Abstract
Rationale: In October 2012, the initial phase of the Hospital Readmission Reduction Program imposed financial penalties on hospitals with higher-than-expected risk-adjusted 30-day readmission rates for Medicare beneficiaries with congestive heart failure, myocardial infarction, and pneumonia. We hypothesized that these penalties may also be associated with decreased readmissions for chronic obstructive pulmonary disease (COPD) in the general population before COPD became a target condition (October 2014).
Objectives: To determine if implementation of the initial financial penalties for other conditions was associated with a decrease in hospital readmissions for COPD.
Methods: We used population-level data to examine patients readmitted for any reason or for COPD within 30 days after an initial hospitalization for COPD. The data source was seven states in the State Inpatient Database. The preimplementation period included calendar years 2006 to 2012. The postimplementation period included 2013 to 2015. Using interrupted time series, the level change was examined, which reflected the difference between the expected and actual readmission rates in 2013. The difference in slopes between the pre- and postimplementation periods was also examined.
Results: We identified 805,764 hospitalizations for COPD from 904 hospitals. Overall, 26% of patients had primary insurance other than Medicare. After the intervention, patients had lower rates of all-cause 30-day readmissions (level change, −0.93%; 95% confidence interval [CI], −1.44% to −0.43%; P = 0.004), which was driven by fewer early readmissions (0–7 d). The postimplementation slope became positive; the difference in slopes was 0.39% (95% CI, 0.28% to 0.50%; P < 0.001). Patients also had lower rates of COPD-related readmissions (level decrease, −0.52%; 95% CI, −0.93% to −0.12%; P = 0.02), which was due to decreases in both early and late (8–30 d) readmissions. The postimplementation slope was negative; the difference in slopes was −0.21% (95% CI, −0.35% to −0.07%; P = 0.009).
Conclusions: In patients with COPD and any insurance status, there was an association between the initial phase of the Hospital Readmission Reduction Program and a decrease in both all-cause and COPD-related readmissions even before COPD became a target diagnosis. The large amount of money at risk to hospitals likely resulted in broad behavioral change. Future research is needed to test which levers can effectively reduce readmission rates for COPD.
Keywords: health policy, COPD, hospital readmissions
In the United States, chronic obstructive pulmonary disease (COPD) accounts for 700,000 hospitalizations, which cost $18 billion annually (1, 2). Approximately 20% of these hospitalizations result in readmissions within 30 days, of which half are respiratory related (3, 4). Some of these readmissions are considered preventable (5), which represents unnecessary healthcare utilization and spending (6).
The Hospital Readmission Reduction Program (HRRP) was created in 2010 to improve value in health care. In October 2012, hospitals began incurring financial penalties if 30-day, all-cause, risk-standardized readmission rates were higher than expected for Medicare beneficiaries discharged with the following three target conditions: congestive heart failure, myocardial infarction, and pneumonia (7). COPD became a target condition in October 2014.
We sought to determine whether the initial penalties were associated with a decrease in 30-day readmissions for patients in the general population admitted with COPD before COPD became a target condition. A few studies have examined the association of the HRRP with readmission rates for target (8, 9) and nontarget conditions (10, 11), but none has examined the effect of early HRRP financial penalties on a COPD-specific cohort (12). Understanding the timing of the effect of financial penalties will help to evaluate the HRRP as a policy as well as inform future programs that have phased penalties. Using population-based data from the State Inpatient Database, we hypothesized that patients hospitalized for COPD in 2013 after implementation of the initial financial penalties would have lower all-cause and COPD-related 30-day readmission rates than the expected rate of readmissions.
Methods
Study Design and Data Source
We performed an interrupted time series. We examined a population-based cohort of patients with any (or no) health insurance to study the broad effects of the policy. We used data from 2006 to 2015 from seven states in the State Inpatient Database (AR, FL, IA, NE, NY, UT, and WA). These data are part of the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project, which contains the largest collection of longitudinal, encounter-based data in the United States (13). The State Inpatient Database contains >90% of the hospital discharges regardless of payer. These seven states were chosen for their high data quality, geographic diversity, and ability to track individual patients over time within a state using a revisit variable (14). Data from these states have been used in previous publications that examined readmissions (15–17). The project was exempted by the Partners Institutional Review Board (2015P001947).
Patient Sample
We studied adults aged ≥40 years who were admitted to the hospital with COPD. This age threshold has been used to help exclude patients with asthma exacerbations (18). The International Classification of Disease, Ninth Revision (ICD-9), Clinical Modification codes used by the Centers for Medicare and Medicaid Services (CMS) were used to define COPD hospitalizations and COPD-related readmissions (see Table E1 in the online supplement). Because the Tenth Revision (ICD-10) was introduced during the last quarter of 2015, we excluded that quarter. Details about the inclusion and exclusion criteria are described in the online supplement.
Key Dates
The preimplementation period spanned calendar years 2006 to 2012. In the last quarter of calendar year 2012, HRRP instituted financial penalties for risk-adjusted hospital-level rates of Medicare patients readmitted to any hospital within 30 days regardless of readmission diagnosis after they were hospitalized for pneumonia, myocardial infarction, or congestive heart failure. The postimplementation period spanned calendar years 2013 to 2015. Financial penalties for COPD as a target condition were announced August 2013 and implemented October 2014. Key dates are outlined in Table E2.
Exposure
The primary exposure was HRRP’s institution of financial penalties for target conditions other than COPD in the last quarter of calendar year 2012.
Outcomes
The primary outcome was the level change in the interrupted time series for all-cause readmissions, which was the difference between the expected and actual readmission rates in 2013. The expected readmission rate was estimated by the linear trend in the preintervention period. We also examined the change in slopes between the preimplementation and postimplementation periods, which are defined above. Although CMS only counts all-cause readmissions in the metric, we also examined COPD-related readmissions, because COPD is the most common readmission diagnosis (19).
Statistical Analysis
In the interrupted time series, we calculated the yearly percentages of 30-day readmissions (0–100%). Yearly percentages were used instead of quarterly because of the known seasonal variation in readmission rate (20). Coefficients for the slopes of the pre- and postintervention periods were estimated using ordinary least squares regression with Newey-West standard errors to adjust for autocorrelation and heteroscedasticity. Using the Baum and Schaffer method (21), we ensured fitting a model that accounted for the correct autocorrelation structure.
In addition to the interrupted time series, we also constructed multivariable logistic regression models with calendar year as exposure and odds of all-cause and COPD-related 30-day readmissions as two different outcomes. Hospitalizations from 2013, 2014, and 2015 calendar years were treated as categories of the exposure and compared with the preimplementation period as the reference group. Patient- and hospital-level covariates used for risk adjustment are listed in the online supplement, along with their definitions. The risk-adjustment strategy was based on a previously published paper (17). We stratified this analysis by early (0–7 d) and late readmission (8–30 d) because of a previous study showing variation in readmission diagnoses over time with inflection point at 7 days (4). We used generalized estimating equations accounting for clustering at the state level because it was the highest sampling unit.
Analyses were performed using SAS 9.4 (SAS Institute). Two-tailed P < 0.05 was the threshold for significance.
Sensitivity Analyses
We performed the following two sensitivity analyses. 1) We repeated the multivariable logistic regression analysis associating calendar year and odds of all-cause and COPD-related readmission in the subgroup of patients aged ≥65 years to determine if the results were consistent in different age groups. 2) We used a different risk-adjustment technique that was based on CMS methodology to confirm the result of the association between calendar year and odds of all-cause readmissions in patients ≥40 years (22).
Results
Of 2,137,468 hospitalizations for COPD, we identified 805,764 index hospitalizations for COPD from 904 hospitals from 2006 to 2015. Baseline characteristics of hospitalizations are shown by year (Table 1). Approximately one-fourth had primary insurance other than Medicare. The number of yearly admissions for COPD and percentage with Medicare insurance remained relatively stable over the time period (Figures E1 and E2, respectively).
Table 1.
Characteristics of index hospitalizations for chronic obstructive pulmonary disease, 2006–2015
| Variables | Preimplementation Period 2006–2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|
| Age, yr, median (interquartile range) | ||||
| 40–64 | 180,625 (32) | 29,355 (33) | 28,674 (35) | 25,004 (33) |
| ≥65 | 379,891 (68) | 59,074 (67) | 53,103 (65) | 50,038 (67) |
| Sex | ||||
| Male | 244,700 (44) | 38,914 (44) | 35,739 (44) | 32,697 (44) |
| Female | 315,816 (56) | 49,515 (56) | 46,038 (56) | 42,345 (56) |
| Primary health insurance | ||||
| Medicare | 412,550 (74) | 65,201 (74) | 59,370 (73) | 55,817 (74) |
| Medicaid | 51,784 (9) | 8,944 (10) | 9,413 (12) | 7,946 (11) |
| Private | 65,386 (12) | 9,171 (10) | 8,469 (10) | 7,433 (10) |
| Self-pay | 15,168 (3) | 2,657 (3) | 2,041 (3) | 1,688 (2) |
| Other | 15,628 (3) | 2,456 (3) | 2,484 (3) | 2,158 (3) |
| Median household income quartile | ||||
| 1 (lowest) | 171,094 (31) | 27,349 (31) | 25,141 (31) | 23,684 (32) |
| 2 | 156,874 (29) | 24,412 (28) | 23,153 (28) | 21,110 (28) |
| 3 | 128,089 (23) | 20,153 (23) | 18,787 (23) | 17,435 (23) |
| 4 (highest) | 89,172 (16) | 14,390 (16) | 12,741 (16) | 11,396 (15) |
| Patient residence | ||||
| Urban area | 457,881 (82) | 72,392 (82) | 68,798 (84) | 63,731 (85) |
| Rural area | 102,522 (18) | 16,026 (18) | 12,971 (16) | 11,307 (15) |
| Hospital length of stay, d | ||||
| 0–1 | 44,732 (8) | 8,228 (9) | 7,716 (9) | 7,189 (10) |
| 2–4 | 282,104 (50) | 46,706 (53) | 44,201 (54) | 40,675 (54) |
| >4 | 233,680 (42) | 33,495 (38) | 29,860 (37) | 27,178 (36) |
| No. of Elixhauser comorbidities | ||||
| 0–1 | 162,547 (29) | 22,177 (25) | 20,251 (25) | 17,460 (23) |
| 2–3 | 254,489 (45) | 40,284 (46) | 37,066 (45) | 33,755 (45) |
| ≥4 | 143,480 (26) | 25,968 (29) | 24,460 (30) | 23,827 (32) |
| Safety-net hospital | ||||
| No | 447,209 (80) | 69,701 (79) | 63,946 (78) | 59,238 (79) |
| Yes | 113,307 (20) | 18,728 (21) | 17,831 (22) | 15,804 (21) |
| Disposition | ||||
| Home | 360,742 (64) | 56,981 (64) | 53,281 (65) | 48,264 (64) |
| Home with services | 106,702 (19) | 17,857 (20) | 16,246 (20) | 15,252 (20) |
| Skilled nursing facility or other facility | 92,607 (17) | 13,513 (15) | 12,200 (15) | 11,439 (15) |
| Hospital state | ||||
| Arkansas | 46,188 (8) | 7,246 (8) | 6,881 (8) | 5,668 (8) |
| Florida | 237,863 (42) | 39,143 (44) | 37,105 (45) | 37,257 (50) |
| Iowa | 15,707 (3) | 4,741 (5) | 4,320 (5) | 3,841 (5) |
| Nebraska | 18,443 (3) | 2,713 (3) | 2,533 (3) | 2,058 (3) |
| New York | 197,874 (35) | 27,977 (32) | 25,380 (31) | 21,348 (28) |
| Utah | 7,628 (1) | 1,106 (1) | 1,036 (1) | 239 (0.32) |
| Washington | 36,813 (7) | 5,503 (6) | 4,522 (6) | 4,631 (6) |
Data are presented as n (%) unless otherwise specified. Safety-net hospitals were defined as those in which the percentage of Medicaid and uninsured discharges fell in the top quartile for that state.
All-Cause 30-Day Readmissions
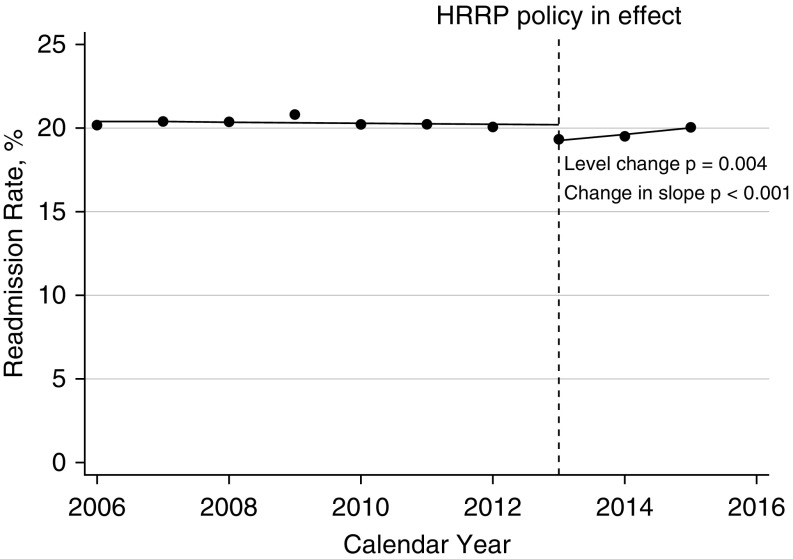
The range of all-cause 30-day readmissions in the baseline period was 20.08% to 20.80% (Figure 1). The level decrease in 30-day readmission rate at the time of the intervention was −0.93% (95% confidence interval [CI], −1.44% to −0.43%; P = 0.004). This represents a significant decrease in all-cause 30-day readmission rate between the observed 2013 readmission rate and the expected rate based on the slope of previous years of data. The postimplementation slope became positive. The difference in slopes (i.e., all-cause 30-day readmission rates) between the pre- and postimplementation periods was 0.39% (95% CI, 0.28–0.50%; P < 0.001).
Figure 1.
Interrupted time series of patients with chronic obstructive pulmonary disease readmitted within 30 days for any reason. The dashed line represents when the financial penalties were implemented for congestive heart failure, myocardial infarction, and pneumonia. The dots represent the actual readmission rate for patients with chronic obstructive pulmonary disease for the calendar year. Patients could be readmitted with any diagnosis. The solid line represents the line of best fit for the preimplementation period and postimplementation period. The level change at the time of the intervention was −0.93% (95% confidence interval [CI], −1.44% to −0.43%; P = 0.004). The postimplementation slope became positive. The change in slopes between the preimplementation period and postimplementation period was 0.39 (95% CI, 0.28% to 0.50%; P < 0.001). HRRP = Hospital Readmission Reduction Program.
The decrease in all-cause readmission rate after the intervention was driven by fewer early (0–7 d) readmissions in 2013, 2014, and 2015 (Table 2). The adjusted odds of being readmitted early (0–7 d) in 2015 compared with the preimplementation period was 0.90 (95% CI, 0.88–0.93; P < 0.001), whereas it was 1.01 (95% CI, 0.98–1.03; P = 0.54) for late (8–30 d) readmissions. The same pattern was observed in the unadjusted comparisons (Table E3) as well as using the alternative risk adjustment technique based on CMS methodology (Table E4).
Table 2.
Adjusted association between year and 30-day all-cause readmission by timing of readmission
| Year | All-Cause Readmission |
All-Cause Early Readmission (0–7 d) |
All-Cause Late Readmission (8–30 d) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted Odds Ratio | 95% CI |
P Value | Adjusted Odds Ratio | 95% CI |
P Value | Adjusted Odds Ratio | 95% CI |
P Value | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
| 2006–2012 | 1 (Reference) |
1 (Reference) |
1 (Reference) |
|||||||||
| 2013 | 0.93 | 0.92 | 0.95 | <0.001 | 0.89 | 0.86 | 0.91 | <0.001 | 0.97 | 0.95 | 1.00 | 0.02 |
| 2014 | 0.94 | 0.92 | 0.96 | <0.001 | 0.89 | 0.86 | 0.92 | <0.001 | 0.98 | 0.96 | 1.00 | 0.09 |
| 2015 | 0.96 | 0.95 | 0.99 | <0.001 | 0.90 | 0.88 | 0.93 | <0.001 | 1.01 | 0.98 | 1.03 | 0.54 |
Definition of abbreviation: CI = confidence interval.
Covariates used in risk adjustment were patient- and hospital-level characteristics, including age, sex, health insurance, median household income quartile, patient residence (urban vs. rural), hospital length of stay, number of Elixhauser comorbidities, safety net hospital, and hospital state.
COPD-related 30-Day Readmissions
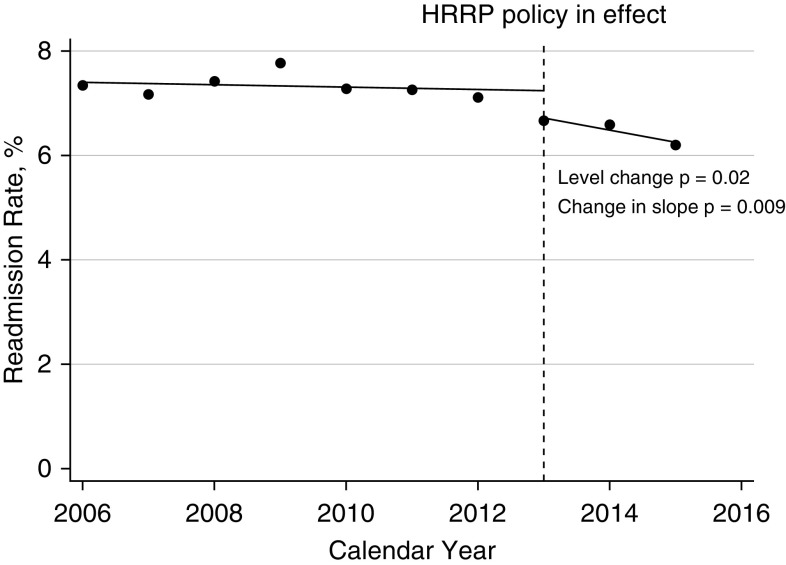
The range of COPD-related 30-day readmissions in the baseline period was 7.10% to 7.76% (Figure 2). The level decrease in 30-day readmission rate in 2013 was −0.52% (95% CI, −0.93% to −0.12%; P = 0.02). The postimplementation slope remained negative. The difference in slopes between the pre- and postimplementation periods was −0.21% (95% CI, −0.35% to −0.07%; P = 0.009).
Figure 2.
Interrupted time series of patients with chronic obstructive pulmonary disease (COPD) readmitted within 30 days for COPD. The dashed line represents when the financial penalties were implemented for congestive heart failure, myocardial infarction, and pneumonia. The dots represent the actual readmission rate for patients with COPD for the calendar year. Patients had to be readmitted for COPD. The solid line represents the line of best fit for the preimplementation period and postimplementation period. The level change at the time of the intervention was −0.52% (95% confidence interval [CI], −0.93% to −0.12%; P = 0.02). The postimplementation slope was negative. The change in slopes between the preimplementation period and postimplementation period was −0.21 (95% CI, −0.35 to −0.07; P = 0.009). HRRP = Hospital Readmission Reduction Program.
Contrary to all-cause readmissions, there were sustained decreases in both early and late COPD-related readmissions (Table 3). The adjusted odds of being readmitted early in 2015 compared with the preimplementation reference group was 0.80 (95% CI, 0.75–0.84; P < 0.001). The odds of being readmitted late were 0.86 (95% CI, 0.83–0.90; P < 0.001). Again, the unadjusted results were similar (Table E5).
Table 3.
Adjusted association between year and 30-day readmission related to chronic obstructive pulmonary disease by timing of readmission
| Year | COPD-related Readmission |
COPD-related Early Readmission (0–7 d) |
COPD-related Late Readmission (8–30 d) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted Odds Ratio | 95% CI |
P Value | Adjusted Odds Ratio | 95% CI |
P Value | Adjusted Odds Ratio | 95% CI |
P Value | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
| 2006–2012 | 1 (Reference) |
1 (Reference) |
1 (Reference) |
|||||||||
| 2013 | 0.91 | 0.88 | 0.94 | <0.001 | 0.88 | 0.84 | 0.93 | <0.001 | 0.93 | 0.90 | 0.97 | <0.001 |
| 2014 | 0.90 | 0.87 | 0.92 | <0.001 | 0.81 | 0.77 | 0.86 | <0.001 | 0.94 | 0.91 | 0.98 | 0.002 |
| 2015 | 0.84 | 0.81 | 0.86 | <0.001 | 0.80 | 0.75 | 0.84 | <0.001 | 0.86 | 0.83 | 0.90 | <0.001 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease, CI = confidence interval.
Readmissions in Patients Aged ≥65 Years
A similar overall pattern was seen in the subgroup of patients aged ≥65 years. There was a significant decrease in all-cause 30-day readmissions in 2013 (level change, −0.86%; 95% CI, −1.55% to −0.17%; P = 0.02; Figure E3). The postimplementation slope was positive. There was a significant difference in slopes between the pre- and postimplementation periods (0.25%; 95% CI, 0.04–0.47%; P = 0.03).
There was also a significant decrease in COPD-related 30-day readmission rate in 2013 (level change, −0.60%; 95% CI, −1.19% to −0.007%; P = 0.048). The postimplementation slope was negative. The difference in slopes between the pre- and postimplementation periods was not significant (−0.17%; 95% CI, −0.37% to 0.03%; P = 0.09; Figure E4). The results of the timing of readmission were similar compared with the larger cohort. The decrease in all-cause readmission was driven by early readmissions (Table E6), whereas the decrease in COPD-related readmissions was attributed to decreases in both early and late readmissions (Table E7).
Discussion
We found that the rate of all-cause and COPD-related readmissions in a broad cohort of patients with mixed insurance statuses declined at the same time that initial financial penalties of the HRRP were implemented for target conditions other than COPD. Previous articles have confirmed that the HRRP decreased readmission rates for target (8, 11) and nontarget conditions (10) as well as for patients with insurance carriers other than Medicare (8, 23). Our results extend the current literature, because COPD uniquely became a target condition years after these decreases in readmissions. Besides highlighting the utility of interim analyses of phased penalties to evaluate for unintended effects, we show that the initial threat of financial penalties for readmissions of target conditions likely prompted broad changes in care delivery across the country to influence patients with COPD before it became a target condition.
We believe behavioral economics may help to explain why the early HRRP penalties decreased hospital readmissions for patients with COPD. The amount of money at risk in the program was quite high. Hospitals could lose up to 3% of their total fee-for-service Medicare payments because of poor performance on the readmission metric, not just the reimbursements for the patients who were readmitted. Loss aversion was almost certainly at play. Loss aversion is the behavioral economic principle in which humans are more motivated to avoid losses than seek gains (24). To preserve their annual reimbursements, hospitals responded quickly to prevent readmissions regardless of the diagnosis. In addition, there is literature documenting how financial penalties in health care (i.e., “sticks”) are more effective than incentive programs (i.e., “carrots”) to change behavior (25–28). Fearing lost revenue, hospitals likely implemented programs to curb their readmission rates. Even if the programs were initially funded to address target conditions, increased awareness about discharge planning or hospital-wide changes in the discharge process could influence the care of patients with nontarget conditions.
We can only speculate about the exact mechanisms of such programs. Programs could have incentivized individual physician behavior or could have been hospital-wide discharge tools. Any of these, or a combination, could explain the effect seen in nontarget conditions. For example, an effort for providers to document an ambulatory saturation before discharge for patients admitted with pneumonia may prompt providers to check it in patients with other respiratory conditions, like COPD, at the time of discharge. Generic discharge planning tools that are agnostic to diagnosis could facilitate getting follow-up appointments for patients regardless of their discharge diagnosis.
Evidence from Rinne and colleagues suggested that hospital-level practices likely influence patients’ likelihood of readmission (29). Using Medicare data, they showed that there were significant correlations between hospital rates of 30-day readmissions for COPD and other medical conditions, including heart failure, myocardial infarction, and pneumonia (29). Future research is needed to explore what proportion of the effect on readmissions is explained by patient- versus hospital-level effects.
Our results are especially interesting given the fact that several studies attempting to decrease readmissions after a hospitalization for COPD have reported null findings (30–32). Aboumatar and colleagues performed a randomized controlled trial testing a 3-month program to help patients self-manage COPD in the outpatient setting after an admission (31). They found a paradoxical increase in acute care use in the intervention group at 6 months (31). Jennings and colleagues performed a randomized controlled trial testing a predischarge bundle for patients admitted with COPD (32). They found no difference in acute care use at 30 days (32). Finally, as part of a voluntary bundled payment incentive program through CMS, Bhatt and colleagues performed a pre–post intervention study to determine whether implementing a comprehensive program for patients admitted with COPD would decrease risk of readmission (30). The program consisted of standardized inpatient care including antibiotics and steroids, patient education materials, tobacco cessation counseling, outpatient follow-up within 2 weeks, and referrals to pulmonary rehabilitation, palliative care, and hospice where appropriate. The program was not associated with reduced all-cause 30-day readmissions or postdischarge care costs (30). Going forward, we believe that testing interventions that leverage the principle of loss aversion at the individual physician level in the outpatient setting may successfully decrease readmission rates.
Barriers to optimal care transitions for patients with COPD have been identified (33). These include 1) poor communication of discharge instructions, 2) poor access to outpatient medications, and 3) lack of or delayed outpatient follow-up. Our results related to the timing of readmissions could inform future prevention efforts to try to overcome these barriers. We found that the decline in all-cause readmissions appeared to be driven by a decrease in early readmissions (Days 0–7), rather than late readmissions (Days 8–30). Jacobs and colleagues similarly reported that a third of all-cause readmissions in a COPD-specific cohort occurred in the first week, using data from the National Readmissions Database (19). Given these findings, examples of crucial time points to prevent later readmission might be the first medication renewal after discharge or the first in-person follow-up appointment after discharge.
Identifying readmission risk factors in real time may also shape future prevention efforts. A study published in 2018 by Jacobs and colleagues used claims data from the National Readmissions Database to identify patient- and hospital-level predictors of 30-day readmissions after hospitalizations for COPD (19). They demonstrated that the strongest predictors were prolonged length of stay (>5 d), multiple comorbidities (>7), and discharge location being home health care or skilled nursing facility (19). Going forward, machine-learning algorithms running via the electronic health record at the time of discharge may provide more contextualized, real-time assessments of readmission risk (34).
Meanwhile, the overarching benefit of HRRP continues to be controversial. Wadhera and colleagues raised concern that the rate of 30-day mortality without readmission increased for Medicare patients with heart failure and pneumonia during HRRP implementation (9). This may be attributed to shifts in hospital priorities to avoid readmissions. Now that COPD has become a target condition, we hope studies assessing 30-day mortality for patients admitted with COPD will be performed.
The results of this study must be interpreted in the context of the study design, which has several limitations. First, our results rely partially on administrative coding of claims. The specificity of ICD-9 codes for COPD is >90%, but the sensitivity can be only ∼25% (35). However, CMS uses administrative codes to calculate the HRRP penalties, so our analysis at least reflects the codes used in the actual program. We also only used one system of administrative coding (ICD-9) throughout the study period, so the level change seen in 2013 cannot be explained by universal shifts in the diagnosis coding system. Second, accounting for death as a competing risk could lessen some of the decrease we saw in postimplementation readmission rate if there was an increase in mortality as a result of the program. Again, we followed CMS methodology by not accounting for death as a competing risk. Third, there are several limitations to the data set. Including as many states as possible with the revisit variable meant that we had to use calendar year, instead of restricting to only states with data at quarterly intervals. These data do not link across states, although the benefit of using non-Medicare data is that they represent the general population, so broader policy effects can be studied. An alternative data source, such as the National Readmission Database, could not be used because it does not contain data before 2010, and more preimplementation data were needed to estimate the baseline slope accurately. Fourth, claims-based data do not contain granular clinical information important for risk adjustment, such as pulmonary function testing parameters.
Despite the above limitations, the current study has several strengths. First, the number of index admissions and Medicare patients in the sample were relatively stable over time. This is reassuring that there were not decreases in the number of COPD hospitalizations being coded, (i.e., hospitals dodging the penalty either by coding the discharge condition differently or avoiding admitting Medicare patients with COPD altogether). Second, the magnitude of the level change for all-cause readmissions is sizeable (∼1%) compared with the narrow range in rates across the 7-year preintervention period (0.72%). Third, the inversion of the slope for all-cause readmissions from negative in the preintervention period to positive in the postintervention period further supports the presence of an effect on all-cause readmissions at the time of the intervention.
In summary, we intended to study the broad impact of the initial implementation of the HRRP in a cohort of patients admitted with COPD who had multiple insurance types. In this way, we used a large data set from geographically diverse states that crossed insurance types. We studied the level change in 2013, given our hypothesis about the initial phase of the HRRP. We also examined the timing and type of readmission to provide granular information beyond the numeric trends.
Conclusions
Patients with mixed insurance statuses admitted to the hospital with COPD had lower all-cause and COPD-related readmission in 2013 after HRRP implemented financial penalties for target conditions other than COPD, compared with the expected rate based on trends of previous years of data (2006–2012). We believe that the HRRP had implications for patients with COPD in the general population before financial penalties were imposed for this condition.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Ashley Sullivan, M.S., M.P.H., for her numerous contributions as Director of the EMNet Coordinating Center (Massachusetts General Hospital, Boston, MA).
Footnotes
Supported by grant R01 HS-023305 (C.A.C.) from the Agency for Healthcare Research and Quality.
Author Contributions: L.C.M. helped to design the study, interpreted the results, and drafted the manuscript. M.K.F. performed the statistical programming and critically reviewed the manuscript. K.H. helped to design the study, interpreted the results. and critically reviewed the manuscript. N.A.H. provided expert opinion on interpreting the results and critically reviewed the manuscript. C.A.C. oversaw the statistical analysis, interpreted the results, critically reviewed the manuscript, had full access to the data, and takes full responsibility for the accuracy of the analysis.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147:989–998. doi: 10.1378/chest.14-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perera PN, Armstrong EP, Sherrill DL, Skrepnek GH. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. COPD. 2012;9:131–141. doi: 10.3109/15412555.2011.650239. [DOI] [PubMed] [Google Scholar]
- 3.Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147:1219–1226. doi: 10.1378/chest.14-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goto T, Faridi MK, Camargo CA, Jr, Hasegawa K. Time-varying readmission diagnoses during 30 days after hospitalization for COPD exacerbation. Med Care. 2018;56:673–678. doi: 10.1097/MLR.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Walraven C, Jennings A, Taljaard M, Dhalla I, English S, Mulpuru S, et al. Incidence of potentially avoidable urgent readmissions and their relation to all-cause urgent readmissions. CMAJ. 2011;183:E1067–E1072. doi: 10.1503/cmaj.110400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 7.CMS.gov. Hospital Readmissions Reduction Program. Baltimore, MD: Centers for Medicare and Medicaid Services; 2019 [updated 2019 Jul 31; accessed 2019 May 14]. Available from: https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html.
- 8.Wasfy JH, Zigler CM, Choirat C, Wang Y, Dominici F, Yeh RW. Readmission rates after passage of the Hospital Readmissions Reduction Program: a pre-post analysis. Ann Intern Med. 2017;166:324–331. doi: 10.7326/M16-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the Hospital Readmissions Reduction Program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320:2542–2552. doi: 10.1001/jama.2018.19232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374:1543–1551. doi: 10.1056/NEJMsa1513024. [DOI] [PubMed] [Google Scholar]
- 11.Desai NR, Ross JS, Kwon JY, Herrin J, Dharmarajan K, Bernheim SM, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316:2647–2656. doi: 10.1001/jama.2016.18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah T, Press VG, Huisingh-Scheetz M, White SR. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150:916–926. doi: 10.1016/j.chest.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Healthcare Cost and Utilization Project. Overview of state inpatient databases (SID). Rockville, MD: Agency for Healthcare Quality and Research; 2019 [updated 2019 Nov 12; accessed 2019 May 14]. Available from: https://www.hcup-us.ahrq.gov/sidoverview.jsp.
- 14.Healthcare Cost and Utilization Project. Data element availability map: HCUP revisit variables. Rockville, MD: Agency for Healthcare Quality and Research; 2019 [updated 2019 Sep 9; accessed 2019 May 23]. Available from: https://www.hcup-us.ahrq.gov/maps/revisit.jsp.
- 15.Spataro E, Branham GH, Kallogjeri D, Piccirillo JF, Desai SC. Thirty-day hospital revisit rates and factors associated with revisits in patients undergoing septorhinoplasty. JAMA Facial Plast Surg. 2016;18:420–428. doi: 10.1001/jamafacial.2016.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis JD, Olsen MA, Bommarito K, LaRue SJ, Saeed M, Rich MW, et al. All-payer analysis of heart failure hospitalization 30-day readmission: comorbidities matter. Am J Med. 2017;130:93.e9–93.e28. doi: 10.1016/j.amjmed.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto T, Faridi MK, Gibo K, Toh S, Hanania NA, Camargo CA, Jr, et al. Trends in 30-day readmission rates after COPD hospitalization, 2006-2012. Respir Med. 2017;130:92–97. doi: 10.1016/j.rmed.2017.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta AB, Douglas IS, Walkey AJ. Hospital noninvasive ventilation case volume and outcomes of acute exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13:1752–1759. doi: 10.1513/AnnalsATS.201603-209OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs DM, Noyes K, Zhao J, Gibson W, Murphy TF, Sethi S, et al. Early hospital readmissions after an acute exacerbation of chronic obstructive pulmonary disease in the Nationwide Readmissions Database. Ann Am Thorac Soc. 2018;15:837–845. doi: 10.1513/AnnalsATS.201712-913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blecker S, Kwon JY, Herrin J, Grady JN, Horwitz LI. Seasonal variation in readmission risk for patients hospitalized with cardiopulmonary conditions. J Gen Intern Med. 2018;33:599–601. doi: 10.1007/s11606-017-4299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baum CF, Schaffer ME. ACTEST: Stata module to perform Cumby-Huizinga general test for autocorrelation in time series. Chestnut Hill, MA: Boston College Department of Economics; 2019 [updated 2015 Jan 24; accessed 2019 May 5]. Available from: https://ideas.repec.org/c/boc/bocode/s457668.html.
- 22.Grosso LM, Lindenauer P, Wang C, Savage S, Potteiger J, Abedin Z, et al. Hospital-level 30-day readmission following admission for an acute exacerbation of chronic obstructive pulmonary disease.New Haven, CT: Yale-New Haven Health Services Corporation/Center for Outcomes Research and Evaluation; 2011 [Google Scholar]
- 23.Ferro EG, Secemsky EA, Wadhera RK, Choi E, Strom JB, Wasfy JH, et al. Patient readmission rates for all insurance types after implementation of the Hospital Readmissions Reduction Program. Health Aff (Millwood) 2019;38:585–593. doi: 10.1377/hlthaff.2018.05412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263–292. [Google Scholar]
- 25.Doran T, Maurer KA, Ryan AM. Impact of provider incentives on quality and value of health care. Annu Rev Public Health. 2017;38:449–465. doi: 10.1146/annurev-publhealth-032315-021457. [DOI] [PubMed] [Google Scholar]
- 26.Khullar D, Chokshi DA, Kocher R, Reddy A, Basu K, Conway PH, et al. Behavioral economics and physician compensation--promise and challenges. N Engl J Med. 2015;372:2281–2283. doi: 10.1056/NEJMp1502312. [DOI] [PubMed] [Google Scholar]
- 27.Emanuel EJ, Ubel PA, Kessler JB, Meyer G, Muller RW, Navathe AS, et al. Using behavioral economics to design physician incentives that deliver high-value care. Ann Intern Med. 2016;164:114–119. doi: 10.7326/M15-1330. [DOI] [PubMed] [Google Scholar]
- 28.Torchiana DF, Colton DG, Rao SK, Lenz SK, Meyer GS, Ferris TG. Massachusetts General Physicians Organization’s quality incentive program produces encouraging results. Health Aff (Millwood) 2013;32:1748–1756. doi: 10.1377/hlthaff.2013.0377. [DOI] [PubMed] [Google Scholar]
- 29.Rinne ST, Castaneda J, Lindenauer PK, Cleary PD, Paz HL, Gomez JL. Chronic obstructive pulmonary disease readmissions and other measures of hospital quality. Am J Respir Crit Care Med. 2017;196:47–55. doi: 10.1164/rccm.201609-1944OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatt SP, Wells JM, Iyer AS, Kirkpatrick DP, Parekh TM, Leach LT, et al. Results of a Medicare Bundled Payments for Care Improvement initiative for chronic obstructive pulmonary disease readmissions. Ann Am Thorac Soc. 2017;14:643–648. doi: 10.1513/AnnalsATS.201610-775BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aboumatar H, Naqibuddin M, Chung S, Chaudhry H, Kim SW, Saunders J, et al. Effect of a hospital-initiated program combining transitional care and long-term self-management support on outcomes of patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA. 2019;322:1371–1380. doi: 10.1001/jama.2019.11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jennings JH, Thavarajah K, Mendez MP, Eichenhorn M, Kvale P, Yessayan L. Predischarge bundle for patients with acute exacerbations of COPD to reduce readmissions and ED visits: a randomized controlled trial. Chest. 2015;147:1227–1234. doi: 10.1378/chest.14-1123. [DOI] [PubMed] [Google Scholar]
- 33.Press VG, Au DH, Bourbeau J, Dransfield MT, Gershon AS, Krishnan JA, et al. Reducing chronic obstructive pulmonary disease hospital readmissions: an official American Thoracic Society workshop report. Ann Am Thorac Soc. 2019;16:161–170. doi: 10.1513/AnnalsATS.201811-755WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan DJ, Bame B, Zimand P, Dooley P, Thom KA, Harris AD, et al. Assessment of machine learning vs standard prediction rules for predicting hospital readmissions. JAMA Netw Open. 2019;2:e190348. doi: 10.1001/jamanetworkopen.2019.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein BD, Bautista A, Schumock GT, Lee TA, Charbeneau JT, Lauderdale DS, et al. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141:87–93. doi: 10.1378/chest.11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.