Abstract
COVID-19 pandemic is a major challenge for global and national healthcare providers. Number of new cases is continuously increasing with an emerging trend showing worse prognosis in males in comparison to females. Based on this observation, our proposed hypothesis is that 5-alpha-reductase inhibitors, that are commonly used for BPH treatment, may be one of the factors contributing to poorer prognosis in males.
Background
With increasing number of COVID-19 cases, an evident sex-dependent difference in disease outcomes can be observed. Based on published studies with short term follow-up, males have 65% higher mortality rate [1]. The question remains, whether long term observational studies will confirm improved recovery in females.
The major alleged cause of this phenomenon, is a general poorer male health condition, related mostly to a higher cigarette consumption and more common heart disease [2]. In spite of these reports, there have been other published opinions, raising the possibility of an intrinsic protective immunomodulation mediated by estrogen receptor pathway. Indeed, the sex dependent susceptibility to COVID-19 infection, is a result of many not yet identified factors. These however, should be recognized as soon as possible, in order to improve an accurate management of the disease.
Hypothesis
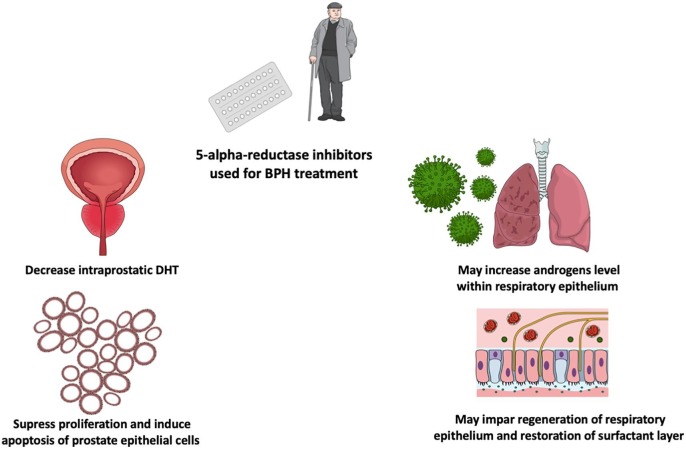
From urologist’s perspective, a potential explanation might be related to the BPH (Benign Prostatic Hyperplasia) treatment with finasteride and dutasteride. Both of these members of 5-alpha-reductase inhibitors are used to reduce prostate volume pharmacologically [3]. Therapeutic effect is mediated by decreasing intraprostatic level of DHT (Dihydrotestosterone). DHT is more potent than testosterone at maintaining normal prostate weight and stroma volume [4]. The influence of 5-alpha-reductase inhibitors on prostate is well established but 5-alpha-reductase is also an enzyme associated with androgen metabolism in many organs. Consequently, we introduce hypothesis that 5-alpha-reductase inhibitors may disrupt androgens metabolism in lungs, which in turn may have a negative impact on course of COVID-19 infection (See Fig. 1 ).
Fig. 1.
5-alpha-reductase inhibitors therapy due to BPH may increase the risk of COVID-19 infection unfavorable outcome.
Evaluation of the hypothesis and discussion
Finasteride and less selective dutasteride both block 5-alpha-reductase isoform 3, which is expressed in respiratory epithelium and fibroblasts [5]. The regulative role of androgens in adult human lungs function is an underdeveloped field. Nevertheless, there is limited scientific data indicating that androgens are involved in proper function of respiratory epithelium. Importantly, maintenance and restoration of surfactant layer may be controlled by androgens’ metabolism, where 5-alpha-reductase plays a key role [6]. During alveolar fetal development and alveolar repair after inflammatory lung disease close contacts needs to be established between fibroblasts and lung epithelial cells through gaps in the basement membrane [7]. It was documented that androgens including DHT disrupt communication between fibroblasts and alveolar type II cells by a mechanism involving TGFβ (Transforming Growth Factor beta) and EGF (Epidermal Growth Factor) receptor signaling pathways [8], [9].
During human fetal lung development, DHT slows down epithelial layer maturation.
In animal studies supplementation with DHT inhibited surfactant phospholipid production during fetal lung development whereas application of antiandrogen flutamide increased surfactant phospholipid production [8], [10]. It is likely that in mature lung tissue, the androgen regulative pathways analogously to fetal ones are also active. The expression of major regulative enzymes 17 beta-hydroxysteroid dehydrogenase and 5 alpha-reductase was detected in adult lung tissue. Interestingly, it was shown that balanced interplay of both enzymes is physiologically adjusted to obtain precisely regulated androgen inactivation within lungs. Therefore, in contrast to prostate, the physiological function of 5 alpha-reductase in lungs is to locally minimalize androgen potential [11]. This action profile is a result of low 5 alpha-reductase affinity to testosterone and high to androstadiene with respiratory epithelium.
The respiratory epithelium is characterized with capacity of spontaneous regeneration. Despite being quiescent tissue, lungs’ regeneration mechanism are activated in pneumonia related injuries [12]. Regeneration processes are universal and mimic organogenesis stages, in this situation, restoration of proper respiratory epithelial layer may be dependent on androgens metabolism. Under this assumption, 5-alpha-reductase inhibitors might increase androgen concentration in lungs hampering their regeneration. Interstitial pneumonia is the main cause of life-threatening respiratory disorders at the severe stage of COVID-19 infection [13]. Complex lung damage including lung alveolar epithelial cells and fibroblasts is a major hurdle to recovery in those patients. Therefore, inhibition of 5 alpha-reductase might result in impairment of spontaneous regeneration capacity and prolonged or deteriorated recovery prognosis.
Consequences of the hypothesis
Due to high prevalence of 5-alpha-reductase inhibitor in BPH treatment, its potential negative influence on recovery after COVID-19 infection, should be established. According to presented hypothesis, patients receiving 5-alpha-reductase inhibitors, might be vulnerable to COVID-19 infection with poorer prognosis. In such dynamic situation as COVID-19 pandemic, an interdisciplinary analysis of patients may deliver plenty of clues for development of treatment protocols optimization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.109751.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Li L.-Q., Huang T., Wang Y.-Q., Wang Z.-P., Liang Y., Huang T.-B. 2019 novel coronavirus patients’ clinical characteristics, discharge rate and fatality rate of meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet. Respir Med. 2020 doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim E.H., Brockman J.A. Andriole GL. The use of 5-alpha reductase inhibitors in the treatment of benign prostatic hyperplasia. Asian. J Urol. 2018 doi: 10.1016/j.ajur.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carson C., Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003 doi: 10.1016/s0090-4295(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 5.Azzouni F., Godoy A., Li Y., Mohler J. The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases. Adv Urol. 2012 doi: 10.1155/2012/530121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Provost P.R., Simard M., Tremblay Y. A link between lung androgen metabolism and the emergence of mature epithelial type II cells. Am J Respir Crit Care Med. 2004 doi: 10.1164/rccm.200312-1680OC. [DOI] [PubMed] [Google Scholar]
- 7.Griffin M., Bhandari R., Hamilton G., Chan Y.C., Powell J.T. Alveolar type II cell-fibroblast interactions, synthesis and secretion of surfactant and type I collagen. J Cell Sci. 1993 doi: 10.1242/jcs.105.2.423. [DOI] [PubMed] [Google Scholar]
- 8.Bresson E., Seaborn T., Côté M., Cormier G., Provost P.R., Piedboeuf B. Gene expression profile of androgen modulated genes in the murine fetal developing lung. Reprod Biol Endocrinol. 2010 doi: 10.1186/1477-7827-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhaskaran M., Kolliputi N., Wang Y., Gou D., Chintagari N.R., Liu L. Trans-differentiation of alveolar epithelial type II cells to type I cells involves autocrine signaling by transforming growth factor β1 through the Smad pathway. J Biol Chem. 2007 doi: 10.1074/jbc.M609060200. [DOI] [PubMed] [Google Scholar]
- 10.Card J.W., Zeldin D.C. Hormonal influences on lung function and response to environmental agents: Lessons from animal models of respiratory disease. Proc Am Thor Soc. 2009 doi: 10.1513/pats.200904-020RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provost P.R., Blomquist C.H., Drolet R., Flamand N., Tremblay Y. Androgen inactivation in human lung fibroblasts: Variations in levels of 17β-hydroxysteroid dehydrogenase type 2 and 5α-reductase activity compatible with androgen inactivation. J Clin Endocrinol Metab. 2002 doi: 10.1210/jcem.87.8.8764. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad S Ahmad A. Epithelial Regeneration and Lung Stem Cells. In: V S, M K, editors. Lung Epithelial Biology in the Pathogenesis of Pulmonary Disease. Elsevier Inc.; 2017.
- 13.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.