Abstract
A novel virus named 2019 novel coronavirus (2019-nCoV/SARS-CoV-2) causes symptoms that are classified as coronavirus disease (COVID-19). Respiratory conditions are extensively described among more serious cases of COVID-19, and the onset of acute respiratory distress syndrome (ARDS) is one of the hallmark features of critical COVID-19 cases. ARDS can be directly life-threatening because it is associated with low blood oxygenation levels and can result in organ failure. There are no generally recognized effective treatments for COVID-19, but treatments are urgently needed. Anti-viral medications and vaccines are in the early developmental stages and may take many months or even years to fully develop. At present, management of COVID-19 with respiratory and ventilator support are standard therapeutic treatments, but unfortunately such treatments are associated with high mortality rates. Therefore, it is imperative to consider novel new therapeutic interventions to treat/ameliorate respiratory conditions associated with COVID-19. Alternate treatment strategies utilizing clinically available treatments such as hyperbaric oxygen therapy (HBOT), packed red blood cell (pRBC) transfusions, or erthropoiesis-stimulating agent (ESA) therapy were hypothesized to increase oxygenation of tissues by alternative means than standard respiratory and ventilator treatments. It was also revealed that alternative treatments currently being considered for COVID-19 such as chloroquine and hydroxychloroquine by increasing hemoglobin production and increasing hemoglobin availability for oxygen binding and acetazolamine (for the treatment of altitude sickness) by causing hyperventilation with associated increasing levels of oxygen and decreasing levels of carbon dioxide in the blood may significantly ameliorate COVID-19 respiratory symptoms. In conclusion, is recommend, given HBOT, pRBC, and ESA therapies are currently available and routinely utilized in the treatment of other conditions, that such therapies be tried among COVID-19 patients with serious respiratory conditions and that future controlled-clinical trials explore the potential usefulness of such treatments among COVID-19 patients with respiratory conditions.
Keywords: 2019-nCoV, EPO, Pulmonary, SARS-CoV-2
Introduction
A novel virus named 2019 novel coronavirus (2019-nCoV/SARS-CoV-2) is the cause of a syndrome of symptoms that are classified as coronavirus disease (COVID-19) [1]. COVID-19 was first described among a case-series of patients that visited a local market in the Chinese city of Wuhan in December 2019 and the virus was first isolated on 7 January 2020 [2]. Since then, COVID-19 has spread around the world with the most recent estimates, as of 10 April 2020 revealing that there are currently 1,631,310 confirmed cases and 98,400 deaths [3].
A recently published meta-analysis examined the frequency and symptoms of COVID-19 in humans [4]. These investigators described that among the most common COVID-19 symptoms were fever (82%), cough (61%), muscle aches/fatigue (36%), dyspnea (26%), headache (12%), sore throat (10%), and gastrointestinal symptoms (9%).
In addition to the aforementioned common clinical symptoms of COVID-19, these investigators described detailed chest imaging results [4]. Among those with chest radiologic examinations, the most common abnormalities were opacities (bilateral or unilateral, with or without pleural effusion), multiple ground-glass opacities, and infiltrate. Among those undergoing computer tomography (CT) scans, the most common abnormalities observed were ground-glass opacities (accompanied or not by septal thickening), infiltration abnormalities, and parenchymal consolidation. Only a small number of persons were observed to have normal chest radiographical or CT findings. Other investigators described that radiological examinations revealed ground-glass opacities in up to 86% of COVID-19 patients with 76% of COVID-19 patients presenting with bilateral distribution and 33% peripheral distribution [5]. Interestingly, COVID-19 patients were not observed to present with lung cavitations, discrete pulmonary nodules, pleural effusions, or lymphadenopathy [6]. Finally, COVID-19 patients undergoing autopsy showed bilateral diffuse alveolar damage associated with pulmonary edema, pro-inflammatory concentrates, and indications of early-phase acute respiratory distress syndrome (ARDS) [7].
Clinical examination of severe cases of COVID-19 revealed a decreased ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2:FiO2 ratio) with concomitant hypoxia and tachypnea [8]. In addition, investigators have described low carbon dioxide (CO2) carbon dioxide levels in COVID-19 as the median partial pressure of carbon dioxide (PaCO2) level was 34 mmHg [9]. In short, hypoxia and hypocapnia are seen in severe COVID-19 cases.
It was even postulated recently, based upon analyzing clinical data reported in published studies, that there was a striking similarity between high altitude pulmonary edema (HAPE) as manifested during the acute hypoxic ventilatory response and COVID-19 [10]. This researcher observed the following similarities: arterial oxygen partial pressure to fractional inspired oxygen ratio (decreased), hypoxia (present), tachypnea (increased), partial pressure of carbon dioxide level (decreased), ground glass opacities on chest CT (present), patchy infiltrates on chest x-ray (present), fibrinogen levels/fibrin formation (increased), alveolar comprise (present), and ARDS development in severe disease (present).
There are currently no generally recognized effective treatments for COVID-19, but are urgently needed given the breadth and scope of the disease. At present, anti-viral medications and vaccines are in the early developmental stages and may take many months or even years to fully develop [11]. As a result, it is imperative to consider novel new therapeutic interventions that do not necessarily cure the underlying disease, but, instead provide supportive care to help assist patients survive COVID-19, especially given its potential to induce respiratory conditions associated with significant mortality [12]. Respiratory conditions in COVID-19 are of such importance that it was described one of the hallmarks of a critical course of COVID-19 is the development of ARDS [13].
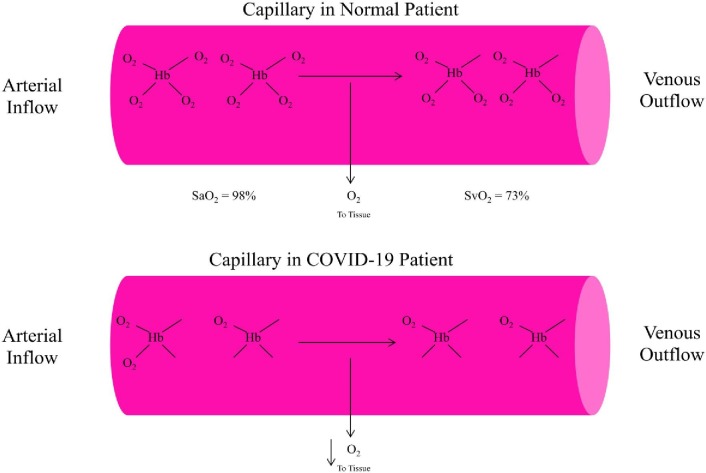
ARDS leads to low blood oxygenation levels and can be directly life-threatening because of the body's organs dependence upon adequately oxygenated blood. In order to more fully understand ARDS, it is important to consider how the lungs function. As one breathes, air is taken in through the nose and mouth and passes down the windpipe into alveoli within the lungs. It is in the capillaries that run through the alveoli that oxygen passes into the blood stream and is carried to all parts of the body. In ARDS, the capillaries within the lungs leak more fluid than normal into the alveoli and prevent the lungs from delivering enough oxygen into the blood stream. The consequence is that the body's organs work poorly or not at all. Fig. 1 summarizes the delivery of hemoglobin-based oxygen molecules to peripheral tissues in a normal patient's capillary and in a COVID-19 patient's capillary [14].
Fig. 1.
A summary of hemoglobin-based delivery of oxygen molecules to peripheral tissues in a normal patient's capillary and in a COVID-19 patient's capillary. Hb = hemoglobin; O2 = oxygen; SaO2 = arterial oxygen saturation; SvO2 = venous oxygen saturation. The oxygen tension of tissues will dedictate whether one of several oxygen molecules will be released from oxyhemoglobin (HbO2) and diffuse from the capillary (pink area) into the tissue. The fraction of HbO2 relative to total Hb in arterial blood is the SaO2, which is normally > 95%. Normal mixed SvO2 is about 65–75%. A decrease in SvO2 usually indicates low tissue oxygen tension, resulting in increased extraction of oxygen molecules from Hb.
As such, supportive management of COVID-19 with respiratory and ventilator support are standard therapeutic treatments [15]. A recent meta-analysis identified that the use of supplementary oxygen therapy (38.9%), invasive ventilation (28.7%), and even extracorporeal membrane oxygenation (ECMO) (0.9%) treatments were surprisingly high among 1,876 identified patients in which any kind of pharmacological and/or supportive intervention was reported [4].
Unfortunately, regarding the standard ventilator support of COVID-19 patients, it was observed in a cohort of patients admitted to an intensive care unit (ICU) that 56% were given non-invasive ventilation at ICU admission, of whom 76% required further orotracheal intubation and invasive mechanical ventilation. The ICU mortality rate among those who required non-invasive ventilation was 79% and among those who required invasive mechanical ventilation the morality rate was 86% [16].
The current standard respiratory treatments employed among COVID-19 patients are based upon long-established treatment protocols for viral pneumonia. Since, the pathology observed in COVID-19 patients is very unlike other viral pneumonias, it is understandable that the currently employed therapies are highly ineffective. In the following section, it is hypothesized that a series of alternative treatment strategies for treating respiratory conditions will significantly improve tissue oxygenation among COVID-19 patients.
Alternate treatment strategies for respiratory conditions in COVID-19
Hyperbaric oxygen therapy
Hyperbaric oxygen therapy (HBOT) is treatment designed to increase the oxygen level in the blood. The patient either sits or lies down in an enclosed chamber. Then the pressure is raised in the chamber and 100% oxygen is administered [17]. For HOBT, pressure is expressed in multiples of the atmospheric pressure at sea level, which is 1 atmosphere (1 atmosphere = 14.7 psi, 1 Kg per cm2, 101.3 kPa, 760 torr, or 760 mm Hg). At sea level the blood (plasma) oxygen concentration is 0.3 mL per deciliter [18], [19]. Tissues at rest extract 5 to 6 mL of oxygen per deciliter of blood, assuming normal perfusion [18], [20]. Administering 100 percent oxygen at ambient pressure increases the amount of oxygen dissolved in the blood 5-fold to 1.5 mL per deciliter, and at 3 atmospheres, the dissolved-oxygen content is approximately 6 mL per deciliter. This latter level is more than adequate to meet resting cellular requirements without any contribution from oxygen bound to hemoglobin.
As such, HBOT may provide a novel means of treating/ameliorating respiratory conditions associated with COVID-19. It is theoretically possible that such therapy may have the potential to provide adequate blood oxygenation levels almost in the complete absence of lung-blood interaction. Further, it is currently estimated that more than 1,350 hospitals in the US offer HBOT services and Medicare covers HBOT for more than a dozen conditions [21]. As a result, there presently exists a large-scale infrastructure to administered HBOT to COVID-19 patients.
Packed red blood cell transfusions
It was previously described that packed red blood cell (pRBC) transfusions are a means to enhance intravascular oxygen-carrying capability, and, thus, increase the oxygenation of tissues within the body [22]. A number of previous studies demonstrated that pRBC transfusions resulted in significant increases in the oxygenation of various body tissues [23], [24], [25].
As such, pRBC transfusions may provide another novel means of treating/ameliorating respiratory conditions associated with COVID-19. It is theoretically possible that such therapy may significantly increase blood oxygenation levels. Further, pRBC transfusions are a common medical procedure, especially among patients receiving critical care [26]. Among patients not diagnosed with COVID-19, it was previously estimated that about 40% of all patients admitted to the ICU receive pRBC transfusions (with a mean of 5 units per patient) [27]. The widespread availability of pRBC provides a means to be able to rapidly and efficiently transfuse COVID-19 patients.
Erthropoiesis-stimulating agents
The kidney produces erthropoietin (EPO) a circulating hormone that stimulates erythropoiesis (the production of RBCs) by binding and activating the EPO receptors (EPOR) on erythroid progenitor cells [28]. The cloning of the EPO gene allowed for the development of treatments to stimulate erthropoiesis by erthropoiesis-stimulating agents (ESAs) [29]. ESAs given by injection were observed to significantly increase the numbers of RBCs with increased tissue perfusion and oxygenation over several weeks [30]. In addition, given the ability of ESAs to increase RBC synthesis, it was observed that the supply of iron may not be able to keep pace with RBC synthesis, and, hence, it may be necessary to provide iron supplementation [31].
At present, there are multiple forms of ESA available on the US market that are defined by differences in glycosylation patterns giving rise to alpha, beta, delta, and omega forms, and ESAs are covered by Medicare [32]. The widespread availability of ESAs provides another means to be able to rapidly and efficient treat COVID-19 patients.
Other considerations
Chloroquine/hydroxychloroquine treatments
It was recently suggested that chloroquine (CQ) and hydroxychloroquine (HCQ) may be useful in the treatment of COVID-19, but presently there is inadequate evidence to support their safe and effective treatment of COVID-19 [33]. There is emerging evidence suggesting that CQ and HCQ may directly impact cornavirus attachment to respiratory cells [34], but there is also interesting potential modes of action for these drugs with respect to respiratory conditions associated with COVID-19. Namely, it was revealed that CQ and HCQ have the ability to significantly increase cellular hemoglobin production [35] and it was suggested that such drugs may have a therapeutic potential to raise hemoglobin levels in patients [36]. In addition, a recent meta-analysis of clinical trials revealed that HCQ treatment among patients diagnosed with diabetes mellitus significantly reduced their levels of hemoglobin A1c (glycated hemoglobin decreases the oxygen carrying capacity and limits oxygen tissue delivery) [37]. As a result, CQ and HCQ treatments may help to improve respiratory conditions related COVID-19 by a mechanism consistent with the various previously considered treatments.
Acetazolamide treatment
It was also recently suggested that acetazolamide may useful in the treatment of respiratory conditions in COVID-19 patients [10]. Acetazolamide is a carbonic anhydrase inhibitor. Carbonic anhydrase is found in the proximal tubule of the kidney and allows for the re-absorption of bicarbonate, sodium, and chloride. Acetazolamide inhibits the re-absorption of these ions, and hence, by increasing the blood level of bicarbonate, the blood becomes acidic. This results in a compensatory hyperventilation (Kussmaul respiration) with increased blood oxygen levels and decreased blood carbon dioxide levels [38]. As a result, acetazolamide treatment may help to improve respiratory conditions related COVID-19 by a mechanism consistent with the various previously considered treatments.
Future directions
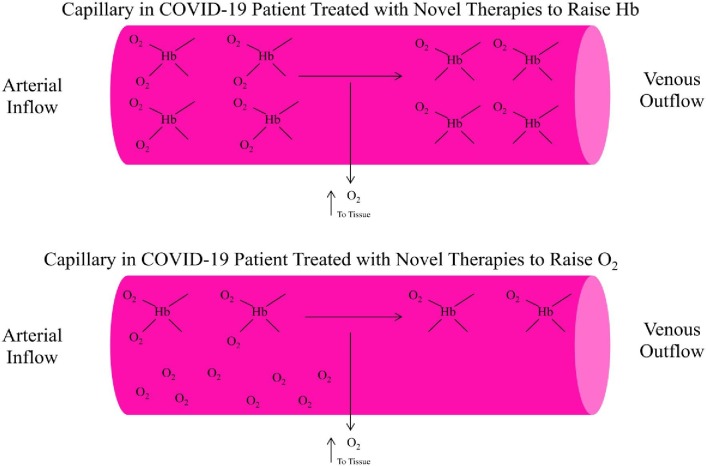
In conclusion, it is apparent that COVID-19 is global disease associated with significant morbidity and mortality. A significant percentage of the mortality associated with COVID-19 is related to respiratory conditions. At present, respiratory and ventilator support are standard therapeutic treatments for respiratory conditions associated with COVID-19. Unfortunately, despite such treatments of COVID-19 patients with respiratory conditions, a high mortality rate is still being observed. It is hypothesized that there are underlying pathological changes among COVID-19 patients that are compatible with ARDS. A series of alternate treatment strategies utilizing clinical available treatments such as HBOT, pRBC transfusions, or ESAs were hypothesized to deliver increased oxygen to peripheral tissues by alternative means than standard respiratory and ventilator support therapeutic treatments. Fig. 2 provides an overview of the mechanism of action for the various novel treatment strategies for respiratory conditions in COVID-19 patients. It is recommend, given such alternative therapies are currently available and routinely utilized in the treatment of other conditions, that such therapies be tried among COVID-19 patients with serious respiratory conditions. It is also recommend that future controlled-clinical trials explore the potential usefulness of such treatments among COVID-19 patients with respiratory conditions.
Fig. 2.
A summary of novel treatments considered to improve hemoglobin-based delivery of oxygen molecules to peripheral tissues in a COVID-19 patient's capillary. Hb = hemoglobin; O2 = oxygen. It is hypothesized that hyperbaric oxygen therapy will significantly increase O2 levels in the blood independently of Hb levels and improve tissue oxygenation. It is also hypothesized that packed red blood cell transfusions or injected erthropoiesis-stimulating agents will significantly raise blood Hb levels by increased numbers of red blood cells and improve tissue oxygenation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.World Health Organization. Naming the Coronavirus Disease (COVID-2019) and the Virus That Causes It. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed on 1 March 2020).
- 2.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johns Hopkins University and Medicine Coronavirus Resource Center. Available at: coronavirus.jhu.edu (accessed on 10 April 2020).
- 4.Borges do Nascimento I.J., Cacic N., Abdulazeem H.M., von Groote T.C., Jayarajah U., Weerasekara I. Novel Coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med. 2020;9:E941. doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanne J.P. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020;295 doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. CT imaging features of 2019 novel coronavirus (2019- nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M., Zhou Y., Zong Z., Liang Z., Cao Y., Tang H. A precision medicine approach to managing 2019 novel coronavirus pneumonia. Precis Clin Med. 2020;3:14–21. doi: 10.1093/pcmedi/pbaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solaimanzadeh I. Acetazolamide, nifedipine and phophodiesterase inhibitors: rationale for their utilization as adjunctive countermeasures in the treatment of Coronavirus disease 2019 (COVID-19) Cureus. 2020;12 doi: 10.7759/cureus.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 12.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel 2020 coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutje S, Marinova M, Jutting D, Attenberger U, Essler M, Bundschuh RA. Nuclear medicine in SARS-CoV-2 pandemia: 18F-FDG-PET/CT to visualize COVID-19. Nuklearmedizin (in press). [DOI] [PubMed]
- 14.Chan Y.L., Han S.T., Li C.H., Wu C.C., Chen K.F. Transfusion of red blood cells to patients with sepsis. Int J Mol Sci. 2017;18:E1946. doi: 10.3390/ijms18091946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arabi Y.M., Fowler R., Hayden F.G. Critical care management of adults with community acquired severe respiratory viral infection. Intensive Care Med. 2020;46:315–328. doi: 10.1007/s00134-020-05943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lance Respir Med (in press). [DOI] [PMC free article] [PubMed]
- 17.Tibbles P.M., Edelsberg J.S. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334:1642–1648. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]
- 18.Lambertsen C.J., Kough R.H., Cooper D.Y., Emmel G.L., Loeschcke H.H., Schmidt C.F. Oxygen toxicity: effects in man of oxygen inhalation at 1 and 3.5 atmospheres upon blood gas transport, cerebral circulation and cerebral metabolism. J Appl Physiol. 1953;5:471–486. doi: 10.1152/jappl.1953.5.9.471. [DOI] [PubMed] [Google Scholar]
- 19.Boerema I., Meyne N.G., Brummelkamp W.K. Life without blood: a study of the influence of high atmospheric pressure and hypothermia on dilution of the blood. J Cardiovasc Surg. 1960;1:133–146. [Google Scholar]
- 20.Kety S.S., Schmidt C.F. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser Health News. Available at: https://khn.org/news/hospitals-put-more-stock-in-hyperbaric-therapy-for-diabetics-despite-concerns/. Accessed on 8 April 2020.
- 22.Vermeulen Windsant I.C., de Wit N.C., Sertorio J.T., Beckers E.A., Tanus-Santos J.E., Jacobs M.J. Blood transfusions increase circulating plasma free hemoglobin levels and plasma nitric oxide consumption: a prospective observational pilot study. Crit Care. 2012;16:R95. doi: 10.1186/cc11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith M.J., Stiefel M.F., Magge S., Frangos S., Bloom S., Gracias V. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med. 2005;33:1104–1108. doi: 10.1097/01.ccm.0000162685.60609.49. [DOI] [PubMed] [Google Scholar]
- 24.Aktas S., Ergenekon E., Ozcan E., Aksu M., Unal S., Hirfanoglu I.M. Effects of blood transfusion on regional tissue oxygenation in preterm newborns are dependent on the degree of anaemia. J Paediatr Child Health. 2019;55:1209–1213. doi: 10.1111/jpc.14378. [DOI] [PubMed] [Google Scholar]
- 25.Fabricant L., Kiraly L., Wiles C., Differding J., Underwood S., Deloughery T. Cryopreserved deglycerolized blood is safe and achieve superior tissue oxygenation compared with refrigerated red blood cells: a prospective randomized pilot study. J Trauma Acute Care Surg. 2013;74:371–376. doi: 10.1097/TA.0b013e31827e1d40. [DOI] [PubMed] [Google Scholar]
- 26.Shaz B.H., Hillyer C.D. Is there transfusion-related acute renal injury. Anesthesiology. 2010;113:1012–1013. doi: 10.1097/ALN.0b013e3181f710b8. [DOI] [PubMed] [Google Scholar]
- 27.Napolitano L.M., Kurek S., Luchette F.A., Corwin H.L., Barie P.S., Tisherman S.A. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37:3124–3157. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 28.Elliott S., Sinclair A.M. The effect of erythropoietin on normal and neoplastic cells. Biologics: Targets. Therapy. 2012;6:163–189. doi: 10.2147/BTT.S32281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin F.K., Suggs S., Lin C.H., Browne J.K., Smalling R., Egrie J.C. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci USA. 1985;82:7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contaldo C., Elsherbiny A., Lindenblatt N., Plock J.A., Trentz O., Giovanoli P. Erthropoietin enhances oxygenation in critically perfused tissue through modulation of nitric oxide synthase. Shock. 2009;31:599–606. doi: 10.1097/SHK.0b013e31818b9cc4. [DOI] [PubMed] [Google Scholar]
- 31.Rodgers G.M., Gilreath J.A. The role of intravenous iron in the treatment of anemia associated with cancer and chemotherapy. Acta Haematol. 2019;142:13–20. doi: 10.1159/000496967. [DOI] [PubMed] [Google Scholar]
- 32.Mitka M. High-cost drugs account for most of Medicare Part B spending. JAMA. 2013;310:572. [Google Scholar]
- 33.Gbinigie K, Frie K. Should chloroquine and hydroxychloroquine be used to treat COVID-19? A rapid review. BJGP Open (in press). [DOI] [PMC free article] [PubMed]
- 34.Fantini J, Scala CD, Chahinian H, Yahi N. Structural and molecular modeling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents (in press). [DOI] [PMC free article] [PubMed]
- 35.Iyamu E., Perdew H., Woods G. Growth inhibitory and differention effects of chloroquine and its analogue on human leukemic cells potentiate fetal hemoglobin production by targeting the polyamine pathway. Biochem Pharmacol. 2009;77:1021–1028. doi: 10.1016/j.bcp.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Ben-Zvi I., Kivity S., Langevitz P., Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42:145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wondafrash D.Z., Desalegn T.Z., Yimer E.M., Tsige A.G., Adamu B.A., Zewdie K.A. Potential effects of hydroxychloroquine in diabetes mellitus: a systematic review on preclinical and clinical trial studies. J Diabetes Res. 2020;2020:5214751. doi: 10.1155/2020/5214751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leaf D.E., Goldfarb D.S. Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J Appl Physiol. 2007;102:1313–1322. doi: 10.1152/japplphysiol.01572.2005. [DOI] [PubMed] [Google Scholar]