Abstract
Resident memory T (Trm) cells stably occupy tissues and cannot be sampled in superficial venous blood. Trm cells are heterogeneous but collectively constitute the most abundant memory T cell subset. Trm cells form an integral part of the immune sensing network, monitor for local perturbations in homeostasis throughout the body, participate in protection from infection and cancer, and likely promote autoimmunity, allergy, and inflammatory diseases and impede successful transplantation. Thus Trm cells are major candidates for therapeutic manipulation. Here we review CD8+ and CD4+ Trm ontogeny, maintenance, function, and distribution within lymphoid and nonlymphoid tissues and strategies for their study. We briefly discuss other resident leukocyte populations, including innate lymphoid cells, macrophages, natural killer and natural killer T cells, nonclassical T cells, and memory B cells. Lastly, we highlight major gaps in knowledge and propose ways in which a deeper understanding could result in new methods to prevent or treat diverse human diseases.
Keywords: T lymphocytes, memory, residence, immunity, lymphoid cells
INTRODUCTION
Blood and lymphoid tissues were the primary anatomic compartments in which lymphocyte biology was initially defined, which in turn provided the foundation for our modern conceptualization of how adaptive immunity works. However, much of the immune system does not traffic through blood or lymphoid organs and has thus remained hidden from many analyses. These less accessible, or resident, cell populations play critical roles in immune surveillance, protective immunity, and organ homeostasis. Further understanding their biology represents an opportunity for those wishing to prime immune responses against infection and cancer, and for those endeavoring to quiet pathological responses. To date, much of the emphasis on resident lymphocyte biology has focused on T cells.
T cell biology is shaped by their primary objective: target localization. In the 1960s, James Gowans demonstrated that many of the abundant populations of small lymphocytes that enter blood each day from the thoracic duct lymph recirculate through lymph nodes (LNs) (1, 2). We now understand that naive T cells are clonally diverse and there are only a handful of naive T cells specific for a particular foreign antigen. Moreover, T cells are tactile and require host cell contact for antigen recognition. In the setting of infection, rapid target recognition becomes a unique biological problem, as the infection could be contained among any of the ~40 trillion cells throughout the body (3). It thus makes sense that naive T cells pursue a restricted yet nomadic recirculation program that (a) takes advantage of the antigen collecting properties of secondary lymphoid organs (SLOs), which drain peripheral tissues or filter blood, (b) reduces the area needed for immune surveillance, because antigens come to the naive T cell, and also (c) permits transient sampling of different SLOs that drain each region of the body.
While naive immune surveillance strategies increase efficiency, primary responses are still relatively slow to develop because naive T cells specific for a particular antigen (a) are very scarce, (b) have to wait until peripheral antigen comes to them in the SLO, (c) must undergo extensive proliferation to be numerically relevant, and (d) must migrate to relevant sites to exert effector functions. In the context of primary infection, productive activation of naive T cells induces profound clonal expansion. Cells undergo upwards of 20 cell divisions within a few days. This provides the large army of specific effectors required to scan the vast volume of the organism to locate host cells harboring pathogen. After infected cells are eliminated, most of the expanded T cell population is lost. However, a functionally and phenotypically diverse fraction of cells survives and is maintained. These so-called memory cells are more abundant than their naive counterparts prior to infection, and this numerical expansion affords the luxury of broader surveillance.
At the time of Gowans & Knight’s (1) and Gowans’ (2) studies, no distinction was made between naive and memory T cells. Some hint of heterogeneity among lymphocyte recirculation pathways was provided by Cahill et al. (4), who collected lymphocytes from the afferent and efferent lymph of sheep and observed that, when injected back into the blood, transferred cells were more likely to be recovered from the same cannula. Mackay et al. (5, 6) extended these findings by showing that while the popliteal efferent lymph contained both naive and antigen-experienced T cells, only antigen-experienced cells could be recovered from the afferent lymph. Since the popliteal lymph node drains the sheep hindquarter, which lacks upstream SLOs, these studies were interpreted to signify that memory T cells have the capacity to constitutively exit peripheral nonlymphoid tissues via lymphatic vessels and, presumably, recirculate back using blood as a conduit.
Through much of the 1980s and early 1990s, elegant studies deepened our understanding of how different T cell populations use combinations of selectins, chemokine receptors, and integrins to target their entry into specific tissues. LN entry was found to depend on CD62L and CCR7 (7, 8). CLA+ cells were rare in blood but abundant at sites of skin inflammation (9), leading to the identification of a role for CLA in migration to skin (10). Homing to the small intestine was found to depend on α4β7, and later CCR9 (11, 12). This culminated in the elegant hypothesis that T cell trafficking could be meticulously controlled through combinatorial expression of a variety of homing molecules on the surface of lymphocytes. Every tissue could have a specific “area code,” and T cells could be dialed in to traffic to, and recirculate through, very specific regions of the body (13, 14). Such biasing of immune surveillance could increase efficiency by focusing on previous sites of infection rather than uninvolved sites (15, 16).
Sallusto et al. (17) built on these concepts in a study that further shaped conceptualization of T cell immune surveillance. They divided antigen-experienced CD8 and CD4 T cells on the basis of the expression of the SLO homing molecule CCR7 and compared their phenotype and function. CD45RA−CCR7+ cells, referred to as central memory T (Tcm) cells, were adept at proliferation, but not effector functions. CCR7− cells, referred to as effector memory T (Tem) cells, maintained rapidly executable effector functions and increased expression of putative peripheral homing chemokine receptors and integrins, but they exhibited shortened telomeres (17). These observations were synthesized into a highly influential model in which T cell responses were diver-sified into subsets with functional specialization. One interpretation is that those cells collected by Mackay et al. (5), from afferent lymph, were Tem cells, responsible for peripheral immune surveillance and immediate protective functions. Tcm cells, in contrast, putatively constituted a reserve force that patrolled SLOs, and retained the capacity to proliferate and expand the effector or Tem pool if called upon during reinfection. Thus, this study insightfully proposed relationships between migration patterns, function, stimulation history, and developmental plasticity.
Importantly, Sallusto et al. (17) limited analysis to human blood; they did not test migration to (or through) nonlymphoid tissues. Subsequent studies identified abundant memory T cells of known specificity within nonlymphoid tissues of mice (18–22). And not only because they lacked the SLO homing receptor CD62L, but because they expressed enhanced effector functions relative to their counterparts isolated from SLOs (including memory cells in spleen that lacked CD62L expression), they were referred to as Tem cells (19, 20). Of note, phenotypic discrepancies between memory CD8 T cells isolated from different locations indicated that peripheral immune surveillance could not be extrapolated from the phenotype of blood-borne lymphocytes. As discussed throughout the remainder of this review, we now understand that most T cells within nonlymphoid tissues are parked within the tissue, do not recirculate, and thus constitute a pool separate from those found in venous blood (Figure 1, Table 1). Nonrecirculating memory T cells are now referred to as resident memory T cells (often abbreviated Trm cells) to remove ambiguous conflation with recirculating Tem cells. Many other leukocyte lineages have the capacity for residence, including innate lymphoid cells (ILCs), macrophages, natural killer (NK) cells, memory B cells, and plasma cells. Indeed, for many leukocyte subsets, residence is more the norm than a lifestyle of recirculation.
Figure 1.
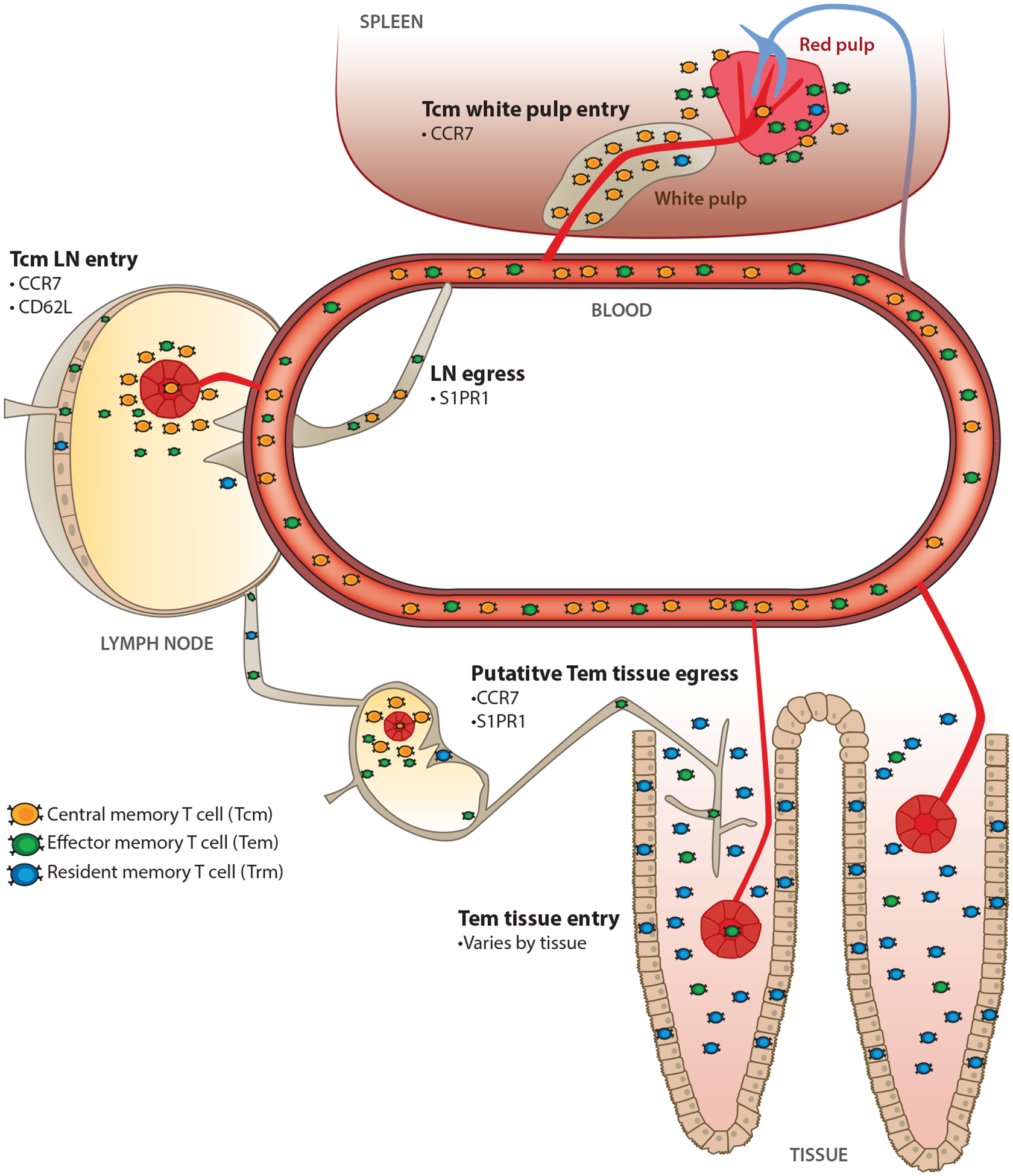
Memory T cell migration properties. Memory T cells can be classified into subsets based on their migration patterns. Central memory T (Tcm) cells recirculate through blood and constitutively migrate through lymph nodes (LNs) using high endothelial venules. Effector memory T (Tem) cells are also found in blood and exhibit diverse migration properties, but they do not enter uninflamed lymph nodes via high endothelial venules. Resident memory T (Trm) cells do not recirculate and remain local occupants of tissues during the steady state. See Table 1 for distinct and overlapping characteristics of Tcm, Tem, and Trm cells.
Table 1.
Distinct and overlapping characteristics of Tcm, Tem, and Trm cells
| Can cross HEV | Can be sampled in blood | Migrate to inflamed NLT | Recirculate through uninflamed NLT | Dominant surveyor of NLT | |
|---|---|---|---|---|---|
| Tcm | Yes | Yes | Yes | Limited evidence, but likely rare | No |
| Tem | No | Yes | Yes | Yes (some subsets) | No |
| Trm | No | No | No | No | Yes |
Abbreviations: HEV, high endothelial venule; NLT, nonlymphoid tissue; Tcm, central memory T; Tem, effector memory T; Trm, resident memory T.
The implication of residence is that most of the immune system remains hidden from view if we restrict studies to easily accessible blood. Moreover, we could view the immune system as an integrated component of almost any given tissue or organ. As such, leukocytes must be in tune with distinct local environments, which vary in available nutrients, O2, pH, blood supply, matrix proteins, parenchymal cell types, and myriad other relevant physiological factors. In turn, durable occupancy provides an opportunity for cells to adapt to and monitor distinct locales. This surveillance role may extend beyond detection of infections and include monitoring for other perturbations of homeostasis. This perspective more broadly encompasses the immune system’s involvement in metabolism, inflammatory disease, cancer, wound repair, and vascular and cognitive diseases, as well as a conventional role in control of foreign agents. Understanding the biology of resident leukocyte populations, how to tractably measure them, and strategies to manipulate their formation, function, or retention should create opportunities for treatment of a range of diseases. This field is in its relative infancy but is currently witnessing very rapid growth. This review summarizes major concepts and discoveries, with an emphasis on T cells but inclusion of other resident immune populations, and highlights critical knowledge gaps and future directions.
EVIDENCE FOR RESIDENT MEMORY T CELLS
It was long known that lymphocytes are abundant in the intestinal mucosa, which is also a site of prodigious antibody secretion. Here, T cells appear effector-like morphologically, exhibit activity in a redirected cytolytic assay, and express CD69, which was generally thought to indicate recent T cell receptor (TCR) stimulation (23, 24). Based on these observations, it was proposed that CD8αβ TCRαβ intestinal T cells were recently activated effectors, engaged in ongoing immune responses against the microbiota and intestinal pathogens, or that the gut constituted a graveyard where effector T cells throughout the body went to die (25). However, direct ex vivo methods for detecting antigen-specific T cells (e.g., MHC-I tetramers or adoptive transfer of TCR transgenic T cells) allowed one to address context and indicated that pathogen-specific T cells persisted in the intestine long after pathogen had putatively been cleared. Thus, these cells fit the operational definition of memory cells. However, they still expressed constitutive ex vivo cytolytic activity and maintained CD69 and granzyme B expression, so either they were masquerading as recently stimulated cells or they were constitutively exposed to cross-reactive antigen.
Virus-specific TCRαβ CD8αβ memory T cells isolated from mouse intestinal epithelium also expressed high levels of CD103 and β7 integrin and low levels of IL-15Rβ, Ly6C, CD62L, and CD27; had constitutive cytolytic activity; and underwent minimal homeostatic proliferation (18, 20, 26). Of particular note, there was no cell that existed in blood or spleen that expressed this panoply of differentiation markers, even among the CD62L− cells that later became the signature semantic definition of Tem cells in mice. The unique intestinal phenotype indicated that either cells were recirculating and constantly changing their phenotype every time they entered or left the intestinal mucosa or, far more likely, they were in fact resident. Nonlymphoid memory CD8 T cells were further indicated to represent a separate resident pool on the basis of distinct priming requirements or preferential dependence on VLA-1 (26, 27).
Evidence for T cell residence also came from skin. Fixed drug eruption is a T cell–mediated allergic response that can be observed in skin, often recurring repeatedly at the same location (28). A mouse model of human psoriasis provided evidence that T cells in the tissue can mediate effector function without any contribution from circulating cells (29). When human skin from a psoriasis patient was transplanted onto an immunocompromised mouse, the skin developed symptoms that mirror psoriasis. The skin pathology was T cell mediated and developed without apparent T cell egress. Further evidence in human skin suggested the persistence of local functional T cell memory(30).
Direct tests of residence typically depend on migration assays. In mouse, virus-specific memory CD8 T cells isolated from the lamina propria of the small intestine failed to migrate back to the lamina propria after transfer to a congenic recipient, indicating that cells in the gut were residents (31, 32). However, transferred memory CD8 T cells could be recovered from lung and liver: perceived migration that in hindsight was likely due to vascular contamination, even though the mice were perfused (31) (see the next section). Subsequently, Klonowksi et al. (33), used parabiosis surgery to conjoin the vasculatures of congenically distinct mice to look for the movement of memory CD8 T cells via the blood into tissues of the partner parabiont. This study also had issues of vascular contamination that were unknown at that time and led to overestimations of recirculation through nonlymphoid tissues. Importantly, however, CD8 T cells showed delayed and incomplete mixing in the brain and the small intestines. Interestingly, partner-derived cells expressed CD69 within the intestines, indicating true migration and suggesting that cells were eventually equilibrating and adopting tissue-specific signatures (33).
In HSV-1-infected mice, evidence was provided that CD8 T cells were maintained within the trigeminal ganglia, where they restrained reactivating virus in latently infected sensory neurons(34). When infected ganglia were cotransplanted at different sites under the kidney capsule, virus successfully recrudesced and local CD8 T cells were induced to proliferate, although there was little intermixing of CD8 T cell populations between grafts (35). These studies highlight a role for local immunity in maintaining control over persistent infections.
Gebhardt et al. (36) elegantly showed that when mice were infected with HSV-1 on one flank, they exhibited superior protective immunity against rechallenge at that site versus the contralateral flank. Furthermore, when previously infected skin was transplanted onto naive mice, HSV-1-specific CD8 T cells were retained within the transplant, presumably in the absence of antigen (36). After acute viral infections in mice and humans, CD8 T cells express mucosal entry molecules only during the recently activated stage of differentiation, and downregulate them during the memory phase of differentiation (31, 32). Likewise, only recently activated cells, not memory CD8 T cells, could migrate to the intestine after transfer to naive recipients. FTY720 and intestinal grafting experiments further confirmed the interpretation that intestinal mucosal memory constituted a distinct resident pool (32). By locally labeling cells within the brain after virus clearance, Wakim et al. (37) showed that antigen-specific memory CD8 T cells persist within the brain with no detected egress from the tissue or infiltration of the tissue from the blood. Some proposed a dichotomy in which nonlymphoid CD8 T cells preferentially differentiate into Trm cells whereas CD4 T cells recirculate (38, 39). While this issue remains to be settled, work from numerous labs employing diverse infectious models indicate that Trm cells typically dominate immune surveillance of most nonlymphoid tissues (37, 40–47).
TOOLS FOR IDENTIFYING RESIDENT MEMORY T CELLS: APPROACHES AND CAVEATS
This section summarizes the strengths and weaknesses of the most common or stringent approaches to assess residence and highlights caveats that may impede interpretation (Figure 2).
Figure 2.

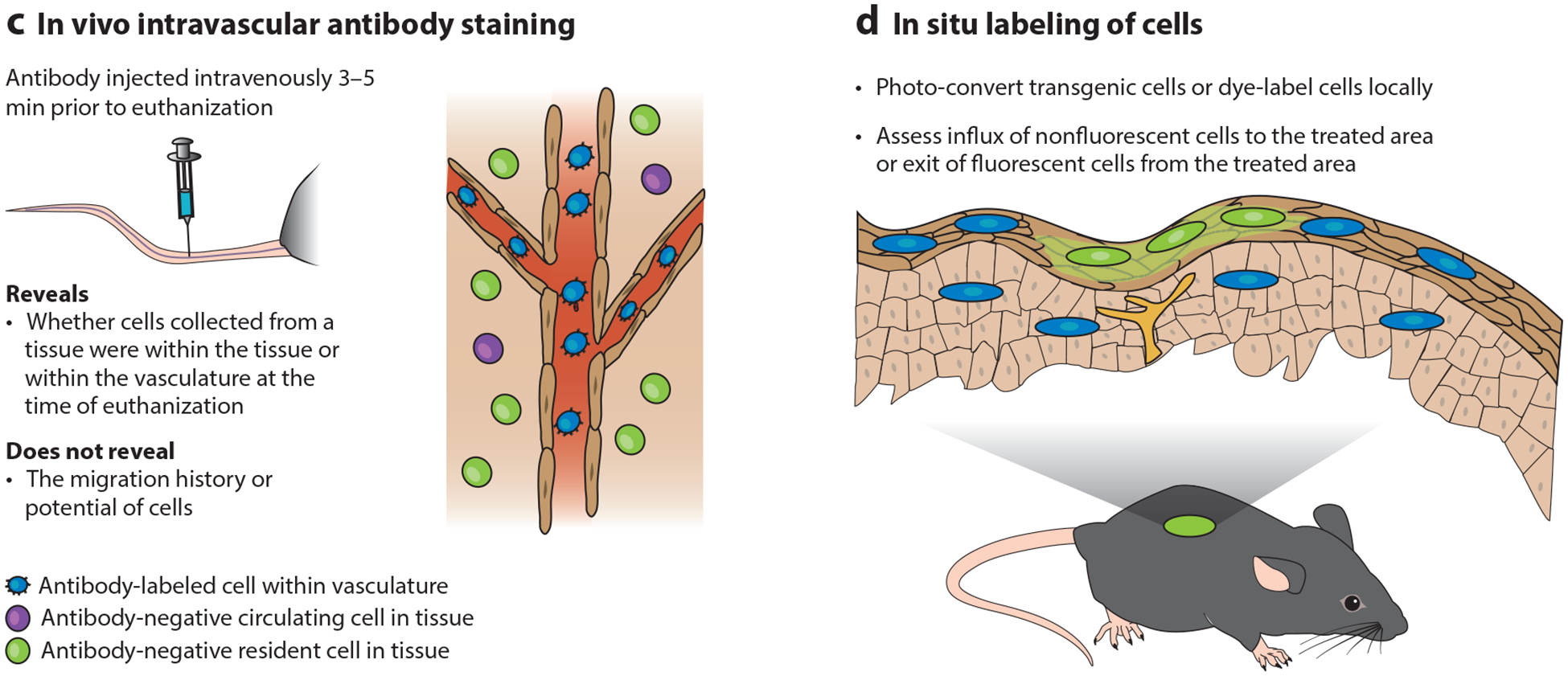
Tools for studying resident memory T (Trm) cells. Trm cells are defined by migration properties, not cell surface markers, which makes them difficult to identify. Four common techniques, along with their strengths and weaknesses for interpretation, are summarized here: (a) parabiosis surgery, (b) transplantation, (c) intravascular staining, and (d) in situ labeling.
Unlike cells in blood, most Trm cells are CD69+, and particularly among CD8 T cells many are CD103+. This phenotypic disparity provided early evidence of resident T cell populations. Due to its simplicity, evaluating residence solely on the basis of CD69 and CD103 expression is the most common approach. However, in isolation, this method has significant limitations. CD69−Trm cells have been reported, and most Trm cells in the body likely lack CD103 expression (45). So the absence of these Trm signatures is not disqualifying. More importantly, CD69 can be transiently expressed by recirculating T cells. This occurs in response to TCR stimulation or a variety of cytokines (48). While perhaps less confounding in specific-pathogen-free mice, this issue may severely limit the interpretable value of CD69 expression in analyses of humans or animals with normal microbial exposure. Indeed, “dirty” mice, laboratory mice that acquire microbial experience by being co-housed with pet shop mice, exhibit partial equilibration of certain CD69+ T cell populations during parabiosis (49, 50). Definitively categorizing lymphocyte populations still requires migration assays, and identifying stringently reliable markers remains an important goal for the field.
In animal models, transfer studies allow one to test whether establishment of local memory occurs during a narrow developmental window shortly after stimulation. For instance, lymphocytic choriomeningitis virus (LCMV)-specific CD8 T cells isolated from SLOs or blood transiently express intestine homing molecules. Transfer of this recently activated population confirms that they have the capacity to migrate to the gut in this assay. However, this migration potential is not maintained past the effector phase of the immune response, providing evidence that the tissue was seeded early during the response and that local memory is resident (31, 32). Transfer of recently activated versus resting memory T cells has revealed that Trm cells populate other tissues, including the salivary gland (51). Limitations of this approach are technical challenges, potential limitation of cell yields, and dependence on isolation and ex vivo manipulation.
Parabiotic surgery involves the conjoining of two, typically inbred, congenic mice and results in anastomosis of the vasculature within approximately one week. Recirculating lymphocyte populations equilibrate between each parabiont because the blood supply is shared. The failure of a population to equilibrate between corresponding tissues in each mouse is strong evidence for residence. This technique is very powerful because recirculation potential can be tested over many weeks and the assay captures migration properties of all organismal cell populations (e.g., it does not depend on isolating cells from a subset of tissues) (52). However, when a T cell population is mixed (originating from both parabionts) within a tissue, interpretation is less clear: It could represent bona fide recirculation (entry and exit from the tissue) or a continuous stream of one-way trips (entry but no exit).
Transplantation can be a powerful approach because it can test for both cell ingress and cell egress. For example, grafting an organ containing a known trackable lymphocyte population onto a recipient allows one to examine infiltration of the graft and also egress of graft-localized donor cells into the recipient recirculation (32, 36). The technical challenges vary by type of graft (e.g., skin is relatively easy, small intestine is highly challenging), and inflammation induced by the grafting surgery could overestimate steady-state cellular infiltration or exit.
Techniques have been developed to label cells at specific locations, which can allow their movements to be monitored. A sophisticated approach exploits transgenic cells expressing photoconvertible proteins. For example, Kaede is a coral-derived fluorescent protein that irreversibly changes emission from green to red fluorescence after exposure to violet light (53). Photoconversion allows in situ labeling of transgenic cells within any accessible tissue. One limitation is labeling transience. Because of protein turnover, converted fluorescent signal is lost within a week, and much faster if cells are proliferating. An alternative approach is local labeling with an injected fluorescent dye, such as CFSE (37, 54). This approach may suffer from dye diffusion (and labeling) away from the intended site, indiscriminate and incomplete labeling of local cells, induction of cell death, poor or varied labeling intensity, and rapid loss of label through proliferation.
Historically, there were many observations that were incompatible with the residence hypothesis. Naive B and T cells have been reported to recirculate through the lung and liver (55–59). As noted, there is rapid equilibration in these organs of memory T cells between parabionts, and it was reported that Gi protein–coupled chemokine receptors were not involved in their entry(33). Because mice were perfused to remove most red blood cells, the reasonable presumption was that vascular lymphocytes were largely removed as well. These data supported the contention, for instance, that lung parenchyma T cells composed a shared pool with their blood-borne counterparts and that memory T cells were maintained in the airways via constant recruitment from blood(54).
It was shown that intravascular antibody labeling of T cells could differentially label transferred T cell populations cultured under different conditions, and isolated from the lung (60). Subsequent studies showed that intravascular staining discriminated between T cells in vascular contiguous compartments versus those outside intact endothelium (e.g., the parenchyma, epithelium, airways), and the technique could be applied to many other organs (61). The surprising finding was that many of the leukocytes isolated from perfused organs were actually in the blood. The major implication was that much of the previous recirculation data were subject to misinterpretation because tissue-resident and blood-borne leukocyte populations were conflated. This issue varies by location and context; for example, after Mycobacterium tuberculosis infection of mice, about half of antigen-specific CD4 and CD8 T cells isolated from perfused lung are actually in the blood, and their phenotype and function are quite distinct from those of cells in tissue (62).
Why does perfusion fail to remove leukocytes when red blood cells are clearly removed? An explanation may come from the work of Hogg and colleagues (63), who showed that it takes neutrophils much more time to migrate through small lung vessels compared to red blood cells. As a result, the concentration of neutrophils increases by a factor of ~100 within lung capillaries, and they are just too large to be removed by perfusion. Lymphocytes, which have at least three times greater volume than red blood cells, appear to suffer from the same issue (61, 62). Thus, perfusion is ineffective and intravascular staining preserves blood-borne populations (which may be of interest) and allows discrimination from those outside of the vasculature. It should be emphasized that while intravascular staining is sometimes misinterpreted as a test of residence, it does not address migration properties and only helps to define anatomic location.
Standard approaches for analyzing leukocyte populations in nonlymphoid tissues include optimized mechanical and enzymatic digestion methods. These produce single-cell suspensions that can be subjected to multiple-parameter flow cytometry or ex vivo functional assays (often necessary to interrogate T cell antigen specificity). However, extraction methods typically underestimate nonlymphoid-tissue populations, sometimes quite significantly (by up to a factor of 70) (30, 45, 64). Trm cells in particular, rather than tissue-recirculating or vascular cells, appear under-represented, including those Trm cells that lack CD103 expression (45). Indeed, one reason that vascular contamination appears so high in lung is that the vascular cells were more efficiently liberated than those in the parenchyma. Thus, isolation inefficiency can distort perceived representation of different cell subsets. Imaging-based approaches can overcome this issue; however, they suffer from technical barriers (antibody compatibility, specimen preparation, etc.), fewer parameters can be assessed than with flow cytometry, and one may have to extrapolate from a small 2-D region of tissue to minimize labor intensiveness. However, imaging tools will likely see continued improvements. For instance, histocytometry provides a platform for combining spatial imaging data with multiple-parameter phenotypic analyses (65). Tissue-clearing approaches that reduce light scattering in tissues by minimizing refractive index differences between tissue constituents and the immersion medium now offer opportunities to study immune cell positioning in the context of a complex 3-D organ structure (66).
RESIDENT MEMORY T CELL MAINTENANCE
Recirculation through lymph nodes and blood provides access to survival factors, including stromal IL-7 and S1P. Resident cells dwell constitutively in tissues and organs. And each anatomic compartment can vary widely in available metabolites, cytokines, cell interactions, and matrix proteins. To cite but one example, oxygen tension varies widely: 19% in the upper airways, 3–4% in spleen, and near 0% in the intestinal epithelium (67). Thus, residents need to make adaptations to unique local environments. These accommodations likely impact Trm cell metabolism, function, phenotype, proliferation, longevity, and maintenance requirements. Survival factors may include not only cytokines and nutrients but also molecules associated with physical retention within tissues. And tissue-to-tissue variations in available survival factors might influence the carrying capacity of distinct organs for the size of the total local memory T cell population, although this important issue has not been well investigated.
T cell metabolism correlates with differentiation state. Naive T cells are quiescent and depend on glucose and fatty acid oxidation (FAO) for oxidative phosphorylation (OXPHOS). Shortly after activation, they undergo a glycolytic switch (aerobic glycolysis) that sacrifices the efficiency of ATP production in favor of producing metabolites that promote cell growth. Recirculating memory T cells resume a quiescent state and favor OXPHOS, but they make their own fatty acids to drive FAO (68). Recent characterization of skin Trm cells revealed that they express more of the fatty acid–binding receptors Fabp4 and Fabp5 than Tcm and Tem cells (69). These receptors allow for a more direct pathway of free fatty acid uptake, and deletion of these receptors induced preferential loss of skin Trm cells after vaccinia infection without impairing Tcm cell and Tem cell survival. This illuminating study illustrates that different metabolic strategies may be relied upon for survival in different locations. The hypothesis that different Trm populations might be subject to tissue-specific metabolic programs remains an exciting possibility.
Recirculating T cells chemotactically migrate toward lymphatics, and thus egress from tissues, including SLOs, in response to sphingosine-1-phosphate recognition by the receptor S1PR1 expressed on the cell surface. CD69 is a C-type lectin that posttranslationally antagonizes S1PR1. When T cells are stimulated in SLOs, CD69 is rapidly upregulated and maintained for a few days. This is thought to temporarily prevent S1PR1-mediated T cell egress in order to optimize time for the programming of immune responses. Transient SLO retention and CD69 expression can be induced either by TCR stimulation or by signaling through inflammatory cytokines(70).
Stable maintenance of CD69 by Trm cells is often interpreted as a stop signal that permanently prevents tissue egress. Support for this model comes from HSV infections in mouse skin, in which CD69−/− CD8 T cells have diminished capacity to establish Trm (71). However, whether CD69 has to be stably expressed is uncertain. KLF2 is a transcription factor required for genes involved in SLO recirculation, including S1PR1. KLF2 is downregulated by Trm cells, meaning that the lack of surface S1PR1 expression can be transcriptionally regulated rather than be dependent upon antagonization by CD69 (72). Moreover, lack of S1PR1 expression could allow surface localization of CD69 regardless of CD69’s functional activity. Interestingly, Trm populations that lack CD69 expression have been identified. And the fact that non-Trm cells transiently express CD69 under a variety of circumstances limits the interpretable value of this marker. Compared to recirculating T cells, Trm cells also underexpress another S1P receptor, S1PR5. While the function of S1PR5 is unclear, surface localization is not antagonized by CD69 (73–75).
In addition to KLF2 downregulation, some Trm cells express low levels of Eomes and intermediate levels of T-bet and Blimp-1 (76). Hobit, which suppresses KLF2, has been proposed as a master regulator of resident cells in mice, including Trm cells and NK T cells (see below)(77). Interestingly, some reports indicate that Hobit is not differentially upregulated by resident-phenotype lymphocytes isolated from humans (78–80). This could suggest species-specific differences in Trm ontogeny or an inability to purify human Trm cells based on markers that have caveats, or highlight the heterogeneity of Trm cells, which limits our ability to generalize conclusions broadly between different tissues or infectious contexts.
Indeed, the maintenance requirements for Trm cells are known to be quite heterogeneous. For instance, CD8+ Trm cells require IL-15 for survival within the skin after HSV infection, and in the salivary gland and kidney after LCMV infection (73, 81). However, this is not the case for LCMV-specific CD8+ Trm cells that occupy SLOs, the female reproductive tract, pancreas, thymus, or small intestine (81). CD103 (also called αe or αiel) pairs with β7 integrin and binds E-cadherin that is highly expressed by epithelial cells. CD4+ Trm cells rarely express CD103 and are usually not positioned within epithelium. Some, but not all, epithelium-localized CD8+ Trm cells express CD103, and many require this expression for maintenance (82). However, there are Trm cells localized to both type I and type II epithelial surfaces that lack CD103 expression, and the proportion of epithelial CD103− Trm cells correlates with T cell stimulation history (45, 82, 83). CD103 may also be expressed by a fraction of Trm cells in certain stromal tissues, such as the lamina propria of the mouse small intestine, but here it does not appear to be required for maintenance (82). It is not entirely understood whether CD103 simply facilitates physical adherence that prevents physical displacement (in which case other integrins might suffice) or whether CD103 signaling promotes cell survival. It will be interesting to compare the biology and functional roles of CD103− and CD103+ Trm subsets. It should also be noted that Trm cells might express E-cadherin, which could promote fraternal interactions with other Trm cells (51,84).
Because Trm cells share phenotypic qualities with recently activated effector T cells, there is an expectation that Trm cells are rather temporary. The half-life of Trm cells has not been rigorously addressed, nor compared to the half-life of recirculating memory T cell populations, but available evidence indicates that Trm cells are probably reasonably durable in most tissues (20, 41, 85–87). However, longevity is likely to be tissue and context dependent. Many studies have documented the transience of memory T cells in the lung (88). One model posits that after respiratory infections, lung airway T cells are maintained by continued low-level migration from blood that gradually declines when antigen depots are eliminated, or when recirculating Tem cells that retain lung homing capacity are lost or are converted to Tcm cells (54, 61, 89). Alternatively, elegant work demonstrated the presence of memory T cell depots at sites of previous lung damage, which might gradually egress into the lung airways (90). Either way, T cells are thought to take a one-way trip to the lung airways and to be short-lived upon arrival. Trm cells within the lung stroma and parenchyma are also typically short-lived in mouse, and seemingly after respiratory syncytial virus (RSV) infection in humans (89, 91, 92).
In summary, various populations of Trm cells utilize CD69, CD103, and IL-15 for maintenance and survival. However, these requirements are far from ubiquitous, and Trm cells within different locations or those that have experienced different histories of stimulation may vary in their survival needs and longevity. As such, generalizing findings from one infectious model or one location to all Trm cells merits restraint.
MIGRATION, RESIDENT MEMORY T CELL ONTOGENY, AND NONLYMPHOID RECIRCULATORS
Nonlymphoid Trm differentiation programs are at least partially executed after migration. Here, we review how T cell distribution is a collective consequence of the activation-induced adoption of a somewhat promiscuous nonlymphoid homing program, site-directed homing instructions encoded within specific LNs, recruitment into tissues due to inflamed endothelium, and encounter of antigen and other accessory signals within tissues. We then discuss inductive cues that influence Trm differentiation, tissue-specific heterogeneity in Trm development, and outstanding gaps in our understanding of Trm ontogeny.
Leukocyte extravasation is a highly regulated process involving selectin- or integrin-mediated rolling (to overcome shear forces in blood), chemokine signaling (to induce integrin activation), and integrin-mediated arrest (to enable diapedesis). Entry into resting LNs typically requires the expression of both CD62L [L-selectin, which mediates rolling on peripheral node addressins (PNAd)] and CCR7 (which binds CCL21 within high endothelial venules), whereas S1PR1 is required for egress. Expression of SLO homing molecules is maintained by naive T cells and Tcm cells. A large fraction of recently activated T cells alter their homing properties, enabling migration to nonlymphoid tissues. One dominant model adheres to the hypothesis that effector T cell migration is programmed within regional lymphoid tissue to target specific nonlymphoid tissues. This has been well demonstrated for T cell activation within gastrointestinal associated lymphoid tissue. Here, dendritic cells (DCs) that emigrate from the intestine collaborate with TGF-β and retinoic acid to preferentially induce α4β7 and CCR9 (11), which promotes gut migration. In contrast, DCs isolated from lung or skin preferentially induce CCR4, CCR10, and/or PSGL-1-binding ligand (93, 94). These data illustrate the concept that the site of priming can imprint a regional migration program. However, this mechanism is unlikely to account for the regulation of most T cell distribution.
Most antigen-experienced T cells are poised to migrate to sites of inflammation. Damage-associated or microbe-associated molecular patterns (DAMPs or MAMPs) and cytokines induced at sites of tissue injury or infection induce upregulation of vascular adhesion molecules, such as VCAM-1 (recognized by α4β1), on endothelial cells as well as local presentation of chemokines. This serves to encourage extravasation of recirculating T cells, including Tcm cells, into nonlymphoid compartments (45). Lastly, recently activated T cells may be transiently endowed with the capacity to enter uninflamed resting tissues, in what is likely a relatively promiscuous peripheral migration program (31, 32, 95–97). In summary, the calculus governing T cell distribution is multifactorial and involves promiscuous homing, programmed migration, and inflammation-induced recruitment. Thus, T cell localization can be biased toward nonlymphoid sites of infection, sometimes quite significantly, but this biasing may not be absolute for most visceral organs. However, it is likely that some immune privileged tissues are refractory to migration in the absence of local inflammation and/or fail to induce resident T cell populations without local antigen presentation (37, 90, 98, 99).
Upon arrival to a nonlymphoid organ, recently activated T cells undergo putatively site-directed changes. The role of local TGF-β in driving induction of CD103 expression by CD8+ Trm precursors is well documented (82, 87), although other developmental cues have not been well defined. Not all immigrants are equipotent, and some activated T cells appear too terminally differentiated to acquire Trm characteristics. Indeed, boosted recirculating cells are refractory to acquiring the CD103hiLy6Clo differentiation program after migrating to the intestinal mucosa(85), and KLRG1+ cells, typically correlating with more differentiation, fail to express CD103 (73, 100). Even though Trm cells masquerade as effectors in many tissues, they can maintain this differentiation state in the absence of persistent antigen (82, 87). That said, local antigen recognition can have a pronounced influence on the differentiation of Trm cells, and in situ detection during the effector phase of the immune response promotes Trm cell accumulation in certain tissues including brain, skin, lung, and the female reproductive tract (37, 47, 90, 99, 101, 102).
It is important to remember that since Trm cells position themselves within distinct organs, they are subject to distinct environments. Thus, it would not be surprising to learn that lifestyle choices, and thus Trm differentiation, vary with location in ways that have not yet been discerned. If true, this might be favorable in the event that modalities are desired for site-specific depletion of Trm cells, perhaps in the setting of immunopathological disease or autoimmunity.
Lastly, it should be emphasized that some populations of T cells retain a nonlymphoid tissue recirculation program. This further highlights that mechanisms exist to allow T cell migration to uninflamed tissues, and the degree of tissue specificity (and associated molecular mechanisms) to these recirculation pathways remains an important knowledge gap. However, at least in specific-pathogen-free mice after acute infections, nonlymphoid recirculating T cells appear rare relative to Trm cells in most nonlymphoid tissues. Elegant work suggests that nonlymphoid recirculators preferentially express intermediate levels of CX3CR1, and defining the regulation and ontogeny of this population remains an important goal (103). Indeed, to recirculate through nonlymphoid tissues, a cell would need to retain the effector-like capacity to migrate into uninflamed tissues yet putatively lack CD62L expression. Contemporaneously, these cells presumably retain the naive or Tcm-like capacity for egress via S1PR1, or perhaps CCR7—molecules thought to be coregulated with the expression of CD62L under transcriptional control of KLF2. Perhaps epigenetic silencing of LN entry receptors allows these expression patterns to coexist.
Trm Functions: Protection from Infections, Tumor Control, and the Fundamental Role of Monitoring Organ Homeostasis
The immune system is often conceptualized from an infection-defense viewpoint, but it has a more general role in monitoring local tissue homeostasis. Perceived perturbations include cancer, stress, changes in metabolism, pH, oxygen tension, and cell injury or death, as well as stimulus with a diversity of prokaryotic, eukaryotic, or viral microbes or microbial components. Such disturbances are communicated between conventional components of both the innate and adaptive immune systems, as well as parenchymal and stromal cells using cytokines, neuropeptides, lipid mediators, and cell-cell contact. Information regarding major breaches is relayed to the nodes (as well as to other sensory entities, including the nervous systems, vasculature, and epithelia), but the perception of the perturbation is local, so cell positioning is important. Trm cells represent one fundamental component of the broadly distributed immune sensory network whose major function is to monitor regional homeostasis (Figure 3). Here, we summarize the well-described role of Trm cells in accelerating protection against recrudescent, latent, or reencountered infections. However, brief consideration will also be paid to the role of Trm cells in monitoring cancer, and also their less desirable role in contributing to tissue homeostasis disruptions, as occurs in autoimmunity, allergy, inflammatory diseases, and histoincompatability.
Figure 3.
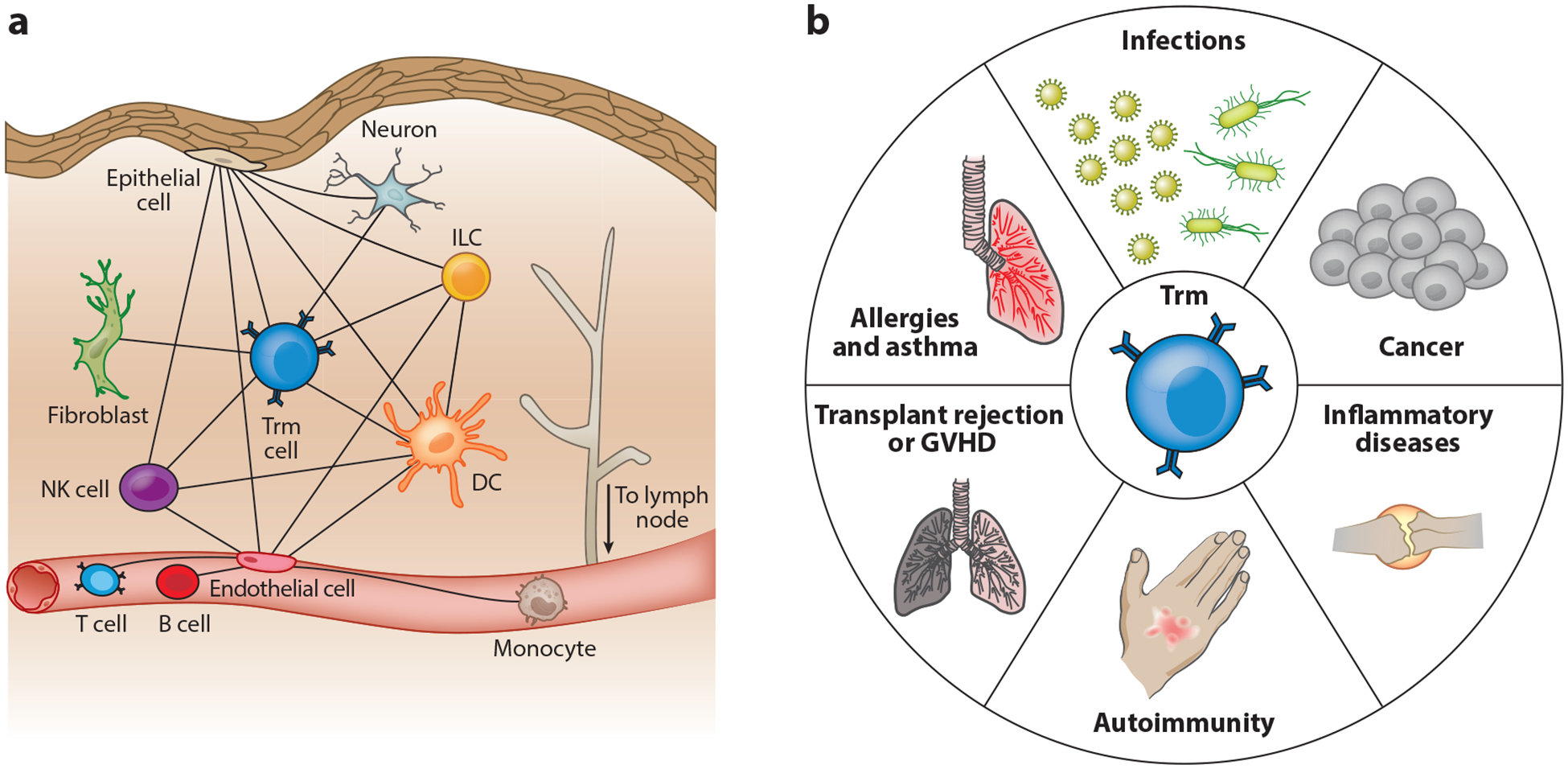
Trm cells form part of an integrated tissue surveillance network that participates in tissue monitoring and disease. (a) Trm cells signal disruptions in tissue homeostasis and communicate with the diverse motley crew of host resident and circulating parenchymal, stromal, and leukocyte populations. (b) Trm cells are thus putative contributors to (and perhaps instigate or sustain) responses in tissues, where they may play favorable or unfavorable roles in diverse human disease conditions. Abbreviations: DC, dendritic cell; GVHD, graft-versus-host disease; ILC, innate lymphoid cell; NK, natural killer; Trm, resident memory T.
In reductionist experiments in mice, Trm cells have been shown to accelerate control against all major classes of microbial pathogens: viruses, bacteria, fungi, and parasites (36, 40–44, 46, 47, 87, 91, 104–107). Sites that Trm cells patrol and protect include barrier tissues, where pathogens are most commonly transmitted (mucosal tissues and skin), and also internal organs, which of course may serve as important sites of pathogen replication. Many of the protection data have been attributed to CD8+ Trm cells, and correlations between CD8+ Trm abundance and protective immunity against RSV or suppression of HSV-1 reactivation have also been made in elegant human studies (92, 108, 109). Experiments in mice revealed that CD4+ Trm cells can also play important roles in protective immunity against diverse pathogens, including Chlamydia trachomatis encountered at the reproductive mucosa (47), Streptococcus pneumoniae (105) in the lung, Leishmania parasites in the skin (42), and HSV-2 and influenza infections at mucosal surfaces (40, 44), and may be an essential protective correlate of a preclinical tuberculosis vaccine (106).
Trm cells patrol tissues, scanning host cells for evidence of cognate antigen. Intravital microscopy studies in mice indicate that immune surveillance rates vary based on location. Trm cells in the compact epidermal compartment are almost sessile and adopt a dendritic morphology that interdigitates among basal keratinocytes (38, 110). In the myometrium of the female reproductive tract stroma, CD8+ Trm motility is approximately 10 μm/min, similar to Tcm cells in LNs or Trm cells in liver sinusoids (111–113). However, Trm motility rate is reduced to 5 μm/min in the collagen-dense perimetrium of the reproductive tract, suggesting that local anatomy might dictate Trm surveillance rates, which might be relevant to the density required to rapidly detect pathogens encountered at different locations (111).
Recognition of infected cells leads to cytokine production that might have direct anti-pathogen activity but that also serves to alarm the neighborhood (sometimes referred to as a sensing-and-alarm function). For instance, reactivated Trm cells express IFN-γ, TNF-α, and IL-2, trigger interferon-stimulated genes in neighboring cells that allow them to resist infection, activate endothelium to recruit leukocytes (including Tem and Tcm cells), and activate DCs, NK cells, and bystander functions of CD8 T cells (42, 114–117) (Figure 4). Evidence supports that recently activated CD8 T cells kill infected targets in nonlymphoid tissues, and it is likely that at least some populations of Trm cells remain particularly poised to express cytolytic function. For example, Trm cells in the small intestine retain constitutive expression of granzyme B (20). This has led some to speculate that Trm cells are trapped in a state of suspended effector differentiation and perhaps comprise a terminally differentiated subset. However, this model conflicts with evidence supporting Trm plasticity.
Figure 4.

Trm ontogeny and function. (a) Naive T cells are primed to proliferate in secondary lymphoid organs. Some, but likely not all, daughter cells that migrate to nonlymphoid tissues possess the developmental potential to differentiate into Trm cells. This occurs under the influence of developmental cues encountered after migration. Some cues may be tissue specific, imparting regional differences between Trm cells occupying different locations. (b) When established Trm cells recognize cognate antigen, they communicate this information to neighboring cells and also mount regionalized anamnestic responses. Outcomes include cytokine production; killing antigen-bearing host cells; activation of endothelium to recruit leukocytes; activation of local DCs, NK cells, and bystander T cells; and egress of DCs to draining lymph nodes. Trm cells also undergo in situ proliferation that can result in the amplification of local memory (independent of Tcm cells) and progeny that egress out of nonlymphoid tissue. Some of those progeny remain Trm cells within the draining lymph node. Abbreviations: DC, dendritic cell; NK, natural killer; Tcm, central memory T; Tem, effector memory T; Trm, resident memory T.
The fundamental properties of a classical anamnestic response, the ability to proliferate, differentiate, and migrate (to colonize or patrol new compartments), are typically relegated to Tcm cells. These are also qualities of naive T cells during a primary response. In contrast, Trm cells are often assumed to be effector-like, terminally differentiated cells. However, Trm cells do have the capacity to proliferate, and in one study in reproductive mucosa and skin, they had the potential to drive their own expansion and contribute to quantitatively increased secondary memory populations (111, 118). Furthermore, Trm cells have the potential to redistribute to draining SLOs(50). Some evidence also indicates that the progeny of reactivated Trm cells have the capacity to rejoin the recirculating memory pool and differentiate into subsets that would be defined as Tcm and Tem on the basis of phenotype (85). Whether these ex-Trm cells are endowed with unique properties (e.g., advantaged to repopulate the tissue of origin in the event of a recall infection) has not yet been reported. Nevertheless, Trm cells do appear to possess the classic memory abilities of population expansion, migration, and developmental plasticity. They also undergo self-renewal, although rates and IL-15 dependence of homeostatic division vary by tissue (81).
A critical question is whether Trm cells could be leveraged to generate more effective vaccines against recalcitrant pathogens. These include M. tuberculosis, HIV, and Plasmodium spp., which might be vulnerable to immediate responses that do not have to wait for T cell activation in draining SLOs, followed by T cell proliferation, differentiation, and migration. Encouraging evidence indicates that malaria vaccines that generate sufficiently abundant Trm cells within the liver might prevent blood-stage parasitemia (46). Moreover, Trm cells elicited by a cytomegalovirus-vectored vaccine might contribute to protection against tuberculosis in rhesus macaques (106).
TCR sequence comparisons between primary tumors and metastases indicate unique populations, suggesting that tumors are patrolled by Trm cells. Evidence from numerous studies indicates that the abundance of CD103+ tumor-infiltrating lymphocytes (rather than total tumor-infiltrating lymphocytes) correlates favorably with prognosis in many forms of cancer (119–127). These data strongly suggest that Trm cells might play an important role in controlling neoplasms. Indeed, one study using mice showed that CD103+ CD8 Trm cells that recognize a tumor antigen protect against an exogenous tumor challenge (128). Furthermore, Runx3 was defined as a transcription factor important for supporting Trm development, and T cells with experimentally diminished Runx3 expression showed an impaired capacity to control tumors in a mouse melanoma model (129). Manipulating Trm cells to promote tumor clearance represents a nascent opportunity that has not yet been intentionally leveraged in the clinic.
Trm Functions: The Dark Side
While Trm cells are most often thought to promote favorable immunity, it seems quite likely that they play pivotal roles in many diseases with an immunological component. When Trm cells recognize innocuous antigens or self-antigens, their activation can cause severe damage that may be both long-lasting and recurring. Human fixed drug eruption is now thought to be a Trm-mediated allergy, which explains site-specific recurrence. CD4+ Trm cells were recently shown to drive experimental allergic airway disease (modeling asthma in mice) and putatively contribute to delayed type hypersensitivity reactions at barrier sites (130). It would be surprising if Trm cells were not involved in the inception, maintenance, or amplification of other T cell–dependent allergic diseases.
Growing evidence also points to a role for Trm cells in autoimmunity at diverse anatomical sites, including alopecia areata, inflammatory bowel disease, psoriasis, and multiple sclerosis (131–134). Given the well-described ability of Trm cells to proliferate locally, produce proinflammatory cytokines, induce DC maturation, and recruit circulating cells, it is reasonable to suspect that Trm cells play a pivotal role in triggering autoimmune relapses and perpetuating disease. If so, elimination of local Trm cells might calm inflamed tissues and close those tissues to routine immune surveillance, allowing for remission that might be long-lasting. In such a scenario, biologics or small molecules that target Trm cells could lead to novel therapies to treat a range of autoimmune diseases. This underscores the need to define Trm-specific markers that might serve as targets for Trm cell elimination.
Such therapies of Trm cell depletion may have broader applications as well. In the context of transplantation, for instance, Trm that are transferred with the graft could trigger sensing and alarm upon contact with infiltrating host cells to exacerbate solid organ rejection. Additionally, some evidence suggests that Trm can be mobilized upon activation (50), and that Trm cells within transplanted tissue can exit the tissue within a new host and contribute to graft-versus-host disease, particularly if the mobilized Trm cells demonstrate proliferative potential and developmental plasticity.
Thus, while much Trm cell–focused research has highlighted their potential to prevent or eliminate infections, a more thorough understanding of how Trm cells can cause damage when mistargeted is needed. Such research will likely inform medical interventions that may allow for sustained recovery from a diverse array of allergies, inflammatory diseases, and autoimmune diseases.
Other Resident Leukocytes
Early studies on thoracic duct lymphocytes described the unique propensity of lymphocytes to recirculate. This was poetically likened by Sir Peter Medawar to the chorus in a provincial production of Faust, wherein actors continuously disappear behind the scenes and later reenter from another route (135). However, for most cells in the organism, including most leukocyte lineages, residence is the norm and thus not a peculiar property of Trm cells (perhaps recirculation is more peculiar). Here, we briefly highlight other leukocyte populations that make adaptions to local tissue microenvironments and appear to be resident, at least under homeostatic conditions.
ILCs are a recently described cell population that arises from a common lymphoid progenitor yet lack a TCR. ILCs are established perinatally, most abundantly at barrier sites, and comprise distinct subsets that share functions with T cells. ILCs respond to innate cytokines that detect perturbations in tissue microenvironments, communicate this information to diverse cell types, and may be a placeholder in tissues to monitor homeostasis before local adaptive immunity is established (136). However, ILCs are still abundant in dirty mice that have abundant Trm cells, so it also seems likely that ILCs execute lineage-specific functions that have not been fully elucidated(49). While ILCs may become mobilized under inflammatory conditions, evidence indicates that they are largely resident (137, 138).
Indeed, most cells found in tissues are biased toward stable residence. For instance, migration of monocytes from blood into tissues induces adaption to the local microenvironment and differentiation into macrophages that remain resident under homeostatic conditions (139). Many nonclassical T cells that are enriched at mucosal and epithelial surfaces, such as TCRγδ+ T cells and CD8αα TCRαβ+ T cells, are resident. Through oligoclonal TCRs that recognize conserved microbial products or host stress molecules, they recognize perturbations in homeostasis. But unlike conventional Trm cells, they are developmentally programmed and do not require priming within SLOs; rather, they appear early during development (140). Memory B cells and plasma cells may also reside within tissues (141, 142). Thus, it seems as though residence will be a dominant property of most mature immune cells, although such residence is not always terminal. And more broadly, fibroblasts, endothelial cells, epithelial cells, and most other differentiated cell types at any location in the body (all of which are highly integrated with the immune system) are typically stable denizens.
In summary, diverse cell types monitor tissue homeostasis and coordinate responses to perturbations. This integrated network collectively accomplishes antigen presentation, cytotoxicity, antimicrobial responses, and amplification of danger signals. Unlike other residents, Trm cells are not seeded developmentally but have to be selected based on specificity and primed, and this in turn invokes a nomadic developmental phase that involves recirculation through SLOs. Trm cells have a sensitive TCR that is linked to a potent capacity for cytokine expression and other effector functions, and they exert robust control over tissue responses. Moreover, an enhanced capacity for memory compared to innate cells, a rearranged TCR as well as somatically encoded sensing receptors, the ability to achieve remarkable variability in population size, and significant developmental plasticity that equates with the capacity to promote or prevent distinct flavors of inflammation or to execute tissue damage or promote tolerance place Trm cells in a pivotal role in tissue monitoring (Figure 3). So while Trm cells function in concert with their neighbors, they represent a key node in the propagation, amplification, and maintenance of signals throughout the network.
CONCLUSIONS
Trm cells constitute the most abundant major T cell lineage and dominate peripheral immune surveillance, yet they remain sequestered in tissues under homeostatic conditions. Residence provides a mechanism for the host to regionalize immune surveillance precisely, and independently of tissue-specific recirculation programs. However, this strategy poses technical challenges for scientists. At one time, it was hoped that peripheral immune surveillance could be captured simply by analyses of blood, at least once predictive homing markers were discerned. However, it is now appreciated that tissues have to be sampled directly. Attendant difficulties include labor intensiveness, sample availability, blood contamination, poor isolation efficiencies, lack of reliable markers for Trm cells, and potentially compromised viability of those cells that are isolated.
The residence strategy also engenders clinical challenges. For example, developing carcinomas, arising from cells of epithelial origin and collectively the most common type of cancer, would likely lie beyond the scope of routine recirculating immune surveillance. One will have to ensure that adoptive cell therapies directed against solid tumors, including transferred chimeric antigen receptor (CAR) T cells, can locate their target (143). And perhaps investigations into HIV reservoirs will have to go beyond analyses of blood: CD4+ Trm cells are abundant in tissues, and if they harbor latent virus, any strategies that target reservoir elimination will be ineffective unless they pervade all relevant anatomic compartments.
But the recognition of Trm cells as the dominant peripheral surveyor also creates new opportunities. Trm cells accelerate protection from infection, and learning the rules of their ontogeny could pay dividends for vaccine development. Issues to investigate will include vaccination route, antigen persistence, and the role of immune stimulatory signals in optimizing Trm establishment at desired locations. Despite past efforts, there is significant room for applicable discovery here.
And while investigations are still nascent, it is almost certain that Trm cells contribute to many human diseases. While Trm cells may be only one player in a multifactorial and integrated process, they express potent effector functions, communicate with diverse cell types, recruit leukocytes to tissues, proliferate in situ, can be self-amplifying in tissue without input from recirculating cells, and possess the property of memory. As such, Trm cells may provide an attractive target to break the cycle of chronic or relapsing/remitting disease. Moreover, local clearance of Trm cells could leave systemic immunity intact. This goal supports the impetus for defining Trm markers (which remains poorly addressed at this time), including those associated with particular tissues or antigen experiences, that might create new therapeutic targets. In addition to employing Trm cells to fight cancer and infectious disease, selective removal of disease-causing Trm cells might ultimately be one of the most clinically impactful outcomes of Trm cell discovery.
Our current understanding of Trm cells remains inchoate. Critical issues to address include longevity and plasticity. Provocative evidence indicates that Trm cells exhibit significant developmental plasticity, and long-lived circulating cells might include ex-Trm cells that left tissues and underwent re-differentiation. Such cells may exhibit properties distinct from those of conventional Tcm and Tem cells, although this remains to be validated. An accepted alternative view is that like effector T cells, Trm cells are relatively short-lived and have lost developmental plasticity. Dissecting Trm heterogeneity is a related concept that merits more focus. This in turn relates to ontogeny and questions of potential roles for signal strength, asymmetric division, location of priming, and migration and tissue-specific developmental cues in determining Trm fate. Additional questions include the regulation of Trm homeostasis and how abundance relates to infection control. Are the numbers of Trm cells stringently regulated, as observed for Tcm cells in SLOs? Or does the population exhibit a dynamic range dependent on infectious experience, as indicated by examination of pet shop mice (49)? If so, is there competition between Trm populations that would prevent T cell vaccines that establish abundant tissue memory from being practical? Or, have we only begun to tap the potential of Trm cells to protect against some of the world’s most intractable pathogens?
Since their recognition as a major determinant of health and disease, research on Trm cells has steadily intensified, and justifiably so. There are numerous unrealized opportunities for discovery that are tangible and tractable, and whose elucidation could have a fairly immediate impact on treating or preventing human illness.
ACKNOWLEDGMENTS
This work was supported by R01 AI111671 and R01 AI084913 (D.M.) and T32HL007741 (A.G.S.)
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Gowans JL, Knight EJ. 1964. The route of re-circulation of lymphocytes in the rat. Proc. R. Soc. Lond. B 159(975):257–82 [DOI] [PubMed] [Google Scholar]
- 2.Gowans JL. 1959. The recirculation of lymphocytes from blood to lymph in the rat. J. Physiol 146(1):54–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, et al. 2013. An estimation of the number of cells in the human body. Ann. Hum. Biol 40(6):463–71 [DOI] [PubMed] [Google Scholar]
- 4.Cahill RN, Poskitt DC, Frost DC, Trnka Z. 1977. Two distinct pools of recirculating T lymphocytes: migratory characteristics of nodal and intestinal T lymphocytes. J. Exp. Med 145(2):420–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackay CR, Kimpton WG, Brandon MR, Cahill RN. 1988. Lymphocyte subsets show marked differences in their distribution between blood and the afferent and efferent lymph of peripheral lymph nodes. J. Exp. Med 167(6):1755–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay CR, Marston WL, Dudler L. 1990. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med 171(3):801–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallatin WM, Weissman IL, Butcher EC. 1983. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature 304(5921):30–34 [DOI] [PubMed] [Google Scholar]
- 8.Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, et al. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99(1):23–33 [DOI] [PubMed] [Google Scholar]
- 9.Picker LJ, Michie SA, Rott LS, Butcher EC. 1990. A unique phenotype of skin-associated lymphocytes in humans: preferential expression of the HECA-452 epitope by benign and malignant T cells at cutaneous sites. Am. J. Pathol 136(5):1053–68 [PMC free article] [PubMed] [Google Scholar]
- 10.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. 1997. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature 389(6654):978–81 [DOI] [PubMed] [Google Scholar]
- 11.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, et al. 2003. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature 424(6944):88–93 [DOI] [PubMed] [Google Scholar]
- 12.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, et al. 1993. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74(1):185–95 [DOI] [PubMed] [Google Scholar]
- 13.Springer TA. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76(2):301–14 [DOI] [PubMed] [Google Scholar]
- 14.Kunkel EJ, Butcher EC. 2002. Chemokines and the tissue-specific migration of lymphocytes. Immunity 16(1):1–4 [DOI] [PubMed] [Google Scholar]
- 15.Butcher EC, Picker LJ. 1996. Lymphocyte homing and homeostasis. Science 272(5258):60–66 [DOI] [PubMed] [Google Scholar]
- 16.von Andrian UH, Mackay CR. 2000. T-cell function and migration: two sides of the same coin. N. Engl.J. Med 343(14):1020–34 [DOI] [PubMed] [Google Scholar]
- 17.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401(6754):708–12 [DOI] [PubMed] [Google Scholar]
- 18.Kim SK, Schluns KS, Lefrançois L. 1999. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J. Immunol 163(8):4125–32 [PubMed] [Google Scholar]
- 19.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410(6824):101–5 [DOI] [PubMed] [Google Scholar]
- 20.Masopust D 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291(5512):2413–17 [DOI] [PubMed] [Google Scholar]
- 21.Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, et al. 2001. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol 166(3):1813–22 [DOI] [PubMed] [Google Scholar]
- 22.Wiley JA, Hogan RJ, Woodland DL, Harmsen AG. 2001. Antigen-specific CD8+ T cells persist in the upper respiratory tract following influenza virus infection. J. Immunol 167(6):3293–99 [DOI] [PubMed] [Google Scholar]
- 23.Zeitz M, Greene WC, Peffer NJ, James SP. 1988. Lymphocytes isolated from the intestinal lamina propria of normal nonhuman primates have increased expression of genes associated with T-cell activation. Gastroenterology 94(3):647–55 [DOI] [PubMed] [Google Scholar]
- 24.Kim SK, Reed DS, Heath WR, Carbone F, Lefrançois L. 1997. Activation and migration of CD8 T cells in the intestinal mucosa. J. Immunol 159(9):4295–4306 [PubMed] [Google Scholar]
- 25.Bunting CH, Huston J. 1921. Fate of the lymphocyte. J. Exp. Med 33(5):593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masopust D, Jiang J, Shen H, Lefrançois L. 2001. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol 166(4):2348–56 [DOI] [PubMed] [Google Scholar]
- 27.Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, et al. 2004. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 20(2):167–79 [DOI] [PubMed] [Google Scholar]
- 28.Teraki Y, Moriya N, Shiohara T. 1994. Drug-induced expression of intercellular adhesion molecule-1 on lesional keratinocytes in fixed drug eruption. Am. J. Pathol 145(3):550–60 [PMC free article] [PubMed] [Google Scholar]
- 29.Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. 2004. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J. Exp. Med 199(5):731–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K-I, et al. 2006. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol 176(7):4431–39 [DOI] [PubMed] [Google Scholar]
- 31.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, et al. 2004. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol 172(8):4875–82 [DOI] [PubMed] [Google Scholar]
- 32.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, et al. 2010. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med 207(3):553–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrançois L. 2004. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20(5):551–62 [DOI] [PubMed] [Google Scholar]
- 34.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18(5):593–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakim LM, Waithman J, Van Rooijen N, Heath WR, Carbone FR. 2008. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319(5860):198–202 [DOI] [PubMed] [Google Scholar]
- 36.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol 10(5):524–30 [DOI] [PubMed] [Google Scholar]
- 37.Wakim LM, Woodward-Davis A, Bevan MJ. 2010. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. PNAS 107(42):17872–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, et al. 2011. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477(7363):216–19 [DOI] [PubMed] [Google Scholar]
- 39.Collins N, Jiang X, Zaid A, Macleod BL, Li J, et al. 2016. Skin CD4+ memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat. Commun 7:11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. 2011. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol 187(11):5510–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. 2012. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483(7388):227–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. 2015. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J. Exp. Med 212(9):1405–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin H, Iwasaki A. 2012. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491(7424):463–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iijima N, Iwasaki A. 2014. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346(6205):93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, et al. 2015. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161(4):737–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez-Ruiz D, Ng WY, Holz LE, Ma JZ, Zaid A, et al. 2016. Liver-resident memory CD8+ T cells form a front-line defense against malaria liver-stage infection. Immunity 45(4):889–902 [DOI] [PubMed] [Google Scholar]
- 47.Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, et al. 2015. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 348(6241):aaa8205–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiow LR, Rosen DB, Brdičková N, Xu Y, An J, et al. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440(7083):540–44 [DOI] [PubMed] [Google Scholar]
- 49.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, et al. 2016. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532(7600):512–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beura LK, Wijeyesinghe S, Thompson EA, Macchietto MG, Rosato PC, et al. 2018. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity 48(2):327–38.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hofmann M, Pircher H. 2011. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. PNAS 108(40):16741–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finerty JC. 1952. Parabiosis in physiological studies. Physiol. Rev 32(3):277–302 [DOI] [PubMed] [Google Scholar]
- 53.Tomura M, Yoshida N, Tanaka J, Karasawa S, Miwa Y, et al. 2008. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. PNAS 105(31):10871–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ely KH, Cookenham T, Roberts AD, Woodland DL. 2006. Memory T cell populations in the lung airways are maintained by continual recruitment. J. Immunol 176(1):537–43 [DOI] [PubMed] [Google Scholar]
- 55.Luettig B, Pape L, Bode U, Bell EB, Sparshott SM, et al. 1999. Naive and memory T lymphocytes migrate in comparable numbers through normal rat liver: Activated T cells accumulate in the periportal field. J. Immunol 163(8):4300–7 [PubMed] [Google Scholar]
- 56.Cose S, Brammer C, Khanna KM, Masopust D, Lefrançois L. 2006. Evidence that a significant number of naive T cells enter non-lymphoid organs as part of a normal migratory pathway. Eur. J. Immunol 36(6):1423–33 [DOI] [PubMed] [Google Scholar]
- 57.Harp JR, Onami TM. 2010. Naïve T cells re-distribute to the lungs of selectin ligand deficient mice. PLOS ONE 5(6):e10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caucheteux SM, Torabi-Parizi P, Paul WE. 2013. Analysis of naïve lung CD4 T cells provides evidence of functional lung to lymph node migration. PNAS 110(5):1821–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertolino P, Bowen DG, McCaughan GW, Fazekas de St Groth B. 2001. Antigen-specific primary activation of CD8+ T cells within the liver. J. Immunol 166(9):5430–38 [DOI] [PubMed] [Google Scholar]
- 60.Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. 2005. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J. Clin. Investig 115(12):3473–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson KG, Sung H, Skon CN, Lefrançois L, Deisinger A, et al. 2012. Cutting edge: Intravascular staining redefines lung CD8 T cell responses. J. Immunol 189(6):2702–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, et al. 2014. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc 9(1):209–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doerschuk CM, Beyers N, Coxson HO, Wiggs B, Hogg JC. 1993. Comparison of neutrophil and capillary diameters and their relation to neutrophil sequestration in the lung. J. Appl. Physiol 74(6):3040–45 [DOI] [PubMed] [Google Scholar]
- 64.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. 2011. Resident memory T cells (TRM) are abundant in human lung: diversity, function, and antigen specificity. PLOS ONE 6(1):e16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN. 2012. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37(2):364–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li W, Germain RN, Gerner MY. 2017. Multiplex, quantitative cellular analysis in large tissue volumes with clearing-enhanced 3D microscopy (Ce3D). PNAS 114(35):E7321–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNamee EN, Korns Johnson D, Homann D, Clambey ET. 2013. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol. Res 55(1–3):58–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buck MD, O’Sullivan D, Pearce EL. 2015. T cell metabolism drives immunity. J. Exp. Med 212(9):1345–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pan Y, Tian T, Park CO, Lofftus SY, Mei S, et al. 2017. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543(7644):252–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freeman BE, Hammarlund E, Raué H-P, Slifka MK. 2012. Regulation of innate CD8+ T-cell activation mediated by cytokines. PNAS 109(25):9971–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, et al. 2015. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol 194(5):2059–63 [DOI] [PubMed] [Google Scholar]
- 72.Skon CN, Lee J-Y, Anderson KG, Masopust D, Hogquist KA, Jameson SC. 2013. Transcriptional down-regulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol 14(12):1285–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, et al. 2013. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol 14(12):1294–301 [DOI] [PubMed] [Google Scholar]
- 74.Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, et al. 2012. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J. Immunol 189(7):3462–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, et al. 2009. T-bet–dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J. Exp. Med 206(11):2469–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, et al. 2015. T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 43(6):1101–11 [DOI] [PubMed] [Google Scholar]
- 77.Mackay LK, Minnich M, Kragten NAM, Liao Y, Nota B, et al. 2016. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352(6284):459–63 [DOI] [PubMed] [Google Scholar]
- 78.Li J, Olshansky M, Carbone FR, Ma JZ. 2016. Transcriptional analysis of T cells resident in human skin. PLOS ONE 11(1):e0148351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hombrink P, Helbig C, Backer RA, Piet B, Oja AE, et al. 2016. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat. Immunol 17(12):1467–78 [DOI] [PubMed] [Google Scholar]
- 80.Kumar BV, Ma W, Miron M, Granot T, Guyer RS, et al. 2017. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep 20(12):2921–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schenkel JM, Fraser KA, Casey KA, Beura LK, Pauken KE, et al. 2016. IL-15-independent maintenance of tissue-resident and boosted effector memory CD8 T cells. J. Immunol 196(9):3920–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, et al. 2012. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol 188(10):4866–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang N, Bevan MJ. 2013. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39(4):687–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hofmann M, Oschowitzer A, Kurzhals SR, Krüger CC, Pircher H. 2013. Thymus-resident memory CD8+ T cells mediate local immunity. Eur. J. Immunol 43(9):2295–304 [DOI] [PubMed] [Google Scholar]
- 85.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. 2006. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol 176(4):2079–83 [DOI] [PubMed] [Google Scholar]
- 86.Li H, Liu J, Carville A, Mansfield KG, Lynch D, Barouch DH. 2011. Durable mucosal simian immunodeficiency virus-specific effector memory T lymphocyte responses elicited by recombinant adenovirus vectors in rhesus monkeys. J. Virol 85(21):11007–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, et al. 2012. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. PNAS 109(18):7037–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang S, Mozdzanowska K, Palladino G, Gerhard W. 1994. Heterosubtypic immunity to influenza type A virus in mice: effector mechanisms and their longevity. J. Immunol 152(4):1653–61 [PubMed] [Google Scholar]
- 89.Slütter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, Harty JT. 2017. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci. Immunol 2(7):eaag2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, et al. 2016. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J. Exp. Med 213(13):3057–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu T, Hu Y, Lee Y-T, Bouchard KR, Benechet A, et al. 2014. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol 95(2):215–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, et al. 2015. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun 6:10224. [DOI] [PMC free article] [PubMed] [Google Scholar]; Erratum. 2016. Nat. Commun 7:11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campbell DJ, Butcher EC. 2002. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med 195(1):135–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mikhak Z, Strassner JP, Luster AD. 2013. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. J. Exp. Med 210(9):1855–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaufman DR, Liu J, Carville A, Mansfield KG, Havenga MJE, et al. 2008. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J. Immunol 181(6):4188–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Agrewala JN, Brown DM, Lepak NM, Duso D, Huston G, Swain SL. 2007. Unique ability of activated CD4+ T cells but not rested effectors to migrate to non-lymphoid sites in the absence of inflammation. J. Biol. Chem 282(9):6106–15 [DOI] [PubMed] [Google Scholar]
- 97.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. 2006. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity 25(3):511–20 [DOI] [PubMed] [Google Scholar]
- 98.Wakim LM, Smith J, Caminschi I, Lahoud MH, Villadangos JA. 2015. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol 8(5):1060–71 [DOI] [PubMed] [Google Scholar]
- 99.McMaster SR, Wein AN, Dunbar PR, Hayward SL, Cartwright EK, et al. 2018. Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal Immunol 11(4):1071–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sheridan BS, Pham Q-M, Lee Y-T, Cauley LS, Puddington L, Lefrançois L. 2014. Oral infection drives a distinct population of intestinal resident memory CD8+ T cells with enhanced protective function. Immunity 40(5):747–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muschaweckh A, Buchholz VR, Fellenzer A, Hessel C, König P-A, et al. 2016. Antigen-dependent competition shapes the local repertoire of tissue-resident memory CD8+ T cells. J. Exp. Med 213(13):3075–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan TN, Mooster JL, Kilgore AM, Osborn JF, Nolz JC. 2016. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J. Exp. Med 213(6):951–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gerlach C, Moseman EA, Loughhead SM, Alvarez D, Zwijnenburg AJ, et al. 2016. The Chemokine receptor CX3CR1 defines three antigen-experienced CD8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity 45(6):1270–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kinnear E, Lambert L, McDonald JU, Cheeseman HM, Caproni LJ, Tregoning JS. 2018. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol 11(1):249–56 [DOI] [PubMed] [Google Scholar]
- 105.Smith NM, Wasserman GA, Coleman FT, Hilliard KL, Yamamoto K, et al. 2018. Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia. Mucosal Immunol 11(1):220–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hansen SG, Zak DE, Xu G, Ford JC, Marshall EE, et al. 2018. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat. Med 24(2):130–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Conti HR, Peterson AC, Brane L, Huppler AR, Hernández-Santos N, et al. 2014. Oral-resident natural Th17 cells and γδT cells control opportunistic Candida albicans infections. J. Exp. Med 211(10):2075–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, et al. 2013. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature 497(7450):494–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, et al. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med 204(3):595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, et al. 2012. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. PNAS 109(48):19739–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beura LK, Mitchell JS, Thompson EA, Schenkel JM, Mohammed J, et al. 2018. Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat. Immunol 19(2):173–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McNamara HA, Cai Y, Wagle MV, Sontani Y, Roots CM. 2017. Up-regulation of LFA-1 allows liver-resident memory T cells to patrol and remain in the hepatic sinusoids. Sci. Immunol 2:eaaj1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miller MJ, Wei SH, Parker I, Cahalan MD. 2002. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296:1869–73 [DOI] [PubMed] [Google Scholar]
- 114.Schenkel JM, Fraser KA, Vezys V, Masopust D. 2013. Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol 14(5):509–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. 2014. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346(6205):98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, et al. 2014. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 346(6205):101–5 [DOI] [PubMed] [Google Scholar]
- 117.Tan H-X, Wheatley AK, Esterbauer R, Jegaskanda S, Glass JJ, et al. 2018. Induction of vaginal-resident HIV-specific CD8 T cells with mucosal prime-boost immunization. Mucosal Immunol 11(3):994–1007 [DOI] [PubMed] [Google Scholar]
- 118.Park SL, Zaid A, Hor JL, Christo SN, Prier JE, et al. 2018. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol 19(2):183–91 [DOI] [PubMed] [Google Scholar]
- 119.Wang B, Wu S, Zeng H, Liu Z, Dong W, et al. 2015. CD103+ tumor infiltrating lymphocytes predict a favorable prognosis in urothelial cell carcinoma of the bladder. J. Urol 194(2):556–62 [DOI] [PubMed] [Google Scholar]
- 120.Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, et al. 2015. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J. Immunol 194(7):3475–86 [DOI] [PubMed] [Google Scholar]
- 121.Boddupalli CS, Bar N, Kadaveru K, Krauthammer M, Pornputtapong N, et al. 2016. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight 1(21):e88955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ganesan A-P, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, et al. 2017. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat. Immunol 18(8):940–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koh J, Kim S, Kim M-Y, Go H, Jeon YK, Chung DH. 2017. Prognostic implications of intratumoral CD103+ tumor-infiltrating lymphocytes in pulmonary squamous cell carcinoma. Oncotarget 8(8):13762–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. 2014. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin. Cancer Res 20(2):434–44 [DOI] [PubMed] [Google Scholar]
- 125.Ademmer K, Ebert M, Müller-Ostermeyer F, Friess H, Büchler MW, et al. 1998. Effector T lymphocyte subsets in human pancreatic cancer: detection of CD8+CD18+ cells and CD8+CD103+ cells by multi-epitope imaging. Clin. Exp. Immunol 112(1):21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang H-G, Chen H-S, Peng J-R, Shang X-Y, Zhang J, et al. 2007. Specific CD8+ T cell responses to HLA-A2 restricted MAGE-A3 p271–279 peptide in hepatocellular carcinoma patients without vaccination. Cancer Immunol. Immunother 56(12):1945–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nizard M, Roussel H, Tartour E. 2016. Resident memory T cells as surrogate markers of the efficacy of cancer vaccines. Clin. Cancer Res 22(3):530–32 [DOI] [PubMed] [Google Scholar]
- 128.Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, et al. 2017. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci. Immunol 2(10):eaam6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Milner JJ, Toma C, Yu B, Zhang K, Omilusik K, et al. 2017. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 552(7684):253–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hondowicz BD, An D, Schenkel JM, Kim KS, Steach HR, et al. 2016. Interleukin-2-dependent allergenspecific tissue-resident memory cells drive asthma. Immunity 44(1):155–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheuk S, Wikén M, Blomqvist L, Nylén S, Talme T, et al. 2014. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J. Immunol 192(7):3111–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cheuk S, Schlums H, Gallais Sérézal I, Martini E, Chiang SC, et al. 2017. CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immunity 46(2):287–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, et al. 2009. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med 206(3):525–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sasaki K, Bean A, Shah S, Schutten E, Huseby PG, et al. 2014. Relapsing-remitting central nervous system autoimmunity mediated by GFAP-specific CD8 T cells. J. Immunol 192(7):3029–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yednock TA, Rosen SD. 1989. Lymphocyte homing. Adv. Immunol 44:313–78 [DOI] [PubMed] [Google Scholar]
- 136.Kotas ME, Locksley RM. 2018. Why innate lymphoid cells? Immunity 48(6):1081–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. 2015. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350(6263):981–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang Y, Mao K, Chen X, Sun M-A, Kawabe T, et al. 2018. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359(6371):114–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ginhoux F, Guilliams M. 2016. Tissue-resident macrophage ontogeny and homeostasis. Immunity 44(3):439–49 [DOI] [PubMed] [Google Scholar]
- 140.McDonald BD, Jabri B, Bendelac A. 2018. Diverse developmental pathways of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol 18(8):514–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Onodera T, Takahashi Y, Yokoi Y, Ato M, Kodama Y, et al. 2012. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. PNAS 109(7):2485–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Landsverk OJB, Snir O, Casado RB, Richter L, Mold JE, et al. 2017. Antibody-secreting plasma cells persist for decades in human intestine. J. Exp. Med 214(2):309–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lim WA, June CH. 2017. The principles of engineering immune cells to treat cancer. Cell 168(4):724–40 [DOI] [PMC free article] [PubMed] [Google Scholar]


