Summary
Zhou et al. (Nature) and Hoffmann et al. (Cell) identify ACE2 as a SARS-CoV-2 receptor, and the latter show its entry mechanism depends on cellular serine protease TMPRSS2. These results may explain proinflammatory cytokine release via the associated angiotestin II pathway and a possible therapeutic target via the IL-6-STAT3 axis.
Understanding the cell entry mechanism of pandemic coronavirus SARS-CoV-2 is crucial for treating the disease. Zhou et al. (Nature) and Hoffmann et al. (Cell) identify ACE2 as a SARS-CoV-2 receptor, and the latter show its entry mechanism depends on cellular serine protease TMPRSS2. These results suggest several possible therapeutic targets, including the IL-6-STAT3 axis previously associated with cytokine release syndromes.
Main Text
In the past two decades, severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) were transmitted from animals to humans, causing severe respiratory diseases SARS and MERS in endemic areas. In December 2019, another coronavirus was discovered in patients with infectious respiratory disease in Wuhan, Hubei province, China, to have the ability for human-to-human transmission. The disease, now termed coronavirus disease 2019 (COVID-19), has spread rapidly all over the world, resulting in a pandemic. COVID-19 is induced by the pathogenic SARS-coronavirus 2 (SARS-CoV-2) and is associated with 2,165,500 cases and 145,705 deaths as of April 17, 2020 (COVID-19 Map Johns Hopkins University and Medicine). The major phenotype of COVID-19 is severe acute respiratory distress syndrome (ARDS), just like the disease caused by SARS-CoV and MERS-CoV (de Wit et al., 2016, Huang et al., 2020).
Lu et al. and Zhou et al. report the genomic characterization and epidemiology of SARS-CoV-2 (Lu et al., 2020, Zhou et al., 2020). The genome sequence of SARS-CoV-2 is similar to, but distinct from, the two other coronaviruses, as it has about 80% sequence identity with SARS-CoV and about 50% with MERS-CoV. Interestingly, SARS-CoV-2 is about 90% identical at the whole-genome level with two bat coronaviruses, bat-SL-CoVZC45 and bat-SL-CoVZXC21, collected in eastern China. Protein sequence analysis showed that SARS-CoV-2 has seven conserved non-structural domains, just like SARS-CoV, suggesting that the two coronaviruses are related. Furthermore, SARS-CoV-2 has a similar receptor-binding domain structure to that of SARS-CoV, despite amino acid variation at some key residues. Thus, it is possible that SARS-CoV-2 uses the same cell entry receptor—angiotensin converting enzyme II (ACE2)—as SARS-CoV (Kuba et al., 2005, Li et al., 2003).
Considering the high mortality rate of COVID-19 (6.7% all over the world), the development of effective therapeutics is an urgent issue and requires the identification of quality targets. In this regard, two papers have identified ACE2 as cell entry receptors for SARS-CoV-2 (Hoffmann et al., 2020, Zhou et al., 2020). In addition, Hoffman and colleagues showed that receptor-mediated virus entry was dependent on a serine protease, transmembrane serine protease 2 (TMPRSS2). Of note, clinically approved inhibitors of TMPRSS2 can prevent cell entry by SARS-CoV-2. Because alveola type 2 cells highly express both ACE2 and TMPRSS2 in the steady state, these cells might be the primary entry cells for SARS-CoV-2 in the lung.
Zhou et al. (2020) performed virus infectivity studies using HeLa cells that did or did not express ACE2 protein originating from humans, Chinese horseshoe bats, civet, pigs, and mice. They showed that SARS-CoV-2 can enter cells expressing all ACE2 proteins, except mouse ACE2, but could not enter cells lacking ACE2, suggesting that the virus utilizes ACE2 as its entry receptor. Additionally, SARS-CoV-2 did not enter cells lacking ACE2 that expressed either dipeptidyl peptidase 4 (DPP4) or aminopeptidase N (APN), the entry receptors for MERS-CoV and HCoV-229E, respectively.
It is known that cell entry by the coronavirus requires the binding of the S1 region of the virus spike (S) protein to the cell surface receptor followed by the fusion of the viral and cellular membranes mediated by the S2 subunit of S protein. This process requires S protein priming by host cell proteases, which entails S protein cleavage at the boundary of S1 and S2 proteins or within the S2 subunit. Analyzing which cellular factors are used by SARS-CoV-2 for cell entry should provide insights into the viral transmission and reveal effective therapeutic targets. Hoffmann et al. (2020) performed a detailed analysis of the cell entry mechanism of SARS-CoV-2. They demonstrated that SARS-CoV-2 uses ACE2 for entry and TMPRSS2 and the endosomal cysteine proteases cathepsin B and L (CatB/L) for S protein priming. They also showed that SARS-CoV-2 S protein (SARS-2-S) is efficiently cleaved at the S1/S2 site in 293T cells. Furthermore, utilizing replication-defective vesicular stomatitis virus (VSV) particles bearing either SARS-2-S or SARS-CoV S protein (SARS-S), they showed that either S protein entered the cells with an identical spectrum of cell lines from various animal species, which is consistent with the amino acid residues essential for ACE2 binding by SARS-S being conserved in SARS-2-S (Lu et al., 2020, Zhou et al., 2020). In fact, both SARS-2-S and SARS-S entered ACE2-negative cells such as BHK-21 cells when the expression of human ACE2 or bat ACE2 was forced, but not with the expression of human DPP4 or human APN. Furthermore, an antibody against human ACE2 blocked both the SARS-S- and SARS-2-S-driven entry into human cell lines. These results indicated that SARS-2-S, like SARS-S, uses ACE2 for its cellular entry.
Hoffmann et al. then investigated the protease dependence of the SARS-CoV-2 entry. SARS-2-S-driven entry into 293T cells (TMPRSS2-negative) expressing ACE2 was inhibited by ammonium chloride, an inhibitor of CatB/L, while its entry into Caco-2 cells (TMPRSS2-positive) was less efficiently inhibited. A clinically proven TMPRSS2 inhibitor, camostat mesylate, partially inhibited SARS-2-S-driven entry into Caco-2 cells, while camostat mesylate together with E-64d, an inhibitor of CatB/L, completely inhibited the entry, suggesting that both TMPRSS2 and CatB/L are involved in SARS-CoV-2 entry. However, the forced expression of TMPRSS2 rescued SARS-2-S-dependent entry into CatB/L-suppressed 293T cells, suggesting that the entry of SARS-CoV-2 is induced when cells express TMPRSS2 regardless of CatB/L expression. SARS-2-S-driven entry into lung cells is also inhibited by camostat mesylate. These findings show that SARS-CoV-2 cell entry depends on surface molecules such as ACE2 and TMPRSS2.
Together, Zhou et al. and Hoffmann et al. showed that SARS-CoV-2 uses ACE2 as its cell entry receptor, just as SARS-CoV does. Importantly, these results suggest therapeutic targets for COVID-19. One is the binding between SARS-2-S protein and ACE2, and the other is the serine protease activity of TMPRSS2 for SARS-2-S protein priming.
These therapeutic targets may act on the initial phase of the SARS-CoV-2 infection, but not dominantly on the latter phase of the disease, because the extremely powerful chronic inflammation induced in the latter phase is the main cause of ARDS-mediated death (de Wit et al., 2016). Consistently, ARDS is a lethal syndrome caused by pneumonia, sepsis or aspiration due to “cytokine storms,” in which immune cells and nonimmune cells release large amounts of proinflammatory cytokines that cause damage to the host. Hyper-activation of the NF-κB pathway is involved in the phenotype. One of the major pathways for NF-κB activation after coronavirus infection is the MyD88 pathway through pattern recognition receptors (PRRs), leading to the induction of a variety of pro-inflammatory cytokines, including interleukin-6 (IL-6), tumor necrosis factor alpha (TNFα) and chemokines (de Wit et al., 2016).
ACE2 is a membrane protein and inactivator of angiotensin 2 (AngII). Importantly, ACE2 is endocytosed together with SARS-CoV, resulting in the reduction of ACE2 on cells, followed by an increase of serum AngII (Kuba et al., 2005). Because ACE2 is also downregulated in lung-injury models and recombinant ACE2 suppressed ARDS development (Imai et al., 2005), severe lung inflammation itself might induce dysregulation of the renin-angiotensin pathway followed by ARDS development after SARS-CoV-2 infection. Indeed, SARS-CoV-induced ARDS in an animal model is prevented by inhibitors of angiotensin receptor type 1 (AT1R) (Kuba et al., 2005).
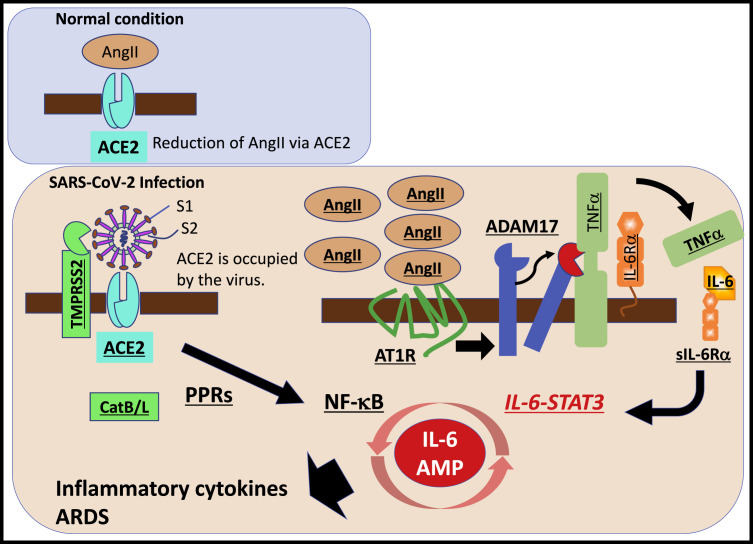
AngII acts not only as a vasoconstrictor but also as a pro-inflammatory cytokine via AT1R (Eguchi et al., 2018). The AngII-AT1R axis also activates NF-κB and disintegrin and metalloprotease 17 (ADAM17), which generates the mature form of epidermal growth factor receptor (EGFR) ligands and TNFα, two NF-κB stimulators (Eguchi et al., 2018). ADAM17 induction also processes the membrane form of IL-6Rα to the soluble form (sIL-6Rα), followed by the gp130-mediated activation of STAT3 via the sIL-6Rα-IL-6 complex in a variety of IL-6Rα-negative nonimmune cells including fibroblasts, endothelial cells, and epithelial cells (Murakami et al., 2019). STAT3 is required for full activation of the NF-κB pathway, and the main stimulator of STAT3 in vivo is IL-6, especially during inflammation, although there are nine other members of IL-6 family cytokines that can activate STAT3, at least in vitro (Murakami et al., 2019). Therefore, SARS-CoV-2 infection in the respiratory system can activate both NF-κB and STAT3, which in turn can activate the IL-6 amplifier (IL-6 Amp), a mechanism for the hyper-activation of NF-κB by STAT3, leading to multiple inflammatory and autoimmune diseases (Murakami et al., 2019). The IL-6 Amp induces various pro-inflammatory cytokines and chemokines, including IL-6, and recruits lymphoid cells and myeloid cells, such as activated T cells and macrophages, in the lesion to strengthen the IL-6 Amp in a positive feedback loop (Figure 1 ). Importantly, because IL-6 is a major functional marker of cellular senescence, the age-dependent enhancement of the IL-6 Amp might correspond to the age-dependent increase of COVID-19 mortality.
Figure 1.
Possible therapeutic targets for COVID-19, a cytokine release syndrome.
SARS-CoV-2 uses angiotensin converting enzyme II (ACE2) and transmembrane serine protease 2 (TMPRSS2) as cell entry receptors, followed by a cytokine-related syndrome, ARDS, which is induced by the hyper-activation of the transcription factor NF-κB, most likely in nonimmune cells including lung epithelial cells. ACE2 molecules on the cell surface are occupied by SARS-CoV-2. Angiotensin 2 (AngII) then increases in the serum due to a reduction of ACE2-mediated degradation. SARS-CoV-2 itself activates NF-κB via pattern recognition receptors (PPRs), and the accumulated AngII induces inflammatory cytokines including TNFα and IL-6-soluble (s)IL-6R via disintegrin and metalloprotease 17 (ADAM17), followed by activation of the IL-6 amplifier (IL-6 AMP), which describes enhanced NF-κB activation machinery via the coactivation of NF-κB and transcription factor STAT3. The molecules underlined indicate possible therapeutic targets for COVID-19, which is a cytokine release syndrome (CRS).
Indeed, the ARDS seen with SARS-CoV-2 infection is a cytokine release syndrome (CRS), which is a disorder induced by cytokine storms. The lethal side effect of CRS found with chimeric antigen receptor (CAR)-T cell therapies for leukemia and lymphoma is also associated with elevated inflammatory cytokines (Neelapu et al., 2018). It is possible that the enhanced pro-inflammatory cytokines are induced by the IL-6 Amp. Considering that the anti-IL-6R antibody tocilizumab is an effective treatment for CRS in CAR-T cell therapies (Neelapu et al., 2018), researchers might want to consider drugs with a similar mechanism of action for CRS in COVID-19.
Taken together, the demonstration of ACE2 as the SARS-CoV-2 receptor for cellular entry provides a key target for therapeutic development during the initial phase of the infection. In its later phase, the potential dysregulation of the AngII-AT1R pathway downstream of ACE2 could lead to cytokine release syndrome as observed in COVID-19 patients that may require targeting of cytokine pathways, particularly IL-6-STAT3 axis.
References
- de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi S., Kawai T., Scalia R., Rizzo V. Understanding Angiotensin II Type 1 Receptor Signaling in Vascular Pathophysiology. Hypertension. 2018;71:804–810. doi: 10.1161/HYPERTENSIONAHA.118.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Kamimura D., Hirano T. Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity. 2019;50:812–831. doi: 10.1016/j.immuni.2019.03.027. [DOI] [PubMed] [Google Scholar]
- Neelapu S.S., Tummala S., Kebriaei P., Wierda W., Gutierrez C., Locke F.L., Komanduri K.V., Lin Y., Jain N., Daver N. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]