Abstract
Treatment with the mechanistic target of rapamycin (mTOR) inhibitor, rapamycin (RAP), alone and in combination with the antidiabetic drug, metformin (RAP+MET), extends lifespan in mice. The mechanisms underlying lifespan extension are unclear. One possibility is improved capacity for proteostatic maintenance. We have previously characterized peripheral protein synthesis rates following treatment with RAP. However, it is unknown if RAP+MET elicits similar changes, or if either treatment affects protein synthesis in the brain. We hypothesized that 8 weeks of treatment with RAP and RAP+MET would alter brain protein synthesis rates to reflect proteostatic processes. Using the stable isotopic tracer, deuterium oxide (D2O), we demonstrate in UM-HET3 mice that protein synthesis rates measured in whole brain were unaffected by treatment in young male mice, whereas RAP+MET decreased mitochondrial protein synthesis in young females. Conversely, RAP increased mitochondrial protein synthesis rates in older females. Activity through the AMPK/mTOR pathway was affected in a sex-specific manner in young mice, and minimal changes were observed in the older cohort. Thus, we establish D2O for measurements of biogenesis in the brain. These results provide initial insights into the effects of RAP and RAP+MET on brain protein synthesis. Additionally, these data emphasize that responses to slowed aging treatments vary with sex and age.
Keywords: Biology of aging, Deuterium oxide, Mitochondria, mTOR, Protein
The Interventions Testing Program (ITP) has shown that the mechanistic target of rapamycin (mTOR) inhibitor, rapamycin (RAP) (1), used alone or in combination with the antidiabetic drug metformin (RAP+MET) (2), extends lifespan in both sexes of the genetically heterogeneous UM-HET3 mouse. The mechanisms of lifespan extension following treatment with RAP and RAP+MET have yet to be completely elucidated, although one candidate factor is the ability to maintain protein homeostasis (proteostasis) across the lifespan.
Maintenance of proteostasis is crucial for cell and organismal function (3). The loss of proteostasis is a characteristic of aging and age-related diseases (4), and the capacity to maintain proteostasis is diminished with age (5). Thus, treatments which promote proteostatic maintenance may slow aging and improve healthspan (6). Our studies have assessed the contribution of protein synthesis to the maintenance of proteostasis, where we have demonstrated higher rates of protein synthesis dedicated to maintaining proteostasis in multiple models of slowed aging (7–9), including RAP (8). Whether or not the addition of MET to RAP has similar beneficial effects is not known.
Treatments that extend lifespan also presumably extend healthspan (10). However, the therapeutic potential of promising slowed aging treatments for specific age-related diseases is understudied. Neurological disorders are tightly linked with aging (11), and thus potentially amenable to slowed aging interventions. Since the number of individuals with cognitive decline is rising with the aging population, treatments that slow the progression or delay the onset of age-related neuronal function and cognitive decline are increasingly important (12).
There is a strong association between neurodegenerative diseases and loss of neuronal proteostasis (13). For example, the debilitating and prevalent neurological disorder, Alzheimer’s disease (AD), for which there is no prevention or treatment, is thought to be caused by proteotoxicity resulting from the accumulation of aggregated proteins (14). The prevailing theory is that AD symptomology may be caused by intracellular, insoluble amyloid-beta plaques (Aβ) and neurofibrillary tangles of tau protein (15). In AD, the impaired clearance of damaged proteins, at least in part by decreased protein turnover, compromises neuronal function and contributes to cognitive decline with age (16).
Both RAP and metformin have been shown to provide neuroprotection in transgenic models of neurodegenerative disease. In both the 3xTg-AD (17) and PDAPP (18) mouse models of AD, RAP improves cognitive outcomes and promotes autophagy in the brain while reducing levels of Aβ. RAP also improves outcomes in a mouse model of Parkinson’s disease through induction of mitophagy (19). On the other hand, studies of the effect of metformin on prevention of neurodegeneration are somewhat conflicting. For example, metformin extends lifespan of male mice in a model of Huntington’s disease (20), but increases the production of insoluble tau aggregate in the P301 second model of tauopathy (21). Similarly, in vitro studies evaluating the effect of metformin on the Aβ-producing enzyme BACE1 have yielded inconsistent results showing both stimulation (22) and inhibition (23).
Successful compounds from the ITP often have sex-specific lifespan-extending effects (1,2,24,25). Female mice have elevated blood levels of RAP at a given dose compared to males (25) and late-life treatment with RAP increased median lifespan 18% in females compared to 10% in males (24). In addition, RAP increased the expression of genes involved in slowed aging and protein turnover in the livers of female but not male C57BL/6 mice (26). In contrast, treatment with RAP+MET results in similar extension of lifespan in both sexes (2). However, recent work on detailed effects of RAP+MET in vivo has revealed disparities in glucose tolerance between male and female mice (27). Because of these discrepancies, studies directly comparing the effects of RAP and RAP+MET between sexes are needed.
We sought to determine whether treatment with RAP or RAP+MET affected brain protein synthesis rates in both sexes of genetically heterogeneous UM-HET3 mice, and to characterize whether such changes are dependent on age. To do so required characterizing protein synthesis of subcellular fractions from whole brain tissue. In addition, we measured mTOR and AMPK signaling to determine whether changes in activation of these pathways were similar to other tissues. We hypothesized that treatment with RAP and RAP+MET would alter protein synthesis in a manner consistent with increased proteostatic responses, and that this effect would be accompanied by mTOR inhibition in both treatments. From these studies, we conclude that there are sex differences in protein synthesis rates in untreated adult mice, and sex differences in the responses to RAP and RAP+MET treatment.
Methods
Animal Care
Male and female UM-HET3 mice were housed at the University of Michigan and kept on a 12-hour light/dark cycle. All procedures met the standards for facilities housing animals described in the Animals Welfare Act regulations, the Guide for the Care and Use of Laboratory Animals, and the Guide for Care and Use of Agricultural Animals in Agricultural Research and Teaching. Control and treatment diets were fed ad libitum for 8 weeks, starting at 4 (young) or 17 (old) months of age. Treatment diets included a standard control diet (Purina 5LG6, CON), standard chow supplemented with RAP (Purina 5LG6 with 14 parts per million RAP), and standard chow supplemented with RAP and metformin (Purina 5LG6 with 14 parts per million RAP and 1000 parts per million metformin, RAP+MET). Drug dosages in treatments were consistent with those used in the National Institute on Aging’s (NIA) ITP.
Stable Isotope Labeling
We labeled newly synthesized macromolecules with deuterium oxide (D2O) using our previously published procedures (28). After an intraperitoneal (i.p) bolus injection of 99% D2O, mice received 8% D2O enriched drinking water for the duration of the labeling period. Young male and female mice were sacrificed at 1, 4, 8, 15, or 32 days of labeling (n = 3 per group at each timepoint except female RAP and RAP+MET, n = 14 total in female RAP and RAP+MET and n = 15 total in all other groups). Administering of treatment diet was staggered so that all mice were sacrificed at exactly 8 weeks of treatment. In the older cohort, we were only able to obtain data on female mice that received labeling for 1, 4, and 15 days (n = 3 per group at each timepoint, n = 9 total per group). Therefore, data from stable isotopic measurements are not presented for male mice in the older cohort. Mice were euthanized by carbon dioxide following 12 hours of fasting. We collected whole brain tissue, as well as whole blood and bone marrow. All tissues were frozen immediately on liquid nitrogen and subsequently stored at −80°C.
Protein Synthesis
Frozen whole brain tissue was pulverized using liquid nitrogen-cooled mortar and pestle. In brief, tissue was fractionated into subcellular compartments by differential centrifugation as previously described (28,29) to obtain mitochondrial (MITO), cytosolic (CYTO), and mixed (MIX) protein fractions. In addition, plasma was prepared according to our previously published procedures (28,29) to determine the precursor pool enrichment. Protein fractions and water were derivatized for analysis of deuterium enrichment by gas chromatography-mass spectrometry (GC-MS, Agilent Technologies, GC 5975C/MS7890A). Deuterium enrichment of protein was used to calculate protein fraction new. From this data, one-phase associations were generated and two parameters of interest were calculated. The rate parameter (k) reflects the protein synthetic rate calculated as the slop of the regression curve. The plateau of the fraction new is representative of the proportion of the protein pool (with 1.0 equal to 100% of the protein pool) that is turning over. For a detailed description of the procedures see the Supplementary Material.
Calculations
The precursor alanine enrichment was calculated using the measured plasma deuterium enrichment adjusted by mass isotopomer distribution analysis (MIDA) (30). The protein fraction new at each time point was calculated using the enrichment of protein hydrolysates (product) divided by free alanine enrichment (precursor). From the fraction new, the rate of synthesis (k, 1 per day) was calculated using curve fitting. Since these were the first measurements using this methodology in brain, we analyzed the lines by both a linear increase, and a one-phase association. It was determined that one-phase associations best predicted the line characteristics. The fraction new of protein at which the curve plateaued, which reflects the portion of the protein pool subject to turnover, was determined using nonlinear regressions.
Western Blotting
See Supplementary Materials for a complete description of the Western blotting procedure. Briefly, whole brain tissue was lysed and quantified for protein content, diluted to consistent protein concentration, then 30 μg of protein was loaded and separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Samples were then transferred, blocked and incubated with 1:1000 primary antibody (Cell Signaling Technologies). Secondary antibodies were applied at a concentration of 1:5000 in 5% milk in TBST for 1 hour. Chemiluminescence images were taken using a FluorChem E imager (ProteinSimple, San Jose, CA). Membranes were then stripped (Restore Western Blot Stripping Buffer, Thermo Scientific, Rockford, IL), checked for effective stripping, and re-probed. Data are presented as the ratio of phosphorylated to total (P/T) protein (n = 6 per group).
Protein Aggregation
Protein aggregation was measured in lysed, whole brain homogenates (n = 3 per group) using a PROTEOSTAT protein aggregation assay (ENZ-51023, Enzo Life Sciences, Inc., Farmingdale, NY) prepared as instructed by the manufacturer. For a detailed description of the procedure see the Supplementary Material.
Statistics
Data are presented as means ± SEM. Differences in young mice were determined using a two-way (treatment by sex) analysis of variance and Sidak’s multiple comparisons post hoc test. In the older female cohort analysis was performed using a one-way analysis of variance and Tukey’s multiple comparison post hoc test. Significance was set a priori at p < .05.
Results
Protein Synthesis
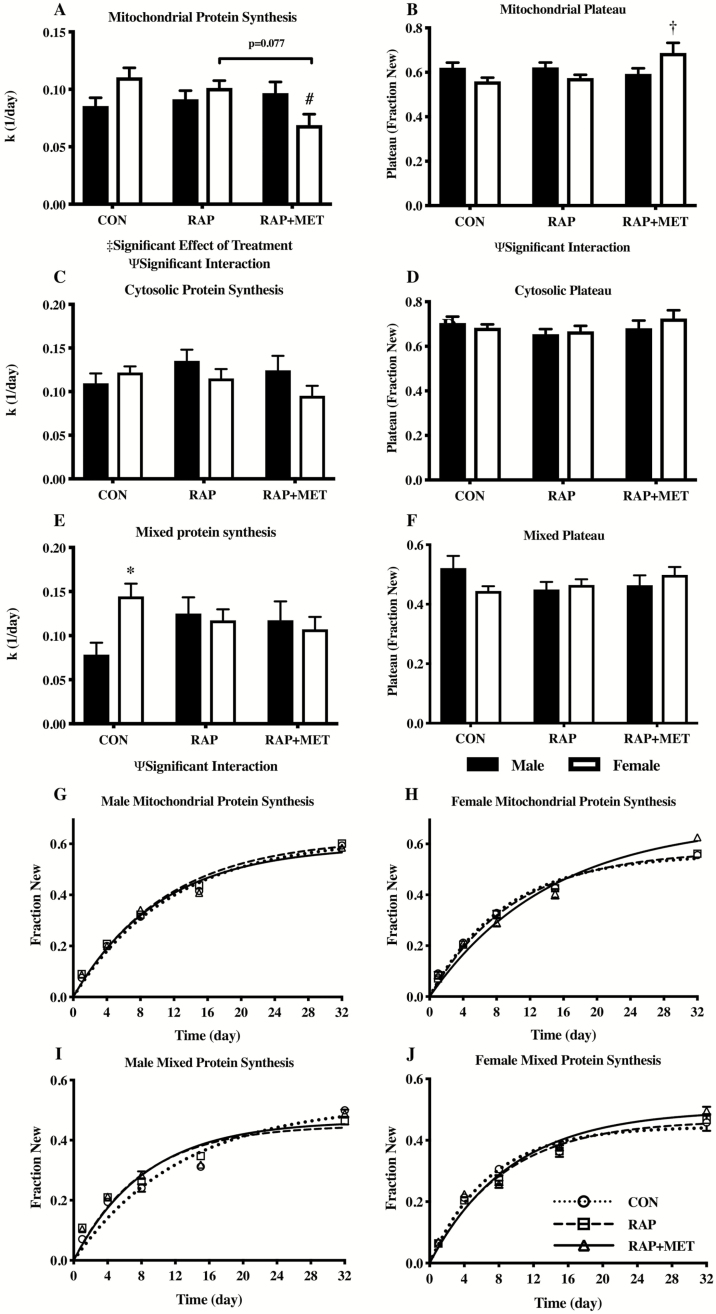
In the young cohort there was a significant effect of treatment and a significant interaction (sex by treatment) in the rate of synthesis in the mitochondrial protein fraction (Figure 1A). Mitochondrial protein synthesis was lower in female RAP+MET compared to female CON, as well as a trend towards decreased synthesis rates (p = .077) compared to female RAP (Figure 1A and H). In the female group, the plateau of fraction new mitochondrial protein, an indicator of the size of the mitochondrial protein pool which was subject to turnover within the measurement period, was significantly greater in RAP+MET compared to CON and RAP (Figure 1B and H). In contrast, no statistically significant differences in mitochondrial protein synthesis or plateau fraction new were detected between treatment groups in the male cohort. Finally, a significant sex by treatment interaction was also present in the plateau of mitochondrial fraction new (Figure 1B). There were no statistically significant differences between sex or treatment group in the rate of synthesis of the cytosolic protein fraction (Figure 1C). Additionally, no differences were observed in the plateau for fraction new cytosolic protein (Figure 1D). In the mixed protein fraction, there was a significant sex by treatment interaction in synthesis rates, and mixed protein synthesis was greater in females compared to males within CON (Figure 1E). However, there were no differences in the rate of mixed protein synthesis between treatment groups within either sex. The plateau for fraction new mixed protein was not different between any treatment groups or either sex (Figure 1F). Nonlinear regressions for mean values of fraction new in the mixed (Figures 1I and J) and mitochondrial (Figure 1G and H) protein fractions for young male and female mice are shown as representative figures. Regression lines for each subcellular protein fraction for both sexes in the young cohort are available in the supplemental materials (Supplementary Figure S1).
Figure 1.
Protein synthesis rates (k) and plateau of fraction new of mitochondrial (A and B), cytosolic (C and D), and mixed (E and F) protein in young male and female mice. Nonlinear regressions of protein synthesis rates from the mitochondrial (G and H) mixed (I and J) protein fractions in young males and females. Ψ Significant interaction effect (sex and treatment). ‡Significant effect of treatment. #Significantly different from CON within sex. *Significantly different from male within treatment group. †Significantly different from CON and RAP within sex. Black bars and white bars indicate males and females, respectively. n = 15 in all groups except female RAP and RAP+MET (n = 14). All values expressed as mean ± SEM.
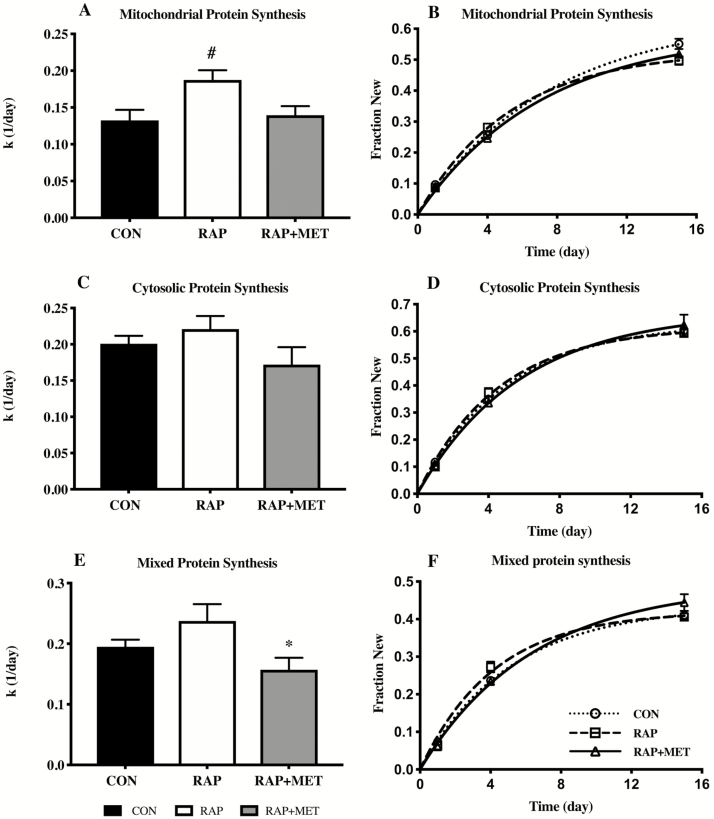
In the older female cohort, RAP increased the rate of mitochondrial protein synthesis rates compared to both CON and RAP+MET (Figure 2A and B). No significant differences were observed in the cytosolic protein fraction (Figure 2C and D). However, the rate of mixed protein synthesis was lower in RAP+MET relative to RAP (Figure 2E and F).
Figure 2.
Protein synthesis rates (k) and nonlinear regression plots of mitochondrial (A and B), cytosolic (C and D), and mixed (E and F) protein fraction new in older female mice. *Significantly different from RAP+MET. #Significantly different from CON and RAP+MET. n = 9 per group. All values expressed as mean ± SEM.
mTOR and AMPK Signaling
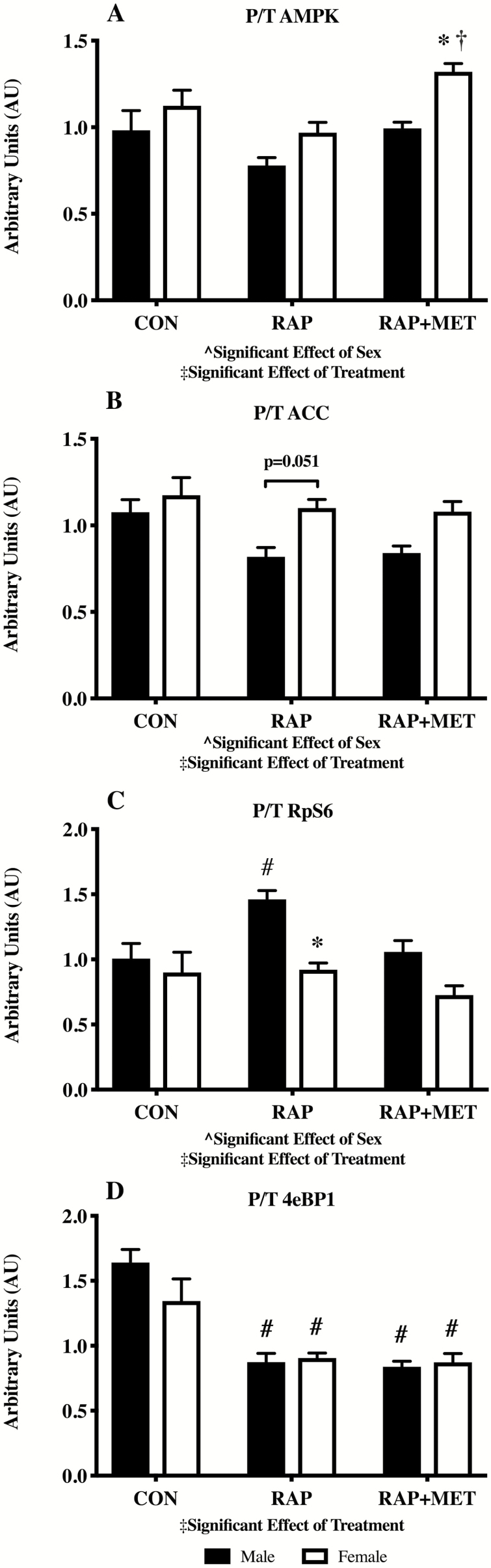
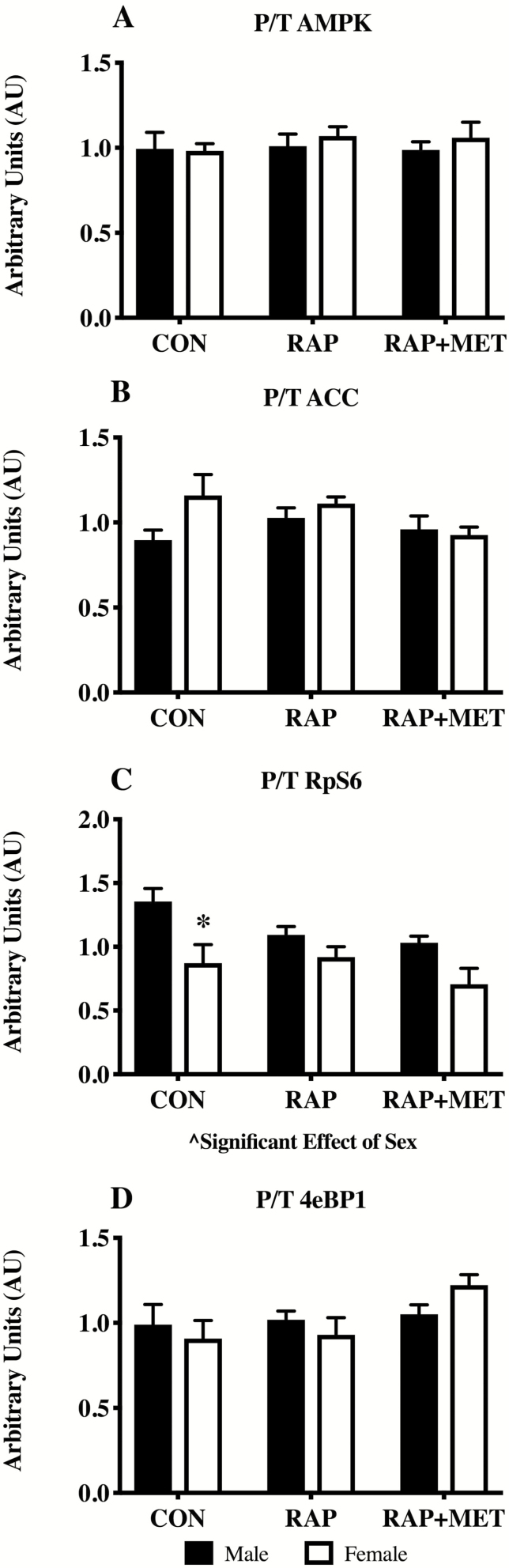
In the younger mice there was an overall significant effect of treatment on the phosphorylated protein/total protein (P/T) ratio of AMPK, ACC, RpS6, and 4eBP1 (Figure 3A–D). There was also a significant effect of sex on P/T for AMPK, ACC, and P/T RpS6. P/T for AMPK was greater in female RAP+MET compared female RAP and male RAP+MET (Figure 3A). No statistically significant differences were observed in pairwise comparisons between sex or treatment in P/T ACC although there was a trend for significance (p = .051) between RAP males and females (Figure 3B). The P/T ratio of RpS6 was greater in male mice treated with RAP compared to CON (Figure 3C) while P/T RpS6 was lower in female mice compared to males within RAP. In both sexes, RAP and RAP+MET decreased P/T 4eBP1 compared to CON (Figure 3D). In the older cohort there were no statistically significant differences in P/T AMPK, ACC, or 4eBP1 (Figure 4A, B, and D). However, there was a significant effect of sex in P/T RpS6 (Figure 4C), and P/T RpS6 was lower in female mice compared to males within CON (Figure 4C). Representative western blot images are provided in the supplemental figures for young (Supplementary Figure S2) and older (Supplementary Figure S3) mice.
Figure 3.
Western blot analysis of AMPK and mTOR signaling in young mice. Activation of AMPK (A), ACC (B), RpS6 (C), and 4eBP1 (D) expressed as ratios of phosphorylated to total protein content (P/T). ^Significant effect of sex. ‡Significant effect of treatment. *Significantly different from male within treatment group. †Significantly different from RAP within sex. #Significantly different from CON within sex. *Significantly different from male within treatment. n = 6 per group. All values expressed as mean ± SEM.
Figure 4.
Western blot analysis of AMPK and mTOR signaling in older mice. Activation of AMPK (A), ACC (B), RpS6 (C), and 4eBP1 (D) expressed as ratios of phosphorylated to total protein content (P/T). ^Significant effect of sex. *Significantly different from male within treatment. n = 6 per group. All values expressed as mean ± SEM.
Protein Aggregation
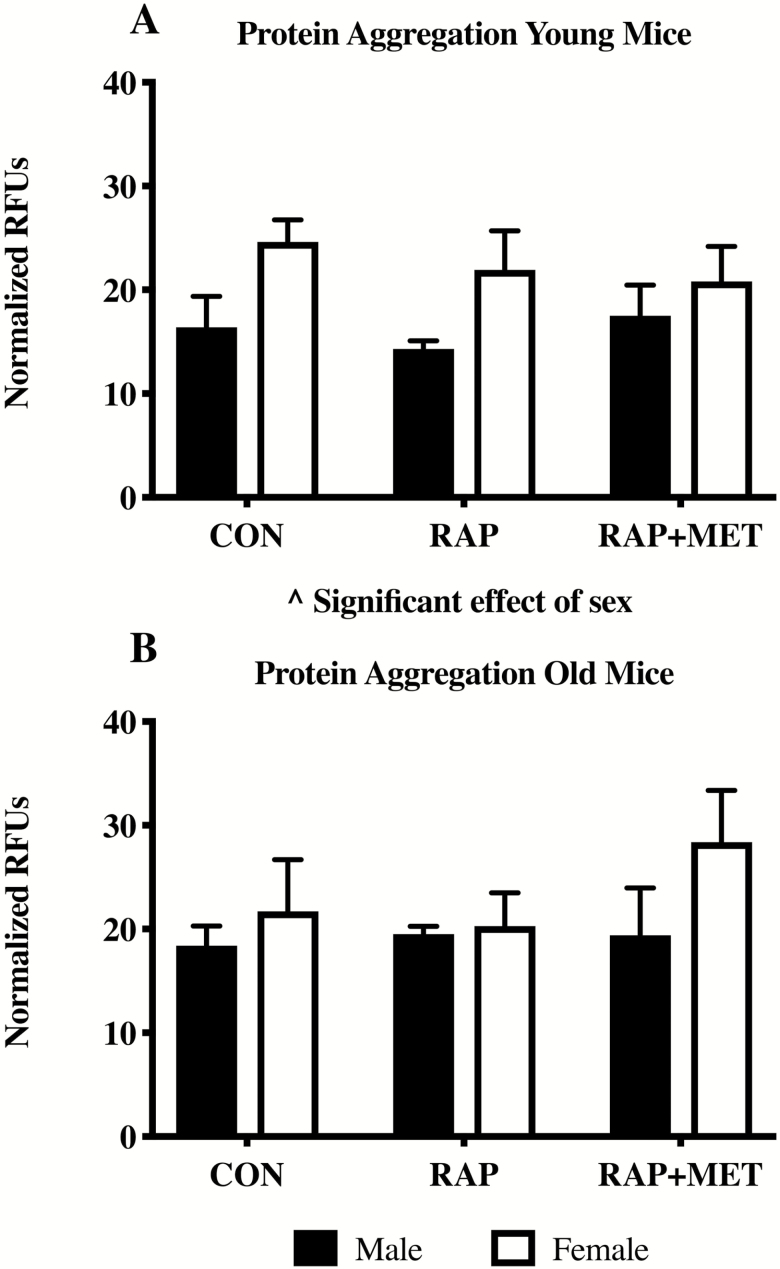
There were no treatment differences in protein aggregation in young or older mice (Figure 5A and B). However, in the young animals there was a significant effect of sex where female mice showed greater overall levels of protein aggregation (Figure 5A).
Figure 5.
Protein aggregation in young and old mice. Relative fluorescence units (RFUs) were normalized to total protein in each sample. ^Significant effect of sex (p < .05). n = 3 per group. All values expressed as mean ± SEM.
Discussion
The purpose of this study was to make long-term measurements of brain protein synthesis rates in mice treated with compounds used in the National Institute of Aging’s ITP. Our primary finding is that subcellular protein synthesis rates measured in whole brain homogenates differ between male and female UM-HET3 mice, with and without treatment with RAP and RAP+MET. Similarly, the effects of treatment on activity through the AMPK/mTOR signaling pathway were both sex and age-dependent. These data are important because they provide evidence that protein turnover in the brain is affected by treatments which have extended lifespan in the ITP. Additionally, our results emphasize the importance of considering sex differences and age in study design and interpretation of responses to lifespan-extending treatments.
D2O for Measurements of Biogenesis in the Brain
We have previously established the use of D2O for measurements of biogenesis in vitro (31) and in skeletal muscle, heart, and liver (8,9,32). We now add D2O methodologies for the determination of protein synthesis rates in the brain. Valid measures of the relationship between proteostasis, aging, and chronic disease are essential for gerontological research. Measurement of protein content is a limited proxy for biogenesis (7), since content by itself does not indicate turnover. In addition, transcriptional outcomes are limited because of the extensive post-transcriptional mechanisms that influence protein synthesis (33) Assessments made using D2O overcome these limitations and appropriately reflect the dynamic nature of protein synthesis over the long term. Therefore, these measurements provide the groundwork for future studies aiming to examine the interplay between proteostasis, aging, and neurodegeneration.
Brain Protein Synthesis Rates With Slowed Aging Treatments
Comparable data on the effects of RAP+MET on protein synthesis in brain or other tissues have yet to be published. However, our finding that RAP did not influence mitochondrial protein synthesis in young mice is in contrast with similar work investigating the effects of RAP on turnover of electron transport chain proteins in brain tissue from C57BL/6 mice (34). Karunadharma and colleagues (34). found that treatment with RAP modestly increased average brain electron transport chain protein half-life in young female mice. Additionally, the same study found that overall electron transport chain half-life was not affected by RAP in an older group of female mice, which is inconsistent with our finding that RAP increased mitochondrial protein synthesis in older females. Several minor differences in study design such as mouse strain, isotopic tracer used, duration of labeling at each timepoint, and number of timepoints included may have played a role in the discrepant findings. One important distinction between the two studies is the measurement of bulk mitochondrial protein synthesis in the current study compared to a targeted proteomic approach utilized by Karunadharma and colleagues. The targeted proteomic approach analyzes the turnover of individual electron transport chain complexes and their subunits, whereas our measurement of bulk mitochondrial protein synthesis likely includes additional mitochondrial proteins which may underlie the difference in results.
It is important to contextualize results from the current study with previous stable isotopic tracer work that has revealed variable responses between tissues with slowed aging interventions. In our study, we found that RAP had no effect on mitochondrial biogenesis in brain from younger female UM-HET3 mice. In comparably aged female C57BL/6 mice RAP increased mitochondrial protein half-life to a greater degree in skeletal muscle compared to other tissues including brain (34). However, a similar study by our group that utilized D2O in young UM-HET3 mice found reduced rates of mitochondrial biogenesis in heart but not skeletal muscle following treatment with RAP (8). In addition, others have found that RAP decreased rates of hepatic global and mitochondrial protein turnover in C57BL/6 mice at both young and old ages (35), a finding which differs from our results in brain from UM-HET3 mice. Although the potential influence of differing study designs must be acknowledged, when considered with prior studies on slowed aging treatments our findings underscore the importance of tissue specificity in measurements of protein synthesis.
Reduced protein synthesis is widely considered to be a shared characteristic of models of slowed aging (36). However, our group has advocated that where protein synthesis is directed, for example, proliferation or somatic maintenance, is an important consideration (37). Because we were not able to measure rates of proliferation in the current study, we were unable to make inferences about somatic maintenance with slowed aging treatments in the brain. The lower rates of mitochondrial protein synthesis observed in the young female RAP+MET group would be in line with decreased protein synthesis with slowed aging treatments. However, it is possible that reduced rates of cell proliferation are responsible for this outcome.
If the rate of cell division is a primary determinant in our measurements of protein synthesis in the brain, then the increased mitochondrial protein synthesis in the older female RAP group is somewhat puzzling given that RAP is a strong cell cycle inhibitor (38). Although mTOR inhibition decreases global protein synthesis through inhibition of cap-dependent translation, certain mitochondrial proteins are preferentially translated (39). Additionally, cellular proliferation in the brain decreases with age (40), which may reduce the effect of RAP on cell division in the brain of older mice. Therefore, selective translation of mitochondrial proteins in combination with a diminished effect of RAP on cell proliferation may have contributed to the increased mitochondrial protein synthesis in older female RAP-treated mice.
Heterogeneity of Outcomes With Treatment, Sex, and Age
Sex differences in response to slowed aging treatments are well documented (1,2,24,25). We also observed sex-specific changes in our measurements of proteostatic maintenance. One unexpected finding was that in a few cases, such as mixed protein synthesis in the young cohort, males and females were different in the absence of treatment. This observation highlights the importance of considering sex in study design and interpretations of findings. Comparisons between sexes in untreated groups are not common in gerontological research, so it is difficult to contextualize our data. However, it appears that, in general, untreated female UM-HET3 mice live longer than their male counterparts (2,25,41). For instance, Strong et al. (2016) reported that median lifespan of untreated UM-HET3 from pooled groups across multiple testing sites was 874 days in female mice and 780 days in male mice (an 11.4% difference) (2). It is possible that subtle physiological differences in proteostatic processes contribute to this discrepancy.
Increased P/T RpS6 in young male mice was the only response to RAP that was unique to either sex in our study. Lack of sexual divergence in response to treatment with RAP is somewhat atypical (25,26) and it has been shown that female mice have greater levels of RAP in the blood compared to males at a given dose (25). However, our findings may be explained by the use of an 8-week treatment in the current study rather than lifelong treatment. It is worth noting that others have reported sex-specific effects of RAP after only 3 months of treatment (42), albeit at much higher doses (daily i.p. injection equivalent of approximately 378 ppm RAP) than those in our study.
In contrast, several outcomes were affected by RAP+MET in female mice only. Specifically, we found in the young cohort of female mice that mitochondrial protein synthesis and mitochondrial plateau fraction new were decreased and increased, respectively, by RAP+MET. Similarly, P/T AMPK was increased by RAP+MET in females compared to female CON and male RAP+MET. That RAP+MET elicited sexually divergent responses is somewhat unexpected given that the ITP found RAP+MET to increase lifespan nearly identically in male and female mice (2). However, these findings fall in line with recent work which showed that female UM-HET3 mice treated with RAP+MET have improved glucose tolerance compared to similarly treated males (27).
Decreased mitochondrial protein synthesis in female RAP+MET was the only significant effect of either RAP or RAP+MET on protein synthesis rates in the young group. The likely explanation for the reduced protein synthesis with RAP+MET is decreased cell proliferation. Although neurons are thought to be post-mitotic, there are several proliferative cell types in our tissue preparation including progenitor, glial, astrocytic, and endothelial cells. Some have estimated a high capacity for cell turnover in mouse brain, and that as many as 180,000 new cells may be produced daily in 4-month-old mice (40). We have previously demonstrated decreased rates of proliferation in peripheral tissue in response to treatment with RAP (8) as well as other slowed aging treatments (29,32). It also has been shown that RAP and metformin can act in an additive manner to inhibit cell division (43). Finally, although the broad effects of AMPK activation on proliferative cell types in the brain have not been directly investigated, stimulation of AMPK decreases proliferation in cultured neural stem cells through cell cycle arrest at G1/G0 (44). Therefore, it is likely that decreased proliferation following treatment with RAP+MET underlies the significantly decreased mitochondrial protein synthesis, and that treatment with RAP alone was not a sufficient stimulus for similar results. This explanation would be congruent with an increased sensitivity of female mice to RAP compared to males (25), potentially explaining the lack of a similar effect in male mice.
We report the plateau of fraction new since this represents the proportion of the protein pool that is synthesizing (45). In most cases these values ranged from 40 to 50%, indicating that a substantial portion of the protein pool is not turning over, or is very slowly. This novel characterization can be used to measure how much of the brain protein pool is resistant to turnover, which can increase due to impaired proteostatic processes in neurodegenerative disease. In some scenarios proteins which do not incorporate label may be insoluble protein aggregates. Our initial measurements of protein aggregation in this study do not support that there was a change in aggregation with treatment in either group, although a smaller number of replicates used in that assay may be a contributing factor. In young female RAP+MET the plateau of fraction new was increased compared to both RAP and CON, indicating that a larger proportion of the pool was turning over. Although the fraction new in young female RAP+MET was lower than RAP and CON within sex, since a larger total protein pool was turning over, the absolute protein turnover was likely not changed from other groups. Determining the differences in pool sizes and fractions of pools turning over is an intriguing future direction using the current methods.
Another consistent theme was that several effects of RAP and RAP+MET were not shared. Given that treatment with RAP (24) or RAP+MET (2) results in different degrees of lifespan extension, the notion that RAP and RAP+MET differently alter cellular processes may be expected to some extent. Experimental interventions which extend lifespan may uniquely impact cellular or biological processes. For instance, the effects of RAP can be distinguished from those of dietary restriction (25). As an example, fasting levels of insulin, IGF-1, and fibroblast growth factor 21 (FGF-21) are decreased by dietary restriction but not by RAP (25). Additionally, some differences in treatment effect that we observed were expected. For example, that RAP+MET increased P/T AMPK whereas RAP failed to invoke a similar change is not surprising considering that metformin is a potent activator of AMPK, while RAP has no reported similar effects. However, the causes of the sex-specific response in AMPK activation to RAP+MET require further investigation.
Finally, we also observed treatment effects that differed by age groups. One example is that RAP stimulated mitochondrial protein synthesis in older female mice, but this effect was not seen in the young group. One potential explanation for the disparate findings in mitochondrial protein synthesis between age groups in RAP-treated female mice is that different portions of the proteome are represented by the different timepoints used. However, analysis of data from the young cohort which included only 1-,4-, and 15-day timepoints revealed no statistically significant differences between treatment groups (data not shown). Therefore, it is unlikely that differences in sampling timepoints explain the increased mitochondrial protein synthesis in old but not young RAP-treated female mice. One comprehensive analysis of the effects of RAP on whole body physiology and function concluded that treatment effects were largely shared between young and old mice (46). However, that study used male C57BL/6 mice and it is possible that age-dependent changes in response to RAP are sex-specific. In the current study, we observed that treatment with RAP differently affected young and old female mice. Unfortunately, in the current study, we were not able to obtain similar data on older male mice to make sex by age comparisons. From our measurements in this study, we cannot determine why young and old mice respond differently to treatment. Future studies that make similar measurements in a region-specific manner or determine the effect of treatment on individual proteins may help to resolve these discrepant results.
AMPK/mTOR Signaling
Some of the effects of RAP and RAP+MET on AMPK/mTOR signaling were somewhat unexpected. RAP+MET increased activation of AMPK in the young female cohort but failed to do so in young males or in either sex in the older cohort. Although in vivo data on the effects of RAP+MET are limited, one study showed that RAP+MET had marginal effects on AMPK in liver (27), an organ which is particularly sensitive to metformin. Interestingly, the same study found that mice treated with RAP+MET had lower blood levels of metformin than mice treated only with metformin and not RAP (27). It is possible a similar effect underlies the lack of increased AMPK activation by RAP+MET in the current study. Finally, treatment with metformin alone increases P/T AMPK in brain of male C57BL/6J mice in a region-specific manner (47), which would not be reflected in our results due to the use of whole brain homogenates. Differences between sexes in activation of AMPK in the brain following treatment with RAP+MET may implicate differences in proteostatic control and should be further investigated.
Another unexpected finding was that RAP increased P/T RpS6 in young male mice, despite decreased P/T 4eBP1 in both sexes of each treatment group. RpS6 is a regulated by mTOR through S6K1 (38) and is a common marker for mTOR activation. On the other hand, 4eBP1 is directly inhibited by mTORC1. It has been shown that in under some conditions P/T 4eBP1 is more closely associated with mTOR activation than RpS6 (48). The discrepancy between phosphorylation status of the two proteins in the younger male group may be due to the fact that RpS6 has multiple upstream effectors through S6K (49), whereas phosphorylation of 4eBP1 is under direct control of mTOR (38). Additionally, it has been previously demonstrated that reduction of P/T RpS6 following treatment with RAP is not uniform throughout all tissues (8). Thus, we interpret our finding of decreased P/T 4eBP1 in response to RAP and RAP+MET to indicate mTOR inhibition.
In the older cohort activity through AMPK/mTOR pathway was essentially unaffected by either RAP or RAP+MET. One possible explanation is that older mice are less sensitive to a given dose of RAP or RAP+MET, possibly due to differences in bioavailability, tissue drug uptake, or decreased responsiveness of drug receptor and downstream intracellular signaling. Although it is known that late-life RAP administration increases lifespan in mice (1) brain-specific outcomes have not previously been assessed. Therefore, the age of onset of treatment has potential implications for effectiveness against neurodegenerative disease.
Implications
Sex differences in responses to lifespan-extending treatments in organisms of varying complexity have previously been discussed (41). To this body of knowledge, we add sex differences in response to treatment with RAP+MET in the UM-HET3 mouse. In addition, these data show that measurements of protein synthesis in the brain are differently affected by RAP and RAP+MET and that these effects are age-dependent. A key point is that determination of the processes that underlie aging and slowed aging interventions are complicated by sex, age, and treatment, even within a given model organism. Studies that examine only one sex or age group may miss important treatments effects or overgeneralize findings. Importantly, these data also show that treatments currently used in the ITP have demonstrable effects on brain protein synthesis rates and cellular signaling. These findings justify future research to examine effects of RAP and RAP+MET in models of neurodegenerative disease. However, it is important to note that the cognitive and functional significance of these findings in the UM-HET3 mouse is currently unknown. More research is needed to understand the mechanisms and relevance of brain mitochondrial biogenesis in response to RAP and RAP+MET, as well how those processes relate to cognitive function.
Limitations
There are several limitations to the current study. First, a lack of functional outcomes to determine the effect of treatment on cognition limits our interpretation of changes in brain protein synthesis rates and cellular signaling. Inclusion of such measurements would greatly enhance our understanding of the relationship between proteostatic maintenance, cognitive function, and slowed aging treatments. In addition, we did not observe differences in response to treatment in protein aggregation in samples from whole brain homogenates in young or old mice. Typically, genetic intervention is required for studies of human-like neurodegeneration in mice. As a result, these findings may poorly reflect the potential effects of RAP and RAP+MET in human neurodegeneration. It should also be noted that the inability to measure cell-specific changes is an important limitation, and futures studies should investigate the effects of RAP and RAP+MET on specific cell populations within the CNS. Lastly, the addition of protein synthesis data from an older male cohort would provide further insight into the influence of age and sex in response to treatment.
Conclusions
Treatment with RAP and RAP+MET extends lifespan in both male and female mice (1,2). Results from the current study demonstrate the effects of RAP and RAP+MET on various aspects of proteostatic maintenance in the brain of the UM-HET3 mouse. Our primary findings are that (a) young showed different rates of brain protein synthesis with and without treatment, (b) RAP+MET decreases mitochondrial protein synthesis only in young female mice that are likely reflective of decreased cellular proliferation, and that (c) the effects of RAP on protein synthesis in female mice are not shared between young and old mice. Additionally, we show that D2O can be used as a stable isotopic tracer to measure protein synthesis rates and the fraction of the pool that is turning over in the brain. Future studies should address these measurements as they relate to functional outcomes. Finally, comparable measurements made in transgenic models of neurodegenerative disease will increase our understanding of human neurodegeneration.
Funding
This work was supported by the National Institute of Aging at the National Institute of Health (R01 AG042569-05 to K.L.H. and B.F.M.). The work at Michigan was supported by the Paul F. Glenn Center for Biology of Aging Research.
Conflict of interest statement
None reported.
Supplementary Material
Acknowledgments
B.F.M., K.L.H., and R.A.M. developed study concept and design. R.A.M. oversaw animal care and tissue collection. J.J.R. and M.A.L. collected and analyzed protein synthesis, protein aggregation, and Western blot data. F.F.P. III assisted with GC-MS data collection and analysis. J.J.R. wrote the manuscript and all other authors provided feedback. We thank Lori Roberts, Roxann Alonzo, and Natalie Perry for animal husbandry assistance.
References
- 1. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strong R, Miller RA, Antebi A, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15:872–884. doi: 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448 [DOI] [PubMed] [Google Scholar]
- 4. Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84:435–464. doi: 10.1146/annurev-biochem-060614-033955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844 [DOI] [PubMed] [Google Scholar]
- 7. Miller BF, Drake JC, Naylor B, Price JC, Hamilton KL. The measurement of protein synthesis for assessing proteostasis in studies of slowed aging. Ageing Res Rev. 2014;18:106–111. doi: 10.1016/j.arr.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drake JC, Peelor FF III, Biela LM, et al. Assessment of mitochondrial biogenesis and mTORC1 signaling during chronic rapamycin feeding in male and female mice. J Gerontol A Biol Sci Med Sci. 2013;68:1493–1501. doi: 10.1093/gerona/glt047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drake JC, Bruns DR, Peelor FF III, et al. Long-lived Snell dwarf mice display increased proteostatic mechanisms that are not dependent on decreased mTORC1 activity. Aging Cell. 2015;14:474–482. doi: 10.1111/acel.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol. 2016;594:2001–2024. doi: 10.1113/jphysiol.2014.282665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erkkinen MG, Kim M-O, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018;10(4):a033118. doi: 10.1101/cshperspect.a033118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381 [DOI] [PubMed] [Google Scholar]
- 13. Sweeney P, Park H, Baumann M, et al. Protein misfolding in neurodegenerative diseases: implications and strategies. Transl Neurodegener. 2017;6:6. doi: 10.1186/s40035-017-0077-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers. 2015;1:15056. doi: 10.1038/nrdp.2015.56 [DOI] [PubMed] [Google Scholar]
- 15. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- 16. Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-β Levels in a Mouse Model of Alzheimer’s Disease. Ferrari PF, ed. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siddiqui A, Bhaumik D, Chinta SJ, et al. Mitochondrial Quality Control via the PGC1α-TFEB Signaling Pathway Is Compromised by Parkin Q311X Mutation But Independently Restored by Rapamycin. J Neurosci. 2015;35:12833–12844. doi: 10.1523/JNEUROSCI.0109-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma TC, Buescher JL, Oatis B, et al. Metformin therapy in a transgenic mouse model of Huntington’s disease. Neurosci Lett. 2007;411:98–103. doi: 10.1016/j.neulet.2006.10.039 [DOI] [PubMed] [Google Scholar]
- 21. Barini E, Antico O, Zhao Y, et al. Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Mol Neurodegener. 2016;11:16. doi: 10.1186/s13024-016-0082-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y, Zhou K, Wang R, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009;106:3907–3912. doi: 10.1073/pnas.0807991106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hettich MM, Matthes F, Ryan DP, et al. The anti-diabetic drug metformin reduces BACE1 protein level by interfering with the MID1 complex. Paudel HK, ed. PLoS One. 2014;9(7):e102420. doi: 10.1371/journal.pone.0102420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller RA, Harrison DE, Astle CM, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu Z, Sunchu B, Fok WC, Alshaikh N, Pérez VI. Gene expression in the liver of female, but not male mice treated with rapamycin resembles changes observed under dietary restriction. Springerplus. 2015;4:174. doi: 10.1186/s40064-015-0909-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weiss R, Fernandez E, Liu Y, Strong R, Salmon AB. Metformin reduces glucose intolerance caused by rapamycin treatment in genetically heterogeneous female mice. Aging (Albany NY). 2018;10(3):386. doi: 10.18632/aging.101401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller BF, Robinson MM, Reuland DJ, et al. Calorie restriction does not increase short-term or long-term protein synthesis. J Gerontol A Biol Sci Med Sci. 2013;68:530–538. doi: 10.1093/gerona/gls219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Drake JC, Bruns DR, Peelor FF III, et al. Long-lived crowded-litter mice have an age-dependent increase in protein synthesis to DNA synthesis ratio and mTORC1 substrate phosphorylation. Am J Physiol Endocrinol Metab. 2014;307:E813–E821. doi: 10.1152/ajpendo.00256.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am J Physiol. 1999;276(6 Pt 1):E1146–1170. http://www.ncbi.nlm.nih.gov/pubmed/10362629. Accessed February 5, 2018. [DOI] [PubMed] [Google Scholar]
- 31. Miller BF, Wolff CA, Peelor FF, Shipman PD, Hamilton KL. Modeling the contribution of individual proteins to mixed skeletal muscle protein synthetic rates over increasing periods of label incorporation. J Appl Physiol. 2015;118(6):655–661. doi: 10.1152/japplphysiol.00987.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller BF, Robinson MM, Bruss MD, Hellerstein M, Hamilton KL. A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell. 2012;11:150–161. doi: 10.1111/j.1474-9726.2011.00769.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwanhäusser B, Busse D, Li N, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098 [DOI] [PubMed] [Google Scholar]
- 34. Karunadharma PP, Basisty N, Chiao YA, et al. Respiratory chain protein turnover rates in mice are highly heterogeneous but strikingly conserved across tissues, ages, and treatments. FASEB J. 2015;29:3582–3592. doi: 10.1096/fj.15-272666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karunadharma PP, Basisty N, Dai DF, et al. Subacute calorie restriction and rapamycin discordantly alter mouse liver proteome homeostasis and reverse aging effects. Aging Cell. 2015;14:547–557. doi: 10.1111/acel.12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Basisty N, Meyer JG, Schilling B. Protein turnover in aging and longevity. Proteomics. 2018;18(5–6):e1700108. doi: 10.1002/pmic.201700108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hamilton KL, Miller BF. Mitochondrial proteostasis as a shared characteristic of slowed aging: the importance of considering cell proliferation. J Physiol. 2017;595:6401–6407. doi: 10.1113/JP274335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–3594. doi: 10.1242/jcs.051011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zid BM, Rogers AN, Katewa SD, et al. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bordiuk OL, Smith K, Morin PJ, Semënov MV. Cell proliferation and neurogenesis in adult mouse brain. PLoS One. 2014;9:e111453. doi: 10.1371/journal.pone.0111453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Austad SN, Fischer KE. Sex Differences in Lifespan. Cell Metab. 2016;23:1022–1033. doi: 10.1016/j.cmet.2016.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bitto A, Ito TK, Pineda VV, et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. Elife. 2016;5: pii: e16351. doi: 10.7554/eLife.16351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang JW, Zhao F, Sun Q. Metformin synergizes with rapamycin to inhibit the growth of pancreatic cancer in vitro and in vivo. Oncol Lett. 2018;15:1811–1816. doi: 10.3892/ol.2017.7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zang Y, Yu LF, Nan FJ, Feng LY, Li J. AMP-activated protein kinase is involved in neural stem cell growth suppression and cell cycle arrest by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside and glucose deprivation by down-regulating phospho-retinoblastoma protein and cyclin D. J Biol Chem. 2009;284:6175–6184. doi: 10.1074/jbc.M806887200 [DOI] [PubMed] [Google Scholar]
- 45. Zhou H, Wang SP, Herath K, et al. Tracer-based estimates of protein flux in cases of incomplete product renewal: evidence and implications of heterogeneity in collagen turnover. Am J Physiol Endocrinol Metab. 2015;309:E115–E121. doi: 10.1152/ajpendo.00435.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neff F, Flores-Dominguez D, Ryan DP, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–3291. doi: 10.1172/JCI67674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Picone P, Vilasi S, Librizzi F, et al. Biological and biophysics aspects of metformin-induced effects: cortex mitochondrial dysfunction and promotion of toxic amyloid pre-fibrillar aggregates. Aging (Albany NY). 2016;8:1718–1734. doi: 10.18632/aging.101004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Clark C, Shah S, Herman-Ferdinandez L, et al. Teasing out the best molecular marker in the AKT/mTOR pathway in head and neck squamous cell cancer patients. Laryngoscope. 2010;120:1159–1165. doi: 10.1002/lary.20917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.