Abstract
Extracervical, scarless in-the-neck endoscopic thyroidectomy (SET) is a relatively new offshoot of minimal access neck surgery which is gaining popularity rapidly. Among all the approaches described, hybrid approaches such as axillary-breast and bilateral axillo-breast (BABA) are most practiced world over. We have performed more than 130 cases of SET using various approaches (ABA, BABA and transoral vestibular approach). We find BABA most suitable for patients who present with larger goitres (≥6cm), toxic glands or low-grade thyroid cancers and are desirous of SET. Here, we describe the surgical technique of BABA, its pros and pitfalls based on our experience.
Keywords: Bilateral axillo-breast approach, extracervical approach, scarless (in the neck) endoscopic thyroidectomy
INTRODUCTION
Huscher performed the first endoscopic thyroid lobectomy in 1997.[1] This paved the way for minimal access neck surgery. Since then, innumerable approaches have been described with the common goal of minimising/avoiding ‘visible neck scar’ without compromising treatment efficacy. According to the site of port placement, these are broadly classified into cervical or minimal neck incision thyroidectomy (access to thyroid through the neck) and extracervical SET (access to thyroid remote from neck).[2] The most popular cervical approach is minimally invasive video-assisted thyroidectomy described by Micolli.[3] Extracervical approaches can be divided into - anterior chest wall,[4] axillary,[5] breast[6] and hybrid approaches such as axillary-bilateral breast,[7] bilateral axillo-breast (BABA)[8] and postauricular and axillary approach.[9] Here, we describe the technique of BABA, which simulates conventional/open thyroidectomy (ConT) and hence is suitable for all thyroid nodules (TN) that are eligible for endoscopic thyroidectomy (ET) including toxic goitres and low grade differentiated thyroid cancers. BABA was developed by Choe et al. from Korea[8] who found it suitable for benign TN <5 cm and low-risk thyroid malignancies (age <45 years, size ≤2 cm with no evidence of local/regional invasion or metastasis).
Indications
We started performing ET in our department from 2012. Initially, we strictly adhered to the inclusion criteria described by experts,[9,10] however, as our experience increased we attempted benign TN ≤8 cm, toxic goitres, Graves’ disease and low-grade malignancies <4 cm for patients’ desirous of SET.
Pre-operative preparation
Similar to ConT: Serum thyroid stimulating hormone estimation, ultrasound neck, aspiration cytology from nodule, vocal cord evaluation and investigations for anaesthesia fitness. Optimisation of hypo/hyperthyroidism and pre-existing chronic conditions may be required.
In addition, a clinical breast examination is mandatory to screen for pre-existing breast lumps/lesions.
SURGICAL TECHNIQUE
Instruments
Theatre set-up and instruments are similar to those required for advanced laparoscopic procedures, including ultrasonic energy devices, high definition camera system and high flow CO2 insufflators. We have recently started using three-dimension technology which aids in identifying vital structures such as recurrent laryngeal nerve (RLN) and parathyroid glands (PTH). Hand instruments required are those used in basic/conventional laparoscopic procedures, except for a tunneler to raise flaps (described later).
Anesthesia
General anaesthesia using armored flexometallic endotracheal tube. As a policy, we administer single dose intravenous (IV) antibiotic prophylaxis, (Amoxycyllin+Clavulanate) at the time of induction.
Position of patient
The patient is supine with a sandbag under the shoulders and 30° headend elevation (Reverse Trendelenburg) which provides neck extension. Arms are kept by the patients’ side, slightly abducted at shoulders and semi-flexed at elbows to expose the axillae [Figure 1].
Figure 1.
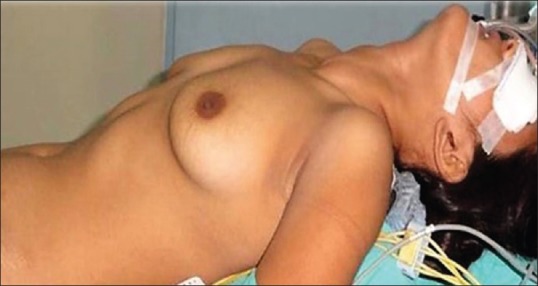
Patient position: Supine with sand bag under shoulders and 30° elevation of head-end with arms abducted at shoulders and semi flexed at elbows, to expose the breast-axilla fold
Operative steps
Surface marking
Chlorhexidine and povidone-iodine solution is used for sterile preparation (chin to umbilicus and axillae). Anatomical landmarks: clavicle, medial border of sternocleidomastoid, sternal and thyroid notch, hyoid bone and a line two fingerbreadths below and parallel line to the clavicle and port sites are marked.
Tangential lines are drawn from all 4-port site markings towards midline in the direction of hyoid bone which define the direction of flaps. A curved line is drawn from the hyoid which connects the tangential lines at the lateral end of the clavicle. This defines the extent of flaps.
The operating surgeon (OS) stands opposite to the side of lesion/larger nodule. The first (AS1) and second assistant (AS2) stand opposite the OS. AS2 aids in retraction through the 5 mm port. Figure 2 shows the operation theatre layout and surface markings. Thus, the OS has both working instruments on the same side (10 mm breast and axilla), and the camera is on the opposite side. This arrangement differs from the principle of triangulation of camera and working instruments described for most laparoscopic procedures, thus adaptation to this orientation takes time.
Figure 2.
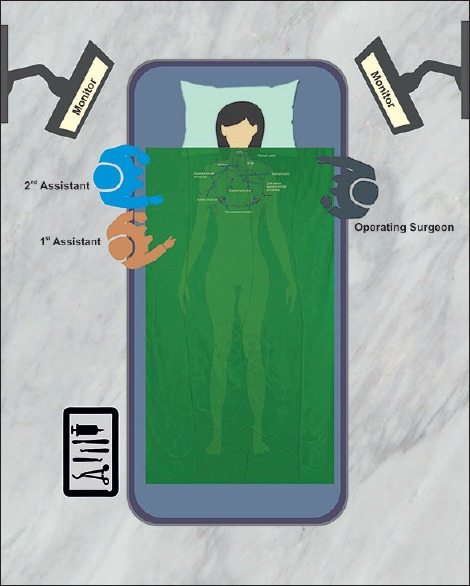
Illustration showing operation theater layout while performing bilateral axillo-breast for a right-sided solitary nodule (right hemithyroidectomy). Position of operating surgeon, assistants and important surface landmarks are marked with arrows. SCM: Sternocleidomastoid muscle
Adrenal saline infiltration
A volume of 60–80 ml of reconstituted 2% lignocaine + adrenal in saline solution is infiltrated (500 ml normal saline+1amp adrenaline+40ml lignocaine) in the subcutaneous and subplatysmal plane over the chest and neck, respectively, which causes hydrodissection, reduces vascularity and provides post-operative analgesia. Infiltration of the excessive amount can hamper dissection and vision while using energy devices by producing excessive smoke causing charring and frequent fogging of the camera.
Creation of blind space over the chest wall
The skin incision is placed over marked supra-areolar sites. A blind subcutaneous tunnel is created initially using a straight medium sized haemostat forceps along the tangential lines directed towards the neck. After this, a large haemostat is inserted along the same tract followed by a custom-made metallic tunneler [Figure 3a]. Using to-and-fro motion at the elbow, a plane is created over the chest and neck in the marked area. A similar procedure is repeated on the opposite side. This is a blind procedure and one should be careful not to go too deep into the breast parenchyma or too superficial into the dermis which causes bruising.
Figure 3.
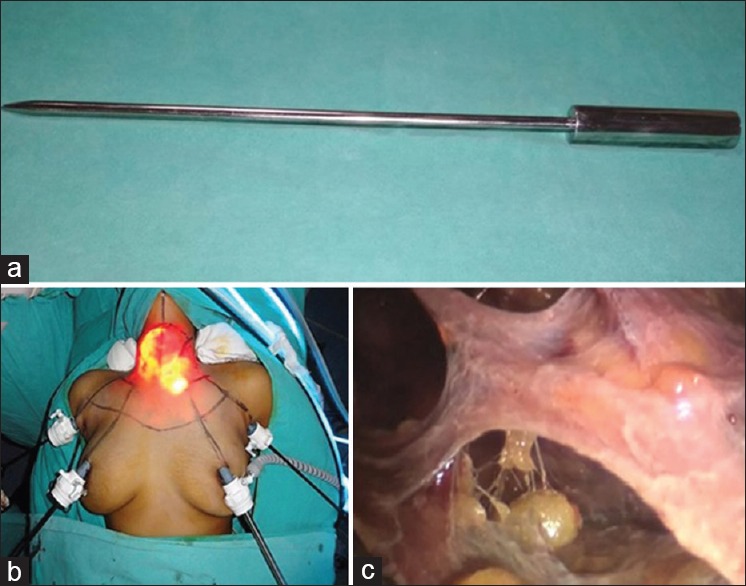
(a) Custom made metallic tunneler used for creating plane over chest wall and neck (b) after placement of all 4 ports (c) honeycomb appearance of the subcutaneous plane over the chest created using the tunneler
Port placement
Two 10 mm ports, are placed over bilateral supra-areolar margins, 12-3 ‘o’clock on the right and 9–12 ‘o’clock on the left, serve as the camera and main working port and CO2 insufflation started. Another 10 mm port is placed at axillary skin fold on the side of OS which serves as second working port. Specimen is retrieved through this port. Finally, a 5 mm port is placed at the opposite axillary skin fold [Figure 3b]. We use flow rate at 7 L/min and maintain pressure at 7mm Hg. As soon as, the telescope is inserted a honeycomb appearance of the subcutaneous plane over the chest is seen [Figure 3c]. Dissection using ultrasonic energy device is started, and fibres over chest are divided. After this axillary ports are inserted: 10mm and 5mm on the OS and AS1 side respectively.
Creation of subplatysmal plane
Once all ports are in, dissection proceeds towards the neck extending from sternocleidomastoids on either side laterally, superiorly till hyoid and inferiorly till suprasternal notch [Figure 4a]. One must err on the side of raising thick flaps because skin necrosis/perforation will defeat the purpose of superior cosmesis. Instrument through 5 mm axillary port is used to lift the skin flap.
Figure 4.
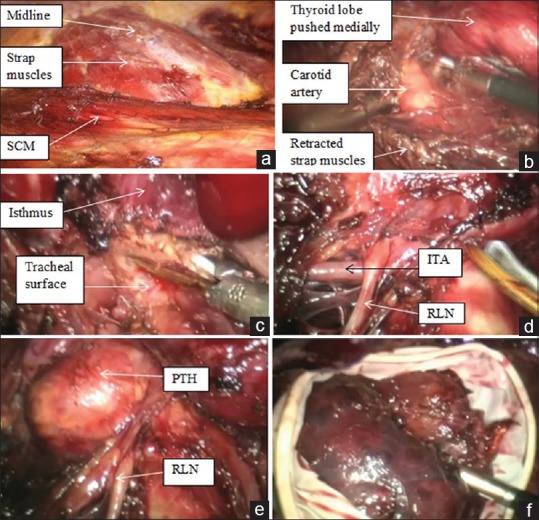
Key operative steps: (a) Extent of cervical flap superior up to hyoid bone, inferiorly up to suprasternal notch and sternocleidomastoid muscles on either sides laterally (b) completion of lateral dissection marked by identification of carotid artery (c) isthmusectomy (d) Identification of recurrent laryngeal nerve, seen coursing over the inferior thyroid artery, (e) Inferior parathyroid gland easily identified due to high magnification, (f) surgical glove serves as endobag
Division of midline and dissection of strap muscles
Similar to ConT, after raising flaps, midline is identified and opened using monopolar electrocautery hook. Strap muscles are separated from the visceral surface of gland, followed by lateral dissection and separation from medial border of sternocleidomastoid marked by identification of carotid artery [Figure 4b]. The middle thyroid vein is identified and secured and strap muscles are retraced laterally using S-shaped retractor.
Isthmusectomy and dissection of the poles
Medially dissection is continued till the identification of trachea, and here, the isthmus is identified and dissected off anterior tracheal surface. In smaller nodules (≤4cm), Isthmusectomy [Figure 4c] is the initial step which helps in providing traction while dissecting the lobe.
Unlike ConT, in chest wall approaches, the inferior pole is secured first. The RLN and inferior PTH are identified using blunt dissecting forceps [Figure 4d and e]. One must be extremely cautious and avoid using energy devices in this area. After RLN has been identified, inferior thyroid vessels can be divided using ultrasonic devices or sharp division between lilac lips. After this, lobe can be pulled down and rotated medially which helps in visualising superior pole. The superior PTH and external branch of the superior laryngeal nerve are identified and secured, and lobe is dissected off the trachea by dividing Berry's ligament. For total thyroidectomy, the same procedure is repeated on the opposite side. We routinely do central compartment lymph node dissection for papillary thyroid cancers with special attention to inferior PTH and RLN.
Specimen extraction
A surgical glove serves as an economical retrieval bag [Figure 4f]. Larger specimens are extracted by enlarging the 10-mm axillary port.
Haemostasis and closure
The cavity is irrigated, haemostasis ensured, an absorbable haemostat (SURGICEL) and Jackson-Pratt drain are placed in the thyroid bed. External end is taken out through a separate skin puncture close to axillary port.
Midline is approximated using absorbable (polyglactin 3-0) continuous suture, ports in 2 layers using absorbable sutures. A pressure dressing is applied [Figure 5a] which helps in preventing seroma formation (due to extensive flap dissection) and acts as breast support which alleviates post-operative pain. Our policy is to keep the drain in situ until output over 24 h is ≤20ml.
Figure 5.
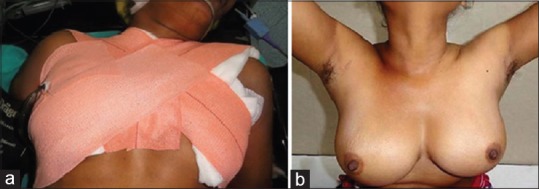
(a) Pressure dressing applied at end of procedure (b) cosmetic outcome at 6 months
Post-operative care
Liquid to soft diet is allowed as tolerated from the evening of surgery. The wound is inspected on the second post-operative day. Post total thyroidectomy, l-thyroxine is started and serum calcium is monitored daily. Biochemical/clinical hypocalcemia are corrected by oral/IV calcium and vitamin D supplements. Patients can be discharged once eucalcaemic and drain removed. Post-hemithyroidectomy patients are discharged after drain removal. Oral antibiotics are continued for 5 days. Figure 5b shows the 6-month post-operative outcome with almost invisible scars.
CONCLUSION
Most of our patients present with larger goitres possibly due to delay in seeking medical attention, however, the demand for superior cosmesis is ever increasing. The superior ergonomics, a similitude to ConT and excellent cosmesis make BABA the most suitable approach of SET for such patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/ have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank Mr. Amit Yadav, School of Telemedicine and Bioinformatics, SGPGIMS, Lucknow, for providing technical support with illustrations.
REFERENCES
- 1.Hüscher CS, Chiodini S, Napolitano C, Recher A. Endoscopic right thyroid lobectomy. Surg Endosc. 1997;11:877. doi: 10.1007/s004649900476. [DOI] [PubMed] [Google Scholar]
- 2.Dralle H, Machens A, Thanh PN. Minimally invasive compared with conventional thyroidectomy for nodular goitre. Best Pract Res Clin Endocrinol Metab. 2014;28:589–99. doi: 10.1016/j.beem.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Miccoli P, Berti P, Bendinelli C, Conte M, Fasolini F, Martino E, et al. Minimally invasive video-assisted surgery of the thyroid: A preliminary report. Langenbecks Arch Surg. 2000;385:261–4. doi: 10.1007/s004230000141. [DOI] [PubMed] [Google Scholar]
- 4.Cho YU, Park IJ, Choi KH, Kim SJ, Choi SK, Hur YS, et al. Gasless endoscopic thyroidectomy via an anterior chest wall approach using a flap-lifting system. Yonsei Med J. 2007;48:480–7. doi: 10.3349/ymj.2007.48.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda Y, Takami H, Niimi M, Kan S, Sasaki Y, Takayama J, et al. Endoscopic thyroidectomy by the axillary approach. Surg Endosc. 2001;15:1362–4. doi: 10.1007/s004640080139. [DOI] [PubMed] [Google Scholar]
- 6.Ohgami M, Ishii S, Arisawa Y, Ohmori T, Noga K, Furukawa T, et al. Scarless endoscopic thyroidectomy: Breast approach for better cosmesis. Surg Laparosc Endosc Percutan Tech. 2000;10:1–4. [PubMed] [Google Scholar]
- 7.Shimazu K, Shiba E, Tamaki Y, Takiguchi S, Taniguchi E, Ohashi S, et al. Endoscopic thyroid surgery through the axillo-bilateral-breast approach. Surg Laparosc Endosc Percutan Tech. 2003;13:196–201. doi: 10.1097/00129689-200306000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Choe JH, Kim SW, Chung KW, Park KS, Han W, Noh DY, et al. Endoscopic thyroidectomy using a new bilateral axillo-breast approach. World J Surg. 2007;31:601–6. doi: 10.1007/s00268-006-0481-y. [DOI] [PubMed] [Google Scholar]
- 9.Lee KE, Kim HY, Park WS, Choe JH, Kwon MR, Oh SK, et al. Postauricular and axillary approach endoscopic neck surgery: A new technique. World J Surg. 2009;33:767–72. doi: 10.1007/s00268-009-9922-8. [DOI] [PubMed] [Google Scholar]
- 10.Choi JY, Lee KE, Chung KW, Kim SW, Choe JH, Koo do H, et al. Endoscopic thyroidectomy via bilateral axillo-breast approach (BABA): Review of 512 cases in a single institute. Surg Endosc. 2012;26:948–55. doi: 10.1007/s00464-011-1973-x. [DOI] [PubMed] [Google Scholar]


