Abstract
In Major ABO mismatched allogeneic stem cell transplantation (SCT) persistence of anti-donor isohemagglutinins leads to pure red cell aplasia (PRCA). To investigate severe pancytopenia noted in a previous study of PRCA,1 we analyzed all major ABO-mismatched HCT between January 2003 and December 2012.Of 83 PRCA patients, 13 (16%) PRCA patients had severe pancytopenia.Severe pancytopenia was defined as Absolute Neutrophil Count (ANC) < 1.5 K/UL or requiring G-CSF, Platelets < 50 K/UL or transfusion dependent, and PRCA with red cell transfusion dependence at post-transplant day 90. In 6 (46%) patients severe pancytopenia resolved after PRCA resolution.2 (15%) patients received a second transplant due to persistent pancytopenia/secondary graft failure. 1 (8%) died from secondary graft failure despite a stem cell boost. 1 (8%) patient did not recover his platelet counts despite red cell/ANC recovery and 3 (23%) patients died from disease relapse.We found that severe pancytopenia is frequently associated with PRCA in 16% of major ABO incompatible HCT with a higher incidence in males and pancytopenia resolved with resolution of PRCA in 46 % of patients..
Keywords: Pure red cell aplasia, Hematopoietic Stem cell Transplant, Pancytopenia
Introduction
The persistence of anti-donor isohemagglutinins in Major ABO mismatched allogeneic stem cell transplantation (HCT) leads to pure red cell aplasia (PRCA) in a small proportion of patients.1,2 In a previous review of 596 major ABO mismatched HSCT patients, PRCA occurred in 7.5% of Major ABO mismatched HCTs and pancytopenia was observed in one patient which resolved upon resolution of PRCA.3 To further investigate this observation, we retrospectively analyzed a larger cohort of major ABO mismatched allogeneic SCT patients to determine the frequency of pancytopenia in PRCA and the resolution of pancytopenia in patients with PRCA after major ABO incompatible HCT.
Patients, material and methods
This retrospective study was approved by the institutional review board of MD Anderson Cancer Center.
Patients
The study group consisted of 707 patients who received a major ABO-mismatched allogeneic stem cell transplant between January 2003 and December 2012.The patients were identified from the Stem Cell Transplantation and Cellular Therapy database and transplant and transfusion data was collected from the Patients’ electronic medical record and Blood Bank data base.
Definition of Pure Red Cell Aplasia
Pure red cell aplasia was determined to be present when the bone marrow biopsy on post-transplant day 30 demonstrated adequate myeloid, lymphoid and megakaryocyte populations in the setting of severe erythroid hypoplasia defined by <5% precursors in bone marrow biopsies with absence of donor red cells on forward red cell typing of the recipient and the recipient being red cell transfusion dependent.
ABO Incompatibility
A major ABO incompatibility existed when the recipient had naturally occurring isohemagglutinins against the red cell antigen(s) present on the surface of the donor’s red cells. This occurred between ABO blood groups, A, B or AB donors and group O recipients (A→O, B→O, AB→O) and between group AB donors and group A or B recipients (AB→B, AB→A). Patients with bidirectional mismatches(A→B and B→A) were also included with the major ABO incompatible group.
Blood Group Serology
ABO forward and reverse typing was determined serologically using both the solid-phase and tube methods.(Immuncor, Norcross, GA, USA)
Engraftment
White cell and Platelet Engraftment
The day of neutrophil engraftment was defined as the first of 3 consecutive days on which the patient’s absolute neutrophil count was > 500 k/uL. The day of platelet engraftment was defined as the first of 7 consecutive days on which the patient’s platelet count was > 20,000 k/uL without platelet transfusion.
Red Cell Engraftment
The day of red cell engraftment was defined as 30 days after SCT for patients who did not require red cell transfusions. For patients who were red cell transfusion-dependent, the day on which the donor red cells appeared on forward red cell typing was taken as the day of red cell engraftment. For patients who did not have ABO typing performed, the day of red cell engraftment was the last day of red cell transfusion. Red cell type and screens were performed every third day for those inpatients who required red cell transfusions.
Severe Pancytopenia
Severe pancytopenia was defined as ANC < 1.5 k/uL or requiring G-CSF, Platelets < 50,000 k/uL and PRCA with red cell transfusion dependent at 90 days post allogeneic SCT.
Results
Of a total of 707 (428 [71%] males: 279 [39%] females) major ABO mismatched HCTs, there were 83 (11.7%) PRCA patients (29 [35%] males: 54 [65%] females, median age 50 [range 15–69 years]).Severe pancytopenia was noted in 13 (16%) PRCA patients (10[77%] males: 3 [23%] females, median age 53 [range 27–66 years]) at 90 days after transplant. 7 (53%) received allogeneic hematopoietic peripheral stem cells. 6 out the 7 donors (2 related vs. 5 unrelated donors) were HLA matched. Of the 6 (46%) patients who received allogeneic hematopoietic bone marrow cells, all of the donors were unrelated with 5 donors being a 7/8 HLA match. Bone marrow cellularity was a median of 5% (range 5–90%) at post-transplant day median 91 (range 52–128). There was a female preponderance of PRCA patients; however a male predominance (10M/3F) was noted in PRCA patients with severe pancytopenia and this was found to be statistically significant (odds ratio 8.9,p=0.001 Fisher’s exact test).All patients were red cell and platelet transfusion dependent and all patients received intermittent G-CSF. (Table-1) None of the patients had any other apparent reason for persistent pancytopenia such as CMV or other viral infection or use of drugs like ganciclovir or disease recurrence.
Table 1.
Patient Characteristics
| # | Age Patient/Donor | Gender Patient/Donor | ABO Patient/Donor | Diagnosis | Conditioning regimen | Donor* | Graft Source* | GVHD Prophylaxis | CD34 dose x10e6/kg | TNC dose x10e8/kg | MC^ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59/36 | M/F | O/A | Lymphoma | Flu/Mel/Thio/Rituximab | 7/8 ** Matched Unrelated | Bone Marrow | T a cro/Cytoxa n/MMF | 0.703 | 0.39 | No |
| 2 | 38/39 | M/M | O/A | AA/HIV | Flu/Mel/ATG | 8/8 Matched Unrelated | Peripheral Blood | Tacro/MTX | 7.799 | 14.18 | No |
| 3 | 56/61 | M/F | O/A | CML | Flu/Mel/Rituximab | 8/8 Matched Unrelated | Peripheral Blood | Tacro/MTX | 3.95 | 8.94 | No |
| 4 | 53/30 | M/F | O/A | MDS | Flu/Bu/ATG | 7/8 *** Matched Unrelated | Bone Marrow | Tacro/MTX | 2.281 | 0.56 | No |
| 5 | 27/45 | F/F | O/AB | MDS | Flu/Bu | 8/8 Matched Unrelated | Peripheral Blood | Tacro/MTX | 3.73 | 4.28 | No |
| 6 | 66/67 | M/M | O/AB | AML | Flu/Bu | 8/8 Matched Unrelated | Peripheral Blood | Tacro/MTX | 4.51 | 16.11 | No |
| 7 | 50/38 | M/M | O/A | ALL | Bu/Clo/ATG | 7/8 **** Matched Unrelated | Bone Marrow | Tacro/MTX | 0.941 | 0.75 | No |
| 8 | 56/55 | M/F | O/A | AML | Fu/Mel/Thio/Post Cy | 8/8 Matched Unrelated | Bone Marrow | T a cro/Cytoxa n/MMF | 1.89 | 0.53 | No |
| 9 | 62/19 | M/F | B/O | AML | Flu/Bu/ATG | 8/8 Matched Unrelated | Bone Marrow | Tacro/MTX | 0.178 | 0.21 | Yes |
| 10 | 35/35 | M/F | A.AB | AML | Flu/Bu/ATG | 8/8 Matched Unrelated | Peripheral Blood | Tacro/MTX | 3.18 | 7.57 | No |
| 11 | 56/65 | F/F | O/AB | CLL | Flu/Cy/Rituximab | 8/8 Matched Related | Peripheral Blood | Tacro/MTX | 8.22 | 17.27 | No |
| 12 | 42/23 | M/M | O/A | MDS | Flu/Bu/ATG | 8/8 Matched Unrelated | Peripheral Blood | Tacro/MTX | 1.88 | 1.01 | Yes |
| 13 | 27 | 57 | F/M | ALL | Bu/Clo/ATG | 8/8 Matched Unrelated | Bone Marrow | Tacro/MTX | 1.13 | 1.36 | No |
Donor HLA Matching: HLA-A/-B/-C/-DRB1
1 HLA-DRB1 allele level mismatch
1 HLA-A mismatch
1 HLA-C mismatch
G-CSF= Granulocyte Stimulating Factor; RBC=Red Blood Cell; ANC=Absolute Neutrophil Count; AML+ Acute Myeloid Leukemia; AA= Aplastic; MDS=Myelodysplastic Syndrome; CLL=Chronic Lymphocytic Leukemia; ALL=Acute Lymphoblastic Leukemia
MC= Mixed Chimerism
Severe Pancytopenia resolved after the resolution of PRCA in 6 (46%) patients, all of whom are alive at last follow up at a median of 1283 days post-transplant (range 870–4064). One patient received weekly Procrit on D+89 thru 106 and the second patient received Procrit on D+138 and D+145. No other specific measures were instituted and the patients were observed and supported with transfusions. None of the patients underwent therapeutic plasma exchanges or were treated with IVIG. 2 (15%) patients received a second transplant due to persistent pancytopenia/secondary graft failure (115 and 267 days after transplant) and of the two patients, one is alive. 1 (8%) patient died from secondary graft failure despite a stem cell boost on 2611 days after transplant. 1 (8%) patient did not recover his platelet count despite red cell recovery and died of a myocardial infarct on post-transplant day 303. 3 (23%) patients died from disease relapse which occurred on 205/500/1455 days after transplant. Table-2 Of the 13 patients with severe pancytopenia, red cell recovery in 10 (77%) patients was a median of 160 (95–587) days from transplant, ANC recovery >1.5 K/uL in 7 (54%) patients was a median of 296 (145–777) days from transplant and platelet recovery > 50 K/uL in 6(46%) patients was median of 1283 (870–3334) days from transplant.(Graph 1; Table 2; Figures 1–2).It was noted that patients with PRCA with severe pancytopenia had platelet and white cell recovery after resolution of their PRCA.
Table 2.
Patient Outcomes
| # | Require G-CSF at Day 90 | Day 90 - ANC K/uL | Day 90 -Platelet count K/uL | Platelet Transfusion Dependent | RBC Recovery- (Days from transplant) | ANC Recovery 1.5K/uL- (Days from transplant) | Platelet Count >50K/uL without transfusion, (Days from Transplant) | Platelet Recovery >50K/uL- (Days from Transplant) | Alive/Died (Days from Transplant) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | 2.8 | 14 | Yes | 188 | 145 | 190 | Yes | Alive (978) |
| 2 | Yes | 1.5 | 21 | Yes | 349 | 286 | 429 | Yes | Alive (870) |
| 3 | Yes | 1.29 | 25 | Yes | 97 | 296 | 296 | Yes | Alive (3614) |
| 4 | Yes | 0.43 | 10 | Yes | 133 | 374 | 259 | Yes | Alive (1305) |
| 5 | Yes | 0.46 | 6 | Yes | 549 | 743 | 1250 | Yes | Alive (1262) |
| 6 | Yes | 0.97 | 13 | Yes | 95 | 777 | 3334** | Yes | Alive (4064) |
| 7 | Yes | 0.88 | 17 | Yes | 211 | 179 | Did not recover | Did not recover | Died - MI (303) |
| 8 | Yes | 0.32 | 11 | Yes | 587 | Disease relapse (1098) | Did not recover | Did not recover | Died-Recurrence (1455) |
| 9 | Yes | 1.04 | 10 | Yes | 120 | Disease relapse (313) | Disease relapse | Disease relapse | Died - Recurrence (500) |
| 10 | Yes | 0.93 | 10 | Yes | 122 | Disease relapse (143) | Disease relapse | Disease relapse | Died - Recurrence (205) |
| 11 | Yes | 1.7 | 32 | Yes | Secondary Graft Failure (Stem cell Boost) | Secondary Graft Failure (2611) | Secondary Graft Failure | Secondary Graft Failure | Died-Unknown (3112) |
| 12 | Yes | 0.72 | 8 | Yes | Secondary Graft Failure | Secondary Graft Failure (115) Second transplant | Secondary Graft Failure | Secondary Graft Failure | Died- Infection after second transplant |
| 13 | Yes | 1.3 | 14 | Yes | Secondary Graft Failure | Secondary Graft Failure (267)Second transplant | Secondary Graft Failure | Secondary Graft Failure | Alive after second transplant |
Lost to follow-up for 3.5 years
Graph 1.
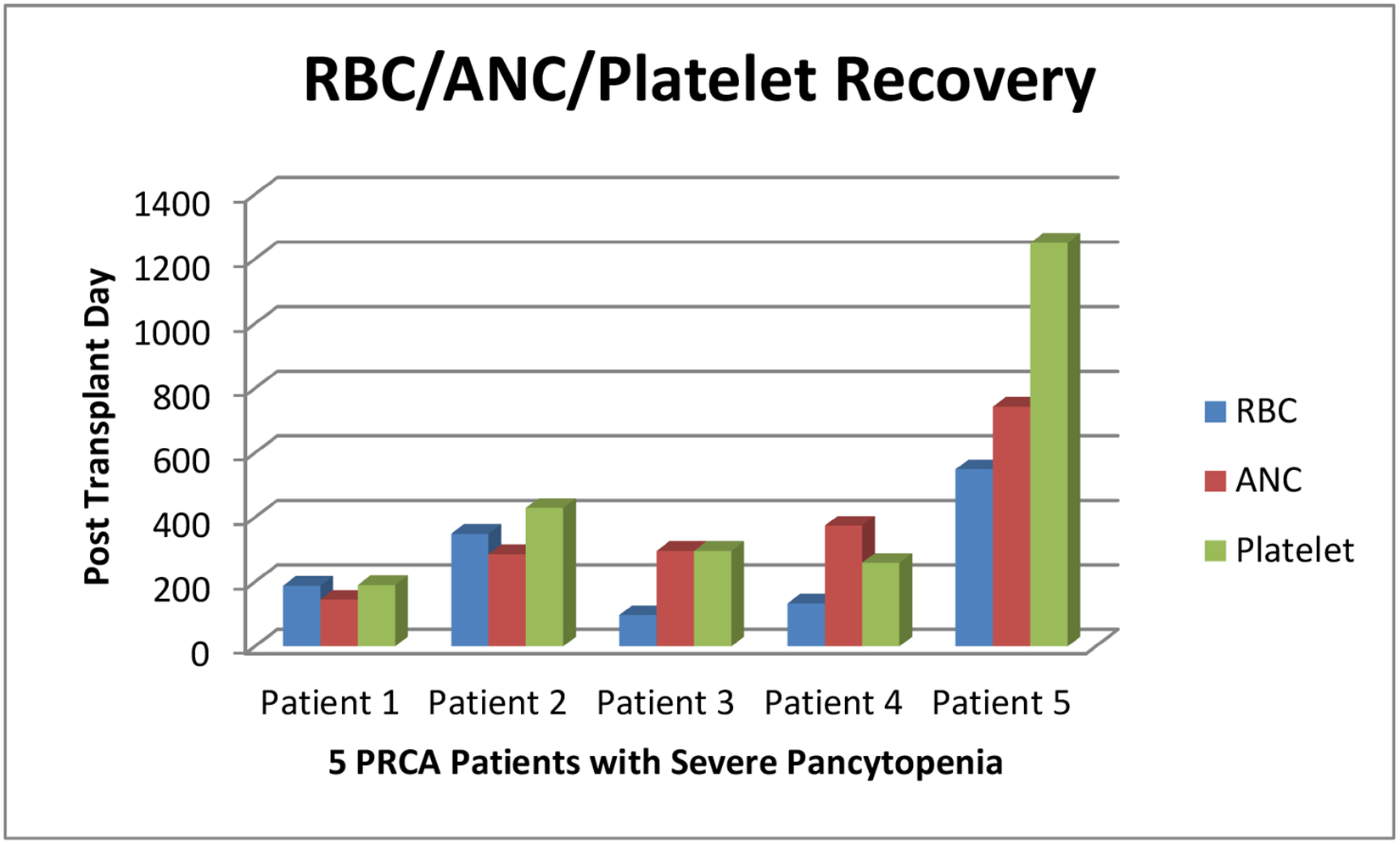
RBC/ANC/Platelet Recovery of 5 PRCA Patients with Severe Pancytopenia
Figure 1: PRCA (Hypocellular) Bone Marrow.
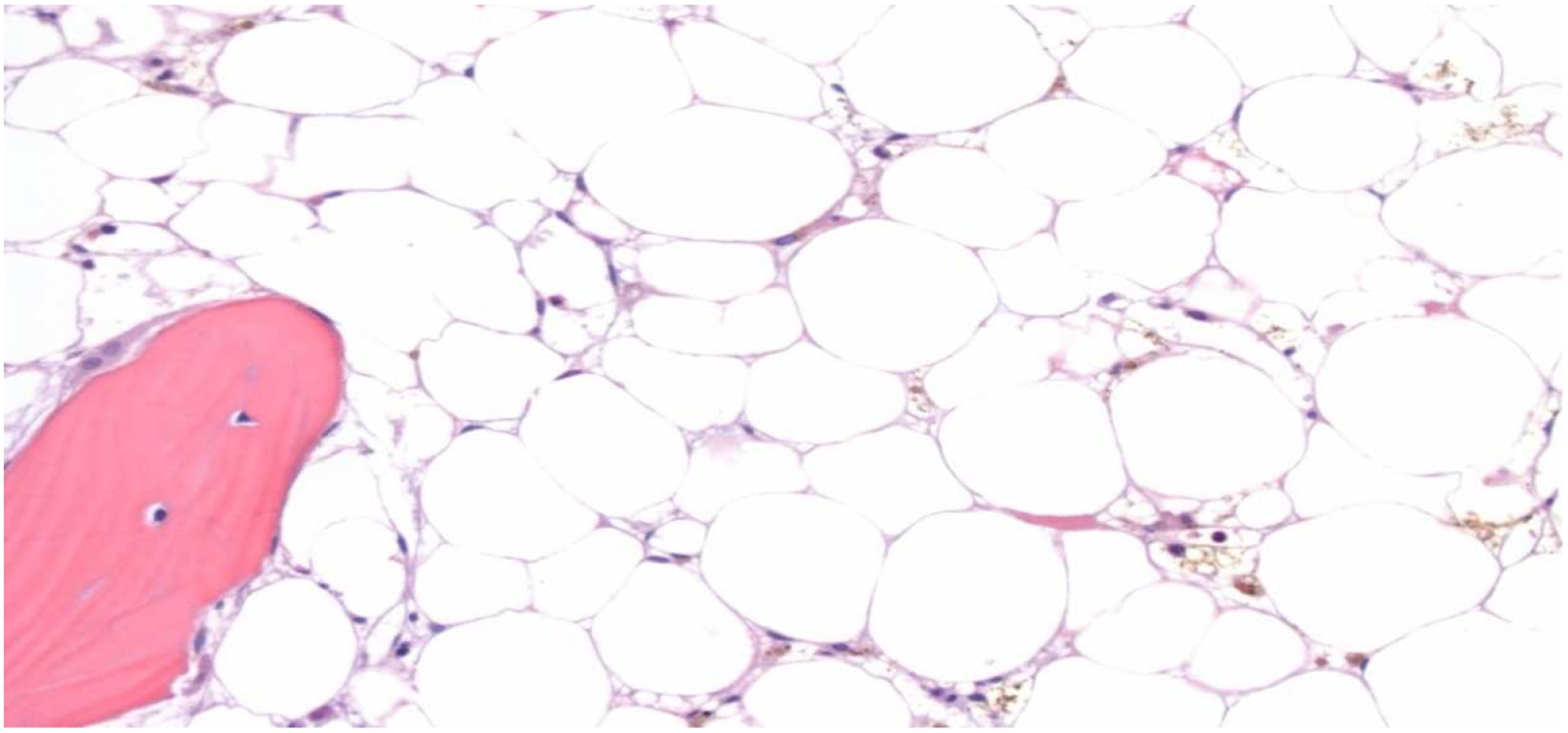
The core biopsy shows a markedly hypocellular (<5%) bone marrow with trilineage hypoplasia (H&E, 200x)
Figure 2: Bone Marrow after PRCA Recovery.
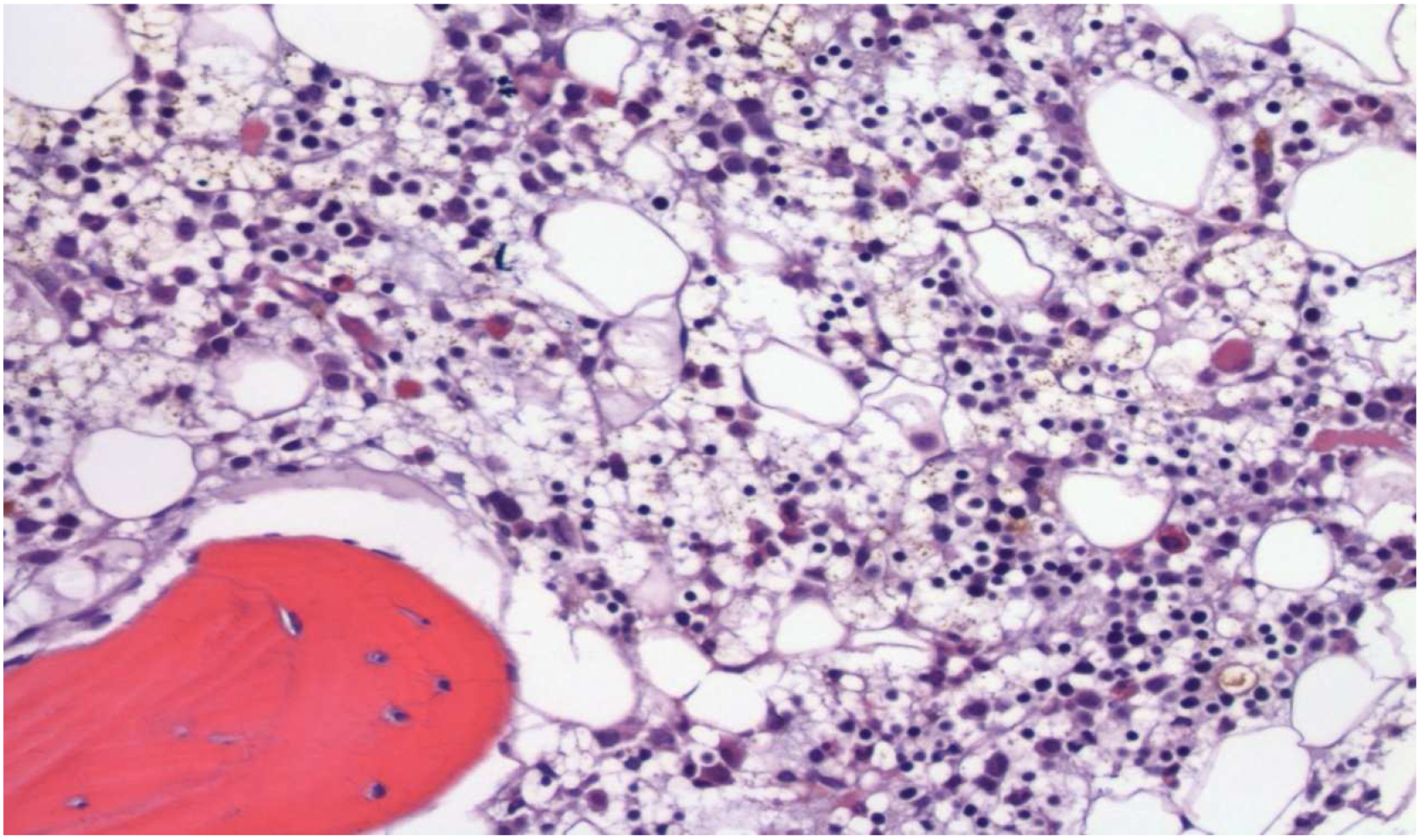
The core biopsy shows a cellular (20–30%) bone marrow with trilineage hematopoiesis (H&E, 200x)
Discussion
The occurrence of PRCA due to the persistence of donor isohemagglutinins has been well described by many authors.1, 2, 3 The immunodominant structure of A and B antigens also called histo-blood group antigens are present as portions of glycoproteins and glycolipids as well as free oligosaccharides. In addition, the expression of these antigens occurs not only in RBCs but also in other types of cells (mostly epithelial and endothelial cells).4 The naturally occurring anti-A and anti-B antibodies are predominantly IgM, although variable amounts of IgG may also be present. Certain individuals possess higher titers of ABO hemagglutinins, stimulated by pregnancy, immunizations or the ingestion of bacteria in the form of probiotics.7 Individuals with blood group O are most likely to produce high titers of anti-A IgG, anti-B IgG and anti-A,B IgG, and in this setting host-versus-graft (HvG) reactions may occur leading to delayed RBC and neutrophil engraftment.5, 6Interestingly all of our patients were of the blood group O.ABO titers were not performed in any of the patients pre- or post-transplant.
Platelets are known to express ABH antigens on their surface and also adsorbed soluble A and B antigens from plasma.A and H antigen expression on platelets vary according to genotype and several studies have demonstrated that individual platelets express variable levels of A and B antigens in the same person. The blood group ABO antigens are expressed by several platelet Glycoproteins (GPIb, GPIIb, GPIIIa and platelet endothelial cell adhesion molecule [PECAM]). These glycoproteins are also constitutively expressed in tissues other than megakaryocytes and platelets and may play a role in major ABO incompatible HSCT. Family studies have determined that the expression of ABH antigens on platelets to be genetically determined. Elevated levels of A and/or B antigens are expressed in 4–7% of individuals and a subset of this group termed “type II high expressers” has more than 20 times the normal levels of A and B antigens. ABO antibodies reacting with high expresser platelets have been implicated in conditions such as multitransfusion platelet refractoriness..7–12 Thus pancytopenia is likely in the presence of high titers of ABO antibodies and/or high expression of A or H antigen on blood cells in Major ABO mismatched SCTs with PRCA and pancytopenia. Although unlikely, donor isohemagglutinins from apheresis or pooled platelet products the patients received could have contributed to the pancytopenia and cannot be completely excluded.
Seebach et al.7, 8 proposed that the delay in neutrophil engraftment to be a direct effect of recipient isoagglutinins possibly by adsorption onto the cell surface on donor granulopoiesis or the presence of neutrophil antibodies. The presence of generation of anti-donor A and anti-B antibodies by persisting B cells were due to incomplete eradication during conditioning.There is however, a lack of consensus in the literature concerning the occurrence of major ABH antigens on leukocytes,13–15which has a proposed mechanism similar to platelets that are known to express and adsorb ABH antigens on their surface.
Rowley et al.16 described that classification of patients by ABO phenotype ignoring the allelic differences of these antigens may obscure the effect of red cell incompatible transplantation on transplant outcomes. However, this finding has yet to be confirmed, and we do not know whether the differences in other clinically significant red cell antigens between donor and recipient may play a role in PRCA in major ABO incompatible SCTs.
Graft failure developing in two of our patients’ raises the questions as to whether anti-A or anti-B isohemagglutinins caused graft failure or was it related to total nucleated cell (TNC) dose received or other factors. One patient received a bone marrow graft, TNC dose 1.36 ×10e8/kg (CD34 dose 1.13×10e6/kg) from a 57 year old male donor with a single allele mismatch in HLA-DP with Busulfan/Clofarabine/ATG conditioning and the second patient was below the age of 30, received peripheral stem cells, TNC dose 1.01×10e8/kg (CD34 dose 4.88×10e6/kg) from a fully HLA matched (HLA-A/B/C/DRB1/DQB1/DPB1) 23 year old male donor with Busulfan/Clofarabine conditioning. Both grafts were previously cryopreserved and major ABO incompatible. HLA mediated graft failure has been described in patients receiving Haploidentical transplantation,17 but published studies18–21 are small and do not have the power to detect the difference in a rare event like graft failure. However, a most recent study of a large retrospective analysis by Olsson et al. 27 reported that major ABO incompatibility was associated with the increased risk of primary graft failure.
We do not believe that mixed chimerism played a major role in pancytopenia as there were only two patients with mixed chimerism. One patient died from disease recurrence and the second patient had graft failure. Our analysis revealed that pancytopenia resolved when red cell recovery occurred. Thus, one therapeutic option is to continue supportive care awaiting spontaneous resolution of PRCA or resolution of PRCA after tapering of tacrolimus or cyclosporine. We believe that the comprehensive supportive care provided to the patients has kept the death rate lower. If treatment is felt to be necessary, it should be directed against antibody producing cells to reduce donor specific ABO isoagglutinins. Although our numbers are small we believe that this is a significant finding in PRCA patients who have persistent pancytopenia on post-transplant day 90. In summary, severe pancytopenia is seen in 16% of patients with pure red cell aplasia due to major ABO incompatible hematopoietic cell transplantation, and it resolves in approximately half of the patients after resolution of PRCA.
Key Points.
The majority of PRCA patients with persistent severe pancytopenia after major
ABO mismatched SCT resolve after resolution of PRCA.
HIghlights for Review.
The majority of PRCA patients with persistent severe pancytopenia after major
ABO mismatched SCT resolve after resolution of PRCA.
Acknowledgments
The authors wish to thank scientific editor Elizabeth Hess for her assistance in editing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure:
The authors declare no relevant financial conflict of interests.
REFERENCES:
- 1.Aung FM, Lichtiger B, Bassett R, et al. Incidence and natural history of pure red cell aplasia in major ABO-mismatched haematopoietic cell transplantation. Br J Haematol. 2013; 160:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mielcarek M, Leisenring W, Torok-Storb B, et al. Graft-versus-host disease and donor-directed hemagglutinin titers after ABO-mismatched related and unrelated marrow allografts: evidence for a graft-versus-plasma cell effect. Blood 2000; 96:1150–1156. [PubMed] [Google Scholar]
- 3.Griffith LM, McCoy JP Jr, Bolan CD, et al. Persistence of recipient plasma cells and anti-donor isohaemagglutinins in patients with delayed donor erythropoiesis after Major ABO incompatible non-myeloablative haematopoietic cell transplantation. Br J Haematol. 2005; 128:668–675. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto F, Clausen H, White T, et al. Molecular basis of the histo-blood group ABO system, Nature 1990; 345: 229–33. [DOI] [PubMed] [Google Scholar]
- 5.Stussi Georg, Halter Jörg, Schanz Urs, et al. ABO-histo blood group incompatibility in hematopoietic stem cell and solid organ transplantation. Transfusion and Apheresis Science 2006; 35: 59–69. [DOI] [PubMed] [Google Scholar]
- 6.Seebach JD, Stussi G, Passweg JR, et al. ABO blood group barrier in allogeneic bone marrow transplantation revisited Biol Blood Marrow Transplant, 2005; 11: 1006–1013. [DOI] [PubMed] [Google Scholar]
- 7.Quillen K Hemolysis from platelet transfusion: call to action for an underreported reaction. Transfusion 2012; 52: 2072–2074. [DOI] [PubMed] [Google Scholar]
- 8.Ogasawara K, Ueki J, Takenaka M, et al. Study on the Expression of ABH Antigens on Platelets. Blood 1993; 82: 993–999. [PubMed] [Google Scholar]
- 9.Curtis BR, Edwards JT, Hessner MJ, et al. Blood group A and B antigens are strongly expressed on platelets of some individuals. Blood 2000; 96:1574–1581. [PubMed] [Google Scholar]
- 10.Dunstan RA. Use of fluorescence flow Cytometry to study the binding of various ligands to platelets, J Histochem Cytochem 1985; 33:1176–1179. [DOI] [PubMed] [Google Scholar]
- 11.Kao K, Scornik JC, McQueen CF. Evaluation of individual specificities of class I HLA on platelets by a newly developed monoclonal antibody. Hum Immunology 1990; 27:285–297. [DOI] [PubMed] [Google Scholar]
- 12.Dunstan RA. The expression of ABH antigens during in vitro megakaryocyte maturation: origin of heterogeneity of antigen density. Br J of Hematol. 1985; 62:587–593. [DOI] [PubMed] [Google Scholar]
- 13.Dunstan RA, Simpson MB, Borowitz M. Absence of ABH antigens on neutrophils. Br J Hematol 1985; 60: 651–657. [DOI] [PubMed] [Google Scholar]
- 14.Kelton JG, Bebenek G. Granulocytes do not have surface ABO antigens. Transfusion 1985; 25: 567–569. [DOI] [PubMed] [Google Scholar]
- 15.Dunstan RA. Status of major red cell blood group antigens on neutrophils, lymphocytes and monocytes. Br J Hematol. 1986; 62: 301–309. [DOI] [PubMed] [Google Scholar]
- 16.Rowley SD, Donato MI and Bhattacharyya P. Red Blood cell-incompatible allogeneic hematopoietic progenitor cell transplantation. Bone Marrow Transplantation 2011; 46: 1167–1185. [DOI] [PubMed] [Google Scholar]
- 17.Bayraktar UD, Champlin RE, Ciurea SO, et al. Progress in Haploidentical Stem Cell Transplantation. Am Soc Bld and Marrow Transplantation. 2012; 18: 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canals C, Muniz-Diaz E, Martinez C.et al. Impact of ABO incompatibility on allogeneic peripheral blood progenitor cell transplantation after reduced intensity conditioning. Transf 2004; 44:1603–1611. [DOI] [PubMed] [Google Scholar]
- 19.Ozkurt ZN, Yegin ZA, Yenicesu I, et al. Impact of ABO-incompatible donor on early and late outcome of hematopoirtic stem cell transplantation. Transplant Proc. 2009; 41: 3851–3858. [DOI] [PubMed] [Google Scholar]
- 20.Blin N, Traineau R, Houssin S, et al. Impact of donor-recipient ABO mismatch on allogeneic transplantation outcome according to stem cell source. Biol Blood Marrow Transplant. 2010; 16: 1315–1323. [DOI] [PubMed] [Google Scholar]
- 21.Helbig G, Stella-Holowiecka B, Wojnar J, et al. Pure red-cell aplasia following major and bi-directional ABO-incompatible allogeneic stem cell transplantation: recovery of donor derived erythropoiesis after long-term treatment using different therapeutic strategies. Ann Hematol 2007; 86: 677–683. [DOI] [PubMed] [Google Scholar]
- 22.Olsson RF, Logan BR, Chaudhury S, et al. Primary Graft Failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia 2015; 29: 1754–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]


