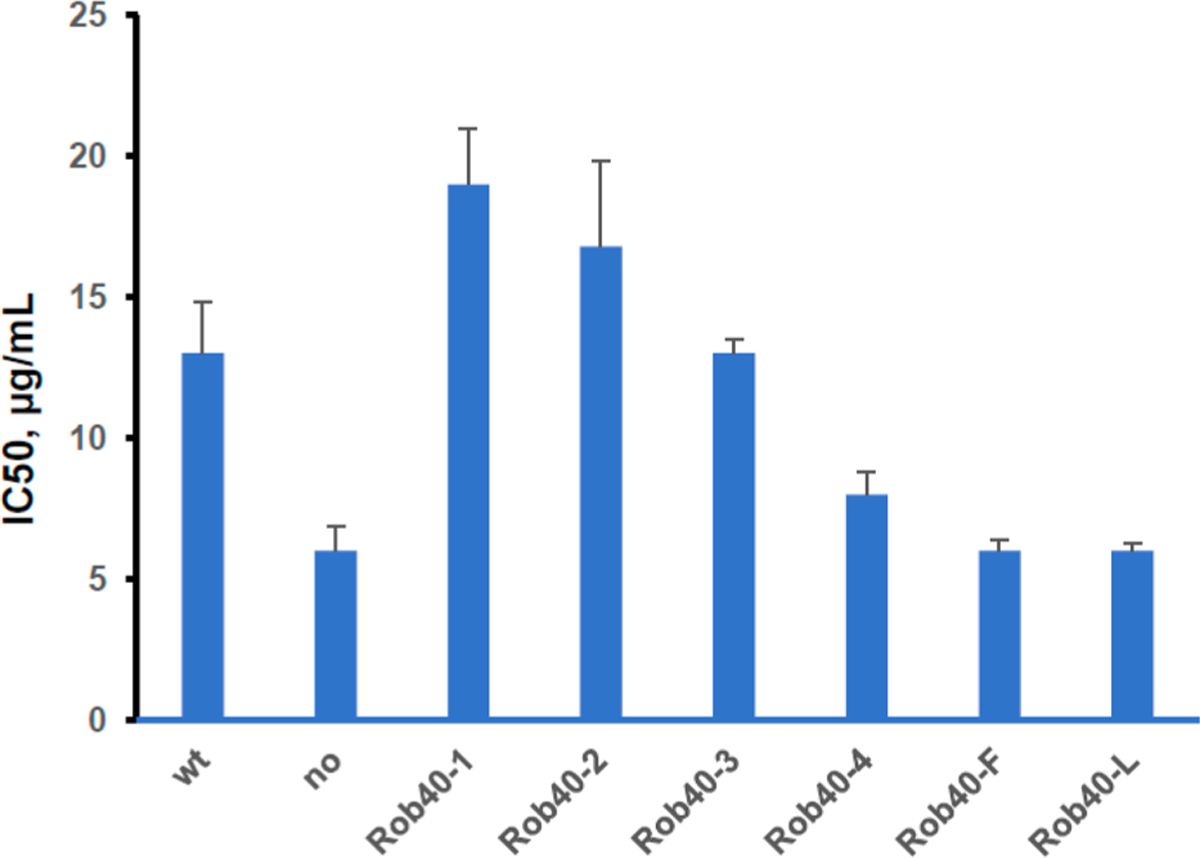
Bacterial growth inhibition by roxithromycin (pH 8.9) in cultures overexpressing Rob-wt protein or suppression of a Rob gene having a TAG codon at protein position 40 by a specific amino acid. Abbreviations: wt, wild type; no, no Rob protein sequence in the vector; Rob40–1, Rob40–2, Rob40–3, Rob40–4, Rob40–F, and Rob40–L, Rob40 with nucleobase amino acids 1, 2, 3, 4, Phe, and Leu at position 40 instead of Arg, respectively. Rob40–1 vs Rob-wt: p < 0.05. Rob40–2 vs Rob-wt: p > 0.05.
Wild-type Rob overexpression resulted in an ~ 2-fold decreased sensitivity of E. coli to Rox [IC50 of 15 ± 2 μg/mL vs 7 ± 0.8 μg/mL (Figure 4)]. The greatest Rox resistance occurred for BL-21(DE-3)-Pyl-Rob40–1, which overexpressed modified Rob containing amino acid 1 (IC50 of 19 ± 2 μg/mL vs an IC50 of 13 ± 2 μg/mL for Rob-wt). Cultures overexpressing Rob40–2 and Rob40–3 had Rox sensitivity comparable to that of Rob-wt (IC50 values of 16 ± 5 and 13 ± 0.5 μg/mL, respectively) but ~ 2-fold lower than that of a control incapable of Rob overexpression. This correlated with the EMSA binding data for micF and tolCP3 substrates (Table 1). The culture containing Rob40–2 was also more resistant to the effect of clarithromycin than Rob-wt (Figure S7), and more substantial resistance was apparent versus ampicillin (Figure S8). While some of these differences are seemingly small, it should be appreciated that limitations in nonsense codon suppression by suppressor tRNAs (e.g., tRNAPyl) are such that the cellular concentrations of the modified Rob proteins containing 1–4 were almost certainly several-fold lower than those containing proteinogenic amino acids (Table S2).
Three other tested cultures, overexpressing Rob40–4, Rob40–Leu, and Rob40–Phe, were all sensitive to Rox (IC50 values of 6 ± 0.4, 8 ± 0.8, and 6 ± 0.3 μg/mL, respectively), comparable to the culture incapable of Rob overexpression. Thus, the data from the in vivo experiments differ from the in vitro EMSA data, in which Rob40–4 and Rob40–Phe may have interacted with tolCP3 (Rob40–4) and micF (both) by weak stacking interactions. It may be noted that the in vivo structures of DNA promoters, responsible for Rob activation of macrolide resistance, are different from those of the in vitro DNA substrate.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Prof. Dieter Söll for plasmid pTECH-Pyl-OP.
Funding
This study was supported by National Institute of General Medical Sciences Grant GM12367.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.biochem.0c00103
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.biochem.0c00103.
Tables, figures, and experimental methods for preparing and studying protein with nucleobase amino acids (PDF)
Accession Codes
UniProtKB entry for E. coli Rob, P0AC10.
REFERENCES
- (1).Martin RG, and Rosner JL (2002) Genomics of the marA/soxS/rob regulon in Escherichia coli: identification of directly activated promotors by application of molecular genetics and informatics in microarray data. Mol. Microbiol 44, 1611–1624. [DOI] [PubMed] [Google Scholar]
- (2).Tobes R, and Ramos JL (2002) AraC-XylS database: a family of positive transcriptional regulators in bacteria. Nucleic Acids Res. 30, 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Skarstad K, Thony B, Hwang DS, and Kornberg A (1993) A novel binding protein of the origin of the Escherichia coli chromosome. J. Biol. Chem 268, 5365–5370. [PubMed] [Google Scholar]
- (4).Jain K, and Saini S (2016) MarRA, SoxSR and Rob encode a signal dependent regulatory network in Escherichia coli. Mol. BioSyst 12, 1901–1912. [DOI] [PubMed] [Google Scholar]
- (5).Chubiz LM, Glekas GD, and Rao CV (2012) Transcriptional cross talk within the mar-sox-rob regulon in Escherichia coli is limited to the rob and marRAB operons. J. Bacteriol 194, 4867–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Martin RG, and Rosner JL (2001) The AraC transcriptional activators. Curr. Opin. Microbiol 4, 132–137. [DOI] [PubMed] [Google Scholar]
- (7).Ariza RR, Li Z, Ringstad N, and Demple B (1995) Activation of multiple antibiotic resistance and binding of stress-inducible promoters by Escherichia coli Rob protein. J. Bacteriol 177, 1655–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Martin RG, Gillette WK, Rhee S, and Rosner JL (1999) Structural requirements for marbox function in transcriptional activation of mar/sox/rob regulon promoters in Escherichia coli: sequence, orientation and spatial relationship to the core promoter. Mol. Microbiol 34, 431–441. [DOI] [PubMed] [Google Scholar]
- (9).Li XZ, and Demple B (1996) Sequence specificity for DNA binding by Escherichia coli SoxS and Rob proteins. Mol. Microbiol 20, 937–945. [DOI] [PubMed] [Google Scholar]
- (10).Martin RG, Bartlett ES, Rosner JL, and Wall ME (2008) Activation of the Escherichia coli marA/soxS/rob regulon in response to transcriptional activator concentration. J. Mol. Biol 380, 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Asako H, Nakajima H, Kobayashi K, Kobayashi M, and Aono R (1997) Organic solvent tolerance and antibiotic resistance increased by overexpression of marA in Escherichia coli. Appl. Environ. Microbiol 63, 1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Rosner JL, Dangi B, Gronenborn AM, and Martin RG (2002) Posttranscriptional activation of the transcriptional activator Rob by dipyridyl in Escherichia coli. J. Bacteriol 184, 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Rosenberg EY, Bertenthal D, Nilles ML, Bertrand KP, and Nikaido H (2003) Bile salts and fatty acid induce expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol. Microbiol 48, 1609–1619. [DOI] [PubMed] [Google Scholar]
- (14).Pomposiello PJ, Bennik MH, and Demple B (2001) Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J. Bacteriol 183, 3890–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Ali Azam T, Iwata A, Nishimura A, Ueda S, and Ishihama A (1999) Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J. Bacteriol 181, 6361–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Barbosa TM, and Levy SB (2000) Differential expression of over 60 chromosomal genes in Escherichia coli by constitutive expression of MarA. J. Bacteriol 182, 3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Okusu H, Ma D, and Nikaido H (1996) AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol 178, 306–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Clubiz LM, and Rao CV (2011) Role of the mar-sox-rob regulon in regulating outer membrane porin expression. J. Bacteriol 193, 2252–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Griffith KL, Fitzpatrick MM, Keen EF III, and Wolf RE (2009) Two functions of the C-terminal domain of Escherichia coli Rob: mediating “sequestration-dispersal” as a novel off-on switch for regulating Rob’s activity as transcription activator and preventing degradation of Rob by Lon protease. J. Mol. Biol 388, 415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Kwon HJ, Bennik MH, Demple B, and Ellenberger T (2000) Crystal structure of the Escherichia coli Rob transcriptional factors in complex with DNA. Nat. Struct. Biol 7, 424–430. [DOI] [PubMed] [Google Scholar]
- (21).Rhee S, Martin RG, Rosner JL, and Davies DR (1998) A novel DNA binding motif in Mar A: the first structure for an AraC family transcriptional activator. Proc. Natl. Acad. Sci. U. S. A 95, 10413–10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Delihas N, and Forst S (2001) MicF: an antisense RNA gene involved in response of Escherichia coli to global stress factors. J. Mol. Biol 313, 1–12. [DOI] [PubMed] [Google Scholar]
- (23).Vogel J, and Papenfort K (2006) Small non-coding RNAs and the bacterial outer membrane. Curr. Opin. Microbiol 9, 605–611. [DOI] [PubMed] [Google Scholar]
- (24).Nikaido H (1996) Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol 178, 5853–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Pratt LA, Hsing W, Gibson KE, and Silhavy TJ (1996) From acids to osmZ: multiple factors influence synthesis on the OmpF and OmpC porins in Escherichia coli. Mol. Microbiol 20, 911–917. [DOI] [PubMed] [Google Scholar]
- (26).Tikhonova EB, and Zgurskaya HI (2004) AcrA, AcrB, and TolC of Escherichia coli form a stable intermembrane multidrug efflux complex. J. Biol. Chem 279, 32116–32124. [DOI] [PubMed] [Google Scholar]
- (27).Zhang A, Rosner JL, and Martin RG (2008) Transcriptional activation by MarA, SoxS and Rob of two tolC promotors using one binding site: a complex promoter configuration for tolC in Escherichia coli. Mol. Microbiol 69, 1450–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Bai X, Talukder P, Daskalova SM, Roy B, Chen S, Li Z, Dedkova LM, and Hecht SM (2017) Enhanced binding affinity for an i-motif DNA substrate exhibited by a protein containing nucleobase amino acids. J. Am. Chem. Soc 139, 4611–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Tikhonova EB, Dastidar V, Rybenkov VV, and Zgurskaya HI (2009) Kinetic control of TolC recruitment by multidrug efflux complexes. Proc. Natl. Acad. Sci. U. S. A 106, 16416–16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


