Abstract
Background
People with non‐cystic fibrosis bronchiectasis commonly experience chronic cough and sputum production, features that may be associated with progressive decline in clinical and functional status. Airway clearance techniques (ACTs) are often prescribed to facilitate expectoration of sputum from the lungs, but the efficacy of these techniques in a stable clinical state or during an acute exacerbation of bronchiectasis is unclear.
Objectives
Primary: to determine effects of ACTs on rates of acute exacerbation, incidence of hospitalisation and health‐related quality of life (HRQoL) in individuals with acute and stable bronchiectasis.
Secondary: to determine whether:
• ACTs are safe for individuals with acute and stable bronchiectasis; and
• ACTs have beneficial effects on physiology and symptoms in individuals with acute and stable bronchiectasis.
Search methods
We searched the Cochrane Airways Group Specialised Register of trials from inception to November 2015 and PEDro in March 2015, and we handsearched relevant journals.
Selection criteria
Randomised controlled parallel and cross‐over trials that compared an ACT versus no treatment, sham ACT or directed coughing in participants with bronchiectasis.
Data collection and analysis
We used standard methodological procedures as expected by The Cochrane Collaboration.
Main results
Seven studies involving 105 participants met the inclusion criteria of this review, six of which were cross‐over in design. Six studies included adults with stable bronchiectasis; the other study examined clinically stable children with bronchiectasis. Three studies provided single treatment sessions, two lasted 15 to 21 days and two were longer‐term studies. Interventions varied; some control groups received a sham intervention and others were inactive. The methodological quality of these studies was variable, with most studies failing to use concealed allocation for group assignment and with absence of blinding of participants and personnel for outcome measure assessment. Heterogeneity between studies precluded inclusion of these data in the meta‐analysis; the review is therefore narrative.
One study including 20 adults that compared an airway oscillatory device versus no treatment found no significant difference in the number of exacerbations at 12 weeks (low‐quality evidence). Data were not available for assessment of the impact of ACTs on time to exacerbation, duration or incidence of hospitalisation or total number of hospitalised days. The same study reported clinically significant improvements in HRQoL on both disease‐specific and cough‐related measures. The median difference in the change in total St George's Respiratory Questionnaire (SGRQ) score over three months in this study was 7.5 units (P value = 0.005 (Wilcoxon)). Treatment consisting of high‐frequency chest wall oscillation (HFCWO) or a mix of ACTs prescribed for 15 days significantly improved HRQoL when compared with no treatment (low‐quality evidence). Two studies reported mean increases in sputum expectoration with airway oscillatory devices in the short term of 8.4 mL (95% confidence interval (CI) 3.4 to 13.4 mL) and in the long term of 3 mL (P value = 0.02). HFCWO improved forced expiratory volume in one second (FEV1) by 156 mL and forced vital capacity (FVC) by 229.1 mL when applied for 15 days, but other types of ACTs showed no effect on dynamic lung volumes. Two studies reported a reduction in pulmonary hyperinflation among adults with non‐positive expiratory pressure (PEP) ACTs (difference in functional residual capacity (FRC) of 19%, P value < 0.05; difference in total lung capacity (TLC) of 703 mL, P value = 0.02) and with airway oscillatory devices (difference in FRC of 30%, P value < 0.05) compared with no ACTs. Low‐quality evidence suggests that ACTs (HFCWO, airway oscillatory devices or a mix of ACTs) reduce symptoms of breathlessness and cough and improve ease of sputum expectoration compared with no treatment (P value < 0.05). ACTs had no effect on gas exchange, and no studies reported effects of antibiotic usage. Among studies exploring airway oscillating devices, investigators reported no adverse events.
Authors' conclusions
ACTs appear to be safe for individuals (adults and children) with stable bronchiectasis and may account for improvements in sputum expectoration, selected measures of lung function, symptoms and HRQoL. The role of these techniques in acute exacerbation of bronchiectasis is unknown. In view of the chronic nature of bronchiectasis, additional data are needed to establish the short‐term and long‐term clinical value of ACTs for patient‐important outcomes and for long‐term clinical parameters that impact disease progression in individuals with stable bronchiectasis, allowing further guidance on prescription of specific ACTs for people with bronchiectasis.
Plain language summary
Airway clearance techniques in bronchiectasis
Bottom line: We reviewed the evidence to determine whether airway clearance techniques (ACTs) are helpful for people with bronchiectasis. The sparse data that we found suggest that their effect on the number of exacerbations (flare‐ups) is unknown. Airway clearance techniques seem to be safe, and two small studies indicate that they may improve quality of life. Airway clearance techniques also led to clearance of more mucus from the lungs and some improvement in defined measures of lung function, but no change in oxygenation. A reduction in symptoms of breathlessness, cough and mucus was found in one study. Other outcomes of interest were hospitalisation and prescription of antibiotics, but these were not yet reported. Overall, the impact of ACTs on individuals experiencing chest infection is unknown. On the basis of this information, current guidelines for treating bronchiectasis recommend routine assessment for ACTs and prescription as required.
What evidence did we find and how good was it? Seven studies included 105 people with bronchiectasis. Only two of these studies lasted for six months; the others were completed over a shorter time frame or involved a single treatment session. From these, it is difficult to know whether any improvement would be maintained over a longer term. The methods used to conduct these trials were not well reported; therefore, we believe that overall the evidence was of low quality. Three studies were funded by research institutions or governmental organisations; the other four studies did not report any funding.
What is bronchiectasis? Bronchiectasis is a lung condition in which the airways become abnormally widened, leading to a build‐up of excess mucus. People with bronchiectasis frequently report symptoms of cough, excessive mucus production and breathlessness and are at risk of chest infection.
What are airway clearance techniques? Physiotherapy treatment in the form of ACTs is often prescribed to help people clear mucus from their lungs.
Summary of findings
Summary of findings for the main comparison. Airway clearance techniques for individuals with stable bronchiectasis.
| ACTs for individuals with stable bronchiectasis | ||||||
|
Patient or population: individuals with stable bronchiectasis
Settings: hospital (outpatient department)
Intervention: airway clearance techniques (ACTs) Control: no intervention, sham intervention (placebo) or coughing alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Airway clearance techniques (ACTs) | |||||
| Number of exacerbations of bronchiectasis Frequency of acute exacerbations of bronchiectasis Follow‐up: mean 3 months | 35 per 100a | 25 per 100 (8 to 79)a | RR 0.71 (0.23 to 2.25) | 20 (1 study) | ⊕⊕⊝⊝ lowb,c | Duration of each intervention was 3 months of PEP‐based ACT |
| Hospitalisations | See comment | See comment | See comment | See comment | See comment | Not reported |
| Health‐related quality of life (disease‐specific) Scale from 0 to 100; lower score indicates better HRQoL. SGRQ total score consists of weighted scores from 3 domains Follow‐up: mean 3 months | Median health‐related quality of life (disease‐specific) in control groups was ‐0.7 pointsa | Median health‐related quality of life (disease‐specific) in intervention groups was 7.5 lowera | 20 (1 study) | ⊕⊕⊝⊝ lowb,d | Lower score post intervention was favourable, indicating improvement in HRQoL | |
|
Health‐related quality of life (cough‐specific)
Leicester Cough Questionnaire Scale from 0 to 133; higher score indicates better HRQoL. Contains 19 questions from 3 domains on a Likert scale Follow‐up: mean 3 months |
Median HRQoL (cough‐specific) in control groups was 0.0 pointsa | Median HRQoL (cough‐specific) in intervention groups was 1.3 highera | 20 (1 study) | ⊕⊕⊝⊝ lowb,e | Higher score post intervention was favourable, indicating improvement in HRQoL cough‐related | |
|
Health‐related quality of life (health status) COPD Assessment Tool. Scale from 0 to 5; higher score indicates worse HRQoL Contains 10 questions with 5‐point Likert scale Follow‐up: mean 15 days |
Mean health status score in control group was 9.9 points | Mean health status score in intervention groups was 14.8 points lower (11.6 to 18 points lower) | MD ‐14.8 (95% CI ‐18.0 to ‐11.6) | 30 (1 study) | ⊕⊕⊝⊝ lowb,e |
Two interventions (high‐frequency chest wall oscillation and mixed ACTs) compared with control. Lower score post intervention was favourable, indicating improvement in health status |
|
Respiratory symptoms (symptoms) Breathlessness, Cough and Sputum Scale Scale from 0 to 12. Lower score indicates fewer symptoms Follow‐up: mean 15 days |
Mean respiratory symptom score was 3.1 points | Mean respiratory symptom score in intervention groups was 4.4 points lower (3.2 to 5.5 points lower) | MD ‐4.4 (95% CI ‐5.5 to ‐3.2) | 30 (1 study) | ⊕⊕⊝⊝ lowb,e |
Two interventions (high‐frequency chest wall oscillation and mixed ACTs) compared with control |
| Adverse events (AEs) | See comment | See comment | See comment | See comment | See comment | 2 studies reported no AEs related to use of PEP‐based ACTs. 2 studies reported no AEs |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACTs: airway clearance techniques; CI: confidence interval; HRQoL: health‐related quality of life; PEP: positive expiratory pressure; RR: risk ratio; SGRQ: St George's Respiratory Questionnaire. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aStudy was a randomised cross‐over trial bAbsence of allocation concealment; participants and therapists unblinded (risk of bias ‐1) cSmall number of participants and few events evident (imprecision ‐1) dTwo studies assessed this outcome; only 1 study provided sufficient data for pooling (imprecision ‐1)
eSmall number of participants (imprecision ‐1)
Background
People with non‐cystic fibrosis (non‐CF) bronchiectasis commonly cough up mucus, have low fitness levels and are at risk of chest infection.. The optimal treatment for reducing these symptoms is unknown.
Description of the condition
Non‐CF bronchiectasis is a chronic and progressive respiratory condition characterised by irreversible and abnormal dilation of the bronchial lumen (Barker 2002; Chang 2008). Multiple causes of bronchiectasis range from immunological disorders to systemic and other respiratory conditions (McShane 2013). Although the global prevalence of this condition is not well described, it remains a major source of morbidity, with increased hospitalisation and medical therapy (AIHW 2005; King 2005; King 2006a; Weycker 2005), and the mortality rate has been reported to range from 10% to 16% over a four‐year period (Goeminne 2012; Loebinger 2009; Onen 2007). Bronchiectasis commonly develops following an infective insult to the airways, often against a background of genetic susceptibility, and may affect both children and adults. Subsequent impairment of mucociliary clearance contributes to persistent presence of micro‐organisms in the bronchial tree and subsequent colonisation (Cole 1986; Neves 2011). This leads to chronic inflammation, which further disrupts mucociliary function, resulting in tissue damage and remodelling (Gaga 1998), and a cycle of infection and inflammation that is characterised by frequent acute exacerbations (Chang 2008; King 2005). Sputum retention can result in mucous plugs, which reduce the diameter of airways and contribute to airflow obstruction (McShane 2013; van der Schans 2002). A decline in respiratory function and frequent acute exacerbations are independent predictors of poor prognosis in bronchiectasis (Loebinger 2009; Martinez‐Garcia 2007). Clinically, this underlying pathophysiology is characterised by chronic cough with purulent sputum production as well as dyspnoea and fatigue ‐ symptoms that contribute to diminished health‐related quality of life (HRQoL) (King 2006b; Martinez‐Garcia 2005; Tsang 2009; Wilson 1997). This profile of sputum retention combined with airway damage lends support to the application of treatment techniques that seek to facilitate sputum removal (McShane 2013; Pasteur 2010).
Description of the intervention
Airway clearance techniques (ACTs) are non‐pharmacological interventions that facilitate removal of secretions from the lungs (McCool 2006). A myriad of ACTs are applied in clinical practice, including positioning, gravity‐assisted drainage, manual techniques, various breathing strategies, directed coughing, positive expiratory pressure (PEP) devices, airway oscillating devices and mechanical tools that are applied to the external chest wall. These ACTs may be used as isolated techniques or in combination.
How the intervention might work
The rationale by which ACTs may improve sputum clearance includes changes in lung volume, pulmonary pressure and expiratory flow; use of gravity; and the application of directly compressive or vibratory forces. These mechanisms may modify the viscoelastic properties of pulmonary secretions and may augment gas‐liquid interactions (Kim 1986; Kim 1987) and cilial beat frequency (Hansen 1994) to enhance sputum clearance. Limited evidence suggests that ACTs have a beneficial effect on sputum clearance (Bateman 1981; Sutton 1983; Sutton 1985) and sputum volume (Eaton 2007; Gallon 1991) in people with bronchiectasis. Whether the beneficial effects of ACTs extend to other outcomes, leading to fewer acute exacerbations, reduced incidence of hospitalisation and improved HRQoL in this population, remains unknown.
Why it is important to do this review
Current guidelines for non‐CF bronchiectasis advocate for prescription of ACTs, irrespective of clinical state (Chang 2015; Pasteur 2010). However, the clinical utility of ACTs in bronchiectasis is unclear (Tsang 2004). Previous research has reported conflicting results, for instance, gravity‐assisted drainage with or without breathing exercises improved transport of mucus and production of sputum in some studies (Eaton 2007; Gallon 1991; Kaminska 1988; Sutton 1983) but had no effect in others (Tsang 2003; van Henstrum 1988). The difficulty involved in determining efficacy of ACTs in bronchiectasis may be related to the heterogeneity of the disease, including variability in sputum volume, and in the extent of ventilatory defects (Tsang 2004). In addition, clinical status may be of significance, with response to treatment influenced by severity of respiratory symptoms, degree of inflammation and infection and extent of airflow obstruction. Finally, ACTs that apply PEP to the airways may have different physiological effects when compared with those that do not use positive pressure, such as augmentation of lung volumes (Garrard 1978) and prevention of early airway closure during expiration (Oberwaldner 1986).
This review has been conducted to summarise the results of literature evaluating the safety and efficacy of ACTs in people with acute and stable bronchiectasis, and to determine the effects of ACTs on rates of acute exacerbation, incidence of hospitalisation and HRQoL. This review is an update of a previous version analysing the effects of ACTs in bronchiectasis (Lee 2013), which originated from an earlier review conducted to analyse the effects of ACTs in both chronic obstructive pulmonary disease (COPD) and bronchiectasis (Jones 1998).
Objectives
Primary
To determine effects of ACTs on rates of acute exacerbation, incidence of hospitalisation and HRQoL in individuals with acute and stable bronchiectasis.
Secondary
To determine whether:
ACTs are safe for individuals with acute and stable bronchiectasis; and
ACTs have beneficial effects on physiology and symptoms among individuals with acute and stable bronchiectasis.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials in which a prescribed ACT regimen was compared with no intervention, sham intervention or coughing alone in individuals with acute or stable bronchiectasis. Both parallel‐group and cross‐over designs were eligible for inclusion.
Types of participants
Adults and children with bronchiectasis of any origin, diagnosed according to the investigator's definition based on plain‐film chest radiography, bronchography, high‐resolution computed tomography (HRCT) or physician diagnosis of bronchiectasis, were included. No exclusions were based on age, gender or physiological status. Participants were considered to have an exacerbation of bronchiectasis if they had an exacerbation of symptoms (dyspnoea, increased cough or sputum production) requiring medical treatment, including hospitalisation. Participants were considered to have stable bronchiectasis if they were free from an exacerbation requiring medical treatment for a period of four weeks (O'Donnell 1998), or as defined by investigators. We planned to analyse studies involving participants with acute bronchiectasis separately from studies involving participants with stable bronchiectasis; however, we identified no eligible studies of participants with acute bronchiectasis. We excluded studies of participants with bronchiectasis who also had documented evidence of COPD, chronic bronchitis, cystic fibrosis (CF) or asthma (baseline forced expiratory volume in one second (FEV1) > 15% reversibility in more than 50% of participants), or who were breathing via an artificial airway.
Types of interventions
Intervention: We considered any technique used with the primary intent of clearing sputum from the airways, including, but not restricted to, 'conventional techniques', breathing exercises, PEP or airway oscillating devices and mechanical devices, but excluding suctioning. We did not include inhalation therapy, as its mechanism of action for clearing mucus differs from that of ACTs. We included interventions consisting of a single short‐term (less than seven days) or long‐term (longer than seven days) treatment.
Control: This comprised no intervention, sham intervention (placebo) or coughing alone.
To be eligible for inclusion, a study had to compare an ACT versus a control condition. When multiple ACTs were investigated in a single study, data had to reflect independent comparisons of each ACT versus the control condition. We did not include studies comparing only one ACT versus another.
Types of outcome measures
Primary outcomes
Rate of, duration of or time to acute exacerbation of bronchiectasis, as defined by investigators. The minimal time over which data related to rates of acute exacerbation were obtained was one month, during which ACTs may have continued.
-
Incidence of hospitalisation for bronchiectasis:
for stable bronchiectasis ‐ time to hospitalisation, number of hospital admissions or hospital days; or
for exacerbation of acute bronchiectasis ‐ length of hospital stay, time to re‐admission, number of hospital admissions or hospital days.
Quality of life, as measured by a generic or disease‐specific HRQoL instrument.
Secondary outcomes
Pulmonary function (e.g. FEV1, forced vital capacity (FVC), FEV1/FVC, forced expiratory flow between 25% and 75% of vital capacity (FEF25‐75%), peak expiratory flow rate (PEFR), total lung capacity (TLC), residual capacity (RC), functional residual capacity (FRC)).
Gas exchange (e.g. blood oxygen saturation (SpO2), partial pressure of oxygen in the blood (PaO2), partial pressure of carbon dioxide in the blood (PaCO2)).
Symptoms (e.g. dyspnoea, cough), with all measures of symptoms eligible for inclusion.
Clearance and expectoration of mucus (e.g. mucociliary transport, sputum weight (dry and wet), sputum volume).
Days of antibiotic usage.
Adverse events (decline in lung function, desaturation, haemoptysis, arrhythmia, tachypnoea).
Mortality (all‐cause).
Participant withdrawal.
Search methods for identification of studies
Electronic searches
The previously published version pf this review included searches up to October 2012. The search period for this update is October 2012 through November 2015.
We identified studies using the Cochrane Airways Group Specialised Register of Trials (CAGR), which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and from handsearching of respiratory journals and meeting abstracts (see Appendix 1 for details of sources and search methods). We searched all records in the CAGR using the search strategy in Appendix 2, and the PEDro database using the terms provided in Appendix 3.
Both databases were searched from the period of their inception to November 2015 (CAGR) and again in March 2015 (PEDro), with no language or publication restriction.
Searching other resources
We handsearched the reference lists of all primary studies and review articles for additional references and reviewed all relevant conference abstracts to identify additional articles. We contacted the authors of identified trials and experts in the field to identify other published and unpublished studies when possible. We checked clinical trials registries for current studies and those that might have been recently completed.
Data collection and analysis
Selection of studies
Two review authors independently coded studies identified in the literature searches for relevance by examining titles, abstracts and keyword fields as follows.
INCLUDE: Study met all review criteria.
UNCLEAR: Study appeared to meet some review criteria, but available information was insufficient for categorical determination of relevance; or
EXCLUDE: Study did not categorically meet all review criteria.
Two review authors (AL, AB) used a full‐text copy of studies in categories INCLUDE and UNCLEAR to decide on study inclusion. We resolved disagreements by consensus and kept a full record of decisions for calculation of simple agreement and a kappa statistic.
Data extraction and management
Two review authors (AL, AB) independently extracted data using a prepared checklist. We compared the generated data and resolved discrepancies by consensus. One review author (AL) entered data into RevMan 5.1 (RevMan 2015) and performed random checks on accuracy. We contacted the authors of included studies to verify data extracted from their study when possible and to request details of missing data when applicable.
Assessment of risk of bias in included studies
Two review authors (AL, AB) conducted a 'Risk of bias' assessment in accordance with recommendations outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed risk of bias according to six domains of potential sources of bias (sequence generation, allocation concealment, blinding, loss to follow‐up, selective outcome reporting and other possible bias). We graded bias as low, high or unclear, and resolved discrepancies by consensus. We summarised results in a 'Risk of bias' table.
Measures of treatment effect
We summarised the findings of all included studies. For continuous variables, we recorded median or mean differences from baseline and post‐intervention values with interquartile ranges (IQRs) or standard deviations. When possible, for pooled analyses, we used RevMan 5.3 to calculate mean differences (MDs) for outcomes measured with the same metrics, or standardised mean differences (SMDs) for outcomes measured with different metrics, along with 95% confidence intervals (CIs).
Unit of analysis issues
We had intended to analyse exacerbations and hospitalisations as dichotomous (yes/no) or ratio (rate, frequency) data by analysing scores from instruments measuring quality of life and symptoms as continuous or ordinal data. As quantitative data were analysed using non‐parametric tests from cross‐over trials (Guimaraes 2012; Kurz 1997; Murray 2009; Sutton 1988), with results available in median (IQR), we could not undertake analysis using RevMan 2015. Therefore, we reported these results in a narrative format. For quantitative data from cross‐over trials that had been analysed by trial authors using parametric tests (Figueiredo 2010; Svenningsen 2013), we performed analysis using the generic inverse variance method in RevMan 2015 when data were available in an appropriate form, with the first arm used for analysis. For cross‐over trials, we calculated paired MDs between interventions and their standard errors (SEs) by using MDs between interventions and their SDs, or by using P values reported in the manuscript.
Dealing with missing data
We contacted the authors of studies with missing data and asked them to provide data if possible.
Assessment of heterogeneity
If we are able to include sufficient data, we will assess heterogeneity within each outcome between comparisons by calculating I2 statistics.
Assessment of reporting biases
We intended to use visual inspection of funnel plots to assess publication bias; however, the number of included trials was too small (10 or more studies are needed).
Data synthesis
We intended to analyse data from studies on exacerbations of acute bronchiectasis separately from data derived from studies of stable bronchiectasis; however, we identified no studies pertaining to people with an acute exacerbation of bronchiectasis. We reported studies according to the duration of post‐intervention follow‐up, as follows: 'immediate' (within 24 hours) and 'long‐term' (greater than 24 hours). Within each participant group, we pooled data that were both clinically and statistically homogeneous by using a fixed‐effect model. We pooled data that were clinically homogeneous but statistically heterogeneous by using a random‐effects model. We did not pool data that were clinically heterogeneous. We used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach to interpret findings (Langendam 2013; Schunemann 2008), and we used the GRADE profiler (GRADEPRO) to import data from Review Manager 5.3 (RevMan 2015) to create 'Summary of findings' tables. These tables provide outcome‐specific information related to the overall quality of evidence generated by studies included in the comparison and the sum of available data on the outcomes considered. We included the following outcomes in the 'Summary of findings' tables: numbers of exacerbations, hospitalisations, HRQoL (disease‐specific, generic and cough‐related), symptoms and adverse events. We calculated risk ratios by using GRADEPRO. For assessments of the quality of evidence for each outcome, we downgraded evidence from 'high quality' by one level for serious study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Subgroup analysis and investigation of heterogeneity
We planned to conduct one subgroup analysis, specified a priori, to identify potential influences on pooled results.
PEP devices: ACTs using PEP may have differing physiological effects and outcomes compared with ACTs not using PEP.
Sensitivity analysis
We planned to perform a sensitivity analysis to analyse the effects of allocation concealment, assessor blinding and intention‐to‐treat analysis on results. However, insufficient numbers of included studies precluded this analysis. We will perform sensitivity analyses in future updates if additional trials become available.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification for complete details.
Results of the search
In the original version of this review, review authors identified 63 records through the initial database search, and an additional three records through review of reference lists and clinical trial registries. From these studies, they retrieved nine full‐text papers for further review. Agreement on these full‐text papers between review authors had a kappa = 0.97, indicating excellent agreement. The original review included five studies. The 2015 updated search of databases yielded 91 references (CAGR and PEDro databases) and an additional 13 studies obtained from clinical trial registries of potential studies, yielding 104 studies. After discarding duplicates, we included a total of 96 studies. We made attempts to contact the authors of two studies rated 'unclear' to determine their suitability for inclusion in the review, and obtained two responses. We excluded 83 studies on the basis of title and abstract, including three studies awaiting classification and nine that were ongoing; we could not included these in the analysis. We assessed 13 studies for eligibility via full‐text review. We excluded 11 studies, as they did not meet the review criteria. Two studies from the updated search met the inclusion criteria. Review authors agreed on 12 out of 13 articles following full‐text review (92%), with kappa = 0.82, indicating substantial agreement. We included in this review a total of seven studies from the original and updated searches (Figure 1). Common reasons for exclusion were that studies did not include a control group (n = 29), did not undertake ACTs (n = 28) or included a mixed disease group (n = 9). We outlined full details of excluded studies and those awaiting classification in the Characteristics of excluded studies and Characteristics of studies awaiting classification tables.
1.
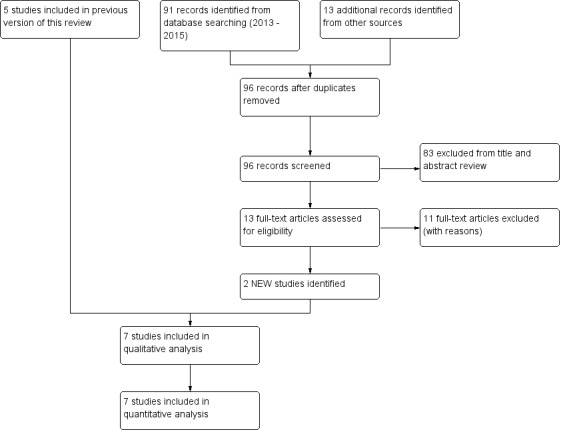
Study flow diagram.
Included studies
Refer to Characteristics of included studies.
Design
This review comprises six randomised cross‐over trials and one randomised controlled trial.
Participants
The seven included studies involved 102 participants and sample sizes ranging from eight to 37 participants. Six studies examined clinically stable adult participants (Figueiredo 2010; Guimaraes 2012; Murray 2009; Nicolini 2013; Sutton 1988; Svenningsen 2013), and one study focused on clinically stable children (Kurz 1997). Six studies diagnosed bronchiectasis on the basis of HRCT (Figueiredo 2010; Guimaraes 2012; Murray 2009; Nicolini 2013; Sutton 1988; Svenningsen 2013), and one study did not specify the diagnostic criteria used (Kurz 1997). Among adults, the age of participants ranged from 36 to 75 years in four studies (Murray 2009; Nicolini 2013; Sutton 1988; Svenningsen 2013), and between 47 and 56 years in two studies (Figueiredo 2010; Guimaraes 2012), and the age range of children with bronchiectasis was six to 16 years (Kurz 1997). Of the six studies that documented disease severity, lung function ranged from FEV1 of 1.2 L to 1.7 L (Nicolini 2013; Sutton 1988), 53% to 65% predicted (Figueiredo 2010; Guimaraes 2012) and 69% to 76% predicted (Murray 2009; Svenningsen 2013). Two studies commented that participants were naïve to the forms of airway clearance therapy included in the trial (Figueiredo 2010; Murray 2009).
Intervention
The duration of each intervention ranged from a single treatment in three studies (Figueiredo 2010; Guimaraes 2012; Sutton 1988) to longer‐term treatment over 15 to 21 days in two studies (Nicolini 2013; Svenningsen 2013) to a six‐month treatment duration, with 90 days (12 weeks) for each arm, in two other studies (Kurz 1997; Murray 2009). Three studies detailed the duration of the washout period between interventions, which ranged from one week in two short‐term studies (Figueiredo 2010; Guimaraes 2012) to one month in the longer‐term study (Murray 2009), with outcome measures collected only during the study, and no long‐term follow‐up beyond the intervention.
Two studies compared three techniques: Guimaraes 2012 compared airway oscillating devices versus slow expirations with the glottis open from the FRC to the residual volume (RV), performed in the lateral decubitus position, with the affected lung in the dependent position ('L'expiration Lente Totale Glotte Ouverte en Decubitus Lateral' (ELTGOL)) versus resting, and Nicolini 2013 examined high‐frequency chest wall oscillation (HFCWO) versus chest physiotherapy (gravity‐assisted drainage with slow expiration in the lateral decubitus position or PEP therapy or oscillating PEP therapy) versus usual medical care. One study compared gravity‐assisted drainage and forced expiratory technique (FET) versus resting (Sutton 1988). Two studies compared airway oscillating devices versus sham PEP, with sham therapy consisting of a flutter with the steel ball removed (Figueiredo 2010; Kurz 1997), with no modification made to the size of the orifice to minimise the opportunity of achieving therapeutic PEP (Tambascio 2011), and two studies examined the effects of airway oscillating devices versus a suitable control of standard care (Murray 2009; Svenningsen 2013).
It was not possible to pool data for meta‐analyses from studies because of the heterogeneity of study designs (differing types of interventions) and differing time frames and outcomes.
Excluded studies
We excluded a total of 75 studies. Most excluded studies compared different ACTs versus each other without including a suitable control, or used interventions that were not ACTs. Other reasons for exclusion were lack of randomisation, inclusion of mixed disease groups, for which data exclusively for participants with bronchiectasis were not available, and use of outcome measures that were not of interest for this review. Full details of reasons for exclusion are provided in the Characteristics of excluded studies table. Nine studies are ongoing (Characteristics of ongoing studies), and three are awaiting classification and will be assessed for eligibility in future updates of this review.
Risk of bias in included studies
We noted some variation in risk of bias across included studies. Some judgements were limited by inadequate reporting, which made determining the true quality of the study design difficult. Refer to the Characteristics of included studies table for full details of risk of bias across all studies, and to Figure 2 for a summary of our judgements on potential risks of bias across studies.
2.
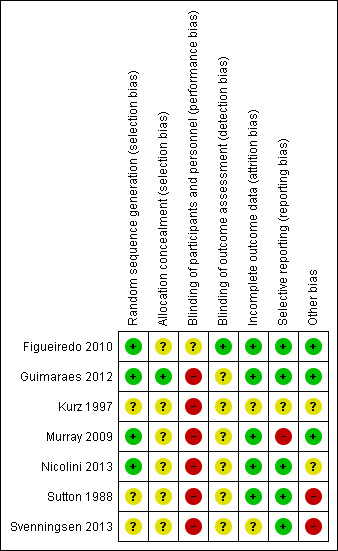
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four studies reported sufficient detail to confirm low risk of bias for randomisation sequence (Figueiredo 2010; Kurz 1997; Murray 2009; Nicolini 2013), and three studies were unclear (Guimaraes 2012; Sutton 1988; Svenningsen 2013). Only one study provided evidence of allocation concealment (Guimaraes 2012).
Blinding
All seven included studies were rated as having high risk of bias due to inadequate blinding of participants. Two studies attempted to blind participants to knowledge of the intervention by using a sham ACT (Figueiredo 2010; Kurz 1997); however, this did not eliminate the possibility that specific outcomes would not be affected. One study was rated as having low risk of detection bias; although blinding of all assessors could not be guaranteed, the objective outcomes are not likely to be influenced by knowledge of the intervention (Figueiredo 2010). The remaining six studies did not report sufficient detail to reveal the level of risk of detection bias (Guimaraes 2012; Kurz 1997; Murray 2009; Nicolini 2013; Sutton 1988; Svenningsen 2013).
Incomplete outcome data
We rated six included studies as having low risk of bias due to complete outcome data (Figueiredo 2010; Guimaraes 2012; Murray 2009; Nicolini 2013; Sutton 1988; Svenningsen 2013). One study did not report the number of dropouts; in this case, the risk of bias was unclear (Kurz 1997).
Selective reporting
Two studies were registered on a clinical trial registry (Murray 2009; Nicolini 2013). Five studies rated well and were assigned low risk of bias (Figueiredo 2010; Guimaraes 2012; Nicolini 2013; Sutton 1988; Svenningsen 2013), with documented findings for all pre‐specified outcomes. One study was rated as having high risk of bias because not all outcome measures were reported (Murray 2009), and one had unclear risk (Kurz 1997).
Other potential sources of bias
Six cross‐over trials used appropriate statistical analyses. One study did not include a washout period between oscillating PEP and no treatment (Svenningsen 2013). Three studies employed adequate washout periods (Figueiredo 2010; Guimaraes 2012; Murray 2009); however, two studies did not specify the length of the washout period (Kurz 1997; Sutton 1988).
Effects of interventions
See: Table 1
We were able to include data from seven studies including 105 participants in a quantitative and narrative synthesis, six of which were conducted in adult participants with stable bronchiectasis (Figueiredo 2010; Guimaraes 2012; Murray 2009; Nicolini 2013; Sutton 1988; Svenningsen 2013) and one in children (Kurz 1997).
Primary outcome: exacerbations and hospitalisations
One study evaluated the long‐term impact of an airway oscillatory device on the frequency of exacerbations in 20 adults (Murray 2009) and found no significant differences between groups at 12 weeks (five exacerbations with ACT vs seven exacerbations without ACT; P value = 0.48). No available data showed the impact of the airway oscillating device on time to exacerbation, duration of or incidence of hospitalisation or total number of hospitalised days.
Primary outcome: quality of life
Three studies explored the effects of airway clearance techniques on HRQoL (Murray 2009; Nicolini 2013; Svenningsen 2013). One study (Murray 2009) of 20 participants investigated the effect of an airway oscillatory device on HRQoL, providing long‐term data that show significantly lower (better) cough‐related quality of life according to Leicester Cough Questionnaire (LCQ) total scores following 12 weeks of twice‐daily ACTs compared with control (median difference of 1.3; P value = 0.002). This improvement is equivalent to the minimal important difference for the LCQ total score (Raj 2009). Significant improvement in the physical (P value = 0.002), psychological (P value < 0.0001) and social (P value = 0.02) impact of coughing was also reported. This airway oscillatory device also showed significant improvement in median values in disease‐specific HRQoL according to St George's Respiratory Questionnaire (SGRQ) total scores (intervention median ‐7.8 (IQR ‐14.5 to ‐0.99); control ‐0.7 (IQR ‐2.3 to 0.05); median difference 8.5 units in favour of improvement; P value = 0.005 (Wilcoxon)). This exceeds the minimal important difference for the SGRQ (Jones 2005). In contrast, using an airway oscillation device for 15 days did not improve disease‐specific HRQOL when the same outcome measure was used (P value > 0.05) (Svenningsen 2013). HFCWO improved HRQoL better than control (MD ‐18, 95% CI ‐21 to ‐15) (Analysis 1.1), according to CAT (the chronic obstructive pulmonary disease assessment tool) (Nicolini 2013). A mix of ACTs in the same study also improved HRQoL better than control (MD ‐10, 95% CI ‐14 to ‐5) (Analysis 2.1). However, our confidence in these patient‐reported outcomes is reduced by lack of blinding in the included trials.
1.1. Analysis.
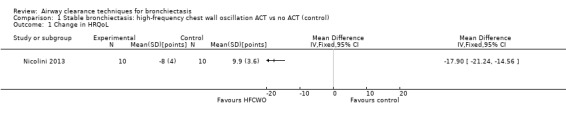
Comparison 1 Stable bronchiectasis: high‐frequency chest wall oscillation ACT vs no ACT (control), Outcome 1 Change in HRQoL.
2.1. Analysis.
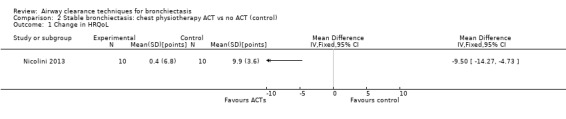
Comparison 2 Stable bronchiectasis: chest physiotherapy ACT vs no ACT (control), Outcome 1 Change in HRQoL.
Secondary outcome: pulmonary function
Six studies evaluated pulmonary function (Guimaraes 2012; Kurz 1997; Murray 2009; Nicolini 2013; Sutton 1988; Svenningsen 2013). Quantitative analysis of data from one study showed no significant differences between ACTs (gravity‐assisted drainage and breathing technique) and control in FEV1 or FVC immediately following treatment (Sutton 1988). Similarly, Guimaraes 2012 found no significant differences between an ACT (expiration with the glottis open in the lateral posture (ELTGOL)) and control in FEV1, FEV1/FVC, FEF25‐75%, FVC, inspiratory capacity (IC) and RV/total lung capacity (TLC) immediately following a single session. However, a significant reduction in FRC and TLC was demonstrated immediately following ELTGOL versus control (median difference in FRC of 18.7%; P value < 0.05; median difference in TLC of 14.29%; P value < 0.05). In the same study, an airway oscillating device significantly reduced FRC (median difference of 30.07%; P value < 0.05), TLC (median difference of 22.9%; P value < 0.05), IC/TLC ratio (median difference of 16.07%; P value < 0.05) and RV (median difference of 26.7%; P value < 0.05) versus control. However, no significant difference in FEV1, FEV1/FVC, FVC, FEF25‐75%, IC or RV/TLC ratio was demonstrated between an airway oscillating device and control (Guimaraes 2012). Following 15 days of treatment, HFCWO was associated with significant improvement in FEV1 (MD 156.1 mL, 95% CI 95.6 to 217.4 mL) (Analysis 1.2) and FVC (MD 229.1 mL, 95% CI 174.5 to 283.7 mL) (Analysis 1.3) compared with control (Nicolini 2013). A significant reduction in TLC (MD ‐703 mL, 95% CI ‐1380.5 to ‐25.5 mL) (Analysis 1.4) with HFCWO compared with control was evident, although no difference in RV was observed (MD ‐645 mL, 95% CI ‐1338.9 mL to 48.9 mL) (Analysis 1.5). In the same study, ACTs (gravity‐assisted drainage with expiratory technique or PEP therapy or airway oscillation device) were not associated with a significant difference compared with control in FEV1 (MD ‐73 mL, 95% CI ‐154.8 to 8.76 mL) (Analysis 2.2) nor in FVC (MD 91.5 mL, 95% CI ‐6.2 to 189.2 mL) (Analysis 2.3) (Nicolini 2013). ACTs did reduce RV compared with control (MD ‐21 mL, 95% CI ‐416.4 to ‐3.62 mL) (Analysis 2.5) but had no effect on TLC (MD ‐134 mL, 95% CI ‐336.5 to 68.5 mL) (Analysis 2.4) (Nicolini 2013).
1.2. Analysis.
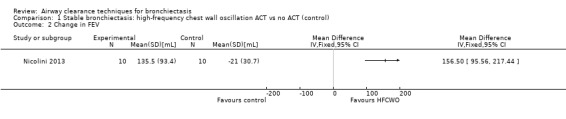
Comparison 1 Stable bronchiectasis: high‐frequency chest wall oscillation ACT vs no ACT (control), Outcome 2 Change in FEV.
1.3. Analysis.
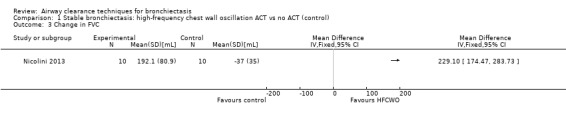
Comparison 1 Stable bronchiectasis: high‐frequency chest wall oscillation ACT vs no ACT (control), Outcome 3 Change in FVC.
1.4. Analysis.
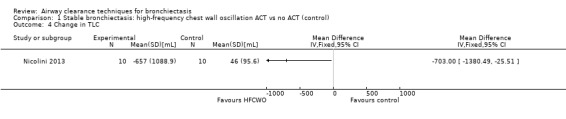
Comparison 1 Stable bronchiectasis: high‐frequency chest wall oscillation ACT vs no ACT (control), Outcome 4 Change in TLC.
1.5. Analysis.
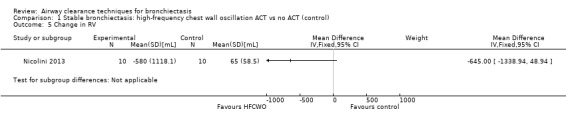
Comparison 1 Stable bronchiectasis: high‐frequency chest wall oscillation ACT vs no ACT (control), Outcome 5 Change in RV.
2.2. Analysis.
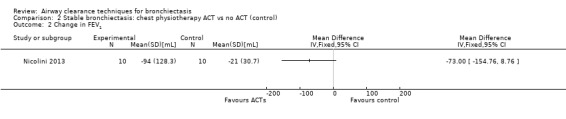
Comparison 2 Stable bronchiectasis: chest physiotherapy ACT vs no ACT (control), Outcome 2 Change in FEV1.
2.3. Analysis.
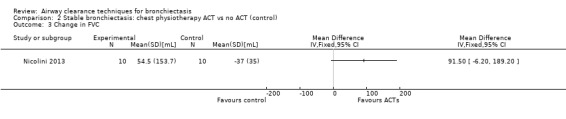
Comparison 2 Stable bronchiectasis: chest physiotherapy ACT vs no ACT (control), Outcome 3 Change in FVC.
2.5. Analysis.
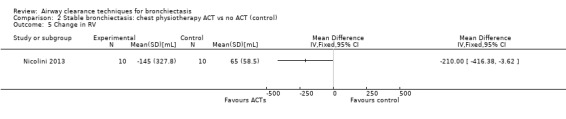
Comparison 2 Stable bronchiectasis: chest physiotherapy ACT vs no ACT (control), Outcome 5 Change in RV.
2.4. Analysis.
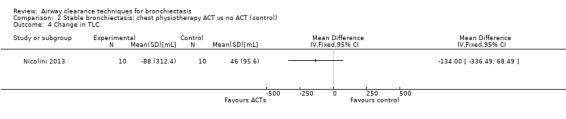
Comparison 2 Stable bronchiectasis: chest physiotherapy ACT vs no ACT (control), Outcome 4 Change in TLC.
Following a long‐term study in adults, Murray 2009 reported no significant differences in FEV1 (median difference 0.00 L; P value = 0.7), FVC (median difference 0.07 L; P value = 0.9) or FEF25‐75% (median difference 0.06 L; P value = 0.6) between 12 weeks of an airway oscillating device and no ACT. When applied for 15 days, an airway oscillation device was not associated with a significant change in FEV1, FVC or TLC (all P values > 0.05) compared with no treatment (Svenningsen 2013).
In children, Kurz 1997 reported differences in FEV1 of 8.86%, in PEF of 6% and in FVC of 4%, and a reduction in FRC of 6%, in favour of the longer‐term airway oscillating device over sham therapy (no P values provided).
Secondary outcome: gas exchange
One study reported effects of ACTs on measures of gas exchange (Nicolini 2013). HFCWO had no effect on PaO2 (MD 3.1 mmHg, 95% CI ‐2.8 to 8.9 mmHg) (Analysis 1.6) nor on PaCO2 (MD ‐1.8 mmHg, 95% CI 3.8 to 0.21 mmHg) (Analysis 1.7) compared with control. In the same study, ACTs had no effect on PaO2 (MD 2.2 mmHg, 95% CI ‐4.1 to 8.5 mmHg) (Analysis 2.6) nor on PaCO2 (MD 0.2 mmHg, 95% CI ‐1.7 to 2.1 mmHg) (Analysis 2.7) compared with control.
1.6. Analysis.
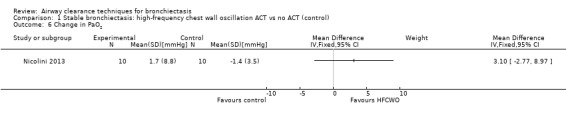
Comparison 1 Stable bronchiectasis: high‐frequency chest wall oscillation ACT vs no ACT (control), Outcome 6 Change in PaO2.
1.7. Analysis.
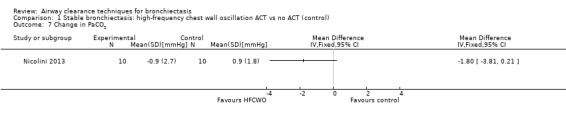
Comparison 1 Stable bronchiectasis: high‐frequency chest wall oscillation ACT vs no ACT (control), Outcome 7 Change in PaCO2.
2.6. Analysis.
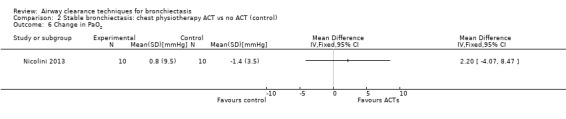
Comparison 2 Stable bronchiectasis: chest physiotherapy ACT vs no ACT (control), Outcome 6 Change in PaO2.
2.7. Analysis.
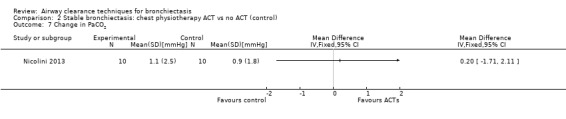
Comparison 2 Stable bronchiectasis: chest physiotherapy ACT vs no ACT (control), Outcome 7 Change in PaCO2.
Secondary outcome: symptoms
Two studies reported on symptoms (Nicolini 2013; Svenningsen 2013). Based on a combined measure of the Breathlessness, Cough and Sputum scale (BCSS), both HFCWO (MD 5.8, 95% CI ‐7.2 to ‐4.4) (Analysis 1.8) and ACTs (MD ‐2.9, 95% CI ‐4.3 to ‐1.5) (Analysis 2.8) significantly reduced symptoms of dyspnoea, cough and sputum compared with control (Nicolini 2013). A similar effect was demonstrated in the same study for functional dyspnoea for HFCWO (MD ‐1.7, 95% CI ‐2.4 to ‐1.0) (Analysis 1.9) and for ACTs (MD ‐1.5, 95% CI ‐2.3 to ‐0.7) (Analysis 2.9) compared with no treatment. According to the patient evaluation questionnaire, 15 days with the airway oscillation device significantly improved the ease of expectorating secretions (P value < 0.001) and reduced cough frequency (P value = 0.003) compared with no treatment, but had no effect on cough severity, chest discomfort or dyspnoea (P value > 0.05) (Svenningsen 2013).
1.8. Analysis.
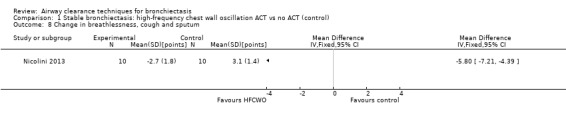
Comparison 1 Stable bronchiectasis: high‐frequency chest wall oscillation ACT vs no ACT (control), Outcome 8 Change in breathlessness, cough and sputum.
2.8. Analysis.
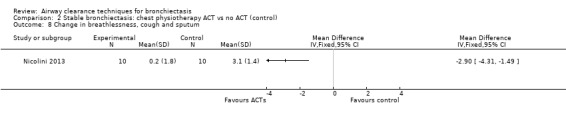
Comparison 2 Stable bronchiectasis: chest physiotherapy ACT vs no ACT (control), Outcome 8 Change in breathlessness, cough and sputum.
1.9. Analysis.
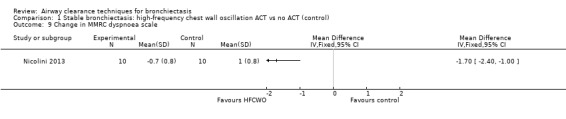
Comparison 1 Stable bronchiectasis: high‐frequency chest wall oscillation ACT vs no ACT (control), Outcome 9 Change in MMRC dyspnoea scale.
2.9. Analysis.
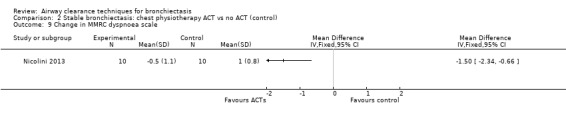
Comparison 2 Stable bronchiectasis: chest physiotherapy ACT vs no ACT (control), Outcome 9 Change in MMRC dyspnoea scale.
Secondary outcome: sputum clearance
Five studies measured the effect of treatment on sputum yield (Figueiredo 2010; Guimaraes 2012; Murray 2009; Nicolini 2013; Sutton 1988). A significant increase in the volume of sputum produced was noted with an airway oscillating device compared with sham therapy in Figueiredo 2010 (MD 8.40 mL, 95% CI 3.40 to 13.40 mL) (Analysis 3.1). A significant increase in 24‐hour sputum volume was reported in a long‐term study of an airway oscillating device compared with control (Murray 2009) (MD 3 mL; P value = 0.02). The third study reported a greater sputum yield with ACT (gravity‐assisted drainage and breathing technique) compared with control (22 g on ACT vs 5 g on control; P value < 0.01) (Sutton 1988). After 15 days of treatment with HFCWO, significant improvement in sputum volume was evident compared with no treatment (12.5 mL vs 3 mL; P value = 0.001), and a mix of ACTs over the same duration yielded greater sputum expectoration compared with control (7.5 mL vs 3 mL; P value = 0.04) (Nicolini 2013). In one study in which investigators measured dry sputum weight, a single session of ACT (ELTGOL) was associated with greater sputum production compared with control (0.38 g on ACT vs 0.14 g on control; P value < 0.05) (Guimaraes 2012). In the same study, no significant difference in sputum yield was reported with an airway oscillating device compared with control (0.15 g on ACT vs 0.14 g on control; P value > 0.05). According to other measures of sputum clearance, no significant improvement in mucociliary clearance was found upon radioaerosol imaging with ACTs compared with control (gravity‐assisted drainage and breathing technique) in terms of whole lung radioaerosol clearance (20% on ACT vs 17% on control; P value = not significant) nor in terms of regional (inner and outer) clearance (12% vs 11% inner region and 3% vs 2% outer region; P value > 0.05) (Sutton 1988).
3.1. Analysis.
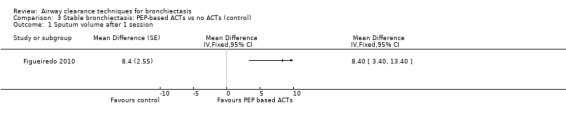
Comparison 3 Stable bronchiectasis: PEP‐based ACTs vs no ACTs (control), Outcome 1 Sputum volume after 1 session.
Secondary outcome: days of antibiotic usage
No data were available for analysis.
Secondary outcome: adverse events
Three studies reported absence of adverse events during short‐term (Figueiredo 2010) and longer‐term (Murray 2009; Svenningsen 2013) use of airway oscillating devices compared with control, which reflects the safety of these types of techniques. Four studies did not report on the occurrence of adverse events (Guimaraes 2012; Kurz 1997; Nicolini 2013; Sutton 1988).
Secondary outcome: mortality
No data were available for analysis.
Secondary outcome: participant withdrawal
Three studies reported that all participants completed the study, and no withdrawals were related to use of ACTs (Figueiredo 2010; Guimaraes 2012; Murray 2009). One study reported that three children with bronchiectasis refused to use the sham therapy, but it is unclear whether this refusal occurred during the trial (withdrawal) or after the trial (refusal after participation) (Kurz 1997).
Discussion
This review aimed to determine the effects of airway clearance techniques (ACTs) compared with no treatment in individuals with bronchiectasis. Results from seven studies of 105 participants were mixed. Medium‐ or longer‐term use of specific ACTs or a combination of techniques may have clinically important effects on HRQoL in adults with stable bronchiectasis. A summary of data can be found in Table 1. Selected ACTs may increase the volume of sputum expectorated. However, their effects on lung function are variable. The impact on dynamic lung function may be specific to the types of ACTs applied and the duration of treatment. Selected non‐PEP therapy and airway oscillatory devices may reduce the degree of pulmonary hyperinflation. Reduction in specific respiratory symptoms was apparent with some types of ACTs. It is difficult to determine the clinical impact and value of ACTs across the disease spectrum of this condition because of the absence of studies in participants with an acute exacerbation of bronchiectasis, the small numbers of participants overall and the limited number of ACTs tested. Pooling of data for meta‐analysis was limited by heterogeneity between studies, including differing types of ACTs applied, variation in treatment session duration and differing methods of outcome measurement applied. This prevented analysis of publication bias through funnel plots.
Symptoms of cough, sputum production and dyspnoea are typical of bronchiectasis, and HRQoL is closely linked to these symptoms (Martinez‐Garcia 2005). The positive effects of high‐frequency chest wall oscillation (HFCWO) and of a mix of ACTs on sputum production and dyspnoea as well as HRQoL (Nicolini 2013) and improved disease‐specific and cough‐related HRQoL with twice‐daily treatment with an airway oscillatory device for three months are important clinical outcomes and may influence patient compliance with treatment (Prasad 2007). These beneficial effects were apparent only after a treatment duration of 15 days or longer, suggesting that the influence of specific ACTs on HRQoL and on symptoms may be determined by treatment duration. Although only two studies included symptoms as an outcome, the positive effects of HFCWO and of a mix of PEP and non‐PEP therapy or airway oscillatory devices lend support to the physiological rationale for these techniques. However, with two studies using a cross‐over design (Murray 2009; Svenningsen 2013), absence of a placebo group suggests that these results should be viewed with caution. Lack of information regarding inclusion of a blinded assessor for this outcome in these studies potentially introduces a degree of bias in the results. Although no effect on exacerbation rate was noted in this longer‐term study (Murray 2009), absence of a follow‐up period after completion of treatment during which exacerbations may be collated limits the ability of investigators to accurately interpret the impact of airway oscillatory devices on exacerbation rates. In addition, the effect of this form of ACT on the incidence of hospitalisation is unknown and has not been explored. As both of these primary outcomes are of relevance to the clinical profile, disease progression and the prognosis of this condition (Loebinger 2009), this important area requires further examination.
Although acute improvement in lung function following ACTs is likely to reflect secretion displacement within the airways (from the periphery to the proximal airways), change in lung function over time in individuals with bronchiectasis (King 2005; Martinez‐Garcia 2007) is influenced by the frequency and severity of acute exacerbations (Elborne 2007). The short‐term impact of ACTs on lung function has remained variable. It appears that HFCWO did have a positive influence on dynamic lung function; however, this did not translate into an advantage in terms of gas exchange. Improved forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) in cystic fibrosis (CF) with this type of ACT have been previously demonstrated and were accompanied by a reduction in functional residual capacity (FRC) (Braveman 2007; Kempainen 2010). The rationale is that when delivered with 10 to 15 Hz, this technique maximises oscillatory airflow while minimising small airway closure (Braveman 2007; Kempainen 2010). In contrast, this effect was not evident with other techniques, with no change in dynamic lung function observed with gravity‐assisted drainage and breathing techniques compared with no treatment (Murray 2009; Sutton 1988), and the effects of airway oscillatory devices were varied, with some reports of improvement with long‐term treatment (Kurz 1997) and other reports of no benefit (Guimaraes 2012; Murray 2009). Although this may suggest that the clinical value of these techniques for short‐ or long‐term management is unclear, findings are predominantly consistent with previous reports demonstrating that measures of airflow obstruction did not reflect changes in sputum transport and are considered an insensitive reflection of the effectiveness of ACTs (Cochrane 1977). Any changes in lung function do not appear to be reflected by gas exchange measures, despite the rationale that HFCWO improves alveolar ventilation homogenisation (Braveman 2007; Isabey 1984). In contrast, a mix of techniques appear to have a positive impact on pulmonary hyperinflation (Guimaraes 2012; Kurz 1997; Nicolini 2013), which is a feature of non‐CF bronchiectasis (Koulouris 2003; Martinez‐Garcia 2007a). It has been suggested that in people with CF, measures of static lung volume rather than dynamic lung volume may provide a clearer reflection of the impact of ACTs on sputum transport, although the precise mechanism is not clear (Regnis 1994). In addition, their effect on static lung volumes may be dependent on the type of ACT provided, with some studies demonstrating a positive effect on total lung capacity (TLC) with airway oscillatory devices (Guimaraes 2012; Nicolini 2013) that is greater than with ELTGOL (Guimaraes 2012), and others reporting benefit with a mix of ACTs (Nicolini 2013). One study showed considerable baseline variation in both static lung volume measures (residual volume (RV) and TLC) between groups undertaking HFCWO, a mix of ACTs and no treatment (Nicolini 2013). No adjustment for this variation was included within the statistical comparison between groups, and for this reason, the effects of both HFCWO and a mix of ACTs on RV and TLC compared with no treatment should be interpreted with caution, with the magnitude of the difference between groups likely to be smaller than reported. Even in consideration of this limitation, the differing degree of impact on hyperinflation suggests that the mechanism behind this effect may vary between techniques and may be influenced by the baseline static lung volume of participants. The positive pressure generated during expiration is designed to provide a splinting effect on the airways, to prevent dynamic airway collapse and to enhance expiratory flow (Flude 2012). In contrast, ELTGOL (Guimaraes 2012) may target the specific lung volumes associated with pulmonary hyperinflation.
Several studies found a significant increase in a key clinical outcome of sputum expectoration immediately following treatment up to one hour post treatment or over 24 hours with airway oscillatory devices compared with no intervention (Figueiredo 2010; Murray 2009; Nicolini 2013) or with ELTGOL ACTs (Guimaraes 2012; Sutton 1988). One potential confounding factor is the difference between studies in the methods of measurement of sputum volume used, with three studies using wet volume (Figueiredo 2010; Murray 2009; Sutton 1988) and one study using dry weight (Guimaraes 2012). The quantification of wet volume may be influenced by a person's reticence to expectorate, saliva contamination or swallowing of secretions (Arens 1994; Williams 1994), which may lead to incorrect estimation of the outcome. In contrast, contamination with saliva may be corrected in part by drying sputum and measuring dry weight (van der Schans 2002), which may lend greater support to selected ACTs that applied this method of evaluation. Even with the approach of using dry weight of sputum, the sputum density of individual people may demonstrate diurnal variation (van der Schans 2002) and may be influenced by hydration levels (Boucher 2010). Despite these limitations, these studies provide preliminary support of ACTs by addressing a symptom commonly associated with bronchiectasis.
Study of the contrasting effects of airway oscillatory devices versus ELTGOL on sputum production has been limited, with only one study demonstrating an immediately greater sputum yield with ELTGOL (Guimaraes 2012). The physiological rationale for this form of ACT involves promoting two‐phase gas‐liquid interaction to facilitate mucociliary clearance; in contrast, the primary mechanism of the airway oscillatory device involves providing a splinting effect on the airways, improving collateral ventilation and altering sputum rheology (Tambascio 2011). This may account for the different clinical effect.
Each ACT applied in the included studies is comparable with those frequently prescribed in people with CF (Flume 2009; van der Schans 2000), suggesting that the rationale behind the prescription is considered to be similar between different patient populations (Bott 2009). However, compared with studies in CF, fewer types of ACTs have been compared with a control condition. Despite this fact, selected effects of these treatments are similar, and they lead to improvement in sputum production over the short term (van der Schans 2000). Direct comparison of the benefits of ACTs for bronchiectasis versus CF with other outcome measures incorporated into this review is limited because ACT is considered to be a cornerstone of management in CF, and, for this reason, long‐term trials in this patient population are lacking. Recently, clinical studies of ACTs in bronchiectasis have focused on comparing the effects of different types of techniques over the short and long term (Eaton 2007; Patterson 2004; Patterson 2005; Patterson 2007; Savci 1998; Thompson 2002; Tsang 2003), an approach that is similar in CF. However, this review did not incorporate results from these studies comparing the effects of different types of ACTs only, without a control group. The approach in the current review was to establish the value of ACTs compared with no ACTs in individuals with exacerbation of acute or stable bronchiectasis, rather than comparing the effects of specific techniques whose physiological rationale may differ. With current clinical practice guidelines and recommendations for bronchiectasis advocating for ACTs as part of management (Chang 2015; Hill 2011; King 2010; Pasteur 2010), comparison and contrast of the effects of different types of ACTs in this patient group are needed to clarify the role of routine ACT prescription in bronchiectasis. Current findings suggest benefit, but further research is needed to establish the physiological and clinical effects of different types of ACTs to inform clinical practice.
Summary of main results
In individuals with stable bronchiectasis, data from a small number of studies show that ACTs result in improvement in HRQoL, fewer respiratory symptoms, greater sputum expectoration and improvement in pulmonary hyperinflation. Preliminary work suggests lack of change in gas exchange with ACTs, but future studies are needed to confirm the magnitude of these effects and the impact on dynamic measures of lung function across a range of ACTs. No effect of ACTs on exacerbation frequency was evident. Future studies with blinded assessors are required to investigate the effects of ACTs on rates of exacerbation, incidence of hospitalisation and antibiotic usage. The impact of ACTs on individuals with an acute exacerbation of bronchiectasis has not been established.
Overall completeness and applicability of evidence
Most studies have not specified the underlying cause of bronchiectasis nor the extent of bronchiectasis or functional level. Therefore, the influence of these factors on the effectiveness of specific ACTs is difficult to determine. From the two studies evaluating single treatment sessions, it is difficult to generalise findings to the overall population. We hypothesised that the effect of ACTs may differ in those experiencing an acute exacerbation of bronchiectasis compared with those in a stable state. However, with all studies conducted in individuals in a stable clinical state, the effects of these techniques during an acute exacerbation remain unclear and require further study.
Quality of the evidence
The quality of the seven studies included in this review is mixed, with small sample sizes and often unclear or high risk of bias, particularly with regard to blinding of assessors and inclusion of concealed allocation. Available data for quantitative analysis were limited by small effect size, and differing time frames and types of interventions limited meta‐analysis. These publications provided limited data on the primary outcomes of interest, and obtaining additional data from study authors was not consistently possible. The physical nature of these interventions limited the ability of investigators to blind participants and therapists, and only one study incorporated assessor blinding (Figueiredo 2010). All review outcomes were determined to be of low quality, primarily as the result of risk of bias and imprecise results, small participant numbers, small numbers of studies for all outcome and few events for specific outcomes.
Potential biases in the review process
A broad search incorporated handsearching of conference abstracts and trial registries, with inclusion of studies published only in abstract form. When clarification was necessary, study authors were contacted to confirm details of study design or to provide additional data. However, additional data were not consistently available. This may have affected some judgements regarding risk of bias and may have limited included data. Two review authors independently extracted data and resolved disagreements via discussion. These two review authors independently rated risk of bias.
Agreements and disagreements with other studies or reviews
This is an update of a previous review, published in 2013. In the previous review, data suggested improvement with ACTs in HRQoL, sputum expectoration and pulmonary hyperinflation. This update also suggests positive benefit for respiratory symptoms of cough, sputum expectoration and dyspnoea with ACTs. With current clinical practice guidelines for bronchiectasis advocating for ACTs as part of management (Chang 2015; Hill 2011; Pasteur 2010), these are important clinical outcomes, which may account for the change in HRQoL reported in previous studies. The positive influence of a specific type of ACT on dynamic lung function suggests the need for better understanding of the physiological mechanisms behind each type of technique when applied in people with bronchiectasis.
Authors' conclusions
Implications for practice.
Airway clearance techniques appear to be safe for individuals (adults and children) with stable bronchiectasis and may lead to improvements in sputum expectoration, selected measures of lung function, patient symptoms and HRQoL. However, additional data are needed to establish the clinical value of ACTs over the short and the long term for participant‐important outcomes, including clinically important long‐term parameters that impact disease progression in individuals with stable bronchiectasis. The role of these techniques in people with an acute exacerbation of bronchiectasis remains unknown.
Implications for research.
The chronic nature of bronchiectasis makes clear the value of examining the longer‐term effectiveness of ACTs compared with no treatment, rather than effects within a single isolated treatment session. Additional adequately powered long‐term studies that include physiological outcomes such as measurement of small airway function, radioaerosol clearance and the lung clearance index may be more sensitive to change and may be more clinically useful (Horsley 2008). Inclusion of these measures together with clinically meaningful outcomes such as exacerbation rate and hospitalisation may clarify the rationale and mechanism of action of each technique. Clinical guidelines recommend that ACTs should be prescribed for individuals with clinical symptoms of cough, sputum production and radiological signs of mucus plugging (Chang 2015; Pasteur 2010). In view of this, future studies should include cross‐over study designs and blinded assessors to minimise potential bias in outcome measurement,. with the goal of providing further guidance on specific ACT prescription for people with bronchiectasis. In addition, lack of controlled trials for acute exacerbations of bronchiectasis is a matter of concern, as exacerbation is a key clinical characteristic of the condition (King 2005; Loebinger 2009). It may also be important to establish the comparative effects of different types of ACTs in individuals with bronchiectasis.
What's new
| Date | Event | Description |
|---|---|---|
| 11 November 2015 | New search has been performed | New literature search run |
| 13 July 2015 | New citation required but conclusions have not changed | 2 new studies added Elements of Background and Discussion redrafted ACTs are associated with improvements in generic HRQOL and respiratory symptoms |
Acknowledgements
Thank you to Elizabeth Stovold for assistance with developing the search strategy, and to Emma Welsh and Chris Cates for providing ongoing support throughout the review process.
Chris Cates was the Editor for this review and commented critically on the review.
The Background and Methods sections of this review are based on a standard template used by the Cochrane Airways Group.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (T he Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Bronchiectasis search
1. exp Bronchiectasis/
2. bronchiect$.mp.
3. bronchoect$.mp.
4. kartagener$.mp.
5. (ciliary adj3 dyskinesia).mp.
6. (bronchial$ adj3 dilat$).mp.
7. or/1‐7
Filter to identify randomised controlled trials
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and randomised controlled trial filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to retrieve relevant trial reports from the CAGR
2015 search (via the Cochrane Register of Studies)
#1 BRONCH:MISC1
#2 MeSH DESCRIPTOR Bronchiectasis Explode All
#3 bronchiect*
#4 #1 or #2 or #3
#5 physiotherap*
#6 physical* NEXT therap*
#7 "bronchopulmonary hygiene"
#8 "tracheobronchial clearance"
#9 airway* NEXT clearance
#10 (chest* or lung* or sputum* or mucus*) NEAR3 (clearance*)
#11 "active cycle"
#12 ACBT
#13 deep NEXT breath*
#14 DBE
#15 "thoracic expansion"
#16 TEE
#17 sustained NEXT maximal NEXT inspirat*
#18 SMI
#19 breathing NEXT exercise*
#20 "postural drainage"
#21 "gravity assisted drainage"
#22 "gravity‐assisted drainage"
#23 "autogenic drainage"
#24 GAD
#25 CCPT
#26 ELTGOL
#27 FET
#28 "forced expiratory technique"
#29 huff*
#30 PEP
#31 PEEP
#32 resistance NEXT breath*
#33 "positive expiratory pressure"
#34 hi‐PEP
#35 bubble‐PEP
#36 bottle‐PEP
#37 oscillat*
#38 mouthpiece‐PEP
#39 pari‐PEP
#40 VRP1
#41 flutter*
#42 desitin
#43 cornet
#44 acapella
#45 scandipharm
#46 percuss*
#47 vibrat*
#48 vest
#49 HFCWO
#50 OHFO
#51 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50
#52 #4 and #51
[Note: in search line #1, MISC1 denotes the field where the reference has been coded for condition, in this case, bronchiectasis]
Previous search (via Procite software)
physiotherap* or "physical therap*" or "bronchopulmonary hygiene" or "tracheobronchial clearance" or "airway* clearance" or "chest clearance" or "lung clearance" or "sputum clearance" or "mucus clearance" or "active cycle" or ACBT or "deep breath*" or DBE or "thoracic expansion" or TEE or "sustained maximal inspirat*" or SMI or "breathing exercise*" or "postural drainage" or "gravity assisted drainage" or "gravity‐assisted drainage" or "autogenic drainage" or GAD or CCPT or ELTGOL or FET or "forced expiratory technique*" or huff* or *PEP or PEEP or "resistance breath*" or "positive expiratory pressure" or "hi‐PEP" or "bubble‐PEP" or "bottle‐PEP" or "oscillat*‐PEP" or "mouthpiece‐PEP" or "pari‐PEP" or VRP1 or flutter or desitin or cornet or acapella or scandipharm or percuss* or vibrat* or vest or HFCWO or OHFO or "chest wall oscillat*" or "oral oscillat*" or "thoracic oscillat*".
[Limited to records coded as 'bronchiectasis']
Appendix 3. Search terms for PEDro (http://www.pedro.org.au/)
Bronchiectasis" or "Kartagener*" or "Agammaglobulinaemia"
Data and analyses
Comparison 1. Stable bronchiectasis: high‐frequency chest wall oscillation ACT vs no ACT (control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in HRQoL | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Change in FEV | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in FVC | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Change in TLC | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Change in RV | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Change in PaO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Change in PaCO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Change in breathlessness, cough and sputum | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Change in MMRC dyspnoea scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Stable bronchiectasis: chest physiotherapy ACT vs no ACT (control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in HRQoL | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Change in FEV1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in FVC | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Change in TLC | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Change in RV | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Change in PaO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Change in PaCO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Change in breathlessness, cough and sputum | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Change in MMRC dyspnoea scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Stable bronchiectasis: PEP‐based ACTs vs no ACTs (control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sputum volume after 1 session | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Figueiredo 2010.
| Methods | Randomised cross‐over trial Study setting: medical department, Brazil Study duration: 9 days |
|
| Participants | 8 people (mean age 47.4 years) with stable bronchiectasis productive of 47.8 mL sputum per day, diagnosed by HRCT (lobes affected or disease severity not stated), mean FEV1 65% predicted. Functional level not stated | |
| Interventions | Intervention: 1 session of oscillating PEP therapy using flutter, performed in seated position for 15 minutes' duration and 5 minutes' coughing after intervention Control: 1 session of sham oscillating PEP (using flutter valve without sphere or lid, with no modification of the size of the orifice). Same sequence of therapy applied 1‐Week washout period between interventions |
|
| Outcomes | Sputum volume (mL) Measurement was recorded immediately following completion of the intervention |
|
| Notes | No adverse events Funding: supported by Centers of Excellence Program (PRONEXFAPERJ), Brazilian Council for Scientific and Technological Development (CNPq), Financing for Studies and Projects (FINEP) and Rio de Janeiro State Research Supporting Foundation (FAPERJ) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This was a randomised, blinded and crossover study. Randomization defined the order of interventions in accordance with a computer‐generated sequence using a block size of 4" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The protocol to be applied (Flutter valve or Sham Flutter) was revealed to the investigator only at the onset of each experimental sequence..." Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "One week before the testing protocol, each subject visited the laboratory for an introductory session to become familiar with the equipment and the procedures...In the Sham Flutter intervention, the subjects followed the same sequence of the Flutter Valve intervention, but the metallic sphere and the cover lid of the device were removed (Sham Flutter). Since the patients were not acquainted with the valve, they did not know its proper assembly" Comment: As participants received training in the intervention, they may have been able to identify differences in the sham intervention. Personnel were not blinded during delivery of the intervention (see above) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...a second examiner blinded to the applied intervention processed the signals" Comment: As other examiners were unblinded, blinding of the second examiner could have been compromised, but outcomes were objectively measured and were likely to be resistant to any issues with blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "...all completed the study" Comment: Complete data were available for reported outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes were reported as outlined in the Methods section. Study protocol was not available |
| Other bias | Low risk | Quote: "After washout period and prior to the second intervention, the patients were re‐examined by a physician to ensure that they remained stable" Quote: "Impedance values preceding Sham Flutter and Flutter Valve interventions were similar" Comment: No carryover effect was observed. No other risks were identified |
Guimaraes 2012.
| Methods | Randomised cross‐over trial Study setting: medical department, Brazil Study duration: 24 days |
|
| Participants | 10 people (mean age 55.9 years) with stable bronchiectasis with a persistent productive cough, diagnosis by radiological findings (number of lobes or severity not stated), mean FEV1 53% predicted, functional level not stated | |
| Interventions | Intervention 1: single session of oscillating PEP therapy using flutter, performed in seated position for 15 minutes' duration (breathing from total lung capacity until cough occurred) followed by 5 minutes' coughing Intervention 2: single session of ELTGOL: performed in lateral decubitus position with affected lung in the dependent position and the individual performing slow expirations with the glottis open from functional residual capacity to residual volume Control: 1 session: participants in seated position, no manoeuvres performed for 15 minutes followed by 5 minutes' coughing 1‐Week washout period between interventions Before control and intervention sessions, salbutamol and a series of 5 minutes' voluntary coughing were administered |
|
| Outcomes | Sputum volume (dry weight) (g) Spirometry (FEV1, FVC, FEV1/FVC, FEF25‐75%) Static lung volumes (IC, VC, TLC, FRC, RV, RV/TLC, IC/TLC) All measures were recorded immediately following completion of the intervention |
|
| Notes | No adverse events Funding: Sponsors and collaborators were at Federal University of Rio de Janeiro Centro Universitário Augusto Motta (from clinicaltrials.gov) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were submitted to the control protocol and two interventions in a random order. Block randomisation sequences were created by a researcher not involved with recruitment, selection and assessments" |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed opaque envelopes containing patients' assignments were opened at the time of first treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information was provided Comment: Participants and treating therapist(s) were not likely to be blinded to group allocation. This may influence outcome measures (spirometry, static lung volumes, sputum dry weight) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided Comment: Outcome assessor(s) were not likely to be blinded to group allocation. As outcomes were objectively measured (spirometry, static lung volumes, sputum dry weight), it is likely they were resistant to issues with blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All individuals tolerated and completed the steps in this study" Complete data were available for reported outcomes |
| Selective reporting (reporting bias) | Low risk | Protocol was available, and all specified outcomes were reported |
| Other bias | Low risk | Quote: "Measurements of static lung volumes were conducted before the spirometry to avoid any residual effect of dynamic compression of the airways in the plethysmography results" Comment: Attempts were made to minimise the impact of multiple measures of lung function |
Kurz 1997.
| Methods | Randomised cross‐over trial Study setting: medical department, Germany Study duration: 6 months |
|
| Participants | 9 children with bronchiectasis (aged 6 to 16 years), with stable bronchiectasis (7 participants had a primary diagnosis of primary ciliary dyskinesia but were classified as bronchiectasis), diagnosed by HRCT (lobes affected or disease severity not stated), functional level not stated | |
| Interventions | Intervention: oscillating PEP using flutter, 3 times a day, breathing out 60 times Control: sham oscillating PEP using flutter (without metal ball, no modification to size of orifice). Same prescription of therapy applied |
|
| Outcomes | Spirometry (VC, FEV1, PEFR) Static lung volumes (FRC) Measurements were recorded after a 3‐month intervention |
|
| Notes | No specified run‐in period, no details of washout period between interventions Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Nothing is stated other than it was randomised Insufficient information was provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: Nothing is stated regarding concealment of assignment of participants to treatment order Insufficient information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Study authors noted that it was possible that participants were aware of the differences between devices when they were used, as they did not oscillate during the control phase. No comment was made on the blinding of personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided Comment: Outcome assessor(s) were not likely to be blinded to group allocation. As outcomes were objectively measured (spirometry, static lung volumes), it is likely they were resistant to issues with blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Report states that 3 participants refused sham therapy, but it is unclear whether this occurred during the trial period (withdrawal) or afterwards (refusal after participation) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available. Insufficient information was provided |
| Other bias | Unclear risk | Duration of washout was not reported, and this may influence outcomes. Insufficient information was provided |
Murray 2009.
| Methods | Randomised cross‐over trial Study setting: Edinburgh Study duration: 7 months |
|
| Participants | 20 people, median age 73 years, clinically stable (no prescription of antibiotics in 4 weeks before participation). Diagnosed by HRCT, with number of lobes affected being median of 4 (IQR 3 to 4.75), with 15 participants with varicose or cystic dilatation affecting ≥ 1 lobe. Regular chest physiotherapy was not performed, and functional level was not stated | |
| Interventions | Intervention: Oscillating PEP with Acapella device (regimen of 3 sets of 10 breaths, 2 to 3 huffs and cough, resistance set at 3) undertaken twice daily (duration of 20 to 30 minutes) for 3 months Control: Standard medical care was provided with no physiotherapy intervention (oscillating PEP device was retained by physiotherapist during the non‐treatment phase) 1‐Month washout period was reported between interventions |
|
| Outcomes | Number of exacerbations Cough‐related quality of life (LCQ) HRQoL: SGRQ 24‐Hour sputum volume Spirometry (FEV1, FVC, FEF25‐75%) |
|
| Notes | No adverse events were reported No other interventions or changes to care were provided during the study Funding: This study was funded by a Small Project Grant (NHS Lothian Research and Development Fund, Edinburgh, UK). M. Murray was funded by the Chief Scientist Office (Edinburgh) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was determined by computer generation..." |
| Allocation concealment (selection bias) | Unclear risk | No information was provided Unclear whether allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information was provided Comment: Participants and treating therapists were not likely to be blinded to group allocation. This may have affected outcomes (HRQoL, FEV1, FVC, FEF25‐75%, number of exacerbations) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided Comment: Outcome assessor(s) were not likely to have been blinded to group allocation (presumably the same therapist that prescribed the intervention). This may have affected primary (HRQoL, sputum volume) or secondary (FEV1, FVC, FEF25‐75%, number of exacerbations) outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data were available for reported outcomes |
| Selective reporting (reporting bias) | High risk | Protocol was available. Not all outcomes were reported |
| Other bias | Low risk | Adequate washout period (1 month) was applied |
Nicolini 2013.
| Methods | Randomised controlled trial Study setting: Italy Study duration: 15 days |
|
| Participants | 37 people, ranging from 72 to 75 years of age, clinically stable (no change in medication within 1 week before enrolment), diagnosed by HRCT (lobes affected or disease severity not stated), daily sputum volume ≥ 20 mL daily ≥ 3 consecutive days, mean FEV1 ranging from 1.0 to 1.7 L, baseline dyspnoea 2.1 to 2.3 on Modified Medical Research Council (MMRC) Dyspnoea Scale | |
| Interventions | Intervention: high‐frequency chest wall oscillation (HFCWO) with oscillating frequency of 13 to 15 Hz, with pressure of 2 to 5 cmH2O, for 30 minutes, given twice daily, 5 days a week for 15 days Intervention: slow expiratory with glottis opened in lateral position (ELTGOL) or PEP therapy (mask), or PEP therapy (bottle) or oscillating PEP with Acapella device, for 45 minutes, given twice daily, 5 days a week for 15 days Control: medical therapy only |
|
| Outcomes | HRQoL (Breathlessness, Cough and Sputum Scale and COPD Assessment Test) Dyspnoea: MMRC Sputum volume during treatment and any collected if patient felt the need to continue coughing Spirometry (FEV1, FVC) Static lung volumes (TLC, RV) Gas exchange (PaO2, PaCO2) |
|
| Notes | No adverse events were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Every patient was assigned following a computed randomisation list..." |
| Allocation concealment (selection bias) | Unclear risk | No information was provided Unclear whether allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information was provided Comment: Participants and treating therapists were not likely to be blinded to group allocation. This may have affected outcomes (HRQoL, symptoms of dyspnoea, FEV1, FVC, TLC, RV) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided This may have affected primary (HRQoL, sputum volume) or secondary (FEV1, FVC) outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: It is stated that all participants assigned to airway clearance sessions completed their sessions, with no withdrawals. Complete data were available for all outcomes |
| Selective reporting (reporting bias) | Low risk | Protocol was available. All specified outcomes were reported |
| Other bias | Unclear risk | No other bias was noted |
Sutton 1988.
| Methods | Randomised cross‐over trial Study setting: UK Study duration: 4 occasions |
|
| Participants | 8 people, age range of 36 to 71 years, mean sputum production of 30 g, diagnostic criteria not stated, functional level not stated | |
| Interventions | Intervention: PD (position not disclosed) with forced expiration technique for 20 minutes Control: resting sitting position No bronchodilator or normal airway clearance was provided on study morning before treatment |
|
| Outcomes | Spirometry (FEV1, FVC) Sputum wet weight (g) (during and 30 minutes post treatment) Radioaerosol clearance (whole lung and inner/outer region clearance) |
|
| Notes | No bronchodilator or normal airway clearance was provided on study morning before treatment Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the treatment schedules, given in randomised order..." Comment: unclear whether this was adequate |
| Allocation concealment (selection bias) | Unclear risk | No information was provided Comment: unclear whether this was adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information was provided Comment: Participants and therapists were not likely to have been blinded to group allocation. This may have affected outcome measures (FEV1, FVC, sputum weight and radioaerosol clearance) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided Comment: Outcome assessors were not likely to be blinded to group allocation. This may have affected outcome measures (FEV1, FVC, sputum weight and radioaerosol clearance) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data were available for reported outcomes |
| Selective reporting (reporting bias) | Low risk | Complete data were available for reported outcomes |
| Other bias | High risk | Washout period was unclear (presumably consecutive days) |
Svenningsen 2013.
| Methods | Randomised cross‐over trial Setting: Canada Study duration: 6 weeks |
|
| Participants | 14 people, clinically stable, diagnosed by HRCT (lobes affected or disease severity not stated), mean age 70 years, mean FEV1 69% predicted, mean 6MWD 410 m | |
| Interventions | Intervention: oscillating PEP therapy using Aerobika®, consisting of 10 to 20 blows through device, followed by 2 to 3 huff coughs, 4 times daily for 3 weeks Control: no airway clearance therapy. |
|
| Outcomes | HRQoL: SGRQ Exercise capacity: 6‐minute walk distance Spirometry (FEV1, FVC, FEV1/FVC) Static lung volumes (TLC) Symptoms (patient evaluation questionnaire) |
|
| Notes | No adverse events Unclear as to consistency of other medical care provided during study period Funding: This study was funded by Canadian Institute of Health Research and Trundell Medical International through a grant‐in‐aid |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects with non‐CF bronchiectasis were randomized to perform OPEP four‐times daily in a cross‐over controlled study" Comment: unclear whether this was adequate |
| Allocation concealment (selection bias) | Unclear risk | No information was provided Comment: unclear whether this was adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information was provided Comment: Cross‐over nature of the trial limits ability to blind participants and therapists not likely to have been blinded to group allocation. For participants, likely to have affected outcome measures (FEV1, FVC, HRQoL measures, exercise capacity and patient evaluation of symptoms) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided Comment: Outcome assessors were not likely to be blinded to group allocation. This may have affected outcome measures (FEV1, FVC, exercise capacity, HRQoL and symptoms) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete data were available for reported outcomes |
| Selective reporting (reporting bias) | Low risk | Protocol was provided. Complete data were available for reported outcomes |
| Other bias | High risk | No washout period was provided |
6MWD: 6‐minute walk test; COPD: chronic obstructive pulmonary disease; ELTGOL: expiration with the glottis open in the lateral posture; FEF25‐75%: forced expiratory flow between 25% and 75% of vital capacity; FEV1: forced expiratory volume in 1 second; FRC: functional residual capacity; FVC: forced vital capacity; HFCWO: high‐frequency chest wall oscillation; HRCT: high‐resolution computed tomography; HRQoL: health‐related quality of life; IC: inspiratory capacity; IQR: interquartile range; LCQ: Leicester Cough Questionnaire; MMRC: Modified Medical Research Council; PaO2: partial pressure of oxygen in the blood; PaCO2: partial pressure of carbon dioxide in the blood; PD: postural drainage; PEFR: peak expiratory flow rate; PEP: positive expiratory pressure; RV: residual volume; SGRQ: St George's Respiratory Questionnaire; TLC: total lung capacity; VC: vital capacity.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altiay 2012 | No appropriate control |
| Ambrosino 1995 | Mixed disease sample, bronchiectasis data not available (via correspondence with study author) |
| Antunes 2001 | No appropriate control |
| Bernabeu Lledo 2014 | No appropriate control |
| Bilton 2013 | Not an ACT |
| Bradley 2006 | Not an ACT |
| Briffa 2011 | Not an ACT |
| Camargo 2013 | Not an ACT |
| Cecins 1999 | Mixed disease sample, bronchiectasis data not available (via correspondence with study author) |
| Cegla 1993 | No appropriate control |
| Clinkscale 2012 | No appropriate control |
| Conway 1992 | Not an ACT |
| Crisafulli 2007 | Not an ACT |
| Currie 1988 | Not an ACT, not a randomised trial |
| Daviskas 2008 | Not an ACT |
| De Diego 2013 | Not an ACT |
| Eaton 2007 | No appropriate control |
| Gallon 1991 | No appropriate control |
| Gastaldi 2011 | No appropriate control |
| Gokdemir 2014 | No appropriate control |
| Gurses 2013 | Not an ACT |
| Hartsell 1987 | Mixed disease sample, unable to contact study author for bronchiectasis data |
| Hasani 1994 | Mixed disease sample, data for bronchiectasis not available (via correspondence with study author) |
| Hasani 1995 | Mixed disease sample, data for bronchiectasis not available (via correspondence with study author) |
| Herrero 2011 | No appropriate control |
| Herrero 2014 | No appropriate control |
| Huang 2014 | No ACTs |
| Indinnimeo 2007 | No appropriate control |
| Joshi 2004 | Not an ACT |
| Kaminska 1988 | Not a randomised trial |
| Kellett 2005 | Not an ACT |
| Kellett 2011 | Not an ACT |
| Larroquet 1997 | Not an ACT |
| Lee 2014 | Not an ACT |
| Long 2001 | Mixed disease sample, unable to contact study author for bronchiectasis data |
| Maa 2007 | Not an ACT |
| Mandal 2012 | Not an ACT |
| Masekela 2013 | Not an ACT |
| Mazzocco 1985 | Not a randomised trial |
| Moran 2007 | No appropriate control |
| Morgan 1999 | No appropriate control |
| Mutalithas 2008 | Not a randomised trial, no appropriate control |
| Nakamura 2003 | Not an ACT |
| Naraparaju 2010 | No appropriate control |
| Narayanan 2014 | No appropriate control |
| Newall 2005 | Not an ACT |
| Newton 1979 | Not a randomised trial |
| Noone 1999 | Not an ACT |
| Osadnik 2014 | Not conducted in bronchiectasis, not an ACT |
| Paneroni 2011 | No appropriate control |
| Patterson 2004 | No appropriate control |
| Patterson 2005 | No appropriate control |
| Patterson 2007 | No appropriate control |
| Polverino 2012 | No appropriate control |
| Santamaria 1998 | Not a randomised trial |
| Savci 1998 | Not a randomised trial |
| Su 2012 | No appropriate control |
| Sutton 1983 | Mixed disease sample, unable to contact study author for bronchiectasis data |
| Syed 2009 | No appropriate control |
| Tabernero 2014 | Not an ACT |
| Tabernero Huguet 2015 | No ACTs |
| Tambascio 2011 | No appropriate control |
| Tambascio 2014 | No relevant outcomes reported |
| Thompson 2002 | No appropriate control |
| Tsang 1994 | Not an ACT |
| Tsang 2003 | No appropriate control (via correspondence with study author) |
| Valente 2004 | No relevant outcomes reported |
| Venturelli 2013 | No appropriate control |
| Volker 1972 | Not an ACT |
| Wilson 1995 | Mixed disease group, bronchiectasis data not available (via correspondence with study author) |
| Yilva 2015 | No appropriate control |
ACT: airway clearance technique.
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00700388.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in 2014 search. Further information required to confirm appropriateness |
NCT01112410.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in 2014 search. Further information required to confirm appropriateness |
NCT01209546.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in 2014 search. Further information required to confirm appropriateness |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12610000078055.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Identified in 2014 search. Further information required to confirm appropriateness |
ACTRN12614001072606.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Identified in 2014 search. Further information required to confirm appropriateness |
ACTRN12614001233617.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Identified in 2014 search. Further information required to confirm appropriateness |
NCT00452114.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Identified in 2014 search. Further information required to confirm appropriateness |
NCT01480882.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Identified in 2014 search. Further information required to confirm appropriateness |
NCT01578681.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Identified in 2014 search. Further information required to confirm appropriateness |
NCT01854788.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Identified in 2014 search. Further information required to confirm appropriateness |
NCT01929356.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Identified in 2014 search. Further information required before classification |
NCT02324855.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Identified in 2014 search. Further information required to confirm appropriateness |
Differences between protocol and review
We specified a measure of health status (COPD Assessment Tool) as a reflection of HRQoL in this update. Although originally developed for assessment of health status, it has been subsequently applied as a measure of HRQoL in studies with chronic respiratory disease populations. We specified a subgroup analysis of PEP therapy for this review and reported these measurements separately because of the small number of included studies. We did not perform sensitivity analysis and did not construct funnel plots because of the small number of included studies. If in future updates additional studies are included, we will perform these analyses.
Contributions of authors
Initiated protocol: AH.
Developed protocol: AL, AB and AH.
Undertook literature search for the original version: AL and AB; for the updated version: AL and AB.
Retrieved papers for the original version: AL; for the updated version: AL.
Screened retrieved papers against eligibility criteria for the original version: AL and AB; for the updated version: AL and AB.
Appraised quality for the original version: AL and AB; for the updated version: AL and AB.
Extracted data for the original version: AL and AB; for the updated version: AL and AB.
Wrote to study authors to ask for additional information for the original version: AL; for the updated version: AL.
Entered data into RevMan for the original version: AL; for the updated version: AL.
Performed analysis for the original version: AL and AB; for the updated version: AL.
Wrote the review for the original version: AL, AB and AH; amended the manuscript for the updated version: AL; reviewed the updated version of the manuscript: AB and AH.
Served as guarantor for the review: AH.
Sources of support
Internal sources
The authors declare that no such funding was received for this systematic review, Other.
External sources
The authors declare that no funding was received for this systematic review, Other.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Figueiredo 2010 {published data only}
- Figueiredo PHS, Zin WA, Guimaraes FS. Flutter valve improves respiratory mechanics and sputum production in patients with bronchiectasis. Physiotherapy Research International 2010;17:12‐20. [DOI] [PubMed] [Google Scholar]
Guimaraes 2012 {published data only}
- Guimaraes FS, Moco VJR, Menezes SLS, Dias CM, Salles REB, Lopes AJ. Effects of ELTGOL and Flutter VRP1 on the dynamic and static pulmonary volumes and on the secretion clearance of patients with bronchiectasis [Efeitos da ELTGOL e do Flutter nos volumes pulmonares dinamicos e estaticos e na remocao de secrecao de pacientes com bronquiectasia]. Revista Brasileira de Fisioterapia 2012;16(2):108‐13. [PubMed] [Google Scholar]
Kurz 1997 {published data only}
- Kurz M, Ahrens P, Zielen S, Hofmann D. Physiotherapy with the VRP‐1 device. Objective and subjective results of a comparative study in children with chronic lung diseases [Physiotherapie mit dem VRP1‐Gerat: Objektive und subjektive Ergebnisse einer vergleichenden Studie bei Kindern mit chronischen Lungenerkrankungen]. Pravention und Rehabilitation 1997;9(3):105‐9. [Google Scholar]
Murray 2009 {published data only (unpublished sought but not used)}
- Murray MP, Pentland JL, Hill AT. A randomised crossover trial of chest physiotherapy in non‐cystic fibrosis bronchiectasis. European Respiratory Journal 2009;34(5):1086‐92. [DOI] [PubMed] [Google Scholar]
Nicolini 2013 {published and unpublished data}
- Nicolini A, Cardini F, Landucci N, Lanata S, Ferrari‐Bravo M, Barlascini C. Effectiveness of treatment with high‐frequency chest wall oscillation in patients with bronchiectasis. BMC Pulmonary Medicine 2013;13(21):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sutton 1988 {published data only (unpublished sought but not used)}
- Sutton PP, Gemmell HG, Innes N, Davidson J, Smith FW, Legge JS. Use of nebulised saline and nebulised terbutaline as an adjunct to chest physiotherapy. Thorax 1988;43:57‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Svenningsen 2013 {published and unpublished data}
- Svenningsen S, Paulin G, Wheatley A, Pike D, Suggett J, McCormack D, et al. Oscillating positive expiratory pressure therapy in chronic obstructive pulmonary disease and bronchiectasis. Chest 2013;144(741A):741. [Google Scholar]
References to studies excluded from this review
Altiay 2012 {published data only}
- Altiay G, Uzmezoglu B, Sut N, Tuna H, Hatipoglu ON. Evaluating flutter device and the active cycle of breathing technique in non‐cystic bronchiectasis: the prospective randomised study. European Respiratory Journal 2012;40(56s):449s. [Google Scholar]
Ambrosino 1995 {published data only (unpublished sought but not used)}
- Ambrosino N, Callegari G, Galloni C, Brega S, Pinna G. Clinical evaluation of oscillating positive expiratory pressure for enhancing expectoration in diseases other than cystic fibrosis. Monaldi Archives for Chest Disease 1995;50(4):269‐75. [PubMed] [Google Scholar]
Antunes 2001 {published data only}
- Antunes LC, Carvalho SMF, Borges FD, Assis VL, Godoy I. A study of the conventional chest physiotherapy versus Flutter VRP1 in the treatment of patients carrying bronchiectasis. Salusvita 2001;20(1):23‐30. [Google Scholar]
Bernabeu Lledo 2014 {published data only}
- Bernabeu Lledo M, Atin Arratibel MA, Martinez Tardido M, Gonzalez Cifuentes M, Fuertes Conejo R. Efficacy of respiratory physiotherapy combined with ventilation percussive intrapulmonary (VPI) in stable adult. Annals of Physical and Rehabilitation Medicine 2014;57:e326. [Google Scholar]
Bilton 2013 {published data only}
- Bilton D, Daviskas E, Anderson SD, Kolbe J, King G, Stirling RG, et al. Phase 3 randomized study of the efficacy and safety of inhaled dry powder mannitol for the symptomatic treatment of non‐cystic fibrosis bronchiectasis. Chest 2013;44(1):215‐25. [DOI] [PubMed] [Google Scholar]
Bradley 2006 {published data only}
- Bradley J, Moran F. Pulmonary rehabilitation improves exercise tolerance in patients with bronchiectasis. Australian Journal of Physiotherapy 2006;52(1):65. [DOI] [PubMed] [Google Scholar]
Briffa 2011 {published data only}
- Briffa PJ, Anderson SD, Burton DL, Young IH. Sodium cromoglycate and eformoterol attenuate sensitivity and reactivity to inhaled mannitol in subjects with bronchiectasis. Respirology 2011;16(5):161‐6. [DOI] [PubMed] [Google Scholar]
Camargo 2013 {published data only}
- Camargo AA, Lanza FC, Tupinamba T, Corso SD. Reproducibility of step tests in patients with bronchiectasis. Brazilian Journal of Physical Therapy 2013;17(3):255‐62. [DOI] [PubMed] [Google Scholar]
Cecins 1999 {published data only}
- Cecins N, Jenkins SC, Pengelley J, Ryan G. The active cycle of breathing techniques ‐ to tip or not to tip?. Respiratory Medicine 1999;93(9):660‐5. [DOI] [PubMed] [Google Scholar]
Cegla 1993 {published data only}
- Cegla UH, Retzow A. Physiotherapy in chronic obstructive lung diseases ‐ experiences with the VRP1 Desitin in a multicentre comparative study [Physiotherapie mit dem VRP1 bei chronisch obstruktiven Atemwegserkrankungen ‐ Ergebnisse einer multizentrischen vergleichsstudie]. Pneumologie 1993;47:636‐9. [PubMed] [Google Scholar]
Clinkscale 2012 {published data only}
- Clinkscale D, Spihlman K, Watts P, Rosenbluth D, Kollef MH. A randomised trial of conventional chest physical therapy versus high frequency chest wall compressions in intubated and non‐intubated adults. Respiratory Care 2012;57(8):221‐8. [DOI] [PubMed] [Google Scholar]
Conway 1992 {published data only}
- Conway JH, Fleming JS, Perring S, Holgate ST. Humidification as an adjunct to chest physiotherapy in aiding tracheo‐bronchial clearance in patients with bronchiectasis. Respiratory Medicine 1992;86(2):109‐14. [DOI] [PubMed] [Google Scholar]
Crisafulli 2007 {published data only}
- Crisafulli E, Coletti O, Costi S, Zanasi E, lorenzi C, Lucic S, et al. Effectiveness of erdosteine in elderly patients with bronchiectasis and hypersecretion: a 15 days prospective, parallel, open‐label, pilot study. Clinical Therapeutics 2007;29(9):2001‐9. [DOI] [PubMed] [Google Scholar]
Currie 1988 {published data only}
- Currie DC, Greenstone M, Pavia D, Agnew JE, Pellow P, Clarke SW, et al. Efficacy of 'mucoregulatory' agents in Young's syndrome. Thorax 1988;43(6):480‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daviskas 2008 {published data only}
- Daviskas E, Anderson SD, Eberl S, Young IH. Effect of increasing doses of mannitol on mucus clearance in patients with bronchiectasis. European Respiratory Journal 2008;31(4):765‐72. [DOI] [PubMed] [Google Scholar]
De Diego 2013 {published data only}
- Diego A, Milara J, Martinez‐Moragon E, Palop M, Leon M, Cortijo J. Effects of long‐term azithromycin therapy on airway oxidative stress markers innon‐cystic fibrosis bronchiectasis. Respirology 2013;18:1056‐62. [DOI] [PubMed] [Google Scholar]
Eaton 2007 {published data only}
- Eaton T, Young P, Zeng I, Kolbe J. A randomised evaluation of the acute efficacy, acceptability and tolerability of Flutter and active cycle of breathing with and without postural drainage in non‐cystic fibrosis bronchiectasis. Chronic Respiratory Disease 2007;4(1):23‐30. [DOI] [PubMed] [Google Scholar]
Gallon 1991 {published data only}
- Gallon A. Evaluation of chest percussion in the treatment of patients with copious sputum production. Respiratory Medicine 1991;85(1):45‐51. [DOI] [PubMed] [Google Scholar]
Gastaldi 2011 {published data only}
- Gastaldi A, Tambascio J, Lisboa R, Passarelli R, Tatiana L. Effect of the components of Flutter VRP1 on the transport of respiratory secretions in patients with bronchiectasis. World Physical Therapy Conference proceedings 2011;97:eS395‐eS396. [Google Scholar]
Gokdemir 2014 {published data only}
- Gokdemir Y, Karadag‐Saygi E, Erdem E, Bayindir O, Ersu R, Karadag B, et al. Comparison of conventional pulmonary rehabilitation and high‐frequency chest wall oscillation in primary ciliary dyskinesia. Pediatric Pulmonology 2014;49(6):611‐6. [DOI] [PubMed] [Google Scholar]
Gurses 2013 {published data only}
- Gurses HN, Ayhan B, Demir R, Ozyilmaz S. The effects of inspiratory muscle training on respiratory muscle strength and pulmonary functions in children with bronchiectasis. European Respiratory Journal 2013;47(Suppl 57):5064. [Google Scholar]
Hartsell 1987 {published data only}
- Hartsell MB. Chest physiotherapy and mechanical vibration. Journal of Paediatric Nursing 1987;2(2):135‐7. [PubMed] [Google Scholar]
Hasani 1994 {published data only}
- Hasani A, Pavia D, Agnew JE, Clarke SW. Regional lung clearance during cough and forced expiration technique (FET): effects of flow and viscoelasticity. Thorax 1994;49(6):557‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasani 1995 {published data only}
- Hasani A, Toms N, Agnew JE, Pavia D, Clarke SW. Regional mucus clearance following cough and forced expiration technique in patients with asthma, chronic bronchitis and bronchiectasis. European Respiratory Journal Supplement 1995;8:19297. [Google Scholar]
Herrero 2011 {published data only}
- Herrero B, Polverino E, Romeu DM, Vilaro J. Efficacy of airway clearance therapy with different autonomy degrees in nonCF‐bronchiectasis: randomised cross‐over trial. European Respiratory Society 21st Annual Congress; 2011 Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 3:540s (2977).
Herrero 2014 {published data only}
- Herrero B, Polverino E, SanMiguel M, Alcaraz V, Marti D, Vilaro J, Torres A. Wet sputum as an objective outcome in a randomized crossover trial in NCF‐BE: Reliability and responsiveness. European Respiratory Journal 2014;44:P4274. [Google Scholar]
Huang 2014 {published data only}
- Huang H, Yang P, Xue J, Tang J, Ding L, Ma Y, Wang J, Guyatt GH, Vannivasingam T, Zhang Y. Evaluating the individualized treatment of traditional Chinese medicine: A pilot study of N‐of‐1 trials.. Evidence‐based Complementary and Alternative Medicine 2014;2014:148730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Indinnimeo 2007 {published data only}
- Indinnimeo L, Tancredi G, Barreto M, Castro G, Zicari AM, Monaco F, et al. Effects of a program of hospital‐supervised chest physical therapy on lung function tests in children with chronic respiratory disease: 1‐year follow‐up. International Journal of Immunopathology and Pharmacology 2007;20(4):841‐5. [DOI] [PubMed] [Google Scholar]
Joshi 2004 {published data only}
- Joshi JM, Sundaram P. Role of inhaled steroids in stable bronchiectasis. Indian Practitioner 2004;57(4):243‐5. [Google Scholar]
Kaminska 1988 {published data only}
- Kaminska TM, Pearson SB. A comparison of postural drainage and positive expiratory pressure in the domiciliary management of patients with chronic bronchial sepsis. Physiotherapy 1988;74(5):251‐4. [Google Scholar]
Kellett 2005 {published data only}
- Kellett F, Redfern J, Niven RM. Evaluation of nebulised hypertonic saline (7%) as an adjunct to physiotherapy in patients with stable bronchiectasis. Respiratory Medicine 2005;99(1):27‐31. [DOI] [PubMed] [Google Scholar]
Kellett 2011 {published data only}
- Kellett F, Robert NM. Nebulised 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respiratory Medicine 2011;105(4):1831‐5. [DOI] [PubMed] [Google Scholar]
Larroquet 1997 {published data only}
- Larroquet M, Balquet P, Gruner M. Video‐assisted thorascopic surgery for bronchiectasis. Pediatric Pulmonology 1997;24 Suppl 16:180. [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee AL, Hill CJ, Cecins N, Jenkins S, McDonald CF, Burge AT, et al. The short and long term effects of exercise training in non‐cystic fibrosis bronchiectasis ‐ a randomised controlled trial. Respiratory Research 2014;15(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Long 2001 {published data only}
- Long C. The Effects of Manual Chest Percussion in Patients with Chronic Obstructive Pulmonary Disease and Bronchiectasis. East Sussex Hospitals NHS Trust 2001.
Maa 2007 {published data only}
- Maa SH, Tsou TS, Wang KY, Wang CH, Lin HC, Huang YH. Self‐administered acupressure reduces the symptoms that limit daily activities in bronchiectasis patients: pilot study findings. Journal of Clinical Nursing 2007;16(4):794‐804. [DOI] [PubMed] [Google Scholar]
Mandal 2012 {published data only}
- Mandal P, Sidhu MK, Kope L, Pollock W, Stevenson LM, Pentland JL, et al. A pilot study of pulmonary rehabilitation and chest physiotherapy versus chest physiotherapy alone in bronchiectasis. Respiratory Medicine 2012;106(12):1647‐54. [DOI] [PubMed] [Google Scholar]
Masekela 2013 {published data only}
- Masekela R, Anderson R, Gongxeka H, Steel HC, Becker PJ, Green RJ. Lack of efficacy of an immunomodulatory macrolide in childhood HIV related bronchiectasis: a randomised, placebo‐controlled trial. Journal of Antivirals and Antiretrovirals 2013;5(2):44‐9. [Google Scholar]
Mazzocco 1985 {published data only}
- Mazzocco MC, Owens GR, Kirilloff LH, Rogers RM. Chest physiotherapy and postural drainage in patients with bronchiectasis. Chest 1985;88(3):360‐3. [DOI] [PubMed] [Google Scholar]
Moran 2007 {published data only}
- Moran FM, Elborn SE, Bradbury I, Piper A, Hewitt O, Bradley JM. Airway clearance in bronchiectasis: is non‐invasive ventilation a useful adjunct in moderate to severe disease?. Thorax 2007;62 Suppl III:A87. [Google Scholar]
Morgan 1999 {published data only}
- Morgan LC, Maxwell L, Longhi R, Peters MJ, Rutland J. Comparison of Flutter device (FD) with postural drainage (PD) in adult patients with non‐CF bronchiectasis. Proceedings of the Thoracic Society of Australia and New Zealand 1999;52:P93. [Google Scholar]
Mutalithas 2008 {published data only}
- Mutalithas K, Watkin G, Willig B, Wardlaw A, Pavord ID, Birring SS. Improvement in health status following bronchopulmonary hygiene physical therapy in patients with bronchiectasis. Respiratory Medicine 2008;102(8):1140‐4. [DOI] [PubMed] [Google Scholar]
Nakamura 2003 {published data only}
- Nakamura S, Hashimoto Y, Mikami M, Yamanaka E, Soma T, Hino M, et al. Effect of the proteolytic enzyme serrapeptase in patients with chronic airway disease. Respirology 2003;8(3):316‐20. [DOI] [PubMed] [Google Scholar]
Naraparaju 2010 {published data only}
- Naraparaju S, Vaishali K, Venkatesan P, Acharya V. A comparison of the Acapella and a threshold inspiratory muscle trainer for sputum clearance in bronchiectasis ‐ a pilot study. Physiotherapy Theory and Practice 2010;26(6):353‐7. [DOI] [PubMed] [Google Scholar]
Narayanan 2014 {published data only}
- Narayanan A, Meng O, Hyder Ali I A, Izmi M, Ahmad I, Rehab M, et al. A pilot randomized control cross over study evaluating the effectiveness and safety of mechanical percussor compared with conventional chest physiotherapy in adults with productive cough. Medical Journal of Malaysia 2014;69(1):16‐20. [PubMed] [Google Scholar]
Newall 2005 {published data only}
- Newall C, Stockley RA, Hill SL. Exercise training and inspiratory muscle training in patients with bronchiectasis. Thorax 2005;60(11):943‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newton 1979 {published data only}
- Newton DA. Chest physiotherapy under scrutiny. Lancet 1979;1(8106):49‐50. [DOI] [PubMed] [Google Scholar]
Noone 1999 {published data only}
- Noone PG, Bennett WD, Regnis JA, Zeman KL, Carson JL, King M, et al. Effect of aerosolized uridine‐5'‐triphosphate on airway clearance with cough in patients with primary ciliary dyskinesia. American Journal of Respiratory and Critical Care Medicine 1999;160(1):144‐9. [DOI] [PubMed] [Google Scholar]
Osadnik 2014 {published data only}
- Osadnik C, Stuart‐Andrews C, Ellis SE, Thompson B, McDonald CF, Holland AE. Positive expiratory pressure via mask does not improve ventilation inhomogeneity more than huffing and coughing in individuals with stable chronic obstructive pulmonary disease and chronic sputum expectoration. Respiration 2014;87(1):38‐44. [DOI] [PubMed] [Google Scholar]
Paneroni 2011 {published data only}
- Paneroni M, Clini E, SImonelli C, Bianchi L, Degli Antoni F, Vitacca M. Safety and efficacy of short‐term intrapulmonary percussive ventilation in patients with bronchiectasis. Respiratory Care 2011;56(7):984‐8. [DOI] [PubMed] [Google Scholar]
Patterson 2004 {published data only}
- Patterson JE, Bradley JM, Elborn JS. Airway clearance in bronchiectasis: a randomized crossover trial of active cycle of breathing techniques (incorporating postural drainage and vibration) versus test of incremental respiratory endurance. Chronic Respiratory Disease 2004;1(3):127‐30. [DOI] [PubMed] [Google Scholar]
Patterson 2005 {published data only}
- Patterson JE, Bradley J, Hewitt O, Bradbury I, Elborn JS. Airway clearance in bronchiectasis: a randomised cross‐over trial of active cycle of breathing techniques versus acapella. Respiration 2005;72:239‐42. [DOI] [PubMed] [Google Scholar]
Patterson 2007 {published data only (unpublished sought but not used)}
- Patterson JE, Hewitt O, Kent L, Bradbury I, Elborn JS, Bradley JM. Acapella versus 'usual airway clearance' during acute exacerbation in bronchiectasis: a randomised crossover trial. Chronic Respiratory Disease 2007;4(2):67‐74. [DOI] [PubMed] [Google Scholar]
Polverino 2012 {published data only}
- Polverino E, Herrero B, Vilaro J, Marti D. Efficacy of airway clearance therapy with different autonomy degrees in non‐CF bronchiectasis: a randomised cross‐over trial. American Journal of Respiratory and Critical Care Medicine 2012;185(1):A4876. [Google Scholar]
Santamaria 1998 {published data only}
- Santamaria F, Celentano L, Buonpensiero P, Sarnelli P, Caterino M, Raia V. Positive expiratory pressure treatment: efficacy in pulmonary diseases. Journal of Pediatrics 1998;133(5):717‐8. [DOI] [PubMed] [Google Scholar]
Savci 1998 {published data only}
- Savci S, Inal D, Arikan H. A comparison of the active cycle of breathing technique with and without manual techniques in patients with bronchiectasis. European Respiratory Journal 1998;1 Suppl 12:28212S. [Google Scholar]
Su 2012 {published data only}
- Su CL, Chang CC, Lin YK, Lee KT, Lee CN, Chiang LL. Randomised crossover study of lung expansion therapy using negative pressure and positive pressure in bronchiectasis. Journal of Experimental and Clinical Medicine 2012;4(7):149‐53. [Google Scholar]
Sutton 1983 {published data only (unpublished sought but not used)}
- Sutton PP, Parker RA, Webber BA, Newman SP, Garland N, Lopez Vidriero MT, et al. Assessment of the forced expiration technique, postural drainage and directed coughing in chest physiotherapy. European Journal of Respiratory Diseases 1983;64(1):62‐8. [PubMed] [Google Scholar]
Syed 2009 {published data only}
- Syed N, Maiya AG, Siva Kumar T. Active cycles of breathing technique (ACBT) versus conventional chest physical therapy on airway clearance in bronchiectasis ‐ crossover trial. Advances in Physiotherapy 2009;11(4):193‐8. [Google Scholar]
Tabernero 2014 {published data only}
- Tabernero A, Gil P, Alkiza R, Garros J, Hernandez A, Artola JL. Inhaled colistin in elderly patients with non‐cystic fibrosis bronchiectasis and chronic Pseudomonas aeruginosa (PA) bronchial infection. Chest 2014;145(Suppl 3):431A. [Google Scholar]
Tabernero Huguet 2015 {published data only}
- Tabernero Huguet E, Gil Alana P, Alkiza Basanez R, Hernandez Gil A, Garros Garay J, Artola Igarza JL. Inhaled colistin in elderly patients with non‐cystic fibrosis bronchiectasis and chronic Pseudomonas aeruginosa bronchial infection [Tratamiento inhalado con colistina a largo plazo en pacientes ancianos con infeccion cronica por Pseudomonas aeruginosa y bronquiectasias]. Revista Espanola de Geriatria y Gerontologia 2015;50:111‐5. [DOI] [PubMed] [Google Scholar]
Tambascio 2011 {published data only}
- Tambascio J, Souza LT, Lisboa RMh, Passarelli RDCV, Souza HCD, Gastaldi AC. The influence of Flutter VRP1 components of mucus transport of patients with bronchiectasis. Respiratory Medicine 2011;105(9):1316‐21. [DOI] [PubMed] [Google Scholar]
Tambascio 2014 {published data only}
- Tambascio J, Baddini‐Martinez JA, Passarelli CV, Goncalves EC, Miranda APB, Martinez R, Gastaldi AC. Effects of Flutter ® VRP1 on inflammatory profile, microbiology and transport indexes of respiratory secretions of patients with bronchiectasis.. American Journal of Respiratory and Critical Care Medicine 2014;189:A6260. [Google Scholar]
Thompson 2002 {published data only}
- Thompson CS, Harrison S, Ashley J, Day K, Smith DL. Randomised crossover study of the Flutter device and the active cycle of breathing technique in non‐cystic fibrosis bronchiectasis. Thorax 2002;57(5):446‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsang 1994 {published data only}
- Tsang KWT, Roberts P, Read RC, Kees R, Wilson R, Cole PJ. The concentrations of clarithromycin and its 14‐hydroxy metabolite in sputum of patients with bronchiectasis following single dose administration. Journal of Antimicrobial Chemotherapy 1994;33(2):289‐97. [DOI] [PubMed] [Google Scholar]
Tsang 2003 {published data only (unpublished sought but not used)}
- Tsang SMH, Jones AYM. Postural drainage or flutter device in conjunction with breathing and coughing compared to breathing and coughing alone in improving secretion removal and lung function in patients with acute exacerbation of bronchiectasis. A pilot study. Hong Kong Physiotherapy Journal 2003;21:29‐36. [Google Scholar]
Valente 2004 {published data only}
- Valente AM, Gastaldi AC, Cravo SL, Afonso JL, Sologuren MJJ, Guimaraes RC. The effect of two techniques on the characteristics and transport of sputum in patients with bronchiectasis. A pilot study. Physiotherapy 2004;90(3):158‐64. [Google Scholar]
Venturelli 2013 {published data only}
- Venturelli E, Crisafulli E, deBiase A, Righi D, Berrighi D, Cavicchioli PP, et al. Efficacy of temporary positive expiratory pressure (TPEP) in patients with lung diseases and chronic mucus hypersecretion. The UNIKO(R) project: a multicentre randomized controlled trial. Clinical Rehabilitation 2013;27(4):336‐46. [DOI] [PubMed] [Google Scholar]
Volker 1972 {published data only}
- Volker C. Objectivation of treatment effects in children with respiratory tract diseases using spirometric examinations in the people's saline bath resort, Kosen. Zeitschrift Fur Physiotherapie 1972;24(5):383‐90. [PubMed] [Google Scholar]
Wilson 1995 {published data only}
- Wilson GE, Baldwin AL, Walshaw MJ. A comparison of traditional chest physiotherapy with the active cycle of breathing in patients with chronic suppurative lung disease. European Respiratory Journal 1995;8 Suppl 19:171S. [Google Scholar]
Yilva 2015 {published data only}
- Silva Y, Greer T, Farah C, Li F, Morgan L. Lung flute is comparable to flutter device for sputum clearance in adults with non‐cystic fibrosis bronchiectasis. Respirology 2015;20:114(TP 143). [Google Scholar]
References to studies awaiting assessment
NCT00700388 {published data only}
- NCT00700388. Efficacy of temporary positive expiratory pressure (TPEP) in chronic hypersecretion (UNIKO). https://clinicaltrials.gov/ct2/show/ (accessed 10 April 2015).
NCT01112410 {published data only}
- NCT01112410. Hypertonic saline (6%) versus isotonic saline (0.9%) in bronchiectasis. https://clinicaltrials.gov/ct2/show/NCT01112410 (accessed 10 April 2015).
NCT01209546 {published data only}
- NCT01209546. Study of the effect of FLUTTER® VRP1 (PEP and Oscillating High Frequency). (FLUTTER®VRP1). https://clinicaltrials.gov/ct2/show/NCT01209546 (accessed 10 April 2015).
References to ongoing studies
ACTRN12610000078055 {published data only}
- ACTRN12610000078055. Acute effect of slow expiration with the glottis open in lateral posture ("expiration lente totale glotte ouverte en infralatéral", ELTGOL) and huff on physical properties of sputum from patients with bronchiectasis: a pilot study. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335040 (accessed 2 July 2015).
ACTRN12614001072606 {published data only}
- ACTRN12614001072606. Evaluating the ease of secretion clearance with flutter device compared to lung Flute in patients with bronchiectasis. WHO Clinical Trials 2013.
ACTRN12614001233617 {published data only}
- ACTRN12614001233617. Does the bubble‐positive expiratory pressure (PEP) device improve secretion clearance compared to the active cycle of breathing technique (ACBT) or no intervention (control) in people with non‐cystic fibrosis bronchiectasis?. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614001233617 (accessed 3 July 2015).
NCT00452114 {published data only}
- NCT00452114. In‐exsufflator cough assist device in patients with symptomatic bronchiectasis. https://clinicaltrials.gov/ct2/show/NCT00452114 (accessed 10 April 2015).
NCT01480882 {published data only}
- NCT01480882. Efficacy and safety study of a percussion device to mobilise sputum from respiratory passage (LEGA). https://clinicaltrials.gov/ct2/show/NCT01480882 (accessed 10 April 2015).
NCT01578681 {published data only}
- NCT01578681. ELTGOL and bronchiectasis. Respiratory therapy (ELTGOLBQ). https://clinicaltrials.gov/ct2/show/NCT01578681 (accessed 10 April 2015).
NCT01854788 {published data only}
- NCT01854788. 3 airway clearance techniques in non cystic fibrosis bronchiectasis. https://clinicaltrials.gov/ct2/show/NCT01854788 (accessed 10 April 2015).
NCT01929356 {published data only}
- NCT01929356. Chest physiotherapy and lung function in primary ciliary dyskinesia. https://clinicaltrials.gov/ct2/show/NCT01929356 (accessed 10 April 2015).
NCT02324855 {published data only}
- NCT02324855. Long‐term airway clearance therapy in non‐cystic fibrosis bronchiectasis. https://clinicaltrials.gov/ct2/show/NCT02324855 (accessed 10 April 2015).
Additional references
AIHW 2005
- Australian Institute of Health and Welfare. Chronic Respiratory Diseases in Australia: Their Prevalence, Consequences and Prevention. http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=6442459693 (accessed 3 July 2015).
Arens 1994
- Arens RA, Gozal D, Omlin KJ, Vega J, Boyd KP, Keens TG, et al. Comparison of high frequency chest compression and conventional chest physiotherapy in hospitalised patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 1994;114:171‐7. [DOI] [PubMed] [Google Scholar]
Barker 2002
- Barker A. Medical progress: bronchiectasis. New England Journal of Medicine 2002;346(18):1383‐93. [DOI] [PubMed] [Google Scholar]
Bateman 1981
- Bateman J, Newman S, Daunt K, Sheahan N, Pavia D, Clarke S. Is cough as effective as chest physiotherapy in the removal of excessive tracheobronchial secretions?. Thorax 1981;36:683‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bott 2009
- Bott J, Blumenthal S, Buxton M, Ellum S, Falconer C, Garrod R, et al. Guidelines for the physiotherapy management of the adult medical, spontaneously breathing patient. Thorax 2009;64 Suppl 1:i1‐i51. [DOI] [PubMed] [Google Scholar]
Boucher 2010
- Boucher RC. Bronchiectasis: a continuum of ion transport dysfunction or multiple hits?. American Journal of Respiratory and Critical Care Medicine 2010;181:1017‐9. [DOI] [PubMed] [Google Scholar]
Braveman 2007
- Braveman J, Nozzarella M. High‐frequency chest compression advanced therapy for obstructive lung disease. Respiratory Therapy 2007;2(48):51. [Google Scholar]
Chang 2008
- Chang A, Bilton D. Exacerbations of cystic fibrosis: 4 Non‐cystic fibrosis bronchiectasis. Thorax 2008;63:269‐76. [DOI] [PubMed] [Google Scholar]
Chang 2015
- Chang AB, Bell SC, Torzillo PJ, King PT, Maguire GP, Byrnes CA, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand Thoracic Society of Australia and New Zealand guidelines. Medical Journal of Australia 2015;202(1):21‐3. [DOI] [PubMed] [Google Scholar]
Cochrane 1977
- Cochrane GM, Webber BA, Clarke SW. Effect of sputum on pulmonary function. British Medical Journal 1977;2:1181‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cole 1986
- Cole PJ. Inflammation: a two‐edged sword ‐ the model of bronchiectasis. European Journal of Respiratory Disease ‐ Supplement 1986;147:6‐15. [PubMed] [Google Scholar]
Elborne 2007
- Elborne JS, Bell S. Pulmonary exacerbations in cystic fibrosis and bronchiectasis. Thorax 2007;62(4):288‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flude 2012
- Flude LJ, Agent P, Bilton D. Chest physiotherapy techniques in bronchiectasis. Clinics in Chest Medicine 2012;33(2):351‐61. [DOI] [PubMed] [Google Scholar]
Flume 2009
- Flume PA, Robinson KA, O'Sullivan BP, Finder JD, Vender RL, Willey‐Courand DB. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respiratory Care 2009;54:522‐37. [PubMed] [Google Scholar]
Gaga 1998
- Gaga M, Bentley AM, Humbert M, Barkans J, O'Brien F, Wathens CG, et al. Increases in CD4+ T lymphocytes, macrophages, neutrophils and interleukin 8 positive cells in the airways of patients with bronchiectasis. Thorax 1998;53(8):685‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garrard 1978
- Garrard CS, Shah M. The effects of expiratory positive airway pressure on functional residual capacity in normal subjects. Critical Care Medicine 1978;6:320‐2. [DOI] [PubMed] [Google Scholar]
Goeminne 2012
- Goeminne PC, Scheers H, Decraene A, Seys S, Dupont LJ. Risk factors for morbidity and death in non‐cystic fibrosis bronchiectasis: a retrospective cross‐sectional analysis of CT diagnosed bronchiectatic patients. Respiratory Research 2012;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 1994
- Hansen LG, Warwick WJ, Hansen KL. Mucus transport mechanisms in relation to the effect of high frequency chest compression (HFCC) on mucus clearance. Pediatric Pulmonology 1994;17:113‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins J, Altman D (editors). Chapter 8: Assessing risk of bias in included studies. In: HigginsJPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hill 2011
- Hill AT, Pasteur M, Cornford C, Welham S, Bilton D. Primary care summary of the British Thoracic Society guideline on the management of non‐cystic fibrosis bronchiectasis. Primary Care Respiratory Journal 2011;20(2):135‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horsley 2008
- Horsley A, Gustafsson P, Macleod K, Saunders C, Greening AP, Porteus DJ, et al. Lung clearance index is a sensitive, repeatable and practical measure of airway disease in adults with cystic fibrosis. Thorax 2008;63:135‐40. [DOI] [PubMed] [Google Scholar]
Isabey 1984
- Isabey D, Harf A, Chang HK. Alveolar ventilation during high‐frequency oscillation: core dead space concept. Journal of Applied Physiology 1984;56:700‐7. [DOI] [PubMed] [Google Scholar]
Jones 2005
- Jones PW. St George's Respiratory Questionnaire. Journal of Chronic Obstructive Pulmonary Disease 2005;2(11):75‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kempainen 2010
- Kempainen RR, Milla C, Dunitz J, Savik K, Hazelwood A, Williams C, et al. Comparison of settings used for high‐frequency chest wall compression in cystic fibrosis. Respiratory Care 2010;55:695‐701. [PubMed] [Google Scholar]
Kim 1986
- Kim C, Rodriguez C, Eldridge M, Sackner M. Criteria for mucus transport in the airways by two‐phase gas‐liquid flow mechanism. Journal of Applied Physiology 1986;60(3):901‐7. [DOI] [PubMed] [Google Scholar]
Kim 1987
- Kim C, Iglesias A, Sackner M. Mucus clearance by two‐phase gas‐liquid flow mechanism: asymmetric periodic flow model. Journal of Applied Physiology 1987;62(3):957‐71. [DOI] [PubMed] [Google Scholar]
King 2005
- King P, Holdsworth SR, Freezer NJ, Villanueva E, Gallagher M, Holmes PW. Outcome in adult bronchiectasis. Journal of Chronic Obstructive Pulmonary Disease 2005;2:27‐34. [DOI] [PubMed] [Google Scholar]
King 2006a
- King P, Holdsworth SR, Freezer NJ, Holmes PW. Bronchiectasis. Internal Medicine Journal 2006;36:729‐37. [DOI] [PubMed] [Google Scholar]
King 2006b
- King P, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respiratory Medicine 2006;100(12):2183‐9. [DOI] [PubMed] [Google Scholar]
King 2010
- King PT, Daviskas E. Management of bronchiectasis. Breathe 2010;6:353‐60. [Google Scholar]
Koulouris 2003
- Koulouris NG, Retsou S, Kosmas E, Dimakou K, Malagari K, Mantzikopoulos G, et al. Tidal expiratory flow limitation, dyspnoea and exercise capacity in patients with bilateral bronchiectasis. European Respiratory Journal 2003;21:743‐8. [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam M, Akl EA, Dahm P, Glasziou P, Guyatt G, Schunemann HJ. Assessing and presenting summaries of evidence in Cochrane reviews. Systematic Reviews 2013;2(81):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loebinger 2009
- Loebinger M, Wells A, Hansell D, Chinyanganya N, Devaraj A, Meister M, et al. Mortality in bronchiectasis: a long‐term study assessing the factors influencing survival. European Respiratory Journal 2009;34:843‐9. [DOI] [PubMed] [Google Scholar]
Martinez‐Garcia 2005
- Martinez‐Garcia MA, Perpina‐Tordera M, Roman‐Sanchez P, Soler‐Cataluna JJ. Quality‐of‐life determinants in patients with clinically stable bronchiectasis. Chest 2005;128(2):739‐45. [DOI] [PubMed] [Google Scholar]
Martinez‐Garcia 2007
- Martinez‐Garcia MA, Soler‐Cataluna JJ, Perpina‐Tordera M, Roman‐Sanchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non‐cystic fibrosis bronchiectasis. Chest 2007;132:1565‐72. [DOI] [PubMed] [Google Scholar]
Martinez‐Garcia 2007a
- Martinez‐Garcia MA, Perpina‐Tordera M, Soler‐Cataluna JJ, Roman‐Sanchez P, Lloris‐Bayo A, Gonzalez‐Molina A. Dissociation of lung function, dyspnoea ratings and pulmonary extension in bronchiectasis. Respiratory Medicine 2007;101:2248‐53. [DOI] [PubMed] [Google Scholar]
McCool 2006
- McCool FD, Rosen MJ. Nonpharmacologic airway clearance therapies: ACCP evidence‐based clinical practice guidelines. Chest 2006;129 Suppl 1:250S‐259S. [DOI] [PubMed] [Google Scholar]
McShane 2013
- McShane PJ, Naureckas ET, Tino G, Strek ME. Non‐cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2013;188:647‐56. [DOI] [PubMed] [Google Scholar]
Neves 2011
- Neves PC, Guerra M, Ponce P, Miranda J, Vouga L. Non‐cystic fibrosis bronchiectasis. Interactive Cardiovascular and Thoracic Surgery 2011;13:619‐25. [DOI] [PubMed] [Google Scholar]
O'Donnell 1998
- O'Donnell AE, Barker AF, Ilowite JS, Fick RB and the rhDNAse Study Group. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNAse I. rhDNAse Study Group. Chest 1998;113:1329‐34. [DOI] [PubMed] [Google Scholar]
Oberwaldner 1986
- Oberwaldner B, Evans JC, Zach MS. Forced expirations against a variable resistance: a new physiotherapy method in cystic fibrosis. Pediatric Pulmonology 1986;2:358‐67. [DOI] [PubMed] [Google Scholar]
Onen 2007
- Onen ZP, Gulbay EB, Sen E, Yildiz OA, Saryal S, Acican T, Karabiyikoglu G. Analysis of the factors related to mortality in patients with bronchiectasis. Respiratory Medicine 2007;101:1390‐7. [DOI] [PubMed] [Google Scholar]
Pasteur 2010
- Pasteur MC, Bilton D, Hill AT, British Thoracic Society Bronchiectasis non‐CF Group. British Thoracic Society guideline for Non‐CF Bronchiectasis. Thorax 2010;65 Suppl 1:i1‐58. [DOI] [PubMed] [Google Scholar]
Prasad 2007
- Prasad SA. Airway clearance techniques. Which one?. Chronic Respiratory Disease 2007;4:65‐6. [DOI] [PubMed] [Google Scholar]
Raj 2009
- Raj AA, Pavoord DI, Birring SS. Clinical cough IV: What is the minimal important difference for the Leicester Cough Questionnaire?. Handbook of Experimental Pharmacology 2009;187:311‐20. [DOI] [PubMed] [Google Scholar]
Regnis 1994
- Regnis JA, Robinson M, Bailey DL, Cook P, Hooper P, Chan HK, et al. Mucociliary clearance in patients with cystic fibrosis and in normal subjects. American Journal of Respiratory and Critical Care Medicine 1994;150:66‐71. [DOI] [PubMed] [Google Scholar]
RevMan 2015 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2015.
Schunemann 2008
- Schunemann JH, Oxman AD, Vist GE, Higgins J, Deeks J, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: HigginsJPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Sutton 1985
- Sutton P, Lopez‐Vidriero M, Pavia D, Newman S, Clay M, Webber B, et al. Assessment of percussion, vibratory‐shaking and breathing exercises in chest physiotherapy. European Journal of Respiratory Disease 1985;66(2):147‐52. [PubMed] [Google Scholar]
Tsang 2004
- Tsang K, Tipoe G. Bronchiectasis: not an orphan disease of the East. International Journal of Tuberculosis and Lung Disease 2004;8(6):691‐702. [PubMed] [Google Scholar]
Tsang 2009
- Tsang KW, Bilton D. Clinical challenges in managing bronchiectasis. Respirology 2009;14:637‐50. [DOI] [PubMed] [Google Scholar]
van der Schans 2000
- Schans CP, Prasad A, Main E. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001401] [DOI] [PubMed] [Google Scholar]
van der Schans 2002
- Schans CP. Airway clearance: assessment of techniques. Paediatric Respiratory Reviews 2002;3:110‐4. [DOI] [PubMed] [Google Scholar]
van Henstrum 1988
- Henstrum M, Festen J, Beurskens C, Hankel M, Beekman F, Corstens F. Conventional physiotherapy and forced expiration manoeuvres have similar effects on tracheobronchial clearance. European Respiratory Journal 1988;1:758‐61. [PubMed] [Google Scholar]
Weycker 2005
- Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clinical Pulmonary Medicine 2005;12(4):205‐9. [Google Scholar]
Williams 1994
- Williams MT. Chest physiotherapy and cystic fibrosis. Why is the most effective form of treatment still unclear?. Chest 1994;1006:1872‐82. [DOI] [PubMed] [Google Scholar]
Wilson 1997
- Wilson C, Jones P, O'Leary CJ, Cole P, Wilson R. Validation of the St George's Respiratory Questionnaire in bronchiectasis. American Journal of Respiratory and Critical Care Medicine 1997;156:536‐41. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jones 1998
- Jones AP, Rowe BH. Bronchopulmonary hygiene physical therapy for chronic obstructive pulmonary disease and bronchiectasis. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD000045] [DOI] [PubMed] [Google Scholar]
Lee 2013
- Lee AL, Burge AT, Holland AE. Airway clearance techniques for bronchiectasis. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD008351.pub2] [DOI] [PubMed] [Google Scholar]


