Abstract
Alarming situation has been caused due to the emergence of COVID-19 infection around the world. There is an urgency of developing a therapeutic strategy in order to control the spread of COVID-19. Towards that initiative, potential drugs like hydroxychloroquine, ivermectin and azithromycin have been tested by diverse group of researchers worldwide for their potential against novel coronavirus. The present report presents together the comprehensive knowledge derived from the major researches about the above drugs altogether in context of the current health emergency around the world. Hydroxychloroquine and ivermectin were known to act by creating the acidic environment and inhibiting the importin (IMPα/β1) mediated viral import. Azithromycin was found to act similar to the hydroxychloroquine as an acidotropic lipophilic weak base. All the three categories of drugs seemed to potentially act against novel coronavirus infection. However, their efficacies need to be studied in detail individually and in combination in-vivo in order to combat COVID-19 infection.
Keywords: Azithromycin, coronavirus, COVID-19, hydroxychloroquine, ivermectin
Introduction
COVID-19 infection has become pandemic after its first outbreak in Wuhan, China in 2019. This infection is caused by novel SARS-CoV-2 virus, which is distinct from its related type (SARS-CoV). The World Health Organization (WHO) has termed this disease as COVID-19 (Coronavirus disease-2019). Upto 09thApril 2020, 1,496,055 cases have been confirmed globally with 89,435 deaths while 336,780 people got recovered across the world. The most number of infected cases were from United States (432,438 confirmed cases, 14,808 deaths and 24,125 recovery of the patients) followed by Spain (152,446 confirmed cases, 15,238 deaths and 52,165 recovery of the patients), Italy (139,422 confirmed cases, 17,669 deaths and 26,491 recovery of the patients), Germany (113,296 confirmed cases, 2349 deaths and 46,300 recovery of the patients), France (83,080 confirmed cases, 10,887 deaths and 21,461 recovery of the patients) and China (82,883 confirmed cases, 3339 deaths and 77,678 recovery of the patients) (Fig. 1), latter being the first epicentre of this disease. This data suggests that infection due to COVID-19 is spreading exponentially in new hotspots as compared to the first epicentre Wuhan, where new cases are in constant decline [1]. At this point of time, there is an urgent need of therapeutic strategy in order to control the spread of COVID-19 as a disease. Numerous researchers across the world are working on finding the cure and chemoprophylaxis of this disease with many of them are even putting their efforts to develop the vaccine. Recently some of the reports on Hydroxychloroquine [[2], [3], [4]], Ivermectin [5] and Azithromycin [6], have shown therapeutic effects against novel coronavirus infection. However, it was not reported that which drug has better efficacy in comparison to other or a combination of them can give life saving results. Therefore, the present report has been able to provide the comprehensive view of combining the knowledge of these drugs altogether in the context of current health emergency around the world.
Fig. 1.
Data obtained from coronavirus resource centre of John Hopkins University of Medicine (1).
Hydroxychloroquine
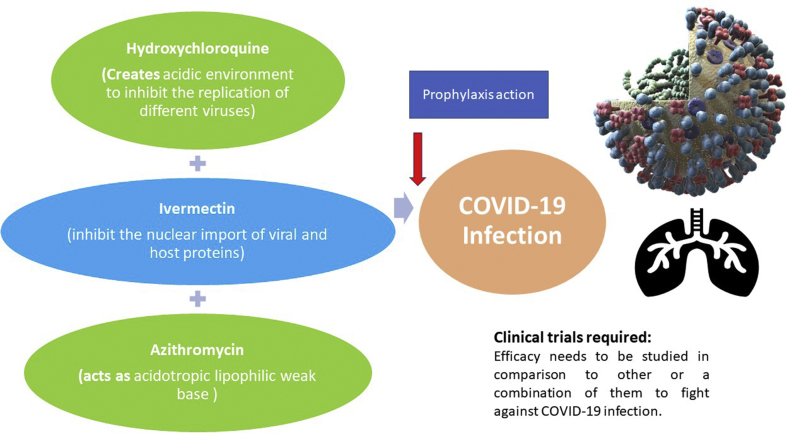
The first category of drug was Chloroquine and its safer derivative hydroxychloroquine which may act as a therapeutic agent against COVID-19 infection. Earlier both have been used widely for the treatment of rheumatoid arthritis and systemic lupus erythematosus. Chloroquine was initially used for the treatment of malaria, but Plasmodium falciparum substantially developed resistance against it. With the subsequent development of new antimalarials, this drug is now being used for the prophylaxis of malaria. In 1946, by the introduction of hydroxyl group into chloroquine, a derivative was produced known as Hydroxychloroquine and was found to have less acute poisoning than the former one [7]. Both the drugs otherwise, share a similar mechanism of action and structure. These drugs tend to increase the pH within intracellular vacuoles and act as weak base. In addition, they are known to alter processes such as protein degradation by acidic hydrolases in the lysosome, assembly of macromolecules in the endosomes, and post translation modification of proteins in the Golgi apparatus [8]. Over the past few decades this drug has received wider attention, as a potential antiviral drug. Chang and his colleagues in 2014 revealed that hydroxychloroquine activates the host anti-viral innate immunity [9]. This drug accumulates in the cellular organelles creating acidic environment to inhibit the replication of different viruses by interfering with endosome/lysosome trafficking or viral protein maturation during virions maturation (Fig. 2). During the recent pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), hydroxychloroquine was found to act as a potential drug in fighting against COVID-19. Some of the in vitro and poorly controlled or uncontrolled clinical trials revealed that this drug has activity against severe acute respiratory syndrome–coronavirus 2 (SARS–CoV-2) [[2], [3], [4]]. In China, clinical trials of hydroxychloroquine are further in-progress for the treatment and management of COVID-19 disease (NCT04261517 and NCT04307693).
Fig. 2.
Illustration showing each drug mode of action against COVID-19 infection.
Ivermectin
Another study revealed that ivermectin which is a broad spectrum anti-parasitic drug demonstrated its efficacy against COVID-19 which comes as a second line of drug [5]. Ivermectin is FDA approved drug, known to have wide-spectrum antiviral activity against number of viruses under in vitro conditions [[10], [11], [12], [13]]. SARS-CoV-2 (causative agent of COVID-19) is a single stranded RNA virus (positive sense) which is closely related to SARS coronavirus (SARS-CoV). Recent study on ivermectin against SARS-CoV-2 under in vitro conditions revealed that it can inhibit the viral replication. The single treatment of this drug was able to reduce the virus up to 5000-fold in culture within 48h. However, no further reduction was reported with further increase in time period i.e up to 72h. Moreover, no toxicity was seen with the drug at any point of time [5]. Mechanism by which ivermectin responded against the CoV-19 virus is not known and was believed to be working similarly as it acted on other viruses. It was known to inhibit the nuclear import of viral and host proteins. Integrase protein of viruses and the importin (IMP) α/β1 heterodimer was responsible for IN nuclear import which further increases the infection (Fig. 2). As most of the RNA viruses are dependent upon IMPα/β1 during infection, Ivermectin acts on it and inhibits the import with the increase in antiviral response [5, 14].
Azithromycin
Third category of therapeutic drug is Azithromycin, which is a class of antibiotics known as macrolide, used to treat infections like bronchitis, pneumonia and MAC (Mycobacterium avium complex) infection. With the spread of the SARS-CoV-2 viral pneumonia, which started in Wuhan, China, many countries of the world started developing countermeasures in order to decrease the spread of the disease. Researchers found that apart from hydroxychloroquine, another FDA approved drug known as Azithromycin was shown to have therapeutic effects against COVID-19 in a study done by a research group at New Mexico University. The researchers were able to prove that azithromycin acted as an acidotropic lipophilic weak base which modulate the pH of endosomes and trans-Golgi network (Fig. 2). This further led to in vitro effects on intracellular organelles similar to the one as conferred by hydroxychloroquine [6]. This further indicates that this antimicrobial drug has an immense therapeutic value as far as the treatment of COVID-19 patients is concerned. Clinical trials need to be carried out with this drug as it can act as a prophylaxis for declining the infection rate.
Challenges
These drugs have been shown to have a potential broad-spectrum antiviral response in vitro against many viruses including coronaviruses. However, hydroxychloroquine has been found to be associated with dangerous side-effects in the past if the dosage is not carefully controlled. There have been many cases of chloroquine poisoning reported in Nigeria and the USA as well. Due to sudden rapid increase in demand of the above broad-spectrum drugs, may lead to significant shortage for patients who rely on these drugs to treat their other disorders like malaria, rheumatoid arthritis among others. Therefore, adequate buffer stocks should be maintained for these drugs to overcome potential scarcity of the above-mentioned drugs.
Future perspectives
All three categories of drugs seem to act against novel coronavirus infection. However, further research using large cohort of samples with randomized controlled clinical trials are urgently required for each drug alone and in-combination in order to find a concrete solution against COVID-19 infection.
Conflict of interest
The author has no conflict of interest to declare.
Acknowledgements
The authors express sincere thanks to the Chancellor, Maharishi Markandeshwar deemed to be University, Mullana for providing necessary facilities for writing on pandemic research.
Contributor Information
R. Choudhary, Email: rc_07@live.in.
A.K. Sharma, Email: anibiotech18@gmail.com.
References
- 1.Center J.H.C.R. https://coronavirus.jhu.edu/map.html
- 2.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.X Yao, F Ye, M Zhang, C Cui, B Huang, P Niu. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautret P., Lagier J.C., Parola P., Meddeb L., Mailhe M., Doudier B. Hydroxychloroquine and azithromycin as a treatment of COVID19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:n105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 3 April 2020:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poschet J.F., Perkett E.A., Timmins G.S., Deretic Vojo. Azithromycin and ciprofloxacin have a chloroquine-like effect on respiratory epithelial cells. bioRxiv. 2020;3(29) doi: 10.1101/2020.03.29.008631. [DOI] [Google Scholar]
- 7.Weniger H. Review of side effects and toxicity of chloroquine. Bull World Health. 1979;79:906. [Google Scholar]
- 8.Fox R.I. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum. 1993;23(2):82–91. doi: 10.1016/s0049-0172(10)80012-5. [DOI] [PubMed] [Google Scholar]
- 9.Chang T.H., Wang L.F., Lin Y.S., Yang C.S., Yu C.Y., Lin Y.L. Hydroxychloroquine activates host antiviral innate immunity. Cytokine. 2014;70(1):33–34. [Google Scholar]
- 10.Azeem S., Ashraf M., Rasheed M.A., Anjum A.A., Hameed R. Evaluation of cytotoxicity and antiviral activity of ivermectin against Newcastle disease virus. Pak J Pharm Sci. 2015;28(2):597–602. [PubMed] [Google Scholar]
- 11.Mastrangelo E., Pezzullo M., De Burghgraeve T., Kaptein S., Pastorino B., Dallmeier K. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemother. 2012;67(8):1884–1894. doi: 10.1093/jac/dks147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Götz V., Magar L., Dornfeld D., Giese S., Pohlmann A., Höper D. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci Rep. 2016;6:23138. doi: 10.1038/srep23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundberg L., Pinkham C., Baer A., Amaya M., Narayanan A., Wagstaff K.M. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication. Antivir Res. 2013;100(3):662–672. doi: 10.1016/j.antiviral.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Jans D.A., Martin A.J., Wagstaff K.M. Inhibitors of nuclear transport. Curr Opin Cell Biol. 2019;58:50–60. doi: 10.1016/j.ceb.2019.01.001. [DOI] [PubMed] [Google Scholar]