Abstract
Background
Extensive work in basic and clinical science suggests that biological mechanisms of aging are causally related to the development of disease and disability in late life. Modulation of the biological mechanisms of aging can extend both life span and health span in animal models, but translation to humans has been slow.
Methods
Summary of workshop proceedings from the 2018–2019 Epidemiology of Aging Workshop hosted by the Intramural Research Program at the National Institute on Aging.
Results
Epidemiologic studies play a vital role to progress in this field, particularly in evaluating new risk factors and measures of biologic aging that may influence health span, as well as developing relevant outcome measures that are robust and relevant for older individuals.
Conclusions
Appropriately designed epidemiological studies are needed to identify targets for intervention and to inform study design and sample size estimates for future clinical trials designed to promote health span.
Keywords: Epidemiology, Longevity, Successful aging
The successes of medical therapy, preventive medicine, and public health have resulted in unprecedented increases in the numbers of individuals reaching old age. Although the health of older adults may be improving, the even greater rise in longevity has resulted in expansion in the absolute number of older persons affected by multiple chronic diseases and disabilities. These demographic trends are outstripping gains in health, with greater numbers of older people in need of major, often costly, medical and surgical treatments and long-term care (1). Thus, identification and amelioration of modifiable factors (e.g. following a healthy diet, not smoking, regular physical activity, and avoidance of environmental stresses) that could help to compress morbidity and increase health span is a public health priority more urgent than ever before.
A new generation of studies is addressing the underlying biology of the aging process in the hope of identifying new targets for interventions that could increase health span. This concept has broad appeal but its translation to care is more complex than is commonly appreciated. Currently, we do not know the best and most efficient approaches to define and operationalize health span, which is a necessary requirement in identifying pathways, dimensions, and/or risk factors that could be targeted for its expansion. Health span studies focusing on individual diseases fail to address geriatric syndromes, such as delirium, sarcopenia, and incontinence, as well as changes in physical and cognitive function. Hence, the science of designing clinical trials that target health span expansion is still in its early stages.
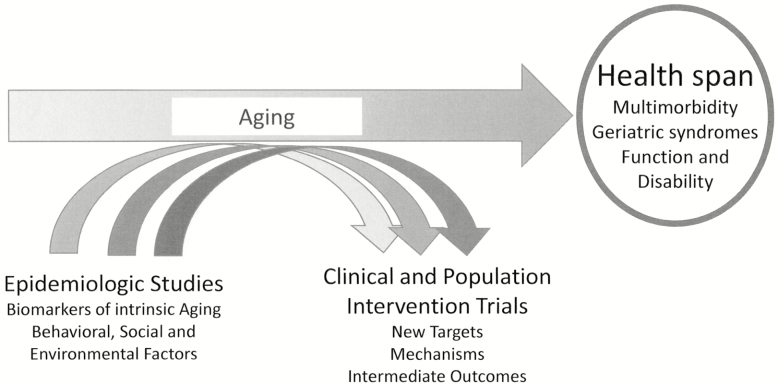
New approaches in the epidemiology field are addressing the issue of adequately measuring and defining health span. Global biomarkers of phenotypical and biological aging have been developed by combining health dimensions captured in large epidemiological studies using existing or new biological measures performed on biorepository specimens (2–4). These efforts have demonstrated that single or aggregate biomarkers/indices of global susceptibility to disease can predict the risk of impending deterioration of health status and multimorbidity, dimensions that can be directly connected to health span. Although these methods require refinement, especially validation from a longitudinal perspective, the preliminary results suggest that using these tools in future epidemiological studies may facilitate in identifying mechanisms that can be targeted by clinical or population-based intervention trials aimed at expanding health span before physiologic reserve has been exhausted (Figure 1).
Figure 1.
Linking epidemiology to clinical trials to increase health span.
Are there innovative designs for epidemiologic studies that can maximize the effectiveness of clinical or population-based intervention trials in measuring aging outcomes? What outcomes are most relevant and impactful to aging populations? Are there new approaches to assessing risk? These questions were recently discussed in workshop organized in Baltimore by the Intramural Research Program of the National Institute on Aging. This manuscript is an attempt to summarize the premises, topics, controversies, and recommendations that emerged during the meeting.
Study Design
Life Course Approach
The course of aging is the product of many life events and exposures, which begin as early as conception; however, many epidemiologic studies of aging only begin in old age. The rare cohorts that have been followed from birth to old age are starting to reveal trajectories of health dimensions and critical time points in those trajectories that can guide potential preventive interventions earlier in the aging process (5,6). The availability of biological samples from these cohorts is even more limited revealing an important scientific gap that should be addressed in future studies. According to the theory of developmental origins of health and disease (DOHAD), experiences in early life modulate physiological changes that condition health trajectories later in life, but the biological mechanisms that sense these inputs and encode them into epigenetic modifications that cause persistent shifts in the cellular composition of tissues are unknown. Knowledge about these mechanisms would be highly translatable to clinical applications and public health policy. A life course approach can be added into existing cohorts using proxy measures of early-life exposures, such as the North American Adult Reading Test (NART) to measure premorbid IQ (7), electronic health records (EHRs) to obtain past medical history, or geocoding of the environment from early-life home address (8,9). Combinations of cohorts of various ages have also been put together to create synthetic or “knitted” cohorts, revealing a life course perspective (10). Early-life exposures, such as birth weight and educational attainment, should be examined not only as life course exposures but also as stratification variables to assess interactions with current exposures (11). For example, the association of body mass index (BMI) with mortality in old age depends on the life trajectory of weight change (12), while the benefits of lowering blood pressure could vary based on when in the life course the blood pressure was measured (13).
Big Data
The trend towards mega-data comes with the hope that larger, simpler study designs that leverage existing clinical data will be more cost-effective and efficient in identifying both exposures and outcomes. Large datasets have been assembled using administrative records, EHRs, and/or combined cohort studies. Several advantages relevant to aging studies have been documented. For example, large EHR databases have allowed for infrequent events, such as delirium (14), to be identified in larger numbers and studied with adequate power, although validation work suggests that sensitivity and specificity are limited in many situations (15). A major disadvantage to EHR-based studies is the lack of unbiased, reliable assessments of physical and cognitive performance, geriatric syndromes, and common symptoms (eg, pain, fatigue, and sleep disorders) in clinical settings. For these important issues in aging, planned cohort studies remain the best option. However, records may include long-term follow-up (>10 years), allowing the latency period for many chronic diseases to be revealed. For example, obesity may be a risk factor for dementia in the long term, but is confounded by weight loss caused by the developing dementia pathology when examined in the shorter term (16).
Studies conducted in mega-cohorts (e.g. the UK Biobank) and in consortia of cohorts have demonstrated that large sample sizes are required to identify genetic variants that have very small effects on health. Association studies looking at traditional risk factors provide dubious results because of the lingering possibility of reverse causation and confounding. Instead, genetic polymorphisms already present at birth can provide near-causal estimates of association between variants and outcomes (17). For example, hemochromatosis C282Y homozygotes experience greater penetrance to clinical disease and symptoms than previously recognized when better-powered assessments are performed using a more prospective design, as well as when older groups are included (14,18).
A clear advantage of using a mega-cohort is that studies that examine associations of interest within and across pre-defined strata of the population have a sufficient sample size to compute reliable estimates and interactions between stratification and exposure variables. For example, a recent mega-cohort study revealed that hypertension treatment appears maximally effective at young age and diminishes in effectiveness for cardiovascular disease protection with older age, although these treatments benefit even the oldest old (19). However, there is usually a trade-off between having a large sample size and the quality of the data collected and the choice may depend on the scientific question to be addressed. Access to clinical tests, imaging, and electronic record data will assist in developing clinically valid prediction models and support software development to alert clinicians to potentially actionable factors, aiding in translation of research to practical applications.
Deep Phenotyping
New methods in imaging, personal monitoring, and cellular/tissue isolation techniques are constantly being developed and could uncover previously uncharacterized mechanisms, as well as novel intervention targets. Such methods should be carefully assessed for longitudinal reproducibility. Some biobank studies are linked to existing clinical information, but health records usually do not include important information defining the health and function of older adults and biobanked material may not be appropriate for new hypotheses. Dementia studies benefit from the availability of neuroimaging data, including recent quantification of amyloid and tau protein in the brain, as well as specific brain and other tissue biomarkers—including blood and CSF exosomes—and a detailed assessment of type, severity, and longitudinal emergence of cognitive and non-cognitive symptoms. Recent evidence indicates that different types of dementia can be distinguished by longitudinal patterns of symptom development that are generally not accurately ascertained using routine health records, suggesting that standardizing the data collected in medical records, as is performed currently in large epidemiologic cohort studies, may improve the quality of research using medical records (20).
New observational studies and clinical trials must be forward-thinking when collecting biological samples, cognizant of emerging technology, and that many of the new generation biomarkers will require specialized isolation and storage of cells/tissues. For example, an emerging frontier in the study of biomarkers is the use of “liquid biopsies” that use extracellular vesicles, which could be particularly valuable to discriminate between biomarkers coming from different tissues (21). Extracellular vesicles can be isolated from frozen blood samples, although there is limited information on whether long-term storage or repeated cycles of thawing and freezing affect their informational content. In contrast, isolating small numbers of hematopoietic stem cells or producing induced pluripotent stem cells is best performed using freshly collected samples. Assessments that examine proteomics, metabolomics, gene expression, and circulating nucleic acids are additional examples of methods that are optimally effective when samples are collected and stored according to specific, dedicated protocols.
Clinical Trials
Advances in aging biology suggest that aging itself is more modifiable than traditionally thought. Preventive interventions that target biological aging are being tested in humans (eg, rapamycin (22), metformin (23), canakinumab (24), caloric restriction (25)), although generally on a small scale. If the intervention requires sustained treatment over life, then the effects of these interventions cannot easily be evaluated for longevity—the most direct biomarker of biological aging—as this would require decades of follow-up. However, some types of interventions, such as senolytic therapy, may require shorter follow-up to demonstrate effects on health span. Surrogate biomarkers of biological and phenotypic aging are a main focus of current research but have not been fully developed for human studies. Ideally, interventions aimed at maximizing health span should be able to modify one or more of the hypothetical biological mechanisms of aging, and the effectiveness of these therapies should be evaluated by objective health outcomes that are anchored to perceived clinical improvement. Inclusion of assessments that are practical for clinical settings should be considered when designing epidemiologic studies to best enable translation from epidemiology to clinical trials. Ultimately, this approach would translate to more rapidly to clinical practices that directly address the underlying causes of aging (26).
Epidemiologic research has played a key role in the pathway to clinical trial conceptualization and design. Isolated systolic hypertension was first identified as a major risk factor for stroke in the Framingham cohort (27). This led to the proposal for the Systolic Hypertension in the Elderly Program trial that examined antihypertensive therapy targeting of isolated systolic hypertension, which demonstrated a reduction in stroke (28) but did not show benefit for dementia or disability, potentially due to differential drop out (29). Study designs have since evolved to include follow-up in home or nursing home settings, as well as detailed proxy assessment protocols.
Several observational studies had identified low physical activity as a modifiable risk factor and mobility disability as a common outcome. Planned using these observations, the LIFE clinical trial was conducted to increase walking, successfully reducing mobility disability in study participants (30). Simulated from existing studies, the population at risk was defined using the Short Physical Portable Performance Battery developed in the Established Populations for Epidemiologic Studies of the Elderly cohort (31). In this cohort, individuals were also at high risk for multimorbidity, which was a challenge for maintaining the interventions and complicated the follow-up (32). Better estimates of such competing risks for multimorbidity are needed for future trial planning.
Cohort studies that utilize repeated measurements of specific biomarkers are essential for providing estimates of expected changes that might be seen in trial participants. In particular, cohort studies also provide data about the variability (ie, the standard deviation in the population and the variability within individuals) of these markers, which is essential in planning sample sizes for trials that use changes in a biomarker as the primary endpoint. Furthermore, the variability per se may be a valuable predictor, for example, blood pressure variability predicts vascular risk over and above mean blood pressure perhaps serving as a proxy for arterial stiffness (33).
Diversity and Birth Cohorts
The U.S. population is increasingly diverse, with increases in racial and ethnic groups being most dramatic in the oldest old. Subgroups may vary in risk for disease, disability, or dementia and may be exposed to unique environmental and genetic risk factors that influence their aging. Globally, aging is most rapid in less developed countries, providing opportunities to observe the impact of social changes on aging in a shorter time period. In the United States, diverse cohorts recruited for studying specific diseases in early-to-midlife should be followed to understand their aging outcomes. Studies of the oldest old are especially needed, thus researchers should not apply an upper age limit in aging research, a view consistent with a new NIH policy that requires justification for not including older adults in relevant research (34,35).
A central question remains: Is aging changing over time? A challenge to epidemiologic studies of aging is the lack of a solid method for distinguishing between birth cohort effects and secular trends. Vast differences exist between previously studied cohorts and more recent cohorts based largely on societal changes, which could translate into major differences in disease and disability in late life. For example, more recent birth cohorts have higher caloric intake, lower levels of activity, and greater obesity than earlier birth cohorts. They also have later exposure to common viruses and more frequent exposure to antibiotic therapy. The extent to which these differences will translate to greater or lesser disease and disability in old age is still uncertain.
Outcomes
The quality of the remaining years of life is the ultimate measure of success in improving aging outcomes. An older adult’s perception of the quality of life is based on individual, personal priorities. Patient-reported outcomes usually refer to these perceptions, which are assessed by self-report of overall health, specific symptoms, or perceived satisfaction and quality of life (36). These outcomes complement other common age-oriented outcomes, such as multimorbidity, geriatric syndromes, and disability.
Multimorbidity
Epidemiologic research has traditionally considered multimorbidity, geriatric syndromes, and types of disability as important outcomes. Conceptually, multimorbidity is a summary of the total number of conditions affecting a person, whereas co-morbidity assumes a disease-centered focus regarding a primary condition of interest with consideration for additional conditions (37). Multimorbidity can include conditions that are linked pathophysiologically or have underlying risk factors in common—such as “cardiovascular morbidity,” which encompasses myocardial infarction, stroke, and heart failure—or diabetes, hypertension, and arthritis in obese people. It can also include conditions that are unrelated in pathogenesis or management (38). A recent NIA conference on multimorbidity proposed that research priorities to accomplish progress in this field include: standardizing the universe of morbidity to be included in practical tools, deciding how to weight conditions and for which outcomes, determining if information on duration and severity should be considered in diagnosis, highlighting opportunities and limitations of EHRs, and innovating standard and new analytic techniques (39). Accumulation of multimorbidity appears to be a fundamental feature of the aging process, thus, the accumulation rate has been proposed to be a composite endpoint of interventions that target aging (40). This outcome has gained some traction with many stakeholders as a global measure of the aging process.
Geriatric Syndromes
Geriatric syndromes are often either included as multimorbid conditions or examined as distinct outcomes/phenotypes. They are by definition highly multifactorial, with interacting pathogenic etiologies and mechanisms (41). Common geriatric syndromes—such as incontinence, delirium, frailty, or falls—are not always assessed in administrative data or epidemiologic studies. These syndromes may be assessed differently from one study to the next and, if assessed, risk factor assessment may also vary. For example, emerging evidence suggests that influenza is a major risk factor for both catastrophic and progressive disability (42), yet information regarding both influenza infection and muscle performance are rarely available in the same dataset. Some multifactorial syndromes, such as delirium, may be limited to inpatient records and are not assessed with standard methods. Accessing information from large EHRs has the potential to capture infrequent events, such as delirium, with high specificity but low sensitivity (43). Frailty has been conceptualized as a geriatric syndrome with a defined set of signs and symptoms (phenotype), but also as an index of accumulated deficits, similar to multimorbidity (44,45). Frailty, whether assessed in trials as a syndrome or as an index of deficits, has been useful as an outcome in clinical trials (46,47).
Disability and Function
Health span can also be characterized as disability-free survival. Disability endpoints include loss of ability to perform activities of daily living, loss of independence or active life expectancy, or loss of mobility. In older adults, disability is often linked to disease and is progressive in contrast to earlier onset disability, which is often more fixed. Consequences of disability include loss of social engagement and increased cost of assistance or care. Overall survival has increased more rapidly than survival free of disability, especially in men, although years with severe disability seem stable (48). Harmonized measures of health, such as the Patient-Reported Outcomes Measurement Information System, provide methods for patient-reported outcomes of physical function, as well as psychological and social function. Unfortunately, there is no standard assessment of disability that fits all purposes at present, and conceptual frameworks have been shifting. The Nagi model, which is most often used in the aging literature, distinguishes impairments or limitations in functions and disabilities as distinct constructs, but does not address social participation and environmental context, which are features of the International Classification of Functioning, Disability, and Health model (49). Severity is sometimes captured by reported degree of difficulty, but scales can vary (50). Finally, fluctuations in function illustrate that late-life disability is a dynamic process (51).
Regardless of methodology, the loss of function with age appears to be an index of the aging process itself. Gait speed and grip strength are remarkably robust in their ability to capture change over time, which lends them to be usefully translated as intermediate outcomes in clinical trials. In recent clinical trials, disability outcomes have been defined by either self-report or performance, such as reduction in ability to walk 400 m to operationalize mobility disability (52). Meaningful changes in performance have been defined by anchoring change to self-reported improvement or worsening in function (53). Standardization of both self-report and performance measures within a conceptual framework should be applied to epidemiologic studies in order to inform appropriate outcomes and degrees of meaningful change for clinical trials.
Exposures
Physical Activity
Low physical activity is an important risk factor for morbidity and mortality in older adults and is also an indicator of functional capacity. Self-reported physical activity is subject to bias and does not capture low levels of activity, but self-report adds a valuable subjective dimension to personal activity monitoring (54). Accelerometry uses sensors to detect acceleration in three planes and provides raw activity counts over days to weeks, which can be summarized as duration, intensity, and frequency. More recently, patterns of activity across the day have indicated that older adults have a lower total volume of activity that tends to decrease earlier in the day in comparison to younger adults (55). This earlier reduction in volume is associated with perceived fatigability. Additional information on activity behavior can be gathered by studying patterns of peak activity that vary in groups characterized by different balance performance, as well as frequency and type of fall (56,57). With advanced age and frailty, bouts of activity are shorter and increasingly fragmented and can predict higher mortality risk. In randomized controlled trials, increased walking lead to increased physical activity and reduced risk of disability (30), but these behaviors are challenging to sustain. New questions address if sedentary time and low levels of activity are distinct risk factors that require specific interventions, as well as whether low activity is a marker of an early stage in the path to disability. Stratification of patients based on patterns of activity may be useful in order to better identify appropriate populations and targets for intervention.
Nutrition
Epidemiologic studies have helped to identify important health, environmental, and social factors that affect nutrient intake, absorption, and metabolism. Observational dietary assessment has been used to self-report the intake of specific foods or frequency of intake of various food groups. Blood biomarkers of specific nutrients can supplement these assessments, as previously reported in assessing vitamin D intake and metabolism (58). Metabolomic profiles may also be used to monitor useful biomarkers for assessing diet and nutritional interventions. Several trials have demonstrated different metabolomic biomarker patterns that can distinguish between types of diet (59,60), although they do not always have the necessary sensitivity or specificity. Some metabolites can also reflect short-term intakes, medication effects, or intermediate metabolism by gut microbiota (61), but few existing epidemiologic studies of aging have incorporated microbiome and metabolomics assessments (62). There have been attempts to better quantify dietary intake using smart phone applications, but these have yet to be established as superior to standard approaches, such as food frequency or food diaries (63). New approaches to assessing diet and nutrition hold promise in better informing targets for intervention in older adults (64).
Social Factors
The interplay between social factors and health in old age is substantial. Social factors can influence activity and diet, reduce accessibility to resources needed to improve health, or directly impact biological status. Adverse socioeconomic experience across an individual’s life course influences subsequent health outcomes (65), which can then adversely impact social activity and relationships later in life. Epidemiologic studies of older adults can be used to assess historical factors with respect to current social status. For example, parental relationships with their children interact to shape those children’s health in adulthood (66) and can inform interventions for subsequent generations. Marriage, widowhood, social networks, and social isolation can promote or buffer adverse health events, such as depression (67), although the biologic mechanisms of these effects are just beginning to be understood (68). Social factors have been shown to impact epigenetic age (69), gene expression (70,71), and telomere length (72,73), suggesting that there are biologic pathways whereby stress may modify aging via biological pathways. These biomarkers may prove useful in judging the effects of social determinant interventions to improve health.
Environmental Exposures
Ecological studies examining the co-occurrence of diseases with environmental monitors (e.g. air quality monitors) are rapidly being supplanted by more direct personal exposure assessments. Extracellular vesicles, which are membrane-bound nano-vesicles, represent a previously hidden signaling system that can serve as direct “biosensors” of individual environmental exposures (74). Air particulate inhalation and gastrointestinal absorption of toxic metals can be detected through the expression of extracellular vesicle-encapsulated microRNAs (75). As a transport and signaling mechanism, these vesicles may explain the mechanisms by which environmental exposures affect diverse and distant tissues, such as the brain (75). Simultaneously, sophisticated mapping of geospatial environments can provide more accurate, direct estimates of personal exposures (76) and link these to age-related outcomes (77). New epidemiologic studies should also collect more detail on geospatial locations and collect biologic fluids that can be assessed for the presence of extracellular vesicles.
While traditional environmental epidemiologic studies have focused on one type of exposure at a time (e.g. one single chemical or a class of chemicals), individuals are continuously exposed to multiple chemical inputs. To address this, the field has made substantial progress in developing both experimental and computational approaches to evaluate the effects of multiple chemicals. For instance, new technologies that measure thousands of chemicals simultaneously have propelled the concept of the exposome (78)—the environmental equivalent of the genome—that can pair with emerging data approaches to investigate chemical patterns and synergistic effects (79). These new methods are ushering in a new era that can help pinpoint the environmental drivers of aging with greater accuracy.
Intrinsic Aging
Aging can be defined as a complex balance between intrinsic and extrinsic damage and repair processes. Many of the aforementioned categories of risk are thought to influence the underlying biology of aging itself. For example, air pollution might influence aging by its association with changes in epigenetic age. The fundamental biologic mechanisms of aging include genomic instability, DNA methylation, telomere shortening, proteostasis, cell senescence, mitochondrial dysfunction, and stem cell exhaustion (80). These and related mechanisms have been robustly linked to aging in cells and animal models but are challenging to measure in free-living humans. Changes in these basic biological functions could serve as intermediate biomarkers of aging. Epidemiologic studies could be used to develop robust methods in order to characterize these changes in humans, determine key external drivers, and assess which methods are most critical for measuring key outcomes of interest. Some techniques may be more relevant for specific diseases that are strongly age-related, such as some cancers or Alzheimer’s disease, while others may be related to fundamental age-related declines in function, such as diminishing gait speed, endurance, and muscle strength. Together, these aging processes are thought to promote a pro-inflammatory state and impaired responsiveness to stressors. Long-term cohort studies with biological measures can test whether acute or chronic inflammations are associated with aging traits (81).
Measures of resilience—such as response to exercise or glucose challenge—reveal substantial loss of these integrative functions with age, which may reveal changes in an individual long before loss of physical function is apparent, thus holding promise as early markers of aging (82,83). Studies are underway to evaluate physiologic responses to stressors in clinical settings that could be used as probes of age-related multisystem integrity. Several clinically used stress tests—such as exercise stress tests, immune responsiveness to vaccines, or blood pressure response to cognitive testing—could be evaluated in epidemiologic studies as intermediate markers of health span.
Many epidemiologic studies have generated DNA methylation data at one or more time points in adulthood, which can be used to further our understanding of the contribution of this key epigenetic modification to aging and health span. Widespread DNA methylation changes occur with age that are linked to biologic processes (84,85), and changes in DNA methylation reflect lifestyle factors—including obesity (86) and smoking (87)—that could accelerate age-related disease progression, while diminishing function. Methylation sites associated with BMI are associated with the expression of lipid metabolism genes.
Epigenetic clocks have been developed utilizing CpG sites that correlate with age in blood (71 CpGs) or multiple tissues (353 CpGs) (88,89). These biomarkers of epigenetic age robustly predict mortality, have shown some heritability (90), and are associated with some age-related diseases. Newer epigenetic biomarkers of aging and phenotypic age can predict health span, physical functioning, and Alzheimer’s disease (4). Further, phenotypic DNA methylation age associates with several aging pathways, including inflammation, DNA damage response, and mitochondrial function (4). Thus, these CpG sites have strong potential to be utilized in explaining the fundamental pathways that drive the processes of aging. Many of these methylation sites are highly correlated with chronological and biological aging, and their functions are only just beginning to be understood, but it is clear that most of the age-associated methylations sites are near transcription factor-binding sites and enhancers (91).
Potentially, DNA methylation could serve as a biomarker in clinical trials that target fundamental aging mechanisms. Although the current underlying biology is not clear, methylation clocks have been reproducible and sensitive to change in at least one clinical trial (92).
Other indices of biologic age have been calculated in epidemiologic studies using deviations from expected values for age of several biomarkers, such as hemoglobin levels, cytokine amounts, or physiology, including walking speed or exercise capacity (2–4). Currently, these biomarkers have not been clearly linked to underlying aging biology, genetic mechanisms, or epigenetic mechanisms, although they have been shown to be sensitive for detecting change over time in health dimensions in longitudinal studies with interventions (93,94). In the future, these measures might be useful in detecting impending changes in health before the onset of clinical illness.
Future Studies
Designed improvements in longitudinal epidemiologic studies can advance our understanding of human aging, its causes, and the related consequences. Previous epidemiologic studies have identified and characterized both aging and age-related health outcomes, but only now are we beginning to understand the underlying biology of aging and consider them as potential targets of aging in clinical trials. New observational studies are needed to refine assessments regarding the lifetime of environmental, lifestyle, and social factors that contribute to an optimal health span. These studies can be used to identify earlier intermediate outcomes of aging, such as resilience to stressors or biomarkers of early age-related changes. New studies could refine key aspects of behavioral factors—including diet, physical activity, and environmental exposures—using technologies, such as personal monitoring. Aging is highly heterogeneous in its onset and expression. A new generation of studies would give us finer-grained insights that could personalize care and management. Some key recommendations are highlighted in Table 1.
Table 1.
Recommendations for Future Epidemiologic Studies of Aging
| Recommendation | Rationale |
|---|---|
| Populations | |
| Study more recent birth cohorts | Physical and social environment has changed dramatically |
| Continue to follow existing birth to midlife cohorts | Have most efficient time course to study old age outcomes |
| Include diverse, global populations | Aging is advancing most rapidly in minority and global populations |
| Extend age range to study oldest old | Exemplars of health span—underrepresented in past studies |
| Use clinically relevant screening and assessment | Can use to simulate clinical trial target populations |
| Assess aging in midlife | Midlife aging has not been assessed but may be ideal point of intervention |
| Exposures | |
|---|---|
| Conduct finer-grained assessment of lifestyle and behavior with personal monitoring | Heterogeneity of aging requires more personalized approach to therapies |
| Include multilevel assessment of environment from subcellular to geographic factors | Environment affects large groups; policy change has large impact |
| Evaluate social determinants of health across the life course | Modifiable targets for optimizing health span |
| Repeat measures over life course | Individual peak and decline are highly variable and variability itself may be informative |
| Anticipate future methods in biologic sample preparation, such as extracellular vesicle isolation, genomic expression, pluripotent stem cells | Outcomes take many years to develop after samples are stored |
| Develop biomarkers of aging process that are valid and reproducible, including stress response and imaging biomarkers | Can be targets or intermediate outcomes of health span-promoting therapies, including lifestyle interventions |
| Enhance quality of electronic health records for aging research | Exposure and outcome information is disease focused, rarely includes function |
| Outcomes | |
|---|---|
| Include self-reported health and quality of life | Prioritizes personal values |
| Develop common definition of health span | Improvement is primary goal of aging research |
| Use high quality assessments of physical, cognitive and psychosocial functioning | Function summarizes aging process |
| Improve methods to assess multimorbidity | Composites reflect that most older adults have multi co-occurring and interacting health conditions |
| Include geriatric syndromes | Global, multifactorial syndromes such as delirium, falls, and frailty are highly impactful on function |
| Synthesis | |
|---|---|
| Consider heterogeneity, bias, and competing risk | Given challenges of aging research |
| Set standards for machine-learning approaches | Lack of transparency challenges interpretation |
| Use systems approach | Needed to capture the complexity of aging processes |
Epidemiologic studies are necessary for evaluating if new measures of aging biology are useful in defining biologic age, rate of aging, and for assessing the potential magnitude of an intervention response. The next-generation studies of the epidemiology of aging will need to incorporate technological assessments of behavior and environment, which emphasize a robust understanding of the biologic processes of aging. These assessments may require special imaging, tissues, or cells that are not available in existing biorepositories. Big data cohorts can be complemented by smaller, deeply phenotyped cohorts. Routine collection of standardized measures of performance and geriatric syndromes and storage of blood specimens from clinical care would vastly improve the value of health records for aging research.
Interventions designed to target basic biology of aging mechanisms (geroscience-guided therapies) are on the horizon. It is important to underline that while trials targeting the biology of aging may include targeted drugs, there is substantial indirect evidence that lifestyle and behavioral modification, as well as the management of environmental factors, may be effective in slowing the pace of aging. This theory should be empirically tested, where initial studies could focus on very high-risk individuals in whom assessing effectiveness can be performed over a limited follow-up. Recent studies have indicated that interventions, including physical activity, may be efficacious even very late in life, as very old or sick individuals still show substantial responses to intervention and large absolute decreases in risk. Appropriate target populations can be best-defined using longitudinal, observational studies, which should include laboratory or functional measurements—such as IL-6 levels or gait speed—that can be useful in defining high-risk individuals for planning interventions.
Outcomes for epidemiologic studies should be designed with an understanding of regulatory requirements for clinical trials (95). These can include outcomes reported by clinicians, patients, observers, or based on performance, and all outcomes need to be validated based on content, reliability, and sensitivity in detecting clinically meaningful changes. Some measures, such as gait speed and 6-minute walk test, have been vetted in this manner (53,96). Health span may be the most salient outcome for these intervention trials. When operationalized as a composite of accumulated multiple morbidities, health span has been assessed for reliability in epidemiologic studies and demonstrated to be sufficiently sensitive to changes for use in clinical trials.
Cost and efficiency are major constraints in epidemiologic research. Although some questions can be addressed with large clinical or administrative databases, many cannot. Questions regarding the impact of medical care and medication can be addressed by utilizing EHRs, while studies that focus on the role of basic biologic mechanisms will likely require novel measurements that are rarely used or collected in clinical practice or available in existing biobanks. New deep phenotyping studies may be limited in sample size but also can serve as platforms for deploying many types of novel assessments. Aging studies that focus on midlife or earlier should include very long-term follow-up, at a minimum of 20–30 years. Mechanisms to sustain the infrastructure for these types of studies are needed.
Long-term studies are an ideal training environment for scholars new to the aging field. Widespread data sharing between institutions has allowed for training programs to use the data gathered in these studies to challenge the creative minds of pre- and post-doctoral fellows and train the next generation of scientists in the field of aging research. The addition of novel measures, assays, and imaging can serve to extend the impact of the studies, providing an efficient platform for many career development awards and opportunities for the next generation of new investigators.
While the wealth of information collected in previous studies may help address new questions about aging, new epidemiological studies that directly apply new models and new measures to address the needs of an aging population are required. Novel approaches might include new studies of midlife aging, which may provide information on the early changes in biomarkers and physiologic function that emerge early in the pathways to disease and functional decline, as well as follow-up studies of birth cohorts where early-life exposures have been well defined. Future interventions can be more rapidly deployed by more sharply refocusing epidemiologic studies of aging on health span and its potential mechanistic targets.
Funding
A. B. Newman was supported by National Institute on Aging grants R01 AG059416, U01 AG023744, R01 AG059729, P30 AG024827, U01 DK057002, R01 AG052964, UH2 AG056933, U01 HL130114, and 1U19AG062682. L. Ferrucci and M. Salive were supported by the National Institute on Aging. S. R. Cummings was supported by the National Institute on Aging grant AG023122. G. A. Kuchel received National Institutes of Health funding grants R01 AG048023, R01 AG052608, R35 GM124922, UH2 AG056925, R21 AG060018, and R01 AI142086. J. Schrack was supported by National Institutes of Health grants U01 AG057545 and R21 AG053198. A. Baccarelli was supported by National Institute on Aging grants P30 ES009089 and R01 ES025225. J. M.Murabito was supported by National Institute on Aging grants U01AG023755 and U24AG051129. M. A. Espeland was supported by National Institutes of Health grants DK0 92237-01 and DK0 92237-02S2, while research he reported was also funded by two diversity supplements (3U01 DK057136-19S1 and 3U01 DK057136-19S2) to the parent award National Institute of Diabetes and Digestive and Kidney Diseases 5U01 DK057136-19 and the National Institute on Aging Wake Forest Alzheimer’s Disease Core Center (P30 AG049638-01A1). J. Kirkland was supported by National Institutes of Health grants AG013925, AG062413, and the National Institute on Aging Translational Geroscience Network (AG061456), Robert and Arlene Kogod, the Connor Group, Robert J. and Theresa W. Ryan, and the Ted Nash Long Life and Noaber Foundations. D. Melzer was supported in part by the United Kingdom Medical Research Council (MR/M023095). S. B. Kritchevsky was supported by the National Institute on Aging Wake Forest Older Americans Independence Center (P30 AG021332).
Acknowledgments
The authors would like to thank Dr. Marie Bernard for her insightful advice and contributions during the workshop and in preparing this manuscript. We thank Adam Cornish for his help in editing and proofreading. The participation of these individuals or the materials should not be interpreted as representing the official viewpoint of the U.S. Department of Health and Human Services, the National Institutes of Health, or the National Institute on Aging, except where noted.
Conflict of Interest
Dr. A. B. Newman is Editor-in-Chief of the Journal of Gerontology: Medical Sciences. Dr. D. Melzer is co-Deputy Editor of the Journal of Gerontology: Medical Sciences. Drs. L. Ferrucci, S. B. Kritchevsky, and J. M. Murabito are Associate Editors of the Journal of Gerontology: Medical Sciences. The remaining authors declare no conflicts of interest.
References
- 1. Chatterji S, Byles J, Cutler D, Seeman T, Verdes E. Health, functioning, and disability in older adults—present status and future implications. Lancet. 2015;385:563–575. doi: 10.1016/S0140-6736(14)61462-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci USA. 2015;112:E4104–E4110. doi: 10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanders JL, Arnold AM, Boudreau RM, et al. Association of biomarker and physiologic indices with mortality in older adults: Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2019;74:114–120. doi: 10.1093/gerona/gly075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10:573–591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–293. doi:https://doi.org/10.1093/ije/31.2.285 [PubMed] [Google Scholar]
- 6. Ben-Shlomo Y, Cooper R, Kuh D. The last two decades of life course epidemiology, and its relevance for research on ageing. Int J Epidemiol. 2016;45:973–988. doi: 10.1093/ije/dyw096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crawford JR, Deary IJ, Starr J, Whalley LJ. The NART as an index of prior intellectual functioning: a retrospective validity study covering a 66-year interval. Psychol Med. 2001;31:451–458. doi: 10.1017/s0033291701003634 [DOI] [PubMed] [Google Scholar]
- 8. Elo IT, Mykyta L, Sebastiani P, Christensen K, Glynn NW, Perls T. Age validation in the long life family study through a linkage to early-life census records. J Gerontol B Psychol Sci Soc Sci. 2013;68:580–585. doi: 10.1093/geronb/gbt033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murray ET, Ben-Shlomo Y, Tilling K, et al. Area deprivation across the life course and physical capability in midlife: findings from the 1946 British Birth cohort. Am J Epidemiol. 2013;178:441–450. doi: 10.1093/aje/kwt003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9:e113637. doi: 10.1371/journal.pone.0113637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nguyen TT, Tcshetgen Tchetgen EJ, Kawachi I, et al. Instrumental variable approaches to identifying the causal effect of educational attainment on dementia risk. Ann Epidemiol. 2016;26:71–6.e1. doi: 10.1016/j.annepidem.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Breeze E, Clarke R, Shipley MJ, Marmot MG, Fletcher AE. Cause-specific mortality in old age in relation to body mass index in middle age and in old age: follow-up of the Whitehall cohort of male civil servants. Int J Epidemiol. 2006;35:169–178. doi: 10.1093/ije/dyi212 [DOI] [PubMed] [Google Scholar]
- 13. Liu K, Colangelo LA, Daviglus ML, et al. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels? The Coronary Artery Risk Development in Young Adults (CARDIA) Study and the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc. 2015;4:e002275. doi: 10.1161/JAHA.115.002275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bowman K, Jones L, Pilling LC, et al. Vitamin D levels and risk of delirium: a Mendelian randomization study in the UK Biobank. 2019:92:e1387–e1394. doi: 10.1212/WNL.0000000000007136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hustad E, Skogholt AH, Hveem K, Aasly JO. The accuracy of the clinical diagnosis of Parkinson disease. The HUNT study. J Neurol. 2018;265:2120–2124. doi: 10.1007/s00415-018-8969-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bowman K, Thambisetty M, Kuchel GA, Ferrucci L, Melzer D. Obesity and longer term risks of dementia in 65-74 year olds. Age Ageing. 2019;48:367–373. doi: 10.1093/ageing/afz002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walker VM, Davies NM, Windmeijer F, Burgess S, Martin RM. Power calculator for instrumental variable analysis in pharmacoepidemiology. Int J Epidemiol. 2017;46:1627–1632. doi: 10.1093/ije/dyx090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tamosauskaite J, Atkins JL, Pilling LC, et al. Hereditary hemochromatosis associations with frailty, sarcopenia and chronic pain: evidence from 200,975 older UK biobank participants. J Gerontol A Biol Sci Med Sci. 2019;74:337–342. doi: 10.1093/gerona/gly270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beckett NS, Peters R, Fletcher AE, et al. ; HYVET Study Group Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369 [DOI] [PubMed] [Google Scholar]
- 20. Donaghy PC, O’Brien JT, Thomas AJ. Prodromal dementia with Lewy bodies. Psychol Med. 2015;45:259–268. doi: 10.1017/S0033291714000816 [DOI] [PubMed] [Google Scholar]
- 21. Urbanelli L, Buratta S, Sagini K, Tancini B, Emiliani C. Extracellular vesicles as new players in cellular senescence. Int J Mol Sci. 2016;17. doi: 10.3390/ijms17091408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mannick JB, Del Giudice G, Lattanzi M, et al. mTOR inhibition improves immune function in the elderly. Sci Trans Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892 [DOI] [PubMed] [Google Scholar]
- 23. Espinoza SE, Musi N, Wang CP, et al. Rationale and study design of a randomized clinical trial of metformin to prevent frailty in older adults with pre-diabetes. J Gerontol A: Biol Sci Med Sci. 2019. doi: 10.1093/gerona/glz078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 25. Kraus WE, Bhapkar M, Huffman KM, et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabet Endocrinol. 2019;7:673–683. doi: 10.1016/S2213-8587(19)30151-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guralnik JM, Kritchevsky SB. Translating research to promote healthy aging: the complementary role of longitudinal studies and clinical trials. J Am Geriatr Soc. 2010;58(Suppl. 2):S337–S342. doi: 10.1111/j.1532-5415.2010.02938.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kannel WB, Wolf PA, McGee DL, Dawber TR, McNamara P, Castelli WP. Systolic blood pressure, arterial rigidity, and risk of stroke. The Framingham study. J Am Med Assoc. 1981;245:1225–1229. doi: 10.1001/jama.1981.03310370017013 [DOI] [PubMed] [Google Scholar]
- 28. Shekelle R, Ostfeld A, Klawans HJ. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. J Am Med Assoc. 1991;265:3255–3264. doi: 10.1001/jama.1991.03460240051027 [DOI] [PubMed] [Google Scholar]
- 29. Di Bari M, Pahor M, Franse LV, et al. Dementia and disability outcomes in large hypertension trials: lessons learned from the Systolic Hypertension in the Elderly Program (SHEP) trial. Am J Epidemiol. 2001;153:72–78. doi: 10.1093/aje/153.1.72 [DOI] [PubMed] [Google Scholar]
- 30. Pahor M, Guralnik JM, Ambrosius WT, et al. ; LIFE Study Investigators Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. J Am Med Assoc. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pahor M GJ, Anton SD, Ambrosius WT, et al. Impact and lessons learned from the LIFE studies. J Am Geriatr Soc. 2019. In press. [Google Scholar]
- 33. Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. [DOI] [PubMed] [Google Scholar]
- 34. Bernard MA, Clayton JA, Lauer MS. Inclusion across the lifespan: NIH policy for clinical research. J Am Med Assoc. 2018;320:1535–1536. doi: 10.1001/jama.2018.12368 [DOI] [PubMed] [Google Scholar]
- 35. Kuchel GA. Inclusion of older adults in research: ensuring relevance, feasibility, and rigor. J Am Geriatr Soc. 2019;67:203–204. doi: 10.1111/jgs.15802 [DOI] [PubMed] [Google Scholar]
- 36. Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2:137–144. doi: 10.4103/2229-3485.86879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boyd CM, Fortin M. Future of multimorbidity research: how should understanding of multimorbidity inform health system design? Pub Health Rev. 2010;32:451–474. doi: 10.1007/BF03391611 [DOI] [Google Scholar]
- 38. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29:725–731. doi: 10.2337/diacare.29.03.06.dc05-2078 [DOI] [PubMed] [Google Scholar]
- 39. Tisminetzky M, Bayliss EA, Magaziner JS, et al. Research priorities to advance the health and health care of older adults with multiple chronic conditions. J Am Geriatr Soc. 2017;65:1549–1553. doi: 10.1111/jgs.14943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Espeland MA, Crimmins EM, Grossardt BR, et al. ; Multimorbidity Clinical Trials Consortium Clinical trials targeting aging and age-related multimorbidity. J Gerontol A Biol Sci Med Sci. 2017;72:355–361. doi: 10.1093/gerona/glw220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bartley JM, Pan SJ, Keilich SR, et al. Aging augments the impact of influenza respiratory tract infection on mobility impairments, muscle-localized inflammation, and muscle atrophy. Aging (Albany NY). 2016;8:620–635. doi: 10.18632/aging.100882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rudolph JL, Doherty K, Kelly B, Driver JA, Archambault E. Validation of a delirium risk assessment using electronic medical record information. J Am Med Dir Assoc. 2016;17:244–248. doi: 10.1016/j.jamda.2015.10.020 [DOI] [PubMed] [Google Scholar]
- 44. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Walston J, Bandeen-Roche K, Buta B, et al. Moving frailty toward clinical practice: NIA intramural frailty science symposium summary. J Am Geriatr Soc. 2019;67:1559–1564. doi: 10.1111/jgs.15928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Puts MTE, Toubasi S, Andrew MK, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017;46:383–392. doi: 10.1093/ageing/afw247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53–61. doi: 10.1016/j.arr.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Freedman VA, Wolf DA, Spillman BC. Disability-free life expectancy over 30 years: a growing female disadvantage in the US population. Am J Public Health. 2016;106:1079–1085. doi: 10.2105/AJPH.2016.303089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Freedman VA. Adopting the ICF language for studying late-life disability: a field of dreams? J Gerontol A: Biol Sci Med Sci. 2009;64:1172–1174; discussion 5–6. doi: 10.1093/gerona/glp095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jacob ME, Marron MM, Boudreau RM, Odden MC, Arnold AM, Newman AB. Age, race, and gender factors in incident disability. J Gerontol A Biol Sci Med Sci. 2018;73:194–197. doi: 10.1093/gerona/glx194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stolz E, Gill TM, Mayerl H, Freidl W. Short-term disability fluctuations in late life. J Gerontol B: Psychol Sci Soc Sci. 2019;74:e135–140. doi: 10.1093/geronb/gbz089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pahor M, Anton SD, Beavers DP, et al. Effect of losartan and fish oil on plasma IL-6 and mobility in older persons. The ENRGISE pilot randomized clinical trial. J. Gerontol. A: Biol. Sci. Med. Sci. 2019;74:1612–1619. doi: 10.1093/gerona/gly277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 54. Ainsworth B, Cahalin L, Buman M, Ross R. The current state of physical activity assessment tools. Prog Cardiovasc Dis. 2015;57:387–395. doi: 10.1016/j.pcad.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 55. Schrack JA, Zipunnikov V, Goldsmith J, et al. Assessing the “physical cliff”: detailed quantification of age-related differences in daily patterns of physical activity. J Gerontol A Biol Sci Med Sci. 2014;69:973–979. doi: 10.1093/gerona/glt199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nastasi AJ, Ahuja A, Zipunnikov V, Simonsick EM, Ferrucci L, Schrack JA. Objectively measured physical activity and falls in well-functioning older adults: findings From the Baltimore Longitudinal Study of Aging. Am J Phys Med Rehabil. 2018;97:255–260. doi: 10.1097/PHM.0000000000000830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schrack JA, Kuo PL, Wanigatunga AA, et al. Active-to-sedentary behavior transitions, fatigability, and physical functioning in older adults. J Gerontol A: Biol Sci Med Sci. 2019;74:560–567. doi: 10.1093/gerona/gly243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7 [DOI] [PubMed] [Google Scholar]
- 59. Rebholz CM, Zheng Z, Grams ME, et al. Serum metabolites associated with dietary protein intake: results from the modification of diet in renal disease (MDRD) randomized clinical trial. Am J Clin Nutr. 2019;109:517–525. doi: 10.1093/ajcn/nqy202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Almanza-Aguilera E, Urpi-Sarda M, Llorach R, et al. Microbial metabolites are associated with a high adherence to a Mediterranean dietary pattern using a 1H-NMR-based untargeted metabolomics approach. J Nutr Biochem. 2017;48:36–43. doi: 10.1016/j.jnutbio.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 61. Guasch-Ferré M, Bhupathiraju SN, Hu FB. Use of metabolomics in improving assessment of dietary intake. Clin Chem. 2018;64:82–98. doi: 10.1373/clinchem.2017.272344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jin Q, Black A, Kales SN, Vattem D, Ruiz-Canela M, Sotos-Prieto M. Metabolomics and microbiomes as potential tools to evaluate the effects of the Mediterranean diet. Nutrients. 2019;11:207. doi:https://doi.org/10.3390/nu11010207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McCullough ML. Dietary assessment in the digital age: the ongoing quest for better methods. Am J Clin Nutr. 2018;107:1–2. doi: 10.1093/ajcn/nqx066 [DOI] [PubMed] [Google Scholar]
- 64. Hu FB, Willett WC. Current and future landscape of nutritional epidemiologic research. J Am Med Assoc. 2018;320:2073–2074. doi: 10.1001/jama.2018.16166 [DOI] [PubMed] [Google Scholar]
- 65. Birnie K, Cooper R, Martin RM, et al. ; HALCyon Study Team Childhood socioeconomic position and objectively measured physical capability levels in adulthood: a systematic review and meta-analysis. PLoS One. 2011;6:e15564. doi: 10.1371/journal.pone.0015564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Andersson MA. Chronic disease at midlife: do parent-child bonds modify the effect of childhood SES? J Health Soc Behav. 2016;57:373–389. doi: 10.1177/0022146516661596 [DOI] [PubMed] [Google Scholar]
- 67. Jadhav A, Weir D. Widowhood and depression in a cross-national perspective: evidence from the United States, Europe, Korea, and China. J Gerontol B Psychol Sci Soc Sci. 2018;73:e143–e153. doi: 10.1093/geronb/gbx021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bennett-Britton I, Teyhan A, Macleod J, Sattar N, Davey Smith G, Ben-Shlomo Y. Changes in marital quality over 6 years and its association with cardiovascular disease risk factors in men: findings from the ALSPAC prospective cohort study. J Epidemiol Community Health. 2017;71:1094–1100. doi: 10.1136/jech-2017-209178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hughes A, Smart M, Gorrie-Stone T, et al. Socioeconomic position and DNA methylation age acceleration across the life course. Am J Epidemiol. 2018;187:2346–2354. doi: 10.1093/aje/kwy155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Levine ME, Crimmins EM, Weir DR, Cole SW. Contemporaneous social environment and the architecture of late-life gene expression profiles. Am J Epidemiol. 2017;186:503–509. doi: 10.1093/aje/kwx147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cole SW, Capitanio JP, Chun K, Arevalo JM, Ma J, Cacioppo JT. Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc Natl Acad Sci USA. 2015;112:15142–15147. doi: 10.1073/pnas.1514249112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry. 2013;73:15–23. doi: 10.1016/j.biopsych.2012.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Puterman E, Gemmill A, Karasek D, et al. Lifespan adversity and later adulthood telomere length in the nationally representative US Health and Retirement Study. Proc Natl Acad Sci USA. 2016;113:E6335–E6342. doi: 10.1073/pnas.1525602113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Machtinger R, Bollati V, Baccarelli AA. Chapter 3-2-miRNAs and lncRNAs as biomarkers of toxicant exposure. In: McCullough SD, Dolinoy DC, eds. Toxicoepigenetics. London, United Kingdom: Academic Press; 2019:237–247. [Google Scholar]
- 75. Bollati V, Angelici L, Rizzo G, et al. Microvesicle-associated microRNA expression is altered upon particulate matter exposure in healthy workers and in A549 cells. J Appl Toxicol. 2015;35:59–67. doi: 10.1002/jat.2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nwanaji-Enwerem JC, Colicino E, Trevisi L, et al. Long-term ambient particle exposures and blood DNA methylation age: findings from the VA normative aging study. Environ Epigenet. 2016;2:dvw006. doi: 10.1093/eep/dvw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cacciottolo M, Wang X, Driscoll I, et al. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry. 2017;7:e1022. doi: 10.1038/tp.2016.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Niedzwiecki MM, Walker DI, Vermeulen R, Chadeau-Hyam M, Jones DP, Miller GW. The exposome: molecules to populations. Annu Rev Pharmacol Toxicol. 2019;59:107–127. doi: 10.1146/annurev-pharmtox-010818-021315 [DOI] [PubMed] [Google Scholar]
- 79. Gibson EA, Goldsmith J, Kioumourtzoglou MA. Complex mixtures, complex analyses: an emphasis on interpretable results. Curr Environ Health Rep. 2019;6:53–61. doi: 10.1007/s40572-019-00229-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kuh D, Cooper R, Sattar N, Welsh P, Hardy R, Ben-Shlomo Y. Systemic inflammation and cardio-renal organ damage biomarkers in middle age are associated with physical capability up to 9 years later. Circulation. 2019;139:1988–1999. doi: 10.1161/CIRCULATIONAHA.118.037332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kirkland JL, Stout MB, Sierra F. Resilience in aging mice. J Gerontol A Biol Sci Med Sci. 2016;71:1407–1414. doi: 10.1093/gerona/glw086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hadley EC, Kuchel GA, Newman AB; Workshop Speakers and Participants Report: NIA workshop on measures of physiologic resiliencies in human aging. J Gerontol A Biol Sci Med Sci. 2017;72:980–990. doi: 10.1093/gerona/glx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bell JT, Tsai PC, Yang TP, et al. ; MuTHER Consortium Epigenome-wide scans identify differentially methylated regions for age and age-related phenotypes in a healthy ageing population. PLoS Genet. 2012;8:e1002629. doi: 10.1371/journal.pgen.1002629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Alisch RS, Barwick BG, Chopra P, et al. Age-associated DNA methylation in pediatric populations. Genome Res. 2012;22:623–632. doi: 10.1101/gr.125187.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mendelson MM, Marioni RE, Joehanes R, et al. Association of body mass index with DNA methylation and gene expression in blood cells and relations to cardiometabolic disease: a Mendelian randomization approach. PLoS Med. 2017;14:e1002215. doi: 10.1371/journal.pmed.1002215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Joehanes R, Just AC, Marioni RE, et al. Epigenetic signatures of cigarette smoking. Circ Cardiovasc Genet. 2016;9:436–447. doi: 10.1161/CIRCGENETICS.116.001506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Robins C, McRae AF, Powell JE, et al. Testing two evolutionary theories of human aging with DNA methylation data. Genetics. 2017;207:1547–1560. doi: 10.1534/genetics.117.300217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bell CG, Xia Y, Yuan W, et al. Novel regional age-associated DNA methylation changes within human common disease-associated loci. Genome Biol. 2016;17:193. doi: 10.1186/s13059-016-1051-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen L, Dong Y, Bhagatwala J, Raed A, Huang Y, Zhu H. Effects of vitamin D3 supplementation on epigenetic aging in overweight and obese African Americans with suboptimal vitamin D status: a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2019;74:91–98. doi: 10.1093/gerona/gly223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Belsky DW, Huffman KM, Pieper CF, Shalev I, Kraus WE. Change in the rate of biological aging in response to caloric restriction: CALERIE biobank analysis. J Gerontol A Biol Sci Med Sci. 2017;73:4–10. doi: 10.1093/gerona/glx096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. O’Connell MDL, Marron MM, Boudreau RM, et al. Mortality in relation to changes in a healthy aging index: the health, Aging and Body Composition Study. J Gerontol A: Biol Sci Med Sci. 2019;74:726–732. doi: 10.1093/gerona/gly114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zarin DA, Tse T, Williams RJ, Carr S. Trial reporting in clinicalTrials.gov—the final rule. N Engl J Med. 2016;375:1998–2004. doi: 10.1056/NEJMsr1611785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23:377–381. doi: 10.1111/jep.12629 [DOI] [PubMed] [Google Scholar]