Abstract
Background
Fatigue is a common and potentially distressing symptom for patients with rheumatoid arthritis (RA), with no accepted evidence‐based management guidelines. Evidence suggests that biologic interventions improve symptoms and signs in RA as well as reducing joint damage.
Objectives
To evaluate the effect of biologic interventions on fatigue in rheumatoid arthritis.
Search methods
We searched the following electronic databases up to 1 April 2014: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, Current Controlled Trials Register, the National Research Register Archive, The UKCRN Portfolio Database, AMED, CINAHL, PsycINFO, Social Science Citation Index, Web of Science, and Dissertation Abstracts International. In addition, we checked the reference lists of articles identified for inclusion for additional studies and contacted key authors.
Selection criteria
We included randomised controlled trials if they evaluated a biologic intervention in people with rheumatoid arthritis and had self reported fatigue as an outcome measure.
Data collection and analysis
Two reviewers selected relevant trials, assessed methodological quality and extracted data. Where appropriate, we pooled data in meta‐analyses using a random‐effects model.
Main results
We identified 32 studies for inclusion in this current review. Twenty studies evaluated five anti‐tumour necrosis factor (anti‐TNF) biologic agents (adalimumab, certolizumab, etanercept, golimumab and infliximab), and 12 studies focused on five non‐anti‐TNF biologic agents (abatacept, canakinumab, rituximab, tocilizumab and an anti‐interferon gamma monoclonal antibody). All but two of the studies were double‐blind randomised placebo‐controlled trials. In some trials, patients could receive concomitant disease‐modifying anti‐rheumatic drugs (DMARDs). These studies added either biologics or placebo to DMARDs. Investigators did not change the dose of the latter from baseline. In total, these studies included 9946 participants in the intervention groups and 4682 participants in the control groups. Overall, quality of randomised controlled trials was moderate with a low to unclear risk of bias in the reporting of the outcome of fatigue. We downgraded the quality of the studies from high to moderate because of potential reporting bias (studies included post hoc analyses favouring reporting of positive result and did not always include all randomised individuals). Some studies recruited only participants with early disease. The studies used five different instruments to assess fatigue in these studies: the Functional Assessment of Chronic Illness Therapy Fatigue Domain (FACIT‐F), Short Form‐36 Vitality Domain (SF‐36 VT), Visual Analogue Scale (VAS) (0 to 100 or 0 to 10) and the Numerical Rating Scale (NRS). We calculated standard mean differences for pooled data in meta‐analyses. Overall treatment by biologic agents led to statistically significant reduction in fatigue with a standardised mean difference of −0.43 (95% confidence interval (CI) −0.38 to −0.49). This equates to a difference of 6.45 units (95% CI 5.7 to 7.35) of FACIT‐F score (range 0 to 52). Both types of biologic agents achieved a similar level of improvement: for anti‐TNF agents, this stood at −0.42 (95% CI −0.35 to −0.49), equivalent to 6.3 units (95% CI 5.3 to 7.4) on the FACIT‐F score; and for non‐anti‐TNF agents, it was −0.46 (95% CI −0.39 to −0.53), equivalent to 6.9 units (95% CI 5.85 to 7.95) on the FACIT‐F score. In most studies, the double‐blind period was 24 weeks or less. No study assessed long‐term changes in fatigue.
Authors' conclusions
Treatment with biologic interventions in patients with active RA can lead to a small to moderate improvement in fatigue. The magnitude of improvement is similar for anti‐TNF and non‐anti‐TNF biologics. However, it is unclear whether the improvement results from a direct action of the biologics on fatigue or indirectly through reduction in inflammation, disease activity or some other mechanism.
Keywords: Humans; Abatacept; Abatacept/therapeutic use; Adalimumab; Adalimumab/therapeutic use; Antibodies, Monoclonal; Antibodies, Monoclonal/therapeutic use; Antibodies, Monoclonal, Humanized; Antibodies, Monoclonal, Humanized/therapeutic use; Antirheumatic Agents; Antirheumatic Agents/therapeutic use; Arthritis, Rheumatoid; Arthritis, Rheumatoid/complications; Arthritis, Rheumatoid/drug therapy; Certolizumab Pegol; Certolizumab Pegol/therapeutic use; Etanercept; Etanercept/therapeutic use; Fatigue; Fatigue/drug therapy; Fatigue/etiology; Fatigue/therapy; Immunosuppressive Agents; Immunosuppressive Agents/therapeutic use; Infliximab; Infliximab/therapeutic use; Interferon‐gamma; Interferon‐gamma/antagonists & inhibitors; Randomized Controlled Trials as Topic; Rituximab; Rituximab/therapeutic use
Plain language summary
Biological interventions for the management of fatigue in rheumatoid arthritis
Background
What is rheumatoid arthritis and what are biologics?
When you have rheumatoid arthritis, your immune system, which normally fights infection, attacks the lining of your joints, causing swelling, stiffness and pain. The small joints of your hands and feet are usually affected first. There is no cure for rheumatoid arthritis at present, so treatments aim to relieve pain and stiffness and improve your ability to move. Biologics are medications that can reduce joint inflammation, improve symptoms and prevent joint damage.
Fatigue is an important symptom in people with rheumatoid arthritis. However, there is no consensus on the most effective management approaches for it. A number of studies have explored the effects of biologic response modifiers (biologics) in the management of rheumatoid arthritis and associated symptoms such as fatigue. We carried out the current review to evaluate the effects of these therapies on fatigue in adults with rheumatoid arthritis.
Study characteristics
We searched for all research published up to 1 April 2014, finding 32 relevant studies. There were 19 studies on five anti‐TNF biologics (adalimumab, certolizumab, etanercept, golimumab and infliximab) and 12 studies on five non‐anti‐TNF biologics (abatacept, canakinumab, rituximab, tocilizumab and an anti‐interferon gamma monoclonal antibody).
Key results
Altogether 9,946 participants received biologics and 4,682 participants received standard therapy. All but two of the studies were randomised placebo‐controlled trials, the gold standard in terms of study quality. We compared the effects of biologics versus placebo. In some studies, participants may have been taking standard therapy for rheumatoid arthritis at the start of the trial. In these studies, investigators added either biologics or placebo treatment to standard therapy. Overall, treatment by biologics led to small to moderate reductions (9 units reduction on a 0‐52 scale) in patient‐reported fatigue compared with 3 units in participants treated by placebo. It is unclear whether this improvement is due to a reduction in overall disease activity, a direct effect of the biologics or some other mechanism.
Quality of the evidence
There may have been some potential bias in the way investigators analysed data, and some studies did not include all randomised individuals, so we judged the quality of the evidence to be only moderate rather than high.
Summary of findings
Summary of findings for the main comparison. All biologics for fatigue in rheumatoid arthritis.
| All biologics for fatigue in rheumatoid arthritis | |||||
|
Patient or population: patients with fatigue in rheumatoid arthritis
Settings: hospital, outpatient clinics
Intervention: all biologics Comparison: placebo or usual care | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Control | Biologics | ||||
| Fatigue continuous measures Follow‐up: median 24 weeks | The mean change in fatigue score from baseline in the control for all biologics ‐ was 3.3 units lower of the FACIT‐F score or 3.9 lower of the SF‐36 vitality. | The standardised mean difference between control and intervention groups at study endpoint for all biologics was 6.45 units lower of the FACIT‐F score or 7.65 units of SF‐36 vitality | 14,628 (30 studies) |
⊕⊕⊕⊕ Moderatea | SMD −0.43 (95% CI −0.49 to −0.38). An SMD of 0.43 would be considered as a moderate effect. This equates to a difference of 6.45 units (95% CI 5.70 to 7.35) of FACIT‐F score (range 0‐52) or 7.65 units (95% CI 6.76 to 8.72) of SF‐36 vitality (range 0‐100). NNTB 5 (95% CI 5 to 6) |
| The mean change in fatigue score from baseline in the control for anti‐TNF biologics ‐ was 3.3 units lower of FACIT‐F score or 3.9 lower of the SF‐36 vitality. | The standardised mean difference between control and intervention groups at study endpoint for anti‐TNF biologics was 6.3 units lower of the FACIT‐F score or 7.5 units of SF‐36 vitality. | 8946 (19 studies) | ⊕⊕⊕⊕ Moderatea | SMD −0.42 (95% CI−0.35 to −0.49). An SMD of 0.42 would be considered as a moderate effect. This equates to a difference of 6.3 units (95% CI: 5.3 to 7.4) of FACIT‐F score (range 0‐52) or 7.5 units (95% CI 6.2 to 8.7) of SF‐36 vitality (range 0‐100). NNTB 6 (95% CI 5 to 7) | |
| The mean change in fatigue score from baseline in the control for non‐anti‐TNF biologics ‐ was 0.5 units lower of FACIT‐F score or 0.59 lower of the SF‐36 vitality. | The standardised mean difference between control and intervention groups at study endpoint for non‐anti‐TNF biologics was 6.9 units lower of FACIT‐F score or 8.19 units of SF‐36 vitality. | 5682 (11 studies) | ⊕⊕⊕⊕ Moderatea | An SMD of 0.46 would be considered as a moderate effect. This equates to a difference of 6.9 units (95% CI 5.85 to 7.95) of FACIT‐F score (range 0‐52) or 8.19 units (95% CI 6.94 to 9.43) of SF‐36 vitality (range 0‐100). NNTB 5 (95% CI 4 to 6) | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; NNTB: number needed to treat for an additional beneficial outcome; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aThe quality of the studies were downgraded from high to moderate because of potential reporting bias (studies included post hoc analysis favouring reporting of positive result and studies did not always include all randomised individuals.
Background
Description of the condition
Rheumatoid arthritis (RA) is an autoimmune, systemic, inflammatory condition causing pain and synovitis in the joints of the hands and feet (Conaghan 1999). Repeated flares of disease activity cause symptoms of pain, fatigue, stiffness and loss of function. People with RA have identified fatigue as a key problem, which they consider harder to manage than pain (Hewlett 2005). Quantitative studies consistently show that significant fatigue occurs in up to 70% of patients in the UK (almost 0.4 million people) and is as common and severe as pain (Department of Health 2006; Wolfe 1996). There is a Cochrane review on the effect of non‐pharmaceutical interventions on fatigue in patients with RA (Cramp 2013).
Description of the intervention
Medication for controlling the inflammatory response (and therefore symptoms) in RA comprises non‐steroidal anti‐inflammatory drugs (NSAIDs), rapid introduction of disease‐modifying anti‐rheumatic drugs (DMARDs), glucocorticoids and biologic therapies to inhibit disease progression (Luqmani 2006). Although there is evidence that biologic interventions can improve symptoms of pain, stiffness, inflammation and loss of function (Blumenauer 2002; Blumenauer 2003; Maxwell 2009; Mertens 2009; Navarro‐Sarabia 2005; Singh 2009), and studies increasingly include fatigue as a secondary outcome, no systematic review has clearly established the evidence for improvement in RA fatigue. Other pharmacological interventions such as anti‐depressants are often also used to improve intractable symptoms of RA such as pain and may also improve fatigue. A separate review is analysing these agents, along with DMARDs and NSAIDs.
How the intervention might work
RA fatigue probably acts through multiple and complex pathways that vary between and within patients over time (Hewlett 2008). Inflammatory activity may directly cause fatigue through systemic effects or indirectly through its effects on pain and function (Pollard 2006). Therefore, biologic agents may improve RA fatigue by reducing the inflammatory components of fatigue, pain and function.
Why it is important to do this review
People with RA have clearly identified fatigue as a common, unmanageable symptom that reduces quality of life (Hewlett 2005), and there is international consensus that all clinical trials should measure it (Kirwan 2007). In addition, ongoing research identifies fatigue as a key symptom associated with disease flare. Although there is no systematic review on the evidence for the effect of pharmacological interventions on RA fatigue, investigators often report the symptom as a secondary outcome. Clinicians need to be able to evaluate the potential (or limitations) of such interventions for reducing RA fatigue in order to reach concordant decisions with patients on treatment options.
Objectives
To evaluate the effect of biologic interventions on fatigue in rheumatoid arthritis.
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria
Randomised controlled trials of biologics in adults with confirmed RA that included fatigue as a primary or secondary outcome measure (and not just an adverse effect) and reported it separately for RA participants (Arnett 1988).
Exclusion criteria
Studies that only investigated non‐biologic interventions or non‐pharmacological interventions.
Types of participants
Adults (usually over 18 years of age) with a diagnosis of RA either confirmed by rheumatologist or using American College of Rheumatology (ACR) criteria (Arnett 1988).
Types of interventions
All recognised biologic interventions. These included anti‐TNF (infliximab, etanercept, adalimumab, certolizumab pegol and golimumab) and non‐anti‐TNF (rituximab, abatacept, tocilizumab, anakinra, canakinumab and anti‐IFN gamma monoclonal antibody) biologic agents.
The comparison arm could have been a placebo, alternative intervention (pharmacological or non‐pharmacological) or usual care, including no specific intervention for fatigue.
Types of outcome measures
Primary outcomes
The primary outcomes for this systematic review were change in self reported fatigue scores using validated measures and adverse events. We defined validated measures as instruments used to assess fatigue in clinical trials or observational studies as detailed in a recent review (Hewlett 2007). We included adverse events in the initial protocol; however, since then a separate Cochrane review has assessed adverse events associated with anti‐TNF and non‐anti‐TNF biologic treatments, so we have referred to this publication rather than conducting a separate analysis (Singh 2011).
Secondary outcomes
In addition to presenting data on the primary outcome of fatigue in the 'Summary of findings' table, we also extracted the secondary outcomes of pain, anxiety and depression.
Search methods for identification of studies
We developed our search strategies in line with recommendations from the Cochrane Musculoskeletal Review Group and present them in Appendix 1. We applied these search strategies to all databases, adapting them appropriately to suit database style.
Electronic searches
We searched the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue no).
MEDLINE (1966 to April 2014).
EMBASE (1983 to April 2014).
Cochrane Database of Systematic Reviews (2007 to April 2014).
Current Controlled Trials Register (USA) (2000 to April 2014).
The National Research Register (NRR) Archive (UK) (2006 to April 2014).
The UKCRN Portfolio Database (UK) (2006 to April 2014).
AMED (1985 to April 2014).
CINAHL (1982 to April 2014).
PsycINFO (1974 to April 2014).
Social Science Citation Index (1990 to April 2014).
Web of Science (1990 to April 2014).
Dissertation Abstracts International (1871 to April 2014).
WHO International Clinical Trials Registry Platform (ICTRP) (Nov 2005 to April 2014).
Searching other resources
In addition, we handsearched the reference lists of included studies and previous review papers to find additional studies, as well as the Topical Review Series on fatigue in musculoskeletal disease (Hewlett 2008). We contacted relevant authors in the field to ask about unpublished research that the search strategies could not have detected.
Data collection and analysis
Selection of studies
Two review authors assessed titles and abstracts for all records identified through the search strategies, retrieving full texts for all those that appeared to meet the inclusion criteria. We also acquired the full reports if there was any uncertainty or disagreement surrounding their inclusion, or if abstracts were not available and it was not possible to exclude the trial on title alone. Two independent review authors screened all full‐text articles for inclusion/exclusion criteria, resolving disagreements by discussion and the involvement of an arbiter where necessary.
Data extraction and management
For data extraction, the review team allocated papers to different authors according to their areas of expertise, and two reviewers independently retrieved the following details for each publication, tabulating them on a standardised form: intervention (including characteristics and duration); details of the participants' health status; assignment to groups (including process used, concealment and comparability of groups); outcome measures; details of outcome measures used for assessing fatigue, timing of measurements; adherence to intervention/control, sample size and statistical analysis methods (including use of intention‐to‐treat principle) as well as power to detect a change in fatigue, adverse events and withdrawals.
Assessment of risk of bias in included studies
The two review authors independently assessed the methodological quality of each trial using individual components of quality from tools such as the one provided by Cochrane. Additionally, two independent review authors assessed the risk of bias of the included studies. As recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008), we assessed the following methodological domains.
Sequence generation.
Allocation concealment.
Blinding of participants, personnel and outcome assessors.
Incomplete outcome data.
Selective outcome reporting.
Other potential threats to validity (e.g. appropriate use of co‐interventions).
We explicitly assessed each of these domains as being at 'low' or 'high' risk of bias; where insufficient information was available, or there was uncertainty over the potential for bias, we rated the study as being at 'unclear' risk of bias in that domain.
We also assessed the power of the study to detect change in RA fatigue by examining the power calculations reported in the studies. Where this was missing, we based our assessment on recent publications focusing on the Patient Acceptable Symptom State or the minimally important differences in RA fatigue (Heiberg 2008; Wells 2007). We also used methods described in (Hewlett 2007) to assess the validity of the fatigue measure.
Measures of treatment effect
As we expected, the identified studies used a range of fatigue outcome measures, so we calculated standardised mean differences (SMD). We recorded the central estimate (mean) and standard deviation (SD). Where the standard deviations were not explicitly stated, we calculated them from the standard error, the different means and their respective confidence intervals (CIs) or P values. Where studies described adverse events as dichotomous data, we had planned to report them as the proportion of participants experiencing the event in each arm and would have made comparisons using the risk ratio (RR) and the corresponding 95% CI. For rare events (< 10%), we planned to report the Peto odds ratio. However, as stated in Primary outcomes, in the end we did not perform any analyses on adverse events since this has already been studied in a separate Cochrane review (Singh 2011).
Unit of analysis issues
Some studies included multiple doses of the same intervention. In these cases, we divided the control group into equal numbers and included pairwise comparisons in the meta‐analysis as recommended in sections 9.3.9 and 16.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Dealing with missing data
Where the change in scores was not available, we sought these data from the authors. Failing that, we imputed them using methods recommended in section 16.1.3.2 of Higgins 2008.
We carried out an intention‐to‐treat analysis in studies that included participants allocated to the intervention arm regardless of whether or not they completed the follow‐up. In these studies we assumed that participants who dropped out of the study had no changes in their outcomes, assigning a conservative assessment of response to treatment. We requested further details from authors in cases where published data were incomplete.
Assessment of heterogeneity
Where appropriate, we formally assessed heterogeneity of the data using the I2 statistic (Higgins 2003). We judged a value greater than 50% to represent substantial heterogeneity. Where we detected this level of heterogeneity and there were sufficient studies available, we conducted subgroup analyses in an attempt to explain the heterogeneity.
Assessment of reporting biases
We used a funnel plot to assess the possibility of publication bias.
Data synthesis
We evaluated the quality of included studies using the GRADE approach (Schünemann 2008), which employs the following rating system: randomised trials (high), downgraded randomised trials (moderate), double‐downgraded randomised trials (low) and triple‐downgraded randomised trials (very low). The quality ratings may be decreased by:
limitations in the design and implementation of available studies, suggesting a high likelihood of bias;
indirectness of evidence (indirect population, intervention, control, outcomes);
unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
imprecision of results (wide confidence intervals); or
high probability of publication bias.
We expected a mixture of changes from baseline and absolute group differences across a variety of measures of RA fatigue. We also anticipated some variation in methods of analysis, including absolute difference compared between groups, and baseline‐adjusted differences between groups. We followed the Cochrane guidelines described in section 9.4.5.2 of Higgins 2008 to decide which group of studies we could include in any meta‐analysis. We imputed the SD if necessary as described in section 16.1.3.
Summary of finding tables
We present the grading and meta‐analyses in a 'Summary of findings' table.
Where there was no heterogeneity, we used a fixed‐effect model, and where there was heterogeneity, we used a random‐effects model. When the outcome used, or the number, quality or heterogeneity of existing trials contraindicated meta‐analysis, we reported and discussed each study individually, using effect sizes for fatigue difference (differences divided by the SD) and Cohen's statistic (0.2 to 0.5 = small effect, 0.5 to 0.8 = moderate, > 0.8 = large effect) (Cohen 1998). We calculated SMDs for pooled data in meta‐analysis. If trials reported more than one outcome measure, such as FACIT and SF‐36 VT, we used the latter. Negative values indicated reduction in the fatigue.
In order to estimate the number needed to treat for an additional beneficial outcome (NNTB) from the SMD, we performed a log transformation of the SMD to an odds ratio (OR) (Chinn 2000). Subsequently, we combined the resulting OR with an assumed control event rate (CER = 0.5) generating an estimated NNTB. These control group risks refer to proportions of people who improved by some (unspecified) amount in the continuous outcome ('responders').
Subgroup analysis and investigation of heterogeneity
Where sufficient studies were available and the data were heterogenous, we carried out separate meta‐analyses for studies according to different biologic agents.
Sensitivity analysis
We planned the following sensitivity analyses a priori in order to explore differences in effect size and to assess whether the conclusions were robust to the decision‐making process.
The effect of risk of bias in included studies ‐ defined as adequate allocation concealment and blinding of outcome assessors.
The effect of imputing missing data or transforming variables.
Results
Description of studies
Results of the search
We undertook a comprehensive literature search, including screening of titles and abstracts (where available). We retrieved 54 full‐text references for further evaluation, including 32 that met the criteria for the current review and excluding the remaining 22. Handsearching of reference lists led to the retrieval of six further full‐text studies; we excluded one because fatigue was not an outcome measure and five because we were unable to obtain necessary data from the authors (Figure 1).
1.
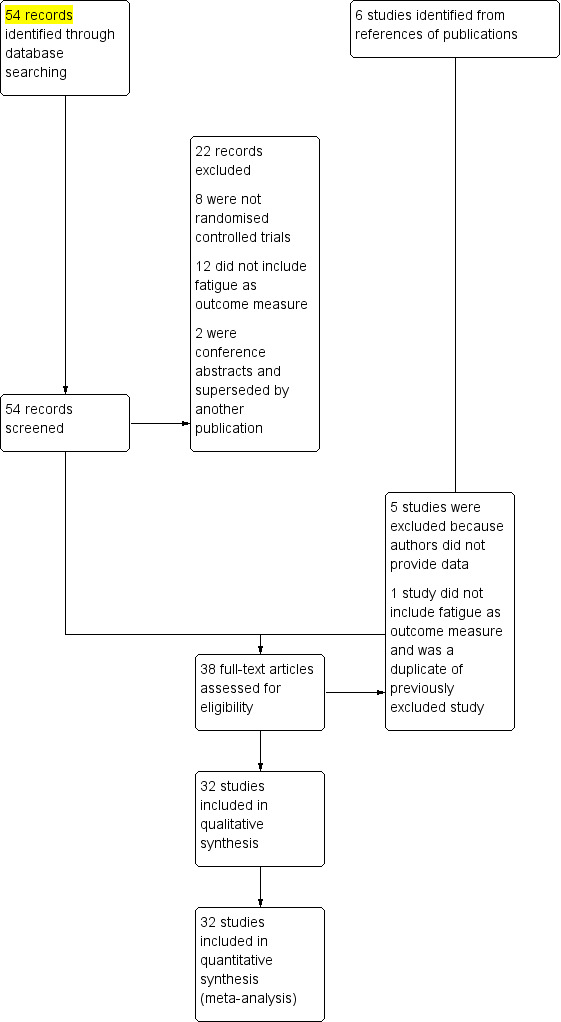
Study flow diagram
Included studies
All the studies recruited participants with established RA who fulfilled ACR criteria (Arnett 1988). There were 20 studies of five anti‐TNF agents: one studied infliximab (Maini 1999), three studied etanercept (Bae 2013; Emery 2008; Moreland 1999), six studied adalimumab (Hørslev‐Petersen 2014; Keystone 2004; Mittendorf 2007; Soubrier 2009; Strand 2012b; Weinblatt 2003), five studied certolizumab pegol (Choy 2012; Fleischmann 2009; Pope 2012; Smolen 2009a; Strand 2009), and five studied golimumab (Emery 2009; Keystone 2009; Li 2013; Smolen 2009b; Weinblatt 2013). All but two were randomised placebo‐controlled trials: Mittendorf 2007 reported the result of a pooled analysis of six randomised placebo‐controlled trials of adalimumab in RA, while Bae 2013 was a randomised open‐label active comparator trial of etanercept. Of the 12 non‐anti‐TNF biologic studies, 4 studied abatacept (Genovese 2005; Kremer 2003; Kremer 2006; Schiff 2008), three studied rituximab (Cohen 2006; Emery 2006; Rigby 2011), three studied tocilizumab (Genovese 2008; Smolen 2008; Strand 2012a), one studied canakinumab (Alten 2011), and one was an early phase trial of an anti‐IFN gamma monoclonal antibody (Lukina 1998). All but two of these studies were randomised placebo‐controlled trials: Bae 2013 compared etanercept with standard DMARD, and Lukina 1998 (which was translated from Russian) compared the effect of anti‐interferon gamma (anti‐IFNγ) monoclonal antibody with anti‐TNF antibodies as well as a combination of anti‐IFNγ and anti‐TNF antibodies. The sample size of Lukina 1998 was not based on statistical estimation; the study only recruited 25 participants and allocated just five to each treatment arm. This study (Lukina 1998) and the study by Maini 1999 did not contribute data to the meta‐analysis as we were unable to obtain precise estimate including standard deviation of change from the authors. In some trials, both active participants and controls could receive concomitant DMARDs. These studies added either biologics or placebo to DMARDs. The dose of the latter did not change from baseline. In total, these studies included 9,946 participants in the intervention groups and 4,682 participants in the control groups.
The primary outcomes of the included studies were disease activity, mostly assessed by ACR response criteria. The only exception is the pooled analyses in Mittendorf 2007, which focused on patient‐reported outcomes. None of the studies used fatigue as their primary outcome. These studies used five different instruments to assess fatigue: the Functional Assessment of Chronic Illness Therapy Fatigue Domain (FACIT‐F), Short Form‐36 Vitality Domain (SF‐36 VT), visual analogue scale (VAS) (0 to 100 or 0 to 10) and the Numerical Rating Scale NRS (0 to 10). The most commonly used instrument was SF‐36 VT, which authors reported in 15 studies. Nine studies used the FACIT‐F, five used a VAS, and three used an NRS. Fatigue measures were taken at the primary endpoints, which for most trials were at 24 weeks or less. One trial assessed fatigue at six weeks (Pope 2012), two trials at three months (Genovese 2005; Kremer 2003), and two studies at week 52 (Keystone 2004; Kremer 2006). Most papers did not provide data on pain, anxiety or depression, hence we were unable to conduct analyses of these secondary outcomes.
Excluded studies
Twenty‐two excluded publications did not meet the review inclusion criteria for the following reasons: 8 were not randomised controlled trials (Cella 2005; Duggan 2009; Frampton 2007; Kavanaugh 2012; Sansonno 2003; Strand 2012; Strand 2014; Yount 2007), 12 did not report fatigue as an outcome measure (Breedveld 2005; Furst 2003; Genovese 2010; Grigor 2004; Haugeberg 2009; Moreland 2000; Moreland 2002; Kavanaugh 2003; Kim 2007; Kremer 2008; Song 2007; Tak 2008), and two papers were conference abstracts superseded by another publication (Dougados 2007; Gnanasakthy 2013). We report details of the excluded studies in the Characteristics of excluded studies tables. Of the additional six studies identified through reference lists, five studies are awaiting classification until enough data is available to make a decision regarding inclusion (Elliott 1994; Kosinski 2000; St Clair 2004; Van der Kooij 2009; Westhovens 2006). We excluded the one remaining study, as fatigue was not an outcome measure and was a duplicate of a previously excluded study (Grigor 2004).
Risk of bias in included studies
Overall, for most of the included studies the risk of bias was low or unclear (Figure 2). However, for Lukina 1998, an early phase II trial of anti‐IFNγ monoclonal antibody, the risk of bias was high. This study did not provide any precision estimates on fatigue, so we did not include its results in the meta‐analyses of this review. Authors of study by Maini 1999 did not provide standard deviation so data from the study were not included in the meta‐analysis. Li 2013 and Pope 2012 were only available as conference abstracts, so carried a potential high risk of bias since details on randomisation, allocation concealment, blinding were not reported. Therefore, details on method of allocation, blinding and completeness of reporting could not be assessed adequately.
2.
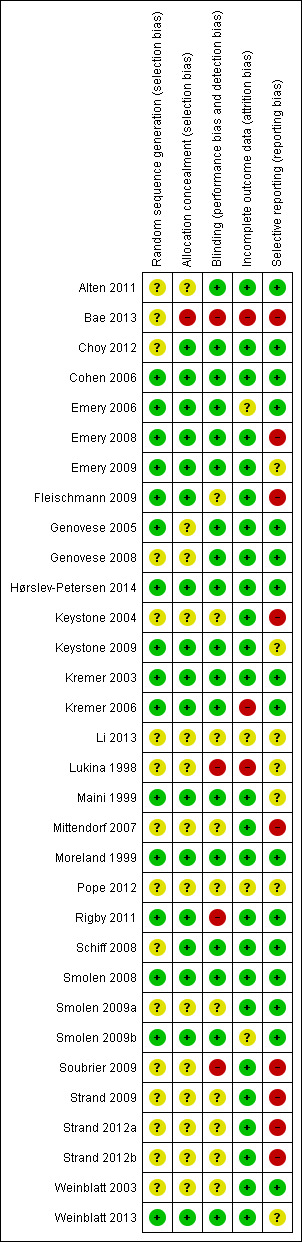
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Investigators described all the studies as randomised controlled trials but did not report the method of randomisation in 12 studies.
Blinding
Investigators described all but two studies as double‐blind, placebo‐controlled trials (Lukina 1998; Bae 2013); however, 10 studies do not provide details.
Incomplete outcome data
Four studies did not provide sufficient details on attrition (Bae 2013; Li 2013; Lukina 1998; Pope 2012), and four studies did not account for a number of the participants who dropped out before the end of the studies (Emery 2006; Kremer 2003; Kremer 2006; Smolen 2009b).
Selective reporting
Selective reporting bias was a concern in four studies that either did not report details of improvement in fatigue or health‐related quality of life (HRQol) or did not report them at the primary endpoint of the trial (Emery 2008; Fleischmann 2009; Keystone 2004; Soubrier 2009) . Four studies described patient‐reported outcomes from previously published RCTs (Bae 2013; Strand 2009; Strand 2012a; Strand 2014).
Other potential sources of bias
In four studies, the lack of information on completeness of data from the questionnaire was a potential risk of bias (Emery 2006; Emery 2008, Lukina 1998; Maini 1999). Maini 1999 reported the second year result of a randomised controlled trial. After the first year, 94 participants had a treatment gap of over eight weeks, while the rest continued immediately into the second year. Those participants with the gap may have received other medications. Furthermore standard deviations of change were not provided by the authors. Lukina 1998 had no placebo control arm and had a very short‐term follow‐up as well as a very small sample size. Furthermore, authors did not provide the statistical analysis method or precision estimates. A funnel plot of all the studies did not suggest significant publication bias (Figure 3).
3.
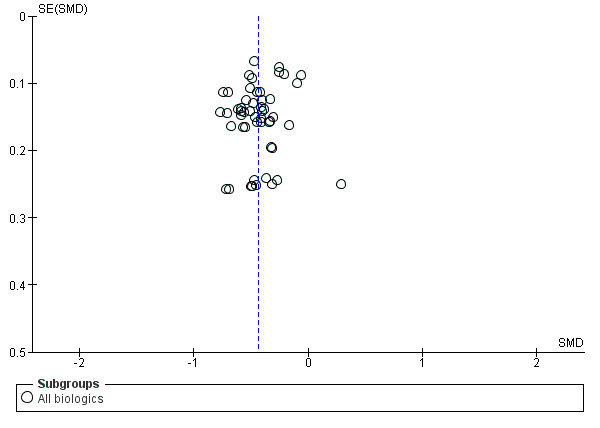
Funnel plot of comparison: 1 All Biologics, outcome: 1.1 All studies ‐ fatigue continuous measures.
Effects of interventions
See: Table 1
Some of the trials were multidose trials and therefore provided at least two comparisons for the purpose of meta‐analyses. Data from Lukina 1998 and Maini 1999 did not contribute to the result of the meta‐analyses because the trial did not provide precision estimates, and there was no placebo control group. All the randomised placebo‐controlled trials reported statistically significant improvement in disease activity as well as pain score in the active treatment groups when compared with controls. Two studies did not use placebo controls (Bae 2013; Lukina 1998).
Primary outcomes
Self reported fatigue
Overall treatment by biologic agents led to a statistically significant reduction in fatigue with an SMD of −0.43 (95% CI −0.49 to −0.38; P < 0.00001; Analysis 1.1; Figure 4). There was statistically significant heterogeneity (I2 = 48%, P < 0.0001). Anti‐TNF biologic agents had an SMD of −0.42 (95% CI −0.49 to −0.35, P < 0.00001; Analysis 2.1; Figure 5) and non‐anti‐TNF agents had an SMD of −0.46 (95% CI −0.53 to −0.39; P < 0.00001; Analysis 4; Figure 5), showing similar effects on fatigue (Table 1). However, there was statistically significant heterogeneity in anti‐TNF trials (I2 = 54%, P = 0.0002). The precise cause of heterogeneity is unclear but may be due to different dosage, participant characteristics (early versus established disease), previous treatment (biologic naive versus failed biologic participants) and comorbidities that are associated with fatigue (e.g. depression).
1.1. Analysis.
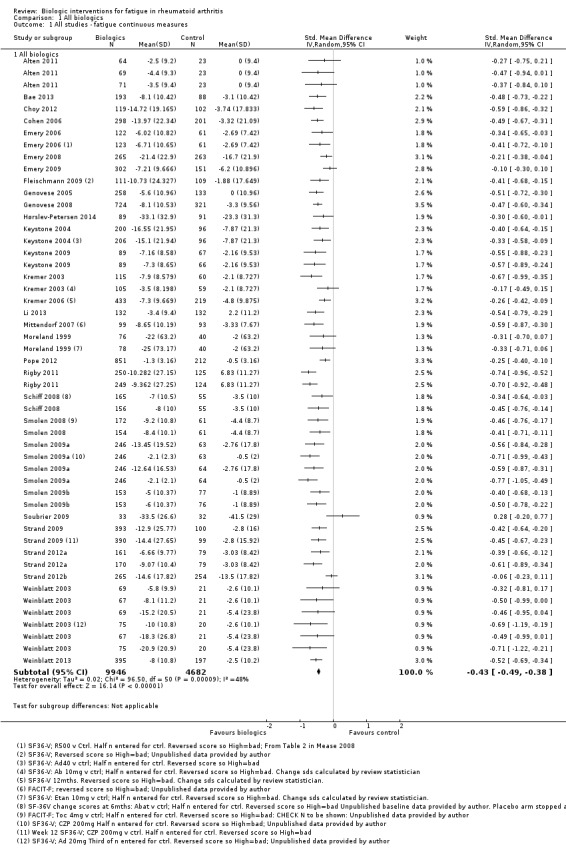
Comparison 1 All biologics, Outcome 1 All studies ‐ fatigue continuous measures.
4.
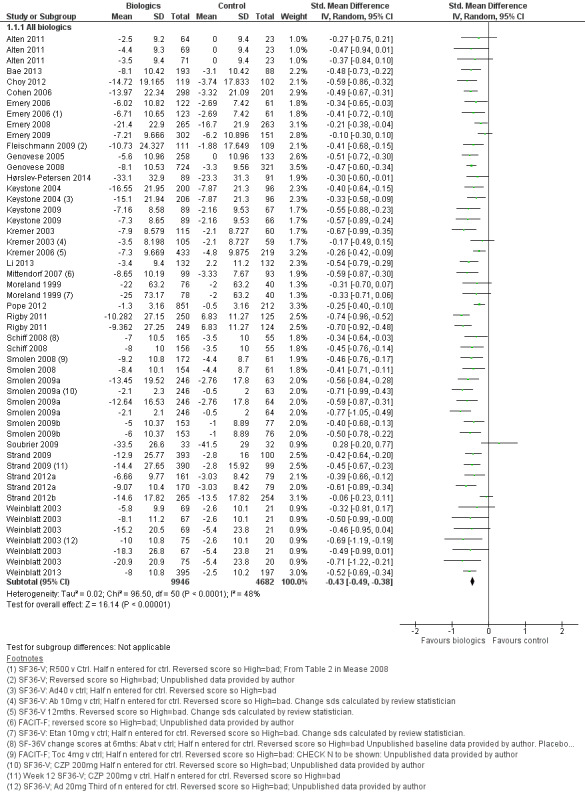
Forest plot of comparison: 1 All Biologics, outcome: 1.1 All studies ‐ fatigue continuous measures.
5.
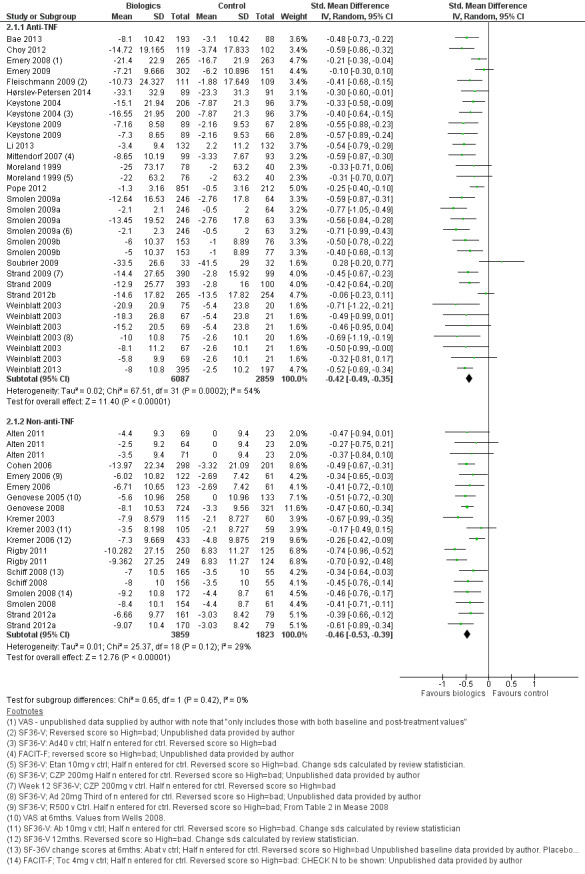
Forest plot of comparison: 4 Subgroup comparison: anti‐TNF vs non anti‐TNF, outcome: 4.1 Anti‐TNF and non anti‐TNF ‐ fatigue continuous measures.
We performed sensitivity analyses to explore the potential cause(s) of heterogeneity. Excluding dose‐ranging studies or trials in participants who had failed previous biologic therapy did not affect heterogeneity. Disease duration was, however, a significant factor. Five studies assessed the effect of anti‐TNF agents in early rheumatoid arthritis (Emery 2008; Hørslev‐Petersen 2014; Moreland 1999; Soubrier 2009; Strand 2012b). Excluding these studies reduced heterogeneity to statistical insignificance in the anti‐TNF meta‐analysis (I2 = 30%, P = 0.08). Most of the studies also reported significant improvement in disease activity as measured by ACR response criteria, disease activity score or both.
Secondary outcomes
Five studies did not report results of pain score, tender and swollen joints, or depression (Emery 2006; Kremer 2006; Maini 1999; Mittendorf 2007; Schiff 2008). All the other studies reported statistically significant reduction in pain score, physical function, and tender and swollen joint counts. However, improvement in pain was reported but data were not provided in many papers. Different pain instruments were used, visual analogue scale, SF‐36 bodily pain, numeric rating scale and percentage of patients with improvement in pain. Consequently, we were unable to pool data on pain for meta‐analysis. Most of the studies did not assess anxiety or depression. We could not determine whether reduction in fatigue is due to reduction in disease activity, pain, depression or a combination of these.
Discussion
Summary of main results
The aim of this review was to provide an overview of the effects of biologic interventions on fatigue in people with RA. The review revealed 32 RCTs investigating biologic interventions and including fatigue as an outcome measure. There were two main categories of biologic interventions: anti‐TNF (20 studies) and non‐anti‐TNF biologics (12 studies). Overall the quality of the evidence was moderate. Both anti‐TNF and non‐anti‐TNF biologic treatments led to a small to moderate reduction in fatigue in participants with RA.
Overall completeness and applicability of evidence
Randomised controlled trials of biologic agents for RA commonly assess fatigue. Clinical trials included in this review included all current biologic agents licensed for the treatment of RA. The magnitude of improvement is similar in the included studies. However, most of the studies were phase III trials conducted for the purpose of registration. Consequently, participants recruited into these trials had high disease activity, and the primary outcome measure was improvement in disease activity; change in fatigue was a secondary outcome measure. As the primary purpose of the interventions was not fatigue reduction, no consideration was given to factors that may confound or explain it, such as depression and reduced haemoglobin. Moreover, it is unclear whether improvement in fatigue was due to a reduction in overall disease activity or due to specific actions of the biologic agent. Analysis of fatigue in these studies did not make any adjustment for possible confounding factors such as change in pain, haemoglobin or mood. The duration of most of the double‐blind randomised controlled trials was 24 weeks or less. It is unclear whether improvement in fatigue is sustained with long‐term therapy. In these trials, recruited participants had highly active disease and moderate to high levels of fatigue at baseline. It is unclear whether biologic interventions improve fatigue in patients with moderate or low level of fatigue.
Quality of the evidence
Almost all the studies included are double‐blind, randomised placebo‐controlled trials. The quality of these trials was moderate, with highly variable reporting of fatigue using different measurement instruments. The SF‐36 VT was the most commonly used instrument. Many of these instruments were not developed specifically for assessing fatigue in RA, although they have been validated for assessing fatigue in other medical conditions and in general health. The use of the SF‐36 VT may also be questionable, as vitality may not be at the opposite end of the spectrum to fatigue. Consistent use of outcomes would simplify pooling of data and allow comparison between interventions.
Potential biases in the review process
There are two trials for which we failed to obtain data on fatigue from the authors; therefore there is some risk of reporting bias.
Agreements and disagreements with other studies or reviews
A recently published systematic review suggested that the biologic agents have a small to moderate effect in improving fatigue in RA, although it only included 10 studies (Chauffier 2012), all of which are included in this review. By including more studies, this Cochrane review reduces the risk of publication bias.
Authors' conclusions
Implications for practice.
Treatment with biologic interventions in patients with active RA and moderate to high levels of fatigue may lead to a small to moderate improvement in fatigue. The magnitude of improvement is similar for anti‐TNF and non‐anti‐TNF biologic agents. However, it is unclear whether the improvement results directly from the biologic interventions on fatigue or indirectly through reduction in inflammation and disease activity.
Implications for research.
Future research needs to determine the mechanisms whereby biologic interventions reduce fatigue in patients with RA, in particular, to assess whether this is a direct or indirect effect of biologic agents through intermediary factors such as disease activity. In addition, it is important to assess whether the improvement in fatigue associated with biologic interventions observed in short‐term randomised controlled trials is maintained in the long term.
Acknowledgements
Louise Falzon, Trials Search Coordinator of the Cochrane Musculoskeletal Group, for providing assistance with the search strategy.
Eugene A. Sushchuk for invaluable help with translating a study from Russian.
Appendices
Appendix 1. MEDLINE search strategy
1. exp arthritis, rheumatoid/
2. ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw.
3. 1 or 2
4. exp Fatigue/
5. fatigue$.tw.
6. (tired$ or weary or weariness or exhaustion or exhausted).tw.
7. ((astenia or asthenic) and syndrome).tw.
8. ((lack or loss or lost) adj3 (energy or vigo?r)).tw.
9. (apath$ or lassitude or weak$ or letharg$).tw.
10. (feel$ adj3 (drained or sleep$ or sluggish)).tw.
11. vitality.tw.
12. or/4‐11
13. randomized controlled trial.pt.
14. controlled clinical trial.pt.
15. randomized.ab.
16. placebo.ab.
17. drug therapy.fs.
18. randomly.ab.
19. trial.ab.
20. groups.ab.
21. or/13‐20
22. (animals not (humans and animals)).sh.
23. 21 not 22
24. and/3,12,23
Appendix 2. EMBASE search strategy
1 exp rheumatoid arthritis/
2 ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw.
3 1 or 2
4 exp fatigue/
5 fatigue$.tw.
6 (tired$ or weary or weariness or exhaustion or exhausted).tw.
7 ((astenia or asthenic) and syndrome).tw.
8 ((lack or loss or lost) adj3 (energy or vigo?r)).tw.
9 (apath$ or lassitude or weak$ or letharg$).tw.
10 (feel$ adj3 (drained or sleep$ or sluggish)).tw.
11 vitality.tw.
12 or/4‐11
13 3 and 12
14 random$.ti,ab.
15 factorial$.ti,ab.
16 (crossover$ or cross over$ or cross‐over$).ti,ab.
17 placebo$.ti,ab.
18 (doubl$ adj blind$).ti,ab.
19 (singl$ adj blind$).ti,ab.
20 assign$.ti,ab.
21 allocat$.ti,ab.
22 volunteer$.ti,ab.
23 crossover procedure.sh.
24 double blind procedure.sh.
25 randomized controlled trial.sh.
26 single blind procedure.sh.
27 or/14‐26
28 exp animal/ or nonhuman/ or exp animal experiment/
29 exp human/
30 28 and 29
31 28 not 30
32 27 not 31
33 13 and 32
Appendix 3. CENTRAL search strategy
#1 MeSH descriptor Arthritis, Rheumatoid explode all trees
#2 ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat* or reumat* or revmarthrit*) near/3 (arthrit* or artrit* or diseas* or condition* or nodule*)):ti,ab
#3 (#1 OR #2)
#4 MeSH descriptor Fatigue explode all trees
#5 fatigue*:ti,ab
#6 (tired* or weary or weariness or exhaustion or exhausted):ti,ab
#7 ((astenia or asthenic) and syndrome):ti,ab
#8 ((lack or loss or lost) near/3 (energy or vigor)):ti,ab
#9 (apath* or lassitude or weak* or letharg*):ti,ab
#10 (feel* near/3 (drained or sleep* or sluggish)):ti,ab
#11 vitality:ti,ab
#12 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11)
#13 (#3 AND #12)
Appendix 4. CINAHL search strategy
S75 S61 and S74
S74 S62 or S63 or S64 or S65 or S66 or S67 or S68 or S69 or S70 or S71 or S72 or S73
S73 TI Allocat* random* or AB Allocat* random*
S72 (MH "Quantitative Studies")
S71 (MH "Placebos")
S70 TI Placebo* or AB Placebo*
S69 TI Random* allocat* or AB Random* allocat*
S68 (MH "Random Assignment")
S67 TI Randomi?ed control* trial* or AB Randomi?ed control* trial*
S66 AB singl* blind* or AB singl* mask* or AB doub* blind* or AB doubl* mask* or AB trebl* blind* or AB trebl* mask* or AB tripl* blind* or AB tripl* mask*
S65 TI singl* blind* or TI singl* mask* or TI doub* blind* or TI doubl* mask* or TI trebl* blind* or TI trebl* mask* or TI tripl* blind* or TI tripl* mask*
S64 TI clinical* trial* or AB clinical* trial*
S63 PT clinical trial
S62 (MH "Clinical Trials+")
S61 S42 and S60
S60 S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 or S59
S59 ti vitality or ab vitality
S58 ab feel* N3 drain* or ab feel* N3 sleep* or ab feel* N3 sluggish
S57 ti feel* N3 drain* or ti feel* N3 sleep* or ti feel* N3 sluggish
S56 ab apath* or ab lassitude or ab weak* or ab letharg*
S55 ti apath* or ti lassitude or ti weak* or ti letharg*
S54 ab lack N3 vigour or abloss N3 vigour or ab lost N3 vigour
S53 ti lack N3 vigour or ti loss N3 vigour or ti lost N3 vigour
S52 ab lack N3 vigor or ab loss N3 vigor or ab lost N3 vigor
S51 ti lack N3 vigor or ti loss N3 vigor or ti lost N3 vigor
S50 ti lack N3 vigor or ti loss N3 vigor or ab lost N3 vigor
S49 ti lack N3 energy or ti loss N3 energy or ti lost N3 energy
S48 ab astenia syndrome or ab asthenic syndrome
S47 ti astenia syndrome or ti asthenic syndrome
S46 ab tired* or weary or weariness or exhaustion or exhausted
S45 ti tired* or weary or weariness or exhaustion or exhausted
S44 ti fatigue* or ab fatigue*
S43 (MH "Fatigue+")
S42 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41
S41 TI reumat* N3 nodule* or AB reumat* N3 nodule*
S40 TI reumat* N3 condition* or AB reumat* N3 condition*
S39 TI reumat* N3 diseas* or AB reumat* N3 diseas*
S38 TI reumat* N3 artrit* or AB reumat* N3 artrit*
S37 TI reumat* N3 arthrit* or AB reumat* N3 arthrit*
S36 TI revmarthrit* N3 nodule* or AB revmarthrit* N3 nodule*
S35 TI revmarthrit* N3 condition* or AB revmarthrit* N3 condition*
S34 TI revmarthrit* N3 diseas* or AB revmarthrit* N3 diseas*
S33 TI revmarthrit* N3 artrit* or AB revmarthrit* N3 artrit*
S32 TI revmarthrit* N3 arthrit* or AB revmarthrit* N3 arthrit*
S31 TI rheumat* N3 nodule* or AB rheumat* N3 nodule*
S30 TI rheumat* N3 condition* or AB rheumat* N3 condition*
S29 TI rheumat* N3 diseas* or AB rheumat* N3 diseas*
S28 TI rheumat* N3 artrit* or AB rheumat* N3 artrit*
S27 TI rheumat* N3 arthrit* or AB rheumat* N3 arthrit*
S26 TI revmatic N3 nodule* or AB revmatic N3 nodule*
S25 TI revmatic N3 condition* or AB revmatic N3 condition*
S24 TI revmatic N3 diseas* or AB revmatic N3 diseas*
S23 TI revmaticN3 artrit* or AB revmatic N3 artrit*
S22 TI revmatic N3 arthrit* or AB revmatic N3 arthrit*
S21 TI rheumatic N3 nodule* or AB rheumatic N3 nodule*
S20 TI rheumatic N3 condition* or AB rheumatic N3 condition*
S19 TI rheumatic N3 diseas* or AB rheumatic N3 diseas*
S18 TI rheumatic N3 artrit* or AB rheumatic N3 artrit*
S17 TI rheumatic N3 arthrit* or AB rheumatic N3 arthrit*
S16 TI revmatoid N3 nodule* or AB revmatoid N3 nodule*
S15 TI revmatoid N3 condition* or AB revmatoid N3 condition*
S14 TI revmatoid N3 diseas* or AB revmatoid N3 diseas*
S13 TI revmatoid N3 artrit* or AB revmatoid N3 artrit*
S12 TI revmatoid N3 arthrit* or AB revmatoid N3 arthrit*
S11 TI reumatoid N3 nodule* or AB reumatoid N3 nodule*
S10 TI reumatoid N3 condition* or AB reumatoid N3 condition*
S9 TI reumatoid N3 diseas* or AB reumatoid N3 diseas*
S8 TI reumatoid N3 artrit* or AB reumatoid N3 artrit*
S7 TI reumatoid N3 arthrit* or AB reumatoid N3 arthrit*
S6 TI rheumatoid N3 nodule* or AB rheumatoid N3 nodule*
S5 TI rheumatoid N3 condition* or AB rheumatoid N3 condition*
S4 TI rheumatoid N3 diseas* or AB rheumatoid N3 diseas*
S3 TI rheumatoid N3 artrit* or AB rheumatoid N3 artrit* *
S2 TI rheumatoid N3 arthrit* or AB rheumatoid N3 arthrit*
S1 (MH "Arthritis, Rheumatoid+")
Appendix 5. PsycINFO search strategy
1. rheumatoid arthritis/
2. ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw.
3. 1 or 2
4. Fatigue/
5. fatigue$.tw.
6. (tired$ or weary or weariness or exhaustion or exhausted).tw.
7. ((astenia or asthenic) and syndrome).tw.
8. ((lack or loss or lost) adj3 (energy or vigo?r)).tw.
9. (apath$ or lassitude or weak$ or letharg$).tw.
10. (feel$ adj3 (drained or sleep$ or sluggish)).tw.
11. vitality.tw.
12. or/4‐11
Appendix 6. AMED search strategy
1. exp arthritis, rheumatoid/
2. ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw.
3. 1 or 2
4. exp Fatigue/
5. fatigue$.tw.
6. (tired$ or weary or weariness or exhaustion or exhausted).tw.
7. ((astenia or asthenic) and syndrome).tw.
8. ((lack or loss or lost) adj3 (energy or vigo?r)).tw.
9. (apath$ or lassitude or weak$ or letharg$).tw.
10. (feel$ adj3 (drained or sleep$ or sluggish)).tw.
11. vitality.tw.
12. or/4‐11
13. 3 and 12
Appendix 7. Web of Science search strategy
Topic=((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat* or reumat* or revmarthrit*) and (arthrit* or artrit* or diseas* or condition* or nodule*)) AND Topic=(fatigue* or tired* or weary or weariness or exhaustion or exhausted or astenia syndrome or asthenic syndrome or apath* or
lassitude or weak* or
(lack or loss or lost) and (letharg* or energy or vigoor* or vigour*) or
Feel* and (drained or sleep* or sluggish)
(trial* or random* or placebo* or control* or double or treble or triple or blind* or mask* or allocat* or prospective* or volunteer*or comparative or evaluation or follow‐up or followup)
Appendix 8. Dissertation Abstracts search strategy
(rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat* or reumat* or revmarthrit*) in citation and abstract
AND (fatigue* or tired* or weary or weariness or exhaustion or exhausted or astenia syndrome or asthenic syndrome or apath* or lassitude or weak* or letharg* or energy or vigoor* or vigour* or drained or sleep* or sluggish) in citation and abstract
Data and analyses
Comparison 1. All biologics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All studies ‐ fatigue continuous measures | 30 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All biologics | 30 | 14628 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.49, ‐0.38] |
Comparison 2. Subgroup comparison : anti‐TNF vs non‐anti‐TNF.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anti‐TNF and non anti‐TNF ‐ fatigue continuous measures | 30 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Anti‐TNF | 19 | 8946 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.49, ‐0.35] |
| 1.2 Non‐anti‐TNF | 11 | 5682 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.53, ‐0.39] |
2.1. Analysis.
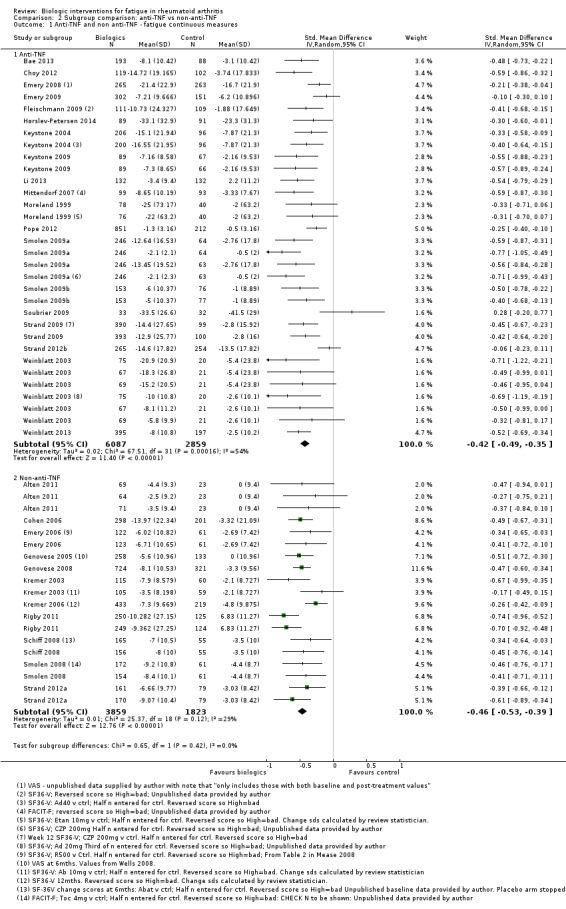
Comparison 2 Subgroup comparison : anti‐TNF vs non‐anti‐TNF , Outcome 1 Anti‐TNF and non anti‐TNF ‐ fatigue continuous measures.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alten 2011.
| Methods | RCT of 12 weeks | |
| Participants | Outpatient clinics, Multicentre international Males or females ≥ 18 years of age Revised 1987 ACR classification criteria for RA, symptoms for ≥ 3 months Active RA, defined as ≥ 6/28 tender and swollen joints with CRP ≥ 10 mg/L, erythrocyte sedimentation ≥ 28 mm or both ACR Functional status classes I, II, or III Treated with methotrexate at the maximum tolerated dose (≤ 25 mg/week) and at a stable dose of ≥ 7.5 mg/week for ≥ 12 weeks. Patients who had failed treatment with any DMARD, including any such agent used in combination with methotrexate as well as any biologic agent, were eligible for participation after an appropriate washout period. Systemic corticosteroids, NSAIDs, including cyclooxygenase‐2 inhibitors or paracetamol, had to have been stable doses for at least 4 weeks. Maximum allowable dose of systemic corticosteroids was ≤ 10 mg/day prednisone or an equivalent for ≥ 4 weeks. Group 1‐4: Percentage Female: 81.2%, 89.1%, 84.5% and 74.3% respectively Age, mean (SD) years: 57.10 (11.899), 61.02 (12.244), 55.62 (11.236), 57.53 (12.121) respectively Exclusion Previous hypersensitivity to the study drug or to molecules with similar structures Intra‐articular therapy for RA within the previous 4 weeks Pregnant or breastfeeding Positive TB skin test without a follow‐up negative chest X‐ray |
|
| Interventions | Group 1: canakinumab 150 mg subcutaneously (SC) every 4 weeks + MTX
Group 2: canakinumab 300 mg SC every 2 weeks + MTX
Group 3: canakinumab 600 mg IV loading dose followed by 300 mg SC every 2 weeks + MTX Group 4: Placebo subcutaneously every 2 weeks + MTX |
|
| Outcomes | Primary outcome: response to treatment according to ACR 50 criteria at 12 weeks
Secondary outcomes:
|
|
| Exclusions | Previous hypersensitivity to the study drug or to molecules with similar structures Intra‐articular therapy for RA within the previous 4 weeks Pregnant or breastfeeding Positive TB skin test without a follow‐up negative chest X‐ray | |
| Fatigue outcomes | FACIT, 0‐52, high = good | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised in a double‐blind fashion |
| Allocation concealment (selection bias) | Unclear risk | Randomised in a double‐blind fashion |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Randomised in a double‐blind fashion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study report |
Bae 2013.
| Methods | RCT of 16 weeks | |
| Participants | Outpatient clinics Multicentre, Hong Kong, India, Malaysia, Philippines, Taiwan, Korea and Thailand. RA, based on 1987 ACR criteria 28‐joint Disease Activity Score [DAS28] ≥3.2, who displayed inadequate response to oral MTX (stable dosing between 7.5 mg/week and 25 mg/week for minimum 3 months ETN + MTX group: 91.4% female, mean age (± SD) 48.4 years ± 12.0 DMARD+MTX group: 88.4% female, mean age (± SD) 48.5 years ± 11.3, Exclusion Not stated |
|
| Interventions | Subcutaneous etanercept (ETN) 25 mg per injection twice weekly was added to methotrexate (MTX). MTX according to local use, orally, weekly mean dose was 12.9 mg (1.9 mg‐25 mg) Patients were randomized to either of two treatment groups in an approximate 2:1 ratio: 1. ETN+MTX (N= 197) or 2. DMARD +MTX (N= 103). DMARD therapy (defined as the addition of DMARD investigator’s choice to MTX) followed the standard of care and approved local label or recommendations; the three most frequently used DMARDs in the study were leflunomide (n = 69), sulfasalazine (n = 23) and hydroxychloroquine (n = 11). |
|
| Outcomes | Primary Outcome: ACR response Secondary Outcomes 1. Health Assessment Questionnaire (HAQ) ranges 0 (best) ‐ 3 (worse). 2. SF‐36 in eight domains: bodily pain, general health, physical functioning, role‐physical, mental health, role‐emotional, social functioning and vitality. SF‐36 scores range from 0 (worst) to 100 (best) for each of the eight domains. 3. Hospital Anxiety and Depression Scale (HADS) ranges 0 (best) to 3 (worst) 4. FACIT‐F Scale. Scores range from 0 to 52, with higher scores indicating less fatigue. 5. Work Productivity and Activity Impairment Questionnaire: General Health (WPAI:GH) measures percentage impairment of usual activities and percentage impairment of work and productivity due to health, with higher scores reflecting higher percentage impairment. All assessments were carried out at baseline, week 8 and 16 |
|
| Exclusions | Not stated | |
| Fatigue outcomes | Fatigue Assessment Scale (FAS) NRS, 0‐10, high = bad, SF‐36 vitality | |
| Notes | This is patient‐reported outcome paper, earlier paper reported the main study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported in the text |
| Allocation concealment (selection bias) | High risk | Open‐label |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | SD are not reported |
| Selective reporting (reporting bias) | High risk | This is patient‐reported outcome paper, earlier paper reported the main study |
Choy 2012.
| Methods | RCT of 24 weeks | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria Aged 18‐75 years Adult‐onset RA of at least 6 months as defined by the 1987 ACR criteria Active disease defined as > 9 tender joints, > 9 swollen joints and at least 1 of the 3 following criteria: > 45 min EMS, ESR > 28 mm/h or CRP > 10 mg/L Receiving MTX for at least 6 months and on a stable dosage of 15‐25 mg/week for at least 8 weeks (10‐15 mg/week was deemed acceptable in cases where a dosage reduction had been necessary because of toxicity). All other DMARDs were to have been discontinued at least 28 days before the first study medication dose. Exclusion Any form of inflammatory arthritis other than RA History of chronic, serious or life‐threatening infection, current infection History or chest X‐ray suggestive of tuberculosis, or positive (defined per local medical practice) purified protein derivative (PPD) skin test (Mantoux) History of infected joint prosthesis IM, IV or IA CSs or IA hyaluronic acid in the 4 weeks preceding the study Prior treatment with any TNF‐ɑ inhibitor Receipt of any experimental, unregistered or biological therapy in the 6 months preceding the study NSAIDs and oral CSs at a dosage of < 10 mg/day prednisone equivalent were allowed if stable for > 4 weeks before study entry and thereafter Analgesics not allowed during the 4 days preceding baseline assessment, except paracetamol, which was not allowed within 24 h Group 1 and 2: Gender (percentage female): 72.2% and 66.1% respectively Age, mean (S.D.), years 53.0 (12.3) and 55.6 (11.7) respectively |
|
| Interventions | SC reconstituted lyophilised CZP 400 mg or placebo every 4 weeks from baseline to week 20. MTX 15‐25 mg/week (10‐15 mg/week was allowed if the dose had been reduced because of toxicity). Group 1: CZP + MTX Group 2: Placebo + MTX |
|
| Outcomes | Primary outcome: ACR 20 response rate at week 24
Secondary outcomes:
Assessments of endpoints at weeks 1, 2, 4, 8, 12, 16, 20, 22 and 24 |
|
| Exclusions | Any form of inflammatory arthritis other than RA History of chronic, serious or life‐threatening infection, current infection History or chest X‐ray suggestive of tuberculosis, or positive (defined per local medical practice) purified protein derivative (PPD) skin test (Mantoux) History of infected joint prosthesis IM, IV or IA CSs or IA hyaluronic acid in the 4 weeks preceding the study Prior treatment with any TNF‐ɑ inhibitor Receipt of any experimental, unregistered or biological therapy in the 6 months preceding the study NSAIDs and oral CSs at a dosage of < 10 mg/day prednisone equivalent were allowed if stable for > 4 weeks before study entry and thereafter Analgesics not allowed during the 4 days preceding baseline assessment, except paracetamol, which was not allowed within 24 h | |
| Fatigue outcomes | SF‐36, 0‐100, high = good | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised on a 1:1 basis via an interactive voice‐response system |
| Allocation concealment (selection bias) | Low risk | Patients were randomised on a 1:1 basis via an interactive voice‐response system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | To preserve the blind to clinical research staff, the study site pharmacist labelled clinical supplies (study medication syringes), and a sorbitol placebo was used to match the viscosity of CZP. Placebo injections |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Efficacy evaluations were carried out in the modified intention‐to‐treat (mITT) population, defined as all randomised patients who had taken at least 1 dose of study medication. For continuous data, missing data were imputed by last observation carried forward (LOCF) analysis. |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study report |
Cohen 2006.
| Methods | RCT of 24 weeks, followed up until week 104 weeks | |
| Participants | Outpatient clinic, multicentre international Inclusion 18‐80 years, active RA (≥ 8 swollen or tender joints), RA minimum 6 months, non‐response to anti‐TNF, taking MTX for minimum 12 weeks prior to screening Exclusion Significant systemic involvement secondary to RA, ACR functional class IV Group 1 and 2: Gender (percentage female): 81% and 81% respecitively Age (mean (SD) years): 52.8 (12.6), 52.2 (12.2) respecitively |
|
| Interventions | Rituximab 1000 mg on days 1 and 15 or placebo
MTX (10‐25 mg/week orally or parenterally), folate (≥ 5 mg/week), IV methylprednisolone (100 mg 30 min before infusion) and oral prednisolone (60 mg on days 2‐7, 30 mg on days 8‐14) Group 1: Placebo + MTX Group 2: RTX + MTX |
|
| Outcomes | Primary outcome: ACR 20 response at week 24
Secondary outcomes:
|
|
| Exclusions | Significant systemic involvement secondary to RA, ACR functional class IV | |
| Fatigue outcomes | FACIT‐F, range 0‐52, high = bad SF‐36 VT, 0‐100, high = good | |
| Notes | Keystone 2008 provided further data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised at a ratio of 3:2" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised at a ratio of 3:2" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Blinded study with the study sponsor, investigators and patients unaware of the treatment assignment of each patient" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT population was defined as all randomised patients who received any part of an infusion of study medication and included patients who withdrew prematurely from the study for any reason and for whom assessments were not made. Last observation carried forward |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study report |
Emery 2006.
| Methods | 3 x 3 factorial RCT of 24 weeks | |
| Participants | Outpatient clinic, Multicentre international Inclusions 18‐80 years, active RA (≥ 8 swollen or tender joints/CRP ≥ 1.5 mg/dL or ESR ≥ 28mm/h) despite MTX. Failed 1‐5 DMARDS or biologic agents, glucocorticoids ≤ 10 mg/day, RF positive Exclusions Significant systemic RA, other illness or lab abnormalities, recurrent significant infections, prior rituximab, allergy to agents Gender Placebo (percentage female): 80% (placebo), 83% (RTX 2x500mg) and 80% (RTX 2x1000mg) Age (mean years): 51.1 (placebo) 51.4 (RTX 2x500mg) and 51.1 (RTX 2x1000mg) |
|
| Interventions | All patients received MTX (10–25 mg/week); no other DMARDs were permitted RTX IV infusion on day 1 and 15. 9 Groups 1. RTX 2 x 500 mg + placebo glucocorticoid on days 1 and 15 2. RTX 2 x 500 mg + 100 glucocorticoid on day 1 and 15 3. RTX 2 x 500 mg + IV methylprednisolone premedication + oral prednisone for 2 weeks 4. RTX 2 x 1000 mg + placebo glucocorticoid on days 1 and 15 5. RTX 2 x 1000 mg + 100 glucocorticoid on days 1 and 15 6. RTX 2 x 1000 mg + intravenous methylprednisolone premedication + oral prednisone for 2 weeks 7. Pacebo 2 x infusion + placebo glucocorticoid on days 1 and 15 8. Placebo 2 x infusion + 100 glucocorticoid on days 1 and 15 9. Placebo 2 x infusion + intravenous methylprednisolone premedication + oral prednisone for 2 weeks |
|
| Outcomes | Primary outcome: ACR 20 at 24 week Secondary outcomes: ACR50, 70 EULAR response HAQ‐DI SF‐36 FACIT‐F safety All assessments at baseline and week 24 |
|
| Exclusions | Significant systemic RA, other illness or lab abnormalities, recurrent significant infections, prior rituximab, allergy to agents | |
| Fatigue outcomes | FACIT‐F, range 0‐52, high = good SF‐36 VT, high = good | |
| Notes | Fatigue was reported in the main study report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised control trial |
| Allocation concealment (selection bias) | Low risk | "Double‐blind, double‐dummy" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind, double‐dummy" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts "failed" |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study report |
Emery 2008.
| Methods | RCT of 52 weeks | |
| Participants | Outpatient clinics Multicentre, international in Europe, Latin America, Asia, and Australia Inclusions 18 years or older, diagnosis of adult‐onset rheumatoid arthritis, disease duration minimum 3 months maximum 2 years, DAS 28 of 3.2 or more, and either Westergren ESR of ≥ 28 mm/h or CRP of ≥ 20 mg/L Exclusions Previous treatment with MTX, etanercept, or another TNF antagonist at any time or treatment with other DMARDs or corticosteroid injections in the 4 weeks before baseline visits. Individuals with important concurrent medical diseases were ineligible, as were those with other relevant comorbidities. Group 1 and 2: Gender (percentage female): 73% and 74% respecitively Age (mean (SD) years): 52.3 (0.8), 50.5 (0.9) respecitively |
|
| Interventions | All participants received oral methotrexate, starting at 7.5 mg once a week. ETN 50 mg by subcutaneous injection once a week for 52 weeks. In patients with tender or swollen joints, the dose was titrated up over 8 weeks to a maximum of 20 mg a week Group 1: Placebo+ MTX Group 2: ETN + MTX |
|
| Outcomes | Primary outcomes:
Secondary outcomes (at weeks 12, 24, 36 and 52):
|
|
| Exclusions | Previous treatment with MTX, etanercept, or another TNF antagonist at any time or treatment with other DMARDs or corticosteroid injections in the 4 weeks before baseline visits. Individuals with important concurrent medical diseases were ineligible, as were those with other relevant comorbidities. | |
| Fatigue outcomes | Fatigue VAS, 0‐100, high = bad SF‐36 VT, 0‐100, high = good | |
| Notes | For all patients stable doses of oral corticosteroids (≤ 10 mg per day of prednisone or an equivalent agent) or a single non‐steroidal anti‐inflammatory drug were permitted if started at least 4 weeks before baseline and kept constant throughout the first 24 weeks of the study. After completion of 24 weeks of treatment, reductions in dose of prednisone or other oral corticosteroid by 1 mg per day or less were allowed every week. Oral corticosteroids were tapered to 3 mg per day or less before the dose of non‐steroidal anti‐inflammatory drug was decreased. All patients received folic acid supplementation of 5 mg twice weekly (not given on the same day as methotrexate) to reduce side effects associated with methotrexate. Fatigue data reported in Kekow 2010. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned with a computerised randomisation and enrolment (CORE) system to generate and implement allocation sequence, manage assignment to treatment groups, and maintain blinding. |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned with a computerised randomisation and enrolment (CORE) system to generate and implement allocation sequence, manage assignment to treatment groups, and maintain blinding. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Data were unblinded only if needed for medical management of patients. Masking was removed for one sponsor biostatistical programmer to do the 52‐week primary analysis for this report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT/LOCF |
| Selective reporting (reporting bias) | High risk | results of employment questionnaire were not reported |
Emery 2009.
| Methods | RCT of 24 week | |
| Participants | Outpatient clinic, Multicentre international Inclusions Adults who had RA, according to ACR criteria for at least 3 months Had not received more than 3 weekly doses of oral MTX as treatment of RA Active RA, with at least 4 swollen joints and at least 4 tender joints AND at least 2 of the following.
Prespecified TB screening criteria. Patients with positive results for TB skin or whole blood interferon‐γ–based QuantiFERON‐TB testing could participate but had to start prophylaxis for latent TB before or simultaneously with administration of the first dose of the study agent. Concurrent use of NSAIDs, other analgesics for RA, and oral corticosteroids (< 10 mg of prednisone/day or equivalent) was allowed if doses were stable for > 2 weeks Patients receiving anakinra could participate 4 weeks after receiving the last dose Patients receiving alefacept or efalizumab could participate 3 months after receiving the last dose Patients receiving an experimental agent could participate after the equivalent of 5 half‐lives of the agent Exclusions Patients who had previously received infliximab, etanercept, adalimumab, rituximab, natalizumab or cytotoxic agents, including chlorambucil, cyclophosphamide, nitrogen mustard, and other alkylating agents, were excluded. Group 1, 2 3, and 4 Gender (percentage female): 83.8%, 84.3%, 84.9% and 78.6% respectively Age, mean (SD) years: 48.6 (12.91), 48.2 (12.85), 50.9 (11.32) and 50.6 (11.58) |
|
| Interventions | Golimumab subcutaneously at week 0 and then every 4 weeks
MTX started at 10 mg/week at week 0 and escalated by 2.5 mg every 2 weeks to 20 mg/week by week 8
Duration of intervention = 24 weeks reported here (but 52 weeks plus 5 years open label extension) Group 1: placebo by SC injection plus MTX Group 2: golimumab 100 mg by SC injection plus placebo capsules Group 3: golimumab 50 mg by SC injection plus MTX capsules Group 4: golimumab 100 mg by SC injection plus MTX capsules |
|
| Outcomes | Primary outcome: difference in the ACR 50 response at week 24 between groups 3 and 4 combined (combined group) versus group 1 and a pairwise comparison (group 3 or group 4 versus group 1).
Secondary outcomes:
|
|
| Exclusions | Patients who had previously received infliximab, etanercept, adalimumab, rituximab, natalizumab or cytotoxic agents, including chlorambucil, cyclophosphamide, nitrogen mustard, and other alkylating agents, were excluded. | |
| Fatigue outcomes | SF‐36 VT. 0‐100, high = good | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An interactive voice response system (IVRS) was used to randomly assign eligible patients to 1 of 4 treatment groups in approximately equal proportions |
| Allocation concealment (selection bias) | Low risk | IVRS was used to randomly assign eligible patients to 1 of 4 treatment groups in approximately equal proportions |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Golimumab and placebo were supplied as sterile liquid (aqueous medium of histidine, sorbitol, and polysorbate 80 (pH 5.5) with or without golimumab) for SC injection. Active and placebo MTX were supplied as double‐blinded, identical opaque capsules (filled with microcrystalline cellulose with or without MTX). An independent assessor at each study centre, who had no access to patient records and no other role in the study, performed the joint assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients (all of those who were entered in the IVRS for randomisation regardless of receipt of study treatment) were analysed by assigned treatment group (ITT) approach. Patients for whom all week 24 (the primary end point visit) ACR component data were missing were considered non‐responders, as were patients meeting predefined treatment failure criteria related to prohibited concomitant medications or discontinuation of the SC study agent due to lack of efficacy. Actual week 24 data were used for patients who discontinued the study agent for reasons other than lack of efficacy but returned for clinical evaluations, but these patients were considered non‐responders if they met any of the treatment failure criteria |
| Selective reporting (reporting bias) | Unclear risk | Main paper, methods section does not say whether fatigue and SF‐36 data are collected, but these are presented in a later paper |
Fleischmann 2009.
| Methods | RCT of 24 weeks | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria 18‐75 years, adult onset RA minimum 6 months, failed ≥1 DMARD, active disease ≥ 9 (out of 68) tender joints and ≥ 9 (out of 66) swollen joints and ≥ 1 of the following: > 45 min of morning stiffness, ESR ≥ 28 mm/h, CRP > 10 mg/L Exclusions Any inflammatory arthritis other than RA, history of chronic, serious or life‐threatening infection, any current infection, history of or a chest x ray suggesting tuberculosis or a positive PPD skin test, patients who received biological therapies for RA within 6 months, prior treatment with TNF‐α inhibitors, intra‐articular, periarticular, intramuscular and intravenous corticosteroids Group 1 and 2 Gender (percentage female): 89%and 78.4% respectively Age, mean (SD) years: 54.9 (11.6) and 52.7 (12.7) respectively |
|
| Interventions | 400 mg SC certolizumab pegol Group 1: Placebo SC Group 2: Certolizumab pegol SC |
|
| Outcomes | Primary outcome: ACR 20 response at 24 weeks Secondary outcomes at week 24:
|
|
| Exclusions | Any inflammatory arthritis other than RA, history of chronic, serious or life‐threatening infection, any current infection, history of or a chest x ray suggesting tuberculosis or a positive PPD skin test, patients who received biological therapies for RA within 6 months, prior treatment with TNF‐α inhibitors, intra‐articular, periarticular, intramuscular and intravenous corticosteroids | |
| Fatigue outcomes | SF‐36 VT, 0‐100, high = good Fatigue Assessment Scale, 11‐point scale, high = bad | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive voice randomisation service |
| Allocation concealment (selection bias) | Low risk | Interactive voice randomisation service |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Intervention and placebo solutions were administered by blinded study personnel. No details given about outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified ITT for all efficacy analyses |
| Selective reporting (reporting bias) | High risk | Health‐related quality of life data not shown but improvements claimed. SF‐36 data not published but have been provided subsequently |
Genovese 2005.
| Methods | RCT of 6 months | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria At least 10 swollen/12 tender joints, RA ≥ 1 year, ≥ 18 years, inadequate response to anti‐TNF (etanercept, infliximab or both), CRP at least 1mg/dL, taking oral DMARD/anakinra for 3vmonths stable for minimum 28 days. If steroids ‐ stable for 28 days Exclusions Not stated Group 1 and 2 Gender (percentage female): 77.1% and 79.9% respectively Age, mean (SD) years: 53.4 (12.4) and 52.7 (11.3)respectively |
|
| Interventions | Abatacept 2:1 stratified for anti‐TNF use prior to trial. 10 mg/kg body weight. IV infusion days 1, 15, 29 then every 28 days to day 141 inclusive Group 1: Abatacept IV infusion Group 2: Placebo IV infusion |
|
| Outcomes | SF‐36, DAS, HAQ | |
| Exclusions | Not stated | |
| Fatigue outcomes | SF‐36 VT, range 0‐100, high = good VAS, range 0‐100, high = bad | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study report |
Genovese 2008.
| Methods | RCT of 24 weeks | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria > 18 years with moderate‐to severe RA of > 6 months' duration Diagnosed according to the ACR criteria Swollen joint count > 6, tender joint > 8, CRP > 1 mg/dL or ESR > 28 mm/h Stable doses of permitted DMARDs (methotrexate, chloroquine, hydroxychloroquine, parenteral gold, sulfasalazine, azathioprine, leflunomide) for > 8 weeks prior to study entry Oral glucocorticoids (< 10 mg/day prednisone or equivalent) and NSAIDs permitted if the doses were stable for > 6 weeks Exclusions Patients who were unsuccessfully treated with an anti‐TNF agent or were previously treated with any cell‐depleting therapy Group 1 and 2 Gender (percentage female): 81% and 84% respectively Age, mean (SD) years: 53 (13) and 54 (13) |
|
| Interventions | Tocilizumab at a dose of 8 mg/kg intravenously as a 60 min infusion every 4 weeks, combined with stable DMARD therapy Duration of intervention was 24 weeks with doses at 0, 4, 8, 12, 16, 20, 24 weeks Group 1: Tocilizumab IV infusion + DMARDs Group 2: Placebo IV infusion + DMARDs |
|
| Outcomes | Primary outcome: proportion of patients who had achieved a response according to ACR 20 at week 24
Secondary outcomes at week 24:
|
|
| Exclusions | Patients who were unsuccessfully treated with an anti‐TNF agent or were previously treated with any cell‐depleting therapy | |
| Fatigue outcomes | SF‐36 VT, range 0‐100, high = good FACIT‐F, 0‐52, high = good | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, double‐blind, placebo‐controlled, international, multicentre |
| Allocation concealment (selection bias) | Unclear risk | Randomised, double‐blind, placebo‐controlled, international, multicentre |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind RCT, drugs administered in blinded fashion Patients were assessed using a dual‐assessor approach for efficacy and safety evaluations, to ensure that blinding was not compromised. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was by ITT. All patients who received at least 1 injection with adalimumab or placebo were included. LOCF was applied for patients who withdrew from the trial before the 12‐month visit. In a post hoc analysis, non‐responder imputation (NRI) of the patients who withdrew from the study was performed for the primary outcome. ITT analysis without LOCF and completers' analysis were also performed and gave similar results (not shown). |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study publication |
Hørslev‐Petersen 2014.
| Methods | RCT of 12 months | |
| Participants | Outpatient clinic, Multicentre Denmark Inclusion criteria Patients (≥ 18 years) with RA according to the ACR classification criteria who have had the diagnose < 6 months Moderate to severe rheumatoid arthritis defined as DAS 28 (CRP‐based) > 3.2. Negative pregnancy test (serum HCG) for women of childbearing potential prior to trial start. (Non‐fertile women are defined as postmenopausal for at least 1 year or surgical sterilisation (bilateral tubal ligation, bilateral oophorectomy or hysterectomy). Fertile women included in the trial should use contraception during the entire trial period (i.e. one of the following methods: oral contraception, intrauterine device (IUD), depot injection of progesterone, subdermal implantation, contraceptive vaginal ring, transdermal depot plaster). In addition, contraception should be used for a period of 150 days after any discontinuation of trial medicine. Ability and willingness to inject the SC injections alone or to have an assistant give the injections Ability and willingness to give written informed consent and to meet the requirements of the trial protocol Exclusions People with latent TB defined with a positive Mantoux test (> 12 mm for vaccinated and 6 mm for non‐vaccinated), positive cultivation of mycobacteria in tissue samples, chest X‐ray indicating TB,or other risk factors for activation of untreated latent TB, and people not been given adequate TB prophylaxis according to the instructions of the department Active or recurrent infections or severe infections requiring hospitalisation or treatment with IV antibiotics within the last 30 days or oral antibiotics within the last 14 days prior to inclusion Positive serology for hepatitis B or C indicating active infection Medical history with a positive HIV status (check of HIV test upon suspicion). Medical history with histoplasmosis or listeriosis Previous cancer or lymph proliferative disease except cases treated radically and have been without relapse for a minimum of 5 years. Patients with previous squamous cell carcinoma, basal cell skin carcinoma or cervical dysplasia, who have been treated successfully and radically can be included. Previous diagnosis or signs of demyelinised disease in the central nervous system (e.g. optic neuritis, visual disorder, disturbed gait, facial paralysis, apraxia) Severe renal insufficiency (creatinine clearance < 35 mL/min ‐ nomogram) Affected liver function: liver enzymes > 2 x above normal limit value Clinically significant drug or alcohol abuse during the past year or current daily alcohol consumption Unstable diabetes, unstable ischaemic heart disease, heart insufficiency (NYHA III‐IV), active chronic inflammatory intestinal disease, recent cerebral apoplexia (within 3 months), chronic leg ulcer or any other condition (e.g. kateter a demeure), which according to the investigator imposes an increased risk to the participant Anticoagulant therapy Pregnancy or breastfeeding Other inflammatory rheumatic diseases Aggressive parvovirus B19 infection Previous treatment with one or more DMARDs Glucocorticosteroid treatment within the last 4 weeks (except nasal and inhalation steroids) Contraindications for trial medicine Group 1 and 2 Gender (percentage female): 63% and 69% respectively Age, mean (range) years: 56.2 (25.8‐77.6) and 54.2 (28.3‐76.7) respectively |
|
| Interventions | Group 1: Adalimumab SC + MTX Group 2: Placebo SC + MTX Adalimumab group: adalimumab 40 mg subcutaneously every second week. Both groups: started oral methotrexate, 7.5 mg/week at baseline, increased to 15 mg/week after 1 month and 20 mg/week after 2 months (or the highest tolerated dose) in combination. Any swollen joint observed at baseline or at a subsequent visit was injected with triamcinolone hexacetonide (40 mg/mL, 0.5–2 mL/joint). Up to 4 joints (max. 4 mL) could be injected per visit. If unacceptable disease activity persisted at the 3 months' visit or thereafter, that is, either DAS 28 CRP ≥ 3.2 + ≥ 1 swollen joint or intra‐articular injection of 4 mL triamcinolone had been given monthly for 3 consecutive months, then hydroxychloroquine (200 mg/day) and sulphasalazine (2 g/day) were added. If the treatment target (low disease activity) was not achieved within an additional 3 months, adalimumab/placebo‐adalimumab was discontinued, the patient was considered a non‐responder and excluded from the study, and open‐label biologics (other than adalimumab) were prescribed at the discretion of the treating physician. |
|
| Outcomes | Primary outcome: proportion of patients in each group that had achieved low disease activity (DAS 28, CRP < 3.2) at 12 months
Secondary outcomes (at 12 months):
The doctor recorded number of swollen and tender joints (N = 40), doctor's global score (VAS) |
|
| Exclusions | People with latent TB defined with a positive Mantoux test (> 12 mm for vaccinated and 6 mm for non‐vaccinated), positive cultivation of mycobacteria in tissue samples, chest X‐ray indicating TB,or other risk factors for activation of untreated latent TB, and people not been given adequate TB prophylaxis according to the instructions of the department Active or recurrent infections or severe infections requiring hospitalisation or treatment with IV antibiotics within the last 30 days or oral antibiotics within the last 14 days prior to inclusion Positive serology for hepatitis B or C indicating active infection Medical history with a positive HIV status (check of HIV test upon suspicion). Medical history with histoplasmosis or listeriosis Previous cancer or lymph proliferative disease except cases treated radically and have been without relapse for a minimum of 5 years. Patients with previous squamous cell carcinoma, basal cell skin carcinoma or cervical dysplasia, who have been treated successfully and radically can be included. Previous diagnosis or signs of demyelinised disease in the central nervous system (e.g. optic neuritis, visual disorder, disturbed gait, facial paralysis, apraxia) Severe renal insufficiency (creatinine clearance < 35 mL/min ‐ nomogram) Affected liver function: liver enzymes > 2 x above normal limit value Clinically significant drug or alcohol abuse during the past year or current daily alcohol consumption Unstable diabetes, unstable ischaemic heart disease, heart insufficiency (NYHA III‐IV), active chronic inflammatory intestinal disease, recent cerebral apoplexia (within 3 months), chronic leg ulcer or any other condition (e.g. kateter a demeure), which according to the investigator imposes an increased risk to the participant Anticoagulant therapy Pregnancy or breastfeeding Other inflammatory rheumatic diseases Aggressive parvovirus B19 infection Previous treatment with one or more DMARDs Glucocorticosteroid treatment within the last 4 weeks (except nasal and inhalation steroids) Contraindications for trial medicine |
|
| Fatigue outcomes | VAS (visual analog scale), range 0–100 mm, high = bad | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised in blocks of 4 from a central, computer‐generated list of study numbers |
| Allocation concealment (selection bias) | Low risk | Patients were randomised in blocks of four from a central, computer‐generated list of study numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Randomised, double‐blind, placebo‐controlled, 2‐armed, parallel‐group, multicentre trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was by ITT. All patients who received at least 1 injection with adalimumab or placebo were included. LOCF was applied for patients who withdrew from the trial before the 12‐month visit. In a post hoc analysis, non‐responder imputation (NRI) of the patients who withdrew from the study was performed for the primary outcome. ITT analysis without LOCF and completers' analysis were also performed and gave similar results (not shown). |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study publication |
Keystone 2004.
| Methods | RCT of 52 weeks | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria ≥18 years of age or older, active RA diagnosed (Arnett 1988), ≥ 9 tender joints, ≥ 6 swollen joints, a CRP concentration over 1 mg/dL, and either rheumatoid factor positivity or ≥ 1 joint erosion on radiographs of the hands and feet. Must have been on MTX therapy for ≥ 3 months at a stable dose of 12.5‐25 mg/week (or ≥ 10 mg/week in patients intolerant to MTX) for ≥ 4 weeks Group 1, 2 3, and 4 Gender (percentage female): 83.8%, 84.3%, 84.9% and 78.6% respectively Age, mean (SD) years: 48.6 (12.91), 48.2 (12.85), 50.9 (11.32) and 50.6 (11.58) Exclusions Prior use of anti‐CD4 antibody therapy or TNF antagonists, a history of an active inflammatory arthritide other than RA, a history of active listeriosis or mycobacterial infection, a history of lymphoma, leukaemia or other malignancy besides non‐melanoma skin cancer within 5 years, a major episode of infection (i.e. infections requiring hospitalisation, treatment with intravenous antibiotics within 30 days prior to screening, or oral antibiotics within 14 days prior to screening), any uncontrolled medical condition, and pregnancy or breastfeeding Groups 1, 2 and 3 Gender (percentage female): 76.3%, 75.5% and 73% respectively Age mean (SD) years: 56.1 (13.5), 57.3 (10.5) and 56.1 (12.0) respectively |
|
| Interventions | Ad40: single self injections (1.6 mL/injection) of adalimumab subcutaneously at 40 mg every other week (with placebo injections on alternate weeks)
Ad20: adalimumab subcutaneously at 20 mg every week
Traditional DMARDs other than MTX were discontinued at least 28 days prior to the study baseline. Oral corticosteroids, if used previously, were allowed at a maximum prednisone‐dose equivalent of 10 mg/day.
At week 16 or thereafter, patients who were not achieving an ACR 20 response (improvements of at least 20% in the ACR core criteria) were allowed to receive rescue treatment with a traditional DMARD at the discretion of their treating physician. Patients commencing other therapies after not achieving an ACR 20 response were considered treatment failures for the purposes of clinical efficacy (determined by the ACR score for level of improvement) from that time onward. Patients who received rescue therapy and continued in the study were included in the radiographic analysis Group 1: Ad40 SC + MTX Group 2: Ad20 SC + MTX Group 3: Placebo SC + MTX |
|
| Outcomes | Primary outcomes: ACR 20 response at week 24 Secondary outcomes:
|
|
| Exclusions | Prior use of anti‐CD4 antibody therapy or TNF antagonists, a history of an active inflammatory arthritide other than RA, a history of active listeriosis or mycobacterial infection, a history of lymphoma, leukaemia or other malignancy besides non‐melanoma skin cancer within 5 years, a major episode of infection (i.e. infections requiring hospitalisation, treatment with intravenous antibiotics within 30 days prior to screening, or oral antibiotics within 14 days prior to screening), any uncontrolled medical condition, and pregnancy or breastfeeding | |
| Fatigue outcomes | SF‐36 VT, range 0‐100, high = good | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomly assigned" but no details |
| Allocation concealment (selection bias) | Unclear risk | "[R]andomly assigned" but no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of personnel not stated, placebo injection given to participants at identical times, X‐ray scorers "blinded to the treatment, chronologic order and clinical response of each patient" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, LOCF |
| Selective reporting (reporting bias) | High risk | Primary endpoint 24 weeks but fatigue only reported at 52 weeks |
Keystone 2009.
| Methods | RCT of 52 weeks | |
| Participants | Outpatient clinic, Multicentre international (Argentina, Australia, Canada, Chile, Germany, Hungary, Mexico, New Zealand, Poland, South Korea, Taiwan and the USA.) Inclusion criteria Swollen joint count > 6, tender joint > 8, CRP > 1 mg/dL or ESR > 28 mm/h ≥ 18 years of age RA according to the revised 1987 ACR criteria for least 3 months Active RA, despite previous MTX therapy, which was defined as ≥ 4 swollen joints (out of 66 total) and ≥ 4 tender joints (of 68) and at least 2 of the following: screening CRP of at least 1.5 mg/dL or ESR of at least 28 mm, morning stiffness of at least 30 min, bone erosion as observed by radiograph or MRI, positive for anti‐CCP or RF Patients must have received a stable dose of MTX (≥ 15 mg/week but not > 25 mg/week) during the 4‐week period immediately preceding screening and to have tolerated a dose ≥ 15 mg/week for at least 3 months Eligible patients had to have met the tuberculosis screening criteria. Patients who were using NSAIDs or other analgesics for RA had to be taking a stable dose for at least 2 weeks before the first dose of study agent. Patients who were taking oral corticosteroids had to have been receiving a stable dose equivalent to 10 mg/day or less of prednisone for ≥ 2 weeks before the first dose of study agent. Group 1, 2 3, and 4 Gender (percentage female): 83.8%, 84.3%, 84.9% and 78.6% respectively Age, mean (SD) years: 48.6 (12.91), 48.2 (12.85), 50.9 (11.32) and 50.6 (11.58) respectively |
|
| Interventions | Group 1: placebo SC + MTX Group 2: golimumab 100 mg by SC injection plus placebo capsules Group 3: golimumab 50 mg by SC injection plus MTX capsules Group 4: golimumab 100 mg by SC injection plus MTX capsules MTX was at participants' pre‐study stable dose | |
| Outcomes | Primary outcomes:
Secondary outcomes at week 14 and 24:
|
|
| Exclusions | Hypersensitivity to human immunoglobulin proteins or other components of golimumab. Any previous use of any anti‐TNF agent, rituximab, natalizumab or cytotoxic agents. Should not have received anakinra; disease‐modifying antirheumatic drugs other than methotrexate; intravenous, intramuscular or intra‐articular corticosteroids within 4 weeks before the first dose of study agent; or alefacept or efalizumab within 3 months before the first dose of the study agent | |
| Fatigue outcomes | FACIT‐F, 0‐52, high = good | |
| Notes | GO FORWARD – FACIT data (replaces original data extraction on Keystone 2009 which had been supplemented by data from authors, this is now the full paper) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified by investigational site and was conducted using a telephone IVRS |
| Allocation concealment (selection bias) | Low risk | Randomisation was stratified by investigational site and was conducted using a telephone IVRS |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Packaging identical, independent assessor used Golimumab and placebo were supplied as sterile liquid for SC injection. Placebo injections contained the same solution as active golimumab but did not contain the monoclonal antibody. Active methotrexate and placebo methotrexate were supplied as identical opaque capsules. Injections were administered every 4 weeks and each patient received 2 injections per dose (0.5 mL and 1.0 mL syringes) to maintain the blind. An independent assessor designated at each study centre performed all joint assessments. The joint assessor had no other contact with the patient, was not the treating physician and was not permitted to review patient medical records, case report forms, or previous joint counts during the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All analyses were based on an ITT principle, and all statistical testing was performed 2‐sided at a significance level of 0.05. An LOCF procedure was used for imputation of missing data and for patients who entered the early escape procedure. |
| Selective reporting (reporting bias) | Unclear risk | GO FORWARD – FACIT data (replaces original data extraction on Keystone 2009 which had been supplemented by data from authors, this is now the full paper) |
Kremer 2003.
| Methods | RCT of 6 months | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria ACR criteria for RA, functional class I, II, III; MTX ≥ 6 months, stable for 28 days, no other DMARDS in last 28 days, ≥ 10 swollen/12 tender joints, CRP ≥ 1mg/dL Exclusions Nursing/pregnant women Group 1, 2 and 3 Gender (percentage female): 66%, 63% and 75% respectively Age, mean (range) years: 54.7 (23‐80), 54.4 (23‐80) and 55.8 (17‐83) respectively |
|
| Interventions | CTLA4Ig infusions intravenously over 30 min period on days 1, 15, 30 and monthly thereafter for total of 6 months. 2 groups: 10 mg/kg and 2 mg/kg. All groups including controls received methotrexate Group 1: Placebo IV infusion + MTX Group 2: Abatacept 2mg/kg IV infusion + MTX Group 3: Abatacept 10mg/kg IV infusion + MTX |
|
| Outcomes | Primary outcome: ACR 20 at 6 months Secondary outcomes at days 1, 15 and 30 and then monthly:
|
|
| Exclusions | Nursing/pregnant women | |
| Fatigue outcomes | SF‐36 VT (0‐100) high = good | |
| Notes | Vitality scale changed most out of all SF‐36 dimensions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A central randomisation procedure was used" |
| Allocation concealment (selection bias) | Low risk | "A central randomisation procedure was used" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, outcome assessors unaware of patients' treatment assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "To account for missing data in the assessment of the ACR responses in the primary, prespecified analysis, we considered patients who discontinued the study because of worsening disease not to have had a response, and we carried forward the values obtained at the last assessment for patients who discontinued the study for any other reason. Thus, all patients were assessed for an ACR response." |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study publication |
Kremer 2006.
| Methods | RCT of 6 months | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria RA, functional class I, II, III; disease duration > 1 year, MTX ≥ 3 months, stable for 28 days, no other DMARDS in last 28 days, ≥ 10 swollen/12 tender joints, CRP ≥ 10 mg/L, ≤ 10 mg prednisolone/day over 25/28 days Exclusions None stated Group 1 and 2 Gender (percentage female): 72.3%, and 70.2% respectively Age, mean (SD) years: 51.5 (12.9) and 50.4 (11.58) respectively |
|
| Interventions | Abatacept IV 10 mg/kg on days 1, 15, 29 and months 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 = 14 doses. MTX ≥ 15 mg/week for 52 weeks Group 1: Abatacept IV infusion + MTX Group 2: Placebo IV infusion + MTX |
|
| Outcomes | Primary outcome: ACR 20 response at 6 months Seconday outcomes
|
|
| Exclusions | None stated | |
| Fatigue outcomes | SF‐36 VT, 0‐100, high= good VAS, 0‐10, high = bad | |
| Notes | Important study showing reduction in fatigue but problems in obtaining data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central by a drug company |
| Allocation concealment (selection bias) | Low risk | Central by a drug company |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients were randomly assigned in a 2:1 ratio to receive either a fixed dose of abatacept, approximately 10 mg/kg of body weight, or placebo. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not clear how many withdrew |
| Selective reporting (reporting bias) | Low risk | We performed all efficacy and safety analyses on a modified intention‐to‐treat population, defined as all randomly assigned patients who received at least 1 dose of study medication. |
Li 2013.
| Methods | RCT of 1 year | |
| Participants | How and where patients were recruited were not reported RA patients with active RA despite MTX Baseline demographics and disease characteristics were generally similar between the groups; median age was 49 yrs and 81.1% of patients were female. |
|
| Interventions | Group 1: Placebo SC every 4 week + MTX Group 2: golimumab 50 mg every 4 weeks + MTX |
|
| Outcomes | Primary outcome: ACR 20 at week 14 Secondary outcomes:
|
|
| Exclusions | Not stated | |
| Fatigue outcomes | FACIT‐F, range 0‐52, high = good | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not stated |
| Selective reporting (reporting bias) | Unclear risk | not stated |
Lukina 1998.
| Methods | RCT of 6 months | |
| Participants |
Group 1, 2 3, and 4 Gender (percentage female): 83.8%, 84.3%, 84.9% and 78.6% respectively Age, mean (SD) years: 48.6 (12.91), 48.2 (12.85), 50.9 (11.32) and 50.6 (11.58) respectively |
|
| Interventions | Group 1: anti‐IFNα IM injection, 5 days, dose 96 mg at 1st injection and 144 mg at days 2, 3, 4 and 5 Group 2: anti‐IFNγ IM injection, 5 days, dose 96 mg at 1st injection and 144 mg at days 2, 3, 4 and 5 Group 3: anti‐TNF IM injection, 5 days, dose 96 mg at 1st injection and 144 mg at days 2, 3, 4 and 5 Group 4: anti‐IFNγ + anti‐TNF IM injection, 5 days, dose 96 mg of each antibody at days 1, 2, 3, 4 and 5 Group 5: anti‐IFNγ + anti‐IFNα + anti‐TNF IM injection, 5 days, dose 96 mg of each antibody at days 1, 2, 3, 4 and 5 | |
| Outcomes |
|
|
| Exclusions | Pregnancy or breastfeeding History of serum disease or any allergic reactions to protein preparations | |
| Fatigue outcomes | VAS, range 0‐100 mm, high = bad | |
| Notes | Translator's notes: I did not discuss the trial with the authors, but they do have expertise in conducting trials, so I believe that true randomisation has been performed. The authors stated that they collected clinical and laboratory data at multiple time points, but only reported data on days 7 and 28; others were not reported. Also they stated that patient global assessment has been performed ‐ but no data reported. No baseline data for outcomes were reported, only mean changes (without SDs). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomised" but no details |
| Allocation concealment (selection bias) | Unclear risk | "[R]andomised" but no details |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Possibly |
Maini 1999.
| Methods | RCT of 54 weeks extended follow up to 104 weeks | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria Diagnosed with rheumatoid (1987 ACR criteria) and had evidence of active disease despite treatment with methotrexate (≥ 6 swollen and tender joints plus 2 of the following: morning stiffness greater than or equal to 45 min, erythrocyte sedimentation rate greater than 28 mm/h, CRP greater than 2 mg/dL). The patients were classified into a functional class (ACR criteria). Patients must also have been receiving oral or parenteral methotrexate for at least 3 months with no break in treatment of more than 2 weeks during this period. The methotrexate dose must have been stable at 12.5 mg/week or more, for at least 4 weeks before screening and the patient must have been on a stable dose of folic acid for the same period. Exclusions Little or no ability for self care; any current inflammatory condition with signs and symptoms that might confound the diagnosis (e.g. connective tissue disease or Lyme disease); used a DMARD other than methotrexate or received IA, IM or IV corticosteroids in the 4 weeks before screening; received any other agent to reduce TNF or had any previous use of cyclophosphamide, nitrogen mustard, chlorambucil or other alkylating agents; or a history of known allergies to murine proteins. Patients were also excluded if they had had infected joint prosthesis during the previous 5 years; serious infections, such as hepatitis, pneumonia, pyelonephritis in the previous 3 months; any chronic infectious disease such as renal infection, chest infection with bronchiectasis or sinusitis; active tuberculosis requiring treatment within the previous 3 years; opportunistic infections such as herpes zoster within the previous 2 months; any evidence of active cytomegalovirus; active Pneumocystis carinii; or drug‐resistant atypical mycobacterial infection. Other exclusions: current signs or symptoms of severe, progressive, or uncontrolled renal, hepatic, haematological, gastrointestinal, endocrine, pulmonary, cardiac, neurological, or cerebral disease; a history of lymphoproliferative disease including lymphoma or signs suggestive of disease, such as lymphadenopathy of unusual size or location (i.e. lymph nodes in the posterior triangle of the neck, infraclavicular epitrochlear, or periaortic areas); splenomegaly; any known malignant disease except basal cell carcinoma currently or in the past 5 years. All patients randomised Gender (percentage female): 78% Age, mean years: 54 |
|
| Interventions | All patients received intravenous infusions at the initiation of treatment (week 0) and at weeks 2 and 6. 2 groups of patients were administered infliximab + MTX (1 group receiving 3 mg/kg and the other receiving 10 mg/kg infliximab) and had subsequent infusions every 4 weeks, whereas 2 other groups of patients received infliximab plus MTX (1 receiving 3 mg/kg and the other receiving 10 mg/kg infliximab) but had subsequent infusions every 8 weeks, with placebo infusions at the interim 4‐week visits. Group 1: Placebo IV infusion + MTX Group 2: Infliximab 3mg/kg IV infusion every 8 weeks + MTX Group 3: Infliximab 3mg/kg IV infusion every 4 weeks + MTX Group 4: Infliximab 10mg/kg IV infusion every 8 weeks + MTX Group 5: Infliximab 10 mg/kg IV infusion every 4 weeks + MTX |
|
| Outcomes | Primary outcomes: ACR 20 at week 52
Secondary outcomes:
From Maini 2004 ‐ HAQ, SF‐36, sharp radiographic damage score, lab evaluations |
|
| Exclusions | Little or no ability for self care; any current inflammatory condition with signs and symptoms that might confound the diagnosis (e.g. connective tissue disease or Lyme disease); used a DMARD other than methotrexate or received IA, IM or IV corticosteroids in the 4 weeks before screening; received any other agent to reduce TNF or had any previous use of cyclophosphamide, nitrogen mustard, chlorambucil or other alkylating agents; or a history of known allergies to murine proteins. Patients were also excluded if they had had infected joint prosthesis during the previous 5 years; serious infections, such as hepatitis, pneumonia, pyelonephritis in the previous 3 months; any chronic infectious disease such as renal infection, chest infection with bronchiectasis or sinusitis; active tuberculosis requiring treatment within the previous 3 years; opportunistic infections such as herpes zoster within the previous 2 months; any evidence of active cytomegalovirus; active Pneumocystis carinii; or drug‐resistant atypical mycobacterial infection. Other exclusions: current signs or symptoms of severe, progressive, or uncontrolled renal, hepatic, haematological, gastrointestinal, endocrine, pulmonary, cardiac, neurological, or cerebral disease; a history of lymphoproliferative disease including lymphoma or signs suggestive of disease, such as lymphadenopathy of unusual size or location (i.e. lymph nodes in the posterior triangle of the neck, infraclavicular epitrochlear, or periaortic areas); splenomegaly; any known malignant disease except basal cell carcinoma currently or in the past 5 years. | |
| Fatigue outcomes | SF‐36 VT, Scale 0‐100, high = good | |
| Notes | Fatigue data reported in Maini 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent organisation did the centralised randomisation" |
| Allocation concealment (selection bias) | Low risk | "An independent organisation did the centralised randomisation" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LOCF |
| Selective reporting (reporting bias) | Unclear risk | Maini 1999 does not mention HAQ/SF‐36 yet these were measured from baseline and reported in Maini 2004 |
Mittendorf 2007.
| Methods | A pooled analysis of 6 RCTs of at least 6 weeks and up to 6 months | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria RA Exclusions Pregnant/breastfeeding women, HIV positive, alcohol or drug abuse in previous 6 months, ongoing/active clinically relevant infection, major episode of infection requiring hospitalisation or IV antibiotics in previous 30 days or oral antibiotics in previous 15 days; underlying cardiac, pulmonary, metabolic, renal, or gastrointestinal conditions, chronic or latent infectious diseases, immune deficiency or abnormal laboratory values All patients randomised Gender (percentage female): 79.8% Age, mean years (range): 54 (19‐80) |
|
| Interventions | Group 1: Adalimumab 40 mg every other week Group 2: Placebo |
|
| Outcomes | Clinical examination, disease activity, pain, HRQoL, SF‐36, FACIT‐F. No primary outcome defined. | |
| Exclusions | Pregnant/breastfeeding women, HIV positive, alcohol or drug abuse in previous 6 months, ongoing/active clinically relevant infection, major episode of infection requiring hospitalisation or IV antibiotics in previous 30 days or oral antibiotics in previous 15 days; underlying cardiac, pulmonary, metabolic, renal, or gastrointestinal conditions, chronic or latent infectious diseases, immune deficiency or abnormal laboratory values | |
| Fatigue outcomes | FACIT‐F, range: 0‐52, high = good SF‐36 VT, 0‐100, high = good BUT data not available | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomised" but no details |
| Allocation concealment (selection bias) | Unclear risk | "[R]andomised" but no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, LOCF |
| Selective reporting (reporting bias) | High risk | Pooled analysis |
Moreland 1999.
| Methods | RCT of 26 weeks | |
| Participants | Outpatient clinic, Multicentre North America Inclusion criteria Over 18 years, not wheelchair user, inadequate response to DMARD, ≥ 10 swollen joints, ≥ 12 painful joints, ESR ≥ 28, CRP > 20 mg/L, EMS > 45 min Exclusions Patients on corticosteroid doses could not exceed the equivalent of 10 mg of prednisone per day Group 1, 2 and 3 Gender (percentage female): 76%, 84%, and 74% respectively Age, mean years: 51, 53, 50.9 and 53 respectively |
|
| Interventions | Etanercept 10 mg or etanercept 25 mg twice weekly for 26 weeks Group 1: Placebo SC Group 2: Etanercept 10mg SC Group 3: Etanercept 25mg SC |
|
| Outcomes | Primary outcomes: 20% and 50% improvement in ACR responses at 3 and 6 months
Secondary outcomes:
|
|
| Exclusions | Patients on corticosteroid doses could not exceed the equivalent of 10 mg of prednisone per day | |
| Fatigue outcomes | HAQ VT (SF‐36 VT scale) range 0‐100, high = good | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned, block randomisation stratified by study site, codes housed by sponsor in locked database |
| Allocation concealment (selection bias) | Low risk | Randomly assigned, block randomisation stratified by study site, codes housed by sponsor in locked database |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo identical, outcome assessors had no knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LOCF ITT |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study publication |
Pope 2012.
| Methods | RCT of 12 weeks | |
| Participants | Outpatient clinic, multicentre international Inclusion criteria Active RA and inadequate response to at least 1 DMARD Group 1, 2 3, and 4 Gender (percentage female): 83.8%, 84.3%, 84.9% and 78.6% respectively Age, mean (SD) years: 48.6 (12.91), 48.2 (12.85), 50.9 (11.32) and 50.6 (11.58) Exclusions not stated Gender and age not reported |
|
| Interventions | Certolizumab (CZP) 400 mg at weeks 0, 2 and 4 followed by 200 mg every 2 weeks added to current therapy Group 1: CZP SC + DMARD Group 2: placebo SC + DMARD |
|
| Outcomes |
|
|
| Exclusions | Not stated | |
| Fatigue outcomes | FACIT‐F, range 0‐52, high = good | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Rigby 2011.
| Methods | RCT of 52 weeks | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria Patients aged 18–80 years with a diagnosis of RA (> 8 weeks to < 4 years prior to baseline) according to the revised 1987 ACR criteria Group 1, 2 3, and 4 Gender (percentage female): 83.8%, 84.3%, 84.9% and 78.6% respectively Age, mean (SD) years: 48.6 (12.91), 48.2 (12.85), 50.9 (11.32) and 50.6 (11.58) Exclusions not stated Group 1, 2 and 3 Gender (percentage female): 77.1%, 81.5% and 84.8% respectively Age, mean (SD) years: 48.1 (12.7), 47.9 (13.4) and 47.9 (13.3) respectively |
|
| Interventions | Rituximab 500 mg on days 1 and 15 by IV; preceded by methylprednisolone 100 mg
MTX 7.5 mg/week titrated to 20 mg/week by week 8
Rituximab 1000 mg on days 1 and 15 by IV, preceded by methylprednisolone 100 mg; MTX 7.5 mg/week titrated to 20 mg/week by week 8
Second course given at 24 weeks if DAS > 2.6 or later if it increased to that value Group 1: Placebo + MTX Group 2: Rituximab 500mg + MTX Group 3: Rituximab 1000mg + MTX |
|
| Outcomes | Primary outcome: Radiographic damage by Genant score at week 52 Secondary outcomes at week 52:
|
|
| Exclusions | None stated | |
| Fatigue outcomes | FACIT, 0‐52, high = good | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule, stratified by region (USA or rest of world) and RF status (positive or negative), was generated by the sponsor and supplied to an IVRS. At randomisation, patients were assigned unique medication and randomisation numbers via the IVRS. |
| Allocation concealment (selection bias) | Low risk | The randomisation schedule, stratified by region (USA or rest of world) and RF status (positive or negative), was generated by the sponsor and supplied to an IVRS. At randomisation, patients were assigned unique medication and randomisation numbers via the IVRS. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The sponsor, investigators and patients were blinded to treatment allocation until week 52, at which time the sponsor was unblinded for the purposes of data analysis (i.e. those undertaking analysis were not blinded) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, LOCF |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study publication |
Schiff 2008.
| Methods | RCT of 6 months | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria ACR criteria for RA. ≥ 18 years old, RA for ≥ 1 year, inadequate response to MTX. Received at least 15 mg MTX per week for at least 3 months prior to randomisation, washed out all DMARDs except MT for 28 days Exclusions Prior experience of abatacept or anti‐TNF therapy; TB Group 1, 2 and 3 Gender (percentage female): 83.3%, 87.3%, and 82.4% respectively Age, mean (SD) years: 49.0 (12.5), 49.4 (11.5) and 49.1 (12.0) |
|
| Interventions | Abatacept: dosed according to weight: < 60 kg received 500 mg; 60‐100kg received 750 mg; > 100kg received 1000 mg. IV infusion on days 1, 15 and 29 and every 28 days thereafter up to and including day 337 (with normal saline received on day 43)
Infliximab: 3mg/kg, administered on days 1,15, 43 and 85 and every 56 days thereafter (normal saline was received at the remaining visits) Group 1: Abatacept IV + MTX Group 2: Placebo IV + MTX Group 3: Infliximab IV + MTX |
|
| Outcomes | Primary outcome: disease activity (DAS 28) at day 197 Secondary outcomes at day 197:
|
|
| Exclusions | Prior experience of abatacept or anti‐TNF therapy; TB | |
| Fatigue outcomes | SF‐36 VT, 0‐100, high = good | |
| Notes | Author contacted to ask for vitality data at 6 months as this was the primary endpoint; placebo participants were reassigned at 6 months ‐ no response received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | "[R]andomised, double‐blind, double‐dummy, placebo‐ and active‐(infliximab) controlled . . ." "randomised by centre" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Assessors, physicians and patients were blinded to the treatment group assignment for 1 year" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Patients who discontinued the study early were considered as non‐responders", used LOCF |
| Selective reporting (reporting bias) | Low risk | Almost full participation to 6 months in all study arms |
Smolen 2008.
| Methods | RCT of 24 weeks | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria Adult patients with moderate‐severe active RA (Arnett ‐swollen joint count of ≥ 6 and tender joint count of ≥ 8 or CRP over 10 mg/L or ESR of 28 mm/h or more) for ≥ 6 months duration with inadequate response to methotrexate. Must have received methotrexate for ≥ 12 weeks before the start of the study (stable dose of 10‐25 mg/week for ≥ 8 weeks). All other DMARDs were discontinued before the start of the study: leflunomide for ≥ 12 weeks (or ≥ 4 weeks after 11 days of standard cholestyramine washout), anakinra for ≥ 1 week, etanercept for ≥ 2 weeks, and infliximab or adalimumab for ≥ 8 weeks. Oral glucocorticoids (≤ 10 mg/day prednisone or equivalent) and non‐steroidal anti‐inflammatory drugs (NSAIDs) were permitted if doses were stable for 6 weeks or more before inclusion. Exclusions Other autoimmune diseases or significant systemic involvement secondary to RA; functional class IV RA; previous or current inflammatory joint disease other than rheumatoid arthritis; active/previous recurrent bacterial, viral, fungal or other infections; clinically significant abnormalities on chest radiograph, hepatitis B and C; or recurrent herpes zoster. Active liver disease, previous unsuccessful treatment with an anti‐TNF agent due to lack of efficacy/significant safety issues. Group 1, 2 and 3 Gender (percentage female): 82%, 85% and 78% respectively Age, mean (SD) years: 51.4 (12.8), 50.8 (11.8) and 50.6 (12.1) respectively |
|
| Interventions | IV tocilizumab 4 weekly: 4mg/kg or 8mg/kg. All patients received drug in combination with weekly administration of their stable dose of methotrexate.
If ACR 20 not achieved by week 16, patients in all arms given rescue therapy with tocilizumab 8 mg/kg and, if necessary, intra‐articular steroids or an increase in oral corticosteroid Group 1: Tocilizumab 4mg/kg IV + MTX Group 2: Tocilizumab 8 mg/kg IV + MTX Group 3: Placebo IV + MTX |
|
| Outcomes | Primary outcome: ACR 20 at week 24 weeks Secondary outcomes:
|
|
| Exclusions | Other autoimmune diseases or significant systemic involvement secondary to RA; functional class IV RA; previous or current inflammatory joint disease other than rheumatoid arthritis; active/previous recurrent bacterial, viral, fungal or other infections; clinically significant abnormalities on chest radiograph, hepatitis B and C; or recurrent herpes zoster. Active liver disease, previous unsuccessful treatment with an anti‐TNF agent due to lack of efficacy/significant safety issues | |
| Fatigue outcomes | FACIT‐F, 0‐52, high = good | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done centrally with an interactive voice response system stratified by site with a randomization list provided by Roche" |
| Allocation concealment (selection bias) | Low risk | "Randomization was done centrally with an interactive voice response system stratified by site with a randomization list provided by Roche" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Physician blinded to treatment, placebo‐controlled intervention, trained assessor with no access to data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT done, LOCF for joints |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study publication |
Smolen 2009a.
| Methods | RCT of 24 weeks | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria Aged > 18 years, diagnosis of RA (ACR) > 6 months' duration but not longer than 15 years, active disease at screening and baseline. Patients had to have received prior MTX for > 6 months (stable dose > 10 mg/week for > 2 months before baseline). Supplementary list of inclusion criteria available at: http://ard.bmj.com/content/vol68/issue6 Exclusions Patients who received any biological agent for RA within 6 months before enrolment (3 months for etanercept and anakinra), previous treatment with a biological agent resulting in a severe hypersensitivity or anaphylactic reaction, or had not initially responded to previous anti‐TNF therapy. Oral corticosteroids (≤ 10 mg/day prednisone equivalent) and non‐steroidal anti‐inflammatory drugs and cyclo‐oxygenase‐2 inhibitors were permitted provided that the doses were stable within 28 and 14 days of baseline, respectively, and remained stable during the study. Patients with history of, or positive chest x‐ray findings for, tuberculosis, or a positive PPD skin test (defined as positive indurations per local medical practice) were excluded. Supplementary list of exclusion criteria available at: http://ard.bmj.com/content/vol68/issue6 Group 1, 2 and 3 Gender (percentage female): 84.3%, 83.7%, and 78% respectively Age, mean (SD) years: 51.5 (11.8), 52.2 (11.1), and 51.9 (11.8) respectively |
|
| Interventions | Patients were randomised 2:2:1 to one of two regimens of subcutaneous liquid certolizumab pegol (400 mg at weeks 0, 2 and 4, followed by 200 or 400 mg every 2 weeks) plus MTX for 24 weeks Group 1: Placebo SC + MTX Group 2: Certolizumab pegol 200 mg SC + MTX Group 2: Certolizumab pegol 400 mg SC + MTX |
|
| Outcomes | Primary outcome: ACR 20 response at 24 weeks
Secondary outcomes at 24 weeks:
|
|
| Exclusions | Patients who received any biological agent for RA within 6 months before enrolment (3 months for etanercept and anakinra), previous treatment with a biological agent resulting in a severe hypersensitivity or anaphylactic reaction, or had not initially responded to previous anti‐TNF therapy. Oral corticosteroids (≤ 10 mg/day prednisone equivalent) and non‐steroidal anti‐inflammatory drugs and cyclo‐oxygenase‐2 inhibitors were permitted provided that the doses were stable within 28 and 14 days of baseline, respectively, and remained stable during the study. Patients with history of, or positive chest x‐ray findings for, tuberculosis, or a positive PPD skin test (defined as positive indurations per local medical practice) were excluded. Supplementary list of exclusion criteria available at: http://ard.bmj.com/content/vol68/issue6 | |
| Fatigue outcomes | SF‐36 VT, range 0‐100, high = good Fatigue analogue scale, range 0‐10, high = bad | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised 2:2:1 |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomised 2:2:1 |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention as to whether placebo looked identical; radiographs were read centrally and blinded (for treatment, visit and patient identification) and independently by 2 experienced readers but not clear if nurses taking joint counts were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study publication |
Smolen 2009b.
| Methods | RCT of 24 weeks | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria Both a swollen joint count (66 joints) and tender joint count (68 joints) of > 8 at screening and at baseline, a CRP level of > 1.0 mg/dL; receiving < 10 mg/day of prednisolone or equivalent (if stable for at least 4 weeks prior to baseline) or NSAIDs (if stable for at least 2 weeks prior to baseline). Exclusions Inflammatory diseases other than rheumatoid arthritis; a serious adverse reaction to a previous TNFα inhibitor (judged by the investigator); had ever received natalizumab or rituximab; had received anakinra less than 4 weeks, or alefacept or efalizumab less than 3 months before the first dose of study drug; had ever received cytotoxic drugs; had a history of latent or active granulomatous infection, except latent tuberculosis, that was treated prophylactically in the past 3 years; had a BCG vaccination less than 12 months before screening; had an opportunistic infection less than 6 months before screening; had a serious infection (judged by the investigator) less than 2 months before screening; had a history of chronic infection, demyelinating disease, congestive heart failure, or severe, progressive, uncontrolled renal, hepatic, haematological, gastric intestinal, endocrine, pulmonary, cardiac, neurological, psychiatric, or cerebral disease; or had a transplanted organ or a malignancy in the past 5 years Group 1, 2 and 3 Gender (percentage female): 85%, 74%, and 80% respectively Age, median (range) years: 54 (46‐645), 55 (46‐63), and 55 (47‐61) respectively |
|
| Interventions | Subcutaneous injections of 50 mg golimumab or 100 mg golimumab every 4 weeks. Every patient received a 0.5 mL and a 1 mL injection every 4 weeks. Patients in the 50 mg group received golimumab in the 0.5 mL syringe and placebo in the 1 mL syringe, whereas those in the 100 mg group received golimumab in the 1 mL syringe and placebo in the 0.5 mL syringe. Total = 4 doses. At week 16, patients in the 50 mg group who had less than 20% improvement from baseline in both tender and swollen joint counts entered a double‐blinded rescue therapy phase to receive 100 mg golimumab. Patients in the 100 mg group who met the criteria for rescue therapy continued to receive the same dose. Change of treatment to rescue therapy was not possible at any other time point. Group 1: Placebo SC Group 2: Golimumab 50mg SC Group 3: Golimumab 100mg SC |
|
| Outcomes | Primary outcome: ACR 20 at week 14 Secondary outcome:
|
|
| Exclusions | Inflammatory diseases other than rheumatoid arthritis; a serious adverse reaction to a previous TNFα inhibitor (judged by the investigator); had ever received natalizumab or rituximab; had received anakinra less than 4 weeks, or alefacept or efalizumab less than 3 months before the first dose of study drug; had ever received cytotoxic drugs; had a history of latent or active granulomatous infection, except latent tuberculosis, that was treated prophylactically in the past 3 years; had a BCG vaccination less than 12 months before screening; had an opportunistic infection less than 6 months before screening; had a serious infection (judged by the investigator) less than 2 months before screening; had a history of chronic infection, demyelinating disease, congestive heart failure, or severe, progressive, uncontrolled renal, hepatic, haematological, gastric intestinal, endocrine, pulmonary, cardiac, neurological, psychiatric, or cerebral disease; or had a transplanted organ or a malignancy in the past 5 years | |
| Fatigue outcomes | FACIT‐F, 0‐52, high = good | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned in a 1:1:1 ratio to receive subcutaneous injections of placebo, 50 mg golimumab, or 100 mg golimumab every 4 weeks. Randomisation was stratified by study site and baseline methotrexate use. Site personnel called a central telephone interactive voice response system (IVRS) to obtain randomisation information for every patient. |
| Allocation concealment (selection bias) | Low risk | Both patients and investigators were masked to treatment assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Including packaging and even when eligible for rescue package |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Patients with missing data were assumed to be non‐responders as were those who dropped out. ITT used. Doesn't say how fatigue scores were calculated where the data were missing: LOCF? Imputed? |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study publication |
Soubrier 2009.
| Methods | RCT of 1 year | |
| Participants | Outpatient clinics, multicentre in France Inclusion criteria Maximum disease duration of 6 months ≥ 18 years of age Active disease defined as a DAS 28 ESR (DAS 28) > 5.1 Screened for tuberculosis; patients who were at high risk for tuberculosis were allowed to enrol in the study after chemoprophylaxis Group 1, 2 3, and 4 Gender (percentage female): 83.8%, 84.3%, 84.9% and 78.6% respectively Age, mean (SD) years: 48.6 (12.91), 48.2 (12.85), 50.9 (11.32) and 50.6 (11.58) respectively Exclusions Previous treatment with MTX or biologics Concomitant treatment with an experimental drug Malignancy within the previous 10 years Cytopaenia (haemoglobin < 9 g/dL in men, 8.5 g/dL in women, leucocytes < 3x109/L, platelets < 150x109/L) Serum aspartate aminotransferase/alanine aminotransferase level more than 1.5 times the upper limit of normal Creatinine clearance level < 50 mL/min Concurrent pregnancy, inadequate contraception Chronic infectious disease Major episode of infection requiring hospitalisation or treatment with IV antibiotics within 30 days or oral antibiotics within 14 days prior to screening Heart disease, multiple sclerosis Group 1 and Group 2: Gender (percentage female): 81.25% and 78.79% respectively Age mean (SD) year: 49.3 (15.2) and 46.3 (16.3) respectively |
|
| Interventions | Initial combination therapy with MTX and ADA (Group 2): 0.3 mg/kg/week, maximum of 20 mg/week
Every 3 months decided whether or not to escalate based on DAS 28. If the patient did not achieve a low disease activity (DAS 28 < 3.2), the treating physician immediately adjusted therapy by proceeding to the next step in the allocated treatment group.
If the DAS 28 was < 3.2 at week 12, ADA was stopped. In the event of remission (DAS 28 < 2.6 for at least 6 months), MTX was tapered (2.5 mg/month) to a maintenance dose of 7.5 mg/week. If disease activity flared after tapering of MTX, the initial dose of MTX was reintroduced. In the event of relapse, patients restarted ADA 40 mg every other week for 12 weeks. If the DAS 28 was > 3.2 after 12 weeks, ADA was stopped.
In the event of inefficacy (DAS 28 > 3.2 after 12 weeks of treatment), ADA was increased (40 mg/week) for 12 weeks. After 12 weeks of effective therapy, ADA was decreased (40 mg every other week) for 12 weeks and stopped if successful. In the event of failure on ADA 40 mg/week, etanercept (25 mg twice a week) was initiated for 12 weeks. If effective, etanercept was stopped and started again for 12 weeks if relapse occurred. If etanercept failed, LEF was initiated. If the treatment was unsuccessful after the initial 12 weeks, the same regimen was applied according to the protocol indicated above. Group 1: MTX Group 2: MTX + ADA |
|
| Outcomes | Primary outcome: proportion of patients in low disease activity at week 12 for whom anti‐TNF was not introduced or reintroduced at 1 year. Secondary outcomes:
|
|
| Exclusions | Previous treatment with MTX or biologics Concomitant treatment with an experimental drug Malignancy within the previous 10 years Cytopaenia (haemoglobin < 9 g/dL in men, 8.5 g/dL in women, leucocytes < 3x109/L, platelets < 150x109/L) Serum aspartate aminotransferase/alanine aminotransferase level more than 1.5 times the upper limit of normal Creatinine clearance level < 50 mL/min Concurrent pregnancy, inadequate contraception Chronic infectious disease Major episode of infection requiring hospitalisation or treatment with IV antibiotics within 30 days or oral antibiotics within 14 days prior to screening Heart disease, multiple sclerosis | |
| Fatigue outcomes | VAS (visual analog scale), range 0–100, high = bad | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients randomised |
| Allocation concealment (selection bias) | Unclear risk | Patients randomised |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not possible, complex escalation regime dependent on response |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients enrolled in the study were included in ITT analyses of efficacy and safety. The LOCF approach was used to handle missing data. |
| Selective reporting (reporting bias) | High risk | This main paper does not give details of all outcomes, but does summarise (e.g. had to get full fatigue data from authors) |
Strand 2009.
| Methods | RCT of 1 year | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria ≥ 18 years of age, diagnosis of RA as defined by the ACR 1987 criteria, active disease was defined as > 9 tender and 9 swollen joints at screening and at baseline, with either an ESR > 30 mm/h or a CRP of > 15 mg/L. Patients were required to have received MTX for > 6 months, with a stable dosage of > 10 mg/week for > 2 months prior to baseline. Exclusions Diagnoses of any other inflammatory arthritis or a secondary non‐inflammatory arthritis that could have interfered with our evaluation of the effects of certolizumab pegol on RA. Patients with a history of tuberculosis or a chest radiograph showing active or latent tuberculosis. Patients with positive PPD skin test were excluded unless the PPD positivity was associated with previous vaccination with BCG. Patients who, in the investigator's opinion, were at a high risk of infection. History of malignancy, demyelinating disease, blood dyscrasias, or severe, progressive, uncontrolled renal, hepatic, hematologic, gastrointestinal, endocrine, pulmonary, cardiac, neurologic or cerebral disease. Patients who had received any biologic therapy within 6 months (or had received etanercept or anakinra within 3 months) of baseline, or any previous biologic therapy that resulted in a severe hypersensitivity or anaphylactic reaction were excluded, as were patients who had previously failed to respond to treatment with an anti‐TNF agent. Group 1, 2 and 3 Gender (percentage female): 83.9%, 82.4% and 83.6% respectively Age, mean (SD) years: 52.2 (11.2), 51.4 (11.6) and 52.4 (11.7) respectively |
|
| Interventions | All patients received stable doses of methotrexate
Subcutaneous injections of certolizumab over 52 weeks
Loading doses of 400 mg at 0, 2 and 4 then 200 mg or 400 mg every other week. Total 27 injections. Group 1: Placebo + MTX Group 2: Certolizumab 200mg + MTX Group 3: Certolizumab 400mg + MTX |
|
| Outcomes | From Keystone 2008: Primary Outcomes:
Secondary Outcomes
|
|
| Exclusions | Diagnoses of any other inflammatory arthritis or a secondary non‐inflammatory arthritis that could have interfered with our evaluation of the effects of certolizumab pegol on RA. Patients with a history of tuberculosis or a chest radiograph showing active or latent tuberculosis. Patients with positive PPD skin test were excluded unless the PPD positivity was associated with previous vaccination with BCG. Patients who, in the investigator's opinion, were at a high risk of infection. History of malignancy, demyelinating disease, blood dyscrasias, or severe, progressive, uncontrolled renal, hepatic, hematologic, gastrointestinal, endocrine, pulmonary, cardiac, neurologic or cerebral disease. Patients who had received any biologic therapy within 6 months (or had received etanercept or anakinra within 3 months) of baseline, or any previous biologic therapy that resulted in a severe hypersensitivity or anaphylactic reaction were excluded, as were patients who had previously failed to respond to treatment with an anti‐TNF agent. | |
| Fatigue outcomes | SF‐36 VT, range 0‐100, high = good Fatigue numerical rating scale, range 0‐10, high = bad | |
| Notes | Patients who failed to achieve a response the ACR 20 at weeks 12 and 14 were designated treatment failures and were withdrawn from the study at week 16. Patients who withdrew at week 16 or who successfully completed the trial were offered enrolment in an open‐label extension study of certolizumab pegol 400 mg every 2 weeks. Patients who withdrew early for reasons other than withdrawal of consent underwent mandatory radiographic assessment at the time of withdrawal and at week 52. Mean change/SDS not reported for NRS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomized 2:2:1" but no details |
| Allocation concealment (selection bias) | Unclear risk | "[R]andomized 2:2:1" but no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT |
| Selective reporting (reporting bias) | High risk | Post hoc analysis from another trial |
Strand 2012a.
| Methods | RCT of 24 weeks | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria ≥ 18 years of age, moderate to severe active RA, failure to respond or intolerance to one or more TNF antagonists within the past year, active RA for 6 months or more, swollen joint count of ≥ 6, tender joint count of ≥ 8, CRP greater than 1.0 mg/dL or ESR greater than 28 mm/h. Patients had to be treated with MTX for 12 weeks or more before baseline (stable dose > 8 weeks) Exclusions Treatment with cell depleting agents Uncontrolled medical conditions Other inflammatory diseases Functional class IV RA History of malignancies or recurrent infections, primary or secondary immunodeficiency Haemoglobin less than 8.5 g/dL, leucopaenia, neutropaenia, thrombocytopaenia Abnormal liver function, triglycerides greater than 10 mmol/L, Active tuberculosis, hepatitis B, or hepatitis C. Group 1, 2 and 3 Gender (percentage female): 79%, 81%, and 84% respectively Age, mean (SD) years: 53.4 (13.3), 50.9 (12.5), and 53.9 (12.7) respectively |
|
| Interventions | 4 mg/kg tocilizumab IV once every 4 weeks
Stable doses (10‐25 mg) of weekly MTX for 24 weeks
or
8 mg/kg tocilizumab IV once every 4 weeks
Stable doses (10‐25 mg) of weekly MTX for 24 weeks. Group 1: Placebo + MTX Group 2: Tocilizumab 4mg/kg + MTX Group 2: Tocilizumab 8mg/kg + MTX |
|
| Outcomes | Primary outcome: ACR 20 response at week 24 Secondary outcomes:
|
|
| Exclusions | Treatment with cell depleting agents Uncontrolled medical conditions Other inflammatory diseases Functional class IV RA History of malignancies or recurrent infections, primary or secondary immunodeficiency Haemoglobin less than 8.5 g/dL, leucopaenia, neutropaenia, thrombocytopaenia Abnormal liver function, triglycerides greater than 10 mmol/L, Active tuberculosis, hepatitis B, or hepatitis C. | |
| Fatigue outcomes | SF‐36 VT, 0‐100, high = good FACIT‐F, range: 0‐52, high = good | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomised, double‐blind" but no details |
| Allocation concealment (selection bias) | Unclear risk | "[R]andomised, double‐blind" but no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Joint counts? Analysis? |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT |
| Selective reporting (reporting bias) | High risk | Post hoc analysis from another trial |
Strand 2012b.
| Methods | RCT of 2 years | |
| Participants | Outpatient clinic, multicentre international Inclusion criteria MTX‐naive patients ≥ 18 years of age with active RA (≥ 8 swollen joints, ≥ 10 tender joints, and an erythrocyte sedimentation rate ≥ 28 mm/h or CRP concentration ≥ 1.5 mg/dL, in addition to rheumatoid factor positivity or ≥ 1 joint erosion) and disease duration < 3 years Exclusions not stated Group 1, 2 and 3 Gender (percentage female): 72%, 73.9% and 77.4% respectively Age, mean (SD) years: 51.9 (14), 52.0 (13.1) and 52.1 (13.5) |
|
| Interventions | Adalimumab (ADA) 40 mg subcutaneously every other week plus weekly oral MTX, 104 weeks Group 1: ADA + MTX Group 2: Placebo + MTX Group 3: ADA + placebo [only extract adalimumab plus methotrexate (group 1) vs methrotrexate (group 2) for review] |
|
| Outcomes | Primary outcomes (at 52 weeks):
Secondary outcomes:
|
|
| Exclusions | — | |
| Fatigue outcomes | FACIT‐F, 0‐52, High good | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomised, double‐blind" but no details |
| Allocation concealment (selection bias) | Unclear risk | "[R]andomised, double‐blind" but no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Patients were randomized to 1 of 3 treatment groups: adalimumab 40 mg subcutaneously every other week plus weekly oral MTX (20 mg/week); adalimumab 40 mg subcutaneously every other week (adalimumab plus placebo); or weekly oral MTX (MTX plus placebo). Hence, all patients received an injection (adalimumab or placebo) and an oral medication (MTX or placebo). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ACR responses were calculated using an ITT analysis, for which patients who discontinued the study prior to reaching the end point were considered to be non‐responders. |
| Selective reporting (reporting bias) | High risk | Post hoc analysis from another trial |
Weinblatt 2003.
| Methods | RCT of 24 weeks | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria ≥ 18 years, RA (1987 revised criteria of ACR); active disease (at least 9/68 tender joints and 6/66 swollen joints). Must have been treated with MTX for ≥ 6 months and taking a stable weekly dose (12.5‐25 mg, or 10 mg if intolerant to higher doses) for ≥ 4 weeks before entering the study. All participants must have failed treatment with at least 1 DMARD besides MTX, but no more than 4 DMARDs Exclusions For all patients: all DMARDs, except MTX, were discontinued 4 weeks before the study. In addition to MTX, concomitant RA therapies permitted during the study included salicylates, NSAIDs and corticosteroids (maximum daily dose of 10 mg of oral prednisone or equivalent). Dosage tapering or changes in the route of administration of the concomitant medications were not permitted during the study. Folic acid or leucovorin was permitted. High potency opioid analgesics (e.g., methadone, hydromorphone or morphine) were prohibited; other analgesics were allowed, although not within 12 h of study visits. Group 1, 2 3, and 4 Gender (percentage female): 82.3%, 75.4%, 74.6% and 75.3% respectively Age, mean (SD) years: 56.0 (10.8), 53.5 (12.4), 57.2 (11.4) and 55.5 (11.7) respectively |
|
| Interventions | Patients were randomised to receive adalimumab at a dosage of 20 mg, 40 mg, or 80 mg subcutaneously every other week as 2 injections of 1.6 mL per injection. Patients were instructed in self injection techniques Group 1: Placebo SC every other week + MTX Group 2: Adalimumab 20mg SC every other week + MTX Group 3: Adalimumab 40mg SC every other week + MTX Group 4: Adalimumab 80mg SC every other week + MTX |
|
| Outcomes | Primary outcome: percentage of patients achieving ACR 20 response at week 24
Secondary outcomes:
|
|
| Exclusions | Standard exclusion criteria used in trials of other biologics in patients with RA, also patients who had received anti‐CD4 therapy or TNF antagonists, had a history of active listeriosis or mycobacterial infection, and had a major episode of infection requiring hospitalisation or treatment with IV antibiotics within 30 days or oral antibiotics within 14 days prior to screening | |
| Fatigue outcomes | SF‐36 VT, 0‐100, high = good FACIT‐F, range: 0‐52, high = good | |
| Notes | For all patients: all DMARDs, except MTX, were discontinued 4 weeks before the study. In addition to MTX, concomitant RA therapies permitted during the study included salicylates, NSAIDs and corticosteroids (maximum daily dose of 10 mg of oral prednisone or equivalent). Dosage tapering or changes in the route of administration of the concomitant medications were not permitted during the study. Folic acid or leucovorin was permitted. High potency opioid analgesics (e.g., methadone, hydromorphone or morphine) were prohibited; other analgesics were allowed, although not within 12 h of study visits. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomized" but no details |
| Allocation concealment (selection bias) | Unclear risk | "[R]andomized" but no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LOCF |
| Selective reporting (reporting bias) | Low risk | Fatigue was reported in the main study publication |
Weinblatt 2013.
| Methods | RCT of 100 weeks | |
| Participants | Outpatient clinic, Multicentre international Inclusion criteria Adults with active RA despite MTX (stable regimen of 15–25 mg/week for ≥ 4 weeks) for ≥ 3 months Active RA was defined by ≥ 6/66 swollen joints and ≥ 6/68 tender joints RF positive or anti‐CCP positive at screening, CRP ≥ 1.0 mg/dL Naive to anti‐TNF treatment Stable (≥ 2 weeks) approved regimens of NSAIDs, oral corticosteroids (≤10 mg/day) or both were allowed. Eligible patients met all relevant TB and clinical laboratory screening criteria. With regard to TB, patients with no history of latent or active TB prior to screening, no signs or symptoms suggestive of active TB upon medical history or physical examination, no recent close contact with a person with active TB, a negative QuantiFERON‐TBÒ Gold In‐Tube 2 test result within 6 weeks of study agent start, and a chest radiograph within 3 months prior to the first administration of study agent and read by a qualified radiologist. Patients with evidence of close contact to active TB or latent TB could enroll in the study if treated for latent TB prior to or simultaneously with the first administration of study agent. Exclusions DMARDs other than MTX or non‐oral corticosteroids within the prior 4 weeks were excluded Prior receipt of: any commercial/investigational TNF‐inhibitor; natalizumab or other alpha‐4‐integrin blockers; or rituximab, abatacept or efalizumab Patients with other inflammatory diseases, a known hypersensitivity to human immunoglobulin proteins or other components of golimumab Prior receipt of any commercial or investigational anti‐TNF therapy History of latent or active granulomatous infection, including histoplasmosis, or coccidioidomycosis prior to screening; had a bacille Calmette‐Guérin vaccination within 12 months of screening; had a chest radiograph within 3 months prior to the first administration of study agent that showed an abnormality suggestive of a malignancy or current active infection, including TB; had a nontuberculous mycobacterial infection or opportunistic infection (e.g. cytomegalovirus, pneumocystosis, aspergillosis) within 6 months prior to screening; or had received, or was expected to receive, any live virus or bacterial vaccination within 3 months prior to the first administration of study agent, during the study, or within 6 months after the last administration of study agent. History of an infected joint prosthesis, receipt of antibiotics for a suspected infection of a joint prosthesis, if that prosthesis has not been removed or replaced; a serious infection (e.g., hepatitis, pneumonia, or pyelonephritis), hospitalisation for an infection, or treatment of an infection with intravenous antibiotics within 2 months prior to the first administration of study agent; a history of, or ongoing, chronic or recurrent (> 3 identical infections/12 months) infectious disease; an open, draining, or infected skin wound; or an ulcer. Patients with a history of known demyelinating diseases such as multiple sclerosis or optic neuritis were excluded, as were patients with a history of, or concurrent, congestive heart failure. Patients with a history of lymphoproliferative disease, including lymphoma, or signs suggestive of possible lymphoproliferative disease such as lymphadenopathy of unusual size or location, or clinically significant splenomegaly were ineligible, as were patients with any known malignancy or a history of malignancy within the previous 5 years (with the exception of a treated nonmelanoma skin cancer with no evidence of recurrence). Group 1 and 2 Gender (percentage female): 79.7% and 82.5% respectively Age, mean (SD) years: 51.4 (11.26) and 51.9 (12.55) respectively |
|
| Interventions | Intravenous golimumab 2 mg/kg at weeks 0 and 4, and then every 8 weeks through week 100. Group 1: Placebo + MTX Group 2: Glimumab 2mg/kg + MTX |
|
| Outcomes | Primary outcome: ACR 20 at week 14 Secondary outcomes:
|
|
| Exclusions | DMARDs other than MTX or non‐oral corticosteroids within the prior 4 weeks were excluded Prior receipt of: any commercial/investigational TNF‐inhibitor; natalizumab or other alpha‐4‐integrin blockers; or rituximab, abatacept or efalizumab Patients with other inflammatory diseases, a known hypersensitivity to human immunoglobulin proteins or other components of golimumab Prior receipt of any commercial or investigational anti‐TNF therapy History of latent or active granulomatous infection, including histoplasmosis, or coccidioidomycosis prior to screening; had a bacille Calmette‐Guérin vaccination within 12 months of screening; had a chest radiograph within 3 months prior to the first administration of study agent that showed an abnormality suggestive of a malignancy or current active infection, including TB; had a nontuberculous mycobacterial infection or opportunistic infection (e.g. cytomegalovirus, pneumocystosis, aspergillosis) within 6 months prior to screening; or had received, or was expected to receive, any live virus or bacterial vaccination within 3 months prior to the first administration of study agent, during the study, or within 6 months after the last administration of study agent. History of an infected joint prosthesis, receipt of antibiotics for a suspected infection of a joint prosthesis, if that prosthesis has not been removed or replaced; a serious infection (e.g., hepatitis, pneumonia, or pyelonephritis), hospitalisation for an infection, or treatment of an infection with intravenous antibiotics within 2 months prior to the first administration of study agent; a history of, or ongoing, chronic or recurrent (> 3 identical infections/12 months) infectious disease; an open, draining, or infected skin wound; or an ulcer. Patients with a history of known demyelinating diseases such as multiple sclerosis or optic neuritis were excluded, as were patients with a history of, or concurrent, congestive heart failure. Patients with a history of lymphoproliferative disease, including lymphoma, or signs suggestive of possible lymphoproliferative disease such as lymphadenopathy of unusual size or location, or clinically significant splenomegaly were ineligible, as were patients with any known malignancy or a history of malignancy within the previous 5 years (with the exception of a treated nonmelanoma skin cancer with no evidence of recurrence). | |
| Fatigue outcomes | FACIT‐F, range: 0‐52, high = good | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly (2:1) assigned, via IVRS |
| Allocation concealment (selection bias) | Low risk | Patients were randomly (2:1) assigned, via IVRS |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind with placebo infusion. Joint evaluations were performed by an independent blinded assessor assigned to each study centre. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patients with prohibited medication usage, who discontinued because of lack of efficacy before week 14 and who lacked all week 14 ACR 20 component data for any reason were considered ACR 20 non‐responders at week 14 through week 24. In these ITT analyses, patients randomised to placebo who early escaped (EE) (n=68/197), and who received golimumab 2 mg/kg infusions at weeks 16 and 20, had week 16 data carried forward for response calculations at weeks 20 and 24. A LOCF procedure was employed to impute missing ACR component data (e.g. swollen or tender joint count, or global assessments of disease) at week 14 if the patient had data for at least one other ACR component at week 14. |
| Selective reporting (reporting bias) | Unclear risk | Didn't mention fatigue data collected, presented as an abstract. What else is missing? |
ACR: American College of Rheumatology; ADA: adalimumab; BCG: bacille Calmette‐Guerin; CCP: anti‐cyclic citrullinated peptide antibody; CDAI: Clinical Disease Activity Index; CORE: computerised randomisation and enrolment; CRP: C‐reactive protein; CS: corticosteroid; CTLA4Ig: cytotoxic T‐lymphocyte‐associated protein 4‐immunoglobulin superfamily; CZP: certolizumab; DAS: Disease Activity Score 28; DMARD: disease‐modifying anti‐rheumatic drugs; ETN: etanercept; EE: early escaped; EMS: electronic muscle therapy; EQ‐5D: EuroQol five dimensions questionnaire; ESR: erythrocyte sedimentation rate; EULAR: European League Against Rheumatism; FACIT‐F: Functional Assessment of Chronic Illness Therapy‐Fatigue; FAS: Fatigue Assessment Scale; HADS: Hospital Anxiety and Depression Scale; HAQ (DI): Health Assessment Questionnaire (disability index); IA: intra‐articular; IM: intramuscular; INF: Infliximab; ITT: intention‐to‐treat; IUD: intrauterine device; IV: intravenous; IVRS: interactive voice response system; LEF: leflumonide; LOCF: last observation carried forward; MTX: methotrexate; NRI: non‐responder imputation; NRS: numeric rating scale; NSAID: non‐steroidal anti‐inflammatory drug; PCS: physical component score; PPD: purified protein derivative; PRO: patient‐reported outcomes; RA: rheumatoid arthritis; RCT: randomised controlled trial;RF: rheumatoid factor; RTX: Rituximab; SC: subcutaneous; SD: standard deviation; SDAI: Simplified Disease Activity Index; SF‐6D: Short Form Six Dimension questionnaire; SF‐12v2: Short Form 12‐item version 2 questionnaire; SF‐36 (VT): Short Form 36‐item questionnaire (vitality); TCZ: Tocilizumab; TB: tuberculosis; TNF: tumour necrosis factor; VAS: visual analogue scale; WPAI: work productivity and activity impairment.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Breedveld 2005 | No fatigue outcome measure |
| Cella 2005 | Not an RCT |
| Dougados 2007 | CRA conference abstract. Full text of trial was published as Genovese 2005 |
| Duggan 2009 | Not an RCT |
| Elliott 1994 | No fatigue outcome measure |
| Frampton 2007 | Not an RCT |
| Furst 2003 | No fatigue outcome measure |
| Genovese 2010 | No fatigue outcome measure |
| Gnanasakthy 2013 | Abstract only, same as published in Strand 2014 |
| Grigor 2004 | No fatigue outcome measure |
| Haugeberg 2009 | No fatigue outcome measure |
| Kavanaugh 2003 | No fatigue outcome measure |
| Kavanaugh 2012 | Not an RCT |
| Kim 2007 | No fatigue outcome measure |
| Kosinski 2000 | No fatigue outcome available |
| Kremer 2008 | No fatigue outcome measure |
| Moreland 2000 | No fatigue outcome measure |
| Moreland 2002 | No fatigue outcome measure |
| Sansonno 2003 | Not an RCT |
| Song 2007 | No fatigue outcome measure |
| Strand 2012 | Reported fatigue was a factor that influenced work productivity but not the result of RCT |
| Strand 2014 | Report the effect of different doses of seculimumab on fatigue before and after treatment but not between active and placebo nor usual care |
| Tak 2008 | No fatigue outcome measure |
| Yount 2007 | Not an RCT |
Characteristics of ongoing studies [ordered by study ID]
St Clair 2004.
| Trial name or title | Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Starting date | |
| Contact information | |
| Notes | — |
Van der Kooij 2009.
| Trial name or title | Patient‐reported outcomes in a randomized trial comparing four different treatment strategies in recent‐onset rheumatoid arthritis |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Starting date | |
| Contact information | |
| Notes | — |
Westhovens 2006.
| Trial name or title | Improved health‐related quality of life for rheumatoid arthritis patients treated with abatacept who have inadequate response to anti‐TNF therapy in a double‐blind, placebo‐controlled, multicentre randomized clinical trial |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Starting date | |
| Contact information | |
| Notes | — |
Differences between protocol and review
In the protocol, we did not include sensitivity analysis. However, as there was statistically significant heterogeneity, we performed sensitivity analyses to examine to explore the potential cause(s) of heterogeneity: dose‐ranging studies trials in participants who had failed previous biologic and disease duration.Robin Christensen has received consulting fees paid to the Parker Institute from Abbott/AbbVie, Axellus A/S, Bristol‐Myers Squibb, Cambridge Weight Plan, Norpharma, Pfizer and Roche; speakers fees paid to the Parker Institute from Axellus A/S, Cambridge Weight Plan, Mundipharma, Roche, and Rottapharm‐MEDA; research grants paid to the Parker Institute from Abbott/AbbVie, Axellus, Bayer HealthCare Pharmaceuticals, Biogen Idec, Bristol‐Myers Squibb, Cambridge Weight Plan, Ipsen, Laboratoires Expanscience, MSD, Mundipharma/Norpharma, Pfizer, and Roche.
Contributions of authors
All authors contributed to the initial draft protocol for the review. Fiona Cramp, Celia Almeida and Sarah Hewlett worked in collaboration on refining all parts of the final protocol, incorporating comments from members of the wider review group (with particular thanks to Dr Jon Pollock). Robin Christensen provided advice and performed statistical analyses. Ernest Choy drafted the review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
At the time of protocol development Sarah Hewlett was in receipt of a small unrestricted educational grant from GlaxoSmithKline to partially fund a PhD studentship on fatigue measurement in RA and also undertaking an RCT of cognitive behavioural therapy (CBT) for the self‐management of RA fatigue, funded by the Arthritis Research Campaign. During the full review process Sarah Hewlett has been undertaking an RCT of CBT for the self‐management of RA fatigue by the clinical team, funded by the National Institutes for Health Research. She has received small consultancy fees from UCB Pharmaceuticals and Bristol Myers Squibb to advise on the translation of the Bristol RA Fatigue Scales, and small, unrestricted educational grants from Pfizer to deliver training days for staff, in which non‐pharmacological management of fatigue was included. These associations reflect our large programme of research in fatigue into RA but do not constitute a conflict of interests. Robin Christensen has received consulting fees paid to the Parker Institute from Abbott/AbbVie, Axellus A/S, Bristol‐Myers Squibb, Cambridge Weight Plan, Norpharma, Pfizer and Roche; speakers fees paid to the Parker Institute from Axellus A/S, Cambridge Weight Plan, Mundipharma, Roche, and Rottapharm‐MEDA; research grants paid to the Parker Institute from Abbott/AbbVie, Axellus, Bayer HealthCare Pharmaceuticals, Biogen Idec, Bristol‐Myers Squibb, Cambridge Weight Plan, Ipsen, Laboratoires Expanscience, MSD, Mundipharma/Norpharma, Pfizer, and Roche. John Kirwan has been a paid adviser to the Royal Pharmaceutical Society and British Medical Association publication the British National Formulary during the time of this review. He also had unconditional educational grants from Horizon Pharma for the cost of attending the American College of Rheumatology Annual Scientific Meeting in 2013 and the cost of travel to the American College of Rheumatology Annual Scientific Meeting in 2014. Ernest Choy and Cardiff University has received research grants from Ferring Pharmaceuticals, Novimmune, Pfizer, Roche, and UCB. Ernest Choy has received payment as member of advisory boards and lecture fees from Amgen, Biogen, BMS, Celgene, Chugai Pharma, Eli Lilly, Ferring Pharmacuetical, Hospita, Jenssen, MSD, Napp, Novimmune, Pfizer, Regeneron, Roche, Sanofi, Tonix and UCB. Trudie Chalder is author of self help books for chronic fatigue and receives some royalties. Celia Almeida, Fiona Camp and Jon Pollock have no conflict of interest to declare.
New
References
References to studies included in this review
Alten 2011 {published data only}
- Alten R, Gomez‐Reino J, Durez P, Beaulieu A, Sebba A, Krammer G, et al. Efficacy and safety of the human anti‐IL‐1β monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12‐week, phase II, dose‐finding study. BMC Musculoskeletal Disorder 2011;12:153‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bae 2013 {published data only (unpublished sought but not used)}
- Bae SC, Gun SC, Mok CC, Khandker R, Nab HW, Koenig AS, et al. Improved health outcomes with etanercept versus usual DMARD therapy in an Asian population with established rheumatoid arthritis. BMC Musculoskeletal Disorder 2013;14:13‐21. [DOI: 10.1186/1471-2474-14-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choy 2012 {published and unpublished data}
- Choy E, McKenna F, Vencovsky J, Valente R, Goel N, Vanlunen B, et al. Certolizumab pegol plus MTX administered every 4 weeks is effective in patients with RA who are partial responders to MTX. Rheumatology 2012;51(7):1226‐34. [DOI] [PubMed] [Google Scholar]
Cohen 2006 {published data only}
- Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti‐tumor necrosis factor therapy: Results of a multicenter, randomized, double‐blind, placebo‐controlled, phase III trial evaluating primary efficacy and safety at twenty‐four weeks. Arthritis and Rheumatism 2006;54(9):2793‐806. [DOI] [PubMed] [Google Scholar]
- Keystone E, Burmester GR, Furie R, Loveless JE, Emery P, Kremer J, et al. Improvement in patient‐reported outcomes in a rituximab trial in patients with severe rheumatoid arthritis refractory to anti‐tumor necrosis factor therapy. Arthritis and Rheumatism 2008;59(6):785‐93. [DOI] [PubMed] [Google Scholar]
Emery 2006 {published data only}
- Emery P, Fleischmann R, Filipowicz‐Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIb randomized, double‐blind, placebo‐controlled, dose‐ranging trial. Arthritis and Rheumatism 2006;54(5):1390‐400. [DOI] [PubMed] [Google Scholar]
- Mease PJ, Revicki DA, Szechinski J, Greenwald M, Kivitz A, Barile‐Fabris L, et al. Improved health‐related quality of life for patients with active rheumatoid arthritis receiving rituximab: results of the Dose‐Ranging Assessment: International Clinical Evaluation of Rituximab in Rheumatoid Arthritis (DANCER) trial. Journal of Rheumatology 2008;35(1):20‐30. [PubMed] [Google Scholar]
Emery 2008 {published data only}
- Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double‐blind, parallel treatment trial. The Lancet 2008;372(9636):375‐82. [DOI] [PubMed] [Google Scholar]
- Kekow J, Moots RJ, Emery P, Durez P, Koenig A, Singh A, et al. Patient‐reported outcomes improve with etanercept plus methotrexate in active early rheumatoid arthritis and the improvement is strongly associated with remission: the COMET trial. Annals of the Rheumatic Diseases 2010;69(1):222‐5. [DOI] [PubMed] [Google Scholar]
Emery 2009 {published data only}
- Emery P, Fleischmann RM, Moreland LW, Hsia EC, Strusberg I, Durez P, et al. Golimumab, a human anti‐tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate‐naive patients with active rheumatoid arthritis: Twenty‐four‐week results of a phase III, multicenter, randomized, double‐blind, placebo‐controlled study of golimumab before methotrexate as first‐line therapy for early‐onset rheumatoid arthritis. Arthritis and Rheumatism 2009;60(8):2272‐83. [DOI] [PubMed] [Google Scholar]
Fleischmann 2009 {published data only}
- Fleischmann R, Vencovsky J, Vollenhoven R F, Borenstein D, Box J, Coteur G, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease‐modifying antirheumatic therapy: the FAST4WARD study. Annals of the Rheumatic Diseases 2009;68(6):805‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Genovese 2005 {published data only}
- Genovese MC, Becker J, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. New England Journal of Medicine 2005;353(11):1114‐23. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Schiff M, Luggen M, Becker JC, Aranda R, Teng J, et al. Efficacy and safety of the selective co‐stimulation modulator abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti‐tumour necrosis factor therapy. Annals of the Rheumatic Diseases 2008;67(4):547‐54. [DOI] [PubMed] [Google Scholar]
- Ostor AJ. Abatacept: a T‐cell co‐stimulation modulator for the treatment of rheumatoid arthritis. Clinical Rheumatology 2008;27(11):1343‐53. Erratum in Clin Rheumatol. 2008 Nov;27(11):1477. [DOI] [PubMed] [Google Scholar]
- Russell A, Dougados M, Li T, Sherrer Y, Becker J, Gandhi M, et al. Abatacept reduces fatigue and pain severity and sleep problems through 2 years in rheumatoid arthritis patients in the AIM and ATTAIN trials. Journal of Rheumatology 2008;35(6):1178. [Google Scholar]
- Russell A, Li T, Genovese M, Pineda C. In rheumatoid arthritis patients with an inadequate response to anti‐TNF therapy in the Attain trial, abatacept effectively reduced pain and fatigue, and improved sleep quality. Journal of Rheumatology 2006;33(2):394. [Google Scholar]
- Russell A, Sherrer Y, Westhovens R, Box J, Pritchard C, Nuamah I, et al. Abatacept is effective at reducing pain and fatigue, and improving sleep quality in rheumatoid arthritis patients with an inadequate response to anti‐TNF therapy in the attain trial. Annals of the Rheumatic Diseases 2005;64:397. [Google Scholar]
- Wells G, Li T, Maxwell L, MacLean R, Tugwell P. Determining the minimal clinically important differences in activity, fatigue, and sleep quality in patients with rheumatoid arthritis. Journal of Rheumatology 2007;34(2):280‐9. [PubMed] [Google Scholar]
- Wells G, Li T, Maxwell L, Maclean R, Tugwell P. Responsiveness of patient reported outcomes including fatigue, sleep quality, activity limitation, and quality of life following treatment with abatacept for rheumatoid arthritis. Annals of the Rheumatic Diseases 2008;67(2):260‐5. [DOI] [PubMed] [Google Scholar]
- Westhovens R, Cole JC, Li T, Martin M, Maclean R, Lin P, et al. Improved health‐related quality of life for rheumatoid arthritis patients treated with abatacept who have inadequate response to anti‐TNF therapy in a double‐blind, placebo‐controlled, multicentre randomized clinical trial. Rheumatology 2006;45(10):1238‐46. [DOI] [PubMed] [Google Scholar]
Genovese 2008 {published data only}
- Genovese MC, McKay JD, Nasonov EL, Mysler EF, Silva NA, Alecock E, et al. Interleukin‐6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease‐modifying antirheumatic drugs: the tocilizumab in combination with traditional disease‐modifying antirheumatic drug therapy study. Arthritis and Rheumatism 2008;58(10):2968‐80. [DOI] [PubMed] [Google Scholar]
Hørslev‐Petersen 2014 {published data only}
- Hørslev‐Petersen K, Hetland ML, Junker P, Pødenphant J, Ellingsen T, Ahlquist P, et al. Adalimumab added to a treat‐to‐target strategy with methotrexate and intra‐articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: an investigator‐initiated, randomised, double‐blind, parallel‐group, placebo‐controlled trial. Annals of the Rheumatic Diseases 2014;73(4):654‐61. [DOI] [PubMed] [Google Scholar]
Keystone 2004 {published data only}
- Keystone E, Kavanaugh A, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical and functional outcomes of treatment with adalimumab (a human anti‐tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy. Arthritis and Rheumatism 2004;50(5):1400‐11. [DOI] [PubMed] [Google Scholar]
Keystone 2009 {published and unpublished data}
- Genovese MC, Han C, Keystone EC, Hsia EC, Buchanan J, Gathany T, et al. Effect of golimumab on patient‐reported outcomes in rheumatoid arthritis: results from the GO‐FORWARD study. Journal of Rheumatology 2012;39(6):1185‐91. [DOI] [PubMed] [Google Scholar]
- Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, et al. Golimumab, a human antibody to tumour necrosis factor (alpha) given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO‐FORWARD Study. Annals of the Rheumatic Diseases 2009;68(6):210‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kremer 2003 {published data only}
- Emery P, Kosinski M, Li T, Martin M, Williams GR, Becker JC, et al. Treatment of rheumatoid arthritis patients with abatacept and methotrexate significantly improved health‐related quality of life. Journal of Rheumatology 2006;33(4):681‐9. [PubMed] [Google Scholar]
- Kremer JM, Westhovens R, Leon M, Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T‐cell activation with fusion protein CTLA4Ig. New England Journal of Medicine 2003;349(20):1907‐15. [DOI] [PubMed] [Google Scholar]
Kremer 2006 {published data only}
- Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud‐Mendoza C, et al. Effects of abatacept in patients with methotrexate‐resistant active rheumatoid arthritis: a randomized trial. Annals of Internal Medicine 2006;144(12):865‐76. [DOI] [PubMed] [Google Scholar]
- Li T, Gignac M, Wells G, Shen S, Westhovens R, Li T, et al. Decreased external home help use with improved clinical status in rheumatoid arthritis: an exploratory analysis of the Abatacept in Inadequate responders to Methotrexate (AIM) trial. Clinical Therapeutics 2008;30(4):734‐48. [DOI] [PubMed] [Google Scholar]
- Russell AS, Wallenstein GV, Li T, Martin MC, Maclean R, Blaisdell B, et al. Abatacept improves both the physical and mental health of patients with rheumatoid arthritis who have inadequate response to methotrexate treatment. Annals of the Rheumatic Diseases 2007;66(2):189‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G, Li T, Maxwell L, MacLean R, Tugwell P. Determining the minimal clinically important differences in activity, fatigue, and sleep quality in patients with rheumatoid arthritis. Journal of Rheumatology 2007;34(2):280‐9. [PubMed] [Google Scholar]
Li 2013 {published data only (unpublished sought but not used)}
- Li Z, Zhang F, Kay J, Fei K, Han C, Zhuang Y, Wu Z. Safety and efficacy of subcutaneous golimumab In Chinese patients with active rheumatoid arthritis despite MTX therapy: results from a randomized, placebo‐controlled, phase 3 trial. Arthritis and Rheumatology 2013;65(Suppl 10):1416. [DOI: 10.1002/art.2013.65.issue-s10] [DOI] [PubMed] [Google Scholar]
Lukina 1998 {published data only}
- Lukina GV, Sigidin IaA, Skurkovich SV, Skurkovich BS. New approaches to biological immunomodulation therapy of rheumatoid arthritis: neutralization of basic cytokines. Terapevticheskiĭ Arkhiv 1998;70(5):32‐7. Russian. [PubMed] [Google Scholar]
Maini 1999 {published data only}
- Maini R, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis and Rheumatism 2004;50(4):1051‐65. [DOI] [PubMed] [Google Scholar]
- Maini R, Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A randomised phase III trial. The Lancet 1999;354(9194):1932‐9. [DOI] [PubMed] [Google Scholar]
Mittendorf 2007 {published data only}
- Mittendorf T, Dietz B, Sterz R, Kupper H, Cifaldi M A, Schulenburg JM. Improvement and longterm maintenance of quality of life during treatment with adalimumab in severe rheumatoid arthritis. Journal of Rheumatology 2007;34(12):2343‐50. [PubMed] [Google Scholar]
- Mittendorf T, Sterz R, Schulenburg J, Kupper H, Cifaldi M, Dietz B. Effects of long‐term adalimumab therapy on health utility and fatigue in patients with long‐standing, severe rheumatoid arthritis (RA) ‐ Results from a 3‐year follow‐up study. Value in Health 2005;8(6):A30. [Google Scholar]
Moreland 1999 {published data only}
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. The New England Journal of Medicine 2000;343(22):1586‐93. [DOI] [PubMed] [Google Scholar]
- Moreland LW, Genovese MC, Sato R, Singh A, Moreland LW, Genovese MC, et al. Effect of etanercept on fatigue in patients with recent or established rheumatoid arthritis. Arthritis and Rheumatism 2006;55(2):287‐93. [DOI] [PubMed] [Google Scholar]
- Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Annals of Internal Medicine 1999;130(6):478‐86. [DOI] [PubMed] [Google Scholar]
- Singh G, Singh A, Wanke L, Sato R. Rapid improvement of fatigue in patients on etanercept compared to those on methotrexate: A double‐blind randomized trial in 632 MTX‐naive patients with early, active rheumatoid arthritis. Annals of the Rheumatic Diseases 2003;62(Supplement 1):157. [Google Scholar]
Pope 2012 {published data only}
- Pope J, Fleischmann R, Dougados M, Bingham CO, Massarotti EM, Wollenhaupt J, et al. Rapid reductions in fatigue and sleep problems and correlation with improvements in patient‐reported outcomes in patients with active rheumatoid arthritis treated with certolizumab pegol in the realistic 12‐week phase IIIb randomized controlled study. Rheumatology 2014;53(Supplement 3):43‐5. [Google Scholar]
Rigby 2011 {published data only}
- Rigby W, Ferraccioli G, Greenwald M, Zazueta‐Montiel B, Fleischmann R, Wassenberg S, et al. Effect of rituximab on physical function and quality of life in patients with rheumatoid arthritis previously untreated with methotrexate. Arthritis Care and Research 2011;63(5):711‐20. [DOI] [PubMed] [Google Scholar]
Schiff 2008 {published data only}
- Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi‐centre, randomised, double‐blind, placebo‐controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Annals of the Rheumatic Diseases 2008;67(8):1096‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenhoven R, Dougados M, Keiserman M, Codding C, Songcharoen S, Berman A, et al. Efficacy of abatacept or infliximab in RA patients with an inadequate response to MTX: Results from a 1‐year double‐blind randomized clinical trial. Scandinavian Journal of Rheumatology 2008;37(Supplment 123):51. [Google Scholar]
Smolen 2008 {published data only}
- Smolen JS, Beaulieu A, Rubbert‐Roth A, Ramos‐Remus C, Rovensky J, Alecock E, et al. Effect of interleukin‐6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double‐blind, placebo‐controlled, randomised trial. The Lancet 2008;371(9617):987‐97. [DOI] [PubMed] [Google Scholar]
Smolen 2009a {published data only}
- Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Annals of the Rheumatic Diseases 2009;68(6):797‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smolen 2009b {published data only}
- Smolen JS, Kay J, Doyle MK, Landewé R, Matteson EL, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO‐AFTER study): a multicentre, randomised, double‐blind, placebo‐controlled, phase III trial. The Lancet 2009;374(9685):210‐21. [DOI] [PubMed] [Google Scholar]
Soubrier 2009 {published data only}
- Soubrier M, Puéchal X, Sibilia J, Mariette X, Meyer O, Combe B, et al. Evaluation of two strategies (initial methotrexate monotherapy vs its combination with adalimumab) in management of early active rheumatoid arthritis: data from the GUEPARD trial. Rheumatology 2009;48(11):1429‐34. [DOI] [PubMed] [Google Scholar]
Strand 2009 {published data only}
- Keystone E, Heijde D, Mason D, Landewe R, Vollenhoven R, Combe B, et al. Certolizumab Pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis. Arthritis and Rheumatism 2008;58(11):3319‐29. [DOI] [PubMed] [Google Scholar]
- Strand V, Mease P, Burmester GR, Nikai E, Coteur G, Vollenhoven R, et al. Rapid and sustained improvements in health‐related quality of life, fatigue, and other patient‐reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial. Arthritis Research and Therapy 2009;11(6):R170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strand 2012a {published data only}
- Strand V, Burmester GR, Ogale S, Devenport J, John A, Emery P. Improvements in health‐related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24‐week randomized controlled RADIATE study. Rheumatology (Oxford) 2012;51(10):1860‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strand 2012b {published data only}
- Strand V, Rentz AM, Cifaldi MA, Chen N, Roy S, Revicki D. Health‐related quality of life outcomes of adalimumab for patients with early rheumatoid arthritis: results from a randomized multicenter study. Journal of Rheumatology 2012;39(1):63‐72. [DOI] [PubMed] [Google Scholar]
Weinblatt 2003 {published data only}
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis and Rheumatism 2003;48(1):35‐45. Erratum in Arthritis Rheum 2003 Mar;48(3):855. [DOI] [PubMed] [Google Scholar]
Weinblatt 2013 {published data only}
- Weinblatt ME, Bingham CO 3rd, Mendelsohn AM, Kim L, Mack M, Lu J, et al. Intravenous golimumab is effective in patients with active rheumatoid arthritis despite methotrexate therapy with responses as early as week 2: results of the phase 3, randomised, multicentre, double‐blind, placebo‐controlled GO‐FURTHER trial. Annals of the Rheumatic Diseases 2013;72(3):381‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Breedveld 2005 {published data only}
- Breedveld FC, Han C, Bala M, Heijde D, Baker D, Kavanaugh AF, et al. Association between baseline radiographic damage and improvement in physical function after treatment of patients with rheumatoid arthritis. Annals of the Rheumatic Diseases 2005;64(1):52‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cella 2005 {published data only}
- Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J, et al. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. Journal of Rheumatology 2005;32(5):811‐9. [PubMed] [Google Scholar]
Dougados 2007 {published data only}
- Dougados M, Russell A, Li T, Sherrer Y, Teng J, McCann T, et al. Abatacept provides sustained improvements in pain, fatigue and sleep quality through 2 years in the treatment of rheumatoid arthritis patients in the aim and attain trials. Journal of Rheumatology 2007;34(7):1629. [Google Scholar]
Duggan 2009 {published data only}
- Duggan ST, Keam SJ. Certolizumab pegol in rheumatoid arthritis. BioDrugs 2009;23(6):407‐17. [DOI] [PubMed] [Google Scholar]
Elliott 1994 {published data only (unpublished sought but not used)}
- Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, et al. Randomised double‐blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. The Lancet 1994;344(8930):1105‐10. [DOI] [PubMed] [Google Scholar]
Frampton 2007 {published data only}
- Frampton JE, Scott LJ, Frampton JE, Scott LJ. Rituximab in rheumatoid arthritis. BioDrugs 2007;21(5):333‐41: discussion 342. [DOI] [PubMed] [Google Scholar]
Furst 2003 {published data only}
- Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti tumor necrosis factor‐alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). Journal of Rheumatology 2003;30(12):2563‐71. [PubMed] [Google Scholar]
Genovese 2010 {published data only}
- Genovese MC, Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, et al. LY2439821, a humanized anti‐interleukin‐17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double‐blind, placebo‐controlled, proof‐of‐concept study. Arthritis and Rheumatism 2010;62(4):929‐39. [DOI] [PubMed] [Google Scholar]
Gnanasakthy 2013 {published data only}
- Gnanasakthy A, Kosinski M, Genovese M, Mallya U, Mpofu S. Health‐related quality of life (HRQOL) benefits associated with reductions in disease activity and pain severity among rheumatoid arthritis (RA) patients treated with secukinumab. Annals of the Rheumatic Diseases 2012;71(Suppl 3):667. [Google Scholar]
Grigor 2004 {published data only}
- Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single blind randomised controlled trial. The Lancet 2004;364(9430):263‐9. [DOI] [PubMed] [Google Scholar]
Haugeberg 2009 {published data only}
- Haugeberg G, Conaghan PG, Quinn M, Emery P. Bone loss in patients with active early rheumatoid arthritis: infliximab and methotrexate compared with methotrexate treatment alone. Explorative analysis from a 12‐month randomised, double‐blind, placebo‐controlled study. Annals of the Rheumatic Diseases 2009;68(12):1898‐901. [DOI] [PubMed] [Google Scholar]
Kavanaugh 2003 {published data only}
- Kavanaugh A, Genovese M, Baughman J, Kivitz A, Bulpitt K, Olsen N, et al. Allele and antigen‐specific treatment of rheumatoid arthritis: a double blind, placebo controlled phase trial. Journal of Rheumatology 2003;30(3):449‐54. [PubMed] [Google Scholar]
Kavanaugh 2012 {published data only}
- Kavanaugh A, Vollenhoven RF, Emery P, et al. Patient‐reported outcomes in early RA patients failing to achieve stable low disease activity: Comparing addition of adalimumab to methotrexate monotherapy with maintenance on adalimumab plus methotrexate.. Arthritis and Rheumatism. 2012; Vol. 64, issue S10:S162‐S3.
Kim 2007 {published data only}
- Kim HY, Lee SK, Song YW, Yoo DH, Koh EM, Yoo B, et al. A randomized, double‐blind, placebo‐controlled, phase III study of the human anti‐tumor necrosis factor antibody adalimumab administered as subcutaneous injections in Korean rheumatoid arthritis patients treated with methotrexate. APLAR Journal of Rheumatology 2007;10(1):9‐16. [Google Scholar]
Kosinski 2000 {published data only}
- Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE Jr. Determining minimally important changes in generic and disease‐specific health‐related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis and Rheumatism 2000;43(7):1478‐87. [DOI] [PubMed] [Google Scholar]
Kremer 2008 {published data only}
- Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud‐Mendoza C, et al. Results of a two‐year follow‐up study of patients with rheumatoid arthritis who received a combination of abatacept and methotrexate. Arthritis and Rheumatism 2008;58(4):953‐63. [DOI] [PubMed] [Google Scholar]
Moreland 2000 {published data only}
- Moreland LW, McCabe DP, Caldwell JR, Sack M, Weisman M, Henry G, et al. Phase I/II trial of recombinant methionyl human tumor necrosis factor binding protein PEGylated dimer in patients with active refractory rheumatoid arthritis. Journal of Rheumatology 2000;27(3):601‐9. [PubMed] [Google Scholar]
Moreland 2002 {published data only}
- Moreland LW, Alten R, Bosch F, Appelboom T, Leon M, Emery P, et al. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose‐finding, double‐blind, placebo‐controlled clinical trial evaluating CTLA‐4Ig and LEA29Y eighty‐five days after the first infusion. Arthritis and Rheumatism 2002;46(6):1470‐9. [DOI] [PubMed] [Google Scholar]
Sansonno 2003 {published data only}
- Sansonno D, Re V, Lauletta G, Tucci FA, Boiocchi M, Dammacco F. Monoclonal antibody treatment of mixed cryoglobulinemia resistant to interferon alpha with an anti‐CD20. Blood 2003;101(10):3818‐26. [DOI] [PubMed] [Google Scholar]
Song 2007 {published data only}
- Song YW, Lee EY, Koh EM, Cha HS, Yoo B, Lee CK, et al. Assessment of comparative pain relief and tolerability of SKI306X compared with celecoxib in patients with rheumatoid arthritis: a 6‐week, multicenter, randomized, double‐blind, double‐dummy, phase III, noninferiority clinical trial. Clinical Therapeutics 2007;29(5):862‐73. [DOI] [PubMed] [Google Scholar]
Strand 2012 {published data only}
- Strand V, Jones TV, Li W, Koenig AS, Kotak S. Factors that impact work productivity in the preserve trial: A randomized controlled trial of combination etanercept‐methotrexate therapy in patients with moderately active rheumatoid arthritis. Arthritis and Rheumatism 2012;64(S10):S777. [Google Scholar]
Strand 2014 {published data only}
- Strand V, Kosinski M, Gnanasakthy A, Mallya U, Mpofu S. Secukinumab treatment in rheumatoid arthritis is associated with incremental benefit in the clinical outcomes and HRQoL improvements that exceed minimally important thresholds. Health and Quality of Life Outcomes 2014;12(1):31‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tak 2008 {published data only}
- Tak PP, Thurlings RM, Rossier C, Nestorov I, Dimic A, Mircetic V, et al. Atacicept in patients with rheumatoid arthritis: Results of a multicenter, phase Ib, double‐blind, placebo‐controlled, dose‐escalating, single‐ and repeated‐dose study. Arthritis and Rheumatism 2008;58(1):61‐72. [DOI] [PubMed] [Google Scholar]
Yount 2007 {published data only}
- Yount S, Sorensen MV, Cella D, Sengupta N, Grober J, Chartash EK. Adalimumab plus methotrexate or standard therapy is more effective than methotrexate or standard therapies alone in the treatment of fatigue in patients with active, inadequately treated rheumatoid arthritis. Clinical and Experimental Rheumatology 2007;25(6):838‐46. [PubMed] [Google Scholar]
References to ongoing studies
St Clair 2004 {published data only}
- Clair EW, Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis and Rheumatism 2004;50(11):3432‐43. [DOI] [PubMed] [Google Scholar]
Van der Kooij 2009 {published data only}
- Kooij SM, Vries‐Bouwstra JK, Goekoop‐Ruiterman YP, Ewals JA, Han KH, Hazes JM, et al. Patient‐reported outcomes in a randomized trial comparing four different treatment strategies in recent‐onset rheumatoid arthritis. Arthritis and Rheumatism 2009;61(1):4‐12. [DOI] [PubMed] [Google Scholar]
Westhovens 2006 {published data only}
- Westhovens R, Cole JC, Li T, Martin M, Maclean R, Lin P, et al. Improved health‐related quality of life for rheumatoid arthritis patients treated with abatacept who have inadequate response to anti‐TNF therapy in a double‐blind, placebo‐controlled, multicentre randomized clinical trial. Rheumatology 2006;45(10):1238‐46. [DOI] [PubMed] [Google Scholar]
Additional references
Arnett 1988
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis & Rheumatism 1988;31(3):315‐24. [DOI] [PubMed] [Google Scholar]
Blumenauer 2002
- Blumenauer BBTB, Judd M, Wells GA, Burls A, Cranney A, Hochberg MC, Tugwell P. Infliximab for the treatment of rheumatoid arthritis. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003785] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blumenauer 2003
- Blumenauer BBTB, Cranney A, Burls A, Coyle D, Hochberg MC, Tugwell P, Wells GA. Etanercept for the treatment of rheumatoid arthritis. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004525] [DOI] [PubMed] [Google Scholar]
Chauffier 2012
- Chauffier K, Salliot C, Berenbaum F, Sellam J. Effect of biotherapies on fatigue in rheumatoid arthritis: a systematic review of the literature and meta‐analysis.. Rheumatology (Oxford) 2012;51(1):60‐8. [DOI] [PubMed] [Google Scholar]
Chinn 2000
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta‐analysis. Statistics in Medicine 2000;19(22):3127‐31. [DOI] [PubMed] [Google Scholar]
Cohen 1998
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum, 1988. [Google Scholar]
Conaghan 1999
- Conaghan PG, Green MJ, Emery P. Established rheumatoid arthritis. Baillières Clinical Rheumatology 1999;13(4):561‐575. [DOI] [PubMed] [Google Scholar]
Cramp 2013
- Cramp F, Hewlett S, Almeida C, Kirwan JR, Choy EH, Chalder T, Pollock J, Christensen R. Non‐pharmacological interventions for fatigue in rheumatoid arthritis. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD008322.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Department of Health 2006
- Department of Health. The Musculoskeletal Services Framework. London: DoH, 2006. [270211] [Google Scholar]
Heiberg 2008
- Heiberg T, Kvien TK, Mowinckel P, Aletaha D, Smolen JS, Hagen KB. Identification of disease activity and health status cut‐off points for the symptom state acceptable to patients with rheumatoid arthritis. Annals of the Rheumatic Diseases 2008;67(7):967‐71.. [DOI] [PubMed] [Google Scholar]
Hewlett 2005
- Hewlett S, Cockshott Z, Byron M, Kitchen K, Tipler S, Pope D, et al. Patients' perceptions of fatigue in RA: Overwhelming, uncontrollable, ignored. Arthritis & Rheumatism (Arthritis Care & Research) 2005;53(5):697‐702. [DOI] [PubMed] [Google Scholar]
Hewlett 2007
- Hewlett S, Hehir M, Kirwan J. Measuring fatigue in rheumatoid arthritis: a systematic review of scales in use. Arthritis & Rheumatism (Arthritis Care & Research) 2007;57(3):429‐39. [DOI] [PubMed] [Google Scholar]
Hewlett 2008
- Hewlett S, Nicklin J, Treharne GJ. Fatigue in musculoskeletal conditions. Reports on the Rheumatic Diseases (Series 6). Topical Reviews 1. Arthritis Research Campaign 2008 Autumn.
Higgins 2003
- Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta‐analysis. British Medical Journal 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Kirwan 2007
- Kirwan J, Minnock P, Adebajo A, Bresnihan B, Choy E, Wit M, et al. Patient Perspective Workshop: Fatigue as a recommended patient‐centred outcome measure in rheumatoid arthritis. Journal of Rheumatology 2007;34(5):1174‐7. [PubMed] [Google Scholar]
Luqmani 2006
- Luqmani R, Hennell S, Estrach C, Birrell F, Bosworth A, Davenport G, et al. British Society for Rheumatology and British Health Professionals in Rheumatology Guideline for the Management of Rheumatoid Arthritis (the first two years). Rheumatology (Oxford) 2006; Vol. 45, issue 9:1167‐9. [DOI] [PubMed]
Maxwell 2009
- Maxwell L, Singh JA. Abatacept for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007277.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mertens 2009
- Mertens M, Singh JA. Anakinra for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005121.pub3] [DOI] [PubMed] [Google Scholar]
Navarro‐Sarabia 2005
- Navarro‐Sarabia F, Ariza‐Ariza R, Hernandez‐Cruz B, Villanueva I. Adalimumab for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005113.pub2] [DOI] [PubMed] [Google Scholar]
Pollard 2006
- Pollard LC, Choy EH, Gonzalez J, Khoshaba B, Scott DL. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology (Oxford) 2006;45(7):885‐9. [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P et al on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008).. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2008.
Singh 2009
- Singh JA, Christensen R, Wells GA, Suarez‐Almazor ME, Buchbinder R, Lopez‐Olivo MA, Tanjong Ghogomu E, Tugwell P. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007848.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2011
- Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, Filippini G, Skoetz N, Francis D, Lopes LC, Guyatt GH, Schmitt J, Mantia L, Weberschock T, Roos JF, Siebert H, Hershan S, Lunn MP, Tugwell P, Buchbinder R. Adverse effects of biologics: a network meta‐analysis and Cochrane overview. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008794.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2007
- Wells G, Li T, Maxwell L, MacLean R, Tugwell P. Determining the minimal clinically important differences in activity, fatigue, and sleep quality in patients with rheumatoid arthritis. Journal of Rheumatology 2007;34(2):280‐9. [PubMed] [Google Scholar]
Wolfe 1996
- Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. Journal of Rheumatology 1996;23(8):1407‐17. [PubMed] [Google Scholar]


