Abstract
Background
Pancreatic resections are associated with high morbidity (30% to 60%) and mortality (5%). Synthetic analogues of somatostatin are advocated by some surgeons to reduce complications following pancreatic surgery; however, their use is controversial.
Objectives
To determine whether prophylactic somatostatin analogues should be used routinely in pancreatic surgery.
Search methods
We searched the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 1), MEDLINE, EMBASE and Science Citation Index Expanded to February 2013.
Selection criteria
We included randomised controlled trials comparing prophylactic somatostatin or one of its analogues versus no drug or placebo during pancreatic surgery (irrespective of language or publication status).
Data collection and analysis
Two review authors independently assessed trials for inclusion and independently extracted data. We analysed data with both the fixed‐effect and random‐effects models using Review Manager (RevMan). We calculated the risk ratio (RR), mean difference (MD) or standardised mean difference (SMD) with 95% confidence intervals (CI) based on an intention‐to‐treat or available case analysis. When it was not possible to perform either of the above, we performed a per protocol analysis.
Main results
We identified 21 trials (19 trials of high risk of bias) involving 2348 people. There was no significant difference in the perioperative mortality (RR 0.80; 95% CI 0.56 to 1.16; n = 2210) or the number of people with drug‐related adverse effects between the two groups (RR 2.09; 95% CI 0.83 to 5.24; n = 1199). Quality of life was not reported in any of the trials. The overall number of participants with postoperative complications was significantly lower in the somatostatin analogue group (RR 0.70; 95% CI 0.61 to 0.80; n = 1903) but there was no significant difference in the re‐operation rate (RR 1.26; 95% CI 0.58 to 2.70; n = 687) or hospital stay (MD ‐1.29 days; 95% CI ‐2.60 to 0.03; n = 1314) between the groups. The incidence of pancreatic fistula was lower in the somatostatin analogue group (RR 0.66; 95% CI 0.55 to 0.79; n = 2206). The proportion of these fistulas that were clinically significant was not mentioned in most trials. On inclusion of trials that clearly distinguished clinically significant fistulas, there was no significant difference between the two groups (RR 0.69; 95% CI 0.38 to 1.28; n = 292).
Authors' conclusions
Somatostatin analogues may reduce perioperative complications but do not reduce perioperative mortality. Further adequately powered trials with low risk of bias are necessary. Based on the current available evidence, somatostatin and its analogues are recommended for routine use in people undergoing pancreatic resection.
Keywords: Humans, Gastrointestinal Agents, Gastrointestinal Agents/therapeutic use, Length of Stay, Octreotide, Octreotide/therapeutic use, Pancreas, Pancreas/metabolism, Pancreas/surgery, Pancreatectomy, Pancreatectomy/adverse effects, Pancreatectomy/mortality, Pancreatic Diseases, Pancreatic Diseases/surgery, Pancreatic Fistula, Pancreatic Fistula/prevention & control, Pancreaticoduodenectomy, Pancreaticoduodenectomy/adverse effects, Pancreaticoduodenectomy/mortality, Postoperative Complications, Postoperative Complications/mortality, Postoperative Complications/prevention & control, Publication Bias, Randomized Controlled Trials as Topic, Reoperation, Reoperation/statistics & numerical data, Sepsis, Sepsis/prevention & control, Somatostatin, Somatostatin/analogs & derivatives
Plain language summary
Somatostatin analogues for reducing complications following pancreatic surgery
Pancreatic resections are associated with high morbidity (30% to 60%) and mortality (5%). It is not clear whether routine, preventive use of synthetic analogues of somatostatin (a hormone that inhibits pancreatic secretions) could reduce complications following pancreatic surgery. We included 21 randomised clinical trials in this review. All trials had high risk of bias ('systematic error'). A total of 2348 people were randomised either to somatostatin analogues or a control in the 21 trials. The overall number of people with postoperative complications was lower by 30% in the somatostatin analogues group but there was no difference in postoperative mortality, re‐operation rate or overall length of hospital stay between the groups. Pancreatic fistula is drainage of pancreatic juice secreted by the remaining pancreas to the exterior. This was lower in the intervention group by 34%. The proportion of these fistulas that resulted in change to the treatment given to the participants is not clear. When we included trials that clearly distinguished fistulas that required change to the treatment given to the participants, there was no difference between the two groups. Participant quality of life was not reported in any of the trials. In conclusion, somatostatin analogues reduce the incidence of pancreatic fistula. Further trials with sufficient participant numbers and a low risk of bias are necessary. Based on the current available evidence, somatostatin and its analogues are recommended for routine use in people undergoing pancreatic resection.
Summary of findings
Summary of findings for the main comparison. Somatostatin analogues for pancreatic surgery.
| Somatostatin analogues for pancreatic surgery | ||||||
| Patient or population: people with pancreatic surgery Settings: secondary or tertiary care Intervention: somatostatin analogues | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Somatostatin analogues | |||||
| Perioperative mortality | 49 per 1000 | 40 per 1000 (28 to 57) | RR 0.8 (0.56 to 1.16) | 2210 (18 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| Treatment withdrawal | 8 per 1000 | 13 per 1000 (5 to 35) | RR 1.55 (0.56 to 4.33) | 1220 (9 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | |
| Re‐operation | 102 per 1000 | 108 per 1000 (70 to 166) | RR 1.06 (0.69 to 1.63) | 687 (7 studies) | ⊕⊝⊝⊝ very low1,2,3,4,5 | |
| Anastomotic leak | 48 per 1000 | 39 per 1000 (25 to 61) | RR 0.81 (0.51 to 1.27) | 1585 (9 studies) | ||
| Pancreatic fistula (clinically significant) | 151 per 1000 | 104 per 1000 (46 to 191) | RR 0.69 (0.38 to 1.29) | 292 (4 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | |
| Infected abdominal collections | 61 per 1000 | 56 per 1000 (40 to 80) | RR 0.93 (0.66 to 1.32) | 1965 (13 studies) | ||
| Shock | 29 per 1000 | 29 per 1000 (14 to 63) | RR 1 (0.46 to 2.15) | 812 (4 studies) | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Most trials were of high risk of bias. 2 Confidence intervals overlap 1 and 0.75 or 1.25. 3 A total of fewer than 300 events in the comparison. 4 Fewer than 10 trials were included for this outcome. So, there is a suspicion of selective outcome reporting, which can indicate publication bias. 5 There was moderate heterogeneity as indicated by I² of 30%, tau² of 0.27; and lack of overlapping of confidence intervals.
Background
Description of the condition
Pancreatic resection is performed to treat pancreatic diseases, including malignancy and chronic pancreatitis. The overall morbidity following pancreatic surgery varies between 30% and 60%, and the mortality rate is about 5% (Alexakis 2004; Gouma 2000; Sohn 2000). The major complication following pancreatic resection is postoperative pancreatic leak or fistula. Reviews have described an incidence of pancreatic leak or fistula of around 37% (Connor 2005). Various methods, such as pancreaticogastrostomy rather than pancreaticojejunostomy after pancreatic resections (McKay 2006) and the use of fibrin sealant (Ohwada 1998), have been suggested to decrease the incidence of pancreatic complications, but one of the most common approaches has been the use of somatostatin or its synthetic analogues. However, their use is controversial and some randomised clinical trials and systematic reviews recommend routine prophylactic somatostatin analogues in pancreatic resections (Buchler 1992; Connor 2005; Montorsi 1995) while others do not (Alghamdi 2007; Hesse 2005; Lange 1992).
Description of the intervention
Somatostatin or its analogues are administered prophylactically during pancreatic surgery.
How the intervention might work
Somatostatin and its analogues inhibit pancreatic exocrine secretions (Lembcke 1987). The theory is that by decreasing the volume of pancreatic secretion, the incidence of pancreatic leak or fistula could be decreased. Considering that pancreatic fistulas can be life‐threatening (Bassi 2005), a decrease in clinically significant pancreatic fistula might decrease perioperative mortality, morbidity and postoperative hospital stay.
Why it is important to do this review
The use of somatostatin and its analogues during pancreatic surgery is controversial. They may potentially decrease morbidity and mortality following pancreatic surgery but it is also possible that they may have no therapeutic benefit and may be associated with negative outcomes.
Objectives
To determine whether prophylactic somatostatin analogues should be used routinely in pancreatic surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials of parallel design, irrespective of blinding, sample size, publication status or language. We excluded quasi‐randomised trials (see below) and other study designs.
Types of participants
People who were undergoing a pancreatic surgical procedure (pancreatic resection, pancreatic duct drainage procedures or cyst drainage procedures) for any pancreatic disease.
Types of interventions
Administration of perioperative somatostatin (or an analogue of this hormone, such as octreotide) versus a comparator of placebo or no intervention.
Types of outcome measures
Primary outcomes
Postoperative mortality.
-
Drug‐related complications.
Treatment withdrawal.
Number with adverse effects due to treatment.
Quality of life.
Secondary outcomes
-
Postoperative complications.
Re‐operation.
Anastomotic leak.
Pancreatic fistula. Pancreatic fistula has been graded as A, B and C by consensus among surgeons (Bassi 2005). We included any pancreatic fistula; however it was defined by the authors, as one of the outcomes. We also included clinically significant pancreatic fistulas as another outcome. For this outcome, we included only trials in which data on grade B or C were available separately from grade A (not clinically significant).
Postoperative pancreatitis.
Sepsis.
Renal failure.
Bleeding.
Abdominal collections.
Infected abdominal collections.
Delayed gastric emptying.
Pulmonary complications.
Shock.
Number of complications.
Number of people with any complications.
-
Hospital stay.
Total hospital stay.
Intensive treatment unit (ITU) stay.
Search methods for identification of studies
Electronic searches
We searched:
the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group Controlled Trials Register (Appendix 1; Forman 2009);
the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 1) (Appendix 1);
MEDLINE via PubMed (1987 to February 2013) (Appendix 2);
EMBASE via OVID SP (1987 to February 2013) (Appendix 3) and
Science Citation Index Expanded (Royle 2003) to February 2013 (Appendix 4).
Searching other resources
We also searched the references of the identified trials to identify further relevant trials.
Data collection and analysis
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group Module (Forman 2009). We imputed the standard deviation from P values according to the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and used the median for the meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or confidence intervals (CIs), we imputed the standard deviation as the highest standard deviation noted for that group under that outcome.
Selection of studies
KG and RK identified the trials for inclusion, independently of each other. We listed the excluded trials with reasons for exclusion.
Data extraction and management
KG and RK extracted the data for the review independently. In addition, we extracted the population characteristics (such as sex, age, proportion of pancreaticoduodenectomies, the disease aetiology) and the interventions used in each trial. We assessed the risk of bias of the trials independently, without masking of the trial names. We sought any unclear or missing information by contacting the authors of the individual trials. If there was any doubt whether the trials shared the same participants ‐ completely or partially (by identifying common authors and centres) ‐ we contacted the authors of the trials to ascertain whether the trial report had been duplicated. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
According to empirical evidence (Kjaergard 2001; Moher 1998; Schulz 1995; Wood 2008) we assessed the risk of bias of the trials based on sequence generation, allocation concealment, blinding (of participants, personnel and outcome assessors), incomplete outcome data, selective outcome reporting and other sources of bias. Quality components were based on the Cochrane Handbook for Systematic Reviews of Interventions (Gurusamy 2009; Higgins 2011).
Sequence generation
Low risk of bias (the method used is either adequate (e.g. computer‐generated random numbers, table of random numbers) or unlikely to introduce confounding).
Uncertain risk of bias (there is insufficient information to assess whether the method used is likely to introduce confounding).
High risk of bias (the method used (e.g. quasi‐randomised trials) is improper and likely to introduce confounding).
Allocation concealment
Low risk of bias (the method used (e.g. central allocation) is unlikely to induce bias in the final observed effect).
Uncertain risk of bias (there is insufficient information to assess whether the method used is likely to induce bias in the estimate of effect).
High risk of bias (the method used (e.g. open random allocation schedule) is likely to induce bias in the final observed effect).
Blinding of participants, personnel and outcome assessors
Low risk of bias (blinding was performed adequately, or the outcome measurement is not likely to be influenced by lack of blinding).
Uncertain risk of bias (there is insufficient information to assess whether the type of blinding used is likely to induce bias in the estimate of effect).
High risk of bias (no blinding or incomplete blinding, and the outcome or the outcome measurement is likely to be influenced by lack of blinding).
Incomplete outcome data
Low risk of bias (the underlying reasons for missing data are unlikely to make treatment effects depart from plausible values, or proper methods have been employed to handle missing data).
Uncertain risk of bias (there is insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data is likely to induce bias in the estimate of effect).
High risk of bias (the crude estimate of effects (e.g. complete case estimate) will clearly be biased due to the underlying reasons for data being missing, and the methods used to handle missing data are unsatisfactory).
Selective outcome reporting
Low risk of bias (the trial protocol is available and all of the trial's pre‐specified outcomes that are of interest in the review have been reported or similar).
Uncertain risk of bias (there is insufficient information to assess whether the magnitude and direction of the observed effect is related to selective outcome reporting).
High risk of bias (not all of the trial's pre‐specified primary outcomes have been reported or similar).
Source of funding bias
Low risk of bias (the trial's source(s) of funding did not come from any parties that might have a conflicting interest (e.g. pharmaceutical company).
Uncertain risk of bias (the source of funding was not clear).
High risk of bias (the trial was funded by a pharmaceutical company).
We considered trials that were classified as low risk of bias in all the above domains as low bias‐risk trials.
Measures of treatment effect
For dichotomous outcomes, we calculated the risk ratio (RR) with 95% CI. For continuous outcomes we calculated mean difference (MD) or standardised mean difference (SMD) with 95% CI. We also calculated the risk difference (RD) with 95% CI but planned to report the results if they were different from the RR. We identified no such results.
Unit of analysis issues
All the trials included in this review were simple parallel group design and the unit of analysis was each person recruited into the trial.
Dealing with missing data
We performed the analysis on an 'intention‐to‐treat' basis (Newell 1992), whenever possible. Otherwise, we adopted the 'available case analysis'.
Assessment of heterogeneity
We explored heterogeneity by Chi² test with significance set at a P value 0.10, and the quantity of heterogeneity was measured by the I² statistic (Higgins 2002). An I² statistic of > 30% was considered statistically significant heterogeneity.
Assessment of reporting biases
We used a funnel plot to explore bias (Egger 1997; Macaskill 2001). We used asymmetry in a funnel plot of trial size against treatment effect to assess bias. We performed the linear regression approach described by Egger et al to determine the funnel plot asymmetry (Egger 1997).
Data synthesis
We performed the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2011). We used the software package Review Manager (RevMan) provided by The Cochrane Collaboration (RevMan 2011). We used a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987). In case of discrepancy between the two models, we reported both results; otherwise we have reported only the results from the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses for the primary outcomes.
Trials with low risk of bias versus those with high risk of bias.
Different interventions (somatostatin and octreotide).
Different aetiologies (malignancy and chronic pancreatitis).
Different procedures (pancreatoduodenectomy, distal pancreatectomy and pancreatic drainage procedures).
Different methods of management of pancreatic stump (pancreatogastrostomy and pancreatojejunostomy).
We performed the Chi² test for subgroup differences, setting a P value of 0.05 to identify any differences.
Sensitivity analysis
In the event that we found 'zero‐event' trials for statistically significant outcomes, we intended to perform a sensitivity analysis with and without empirical continuity correction factors, as suggested by Sweeting et al (Sweeting 2004). However, we did not find any such outcomes.
Results
Description of studies
Results of the search
We identified 1352 references through the electronic searches of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group Controlled Trials Register and CENTRAL in The Cochrane Library (N = 77), MEDLINE (N = 567), EMBASE (N = 356) and Science Citation Index Expanded (N = 352). We have shown the flow of references in Figure 1. We excluded 315 duplicates and 979 clearly irrelevant references through reading abstracts. We retrieved 58 references for further assessment. No references were identified through scanning reference lists of the identified randomised trials. Of the 58 references, we excluded 21 for the reasons listed in the 'Characteristics of excluded studies' table. Four of the 21 references corresponded to two studies (Lowy 1997; Wang 2012), hence the inclusion of only 19 studies in the table. Of the remaining 37 references, 16 were multiple reports resulting in 21 randomised trials that fulfilled the inclusion criteria.
1.
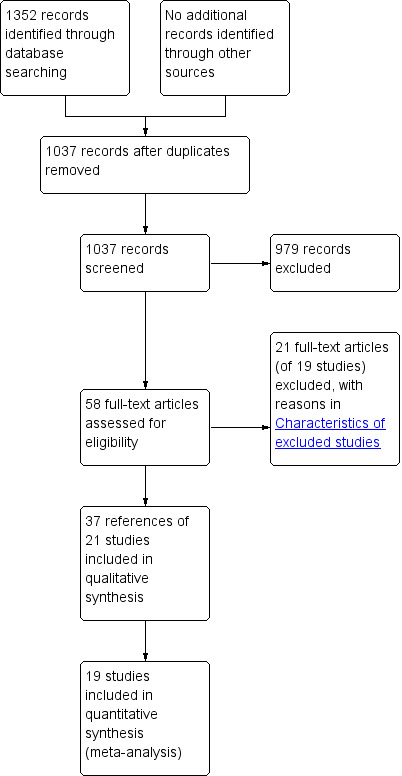
Study flow diagram.
Included studies
All the 21 trials were completed trials and 19 of these provided data for the analyses. Details of the trials are shown in the 'Characteristics of included studies' table. Overall, the 21 trials included 2348 participants. Three hundred and four participants were involved in seven trials comparing somatostatin versus control (Beguiristain 1995; Buccoliero 1992; Gouillat 2001; Katsourakis 2010; Klempa 1991; Shan 2005; Tulassay 1993) and 1702 participants were involved in 13 trials comparing octreotide versus control (Briceño Delgado 1998; Buchler 1992; Fernandez‐Cruz 2012; Friess 1995; Hesse 2005; Kollmar 2008; Kurumboor 2012; Lange 1992; Matheus 2009; Montorsi 1995; Pederzoli 1994; Suc 2004; Yeo 2000). The remaining participants were involved in one trial comparing vapreotide versus control (Sarr 2003). Overall 1608 participants underwent pancreatoduodenectomy, 1196 participants had malignancy and 596 participants had chronic pancreatitis in the trials that reported these characteristics. The mean age of the individuals in the trials varied between 43 and 65 years. The mean proportion of females varied between 15% and 48%. There was no difference in the characteristics of participants in the intervention group or control in any of the trials that reported these baseline characteristics.
Excluded studies
The reasons for exclusion of studies are listed in the 'Characteristics of excluded studies' table. None of these studies were randomised clinical trials.
Risk of bias in included studies
The risk of bias is summarised in the 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary (Figure 3). We classified two trials as low risk of bias (Gouillat 2001; Kollmar 2008).
2.
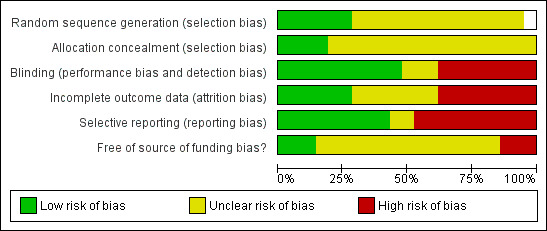
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
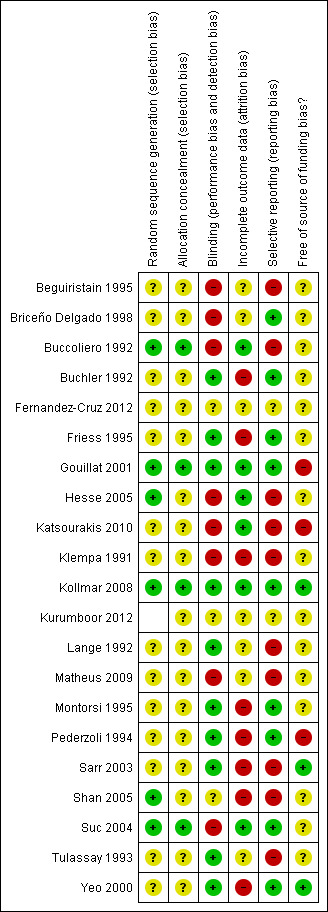
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
The generation of allocation sequence was adequate in five trials (Gouillat 2001; Hesse 2005; Kollmar 2008; Shan 2005; Suc 2004) and allocation concealment was adequate in three trials (Gouillat 2001; Kollmar 2008; Suc 2004).
Blinding
Blinding was adequate in 10 trials (Buchler 1992; Friess 1995; Gouillat 2001; Kollmar 2008; Lange 1992; Montorsi 1995; Pederzoli 1994; Sarr 2003; Tulassay 1993; Yeo 2000).
Incomplete outcome data
Five trials were free from bias due to incomplete outcome data (Gouillat 2001; Hesse 2005; Katsourakis 2010; Kollmar 2008; Suc 2004). In eight trials there were post‐randomisation drop‐outs (Buchler 1992; Friess 1995; Klempa 1991; Montorsi 1995; Pederzoli 1994; Sarr 2003; Shan 2005; Yeo 2000) resulting in a high risk of bias from incomplete outcome data. In these trials with post‐randomisation drop‐outs, a significant proportion of participants underwent pancreatic surgery. There was no information available on these participants. Thus, it was not possible even to perform an available case analysis and we had to perform a per protocol analysis. In the remaining trials, it was not clear whether there were post‐randomisation drop‐outs (Beguiristain 1995; Briceño Delgado 1998; Buccoliero 1992; Katsourakis 2010; Matheus 2009; Lange 1992; Tulassay 1993).
Selective reporting
Nine trials were free from bias due to selective reporting (Briceño Delgado 1998; Buchler 1992; Friess 1995; Gouillat 2001; Kollmar 2008; Montorsi 1995; Pederzoli 1994; Suc 2004; Yeo 2000).
Other potential sources of bias
Three trials were free from other potential sources of bias (Kollmar 2008; Sarr 2003; Yeo 2000).
Effects of interventions
See: Table 1
Somatostatin analogues versus none (Analysis 1.1 to Analysis 1.20)
Primary outcomes
Mortality
There was no difference in the perioperative mortality between the two groups (RR 0.80; 95% CI 0.56 to 1.16).
Drug‐related complications
There was no significant difference in treatment withdrawal (RR 1.55; 95% CI 0.56 to 4.33) between the groups. The number of people with adverse effects due to treatment was significantly higher in the somatostatin analogues group than the control group by the fixed‐effect model (RR 1.36; 95% CI 1.02 to 1.82) but not by the random‐effects model (RR 2.09; 95% CI 0.83 to 5.24).
Quality of life
This was not reported in any of the trials.
Secondary outcomes
Postoperative complications
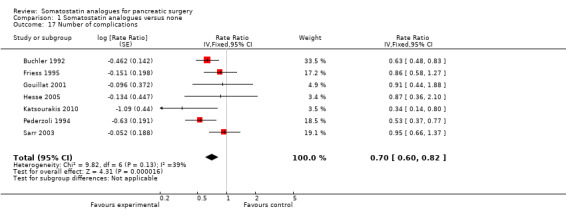
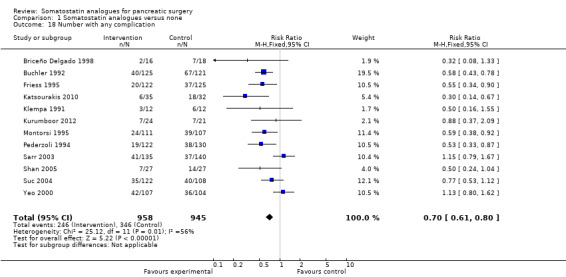
There were statistically significant lower incidences of pancreatic fistula (RR 0.66; 95% CI 0.55 to 0.79) and sepsis (RR 0.42; 95% CI 0.21 to 0.85) in the somatostatin analogues group than in the control group. There was a statistically significant lower number of complications (RR 0.70; 95% CI 0.60 to 0.82) and lower number of participants with any complication (RR 0.70; 95% CI 0.61 to 0.80) in the somatostatin analogues group than the control group. There was no significant difference in the re‐operation rates (RR 1.06; 95% CI 0.69 to 1.63), incidence of anastomotic leak (RR 0.81; 95% CI 0.51 to 1.27), clinically significant pancreatic fistulas (RR 0.69; 95% CI 0.38 to 1.28), postoperative pancreatitis (RR 0.63; 95% CI 0.32 to 1.22), renal failure (RR 0.67; 95% CI 0.25 to 1.77), bleeding (RR 1.00; 95% CI 0.70 to 1.44), abdominal collections (RR 0.76; 95% CI 0.56 to 1.05), infected abdominal collections (RR 0.93; 95% CI 0.66 to 1.32), delayed gastric emptying (RR 0.79; 95% CI 0.51 to 1.23), pulmonary complications (RR 0.89; 95% CI 0.57 to 1.39) or shock (RR 1.00; 95% CI 0.46 to 2.15) between the groups.
Hospital stay
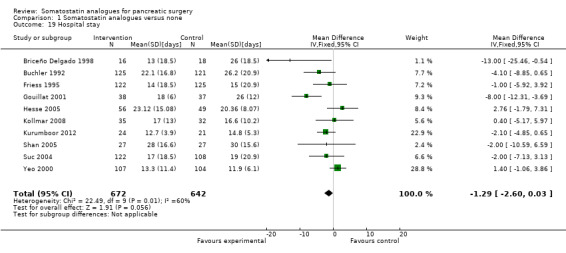
There was no difference in the duration of hospital stay (MD ‐1.29 days; 95% CI ‐2.60 to 0.03) or in the duration of ITU stay (MD 0.90 days; 95% CI ‐1.76 to 3.56) between the groups.
Subgroup analysis
We performed the following planned subgroup analyses.
Different interventions (somatostatin and octreotide).
Different aetiologies (malignancy and chronic pancreatitis).
Different procedures (pancreatoduodenectomy).
We were unable to perform subgroup analysis based on the risk of bias in the trials as only two trials were of low risk of bias. We were unable to perform a planned subgroup analysis of distal pancreatectomy and pancreatic drainage procedures, and the different methods of management of pancreatic stump (pancreatogastrostomy and pancreatojejunostomy), as the outcome data for the different subgroups were not available from the trials.
There was no significant difference in any of the primary outcomes between intervention and control groups in the different subgroups (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 4.1; Analysis 4.2; Analysis 4.3).
2.1. Analysis.
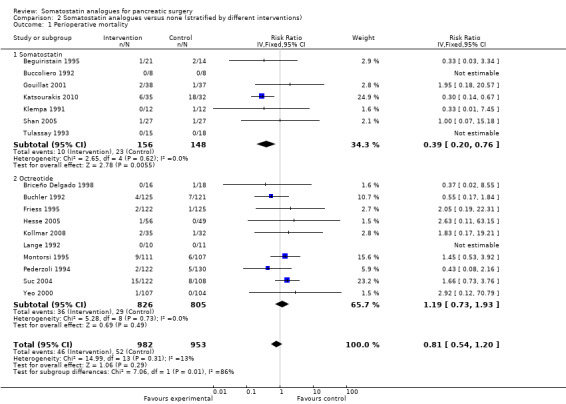
Comparison 2 Somatostatin analogues versus none (stratified by different interventions), Outcome 1 Perioperative mortality.
2.2. Analysis.
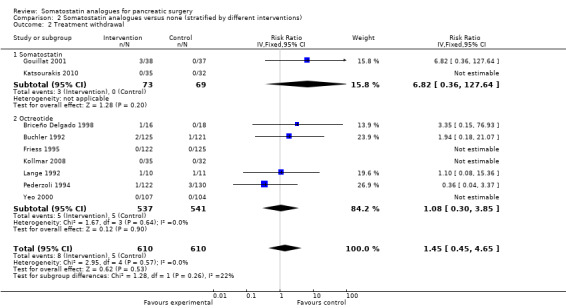
Comparison 2 Somatostatin analogues versus none (stratified by different interventions), Outcome 2 Treatment withdrawal.
2.3. Analysis.
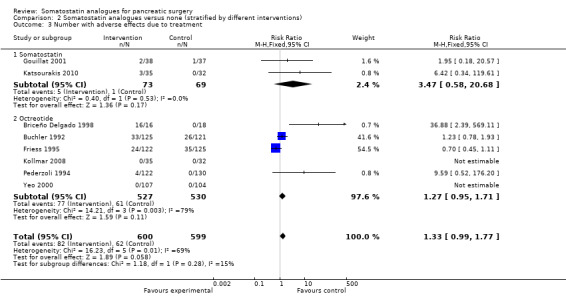
Comparison 2 Somatostatin analogues versus none (stratified by different interventions), Outcome 3 Number with adverse effects due to treatment.
3.1. Analysis.
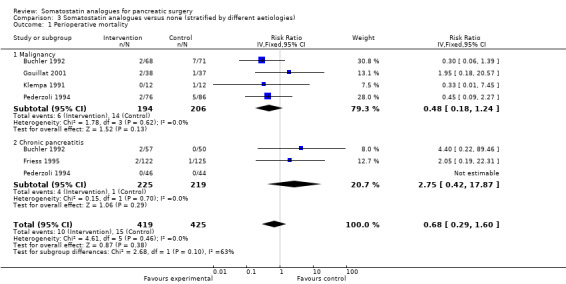
Comparison 3 Somatostatin analogues versus none (stratified by different aetiologies), Outcome 1 Perioperative mortality.
3.2. Analysis.
Comparison 3 Somatostatin analogues versus none (stratified by different aetiologies), Outcome 2 Treatment withdrawal.
3.3. Analysis.
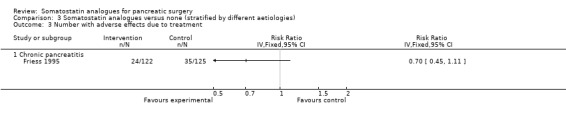
Comparison 3 Somatostatin analogues versus none (stratified by different aetiologies), Outcome 3 Number with adverse effects due to treatment.
4.1. Analysis.
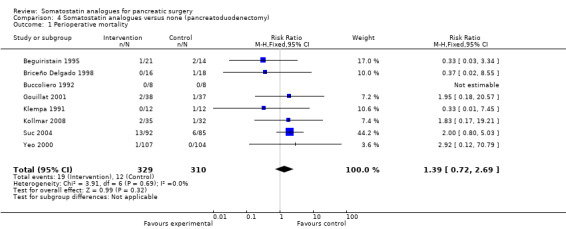
Comparison 4 Somatostatin analogues versus none (pancreatoduodenectomy), Outcome 1 Perioperative mortality.
4.2. Analysis.
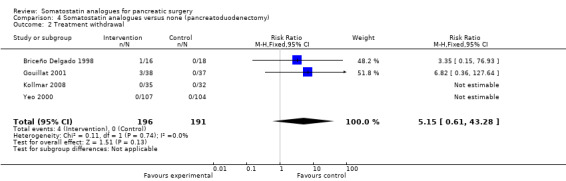
Comparison 4 Somatostatin analogues versus none (pancreatoduodenectomy), Outcome 2 Treatment withdrawal.
4.3. Analysis.
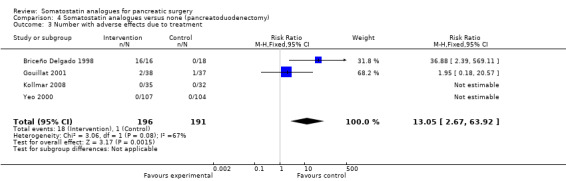
Comparison 4 Somatostatin analogues versus none (pancreatoduodenectomy), Outcome 3 Number with adverse effects due to treatment.
Variations in statistical analysis
We observed no change in results by adopting the random‐effects model or by calculating the RD except for the outcome 'number with adverse effects due to treatment'. We did not perform the sensitivity analysis using empirical continuity correction factors as suggested by Sweeting 2004, as there were no statistically significant outcomes in the main comparison with zero event trials.
Reporting bias
The funnel plot of perioperative mortality did not show any reporting bias (Figure 4). The Egger's linear regression approach to identify publication bias (Egger 1997) did not reveal any bias for the outcome perioperative mortality (P value = 0.7721). We performed the Egger's approach using the statistical software StatsDirect 2.7.2. No funnel plot was calculated for adverse effects of the drug because of the few trials reporting this outcome. None of the trials reported participant quality of life.
4.
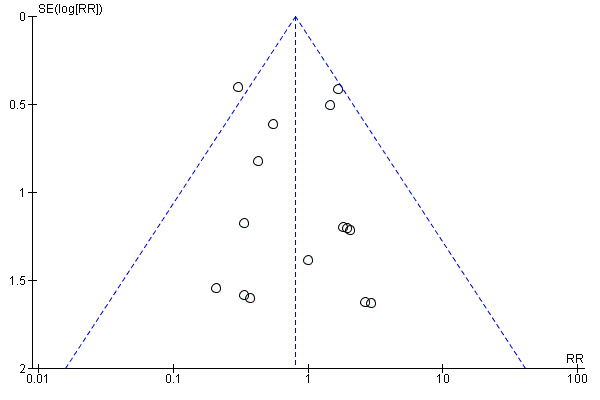
Funnel plot of comparison: 1 Somatostatin analogues versus none, outcome: 1.1 Perioperative mortality.
Discussion
Summary of main results
Somatostatin analogues did not decrease perioperative mortality and re‐operation rates in people undergoing pancreatic surgery.
The main indication for re‐operation is the presence of a pancreatic fistula, associated sepsis or organ dysfunction (Bassi 2005). There is no universal definition of pancreatic fistula or pancreatic leak and the incidence can vary depending on the definition used. An international study group of surgeons have graded postoperative pancreatic fistulas by consensus as A, B and C (Bassi 2005). Grade A is the transient fistula that does not have any clinical impact. Grade B fistulas require alteration in the management of the person. Grade C fistulas require major alterations in the management of the person and usually require re‐operation. Grade B and C fistulas have significant clinical impact and may contribute to increased morbidity and mortality. In this review, we included only trials in which data on grade B or C were available separately from grade A for the outcome clinically significant pancreatic fistula (grades B or C). The overall incidence of pancreatic fistula was lower in the somatostatin analogues group. Only four trials distinguished between any pancreatic fistula and clinically significant pancreatic fistula (Gouillat 2001; Hesse 2005; Kollmar 2008; Kurumboor 2012). There was no difference between the somatostatin analogues group and control in the incidence of clinically significant pancreatic fistula. It is likely that some of the pancreatic fistulas that were reported in the other trials were clinically significant. However, in the absence of data on the proportion of these fistulas that were clinically significant, such trials could not be included for the outcome 'clinically significant pancreatic fistulas' and could be included only for the outcome 'all pancreatic fistulas'. The few trials included under the outcome 'clinically significant pancreatic fistulas' may be the reason for the lack of a statistically significant difference between the somatostatin analogues and control. Alternatively, the lack of a statistically significant difference may be due to the lack of effect.
The overall postoperative complications were significantly lower in the intervention group than the control group. However, there was no significant difference in total hospital stay between the two groups for the main analysis. Possible reasons for the absence of difference in total hospital stay include a lack of effect of somatostatin analogues with regards to the incidence of re‐operation, anastomotic leak or clinically significant pancreatic fistulas; or could be because of the management of the pancreatic fistulas at the person's home (community‐based treatment). Pancreatic fistulas amenable for community‐based treatment might decrease the quality of life of people during the time it takes for these fistulas to close, increase the convalescence period resulting in a later return to work with major cost implications for the person, person's carers and the person's employers, and increase the costs associated with the provision of community‐based treatment, even though they do not prolong hospital stay. In the absence of any information on the quality of life of the participants, it was not possible to assess the impact of pancreatic fistulas that did not prolong hospital stay.
As far as the interventions are concerned, somatostatin has to be administered by continuous intravenous infusion for approximately one week. This can decrease the mobility of the person. Octreotide, in contrast, is administered subcutaneously three times a day allowing good mobility. The other advantage is that it can be administered even in people with difficult venous access thereby increasing compliance. The adverse effects associated with the intervention were mainly minor adverse effects, such as pain at the injection site. There were no serious adverse effects reported in any of the trials. Of the trials that reported the withdrawal of intervention, the treatment was stopped in about 1.3% of the 610 participants. In high‐income countries, the cost of the entire course of octreotide is less than the cost of one additional day at the hospital. There was no difference in the hospital stay between the two groups. The lack of information on pancreatic fistulas (i.e. whether they were clinically significant or not) does not help in reaching a conclusion as to whether the somatostatin analogues should be routinely recommended. However, on a balance, considering the lack of serious adverse effects, low costs and the potential benefit in reducing the proportion of people who developed any complication and in the number of people who developed pancreatic fistulas, somatostatin analogues are recommended routinely for people undergoing pancreatic surgery. Further cost‐effectiveness evaluation of somatostatin analogues in pancreatic surgery is necessary.
Overall completeness and applicability of evidence
This review included people undergoing pancreatic surgery for malignancy and chronic pancreatitis using pancreatoduodenectomy, distal pancreatectomy and pancreatic drainage procedures. Hence, this review is applicable to most people undergoing pancreatic surgery.
Quality of the evidence
Most of the trials were of high risk of bias. Allocation was unclear in most of the trials. Blinding was not performed in some of the trials. Considering that many of the outcomes were subjective, this could have resulted in overestimation of treatment effects (Wood 2008). Some of the trials had a significant proportion of post‐randomisation drop‐outs. A significant proportion of these people underwent pancreatic surgery. Thus, we had to adopt the per protocol analysis for some trials. In spite of all the preoperative staging investigations, such as computerised tomography (CT) scan, endoscopic ultrasound (EUS), diagnostic laparoscopy (DL) or a combination of these investigations, between 8% and 33% of people are found to have unresectable pancreatic cancer on laparotomy (Mayo 2009). Considering that the intervention was started preoperatively in the majority of the people, it appears that post‐randomisation drop‐outs are inevitable. Considering that no pancreatic surgery is carried out in people with unresectable disease (they may undergo palliative bypass procedures such as gastrojejunostomy or cholecystojejunostomy), it is reasonable to exclude these participants from the analysis for outcomes related to postoperative complications or hospital stay. Thus, an available case analysis may be appropriate in such circumstances when the drop‐outs are not related to the intervention (Gurusamy 2009) (we can be sure that octreotide does not cause unresectability). However, exclusion of participants for any other reason will lead to a biased effect estimate. It is also important to report all the pre‐specified outcomes in order to avoid selective reporting. Some of the trials did not report all the pre‐specified outcomes. It will also be useful in reporting the outcomes according to their severity. In spite of its deficiencies, the only validated classification of postoperative complications is the Clavien‐Dindo classification (Clavien 2009; Dindo 2004). It is also important to report the quality of life in participants so that a formal cost‐effectiveness analysis can be undertaken. Reporting of the outcomes stratified by the aetiology, type of pancreatic resection and method of management of pancreatic stump will enable the evaluation of the effect of somatostatin analogues in the different subgroups, as they may be useful in some subgroups but not in others.
Potential biases in the review process
We followed the Cochrane Handbook for Systematic Reviews of Interventions for this review (Higgins 2011). There were no language, publication status or sample size restrictions. Thus, we minimised the bias due to selection of trials. There was no reporting bias in perioperative mortality as suggested by the funnel plot and Egger's linear regression approach (Egger 1997).
We have used per protocol analysis when intention‐to‐treat analysis or available case analysis was not possible. This could lead to bias favouring the intervention. Inadequate reporting of the pancreatic fistulas also make it difficult to judge whether somatostatin analogues are useful.
We have used median for the meta‐analysis when the mean was not available for hospital stay. We have also imputed the standard deviation from P values according to the formulae stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the trials stated a P value < 0.05, we calculated the standard deviation using a P value equal to 0.05. If standard deviation could not be calculated because the trial reports just stated that there was no statistical significance without mentioning the exact P value, we used the highest standard deviation among the other trials included in the outcome. This imputation of standard deviation may have introduced bias. We also performed a sensitivity analysis by excluding the trials that did not report the mean and standard deviation of the hospital stay in the two groups. There was no change in the results either in the overall analysis or in the different subgroup analyses when we performed this sensitivity analysis.
Agreements and disagreements with other studies or reviews
Of the systematic reviews, that by Connor et al concluded that somatostatin and its analogues should be used in pancreatic surgery based on a reduction in the incidence of pancreatic fistula (Connor 2005). The other systematic review by Alghamdi et al concluded that octreotide decreased the incidence of pancreatic fistula, and that further trials are necessary to confirm the findings of the meta‐analysis and to identify subgroups of people undergoing pancreatic surgery who may benefit from octreotide (Alghamdi 2007). We have made a recommendation of routine use of somatostatin analogues in pancreatic surgery because of the low costs of the drug, lack of any serious adverse effects in the postoperative setting and the potential to decrease the complication rates after pancreatic surgery.
Authors' conclusions
Implications for practice.
Somatostatin analogues may reduce perioperative complications but do not reduce perioperative mortality. Further adequately powered trials of low risk of bias are necessary. Based on current available evidence, somatostatin and its analogues are recommended for routine use in people undergoing pancreatic resection.
Implications for research.
Further trials with low risk of bias are necessary.
What's new
| Date | Event | Description |
|---|---|---|
| 16 February 2013 | New search has been performed | Search results updated and two more trials included in the analysis. Numerous subgroup analyses which increase the possibility of type I error have been removed and subgroup analyses have now been performed for primary outcomes only. |
| 30 January 2012 | New citation required but conclusions have not changed | Two trials included in analyses. Conclusions unchanged. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 2, 2010
| Date | Event | Description |
|---|---|---|
| 13 February 2012 | Amended | Contact details updated. |
| 20 September 2010 | Amended | Contact details updated. |
Notes
This review was developed based on a Cochrane protocol written by a different group of authors and published in The Cochrane Library in 2003. The protocol was withdrawn from publication in early 2009, as the original authors were unable to complete the review. The authors of the present review have developed the review based on the original protocol, which has been quality assured during the editorial process. Details of changes to the protocol are noted in Differences between protocol and review. Further revisions to the methods of the review were made according to the guidelines of the most recent version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Details of changes are noted in a sub‐heading in Differences between protocol and review.
Acknowledgements
We would like to thank the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group for the support that they provided for the completion of this review.
We would also like to acknowledge and thank the authors of the original protocol, Eva Morris, Moritz N Wente, Christoph M Seiler, Meg Finch‐Jones, Markus W Büchler and Derek Alderson.
This project was funded by the National Institute for Health Research. Disclaimer of the Department of Health: "The views and opinions expressed in the review are those of the authors and do not necessarily reflect those of the National Institute for Health Research (NIHR), National Health Services (NHS), or the Department of Health".
Appendices
Appendix 1. CENTRAL search strategy
#1 (randomized controlled trial):pt #2 (controlled clinical trial):pt #3 (randomized):ab #4 (placebo):ab #5 MeSH descriptor Drug Therapy, this term only #6 (randomly):ab #7 (trial):ab #8 (groups):ab #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 (pancreas):ti,ab #11 (pancrea*):ti,ab #12 MeSH descriptor Pancreas, this term only #13 MeSH descriptor Islets of Langerhans, this term only #14 MeSH descriptor Pancreas, Exocrine, this term only #15 MeSH descriptor Pancreatic Ducts, this term only #16 (#10 OR #11 OR #12 OR #13 OR #14 OR #15) #17 MeSH descriptor Carcinoma, this term only #18 MeSH descriptor Adenocarcinoma, this term only #19 MeSH descriptor Carcinoma, Ductal, this term only #20 MeSH descriptor Carcinoma, Islet Cell, this term only #21 (cancer) #22 (cancer*) #23 MeSH descriptor Neoplasms explode all trees #24 (tumo*) #25 (#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) #26 (#16 AND #25) #27 MeSH descriptor Pancreatitis explode all trees #28 (pancreatitides) #29 (#26 OR #27 OR #28) #30 MeSH descriptor Surgical Procedures, Operative explode all trees #31 MeSH descriptor General Surgery explode all trees #32 (surger*) #33 (operatio*) #34 (operative therap*) #35 (resection*):kw #36 MeSH descriptor Pancreatectomy explode all trees #37 MeSH descriptor Pancreaticojejunostomy explode all trees #38 MeSH descriptor Pancreaticoduodenectomy explode all trees #39 (pancreaticoduodenectom*):ti,ab #40 (pancreatectomy):ab,ti #41 (pancreaticojejunostomy):ab,ti #42 (duodenopancreatectom*):ab,ti #43 (#30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42) #44 MeSH descriptor Somatostatin explode all trees #45 MeSH descriptor Octreotide explode all trees #46 (somatostatin):ab,ti #47 (octreotide):ab,ti #48 (lanreotide):ab,ti #49 (pasireotide):ab,ti #50 (vapreotide):ab,ti #51 (#44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50) #52 (#9 AND #29 AND #43 AND #51)
Appendix 2. MEDLINE search strategy
1. randomized controlled trial [pt] 2. controlled clinical trial [pt] 3. randomized [tiab] 4. placebo [tiab] 5. drug therapy [sh] 6. randomly [tiab] 7. trial [tiab] 8. groups [tiab] 9. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 10. animals [mh] not (humans [mh] and animals [mh]) 11. #9 not #10 12. Pancreatic Neoplasms [MeSH] 13. Pancreatitis[MeSH] 14. pancreas cancer [tiab] 15. pancreas cancers [tiab] 16. pancreas neoplasm [tiab] 17. pancreas neoplasms [tiab] 18. pancreas carcinoma [tiab] 19. pancreas tumor [tiab] 20. pancreas tumors [tiab] 21. pancreas tumour [tiab] 22. pancreas tumours [tiab] 23. pancreatitis [tiab] 24. pancreatitides [tiab] 25. #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 26. Surgical Procedures, Operative[MeSH] 27. Surgery[MeSH] 28. operation OR operations[tiab] 29. operative therapy [tiab] 30. operative therapies [tiab] 31. surgery OR surgeries [tiab] 32. resection OR resections [tiab] 33. #26 or #27 or #28 or #29 or #30 or #31 or #32 34. #25 and #33 35. Pancreatectomy[MeSH] 36. Pancreaticojejunostomy[MeSH] 37. Pancreaticoduodenectomy[MeSH] 38. pancreaticoduodenectomy [tiab] 39. pancreatectomy [tiab] 40. pancreaticojejunostomy [tiab] 41. pancreaticogastrostomy [tiab] 42. pancreaticoduodenectomies [tiab] 43. duodenopancreatectomy [tiab] 44. duodenopancreatectomies [tiab] 45. #35 or #36 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 46. #34 or #45 47. Somatostatin[Mesh] 48. Octreotide[Mesh] 49. lanreotide [Substance Name] 50. pasireotide[Substance Name] 51. vapreotide [Substance Name] 52. somatostatin [tiab] 53. octreotide [tiab] 54. lanreotide [tiab] 55. pasireotide [tiab] 56. vapreotide [tiab] 57. #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 58. #46 and #57 59. #58 and #11
Appendix 3. EMBASE search strategy
1 randomized controlled trial/ 2 controlled clinical trial/ 3 randomized.ab. 4 placebo.ab. 5 drug therapy/ 6 randomly.ab. 7 trial.ab. 8 groups.ab. (641427) 9 6 or 3 or 7 or 2 or 8 or 1 or 4 or 5 10 animals/ 11 humans/ 12 11 and 10 13 10 not 12 14 9 not 13 15 pancreas.ab,ti. 16 pancrea$.ab,ti. 17 pancreas/ or "islets of langerhans"/ or pancreas, exocrine/ or pancreatic ducts/ 18 16 or 17 or 15 19 carcinoma/ or adenocarcinoma/ or carcinoma, ductal/ or carcinoma, islet cell/ 20 cancer.tw. 21 cancer$.tw. 22 exp neoplasms/ 23 tumo$.tw. 24 22 or 21 or 23 or 19 or 20 25 18 and 24 26 exp Pancreatitis/ 27 pancreatitides.tw. 28 27 or 25 or 26 29 exp Surgical Procedures, Operative/ 30 exp Surgery/ 31 surger$.tw. 32 operatio$.tw. 33 operative therap$.tw. 34 resection$.mp. 35 exp Pancreatectomy/ 36 exp Pancreaticojejunostomy/ 37 exp Pancreaticoduodenectomy/ 38 pancreaticoduodenectom$.ab,ti. 39 pancreatectomy.ab,ti. 40 pancreaticojejunostomy.ab,ti. 41 pancreaticogastrostomy.ab,ti. 42 duodenopancreatectom$.ab,ti. 43 35 or 33 or 32 or 39 or 40 or 36 or 41 or 42 or 38 or 34 or 30 or 37 or 29 or 31 44 exp Somatostatin/ 45 exp Octreotide/ 46 somatostatin.ab,ti. 47 octreotide.ab,ti. 48 lanreotide.ab,ti. 49 pasireotide.ab,ti. 50 vapreotide.ab,ti. 51 50 or 49 or 46 or 45 or 44 or 48 or 47 52 28 and 51 and 43 and 14
Appendix 4. Science Citation Index Expanded search strategy
#1 TS=(randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR drug therapy OR randomly OR trial OR groups) #2 TS=(Pancreatic Neoplasms OR Pancreatitis OR pancreas cancer OR pancreas cancers OR pancreas neoplasm OR pancreas neoplasms OR pancreas carcinoma OR pancreas tumor OR pancreas tumors OR pancreas tumour OR pancreas tumours OR pancreatitis OR pancreatitides) #3 TS=(Operative Surgical Procedures OR Surgery OR operation OR operations OR operative therapy OR operative therapies OR surgery OR surgeries OR resection OR resections) #4 #2 AND #3 #5 (Pancreatectomy OR Pancreaticojejunostomy OR Pancreaticoduodenectomy OR pancreaticoduodenectomy OR pancreatectomy OR pancreaticojejunostomy OR pancreaticogastrostomy OR pancreaticoduodenectomies OR duodenopancreatectomy OR duodenopancreatectomies) #6 #4 OR #5 #7 TS=(Somatostatin OR Octreotide OR lanreotide OR pasireotide OR vapreotide OR somatostatin OR octreotide OR lanreotide OR pasireotide OR vapreotide) #8 #1 AND #6 AND #7
Data and analyses
Comparison 1. Somatostatin analogues versus none.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perioperative mortality | 18 | 2210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.56, 1.16] |
| 2 Treatment withdrawal | 9 | 1220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.56, 4.33] |
| 3 Number with adverse effects due to treatment | 8 | 1199 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [0.83, 5.24] |
| 4 Re‐operation | 7 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.58, 2.70] |
| 5 Anastomotic leak | 9 | 1585 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.51, 1.27] |
| 6 Pancreatic fistula (all) | 17 | 2206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.55, 0.79] |
| 7 Pancreatic fistula (clinically significant) | 4 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.38, 1.28] |
| 8 Postoperative pancreatitis | 11 | 1667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.32, 1.22] |
| 9 Sepsis | 7 | 1092 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.21, 0.85] |
| 10 Renal failure | 5 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.25, 1.77] |
| 11 Bleeding | 14 | 1814 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.70, 1.44] |
| 12 Abdominal collections | 8 | 1589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.56, 1.05] |
| 13 Infected abdominal collections | 13 | 1965 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.32] |
| 14 Delayed gastric emptying | 5 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.23] |
| 15 Pulmonary complications | 9 | 1210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.57, 1.39] |
| 16 Shock | 4 | 812 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.46, 2.15] |
| 17 Number of complications | 7 | Rate Ratio (Fixed, 95% CI) | 0.70 [0.60, 0.82] | |
| 18 Number with any complication | 12 | 1903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.61, 0.80] |
| 19 Hospital stay | 10 | 1314 | Mean Difference (IV, Fixed, 95% CI) | ‐1.29 [‐2.60, 0.03] |
1.1. Analysis.
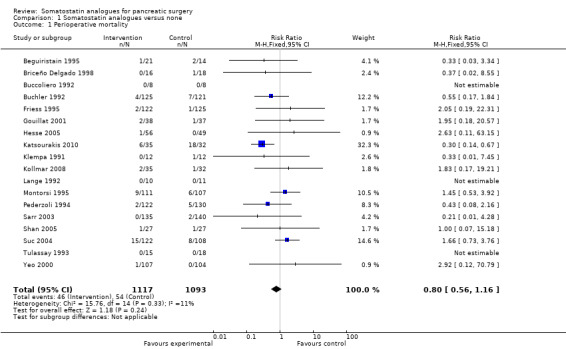
Comparison 1 Somatostatin analogues versus none, Outcome 1 Perioperative mortality.
1.2. Analysis.
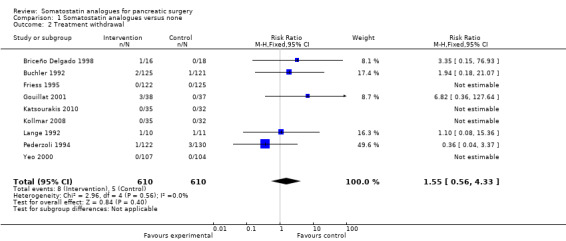
Comparison 1 Somatostatin analogues versus none, Outcome 2 Treatment withdrawal.
1.3. Analysis.
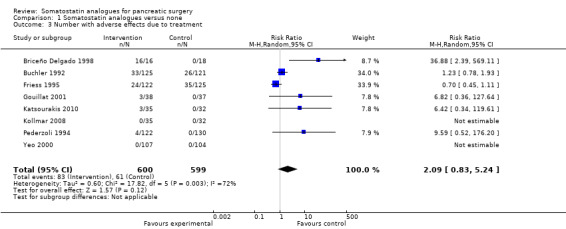
Comparison 1 Somatostatin analogues versus none, Outcome 3 Number with adverse effects due to treatment.
1.4. Analysis.
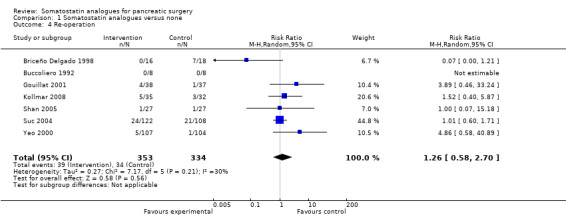
Comparison 1 Somatostatin analogues versus none, Outcome 4 Re‐operation.
1.5. Analysis.
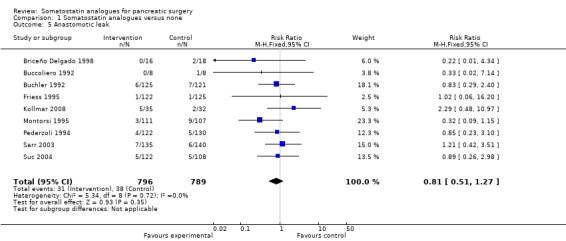
Comparison 1 Somatostatin analogues versus none, Outcome 5 Anastomotic leak.
1.6. Analysis.
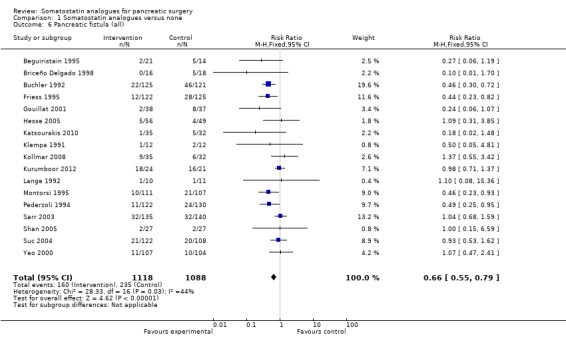
Comparison 1 Somatostatin analogues versus none, Outcome 6 Pancreatic fistula (all).
1.7. Analysis.
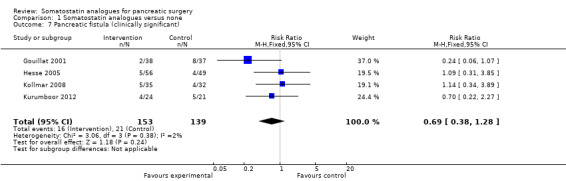
Comparison 1 Somatostatin analogues versus none, Outcome 7 Pancreatic fistula (clinically significant).
1.8. Analysis.
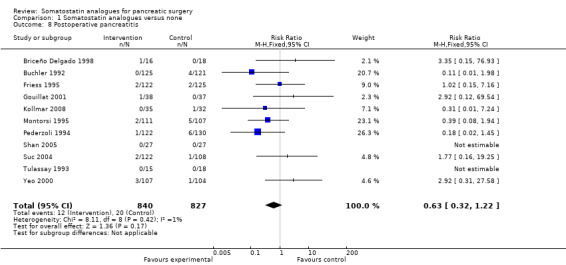
Comparison 1 Somatostatin analogues versus none, Outcome 8 Postoperative pancreatitis.
1.9. Analysis.
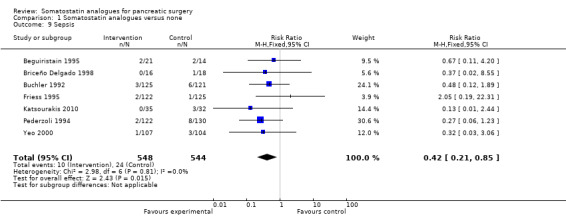
Comparison 1 Somatostatin analogues versus none, Outcome 9 Sepsis.
1.10. Analysis.
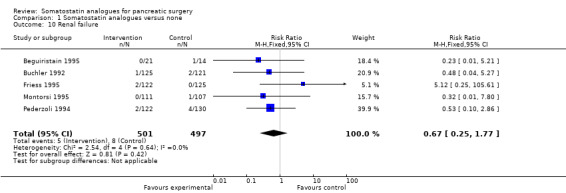
Comparison 1 Somatostatin analogues versus none, Outcome 10 Renal failure.
1.11. Analysis.
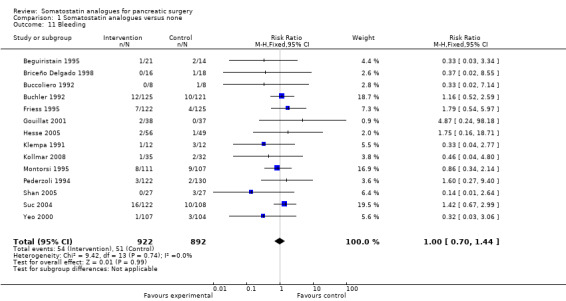
Comparison 1 Somatostatin analogues versus none, Outcome 11 Bleeding.
1.12. Analysis.
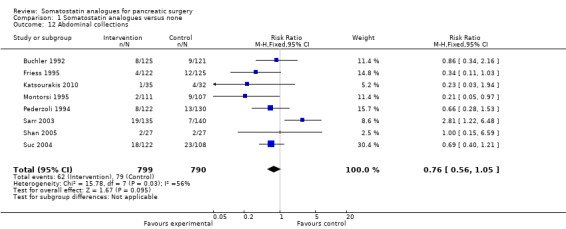
Comparison 1 Somatostatin analogues versus none, Outcome 12 Abdominal collections.
1.13. Analysis.
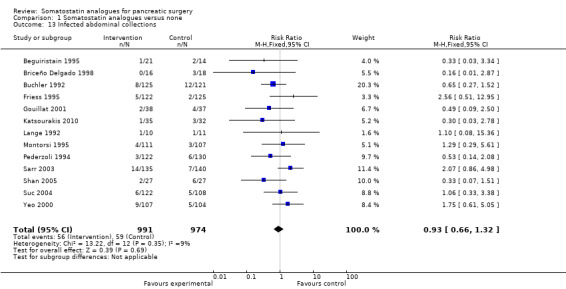
Comparison 1 Somatostatin analogues versus none, Outcome 13 Infected abdominal collections.
1.14. Analysis.
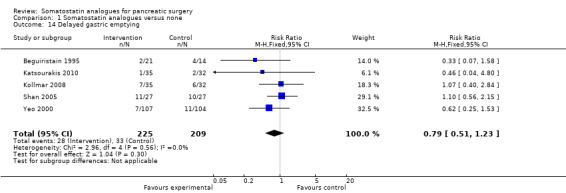
Comparison 1 Somatostatin analogues versus none, Outcome 14 Delayed gastric emptying.
1.15. Analysis.
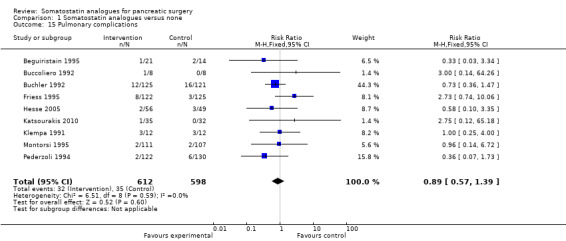
Comparison 1 Somatostatin analogues versus none, Outcome 15 Pulmonary complications.
1.16. Analysis.

Comparison 1 Somatostatin analogues versus none, Outcome 16 Shock.
1.17. Analysis.
Comparison 1 Somatostatin analogues versus none, Outcome 17 Number of complications.
1.18. Analysis.
Comparison 1 Somatostatin analogues versus none, Outcome 18 Number with any complication.
1.19. Analysis.
Comparison 1 Somatostatin analogues versus none, Outcome 19 Hospital stay.
Comparison 2. Somatostatin analogues versus none (stratified by different interventions).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perioperative mortality | 17 | 1935 | Risk Ratio (IV, Fixed, 95% CI) | 0.81 [0.54, 1.20] |
| 1.1 Somatostatin | 7 | 304 | Risk Ratio (IV, Fixed, 95% CI) | 0.39 [0.20, 0.76] |
| 1.2 Octreotide | 10 | 1631 | Risk Ratio (IV, Fixed, 95% CI) | 1.19 [0.73, 1.93] |
| 2 Treatment withdrawal | 9 | 1220 | Risk Ratio (IV, Fixed, 95% CI) | 1.45 [0.45, 4.65] |
| 2.1 Somatostatin | 2 | 142 | Risk Ratio (IV, Fixed, 95% CI) | 6.82 [0.36, 127.64] |
| 2.2 Octreotide | 7 | 1078 | Risk Ratio (IV, Fixed, 95% CI) | 1.08 [0.30, 3.85] |
| 3 Number with adverse effects due to treatment | 8 | 1199 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.99, 1.77] |
| 3.1 Somatostatin | 2 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.47 [0.58, 20.68] |
| 3.2 Octreotide | 6 | 1057 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.95, 1.71] |
Comparison 3. Somatostatin analogues versus none (stratified by different aetiologies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perioperative mortality | 5 | 844 | Risk Ratio (IV, Fixed, 95% CI) | 0.68 [0.29, 1.60] |
| 1.1 Malignancy | 4 | 400 | Risk Ratio (IV, Fixed, 95% CI) | 0.48 [0.18, 1.24] |
| 1.2 Chronic pancreatitis | 3 | 444 | Risk Ratio (IV, Fixed, 95% CI) | 2.75 [0.42, 17.87] |
| 2 Treatment withdrawal | 2 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Malignancy | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Chronic pancreatitis | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with adverse effects due to treatment | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Chronic pancreatitis | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Somatostatin analogues versus none (pancreatoduodenectomy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perioperative mortality | 8 | 639 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.72, 2.69] |
| 2 Treatment withdrawal | 4 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.15 [0.61, 43.28] |
| 3 Number with adverse effects due to treatment | 4 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.05 [2.67, 63.92] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beguiristain 1995.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: Spain
Sample size: 35
Post‐randomisation drop‐out: not stated
Revised sample size: 35
Females: 10 (28.6%)
Mean age: 59.4 years
Malignancy: 30 (85.7%)
Chronic pancreatitis: 3 (8.6%)
Pancreatoduodenectomy: 35 (100%)
Follow‐up: not reported Inclusion criteria: 1. Pancreaticoduodenectomy |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: somatostatin (n = 21) Further details: continuous infusion 4.5 mg/day starting immediately after surgery for 7 days Group 2: control (n = 14) | |
| Outcomes | The outcomes reported were mortality and perioperative morbidity | |
| Notes | Pancreatic fistula definition: drainage of at least 10 mL of fluid with an amylase concentration of more than 5 Somogyi units (grade A) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: blinding was probably not performed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes were not reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Briceño Delgado 1998.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: Spain
Sample size: 34
Post‐randomisation drop‐out: not stated
Revised sample size: 34
Females: 9 (26.5%)
Mean age: 52.5 years
Malignancy: 28 (82.4%)
Chronic pancreatitis: 5 (14.7%)
Pancreatoduodenectomy: 34 (100%)
Follow‐up: not reported Inclusion criteria: 1. Pancreatoduodenectomy |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: octreotide (n = 16) Further details: 0.1 mg sc 3 times a day for 7 days postoperatively Group 2: control (n = 18) | |
| Outcomes | The outcomes reported were mortality, perioperative morbidity and adverse effects of octreotide | |
| Notes | Pancreatic fistula definition: drainage of at least 50 mL a day of fluid rich in amylase for more than 2 weeks (grade A) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: blinding was probably not performed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Buccoliero 1992.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: Italy Sample size: 16 Post‐randomisation drop‐out: not stated Revised sample size: 16 Females: 6 (37.5%) Mean age: 58.2 years Malignancy: not stated Chronic pancreatitis: (0%) Pancreatoduodenectomy: (100%) Follow‐up: not reported Inclusion criteria: 1. Pancreatoduodenectomy for tumour |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: somatostatin (n = 8) Further details: continuous infusion of somatostatin at 250 μg/h for 6 days starting on induction Group 2: control (n = 8) | |
| Outcomes | The outcome reported was postoperative morbidity | |
| Notes | Pancreatic fistula definition: not reported Authors replied to questions related to bias risk assessment in May 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patients sequence was generated according to random number table method" (author replies) |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocation was concealed through the sealed envelope technique" (author replies) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: blinding was probably not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "there were not any drop‐outs in either group" (author replies) |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes were not reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Buchler 1992.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Countries: Germany, Austria Sample size: 322 Post‐randomisation drop‐out: 76 Revised sample size: 246 Females: 72 (29.3%) Mean age: 52 years Malignancy: 111 (45.1%) Chronic pancreatitis: 112 (45.5%) Pancreatoduodenectomy: 200 (81.3%) Follow‐up: 90 days Inclusion criteria: 1. Elective pancreatic resection for tumours or chronic pancreatitis Exclusion criteria: 1. Total pancreatectomy 2. Pancreatic transplantation 3. Pancreatic cyst anastomosis only |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: octreotide (n = 125) Further details: 100 μg sc 3 times daily for 7 days starting at least 1 h preoperatively Group 2: placebo (n = 121) Further details: equal volume, frequency and duration as intervention | |
| Outcomes | The outcomes reported were mortality, perioperative morbidity, adverse effects of octreotide and hospital stay | |
| Notes | Reasons for post‐randomisation drop‐out: unresectable (n = 73); only pancreatic cyst anastomosis done (n = 3) Pancreatic fistula definition: concentration of amylase and lipase in the drainage fluid later than 3 days postoperatively of more than 3 times the serum concentration and a drainage volume of more than 10 mL/h at the same time (grade A) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "on the day before the operation, the patients who satisfied the inclusion criteria were randomly assigned to receive a 7‐day treatment of either 3 X 100 mcg octreotide (1 mL volume) subcutaneously (every 8 hours) or 21 subcutaneous placebo injections (1 mL each) in a double‐blinded fashion" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: post‐randomisation drop‐outs were related to the outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all pre‐defined outcomes were reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Fernandez‐Cruz 2012.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: Spain
Number randomised: 58
Post‐randomisation drop‐outs: not stated
Revised sample size: 58
Average age: not stated
Females: not stated
Malignancy: not stated
Chronic pancreatitis: not stated
Pancreatoduodenectomy: 58 (100%)
Follow‐up: 90 days
Inclusion criteria: 1. People undergoing pancreatoduodenectomy |
|
| Interventions | Participants were randomly assigned to 2 groups Group 1: somatostatin analogue (n = not stated) Further details: octreotide (100 µg sc every 8 h) Group 2: control (n = not stated) Further details: no intervention | |
| Outcomes | The outcomes reported were pancreatic fistula and proportion of people with complications | |
| Notes | Attempts were made to contact the authors in February 2013 The number of participants in each group was not stated. The authors stated that 26.6% of people with octreotide and 33.3% of people without octreotide developed complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Unclear risk | Comment: some important outcomes which will generally be assessed were not reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Friess 1995.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: Germany
Sample size: 280
Post‐randomisation drop‐out: 33
Revised sample size: 247
Females: 53 (21.5%)
Mean age: 48 years
Malignancy: 0 (0%)
Chronic pancreatitis: 247 (100%)
Pancreatoduodenectomy: 124 (50.2%)
Follow‐up: 90 days Inclusion criteria: 1. People with chronic pancreatitis 2. Suitable for pancreatic resection or pancreatic duct anastomosis |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: octreotide (n = 122) Further details: 100 μg sc 3 times daily for 8 days starting at least 1 h preoperatively Group 2: placebo (n = 125) Further details: equal volume, frequency and duration as intervention | |
| Outcomes | The outcomes reported were mortality, perioperative morbidity, adverse effects of octreotide and hospital stay | |
| Notes | Reasons for post‐randomisation drop‐out: confirmation of cancer (n = 17); unresectable because of associated complications (n = 11); cystojejunostomy (n = 3); necrosectomy (n = 2) Pancreatic fistula definition: concentration of amylase and lipase in the drainage fluid later than 3 days postoperatively of more than 3 times the serum concentration and a drainage volume of more than 10 mL/h at the same time (grade A) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "patients who satisfied the inclusion criteria were randomly assigned to receive a 8‐day treatment of either 3 X 100 mcg octreotide (1 ml volume) subcutaneously (every 8 h) or 24 subcutaneous placebo injections (1 ml each) in a double blind manner" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: post‐randomisation drop‐outs were related to the outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all pre‐defined outcomes were reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Gouillat 2001.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: France
Sample size: 75
Post‐randomisation drop‐out: 0
Revised sample size: 75
Females: 24 (32%)
Mean age: 60.2 years
Malignancy: 61 (81.3%)
Chronic pancreatitis: 4 (5.3%)
Pancreatoduodenectomy: 75 (100%)
Follow‐up: not reported Inclusion criteria: 1. Aged 18‐75 years 2. Pancreaticoduodenectomy for presumed tumour 3. No evidence of chronic pancreatitis on preoperative imaging Exclusion criteria: 1. Known allergy to somatostatin or mannitol 2. Received somatostatin or analogues within 3 days of surgery |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: somatostatin‐14 (n = 38) Further details: continuous infusion for 7 days after operation at 6 mg/day Group 2: placebo (n = 37) Further details: mannitol continuous infusion for 7 days after operation at 4 mg/day Additionally, pancreatic juice diversion using a duct inserted into the pancreatic duct was used in both groups | |
| Outcomes | The outcomes reported were mortality, perioperative morbidity, adverse effects of somatostatin and hospital stay | |
| Notes | Pancreatic fistula definition: a clinical pancreatic fistula was defined as the drainage (more than 100 mL/day) of amylase‐rich drainage fluid (greater than 5 times the upper limit of normal for amylase) after day 3, persisting after day 12 or in association with raised temperature (above 38 °C) or other symptoms requiring further surgery, percutaneous drainage or transfer to intensive care (grade B or C) Authors replied to questions related to bias risk assessment in March 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated" Comment: author replies |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelope" Comment: "Author replies" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "during the first 7 days after operation patients received total parenteral nutrition and continuous infusion of either 5‐14 (Somatostatine®; UCB Pharma, Nanterre, France) at a dose of 6 mg per 24 h (days 1 ‐ 6) and 3 mg per 24 h (day 7),or matching placebo (mannitol 4 mg)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported |
| Free of source of funding bias? | High risk | Quote: "this study was supported by a grant from UCB Pharma SA, Nanterre, France" Comment: sponsored by entity with no vested interest in results |
Hesse 2005.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: Belgium
Sample size: 105
Post‐randomisation drop‐out: 0
Revised sample size: 105
Females: 27 (25.7%)
Mean age: 59.5 years
Malignancy: 71 (67.6%)
Chronic pancreatitis: 26 (24.8%)
Pancreatoduodenectomy: 80 (76.2%)
Follow‐up: not reported Inclusion criteria: 1. Aged 16‐86 years 2. Pancreaticojejunostomy |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: octreotide (n = 56) Further details: at the time of operation in a dose of 0.1 mg sc 3 times a day for 7 days Group 2: control (n = 49) Further details: no intervention | |
| Outcomes | The outcomes reported were mortality, perioperative morbidity and hospital stay | |
| Notes | Pancreatic fistula definition: drainage of more than 100 mL/day of amylase rich fluid which had to be more than 5 times the upper limit of normal serum amylase after day 3 and persisting after postoperative day 7 with raising temperature and pre‐septic conditions (grade B) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "was performed according to a computer generated random list" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "single center open label prospectively randomized trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: "There were no post‐randomisation drop‐outs" |
| Selective reporting (reporting bias) | High risk | Comment: important outcomes like re‐operation and adverse effects of octreotide were not reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Katsourakis 2010.
| Methods | Randomised clinical trial | |
| Participants | Country: Greece
Sample size: 67
Post‐randomisation drop‐out(s): not stated
Revised sample size: 67
Females: 28 (41.8%)
Mean age: 61.1 years
Malignancy: 53 (79.1%)
Chronic pancreatitis: 9 (13.4%)
Pancreatoduodenectomy: 13 (19.4%)
Inclusion criteria: 1. People undergoing pancreatic resection |
|
| Interventions | The participants were randomised to 2 groups Group 1: somatostatin analogue (n = 35) Further details: somatostatin 14 intravenously 3.5 μg/kg/h starting 30 minutes before surgery and continued for 7 days Group 2: control (n = 32) | |
| Outcomes | The outcomes reported were postoperative complications and drug‐related adverse events | |
| Notes | Pancreatic fistula: peritoneal amylase levels (drain) > 3 × plasma amylase from day 3 onwards (grade A) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "open‐label, parallel‐group, simple randomized clinical trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes were not reported |
| Free of source of funding bias? | High risk | Quote: "this work was supported in part by a grant from Faran laboratories s.a., Athens, Greece" |
Klempa 1991.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: Germany
Sample size: 30
Post‐randomisation drop‐out: 6
Revised sample size: 24
Females: 9 (37.5%)
Mean age: 56.5 years
Malignancy: 24 (100%)
Chronic pancreatitis: 0 (0%)
Pancreatoduodenectomy: 24 (100%)
Follow‐up: not reported Inclusion criteria: 1. Pancreatic or papillary carcinoma 2. Partial duodenopancreatectomy |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: somatostatin (n = 12) Further details: intravenous 250 μg/h for 6 days starting after operation Group 2: control (n = 12) | |
| Outcomes | The outcomes reported were mortality and perioperative morbidity | |
| Notes | Reasons for post‐randomisation drop‐out: drain falling out or occlusion of drain (n = 4) and because of pancreatitis (n = 2) Pancreatic fistula definition: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: blinding was not performed probably |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: post‐randomisation drop‐outs were related to the outcomes |
| Selective reporting (reporting bias) | High risk | Comment: important outcomes such as re‐operation were not reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Kollmar 2008.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Countries: Germany, Switzerland Sample size: 67 Post‐randomisation drop‐out: 0 Revised sample size: 67 Females: 26 (38.8%) Mean age: 62.8 years Malignancy: 33 (49.3%) Chronic pancreatitis: 16 (23.9%) Pancreatoduodenectomy: 67 (100%) Follow‐up: not reported Inclusion criteria: 1. 18 years or older 2. Undergoing partial duodenopancreatectomy | |
| Interventions | The participants were randomly assigned to 2 groups Group 1: octreotide (n = 35) Further details: 100 μg sc 3 times daily for 7 days Group 2: placebo (n = 32) Further details: equal volume, frequency and duration as intervention | |
| Outcomes | The outcomes reported were mortality, perioperative morbidity, hospital stay and ITU stay | |
| Notes | Pancreatic fistula definition: output via an operatively placed drain (or a subsequently placed percutaneous drain) of any measurable volume of drain fluid on or after postoperative day 3, with an amylase content greater than 3 times the upper normal serum value (grade A) The authors provided further information on the allocation concealment, different grades of pancreatic fistula and adverse reactions to octreotide |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized to either the octreotide or the control group by means of a randomly generated number pattern" |
| Allocation concealment (selection bias) | Low risk | Quote: "octreotide and control saline placebo were prepared in the local investigational drug pharmacy and were identical in appearance, volume and labelling (consecutive numbers), masking the nursing staff, physicians and patients to their contents" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "octreotide and control saline placebo were prepared in the local investigational drug pharmacy and were identical in appearance, volume and labelling (consecutive numbers), masking the nursing staff, physicians and patients to their contents" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported |
| Free of source of funding bias? | Low risk | Quote: "there are no financial and personal relationships with other people or organizations that had inappropriately influenced our work" |
Kurumboor 2012.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: India
Number randomised: 45
Post‐randomisation drop‐outs: not stated
Revised sample size: 45
Average age: not stated
Females: not stated
Malignancy: not stated
Chronic pancreatitis: not stated
Pancreatoduodenectomy: not stated
Inclusion criteria: 1. People with soft pancreas undergoing pancreaticoduodenectomy |
|
| Interventions | Participants were randomly assigned to 2 groups Group 1: somatostatin analogue (n = 24) Further details: octreotide 100 μg sc Group 2: control (n = 21) Further details: no intervention | |
| Outcomes | The outcomes reported were pancreatic fistula, overall complications and hospital stay | |
| Notes | Attempts were made to contact the authors in February 2013 Pancreatic fistula definition: grade A, grade B and grade C reported separately |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Unclear risk | Comment: some important outcomes which will generally be assessed were not reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Lange 1992.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: USA
Sample size: 21
Post‐randomisation drop‐out: not stated
Revised sample size: 21
Females: 10 (47.6%)
Mean age: 46.5 years
Malignancy: (100%)
Chronic pancreatitis: (0%)
Pancreatoduodenectomy: not stated
Follow‐up: not reported Inclusion criteria: 1. Pancreatic neuroendocrine tumours Exclusion criteria: 1. Diabetes |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: octreotide (n = 10) Further details: 50 μg sc every 8 h for first day starting immediately after operation, 100 μg every 8 h on second day, and 150 μg every 8 h continued until 3 days after drain removal Group 2: placebo (n = 11) Further details: saline through the same route and duration | |
| Outcomes | The outcomes reported were mortality and perioperative morbidity | |
| Notes | Pancreatic fistula definition: recurrent pancreatic drainage (grade A) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "after the operation, patients with a pancreatic incision were randomized to receive either octreotide or saline in a double blinded manner" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes were not reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Matheus 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: Brazil Sample size: 35 Post‐randomisation drop‐out: not stated Revised sample size: 35 Females: not stated Mean age: not stated Malignancy: not stated Chronic pancreatitis: 0 (0%) Pancreatoduodenectomy: 35 (100%) Inclusion criteria: 1. People undergoing pancreaticoduodenectomy 2. Abdominal drain fluid containing amylase > 1000 IU/dL on the first postoperative day | |
| Interventions | The participants were randomised to 2 groups Group 1: somatostatin analogue (n = not stated) Further details: octreotide 100 μg sc 3 times daily for 10 days Group 2: control (n = not stated) | |
| Outcomes | The outcomes reported were proportion of participants with pancreatic fistula and hospital stay | |
| Notes | Pancreatic fistula definition: drain output of any measure volume of fluid on or after postoperative day 3 with amylase content greater than 3 times the serum amylase (grade A) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: blinding was probably not performed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes such as clinically significant pancreatic fistulae were not reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Montorsi 1995.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: Italy
Sample size: 278
Post‐randomisation drop‐out: 60
Revised sample size: 218
Females: 87 (39.9%)
Mean age: 58.2 years
Malignancy: 139 (63.8%)
Chronic pancreatitis: 18 (8.3%)
Pancreatoduodenectomy: 143 (65.6%)
Follow‐up: not reported Inclusion criteria: 1. Elective pancreatic resection for neoplastic or chronic inflammatory disease of the pancreas and the periampullary region 2. Aged 18‐75 years 3. ASA I or II Exclusion criteria: 1. People with ongoing acute pancreatitis 2. Treatment with octreotide or native somatostatin within the last 48 h |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: octreotide (n = 111) Further details: 100 μg sc 3 times daily for 7 days starting 1 h before operation Group 2: placebo (n = 107) Further details: equal volume, frequency and duration as intervention | |
| Outcomes | The outcomes reported were mortality and perioperative morbidity | |
| Notes | Reasons for post‐randomisation drop‐out: unresectable (n = 54); protocol violations (n = 6) Pancreatic fistula definition: amylase‐rich fluid (amylase more than 3 times normal serum concentration) collected from the peripancreatic abdominal drainage since the postoperative day 3, with a drainage volume of greater than 10 mL/day (grade A) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "octreotide or placebo was started in a double‐blind fashion, 1 hour before operation, as follows: 1 ml (100 mcg octreotide or placebo) every 8 hours subcutaneously for 7 days" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: post‐randomisation drop‐outs were related to the outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: important outcomes were reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Pederzoli 1994.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: Italy
Sample size: 303
Post‐randomisation drop‐out: 51
Revised sample size: 252
Females: 99 (39.3%)
Mean age: 53.1 years
Malignancy: 162 (64.3%)
Chronic pancreatitis: 90 (35.7%)
Pancreatoduodenectomy: 105 (41.7%)
Follow‐up: until discharge Inclusion criteria: 1. Adults undergoing elective pancreatic surgery Exclusion criteria: 1. Total pancreatectomy 2. Pancreatic transplantation 3. Pancreatic resection combined with substantial intestinal resection |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: octreotide (n = 122) Further details: 100 μg sc 3 times daily for 7 days starting at least 1 h preoperatively Group 2: placebo (n = 130) Further details: equal volume, frequency and duration as intervention | |
| Outcomes | The outcomes reported were mortality, perioperative morbidity and adverse effects of octreotide | |
| Notes | Reasons for post‐randomisation drop‐out: additional surgical procedures (n = 20); unresectable (n = 31) Pancreatic fistula definition: drain output of fluid with amylase content more than 3 times the maximum normal value exceeding 10 mL per 24 h for at least 4 days from day 4 after operation (grade A) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients received octreotide or placebo according to randomization lists balanced in blocks of four that had been drawn up separately for each centre and risk stratum" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients received octreotide or placebo according to randomization lists balanced in blocks of four that had been drawn up separately for each centre and risk stratum" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "on the day of surgery, and for 7 complete days after operation, all patients received octreotide 0.1 mg subcutaneously in 1 ml ampoules or 1 ml placebo under double‐blind conditions" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: post‐randomisation drop‐outs were related to the outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all pre‐defined outcomes were reported |
| Free of source of funding bias? | High risk | Quote: "this study was supported by a grant from Sandoz Prodotti Farmaceutici SPA, Milan, Italy" Comment: sponsored by entity with no vested interest in results |
Sarr 2003.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: USA
Sample size: 381
Post‐randomisation drop‐out: 106
Revised sample size: 275
Females: 95 (34.5%)
Mean age: 62 years
Malignancy: 138 (50.2%)
Chronic pancreatitis: 0 (0%)
Pancreatoduodenectomy: 108 (39.3%)
Follow‐up: 30 days Inclusion criteria: 1. People undergoing an elective, anatomic, proximal or central pancreatectomy with a pancreaticoenteric anastomosis or a distal pancreatectomy with or without a pancreaticoenteric anastomosis 2. At least 18 years of age 3. An elective pancreatic resection because of a presumed pancreatic or periampullary neoplasm Exclusion criteria: 1. Chronic pancreatitis 2. Emergency surgery 3. Total pancreatectomy 4. Duct‐drainage procedures alone without any pancreatic resection 5. Enucleation of neoplasm 6. Enteric drainage of a pancreatic pseudocyst 7. Serum creatinine of twice the local upper limit of normal 8. Serum bilirubin > 20 mg/dL 9. Administration of a somatostatin analogue within the previous month |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: vapreotide (n = 135) Further details: 0.6 mg sc 2 h before operation and then twice daily for 7 days postoperatively Group 2: placebo (n = 140) Further details: same times as intervention | |
| Outcomes | The outcomes reported were mortality and perioperative morbidity | |
| Notes | Reasons for post‐randomisation drop‐out: did not undergo pancreatic resection Pancreatic fistula definition: drainage fluid (beginning on or after postoperative day 5) of both > 30 mL/day and amylase or lipase activity > 5‐fold upper limits of the normal serum value (grade A) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "patients received vapreotide acetate (0.6 mg) or placebo subcutaneously within up to 2 hours before operation and then twice daily for 7 days postoperatively" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "patients not resected were replaced" Comment: this would have necessitated exclusion of the trial if there was no blinding (as replacing recruited patients with other patients introduces selection bias). However, we have treated the patients who were replaced as drop‐outs because of adequate blinding |
| Selective reporting (reporting bias) | High risk | Comment: all pre‐defined outcomes were not reported |
| Free of source of funding bias? | Low risk | Quote: "we would like to thank J‐M Dumont and K Besseghir from Debiopharm SA for their guidance and financial support" Comment: in spite of obtaining financial support from a pharmaceutical company, the authors of this report do not support the use of the drug |
Shan 2005.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: Taiwan
Sample size: 60
Post‐randomisation drop‐out: 6
Revised sample size: 54
Females: 26 (48.1%)
Mean age: 67 years
Malignancy: 45 (83.3%)
Chronic pancreatitis: 0 (0%)
Pancreatoduodenectomy: 54 (100%)
Follow‐up: 60 days Inclusion criteria: 1. People undergoing elective pancreaticoduodenectomy for pancreatic and periampullary lesions and extrapancreatic solid neoplasms involving the duodenum were also included in this study 2. Age more than 16 years 3. ASA I or II |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: somatostatin (n = 27) Further details: intravenous 250 μg/h for 7 days starting after operation Group 2: placebo (n = 27) Further details: normal saline | |
| Outcomes | The outcomes reported were mortality, perioperative morbidity and hospital stay | |
| Notes | Reasons for post‐randomisation drop‐out: direct anastomosis of pancreatic duct to the jejunum (n = 3); obstructive pulmonary disease (n = 1); whole gastrointestinal tract MALToma (n = 1) and pancreatitis in the pancreatic head area (n = 1) Pancreatic fistula definition: amylase‐rich fluid with drain fluid volume greater than 10 mL/day, persistent elevation of the drain amylase level and 3 times higher than the serum level for longer than 7 days (grade A) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients meeting the inclusion criteria were randomized by a random number table before operation to receive either prophylactic somatostatin treatment or placebo" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: further details of placebo were not available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: post‐randomisation drop‐outs were related to the outcomes |
| Selective reporting (reporting bias) | High risk | Comment: all pre‐defined outcomes were not reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Suc 2004.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: France
Sample size: 230
Post‐randomisation drop‐out: 0
Revised sample size: 230
Females: 99 (43%)
Mean age: 56.5 years
Malignancy: 154 (67%)
Chronic pancreatitis: 30 (13%)
Pancreatoduodenectomy: 177 (77%)
Follow‐up: not reported Inclusion criteria: 1. Pancreatic resection was performed for either malignant or benign disease 2. Age more than 18 years Exclusion criteria: 1. Resection for acute pancreatitis or trauma 2. Simple tumour excision 3. Total or central pancreatectomy 4. Pancreatoduodenectomy without immediate pancreatodigestive anastomosis 5. Duodenum‐preserving pancreatectomy |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: octreotide (n = 122) Further details: 100 μg sc during the operation every 8 h for 10 days Group 2: control (n = 108) Further details: no treatment | |
| Outcomes | The outcomes reported were mortality, re‐operation, perioperative morbidity and hospital stay | |
| Notes | Pancreatic fistula definition: either chemically as fluid obtained through drains or percutaneous aspiration containing at least 4 times normal serum values of amylase for 3 days, irrespective of the amount of output and the date of appearance or clinically and radiologically as anastomotic leaks (type A, B, C ‐ not available separately) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "allotment to octreotide or not, as generated by computerized random number tables, was decided by a telephone call to the coordinating center which collected and processed all data" |
| Allocation concealment (selection bias) | Low risk | Quote: "allotment to octreotide or not, as generated by computerized random number tables, was decided by a telephone call to the coordinating center which collected and processed all data" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "patients were not aware of the treatment arm to which they were allotted (octreotide injection was considered as part of postoperative treatment), but the surgeon performing the operation, obviously, was (single‐blind study without placebo). Complications, however, were assessed by a physician who was unaware of the allotted treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: all pre‐defined outcomes were reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Tulassay 1993.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: Hungary
Sample size: 33
Post‐randomisation drop‐out: not stated
Revised sample size: 33
Females: 5 (15.2%)
Mean age: 43 years
Malignancy: 0 (0%)
Chronic pancreatitis: 14 (42.4%)
Pancreatoduodenectomy: 0 (0%)
Follow‐up: not reported Inclusion criteria: 1. Abdominal surgery because of chronic pancreatitis or its complications |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: somatostatin (n = 15) Further details: 125 μg/h continuous infusion started 12 h before surgery and continued for 48 h Group 2: placebo (n = 18) Further details: normal saline | |
| Outcomes | The outcomes reported were postoperative morbidity | |
| Notes | Pancreatic fistula definition: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "one group of 15 patients received somatostatin perioperatively while the control group received a placebo of physiologic saline in continuous infusion"; "the study was carried out in a double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes were not reported |
| Free of source of funding bias? | Unclear risk | Comment: this information was not available |
Yeo 2000.
| Methods | Randomised clinical trial (parallel design) | |
| Participants | Country: USA
Sample size: 383
Post‐randomisation drop‐out: 172
Revised sample size: 211
Females: 100 (47.4%)
Mean age: 64.7 years
Malignancy: 147 (69.7%)
Chronic pancreatitis: 22 (10.4%)
Pancreatoduodenectomy: 211 (100%)
Follow‐up: not reported Inclusion criteria: 1. Anticipated elective pancreaticoduodenal resection Exclusion criteria: 1. Did not undergo pancreatoduodenectomy 2. Total pancreatectomy |
|
| Interventions | The participants were randomly assigned to 2 groups Group 1: octreotide (n = 107) Further details: 100 μg sc 3 times daily for 7 days starting at least 1 h preoperatively Group 2: placebo (n = 104) Further details: equal volume, frequency and duration as intervention | |
| Outcomes | The outcomes reported were mortality, perioperative morbidity, adverse effects of octreotide and hospital stay | |
| Notes | Reasons for post‐randomisation drop‐out: did not undergo pancreaticoduodenectomy (n = 118); total pancreatectomy (n = 14); did not receive at least a 5‐day course of octreotide study drug (n = 40) Pancreatic fistula definition: drainage of greater than 50 mL amylase‐rich fluid (more than 3‐fold elevation above upper limit of normal in serum) per day through the surgically placed drains on or after postoperative day 10, or pancreatic anastomotic disruption demonstrated radiographically (grade A, B, C ‐ not available separately) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "enrolled patients (n = 383) were randomized before surgery to either the octreotide or the control group by means of a randomly generated number pattern" Comment: further details were not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "the octreotide and control saline placebo were prepared in the Investigational Drug Pharmacy and were identical in appearance, volume, and labelling (labelled as “octreotide study drug”), thereby masking the nursing staff, physicians, and patients to their contents" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "after enrolment and randomization, patients were excluded from the study for the following reasons: patient did not undergo pancreaticoduodenectomy (n = 118), patient underwent total pancreatectomy (n = 14), or patient did not receive at least a 5‐day course of octreotide study drug (n = 40)" Comment: post‐randomisation drop‐outs were related to the outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported |
| Free of source of funding bias? | Low risk | Quote: "supported in part by NIH grants RO1‐CA56130 and P50‐CA62924" Comment: sponsored by entity with no vested interest in results |
ASA: American Society of Anesthesiologists Physical Status Classification System; sc: subcutaneous; ITU: intensive treatment unit.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barnett 2004 | Not a randomised clinical trial |
| Bassi 1994 | Review |
| Brennan 2000 | Comment on an included trial |
| Büchler 2001 | Letter to editor about an included trial |
| Droeser 2013 | Not a randomised controlled trial |
| Elhadad 1998 | Unable to obtain this reference identified by reference searching |
| Falconi 2002 | Cross‐over trial |
| Frick 1996 | Letter to editor about an included trial |
| Friess 1989 | Not performed in people undergoing pancreatic surgery |
| Friess 1996 | Review |
| Habib 1998 | Not a randomised clinical trial (the groups were divided according to whether the pancreas was friable or sclerosed) |
| Klempa 1979 | Not a randomised clinical trial |
| Lowy 1997 | Quasi‐randomised study (randomisation based on their number of medical record) |
| Shatverian 2004 | Not a randomised clinical trial |
| Van Berge 1997 | Performed on healthy volunteers and not on people who were about to undergo or had undergone pancreatic resection |
| Van Hee 1998 | Not a randomised clinical trial |
| Wang 2012 | The control group received somatostatin analogue but different regimens were used between intervention and control |
| Woltering 2003 | Comment on an included trial |
| Yeo 1999 | Editorial |
Differences between protocol and review
We have revised the protocol as follows.
The importance of the outcomes has been changed. We have stated primary outcomes as perioperative mortality and re‐operation. The other postoperative complications have been changed to the secondary outcomes.
We have excluded time to fistula closure in people with pancreatic leaks/fistula as one of the outcomes. This is because of the lack of universal definition of pancreatic fistula (grade A fistula is not clinically significant ‐ Bassi 2005) and consider that this outcome is not a significant patient‐oriented outcome.
We have restricted the electronic search to the four main databases, which results in the identification of more than 97% of the randomised clinical trials (Royle 2003).
Assessment of risk of bias in the trials and the statistical methods used have been revised according to the then current version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Differences between previous version and this version
The outcomes have been re‐ordered according to the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in the trials and the statistical methods used have been revised according to the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Numerous subgroup analysis on secondary outcomes have been removed since this will result in error because of the numerous repeated testing performed.
Contributions of authors
KS Gurusamy wrote the review, assessed the trials for inclusion and extracted data on included trials. R Koti wrote sections of the review, independently assessed the trials for inclusion and extracted data on included trials. K Fusai and BR Davidson provided advice for improving the review.
Sources of support
Internal sources
University College London, UK.
External sources
No sources of support supplied
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Beguiristain 1995 {published data only}
- Beguiristain A, Espi A, Balen E, Pardo F, Hernandez Lizoain JL, Alvarez Cienfuegos J. Somatostatin prophylaxis following cephalic duodenopancreatectomy. Revista Espanola de Enfermedades Digestivas 1995;87(3):221‐4. [PubMed] [Google Scholar]
Briceño Delgado 1998 {published data only}
- Briceño Delgado FJ, López Cillero P, Rufián Peña S, Solórzano Peck G, Miño Fugarolas G, Pera Madrazo C. Prospective and randomized study on the usefulness of octreotide in the prevention of complications after cephalic duodeno‐pancreatectomy. Revista Espanola de Enfermedades Digestivas 1998;90(10):687‐94. [PubMed] [Google Scholar]
Buccoliero 1992 {published and unpublished data}
- Buccoliero F, Pansini GC, Mascoli F, Mari C, Donini A, Navarra G. Somatostatin in duodenocephalopancreatectomy for neoplastic pathology. Minerva Chirurgica 1992;47(8):713‐6. [PubMed] [Google Scholar]
Buchler 1992 {published data only}
- Buchler M, Friess H. Inhibition of pancreatic secretion to prevent postoperative complications following pancreatic resection. Acta Gastroenterologica Belgica 1993; Vol. 56, issue 3‐4:271‐8. [PubMed]
- Buchler M, Friess H. Prevention of postoperative complications following pancreatic surgery. Digestion 1993;54(Suppl 1):41‐6. [DOI] [PubMed] [Google Scholar]
- Buchler M, Friess H, Klempa I, Hermanek P, Sulkowski U, Becker H, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. American Journal of Surgery 1992;163(1):125‐31. [DOI] [PubMed] [Google Scholar]
- Friess H, Klempa I, Hermanek P, Sulkowski U, Uhl W, Beger HG, et al. Prophylaxis of complications after pancreatic surgery: results of a multicenter trial in Germany. Digestion 1994; Vol. 55, issue Suppl 1:35‐40. [DOI] [PubMed]
Fernandez‐Cruz 2012 {published data only}
- Fernandez‐Cruz L, Chavarria EJ, Taura P, Vallejos R, Lopez‐Boado MA, Ferrer J. Prospective, randomized trial of the effect of octreotide on pancreatic juice output after pancreatoduodenectomy in relation to histological diagnosis, duct size and leakage. HPB 2012;14:91‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friess 1995 {published data only}
- Buchler MM, Gaus W. Randomized controlled multicentre study of the prevention of complications by octreotide in patients undergoing surgery for chronic pancreatitis. authors' reply. British Journal of Surgery 1996; Vol. 83, issue 3:422‐3. [DOI] [PubMed]
- Friess H, Beger HG, Sulkowski U, Becker H, Hofbauer B, Dennler HJ, et al. Randomized controlled multicentre study of the prevention of complications by octreotide in patients undergoing surgery for chronic pancreatitis. British Journal of Surgery 1995;82(9):1270‐3. [DOI] [PubMed] [Google Scholar]
- Friess H, Beger HG, Sulkowski U, Becker H, Hofbauer B, Dennler HJ, et al. Randomized controlled multicentre study of the prevention of complications by octreotide in patients undergoing surgery for chronic pancreatitis (vol 82, pg 1270, 1995). British Journal of Surgery 1996; Vol. 83, issue 1:126. [DOI] [PubMed]
- Friess H, Hofbauer B, Buchler MW. The role of somatostatin and octreotide in pancreatic surgery and in acute and chronic pancreatitis. Digestive Surgery 1994;11(3‐6):445‐50. [Google Scholar]
Gouillat 2001 {published and unpublished data}
- Gouillat C, Chipponi J, Baulieux J, Partensky C, Saric J, Gayet B. Randomized controlled multicentre trial of somatostatin infusion after pancreaticoduodenectomy. British Journal of Surgery 2001;88(11):1456‐62. [DOI] [PubMed] [Google Scholar]
- Gouillat C, Faucheron JL, Balique JG, Gayet B, Saric J, Partensky C, et al. Natural history of the pancreatic stump after duodenopancreatectomy of the pancreatic head. Annales de Chirurgie 2002;127(6):467‐76. [DOI] [PubMed] [Google Scholar]
Hesse 2005 {published data only}
- Hesse UJ, Decker C, Houtmeyers P, Demetter P, Ceelen W, Pattyn P, et al. Prospectively randomized trial using perioperative low dose octreotide to prevent organ related and general complications following pancreatic surgery and pancreatico‐jejunostomy. Acta Chirurgica Belgica 2005; Vol. 105, issue 4:383‐7. [DOI] [PubMed]
- Hesse UJ, Decker C, Houtmeyers P, Demetter P, Ceelen W, Pattyn P, et al. Prospectively randomized trial using perioperative low‐dose octreotide to prevent organ‐related and general complications after pancreatic surgery and pancreatico‐jejunostomy. World Journal of Surgery 2005;29(10):1325‐8. [DOI] [PubMed] [Google Scholar]
Katsourakis 2010 {published data only}
- Katsourakis A, Oikonomou L, Chatzitheoklitos E, Noussios G, Pitiakoudis M, Polychronidis A, et al. The role of somatostatin in 67 consecutive pancreatectomies: a randomized clinical trial. Clinical and Experimental Gastroenterology 2010;3(1):179‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsourakis A, Zezos P, Noussios G, Kouklakis G, Chatzitheoklitos E, Simopoulos K, et al. Does prophylactic administration of somatostatin decrease the rates of complications after pancreatic resection?: a clinical and electron microscopy study. Pancreas 2013;42(1):37‐41. [DOI] [PubMed] [Google Scholar]
Klempa 1991 {published data only}
- Klempa I, Baca I, Menzel J, Schuszdiarra V. Effect of somatostatin on basal and stimulated exocrine pancreatic secretion after partial duodenopancreatectomy. A clinical experimental study. Der Chirurg 1991; Vol. 62, issue 4:293‐9. [PubMed]
Kollmar 2008 {published and unpublished data}
- Kollmar O, Moussavian MR, Richter S, Roi P, Maurer CA, Schilling MK. Prophylactic octreotide and delayed gastric emptying after pancreaticoduodenectomy: results of a prospective randomized double‐blinded placebo‐controlled trial. European Journal of Surgical Oncology 2008;34(8):868‐75. [DOI] [PubMed] [Google Scholar]
Kurumboor 2012 {published data only}
- Kurumboor P, Kamalesh NP, Pramil K, Vipin IS, Shylesh A, Varma D, et al. A prospective randomized controlled trial on use of octreotide in patients with soft pancreas undergoing pancreaticoduodenectomy: interim analysis. HPB 2012;14:225. [Google Scholar]
Lange 1992 {published data only}
- Lange JR, Steinberg SM, Doherty GM, Langstein HN, White DE, Shawker TH, et al. A randomized, prospective trial of postoperative somatostatin analogue in patients with neuroendocrine tumors of the pancreas. Surgery 1992;112(6):1033‐8. [PubMed] [Google Scholar]
Matheus 2009 {published data only}
- Matheus A, Montagnini A, Abe E, Haddad L, Penteado S, Abdo E, et al. Octreotide in patients with high risk to develop pancreatic fistula after pancreaticoduodenectomy: a prospective randomized trial. HPB 2010;12(Suppl S1):218‐9. [Google Scholar]
- Matheus AS, Montagnini AL, Jukemura J, Abe ES, Penteado S, Haddad LB, et al. Octreotide in patients with high risk to develop pancreatic fistula after pancreaticoduodenectomy ‐ a prospective randomized trial. European Journal of Surgical Oncology 2010;36(9):814. [Google Scholar]
- Matheus AS, Montagnini AL, Jukemura J, Perini MV, Haddad LB, Abe ES, et al. Does octreotide have any beneficial effect in patients with high risk to develop pancreatic fistula after pancreaticoduodenectomy: a prospective randomized trial. Gastroenterology 2009;136(5 Suppl 1):A‐881. [Google Scholar]
Montorsi 1995 {published data only}
- Montorsi M, Zago M, Mosca F, Capussotti L, Zotti E, Ribotta G, et al. Efficacy of octreotide in the prevention of pancreatic fistula after elective pancreatic resections: a prospective, controlled, randomized clinical trial. Surgery 1995;117(1):26‐31. [DOI] [PubMed] [Google Scholar]
Pederzoli 1994 {published data only}
- Bassi C, Falconi M, Lombardi D, Briani G, Vesentini S, Camboni MG, et al. Prophylaxis of complications after pancreatic surgery: results of a multicenter trial in Italy. Italian Study Group. Digestion 1994;55(Suppl 1):41‐7. [DOI] [PubMed] [Google Scholar]
- Pederzoli P, Bassi C, Falconi M, Camboni MG. Efficacy of octreotide in the prevention of complications of elective pancreatic surgery. British Journal of Surgery 1994;81(2):265‐9. [DOI] [PubMed] [Google Scholar]
Sarr 2003 {published data only}
- Ramos‐De la Medina A, Sarr MG. Somatostatin analogues in the prevention of pancreas‐related complications after pancreatic resection. Journal of Hepatobiliary Pancreatic Surgery 2006;13(3):190‐3. [DOI] [PubMed] [Google Scholar]
- Sarr MG. The potent somatostatin analogue vapreotide does not decrease pancreas‐specific complications after elective pancreatectomy: a prospective, multicenter, double‐blinded, randomized, placebo‐controlled trial. Journal of the American College of Surgeons 2003;196(4):556‐65. [DOI] [PubMed] [Google Scholar]
Shan 2005 {published data only}
- Shan YS, Sy ED, Lin PW. Role of somatostatin in the prevention of pancreatic stump‐related morbidity following elective pancreaticoduodenectomy in high‐risk patients and elimination of surgeon‐related factors: prospective, randomized, controlled trial. World Journal of Surgery 2003;27(6):709‐14. [DOI] [PubMed] [Google Scholar]
- Shan YS, Sy ED, Tsai ML, Tang LY, Li PS, Lin PW. Effects of somatostatin prophylaxis after pylorus‐preserving pancreaticoduodenectomy: increased delayed gastric emptying and reduced plasma motilin. World Journal of Surgery 2005;29(10):1319‐24. [DOI] [PubMed] [Google Scholar]
Suc 2004 {published data only}
- Oberlin P, Suc B, Pezet D, Fagniez PL, Hay JM. Prevention of intra‐abdominal complications after pancreatic resection by octreotide. A prospective, multicenter, randomized trial. Gastroenterology 2000;118(4):A463. [Google Scholar]
- Suc B, Msika S, Piccinini M, Fourtanier G, Hay JM, Flamant Y, et al. Octreotide in the prevention of intra‐abdominal complications following elective pancreatic resection: a prospective, multicenter randomized controlled trial. Archives of Surgery 2004;139(3):288‐94. [DOI] [PubMed] [Google Scholar]
Tulassay 1993 {published data only}
- Tulassay Z, Flautner L, Fehervari I, Sandor Z, Nemeth J. Somatostatin in the prevention of postoperative increase of pancreatic enzyme after pancreatic surgery. Orvosi Hetilap 1992;133(13):777‐80. [PubMed] [Google Scholar]
- Tulassay Z, Flautner L, Sandor Z, Fehervari I. Perioperative use of somatostatin in pancreatic surgery. Acta Biomedica Ateneo Parmense 1993;64(5‐6):205‐11. [PubMed] [Google Scholar]
Yeo 2000 {published data only}
- Yeo CJ, Cameron JL, Lillemoe KD, Sauter PK, Coleman J, Sohn TA, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo‐controlled trial. Annals of Surgery 2000;232(3):419‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Barnett 2004 {published data only}
- Barnett SP, Hodul PJ, Creech S, Pickleman J, Arahna GV. Octreotide does not prevent postoperative pancreatic fistula or mortality following pancreaticoduodenectomy. The American Surgeon 2004;70(3):222‐7. [PubMed] [Google Scholar]
Bassi 1994 {published data only}
- Bassi C, Falconi M, Pederzoli P. Role of somatostatin and somatostatin analogs in the treatment of gastrointestinal‐diseases ‐ prevention of complications after pancreatic surgery. Gut 1994;35:S20‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brennan 2000 {published data only}
- Brennan MF, Yeo CJ, Warshaw AL, O'Leary P, Warren KW, Way LW, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo‐controlled trial ‐ discussion. Annals of Surgery 2000;232(3):426‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Büchler 2001 {published data only}
- Büchler MW, Bassi C, Fingerhut A, Klempa I. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy?. Annals of Surgery 2001;234(2):262‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Droeser 2013 {published data only}
- Droeser RA, Jeanmonod P, Schuld J, Moussavian MR, Schilling MK, Kollmar O. Octreotide prophylaxis is not beneficial for biochemical activity and clinical severity of postoperative pancreatic fistula after pancreatic surgery. Digestive Surgery 2013;29(6):484‐91. [DOI] [PubMed] [Google Scholar]
Elhadad 1998 {published data only}
- Elhadad A, Rounanier G, Chippoini J. Octreotide in the prevention of surgical complications after pancreatectomy. A prospective randomised multicenter study. Hepatogastroenterology 1998;45(Suppl 2):152. [Google Scholar]
Falconi 2002 {published data only}
- Falconi M, Contro C, Ballabio M, Bassi C, Salvia R, Pederzoli P. Evaluation of lanreotide effects on human exocrine pancreatic secretion after a single dose: preliminary study. Digestive and Liver Disease 2002;34(2):127‐32. [DOI] [PubMed] [Google Scholar]
Frick 1996 {published data only}
- Frick T. Randomized controlled multicentre study of the prevention of complications by octreotide in patients undergoing surgery for chronic pancreatitis. British Journal of Surgery 1996;83(3):422‐3. [DOI] [PubMed] [Google Scholar]
Friess 1989 {published data only}
- Friess H, Buchler M, Meschenmoser L. Effect of octreotide (SMS 201‐995) on exocrine pancreatic function in man. Pancreas 1989;4:616. [Google Scholar]
Friess 1996 {published data only}
- Friess H, Buchler M. Efficacy of somatostatin and its analogues in pancreatic surgery and pancreatic disorders. Digestion 1996;57 Suppl 1:97‐102. [DOI] [PubMed] [Google Scholar]
Habib 1998 {published data only}
- Habib E, Elhadad A, Fourtanier G, Chipponi J, Faniez P L, Hay JM, et al. Octreotide in prevention of surgical complication after pancreatectomy. Annales de Chirurgie 1998;52(7):671‐2. [Google Scholar]
Klempa 1979 {published data only}
- Klempa I, Schwedes U, Usadel KH. Prevention of postoperative pancreatic complications following duodenopancreatectomy using somatostatin. Der Chirurg 1979;50(7):427‐31. [PubMed] [Google Scholar]
Lowy 1997 {published data only}
- Lowy AM, Lee JE, Davidson BS, Pisters PWT, Evans DB. The role of octreotide in the prevention of pancreatic fistula after pancreaticoduodenectomy for periampullary malignancy: a prospective randomized trial. Gastroenterology 1996;110(4):A1401. [Google Scholar]
- Lowy AM, Lee JE, Pisters PW, Davidson BS, Fenoglio CJ, Stanford P, et al. Prospective, randomized trial of octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Annals of Surgery 1997;226(5):632‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shatverian 2004 {published data only}
- Shatverian GA, Ratnikova NP, Mikhailov Iu E, Bedzhanian AL, Sidorov AN, Fisenko EP, et al. Role octreotide in prevention of complications after pancreatoduodenal resections [Rol' oktreotida v profilaktuke oslozhnenii posle pankreatoduodenal'nykh rezektsii]. Khirurgiia 2004;2:56‐60. [PubMed] [Google Scholar]
Van Berge 1997 {published data only}
- Berge Henegouvven MI, Gulik TM, Akkermans LMA, Jansen JBMJ, Gouma DJ. The effect of octreotide on gastric emptying at a dosage used to prevent complications after pancreatic surgery: a randomised, placebo controlled study in volunteers. Gut 1997;41(6):758‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Hee 1998 {published data only}
- Hee R, Laet I, Salgado R, Ysebaert D. The influence of somatostatin on postoperative outcome after elective pancreatic surgery. Acta Chirurgica Belgica 1998;98(2):62‐5. [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Tian BL, Su AP, Wang WG, Zhang Y. Does preoperative somatostatin administration decrease the rate of postoperative pancreatic fistula following pancreaticoduodenectomy? A randomized placebo‐controlled trial. Journal of Gastroenterology and Hepatology 2012;27 Suppl 5:359. [Google Scholar]
- Wang W, Tian B, Babu SR, Zhang Y, Yang M. Randomized, placebo‐controlled study of the efficacy of preoperative somatostatin administration in the prevention of postoperative complications following pancreaticoduodenectomy. Hepatogastroenterology 2012;60(123):e1. [DOI: 10.5754/hge12669] [DOI] [PubMed] [Google Scholar]
Woltering 2003 {published data only}
- Woltering EA. The potent somatostatin analogue vapreotide does not decrease pancreas‐specific complications after elective pancreatectomy: a prospective, multicenter, double‐blinded, randomized, placebo‐controlled trial ‐ invited commentary. Journal of the American College of Surgeons 2003;196(4):564‐5. [DOI] [PubMed] [Google Scholar]
Yeo 1999 {published data only}
- Yeo CJ. Does prophylactic octreotide benefit patients undergoing elective pancreatic resection?. Journal of Gastrointestinal Surgery 1999;3(3):223‐4. [DOI] [PubMed] [Google Scholar]
Additional references
Alexakis 2004
- Alexakis N, Halloran C, Raraty M, Ghaneh P, Sutton R, Neoptolemos JP. Current standards of surgery for pancreatic cancer. British Journal of Surgery 2004;91(11):1410‐27. [DOI] [PubMed] [Google Scholar]
Alghamdi 2007
- Alghamdi AA, Jawas AM, Hart RS. Use of octreotide for the prevention of pancreatic fistula after elective pancreatic surgery: a systematic review and meta‐analysis. Canadian Journal of Surgery 2007;50(6):459‐66. [PMC free article] [PubMed] [Google Scholar]
Bassi 2005
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138(1):8‐13. [DOI] [PubMed] [Google Scholar]
Clavien 2009
- Clavien PA, Barkun J, Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Annals of Surgery 2009;250(2):187‐96. [DOI] [PubMed] [Google Scholar]
Connor 2005
- Connor S, Alexakis N, Garden OJ, Leandros E, Bramis J, Wigmore SJ. Meta‐analysis of the value of somatostatin and its analogues in reducing complications associated with pancreatic surgery. British Journal of Surgery 2005;92(9):1059‐67. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dindo 2004
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forman 2009
- Forman D, Delaney B, Kuipers E, Malthaner R, Moayyedi P, Gardener E, et al. Cochrane Upper Gastrointestinal and Pancreatic Diseases Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2009, Issue 2. Art. No.: UPPERGI.
Gouma 2000
- Gouma DJ, Geenen RC, Gulik TM, Haan RJ, Wit LT, Busch OR, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Annals of Surgery 2000;232(6):786‐95. [PUBMED: 11088073] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2009
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collsboration, 2011. Available from www.cochrane‐handbook.org.
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lembcke 1987
- Lembcke B, Creutzfeldt W, Schleser S, Ebert R, Shaw C, Koop I. Effect of the somatostatin analogue sandostatin (SMS 201‐995) on gastrointestinal, pancreatic and biliary function and hormone release in normal men. Digestion 1987;36(2):108‐24. [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Mayo 2009
- Mayo SC, Austin DF, Sheppard BC, Mori M, Shipley DK, Billingsley KG. Evolving preoperative evaluation of patients with pancreatic cancer: does laparoscopy have a role in the current era?. Journal of the American College of Surgeons 2009;208(1):87‐95. [DOI] [PubMed] [Google Scholar]
McKay 2006
- McKay A, Mackenzie S, Sutherland FR, Bathe OF, Doig C, Dort J, et al. Meta‐analysis of pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy. British Journal of Surgery 2006;93(8):929‐36. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Ohwada 1998
- Ohwada S, Ogawa T, Tanahashi Y, Nakamura S, Takeyoshi I, Ohya T, et al. Fibrin glue sandwich prevents pancreatic fistula following distal pancreatectomy. World Journal of Surgery 1998;22(5):494‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Sohn 2000
- Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas ‐ 616 patients: results, outcomes, and prognostic indicators. Journal of Gastrointestinal Surgery 2000;4(6):567‐79. [DOI] [PubMed] [Google Scholar]
StatsDirect 2.7.2 [Computer program]
- StatsDirect Ltd. StatsDirect statistical software. Version 2.7.2. StatsDirect Ltd, 06/09/2008.
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Gurusamy 2010
- Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD008370] [DOI] [PubMed] [Google Scholar]
Koti 2010
- Koti RS, Gurusamy KS, Fusai G, Davidson BR. Meta‐analysis of randomized controlled trials on the effectiveness of somatostatin analogues for pancreatic surgery: A Cochrane review. HPB 2010;12(3):155‐65. [DOI: 10.1111/j.1477-2574.2010.00157.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wente 2003
- Morris E, Wente MN, Seiler CM, Finch‐Jones M, Büchler MW, Alderson D. Somatostatin and its analogues in the prevention of complications following pancreatic surgery. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004521] [DOI] [Google Scholar]


