To the Editor:
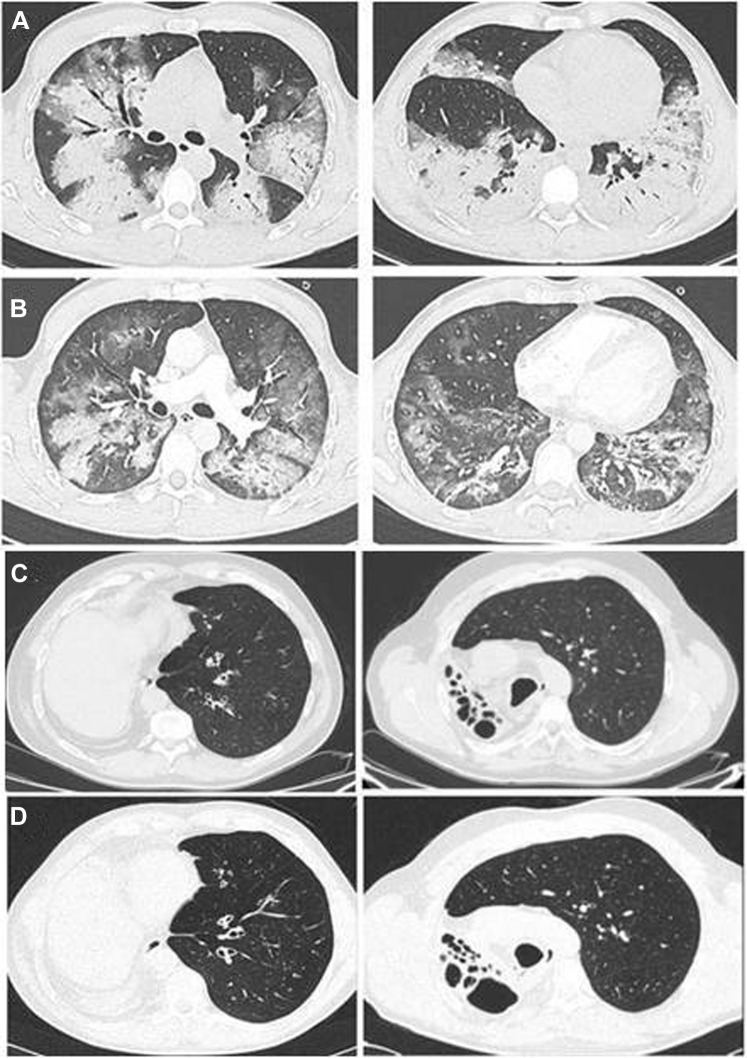
An epidemic of coronavirus SARS-CoV-2 has become the focus of scientific attention.1 The high infectivity of SARS-CoV-2 and rapid rise in the number of patients affected reflects the lack of preexisting immunity as reported by the World Health Organization (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). The clinical presentation of coronavirus disease 2019 (COVID-19) is variable, ranging from lack of symptoms to severe respiratory distress and multiorgan failure requiring intensive care unit admission and mechanical ventilation. Treatment of COVID-19 requires in-depth knowledge of the immune-mediated mechanisms of the disease. To date, we have identified 7 patients with primary antibody deficiencies (PADs) and COVID-19 infection: 5 had common variable immune deficiencies (CVIDs) and 2 had agammaglobulinemia (1 with X-linked agammaglobulinemia and 1 with autosomal recessive agammaglobulinemia).2 All of the patients with PADs had defective antibody production. Patients with agammaglobulinemia lack B lymphocytes, whereas patients with CVID have dysfunctional B lymphocytes. In patients with agammaglobulinemia, the COVID-19 course was characterized by mild symptoms, short duration, and no need for treatment with an immune-modulating drug blocking IL-6, and it had a favorable outcome. In contrast, patients with CVIDs presented with a severe form of the disease requiring treatment with multiple drugs, including antiretroviral agents and IL-6–blocking drugs, as well as mechanical ventilation (Table I ). The strikingly different clinical course of COVID-19 in patients with agammaglobulinemia compared with that in patients with CVIDs cannot be explained by the levels of serum immunoglobulins, which were similarly low in all patients with PADs at diagnosis and were maintained at adequate and comparable levels in all patients by immunoglobulin substitutive therapy (see Table E1 in this article’s Online Repository at www.jacionline.org). A detailed COVID-19 clinical history, laboratory data, type and dosage of administered treatment, and disease timing are provided for each patient in Case Reports in this article’s Online Repository (at www.jacionline.org). The lung high-resolution computed tomography (HRCT) of a patient with CVID at hospital admission for COVID-19 showed extensive ground glass opacities associated with areas of alveolar consolidation in the upper and lower lobes, with the alveolar component predominating over the interstitial component. (Fig 1 , A). On treatment, the lung HRCT showed a reduction in the extent of ground glass opacities and areas of alveolar consolidation. (Fig 1, B). In contrast, the lung HRCT of a patient with agammaglobulinemia performed at the time of COVID-19 was unchanged from lung HRCT performed 1 year earlier and showed bronchiectasis and sequelae of a right lung pneumonectomy done when the patient was 18 years old (Fig 1, C and D). All patients with PADs are equally vulnerable to most bacterial infections because antibodies are important in blocking infectivity and preventing diseases. In addition, antibodies have a role in the immune response to viral infections.3 Patients with agammaglobulinemia are susceptible to a limited number of viral infections only—mainly norovirus and enteroviruses such as polioviruses,4 with an increased incidence of postvaccination poliomyelitis due to the oral attenuated Sabin vaccine.5 CVIDs patients are susceptible to rhinoviruses, noroviruses, and herpesviruses that in turn play a role in driving an underlying inflammatory condition. Because only patients with agammaglobulinemia had a mild course of COVID-19, we speculate on a possible role of B lymphocytes in the SARS-CoV-2–induced inflammation. We have already shown that children appear to better contain SARS-CoV-2 in the early phase of infection, possibly because their B cells are able to generate natural antibodies in a timely manner on encounter with novel pathogens when compared with B cells from adults.6 The role of inflammation in aggravating the clinical picture of subjects with COVID-19 has already been described. Treatment with drugs such as IL-6 inhibitors aimed at reducing the cytokine storm syndrome and lung inflammation associated with a profound increase in level of cytokines such as IL-6 and increased level of ferritin7 have already been carried out, initially on an individual basis and currently within clinical trials. Of note, our patients with CVID who required IL-6–blocking treatment (3 of 5) presented with increased serum ferritin levels (see the Online Repository). It has been demonstrated that B cells produce IL-6 to drive germinal center formation. In patients unable to carry on the physiologic immune response, IL-6 produced by B cells may increase the level of inflammation. Lack of B-cell–derived IL-6 abrogates spontaneous autoimmune germinal center formation in a mouse model, resulting in protection from systemic autoimmunity.8 Thus, it appears that cytokine storm syndrome may play a significant role in the respiratory failure in COVID-19 infection. The role of B cells in determining lung inflammatory disorders is also demonstrated by the observation that granulomatous-lymphocytic interstitial lung disease, which occurs in 10% of patients with CVID, can be treated with B-cell–depleting drugs.9 COVID-19 treatments might contemplate the possibility of dampening the inflammatory functions of B cells and blocking cytokine production by monocytes and dendritic cells. Our data represent the first description of COVID-19 in patients affected with primary antibody defects, offer useful insights to the putative mechanisms underlying the immunologic response to the infection, and suggest possible clues to novel therapeutic targets.
Table I.
Summary of data for the 7 patients with PAD and COVID-19
| Patient No. | PAD | Age (y) | Sex | COVID-19 |
||||
|---|---|---|---|---|---|---|---|---|
| Clinical symptoms | Days | Treatment | ICU | Outcome | ||||
| 1 | ARA | 56 | M | No symptoms | 0 | Hydroxychloroquine, azithromycin, darunavir/cobicistat | No | Recovery |
| 2 | XLA | 34 | M | High fever | 3 | Hydroxychloroquine, ceftriaxone, lopinavir/ritonavir | No | Recovery |
| 3 | CVID | 59 | F | High fever, dyspnea | 20 | Hydroxychloroquine, azithromycin, tocilizumab | Yes | Death |
| 4 | CVID | 32 | F | High fever, dyspnea | 16 | Hydroxychloroquine, darunavir/ritonavir, tocilizumab | No | Recovery |
| 5 | CVID | 57 | M | High fever, dyspnea | 25 | Hydroxychloroquine, lopinavir/ritonavir, remdesivir methylprednisolone. | Yes | Recovery |
| 6 | CVID | 52 | M | High fever, dyspnea | 21 | Hydroxychloroquine, azithromycin, lopinavir/ritonavir, | No | Recovery |
| 7 | CVID | 41 | M | High fever, dyspnea | 19 | Hydroxychloroquine, piperacillin/tazobactam, lopinavir/ritonavir, tocilizumab, remdesivir | Yes | Recovery |
ARA, Autosomal recessive agammaglobulinemia; F, female; ICU, intensive care unit; M, male; XLA, X-linked agammaglobulinemia.
Fig. 1.
Lung HRCT in a patient with CVID at admission, showing extensive ground glass opacities associated with areas of alveolar consolidation in the lower lobes, where the alveolar component predominates over the interstitial component (A [left, mid-upper; right: lower), and after treatment, showing reduction in extent of ground glass opacities and areas of alveolar consolidation (B [left, mid-upper; right, lower]). Lung HRCT in a patient with agammaglobulinemia. Axial sections showing bronchiectasis and sequelae of right lung pneumonectomy (in March 2020 [C] and January 2019 [D]).
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Case Reports
Patient 1
The patient was a 53-year-old male with a diagnosis of agammaglobulinemia at the age of 18 years (in 1985, which was 2 years after a right lung upper lobectomy for bronchiectasis). Since 1985, he has received intravenous immunoglobulin replacement at a cumulative monthly dose of 600 mg/kg. In 2005, a diagnosis of autosomal recessive agammaglobulinemia was made, after exclusion of Bruton tyrosine kinase mutation. As in most patients with autosomal recessive agammaglobulinemia, we did not find any other mutation, including μ-chain mutation. Treatment notwithstanding, he had chronic sinusitis, chronic obstructive pulmonary disease, and bilateral lung bronchiectasis, which progressively worsened. In 2019, therapy was shifted to facilitated subcutaneous immunoglobulins. On March 12, after 2 SARS-CoV-2 infections among family members were ascertained, an oropharyngeal swab was obtained from the patient and tested positive for SARS-CoV-2. At that time, the patient was completely asymptomatic. The contact was likely his wife, who was presumably infected on February 29 by a relative from Milan (an Italian high-risk area), and who in turn infected both her mother (who died on March 20) and the patient. His wife developed anti–SARS-Cov-2 antibodies (according to the testing with the Chemtrue, Novel Coronavirus, 2019-nCoV IgM/IgG Antibody Diagnostic Kit, SIC, Rome, Italy) 15 days after a mild fever with anosmia. The patient never presented with any clinical symptoms of COVID-19, and 1 week after the first swab, a repeated nasopharyngeal swab tested negative. The patient's lymphocyte count stayed within the normal range (1600 cells/mm3), as did his serum C-reactive protein (CRP) (1 mg/L [normal range, <5]), lactate dehydrogenase (LDH) (143 U/L), and IL-6 (1 ng/L) levels. The patient's lung HRCT scan performed on the same day was unchanged with respect to the lung HRCT performed 1 year earlier (Fig 1, A and B). Since March 26, he has been receiving hydroxychloroquine (200 mg twice a day), azithromycin (500 mg once a day), and darunavir/cobicistat (800/150 mg once a day).
Patient 2
The patient was a 34-year-old male with a diagnosis of X-linked agammaglobulinemia established when he was 1 year old. He has since been receiving immunoglobulin replacement treatment at 400 mg/kg per dose every 3 weeks. His clinical history includes recurrent skin and respiratory infections. He developed bronchiectasis at the age of 16 years. Four months before the diagnosis of COVID-19, his differential blood count was normal. On March 13, he presented with fever (temperature >38°C). At home, he received paracetamol, ibuprofen, amoxicillin/clavulanic acid, and levofloxacin. On March 19, he was admitted to the emergency room (ER). The patient did not show respiratory difficulty, and his pulse oxygen saturation was 98%. His initial blood work-up showed mild leukopenia (3840 cells/mm3) and a slightly elevated CRP level (26 mg/L [normal value <5.0]). His chest x-ray showed mild interstitial alveolar infiltrates. His oropharyngeal swab tested positive for SARS-CoV-2. He started receiving lopinavir/ritonavir 200/50 mg, 2 capsules twice a day), hydroxychloroquine (200 mg twice a day), and ceftriaxone (because of a previous allergic reaction to azithromycin). After 2 days, the patient's fever resolved, and he was discharged on March 27.
Patient 3
The patient was a 59-year-old female with a diagnosis of CVID. When she was 48 years old, low serum IgG, IgA and IgM levels were first detected after the occurrence of upper and lower respiratory tract infections; a diagnosis of CVID was made after exclusion of secondary forms of hypogammaglobinemia. Since then, she has been receiving immunoglobulin replacement with intravenous immunoglobulin at a cumulative monthly dose of 400 mg/kg. With time, she has progressively developed chronic sinusitis, chronic bronchitis, and chronic gastritis. In 2009, she shifted to self-administered subcutaneous immunoglobulins. The patient tested positive for SARS-CoV-2 according to a nasopharyngeal swab obtained on March 8, when she was hospitalized because of high fever and dyspnea. The contact was not identified, as her husband and her son became positive 5 days later. Endotracheal intubation was performed in the intensive care unit, and therapy with tocilizumab (8 mg/kg per day) was started; however, she never improved. She died on March 25.
Patient 4
The patient was a 32-year-old female with a diagnosis of CVID established when she was 14 years old, at which time low serum IgG, IgA, and IgM levels were first detected in a routine assessment. A diagnosis of CVID was made after exclusion of secondary forms of hypogammaglobinemia. She refused to begin immunoglobulin replacement. She was never diagnosed with respiratory infections; her clinical history included endometriosis, celiac-like disease, allergy, and skin cancer (melanoma). On March 10, she developed cough and mild fever (temperature ranging from 37.3°C to 38°C). She went to the ER, where nasopharyngeal and oropharyngeal swabs tested negative for SARS-CoV-2. Nine days later (on March 19), her cough and fever worsened and dyspnea and chest pain appeared. She went to the ER again, and a new nasopharyngeal and oropharyngeal swabs tested positive for SARS-CoV-2. Despite respiratory symptoms, her room air oxygen saturation was fair (95%) and she was allowed to go home. After 4 more days (on March 23), her condition worsened, with a high fever (temperature of 39°C) and room air oxygen saturation of 80%. She was admitted to the infectious diseases department. Laboratory tests showed leukopenia without lymphopenia (white blood cell count, 3200 cells/mm3), lymphocyte count of 1480 cells/mm3, CRP level of 7.5 mg/L (normal value <5), ferritin level of 4000 μg/L, and LDH level of 270 U/L. She started receiving oxygen supplementation by O2 mask at a rate of 4 liters per hour. A lung HRCT showed interstitial pneumonia. On day 1, she started therapy with darunavir (800 mg once a day) plus ritonavir (100 mg once a day) for 6 days and hydroxychloroquine (200 mg twice a day) for 6 days. On day 2, administration of intravenous tocilizumab was started (8 mg/kg per day) after a high IL-6 level (10.6 ng/L) was detected. On day 3, her laboratory tests were as follows: white blood cell count, 3600 cells/mm3; lymphocyte count, 1670 cells/mm3; CRP level, 3.9 mg/L; and LDH level, 196 U/L. Her fever abated (her temperature decreased to 37.3°C), and her oxygen saturation increased to 98% (while she was receiving supplemental oxygen at a rate of 4 liters per hour). On day 4, the patient was afebrile, although a nasopharyngeal swab still tested positive for SARS-CoV-2. Her clinical condition further improved, with a reduction in cough, as well as a reduction in respiratory and heart rate. Her oxygen saturation stayed at 98%. As of March 27, she had received 2 doses of tocilizumab.
Patient 5
The patient was a 57-year-old male with a diagnosis of CVID. At the age of 49 years, the patient underwent laboratory investigations because of recurrent upper and lower respiratory tract infections. Low serum IgG, IgA, and IgM levels were then detected, and a diagnosis of CVID was made after exclusion of secondary forms of hypogammaglobinemia. His clinical history included asthma, hypertension, and overweight/obesity (weight, 106 kg; body mass index, 26.77). Since diagnosis, he has begun receiving subcutaneous immunoglobulins at a cumulative monthly dose of 400 mg/kg. The asthma treatment included oral steroids, antihypertensive drugs (calcium antagonist, telmisartan, and ticlopidine), inhalation treatment with steroids, and long-acting β-agonists (fluticasone and salmeterol). On February 21, he developed dry cough, mild-to-moderate fever (temperature ranging from 37.5°C to 38.5°C), and myalgia. His general practitioner ordered levofloxacin (750 mg per day). On February 24, mild dyspnea appeared. On February 26, because of worsening dyspnea, he was admitted to the ER, where nasopharyngeal and oropharyngeal swabs tested positive for SARS-CoV-2. His chest x-ray showed initial evidence of interstitial pneumonia and 2 opacities that were interpreted as alveolar consolidation. The initial laboratory investigations revealed lymphopenia (lymphocyte count, 620 cells/mm3), a CRP level of 120 mg/dL, and an LDH level of 266 U/L. He immediately began receiving high-flow nasal oxygen supplementation and methylprednisolone (80 mg per day intravenously). On February 27, therapy with lopinavir plus ritonavir and hydroxychloroquine (200 mg twice a day) was started. On February 29, laboratory tests showed a decreased lymphocyte count (370 cells/mm3), a CRP level of 29 mg/dL, and an LDH level of 335 U/L. On March 1, his condition worsened, his blood oxygen saturation dropped below 85%, and he underwent endotracheal intubation and was transferred to the intensive care unit. His antiviral treatment was changed to remdesivir (200 mg intravenously once a day) (on the first day) followed by remdesivir (100 mg intravenously once a day) while he continued receiving methylprednisolone (0.8 mg/kg). His bronchoalveolar lavage fluid tested positive for β-d-glucan and Candida. Administration of vancomycin, meropenem, linezolid, caspofungin, and cotrimoxazole was started. On March 3, laboratory tests showed lymphopenia (300 cells/mm3), a CRP level of 120 mg/dL, and an IL-6 level of 11.8 mg/dL. On March 12, his clinical condition appeared markedly improved as his lymphocyte count returned to normal (1300 cells/mm3). He was then extubated and began undergoing noninvasive ventilation, which was maintained until March 17. On March 17, his laboratory investigations showed the following: lymphocyte count, 1940 cells/mm3; LDH level, 189 U/L; and CRP level, 3.8 mg/dL. On March 26, he was discharged in good clinical condition after a nasopharyngeal swab tested negative for SARS-CoV-2. He is currently undergoing home treatment with prednisone (25 mg per day) and subcutaneously injected immunoglobulins.
Patient 6
The patient was a 52-year-old male with a diagnosis of CVID made in 1996. His clinical history included immune thrombocytopenia, polyclonal lymphoproliferation (polydistrectual lymphadenopathy and splenomegaly), recurrent infections (giardiasis and pneumonia due to Haemophilus influenzae), and interstitial lung disease (HRCT finding of macronodules, ground glass opacities, mediastinal lymphadenopathies, and a negative Mycobacterium screening result) with maintained lung function. Since diagnosis, he has begun receiving subcutaneous immunoglobulins at a cumulative monthly dose of 400 mg/kg. On March 12, the patient developed fever (maximum temperature 39.2°C) and a mild exercise-induced dyspnea. One day later, his wife and 1 of his 2 daughters showed milder general symptoms (remittent fever without cough or dyspnea). According to the current Italian guidelines for the management of the COVID-19 epidemic, because symptoms were still present 6 days from their appearance, the patient’s general practitioner arranged for the patient admission to the infectious disease unit appointed to perform the emergency nasopharyngeal swab for SARS-CoV-2 nucleic acid detection and a lung HRCT. The patient's nasopharyngeal swab tested positive for SARS-CoV-2, and his lung HRCT showed a bilateral interstitial pneumonia. Therapy with lopinavir/ritonavir (400/100 mg once a day), azithromycin (500 mg once a day), and hydroxychloroquine (200 mg twice a day) was started. No oxygen supplementation was required during the course of the disease, as his peripheral oxygen saturation was constantly above 90%. The patient's fever and dyspnea completely resolved 5 days after the beginning of the treatment. A new nasopharyngeal swab obtained 9 days after the beginning of therapy tested negative, and no plasma viral replication was detected. As significant improvement of the patient's interstitial pneumonia was documented, he was discharged and a 14-day period of home isolation was ordered.
Patient 7
The patient was a 41-year-old male with a diagnosis of CVID established when he was 14 year old. Secondary causes of hypogammaglobulinemia were excluded. During childhood, he suffered from recurrent respiratory infections and measles-associated pneumonia. His clinical history was complicated by recurrent sinusitis and mild eczema. The patient received immunoglobulin replacement treatment at a rate of 400 mg/kg per dose every 4 weeks with intravenous immunoglobulins administered until 2017, when he switched to facilitated subcutaneous preparations. On March 8, the patient presented with high fever, cough, and dyspnea. At home he received paracetamol, ibuprofen, and amoxicillin/clavulanic acid. On March 16, as his condition deteriorated, he was admitted to the ER. His pulse oxygen saturation was 80%, and he began undergoing noninvasive ventilation with continuous positive airway pressure. His initial blood work-up showed lymphopenia (800 cells/mm3) with an elevated CRP level (315 mg/L [normal value <5.0]). A chest x-ray showed diffuse interstitial alveolar infiltrates. Lung HRCT at admission confirmed extensive infiltrates (Fig 1, A). An oropharyngeal swab tested positive for SARS-CoV-2. He started receiving lopinavir/ritonavir (400/100 mg once a day), hydroxychloroquine (200 mg twice a day), and piperacillin/tazobactam. After admission, his respiratory condition worsened dramatically and he was placed on mechanical ventilation. Laboratory tests showed an increased ferritin level (7200 μg/L [normal value <400]), and increased serum LDH level (495 U/L [normal value <225]). Therapy with tocilizumab (8 mg/kg per day) was started. After 2 days of mechanical ventilation, the patient was switched to remdesivir (200 mg intravenously once a day) (on the first day) followed by remdesivir (100 mg intravenously once a day). His clinical condition and lung HRCT improved (Fig 1, B), and 72 hours later he did not require any more mechanical ventilation. He is still hospitalized, with steady improvement of his clinical condition and laboratory values.
Table E1.
Summary of immunologic data of the 7 patients with PAD collected 1 to 6 months before COVID-19 infection
| Patient No. | Date of last investigation | IgG level (mg/dL) | IgA level (mg/dL) | IgM level (mg/dL) | Lymphocyte count (mm3) | CD19 cell count (mm3) | CD3 cell count (mm3) | CD4 cell count (mm3) | CD8 cell count (mm3) | NK cell count (mm3) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | January 2020 | 750 | 0 | 0 | 1300 | 0 | 1247 | 460 | 739 | 25 |
| 2 | November 2019 | 800 | 0 | 0 | 1700 | 0 | 1600 | 900 | 700 | 28 |
| 3 | January 2020 | 897 | 30 | 33 | 1600 | 400 | 1030 | 672 | 338 | 46 |
| 4 | January 2020 | 500 | 0 | 153 | 2050 | 200 | 1800 | 950 | 850 | 30 |
| 5 | October 2019 | 550 | 40 | 44 | 3400 | 96 | 3200 | 2767 | 1658 | 21 |
| 6 | December 2019 | 662 | 11 | 8 | 890 | 55 | 750 | 274 | 258 | 85 |
| 7 | September 2019 | 700 | 10 | 30 | 1800 | 278 | 1500 | 800 | 700 | 15 |
NK, Natural killer.
References
- 1.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - navigating the uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidel M.G., Kindle G., Gathmann B., Quinti I., Buckland M., van Montfrans J. The European Society for Immunodeficiencies (ESID) Registry working definitions for the clinical diagnosis of inborn errors of immunity. J Allergy Clin Immunol Pract. 2019;7:1763–1770. doi: 10.1016/j.jaip.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Burton D.R. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 4.Jones T.P.W., Buckland M., Breuer J., Lowe D.M. Viral infection in primary antibody deficiency syndromes. Rev Med Virol. 2019;29:e2049. doi: 10.1002/rmv.2049. [DOI] [PubMed] [Google Scholar]
- 5.Lougaris V., Soresina A., Baronio M., Montin D., Martino S., Signa S. Long term follow-up of 168 patients with X-linked agammaglobulinemia reveals increased morbidity and mortality. J Allergy Clin Immunol. 2020;6749:30330–30334. doi: 10.1016/j.jaci.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Grimsholm O., Piano Mortari E., Davydov A.N., Shugay M., Obraztsova A.S., Bocci C. The interplay between CD27dull and CD27bright B cells ensures the flexibility, stability, and resilience of human B cell memory. Cell Rep. 2020;30:2963–2977.e6. doi: 10.1016/j.celrep.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Cron R.Q., Chatham W.W. The rheumatologist's role in Covid-19. J Rheumatol. 2020 Mar 24 doi: 10.3899/jrheum.200334. [Epub ahead of print]. https://doi.org/10.3899/jrheum.200334. [DOI] [PubMed] [Google Scholar]
- 8.Arkatkar T., Du S.W., Jacobs H.M., Dam E.M., Hou B., Buckner J.H. B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J Exp Med. 2017;214:3207–3217. doi: 10.1084/jem.20170580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pecoraro A., Crescenzi L., Galdiero M.R., Marone G., Rivellese F., Rossi F.W. Immunosuppressive therapy with rituximab in common variable immunodeficiency. Clin Mol Allergy. 2019;17:9. doi: 10.1186/s12948-019-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]