Highlights
-
•
The coronavirus disease 2019 (COVID-2019) pandemic has swept in the world.
-
•
There is currently no specific treatment available for patients with COVID-19 infection.
-
•
Clinical trials of numerous potential candidates are underway.
-
•
The current status of potential therapeutic approaches for COVID-19 is summarized.
Keywords: ACE2 blocker, Antimalaria, Antiviral: Chinese traditional medicine, COVID-19, Immunoenhancer, Monoclonal antibody, Vaccine
Abstract
As of April 15, 2020, the ongoing coronavirus disease 2019 (COVID-2019) pandemic has swept through 213 countries and infected more than 1,870,000 individuals, posing an unprecedented threat to international health and the economy. There is currently no specific treatment available for patients with COVID-19 infection. The lessons learned from past management of respiratory viral infections have provided insights into treating COVID-19. Numerous potential therapies, including supportive intervention, immunomodulatory agents, antiviral therapy, and convalescent plasma transfusion, have been tentatively applied in clinical settings. A number of these therapies have provided substantially curative benefits in treating patients with COVID-19 infection. Furthermore, intensive research and clinical trials are underway to assess the efficacy of existing drugs and identify potential therapeutic targets to develop new drugs for treating COVID-19. Herein, we summarize the current potential therapeutic approaches for diseases related to COVID-19 infection and introduce their mechanisms of action, safety, and effectiveness.
1. Introduction
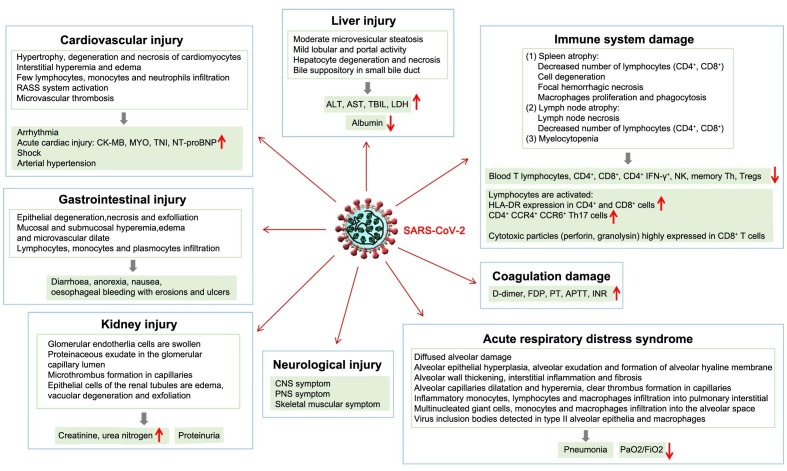
Coronavirus disease 2019 (COVID-2019), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China in December 2019 and has spread rapidly across the world due to its high transmissibility and pathogenicity (Munster et al., 2020, Zhu et al., 2020). SARS-CoV-2 is a distinct clade of beta coronaviruses encompassing severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) (Lu et al., 2020). Although most cases of the disease caused by most pathogenic coronaviruses are mild (Su et al., 2016), the SARS-CoV and MERS-CoV outbreaks in 2002 and 2012, respectively, demonstrated their high lethality (Schoeman and Fielding, 2019). SARS-CoV-2 bears an 82% resemblance to the genomic sequence of SARS-CoV (Chan et al., 2020); however, COVID-19 presents a lower case fatality rate and higher infectiousness than SARS, with mortality rates of approximately 3.7% for COVID-19 and 10% for SARS (WHO, 2003, WHO, 2020a). COVID-19 patients often exhibit mild symptoms, such as fever, cough, myalgia, and fatigue and generally have a good prognosis. However, a large proportion of COVID-19 cases have rapidly progressed to severe types, especially among older men with underlying diseases (Chen et al., 2020c, Huang et al., 2020, Liu et al., 2020c, Wang et al., 2020a). Severe cases can include dyspnea (Lin et al., 2020), shock (Wang et al., 2020a), organ dysfunction [acute respiratory distress syndrome (ARDS)] (Guan et al., 2020, Wang et al., 2020a, Xu et al., 2020, Yang et al., 2020, Yao et al., 2020), acute cardiac injury (Han et al., 2020a, Strabelli and Uip, 2020, Wang et al., 2020a, Yao et al., 2020, Zhou et al., 2020a), acute kidney injury (Guan et al., 2020, Li et al., 2020a, Pan et al., 2020, Wang et al., 2020a, Yao et al., 2020), acute liver injury (Xie et al., 2020, Xu et al., 2020, Yao et al., 2020; Zhang et al., 2020a), neurological injury (Mao et al., 2020, Wu et al., 2020), gastrointestinal injury (Yao et al., 2020), immune injury (Chen et al., 2020b, Guan et al., 2020, Qin et al., 2020, Wang et al., 2020b, Xu et al., 2020, Yao et al., 2020, Liu et al., 2020b, Zheng et al., 2020), coagulation impairment (Han et al., 2020b, Tang et al., 2020a), and even death (Huang et al., 2020, Wang et al., 2020a) (Fig. 1 ). In addition, COVID-19 pandemic has great impact on psychological stress and mental health worldwide (Bao et al., 2020, Kang et al., 2020, Li et al., 2020, Pfefferbaum and North, 2020, Shi et al., 2020a).
Fig. 1.
SARS-CoV-2 infection-induced impairment of multiple organ function Impairment of multiple organ function by SARS-CoV-2 infection includes acute respiratory distress syndrome (ARDS), acute cardiac injury, acute kidney injury, acute liver injury, neurological injury, gastrointestinal injury, immune system injury, and coagulation impairment. Abbreviations: ALT, alanine transaminase; APTT, activated partial thromboplastin time; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; CK-MB, creatine kinase myocardial band; CNS, central nervous system; FDP, fibrinogen degradation products; HLA-DR, human leukocyte antigen DR; INR, international normalized ratio; LDH, lactate dehydrogenase; MYO, myoglobin; NK, natural killer cell; NT-proBNP, N terminal pro-B-type natriuretic peptide; PaO2/FiO2, oxygenation index; PNS, peripheral nervous system; PT, prothrombin time; RAAS, renin-angiotensin-aldosterone system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TBIL, total bilirubin; Th, helper T cell; TNI, troponin I.
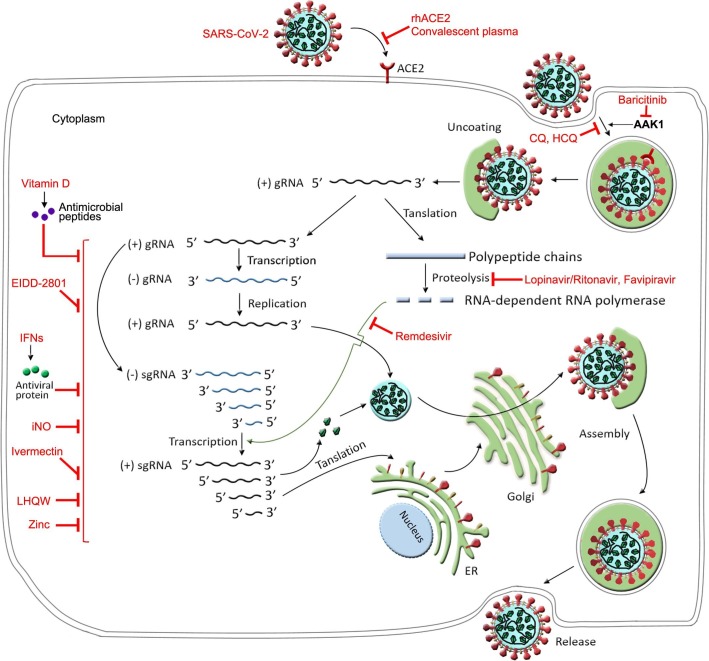
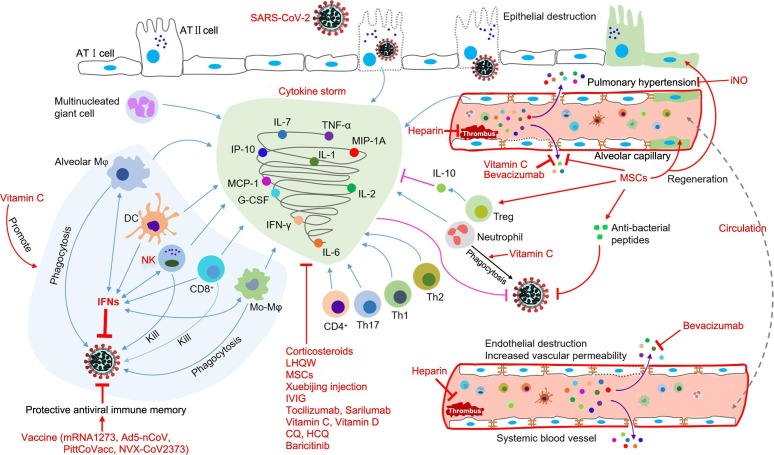
At present, the pathogenesis and etiology of COVID-19 remain unclear, and there are no targeted therapies for COVID-19 patients, who are empirically administered symptomatic treatments, with organ support for those who are seriously ill (Wang et al., 2020a). Given the pandemic spread of COVID-19 and the resulting global economic loss, developing alternative agents to contain SARS-CoV-2 is imperative. In this review, we summarize the potential therapeutic candidates under development for COVID-19 based on clinical trials and describe their potential mechanisms of action (Fig. 2 and Fig. 3 ).
Fig. 2.
The hypothetical replication cycle of SARS-CoV-2 and the possible targets of anti-COVID-19 drugs. SARS-CoV-2 binds to the ACE2 receptor on the surface of cells using the Spike protein, which subsequently triggers endocytosis. On releasing the viral nucleocapsid to the cytoplasm, encapsidated positive-strand genomic RNA [(+)gRNA] serves as a template to translate polypeptide chains, which are cleaved to non-structural proteins including RNA-dependent RNA polymerase. The single negative strand RNA [(-)gRNA] synthesized from (+)gRNA template is employed to replicate more copies of viral RNAs. Subgenomic RNAs (sgRNAs) are synthesized by discontinuous transcription from the (+)gRNA template and then encode viral structural and accessary proteins, which are subsequently assembled with newly synthesized viral RNA to form new virions. The nascent virions are then transported in secretory vesicles to the plasma membrane and released by exocytosis. RhACE2, convalescent plasma and JAK inhibitor baricitinib could dampen the binding of the Spike protein on the surface of the SARS-CoV-2 to ACE2 expressed on the cell surface. Lopinavir/ritonavir and favipiravir inhibit the proteolysis of polypeptide chains. Remdesivir inhibits RNA-dependent RNA polymerase. EIDD-2801 could inhibit SARS-CoV-2 replication. NO and Zinc might inhibit SARS-CoV-2 replication. Vitamin D might induce antimicrobial peptides to reduce SARS-CoV-2 replication. Ivermectin could effectively block SARS-CoV-2 growth. Baricitinib could interrupt the passage of SARS-CoV-2 entering cells through inhibition of AAK1-mediated endocytosis. CQ and HCQ inhibit virus/cell fusion process. LHQW and IFNs could block the process of virus replication (RNAs transcription, protein translation, and post-translational modification). Abbreviations: AAK1, adaptor-associated kinase 1; CQ, chloroquine; ER, endoplasmic reticulum; HCQ, hydroxychloroquine sulfate; IFNs, interferons; iNO, inhaled nitric oxide; JAK, janus kinase; LHQW, Lianhua Qingwen; rhACE2, recombinant human angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Fig. 3.
The hypothetical mechanisms of SARS-CoV-2 infection-induced cytokine storm and antiviral immunity and the possible therapeutic targets of patients with COVID-19 infection. “Cytokine storms” might start with inflammatory cytokine secretion into lung tissue and pulmonary blood vessels from virus-infected alveolar epithelial cells, pulmonary vascular endothelial cells, alveolar macrophages, multinucleated giant cells, and other infiltrated immune cells, which mainly serve to limit the replication and spreading of the virus and to induce downstream immune responses via the blood circulation. Following the recruitment and activation by primary cytokines, systemic immune cells (neutrophils, DCs, Mo-Mφ, NK cells, CD4+ T cells, CD8+ T cells, Th1 cells, Th2 cells, and Th17 cells, etc.) further secrete inflammatory cytokines and promote the cascade of inflammatory processes to eliminate virus and virus-infected cells. Vitamin C might inhibit SARS-CoV-2 and alleviate the illness by decreasing inflammatory cytokines, stimulating IFN production, supporting lymphocyte proliferation, boosting the phagocytic capability of neutrophils, monocytes, and macrophages, protecting lung barrier function and reducing lung vascular injury, increasing IFN secretion from alveolar Mφ, Mo-Mφ, DCs, NK cells, and CD8+ T cells. IFNs could enhance NK cell cytotoxicity, enhance expression of major histocompatibility complex Ⅰ proteins, and promote the production of IFNs and the proliferation of NK cells and Mφ. Bevacizumab could reduce vascular permeability. Vaccines (mRNA1273, Ad5-nCoV, PittCoVacc, and NVX-CoV2373) could induce protective antiviral immune memory, while MSCs could decrease pro-inflammatory cytokines, promote regeneration, secrete multiple paracrine factors and anti-inflammatory cytokines, and enlarge the proportion of Treg cells. iNO could alleviate pulmonary hypertension through its selective pulmonary vasodilation. Corticosteroids, LHQW, Xuebijing, IVIG, tocilizumab, sarilumab, baricitinib, vitamin D, CQ, and HCQ could also reduce inflammation. Heparin blocks the thrombus formation. Abbreviations: AT I, type I alveolar epithelial cell; AT Ⅱ, type Ⅱ alveolar epithelial cell; CQ, chloroquine; DC, dendritic cell; G-CSF, granulocyte-colony stimulating factor; HCQ, hydroxychloroquine sulfate; IFN-γ, interferon gamma; IL, interleukin; iNO, inhaled nitric oxide; IP-10, interferon-inducible protein-10; IVIG, intravenous gamma globulin; LHQW, Lianhua Qingwen; MCP-1, monocyte chemotactic protein 1; MIP-1A, macrophage inflammatory protein-1a; Mo-Mφ, monocyte-macrophage; MSCs, mesenchymal stem cells; NK, natural killer cell; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Th, helper T cell; TNF-α, tumor necrosis factor alpha; Treg, regulatory T cell.
2. Antiviral treatments
2.1. Remdesivir
Remdesivir, a nucleotide prodrug, originally developed to control the Ebola virus (Siegel et al., 2017) and subsequently demonstrating its efficacy in inhibiting coronaviruses such as SARS-CoV and MERS-CoV in vitro (Sheahan et al., 2017), presents antiviral activity by interfering with RNA-dependent RNA polymerase, thereby attacking the virus’s ability to replicate in the body (Fig. 2) (Agostini et al., 2018). A recent study indicated that remdesivir efficiently protected a human cell line against SARS-CoV-2 infection (Wang et al., 2020c). Treatment with intravenous remdesivir successfully improved the clinical state of the first U.S. COVID-19 patient (Holshue et al., 2020). Remdesivir is now being tested in several clinical trials designed to evaluate its efficacy and safety for the treatment of COVID-19. Notably, Gilead Sciences announced the result of a very recent clinical study on the efficacy of remdesivir on COVID-19 (Grein et al., 2020). In this report, 53 patients infected with severe COVID-19 were tracked, and 34 patients of whom were critically ill, with 30 patients requiring mechanical ventilation and 4 patients relying on extracorporeal membrane oxygenation (ECMO). Over a median follow-up of 18 days, 36 patients (68%) presented with improved oxygen-support class. 20 patients of 34 severely ill patients showed improvement in clinical conditions, with 17 of 30 patients stopped receiving invasive mechanical ventilation and 3 of 4 patients stopped receiving ECMO treatment respectively. The treatment of remdesivir limited the mortality rate of seriously ill patients needing invasive ventilation to 18%, and 5% for those who did not needed. Generally, the efficacy of remdesivir reflected in this study is hopeful. However, the sample size of this study was quite small, and definite effectiveness of remdesivir in the treatment of COVID-19 needs to be further verified (Table 1 ).
Table 1.
Potential therapeutic drugs for COVID-19.
| Intervention | Type | Status and mechanisms | Recommendations |
|---|---|---|---|
| Remdesivir | Antiviral | Remdesivir interferes with virus RNA polymerases to inhibit virus replication, and was used for Ebola virus outbreak | Being tested in clinical trials |
| Lopinavir/Ritonavir | Antiviral | Lopinavir/ritonavir are approved protease inhibitors for HIV | Inconsistent results in completed clinical trials |
| Favipiravir | Antiviral | Favipiravir inhibits viral RNA polymerase, thus interfering with viral replication | Proven efficacy in completed clinical trials |
| EIDD-2801 | Antiviral | EIDD-2801 is incorporated during RNA synthesis and then drives mutagenesis, thus inhibiting viral replication | Prepared for clinical trials |
| Convalescent plasma | Antiviral | Convalescent plasma from cured patients provides protective antibody against SARS-CoV-2 | Proven efficacy |
| rhACE2 | ACE2 blocker | rhACE2 completely binds to virus S-protein, thus protects host lungs from virus attack | Being tested in clinical trials |
| Chloroquine Hydroxychloroquine | Antimalaria | Endosomal acidification fusion inhibitor anti-inflammatory activity | Proven efficacy in completed clinical trials (clinical data partly not shown) |
| Tocilizumab | mAb | Humanized mAb targeting IL-6 | Being tested in clinical trials |
| Sarilumab | mAb | Humanized mAb targeting IL-6 | Being tested in clinical trials |
| Bevacizumab | mAb | Humanized mAb targeting VEGF | Being tested in clinical trials |
| Baricitinib | JAK inhibitor | Baricitinib attenuates proinflammatory response by inhibiting JAK and blocks virus entering into host cells through inhibiting AAK1 | Being tested in clinical trials |
| MSCs | Cell therapy | MSCs have regenerative and immunomodulatory properties and protect lungs against ARDS | Proven efficacy in completed clinical trials |
| iNO | Vasodilator | iNO possesses capability of potent and selective pulmonary vasodilation and antimicrobial activity | Being tested in clinical trials |
| LHQW | TCM | LHQW is used for prevention and treatment for infuenza, and has previously been used for SARS outbreak | Being tested in clinical trials |
| Xuebijing injection | TCM | Xuebijing serving as endotoxin antagonist, anti-inflammatory agent and anti-coagulant is used for sepsis | Being tested in clinical trials |
| IFNs | Immunoenhancer | IFNs inhibit viral RNA transcription, protein translation and post translational modifification, thus suppress virus replication | Being tested in clinical trials |
| IVIG | Immunoenhancer | IVIG provides passive immunity and anti-inflammatory effects | Being tested in clinical trials |
| NK cell therapy | Immunoenhancer | NK cells can elicit rapid and robust protective effects in defense against viral infections through direct cytotoxicity and immunomodulatory capability | Being tested in clinical trials |
| Corticosteroids | Corticosteroids | Corticosteroids dampen proinflammatory cytokines and possess antifibrotic property | Still in controversy |
| Heparin | Anticoagulants | Heparin can reverse the hypercoagulability in severe cases of COVID-19 | Proven efficacy in severe types of COVID-19 |
| Vitamin C | Nutritional supportive care | Vitamin C boosts immunity by stimulating IFN production, supplying lymphocyte proliferation and enhancing neutrophil phagocytic capability | Being tested in clinical trials |
| Vitamin D | Nutritional supportive care | Vitamin D induces secretion of antimicrobial peptides and has immunomodulatory property | Being tested in clinical trials |
| Zinc | Nutritional supportive care | Zinc is necessary for the immune system and has anti-viral activities | Being tested in clinical trials |
| Ibuprofen | NSAIDs | Ibuprofen has been widely used to treat fever or pain | Still in controversy |
| mRNA-1273 | Vaccine | mRNA-1273 contains mRNA that can encode S protein of SARS-CoV-2 | Being tested in clinical trials |
| Ad5-nCoV | Vaccine | Ad5-nCoV uses replication-defective adenovirus type 5 as vector to load some gene fragments of SARS-CoV-2 on it to express SARS-CoV-2 S protein | Being tested in clinical trials |
| PittCoVacc | Vaccine | PittCoVacc utilizes microneedle array to deliver pieces of S-protein of SARS-CoV-2 into body | Prepared for phase Ⅰ clinical trials |
| NVX-CoV2373 | Vaccine | NVX-CoV2373 is a stable, prefusion protein developed through the advanced nanoparticle technology | Prepared for phase Ⅰ clinical trials |
Abbreviation: ARDS, acute respiratory distress syndrome; COVID-2019, corona virus disease 2019; HIV, human immunodeficiency virus; IFNs, interferons; IL-6, interleukin 6; iNO, inhaled nitric oxide; IVIG, intravenous gammaglobulin; LHQW, Lianhua Qingwen; mAb, monoclonal antibody; MSCs, mesenchymal stem cells; NK, natural killer cell; NSAIDs, non-steroidal anti-inflammatory drugs; rhACE2, recombinant human angiotensin-converting enzyme 2; SARS, severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TCM, traditional Chinese medicine; VEGF, vascular endothelial growth factor.
2.2. Lopinavir/ritonavir
Lopinavir/ritonavir (LPV-r) is a co-formulated human immunodeficiency virus (HIV)-specific protease inhibitor that serves as first-line therapy for HIV (Barragan and Podzamczer, 2008). Concomitant use of ritonavir could increase the plasma half-life of lopinavir through cytochrome P450 inhibition in the liver. During the 2003 SARS outbreak, LPV-r was reported to have in vitro inhibitory activity against SARS-CoV (Chu et al., 2004a), and combination therapy of LPV-r and ribavirin provided favorable results in treating patients with SARS (Fig. 2) (Chu et al., 2004b). Triple combination therapy with LPV-r, ribavirin, and IFN-α has shown clinical effectiveness for MERS (Kim et al., 2016). Notwithstanding, a recent open-label randomized study with 199 patients in Wuhan showed that LPV-r monotherapy did not produce any therapeutic benefits for COVID-19 patients compared with standard supportive care, which might be caused by the higher throat viral loads in the LPV-r group, concurrent pharmacologic interventions, and late treatment initiation (Table 1) (Cao et al., 2020). The enrolled COVID-19 patients were critically ill, and LPV-r treatment might have been started relatively late. However, in another retrospective cohort study, combination therapy of LPV-r and arbidol was associated with improved pulmonary computed tomography images (Deng et al., 2020). Collectively, the combination therapy of LPV-r and other antiviral agents in early stages of COVID-19 infection might hold promise for treating COVID-19.
2.3. Favipiravir
Favipiravir, also known as Avigan® and originally developed and approved for the influenza virus infection epidemic in Japan, has a broad spectrum of antiviral activity (Furuta et al., 2013). Once it enters cells, favipiravir undergoes phosphorylation to convert into its active phosphorylated form (favipinavir-RTP), which potently inhibits viral RNA polymerase, thereby interfering with viral genome replication (Fig. 2) (Furuta et al., 2005). Favipiravir demonstrated efficacy in inhibiting a wide range of viruses, including resistant influenza viruses and other RNA viruses, such as arenaviruses, bunyaviruses, and filoviruses (Delang et al., 2018). Previous studies have showed that favipiravir is efficacious against Ebola virus in rodent models (Oestereich et al., 2014, Smither et al., 2014), while its effectiveness in humans is unproven (Sissoko et al., 2016). Favipiravir appears to be effective in COVID-19. Two clinical trials involving a total of 340 patients were conducted in Wuhan and Shenzhen. At a press conference in March 17, 2020, Xinmin Zhang, an official of China’s Science and Technology Ministry, stated that favipiravir appeared to be effective in COVID-19 (Hackett, 2020). Preliminary clinical data from 80 patients in the Third People’s Hospital of Shenzhen suggested that favipiravir exerted antiviral effects more potently than LPV-r, with no overt adverse reactions (Third People's, 2020). The other clinical trial in Wuhan showed that, based on conventional therapy, favipiravir demonstrated higher efficacy than arbidol in terms of the 7-day recovery rate and duration of symptom attenuation in patients with moderate COVID-19 infection (Chen et al., 2020a). Taken together, the clinical results of favipiravir are favorable (Table 1); however, further study of favipiravir monotherapy using a large sample size is needed.
2.4. EIDD-2801
Researchers from Emory University, the University of North Carolina, and Vanderbilt University Medical Center tested a new drug, EIDD-2801, for its in vitro efficacy against SARS-CoV-2, and its laboratory results are encouraging (Sheahan et al., 2020). Based on EIDD-1931, an orally bioavailable ribonucleotide analog, EIDD-2801 is designed to optimize oral bioavailability and improve drug uptake in humans and non-human primates (Fig. 2). EIDD-1931 has a variety of antiviral activities against numerous causal agents of deleterious viral infectious diseases, including influenza, chikungunya, Ebola, Venezuelan equine encephalitis, and coronavirus-associated diseases (Agostini et al., 2019, Painter et al., 2019, Reynard et al., 2015, Toots et al., 2019). As a ribonucleotide analog, EIDD-1931 shares similar mechanisms of action with remdesivir and exerts antiviral effects by mimicking a ribonucleotide, thereby mistakenly being incorporated into viral RNA, leading to lethal mutagenesis during virion RNA synthesis and inhibiting the RNA-dependent RNA polymerase encoded by the virus during viral replication (Fig. 2) (Painter et al., 2019, Sheahan et al., 2020). The study suggested that EIDD-1931 robustly blocked the replication of SARS-CoV, MERS-CoV, and the newly emerging SARS-CoV-2 in cell assays. EIDD-2801 demonstrated its prophylactic and therapeutic effects in murine models of SARS and MERS, showing diminished viral lung titers, attenuated weight loss, ameliorated lung damage, and improved pulmonary function, which are dependent on the administered dosage and the treatment initiation time. Compared with remdesivir, EIDD-2801 appears to be favorable. EIDD-2801 has a more convenient oral administration, while remdesivir must rely on intravenous administration by a trained specialist. More importantly, EIDD-2801 showed active antiviral activity against remdesivir-resistant viruses, according to the data presented by the study. Of significant importance, EIDD-1931 appeared to be effective not just for SARS-CoV, MERS-CoV, and SARS-CoV-2, but also other coronavirus species, indicating its potential efficacy for emerging coronaviruses in the future. The U.S. Food and Drug Administration (FDA) has approved an Investigational New Drug application for EIDD-2801, and clinical studies in humans are expected to start as soon as possible (Table 1) (BioSpace, 2020).
3. Convalescent plasma
Convalescent plasma collected from donors who have survived an infectious disease by producing protective antibodies is considered to provide a great degree of protection for recipients affected by the emerging virus (Dodd, 2012). Convalescent plasma has been successfully employed to treat numerous infectious diseases, including the 2003 SARS-CoV-1 epidemic, 2009–2010 H1N1 influenza virus pandemic, and 2012 MERS-CoV epidemic (Dodd, 2012, Hung et al., 2011, Mair-Jenkins et al., 2015), for which modern medicine has no specifically effective treatment. In the absence of specific antiviral agents and vaccines for COVID-19, clinical trials have been conducted aimed at investigating the efficacy of convalescent plasma in treating COVID-19. A very recently published study by Chinese researchers confirmed the efficacy of convalescent plasma in controlling SARS-CoV-2 (Table 1) (Roback and Guarner, 2020). The report suggested that COVID-19 patients showed signs of improvement approximately 1 week after convalescent plasma transfusion. Another clinical study involved 10 critically ill patients infected with COVID-19 from 3 different hospitals in Wuhan suggested high-titer convalescent plasma transfusion can effectively neutralize SARS-CoV-2, leading to impeded inflammatory responses and improved symptom conditions without severe adverse events. All 10 patients receiving convalescent plasma transfusion showed improvement of clinical outcomes or were cured and discharged from hospital (Duan et al., 2020). Given the clinical effectiveness of convalescent plasma, the FDA has granted clinical permission for applying convalescent plasma to the treatment of critically ill COVID-19 patients (FDA, 2020).
4. Spike Protein-Angiotensin-Converting enzyme 2 blockers
4.1. Angiotensin-converting enzyme 2 (ACE2)
ACE2 serves as a negative regulator of the renin angiotensin system (RAS) and is widely distributed among lung tissue and many extrapulmonary tissues, including brain, heart, liver, kidney, endothelium, and intestine (Fig. 2) (Crackower et al., 2002, Ding et al., 2004, Hamming et al., 2004). Of them, alveolar epithelial type Ⅱ cells possess an abundance of ACE2 receptors (Zhao et al., 2020). Unfortunately, ACE2 is also a functional receptor of SARS-CoV (Li et al., 2003) and SARS-CoV-2 (Zhou et al., 2020b). The spike (S) protein of SARS-CoV and SARS-CoV-2 binds to the host ACE2 receptor and then enters target cells (Fig. 2). Critically, SARS-CoV-2 shows higher affinity for ACE2 than SARS-CoV (Wrapp et al., 2020). Moreover, the S protein of SARS-CoV-2 contains a site that can be recognized and activated by a host enzyme called furin, which is highly expressed in the lungs (Coutard et al., 2020). These findings might provide strong evidence for the high pathogenicity of SARS-CoV-2. Due to the pivotal role of widely distributed ACE2 in the entry and replication of viruses, blocking S protein binding to ACE2 might offer some protection against SARS-CoV-2 infection (Fig. 1). A study using a murine model of SARS showed that SARS-CoV S protein binding to ACE2 led to RAS downregulation and consequently contributed to severe lung injury (Imai et al., 2005). Therefore, recombinant human ACE2 (rhACE2) might protect host lungs against infection through an enhanced ACE2 level to neutralize the S protein on the SARS-CoV-2 surface. Clinical evidence has demonstrated the safety of rhACE2 application (Haschke et al., 2013, Khan et al., 2017). A recent in vitro study demonstrated that the fusion protein of rhACE2 with an Fc fragment shows high affinity binding to the receptor-binding domain of SARS-CoV-2 and potently neutralizes SARS-CoV-2 (Lei et al., 2020). Additionally, the findings from a newly online published report in Cell were strongly supportive of potential efficacy of rhACE2 for SARS-CoV-2 infection (Monteil et al., 2020). The study showed that clinical-grade human recombinant soluble ACE2 (hrsACE2) displayed potently inhibitory activity against SARS-CoV-2 in cell cultures and in engineered replicas of human blood vessels and kidneys in a dose-dependent manner. The researchers found that before infecting cells, SARS-CoV-2 co-incubated with hrsACE2, rather than mouse recombinant soluble ACE2, demonstrated dropped activity to infect cells. In the organoid level, administration of hrsACE2 significantly blocked SARS-CoV-2 infection in both engineered human blood vessels and kidneys without observed toxicity. However, note that this study focused on the early stages of SARS-CoV-2 infection, it is still unclear whether hrsACE2 can play a role in the later infection process. Taken together, it is likely that rhACE2 has potentially curative effectiveness in COVID-19. A clinical trial is underway to investigate the efficacy of rhACE2 in the treatment of COVID-19 (NCT04287686) (Table 1).
ACE inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) are widely used for treating cardiovascular diseases. Studies have shown that ACEIs/ARBs can upregulate ACE2 expression (Ferrario et al., 2005, Ishiyama et al., 2004, Karram et al., 2005), which might correlate with the susceptibility to SARS-CoV-2. However, evidence has shown that RAS activation and decreased ACE2 expression contribute to the pathological process of lung injury after SARS-CoV infection (Kuba et al., 2005). Serum angiotensin II levels are markedly elevated and exhibit a linear positive correlation to viral load and lung injury in COVID-19 (Liu et al., 2020d). Consequently, the effects of ACEIs/ARBs on lung-specific ACE2 expression in COVID-19 remain unknown. ACEIs/ARBs might have a dual role in COVID-19. On the one hand, the elevated ACE2 level might increase susceptibility to SARS-CoV-2, while on the other, ACE2 activation and RAS deregulation might ameliorate the acute lung injury, heart injury, and renal damage induced by SARS-CoV-2 (Guo et al., 2020). However, there has been no definite evidence on whether taking ACEIs/ARBs is beneficial or harmful for COVID-19 infected patients.
5. Chloroquine and hydroxychloroquine
Chloroquine (CQ), a drug widely used in treating malarial and autoimmune diseases, also confers considerable broad-spectrum antiviral effects even against SARS-CoV (Table 1) (Keyaerts et al., 2004b, Savarino et al., 2006, Yan et al., 2013). A recent study demonstrated that CQ has anti-SARS-CoV-2 activity in vitro (Fig. 3) (Wang et al., 2020c). A subsequent letter in Bioscience confirmed that CQ is efficacious in treating COVID-19 pneumonia in numerous related clinical trials (Gao et al., 2020). CQ therapy resulted in improved pulmonary lesions, shortened disease course, and good outcomes (Table 1) (Gao et al., 2020). Given the efficiency displayed by CQ in clinical practice, CQ has been included in the Guidelines for the Diagnosis and Treatment of COVID-19 (7th edition) issued by the National Commission of the People’s Republic of China (NHPFC, 2020).
Hydroxychloroquine sulfate (HCQ) shares a similar chemical structure and mechanisms of action with CQ but with lower ocular toxicity (Lim et al., 2009) and has proven efficacious in containing SARS-CoV-2 in vitro (Liu et al., 2020a). CQ and HCQ exert antiviral function through various mechanisms. CQ has been shown to interfere with the glycosylation process of ACE2 in host cells, thereby inhibiting the efficiency of the binding of S protein with ACE2, in turn disrupting the virus/cell fusion process (Fig. 3) (Savarino et al., 2006). CQ can increase the pH of acidic cellular organelles required for virus entry into host cells (Savarino et al., 2003). In addition to its direct antiviral activity, CQ and HCQ can attenuate major “cytokine storms” (an overreaction of the immune system causing inflammatory “storms”) by decreasing cytokine production (interleukin [IL]-1, IL-6, and tumor necrosis factor [TNF], etc.) (Fig. 3) (van den Borne et al., 1997). Notably, high cytokine concentrations have been observed in seriously ill COVID-19 patients (Huang et al., 2020), indicating that over-reactive immune responses exacerbate COVID-19. Therefore, the immune-modulating activity of HCQ might partially account for its efficient control of SARS-CoV-2 infection. CQ and HCQ are therefore promising drugs of choice for large-scale use due to their low cost, wide availability and potential efficacy for treating COVID-19. CQ and HCQ need to be administered with caution when treating COVID-19 infection to prevent toxicity.
A recent open-label study in France showed that HCQ treatment was significantly associated with viral load reduction/disappearance in COVID-19 patients and that its effect was reinforced by azithromycin (Gautret et al., 2020). Although this study is an open-label study using small sample size, the combination of HCQ and azithromycin could be promising candidate for COVID-19 patients. In contrast, a multinational, network cohort and self-controlled case series study demonstrated that short-term HCQ treatment is safe (Lane et al., 2020). However, combination of HCQ and azithromycin may induce an increased risk of 30 day cardiovascular mortality, chest pain/angina, and heart failure (Lane et al., 2020). Therefore, we must pay the attention for the combination therapy in the management of COVID-19 patients.
6. Human monoclonal antibody
6.1. Tocilizumab and sarilumab
Tocilizumab (TCZ) and sarilumab are monoclonal antibodies against IL-6 receptors and have been employed to treat rheumatoid arthritis (Nishimoto et al., 2000, Raimondo et al., 2017). TCZ and sarilumab efficiently inhibit IL-6 through both membrane-bound and soluble IL-6R (Fig. 3) (Raimondo et al., 2017, Sakkas, 2016). TCZ has been approved by the FDA for treating cytokine release syndrome marked by excessive cytokine production and consequently rapid multiorgan damage (lungs, kidney, and heart) (Shimabukuro-Vornhagen et al., 2018). COVID-19 disease severity depends on the increase in pro-inflammatory factors [IL-6, IL-1, IL-2, IL-7, IL-10, granulocyte-colony stimulating factor, interferon-γ-inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein-1 alpha, and TNF-α] (Huang et al., 2020, Ruan et al., 2020, Zhou et al., 2020a), suggesting that cytokine storms are involved in the development of COVID-19. One of the key cytokines involved in the immune system perturbation is IL-6 (Mehta et al., 2020). As IL-6 receptor antagonists, TCZ and sarilumab have therefore been hypothesized to exert suppressive effects on exuberant and dysfunctional systematic inflammation in patients infected with SARS-CoV-2 and further improve patients’ condition. Three clinical trials (ChiCTR2000030196, ChiCTR2000030442, and ChiCTR2000029765) for TCZ have been approved for COVID-19, and the National Health and Family Planning Commission of China has approved the treatment with TCZ in patients with elevated IL-6 level. (Table 1) (NHPFC, 2020). Encouraging results have been seen in Italy where three seriously ill patients administered TCZ showed the signs of improvement (Italian ANSA News Agency, 2020). According to the announcement from Regeneron Pharmaceuticals and Sanofi on March 16, 2020, a phase Ⅱ/III clinical trial in the U.S. for sarilumab has been conducted to assess its therapeutic effects in patients with severe COVID-19 infection (Table 1) (Regeneron, 2020).
6.2. Bevacizumab
Bevacizumab is a monoclonal anti-vascular endothelial growth factor (VEGF) antibody that competes with VEGF receptors on the surface of endothelial cells for VEGF binding, thereby inhibiting the effects caused by binding VEGF to its receptors, such as endothelial cell proliferation and neovascularization (Fig. 3) (Ferrara et al., 2004). VEGF produced by various inflammatory and epithelial cells is a potent vascular permeability inducer (Dvorak et al., 1995). Previous reports have shown that plasma VEGF levels markedly increase in patients with ARDS (Thickett et al., 2001). Given the causal link between ARDS and increased vascular permeability and pulmonary edema (Fanelli and Ranieri, 2015). Bevacizumab might be a potential anti-ARDS therapeutic approach. ARDS is a common complication in severe cases of COVID-19 (Fig. 1). Bevacizumab is therefore likely to be a promising therapy against COVID-19 (Table 1).
7. Janus Kinase inhibitor
7.1. Baricitinib
Baricitinib, a highly selective janus kinase (JAK) inhibitor, has been approved for treating rheumatoid arthritis (Al-Salama and Scott, 2018). JAK-dependent pathways are responsible for producing a variety of cytokines involved in the pathogenesis of the progression process of COVID-19 characterized by elevated levels of inflammatory factors (e.g., IL-6, IL-7, and IL-2) (O'Shea and Plenge, 2012). Baricitinib also serves as an inhibitor of adaptor-associated kinase 1 (AAK1, a member of the numb-associated kinase family), which plays a central role in regulating endocytosis through which the virus invades host cells (Fig. 3) (Richardson et al., 2020). Therefore, baricitinib might exert beneficial effects on COVID-19 pneumonia, not only by impeding overexuberant immune responses by hijacking cytokine signaling pathways but also preventing SARS-CoV-2 from entering host cells. However, blockage of JAK-dependent pathways by baricitinib can also inhibit production of interferons (IFNs) (O'Shea and Plenge, 2012), which might have antiviral activity against SARS-CoV-2 (Fig. 3) (Ströher et al., 2004). Baricitinib treatment is therefore a double-edged sword (Table 1), and choosing the appropriate time to administer baricitinib is vitally important. For severely ill patients with abnormal biomarkers, cautious baricitinib treatment might yield clinically beneficial effects.
8. Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are multipotent cells isolated from diverse mesenchymal tissues (e.g., bone marrow, umbilical cord, adipose tissue) (Lv et al., 2014). By virtue of their low immunogenicity (Chamberlain et al., 2007), proven safety (Zheng et al., 2014), and reparative and immunomodulatory properties (Galipeau and Sensébé, 2018, Mei et al., 2010), MSCs are attractive therapeutic candidates for treating a wide range of diseases [e.g., graft-versus-host disease (Le Blanc et al., 2008), sepsis, and ARDS] (Fig. 3) (Asmussen et al., 2014, Krasnodembskaya et al., 2012). A large body of preclinical data has demonstrated the efficacy of MSCs in treating ARDS, manifested in reduced pulmonary edema, decreased plasma concentrations of pro-inflammatory cytokines, and reduced mortality rates (Chimenti et al., 2012, Devaney et al., 2015, Xu et al., 2007). Although not fully understood, the mechanisms by which MSCs exert protective effects include their direct regenerative ability and secretion of multiple paracrine factors including antibacterial peptides and anti-inflammatory cytokines such as IL-10 (Gupta et al., 2012, Gupta et al., 2007). Another attractive property of MSCs is immune response modulation (Aggarwal and Pittenger, 2005). MSCs have been shown to enlarge the proportion of regulatory T cells and decrease pro-inflammatory factors such as IL-6 and TNF-α (Fig. 3) (Gupta et al., 2012, Li et al., 2012, Prevosto et al., 2007). MSCs showed positive efficacy in a recent study of COVID-19 patients. Treatment with allogenic MSC transplantation at Beijing YouAn Hospital showed significantly improved functional outcomes without obvious adverse effects in 7 COVID-19 patients (Table 1) (Zikuan Leng et al., 2020). Adoptive MSC transfer might therefore be a valuable treatment option for COVID-19.
9. Inhaled nitric oxide (iNO)
Nitric oxide (NO) is a key endogenous molecule implicated in a variety of physiological and pathological processes including smooth muscle relaxation, immune responses and antimicrobial activities (Saura et al., 1999, Schmidt and Walter, 1994). Due to its potent and selective pulmonary vasodilation, iNO has been extensively applied to treat pulmonary hypertension, ARDS and other respiratory diseases with a relative good safety profile (Fig. 3) (Griffiths and Evans, 2005, Pepke-Zaba et al., 1991). Of note, previously published in-vitro studies indicated NO possessed inhibitory effects on SARS-CoV replication (Fig. 2) (Akerström et al., 2005, Keyaerts et al., 2004a). Moreover, a small-sample clinical study testing the efficacy of iNO in the treatment of SARS showed, 6 severely infected patients receiving iNO therapy presented with improved arterial oxygenation and reduction in a need for ventilator support (Chen et al., 2004). In view of the high incidence of pulmonary complications in COVID-19 infected patients, iNO therapy may serve as a promising candidate for treating severe cases of COVID-19 via alleviating lung damage. Currently, a related phase Ⅱ clinical trial testing iNO is underway in COVID-19 infected patients complicated with ARDS (NCT04306393 and NCT04305457).
10. Traditional Chinese medicine treatment
Traditional Chinese medicine (TCM) is a unique and well-established system of medicine widely employed for thousands of years to prevent and treat numerous diseases in China. In the battle to stop the epidemic situation of COVID-19, the integration of traditional Chinese and western medicine is a unique scheme in China. Approximately 85% of Chinese COVID-19 patients underwent TCM treatment as reported by XuNanping, the vice minister of Science and Technology of China (China's State Council, 2020). In clinical practice, several kinds of TCM have demonstrated their beneficial effects in treating patients with COVID-19 pneumonia (Ren et al., 2020). Lianhua Qingwen (LHQW) has a broad-spectrum antiviral effect on a host of influenza viruses, such as influenza A (H1N1), and HPAI A (H7N9) viruses, by inhibiting viral propagation and regulating immune function (Fig. 3) (Ding et al., 2017, Dong et al., 2014). LHQW has been commonly used for treating viral influenza clinically and played a key role in controlling SARS-CoV during the 2003 outbreak. Recently, LHQW was reported to have inhibitory activity against SARS-CoV-2 and anti-inflammatory effects in vitro (Table 1) (Runfeng et al., 2020).
Xuebijing (whose chemical composition includes safflower yellow A, paginin, ferulic acid, and salvianolic acid B) functions as an endotoxin antagonist, anti-inflammatory agent, and anticoagulant (He et al., 2013, Sun et al., 2010, Xu et al., 2009, Zhang et al., 2006) and has been widely used in China as a therapy for sepsis (Fig. 3) (Chen et al., 2018). A clinical trial showed that injecting Xuebijing improved the symptoms of severe pneumonia (Wang et al., 2016). Given the cytokine storms in severe COVID-19 infection, administering Xuebijing could turn critically ill cases into mild ones by attenuating the overactivated immune responses and preventing progressive pathological deterioration. Two clinical trials aimed at testing the efficacy and safety of Xuebijing injection for COVID-19 have been registered in the Chinese Clinical Trial Registry (ChiCTR2000030388 and ChiCTR2000029381) (Table 1).
In addition to LHQW and Xuebijing, the Chinese clinical guidelines for COVID-19 pneumonia treatment have included Jinhua Qinggan granules, Shufeng Jiedu capsules, and Lung Cleansing and Detoxifying Decoction, given their potential efficacy and few adverse effects in clinically treating COVID-19 infection (NHPFC, 2020).
11. Immunoenhancers
11.1. Interferons
IFNs are large families of numerous type Ⅰ species (IFN-α, IFN-β, IFN-ε, IFN-κ, and IFN-ω) and one type Ⅱ species (IFN-γ) (Liu, 2005, Parkin and Cohen, 2001). Type Ⅰ IFNs have demonstrated inhibitory effects on a wide range of viruses including SARS-CoV (Fig. 3) (Minagawa et al., 1987, Sperber and Hayden, 1989, Ströher et al., 2004, Tan et al., 2004). When they encounter viruses, host cells form and release IFNs, which protect the original and adjacent cells against attack. The antiviral state produces its effects by several means, such as inhibiting viral RNA transcription, protein translation, and post-translational modification (Fig. 2 and Fig. 3) (De Andrea et al., 2002). To date, the clinical effect of IFN intervention in COVID-19 infection is ambiguous. Previous evidence confirmed IFN efficacy in inhibiting SARS-CoV (Cinatl et al., 2003, Sainz et al., 2004). IFN-α showed in vitro inhibitory effects on SARS-CoV at concentrations of 1000 IU/ml (Ströher et al., 2004). Interestingly, recombinant human IFN-β1a within a safe dose range exhibited more potent activity on SARS-CoV (Hensley et al., 2004). As a registered agent for chronic hepatitis C, IFN-α2b was reported to protect type 1 pneumocytes against SARS coronavirus infection in macaques (Haagmans et al., 2004). Combined treatment of IFN-α-2a and ribavirin was shown to have antiviral effects on MERS-CoV (Falzarano et al., 2013). In addition to their antiviral activity, IFNs show an immunomodulatory capability; type Ⅰ interferons can enhance natural killer (NK)-cell cytotoxicity, enhance the expression of major histocompatibility complex Ⅰ proteins, as well as promote IFN production and the proliferation of NK cells and macrophages (De Andrea et al., 2002, Goodbourn et al., 2000, Sen, 2001). IFN-α in conjunction with corticosteroids were shown to improve oxygen saturation and facilitate more rapid resolution of radiographic lung abnormalities in SARS infection (Loutfy et al., 2003). A study using a MERS-CoV infection mouse model indicated that early application of exogenous IFN-β facilitated virus clearance and protected against fatal lung infection, while delayed IFN-β administration tended to elevate damaging proinflammatory cytokine responses and led to deleterious outcomes (Channappanavar et al., 2019). Given the inconclusive effects of IFNs in treating viral infectious diseases, IFNs and combined therapy should be prudently employed with COVID-19 infection (Table 1).
11.2. Intravenous gamma globulin
Intravenous gamma globulin (IVIG) as purified IgG products prepared from pooled human plasma has been widely used for treating numerous inflammation-related diseases including heart failure, adult respiratory distress syndrome, and vasculitis (Jolles et al., 2005). IVIG demonstrates its passive immunity and anti-inflammatory effects by supplying idiotypic antibodies (Kaveri et al., 1991), binding to Fc receptors (Fehr et al., 1982), suppressing pathogenic cytokines (Andersson and Andersson, 1990, Andersson et al., 1993), preventing formation of membranolytic attack complexes (Lutz et al., 1996), and modulating T-cell function (Fig. 3) (Marchalonis et al., 1992). Transchromosomic bovine-produced human polyclonal immunoglobulin G antibodies inhibited MERS-CoV in murine models and in vitro assays (Luke et al., 2016). Due to the scarcity of human-derived IgG products (or convalescent plasma), therapeutic immunoglobulin might provide insights for COVID-19 treatment (Table 1). However, IVIG should be carefully administered given its numerous adverse effects (e.g., myalgia, fever, renal failure (Achiron, 1997, Bertorini et al., 1996, Wajanaponsan and Cheng, 2004), and venous thromboembolism (Hoffmann and Enk, 2019). Thromboembolism was found in a third of patients with SARS who underwent IVIG treatment during the SARS outbreak in Singapore (Lew et al., 2003).
11.3. Natural killer (NK) cell therapy
NK cells, a small subset of peripheral white blood cells, serve as an essential part of innate immune response. NK cells can elicit rapid and robust protective effects in defense against viral infections through direct cytotoxicity and immunomodulatory capability (Fig. 3) (Vivier et al., 2008). Once recognizing infected host cells, NK cells trigger targeted cell apoptosis by perforin- and granzyme B-mediated pathways and secrete multiple cytokines involved in regulation of innate and adaptive immune responses, which facilitate viral clearance (Iannello et al., 2008). NK cell-based immunotherapies have long been investigated for treating malignancies, albeit with limited clinical practice due to the difficulty to obtain sufficient cell numbers for adoptive transfer (Lee, 2019). Recently, CYNK-001, an investigational allogeneic NK cell therapy derived from placental hematopoietic stem cells, has passed through an investigational new drug application approved by FDA (Celularity, 2020). As a NK cell product, CYNK-001 harbors the potential for inhibiting viral infection through direct killing SARS-CoV-2 infected host cells and indirect inducing immune responses. However, there exists controversy about whether the elicited strong inflammatory responses are favorable or detrimental for patients infected with severe COVID-19. The efficacy and safety of NK cell therapy for COVID-19 require to be tested by ongoing related clinical trials.
12. Corticosteroids
Due to their excellent anti-inflammatory, antifibrotic properties and ability to suppress collagen deposition, corticosteroids are frequently used to treat ARDS and sepsis (Fig. 3) (Heming et al., 2018, Rhen and Cidlowski, 2005, Thompson, 2003). Despite the long history of administering corticosteroids, their therapeutic effectiveness and safety remain controversial. Several clinical trials and meta-analyses have indicated that corticosteroids are associated with increased mortality, a tendency for requiring mechanical ventilation therapy, and relatively longer hospitalizations for SARS, MERS, and H1N1 infections (Arabi et al., 2018, Han et al., 2011, Stockman et al., 2006). These adverse events are partly due to the suppression of normal host immune responses and impeded viral clearance. Numerous studies have, however, demonstrated corticosteroids’ beneficial effects on physiologic outcomes in virus-associated respiratory diseases, supporting the proper application of corticosteroids in these cases, especially critical cases (Li et al., 2017, Siemieniuk et al., 2015). A retrospective study of 401 patients with SARS showed that corticosteroids reduced case fatality rates and shorten hospital stays (Chen et al., 2006). An accurate conclusion regarding the therapeutic effect of corticosteroids in treating COVID-19 cannot therefore be drawn (Table 1). High corticosteroid doses are closely associated with adverse events such as secondary infections, delayed viral clearance, and emergence of viral resistance. According to the Guidelines for the Diagnosis and Treatment of COVID19 (7th edition) in China (NHPFC, 2020), however, prudent low-to-moderate doses of corticosteroids could yield potential therapeutic benefits for a subset of seriously ill patients with COVID-19 pneumonia, recommendations in line with the interim clinical management guidance for COVID-19 released by the World Health Organization, which advised against routinely administering corticosteroids except for clinical indications such as exacerbated chronic obstructive pulmonary disease and septic shock (WHO, 2020b).
13. Anticoagulants
Tang et al. (2020b) investigated the effects of anticoagulant heparin in patients with severe COVID-19. The D-dimer, prothrombin time and age were positively, and platelet count was negatively correlated with 28-day mortality. There was no difference on 28-day mortality between heparin users and nonusers. However, the 28-day mortality of heparin users was lower than nonusers. This study suggests that anticoagulant therapy with heparin appears to be associated with better prognosis in severe COVID-19 patients (Tang et al., 2020b). Interestingly, patients with severe pneumonia by COVID-19 had higher platelet count than those induced non-COVID-19, and only the former with markedly elevated D-dimer may benefit from anticoagulant therapy (Yin et al., 2020). Future study using a large sample size is needed to confirm the effects of heparin in the management of COVID-19 patients.
A recent study demonstrated the interaction between the SARS-CoV-2 spike S1 protein receptor binding domain (SARS-CoV-2 S1 RBD) and heparin, suggesting the development of heparin-based therapeutics (Microft-West et al., 2020). It is of great interest to develop the novel heparin-based compounds for COVID-19.
14. Vitamins
14.1. Vitamin C
Vitamin C has pleiotropic roles in modulating the immune system (Carr and Maggini, 2017) and is known for its antioxidant properties, and antioxidants are generally accepted as an adjuvant therapy for critically ill patients, whose vitamin C levels are markedly decreased (Carr et al., 2017, Nakano and Suzuki, 1984). Vitamin C exerts positive effects on the immune system by stimulating IFN production, supporting lymphocyte proliferation, and boosting neutrophil phagocytic capability (Fig. 3) (May and Harrison, 2013, Oudemans-van Straaten et al., 2014, Wilson, 2013). In acute lung injury and ARDS, excessive neutrophil accumulation in inflammatory loci results in lung tissue damage through the release of necrotic cell contents (known as neutrophil extracellular traps) (Papayannopoulos, 2018, Weiss, 1989), and vitamin C is reported to prevent this process (Mohammed et al., 2013). In animal models of sepsis, vitamin C was shown to protect lung barrier function and reduce lung vascular injury through diminishing inflammatory responses and coagulant changes (Fisher et al., 2012, Fisher et al., 2011). A phase Ⅰ trial in patients with critically severe sepsis reported that high doses of vitamin C reduced the extent of multiple organ failure and mitigated circulating injury biomarker levels (Fowler et al., 2014). Placebo-controlled trials have shown that vitamin C could reduce the duration of colds (Hemilä and Chalker, 2013). Given that COVID-19 patients frequently present lung damage, vitamin C could be a promising candidate (Table 1).
14.2. Vitamin D
Vitamin D is known to modulate the innate and adaptive immune system, and its deficiency is associated with increased autoimmunity as well as in an increased susceptibility to infection (Aranow, 2011). Grant et al. (2020) pointed the role of vitamin D in reducing the risk of respiratory tract infections by COVID-19. Actions of mechanism of vitamin D include the induction of antimicrobial peptides (i.e., cathelicidins and defensins) that can reduce viral replication rate and impeding pro-inflammatory cytokines. Several clinical trials of vitamin D in patients with COVID-19 (i.e., NCT04334005, NCT04344041) are underway.
15. Non-steroidal anti-inflammatory drugs (NSAIDs)
The non-steroidal anti-inflammatory drugs (NSAIDs) including ibuprofen have been widely used to treat fever or pain. In March 11, 2020, Fang et al. (2020) reported the hypothesis that ibuprofen can increase the risk of developing severe and fatal COVID-19 since ibuprofen is known to upregulate ACE2 receptors. However, there is no evidence indicating that ibuprofen worsens the clinical symptoms of COVID-19 infected patients (FitzGerald, 2002, Kakodkar et al., 2020). In March 23, 2020, US FDA announced that it is not aware of any evidence that NSAIDs such as ibuprofen could worsen COVID-19.
16. Vaccine
There is no specific vaccine currently available for containing COVID-19 infection. To meet the urgent need for an effective vaccine in the context of active SARS-CoV-2 transmission, companies and institutions worldwide have been working on a SARS-CoV-2 vaccine through various approaches, resulting in a series of vaccine candidates (Routley, 2020). One of the most promising one of them is mRNA-1273, which is developed by National Institute of Allergy and Infectious Disease scientists together with biotechnology company Moderna (Fig. 3) (Moderna, 2020a; NIH, 2020). As an mRNA vaccine, mRNA-1273 embedded in lipid nanoparticles encodes viral S proteins of SARS-CoV-2 and then delivers the antigen into human cells to elicit SARS-CoV-2-specific neutralizing antibodies and potent immune responses, thereby protecting healthy individuals against COVID-19 infection (Fig. 3). mRNA vaccine is superior to other conventional vaccines, given its high potency, short production cycles and safety as lack of actual viral genome (Ahn et al., 2020). A phase Ⅰ, open-label, dose-ranging trial of mRNA-1273 was conducted in 45 healthy adult volunteers aged 18 to 55 years with three different doses of mRNA-1273 to assess its efficacy and appropriate effective dose (Table 1) (NCT04283461). On April 16, 2020, Moderna received the award from US Government Agency BRADA for up to $483 million to accelerate development of mRNA-1273 against COVID-19 (Moderna, 2020b).
mRNA-1273 was the first vaccine to be tested in a clinical trial, followed closely by another promising candidate called Ad5-nCoV, which was jointly developed by Tianjin-based biotechnology company Cansino and the Institute of Biotechnology of the Academy of Military Medical Sciences. Developed with Cansino’s adenovirus-based viral vector vaccine technology platform, Ad5-nCoV uses replication-defective adenovirus type 5 as a vector to load SARS-CoV-2 gene fragments onto it to express the SARS-CoV-2 S protein (Fig. 3) (Shi et al., 2020b). According to Cansino, preclinical data in animal models demonstrated that Ad5-nCoV can elicit robust immune responses and a favorable safety profile (Mak, 2020). Currently, the phase Ⅰ clinical trial evaluating the safety and efficacy of Ad5-nCoV has been initiated in Wuhan (Table 1) (Shi et al., 2020b).
A recently published study in the Lancet introduced a newly developed potentially effective SARS-CoV-2 vaccine named PittCoVacc, short for Pittsburgh Coronavirus Vaccine, developed by University of Pittsburgh School of Medicine scientists (Kim et al., 2020). PittCoVacc uses S-protein fragments of SARS-CoV-2 to stimulate the generation of specific antibodies (Fig. 3). PittCoVacc is delivered with a novel technique known as microneedle array, a fingertip-sized patch of 400 tiny needles. This strategy can elicit more potent immune responses than conventional subcutaneous needle injection and has been demonstrated to be sufficiently safe. PittCoVacc has been tested immunogenicity in mice with the emergence of substantial SARS-CoV-2 antibodies within 2 weeks after prime immunization. The research team hoped to test PittCoVacc in humans in clinical trials in the next few months (Table 1) (ScienceDaily, 2020).
The US-based company Novavax has identified a vaccine candidate NVX-CoV2373, a stable, prefusion protein developed through the advanced nanoparticle technology (Table 1 and Fig. 3). The Matrix-M adjuvant will be incorporated with NVX-CoV2373 to enhance immune responses and stimulate increased levels of neutralizing antibodies (Novavax, 2020). A first-in-human trial will be started in May 2020.
Other types of vaccines (e.g., DNA, RNA, vector, whole-cell killed and live-attenuated vaccines) are in the rapid development process (Routley, 2020). A phase 1 study of the novel DNA vaccine INO-4800 (NCT04336410) is underway (Inovio, 2020). Despite the seriousness of the pandemic ravaging the world, researchers should take the time to assess the safety and efficacy of vaccines in animal models and then conduct related human clinical trials to prevent more harm than good from occurring with hastily produced vaccines.
17. Zinc
Zinc, a trace mineral, is necessary for the immune system since zinc-deficient patients had severe immune dysfunctions (Prasad, 2008). Interestingly, there are numerous reports showing the loss of sense of smell and taste in the early stages of COVID-19 infected people (Keyhan et al., 2020, Lechien et al., 2020). It is well known that zinc deficiency is associated with loss of taste, and that zinc supplementation has beneficial effects in subjects with loss of taste (Doty, 2019, Heyneman, 1996, Yagi et al., 2013). Collectively, it is likely that zinc deficiency in patients with COVID-19 infection may be associated with the loss of smell and taste in these patients.
Importantly, zinc is a potent inhibitor of various RNA viruses such as SARS-CoV (te Velthuis et al., 2010). Given the inhibiting action of zinc in the replication of coronavirus, it is possible that zinc may have beneficial effects for COVID-19 infection since zinc supplement is available in the world. First clinical trial of intravenous zinc in COVID-19 patients is underway in Australia (ACTRN12620000454976). Furthermore, several clinical trials of the combination of zinc and other candidates (i.e., HCQ, vitamin D, vitamin C) in patients with COVID-19 are underway (NCT04326725, NCT04351490, NCT04342728).
18. Others
The findings of a recent in vitro study by Australian researchers are notable. They found that ivermectin (an anti-parasitic drug) could effectively block SARS-CoV-2 growth in cell cultures within 48 h, even at a single dose (Fig. 2). However, the mechanism though which ivernectin exerts its antiviral action is unknown. This study only demonstrated the effectiveness of ivernectin for the control of SARS-CoV-2 in vitro (Caly et al., 2020). Further study of its efficacy and safety for inhibiting SARS-CoV-2 in humans or animals needs to be investigated.
19. Conclusion
The COVID-19 pandemic is an unprecedented crisis for public health and world economy and has had a huge impact not only on China but other countries. In the fight against COVID-19, we should unite and understand that benefiting one benefits all, whereas harming one harms all. Scientists worldwide struggle to seek efficacious COVID-19 treatments. Learning from previous experience in coping with SARS and MERS, a series of existing drugs have been used in clinical practice to treat COVID-19 infection and clinical trials evaluating their efficacy and safety for COVID-19 are ongoing. Given the unique viral structure and distinct pathogenesis, there is an urgent need for novel COVID-19-specific therapies, including vaccines and antivirals. As discussed above, there are many different candidates for COVID-19 infected patients. Part of patients with COVID-19 infection have been medicated with other drugs for their illness. Therefore, potential drug- drug interactions should be considered for the combination (Zhang et al., 2020b).
Research teams from China, Singapore and the United States published a new study on the preprint website BioRxiv (Anderson et al., 2020). Through systematic and comprehensive screening of more than 20,000 genes in bat cells, the teams identified MTHFV1, a gene indispensable for viral replication in bat and human cells and found that carolacton, a host protein MTHFV1 inhibitor, could effectively inhibit replication of several RNA viruses including SARS-CoV-2, suggesting that carolacton (a natural bacteria-derived product) could be a novel therapeutic drug for COVID-19.
Finally, further understanding of SARS-CoV-2 mechanisms in humans could help in developing novel therapeutic drugs for COVID-19. We must protect ourselves against COVID-19 infection until the aforementioned candidates for COVID-19 are approved.
20. Authors’ contributions
JZ and BX performed the study design, data collection, data analysis, data interpretation, and writing. KH performed the study design, data interpretation, and writing. All authors approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was partly supported by the grants of Japan Society for the Promotion of Science (to K.H., 17H04243 and 19H05203) and Japan Agency for Medical Research and Development, AMED (to K.H., JP20dm0107119).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2020.04.046.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Achiron A. Complications of intravenous gammaglobulin in neuromuscular diseases. Neurology. 1997;49:899–900. [Google Scholar]
- Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R., Ray A.S., Cihlar T., Siegel D., Mackman R.L., Clarke M.O., Baric R.S., Denison M.R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9:e00221–18. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini M.L., Pruijssers A.J., Chappell J.D., Gribble J., Lu X., Andres E.L., Bluemling G.R., Lockwood M.A., Sheahan T.P., Sims A.C., Natchus M.G., Saindane M., Kolykhalov A.A., Painter G.R., Baric R.S., Denison M.R. Small-molecule antiviral β-d–hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J. Virol. 2019;93:e01348–19. doi: 10.1128/JVI.01348-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn D.G., Shin H.J., Kim M.H., Lee S., Kim H.S., Myoung J., Kim B.T., Kim S.J. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J. Microbiol. Biotechnol. 2020;30:313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerström S., Mousavi-Jazi M., Klingström J., Leijon M., Lundkvist A., Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79:1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Salama Z.T., Scott L.J. Baricitinib: A review in rheumatoid arthritis. Drugs. 2018;78:761–772. doi: 10.1007/s40265-018-0908-4. [DOI] [PubMed] [Google Scholar]
- Anderson, D.E., Cui, J., Ye, Q., Huang, B.Y., Zu, W.H., Gong, J., Liu, W.Q., Kim, S.Y., Yan, B.G., Sigmundsson, K., Lim, X.F., Ye, F., Niu, P.H., Zhou, X.M., Tan, W.J., Wang, L.F., Tan, X., 2020. Orthogonal genome-wide screenings in bat cells identify MTHFD1 as a target of broad antiviral therapy. bioRxiv, 2020.2003.2029.014209. [DOI] [PMC free article] [PubMed]
- Andersson J.P., Andersson U.G. Human intravenous immunoglobulin modulates monokine production in vitro. Immunology. 1990;71:372–376. [PMC free article] [PubMed] [Google Scholar]
- Andersson U.G., Björk L., Skansén-Saphir U., Andersson J.P. Down-regulation of cytokine production and interleukin-2 receptor expression by pooled human IgG. Immunology. 1993;79:211–216. [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., Jose J., Pinto R., Al-Omari A., Kharaba A., Almotairi A., Al Khatib K., Alraddadi B., Shalhoub S., Abdulmomen A., Qushmaq I., Mady A., Solaiman O., Al-Aithan A.M., Al-Raddadi R., Ragab A., Balkhy H.H., Al Harthy A., Deeb A.M., Al Mutairi H., Al-Dawood A., Merson L., Hayden F.G., Fowler R.A. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- Aranow C. Vitamin D and the immune system. J. Investig. Med. 2011;59:881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmussen S., Ito H., Traber D.L., Lee J.W., Cox R.A., Hawkins H.K., McAuley D.F., McKenna D.H., Traber L.D., Zhuo H., Wilson J., Herndon D.N., Prough D.S., Liu K.D., Matthay M.A., Enkhbaatar P. Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax. 2014;69:819–825. doi: 10.1136/thoraxjnl-2013-204980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y., Sun Y., Meng S., Shi J., Lu L. 2019-nCoV epidemic: address mental health care to empower society. Lancet. 2020;395:e37–e38. doi: 10.1016/S0140-6736(20)30309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragan P., Podzamczer D. Lopinavir/ritonavir: a protease inhibitor for HIV-1 treatment. Expert Opin. Pharmacother. 2008;9:2363–2375. doi: 10.1517/14656566.9.13.2363. [DOI] [PubMed] [Google Scholar]
- Bertorini T.E., Nance A.M., Horner L.H., Greene W., Gelfand M.S., Jaster J.H. Complications of intravenous gammaglobulin in neuromuscular and other diseases. Muscle Nerve. 1996;19:388–391. doi: 10.1002/(SICI)1097-4598(199603)19:3<388::AID-MUS20>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- BioSpace. April 07, 2020. FDA clears the way for ridgeback biotherapeutics to begin human testing of a promising potential treatment for COVID-19.
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;104787 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr A.C., Maggini S. Vitamin C and Immune Function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr A.C., Rosengrave P.C., Bayer S., Chambers S., Mehrtens J., Shaw G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care. 2017;21:300. doi: 10.1186/s13054-017-1891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celularity. April 02, 2020. Celularity announces FDA clearance of IND application for CYNK-001 in coronavirus, first in cellular therapy. https://www.prnewswire.com/news-releases/celularity-announces-fda-clearance-of-ind-application-for-cynk-001-in-coronavirus-first-in-cellular-therapy-301034141.html.
- Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S., Yuen K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Zheng J., Wohlford-Lenane C., Abrahante J.E., Mack M., Sompallae R., McCray P.B., Meyerholz D.K., Perlman S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J. Clin. Invest. 2019;130:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C., Huang, J., Cheng, Z., Wu, J., Chen, S., Zhang, Y., Chen, B., Lu, M., Luo, Y., Zhang, J., Yin, P., Wang, X., 2020a. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv, 2020.2003.2017.20037432.
- Chen G., Gao Y., Jiang Y., Yang F., Li S., Tan D., Ma Q. Efficacy and safety of Xuebijing injection combined with Ulinastatin as adjunctive therapy on sepsis: a systematic review and meta-analysis. Front. Pharmacol. 2018;9:743. doi: 10.3389/fphar.2018.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J. Clin Invest. 2020 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Liu P., Gao H., Sun B., Chao D., Wang F., Zhu Y., Hedenstierna G., Wang C.G. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing. Clin. Infect. Dis. 2004;39:1531–1535. doi: 10.1086/425357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., Qiu, Y., Wang, J., Liu, Y., Wei, Y., Xia, J.a., Yu, T., Zhang, X., Zhang, L., 2020c. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507-513. [DOI] [PMC free article] [PubMed]
- Chen R.C., Tang X.P., Tan S.Y., Liang B.L., Wan Z.Y., Fang J.Q., Zhong N. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimenti L., Luque T., Bonsignore M.R., Ramírez J., Navajas D., Farré R. Pre-treatment with mesenchymal stem cells reduces ventilator-induced lung injury. Eur. Respir. J. 2012;40:939–948. doi: 10.1183/09031936.00153211. [DOI] [PubMed] [Google Scholar]
- China's State Council. February 21, 2020. Press conference on scientific and technological innovation to support epidemic prevention and control. http://www.scio.gov.cn/xwfbh/xwbfbh/wqfbh/42311/42568/index.htm.
- Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L., Wong C.L., Guan Y., Peiris J.S., Yuen K.Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-dos-Santos A.J., da Costa J., Zhang L., Pei Y., Scholey J., Ferrario C.M., Manoukian A.S., Chappell M.C., Backx P.H., Yagil Y., Penninger J.M. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- De Andrea, M., Ravera, R., Gioia, D., Gariglio, M., Landolfo, S., 2002. The interferon system: an overview. Eur. J. Paediatr. Neurol. 6 Suppl A, A41-A46 and A55-A58. [DOI] [PubMed]
- Delang L., Abdelnabi R., Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H., Hong Z., Xia J. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaney J., Horie S., Masterson C., Elliman S., Barry F., O'Brien T., Curley G.F., O'Toole D., Laffey J.G. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax. 2015;70:625–635. doi: 10.1136/thoraxjnl-2015-206813. [DOI] [PubMed] [Google Scholar]
- Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Zeng L., Li R., Chen Q., Zhou B., Chen Q., Cheng P.L., Yutao W., Zheng J., Yang Z., Zhang F. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement. Altern. Med. 2017;17:130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd R.Y. Emerging pathogens and their implications for the blood supply and transfusion transmitted infections. Br. J. Haematol. 2012;159:135–142. doi: 10.1111/bjh.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Xia J.W., Gong Y., Chen Z., Yang H.H., Zhang J., He J., Chen X.D. Effect of lianhuaqingwen capsules on airway inflammation in patients with acute exacerbation of chronic obstructive pulmonary disease. Evid. Based Complement Alternat. Med. 2014;2014 doi: 10.1155/2014/637969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty R.L. Treatment for smell and taste disorders: a critical review. Handb. Clin. Neurol. 2019;164:455–479. doi: 10.1016/B978-0-444-63855-7.00025-3. [DOI] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U S A. 2020 doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H.F., Brown L.F., Detmar M., Dvorak A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel beta coronavirus replication by a combination of interferon-alpha2b and ribavirin. Sci. Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli V., Ranieri V.M. Mechanisms and clinical consequences of acute lung injury. Ann. Am. Thorac. Soc. 2015;12(Suppl 1):S3–S8. doi: 10.1513/AnnalsATS.201407-340MG. [DOI] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, March 24, 2020. Investigational covid-19 convalescent plasma-emergency INDs. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasma-emergency-inds.
- Fehr J., Hofmann V., Kappeler U. Transient reversal of thrombocytopenia in idiopathic thrombocytopenic purpura by high-dose intravenous gamma globulin. N. Engl. J. Med. 1982;306:1254–1258. doi: 10.1056/NEJM198205273062102. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Hillan K.J., Gerber H.-P., Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Fisher B.J., Kraskauskas D., Martin E.J., Farkas D., Wegelin J.A., Brophy D., Ward K.R., Voelkel N.F., Fowler A.A., 3rd, Natarajan R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;303:L20–32. doi: 10.1152/ajplung.00300.2011. [DOI] [PubMed] [Google Scholar]
- Fisher B.J., Seropian I.M., Kraskauskas D., Thakkar J.N., Voelkel N.F., Fowler A.A., 3rd, Natarajan R. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit. Care Med. 2011;39:1454–1460. doi: 10.1097/CCM.0b013e3182120cb8. [DOI] [PubMed] [Google Scholar]
- FitzGerald G.A. Misguided drug advice for COVID-19. Science. 2002;367:1434. doi: 10.1126/science.abb8034. [DOI] [PubMed] [Google Scholar]
- Fowler A.A., 3rd, Syed A.A., Knowlson S., Sculthorpe R., Farthing D., DeWilde C., Farthing C.A., Larus T.L., Martin E., Brophy D.F., Gupta S., Fisher B.J., Natarajan R. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral. Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Takahashi K., Kuno-Maekawa M., Sangawa H., Uehara S., Kozaki K., Nomura N., Egawa H., Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005;49:981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gautret, P., Lagier, J.C., Parola, P., Hoang, V.T., Meddeb, L., Mailhe, M., Doudier, B., Coujon, J., Giordanengo, V., Vieira, V.E., Dupont, H.T., Honore, S., Colson, P., Chabriere, E., La Scola, B., Rolain, J.M., Brouqui, P., Raoult D., 2020. Hydrochloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed]
- Goodbourn S., Didcock L., Randall R.E. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:E988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D'Arminio Monforte A., Ismail S., Kato H., Lapadula G., L'Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe COVID-19. N. Engl. J. Med. 2020 doi: 10.1056/NEHMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths M.J.D., Evans T.W. Inhaled nitric oxide therapy in adults. N. Eng. J. Med. 2005;353:2683–2695. doi: 10.1056/NEJMra051884. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Huang Z., Lin L., Lv J. Coronavirus Disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J. Am. Heart. Assoc. 2020;9 doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N., Krasnodembskaya A., Kapetanaki M., Mouded M., Tan X., Serikov V., Matthay M.A. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533–539. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N., Su X., Popov B., Lee J.W., Serikov V., Matthay M.A. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J. Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A., Rimmelzwaan G.F., van Amerongen G., van Riel D., de Jong T., Itamura S., Chan K.H., Tashiro M., Osterhaus A.D. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett, D.W., Mach 17, 2020. China endorses avigan/favipiravir for COVID-19 disease treatment. https://www.precisionvaccinations.com/avigan-favipiravir-t-705-broad-spectrum-inhibitor-viral-rna-polymerase.
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, H., Xie, L., Liu, R., Yang, J., Liu, F., Wu, K., Chen, L., Hou, W., Feng, Y., Zhu, C., 2020a. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J. Med. Virol. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed]
- Han, H., Yang, L., Liu, R., Liu, F., Wu, K.L., Li, J., Liu, X.H., Zhu, C.L., 2020b. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed]
- Han K., Ma H., An X., Su Y., Chen J., Lian Z., Zhao J., Zhu B.P., Fontaine R.E., Feng Z., Zeng G. Early use of glucocorticoids was a risk factor for critical disease and death from pH1N1 infection. Clin. Infect. Dis. 2011;53:326–333. doi: 10.1093/cid/cir398. [DOI] [PubMed] [Google Scholar]
- Haschke M., Schuster M., Poglitsch M., Loibner H., Salzberg M., Bruggisser M., Penninger J., Krähenbühl S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin. Pharmacokinet. 2013;52:783–792. doi: 10.1007/s40262-013-0072-7. [DOI] [PubMed] [Google Scholar]
- He X.D., Wang Y., Wu Q., Wang H.X., Chen Z.D., Zheng R.S., Wang Z.S., Wang J.B., Yang Y. Xuebijing protects rats from sepsis challenged with acinetobacter baumannii by promoting annexin A1 expression and inhibiting proinflammatory cytokines secretion. Evid. Based Complement Alternat. Med. 2013;2013 doi: 10.1155/2013/804940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä, H., Chalker, E., 2013. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. CD000980. doi: 10.1002/14651858.CD000980.pib4. [DOI] [PMC free article] [PubMed]
- Heming N., Sivanandamoorthy S., Meng P., Bounab R., Annane D. Immune effects of corticosteroids in sepsis. Front. Immunol. 2018;9:1736. doi: 10.3389/fimmu.2018.01736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley L.E., Fritz L.E., Jahrling P.B., Karp C.L., Huggins J.W., Geisbert T.W. Interferon-beta 1a and SARS coronavirus replication. Emerg. Infect. Dis. 2004;10:317–319. doi: 10.3201/eid1002.030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyneman C.A. Zinc deficiency and taste disorders. Ann. Pharmacother. 1996;30:186–187. doi: 10.1177/106002809603000215. [DOI] [PubMed] [Google Scholar]
- Hoffmann J.H.O., Enk A.H. High-dose intravenous immunoglobulin in skin autoimmune disease. Front. Immunol. 2019;10:1090. doi: 10.3389/fimmu.2019.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F., To K.K., Lee C.K., Lee K.L., Chan K., Yan W.W., Liu R., Watt C.L., Chan W.M., Lai K.Y., Koo C.K., Buckley T., Chow F.L., Wong K.K., Chan H.S., Ching C.K., Tang B.S., Lau C.C., Li I.W., Liu S.H., Chan K.H., Lin C.K., Yuen K.Y. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin. Infect. Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello A., Debbeche O., Samarani S., Ahmad A. Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J. Leukoc. Biol. 2008;84:1–26. doi: 10.1189/jlb.0907650. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.-C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inovio. press release at March 03, 2020. Inovio Accelerates Timeline for COVID-19 DNA Vaccine INO-4800. http://ir.inovio.com/news-and-media/news/press-release-details/2020/Inovio-Accelerates-Timeline-for-COVID-19-DNA-Vaccine-INO-4800/default.aspx.
- Italian ANSA news agency. March 13, 2020. 3 patients get better on arthritis drug. http://www.ansa.it/english/news/general_news/2020/03/13/3-patients-get-better-on-arthritis-drug_90d4764d-d93f-463e-ab07-168b34b084d0.html.
- Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- Jolles S., Sewell W.A., Misbah S.A. Clinical uses of intravenous immunoglobulin. Clin. Exp. Immunol. 2005;142:1–11. doi: 10.1111/j.1365-2249.2005.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakodkar P., Kaka N., Baig M.N. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 19 (COVID-19) Cureus. 2020;12 doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L., Li Y., Hu S., Chen M., Yang C., Yang B.X., Wang Y., Hu J., Lai J., Ma X., Chen J., Guan L., Wang G., Ma H., Liu Z. The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus. Lancet Psychiatry. 2020;7 doi: 10.1016/S2215-0366(20)30047-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karram T., Abbasi A., Keidar S., Golomb E., Hochberg I., Winaver J., Hoffman A., Abassi Z. Effects of spironolactone and eprosartan on cardiac remodeling and angiotensin-converting enzyme isoforms in rats with experimental heart failure. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1351–H1358. doi: 10.1152/ajpheart.01186.2004. [DOI] [PubMed] [Google Scholar]
- Kaveri S.V., Dietrich G., Hurez V., Kazatchkine M.D. Intravenous immunoglobulins (IVIg) in the treatment of autoimmune diseases. Clin. Exp. Immunol. 1991;86:192–198. doi: 10.1111/j.1365-2249.1991.tb05794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Chen L., Maes P., Hedenstierna G., Van Ranst M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004;8:223–226. doi: 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyhan S.O., Fallahi H.R., Cheshmi B. Dysosmia and dysgeusia due to the 2019 novel coronavirus; a hypothesis that needs further investigation. Maxillofac Plast. Reconstr. Surg. 2020;42:9. doi: 10.1186/s40902-020-00254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., Hall R., Poirier G., Ronco J.J., Tidswell M., Hardes K., Powley W.M., Wright T.J., Siederer S.K., Fairman D.A., Lipson D.A., Bayliffe A.I., Lazaar A.L. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Erdos G., Huang S., Kenniston T.W., Balmert S.C., Carey C.D., Raj V.S., Epperly M.W., Klimstra W.B., Haagmans B.L., Korkmaz E., Falo L.D., Jr., Gambotto A. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020;102743 doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U.J., Won E.J., Kee S.J., Jung S.I., Jang H.C. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for middle east respiratory syndrome. Antivir. Ther. 2016;21:455–459. doi: 10.3851/IMP3002. [DOI] [PubMed] [Google Scholar]
- Krasnodembskaya A., Samarani G., Song Y., Zhuo H., Su X., Lee J.W., Gupta N., Petrini M., Matthay M.A. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;302:L1003–L1013. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, J.C.E., Weaver, J., Kostka, K., Duarte-Salles, T., Abrahao, M.T.F., Alghoul, H., Alser, O., Alshammari, T.M., Biedermann, P., Burn, E., Casajust, P., Conover, M., Culhane, A.C., Davydov, A., DuVall, S.L., Dymshyts, D., Bertolín, S.F., Fišter, K., Hardin, J., Hester, L., Hripcsak, G., Kent, S., Khosla, S., Kolovos, S., Lambert, C.G., ver der Lei, J., Londhe, A.A., Lynch, K.E., Makadia, R., Margulis, A.V., Matheny, M.E., Mehta, P., Morales, D.R., Morgan-Stewart, H., Mosseveld, M., Newby, D., Nyberg,F., Ostropolets, A., Park, R.W., Prats-Uribe, A., Rao, G.A., Reich, C., Reps, J., Rijnbeek, P., Sathappan, S.M.K., Schuemie, M., Seager, S., Sena, A., Shoaibi, A., Spotnitz, M., Suchard, M.A., Swerdel, J., Torre, C.O., Vizcaya, D., Wen, H., de Wilde, M., You, S.C., Zhang, L., Zhuk, O., Ryan, P., Prieto-Alhambra, D., 2020., Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study. medRxiv doi.org/10.1101/2020.04.08.20054551.
- Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M., Dini G., Egeler R.M., Bacigalupo A., Fibbe W., Ringdén O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., Dequanter D., Blecic S., El Afia F., Distinguin L., Chekkoury-Idrissi Y., Hans S., Delgado I.L., Calvo-Henriquez C., Lavigne P., Falanga C., Barillari M.R., Cammaroto G., Khalife M., Leich P., Souchay C., Rossi C., Journe F., Hsieh J., Edjlali M., Carlier R., Ris L., Lovato A., De Filippis C., Coppee F., Fakhry N., Ayad T., Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.A. Cellular therapy: Adoptive immunotherapy with expanded natural killer cells. Immunol. Rev. 2019;290:85–99. doi: 10.1111/imr.12793. [DOI] [PubMed] [Google Scholar]
- Lei, C., Fu, W., Qian, K., Li, T., Zhang, S., Ding, M., Hu, S., 2020. Potent neutralization of 2019 novel coronavirus by recombinant ACE2-Ig. bioRxiv, 2020.2002.2001.929976.
- Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., Fan J., Wang W., Deng L., Shi H., Li H., Hu Z., Zhang F., Gao J., Liu H., Li X., Zhao Y., Yin K., He X., Gao Z., Wang Y., Yang B., Jin R., Stambler I., Lim L.W., Su H., Moskalev A., Cano A., Chakrabarti S., Min K.J., Ellison-Hughes G., Caruso C., Jin K., Zhao R.C. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew T.W.K., Kwek T.-K., Tai D., Earnest A., Loo S., Singh K., Kwan K.M., Chan Y., Yim C.F., Bek S.L., Kor A.C., Yap W.S., Chelliah Y.R., Lai Y.C., Goh S.-K. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- Li H., Yang S.G., Gu L., Zhang Y., Yan X.X., Liang Z.A., Zhang W., Jia H.Y., Chen W., Liu M., Yu K.J., Xue C.X., Hu K., Zou Q., Li L.J., Cao B., Wang C. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza Other Respir. Viruses. 2017;11:345–354. doi: 10.1111/irv.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Li D., Liu X., Tang S., Wei F. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J. Inflamm. (Lond) 2012;9:33. doi: 10.1186/1476-9255-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wu M., Yao J., Guo J., Liao X., Song S., Li J., Duan G., Zhou Y., Wu X., Zhou Z., Wang T., Hu M., Chen X., Fu Y., Lei C., Dong H., Xu C., Hu Y., Han M., Zhou Y., Jia H., Chen X., Yan J. Caution on kidney dysfunctions of COVID-19 patients. medRxiv. 2020 2020.2002.2008.20021212. [Google Scholar]
- Li Z., Ge J., Yang M., Feng J., Qiao M., Jiang R., Bi J., Zhan G., Xu X., Wang L., Zhou Q., Zhou C., Pan Y., Liu S., Zhang H., Yang J., Zhu B., Hu Y., Hashimoto K., Jia Y., Wang H., Wang R., Liu C., Yang C. Vicarious traumatization in the general public, members, and non-members of medical teams aiding in COVID-19 control. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H.S., Im J.S., Cho J.Y., Bae K.S., Klein T.A., Yeom J.S., Kim T.S., Choi J.S., Jang I.J., Park J.W. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax. Antimicrob. Agents Chemother. 2009;53:1468–1475. doi: 10.1128/AAC.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Jiang X., Zhang Z., Huang S., Zhang Z., Fang Z., Gu Z., Gao L., Shi H., Mai L., Liu Y., Lin X., Lai R., Yan Z., Li X., Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020 doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Liu, Y., Xiang, P., Pu, L., Xiong, H., Li, C., Zhang, M., Tan, J., Xu, Y., Song, R., Song, M., Wang, L., Zhang, W., Han, B., Yang, L., Wang, X., Zhou, G., Zhang, T., Li, B., Wang, Y., Chen, Z., Wang, X., 2020b. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv, 2020.2002.2010.20021584. [DOI] [PMC free article] [PubMed]
- Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., Xiao W., Wang Y.N., Zhong M.H., Li C.H., Li G.C., Liu H.G. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. (Engl). 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Loutfy M.R., Blatt L.M., Siminovitch K.A., Ward S., Wolff B., Lho H., Pham D.H., Deif H., LaMere E.A., Chang M., Kain K.C., Farcas G.A., Ferguson P., Latchford M., Levy G., Dennis J.W., Lai E.K., Fish E.N. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke T., Wu H., Zhao J., Channappanavar R., Coleman C.M., Jiao J.A., Matsushita H., Liu Y., Postnikova E.N., Ork B.L., Glenn G., Flyer D., Defang G., Raviprakash K., Kochel T., Wang J., Nie W., Smith G., Hensley L.E., Olinger G.G., Kuhn J.H., Holbrook M.R., Johnson R.F., Perlman S., Sullivan E., Frieman M.B. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS-CoV in vivo. Sci. Transl. Med. 2016;8:326ra321. doi: 10.1126/scitranslmed.aaf1061. [DOI] [PubMed] [Google Scholar]
- Lutz H.U., Stammler P., Jelezarova E., Nater M., Späth P.J. High doses of immunoglobulin G attenuate immune aggregate-mediated complement activation by enhancing physiologic cleavage of C3b in C3bn-IgG complexes. Blood. 1996;88:184–193. [PubMed] [Google Scholar]
- Lv F.-J., Tuan R.S., Cheung K.M.C., Leung V.Y.L. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.M., Lim W.S., Makki S., Rooney K.D., Nguyen-Van-Tam J.S., Beck C.R. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J. Infect. Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, E. March 18, 2020. China approves first homegrown COVID-19 vaccine to enter clinical trials. https://www.bioworld.com/articles/433791-china-approves-first-homegrown-covid-19-vaccine-to-enter-clinical-trials.
- Mao, L., Wang, M., Chen, S., He, Q., Chang, J., Hong, C., Zhou, Y., Wang, D., Li, Y., Jin, H., Hu, B., 2020. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a retrospective case series study. medRxiv, 2020.2002.2022.20026500.
- Marchalonis J.J., Kaymaz H., Dedeoglu F., Schluter S.F., Yocum D.E., Edmundson A.B. Human autoantibodies reactive with synthetic autoantigens from T-cell receptor beta chain. Proc. Natl. Acad. Sci. USA. 1992;89:3325–3329. doi: 10.1073/pnas.89.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J.M., Harrison F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox. Signal. 2013;19:2068–2083. doi: 10.1089/ars.2013.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei S.H.J., Haitsma J.J., Dos Santos C.C., Deng Y., Lai P.F.H., Slutsky A.S., Liles W.C., Stewart D.J. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- Microft-West, C., Su, D., Elli, S., Guimond, S., Miller, G., Turnbull, J., Yates, E., Guerrini, M., Fernig, D., Lima, M., Skidmore, M., 2020. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 receptor domain undergoes conformational change upon heparin binding. bioRxiv doi:10.1101/2020.02.29.971093.
- Minagawa H., Takenaka A., Mohri S., Mori R. Protective effect of recombinant murine interferon beta against mouse hepatitis virus infection. Antiviral Res. 1987;8:85–95. doi: 10.1016/0166-3542(87)90079-9. [DOI] [PubMed] [Google Scholar]
- Moderna. Press release at March 16, 2020a. Moderna announces first participant dosed in NIH-led phase 1 study of mRNA vaccine (mRNA-1273) against novel coronavirus. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-first-participant-dosed-nih-led-phase-1-study.
- Moderna. Press release at April 16, 2020b. Moderna announces award from U.S. government agency BARDA for up to $483 million to accelerate development of mRNA vaccine (mRNA-1273) against novel coronavirus. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-award-us-government-agency-barda-483-million.
- Mohammed B.M., Fisher B.J., Kraskauskas D., Farkas D., Brophy D.F., Fowler A.A., 3rd, Natarajan R. Vitamin C: a novel regulator of neutrophil extracellular trap formation. Nutrients. 2013;5:3131–3151. doi: 10.3390/nu5083131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E. A novel coronavirus emerging in China - key questions for impact assessment. N. Engl. J. Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- Nakano K., Suzuki S. Stress-induced change in tissue levels of ascorbic acid and histamine in rats. J. Nutr. 1984;114:1602–1608. doi: 10.1093/jn/114.9.1602. [DOI] [PubMed] [Google Scholar]
- National Health and Family Planning Commission of China (NHPFC). March 04, 2020. Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment (7th edition). http://kjfy.meetingchina.org/msite/news/show/cn/3337.html.
- National Institutes of Health (NIH). March 16, 2020. NIH clinical trial of investigational vaccine for COVID-19 begins. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins. http://tribuneonlineng.com/china-approves-use-of-roche-drug-in-battle-against-coronavirus-complications/.
- Nishimoto N., Kishimoto T., Yoshizaki K. Anti-interleukin 6 receptor antibody treatment in rheumatic disease. Ann. Rhum. Dis. 2000;59(Suppl 1):i21–i27. doi: 10.1136/ard.59.suppl_1.i21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novavax, Press Release April 08, 2020. Novavax identifies coronavirus vaccine candidate: accelerates initiation of first-in-human trial to mid-May. https://ir.novavax.com/news-releases/news-release-details/novavax-identifies-coronavirus-vaccine-candidate-accelerates.
- O'Shea J.J., Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestereich L., Lüdtke A., Wurr S., Rieger T., Muñoz-Fontela C., Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res. 2014;105:17–21. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Oudemans-van Straaten H.M., Spoelstra-de Man A.M., de Waard M.C. Vitamin C revisited. Crit. Care. 2014;18:460. doi: 10.1186/s13054-014-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter G.R., Bowen R.A., Bluemling G.R., DeBergh J., Edpuganti V., Gruddanti P.R., Guthrie D.B., Hager M., Kuiper D.L., Lockwood M.A., Mitchell D.G., Natchus M.G., Sticher Z.M., Kolykhalov A.A. The prophylactic and therapeutic activity of a broadly active ribonucleoside analog in a murine model of intranasal venezuelan equine encephalitis virus infection. Antiviral Res. 2019;171 doi: 10.1016/j.antiviral.2019.104597. [DOI] [PubMed] [Google Scholar]
- Pan X.W., Xu D., Zhang H., Zhou W., Wang L.H., Cui X.G. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- Parkin J., Cohen B. An overview of the immune system. Lancet. 2001;357:1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- Pepke-Zaba J., Higenbottam T.W., Dinh-Xuan A.T., Stone D., Wallwork J. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet. 1991;338:1173–1174. doi: 10.1016/0140-6736(91)92033-x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum B., North C.S. Mental Health and the Covid-19 pandemic. N. Eng. J. Med. 2020 doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]
- Prasad A.S. Zinc in human health: effect of zinc on immune cells. Mol. Med. 2008;14:353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevosto C., Zancolli M., Canevali P., Zocchi M.R., Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo M.G., Biggioggero M., Crotti C., Becciolini A., Favalli E.G. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des. Devel. Ther. 2017;11:1593–1603. doi: 10.2147/DDDT.S100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regeneron. March 16, 2020. REGENERON AND SANOFI BEGIN GLOBAL KEVZARA® (SARILUMAB) CLINICAL TRIAL PROGRAM IN PATIENTS WITH SEVERE COVID-19. https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-begin-global-kevzarar-sarilumab-clinical/.
- Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynard O., Nguyen X.N., Alazard-Dany N., Barateau V., Cimarelli A., Volchkov V.E. Identification of a new ribonucleoside inhibitor of Ebola Virus replication. Viruses. 2015;7:6233–6240. doi: 10.3390/v7122934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T., Cidlowski J.A. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N. Engl. J. Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roback J.D., Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020 doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- Routley, N. April 01, 2020. Every vaccine and treatment in development for COVID-19, so far. https://www.visualcapitalist.com/every-vaccine-treatment-covid-19-so-far/.
- Ruan, Q., Yang, K., Wang, W., Jiang, L., Song, J., 2020. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed]
- Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., Chufang L., Jin Z., Zhenhua J., Haiming J., Kui Z., Shuxiang H., Jun D., Xiaobo L., Xiaotao H., Lin W., Nanshan Z., Zifeng Y. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020 doi: 10.1016/j.phrs.2020.104761. 104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz B., Jr., Mossel E.C., Peters C.J., Garry R.F. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) Virology. 2004;329:11–17. doi: 10.1016/j.virol.2004.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkas L.I. Spotlight on tocilizumab and its potential in the treatment of systemic sclerosis. Drug Des. Devel. Ther. 2016;10:2723–2728. doi: 10.2147/DDDT.S99696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura M., Zaragoza C., McMillan A., Quick R.A., Hohenadl C., Lowenstein J.M., Lowenstein C.J. An antiviral mechanism of nitric oxide: inhibition of a viral protease. Immunity. 1999;10:21–28. doi: 10.1016/S1074-7613(00)80003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect. Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H.H., Walter U. NO at work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ScienceDaily. April 02, 2020. COVID-19 vaccine candidate shows promise, research shows. https://www.sciencedaily.com/releases/2020/04/200402144508.htm.
- Sen G.C. Viruses and interferons. Annu Rev Microbiol. 2001;55:255–281. doi: 10.1146/annurev.micro.55.1.255. [DOI] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9:eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan, T.P., Sims, A.C., Zhou, S., Graham, R.L., Pruijssers, A.J., Agostini, M.L., Leist, S.R., Schäfer, A., Dinnon, K.H., Stevens, L.J., Chappell, J.D., Lu, X., Hughes, T.M., George, A.S., Hill, C.S., Montgomery, S.A., Brown, A.J., Bluemling, G.R., Natchus, M.G., Saindane, M., Kolykhalov, A.A., Painter, G., Harcourt, J., Tamin, A., Thornburg, N.J., Swanstrom, R., Denison, M.R., Baric, R.S., 2020. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020 Apr 6. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed]
- Shi Y., Wang J., Yang Y., Wang Z., Wang G., Hashimoto K., Zhang K., Liu H. Knowledge and attitudes of medical staff in Chinese psychiatric hospitals regarding COVID-19. Brain Behav. Immun. Health. 2020;4 doi: 10.1016/j.bbih.2020.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Wang N., Zou Q.M. Progress and challenge of vaccine development against 2019 novel coronavirus (2019-nCoV) Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54:E029. doi: 10.3760/cma.j.cn112150-20200317-00366. [DOI] [PubMed] [Google Scholar]
- Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M., Kochanek M., Böll B., von Bergwelt-Baildon M.S. Cytokine release syndrome. J. Immunother. Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D., Hui H.C., Doerffler E., Clarke M.O., Chun K., Zhang L., Neville S., Carra E., Lew W., Ross B., Wang Q., Wolfe L., Jordan R., Soloveva V., Knox J., Perry J., Perron M., Stray K.M., Barauskas O., Feng J.Y., Xu Y., Lee G., Rheingold A.L., Ray A.S., Bannister R., Strickley R., Swaminathan S., Lee W.A., Bavari S., Cihlar T., Lo M.K., Warren T.K., Mackman R.L. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J. Med. Chem. 2017;60:1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- Siemieniuk R.A.C., Meade M.O., Alonso-Coello P., Briel M., Evaniew N., Prasad M., Alexander P.E., Fei Y., Vandvik P.O., Loeb M., Guyatt G.H. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann. Intern. Med. 2015;163:519–528. doi: 10.7326/M15-0715. [DOI] [PubMed] [Google Scholar]
- Sissoko D., Laouenan C., Folkesson E., M'Lebing A.B., Beavogui A.H., Baize S., Camara A.M., Maes P., Shepherd S., Danel C., Carazo S., Conde M.N., Gala J.L., Colin G., Savini H., Bore J.A., Le Marcis F., Koundouno F.R., Petitjean F., Lamah M.C., Diederich S., Tounkara A., Poelart G., Berbain E., Dindart J.M., Duraffour S., Lefevre A., Leno T., Peyrouset O., Irenge L., Bangoura N., Palich R., Hinzmann J., Kraus A., Barry T.S., Berette S., Bongono A., Camara M.S., Chanfreau Munoz V., Doumbouya L., Souley H., Kighoma P.M., Koundouno F.R., Réné L., Loua C.M., Massala V., Moumouni K., Provost C., Samake N., Sekou C., Soumah A., Arnould I., Komano M.S., Gustin L., Berutto C., Camara D., Camara F.S., Colpaert J., Delamou L., Jansson L., Kourouma E., Loua M., Malme K., Manfrin E., Maomou A., Milinouno A., Ombelet S., Sidiboun A.Y., Verreckt I., Yombouno P., Bocquin A., Carbonnelle C., Carmoi T., Frange P., Mely S., Nguyen V.K., Pannetier D., Taburet A.M., Treluyer J.M., Kolie J., Moh R., Gonzalez M.C., Kuisma E., Liedigk B., Ngabo D., Rudolf M., Thom R., Kerber R., Gabriel M., Di Caro A., Wölfel R., Badir J., Bentahir M., Deccache Y., Dumont C., Durant J.F., El Bakkouri K., Gasasira Uwamahoro M., Smits B., Toufik N., Van Cauwenberghe S., Ezzedine K., D'Ortenzio E., Pizarro L., Etienne A., Guedj J., Fizet A., de Sainte Barte, Fare E., Murgue B., Tran-Minh T., Rapp C., Piguet P., Poncin M., Draguez B., Allaford Duverger T., Barbe S., Baret G., Defourny I., Carroll M., Raoul H., Augier A., Eholie S.P., Yazdanpanah Y., Levy-Marchal C., Antierrens A., Van Herp M., Günther S., de Lamballerie X., Keïta S., Mentre F., Anglaret X., Malvy D. Experimental treatment with favipiravir for Ebola Virus disease (the JIKI Trial): A historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smither S.J., Eastaugh L.S., Steward J.A., Nelson M., Lenk R.P., Lever M.S. Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model. Antiviral Res. 2014;104:153–155. doi: 10.1016/j.antiviral.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Sperber S.J., Hayden F.G. Comparative susceptibility of respiratory viruses to recombinant interferons-alpha 2b and -beta. J. Interferon Res. 1989;9:285–293. doi: 10.1089/jir.1989.9.285. [DOI] [PubMed] [Google Scholar]
- Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strabelli, T.M.V., Uip, D.E., 2020. COVID-19 and the Heart. Arq. Bras. Cardiol. doi: 10.36660/abc.20200209. [DOI] [PubMed]
- Ströher U., DiCaro A., Li Y., Strong J.E., Aoki F., Plummer F., Jones S.M., Feldmann H. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon- alpha. J. Infect. Dis. 2004;189:1164–1167. doi: 10.1086/382597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Xue Q., Guo L., Cui L., Wang J. Xuebijing protects against lipopolysaccharide-induced lung injury in rabbits. Exp. Lung Res. 2010;36:211–218. doi: 10.3109/01902140903312123. [DOI] [PubMed] [Google Scholar]
- Tan E.L., Ooi E.E., Lin C.Y., Tan H.C., Ling A.E., Lim B., Stanton L.W. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg. Infect. Dis. 2004;10:581–586. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velthuis A.J.W., van den Worm S.E.V., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickett D.R., Armstrong L., Christie S.J., Millar A.B. Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001;164:1601–1605. doi: 10.1164/ajrccm.164.9.2011071. [DOI] [PubMed] [Google Scholar]
- Third People's Hospital of Shenzhen. February 14, 2020. Familavir is more effective than Lopinavir/ritonavir in the treatment of COVID-19 (in Chinese). http://www.szdsyy.com/News/0a6c1e58-e3d0-4cd1-867a-d5524bc59cd6.html.
- Thompson B.T. Glucocorticoids and acute lung injury. Crit. Care Med. 2003;31:S253–S257. doi: 10.1097/01.CCM.0000057900.19201.55. [DOI] [PubMed] [Google Scholar]
- Toots M., Yoon J.-J., Cox R.M., Hart M., Sticher Z.M., Makhsous N., Plesker R., Barrena A.H., Reddy P.G., Mitchell D.G., Shean R.C., Bluemling G.R., Kolykhalov A.A., Greninger A.L., Natchus M.G., Painter G.R., Plemper R.K. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 2019;11:eaax5866. doi: 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Borne B.E., Dijkmans B.A., de Rooij H.H., le Cessie S., Verweij C.L. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J. Rheumatol. 1997;24:55–60. [PubMed] [Google Scholar]
- Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Del Pozo C.H., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Wajanaponsan N., Cheng S.-F. Acute renal failure resulting from intravenous immunoglobulin therapy. Hawaii Med. J. 2004;63:266–267. [PubMed] [Google Scholar]
- Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., Wang, B., Xiang, H., Cheng, Z., Xiong, Y., Zhao, Y., Li, Y., Wang, X., Peng, Z., 2020a. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel coronavirus-infected pneumonia in Wuhan, China. JAMA doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed]
- Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Song S., Ma Z., Mo P., Zhang Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Song Y., Liu Z., Wang H., Zheng W., Liu S., Feng Z., Zhai J., Yao C., Ren M., Bai C., Shang H. Xuebijing injection in the treatment of severe pneumonia: study protocol for a randomized controlled trial. Trials. 2016;17:142. doi: 10.1186/s13063-016-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- World Health Organization(WHO). December 31, 2003. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. https://www.who.int/csr/sars/country/table2004_04_21/en/.
- World Health Organization(WHO). March 12, 2020a. Coronavirus disease 2019 (COVID-19) situation report – 52. https://www.who.int/docs/default-source/coronaviruse/20200312-sitrep-52-covid-19.pdf?sfvrsn=e2bfc9c0_2.
- World Health Organization(WHO). March 30, 2020b. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html.
- Wilson J.X. Evaluation of vitamin C for adjuvant sepsis therapy. Antioxid. Redox. Signal. 2013;19:2129–2140. doi: 10.1089/ars.2013.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y., Xu, X., Chen, Z., Duan, J., Hashimoto, K., Yang, L., Liu, C., Yang, C., 2020.Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed]
- Xie H., Zhao J., Lian N., Lin S., Xie Q., Zhuo H. Clinical characteristics of Non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A Retrospective study. Liver Int. 2020 doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D.M., Ma X.H., Ai Y.H. Effect of Xuebijing injection pretreatment on expression of pulmonary surfactant-associated protein A in rat alveolar type II epithelial cells induced by lipopolysaccharide. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009;21:690–691. [PubMed] [Google Scholar]
- Xu J., Woods C.R., Mora A.L., Joodi R., Brigham K.L., Iyer S., Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293:L131–L141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T., Asakawa A., Ueda H., Ikeda S., Miyawaki S., Inui A. The role of zinc in the treatment of taste disorders. Recent Pat. Food Nutr. Agric. 2013;5:44–51. doi: 10.2174/2212798411305010007. [DOI] [PubMed] [Google Scholar]
- Yan Y., Zou Z., Sun Y., Li X., Xu K.F., Wei Y., Jin N., Jiang C. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23:300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X., Yu, Y., Xu, J., Shu, H., Xia, J.a., Liu, H., Wu, Y., Zhang, L., Yu, Z., Fang, M., Yu, T., Wang, Y., Pan, S., Zou, X., Yuan, S., Shang, Y., 2020. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed]
- Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., Mou H.M., Wang L.H., Zhang H.R., Fu W.J., Luo T., Liu F., Chen C., Xiao H.L., Guo H.T., Lin S., Xiang D.F., Shi Y., Li Q.R., Huang X., Cui Y., Li X.Z., Tang W., Pan P.F., Huang X.Q., Ding Y.Q., Bian X.W. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis. 2020 doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.W., Sun C.D., Wen Y., Yin C.H. Effect of treatment with Xuebijing injection on serum inflammatory mediators and Th1/2 of spleen in rats with sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2006;18:673–676. [PubMed] [Google Scholar]
- Zhao, Y., Zhao, Z., Wang, Y., Zhou, Y., Ma, Y., Zuo, W., 2020. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv, 2020.2001.2026.919985.
- Zhang Y., Zheng L., Liu L., Zhao M., Xiao J., Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver Int. 2020 doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- Zhang K., Zhou X., Liu H., Hashimoto K. Treatment concerns for psychiatric symptoms in COVID-19-infected patients with or without psychiatric disorders. Br. J. Psychiatry. 2020 doi: 10.1192/bjp.2020.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Huang L., Tong H., Shu Q., Hu Y., Ge M., Deng K., Zhang L., Zou B., Cheng B., Xu J. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir. Res. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, M., Gao, Y., Wang, G., Song, G., Liu, S., Sun, D., Xu, Y., Tian, Z., 2020. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. doi: 10.1038/s4123-020-0402. [DOI] [PMC free article] [PubMed]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.