Abstract
Background:
Physicians are cautious to prescribe antihypertensive drugs in frail older adults because of the potential adverse effects, especially in those with cognitive complaints. Lifestyle aspects might provide safe targets to lower blood pressure in older adults.
Objective:
Our goal was to evaluate the associations between activity patterns and blood pressure in memory clinic patients.
Methods:
We used an observational cross-sectional study to measure activity patterns with the ActivPAL accelerometer, and simultaneous home blood pressure levels in memory clinic patients (age range 51–87 years old). Office blood pressure was assessed during routine clinical practice.
Results:
41 patients (mean age of 74.3 (7.7) years of age, 46% female) were included. Sedentary parameters were associated with higher mean home blood pressure, with the strongest correlation between more prolonged sitting bouts and higher SBP (r = 0.58, p < .0001). Physical activity parameters were negatively associated with mean home blood pressure. Adjusted regression estimates remained significant, showing, e.g., a 4.5 (95% CI = 1.6;7.4) mmHg increase in SBP for every hour of sitting per day and a –1.0 (95% CI = –1.8;–0.2) mmHg decrease in DBP for every additional 1000 steps per day. No strong correlations were found between any of the activity pattern variables and office blood pressure.
Conclusion:
Associations between activity pattern variables and blood pressure were only found with home blood pressure measurements, not with office measurements. Longitudinal evaluations of these associations are now needed to explore if reducing prolonged sedentary bouts and increasing step count indeed serve as safe targets to lower blood pressure.
Keywords: Blood pressure, cognitive decline, dementia, hypertension, physical activity, sedentary behavior
INTRODUCTION
It is widely accepted that cardiovascular risk factors, such as high blood pressure (BP), especially during mid-life, are associated not only with increased risk of vascular disease, but also with increased dementia risk [1]. The proposed underlying mechanisms relating high BP to cognitive function are modifications in brain structure and morphology, such as white matter hyperintensities [2]. While this underscores the importance to lower BP, physicians are cautious to prescribe antihypertensive drugs in older adults because of the potential for adverse effects, especially in those with frailty associated with cognitive impairment or dementia [3]. These controversies around antihypertensive drugs emphasize the importance of non-pharmacological lifestyle interventions as BP-lowering strategies, such as the current recommendation to perform regular aerobic exercise [4].
Physical inactivity is not only linked to increased BP, but may be directly and indirectly responsible for an estimated 20% of all Alzheimer’s disease (AD) cases [5]. While effects of exercise on BP and cognition have been well studied, practical feasibility of regular exercise is highly challenging in older, frail adults. Alternatively, focusing on the other end of the physical inactivity spectrum, i.e., reducing sedentary behavior (SB), could be a promising target for BP control. Studies on the short-term effects of SB indeed show increases in BP over seven hours that could be counteracted by short light-intensity walking breaks [6]. The potential physiological mechanisms that underlie this acute BP raising effect of SB may relate to a lower metabolic demand during sitting [7] or alterations in vascular tone during sitting [8], which result in higher peripheral resistance that increases BP.
Observational studies on SB and BP on the somewhat longer time scale of weeks are limited, especially in the older population, while these are particularly relevant for the question if SB may be a non-pharmacological target for BP management. This may relate to the difficulty to objectively and reliably assess both BP and SB in daily living. In particular in older adults, self-reported assessment of activity patterns is bound to be subject to recall bias and misinterpretation [9, 10]. Additionally, office BP measurements are not ideal due to their inaccuracy caused by the combination of white-coat and masked hypertension [4], which is even more pronounced in people with cognitive impairment [11]. Despite the fact that the association between SB and BP has been widely studied, most studies use these subjective assessments of SB and unreliable single office BP measures. Additionally, the population of interest, older adults with memory complaints, is not researched extensively. Therefore, we aimed to investigate the association between objectively measured activity patterns and home BP measurements (HBPM), with routine office BP measurements as comparison, in memory clinic patients.
METHODS
Participants
We included patients who were referred to the outpatient memory clinic of a university hospital from August 2018 to July 2019. In total 246 patients visited the memory clinic during that time. People not living within reasonable geographical proximity (>30 km) were excluded based on logistical reasons. Remaining patients were pre-screened by the treating physician or nurse on their capability to participate in the study. This capability to participate was judged based on the mastery of the Dutch language, ability to understand and perform the measurements at home, and presence of an informal caregiver at home and during the appointment. There were no additional in- or exclusion criteria used. The study was approved by the medical ethics committee (#2016-2753) and used an opt-out consent approach for the use of medical records. The investigation was conducted in accordance with the Declaration of Helsinki.
Measurements
Activity patterns were assessed for seven full consecutive 24 h periods with an accelerometer (ActivPAL™ micro, PAL Technologies, Glasgow, UK) attached by a research assistant to the upper right leg [12]. This accelerometer is able to distinguish between sitting/lying, standing, and stepping (activity) based on posture. In order to differentiate sitting and sleep, participants were asked to keep a sleep/wake diary, including naps. PALanalysis software v8 (PAL Technologies, Glasgow, UK) was used to visually inspect the data to confirm these diaries. With a modified version of the script by Winkler et al. [13], mean sleep, sitting, standing, and stepping (activity) time were calculated per day. Prolonged bouts were defined as uninterrupted periods of sitting of ≥30 min. The ActivPAL also estimates energy expenditure expressed in metabolic equivalents (METs). Based on the validated cut-off point of ≥3 METs, moderate-to-vigorous physical activity (MVPA) was defined [14]. Days were excluded, based on the criteria of Winkler et al. [13], if less than 10 h of awake time was registered, if one of the three activities (sitting/standing/stepping) would take up 95% of the total awake time, or if the number of steps was lower than 500. The average time or count per day was calculated over the whole period that the device was worn.
During the same period, patients were asked to perform HBPM with an automatic oscillometric device (Microlife WatchBP Home, Microlife, Heerbrugg, Switzerland). Patients received a demonstration and written instructions indicating that patients needed to sit in a straight chair and support their arm using a table for at least 5 min before starting the measurement. As recommended by the European Society of Hypertension, patients were asked to measure in duplicate both in the morning (06 : 00–10 : 00 h) and evening (17 : 00–21 : 00 h) [15, 16]. Of these 28 single measurements, data from day 1 was discarded, and mean systolic (SBP) and diastolic (DBP) blood pressure values over the remainder of the week were calculated. A minimum of eight duplicate measurements was set for the data to be valid and included [15].
Demographic and clinical information, including medication use, was collected during the geriatric assessment by the treating physician. This included a cognitive assessment with the Montreal Cognitive Assessment (MoCA) tool, body mass index (BMI, kg/m2) assessment, and supine office BP measurement with a manual sphygmomanometer. Level of education was asked and coded as proposed by Verhage [17]. Cognitive diagnoses were established during the memory clinic’s multidisciplinary meeting of geriatricians and neuropsychologists based on medical history and physical examination, cognitive evaluation and when indicated other measurements including MRI. Hypertension was defined as mean HBPM≥135/85 mmHg or antihypertensive drug use [4]. A vascular comorbidity score (VCS) was established as a continuous measure based on a history of chronic heart failure, coronary heart disease, cardiac arrhythmias, cerebrovascular disorder, peripheral artery disease, diabetes, antiplatelet, anticoagulant, and statin drug use, and hypertension diagnosis [18].
Statistical analysis
Continuous variables are presented as mean (SD), categorical variables as percentages (n). Pearson and Spearman correlation coefficients were calculated between the activity and BP measures for normally and non-normally distributed variables, assessed by the Shapiro-Wilk test, respectively. Linear regression was used to assess cross-sectional corrected associations (reported as unstandardized regression estimates) between individual-mean activity and individual-mean BP measures. Covariates that were considered were age, sex, education, BMI, and VCS. Since BMI and VCS might partly mediate the association between SB and BP, these were included in a separate model. All covariates, except for sex, were used as continuous measures. Q-Q plots used to assess normality of the residuals of the regression analysis did not show indications for non-normality. Secondary analysis entailed a multi-level model with individual time-varying daily activity pattern variables, corrected for individual week-mean values, and daily evening BP values, to get an impression of the within-subject associations as well. Only evening BP values were taken into account here, to make sure the outcome measure was not preceding the explanatory daily activity patterns. All analyses were performed in SAS 9.4, with two-sided testing with p values less than 0.05 considered significant.
RESULTS
Of the 51 patients who received an accelerometer and home BP device, 41 had valid data for both outcomes (Supplementary Figure 1). These 41 patients were on average 74.3 (7.7) years of age, and 46% were female (Table 1). Mean sitting time was 9.4 (1.6) h/day and home BP was on average 136 (15)/77 (9) mmHg, which was lower than the office BP.
Table 1.
Participant characteristics
| Characteristic | Mean (SD)/% (n) |
| Age (y, (SD)) | 74.3 (7.7) |
| (range, y) | 51–87 |
| Sex (% female, (n)) | 46% (19) |
| BMI (SD)1 | 26.5 (4.0) |
| MoCA (score, (SD))2 | 21.8 (4.1) |
| Medication use (%, (n)) | |
| – Antihypertensive drugs | 42% (17) |
| ∘ ARB | 17% (7) |
| ∘ ACE inhibitor | 15% (6) |
| ∘ Diuretic | 10% (4) |
| ∘ Beta blocker | 24% (10) |
| ∘ Calcium channel blocker | 7% (3) |
| – Statins | 32% (13) |
| – Antithrombotic agents | 37% (15) |
| Diagnosis (%, (n)) | |
| – Dementia | 29% (12) |
| – Mild cognitive impairment | 39% (16) |
| – Subjective cognitive complaints | 24% (10) |
| – Other neurological condition | 7% (3) |
| Sitting time (h/day) | 9.4 (1.6) |
| Prolonged bouts (count/day) | 5.3 (1.3) |
| Steps (count/day) | 8706 (3603) |
| MVPA (min/day) | 68.9 (29.4) |
| Sleep time (h/day) | 8.5 (0.8) |
| Home systolic blood pressure (mmHg) | 136 (15) |
| Home diastolic blood pressure (mmHg) | 77 (9) |
| Office systolic blood pressure (mmHg) | 157 (24) |
| Office diastolic blood pressure (mmHg) | 81 (12) |
ARB, angiotensin II receptor blocker; ACE, angiotensin-converting-enzyme. 1N = 39 due to two missing values. 2N = 40 due to one missing value.
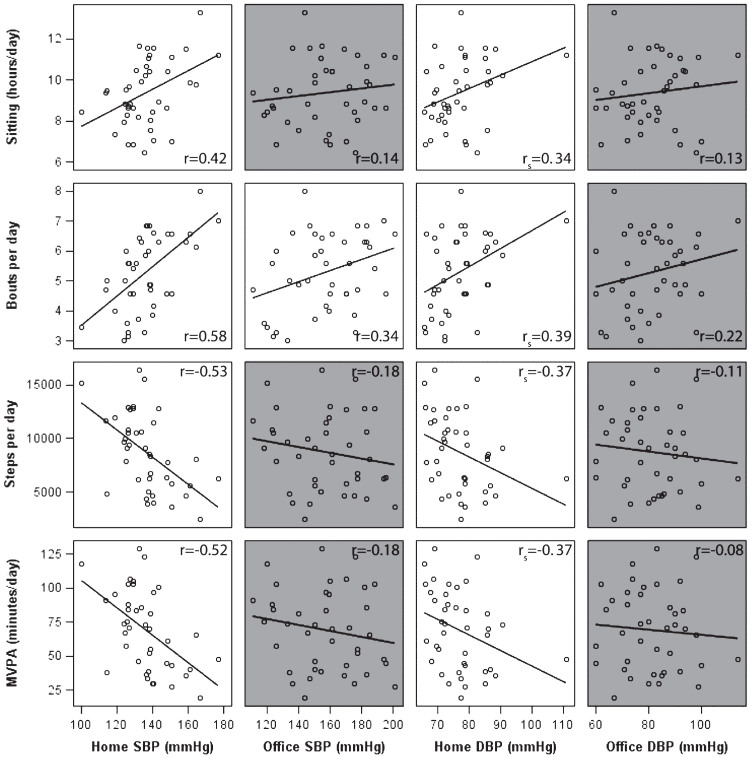
Longer sitting time and more prolonged bouts were associated with higher home BP (Fig. 1). The strongest association was present between prolonged bouts and home SBP (r = 0.58, p < 0.0001). Higher step count and MVPA were associated with lower home BP. In contrast to home BP, no statistically significant correlations were found between activity patterns and office BP, except for a weak correlation between office SBP and prolonged bouts. Sleep time was only associated with home DBP (rs = 0.48, p = 0.002, data not shown).
Fig.1.
Scatter plots with uncorrected correlation coefficients between activity pattern measures and both home and office blood pressure measures in memory clinic patients. MVPA, moderate-to-vigorous physical activity; SBP, systolic blood pressure; DBP, diastolic blood pressure. Note: Correlations that were non-significant (p > 0.05) are shown in grey, Pearson (or Spearman’s for home DBP) correlations are shown in the right corners per association.
When adjusting for age, sex, and education, all associations remained significant (Table 2). For example, an increase of one hour of sitting per day was related to 4.5 mmHg increase in SBP. There was no statistically significant interaction with either antihypertensive drug use or physical activity in all models (data not shown). Additional correction for the potential mediators BMI and VCS did not fully explain this effect (Table 2).
Table 2.
Adjusted unstandardized regression estimates between activity patterns on home and office blood pressure measures in outpatient memory clinic patients
| Home SBP | Office SBP | Home DBP | Office DBP | |
| Sitting time (h/day) | ||||
| Model 1 | 4.5 (1.6;7.4) | 1.7 (–3.4;6.8) | 2.1 (0.2; 3.9) | 0.3 (–2.4;3.0) |
| Model 2 | 4.0 (0.8;7.3) | 2.1 (–0.1;4.2) | ||
| Prolonged bout count | ||||
| Model 1 | 7.4 (4.1;10.7) | 6.6 (0.5;12.7) | 3.1 (0.8;5.4) | 1.5 (1.9;4.9) |
| Model 2 | 6.8 (3.2;10.4) | 3.0 (0.5;5.5) | ||
| Step count (1000 steps/day) | ||||
| Model 1 | –2.2 (–3.5;–1.0) | –0.4 (–2.7;1.9) | –1.0 (–1.8;–0.2) | –0.3 (–1.5;0.9) |
| Model 2 | –2.1 (–3.8;–0.4) | –1.3 (–2.3;–0.2) | ||
| MVPA (min/day) | ||||
| Model 1 | –0.26 (–0.41;–0.10) | –0.06 (–0.33;0.22) | –0.12 (–0.22;–0.02) | –0.03 (–0.18;0.11) |
| Model 2 | –0.24 (–0.44;–0.04) | –0.14 (–0.27;–0.01) |
Estimates are shown with 95%CI. MVPA, moderate-to-vigorous physical activity; SBP, systolic blood pressure; DBP, diastolic blood pressure. Model 1 was corrected for age, sex, and education. Model 2 shows the effect on the home SBP/DBP values additionally corrected for BMI, and vascular comorbidity score with N = 39 due to two missing values on BMI.
The secondary multi-level model analysis with individual daily activity patterns and daily evening BP values resulted in smaller associations between the activity patterns and BP values and a loss of statistical significance, e.g., the estimate of one additional prolonged sedentary bout was 0.7 (95% CI = –0.2;1.7) mmHg on SBP (Table 3).
Table 3.
Adjusted unstandardized multi-level regression estimates between time-varying daily activity patterns on daily evening home blood pressure measures in outpatient memory clinic patients
| Home evening SBP | Home evening DBP | |
| Sitting time (h/day) | 0.7 (–0.4;1.9) | 0.3 (–0.3;1.0) |
| Prolonged bout count | 0.7 (–0.2;1.7) | 0.3 (–0.2;0.8) |
| Step count (1000 steps/day) | –0.7 (–1.8;0.4) | –0.1 (–0.7;0.5) |
| MVPA (min/day) | –0.04 (–0.11;0.03) | –0.01 (–0.05;0.03) |
Estimates are shown with 95% CI, multi-level model with random intercept was corrected for age, sex, education, and individual week-mean values. MVPA, moderate-to-vigorous physical activity; SBP, systolic blood pressure; DBP, diastolic blood pressure. N = 39 due to two measures with insufficient overlap in daily activity and blood pressure measurements.
DISCUSSION
To date only limited observational evidence exists for the association between objectively measured activity patterns and home BP, especially in the older population. In this study, we showed that higher mean sedentary levels were associated with a higher mean BP, and that being more physically active was associated with a lower BP. Interestingly, these associations only became apparent when BP was estimated with repeated measurements at home, and not with single office BP measurements.
Home BP values [19] and sitting patterns [20] were comparable to previous research in older adults. The fact that higher BP levels were seen for higher sedentary levels is in line with the proposed acute mechanism that SB causes increased peripheral resistance which drives an increase in BP in the short-term [7, 8]. The current hypothesis states that sitting time accumulated in prolonged bouts might have more detrimental health effects compared to total sitting time [6]. The even stronger association of prolonged sedentary bouts with SBP could reflect this phenomenon. Combining the knowledge of these acute effects of SB with the current observation that this relationship is also found in a cross-sectional analysis over an individual week-average, might indicate that these acute effects have chronic implications as well.
Our primary analysis consisted of mean-week activity pattern and BP values to increase the validity of these measurements. Despite the fact that no significant associations remained in our secondary multi-level analysis, which used individual daily activity pattern and BP values, we believe that especially for SBP this could mainly be caused by a lack of power. Besides the fact that this might indicate the presence of within-subject correlations, it also shows that the effect size found in our main regression analysis might be an overestimation. In a meta-analysis, Lee and Wong found a pooled effect of a 0.06 mmHg increase in SBP and a 0.20 mmHg increase in DBP for each additional hour of SB [22]. Even though our multi-level analysis has its limitations due to the use of daily values instead of the more valid week-averages, the effect sizes found in our multi-level analysis seem to be more in line with the results from the meta-analysis. However, the meta-analysis included studies with much younger age groups, which may also explain this smaller effect size. Nevertheless, the effect sizes found in our main regression analysis should be interpreted with caution.
In addition to the strong link between SB and BP, associations were found between higher mean step count and MVPA with lower mean BP. Exercise is known to have downregulating effects on BP [23], but given our observations, even increasing step count might be beneficial. The cross-sectional nature of these observations carries the risk of confounding, despite the correction for VCS and BMI. However, considering the established acute effects of SB on BP, it could be true that health benefits might be gained by targeting step count and prolonged sedentary bouts. This would create a larger window of opportunity in relation to feasibility, to change behavior accordingly in older adults. Increasing step count, compared to MVPA, and reducing prolonged sedentary bouts, in contrast to total sitting time, both come with less practical obstacles to perform. This is particularly the case in the older adult population with memory complaints that was of interest here. MVPA might counteract the negative effects of SB, as was seen for some other health effects in previous research [24]. However, in a group that might have physical difficulties in increasing MVPA, reducing SB can still be a very feasible objective to gain improvements.
The associations between the activity pattern variables and BP were only observed for the home BP and not for the office BP. Previous literature already indicated reduced accuracy of office BP measures, especially in memory clinic patients [11]. White-coat and masked hypertension distort the real BP values [4], making an existing association weaker or even disappear. Therefore, our results stress the importance, especially in a research setting in older subjects with cognitive impairment, to include HBPM or 24 h ambulatory BP monitoring instead of office BP measures.
A major strength of this study is the assessment of exposure and outcome. The HBPM are more valid compared to singular office BP measurements. Additionally, objective assessment of the activity patterns is preferred above the self-reported assessment of SB [25]. Limitations of this study are foremost related to the cross-sectional nature of the research. Although reverse causation is not likely to be in play here because hypertension per se is asymptomatic, confounding could be caused by SB and hypertension sharing a common cause. Despite proper confounder correction, it is therefore not possible to make causal claims based on these associations. As our secondary multi-level analysis might have suffered from a lack of power, future research should investigate this further by studying, both in the short- and long-term, the within individual longitudinal associations between changes in SB and changes in BP. This would also provide information on the true effect size of SB on BP. Additionally, it should be mentioned that the research sample used here is not representative for the whole group of outpatient memory clinic patients as a selection was made based on capability to perform in the study. Caution should therefore be taken when generalizing the results to a broader population. Furthermore, future studies including a larger sample size are warranted to stratify by type of cognitive impairment.
Overall, these results indicate associations between more prolonged sedentary bouts and lower step counts with higher BP levels. The fact that high BP is a risk factor for cognitive decline [2], and that antihypertensive drugs carry risks of adverse effects in older adults [3], highlight the clinical relevance for these measures as secondary prevention targets in BP control. This would particularly be beneficial for older adults with memory complaints for whom the hesitation of prescribing antihypertensive drugs and the practical obstacles of increasing exercise are even more pronounced. Research enabling causal claims on these associations is therefore needed to investigate the true effect size and make specific recommendations on these safe targets. In that way, reducing SB and increasing activity levels might be used, when contra-indications are present for antihypertensive drugs, to control BP and potentially delay cognitive decline.
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-1310r1).
Supplementary Material
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-191310.
REFERENCES
- [1]. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K (2005) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64, 277–281. [DOI] [PubMed] [Google Scholar]
- [2]. Swan GE, DeCarli C, Miller B, Reed T, Wolf P, Jack L, Carmelli D (1998) Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology 51, 986–993. [DOI] [PubMed] [Google Scholar]
- [3]. Hajjar I, Miller K, Hirth V (2002) Age-related bias in the management of hypertension: A national survey of physicians’ opinions on hypertension in elderly adults. J Gerontol A Biol Sci Med Sci 57, M487–M491. [DOI] [PubMed] [Google Scholar]
- [4]. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, De Simone G, Dominiczak A (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 39, 3021–3104. [DOI] [PubMed] [Google Scholar]
- [5]. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C (2014) Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol 13, 788–794. [DOI] [PubMed] [Google Scholar]
- [6]. Larsen RN, Kingwell BA, Sethi P, Cerin E, Owen N, Dunstan DW (2014) Breaking up prolonged sitting reduces resting blood pressure in overweight/obese adults. Nutr Metab Cardiovasc Dis 24, 976–982. [DOI] [PubMed] [Google Scholar]
- [7]. Dempsey PC, Larsen RN, Dunstan DW, Owen N, Kingwell BA (2018) Sitting less and moving more: Implications for hypertension. Hypertension 72, 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Carter S, Hartman Y, Holder S, Thijssen DH, Hopkins ND (2017) Sedentary behavior and cardiovascular disease risk: Mediating mechanisms. Exerc Sport Sci Rev 45, 80–86. [DOI] [PubMed] [Google Scholar]
- [9]. Herbolsheimer F, Riepe MW, Peter R (2018) Cognitive function and the agreement between self-reported and accelerometer-accessed physical activity. BMC Geriatr 18, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Van Uffelen JG, Heesch KC, Hill RL, Brown WJ (2011) A qualitative study of older adults’ responses to sitting-time questions: Do we get the information we want? BMC Public Health 11, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. de Heus RA, Tumelaire MV, Olde Rikkert MG, Claassen JA (2019) Diagnostic accuracy of office blood pressure compared to home blood pressure in patients with mild cognitive impairment and dementia. Eur J Cardiovasc Nurs 18, 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Dowd KP, Harrington DM, Donnelly AE (2012) Criterion and concurrent validity of the activPAL professional physical activity monitor in adolescent females. PLoS One 7, e47633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Winkler EA, Bodicoat DH, Healy GN, Bakrania K, Yates T, Owen N, Dunstan DW, Edwardson CL (2016) Identifying adults’ valid waking wear time by automated estimation in activPAL data collected with a 24h wear protocol. Physiol Meas 37, 1653. [DOI] [PubMed] [Google Scholar]
- [14]. Lyden K, Keadle SK, Staudenmayer J, Freedson PS (2017) The activPAL™ accurately classifies activity intensity categories in healthy adults. Med Sci Sports Exerc 49, 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Stergiou GS, Giovas PP, Gkinos CP, Patouras JD (2007) Validation of the Microlife WatchBP Home device for self home blood pressure measurement according to the International Protocol. Blood Press Monit 12, 185–188. [DOI] [PubMed] [Google Scholar]
- [16]. Parati G, Stergiou GS, Asmar R, Bilo G, De Leeuw P, Imai Y, Kario K, Lurbe E, Manolis A, Mengden T (2008) European Society of Hypertension guidelines for blood pressure monitoring at home: A summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens 26, 1505–1526. [DOI] [PubMed] [Google Scholar]
- [17]. Verhage F (1964) Intelligentie en leeftijd: Onderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar, Van Gorcum, Assen.
- [18]. de Heus RA, de Jong DL, Sanders ML, van Spijker GJ, Oudegeest-Sander MH, Hopman MT, Lawlor BA, Olde Rikkert MG, Claassen JA (2018) Dynamic regulation of cerebral blood flow in patients with Alzheimer disease. Hypertension 72, 139–150. [DOI] [PubMed] [Google Scholar]
- [19]. Nishinaga M, Takata J, Okumiya K, Matsubayashi K, Ozawa T Doi Y (2005) High morning home blood pressure is associated with a loss of functional independence in the community-dwelling elderly aged 75 years or older. Hypertens Res 28, 657. [DOI] [PubMed] [Google Scholar]
- [20]. Watts A, Gardiner P, Garnier-Villarreal M (2017) Sitting time in older adults with and without Alzheimer’s disease. Innov Aging 1, 1051. [Google Scholar]
- [21]. Zanchetti A, Thomopoulos C, Parati G (2015) Randomized controlled trials of blood pressure lowering in hypertension: A critical reappraisal. Circ Res 116, 1058–1073. [DOI] [PubMed] [Google Scholar]
- [22]. Lee PH, Wong FK (2015) The association between time spent in sedentary behaviors and blood pressure: A systematic review and meta-analysis. Sports Med 45, 867–880. [DOI] [PubMed] [Google Scholar]
- [23]. Whelton SP, Chin A, Xin X, He J (2002) Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann Intern Med 136, 493–503. [DOI] [PubMed] [Google Scholar]
- [24]. Stamatakis E, Gale J, Bauman A, Ekelund U, Hamer M, Ding D (2019) Sitting time, physical activity, and risk of mortality in adults. J Am Coll Cardiol 73, 2062–2072. [DOI] [PubMed] [Google Scholar]
- [25]. Ekelund U, Tarp J, Steene-Johannessen J, Hansen BH, Jefferis B, Fagerland MW, Whincup P, Diaz KM, Hooker SP, Chernofsky A (2019) Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. BMJ 366, l4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.