Abstract
Background:
Although minimally invasive surgery (MIS) of the liver is increasingly widespread, its role in the treatment of colorectal liver metastasis (CRLM) remains uncertain. In this setting, the role of robotic-assisted surgery (RAS) has not been significantly evaluated yet. The aim of this study was to report our experience with RAS for treatment of CRLM.
Material and Methods:
Prospectively collected surgical and oncologic data on all of the robotic-assisted liver resections for CRLM performed at our centre were retrieved from the institutional database and retrospectively analysed. Intra-operative ultrasound (US) was obtained with a dedicated robotic probe using the TilePro™ function.
Results:
Twenty patients underwent robotic-assisted resection of CRLM between May 2012 and April 2018. Six patients (30%) had multiple synchronous CRLM resections (median = 2; range 2–4). The tumour size averaged 3.0 ± 1.8 cm. All of the lesions were removed using a parenchymal-sparing approach, with R0 resection margins. Mean hospital stay was 4.7 ± 1.8 days. The mean follow-up was 22.5 ± 19.5 months. During the study period, there were no local recurrences, while 9 patients (45%) developed new systemic metastasis. All patients are still alive as of September 2018 with 1- and 3-year disease-free survival of 89.5% and 35.8%, respectively.
Conclusions:
In our experience, RAS for CRLM surgical treatment was feasible and played a positive role even in patients with multiple metastases and previous or synchronous surgery. RAS seemed to be oncologically effective in this setting, as no patients experienced local relapse in the treated area.
Keywords: Colorectal metastasis, da Vinci, intra-operative ultrasound, minimally invasive surgery, robotic-assisted
INTRODUCTION
The advent of new systemic chemotherapy agents has led to improved survival in patients with colorectal liver metastasis (CRLM).[1,2] In this multidisciplinary setting, the role of surgery has drastically changed in the last 20 years, and liver resection with R0 margins is associated with long-term survival. Aggressive surgical resection has been replaced with more conservative surgery in order to maximise liver function, reduce post-operative morbidity, increase recovery and consequently, allow potential adjuvant treatment to be started more quickly post-operatively.[3] The philosophy of ‘parenchymal-sparing surgery,’ in contrast to major hepatic resection or the need for complex strategies to induce liver hypertrophy, have gained popularity and recent data demonstrate equal oncologic outcomes compared to standard treatment.[4] Accurate pre-operative planning and intra-operative ultrasound (US) to map blood vessels and evaluate the extent of the lesion are key factors for the success of this approach. While open surgery is still considered the gold standard in the surgical treatment of CRLM, in particular, when there is a substantial liver disease burden or for patients who previously underwent repeated hepatic resections,[5] a minimally invasive approach is increasingly widespread in the case of metastases located in anterior segments and in patients with oligo-metastases.[6] Although in recent years, the use of minimally invasive surgery (MIS) has been expanding in all surgical specialities and in the management of benign and malignant liver lesions, its specific role in the treatment of CRLM remains uncertain. Issues regarding feasibility and oncologic safety of MIS for CRLM are as follows: difficult locations, multiple locations, limitation of the laparoscopic approach in performing a complete study of the liver, presence of primary tumour requiring resection or previous surgery.
Robotic-assisted surgery (RAS) was considered a type of MIS with the potential to overcome some limitations of standard laparoscopy, but to date, there are very little data about its specific role in the treatment of CRLM.[7] The aim of this study is to report our experience with RAS for treatment of CRLM in a single centre.
MATERIAL AND METHODS
Prospectively collected data on all patients scheduled for robotic-assisted CRLM resections performed at our centre, between May 2012 and April 2018, were retrieved from the institutional database and retrospectively analysed. Demographic data, operative time, blood loss (difference between infused and suctioned fluids) and the number of lesions removed were recorded. Information on post-operative complications using the Clavien–Dindo classification and the length of hospital stay (LOS) was also available. Details on pathology, with particular attention to microscopic resection margins, were collected. Patients were followed after discharge with periodic clinical evaluations, laboratory analyses and radiologic imaging. The da Vinci Si was the first robot used, while the latest model, da Vinci Xi, became available in January 2015. Contraindications to using a robotic approach included extensive liver involvement, previous major upper abdominal surgery and difficulties in maintenance of a pneumoperitoneum. From an oncologic standpoint, local and systemic recurrence rate, recurrence site and 1- and 3-year overall and disease-free survival (DFS) were analysed.
Surgical technique
Patient position was defined according to the pre-operative lesion location and number of lesions as well as the need for synchronous primary tumour resection. Exclusive metastasis in the VII or VIII segments was approached preferably with a left flank position, whereas in case of multiple locations or combined colonrectal surgery, patients were positioned supine with legs parted. After establishing the pneumoperitoneum using a Veress needle, five trocars were inserted in a curvilinear fashion (da Vinci Si) or horizontally in (da Vinci Xi) to maintain optimal triangulation and limit collisions. For the right and left anterior resections, the camera port was generally centred on the homo-lateral pararectal line in a supraumbilical position. Operative robotic trocars were placed cranially and laterally with respect to the camera. The surgical cart was positioned at the patient's head. For the right lateral management, all the trocars were located close to the subcostal line; in this case, the cart is carry on to patient’ right shoulder. The latest robotic platform has new functions which make multi-quadrant surgery possible and the docking phase faster and easier. The first phase of the operation was the exploration of the abdominal cavity and then all of the liver segments to check for occult metastasis and to localise the known lesions. We used a dedicated intra-operative US probe (12-5 MHz, curved linear array, BK Medical APS, Peabody MA, USA) inserted through the assistant port and designed to fit robotic instruments for maximum direct control. US exam was displayed on the console in a real-time manner as a picture-in-picture image, using the TilePro™ function. The relationship of all tumours with blood vessels and the distance from the surface was extensively evaluated. Deep tumours were visualised and the resection area drawn on the Glissonian capsule with electrocautery. The liver was resected using bipolar Maryland forceps or Gyrus PK SuperPulse Generator in the left hand and monopolar scissors in the right one. The assistant used the trocar for suction, irrigation and clipping major vessels if needed. The liver was mobilised to make the target area clearly visible and under control. During transaction, the correct plane was verified with contact US in order to give instant feedback for possible changes of direction. Specimens for extemporaneous pathologic examination were placed in an endobag and retrieved. Haemostatic agents were used if necessary and drains were left near the resection.
Statistical analysis
SPSS version 21.0 (IBM Corp., Armonk, NY, USA) and STATA version 13 (STATA Corp., TX, USA) software were employed for statistical analysis. Continuous variables are given as mean ± standard deviation and compared using Student's t-test; P < 0.05 was considered statistically significant. Variables with a non-normal distribution are expressed as median and compared using the Wilcoxon test. Statistical significance was set at 5%. Overall survival was defined as the time between the date of diagnosis and the date of death or last follow-up visit, and DFS time was defined as the time elapsed between the date of diagnosis and tumour progression. Patients, who died from other causes or were alive at the most recent follow-up, were treated as censored in the analysis of DFS time. Survival curves and disease-free intervals were obtained using the Kaplan–Meier method.
RESULTS
Data from 20 patients were included in this study, 13 males and 7 females. Mean age was 66 ± 12 years and the mean body mass index was 24.4 ± 2.7 kg/m2 [Table 1]. Six patients (30%) had multiple CRLM with a median of two lesions (range: 2–4); moreover, four cases had bilobar metastasis, one in the V and III segment, one in the VII and III segment, one in VI and III segment and one in VII plus I, II and III segment. All cases were wedge resections. No formal lobectomies or segmentectomies were performed. Operative and pathological data are summarised in Table 2. In three cases, with the aid of the da Vinci Xi, a combined MIS with synchronous colon cancer resection was performed: two right colectomies and one abdominal–perineal resection. In the remaining cases, the primary lesion had already been removed; in 10/17 (59%) patients, in a minimally invasive fashion and in 7/17 (41%) with a traditional approach. Apart the primary colorectal cancer resection, a combined procedure was necessary in other two cases: splenectomy for metachronous splenic metastasis and colostomy closure in the other patient. The left flank position was used in seven patients; in the others, the supine position was used to facilitate resection of multiple CRLM or for multi-quadrant surgery. The mean operative time was 198.5 ± 98.0 min and the mean blood loss was 250 ml (range: 200–300 ml). No intra-operative complications or conversion to laparoscopy or open surgery were reported. Only two patients required blood transfusion during surgery, both of them received two units of blood. On pathological examination, the mean nodule size was 3.0 ± 1.8 mm, and in all cases, a parenchymal-sparing approach was successfully used, with R0 resection margins.
Table 1.
Patient characteristics
| Variables | Values |
|---|---|
| Number of patients | 20 |
| Age (year), mean±SD | 66.1±11.8 |
| Sex ratio (male: female) | 13:7 |
| BMI (kg/m), mean±SD | 24.4±2.7 |
| Patients with multiple CRLM (%) | 6 (30) |
| Previous surgery MIS (%) | 10/17 (59) |
| Previous surgery open (%) | 7/17 (41) |
CRLM: Colorectal liver metastasis; MIS: Minimally invasive surgery; BMI: Body mass index; SD: Standard deviation
Table 2.
Operative and pathological data
| Variables | Values |
|---|---|
| Combined primary colorectal cancer resection (%) | 3 (15) |
| Operative time (min), mean±SD | 198.5±98.0 |
| Mean estimate blood loss (ml), range | 250 (200-300) |
| Intra-operative complication, n | 0 |
| Conversion to laparoscopic or open approach, n | 0 |
| Nodule size (cm), mean±SD | 3.0±1.8 |
| Complete (R0) resection, n (%) | 20 (100) |
SD: Standard deviation
Post-operative and follow-up data are summarised in Table 3. The mean hospital stay was 4.7 ± 1.8 days. Overall complication rate was 25% (4 Clavien–Dindo Grade II and 1 Clavien–Dindo Grade I). No surgical complications or in-hospital mortality were reported. The mean follow-up was 22.5 ± 19.5 months. Twelve patients (60%) were treated with pre-operative chemotherapy, whereas eleven cases (55%) underwent post-operative chemotherapy. During the study period, there were no local recurrences at the cutting edge, while 9 patients (45%) developed new systemic metastasis. The first systemic metastatic site was the lung (8/20 patients, 40%) followed by the liver in other segments (3/20 patients, 15%) and then peritoneum (1/20 patients, 5%). Liver only relapse was not reported and liver plus another site relapse was diagnosed in 3/20 (15%) patients. In two patients, additional surgery was considered suitable after chemotherapy for the treatment of liver metastasis recurrence: in one case, an open approach was used to perform hepatic and peritoneal resection, whereas in the remaining case, a repeated robotic approach was used. As of September 2018, all patients are still alive with 1- and 3-year DFS of 89.5% and 35.8%, respectively [Figure 1].
Table 3.
Postoperative and follow-up data
| Variables | Values |
|---|---|
| Hospital stay (days), mean±SD | 4.7±1.8 |
| Overall complication, n (%) | 5 (25) |
| Clavien-Dindo I | 1 (5) |
| Clavien-Dindo II | 4 (20) |
| Reoperation rate, n (%) | 0 |
| In-hospital mortality, n (%) | 0 |
| Mean follow-up (months), mean±SD | 22.5±19.5 |
| Local recurrence, n (%) | 0 |
| Systemic metastasis recurrence, n (%) | 9 (45) |
| Lung metastasis, n (%) | 8 (40) |
| Liver metastasis, n (%) | 3 (15) |
| Peritoneal carcinomatosis, n (%) | 1 (5) |
SD: Standard deviation
Figure 1.
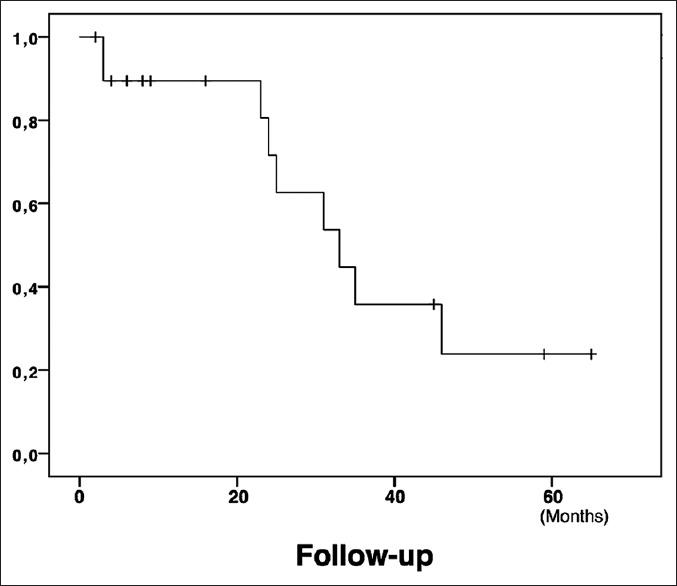
Kaplan–Meier curve showing disease-free survival trend
DISCUSSION
The minimally invasive approach is gaining widespread acceptance in the field of liver surgery. Several studies have shown the feasibility and safety of a minimally invasive hepatic resection over an open procedure in terms of reduced LOS and improved pain control.[8] Recent case series demonstrated that for minor liver resections, laparoscopy and RAS are equivalent in terms of surgical results, while in major liver resections or in challenging cases, the robotic technique can add some technical benefits.[9,10] The case of CRLM is somewhat of an exception as they pose several challenges mainly related to the clear identification of all lesions in the case of multifocal spread, the non-anatomical dissection planes necessary to perform parenchymal-sparing surgery, the synchronous resection of primary colorectal carcinoma and the combination of surgical treatment with chemotherapy. In fact, the liver injury that can be caused by chemotherapy and the expansion of what is considered a resectable lesion, in combination with the significant reduction in what is considered a safe tumour margin, are the principal motivations of parenchymal-sparing liver surgery in management of CRLM. For these reasons today this approach is considered the gold standard associated with less surgical strain and without compromising cancer-related outcomes.[11] With CRLM, the presence of multiple metastases is common and the ability to intra-operatively detect all the metastases is as important as it is limited in MIS because it does not have direct manual control.
Another challenge when using MIS for CRLM is the non-anatomical nature of the parenchymal-sparing approach that may lead to increased blood loss and consequently more post-operative complications; this blood loss may become relevant during laparoscopic CRLM surgery because of the intrinsic drawbacks of this method, in particular, parenchymal dissection carried out with straight instruments.[12] The seepage from cut surfaces, the difficulties in controlling bleeding with compression and the curvilinear line in the posterior portion of the right lobe makes laparoscopic multiple wedge resections challenging in several situations.
An effective laparoscopic intra-operative US would be particularly helpful to compensate for some of these limitations, and in experienced hands, could allow the surgeon to detect multiple metastases, guide the exact resection margin, locate the major vessels in order to reduce bleeding risk and visualise the deeper portions providing real-time guidance during minimally invasive wedge resections.[13,3] Laparoscopic US guarantees real-time guidance in non-anatomical resection, but several barriers exist to its use mainly related to the limited motion of the laparoscopic probe, difficult eye–hand coordination and adequate liver surface and angulation which limit a complete study of the liver or require additional 10-mm trocar insertion.[14] In fact, laparoscopic intra-operative probes are bulky, with limited motion and less adherence to the surface; hence, especially for the right lateral segments, it may become difficult to explore the liver in a complete manner and follow the resection in real time.
With respect to both of these issues, RAS may add some technical innovations that can improve quality in CRLM surgery. Due to the recent introduction of three-dimensional (3D) laparoscopic cameras, 3D vision is no longer a significant advantage for RAS over pure laparoscopy; however, the fine dissection offered by the EndoWrist robotic instruments is a clear upgrade during liver surgery[15,16] as well as in various other surgical indications. In particular, the dexterity of intra-corporeal robotic manipulation may eliminate a certain degree of adjustment as the dissection into the hepatic parenchyma deepens, thus keeping the correct resection plane, as well as helping the surgeon work in difficult locations such as the posterolateral segments. Furthermore, the use of special robotic instruments, such as monopolar scissors, Gyrus PK and Maryland bipolar and needle drivers, can be an advantage during precise manoeuvres such as isolating, sealing or suturing vessels or bile ducts, as well as during parenchymal transection. This technologic progress can translate into surgical benefits.
Apart from the intrinsic improvements of the platform itself, the development of a specific robotic US probe has further enhanced surgeons’ direct control similar to the control in open surgery. In fact, the robotic US probe can give more flexibility than those used in pure laparoscopy because the surgeon seated at a console, thanks to the flexible cable and the small surface, can manipulate the tip of the probe directly with the dominant hand, easily reaching difficult angles while maintaining perpendicular contact with the liver and providing a high grade of accuracy in defining the number and volume of lesions. The TilePro™ function can superimpose US imaging on the console screen, thus eliminating several problems related to hand–eye coordination and uninterrupted surgical dissection.[17]
Another issue regarding the treatment of CRLM is the frequent need for a wide-angle operative field because of bilobar presentation, previous surgery or combined primary tumour resection. Even with the limited number of patients with CRLMs in our institution, we observed, in line with results obtained in other indications, that in this setting the use of the da Vinci Xi can offer further advantages, thanks to its increased flexibility and user-friendly interface. In fact, thanks to camera hopping, the ‘FLEX’ function and integrated table motion,[18,19,20] the da Vinci Xi permits multi-quadrant robotic surgery to treat multiple hepatic metastases or to plan a colonic resection with an oligo-metastatic liver situation.[21,22,23] In our experience, all cases in which liver resection was combined with extra-hepatic surgery were successfully completed robotically and no problems were encountered with instrument traffic or setting that lengthened surgical time. Moreover, this new robot may be particularly useful in patients who have had previous open abdominal surgery and require adhesiolysis because the increased flexibility and reduced collisions between arms allow the surgeon to work in narrow spaces that are frequently observed in this situation and subsequently to work in the target area even when the trocar position is not optimal.[24]
However, although the surgical results were satisfactory, the post-operative course did not translate in a shorter LOS respect than expected for a laparoscopic liver wedge resection. This may be cultural, as in Italy patients expect to leave hospital only when fully recovered and needing little outpatient care. In this regard, Giulianotti[25] reported a difference of 16 days in the median LOS between patients in Italy and the USA on pancreatic resections.
The oncologic outcome of a minimally invasive approach of CRLM has not been extensively evaluated in the literature. Some clinical and case match studies have shown the feasibility of laparoscopic versus open resection with less surgical stress and an improved post-operative course. However, doubts remain about laparoscopic CRLM resection because of the technical complexity and concerns around oncologic safety mainly related to the adequacy of surgical margins and clear identification of all lesions.[26] While only small series have dealt in detail with robotic-assisted CRLM treatment,[7] to the best our knowledge, no one has described the oncologic appropriateness of the procedure. The absence of local recurrence in our series and the DFS comparable to that described in the literature for open surgery[27] showed the positive oncologic result of robotic CRLM resection in the variable scenarios that we have described, thus demonstrating its versatility.
The main limitations of the present study are related to the limited numbers of patients, and to the lack of a control group, however, to the best of our knowledge, this manuscript reports on the largest series to date on robotic CRLM treatment and is the first to report on mid-term surgical and oncologic results.
CONCLUSIONS
The present study has shown that RAS for CRLM surgical treatment was feasible, and in selected cases, played a positive role even in patients’ multiple metastases and previous or synchronous surgery. The availability of a dedicated intra-operative US probe, managed directly with the dominant hand of the surgeon, similarly to the open approach, could add additional safety in the management of multiple metastases. The da Vinci Xi adds the ability to perform multi-quadrant surgery, which is particularly useful when synchronous resection of primary tumour is required. RAS appeared to be oncologically safe in this setting, as no patients experienced local relapse in the treated area. However, given the relatively small experience and lack of objective advantages of robotic liver resections, RAS for the treatment of CRLM should be viewed only as an additional option to conventional techniques. Further studies with larger numbers of patients are necessary to elucidate exactly the value of RAS in the management of CRLM.
Financial support and sponsorship
The study was financially supported by the ARPA Foundation.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would thank to thank Sabrina L. Maurer, PharmD, CMPP, for providing language editing.
REFERENCES
- 1.Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M, et al. Surgical resection of hepatic metastases from colorectal cancer: A systematic review of published studies. Br J Cancer. 2006;94:982–99. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Julien LA, Thorson AG. Current neoadjuvant strategies in rectal cancer. J Surg Oncol. 2010;101:321–6. doi: 10.1002/jso.21480. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez FA, Sanchez Claria R, Oggero S, de Santibañes E. Parenchymal-sparing liver surgery in patients with colorectal carcinoma liver metastases. World J Gastrointest Surg. 2016;8:407–23. doi: 10.4240/wjgs.v8.i6.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA, et al. Selection of patients for resection of hepatic colorectal metastases: Expert consensus statement. Ann Surg Oncol. 2006;13:1261–8. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 5.Welsh FK, Tekkis PP, John TG, Rees M. Open liver resection for colorectal metastases: Better short- and long-term outcomes in patients potentially suitable for laparoscopic liver resection. HPB (Oxford) 2010;12:188–94. doi: 10.1111/j.1477-2574.2009.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halls MC, Cipriani F, Berardi G, Barkhatov L, Lainas P, Alzoubi M, et al. Conversion for unfavorable intraoperative events results in significantly worse outcomes during laparoscopic liver resection: Lessons learned from a multicenter review of 2861 cases. Ann Surg. 2018;268:1051–7. doi: 10.1097/SLA.0000000000002332. [DOI] [PubMed] [Google Scholar]
- 7.Sunil S, Restrepo J, Azin A, Hirpara D, Cleary S, Cleghorn MC, et al. Robotic simultaneous resection of rectal cancer and liver metastases. Clin Case Rep. 2017;5:1913–8. doi: 10.1002/ccr3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiffman SC, Kim KH, Tsung A, Marsh JW, Geller DA. Laparoscopic versus open liver resection for metastatic colorectal cancer: A metaanalysis of 610 patients. Surgery. 2015;157:211–22. doi: 10.1016/j.surg.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Croner RS, Perrakis A, Hohenberger W, Brunner M. Robotic liver surgery for minor hepatic resections: A comparison with laparoscopic and open standard procedures. Langenbecks Arch Surg. 2016;401:707–14. doi: 10.1007/s00423-016-1440-1. [DOI] [PubMed] [Google Scholar]
- 10.Kingham TP, Leung U, Kuk D, Gönen M, D’Angelica MI, Allen PJ, et al. Robotic liver resection: A case-matched comparison. World J Surg. 2016;40:1422–8. doi: 10.1007/s00268-016-3446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moris D, Ronnekleiv-Kelly S, Rahnemai-Azar AA, Felekouras E, Dillhoff M, Schmidt C, et al. Parenchymal-sparing versus anatomic liver resection for colorectal liver metastases: A Systematic review. J Gastrointest Surg. 2017;21:1076–85. doi: 10.1007/s11605-017-3397-y. [DOI] [PubMed] [Google Scholar]
- 12.Nassour I, Polanco PM. Minimally invasive liver surgery for hepatic colorectal metastases. Curr Colorectal Cancer Rep. 2016;12:103–12. doi: 10.1007/s11888-016-0316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langella S, Russolillo N, D’Eletto M, Forchino F, Lo Tesoriere R, Ferrero A, et al. Oncological safety of ultrasound-guided laparoscopic liver resection for colorectal metastases: A case-control study. Updates Surg. 2015;67:147–55. doi: 10.1007/s13304-015-0325-0. [DOI] [PubMed] [Google Scholar]
- 14.Ferrero A, Lo Tesoriere R, Russolillo N, Viganò L, Forchino F, Capussotti L, et al. Ultrasound-guided laparoscopic liver resections. Surg Endosc. 2015;29:1002–5. doi: 10.1007/s00464-014-3762-9. [DOI] [PubMed] [Google Scholar]
- 15.Ocuin LM, Tsung A. Robotic liver resection for malignancy: Current status, oncologic outcomes, comparison to laparoscopy, and future applications. J Surg Oncol. 2015;112:295–301. doi: 10.1002/jso.23901. [DOI] [PubMed] [Google Scholar]
- 16.Wu YM, Hu RH, Lai HS, Lee PH. Robotic-assisted minimally invasive liver resection. Asian J Surg. 2014;37:53–7. doi: 10.1016/j.asjsur.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Guerra F, Amore Bonapasta S, Annecchiarico M, Bongiolatti S, Coratti A. Robot-integrated intraoperative ultrasound: Initial experience with hepatic malignancies. Minim Invasive Ther Allied Technol. 2015;24:345–9. doi: 10.3109/13645706.2015.1022558. [DOI] [PubMed] [Google Scholar]
- 18.Morelli L, Palmeri M, Simoncini T, Cela V, Perutelli A, Selli C, et al. A prospective, single-arm study on the use of the da Vinci ® Table Motion with the Trumpf TS7000dV operating table. Surg Endosc. 2018;32:4165–72. doi: 10.1007/s00464-018-6161-9. [DOI] [PubMed] [Google Scholar]
- 19.Palmeri M, Gianardi D, Guadagni S, Di Franco G, Bastiani L, Furbetta N, et al. Robotic colorectal resection with and without the use of the new da Vinci Table Motion: A Case-matched study. Surg Innov. 2018;25:251–7. doi: 10.1177/1553350618765540. [DOI] [PubMed] [Google Scholar]
- 20.Morelli L, Palmeri M, Guadagni S, Di Franco G, Moglia A, Ferrari V, et al. Use of a new integrated table motion for the da Vinci Xi in colorectal surgery. Int J Colorectal Dis. 2016;31:1671–3. doi: 10.1007/s00384-016-2609-3. [DOI] [PubMed] [Google Scholar]
- 21.Morelli L, Guadagni S, Di Franco G, Palmeri M, Caprili G, D’Isidoro C, et al. Use of the new da Vinci Xi during robotic rectal resection for cancer: Technical considerations and early experience. Int J Colorectal Dis. 2015;30:1281–3. doi: 10.1007/s00384-015-2350-3. [DOI] [PubMed] [Google Scholar]
- 22.Morelli L, Guadagni S, Di Franco G, Palmeri M, Caprili G, D’Isidoro C, et al. Use of the new da Vinci Xi® during robotic rectal resection for cancer: A pilot matched-case comparison with the da Vinci Si®. Int J Med Robot. 2017:13. doi: 10.1002/rcs.1728. [DOI] [PubMed] [Google Scholar]
- 23.Morelli L, Di Franco G, Guadagni S, Palmeri M, Gianardi D, Bianchini M, et al. Full robotic colorectal resections for cancer combined with other major surgical procedures: Early experience with the da Vinci Xi. Surg Innov. 2017;24:321–7. doi: 10.1177/1553350617697183. [DOI] [PubMed] [Google Scholar]
- 24.Martens TP, Morgan JA, Hefti MM, Brunacci DA, Cheema FH, Kesava SK, et al. Adhesiolysis is facilitated by robotic technology in reoperative cardiac surgery. Ann Thorac Surg. 2005;80:1103–5. doi: 10.1016/j.athoracsur.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Giulianotti PC, Sbrana F, Bianco FM, Elli EF, Shah G, Addeo P, et al. Robot-assisted laparoscopic pancreatic surgery: Single-surgeon experience. Surg Endosc. 2010;24:1646–57. doi: 10.1007/s00464-009-0825-4. [DOI] [PubMed] [Google Scholar]
- 26.Montalti R, Berardi G, Laurent S, Sebastiani S, Ferdinande L, Libbrecht LJ, et al. Laparoscopic liver resection compared to open approach in patients with colorectal liver metastases improves further resectability: Oncological outcomes of a case-control matched-pairs analysis. Eur J Surg Oncol. 2014;40:536–44. doi: 10.1016/j.ejso.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Allard MA, Cunha AS, Gayet B, Adam R, Goere D, Bachellier P, et al. Early and long-term oncological outcomes after laparoscopic resection for colorectal liver metastases: A Propensity score-based analysis. Ann Surg. 2015;262:794–802. doi: 10.1097/SLA.0000000000001475. [DOI] [PubMed] [Google Scholar]


