Abstract
L-glutamate is the main excitatory neurotransmitter in the brain, with postsynaptic responses to its release predominantly mediated by AMPA-type glutamate receptors (AMPARs). A critical component of synaptic plasticity involves changes in the number of responding postsynaptic receptors, which are dynamically recruited to and anchored at postsynaptic sites. Emerging findings continue to shed new light on molecular mechanisms that mediate AMPAR postsynaptic trafficking and localization. Accordingly, unconventional secretory trafficking of AMPARs occurs in dendrites, from the endoplasmic reticulum (ER) through the ER-Golgi intermediary compartment directly to recycling endosomes, independent of the Golgi apparatus. Upon exocytosis, AMPARs diffuse in the plasma membrane to reach the postsynaptic site, where they are trapped in order to contribute to transmission. This trapping occurs through a combination of both intracellular interactions, such as TARP (transmembrane AMPAR regulatory protein) binding to α-actinin-stabilized PSD-95, and extracellular interactions through the receptor N-terminal domain. These anchoring mechanisms may facilitate precise receptor positioning with respect to glutamate release sites to enable efficient synaptic transmission.
Introduction
The most prevalent neurotransmitter in the brain is glutamate (1), which predominantly activates AMPA-type glutamate receptors (AMPARs) (2). AMPARs consist of four homologous pore-forming subunits (GluA1–4), which mostly assemble into heteromers. For example, in the hippocampal CA1 area, GluA1/GluA2 and GluA2/GluA3 heteromers account for ~80 and ~20% of the postsynaptic AMPAR response under basal conditions, respectively (2). However, cAMP selectively increases the activity of GluA3-containing AMPARs in a PKA- and Ras-dependent manner (3). AMPAR organization is modular: their extracellular region consists of an N-terminal domain and a ligand binding domain, followed by the transmembrane, which forms the ion-conducting pore, and the cytosolic C-terminal domain (Figure 1) (4, 5).
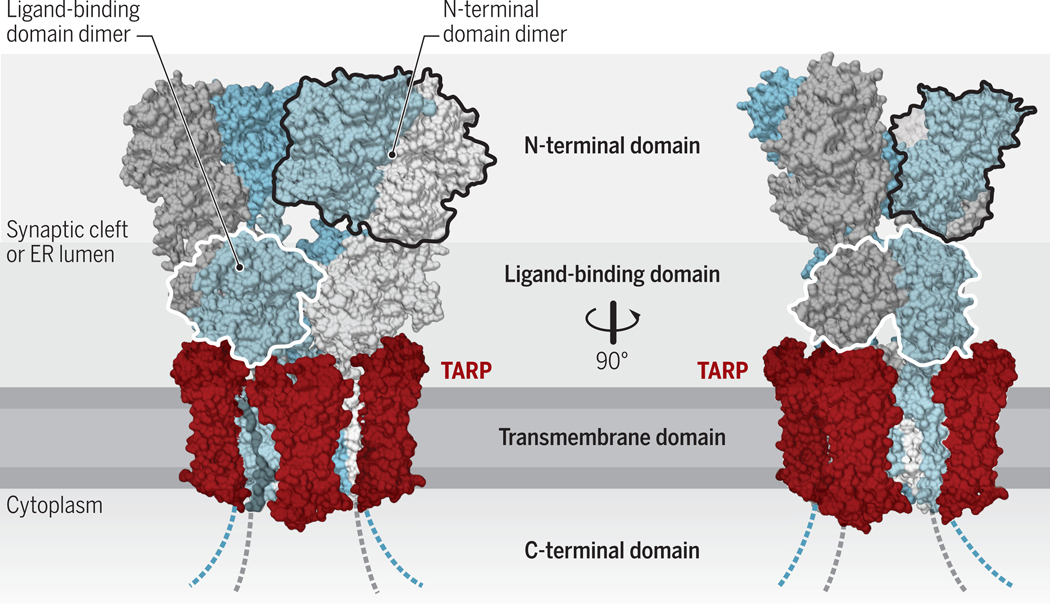
Figure 1. Structural architecture of AMPARs.
AMPARs are formed by four subunits, which are conformationally (and functionally) distinct (‘pore-proximal’ subunits are in grey and ‘pore-distal subunits’ in blue). These subunits consist of an extracellular N-terminal domain, the ligand binding domain, an integral membrane domain, and an intracellular C-terminus domain, and form tetrameric receptors (chains A-D). The large extracellular region faces the ER-lumen during receptor biogenesis and ultimately projects into the synaptic cleft. The transmembrane AMPAR regulatory proteins (TARPs) interact with the receptor at up to four positions around the transmembrane domain (two non-equivalent positions indicated in red; structure reproduced from PDB:5WEO). Credit: Adapted by A. Kitterman/Science Signaling
AMPARs are associated with various auxiliary subunits, which both influence receptor trafficking and modulate channel function (5). Among those, transmembrane AMPAR regulatory proteins (TARPs) are the most intensively studied. TARPs mediate postsynaptic receptor localization, which is best characterized for TARP γ2 (also known as stargazin) and γ8 due to their predominance in well studied brain areas, the cerebellum and hippocampus (6, 7). For this purpose, TARPs bind with their cytoplasmic C-termini to the first two PDZ domains of PSD-95, an abundant postsynaptic scaffolding protein. This TARP-mediated ‘slotting’ into the PSD scaffold has been recognized as a major AMPAR anchoring mechanism (6, 8–12) (Figure 1). Direct trapping of the receptor through its N-terminal domain, which protrudes into the synaptic cleft (Figure 1), has been described as an additional synaptic anchoring mechanism (13, 14). Here, we discuss such new mechanistic insights into AMPAR synaptic traffic and anchorage.
Secretory trafficking of AMPARs
AMPARs are synthesized in the endoplasmic reticulum (ER), where subunits assemble mainly into heterotetramers by first forming dimers and then dimers of dimers (15). The assembly of the initial dimers is driven by their N-terminal domains (16) (Figure 1), which have higher affinities for N-terminal domains of other subunits than their own (17). For instance, the GluA1 N-terminal domain has a more than 100-fold higher affinity for the GluA2 N-terminal domain than for another GluA1 N-terminal domain in a heterologous expression system, giving rise to predominantly heteromeric receptors. Quality control steps before AMPAR release from the ER are complex and poorly understood. These involve association with a select set of AMPAR-interacting proteins (18), sensing of Ca2+ release through ER-based IP3 and ryanodine receptors (19) and sensing of conformations underlying gating functions (20). In the neuronal soma, AMPARs then traffic through the Golgi apparatus for maturation by posttranslational modifications, including a change from high mannosylation to complex glycosylation and ultimately the trans-Golgi network before being transported along microtubules into dendrites (21, 22). This AMPAR transport, at least in Drosophila, requires activity of the Ca2+- and calmodulin-dependent protein kinase CaMKII (23, 24). This function is just one of various critical CaMKII functions, which, likely through additional molecular signaling mechanisms, plays a central role in the induction of long-term potentiation (LTP) (25, 26), which is thought to underlie learning and memory (27, 28).
AMPARs are also synthesized in dendrites, which appear to mostly lack the Golgi apparatus although a modified Golgi-related compartment, the Golgi outpost, has been described in dendrites for trafficking secretory cargo, which includes NMDA-type glutamate receptors (NMDARs) (29, 30). New work now reports that GluA1-containing AMPARs can traffic from dendritic ER through the ER-Golgi intermediary compartment directly to recycling endosomes, independently from the Golgi apparatus (31) (Figure 2). This secretory pathway contrasts with AMPAR trafficking in the soma, where AMPARs pass through the Golgi apparatus (31). In this work, the addition of an FK binding protein tag (3xFM) retained GluA1 in the ER until a de-dimerizing compound was added. Upon release, GluA1 appeared in recycling endosomes before it was detectable at the dendritic surface. Furthermore, disruption of recycling endosomes by expression of a dominant negative form of Rab11 reduced surface expression of 3xFM/mCherry GluA1 2h and 4h after the addition of de-dimerizer. Expression of a dominant negative form of Rab8, which disrupts the Golgi apparatus, affected the surface expression of 3xFM/mCherry GluA1 at 4h but not at 2h. These findings imply that secretion of GluA1 through the dendritic ER – ER-Golgi intermediary compartment – recycling endosomes route is faster than through the somatic ER – ER-Golgi intermediary compartment – Golgi apparatus route. This secretory pathway was further supported by an elegant combination of blocking exit from the ER-Golgi intermediary compartment (but not exit from the ER) at 20oC and subsequently blocking Golgi apparatus function with brefeldin A. GluA1 reached the cell surface after the temperature was raised to 37oC even if brefeldin A was added to block Golgi apparatus-mediated secretion. Roughly half of GluA1 and GluA2 on the cell surface possessed a high mannose glycosylation pattern typical for proteins that have not been processed in the Golgi apparatus, which reflects the proteins bypassing the Golgi apparatus during secretory trafficking (31, 32). Strikingly, the auxiliary TARP subunit γ8 only shows complex glycosylation when at the cell surface. It is possible that in dendrites, other auxiliary subunits are synthesized alongside AMPAR core subunits to enable secretory trafficking to ER-Golgi intermediary compartment and recycling endosomes, such as cornichon proteins (33) or SynDIG4 (34). Alternatively, auxiliary and core subunits might be synthesized and travel independently to associate after glycosylation processing in a late secretory compartment (for example, recycling endosomes) or on the cell surface. These studies raise interesting questions about the essential requirement for TARPs in AMPAR forward trafficking, and the percentage of receptors that are TARP-associated throughout their life-cycle. Indeed, different populations of synapses, suggested to contain different levels of TARP-association, have been recorded in Purkinje neurons of the cerebellum (35); however, PDZ interactions of TARPs appear to be essential for all AMPAR postsynaptic anchoring in hippocampal CA1 cells (36).
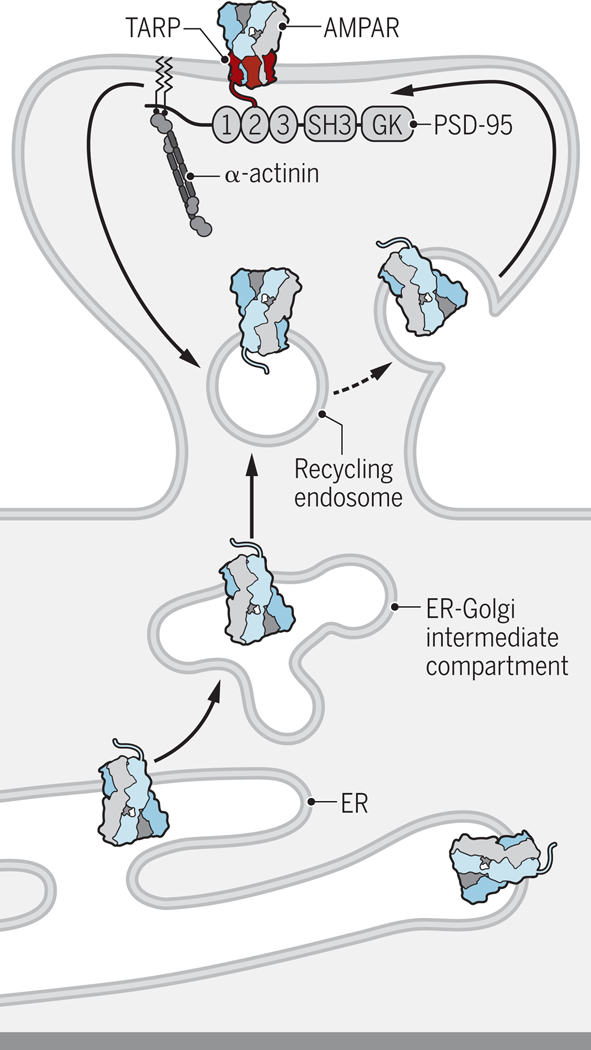
Figure 2. Dendritic AMPAR trafficking.
AMPARs are synthesized either in the soma (not depicted) or dendritic shaft in the endoplasmic reticulum (ER). From the dendritic ER AMPARs traffic through the ER–Golgi intermediate compartment to recycling endosomes, which mediate surface insertion of AMPARs (31). It is unclear where exactly exocytosis occurs but it is likely either in the dendritic shaft near dendritic spines or in dendritic spines outside the postsynaptic density (PSD). AMPARs then move through lateral diffusion to the PSD, where they are trapped by PSD-95 and its homologues through their binding to the C-termini of TARPs. PSD-95 is anchored at postsynaptic sites by α-actinin. When and where TARPs, which are mostly if not exclusively translated in the soma (31), associate with AMPARs and especially those synthesized in dendrites is unknown. Credit: Kellie Holoski/Science Signaling
The possibility of direct entry of AMPARs into recycling endosomes upon their synthesis in the ER without undergoing surface delivery and recycling has functional consequences because AMPAR trafficking through recycling endosomes is critical for LTP (37, 38). Accordingly, newly synthesized AMPARs can enter the LTP-supporting pool of AMPARs without prior surface insertion and endocytosis. That LTP requires stimulated exocytosis beyond basal surface delivery of plasma membrane proteins is also consistent with work demonstrating that LTP is prevented by clostridial toxins and other manipulations that interfere with the Ca2+-triggered exocytosis machinery (39, 40). These findings have been extended to show that both receptor exocytosis and surface diffusion are differentially required for increasing the synaptic AMPAR content in LTP (41).
Regulation of surface delivery of AMPARs
Stimulation of the cAMP-dependent protein kinase PKA augments surface expression of AMPARs by increasing the rate of surface insertion or re-insertion (42–44) and decreasing endocytosis (42). Furthermore, weak (but not strong) paradigms of LTP induction require cAMP signaling and PKA (45–50). The PKA-dependency of LTP is also age-dependent. For instance, LTP induced by a single 1 s long tetanus of 100 Hz is blocked by inhibiting PKA in mice that are 7–12 weeks but not in mice that are 3–4 weeks old (46). PKA activation through dopaminergic signaling can also convert the induction of spike timing-dependent synaptic depression into potentiation (51), which may be mechanistically underpinned by dopaminergic activation causing PKA-dependent AMPAR surface trafficking, as has been previously reported (44).
Stimulation of PKA renders AMPARs more readily available to contribute to and increase synaptic transmission, such as during LTP. This increase in AMPAR availability occurs because PKA stimulation promotes insertion of AMPARs into the neuronal surface (52, 53), particularly into the perisynaptic space (54–57) from where they can readily move to the actual postsynaptic site (Figure 3). The perisynaptic space is thought to be located somewhere on dendritic spines between the postsynaptic sites and the dendritic shaft although the precise localization is unclear and could also be on the shaft (but see below). It is functionally defined as containing AMPARs that become detectable during electrophysiological recording of postsynaptic responses to presynaptic electrical stimulation when glutamate reuptake is inhibited and thus a higher concentration of glutamate can reach the space surrounding the postsynaptic site upon presynaptic glutamate release. Given the arrangement of synaptic AMPARs opposite presynaptic release sites (58), and the non-saturation of synaptic AMPARs during transmission (59, 60), it is possible that this perisynaptic pool is localized at the postsynaptic density yet consists of receptors that are not aligned with vesicle release, and therefore do not contribute to synaptic transmission.
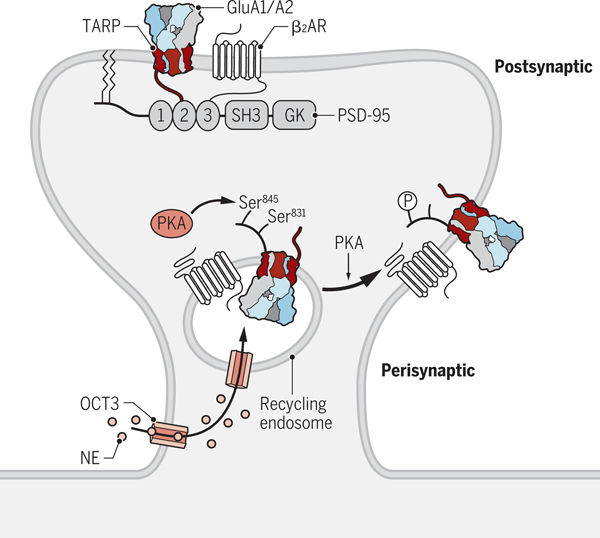
Figure 3. Regulation of perisynaptic AMPAR trafficking.
We propose that norepinephrine (NE) is shuttled by the amino acid transporter OCT3 localized in the plasma membrane from the cell exterior into the cytosol and then by OCT3 localized in recycling endosomes into their lumen. Here, NE stimulates the β2 adrenergic receptor (β2AR) associated with GluA1, which induces PKA activation and phosphorylation of Ser845 in GluA1. This phosphorylation event increases surface delivery of AMPARs from recycling endosomes. Lateral diffusion allows AMPARs to reach the PSD, where they are trapped by binding of the C_termini of TARPs to PSD-95. Credit: Kellie Holoski/Science Signaling
There is also an apparent connection between PKA-dependence of LTP and the requirement for Ca2+-permeable AMPARs, because PKA promotes synaptic delivery of Ca2+-permeable AMPARs during LTP (46, 61). Moreover, the dependence of potentiation on both PKA and Ca2+-permeable AMPARs has been separated by two LTP induction protocols (62). When multiple weak, spaced stimulations are employed, LTP requires both PKA and Ca2+-permeable AMPARs, whereas a single strong induction stimulus requires neither. Corresponding well with previous data (54–57), the authors suggest that PKA drives perisynaptic accumulation of Ca2+-permeable AMPARs during spaced stimulation, which are then required for long-term stability of potentiation.
PKA-mediated accumulation of AMPARs at perisynaptic sites depends on phosphorylation of the AMPAR GluA1 subunit on Ser845 in its cytosolic C-terminus (54, 63), which is a phosphorylation site for PKA (64). How PKA augments AMPAR trafficking to the perisynaptic space is unclear but could be through intracellular activation of the β2 adrenergic receptor– cAMP–PKA signaling cascade. The β2 adrenergic receptor forms a complex with AMPARs by binding with its extreme C-terminus to the third PDZ domain of PSD-95 (65), which in turn binds with its first two PDZ domains to the extreme C-termini of TARPs, thereby anchoring AMPARs at postsynaptic sites (Figure 4). This complex also contains all the other elements of the β2 adrenergic receptor– cAMP–PKA signaling cascade – namely, the trimeric stimulatory Gαs protein, adenylyl cyclase, and PKA – for efficient and localized regulation of AMPAR phosphorylation and surface expression (65). Only GluA1 associated with the β2 adrenergic receptor becomes phosphorylated on Ser845 upon stimulation of the receptor. At the same time, β2 adrenergic receptor stimulation increases the surface localization of GluA1 in dendritic shafts and spines within minutes, an effect that is inhibited when the β2 adrenergic receptor is acutely displaced from AMPARs by peptides that block the interaction (65). Collectively, these results indicate that β2 adrenergic receptor stimulation mediates plasma membrane insertion of pre-existing β2 adrenergic receptor–GluA1 complexes. Such findings raise the question how the endogenous β2 adrenergic receptor agonist norepinephrine (NE) can reach these complexes inside neurons, given that NE typically acts upon its release from norepinephrinergic neurons on β adrenergic receptors at the cell surface. NE can enter the cell interior through the transporter OCT3 and stimulate β adrenergic receptors inside cells (66, 67). We hypothesize that NE accesses the lumen of recycling endosomes where it stimulates β2 adrenergic receptors that form signaling complexes with AMPARs to trigger phosphorylation of Ser845. This phosphorylation event then increases surface expression of AMPARs through unknown mechanisms (Figure 5).
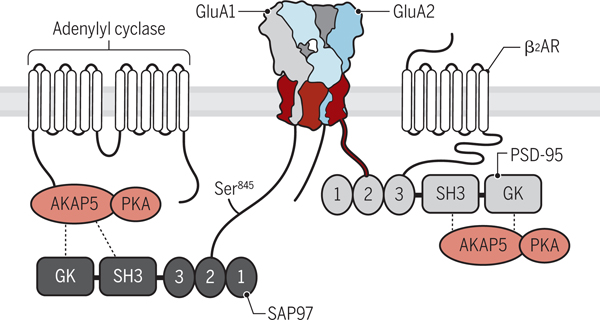
Figure 4. The AMPAR–β2 adrenergic receptor signaling complex.
The β2 adrenergic receptor binds through its extreme C-terminus to the third PDZ domain of PSD-95. In turn, the first two PDZ domains of PSD-95 bind to the C-termini of TARPs (red) including γ2 and γ8. Adenylyl cyclase binds through its N-terminus to the N-terminus of AKAP5 (also known as AKAP79 in humans, AKAP75 in cow, and AKAP150 in rodents), which binds through its C-terminus to PKA. AKAP5 is connected to AMPARs through SAP97, which binds to the C-terminus of GluA1, and potentially also through PSD-95. How Gs is linked to the β2 adrenergic receptor (β2AR) –AMPAR complex is unknown but could be through pre-association with the β2 adrenergic receptor. Credit: Kellie Holoski/Science Signaling
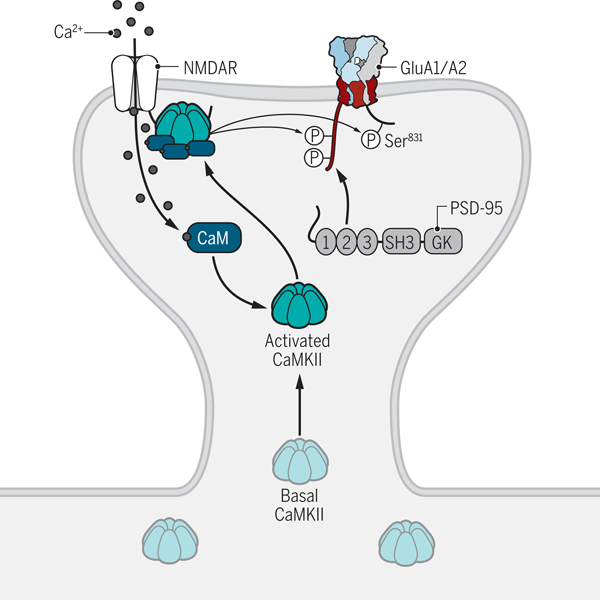
Figure 5. Regulation of postsynaptic AMPAR trafficking.
LTP-inducing stimuli trigger the influx of Ca2+ through NMDARs. Ca2+ binds to CaM and stimulates the activity of CaMKII. CaMKII is then recruited to the NMDAR complex by binding to the C-terminus of the GluN2B subunit. It subsequently phosphorylates the C-termini of TARPs including γ2 and γ8, which may lead to AMPAR trapping at the PSD. Phosphorylation of GluA1 on Ser831 by CaMKII also augments its channel activity. Credit: Kellie Holoski/Science Signaling
Regulation of postsynaptic AMPAR content
PKA activity and the phosphorylation of GluA1 on its PKA site Ser845 are not always required for LTP (46, 68) and are not sufficient to increase postsynaptic AMPAR content. This increase also requires Ca2+ influx and activation and signaling by CaMKII (25, 44, 63, 69–73). CaMKII acts in part by phosphorylating the AMPAR auxiliary TARP subunits γ2 (74–76) and/or γ8 (77) on multiple sites. These phosphorylation events have been suggested to strengthen binding of γ2 and γ8 to PSD-95, which enhances trapping of AMPARs at postsynaptic sites (Figure 5) (6, 8, 10–12, 76). However conflicting reports have suggested a primary requirement for either phosphorylation or PDZ anchoring of γ8, with little influence of the other (7, 36, 77). These reports require reconciliation.
How is surface delivery of AMPARs stimulated in those forms of LTP that do not require PKA? Perhaps the high levels of Ca2+ influx that occur during strong stimulus paradigms drive acute AMPAR surface delivery through synapotagmin-1– and synapotagmin-7– mediated acute exocytosis (40). Alternatively, strong stimulation paradigms of LTP might activate CaMKII more so than weaker ones (for example, two compared to one 100 Hz tetanus) so that CaMKII can compensate for lack of PKA signaling by phosphorylating Ser831 in the C-terminal domain of GluA1 upon stronger stimulation. Ser831 is just 14 residues upstream of Ser845 and is a prominent phosphorylation site for CaMKII (64, 78). In support of this hypothesis, LTP is absent in GluA1 S831A/S845A double knock-in mice (79) but is not affected in mice with single S831A and S845A knock-ins (80). It appears that one site but not both sites are required for LTP. It is conceivable that surface delivery of GluA1-containing AMPARs to the perisynaptic space can be stimulated by phosphorylation of GluA1 on either Ser831 by CaMKII or Ser845 by PKA. In fact, a contributory role by CaMKII in surface insertion of GluA1 had been reported earlier (44). However, LTP can also be induced when GluA1, GluA2, and GluA3 are completely eliminated and are replaced by a GluA1 mutant lacking its C-terminal domain from residues 824–906 (and thus cannot be phosphorylated at Ser831 and Ser845) (68). Furthermore, LTP can still be induced when Ser816 and Ser818 are replaced with Ala residues in the truncation mutant (68). Ser816 and Ser818 are phosphorylated by PKC and function as additional regulatory sites for surface expression and postsynaptic AMPAR targeting (81). These findings indicate that LTP and consequently an increase in postsynaptic glutamate receptor content can occur independently of these phosphorylation sites and that AMPARs can be anchored entirely by TARP PDZ interactions (36). However, it is important to note that these findings do not show that phosphorylation of Ser831 and Ser845 would not contribute to the regulation of AMPAR trafficking and LTP under normal conditions, such as in wild type mice with all AMPARs subunits present. This notion is supported by the finding discussed above that LTP is impaired in S831A/S845A double knock-in mice (79). Similarly, LTP induced by a 5 Hz tetanus that lasts 180 s and requires co-stimulation of the β2 adrenergic receptor is absent in single S845A knock-in mice (47). To clarify the confusion regarding the requirement for the GluA1 C-terminus in LTP, mice were engineered in which the GluA1 and GluA2 C-termini were exchanged, either individually, or simultaneously (82). In a GluA1-[GluA2 C-terminal domain] mouse, which lacks any GluA1 C-terminus, LTP is abolished but can be restored by knock-in of a form of GluA2 with the GluA1 C-terminal sequence (82). These data indicate that AMPAR trafficking in LTP requires the GluA1 C-terminal domain, likely through the aforementioned surface delivery mechanisms.
Knock-out of GluA1 (leaving GluA2 and GluA3 intact) impairs both surface expression of AMPARs and LTP (68, 83). Both AMPAR surface expression and LTP are impaired upon expression of C-terminal domain-lacking GluA1 or GluA2 on an AMPAR null background (68). Because of this coincidence of impaired surface expression and impaired LTP, the authors conclude that LTP requires an extrasynaptic pool of AMPARs at the cell surface (68). It is important to note that a role of extrasynaptic surface AMPARs does not exclude an equally important role of the pool of AMPARs in recycling endosomes (which could also be affected by these truncations). In fact, evidence for the requirement of both exocytosis and subsequent lateral diffusion to support the increase in postsynaptic AMPARs accumulation during LTP has been obtained with forms of GluA1 or GluA2 tagged on their extracellular N-termini by biotinylation in the ER (41). Cross-linking with tetrameric biotin binding proteins at the neuronal surface prevents short-term potentiation and impairs LTP in hippocampal slices. Accordingly, lateral diffusion of AMPARs present at the cell surface is required for LTP. A slowly developing potentiation that remains upon cross-linking is blocked by co-application of tetanus toxin, which inhibits exocytosis that is triggered by Ca2+ influx. In contrast, tetanus toxin does not block the short-term potentiation. Thus, the late phase of potentiation is driven by acute exocytosis, whereas the early phase within the first 2–3 min after the induction of LTP depends on lateral diffusion and not acute exocytosis.
The importance of precise postsynaptic localization of AMPARs
Because the affinity of AMPARs for glutamate is relatively low (high μM range), it had been predicted that only AMPARs that are precisely juxtaposed to presynaptic release sites are effectively activated (84, 85). Indeed, AMPARs are enriched in clusters that are ~80 nm in diameter (86, 87) and those clusters appear to be aligned with presynaptic release sites for fast and efficient synaptic transmission (58, 88). This arrangement has interesting consequences when considering synaptic potentiation. Does LTP involve enlargement of this trans-synaptic ‘nanocolumn’, addition of multiple aligned columns (89), or increased AMPAR clustering within a nanodomain? Enrichment of PSD-95 within nanodomains has been observed using chemical LTP induction (58). Functional evidence for activation of a subset of AMPARs within individual dendritic spines and perhaps within postsynaptic sites has so far been lacking, but is in line with data demonstrating that postsynaptic AMPARs are not saturated by glutamate release (59, 60).
To address this question, the light-induced dimerization of the plant photoreceptor cryptochrome with its binding partner CIB1 has been harnessed to enable the optogenetic recruitment of cryptochrome-tagged GluA1 to synapses through binding to CIB1-tagged PSD-95 or Homer 1c (90). This approach leads to an increase in frequency but not the average amplitude of mini-EPSCs occurring through spontaneous transmission (90), whereas uncaging of glutamate, which activates all receptors in an individual dendritic spine, results in an increase in AMPAR response amplitudes at nearly all spines upon light exposure. These data have been interpreted as showing ‘functional’ delivery of AMPARs only at weak or silent synapses, with little effect on established connections, despite ‘physical’ delivery of AMPARs to all synapses. Although this is an exciting interpretation that supports the ‘functionally clustered’ arrangement of the synapse (58), interpretation of mEPSC data requires more detailed analysis due to the number of events hidden below the noise level, which may contribute to the observed effects.
Postsynaptic anchoring of AMPARs by α-actinin
Knock-down and knock-out of PSD-95 reduces postsynaptic AMPAR responses by ~40%, suggesting that PSD-95 mediates postsynaptic localization of ~40% of the AMPARs in pyramidal cells of the hippocampal CA1 region (8, 10–12). Another ~40% of AMPAR postsynaptic localization depends on PSD-93 and most of the rest on SAP102 (10, 11). How PSD-95 itself docks onto postsynaptic sites has been unclear. Ephrin B3 has been previously implicated in this process (91), although it is unclear if it would be present at high enough levels to mediate postsynaptic anchoring of the highly abundant PSD-95. Instead, postsynaptic anchoring of PSD-95 and consequently of AMPARs has been shown to require α-actinin, which is highly enriched in spines and binds to the N-terminal 13 residues of PSD-95 (92). Knock down of all three α-actinin isoforms that are present in neurons reduces the density of synapses by ~40% but AMPAR content in the remaining synapses is comparable to control conditions, which phenocopies PSD-95 knock down. Those AMPARs not affected by loss of postsynaptic PSD-95 through knock-down of α-actinin are presumably anchored by PSD-93 and SAP102, which do not show any detectable binding to α-actinin (92).
Ca2+ influx through NMDARs leads to diffusion of a portion of PSD-95 out of spines (93, 94). This displacement of PSD-95 is mediated by calmodulin (CaM), which binds in the presence of Ca2+ to the extreme N-terminus of PSD-95 (95, 96). Ca2+/CaM promotes the depalmitoylation of the N-terminus of PSD-95 (95, 96), a posttranslational modification that is required for postsynaptic PSD-95 targeting (97, 98), and displaces α-actinin from PSD-95 (92), both of which contribute to the loss of PSD-95 from spines. During LTP, this PSD-95 displacement appears transient and to have a role in synaptic rearrangements that accompany stabilization of spine growth, which poses interesting questions about the role of PSD-95 in the initial potentiation of AMPAR currents. Ca2+/CaM binding to the PSD-95 N-terminus is also required for homeostatic synaptic downscaling upon a chronic increase in network activity in dissociated hippocampal cultures (96) because mutating Glu17 in the PSD-95 N-terminus to Arg (E17R) prevents Ca2+/CaM binding and downscaling. Furthermore, both effects can be rescued by a form of CaM with mutation of the positively charged Arg126, which forms an electrostatic interaction with the negatively charged Glu17, to Glu (96). The same E17R mutation in PSD-95 also prevents LTD (99), indicating that NMDAR-dependent LTD is also driven by Ca2+/CaM binding to the N-terminus of PSD-95 and its displacement from α-actinin and thereby from spines.
The role of N-terminal domains in postsynaptic anchoring of AMPARs
Synaptic anchoring of AMPARs does not depend only on the interaction of TARP PDZ-binding motif interactions with PSD-95, but also requires the PDZ-ligand in the C-terminal domain in the AMPAR GluA1 subunit (71, 100). Although such C-terminal domain interactions appear to not be essential for AMPAR clustering (86, 101) and their true influence on receptor anchoring are unclear (68, 102, 103), their predominant role may lie in the delivery of AMPARs to the surface, rather than stabilization at the synapse. Consistent with this notion, SAP97 recruits the A-kinase anchoring protein AKAP5 and with it PKA and adenylyl cyclase to GluA1 through binding to its C-terminal PDZ ligand motif (Figure 4) (104, 105), which is important for phosphorylation of Ser845 (105, 106), which in turn promotes surface expression of GluA1 as discussed above.
The influence of the N-terminal domains of AMPAR subunits in organizing functional synapses has been described in several reports. For instance, the N-terminal domain of GluA2 has been suggested to induce spine formation (107, 108), although other studies did not observe an effect of GluA2 on spine density (2, 13, 108) or even directly refuted this finding (109). The N-terminal domains of GluA2 has been reported to exert retrograde effects on presynaptic stabilization (108, 110). Moreover, the N-terminal domains of GluA subunits mediate the assembly of heterotetrameric AMPARs as discussed above (16, 17). Interactions of the N-terminal domains of GluA1 and GluA2 have been now reported to control the anchoring of AMPARs at postsynaptic sites (13). Utilizing the electrophysiological tagging method introduced by Malinow and his co-workers (71), Watson et al. ectopically expressed GluA2 in its unedited ‘R586Q’ form (‘GluA2Q’), which leads to the formation of homomeric AMPARs whose pores can be blocked by intracellular polyamines when the membrane potential is positive inside the cell (100,111). Using this indicator, GluA2Q expression was detected at synapses and contributed to transmission (13). However, removal of the N-terminal domain (ΔNTD) did not affect the rectification seen upon expression of GluA2Q, indicating that GluA2QΔNTD can accumulate at postsynaptic sites (13). However, expression of GluA2QΔNTD substantially reduced EPSC amplitudes and increased receptor mobility, causing an apparent reduction in the number of postsynaptic AMPARs. Thus, the N-terminal domain aids in the accumulation of AMPARs at postsynaptic sites presumably by fostering interactions with other synaptic proteins, which are abundant in the synaptic cleft (112).
Similar to GluA2Q expression, expression of full length GluA1 also results in inwardly rectifying AMPAR currents (100,111) and inwardly rectifying postsynaptic AMPAR responses (13). However, expression of N-terminally deleted ‘GluA1ΔNTD’ did not have these effects (13). Accordingly, postsynaptic accumulation of GluA1 strictly requires its N-terminal domain whereas the N-terminal domain of GluA2 only augments its postsynaptic localization. LTP is impaired in cells expressing a form of GluA1 lacking the N-terminal domain indicating that AMPAR anchoring during LTP also depends on this domain (100) (14).
The N-terminal domains of GluA1 and GluA2 facilitate postsynaptic AMPAR localization presumably by mediating or augmenting interactions with other synaptic proteins, which will need to be identified in future studies. Although TARPs interact with the N-terminal domain and would be at first glance candidates for this critical interaction (113), N-terminal deletion does not appear to alter TARP association (13).
The reduction of EPSC amplitude by ectopic expression of GluA2QΔNTD (see above) is most parsimoniously explained by GluA2QΔNTD acting in a dominant negative manner by competing with endogenous AMPARs for other proteins that are important for postsynaptic targeting. The C-terminus of GluA2 interacts with various proteins (114–116) and is a prime contender of mediating such interactions that are also important for postsynaptic targeting in addition to the presumed N-terminal domain interactions. In support of this notion, replacement of the GluA2 C-terminal domain with that of GluA1 alleviates the dominant negative effect of GluA2QΔNTD. Such a role for the GluA2 C-terminal domain in basal transmission fits well with a previous model (100), and likely reflects the role of the domain in receptor recycling with intracellular pools (115, 116).
What are potential interaction partners for the N-terminal domains of GluA1 and GluA2? N-cadherin has been implicated in the spine-inducing effect of the N-terminal 92 residues of GluA2 mentioned above (107). At the same time, glycosylation of the N-terminus of GluA2 on residue Asn370 is important for secretory trafficking (117). The neuronal pentraxin family of proteins interacts with the AMPAR N-terminal domain (118), with critical roles in maintaining synaptic AMPAR content in inhibitory interneurons (119) and retinal ganglion cells (120), but not CA1 pyramidal neurons (121). Neuropilin-2 binds to the extracellular N-terminus of GluA1 through its two CUB domains (122), which is noteworthy because two different CUB domain-containing proteins, SOL-1 and SOL-2, have been identified as critical for functional surface expression of AMPARs in Caenorhabditis elegans (123, 124). Moreover, two other CUB domain-containing proteins, NETO-1 and NETO-2, are auxiliary subunits of kainate receptors, which constitute another group of ionotropic glutamate receptors homologous AMPARs (125, 126). The neuropilin-2–GluA1 interaction is disrupted upon increased neuronal network activity in hippocampal cultures due to the activity-induced secretion of the neuropilin-2 agonist Semaphorin 3F (122). This reduction in postsynaptic response depends on the Ras GTPase activating protein (GAP) activity of the cytosolic C-terminus of PlexinA3, which dimerizes with neuropilin-2 in the AMPAR complex.
The PlexinA3-related PlexinA4 can directly interact with GluA2 (127) largely through the immunoglobulin-like transcription factor (IPT) domain of PlexinA4, which is extracellular and thus likely interacts with the N-terminus of GluA2. This interaction is induced by Semaphorin 3A, an endogenous ligand for PlexinA4 that causes retrograde transport of PlexinA4 from axons to the neuronal cell body where it binds GluA2 and stimulates its anterograde transport to distal dendrites.
Conclusions and Perspectives
Much has been learned about how AMPARs are trafficked and anchored at postsynaptic sites. This is a critical issue because synaptic strength is mostly determined by the number and functional availability of AMPARs, which is essential for normal learning of, for instance, declarative content, as well as pathological forms of learning such as drug addiction and posttraumatic stress disorder. Much more remains to be discovered about postsynaptic AMPAR localization in order to understand various forms of learning, which will inform strategies for the development of treatments for the pathological forms.
Gloss.
Learning and memory are thought to be supported by experience-dependent neuronal plasticity, which on a cellular level is expressed as long-term changes (such as potentiation or depression) of synaptic responses. Glutamate-gated ion channels known as AMPA receptors mediate basal neurotransmission. Their postsynaptic functional availability can be selectively modulated in correlation with a given stimulus. This review discusses the molecular basis of AMPA receptor trafficking to and anchoring at excitatory postsynaptic sites and their regulation by protein kinases.
Acknowledgments
Funding: Work in the laboratories of the authors was supported by NIH R01 MH097887, R01 NS078792, R01 AG055357 (to JWH) and the MRC MC_U105174197 and the BBSRC BB/N002113/1 (to IHG).
REFERENCES AND NOTES
- 1.Micheva KD, Busse B, Weiler NC, O’Rourke N, Smith SJ, Single-synapse analysis of a diverse synapse population: proteomic imaging methods and markers. Neuron 68, 639–653 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu W et al. , Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62, 254–268 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renner MC et al. , Synaptic plasticity through activation of GluA3-containing AMPA-receptors. eLife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traynelis SF et al. , Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62, 405–496 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greger IH, Watson JF, Cull-Candy SG, Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron 94, 713–730 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Chen L et al. , Stargazing regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 408, 936–943. (2000). [DOI] [PubMed] [Google Scholar]
- 7.Sumioka A et al. , PDZ binding of TARPgamma-8 controls synaptic transmission but not synaptic plasticity. Nature Neurosci 14, 1410–1412 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnell E et al. , Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA 99, 13902–13907 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS, PSD-95 involvement in maturation of excitatory synapses. Science 290, 1364–1368 (2000). [PubMed] [Google Scholar]
- 10.Elias GM et al. , Synapse-Specific and Developmentally Regulated Targeting of AMPA Receptors by a Family of MAGUK Scaffolding Proteins. Neuron 52, 307–320 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA, Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc Natl Acad Sci USA 105, 20953–20958 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schluter OM, Xu W, Malenka RC, Alternative N-terminal domains of PSD-95 and SAP97 govern activity-dependent regulation of synaptic AMPA receptor function. Neuron 51, 99–111 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Watson JF, Ho H, Greger IH, Synaptic transmission and plasticity require AMPA receptor anchoring via its N-terminal domain. eLife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz-Alonso J et al. , Subunit-specific role for the amino-terminal domain of AMPA receptors in synaptic targeting. Proc Natl Acad Sci USA 114, 7136–7141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herguedas B, Krieger J, Greger IH, Receptor heteromeric assembly-how it works and why it matters: the case of ionotropic glutamate receptors. Prog Mol Biol Transl Sci 117, 361–386 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Ayalon G, Stern-Bach Y, Functional assembly of AMPA and kainate receptors is mediated by several discrete protein-protein interactions. Neuron 31, 103–113 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Rossmann M et al. , Subunit-selective N-terminal domain associations organize the formation of AMPA receptor heteromers. EMBO J 30, 959–971 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brechet A et al. , AMPA-receptor specific biogenesis complexes control synaptic transmission and intellectual ability. Nature communications 8, 15910 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pick JE, Ziff EB, Regulation of AMPA receptor trafficking and exit from the endoplasmic reticulum. Mol Cell Neurosci, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penn AC, Williams SR, Greger IH, Gating motions underlie AMPA receptor secretion from the endoplasmic reticulum. EMBO J 27, 3056–3068 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greger IH, Khatri L, Ziff EB, RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron 34, 759–772 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Hanus C, Ehlers MD, Specialization of biosynthetic membrane trafficking for neuronal form and function. Current opinion in neurobiology 39, 8–16 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Hoerndli FJ et al. , Kinesin-1 regulates synaptic strength by mediating the delivery, removal, and redistribution of AMPA receptors. Neuron 80, 1421–1437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoerndli FJ et al. , Neuronal Activity and CaMKII Regulate Kinesin-Mediated Transport of Synaptic AMPARs. Neuron 86, 457–474 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huganir RL, Nicoll RA, AMPARs and synaptic plasticity: the last 25 years. Neuron 80, 704–717 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hell JW, CaMKII: Claiming Center Stage in Postsynaptic Function and Organization. Neuron 81, 249–265 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris RG, NMDA receptors and memory encoding. Neuropharmacology 74, 32–40 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Whitlock JR, Heynen AJ, Shuler MG, Bear MF, Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Jeyifous O et al. , SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nature Neurosci 12, 1011–1019 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horton AC et al. , Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron 48, 757–771 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Bowen AB, Bourke AM, Hiester BG, Hanus C, Kennedy MJ, Golgi-independent secretory trafficking through recycling endosomes in neuronal dendrites and spines. eLife 6, e27362 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanus C et al. , Unconventional secretory processing diversifies neuronal ion channel properties. eLife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwenk J et al. , Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science 323, 1313–1319 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Matt L et al. , SynDIG4/Prrt1 Is Required for Excitatory Synapse Development and Plasticity Underlying Cognitive Function. Cell reports 22, 2246–2253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devi SP, Howe JR, Auger C, Train stimulation of parallel fibre to Purkinje cell inputs reveals two populations of synaptic responses with different receptor signatures. The Journal of physiology 594, 3705–3727 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheng N et al. , LTP requires postsynaptic PDZ-domain interactions with glutamate receptor/auxiliary protein complexes. Proc Natl Acad Sci USA 115, 3948–3953 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD, Recycling endosomes supply AMPA receptors for LTP. Science 305, 1972–1975 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Park M et al. , Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron 52, 817–830 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lledo P-M, Zhang X, Sudhof TC, Malenka RC, Nicoll RA, Postsynaptic membrane fusion and long-term potentiation. Science 279, 399–403 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Wu D et al. , Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP. Nature 544, 316–321 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penn AC et al. , Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature 549, 384–388 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehlers MD, Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28, 511–525 (2000). [DOI] [PubMed] [Google Scholar]
- 43.Sun X, Zhao Y, Wolf ME, Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci 25, 7342–7351 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao C, Sun X, Wolf ME, Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J Neurochem 98, 1664–1677 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Blitzer RD, Wong T, Nouranifar R, Iyengar R, Landau EM, Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron 15, 1403–1414 (1995). [DOI] [PubMed] [Google Scholar]
- 46.Lu Y et al. , Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO J. 26, 4879–4890 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qian H et al. , β2 Adrenergic Receptor Supports Prolonged Theta Tetanus - induced LTP. J Neurophysiol 107, 2703–2712 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian H et al. , Phosphorylation of Ser1928 mediates the enhanced activity of the L-type Ca2+ channel Cav1.2 by the beta2-adrenergic receptor in neurons. Sci Signal 10, eaaf9659 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gelinas JN, Nguyen PV, Beta-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci 25, 3294–3303 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas MJ, Moody TD, Makhinson M, O’Dell TJ, Activity-dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron 17, 475–482 (1996). [DOI] [PubMed] [Google Scholar]
- 51.Brzosko Z, Schultz W, Paulsen O, Retroactive modulation of spike timing-dependent plasticity by dopamine. eLife 4, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oh MC, Derkach VA, Guire ES, Soderling TR, Extrasynaptic Membrane Trafficking Regulated by GluR1 Serine 845 Phosphorylation Primes AMPA Receptors for Long-term Potentiation. J Biol Chem 281, 752–758 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Man H-Y, Sekine-Aizawa Y, Huganir R, Regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci USA 104, 3579–3584 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He K et al. , Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci USA 106, 20033–20038 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y, Wang XB, Frerking M, Zhou Q, Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation. Proc Natl Acad Sci USA 105, 11388–11393 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Y, Wang XB, Frerking M, Zhou Q, Spine expansion and stabilization associated with long-term potentiation. J Neurosci 28, 5740–5751 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y, Wang XB, Zhou Q, Perisynaptic GluR2-lacking AMPA receptors control the reversibility of synaptic and spines modifications. Proc Natl Acad Sci USA 107, 11999–12004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang AH et al. , A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature 536, 210–214 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McAllister AK, Stevens CF, Nonsaturation of AMPA and NMDA receptors at hippocampal synapses. Proc Natl Acad Sci USA 97, 6173–6178 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu G, Choi S, Tsien RW, Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron 22, 395–409 (1999). [DOI] [PubMed] [Google Scholar]
- 61.Sanderson JL, Gorski JA, Dell’Acqua ML, NMDA Receptor-Dependent LTD Requires Transient Synaptic Incorporation of Ca(2+)-Permeable AMPARs Mediated by AKAP150-Anchored PKA and Calcineurin. Neuron 89, 1000–1015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park P et al. , Calcium-Permeable AMPA Receptors Mediate the Induction of the Protein Kinase A-Dependent Component of Long-Term Potentiation in the Hippocampus. J Neurosci 36, 622–631 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Esteban JA et al. , PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nature Neurosci 6, 136–143 (2003). [DOI] [PubMed] [Google Scholar]
- 64.Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL, Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16, 1179–1188 (1996). [DOI] [PubMed] [Google Scholar]
- 65.Joiner ML et al. , Assembly of a beta(2)-adrenergic receptor-GluR1 signalling complex for localized cAMP signalling. EMBO J 29, 482–495 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsvetanova NG, von Zastrow M, Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat Chem Biol 10, 1061–1065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Irannejad R et al. , Functional selectivity of GPCR-directed drug action through location bias. Nat Chem Biol 13, 799–806 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA, LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature 493, 495–500 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malinow R, Schulman H, Tsien RW, Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245, 862–866 (1989). [DOI] [PubMed] [Google Scholar]
- 70.Malenka RC et al. , An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature 340, 554–557 (1989). [DOI] [PubMed] [Google Scholar]
- 71.Hayashi Y et al. , Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267 (2000). [DOI] [PubMed] [Google Scholar]
- 72.Herring BE, Nicoll RA, Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annual review of physiology 78, 351–365 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Halt AR et al. , CaMKII binding to GluN2B is Critical During Memory Consolidation EMBO J 31, 1203–1216 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sumioka A, Yan D, Tomita S, TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron 66, 755–767 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS, Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron 45, 269–277 (2005). [DOI] [PubMed] [Google Scholar]
- 76.Hafner AS et al. , Lengthening of the Stargazin Cytoplasmic Tail Increases Synaptic Transmission by Promoting Interaction to Deeper Domains of PSD-95. Neuron 86, 475–489 (2015). [DOI] [PubMed] [Google Scholar]
- 77.Park J et al. , CaMKII Phosphorylation of TARPgamma-8 Is a Mediator of LTP and Learning and Memory. Neuron 92, 75–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mammen AL, Kameyama K, Roche KW, Huganir RL, Phosphorylation of the a-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J. Biol. Chem. 272, 32528–32533 (1997). [DOI] [PubMed] [Google Scholar]
- 79.Lee HK et al. , Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112, 631–643 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Lee HK, Takamiya K, He K, Song L, Huganir RL, Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. Journal of neurophysiology 103, 479–489 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin DT et al. , Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nature neuroscience 12, 879–887 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou Z et al. , The C-terminal tails of endogenous GluA1 and GluA2 differentially contribute to hippocampal synaptic plasticity and learning. Nature Neurosci 21, 50–62 (2018). [DOI] [PubMed] [Google Scholar]
- 83.Zamanillo D et al. , Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284, 1805–1811 (1999). [DOI] [PubMed] [Google Scholar]
- 84.Lisman J, Raghavachari S, A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci STKE 2006, re11 (2006). [DOI] [PubMed] [Google Scholar]
- 85.Franks KM, Stevens CF, Sejnowski TJ, Independent sources of quantal variability at single glutamatergic synapses. J Neurosci 23, 3186–3195 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacGillavry HD, Song Y, Raghavachari S, Blanpied TA, Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron 78, 615–622 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nair D et al. , Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J Neurosci 33, 13204–13224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Biederer T, Kaeser PS, Blanpied TA, Transcellular Nanoalignment of Synaptic Function. Neuron 96, 680–696 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lisman J, Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: long-term potentiation, long-term depression, short-term potentiation and scaling. Philos Trans R Soc Lond B Biol Sci 372, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sinnen BL et al. , Optogenetic Control of Synaptic Composition and Function. Neuron 93, 646–660 e645 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hruska M, Henderson NT, Xia NL, Le Marchand SJ, Dalva MB, Anchoring and synaptic stability of PSD-95 is driven by ephrin-B3. Nature neuroscience 18, 1594–1605 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matt L et al. , alpha-Actinin Anchors PSD-95 at Postsynaptic Sites. Neuron 97, 1094–1109 e1099 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steiner P et al. , Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron 60, 788–802 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nelson CD, Kim MJ, Hsin H, Chen Y, Sheng M, Phosphorylation of Threonine-19 of PSD-95 by GSK-3beta is Required for PSD-95 Mobilization and Long-Term Depression. J Neurosci 33, 12122–12135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y et al. , Capping of the N-terminus of PSD-95 by calmodulin triggers its postsynaptic release. EMBO J 33, 1341–1353 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chowdhury D et al. , Ca(2+)/calmodulin binding to PSD-95 mediates homeostatic synaptic scaling down. EMBO J 37, 122–138 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.El-Husseini Ael D et al. , Synaptic strength regulated by palmitate cycling on PSD-95. Cell 108, 849–863. (2002). [DOI] [PubMed] [Google Scholar]
- 98.Fukata Y et al. , Local palmitoylation cycles define activity-regulated postsynaptic subdomains. The Journal of cell biology 202, 145–161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu W et al. , Molecular dissociation of the role of PSD-95 in regulating synaptic strength and LTD. Neuron 57, 248–262 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shi S, Hayashi Y, Esteban JA, Malinow R, Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105, 331–343 (2001). [DOI] [PubMed] [Google Scholar]
- 101.Bats C, Groc L, Choquet D, The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53, 719–734 (2007). [DOI] [PubMed] [Google Scholar]
- 102.Kim CH et al. , Persistent hippocampal CA1 LTP in mice lacking the C-terminal PDZ ligand of GluR1. Nature Neurosci 8, 985–987 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Boehm J, Ehrlich I, Hsieh H, Malinow R, Two mutations preventing PDZ-protein interactions of GluR1 have opposite effects on synaptic plasticity. Learning & memory 13, 562–565 (2006). [DOI] [PubMed] [Google Scholar]
- 104.Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW, SAP97 is associated with the a-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J. Biol. Chem. 273, 19518–19524 (1998). [DOI] [PubMed] [Google Scholar]
- 105.Zhang M et al. , Adenylyl Cyclase Anchoring by a Kinase Anchor Protein AKAP5 (AKAP79/150) Is Important for Postsynaptic beta-Adrenergic Signaling. The J Biol Chem 288, 17918–17931 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tavalin SJ et al. , Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J Neurosci 22, 3044–3051 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saglietti L et al. , Extracellular Interactions between GluR2 and N-Cadherin in Spine Regulation. Neuron 54, 461–477 (2007). [DOI] [PubMed] [Google Scholar]
- 108.Tracy TE, Yan JJ, Chen L, Acute knockdown of AMPA receptors reveals a trans-synaptic signal for presynaptic maturation. EMBO J 30, 1577–1592 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Biou V, Bhattacharyya S, Malenka RC, Endocytosis and recycling of AMPA receptors lacking GluR2/3. Proceedings of the National Academy of Sciences of the United States of America 105, 1038–1043 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ripley B, Otto S, Tiglio K, Williams ME, Ghosh A, Regulation of synaptic stability by AMPA receptor reverse signaling. Proc Natl Acad Sci USA 108, 367–372 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bowie D, Mayer ML, Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15, 453–462 (1995). [DOI] [PubMed] [Google Scholar]
- 112.Perez de Arce K et al. , Topographic Mapping of the Synaptic Cleft into Adhesive Nanodomains. Neuron 88, 1165–1172 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cais O et al. , Mapping the interaction sites between AMPA receptors and TARPs reveals a role for the receptor N-terminal domain in channel gating. Cell reports 9, 728–740 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dong H et al. , GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 386, 279–284 (1997). [DOI] [PubMed] [Google Scholar]
- 115.Braithwaite SP, Xia H, Malenka RC, Differential roles for NSF and GRIP/ABP in AMPA receptor cycling. Proc Natl Acad Sci USA 99, 7096–7101 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hanley JG, Khatri L, Hanson PI, Ziff EB, NSF ATPase and alpha-/beta-SNAPs disassemble the AMPA receptor-PICK1 complex. Neuron 34, 53–67 (2002). [DOI] [PubMed] [Google Scholar]
- 117.Takeuchi Y, Morise J, Morita I, Takematsu H, Oka S, Role of Site-Specific N-Glycans Expressed on GluA2 in the Regulation of Cell Surface Expression of AMPA-Type Glutamate Receptors . PLoS One 10, e0135644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sia GM et al. , Interaction of the N-terminal domain of the AMPA receptor GluR4 subunit with the neuronal pentraxin NP1 mediates GluR4 synaptic recruitment. Neuron 55, 87–102 (2007). [DOI] [PubMed] [Google Scholar]
- 119.Pelkey KA et al. , Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 85, 1257–1272 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Farhy-Tselnicker I et al. , Astrocyte-Secreted Glypican 4 Regulates Release of Neuronal Pentraxin 1 from Axons to Induce Functional Synapse Formation. Neuron 96, 428–445 e413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang MC et al. , Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nature Neurosci 13, 1090–1097 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Q et al. , Neuropilin-2/PlexinA3 Receptors Associate with GluA1 and Mediate Sema3F-Dependent Homeostatic Scaling in Cortical Neurons. Neuron 96, 1084–1098 e1087 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zheng Y, Mellem JE, Brockie PJ, Madsen DM, Maricq AV, SOL-1 is a CUB-domain protein required for GLR-1 glutamate receptor function in C. elegans. Nature 427, 451–457 (2004). [DOI] [PubMed] [Google Scholar]
- 124.Wang R et al. , The SOL-2/Neto auxiliary protein modulates the function of AMPA-subtype ionotropic glutamate receptors. Neuron 75, 838–850 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang W et al. , A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron 61, 385–396 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Straub C et al. , Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nature Neurosci 14, 866–873 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yamashita N et al. , Plexin-A4-dependent retrograde semaphorin 3A signalling regulates the dendritic localization of GluA2-containing AMPA receptors. Nature communications 5, 3424 (2014). [DOI] [PubMed] [Google Scholar]