Abstract
Factors associated with negative conversion of SARS-CoV-2 RNA in hospitalized patients have not yet been systematically determined. We conducted a retrospective cohort study of COVID-19 patients in Qingdao, China. Both univariate and multivariate analysis were performed to identify independent factors for time to viral RNA negative conversion. Data on patients with re-detectable viral RNA after showing negative on RT-PCR test (intermittent negative status) were also analyzed. A total of 59 patients confirmed with COVID-19 were included in this study, with a median duration of 1 (interquartile range, IQR: 0–2) day from symptom onset to hospital admission. Median communicable period (from first day of positive nucleic acid test to first day of consecutive negative results) was 14 (IQR: 10–18) days, and 7 (IQR: 6–10) days for 10 patients with intermittent negative results. Age older than 45 years (hazard ratio, HR: 0.378; 95% confidence interval, CI: 0.205–0.698) and chest tightness (HR: 0.290; 95%CI: 0.091–0.919) were factors independently affecting negative conversion of SARS-CoV-2 RNA. Headache (odds ratio: 7.553; 95%CI: 1.011–28.253) was significantly associated with intermittent negative status, with a predicted probability of 60%. Older age and chest tightness were independently associated with delayed clearance of SARS-CoV-2 RNA in hospitalized patients. These predictors would provide a new perspective on early identification of patients with prolonged viral shedding and facilitate optimal isolation protocols and treatment strategies.
Keywords: Coronavirus disease 2019 (COVID-19), Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Negative conversion, Hazard ratio
Graphical abstract
1. Introduction
On December 12, 2019, 27 cases of pneumonia of unknown causes were reported in Wuhan, Hubei province, China (WMHC, 2020). On February 11, 2020, the world health organization (WHO) officially named this emerging disease as coronavirus disease 2019 (COVID-19), and the etiological agent was subsequently identified and renamed as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the Coronavirus Study Group (CSG) of the International Committee on Taxonomy of Viruses (CSGICTV, 2020; WHO, 2020). With the evolving pandemic, COVID-19 has wreaked havoc in China and spread rapidly to around 210 countries and regions all over the world, constituting a global threat with the highest risk impact (Our World in Data, 2020).
Since the identification of the first few cases of COVID-19, numerous studies have mushroomed with a focus on the epidemiological, clinical, laboratory, and radiological characteristics of infected patients, prompting the development of diagnosis and treatment strategies (Ai et al., 2020; Chen et al., 2020; Cortegiani et al., 2020; Huang et al., 2020; Xing et al., 2020; Zhu et al., 2020). However, there is limited data regarding the potential predictors of negative conversion with COVID-19 patients. Possible reasons underlying this research gap may be due to the lack of detailed sequential virology profiles at the early stage of the disease. Another possible reason is that some cases were admitted to hospital after relatively long period of illness onset, making it difficult to determine the start point for the negative conversion of SARS-CoV-2.
Health authorities in Qingdao, a city located in the northeast of China beyond the epidemic center, took a rapid response in the face of COVID-19 outbreak. The local government and municipal center for disease control and prevention in Qingdao has launched series of aggressive measures to screen, quarantine, diagnose, treat and monitor suspected patients and their close contacts (Jia et al., 2020; Xing et al., 2020). COVID-19 cases in Qingdao were identified and admitted to designated hospitals at an early stage, providing a strong foundation for our research. This study aimed to ascertain viral clearance time and factors associated with SARS-CoV-2 RNA negative-conversion in upper respiratory specimens. We described the dynamic changes of viral shedding in laboratory-confirmed COVID-19 patients on presentation till hospital discharge.
2. Methods
2.1. Study design and data collection
This retrospective cohort study included patients with confirmed COVID-19 hospitalized in Qingdao, Shandong Province, China. All patients were diagnosed with COVID-19 based on New Coronavirus Pneumonia Diagnosis and Treatment Plan (National Health Commission of the People's Republic of China (NHCPRC), 2020a, National Health Commission of the People's Republic of China (NHCPRC), 2020b, National Health Commission of the People's Republic of China (NHCPRC), 2020c), and were discharged between Jan 29, 2020 and Mar 12, 2020.
Upon identification of a suspected case of COVID-19, the joint expert team comprising members from the Qingdao Municipal Center for Disease Control and Prevention (Qingdao CDC) together with prefecture health authorities would initiate detailed field investigations and collect respiratory specimens for centralized testing at Qingdao CDC.
Data were collected onto standardized forms through interviews of infected persons. Epidemiological, demographic, clinical, laboratory, treatment, and outcome data were extracted from the interview as well as field reports.
This study was approved by the Ethics Commission of Municipal Centre of Disease Control and Prevention of Qingdao and written informed consent was waived considering the emergency of infectious disease.
2.2. Definitions of basic concepts
A case was confirmed by a positive result of real-time reverse-transcription-polymerase-chain-reaction (RT-PCR) analysis for SARS-CoV-2 in nasopharyngeal swabs. A severe case is defined as a patient who fulfills one of the following criteria: respiratory rate ≥ 30 breaths/min; oxygen saturation ≤ 93% at a rest state; arterial partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) ≤300 mmHg. Patients were discharged based on the clinical guideline such as respiratory samples consecutively negative for nucleic acid testing with an interval of at least one day (National Health Commission of the People's Republic of China (NHCPRC), 2020a, National Health Commission of the People's Republic of China (NHCPRC), 2020b, National Health Commission of the People's Republic of China (NHCPRC), 2020c).
Some novel definitions were developed to describe the outcomes and time to RT-PCR negative conversion during hospitalization. Communicable period is defined as the duration from the first day of positive RT-PCR result till the first day showing negative on nucleic acid testing successively, as proposed by a previous study on a similar topic (Gao and Li, 2020). Intermittent negative status is defined as patients with re-detectable viral RNA after showing negative on RT-PCR test, with a duration from the first day of showing negative on nucleic acid test till the beginning of consecutive negative tests.
2.3. Laboratory procedures
SARS-CoV-2 detection in respiratory specimens was conducted by Qingdao CDC using real-time RT-PCR targeted at open reading frame 1ab (ORF1ab) and nucleocapsid protein (N) genes. Tests were carried out in biosafety level 2 facilities, using a commercial kit approved by the China Food and Drug Administration. Nasopharyngeal swabs were obtained from patients on a daily basis within 3 days after illness onset. RNA was extracted from the samples and then underwent real-time RT-PCR with SARS-CoV-2-specific primers and probes. A cycle threshold (Ct) value were used to approximately reflect the viral loads (inversely related to Ct-value) in the respiratory tract, a method as suggested by previous studies (Zhu et al., 2020; Xu et al., 2020). A Ct value <37 was defined as a positive test, and a Ct value of 40 or more was considered as a negative test. An equivocal result, defined as a Ct value between 37 and 40, required confirmation by retesting. If the repeated Ct value was <40 and an obvious peak was observed, or if the repeated Ct value was <37, the result was deemed positive.
2.4. Statistical analysis
In our study, negative conversion of viral RNA during the communicable period, as time-to-event data, was the outcome measure and presented with Kaplan-Meier curves. In order to detect the independent predictors of RNA negative conversion, the univariate and multivariate analysis were performed. The log-rank test was first conducted with a total of 25 factors, such as virological, demographic, epidemiological, clinical, radiological and laboratory variables. Multivariate Cox regression model was then performed with the significant factors selected by univariate analysis to tease out the independent predictors for RNA negative conversion. The association between independent predictors and negative conversion was quantified by hazard ratio (HR). As negative conversion of viral RNA is a beneficial event, HR value >1 would promote virological clearance, whereas HR value <1 means the independent predictor would delay negative conversion.
Additionally, we investigated factors associated with intermittent negative status. Mann–Whitney U test, χ2 test, or Fisher's exact test was first conducted to compare differences between groups with and without intermittent negative status. Next, factors with statistically significance (P < 0.05) were further analyzed using the logistic regression model, and odds ratio (OR) and predicted probability were calculated.
Continuous and categorical variables were presented as median (interquartile range, IQR) and n (%), respectively. A P value <0.05 (two-tailed) was considered statistically significant. Analyses were performed using SPSS software (version 20.0) and R software (version 3.6.2).
3. Results
3.1. Demographical and epidemiological characteristics of study population
A total of 59 patients hospitalized with COVID-19 were included in this study, of whom 43 were cluster cases from eleven outbreaks, including 33 family members and 10 close contacts. Eighteen patients had a travel history to Hubei Province before illness onset, and 11 patients had been to Wuhan in January 2020. Female accounted for 52.5% (31) of confirmed cases and 47.5% (28) were male. The majority of patients (48) were local residents in Qingdao, and 11 patients were floating population. The median age of patients was 46 years (IQR: 33–57). More than a quarter of patients (17) had underlying diseases, such as cardiovascular and cerebrovascular diseases (9), endocrine disease (3), gastrointestinal disease (3), respiratory disease (1) and neurological disorder (1). Detailed demographical and epidemiological information was represented in Table 1 .
Table 1.
Univariate analysis among 59 cases with negative conversion.
| Factors | Count (%) | Negative conversion rate |
X2 value | P value | ||
|---|---|---|---|---|---|---|
| 7 days | 14 days | 21 days | ||||
| Overall | 59 | 10.2 | 62.7 | 91.2 | ||
| Age | ||||||
| <45 | 28 (47.5) | 7.2 | 82.2 | 96.5 | 6.9 | 0.009 |
| ≥45 | 31 (52.5) | 12.9 | 45.2 | 87.1 | ||
| Sex | ||||||
| Male | 28 (47.5) | 7.2 | 67.9 | 96.5 | 0.8 | 0.40 |
| Female | 31 (52.5) | 12.9 | 58.1 | 87.1 | ||
| Disease severity | ||||||
| Non-severe | 49 (83.1) | 2.5 | 66.7 | 93.8 | 1.7 | 0.20 |
| Severe | 10 (16.9) | – | 44.5 | 81.9 | ||
| Residence | ||||||
| External | 11 (18.6) | – | 91.7 | 100 | 4.2 | 0.06 |
| Local | 48 (81.4) | 23.9 | 55.4 | 89.4 | ||
| Cluster case | ||||||
| Yes | 43 (72.9) | 9.3 | 51.2 | 90.7 | 5.3 | 0.02 |
| No | 16 (27.1) | 12.5 | 93.8 | 93.8 | ||
| Wuhan Hubei | ||||||
| Yes | 18 (30.5) | 5.6 | 83.4 | 94.5 | 2.1 | 0.15 |
| No | 42 (71.2) | 12.1 | 53.6 | 90.3 | ||
| Fever | ||||||
| Yes | 34 (71.2) | 12.1 | 63.6 | 99.1 | 0.2 | 0.66 |
| No | 25 (28.8) | 7.7 | 61.6 | 92.4 | ||
| Non-productive cough | ||||||
| Yes | 18 (30.5) | 15.6 | 55.6 | 88.9 | 0.2 | 0.62 |
| No | 41 (69.5) | 12.2 | 65.9 | 92.7 | ||
| Sputum production | ||||||
| Yes | 7 (11.9) | – | 85.7 | 85.7 | 0.1 | 0.97 |
| No | 52 (88.1) | 11.5 | 59.6 | 92.3 | ||
| Fatigue | ||||||
| Yes | 7 (11.9) | 16.7 | 50.0 | 66.7 | 3.4 | 0.07 |
| No | 52 (88.1) | 9.7 | 65.4 | 94.3 | ||
| Myalgia | ||||||
| Yes | 4 (6.8) | 25.0 | 25.0 | 75.0 | 1.1 | 0.29 |
| No | 55 (93.2) | 9.2 | 66.7 | 92.6 | ||
| Headache | ||||||
| Yes | 6 (10.2) | – | 60.0 | 80.0 | 0.6 | 0.46 |
| No | 53 (89.8) | 11.4 | 64.2 | 92.5 | ||
| Chest tightness | ||||||
| Yes | 5 (8.5) | – | 40.0 | 60.0 | 3.9 | 0.04 |
| No | 54 (91.5) | 13.0 | 64.9 | 94.5 | ||
| Leucocyte count | ||||||
| <4 × 109/L | 7 (11.9) | 14.3 | 100 | 100 | 5.4 | 0.02 |
| ≥4 × 109/L | 52 (88.1) | 10.6 | 53.2 | 89.4 | ||
| Neutrophil percentage | ||||||
| <50% | 6 (10.2) | 16.7 | 33.3 | 100 | 0.1 | 0.87 |
| ≥50% | 53 (89.8) | 9.4 | 66.1 | 90.6 | ||
| Neutrophil percentage | ||||||
| ≤70% | 16 (27.1) | 13.7 | 68.2 | 93.2 | 3.5 | 0.06 |
| >70% | 44 (72.9) | – | 46.7 | 86.7 | ||
| Lymphocyte percentage | ||||||
| <20% | 16 (27.1) | 5.6 | 52.8 | 88.2 | 1.7 | 0.19 |
| ≥20% | 43 (72.9) | 14.3 | 66.7 | 92.9 | ||
| Lymphocyte percentage | ||||||
| ≤40% | 46 (78.0) | 8.7 | 60.9 | 89.2 | 1.9 | 0.17 |
| >40% | 13 (22.0) | 15.4 | 69.3 | 100 | ||
| CT imaging | ||||||
| Normal | 11(18.6) | 8.4 | 58.4 | 91.7 | 0.1 | 0.94 |
| Abnormal | 48 (81.4) | 11.8 | 63.6 | 91.5 | ||
| Involved lung field | ||||||
| Unilateral | 17 (35.4) | 27.8 | 61.2 | 94.5 | 3.1 | 0.08 |
| Bilateral | 31 (64.6) | 3.3 | 62.0 | 81.1 | ||
| Radiological characteristics | ||||||
| Ground-grass opacities | 17 (35.4) | 5.9 | 53.0 | 94.2 | 0.1 | 0.95 |
| High density shadow | 31 (64.6) | 15.7 | 65.7 | 87.5 | ||
| Taking medicine before hospital admission | ||||||
| Yes | 15 (25.4) | 13.4 | 86.7 | 86.7 | 0.9 | 0.35 |
| No | 44 (74.6) | 11.3 | 54.5 | 93.2 | ||
| Comorbidities | ||||||
| Yes | 17 (28.8) | 11.5 | 52.8 | 88.2 | 1.1 | 0.32 |
| No | 42 (71.2) | 11.9 | 66.7 | 92.9 | ||
| Ct-values of ORF | ||||||
| <30 | 41 (55.9) | 4.9 | 63.4 | 92.9 | 0.1 | 0.7 |
| ≥30 | 18 (44.1) | 22.2 | 61.1 | 88.9 | ||
| Ct-values of N | ||||||
| <30 | 33 (55.9) | 6.1 | 63.6 | 93.9 | 0.2 | 0.7 |
| ≥30 | 26 (44.1) | 11.5 | 61.5 | 88.5 | ||
X2 value and P values were calculated by Log rank test.
3.2. Clinical presentations, laboratory and radiological findings
The median duration from disease onset to hospital admission was 1 (IQR: 0–2) day, with a median of 19 days from illness onset to hospital discharge (IQR: 16–25). As shown in Table 1, 83.1% of the 59 COVID-19 cases in Qingdao were non-severe (mild to moderate cases), and 16.9% were severe cases. Most common symptoms at onset of the disease were fever and dry cough. Other symptoms included fatigue, headache, chest tightness, myalgia, and sputum production. All patients received standard care including supportive and anti-viral treatment according to the latest clinical guidelines (National Health Commission of the People's Republic of China (NHCPRC), 2020a, National Health Commission of the People's Republic of China (NHCPRC), 2020b, National Health Commission of the People's Republic of China (NHCPRC), 2020c). Antibiotics were only prescribed to patients who were at risk of or presented with bacterial infection. Patients with symptoms of respiratory distress received oxygen therapy. Corticosteroids were used shortly to inhibit inflammatory cascade in patients with progressing disease. Critically ill patients were transferred to intensive care unit.
On admission, 7 patients had leucopenia (white blood cell count <4 × 109/L), 16 patients had increased proportion of neutrophils (>70%), and 13 patients showed elevated lymphocyte percentage (>40%). Abnormalities in chest computed tomograms (CT) were detected in 46 patients (80.0%). The most common pattern of CT changes was high-density shadow over bilateral lungs (54.2%).
3.3. Negative conversion of SARS-CoV-2 RNA
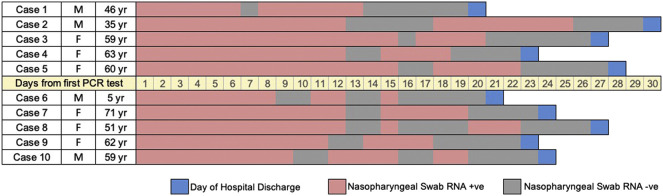
Viral loads in nasopharyngeal swabs were estimated with Ct values by RT-PCR. Median Ct values for RT-PCR on ORF 1ab gene was 26 (IQR: 24–31), and median Ct value for N gene was 28 (IQR: 26–31). All patients were discharged base on two consecutive negative tests at least 24 h apart. Median duration of communicable period was 14 (IQR: 10–18) days, ranging from 4 to 25 days. Moreover, 10 patients were in intermittent negative status after first showing negative for RT-PCR, with a median duration in between of 7 days (IQR: 6–10, Fig. 1 ). The rate of RNA negative conversion within 7 days, 14 days and 21 days among all patients were 10.2% (95% confidence interval, 95%CI: 2.1%–17.5%), 62.7% (48.1%–73.2%), and 91.2% (80.4%–96.4%), respectively (Fig. 2 ).
Fig. 1.
Dynamic profile of intermittent negative status among COVID-19 patients.
Fig. 2.
Overall negative conversion curve in COVID-19 patients.
3.4. Predictors of negative conversion
Table 1 summarized the results of univariate analysis. We evaluated the effect of each factor on negative conversion of COVID-19 patients by Log rank test. Age, cluster cases, chest tightness and leukocyte count were significantly related to RNA negative conversion. Multivariate Cox regression was then performed with the significant factors selected by univariate analysis. Fig. 3 showed that age older than 45 years (HR: 0.378; 95%CI: 0.205–0.698) and chest tightness (HR: 0.290; 95%CI: 0.091–0.919) were independently associated with negative conversion of viral RNA, suggesting that older age and presentation of chest tightness would delay virological clearance in COVID-19 patients (Fig. 4 ).
Fig. 3.
Independent predictors of negative conversion by multivariate Cox regression.
Fig. 4.
Negative conversion curves in COVID-19 patients according to predictors (A: age; B: chest tightness).
Sensitivity analysis was conducted to guarantee robust results. A general liner model (GLM) was performed to quantify the association between four significant factors detected by univariate analysis and time to RNA negative conversion. The effect values were largely consistent, indicating age older than 45 years (RR: 0.553; 95%CI: 0.451–0.869) and chest tightness (RR: 0.444; 95%CI: 0.289–0.978) were inversely correlated with time to negative conversion (Table 2 ).
Table 2.
Results of sensitivity analysis by the general liner model (GLM).
| Variable | RR | 95% CI | P value |
|---|---|---|---|
| Age | |||
| <45 | Reference | ||
| ≥45 | 0.553 | 0.451–0.869 | 0.005 |
| Residence | |||
| Local | Reference | ||
| External | 0.890 | 0.657–1.231 | 0.471 |
| Cluster case | |||
| Yes | Reference | ||
| No | 1.273 | 0.959–1.704 | 0.107 |
| Chest congestion | |||
| Yes | Reference | ||
| No | 0.444 | 0.289–0.978 | 0.043 |
| Leucocyte count | |||
| <4 × 109/L | Reference | ||
| ≥4 × 109/L | 1.177 | 0.850–1.665 | 0.344 |
RR, relative risk; 95%CI, 95% confidence interval.
3.5. Probability of intermittent negative status
After analyzing the variables by Mann–Whitney U test, χ2 test, or Fisher's exact test, there were two variables including headache, days from illness onset to hospital admission, which were significant different (P < 0.05) between groups with and without intermittent negative status. Further logistic regression with the two variables indicated that headache (OR: 7.553; 95%CI: 1.011–28.253) was positively associated with intermittent negative status, rather than days from illness onset to hospital admission (per day increase OR: 1.133; 95%CI: 0.977–1.343). After controlling the effect of days from illness onset to hospital admission, the predicted probability of intermittent negative status in response to headache was 60% as revealed by logistic regression analysis.
4. Discussion
This study retrospectively analyzed demographical, epidemiological, laboratory, clinical and radiological data in a cohort of patients hospitalized with COVID-19 in Qingdao, Shandong Province. To investigate the timeline of RNA negative conversion of COVID-19 patients during the course of disease, some definitions were proposed. Communicable period presents the duration of RT-PCR analysis being positive, which is from the first day of positive RT-PCR test to the first day of showing consecutive negative results. Intermittent negative status is used to describe patients who had re-detectable positive result after showing negative for RT-PCR analysis and RNA negative conversion denotes a time-to-event outcome.
To the best of our knowledge, this is the first study focused on factors associated with SARS-CoV-2 RNA negative conversion. We found that negative conversion of SARS-CoV-2 RNA occurred 14 (IQR: 10–18) days from the first positive RT-PCR test. It has been reported that the viral RNA existed for a median duration of 20.0 (IQR: 17.0–24.0) days in patients survived from COVID-19 (Zhou et al., 2020), while another study demonstrated a median duration of viral shedding of 9.5 (IQR: 6.0–11.0) days (Ling et al., 2020). The discrepancies between studies might attribute to differences in disease severity as well as sampling methods.
Cox regression multivariate analysis revealed that growth of age and chest tightness were correlated to later RT-PCR conversion. Older age has been constantly linked to poor clinical outcomes in COVID-19 patients (Zhou et al., 2020). T-cell numbers and functions are largely compromised with aging, resulting in less controlled viral replication and host inflammatory responses (Webster, 2000; Goronzy et al., 2007). Besides, older patients had more underlying diseases as compared with younger patients infected with SARS-CoV-2 (Liu et al., 2020). Although disease severity and comorbidities were not significant predictors from univariate and multivariate analysis, meaning the two factors had no direct effect on time to RT-PCR conversion, they may indirectly influence viral nucleic acid clearance via effect of age. Further spearman correlation analysis showed that disease severity (r = 0.283, P = 0.03) and comorbidities (r = 0.450, P < 0.001) were positively correlated with age. These age-related defects may make it more difficult for the host cell immunity to eradicate the invasive pathogen, resulting in prolonged viral shedding in the elderly. However, when we compared the 5 patients presented with chest tightness with the other patients, no obvious differences could be found in terms of epidemiological, laboratory and radiological characteristics. A previous study on SARS-CoV (2002−2003) demonstrated that urine positive for RT-PCR analysis on presentation was associated with delayed RNA negative conversion, while treatment with ribavirin and corticosteroid was related to a sooner virological clearance (Chu et al., 2005). In our study, ribavirin was empirically prescribed to all patients confirmed with COVID-19, whereas corticosteroids were rarely given. We could not detect any relationship between treatment strategies and time to RT-PCR conversion in our patients. The clinical significance of these predictors is still unknown and further studies would be warranted. The potential risk factors for a delayed viral nucleic acid clearance would shed light on early recognition of patients with poor prognosis.
In the current study, 10 patients had re-detectable positive results for SARS-CoV-2 RNA test during hospitalization. We found that headache was correlated with intermittent negative results in these patients. Previous studies have reported a proportion of recovered patients who met criteria for hospital discharge showed re-detectable positive results for RT-PCR testing (An et al., 2020; Lan et al., 2020; Ling et al., 2020). In general, patients with intermittent negative RT-PCR were younger and had milder symptoms (r = 0.220, P = 0.041; r = 0.242, P = 0.033, respectively). Cautionary measures should be considered when dealing with patients with recurrence of positive RT-PCR results as SARS-CoV could be transmitted from convalescent patients to others via close contact (Chan et al., 2003). It might be advisable to extend the duration of hospitalization and isolation of patients with intermittent negative RT-PCR results. These findings provide important implications for decision making on quarantine protocols and facilitating optimal antiviral interventions.
Additionally, we analyzed Ct values of RT-PCR test (inversely related to viral load) on admission as accumulating evidence supported the notion that viral loads of SARS-CoV-2 are among the highest in COVID-19 patients early after onset (Pan et al., 2020; To et al., 2020; Zou et al., 2020). A high viral load on presentation was an independent factor contributing to worse prognosis in patients with SARS (Chu et al., 2004). Although we failed to find significant relationship between Ct values and severity of illness, older age was associated with higher viral loads of the RT-PCR test on admission (as reflected by lower Ct values, r = −0.345 P = 0.007). Our findings verified the results from a recent study showing that older age, rather than disease severity, was positively associated with viral RNA copy numbers (To et al., 2020). Studies for SARS-CoV also indicated that increased age was independently associated with higher viral load (Chen et al., 2006).
There are several limitations of this study. First, our sample size is relatively small in comparison with other studies. There were 59 COVID-19 patients in Qingdao during the study period. However, we believe our population was a good preventative of patients outside of the epidemic center. Second, we did not detect the existence of viral RNA in patients' excretions such as urine and fecal specimens. Previous studies indicated the possibility of viral transmission via fomite and viral loads in the stools might be greater than that in the respiratory specimens (Wölfel et al., 2020; Xu et al., 2020). Third, we did not record the dynamic profiles of viral loads (as reflected by Ct values). How the chronological changes in viral loads correlate with disease prognosis merits further investigation.
5. Conclusion
This study identified factors of older age and chest tightness were associated with delayed clearance of viral RNA in patients hospitalized with COVID-19. We hope these predictors could provide clues for early identification of patients with prolonged viral shedding, paving the way for development of optimal isolation protocols and treatment strategies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Xiaowen Hu: Conceptualization, Writing - original draft. Yuhan Xing: Formal analysis, Writing - original draft. Jing Jia: Investigation, Writing - original draft. Wei Ni: Formal analysis, Writing - original draft. Jiwei Liang: Investigation. Dan Zhao: Investigation. Xin Song: Investigation. Ruqin Gao: Investigation, Writing - original draft. Fachun Jiang: Conceptualization, Writing - original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are deeply thankful to all health-care workers involved in the diagnosis and treatment of patients in Qingdao.
References
- Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., Tao Q., Sun Z., Xia L. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J., Liao X., Xiao T., Qian S., Yuan J., Ye H., Qi F., Shen C., Liu Y., Wang L. Clinical characteristics of the recovered COVID-19 patients with re-detectable positive RNA test. medRxiv. 2020 doi: 10.1101/2020.03.26.20044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L.Y., Li P.K., Sung J. Risk of SARS transmission to persons in close contact with discharged patients. Am. J. Med. 2003;115:330. doi: 10.1016/S0002-9343(03)00352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.J., Yang J.Y., Lin J.H., Fann C.S., Osyetrov V., King C.C., Chen Y.M., Chan H.L., Kuo H.W., Liao F. Nasopharyngeal shedding of severe acute respiratory syndrome-associated coronavirus is associated with genetic polymorphisms. Clin. Infect. Dis. 2006;42:1561–1569. doi: 10.1086/503843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Poon L.L., Cheng V.C., Chan K.S., Hung I.F., Wong M.M., Chan K.H., Leung W.S., Tang B.S., Chan V.L. Initial viral load and the outcomes of SARS. CMAJ. 2004;171:1349–1352. doi: 10.1503/cmaj.1040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Leung W.S., Cheng V.C.C., Chan K.H., Lin A.W.N., Chan V.L., Lam J.Y.M., Chan K.S., Yuen K.Y. Duration of RT-PCR positivity in severe acute respiratory syndrome. Eur. Respir. J. 2005;25:12–14. doi: 10.1183/09031936.04.00057804. [DOI] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (CSGICTV) The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020 doi: 10.1016/j.jcrc.2020.03.005. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Li L. Advances on presymptomatic or asymptomatic carrier transmission of COVID-19 (in Chinese) Chin. J. Epidemiol. 2020;41:485–488. doi: 10.3760/cma.j.cn112338-20200228-00207. [DOI] [PubMed] [Google Scholar]
- Goronzy J.J., Lee W.W., Weyand C.M. Aging and T-cell diversity. Exp. Gerontol. 2007;42:400–406. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J., Hu X.W., Yang F., Song X., Dong L., Zhang J., Jiang F., Gao R. Epidemiological characteristics on the clustering nature of COVID-19 in Qingdao City, 2020: a descriptive analysis. Disaster. Med. Public. 2020;31:1–5. doi: 10.1017/dmp.2020.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L., Xu D., Ye G., Xia C., Wang S., Li Y., Xu H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2783. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Xu S.B., Lin Y.X., Tian D., Zhu Z.Q., Dai F.H., Wu F., Song Z.G., Huang W., Chen J. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000774. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.005. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission of the People'’s Republic of China (NHCPRC) Diagnosis and treatment plan of Corona virus disease 2019 (tentative fifth edition) 2020. http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml [Assessed 5 Feb, 2020]. Availbe from.
- National Health Commission of the People'’s Republic of China (NHCPRC), 2020b. Diagnosis and Treatment Plan of Corona Virus Disease 2019 (tentative sixth edition). [Feb 19, 2020]. Availbe from:. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml.
- National Health Commission of the People'’s Republic of China (NHCPRC) Diagnosis and Treatment Plan of Corona Virus Disease 2019 (tentative seventh edition) 2020. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml Availbe from.
- Our World in Data . Oxford Martin School, The University of Oxford, Global Change Data Lab; 2020. Coronavirus Disease (COVID-19) – Statistics and Research.https://ourworldindata.org/coronavirus/ Available from. [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet. Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., Yip C.C., Cai J.P., Chan J.M., Chik T.S. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30196-1. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R.G. Immunity to influenza in the elderly. Vaccine. 2000;18:1686–1689. doi: 10.1016/s0264-410x(99)00507-1. [DOI] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 doi: 10.1038/s41586-020-2196-x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- World Health Organizaiion (WHO) Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. 2020. https://www.who.int/internalpublicationsdetail/clinicalmanagementofsevereacuterespiratoryinfectionwhennovelcoronavirus(ncov)infectionissuspected Availbe from.
- Wuhan Municipal Health Commission (WMHC) Report of clustering pneumonia of unknown etiology in Wuhan City. 2020. http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989 Available from.
- Xing Y.H., Ni W., Wu Q., Li W.J., Li G.J., Wang W.D., Tong J.N., Song X.F., Wong G.W.K., Xing Q.S. Prolonged Viral Shedding in Feces of Pediatric Patients with Coronavirus Disease 2019. J. Microbiol. Immunol. 2020;2020 doi: 10.1016/j.jmii.2020.03.021. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]