Abstract
Background
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the human central nervous system. Statins, prescribed as cholesterol lowering agents, have shown possible effects for treating MS in experimental and preliminary clinical studies.
Objectives
To evaluate the efficacy and safety of statins administered alone or as add‐on to approved treatments for MS.
Search methods
The Trials Search Coordinator searched the Cochrane MS Group Trials Register (1 August 2011). We searched the Chinese National Knowledge Infrastructure (CNKI) (1979 to 1 August 2011), trials registers and conference proceedings. Pharmaceutical companies and authors of included studies were contacted for additional information.There were no language restrictions.
Selection criteria
Randomised controlled trials comparing statins with placebo, or comparing statins in combination with approved treatments alone for patients with MS.
Data collection and analysis
Three review authors independently assessed trial quality and extracted data.
Main results
Four trials involving 458 participants were included. All trials compared statins (two evaluating atorvastatin and two simvastatin) plus interferon beta‐1a with interferon beta‐1a alone for treating MS. The methodological quality was good for three studies and poor for remaining one. None of them showed statistically significant difference between both treatment groups in reducing relapses, preventing disease progression or developing new T2 or gadolinium‐enhanced lesions on MRI after 9, 12, 24 months follow up period. Statins resulted to be safe and well tolerated, no serious adverse effects were reported. Changes on quality of life after receiving statins were not reported in the trials.
Authors' conclusions
There is no convincing evidence to support the use of either atorvastatin or simvastatin as an adjunctive therapy in MS.
Plain language summary
The use of Statins, cholesterol lowering agents, in patients with multiple sclerosis (MS)
MS is an inflammatory demyelinating disease of the human central nervous system and it is thought to be related to abnormal working of the immune system. Preliminary studies have shown that statins, cholesterol lowering agents, have potential immunological regulation effects which may be beneficial for MS. Furthermore, statins are usually orally administered, are less expensive than other MS treatment, and are easily available.
The authors of this review evaluated the efficacy and safety of statins in relapsing‐remitting MS patients. Among the pertinent literature, four studies, involving a total of 458 patients treated with statins as add‐on treatment to interferon beta‐1a were identified.
Two studies took into consideration atorvastatin, while the other two analysed simvastatin. Only three studies were found of good methodological quality.
The authors did not find convincing evidence that either atorvastatin or simvastatin can reduce relapses or prevent disease progression with a follow up at one and two years. No serious adverse events were reported and statins resulted to be safe and well tolerated. At present, the whole data do not support the use of statins as an adjunctive therapy in MS.
Summary of findings
Summary of findings for the main comparison. Statins plus beta interferon versus beta interferon for multiple sclerosis.
| Statins plus beta interferon versus beta interferon for multiple sclerosis | ||||||
| Patient or population: multiple sclerosis Settings: Intervention: Statins plus beta interferon versus beta interferon | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Statins plus beta interferon versus beta interferon | |||||
| Number of participants with relapses at 12 months Number of participants with relapses at 12 months Follow‐up: 12 months | Study population | RR 1 (0.45 to 2.23) | 34 (1 study) | ⊕⊝⊝⊝ very low1,2 | No relevant data | |
| 412 per 1000 | 412 per 1000 (185 to 918) | |||||
| Moderate | ||||||
| 412 per 1000 | 412 per 1000 (185 to 919) | |||||
| Number of participants with relapses at 24 months Number of participants with relapses at 24 months Follow‐up: mean 24 months | Study population | RR 0.7 (0.36 to 1.36) | 45 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 542 per 1000 | 379 per 1000 (195 to 737) | |||||
| Moderate | ||||||
| 542 per 1000 | 379 per 1000 (195 to 737) | |||||
| Number of participants with progression at 12 months3 ‐ not reported | See comment | See comment | Not estimable3 | ‐ | See comment | No relevant data |
| Number of participants with progression at 24 months Number of participants with progression at 24 months Follow‐up: mean 24 months | Study population | RR 0.13 (0.01 to 2.22) | 45 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 167 per 1000 | 22 per 1000 (2 to 370) | |||||
| Moderate | ||||||
| 167 per 1000 | 22 per 1000 (2 to 371) | |||||
| Number of participants with new T2 or GELs at 12 months Number of participants with new T2 or GELs at 12 months Follow‐up: mean 12 months | Study population | RR 1 (0.3 to 3.36) | 34 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 235 per 1000 | 235 per 1000 (71 to 791) | |||||
| Moderate | ||||||
| 235 per 1000 | 235 per 1000 (71 to 790) | |||||
| Number of participants with new T2 or GELs at 24 months3 ‐ not reported | See comment | See comment | Not estimable3 | No relevant data | See comment | No relevant data |
| Changes of EDSS at 12 months Changes of EDSS at 12 months Follow‐up: mean 12 months | The mean changes of EDSS at 12 months in the intervention groups was 0.33 lower (0.92 lower to 0.27 higher) | WMD ‐0.33 (‐0.92 to 0.27) | 387 (2 studies) | ⊕⊕⊕⊝ moderate4,5 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The only one study included was an open‐label controlled randomized trial, did not describe the method used to generate allocation sequence and did not mention allocation concealment. Besides, the drop‐out rate was unbalanced between treatment groups. 2 Only one low quality study contributed to this outcome analysis. The remaining three studies did not report this time point outcome. 3 No studies reported this time point outcome. 4 Two included studies were assessed of good methodological quality. 5 Only two high quality study contributed to this outcome analysis. The remaining two studies did not report this time point outcome.
Background
Description of the condition
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the human central nervous system (CNS) often causing disability in young people, affecting about 2.5 million people in the world, and with a lifetime risk about one in 400 (Compston 2002). Most patients first experience this disorder at the ages of 20 to 50, females are more easily to get MS than male.
Description of the intervention
Statins, inhibitors of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase, are widely used for hypercholestaeremia, have a good safety record (HPS 2002). Experimental studies suggest that statins have potential immunological regulation effects which may be beneficial for MS (Baker 2003). Moreover, statins are usually orally administered, less expensive than other MS treatment, and easily available. There are several prescribed types of statins, such as atorvastatin, simvastatin, lovastatin, fluvastatin, pravastatin, rosuvastatin and pitavastatin, but all of them worked in the same way.
How the intervention might work
Experimental studies indicate that statins can alleviate and reverse the progression of experimental autoimmune encephalomyelitis (EAE), the mouse model of MS (Aktas 2003; Stanislaus 2001; Youssef 2002). Statins may exert protective effects through several different mechanisms of action for MS.
Statins may exert anti‐inflammatory properties by inducing T cells down regulation of pro inflammatory Th1 cytokines release, up regulation of anti‐inflammatory Th2 cytokines secretion, also statins can promote Th1 cells to Th2 cells conversion, stimulate Th0 cells to differentiate into Th2 cells (Nath 2004; Peng 2006; Stanislaus 2002; Youssef 2002).
Statins can decrease the activity of inducible nitric oxide synthase (iNOS), which can catalyze the production of nitric oxide (NO) leading to cytotoxic effects in CNS (Stanislaus 1999).
Statins possess neuroprotective function, can augment remyelination in CNS (Paintlia 2005).
Why it is important to do this review
Statins are considered as a potential treatment for MS after positive results in an experimental model of MS (Aktas 2003; Stanislaus 2001; Youssef 2002). Studies on patients with MS have reported controversial results.Two observational studies reported that lovastatin or simvastatin decreased the relapses, the number and volume of gadolinium‐enhanced lesions in relapsing‐remitting MS (Sena 2003; Vollmer 2004). However, controlled studies did not find a significant reduction of relapses or progression in relapsing‐remitting patients treated with statins alone or added to beta interferon (Lanzillo 2010; Laplaud 2008; Rudick 2009; Sørensen 2007;Öztekin 2008) although statins appeared to be safe and well tolerated. Two additional trials assessed statins in combination with beta interferon in relapsing‐remitting MS. The first showed that combining atorvastatin with beta interferon reduced the number and volume of gadolinium‐enhanced lesions (Paul 2008), the other suggested a harmful effect of atorvastatin compared with placebo increasing both relapses and new lesions (Birnbaum 2008). Further randomized controlled trials assessing statins monotherapy or combined with beta interferon for relapsing‐remitting or secondary‐progressive MS are still ongoing or awaiting publication (http://www.clinicaltrials.gov/) as reported in "Ongoing studies" section. We conducted this systematic review to determine whether or not there is evidence of benefits and safety of statins in the treatment of MS.
Objectives
To evaluate the efficacy and safety of statins used alone or as add‐on to approved treatments for MS.
Methods
Criteria for considering studies for this review
Types of studies
Published or unpublished randomised controlled trials (RCTs) comparing statins with placebo, or comparing statins in combination with approved treatments alone for patients with MS were included.
Types of participants
Male or female patients of all age groups and setting with clinical definite MS according to Poser criteria (Poser 1983), original or revised Mc Donald criteria (McDonald 2001; Polman 2005; Polman 2011). Patients with relapsing‐remitting or secondary‐progressive MS course were included.
Types of interventions
Statins monotherapy or in combination with approved treatments irrespective of duration and dose were included. The control group was placebo, no intervention, or the same co‐intervention of the treated group.
Types of outcome measures
Primary outcomes
(1) The number of participants who had relapses at 6, 12 and 24 months after randomisation and at the end of the follow up.
(2) The number of participants who experienced disease progression, measured by Expanded Disability Status Scale (EDSS) (Kurtzke 1983) at 6, 12 and 24 months after randomisation. Progression was defined as an increase of 0.5 point of disability for patients with baseline EDSS greater than or equal to 5.5 and 1 point of disability for basal EDSS less than or equal 5.0, sustained for six months.
Secondary outcomes
(1) The number of participants who developed new T2 or gadolinium‐enhanced lesions (GELs) on MRI at 6,12 and 24 months after randomisation.
(2) The mean change of EDSS score at 6,12 and 24 months after randomisation.
(3) Quality of life, measured using scales such as Multiple Sclerosis Quality of Life‐54 instrument (MSQOL‐54) (Vickrey 1995).
(4) The number of participants who experienced any of the following adverse effects was evaluated: rhabdomyolysis, elevated liver enzymes (transaminases >3 times upper limit of normal values), elevated creatinine kinase levels (>10 times upper limit of normal values), muscle pain, muscle weakness/fatigue, nausea, headache, dizziness, rash, abdominal pain, diarrhoea.
Search methods for identification of studies
No language restrictions were applied to the search.
Electronic searches
The Trials Search Co‐ordinator searched the Cochrane Multiple Sclerosis Group's Specialised Register (1 August 2011)
The Cochrane Multiple Sclerosis Trials Register is updated regularly and contains trials identified from:
The Cochrane Central Register of Controlled Trials (CENTRAL) (recent issue);
MEDLINE (PubMed) (1966 to date);
EMBASE (Embase.com) (1974 to date);
CINAHL (Ebsco host) (1981 to Feb 2011);
LILACS (Bireme) (1982 to date);
PEDro
clinicaltrials.gov (www.clinicaltrials.gov)
WHO International Clinical Trials Registry Portal (http://apps.who.int/trialsearch/)
Information on the Cochrane Multiple Sclerosis Group's Trials Register and details of search strategies used to identify trials can be found in the 'Specialised Register' section within the Cochrane Multiple Sclerosis Group's module.
The keywords used to search for this review are listed in (Appendix 1). For search methods used in the previous version please see Appendix 2 and Appendix 3.
Additional databases searched by authors:
China Biological Medicine Database (CBM‐disc) (1979 to 1 August 2011) (Appendix 4);
Chinese National Knowledge Infrastructure Database (CNKI) (www.cnki.net, 1979 to 1 August 2011) (Appendix 5).
Searching other resources
We screened reference lists of considered studies and contacted authors of identified trials for unpublished information; we handsearched the abstracts of 23rd and 24th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; contacted pharmaceutical Pfizer company who produce statins to obtain relevant data; we also searched the following trials registers for ongoing trials: ClinicalTrials.gov (http://clinicaltrials.gov/) and Chinese Clinical Trial Register (http://www.chictr.org).
Data collection and analysis
Selection of studies
Three review authors (Wang J, Xiao Y, Luo M) independently read the titles and abstracts of all identified studies. The same review authors independently scrutinised the full texts of the selected studies to decide which trials met the inclusion criteria. There was no disagreement among review authors about the selection of studies for inclusion.
Data extraction and management
Three review authors (Wang J, Xiao Y, Luo M) independently recorded the following information using a data extraction form:
(1) Participants: inclusion/exclusion criteria, number of patients for each group, age, gender, baseline comparability between two groups, follow up, withdrawals and reasons;
(2) Methods: study design, randomisation method, allocation concealment, blinding of participants, personnel and outcome assessors;
(3) Interventions: type of statin, administration method, dosage, duration of treatment, co‐intervention, control intervention;.
(4) Outcomes: primary and secondary outcomes, adverse events;
(5) Others: country and setting, publication year, sources of funding, intention‐to‐treat analysis (ITT);
There were no disagreement about the data extraction among all the review authors.
Assessment of risk of bias in included studies
Three review authors (Wang J, Xiao Y, Luo M) independently assessed the methodological quality of the included studies. We used the quality checklist recommended by Cochrane Handbook for Systematic Reviews of Interventions version 5.1 (Higgins 2011). The quality checklist for assessing the risk of bias contained six specific parameters: (1) sequence generation, (2) allocation concealment, (3) blinding, (4) incomplete outcome data, (5) selective outcome reporting, and (6) other bias. For each entry, the judgement ('Low risk' of bias, 'High risk' of bias, or 'Unclear risk' of bias) is followed by a text box for a description of the design, conduct or observations that underlie the judgement. There was no disagreement in assessing the risk of bias among the review authors. The final results were recorded in Review Manager 5.1 (Review Manager (RevMan) 2011).
Measures of treatment effect
Data was managed according to ITT principle. For dichotomous outcomes (e.g. relapses, disease progression), we used relative risks (RR) with 95% confidence intervals (CI) to express the effect size. For continuous data (e.g. EDSS score), we used mean differences (MD) with 95% CI to analyse the outcomes.
Unit of analysis issues
For studies with more than two intervention groups, only statins intervention versus placebo or no intervention were included. For studies that assessed more than one doses of statins, we grouped the different doses together for analysis.
Dealing with missing data
Authors of identified studies were contacted to obtain additional information. If additional information was not obtained, we analysed the available data.
Assessment of heterogeneity
We evaluated clinical and methodological heterogeneity across included studies by comparing characteristics of participants, interventions and study designs.
We evaluated statistical heterogeneity among included studies using a chi squared test with α of 0.1, and with the I² test. If a p‐value less than 0.1 and I² statistic more than 50%, indicated a substantial statistical heterogeneity (Higgins 2011), the sources of potential clinical and methodological heterogeneity were examined.
Assessment of reporting biases
We could not investigate potential biases of publication using funnel plots according to the approach of Cochrane Handbook for Systematic Reviews of Interventions version 5.1 (Higgins 2011) due to insufficient included studies.
Data synthesis
We used Review Manager 5 (Review Manager (RevMan) 2011) to synthesis the available data. As there was no significant statistical heterogeneity identified from the included trials or no data were able to combined, we then analysed data using a fixed effects model.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroups:
(1) Different dosages of the same statin (e.g. low , high dose);
(2) Different statins (e.g. atorvastatin, simvastatin);
(3) Different duration of treatment (e.g. within 3 months, more than 3 months);
(4) Different types of MS (e.g. relapsing‐remitting, secondary‐progressive);
(5) Different co‐interventions (e.g. steroids, beta interferon);
(6) Different duration of MS (e.g. 5 years, more than 5 years).
However, because of the limited number of studies, we could not perform all.
Sensitivity analysis
We planned to perform the following sensitivity analyses:
Re‐analysis excluding unpublished studies
Re‐analysis excluding large sample size studies
Re‐analysis excluding studies with high risk of bias
However, because of the limited number of studies, we could not perform all.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies
Results of the search
The searches have been updated to 1 August 2011, a further 23 relevant articles (CENTRAL:0, MEDLINE: 12, EMBASE: 6, CBM: 4, CNKI: 0, CRD database: 1, Conference abstracts: 0, Pharmaceutical company: 0, Trials registers: 0) were identified since the last searches in April 2010. The previous version of this review (Wang 2010) identified 211 potentially relevant articles, 193 articles were excluded as they were not relevant, seven trials (eight articles) were excluded as they were not meeting the inclusion criteria, six were ongoing trials or completed trials but results are awaiting publication. Of these 23 newly identified articles, after reviewing the titles and abstracts we excluded 21 articles as they were not relevant, two trials (Sorensen 2011; Togha 2010) were eligible for inclusion which were listed as ongoing or completed trials in the previous version of this review. Therefore, this updated review identified a total of 232 potentially relevant article (CENTRAL:4, MEDLINE: 109, EMBASE: 57, CBM: 38, CNKI: 15, CRD database: 1, Conference abstracts: 4, Pharmaceutical company: 0, Trials registers: 4). As a result, 214 articles were excluded as they were not relevant. Of the remaining 18 articles, we excluded seven trials (eight articles) (Bar‐Or 2007; Laplaud 2008; Paul 2008; Rudick 2009; Sena 2003; Vollmer 2004; Wang 2008). Three were ongoing trials (NCT00429442; NCT00647348; NCT00242268) and have the recruitment status 'Unknown' on clinicaltrials.gov, one of these (NCT00242268) has not been updated since December 2005 and we could not receive data about the advancement of this trial after correspondence with the principal investigator. One trial SWABIMS (NCT00942591) has been completed and the patients are now undergoing an additional 12 month follow up with unchanged medication (NCT01111656). One trial (Öztekin 2008) was presented as an abstract at a conference but because of insufficient data is also awaiting assessment. Four trials (five articles) were finally included (Birnbaum 2008; Lanzillo 2010; Sorensen 2011; Togha 2010) (see Figure 1), two of them compared atorvastatin plus interferon beta‐1a with interferon beta‐1a alone for treating MS (Birnbaum 2008; Lanzillo 2010), the remaining two trials compared simvastatin as an add‐on to interferon beta‐1a with interferon beta‐1a for MS (Sorensen 2011; Togha 2010).
1.

Study flow diagram for selection.
Included studies
Four trials were included (Birnbaum 2008; Lanzillo 2010; Sorensen 2011; Togha 2010). The Lanzillo 2010 included two different time points outcomes at 12 and 24 months, the 12 months results were reported in a conference (Orefice 2007), we then grouped the two parts outcomes together for analysis. The Sorensen 2011 reported results at the end of the follow up with a range of 12 to 36 months. The Togha 2010 did not report all outcomes chosen for this review. Authors were contacted to obtain additional information which may not have been reported in the published articles. We received response only from Birnbaum (Birnbaum 2008) and Sorensen (Sorensen 2011), therefore we could only analyse the available data in both Lanzillo (Lanzillo 2010) and Togha (Togha 2010). Trials awaiting assessment, will be analysed during the next update of this review.
1. Study design
All four trials were RCTs. In Birnbaum 2008, Sorensen 2011 and Togha 2010, double blinding was performed; in Lanzillo 2010, an open‐label design was used meaning that neither the investigators nor the participants were blinded.
2. Participants
All participants were affected by clinically definite relapsing‐remitting MS according to Poser, original or revised Mc Donald criteria (McDonald 2001; Polman 2005; Poser 1983). In Birnbaum 2008, participants were enrolled at clinically stable phase for at least 6 months, while Lanzillo 2010 did not report whether or not participants were in a stable stage prior to entry, the Togha 2010 and Sorensen 2011 only enrolled patients with at least one relapse in the previous 6 or 12 months, besides, the Sorensen 2011 recruited treatment‐naive patients with less disease duration prior to entry than the other three trials. All trials reported a baseline comparability of the characteristics of participants between treatment groups, the number of females enrolled was higher than that of males. All trials included a small sample size except Sorensen 2011, which enrolled a total of 307 participants, was the largest trial in the treatment field of statins for MS. Only Sorensen 2011 carried out a sample size estimation, the other three trials enrolled 26 participants (Birnbaum 2008), 45 participants (Lanzillo 2010), and 80 participants (Togha 2010) respectively.
3. Interventions
Two trials compared atorvastatin administered as adjunctive therapy to interferon beta‐1a with interferon beta‐1a alone. The dose of atorvastatin in Birnbaum 2008 was 40 or 80 mg/d, and in Lanzillo 2010 was 20 mg/d. The treatment duration in Birnbaum 2008 was 6 months with a follow up of 9 months, while in Lanzillo 2010 treatment duration and follow up were 24 months. The remaining two trials compared simvastatin as an add‐on therapy to interferon beta‐1a with interferon beta‐1a. The dose of simvastatin in Sorensen 2011 was 80 mg/d with a treatment duration and follow up of 12 to 36 months, and in Togha 2010 was 40 mg/d with a treatment duration and follow up of 12 months.
4. Outcomes
Our primary outcomes was reported in Birnbaum 2008, Lanzillo 2010 and Sorensen 2011 at different time points. Both Birnbaum 2008 and Sorensen 2011 gave a definite definition of relapses. Birnbaum 2008 defined relapse as new neurologic symptoms accompanied by corresponding neurologic deficits that persisted for greater than 24 hours in the absence of fever or other bodily stressors; Sorensen 2011 divided relapse as documented and undocumented relapse, we grouped them together for analysis. The number of participants suffering disease progression was reported in both Lanzillo 2010 and Sorensen 2011. But in Togha 2010, the authors did not report the primary outcomes chosen for this review, we were unable to extract potentially data after correspondence with the authors.
Our secondary outcomes were partially reported in all included trials. However, changes on quality of life after receiving statins were not reported in any of the trials.
Excluded studies
Seven trials were excluded for the following reasons: Bar‐Or 2007 was a RCT to test combination therapy of atorvastatin with a newly DNA vaccine that did not meet our inclusion criteria; Laplaud 2008 was an abstract reported in a conference proceeding to investigate pravastatin monotherapy for MS but was without available clinical outcome data; Paul 2008 was a baseline‐to‐treatment trial without a control group; Rudick 2009 was a retrospective trial; Sena 2003 and Vollmer 2004 were case series studies; Wang 2008 was a RCT which had an imbalance co‐intervention between treatment groups.
Risk of bias in included studies
See: Overall results of all the risk of bias assessments are summarized in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All four included trials were reported as randomised, but only Sorensen 2011 and Togha 2010 addressed that the random list generated was using a computer‐based blocking method, and the allocation sequence was using central randomization by a third party in Sorensen 2011, while Togha 2010 did not mention allocation concealment used or not. The remaining two trials did not describe the method used to generate allocation sequence. After attempts to contact authors, Birnbaum 2008 confirmed that the sequence generation process used a computer random number generator, in order to ensure adequate allocation concealment and the allocation sequence was stored in sequentially numbered, opaque, sealed envelopes. We were unable to determine whether or not the randomisation and allocation concealment was adequate in Lanzillo 2010 due to the unavailability of sufficient information.
Blinding
In Birnbaum 2008, Sorensen 2011 and Togha 2010, the trial investigators, participants and outcome assessors were blinded to treatment, the intervention drug and placebo were provided with identical appearance, therefore we judged that double blinding was adequate; in Lanzillo 2010, because of the open label design method, we judged double blinding as inadequate.
Incomplete outcome data
All four trials provided enough details about the number of and the reasons for drop out. In Birnbaum 2008, the percentage of drop out was likely balanced between treatment groups (17.6% (3/17) in atorvastatin group, 11.1% (1/9) in control group), we classified the trial as a low risk of incomplete outcome data bias; in Lanzillo 2010, the percentage of drop out was unbalanced between treatment groups (9.5% (2/21) in atorvastatin group, 20.8% (5/24) in control group), we classified the trial as a high risk of incomplete outcome data bias; in Sorensen 2011, the drop‐out rate exceed the trial assumed rate of 21%, we classified the trial as a high risk in this field; in Togha 2010, the drop‐out rate was unbalanced between treatment groups (7.1% (3/42) in simvastatin group, 15.8% (6/38) in control group), we also classified the trial as a high risk in this field.
Selective reporting
It was difficult to ascertain whether all pre‐specified outcomes were reported since we could not obtain protocols from both Birnbaum 2008 and Lanzillo 2010. Considering that all favourable and unfavourable results were reported in Birnbaum 2008, we judged a low risk of selective reporting bias. Considering that only results with statistically significant difference were reported in Lanzillo 2010, we judged an unclear risk of selective reporting bias. After comparing trial publications to protocols being registered (http://www.clinicaltrials.gov/) before start of the study (Dwan 2008; Smyth 2011), we found all pre‐specified outcomes of Sorensen 2011 and Togha 2010 were reported thus we judged them a low risk of bias in this field.
Other potential sources of bias
Both Birnbaum 2008 and Lanzillo 2010, trial drugs were provided by the pharmaceutical company and some authors of Birnbaum 2008 have received honoraria and research support from pharmaceutical companies. Therefore, since conflict of interests may exist, we classified them as an unclear risk of other potential sources of bias. Though the study Sorensen 2011 was sponsored by the pharmaceutical company, Biogen Idec was not involved in the study design, data collection, data analysis and data interpretation, we judged a low risk of bias in this field. We did not identify other potential sources of bias in Togha 2010.
Effects of interventions
See: Table 1
See: Table 1
Although we planed to analysis the results at the time points 6, 12 and 24 months, it was not possible because of the small number of included trials. We then analysed the available time point results as reported in the studies.
Primary outcomes
1. The number of participants who had relapses at 6, 12 and 24 months.
Three of the four included trials contributed to this outcome analysis, including two atorvastatin treated trials (Birnbaum 2008; Lanzillo 2010) and one simvastatin treated trial (Sorensen 2011). Birnbaum 2008 reported results at 9 months, Lanzillo 2010 at 12 and 24 months, Sorensen 2011 reported results at the end of the follow up with a range of 12 to 36 months, the individual results at time point 12, 24 and 36 months were unavailable after correspondence with the original authors. Birnbaum 2008 was a three arm study that reported combination therapy with interferon beta‐1a, comparing 40 or 80 mg/d atorvastatin with placebo, we grouped 40 and 80 mg/d results together in this outcome analysis. No significant difference was detected between both treatment groups in the number of participants who had relapses at 9 months (RR 2.12; 95% CI 0.28 to 16.24), 12 months (RR 1.00; 95% CI 0.45 to 2.23), 24 months (RR 0.70; 95% CI 0.36 to 1.36) and at the end of the follow up with a range from 12 to 36 months (RR 1.27; 95% CI 0.83 to 1.95) (see Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4). When analysing these results together, regardless of doses, statins and follow up period, there was also no significant difference between both groups (RR 1.14; 95% CI 0.80 to 1.62) (see Analysis 1.5), the homogeneity test indicated no significant heterogeneity (I² = 25%), so the fixed effects model was applied. When excluding the poor methodological quality trial (Lanzillo 2010) did not affect our overall results.
1.1. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 1 Number of participants with relapses at 9 months.
1.2. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 2 Number of participants with relapses at 12 months.
1.3. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 3 Number of participants with relapses at 24 months.
1.4. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 4 Number of participants with relapses at the end of follow up (range from 12 to 36 months).
1.5. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 5 Number of participants with relapses.
To investigate the effect of different doses of atorvastatin, a subgroup analysis was performed. Lanzillo 2010 used a dose of 20 mg/d, Birnbaum 2008 used a dose of 40 or 80 mg/d. Subgroup analysis suggested no significant difference between both groups with any of the three different doses (RR 0.70, 95%CI 0.36 to 1.36; RR 2.57, 95%CI 0.29 to 22.93; RR 1.80, 95%CI 0.19 to 16.66; respectively) (see Analysis 1.6). we did not find a definite dose‐dependence relationship among those receiving 20, 40 or 80 mg/d of atorvastatin in addition to interferon beta‐1a.
1.6. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 6 Number of participants with relapses of different doses of atorvastatin.
To investigate the effect of different statins, a subgroup analysis was performed. When added atorvastatin to interferon beta‐1a, results suggested that actively treated group have higher risk to have relapses at 9 months ( (RR 2.12; 95% CI 0.28 to 16.24) similar risk at 12 months ( RR 1.00; 95% CI 0.45 to 2.23)) and fewer risk at 24 months ((RR 0.70; 95% CI 0.36 to 1.36) than placebo treated group but without significant difference; when added simvastatin to interferon beta‐1a, results showed that more participants experienced relapse at the end of the 12 to 36 months follow up (RR 1.27; 95% CI 0.83 to 1.95) in simvastatin treated group than placebo treated group, but also without significant difference.
2. The number of participants who experienced disease progression at 6, 12 and 24 months
Two trials (Lanzillo 2010; Sorensen 2011) contributed to this outcome analysis. Results indicated less participants in atorvastatin treated group experienced disease progression than placebo treated group at 24 months follow up (RR 0.13; 95% CI 0.01 to 2.22) (see Analysis 1.7), more participants experienced disease progression at the end of the 12 to 36 months follow up in simvastatin treated group than placebo treated group (RR 1.20; 95% CI 0.82 to 1.75) (see Analysis 1.8), but both of them did not show significant difference. When analysing these results together, no significant difference was detected between statins treated group and placebo treated group in preventing disease progression (RR 1.09; 95% CI 0.75 to 1.58) (see Analysis 1.9), the homogeneity test indicated no significant heterogeneity (p=0.12), so the fixed effects model was applied.
1.7. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 7 Number of participants with progression (atorvastatin).
1.8. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 8 Number of participants with progression (simvastatin).
1.9. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 9 Number of participants with progression.
Secondary outcomes
1. The number of participants who developed new T2 or gadolinium‐enhanced lesions on MRI at 6,12 and 24 months
Data were available from two atorvastatin treated trials for this outcome. Birnbaum 2008 contributed to result analysis at 9 months and Lanzillo 2010 at 12 months. There was no significant difference in developing new T2 or GELs on MRI between both treatment groups at 9 months (RR 4.24; 95% CI 0.62 to 28.76) and 12 months (RR 1.00; 95% CI 0.30 to 3.36) (see Analysis 1.10; Analysis 1.11). When analysing these results together, there was also no significant difference between both groups (RR 1.80; 95% CI 0.66 to 4.86) (see Analysis 1.12), the homogeneity test indicated no significant heterogeneity (I² = 40%), so the fixed effects model was applied.
1.10. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 10 Number of participants with new T2 or GELs at 9 months.
1.11. Analysis.
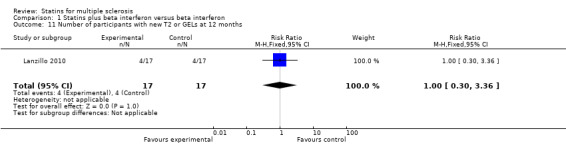
Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 11 Number of participants with new T2 or GELs at 12 months.
1.12. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 12 Number of participants with new T2 or GELs.
To investigate the effect of different doses of atorvastatin, a subgroup analysis was performed. Lanzillo 2010 used a dose of 20 mg/d, Birnbaum 2008 used a dose of 40 or 80 mg/d. Subgroup analysis suggested that there was no significant difference between both groups in developing new T2 or GELs with any of the three different doses, 20 mg/d (RR 1.00; 95%CI 0.30 to 3.36), 40 mg/d (RR 3.86; 95%CI 0.5 to 29.55), 80 mg/d (RR 4.50, 95%CI 0.64 to 31.60) (see Analysis 1.13). Dose‐dependence relationship among those receiving 20, 40 or 80 mg/d of atorvastatin in addition to interferon beta‐1a was not detected.
1.13. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 13 Number of participants with new T2 or GELs of different doses of atorvastatin.
Two simvastatin treated trials (Sorensen 2011; Togha 2010) were not included for this outcome analysis due to they did not report outcomes chosen for this review and further data were unavailable. Sorensen 2011 reported the mean number of new or enlarging T2 lesions in the simvastatin group was 2·96 which was higher than 2·52 of the placebo group. Similarly, Togha 2010 also reported the mean number of new T2 lesions and gadolinium‐enhanced lesions as an important outcome, however, the authors did not show statistically difference between two treatment groups.
2. The mean change of EDSS score at 6,12 and 24 months
Three trials contributed to this outcome analysis, including one atorvastatin treated trial (Lanzillo 2010) and two simvastatin treated trials (Sorensen 2011; Togha 2010). Both Togha 2010 and Sorensen 2011 reported results at 12 months, while Lanzillo 2010 reported at 24 months. There was no significant difference in mean change of EDSS score at 12 months (WMD, ‐0.33; 95%CI, ‐0.92 to 0.27) (see Analysis 1.14), the homogeneity test indicated significant heterogeneity (p=0.07, I² = 69%), so the random effects model was applied. There was also no significant difference at 24 months (WMD, ‐0.40; 95%CI, ‐1.04 to 0.24) (see Analysis 1.15). When analysing these results together, no significant difference was detected between statins treated group and placebo treated group in mean change of EDSS score (WMD, ‐0.21; 95%CI, ‐0.45 to 0.02) (see Analysis 1.16), the homogeneity test indicated no significant heterogeneity (p=0.16), so the fixed effects model was applied.
1.14. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 14 Changes of EDSS at 12 months.
1.15. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 15 Changes of EDSS at 24 months.
1.16. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 16 Changes of EDSS.
To investigate the effect of different doses of simvastatin, a subgroup analysis was performed. Togha 2010 used a dose of 40 mg/d, Sorensen 2011 used a dose of 80 mg/d. Subgroup analysis suggested lower EDSS score was associated with the use of 40 mg/d simvastatin (WMD, ‐0.70; 95%CI, ‐1.31 to ‐0.09) but with no difference of 80 mg/d simvastatin (WMD, ‐0.08; 95%CI, ‐0.36 to 0.20) when compared with placebo treated participants.
3. Quality of life
None of the trials reported changes on quality of life after receiving statins.
4. Adverse effects
All four trials reported adverse effects, no serious adverse effects were reported related to atorvastatin or simvastatin. Two simvastatin treated trials (Sorensen 2011; Togha 2010) provided enough information for synthesis of data. There was no significant differences in the occurrence of fatigue (RR 1.48; 95% CI 0.85 to 2.56) (see Analysis 1.17), muscle pain (RR 0.80; 95% CI 0.32 to 2.01) (see Analysis 1.18) and headache (RR 0.62; 95% CI 0.28 to 1.37) (see Analysis 1.19), the fixed effects model was applied because of no significant heterogeneity was found. The laboratory examinations indicated simvastatin was associated with lower cholesterol action, more frequency of elevated liver enzymes and no differences in serum creatinine kinase (CK). Besides, reported adverse events were mild, and no unexpected adverse events occurred. Unfortunately, we could not combine adverse events from the two atorvastatin trials so that we could only give a description of the results. Addressed adverse effects mainly focused on safety laboratory parameters, including alanine transaminase (ALT), aspartate transaminase (AST), CK, and total cholesterol. No significant difference was found at timing of outcome measures, except a lower cholesterol action was observed in participants receiving atorvastatin (Birnbaum 2008). In Birnbaum 2008, two participants receiving 80 mg/d atorvastatin dropped‐out: one was due to muscle aching and weakness, the other was because of elevated liver enzymes, whereas one subject in the placebo group was advised to drop‐out because of elevated CK. In Lanzillo 2010, one subject dropped out in each group because of depression, meanwhile, another subject receiving 20 mg/d atorvastatin discontinued at 18 months due to amenorrhoea and increased spasticity. However, subcutaneous injection of interferon beta‐1a may also lead to some of these adverse events (Hartung 2009).
1.17. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 17 Adverse events (Fatigue).
1.18. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 18 Adverse events (Muscle pain).
1.19. Analysis.

Comparison 1 Statins plus beta interferon versus beta interferon, Outcome 19 Adverse events (Headache).
Discussion
Summary of main results
Statins, prescribed as cholesterol lowering agents, have shown possible effects for treating MS in experimental and preliminary clinical studies. This systematic review assessed the efficacy and safety of statins used alone or as add‐on to approved treatments for MS. Four RCTs involving 458 participants contributed to the final analysis, two of which reported atorvastatin, 20, 40 or 80 mg/d, administered as adjunctive therapy to subcutaneous interferon beta‐1a 44 μg thrice weekly for relapsing‐remitting MS (Birnbaum 2008; Lanzillo 2010), the remaining two trials reported simvastatin 40 or 80 mg/d as an add‐on therapy to intramuscular interferon beta‐1a, 30 μg weekly for relapsing‐remitting MS (Sorensen 2011; Togha 2010). Three were assessed as having good methodological quality, the remaining one was of poor quality. The results did not show statistically significant difference between both treatment groups in reducing relapses, preventing disease progression or developing new T2 or GELs on MRI after 12 and 24 months follow up period.
When added atorvastatin to interferon beta‐1a, there was no sufficient evidence to support atorvastatin was more effective than placebo due to the small number of included studies and participants and with no statistically difference outcomes analysis; when added simvastatin to interferon beta‐1a, results did not show benefits in favour of simvastatin in terms of relapses, disease progression, new or enlarging T2 lesions on MRI as well as changes of EDSS score, though those differences between both groups without statistical significance. However, statins resulted to be safe and well tolerated when combined with interferon beta‐1a, no serious adverse events were reported.
Since the previous version of this review there is new evidence about simvastatin in the treatment of MS. Two new simvastatin trials have been included in this review, one of which (SIMCOMBIN study) (Sorensen 2011) is the largest statins trial: 307 participants represented 67% of the results. The other trial (Togha 2010) recruited 80 MS patients, but did not report data on the primary outcomes chosen for this review. The authors showed the mean number of relapses was lower in simvastatin treated participants than placebo group. Accordingly, they claimed that the trial provided class 1 evidence with regard to the use of simvastatin 40 mg daily in addition to interferon beta‐1a, however, they did not show (Togha 2010) beneficial effects in favour of simvastatin group in terms of the mean number of new T2 lesions and new gadolinium‐enhanced lesions, besides, more proportion of participants suffered disease exacerbation in simvastatin group than placebo group.
Furthermore, we did not find any evidence supporting statins monotherapy for MS.
Overall completeness and applicability of evidence
It is difficult to give any recommendation for clinical practice of statins for treating MS. Simvastatin should not be routinely recommended as an add‐on therapy to interferon beta‐1a for MS until further data available. In this review, we were unable to answer which therapy, monotherapy or combination treatment were effectively in MS, as well as dose and treatment duration. Besides, not all primary outcomes, secondary outcomes, subgroup analysis or sensitivity analyses could be performed as planned due to the small number of included trials.
All four trials investigated adjunctive therapy effects of atorvastatin or simvastatin to interferon beta‐1a, there was no evidence about statins monotherapy for MS.
Quality of the evidence
There was no sufficient evidence from the small number of included studies. Three of the four trials (Birnbaum 2008; Sorensen 2011; Togha 2010) assessed of good methodological quality, used a double blinding design, has an adequate methods of randomisation and allocation concealment except Togha 2010 with unclear risk of allocation concealment, even so, Birnbaum 2008 did not present the methods of randomisation and allocation concealment in the report but were available from the author. The remaining trial (Lanzillo 2010) was of poor methodological quality, used an open‐label design meaning no blinding was performed, and moreover, addressed the randomization without details, and whether or not allocation concealment was used. No blinding, unclear methods of randomisation and allocation concealment leads to high risk of selection, performance, attrition and detection bias which may result in an overestimation of intervention effects (Boutron 2004; Shulz 1995). Even though the flaws of methodological quality, the lack of outcomes reports chosen for this review made the Togha 2010 trial only contributed to partially secondary outcomes analysis, leading to the simvastatin results analysed mainly based on the largest single trial (Sorensen 2011).
Given the intervention drug atorvastatin was provided by the pharmaceutical company in both atorvastatin treated trials, some potential risk of conflict of interests in performing these trials could not be excluded; on the other hand, simvastatin of the trial Sorensen 2011 was also sponsored by pharmaceutical company, but Biogen Idec was not involved in the study design, data collection, data analysis and data interpretation, hence, a low risk of conflict of interests may exist in conducting this trial.
Other concerns were the statistical power and clinical heterogeneity of the included studies. All trials, except for Sorensen 2011, randomly assigned a relatively small number of patients and did not carry out sample size calculation which may result in an inadequate statistical power. Three trials (Birnbaum 2008; Sorensen 2011; Togha 2010) performed an ITT analysis, the other trial (Lanzillo 2010) did not report it, but in this trial, the authors analysed all patients enrolled since the initial study entry. The clinical heterogeneity was another important concern, the age of the participants, disease duration, statins and statin doses, treatment duration varied substantially among studies.
Potential biases in the review process
Potential biases from the trials
An extensive and comprehensive search was undertaken to limit bias in the review process however a low number of studies were retrieved. We could not confirm whether other studies with negative findings were not identified because trials with negative findings are unpublished more often than trials with positive findings (Hopewell 2009). In addition, we did not receive all additional information required after correspondence with the original authors.
Potential biases from the review authors
There were no potential biases from the review authors in the review process. In the development of this review, three review authors independently read and screened trials retrieved for inclusion, independently completed data extraction and assessed the quality of included trials to minimize potential biases. No conflict of interests were found in relation to the review authors of this review.
Agreements and disagreements with other studies or reviews
Compared with previous version of this review (Wang 2010), the present review increased some new evidence about simvastatin in the treatment of MS which have never been systematically reviewed.
Authors' conclusions
Implications for practice.
We found no convincing evidence from the small number of included trials to support statins (either atorvastatin and simvastatin) administered as an adjunctive therapy or as a monotherapy in MS.
Implications for research.
Future well designed randomized, double blinded, placebo‐controlled trials with larger sample sizes either for mono or combination therapy with statins in all forms of MS are needed.
Trials assessing combination therapy with statins and other approved agents besides interferon beta‐1a are also required.
No more RCTs are suggested on the use of simvastatin until the all awaiting publication and ongoing trials are made available.
Health related quality of life should be addressed for such a disabling disease in future research.
What's new
| Date | Event | Description |
|---|---|---|
| 18 August 2011 | New citation required but conclusions have not changed | Two new trials have been included in this review. The authorship of this review has changed. |
| 1 August 2011 | New search has been performed | Updated search run 1 August 2011. |
Acknowledgements
We thank Dr Xianlong Zhang for drafting the first version of this review. We thank all peer reviewers for their constructive suggestions and comments in this review. We thank Deirdre Beecher, Trials Search Coordinator and Liliana Coco, Managing Editor of the Multiple Sclerosis Review Group for their help in developing this review. We also thank Taixiang Wu, Guanjian Liu,and Youping Li (the Chinese Cochrane centre) for their advice in preparing this review. We are very grateful to the investigators of the trials who provided additional unpublished information.
Appendices
Appendix 1. Keywords used to search Group Specialised Register
{hydroxmethylglutaryl‐CoA reductase inhibitor\*} OR {hydroxymethylglutaryl coenzyme a reductase inhibitor\*} OR {HMG CoA\*} OR {HMG‐CoA+} OR {statin\*} OR {atorvastatin} OR {cerivastatin} OR {compactin} OR {dalvastatin} OR {fluvastatin} OR {fluindostatin} OR {lovastatin} OR {mevastatin} OR {mevinolin} OR {monacolin} OR {meglutol} OR {pitavastatin} OR {pravastatin} OR {rosuvastatin} OR {simvastatin} OR {baycol} OR {crestor} OR {lipitor} OR {lescol} OR {mevacor} OR {pravachol} OR {zocor}
Appendix 2. Search Methods used in the previous version of this review
Search methods for identification of studies
A systematic search without language restriction was conducted to identify all relevant published and unpublished randomised controlled trials using the optimally sensitive strategy developed for the Cochrane Collaboration for the identification of RCTs (Lefebrvre 2011). For additional information about the Group's search strategy please see: Cochrane Multiple Sclerosis Group (D'Amico 2010)
Electronic searches
The following databases were searched:
Cochrane Multiple Sclerosis Group Trials Register (1 August 2011);
The Cochrane Central Register of Controlled Trials (CENTRAL) "The Cochrane Library" (Issue 3, 2011);
MEDLINE (PubMed) (January 1966 to 1 August 2011);
EMBASE (EMBASE.com) (January 1974 to 1 August 2011);
China Biological Medicine Database (CBM‐disc) (1979 to 1 August 2011);
Chinese National Knowledge Infrastructure Database (CNKI) (www.cnki.net, 1979 to 1 August 2011).
Searching other resources
We screened reference lists of considered studies and contacted authors of identified trials for unpublished information; we handsearched the abstracts of 23rd and 24th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; contacted pharmaceutical Pfizer company who produce statins to obtain relevant data; we also searched the following trials registers for ongoing trials: ClinicalTrials.gov (http://clinicaltrials.gov/) and Chinese Clinical Trial Register (http://www.chictr.org).
Appendix 3. Search strategies use for previous version of this review
1 CENTRAL (Issue 3, 2010)
#1MeSH descriptor Multiple Sclerosis explode all trees #2MeSH descriptor Demyelinating Diseases, this term only #3MeSH descriptor Myelitis, Transverse, this term only #4MeSH descriptor Optic Neuritis explode all trees #5MeSH descriptor Encephalomyelitis, Acute Disseminated, this term only #6"multiple sclerosis":ti,ab,kw #7(demyelinating NEXT disease):ti,ab,kw #8(transverse NEXT myelitis):ti,ab,kw #9"neuromyelitis optica":ti,ab,kw #10"optic neuritis":ti,ab,kw #11(devic):ti,ab,kw #12"acute disseminated encephalomyelitis":ti,ab,kw #13(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #14(statin* OR atorvastatin OR cerivastatin OR compactin OR dalvastatin OR fluvastatin OR fluindostatin OR lovastatin OR mevastatin OR mevinolin OR monacolin OR meglutol OR pitavastatin OR pravastatin OR rosuvastatin OR simvastatin OR baycol OR crestor OR lipitor OR lescol OR mevacor OR pravachol OR zocor):ti,ab,kw #15"Hydroxymethylglutaryl‐CoA Reductase Inhibitor*" OR "Hydroxymethylglutaryl Coenzyme a Reductase Inhibitor*" OR " HMG CoA* ":ti,ab,kw #16MeSH descriptor Hydroxymethylglutaryl‐CoA Reductase Inhibitors explode all trees #17(#14 OR #15 OR #16) #18(#13 AND #17)
2 MEDLINE (PubMed) (1966 to April 2010)
(("Hydroxymethylglutaryl‐CoA Reductase Inhibitor*)"[Title/Abstract] OR "(Hydroxymethylglutaryl Coenzyme a Reductase Inhibitor*)"[Title/Abstract] OR "(HMG CoA*)"[Title/Abstract] OR "(HMG‐CoA*)"[Title/Abstract] OR "(statin*)"[Title/Abstract] OR "(atorvastatin)"[Title/Abstract] OR "(cerivastatin)"[Title/Abstract] OR "(compactin)"[Title/Abstract] OR "(dalvastatin)"[Title/Abstract] OR "(fluvastatin)"[Title/Abstract] OR "(fluindostatin)"[Title/Abstract] OR "(lovastatin)"[Title/Abstract] OR "(mevastatin)"[Title/Abstract] OR "(mevinolin)"[Title/Abstract] OR "(monacolin)"[Title/Abstract] OR "(meglutol)"[Title/Abstract] OR "(pitavastatin)"[Title/Abstract] OR "(pravastatin)"[Title/Abstract] OR "(rosuvastatin)"[Title/Abstract] OR "(simvastatin)"[Title/Abstract] OR "(baycol)"[Title/Abstract] OR "(crestor)"[Title/Abstract] OR "(lipitor)"[Title/Abstract] OR "(lescol)"[Title/Abstract] OR "(mevacor)"[Title/Abstract] OR "(pravachol)"[Title/Abstract] OR "(zocor))"[Title/Abstract]) OR ("Hydroxymethylglutaryl‐CoA Reductase Inhibitors"[Mesh])) AND (((("Multiple Sclerosis"[mh]) OR ("Myelitis, Transverse"[mh:noexp]) OR ("Demyelinating Diseases"[mh:noexp]) OR ("Encephalomyelitis, Acute Disseminated"[mh:noexp]) OR ("Optic Neuritis"[mh])) OR (("multiple sclerosis"[Title/Abstract]) OR ("neuromyelitis optica"[Title/Abstract]) OR ("transverse myelitis"[Title/Abstract]) OR ("encephalomyelitis"[Title/Abstract]) OR ("devic"[Title/Abstract]) OR ("optic neuritis"[Title/Abstract]) OR ("demyelinating disease*"[Title/Abstract]) OR ("acute disseminated encephalomyelitis"[Title/Abstract]))) AND ((("Cohort Studies"[Mesh:noexp]) OR (randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT ((animals[mh]) NOT ((animals[mh]) AND (human[mh])))))
3 EMBASE (EMBASE.com) (1974 to 2010)
(('hydroxymethylglutaryl coenzyme a reductase inhibitor'/exp) OR ('hydroxymethylglutaryl‐coa reductase inhibitor':ab,ti) OR ('hydroxymethylglutaryl coenzyme a reductase inhibitor':ab,ti) OR ('hmg coa':ab,ti) OR ('hmg‐coa':ab,ti) OR (statin*:ab,ti) OR (atorvastatin:ab,ti) OR (cerivastatin:ab,ti) OR (compactin:ab,ti) OR (dalvastatin:ab,ti) OR (fluvastatin:ab,ti) OR (fluindostatin:ab,ti) OR (lovastatin:ab,ti) OR (mevastatin:ab,ti) OR (mevinolin:ab,ti) OR (monacolin:ab,ti) OR (meglutol:ab,ti) OR (pitavastatin:ab,ti) OR (pravastatin:ab,ti) OR (rosuvastatin:ab,ti) OR (simvastatin:ab,ti) OR (baycol:ab,ti) OR (crestor:ab,ti) OR (lipitor:ab,ti) OR (lescol:ab,ti) OR (mevacor:ab,ti) OR (pravachol:ab,ti) OR (zocor:ab,ti)) AND ((('encephalomyelitis'/exp) OR ('demyelinating disease'/exp) OR ('multiple sclerosis'/exp) OR ('myelooptic neuropathy'/exp) OR ('multiple sclerosis':ti,ab) OR ('neuromyelitis optica':ab,ti) OR (encephalomyelitis:ab,ti) OR (devic:ti,ab)) AND (('crossover procedure'/exp) OR ('double blind procedure'/exp) OR ('controlled clinical trial'/exp) OR ('single blind procedure'/exp) OR ('randomized controlled trial'/exp) OR (random*:ab,ti) OR (factorial*:ab,ti) OR (crossover:ab,ti) OR (cross:ab,ti AND over:ab,ti) OR (placebo:ab,ti) OR ('double blind':ab,ti) OR ('single blind':ab,ti) OR (assign*:ab,ti) OR (allocat*:ab,ti) OR (volunteer*:ab,ti))) AND [humans]/lim AND [embase]/lim
Appendix 4. CBM (1979 ‐ 1 August 2011)
#1 多发性硬化 (MeSH) #2 多发性硬化 (Ti/Ab/Kw/Tx) #3 视神经脊髓炎 (MeSH) #4 视神经脊髓炎 (Ti/Ab/Kw/Tx) #5 脱髓鞘疾病 (MeSH) #6 脱髓鞘疾病 (Ti/Ab/Kw/Tx) #7 他汀 (Ti/Ab/Kw/Tx) #8 阿托伐他汀 (Ti/Ab/Kw/Tx) #9 辛伐他汀 (Ti/Ab/Kw/Tx) #10 洛伐他汀 (Ti/Ab/Kw/Tx) #11 普伐他汀 (Ti/Ab/Kw/Tx) #12 氟伐他汀 (Ti/Ab/Kw/Tx) #13 瑞舒伐他汀 (Ti/Ab/Kw/Tx) #14 #1 OR #2 OR #3 OR #4 OR #5 OR #6 #15 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 #16 #14 AND #15
Appendix 5. CNKI (1979 ‐ 1 August 2011)
#1多发性硬化 (Ti/Ab/Kw/Tx) AND 他汀 (Ti/Ab/Kw/Tx) #2多发性硬化 (Ti/Ab/Kw/Tx) AND 阿托伐他汀 (Ti/Ab/Kw/Tx) #3多发性硬化 (Ti/Ab/Kw/Tx) AND 辛伐他汀 (Ti/Ab/Kw/Tx) #4多发性硬化 (Ti/Ab/Kw/Tx) AND 洛伐他汀 (Ti/Ab/Kw/Tx) #5多发性硬化 (Ti/Ab/Kw/Tx) AND 普伐他汀 (Ti/Ab/Kw/Tx) #6多发性硬化 (Ti/Ab/Kw/Tx) AND 氟伐他汀 (Ti/Ab/Kw/Tx) #7多发性硬化 (Ti/Ab/Kw/Tx) AND 瑞舒伐他汀 (Ti/Ab/Kw/Tx) #8视神经脊髓炎 (Ti/Ab/Kw/Tx) AND 他汀 (Ti/Ab/Kw/Tx) #9视神经脊髓炎 (Ti/Ab/Kw/Tx) AND 阿托伐他汀 (Ti/Ab/Kw/Tx) #10视神经脊髓炎 (Ti/Ab/Kw/Tx) AND 辛伐他汀 (Ti/Ab/Kw/Tx) #11视神经脊髓炎 (Ti/Ab/Kw/Tx) AND 洛伐他汀 (Ti/Ab/Kw/Tx) #12视神经脊髓炎 (Ti/Ab/Kw/Tx) AND 普伐他汀 (Ti/Ab/Kw/Tx) #13视神经脊髓炎 (Ti/Ab/Kw/Tx) AND 氟伐他汀 (Ti/Ab/Kw/Tx) #14视神经脊髓炎 (Ti/Ab/Kw/Tx) AND 瑞舒伐他汀 (Ti/Ab/Kw/Tx) #15脱髓鞘疾病 (Ti/Ab/Kw/Tx) AND 他汀 (Ti/Ab/Kw/Tx) #16脱髓鞘疾病 (Ti/Ab/Kw/Tx) AND 阿托伐他汀 (Ti/Ab/Kw/Tx) #17脱髓鞘疾病 (Ti/Ab/Kw/Tx) AND 辛伐他汀 (Ti/Ab/Kw/Tx) #18脱髓鞘疾病 (Ti/Ab/Kw/Tx) AND 洛伐他汀 (Ti/Ab/Kw/Tx) #19脱髓鞘疾病 (Ti/Ab/Kw/Tx) AND 普伐他汀 (Ti/Ab/Kw/Tx) #20脱髓鞘疾病 (Ti/Ab/Kw/Tx) AND 氟伐他汀 (Ti/Ab/Kw/Tx) #21脱髓鞘疾病 (Ti/Ab/Kw/Tx) AND 瑞舒伐他汀 (Ti/Ab/Kw/Tx)
Data and analyses
Comparison 1. Statins plus beta interferon versus beta interferon.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with relapses at 9 months | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.28, 16.24] |
| 2 Number of participants with relapses at 12 months | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.45, 2.23] |
| 3 Number of participants with relapses at 24 months | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.36, 1.36] |
| 4 Number of participants with relapses at the end of follow up (range from 12 to 36 months) | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.83, 1.95] |
| 5 Number of participants with relapses | 3 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.80, 1.62] |
| 6 Number of participants with relapses of different doses of atorvastatin | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 20 mg/d atorvastatin | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.36, 1.36] |
| 6.2 40 mg/d atorvastatin | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.29, 22.93] |
| 6.3 80 mg/d atorvastatin | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.19, 16.66] |
| 7 Number of participants with progression (atorvastatin) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.22] |
| 8 Number of participants with progression (simvastatin) | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.82, 1.75] |
| 9 Number of participants with progression | 2 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.75, 1.58] |
| 10 Number of participants with new T2 or GELs at 9 months | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.24 [0.62, 28.76] |
| 11 Number of participants with new T2 or GELs at 12 months | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.30, 3.36] |
| 12 Number of participants with new T2 or GELs | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.66, 4.86] |
| 13 Number of participants with new T2 or GELs of different doses of atorvastatin | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 20 mg/d atorvastatin | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.30, 3.36] |
| 13.2 40 mg/d atorvastatin | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [0.50, 29.55] |
| 13.3 80 mg/d atorvastatin | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.5 [0.64, 31.60] |
| 14 Changes of EDSS at 12 months | 2 | 387 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.92, 0.27] |
| 14.1 Simvastatin 40 mg/d | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.7 [‐1.31, ‐0.09] |
| 14.2 Simvastatin 80 mg/d | 1 | 307 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.36, 0.20] |
| 15 Changes of EDSS at 24 months | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.04, 0.24] |
| 16 Changes of EDSS | 3 | 432 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.45, 0.02] |
| 17 Adverse events (Fatigue) | 2 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.85, 2.56] |
| 18 Adverse events (Muscle pain) | 2 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.01] |
| 19 Adverse events (Headache) | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.28, 1.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Birnbaum 2008.
| Methods | RCT with parallel design, The method of randomisation and allocation concealment was not mentioned in the original article in detail. After correspondence with the author, Dr. Birnbaum quickly responded to our requests, he said patients were randomly allocated using a computer random number generator and the allocation sequence was using sequentially numbered, opaque, sealed envelopes to make sure the participants and investigators unpredict the assignment. Double blinded: all study personnel, participants and outcome assessors were blinded. Drop‐outs: Intervention group: 3 (1 due to muscle aching and weakness, 1 elevation in AST and ALT, 1 for unknown reasons); Control group: 1 elevated CK levels. Losses to follow‐up: 1 in statins group for unknown reasons. Treatment duration: 6 months. Follow‐up period: 9 months. Intention‐to‐treat analysis: yes. | |
| Participants | 26 patients with clinically definite relapsing‐remitting MS according to Poser or McDonald criteria Participants were enrolled to the study only if clinically stable for at least 6 months Characteristics of patients at baseline: similar Sex: G1 F/M: 6/3, G2 F/M: 6/1, G3 F/M: 8/2 Mean age: G1 40.1 ± 9.2 years, G2 38.4 ± 7.5 years, G3 45.1 ± 6.3 years Disease duration: G1 7.7 ± 7 years, G2 6.4 ± 6.7 years, G3 7.2 ± 5.9 years Baseline mean EDSS: G1 2.3 ± 1.4, G2 2.1 ± 0.6, G3 2.0 ± 0.7 | |
| Interventions | Group 1: IFN + 40 mg/d placebo + 40 mg/d placebo Group 2: IFN + 40 mg/d atorvastatin + 40 mg/d placebo Group 3: IFN + 40 mg/d atorvastatin + 40 mg/d atorvastatin atorvastatin and placebo were provided by the manufacturer, Pfizer, with the same shape and size. |
|
| Outcomes | (1) Clinical relapses (2) New or enhancing T2 lesions (3) Laboratory blood testing (4) Safety and tolerability |
|
| Notes | The study was funded by the manufacturer, Pfizer, moreover, Dr. Birnbaum, Dr. Altafullah and Dr. Reder have received honoraria and research support from Pfizer. Relapses were defined as new neurologic symptoms accompanied by corresponding neurologic deficits that persisted for greater than 24 hours in the absence of fever or other bodily stressors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data and reasons were carefully recorded, and balanced between groups. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Conflict of interests may exist |
Lanzillo 2010.
| Methods | An open‐label controlled trial, randomisation was mentioned in the original article but without details, allocation concealment was not mentioned. We could not obtain additional information after correspondence with the authors. Blinding: the study personnel and participants were unblinded. Drop‐outs: Intervention group: 2 (1 for depression, 1 for amenorrhoea and increased spasticity); Control group: 5 (1 for pregnancy, 2 for clinical disease activity, 1 for secondary progression and 1 for depression). Treatment duration: 24 months. Follow‐up period: 24 months. Intention‐to‐treat analysis: no description. | |
| Participants | Country: Italy Setting: outpatients of the MS Center of the Federico II UniversityHospita 45 patients with clinically definite relapsing‐remitting MS according to McDonald criteria 21 in intervention group, F/M: 14/7, mean age 31.5 ± 9 years, disease duration 98.4 ± 68.1 months 24 in control group, F/M: 15/9, mean age 32.9 ± 7 years, disease duration 104.2 ± 64.3 months Characteristics of patients at baseline: similar | |
| Interventions | Intervention group: IFN + 20 mg/d atorvastatin Control group: IFN |
|
| Outcomes | (1) Number of MRI CELs (2) Number of clinical relapses (3) EDSS score variation (4) Safety laboratory parameters |
|
| Notes |
Orefice 2007 reported 34 patients completed 12 months results of Lanzillo 2010, presented in the 23rd Congress of the European Committee for Treatment and Research in Multiple Sclerosis as an abstract. The study drug atorvastatin was donated by the manufacturer, Pfizer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinded. Described as open label in 12 months result of the study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data and reasons were carefully recorded, but unbalanced between groups. |
| Other bias | Unclear risk | Conflict of interests may exist |
Sorensen 2011.
| Methods | A Multi‐centre, Double Blind, Randomised, Placebo Controlled, Parallel Group, Phase 4 Study. Random list generated was using a computer‐based blocking method. Allocation sequence was using central randomization by a third party (aCROnordic, Hørsholm, Denmark). Double blinded: all study personnel, participants and outcome assessors were blinded, the intervention drug and placebo were provided with identical appearance and delivered in sealed containers. Drop‐outs: Intervention group: 34 (22.5%) patients dropped out during the study, of them 12 patients dropped out before 12 months; Control group: 37 (23.7%) patients dropped out during the study, of them 21 patients dropped out before 12 months. Treatment duration: 12‐36 months, mean 20·6 months. Follow‐up period: 12‐36 months, mean 21.7 months. ITT analysis: yes. |
|
| Participants | Setting: 42 neurology department outpatient clinics in Denmark, Sweden, Norway, and Finland.
307 patients with clinically definite relapsing‐remitting MS according to revised McDonald or Poser criteria with an EDSS score of 5.5 or less and at least one documented relapse in the previous 12 months before enrolment.
151 in intervention group, F/M: 113/38, mean age 37.0 ± 8.6 years, disease duration 1.2 ± 2.2 years;
156 in control group, F/M: 108/48, mean age 36.1 ± 8.7 years, disease duration 1.3 ± 3.1 years. Characteristics of patients at baseline: similar |
|
| Interventions | Intervention group: IFN and simvastatin 80 mg/d for 1‐3 years Control group: IFN and placebo for 1‐3 years |
|
| Outcomes | 1. Annual rate of documented relapses 2. The time to first documented relapse 3. Number of new or enlarging lesions on T2‐weighted MRI at 12 months 4. Proportion of patients without disease activity 5. Adverse events |
|
| Notes | The study was sponsored by Biogen Idec with a non‐conditional grant. Biogen Idec was not involved in the study design, data collection, data analysis and data interpretation. A documented relapse was defined as new or exacerbation of existing neurological symptoms or signs in the absence of fever, persisting for more than 48 h after a period of more than 30 days stable or improving. An undocumented relapse was defined as new symptoms or worsening of old symptoms over at least 48 h after 30 days or more of stability where the criteria did not fulfil a documented relapse. Progression was defined as an increase of 1 point or more on EDSS score for at least of 3 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based blocking method |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was using central randomization by a third party |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The reasons of drop‐out and discontinuation were carefully recorded and balanced between groups, but the drop‐out rate exceed the assumed rate of 21%. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | Though the study was sponsored by the manufacturer Biogen Idec, conflict of interests may not exist. |
Togha 2010.
| Methods | A Double Blind, Randomised, Controlled Trial. Random list generated was using a computer‐based blocking method. Allocation sequence was not mentioned, but the authors stated the patients were unaware of their group assignment and all medications were given in similar packages. Double blinded: all study personnel, participants and outcome assessors were blinded, the intervention drug and placebo were provided in similar packages. Drop‐outs: Intervention group: 3 (2 had two relapses, 1 for personal reasons); Control group: 6 (4 had two relapses, 1 with exacerbation, 1 for personal reasons). Treatment duration: 12 months. Follow‐up period: 12 months. ITT analysis: yes. |
|
| Participants | Country: Iran
Setting: the Tehran University of Medical Sciences
85 patients with clinically definite relapsing‐remitting MS according to McDonald criteria, with an EDSS score of 0‐5 and at least one relapse in the previous 6 months before enrolment. Five patients did not receive at least one dose of allocated intervention since the study initial were excluded from the ITT analysis.
42 in intervention group, F/M: 32/10, mean age 29.3 ± 8.2 years, disease duration 45.0 ± 29.9 months
38 in control group, F/M: 27/11, mean age 31.1 ± 7.9 years, disease duration 40.5 ± 31.6 months Characteristics of patients at baseline: similar |
|
| Interventions | Intervention group: IFN and simvastatin 40 mg/d for 12 months Control group: IFN and placebo for 12 months |
|
| Outcomes | 1. The number of the relapses during the 1‐year follow‐up 2. Changes of EDSS 3. The number of new T2 lesions and the number of new gadolinium (Gd)‐enhanced lesions 4. Adverse events |
|
| Notes | Sponsored by Tehran University of Medical Sciences Relapses were defined as new or worsening neurological deficit in the absence of high body temperature that lasted for more than 24 h after 1‐month stability. Exacerbation was defined as the patients who had at least an increase of 1.5 point in their EDSS during the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based blocking method |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned, the authors stated the patients were unaware of their group assignment and all medications were given in similar packages, whether the investigators were aware the assignment was unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All study personnel, participants and outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data and reasons were carefully recorded, but unbalanced between groups. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | Conflict of interests may not exist. |
ALT: alanine transaminase AST: aspartate transaminase CELs: contrast enhanced lesions CK: creatinine kinase EDSS: Expanded Disability Status Scale F/M: female/male G: group IFN : interferon beta‐1a ITT: intention‐to‐treat analysis RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bar‐Or 2007 | RCT comparing atorvastatin add to BHT‐3009 with BHT‐3009, a DNA vaccine that it is not currently approved for MS, did not meet our protocol criteria. |
| Laplaud 2008 | An abstract reported in the 24th Congress of the European Committee for Treatment and Research in Multiple Sclerosis. No useful outcome data for analysis. |
| Paul 2008 | Open‐label baseline‐to‐treatment trial to assess high dose of atorvastatin in relapsing‐remitting MS without a control group. |
| Rudick 2009 | A retrospective analysis of 40 MS patients treated with five different statins. |
| Sena 2003 | Case series study of 7 relapsing‐remitting MS patients treated with 40 mg lovastatin. |
| Vollmer 2004 | Case series study of 30 relapsing‐remitting MS patients treated with 80 mg simvastatin. |
| Wang 2008 | Co‐intervention was not imbalanced between treatment groups, the authors enrolled MS patients only in acute exacerbation stage. |
RCT:randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00942591.
| Methods | Multi‐center, randomized, rater‐blinded, parallel‐group‐study |
| Participants | 80 patients with Relapsing‐Remitting MS (according to McDonald's criteria) At least 1 relapse in the past two year > 3 lesions on spinal or brain‐MRI or both EDSS score between 0 and 3.5 (inclusive) Age between 18 and 55 years Written informed consent Negative pregnancy test results (all women) |
| Interventions | Intervention group: interferon beta‐1b and atorvastatin 40 mg Control group: interferon beta‐1b |
| Outcomes | Primary outcomes: proportion of patients with new T2 lesions on MRI (at 15 months). Secondary Outcomes: Gd‐enhancing lesions on T1‐weighted images, change of total T2 lesion volume, cortical atrophy, clinical disease progression, number of relapse free patients, relapse rate, time to first relapse, and serial levels of neutralizing antibodies (at 15 months). |
| Notes | Sponsored by University Hospital Inselspital, Berne. http://clinicaltrials.gov/ct2/show/NCT00942591 The patients that finished the SWABIMS study are now undergoing for an additional 12 months follow up with unchanged medication (http://clinicaltrials.gov/ct2/show/NCT01111656). Further information received or reported would be analysed in the next update of this review. |
NCT01111656.
| Methods | Randomized single blinded parallel‐group‐study |
| Participants | 28 patients with Relapsing‐Remitting MS (according to McDonald's criteria) Successful completion of the SWABIMS study |
| Interventions | Intervention group: interferon beta‐1b and atorvastatin 40 mg Control group: interferon beta‐1b |
| Outcomes | Primary outcomes: proportion of patients with new lesions on T2‐weighted images after 12 months of treatment. Secondary Outcomes: Gd‐enhancing lesions on T1‐weighted images, change of total T2 lesion volume, cortical atrophy, clinical disease progression, number of relapse free patients, relapse rate, and time to first relapse. |
| Notes | Sponsored by University Hospital Inselspital, Berne. http://clinicaltrials.gov/ct2/show/NCT01111656 |
Öztekin 2008.
| Methods | An open‐label controlled trial, reported in the 24th Congress of the European Committee for Treatment and Research in Multiple Sclerosis as an abstract. Randomisation was mentioned in the abstract but without details, allocation concealment was not mentioned. We could not obtain additional information after correspondence with the authors. Blinding: not used. Drop‐outs: no description. Treatment duration: 24 months. Follow‐up period: 24 months. |
| Participants | 22 patients with clinically definite relapsing‐remitting MS, of them, 18 patients have completed 18 months duration. Age: 20‐55years. Characteristics of patients at baseline: similar |
| Interventions | Intervention group: IFN + 20 mg/d atorvastatin Control group: IFN |
| Outcomes | (1) Clinical relapses (at 12 months) (2) New or enhancing lesions (at 12 months) (3) EDSS variation (at 18 months) (3) Laboratory blood analysis (at 18 months) |
| Notes | Conference abstract, it was difficult to extract primary or secondary outcome specified due to insufficient data available. Further information received or reported would be analysed in an update of this review. |
Characteristics of ongoing studies [ordered by study ID]
NCT00242268.
| Trial name or title | A Safety Study of Combination Treatment With Avonex and Zocor in Relapsing Remitting Multiple Sclerosis |
| Methods | A Randomized, Double‐Blinded, Placebo Controlled Trial |
| Participants | 30 Patients with Relapsing‐remitting MS using the McDonald criteria Age between 18 and 55 years Written informed consent EDSS between 0‐ 5.0 |
| Interventions | Intervention group: interferon beta‐1a and simvastatin 40 mg Control group: interferon beta‐1a and placebo |
| Outcomes | Primary outcomes: new or enlarging T2 lesions on MRI (at 14 months). Secondary Outcomes: relapse rates; disease progression as measured with EDSS and MSFC. |
| Starting date | October 2005 |
| Contact information | William T. White, Pharm.D., Tel: 205‐979‐7555, Email: bwhite@sdr.us |
| Notes | Sponsored by Alabama Neurology Associates, PC and Biogen Idec http://clinicaltrials.gov/ct2/show/NCT00242268 |
NCT00429442.
| Trial name or title | Simvastatin as an Add‐on Treatment to Copaxone for the Treatment of Relapsing Multiple Sclerosis |
| Methods | A Double Blind, Randomised, Placebo Controlled Study |
| Participants | 20 patients with Relapsing‐remitting MS Between the age of 18 and 60 years (both included) EDSS of 6.5 or less 20 Written informed consent |
| Interventions | Intervention group: copaxone and simvastatin 80 mg Control group: copaxone and calcium tablets |
| Outcomes | Primary outcomes: number of new and/or enlarging lesions on T2‐ weighted MRI. Secondary Outcomes: changes in the EDSS and MFSC score, number of documented relapses. |
| Starting date | March 2008 |
| Contact information | Jette L Frederiksen, DMSC, Department of Neurology, Glostrup Hospital, Denmark Tel: +45 43233041; Email: jefr@glo.regionh.dk Anna Tsakiri, MD; Tel: +45 43233524; Email: ants@dadlnet.dk |
| Notes | Sponsored by Sanofi‐Aventis http://clinicaltrials.gov/ct2/show/NCT00429442 |
NCT00647348.
| Trial name or title | Investigation of Simvastatin in Secondary Progressive Multiple Sclerosis (MS‐STAT) |
| Methods | A Phase II Randomised, Placebo‐Controlled Clinical Trial |
| Participants | 140 Patients with Secondary‐Progressive MS EDSS 4.0 ‐ 6.5 inclusive Women of childbearing age will be required to use appropriate methods of contraception Written informed consent 18 ‐ 65 years |
| Interventions | Intervention group: simvastatin 80 mg Control group: placebo |
| Outcomes | Primary outcomes: quantitative MRI analysis to measure cerebral atrophy, and inflammation. Secondary Outcomes: evaluations of disability (EDSS, MSFC, MSIS‐29), quality of life (SF‐36), cognitive and behavioural function tests. |
| Starting date | January 2008 |
| Contact information | David Wilkie, BA, MA, Tel:0044 (0) 208 383 0675 Email: d.wilkie@imperial.ac.uk |
| Notes | Sponsored by Imperial College London http://clinicaltrials.gov/ct2/show/NCT00647348 |
EDSS: Expanded Disability Status Scale
Differences between protocol and review
We analysed the timing of outcome as reported in the original articles instead of as planed in our protocol according to the time points at 6, 12 and 24 months due to small number of included trials. The risk of bias was assessed according to the recently released of Cochrane Handbook for Systematic Reviews of Interventions 5.1 (Higgins 2011).
Contributions of authors
Jin Wang, Yousheng Xiao, Man Luo drafted the update review.
Jin Wang, Yousheng Xiao developed the search strategy.
Jin Wang, Yousheng Xiao, Man Luo selected relevant articles for inclusion.
Jin Wang, Yousheng Xiao, Man Luo extracted the data from included studies.
Jin Wang, Yousheng Xiao, Man Luo assessed the risk of bias in included studies.
Man Luo, Yousheng Xiao entered data to RevMan.
Jin Wang, Yousheng Xiao, Hongye Luo carried out the analysis.
Jin Wang, Yousheng Xiao interpreted the results.
Yousheng Xiao, Jin Wang will update the review.
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Birnbaum 2008 {published data only}
- Birnbaum G, Cree B, Altafullah I, Zinser M, Reder AT. Combining beta interferon and atorvastatin may increase disease activity in multiple sclerosis. Neurology 2008;71(18):1390‐5. [DOI] [PubMed] [Google Scholar]
Lanzillo 2010 {published data only}
- Lanzillo R, Orefice G, Quarantelli M, Rinaldi C, Prinster A, Ventrella G, et al. Atorvastatin combined to interferon to verify the efficacy (ACTIVE) in relapsing‐remitting active multiple sclerosis patients: a longitudinal controlled trial of combination therapy. Multiple Sclerosis 2010;16(4):450‐4. [DOI] [PubMed] [Google Scholar]
- Orefice G, Quarantelli M, Salvatore P, Brunetti A, Lanzillo R, Prinster A, et al. An open label randomised clinical trial of atorvastatin in combination with IFN beta‐1a in active MS patients: 12 months results. Multiple Sclerosis 2007;13:S56‐7. [Google Scholar]
Sorensen 2011 {published data only}
- Sorensen PS, Lycke J, Eralinna JP, Edland A, Wu X, Frederiksen JL, et al. Simvastatin as add‐on therapy to interferon beta‐1a for relapsing‐remitting multiple sclerosis (SIMCOMBIN study): a placebo‐controlled randomised phase 4 trial. Lancet neurology 2011;10(8):691‐701. [DOI] [PubMed] [Google Scholar]
Togha 2010 {published data only}
- Togha M, Ahmadi Karvigh S, Nabavi M, Beladi Moghadam N, Harirchian MH, Sahraian MA, et al. Simvastatin treatment in patients with relapsing‐remitting multiple sclerosis receiving interferon beta 1a: a double blind randomized controlled trial. Multiple Sclerosis 2010;16(7):848‐854. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bar‐Or 2007 {published data only}
- Bar‐Or A, Vollmer T, Antel J, Arnold DL, Bodner CA, Campagnolo D, et al. Induction of antigen‐specific tolerance in multiple sclerosis after immunization with DNA encoding myelin basic protein in a randomized, placebo‐controlled phase 1/2 trial. Archives of Neurology 2007;64(10):1407‐15. [DOI] [PubMed] [Google Scholar]
Laplaud 2008 {published data only}
- Laplaud DA, Lefrere F, Auffray‐Calvier E, Nguyen J, Edan G, Page EL, et al. Safety, tolerance and efficacy of Pravastatin in MS‐STEP in multiple sclerosis: a randomized double‐blind placebo controlled pilot study. ECTRIMS, 24th Congress of the European Committee for the Treatment and Research in Multiple Sclerosis; 2008 Sept 17‐20; Montreal, Canada. 2008.
Paul 2008 {published data only}
- Paul F, Waiczies S, Wuerfel J, Bellmann‐Strobl J, Dorr J, Waiczies H, et al. Oral High‐Dose Atorvastatin Treatment in Relapsing‐Remitting Multiple Sclerosis. PLoS ONE 2008;3(4):e1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rudick 2009 {published data only}
- Rudick R, Pace A, Panzara M, Rani S, Schrock J, Calabresi P, et al. Effects of statins on intramuscular interferon beta‐1afor relapsing‐remitting multiple sclerosis. Multiple Sclerosis 2007;13:S57. [Google Scholar]
- Rudick RA, Pace A, Rani MR, Hyde R, Panzara M, Appachi S, et al. Effect of statins on clinical and molecular responses to intramuscular interferon beta‐1a. Neurology 2009;72(23):1989‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sena 2003 {published data only}
- Sena A, Pedrosa R, Morais MG. Therapeutic potential of lovastatin in multiple sclerosis. Journal of Neurology 2003;250(6):754‐5. [DOI] [PubMed] [Google Scholar]
Vollmer 2004 {published data only}
- Vollmer T, Key L, Durkalski V, Tyor W, Corboy J, Markovic‐Plese S, et al. Oral simvastatin treatment in relapsing‐remitting multiple sclerosis. Lancet 2004;363(9421):1607‐8. [DOI] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang CY, Tang JG, Tan LM, Hu ZP, Zhang HN, Guo JF. The efficacy of Simvastatin and Corticosteroid Combination Therapy in Multiple Sclerosis. Journal of Chinese Physician 2008;10(5):694‐5. [Google Scholar]
References to studies awaiting assessment
NCT00942591 {published data only}
- NCT00942591. Atorvastatin 40 mg in Patients With Relapsing‐Remitting Multiple Sclerosis Treated With Interferon‐Beta‐1b (SWABIMS). clinicaltrials.gov/ct2/show/NCT00942591 (accessed 1 August 2011).
NCT01111656 {published data only}
- NCT01111656. SWABIMS Follow Up‐study. clinicaltrials.gov/ct2/show/NCT01111656 (accessed 1 August 2011).
Öztekin 2008 {published data only}
- Öztekin NS, Öztekin FM, Munis OB. Atorvastatin combined with interferon beta 1a in relapsing‐remitting multiple sclerosis: preliminary results of a 24 month randomized open‐label clinical trial. ECTRIMS, 24th Congress of the European Committee for the Treatment and Research in Multiple Sclerosis. September 17‐20, 2008.
References to ongoing studies
NCT00242268 {published data only}
- NCT00242268. A Safety Study of Combination Treatment With Avonex and Zocor in Relapsing Remitting Multiple Sclerosis. clinicaltrials.gov/ct2/show/NCT00242268 (accessed 1 August 2011).
NCT00429442 {published data only}
- NCT00429442. Simvastatin as an Add‐on Treatment to Copaxone for the Treatment of Relapsing Multiple Sclerosis. clinicaltrials.gov/ct2/show/NCT00429442 (accessed 1 August 2011).
NCT00647348 {published data only}
- NCT00647348. Investigation of Simvastatin in Secondary Progressive Multiple Sclerosis (MS‐STAT). http://clinicaltrials.gov/ct2/show/NCT00647348 (accessed 1 August 2011).
Additional references
Aktas 2003
- Aktas O, Waiczies S, Smorodchenko A, Dorr J, Seeger B, Prozorovski T. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. The Journal of Experimental Medicine 2003;197(6):725‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2003
- Baker D, Adamson P, Greenwood J. Potential of statins for the treatment of multiple sclerosis. Lancet Neurology 2003;2(1):9‐10. [DOI] [PubMed] [Google Scholar]
Boutron 2004
- Boutron I, Tubach F, Giraudeau B, Ravaud P. Blinding was judged more difficult to achieve and maintain in nonpharmacologic than pharmacologic trials. Epidemiology 2004;57(6):543‐50. [DOI] [PubMed] [Google Scholar]
Compston 2002
- Compston A, Coles A. Multiple sclerosis. Lancet 2002;359(9313):1221‐31. [DOI] [PubMed] [Google Scholar]
D'Amico 2010
- D'Amico R, Ebers G, Filippini G, Fredrikson S, Pietrantonj C, Khan F, et al. Cochrane Multiple Sclerosis Group. About The Cochrane Collaboration 2009, issue 3.
Dwan 2008
- Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan AW, Cronin E, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PloS one 2008;3(8):e3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartung 2009
- Hartung HP. High‐dose, high‐frequency recombinant interferon beta‐1ain the treatment of multiple sclerosis. Expert Opinion on Pharmacotherapy 2009;10(2):291‐309. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0[updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.MR000006.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
HPS 2002
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet 2002;360(9326):7‐22. [DOI] [PubMed] [Google Scholar]
Kurtzke 1983
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33(11):1444‐52. [DOI] [PubMed] [Google Scholar]
Lefebrvre 2011
- Lefebrvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT,Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
McDonald 2001
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121‐7. [DOI] [PubMed] [Google Scholar]
Nath 2004
- Nath N, Giri S, Prasad R, Singh AK, Singh I. Potential targets of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitor for multiple sclerosis therapy. Journal of Immunology 2004;172(2):1273‐86. [DOI] [PubMed] [Google Scholar]
Orefice 2007
- Orefice G, Quarantelli M, Salvatore P, Brunetti A, Lanzillo R, Prinster A, et al. An open label randomised clinical trial of atorvastatin in combination with IFN beta‐1a in active MS patients: 12 months results. Multiple Sclerosis 2007;13(2):S56‐7. [Google Scholar]
Paintlia 2005
- Paintlia AS, Paintlia MK, Khan M, Vollmer T, Singh AK, Singh I. HMG‐CoA reductase inhibitor augments survival and differentiation of oligodendrocyte progenitors in animal model of multiple sclerosis. The FASEB journal 2005;19(11):1407‐21. [DOI] [PubMed] [Google Scholar]
Peng 2006
- Peng X, Jin J, Giri S, Montes M, Sujkowski D, Tang Y, et al. Immunomodulatory effects of 3‐hydroxy‐3‐methylglutaryl coenzyme‐A reductase inhibitors, potential therapy for relapsing remitting multiple sclerosis. Journal of Neuroimmunology 2006;178(1‐2):130‐9. [DOI] [PubMed] [Google Scholar]
Polman 2005
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic Criteria for Multiple Sclerosis: 2005 revisions to the Macdonald Criteria. Annals of Neurology 2005;58(6):840‐6. [DOI] [PubMed] [Google Scholar]
Polman 2011
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of neurology 2011;69(2):292‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poser 1983
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227‐31. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Shulz 1995
- Shulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Smyth 2011
- Smyth RM, Kirkham JJ, Jacoby A, Altman DG, Gamble C, Williamson PR. Frequency and reasons for outcome reporting bias in clinical trials: interviews with trialists. BMJ (Clinical research ed.) 2011;342:c7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanislaus 1999
- Stanislaus R, Pahan K, Singh AK, Singh I. Amelioration of experimental allergic encephalomyelitis in Lewis rats by lovastatin. Neuroscience Letters 1999;269(2):71‐4. [DOI] [PubMed] [Google Scholar]
Stanislaus 2001
- Stanislaus R, Singh AK, Singh I. Lovastatin treatment decreases mononuclear cell infiltration into the CNS of Lewis rats with experimental allergic encephalomyelitis. Journal of Neuroscience Research 2001;66(2):155‐62. [DOI] [PubMed] [Google Scholar]
Stanislaus 2002
- Stanislaus R, Gilg AG, Singh AK, Singh I. Immunomodulation of experimental autoimmune encephalomyelitis in the Lewis rats by Lovastatin. Neuroscience Letters 2002;333(3):167‐70. [DOI] [PubMed] [Google Scholar]
Sørensen 2007
- Sørensen PS, Frederiksen JL, Lycke J, Sellebjerg F. Does simvastatin antagonise the effect of interferon beta? Interim safety analysis of the ongoing SIMCOMBIN study. Multiple Sclerosis 2007;13(2):S25. [Google Scholar]
Vickrey 1995
- Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health‐related quality of life measure for multiple sclerosis. Quality of Life Research 1995;4(3):187‐206. [DOI] [PubMed] [Google Scholar]
Wang 2010
- Wang J, Xiao Y, Luo M, Zhang X, Luo H. Statins for multiple sclerosis. Cochrane Database Syst Rev 2010;12(1469‐493X (Electronic), 1361‐6137 (Linking)):CD008386. [DOI] [PubMed] [Google Scholar]
Youssef 2002
- Youssef S, Stüve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG‐CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002;420(6911):78‐84. [DOI] [PubMed] [Google Scholar]


