Abstract
Rationale: Although both type 2 diabetes mellitus (T2DM) and obstructive sleep apnea (OSA) are independently recognized as risk factors for cardiovascular disease, little is known about their interaction.
Objectives: We hypothesized that T2DM and OSA act synergistically to increase vascular risk, and that treatment of OSA would improve vascular reactivity in patients with T2DM plus OSA.
Methods: Cross-sectional study of 141 adults with T2DM, OSA, T2DM plus OSA, and control subjects, followed by a 3-month, parallel-arm, randomized, placebo-controlled trial comparing active and sham continuous positive airway pressure (CPAP) in 53 adults with T2DM plus OSA. Endothelium-dependent macro- and microvascular reactivity (flow-mediated dilation [FMD] of the brachial artery and acetylcholine-induced dilation of forearm microvasculature, respectively) and cardiovascular magnetic resonance to assess left- and right-ventricular mass/volume.
Results: Mean (±SD) FMD was 6.1 (±4.0)%, 7.3 (±3.6)%, 6.8 (±4.5)%, and 4.8 (±2.9)% in control subjects, T2DM only, OSA only, and T2DM plus OSA, respectively. We observed a significant T2DM × OSA interaction on FMD, such that the mean effect of OSA in those with T2DM was 3.1% (95% confidence interval [CI], 0.6 to 5.6) greater than the effect of OSA in those without T2DM. A total of 3 months of CPAP resulted in a mean absolute increase in FMD of 0.3% (95% CI, −1.9 to 2.5; primary endpoint), with a net improvement of 1.1% (95% CI, −1.4 to 3.6) among those with adherence of 4 h/night or greater. A significant T2DM × OSA interaction was found for both left ventricular (LV) and right ventricular end-diastolic volume, such that OSA was associated with a 22.4 ml (95% CI, 3.2 to 41.6) greater LV end-diastolic volume and 23.2 ml (95% CI, 2.6 to 43.8) greater right ventricular end-diastolic volume in those with T2DM compared with the impact of OSA in those without T2DM. We observed a net improvement in LV end-diastolic volume of 8.7 ml (95% CI, −7.0 to 24.4).
Conclusions: The combination of T2DM plus OSA is associated with macrovascular endothelial dysfunction beyond that observed with either disease alone. CPAP for 3 months did not significantly improve macrovascular endothelial function in the intent-to-treat analysis; however, cardiovascular magnetic resonance results suggest that there may be a beneficial effect of CPAP on LV diastolic volume.
Clinical trial registered with www.clinicaltrials.gov (NCT01629862).
Keywords: endothelium, metabolism, cardiovascular, obstructive sleep apnea, type 2 diabetes mellitus
Due to the ongoing obesity pandemic, life expectancy has been predicted to decrease in the future for the first time (1). Obesity is a causal risk factor for both type 2 diabetes mellitus (T2DM) and obstructive sleep apnea (OSA), such that the presence of OSA in those with T2DM is extremely common with estimates as high as 87% (2). Although both conditions are independently recognized as risk factors for cardiovascular disease (3–5), few studies have addressed their interaction (6, 7). If such an interaction exists, then OSA may represent a plausible treatment target to lower cardiovascular complication rates in patients with T2DM. We sought to test the hypotheses that the impact of OSA on cardiovascular function would be worse in those with T2DM compared with those without, and that treatment of OSA in patients with T2DM plus OSA would improve cardiovascular function. By simultaneously assessing the macrocirculation, microcirculation, and heart, combined with a randomized, controlled trial (RCT) of continuous positive airway pressure (CPAP) therapy versus sham CPAP for OSA, our objective was to assess the role of OSA as a reversible risk factor for cardiovascular disease in patients with T2DM.
Methods
Data were collected at Brigham and Women’s Hospital and Beth Israel Deaconess Medical Center. Approval was obtained from the institutional review boards at Partners Healthcare (2011P001308) and the University of Pittsburgh where statistical analyses were undertaken (PRO16020594). All participants provided written informed consent in the presence of a single investigator responsible for enrollment. The trial was registered at clinicaltrials.gov (NCT01629862) on June 28, 2012. Data sharing may be undertaken with institutional review board approval by contacting the corresponding author. The full protocol is also available from the corresponding author. The first participant consented to the trial on July 12, 2012. The final trial visit took place on February 5, 2017.
Design
This study included a cross-sectional component (comparing participants with no T2DM and no OSA (control subjects), T2DM only, OSA only, and T2DM + OSA) followed by a 3-month, single-center, parallel-arm, randomized, placebo-controlled trial comparing active and sham CPAP in participants with T2DM plus OSA. Participants and all investigators, apart from one coinvestigator responsible for randomization and external medical monitors responsible for safety monitoring, were blinded to treatment allocation.
Eligibility Criteria: Cross-Sectional Study
Recruitment took place at local clinics and through community advertisements. Subjects, aged 18–70 years, fluent in English, with no history of sleep disorders other than OSA, no history of home CPAP use, and no current treatment for OSA, were eligible for participation. OSA was defined as an apnea–hypopnea index (AHI) 10 events/h or greater based on the average of 2 nights of home sleep testing (ApneaLink Plus; ResMed), using a 30% reduction in flow and 4% desaturation to define hypopneas. No OSA was defined as AHI less than 10 events/h. T2DM was defined as fasting plasma glucose 126 mg/dL or greater or use of hypoglycemic medication. No T2DM was defined as fasting glucose less than 126 mg/dL, no use of hypoglycemic medication, and HbA1c (glycated hemoglobin) less than 6.5%. Participants with T2DM plus OSA met criteria for both T2DM and OSA; control participants met criteria for no T2DM and no OSA. Exclusion criteria for all four groups included AHI greater than 100 events/h, HbA1c greater than 8.0%, established cardiovascular disease, hematocrit less than 32%, pregnancy, cigarette smoking within 6 months, collagen vascular disease, liver disease, renal disease, pulmonary disease, blood pressure (BP) greater than 180/110 mm Hg or greater than 160/110 mm Hg for those on antihypertensive medication(s), and use of any medication that could affect sleep and/or breathing. Participants with a metallic implant or other contraindication for cardiovascular magnetic resonance (CMR) imaging did not undergo a CMR scan; however, these criteria were not grounds for exclusion. Recruitment of the four groups was performed to try to match on mean age, sex, and body mass index (BMI) across groups.
Eligibility Criteria: RCT
Participants in the T2DM plus OSA group were eligible to enroll in the RCT if they met the following additional inclusion criteria: Epworth Sleepiness Scale score 18/24 or less, no history of motor vehicle crashes or near-misses related to sleepiness within 2 years, and no commercial driver’s license.
Cross-Sectional Study Protocol
Participants were advised to avoid high-nitrate foods and use of phosphodiesterase type 5 inhibitors for the 3 days preceding their data collection visit. Peripheral BP was measured according to American Heart Association guidelines (8). Macrovascular endothelial function was assessed by measuring brachial artery diameter before and after flow-mediated dilation (FMD; endothelium dependent) and nitroglycerin (NTG)-induced dilation (endothelium independent) as previously described and according to standard guidelines (9, 10). After 10 minutes of supine rest, a three-lead electrocardiogram was obtained along with high-resolution ultrasound of the brachial artery of the nondominant arm using a 10.0-MHz linear array transducer (Aloka Prosound α7; Hitachi Aloka Medical), followed by 5-minute inflation of a BP cuff to 50 mm Hg above systolic BP distal to the target artery. A second ultrasound was recorded for at least 90 seconds after cuff deflation. After 20 minutes of supine rest, a third ultrasound of the brachial artery was performed. After administration of 400 μg sublingual NTG, a fourth ultrasound was performed 4 minutes later. Four images from each of the four conditions (pre-cuff inflation, post-cuff inflation, pre-NTG, post-NTG) were obtained coinciding with the peak of the R wave. Two independent investigators measured the brachial artery diameter (media to media) four times. When agreement was within 10%, these eight measurements were averaged. In cases of disagreement, measurements were repeated independently. If disagreement persisted, the two investigators completed the measurements together with a senior adjudicator (A.V.) before averaging the measurements.
Vascular testing was assessed at approximately the same time of day for all participants (beginning at 10:00 a.m.) while in the fasting state in a temperature-controlled environment. Skin blood flow was measured before and after acetylcholine (ACh; endothelium dependent) and sodium nitroprusside (SNP; endothelium independent) (10). A resting skin blood flow measurement was obtained in the forearm not used during macrovascular testing by employing laser Doppler flowmetry (PeriScan PIM II LDPI system; Perimed). An iontophoresis system (MIC1; Moor Instruments Ltd.) was used to deliver a solution of 1% ACh for 60 seconds, followed by a second flowmetry measurement. After 10 minutes of rest, a third flowmetry measurement was obtained, followed by iontophoresis of SNP, then a final flowmetry measurement. The percentage changes in skin blood flow after ACh and SNP administration compared with baseline were used as measures of microcirculatory function.
Participants underwent CMR (Achieva 1.5T, Philips) with a five-element cardiac synergy coil. Breath-hold retrospective electrocardiogram-gated cine balanced steady-state free processing cine images were acquired in the two-chamber and four-chamber horizontal long-axis views, and a short-axis stack covering the entire left ventricle (LV; 8-mm slices with 2-mm gaps). The CMR data for LV and right ventricular (RV) volume, mass, and ejection fraction were measured using standard volumetric techniques and analyzed with commercial software (QMASS v7.4; Medis Inc.) by a blinded investigator. LV and RV endocardial and epicardial borders on cine images were manually planimetered to define the myocardium, taking care to exclude papillary muscles and intertrabecular blood pool.
RCT Protocol
Participants with T2DM plus OSA who met the RCT eligibility criteria entered a 7-day run-in phase during which they were instructed to wear a CPAP mask (without a device) while asleep for at least 1 night. Participants who tolerated the mask were randomized to either active or sham CPAP. The 1:1 randomization sequence was generated by a statistician with a block size of 6, stratified according to BMI (< or ≥ 35 kg/m2). Treatment allocation was concealed by keeping each assignment in a sealed opaque envelope, accessed in sequence by the single unblinded investigator responsible for treatment allocation assignment.
Participants then underwent 7 nights of CPAP autotitration within 4–20 cm H2O using an S9 Autoset device (ResMed), or a mock titration using a sham S9 device, set to a pressure of 4 cm H2O with a flow restrictor placed in the hose and additional exhaust ports in the sham masks to reduce effective pressure. At 1 week later, the active CPAP devices were set to the average 95th percentile pressure collected during the titration period. The settings of the sham devices were not changed.
After 3 months of treatment with either active or sham CPAP, all participants underwent cardiovascular testing identical to the baseline visit. Adherence data were downloaded from the active CPAP devices. For sham devices, adherence was calculated using the device run time.
Power Calculation
Assuming a two-sided null hypothesis and an α of 0.05, 26 subjects per group in the cross-sectional study were required to achieve 80% power to detect a 3.1% difference in FMD (a roughly one-third reduction from normal values) between groups assuming a 3.3% SD based on our preliminary data (effect size, 0.94). For the RCT, we anticipated an effect size (standardized mean difference) of 0.80 or greater, which corresponds to a difference in the change in FMD of 0.97% assuming a 1.20% SD. Assuming a two-sided null hypothesis and an α of 0.05, 25 subjects per arm were required to achieve 80% power.
Statistical Analyses
Before hypothesis testing, we examined outcome distributions to check for potential outliers or other potential violations of regression assumptions. Descriptive statistics were computed to ensure all values were valid and potential outliers were examined and verified. It was decided a priori to exclude outliers defined as values ±4 SD beyond the mean.
The primary outcome measure for the cross-sectional study was FMD (endothelium-dependent macrovascular reactivity). We first tested for interactive effects of T2DM and OSA on FMD using a two-way analysis of covariance (ANCOVA) model adjusted for age, sex, and BMI. If the interaction was not significant, we removed it and tested for additive effects using a two-way ANCOVA model with the main effects of T2DM and OSA, adjusting for age, sex, and BMI.
For the RCT, the primary outcome measure was FMD. We tested for between-arm differences using a one-way ANCOVA model. All analyses, including interactions, were considered statistically significant at a P value of 0.05 or less.
Results
Cross-Sectional Study
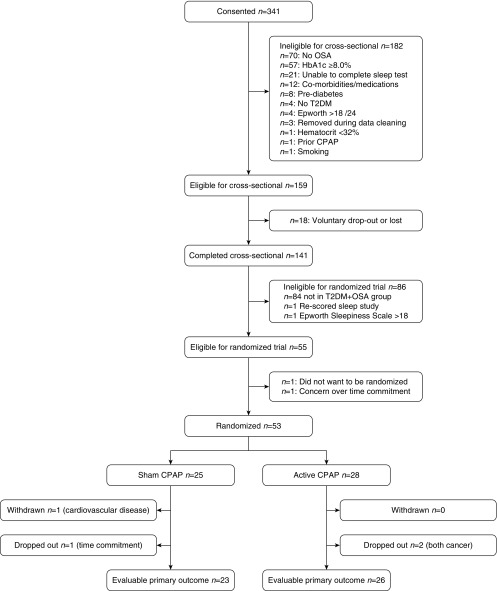
Figure 1 shows the CONSORT (Consolidated Standards of Reporting Trials) diagram encompassing both the cross-sectional study and RCT. Of 341 subjects who consented, 141 completed the cross-sectional study (see Table 1). The average age of the control, T2DM-only, OSA-only, and T2DM-plus-OSA groups fell within a range of approximately 10 years. The average BMI was within 5.4 kg/m2 across groups, with an increased BMI observed in the OSA-only and T2DM-plus-OSA groups. The percentage of males was 44–65% across groups, and, on average, the sample had resting BP in the normal range.
Figure 1.
CONSORT diagram. CPAP = continuous positive airway pressure; CONSORT = Consolidated Standards of Reporting Trials; HbA1c = glycated hemoglobin; OSA = obstructive sleep apnea; T2DM = type 2 diabetes mellitus.
Table 1.
Descriptive characteristics of participants: cross-sectional study
| Variable | Control Subjects (n = 28) | T2DM Only (n = 27) | OSA Only (n = 29) | T2DM + OSA (n = 57) |
|---|---|---|---|---|
| Descriptive characteristics | ||||
| Age, yr | 45.8 ± 14.8 | 50.7 ± 7.8 | 53.0 ± 10.2 | 56.0 ± 9.1 |
| Ethnicity/race, n (%) | ||||
| Non-Hispanic white | 15 (54) | 15 (56) | 17 (59) | 29 (51) |
| All others/did not answer | 13 (46) | 12 (44) | 12 (41) | 28 (49) |
| Male sex, n (%) | 15 (54) | 12 (44) | 15 (52) | 37 (65) |
| BMI, kg/m2 | 29.8 ± 6.3 | 31.0 ± 4.8 | 34.0 ± 6.3 | 35.2 ± 6.6 |
| Neck circumference, cm | 39.3 ± 4.0 | 40.8 ± 4.6 | 40.4 ± 4.3 | 43.2 ± 3.7 |
| Waist circumference, cm | 101.5 ± 14.5 | 109.6 ± 13.7 | 110.0 ± 15.7 | 117.2 ± 14.5 |
| Systolic BP, mm Hg | 120.2 ± 13.4 | 127.9 ± 19.0 | 128.9 ± 14.8 | 134.3 ± 15.9 |
| Diastolic BP, mm Hg | 73.5 ± 10.3 | 76.4 ± 10.2 | 72.5 ± 8.9 | 77.3 ± 10.0 |
| Statins, n (%) | 2 (7.1) | 11 (40.7) | 7 (24.1) | 37 (64.9) |
| Angiotensin-converting enzyme inhibitors, n (%) | 2 (7) | 9 (33) | 1 (4) | 27 (47) |
| Angiotensin II receptor blockers, n (%) | 0 (0) | 2 (7) | 1 (4) | 13 (23) |
| At least one of the above medications, n (%) | 4 (14) | 15 (56) | 7 (24) | 51 (90) |
| T2DM data | ||||
| Fasting plasma glucose, mg/dL | 87.7 ± 11.1 | 138.6 ± 56.9 | 90.1 ± 7.1 | 130.6 ± 34.6 |
| Glycated hemoglobin, % | 5.6 ± 0.5 | 7.1 ± 0.8 | 5.7 ± 0.3 | 6.9 ± 0.6 |
| OSA data | ||||
| Average AHI, events/h | 2.8 ± 2.6 | 4.4 ± 2.8 | 22.7 ± 16.0 | 24.1 ± 16.0 |
| Epworth Sleepiness Scale, /24 | 6.3 ± 4.6 | 6.4 ± 3.9 | 8.8 ± 5.3 | 10.0 ± 5.0 |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; BP = blood pressure; OSA = obstructive sleep apnea; T2DM = type 2 diabetes mellitus.
Data are presented as mean ± SD or number (percentage); all values are unadjusted.
Mean (±SD) FMD was 6.1 (±4.0)%, 7.3 (±3.6)%, 6.8 (±4.5)%, and 4.8 (±2.9)% in control subjects, T2DM only, OSA only, and T2DM plus OSA, respectively (see Table 2). There was a significant T2DM × OSA interaction effect on FMD, such that OSA was associated with an additional mean FMD reduction of 3.1% (95% confidence interval [CI], 0.6 – 5.6) among those with T2DM compared with the impact of OSA in those without T2DM (P = 0.02). In contrast, no significant additive main effects or interactions were identified for endothelium-independent macrovascular function. For the microvascular measurements, no effects of T2DM, OSA, or their interaction were found for either the endothelium-dependent or endothelium-independent measures.
Table 2.
Baseline descriptive characteristics of participants: randomized controlled trial
| CPAP (n = 28) | Sham CPAP (n = 25) | |
|---|---|---|
| Descriptive characteristics | ||
| Age, yr | 58.4 ± 6.7 | 53.8 ± 11.3 |
| Ethnicity/race, n (%) | ||
| Non-Hispanic white | 14 (50%) | 14 (56%) |
| All others/did not answer | 14 (50%) | 11 (44%) |
| Male sex, n (%) | 18 (64%) | 16 (64%) |
| BMI, kg/m2 | 35.0 ± 5.4 | 35.8 ± 8.2 |
| Neck circumference, cm | 43.1 ± 3.3 | 43.2 ± 4.4 |
| Waist circumference, cm | 117.5 ± 10.5 | 117.6 ± 18.8 |
| Systolic BP, mm Hg | 136.5 ± 17.0 | 133.6 ± 15.1 |
| Diastolic BP, mm Hg | 77.9 ± 12.2 | 76.9 ± 8.0 |
| Statins, n (%) | 19 (68%) | 15 (60%) |
| Angiotensin-converting enzyme inhibitors, n (%) | 16 (57%) | 10 (40%) |
| Angiotensin II receptor blockers, n (%) | 7 (25%) | 6 (24%) |
| At least one of the above medications, n (%) | 27 (96%) | 21 (84%) |
| T2DM data | ||
| Fasting plasma glucose, mg/dL | 129.0 ± 36.7 | 130.0 ± 34.7 |
| Glycated hemoglobin, % | 6.7 ± 0.5 | 7.1 ± 0.6 |
| OSA data | ||
| Average AHI, events/h | 21.6 ± 12.3 | 25.6 ± 18.5 |
| Epworth Sleepiness Scale, /24 | 8.9 ± 4.8 | 11.4 ± 4.6 |
| CPAP level, cm H2O | 11.1 ± 2.3 | N/A |
| Randomization strata (BMI ≥ 35 kg/m2), n (%) | 10 (36%) | 9 (36%) |
| Duration of trial excluding titration, d | 95 ± 13 | 102 ± 18 |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; BP = blood pressure; CPAP = continuous positive airway pressure; OSA = obstructive sleep apnea; T2DM = type 2 diabetes mellitus.
Data are presented as mean ± SD or n (%); all values are unadjusted.
CMR was completed in 27 control subjects, 25 T2DM only, 23 OSA only, and 45 participants with T2DM plus OSA. A significant T2DM × OSA interaction was found for both LV end-diastolic volume (P = 0.02) and RV end-diastolic volume (P = 0.03), such that OSA was associated with a 22.4 ml (95% CI, 3.2 – 41.6) greater LV end-diastolic volume and 23.2 ml (95% CI, 2.6 – 43.8) greater RV end-diastolic volume in those with T2DM compared with the impact of OSA in those without T2DM (see Table 3). The same pattern was observed for RV end-systolic volume where OSA was associated with a 14.4 ml (95% CI, 1.9 – 26.9) greater RV end-systolic volume in those with T2DM compared with those without T2DM (P = 0.03).
Table 3.
Vascular reactivity and cardiovascular magnetic resonance results: cross-sectional study
| Variable | Control Subjects (n = 28) | T2DM Only (n = 27) | OSA Only (n = 29) | T2DM + OSA (n = 57) | OSA Main Effect | T2DM Main Effect | OSA × T2DM Interaction |
|---|---|---|---|---|---|---|---|
| Macrovascular reactivity | |||||||
| Flow-mediated dilation, % | 6.1 ± 4.0 | 7.3 ± 3.6 | 6.8 ± 4.5 | 4.8 ± 2.9 | 0.9 (−1.1 to 2.9) | 1.3 (−0.7 to 3.3) | −3.1 (−5.6 to −0.6)* |
| NTG-induced dilation, % | 16.5 ± 8.2 | 17.0 ± 6.9 | 15.3 ± 8.4 | 13.6 ± 7.5 | −1.2 (−4.1 to 1.7) | −0.3 (−2.8 to 2.2) | |
| Microvascular reactivity | |||||||
| ACh-induced dilation, % | 29.9 ± 22.9 | 36.9 ± 29.9 | 44.7 ± 36.1 | 31.2 ± 26.2 | 4.6 (−7.0 to 16.2) | −4.0 (−14.4 to 6.4) | |
| SNP-induced dilation, % | 28.9 ± 30.4 | 31.9 ± 27.0 | 35.7 ± 26.7 | 33.6 ± 29.7 | 9.4 (−2.0 to 20.8) | 2.5 (−7.7 to 12.7) | |
| CMR imaging | |||||||
| LV mass, g | 104.6 ± 24.8 | 96.0 ± 19.3 | 98.0 ± 32.5 | 109.1 ± 32.8 | −4.0 (−13.4 to 5.4) | 1.1 (−7.3 to 9.5) | |
| LV end-diastolic volume, ml | 158.0 ± 32.0 | 135.3 ± 22.9 | 129.5 ± 30.2 | 139.6 ± 33.4 | −27.7 (−43.2 to −12.2)† | −16.8 (−30.9 to −2.7)* | 22.4 (3.2 to 41.6)* |
| LV end-systolic volume, ml | 62.5 ± 18.7 | 52.4 ± 14.3 | 47.9 ± 16.9 | 51.2 ± 19.2 | −6.6 (−13.1 to −0.1)* | −1.9 (−7.6 to 3.8) | |
| LV ejection fraction, % | 61.0 ± 6.5 | 61.6 ± 5.8 | 63.6 ± 6.7 | 64.2 ± 6.6 | 1.0 (−1.5 to 3.5) | 0.0 (−2.4 to 2.4) | |
| RV mass, g | 34.4 ± 7.0 | 29.0 ± 5.1 | 28.8 ± 9.3 | 30.1 ± 8.2 | −2.6 (−5.7 to 0.5) | −1.5 (−4.2 to 1.2) | |
| RV end-diastolic volume, ml | 174.1 ± 31.7 | 144.8 ± 26.5 | 141.4 ± 45.1 | 146.4 ± 32.0 | −32.0 (−49.1 to −14.9)† | −21.6 (−36.5 to −6.7)† | 23.2 (2.6 to 43.8)* |
| RV end-systolic volume, ml | 79.6 ± 20.0 | 61.3 ± 16.2 | 54.5 ± 18.3 | 58.8 ± 21.7 | −20.4 (−30.8 to −10.0)† | −13.0 (−22.0 to −4.0)† | 14.4 (1.9 to 26.9)* |
| RV ejection fraction, % | 54.7 ± 6.1 | 58.1 ± 6.3 | 59.1 ± 8.0 | 60.7 ± 6.7 | 2.2 (−0.5 to 4.9) | 1.8 (−0.6 to 4.2) | — |
Definition of abbreviations: ACh = acetylcholine; CMR = cardiovascular magnetic resonance; LV = left ventricular; NTG = nitroglycerin; OSA = obstructive sleep apnea; RV = right ventricular; SNP = sodium nitroprusside; T2DM = type 2 diabetes mellitus.
Data are presented as mean ± SD; all values are unadjusted. Analysis of covariance models with an OSA ± T2DM interaction term were produced; if the interaction was significant (P × 0.05), the interaction effect and main effects are presented as the unstandardized β (95% confidence interval [CI]). If the interaction was not significant, the interaction term was removed, and the main effects are presented as the unstandardized β (95% CI). All models were adjusted for age, sex, and body mass index. The sample sizes refer to the primary outcome (flow-mediated dilation) for which there was no missing data; sample sizes varied for the other outcomes listed.
P ≤ 0.05.
P ≤ 0.01.
Finally, we repeated statistical comparisons across groups after additionally adjusting for statins, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers (the use of at least one; yes/no), and observed no substantial differences from the models that adjusted only for age, sex, and BMI.
RCT
Of the 57 participants with T2DM plus OSA who completed the cross-sectional study, 53 were eligible and willing to be randomized after run in. During the RCT, one participant was withdrawn from the sham CPAP arm due to previously undiagnosed cardiovascular disease (see Adverse Events); three participants voluntarily withdrew (n = 2 from active CPAP and n = 1 from sham CPAP). There were no crossovers. The trial ended once 53 had been randomized, with the intention of having a final dataset of an n of 50 due to the 3 withdrawals that had taken place by that point; however, the final randomized participant also withdrew, leaving an evaluable dataset for the RCT of an n of 26 and an n of 23 in the active and sham CPAP arms, respectively. Participants were predominantly male, middle aged, and obese (Table 3). The mean (±SD) AHI was 21.6 (±12.3) and 25.6 (±18.5) events/h in the active and sham CPAP groups, indicating moderate OSA. Mean (±SD) adherence to active and sham CPAP during the titration period was 5.0 (±2.6) and 4.8 (±2.7) h/night, respectively. Across the entire 3 months of the trial, adherence was 4.3 (±2.3) and 4.3 (±2.5) h/night, respectively.
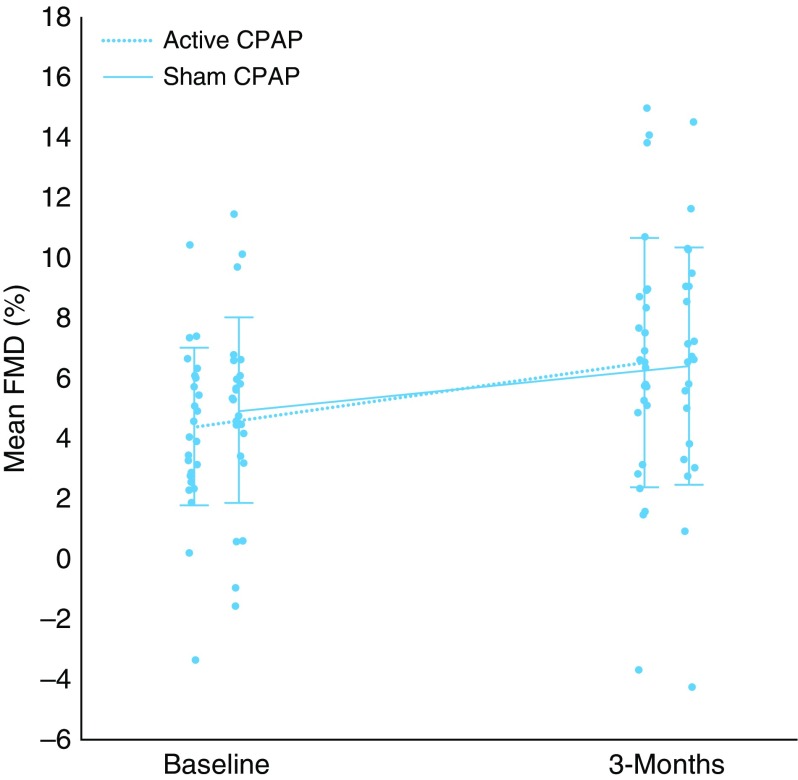
As shown in Table 4 and Figure 2, mean FMD increased by 2.1% in the active CPAP arm (4.5 ± 2.6% to 6.6 ± 4.1%) and 1.5% in the sham CPAP arm (5.0 ± 3.1% to 6.5 ± 3.9%). The mean net improvement in FMD from the ANCOVA analysis was mean 0.3% (95% CI, −1.9 to 2.5). We did not observe substantial differences in microvascular endothelial function between the active and sham CPAP groups.
Table 4.
Vascular reactivity and cardiovascular magnetic resonance imaging results: randomized controlled trial
| Variable | Active CPAP (n = 26) |
Sham CPAP (n = 23) |
β (95% CI); P Value (Between Arms) | ||
|---|---|---|---|---|---|
| Baseline | 3 mo | Baseline | 3 mo | ||
| Macrovascular reactivity | |||||
| Flow-mediated dilation, % | 4.5 ± 2.6 | 6.6 ± 4.1 | 5.0 ± 3.1 | 6.5 ± 3.9 | 0.3 (−1.9 to 2.5); 0.79 |
| NTG-induced dilation, % | 13.3 ± 8.6 | 14.9 ± 7.3 | 14.4 ± 6.5 | 12.2 ± 6.1 | 2.3 (−1.0 to 5.6); 0.20 |
| Microvascular reactivity | |||||
| ACh-induced dilation, % | 25.5 ± 22.0 | 29.1 ± 23.5 | 38.6 ± 30.7 | 28.4 ± 17.0 | 6.7 (−8.2 to 21.6); 0.38 |
| SNP-induced dilation, % | 25.2 ± 24.1 | 27.4 ± 22.6 | 41.0 ± 32.9 | 22.7 ± 16.6 | 7.8 (−3.8 to 19.4); 0.19 |
| Peripheral blood pressure | |||||
| Systolic, mm Hg | 136.5 ± 17.0 | 134.4 ± 15.2 | 133.6 ± 15.1 | 129.7 ± 14.0 | 3.1 (−4.0 to 10.2); 0.39 |
| Diastolic, mm Hg | 77.9 ± 12.2 | 77.0 ± 10.3 | 76.9 ± 8.0 | 74.8 ± 9.9 | 1.4 (−2.7 to 5.5); 0.52 |
| CMR imaging | |||||
| LV mass, g | 115.5 ± 35.3 | 113.8 ± 34.4 | 101.9 ± 31.7 | 97.4 ± 30.3 | −0.7 (−6.8 to 5.4); 0.83 |
| LV end-diastolic volume, ml | 138.9 ± 30.2 | 143.6 ± 27.0 | 143.1 ± 38.9 | 131.1 ± 42.4 | 8.7 (−7.0 to 24.4); 0.28 |
| LV end-systolic volume, ml | 48.0 ± 16.3 | 54.4 ± 15.3 | 55.9 ± 22.1 | 50.1 ± 24.6 | 7.7 (−0.9 to 16.3); 0.09 |
| LV ejection fraction, % | 66.0 ± 6.5 | 62.6 ± 5.6 | 62.1 ± 6.5 | 63.3 ± 8.6 | −2.8 (−6.7 to 1.1); 0.17 |
| RV mass, g | 31.4 ± 8.3 | 30.0 ± 6.7 | 29.3 ± 8.3 | 29.4 ± 7.6 | −1.0 (−3.5 to 1.5); 0.46 |
| RV end-diastolic volume, ml | 148.1 ± 33.4 | 147.5 ± 30.8 | 146.9 ± 32.7 | 135.9 ± 39.8 | 6.8 (−7.7 to 21.3); 0.37 |
| RV end-systolic volume, ml | 59.7 ± 21.7 | 61.9 ± 19.1 | 59.3 ± 22.7 | 56.9 ± 23.5 | 3.8 (−7.0 to 14.6); 0.50 |
| RV ejection fraction, % | 60.5 ± 6.6 | 58.3 ± 6.9 | 60.5 ± 6.8 | 59.0 ± 8.6 | 0.1 (−4.8 to 5.0); 0.98 |
Definition of abbreviations: ACh = acetylcholine; CI = confidence interval; CMR = cardiovascular magnetic resonance; CPAP = continuous positive airway pressure; LV = left ventricular; NTG = nitroglycerin; RV = right ventricular; SNP = sodium nitroprusside.
Data are presented as mean ± SD; all values are unadjusted. Between-arm unstandardized β (95% CI) and P values are based on a one-way analysis of covariance model. The sample sizes refer to the primary outcome (flow-mediated dilation) for which there was no missing data/outliers; sample sizes varied for the other outcomes listed. Data shown are from the intent-to-treat analyses.
Figure 2.
Changes in flow-mediated dilation in the active and sham continuous positive airway pressure (CPAP) groups. Error bars represent the SD. FMD = flow-mediated dilation; SD = standard deviation.
CMR was completed at both baseline and 3 months for an n of 18 in the active CPAP arm and an n of 17 in the sham CPAP arm. Although there were minimal differences in LV and RV mass, we did observe improved LV end-diastolic and end-systolic volume (mean increases of 8.7 ml [95% CI, −7.0 to 24.4] and 7.7 ml [95% CI, −0.9 to 16.3], respectively). We observed a net reduction in LV ejection fraction of 2.8% (95% CI, −6.7 to 1.1). CPAP was associated with minimal changes in RV end-diastolic volume, RV end-systolic volume, and RV ejection fraction.
Finally, we undertook an analysis of our primary outcome, FMD, restricted to the 16 and 12 participants who used their active or sham CPAP device, respectively, for 4 h/night or greater on average throughout the trial. The mean increase in FMD in the active CPAP arm, from 5.0 (±1.6)% to 7.5 (±3.6)%, was 2.5%, whereas the mean increase in the sham CPAP arm, from 5.4 (±3.9)% to 6.5 (±3.3)%, was 1.1%. The mean net improvement in FMD with CPAP compared with sham from ANCOVA was 1.1% (95% CI, −1.4 to 3.6).
Adverse Events
Two adverse events were adjudicated by an independent medical monitor as being serious, unexpected, and related. One participant was diagnosed with coronary artery disease after randomization, underwent coronary artery bypass surgery, and was withdrawn from the trial. One participant in the cross-sectional study experienced a brief episode of syncope after administration of the NTG during vascular reactivity testing, but was able to complete the remaining study procedures once recovered.
Discussion
To our knowledge, this is the first randomized, controlled, prospective trial to assess cardiovascular function comprehensively in patients with T2DM and OSA. Our primary analyses indicated that, although OSA is associated with a greater deleterious effect on the macrovascular endothelium in patients with T2DM compared with those without this disease, as demonstrated by a significant T2DM × OSA interaction on FMD, 3 months of CPAP did not result in substantially improved FMD compared with sham CPAP in patients with T2DM with comorbid OSA. The effect of CPAP on macrovascular reactivity was greater in our per-protocol analysis of participants who used CPAP 4 h/night or greater on average. In this subset, the average FMD at 3 months (7.5%) exceeded the values observed in all groups of the cross-sectional study, although it was below what we have observed in normal weight and overweight participants without OSA or T2DM (9.1% and 8.3%, respectively) (7). The net improvement of 1.1% is comparable to the 1.4% improvement in FMD that we have previously observed with atorvastatin (11), suggesting that CPAP may have a clinically meaningful effect on endothelial function among those who achieve greater adherence to therapy. In addition, our CMR results indicated a greater adverse effect of OSA on ventricular distension among those with T2DM compared with those without, as evidenced by larger differences in both LV and RV end-diastolic volume. We did not observe statistically significant changes in ventricular parameters between the active and sham CPAP groups; however, we noted what may reflect a clinically relevant improvement in LV end-diastolic volume, suggesting improved LV filling and forward flow.
In the cross-sectional component of our study, endothelial-dependent micro- and macrovascular reactivity were higher in the T2DM-only and OSA-only groups compared with control subjects. There were no indications of predisposing cardiovascular disease risk factors in the control group other than the presence of obesity. These findings are in contrast to previous results from our laboratory that showed reduced endothelial function in diabetic patients compared with control subjects (6, 7, 12); however, we and others have not evaluated the role of OSA as a confounding factor in past studies. Our current results indicate that endothelial function in T2DM is affected only in the presence of OSA, and support the stance that OSA should be evaluated as an important predictor of cardiovascular function in future studies of T2DM populations. An additional plausible explanation for our unexpected results is that the majority of our patients with T2DM were prescribed statins, angiotensin-converting enzyme inhibitors, and/or angiotensin II receptor blockers (56% of those with T2DM alone, and 90% of those with OSA + T2DM). These medications have long been proposed to have pleiotropic effects, including improvement of microvascular function, which may have impacted our results (11, 13, 14). We performed sensitivity analyses after adjusting for medication use and observed no substantial changes from the models that only adjusted for age, sex, and BMI; however, the adjustment was based on a dichotomous variable and did not account for dose or adherence. Finally, we only recruited participants with reasonably well-controlled T2DM, as indicated by glycated hemoglobin less than 8%. The rationale behind this inclusion criterion was to allow us to interpret the impact of treating OSA by minimizing fluctuations in glucose control throughout the trial. In doing so, however, we recruited patients who were receiving care that included aggressive control of BP and lipids. It is possible that a greater impact of T2DM and OSA on the vasculature in the cross-sectional study, as well as a larger treatment effect in the interventional study, would have been observed in less well-controlled individuals.
To date, three RCTs of prospective CPAP therapy have assessed FMD as a measure of macrovascular endothelial function in patients without T2DM OSA (15–17), all of which were included in a recent meta-analysis, which reported a pooled absolute mean difference in FMD between CPAP and a nontherapeutic control of 3.96% (18). In contrast, we observed a much smaller effect (0.3%) of CPAP on FMD in T2DM. Few CPAP studies have assessed vascular function in the peripheral microvasculature (19, 20), with the current study being the first randomized, placebo-controlled trial to our knowledge. Our prior nonrandomized study comparing CPAP with bariatric surgery reported an absolute average increase in ACh-induced dilation of 23.5% in the CPAP arm (from 45.6% to 69.1%) (21), compared with 3.6% (from 25.5% to 29.1%) in the active-CPAP arm of the current trial. Our findings in this trial suggest that the impact of CPAP on the microvasculature may not be as robust as those seen in the macrovasculature (22).
In the cross-sectional component of our study, we observed a significant T2DM × OSA interaction for both LV and RV end-diastolic volume, such that OSA was associated with a 22.4 ml greater LV end-diastolic volume and 23.2 ml greater RV end-diastolic volume in those with T2DM compared with the impact of OSA in those without T2DM. In the RCT, we observed a net improvement in LV end-diastolic volume of 8.7 ml, which might suggest improved diastolic function and/or myocardial relaxation. The only other randomized trial of CPAP using CMR that we are aware of did not find any substantial changes in cardiac structure with 6-months of CPAP; however, the study was designed to investigate the impact of CPAP on minimally symptomatic participants with mild-to-moderate OSA (<10 dips in oxygen saturation/h) (22). In our RCT, we also observed a net reduction in LV ejection fraction of 2.8%. Although this difference might be considered meaningful in the appropriate clinical context, the fact that the ejection fraction was within the normal range along with the lack of statistical significance makes this finding unlikely to be of clinical importance (22–26).
Despite a number of strengths, including our robust prospective study design, we acknowledge that our sample size was relatively modest; thus, we may have missed clinically important differences in vascular function. It is also possible that the 3-month duration of CPAP therapy was suboptimal, although prior studies have reported significant results with similar durations (18, 23, 24). Similarly, although we may have observed a greater impact of CPAP with increased adherence, based on the available literature we suspect that an average of 4.3 h/night is adequate. This study was too small and of insufficient duration to assess clinically meaningful events, such as myocardial infarction and stroke; however, we did include CMR, which is the most accurate noninvasive imaging modality of cardiac structure. Despite attempting to match participants in the cross-sectional study on age, sex, and BMI, those with T2DM plus OSA were older, with a higher BMI and a greater proportion of men. As such, we adjusted for these variables in our statistical analyses.
In summary, the combination of T2DM and OSA is associated with macrovascular endothelial dysfunction beyond the impact of either disease alone. Treatment of OSA with 3 months of CPAP did not lead to improvement in this measure compared with a placebo-control arm in the intent-to-treat analysis. Further research is required to elucidate whether this null finding results from an inability of CPAP to reverse endothelial damage associated with OSA in patients with T2DM, and whether there are other populations that may benefit more from treatment, such as those with prediabetes. Furthermore, as very few prior studies assessing cardiovascular risk in T2DM have evaluated the presence of OSA (6, 7), our results suggesting that OSA plays a major role in both large vessel endothelial dysfunction and ventricular hypertrophy emphasize the importance of accounting for OSA in the design of future interventional studies in T2DM.
Supplementary Material
Acknowledgments
Acknowledgment
The authors acknowledge the following contributors for their input and assistance: DSMB (Data and Safety Monitoring Board) members Daniel Gottlieb (Brigham and Women’s Hospital, Harvard Medical School), Gerald Weinhouse (Brigham and Women’s Hospital, Harvard Medical School), Henry Feldman (Boston Children’s Hospital, Harvard Medical School), and Raymond Kwong (Brigham and Women’s Hospital, Harvard Medical School); medical monitors Robert Owens (University of California San Diego) and Katherine Dudley (Brigham and Women’s Hospital, Harvard Medical School); and clinical and technological input from Kraig Kissinger (Beth Israel Deaconess Medical Center), Beth Goddu (Beth Israel Deaconess Medical Center), Sophie Berg (Beth Israel Deaconess Medical Center), Karen McCowen (University of California San Diego), Deborah Wexler (Massachusetts General Hospital, Harvard Medical School), Matthew Kim (Brigham and Women’s Hospital, Harvard Medical School), Gail Adler (Brigham and Women’s Hospital, Harvard Medical School), Susan Redline (Brigham and Women’s Hospital, Harvard Medical School), Kaylee Klingensmith (University of Pittsburgh), and Rebecca De Sensi (University of Pittsburgh).
Footnotes
Supported by National Institutes of Health grants R01HL110350 (A.M., S.R.P., and A.V.) and K24HL127307 (S.R.P.). J.P.B. received support from American Heart Association Scientific Development Grant 14SDG20160000, and A.V. received support from the National Rongxiang Xu Foundation; in addition, this work was conducted with support from Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. ApneaLink diagnostic devices, continuous positive airway pressure (CPAP) machines, and masks were provided by ResMed. CPAP masks were provided by Philips—neither company was involved with the design or conduct of the study.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Author Contributions: Study design—J.P.B., W.J.M., A.M., S.R.P., and A.V.; study conduct and data collection—J.P.B., D.B., F.T., R.H.C., W.J.M., T.G.N., M.H., A.M., S.R.P., and A.V.; analysis and interpretation—J.P.B., D.B., F.T., R.H.C., W.J.M., T.G.N., M.L.W., M.H., A.M., S.R.P., and A.V.; and manuscript preparation—J.P.B., D.B., F.T., R.H.C., W.J.M., T.G.N., M.L.W., M.H., A.M., S.R.P., and A.V.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci USA. 2018;115:957–961. doi: 10.1073/pnas.1716802115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, et al. Sleep AHEAD Research Group. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–1019. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 5.Khaodhiar L, Brennan AM, Lima C, Chan JL, Mantzoros CS, Manning WJ, et al. Effect of valsartan on left ventricular anatomy and systolic function and aortic elasticity. Metabolism. 2009;58:682–688. doi: 10.1016/j.metabol.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Yim-Yeh S, Rahangdale S, Nguyen AT, Jordan AS, Novack V, Veves A, et al. Obstructive sleep apnea and aging effects on macrovascular and microcirculatory function. Sleep. 2010;33:1177–1183. doi: 10.1093/sleep/33.9.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yim-Yeh S, Rahangdale S, Nguyen AT, Stevenson KE, Novack V, Veves A, et al. Vascular dysfunction in obstructive sleep apnea and type 2 diabetes mellitus. Obesity (Silver Spring) 2011;19:17–22. doi: 10.1038/oby.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 9.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 10.Baltzis D, Dushay JR, Loader J, Wu J, Greenman RL, Roustit M, et al. Effect of linagliptin on vascular function: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2016;101:4205–4213. doi: 10.1210/jc.2016-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Economides PA, Caselli A, Tiani E, Khaodhiar L, Horton ES, Veves A. The effects of atorvastatin on endothelial function in diabetic patients and subjects at risk for type 2 diabetes. J Clin Endocrinol Metab. 2004;89:740–747. doi: 10.1210/jc.2003-031116. [DOI] [PubMed] [Google Scholar]
- 12.Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48:1856–1862. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- 13.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23) Suppl 1:III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 14.Shrikhande G, Khaodhiar L, Scali S, Lima C, Hubbard M, Dudley K, et al. Valsartan improves resting skin blood flow in type 2 diabetic patients and reduces poly(adenosine diphosphate-ribose) polymerase activation. J Vasc Surg. 2006;43:760–770, discussion 770–771. doi: 10.1016/j.jvs.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen PK, Katikireddy CK, McConnell MV, Kushida C, Yang PC. Nasal continuous positive airway pressure improves myocardial perfusion reserve and endothelial-dependent vasodilation in patients with obstructive sleep apnea. J Cardiovasc Magn Reson. 2010;12:50. doi: 10.1186/1532-429X-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohler M, Craig S, Pepperell JCT, Nicoll D, Bratton DJ, Nunn AJ, et al. CPAP improves endothelial function in patients with minimally symptomatic OSA: results from a subset study of the MOSAIC trial. Chest. 2013;144:896–902. doi: 10.1378/chest.13-0179. [DOI] [PubMed] [Google Scholar]
- 17.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 18.Ning Y, Zhang TS, Wen WW, Li K, Yang YX, Qin YW, et al. Effects of continuous positive airway pressure on cardiovascular biomarkers in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. Sleep Breath. 2019;23:77–86. doi: 10.1007/s11325-018-1662-2. [DOI] [PubMed] [Google Scholar]
- 19.Lattimore JL, Wilcox I, Skilton M, Langenfeld M, Celermajer DS. Treatment of obstructive sleep apnoea leads to improved microvascular endothelial function in the systemic circulation. Thorax. 2006;61:491–495. doi: 10.1136/thx.2004.039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trzepizur W, Gagnadoux F, Abraham P, Rousseau P, Meslier N, Saumet JL, et al. Microvascular endothelial function in obstructive sleep apnea: impact of continuous positive airway pressure and mandibular advancement. Sleep Med. 2009;10:746–752. doi: 10.1016/j.sleep.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Bakker JP, Balachandran JS, Tecilazich F, Deyoung PN, Smales E, Veves A, et al. Pilot study of the effects of bariatric surgery and continuous positive airway pressure treatment on vascular function in obese subjects with obstructive sleep apnoea. Intern Med J. 2013;43:993–998. doi: 10.1111/imj.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig S, Kylintireas I, Kohler M, Nicoll D, Bratton DJ, Nunn AJ, et al. Effect of CPAP on cardiac function in minimally symptomatic patients with OSA: results from a subset of the MOSAIC randomized trial. J Clin Sleep Med. 2015;11:967–973. doi: 10.5664/jcsm.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magalang UJ, Richards K, McCarthy B, Fathala A, Khan M, Parinandi N, et al. Continuous positive airway pressure therapy reduces right ventricular volume in patients with obstructive sleep apnea: a cardiovascular magnetic resonance study. J Clin Sleep Med. 2009;5:110–114. [PMC free article] [PubMed] [Google Scholar]
- 24.Colish J, Walker JR, Elmayergi N, Almutairi S, Alharbi F, Lytwyn M, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141:674–681. doi: 10.1378/chest.11-0615. [DOI] [PubMed] [Google Scholar]
- 25.Shah RV, Abbasi SA, Heydari B, Farhad H, Dodson JA, Bakker JP, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J. 2014;167:620–626. doi: 10.1016/j.ahj.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neilan TG, Farhad H, Dodson JA, Shah RV, Abbasi SA, Bakker JP, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2:e000421. doi: 10.1161/JAHA.113.000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.