Abstract
Commensal organisms that constitute the skin microbiota play a pivotal role in the orchestration of cutaneous homeostasis and immune competence. This balance can be promptly offset by the expansion of the opportunistic pathogen Staphylococcus aureus, which is responsible for the majority of bacterial skin infections. S. aureus carriage is also known to be a precondition for its transmission and pathogenesis. Recent reports suggest that skindwelling coagulase negative Staphylococci (CoNS) can prime the skin immune system to limit the colonization potential of invaders, and they can directly compete through production of antimicrobial molecules or through signaling antagonism. We review recent advances in these CoNS colonization resistance mechanisms, which may serve to aid development of pharmacologic and probiotic intervention strategies to limit S. aureus skin colonization and disease.
Keywords: quorum sensing, skin microbiota, host-protection, colonization, Staphylococcus aureus, MRSA
Bacterial colonization of the skin
The skin is the body’s largest and most exposed organ system. Recent estimates of the epithelial surface area, accounting for hair follicles and sweat glands, suggest an expanse as large as 30 m2 [1]. This vast region is densely populated with microorganisms that interact and compete with each other in order to colonize and survive. Recent efforts to analyze the human skin microbiome have shed light on the most abundant bacteria, eukaryotes and viruses present in this rich environment. Of the bacteria, the skin is dominated by members of the Staphylococcus, Corynebacterium, Streptococcus, and Propionibacterium genera [2–4]. Within the Staphylococcus genus, the most common skin commensals are members of the coagulase-negative Staphylococci (CoNS), with the species S. epidermidis, S. hominis, S. haemolyticus, S. capitis, S. lugdunensis, and S. warneri being the most frequently isolated [4, 5]. The CoNS are a large and heterogeneous family of staphylococci. As of 2014, 38 species of CoNS have been identified and this number is predicted to grow as more human and animal isolates are collected [6]. The skin of healthy individuals is colonized by a mixture of these abundant CoNS, present at different ratios depending on whether the site is dry, moist, or sebaceous.
Staphylococcus represents a broad genus of Gram-positive bacteria that colonize the skin and mucous membranes of humans and most mammals. S. aureus is the most problematic pathogen of the genus and is known to cause numerous acute and chronic infections [7, 8]. Increasingly, outbreaks of methicillin-resistant S. aureus (MRSA), which had traditionally been confined to hospital settings and limited to immune-compromised patients, have emerged in the community and caused pandemic disease in immune-competent populations [9–11]. An obvious consequence of MRSA’s capacity to perpetrate community outbreaks among healthy individuals is the increased population of human reservoirs, which thereby affords greater opportunity for transmission and infection. Furthermore, S. aureus asymptomatically colonizes approximately 20–30% of the healthy adult population, most often in the anterior nares (nostrils). This translates to 95 million colonized people in the US alone [12]. Surprisingly, S. aureus skin colonization rates are relatively low [13], and abundance levels compared to other bacterial skin colonizers are barely detectable [5]. Despite this, S. aureus is responsible for 76% of all skin and soft tissue infections [14], leading to 500,000 hospital visits and 10 million outpatient visits per year [15].
How can S. aureus cause so much skin disease yet be such a poor skin colonizer compared to other CoNS? The prevailing view is that the natural microbiota like CoNS protect the skin in part by educating the immune system to limit pathogen colonization [16, 17]. It is also increasingly appreciated that the CoNS can directly compete with invading skin pathogens by secreting novel natural products. This overarching concept was collectively termed “colonization resistance” in recent articles [16, 18], a term adopted from the gut microbiota field to describe the inhibition of pathogen colonization [19]. In this review, we will outline the skin as a barrier and we will define the mechanisms used by commensal bacteria to enhance skin immunity to S. aureus opportunistic infection. Lastly, we will summarize the various strategies through which CoNS directly compete with S. aureus to prevent colonization and disease.
The skin as a protective barrier
As the body’s most extensive interface with the outside environment, overall health and homeostasis depends upon the skin’s ability to maintain structural and functional integrity as a protective barrier. To be effective, cutaneous barriers must exercise dynamic control over many complex physiological processes (e.g., thermoregulation and tissue regeneration) and insulate vital internal structures from environmental challenges, including the threat of infection. In recent years, it has become clear that restricting the capacity of pathogenic microbes from both productively dwelling upon and penetrating through epithelial surfaces is the result of extensive cooperation between the skin’s immune system and the communities of commensal microbes that colonize cutaneous surfaces.
The skin’s characteristic acidity, lipid density and lack of nutrients present a seemingly inhospitable terrain, yet the surface of this tissue represents a vibrant ecosystem teaming with abundant and diverse microorganisms, collectively referred to as the skin microbiota. Beginning at birth, the proliferation of microbiota over the entirety of skin’s surface, including inter-follicular appendages (e.g., glands and hair follicles), form a meta-organismal, microbial barrier at the skin’s most superficial aspect. 16s qPCR data obtained from human skin punch biopsies suggests that a square centimeter of skin harbors a million commensal bacteria; multiplying this value by the 30 m2 estimate of the combined surface area of the skin’s interfollicular and follicular territories, this tissue stages a continual dialogue between the mammalian host and ≈ 300 billion microbes [20, 21].
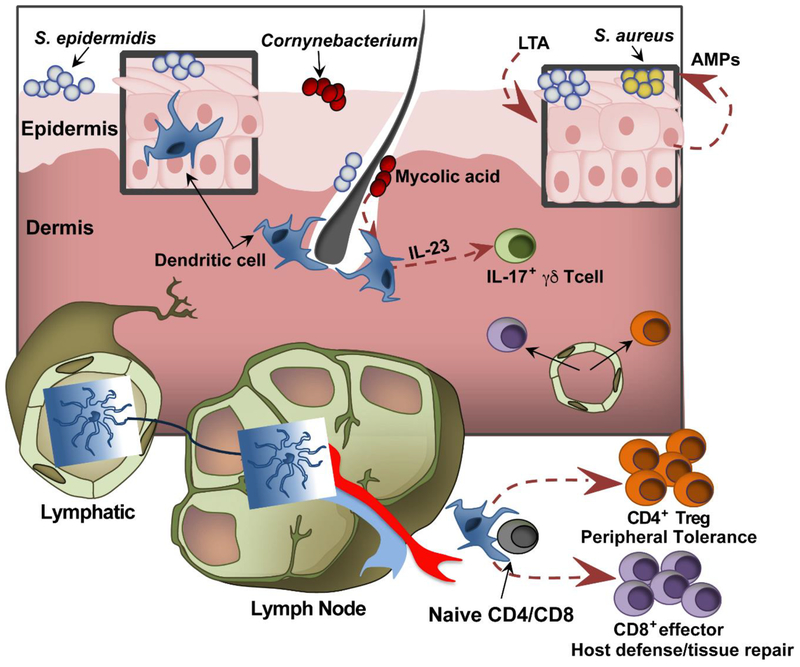
The two principal components of the skin are the epidermis and underlying dermis (Fig. 1). In relation to the skin microbiota, keratinocytes (KCs), which constitute 90–95% of epidermal cellularity, are nearest and most numerous. Arranged in stratified layers according to their differentiation state, KCs are potent purveyors of antimicrobial peptides (AMPs) and cytokine/chemokine signals. As such, these cells are endowed with both direct and indirect means of shaping host/microbe interactions occurring at the skin’s surface. Perhaps the most significant way KCs calibrate these encounters is by orchestrating the formation of the stratum corneum (SC), which physically separates the viable layers of the cutaneous epithelium from surface microbes. This waxy and waterproof composite material of flattened corneocytes and KC-derived lipid/granule content, interlocks at corneocyte interstices to form a tight mechanical barrier that restricts host-microbial engagement. While the underlying mechanisms are continuing to be unraveled, host and microbial elements can traverse the SC barrier to establish a reciprocal cross-talk under steady-state conditions. As evidence of inside-out movement by the host, Langerhans cells, which are uniformly dispersed throughout the epidermis in a lattice-like arrangement and this region’s most abundant antigen presenting cell population, regularly extend their dendrites into the SC for microbial antigen (Ag) sampling [22, 23]. Meanwhile, outside-in movement of microbial components across the SC was revealed by the presence of microbe-derived factors and entire organisms within bona fide dermal territories, which happen to be heavily populated with resident immune cells [24]. That the ongoing crosstalk between cutaneous commensals and resident skin immune cells proceeds in a non-inflammatory manner is quite striking. In this way, the homeostatic nature of the interactions between the resident skin immune cells and commensals resembles the analogous dialogue between the immune cells and microbiota of the gut. In marked contrast, experiments with germ-free mice have shown that whereas the development of gut-associated lymphoid tissue is profoundly stunted without microbial exposure, the absence of skin microbiota does not substantially impact the cellular composition of the cutaneous immune system [25]. Rather, the commensal-immune interactions occurring in the skin principally serve to functionally educate and remodel its cellular compartments. However, more comprehensive studies are needed to fully determine commensal-immune interactions in a greater array of immune cell subsets.
Figure 1. Skin interactions with commensal bacteria.
Enriched in the skin’s epidermal and dermal compartments, resident dendritic cells support cutaneous immune competence by shaping the functional repertoire of the skin’s T cell network. In response to encounters with commensal antigens, these skin dendritic cells migrate to the draining lymph nodes and orchestrate the priming of CD4 and CD8 T cells. The T cells fortify the cutaneous immune system through their respective roles in maintaining peripheral tolerance and mediating host immunity and tissue repair. In addition, commensal derived factors, such as mycolic acid from Corynebacterium sp., have been shown to augment specific modes of inflammation via expanding the cellular mediators of the IL-23/IL-17 inflammatory axis. Apart from altering the function of cutaneous T cells, commensal signals such as lipoteichoic acid (LTA) trigger antimicrobial peptide (AMP) production from the skin’s predominant cellular constituent, keratinocytes. Overall, the sensitivity of both innate and adaptive elements of the cutaneous immune system to specific commensal cues/signals appears to finely tune both effector and regulatory immune responses in a manner that maintains barrier integrity, encourages commensal occupation and opposes pathogen invasion.
Commensal – skin interactions educate host immunity
Forged by a coevolutionary process, the nature of the immune-commensal relationship appears to be an overwhelmingly symbiotic one that dictates mutually beneficial outcomes. Considering that the skin and its resident immune cell network continuously engage compositionally diverse and temporally dynamic microbial communities, the challenge associated with experimentally dissecting the immune-modulatory effects of specific microbes is difficult to overstate. Nonetheless, murine models of experimental association of CoNS, as well as other genera of non-pathogenic skin commensals such as Corynebacterium, have greatly advanced our understanding of the mechanisms through which the microbiota fortify the immunological functions of the skin. Furthermore, these studies have highlighted the capacity of cutaneous T cells, which populate the skin at an estimated density of one million/cm2, to acquire commensal-specific functional signatures that enable cutaneous barriers to uphold three tasks essential to both the establishment and restoration of tissue homeostasis: 1) sustained immunological tolerance to symbiotic commensals, 2) robust immunogenicity to invading pathogens, and 3) efficient tissue restoration/repair following damaging environmental/infectious encounters. Given that the dermatological manifestations of the immune-commensal interactions that are continuously occurring are grossly unremarkable, it seems reasonable to deduce that the majority of these interactions actively support the first task.
Demonstrating the capacity of S. epidermidis colonization to shape the skin’s CD4+ T cell network in a manner that actively promotes homeostatic tolerance to skin commensals, Scharschmidt et al. showed that neonatal exposure to S. epidermidis triggers an influx of CD4+ T regulatory cells that promote homeostasis by mediating tolerogenic responses to commensal Ags [26]. Meanwhile, Naik et al. showed that the mono-association of S. epidermidis functioned as an immunological adjuvant (Fig. 1) by increasing the capacity of cutaneous CD8+ T cells to acquire IL-17 and IFNγ effector functions that translated into heterologous protection against the skin pathogens Candida albicans and Leshmania major [25]. Demonstrating the capacity of a non-CoNS to shape the cutaneous T cell compartment, repeated topical exposure of Corynebacterium accolens, or its membranous derivative mycolic acid, results in the expansion of IL-17 producing dermal γδ T cells [27]. Given the importance of IL-17 to host defense against extracellular pathogens, Corynebacterium exposure may confer immune prophylactic effects against common microbial pathogens such as C. albicans and S. aureus. However, a critical balance of IL-17 must be maintained for protective effects, as strong IL-17 polarization is characteristic of pediatric atopic dermatitis [28] and blocking antibodies to IL-17 is associated with better outcomes for psoriasis patients [29]. Furthermore, Nakagawa et al. recently demonstrated that IL-17 deficient mice have less severe skin disease pathology scores in the context of S. aureus epicutaneous infection than wild type mice even though bacterial burdens remain the same [30] Thus, a definitive pathological or protective role for IL-17 remains elusive.
In addition, exciting new findings show that not only do commensal-specific T cell responses endow the cutaneous immune system with the capacity to protect skin from pathogenic invaders, they also impart a repertoire of tissue repair functions. An implicit intermediary in the dialogue between commensals and skin’s adaptive T cell network are cutaneous dendritic cells (DCs). These DCs serve as the cellular liaison between the skin, where commensal antigens are concentrated, and draining lymph nodes (LNs), where T cell priming is initiated (Fig. 1). Highlighting the ancient origins of this dialogue, studies of S. epidermidis colonization show that the expansion of commensal-specific CD8 T cells is restricted to non-classical MHC-1b (H2-M3) molecules whose emergence predates the evolution of the classical MHC-1a Ag presentation platform. Furthermore, the functional signature imparted by these interactions is impressively pleotropic as it encompasses both heightened immune stimulatory and growth factor expression profiles, which converge to spur both immunity and cutaneous wound repair [31]. Further underscoring the importance of the microbe/T cell axis in the maintenance of cutaneous barrier integrity, recombination activating gene 1 deficient mice, which lack T lymphocytes, show that cutaneous T cell deficiency corresponds with skin bacteria excessively translocating across and dwelling within the skin and draining LNs respectively [32]. It is important to note that commensals have been shown to promote the immunogenicity and tissue repair functions of the skin independently of T cells. For instance, a small molecule of <10 kDa secreted from S. epidermidis increased expression of human β-defensins (hBDs) in murine skin or human keratinocytes [33]. Additionally, S. epidermidis-cell wall components such as lipotechoic acid engage toll-like receptor 2 (TLR2) in a manner that restricts excessive inflammatory responses and promotes AMP production from KCs within the contexts of cutaneous wounding and S. aureus challenge respectively [33] [34]. While cooperative immune-microbe interactions make key contributions to cutaneous homeostasis and host defence, so too do competitive microbe-microbe interactions that abate host exposure to skin pathogens.
Commensal interactions with pathogens
When commensals share an ecological niche with pathogenic microbes that can trigger pathophysiological responses, it could threaten the commensal habitat, and the ability to directly compete with the invader could be a beneficial trait. The dominant colonization site for S. aureus is the anterior nares [35], and many of the initial direct microbe interaction studies with S. aureus have focused on this body site (reviewed in [36]). As some examples, there are reports that artificial implantation of Corynebacterium sp. S. epidermidis pre-administration in a murine nasal model of colonization prevented subsequent S. aureus colonization [39], and S. epidermidis strains releasing a serine protease (Esp) cleared S. aureus nasal colonization in human volunteers [40]. In follow-up studies, it was revealed that the Esp protease degrades both host and bacterial surface proteins associated with colonization [41, 42]. As researchers take a broader sampling of commensal CoNS strain from nasal sites, it is clear that many of them produce antibiotic-like compounds that could aid commensal colonization in a competitive environment [43]. As one prominent example, Staphylococcus lugdunensis was shown to produce a thiazoline-containing cyclic peptide antibiotic, called lugdunin, that inhibits S. aureus in the well-established cotton rat nasal colonization model [44]. Human nasal carriage of S. lugdunensis was negatively correlated with S. aureus colonization, though direct competition was not established in this survey [44]. It seems probable that many more of these antibiotic-like compounds derived from CoNS as well as other nasal commensals remain to be discovered.
Similar to the competitive niche of the anterior nares, skin commensals have also devised strategies to deploy their own arsenal of antimicrobials to aid in colonization (Fig. 2) [43–45]. For instance, S. epidermidis expresses phenol-soluble modulins (PSMs) able to kill both Streptococcus pyogenes (GAS) and S. aureus [46, 47]. Interestingly, S. epidermidis also can produce 6-N-hydroxyaminopurine (6-HAP), which inhibits GAS growth and also impacts host DNA polymerase and thus inhibits skin neoplasia [48]. Nakatsuji et. al recently reported that a Staphylococcus hominis strain produces an AMP that kills S. aureus [45]. Transplantation of this strain has therapeutic efficacy for human patients with atopic dermatitis, a dermato-pathology characterized by excessive S. aureus colonization. Similar to the nasal community, there are increasing examples of skin commensals producing antibiotic-like compounds and many that remain to be identified [45].
Figure 2. The commensal skin microbiota employ diverse strategies to compete with S. aureus.
Healthy skin is populated by a diverse array of coagulase negative staphylococci (CoNS) as well as other commensal flora. CoNS compete with S. aureus on the skin by producing a wide array of small molecule products like antimicrobial peptides (AMPs) and lantibiotics. Another potential mediator of staphylococcal competition is the agr quorumsensing system. While relatively less studied than other small molecules, there is evidence to suggest that interspecies competition exists as shown by the ability of S. epidermidis to make an AIP that inhibits S. aureus quorum sensing. We recently published that another rare skin commensal, S. caprae, also makes an AIP that inhibits all classes of S. aureus quorum sensing with nanomolar potency (further described in Figure 4). Thus, non-cognate AIPs represent a potential source of competition that merits further investigation in more representative models of skin colonization.
Staphylococcal cell-to-cell communication
Extensive interactions occur between commensal bacteria as they compete for resources [49], and new examples of competitive interactions are constantly being uncovered. Not all of these interactions are based on antimicrobials, as signaling cross-talk has been recognized as an effective strategy commensals use to reduce fitness and slow growth of a competitor [49–52]. All staphylococci utilize a system of cell-to-cell communication, also called quorum-sensing (QS) or the accessory gene regulator (agr) system, to coordinate cellular behavior as a function of accumulated cell density [53, 54]. The signal sensed by this regulatory mechanism is an autoinducing peptide (AIP), which is a secreted molecule ranging from 7–12 residues in length, with the last five residues constrained in a cyclic (thio)lactone ring between the terminal carboxylic acid and a cysteine or serine side chain (Fig. 3). Though the fundamental scaffold is conserved amongst all known AIPs, the primary amino acid sequence of these molecules can vary widely across staphylococcal species. It remains unclear why the staphylococci evolved a cyclic quorum-sensing peptide rather than the linear peptide sensed by most other Gram-positives, but it has been demonstrated that the (thio)lactone ring is essential for agr binding and activation (reviewed in [54]). The AIP signal is made and sensed using machinery encoded in the agrBDCA operon present on the chromosome of all staphylococcal strains. The system functions as follows: AgrD is the peptide precursor and AgrB is an integral membrane protease involved in generating AIP. As AIP reaches a critical level, it binds and activates the AgrC histidine kinase, which in turn phosphorylates the response regulator AgrA. Activated AgrA binds the P2 promoter to upregulate agrBDCA transcription as well as to the P3 promoter to produce RNAIII transcript. The RNAIII transcript yields a regulatory RNA molecule that acts as the primary effector of the agr system by upregulating extracellular virulence factors (e.g. toxins in S. aureus or exo-enzymes in all staphylococci).
Figure 3. Schematic of the Staphylococcal agr quorum-sensing system and signaling cross-talk.
The agr locus is conserved in all species of Staphylococci, and is composed of the agrBDCA 4-gene operon divergently transcribed from the RNAIII effector. The AgrB and AgrD proteins build the autoinducing peptide (AIP) signal, and the AgrC histidine kinase is the AIP receptor. When AIP binds to AgrC, phosphoryl transfer to AgrA occurs, inducing the P2 and P3 promoters, resulting in agrBCDA and RNAIII expression. RNAIII transcript is the primary effector of the system and upregulates toxins (in S. aureus) and exo-enzymes (in all staphylococci). The ‘cognate AIP’ shown is AIP-I from S. aureus agr type I strains (e.g. USA300), and the ‘competing AIP’ represents any signal with receptor antagonist activity. These competing AIPs can come from commensal staphylococci. This figure is adapted from [51].
Across the staphylococci, there is divergence within the agr locus that results in subtypes within a species, adding complexity to quorum-sensing interactions. For instance, in S. aureus isolates there are four variants of the agr locus that result in the production of four distinct AIP signal structures (AIP-I, II, III, & IV), each of which require a unique AgrC receptor for productive signaling. Intraspecies cross-talk (Fig. 3) occurs between S. aureus strains (e.g. AIP-I inhibiting an agr type III strain) in a mechanism termed “agr interference” [55], and taking advantage of this mechanism can limit S. aureus skin infection [56, 57]. Similarly, among S. epidermidis isolates there are three types of agr systems and cross-talk has been observed between these types [58]. Among the other CoNS strains, there is not yet enough mechanistic information on whether there is variance in the agr locus within each species that would lead to intraspecies cross-talk. Furthermore, while the agr locus is conserved across all the staphylococci, it is not yet established if all CoNS maintain functional agr systems.
The essential role of agr-quorum sensing to S. aureus skin pathogenesis was elegantly demonstrated in pioneering work by Dr. Richard Novick’s group. Several notable observations in particular established an unequivocal link between quorum sensing and S. aureus skin infection [56]. First, the strength of agr-P3 during the first few hours of infection is roughly proportional to dermo-necrotic injury and therefore serves as a reliable approximation of S. aureus virulence factor induction [56, 57], and this has now been confirmed in multiple examples [51, 59, 60]. Second, whereas sterile skin challenge with culture supernatants conditioned by S. aureus recapitulated the characteristic dermonecrotic injury, analogous challenges prepared from the agr null counterpart failed to induced significant ulceration [56, 57]. Finally, disrupting the respective receptor/ligand interactions between AgrC and its cognate AIP by experimentally administering an excess of inhibitory AIP blunts agr-P3 activation and manifestations of disease [56, 57]. Along these lines, extensive structure/function studies on AIP-based inhibition have led to the development of numerous AIP analogues with S. aureus AgrC antagonistic properties (reviewed in [61]). Together, these findings established the central role of agr signaling in S. aureus skin infection development, and laid the foundation for a new therapeutic paradigm for inhibiting pathogenesis via agr-interference [61–63].
Between staphylococci, interspecies agr cross-talk has only been examined at a limited level (Figs. 2 & 3). The first well-documented example was the S. epidermidis agr type I (making AIP-I) inhibiting S. aureus agr function [58, 64, 65]. Recently, the rare skin commensal Staphylococcus caprae [5] was shown to produce an AIP signal that inhibits all S. aureus agr types with nanomolar potency, providing a second example of interspecies cross-talk [51]. In direct challenge experiments with MRSA during intradermal skin infection, S. caprae provided protection, while a related S. capitis strain (without S. aureus agr inhibiting activity) showed only minimal benefit (Fig. 4). S. caprae could also directly compete with MRSA during skin colonization [51], indicating that MRSA requires a functional agr system to effectively colonize in a competitive environment. A number of animal CoNS species have been recently identified with some inhibitory action against S. aureus agr [66], most notably Staphylococcus schleiferi. Increasingly it is also being observed that agr cross-talk is not limited to the staphylococci, as C. accolens has been shown to release an unknown agent that suppresses quorum-sensing signaling in S. aureus [67].
Figure 4. Commensal competition with MRSA during skin infection.
This figure is adapted from our recent report demonstrating S. caprae can compete with MRSA in a skin model of infection [51]. S. caprae produces a competing AIP signal (Fig. 2) with nanomolar potency for the MRSA AgrC receptor, completely inhibiting agr function. In contrast, a similar skin commensal, S. capitis, has no ability to produce a competing AIP signal and could not prevent MRSA infection. Briefly: A. Representative images of in vivo bioluminescence induction 3.5 hours after challenge with MRSA agr P3-Lux +/− 10 μg of S. caprae AIP, or equal CFU of the CoNS S. caprae or S. capitis. B. Representative images show dermonecrosis 5 days after the bacterial challenge. C. Time course comparison of in vivo bioluminescence after intradermal challenge with the indicated conditions. Error bars represent SEM. Post test p value (*)=<0.05, (**)=<0.01, (***)=<0.005. D. Time course of dermonecrotic lesion size in the indicated challenge conditions. Error bars represent SEM. Post test p value (*)=<0.05, (**)=<0.01.
Concluding remarks and future perspectives
Mammalian skin has evolved within the context of perpetual engagement with ambient microbes. In exchange for a stable ecological niche, commensals augment their host’s capacity to maintain the robust cutaneous barriers needed for overall health and homeostasis. This partnership is largely a bi-product of the abundance of bacterial mutualists within the microbiome, such as the members of the CoNS. Underlying the host/microbe mutualism that typifies the skin’s baseline state, there is a collaborative effort to fortify joint defenses against aggressive pathogens. For their part, commensals convey immunomodulatory signals that bolster both innate and adaptive anti-microbial effector responses. In addition, commensals employ tactics that directly oppose pathogenic outgrowth of invasive microbes, in part due to natural products that are made and released. We are only just beginning to appreciate the chemical diversity on the skin that is the result of commensal growth [16]. As interest in this area grows, efforts will be needed to identify the bioactive small molecules being produced, and different growth media or ex-vivo models should be tested since laboratory conditions and murine skin models do not match the native skin environment (see Outstanding Questions) [43]. One promising ex-vivo model is porcine skin, which has been shown to be both immunologically and structurally similar to human skin [68].
Owing to its prevalence and capacity to both dominate and destroy the cutaneous ecosystem, there is perhaps no microbe that poses a greater threat to both the host and skin commensals than S. aureus, which is responsible for the majority of bacterial skin infections and figures prominently among the multi-drug resistant pathogens. The lapsing effectiveness of conventional antimicrobial therapies and the rise of community associated MRSA (CA-MRSA) strains underscores the need for new anti-S. aureus interventions and highlights an opportunity to formulate novel modalities that specifically target the invading pathogen while sparing beneficial commensal bystanders. It is important to also consider that S. aureus carriage is often a precondition for its transmission and pathogenesis. Recent advances in our understanding of the mechanisms by which S. aureus mediates the abrupt transition from a state of benign carriage to invasive infection continue to highlight an indispensable role for quorum-sensing in pathogenesis (reviewed in [54]). In particular, the agr system is critically important for S. aureus skin infection, and these phenotypes are amplified in CA-MRSA strains that have higher agr activity and produce more toxins and exo-enzymes. These properties have made the agr system an attractive target of chemical inhibitor development [61–63], and our group and others have identified a number of small molecule inhibitors that suppress signaling and in turn MRSA infection [69–72]. Considering that every staphylococcal strain has a agr system [73] (with varying AIP structures and receptors), it seems possible that the agr system has been conserved throughout the species as a colonization determinant, as evidenced by S. epidermidis on skin explants [58]. Since the staphylococcal quorum-sensing system controls production of many secreted enzymes that are likely required for growth on host substrates [74], agr conservation could be for nutrient acquisition. Additionally, our recent findings with S. caprae suggest quorum-sensing is also a competition system to gain a fitness advantage in an environment densely populated with other microbes [51].
Moving forward, it is clear that we are only at the early stages of understanding how the skin microbiota interact with each other and the host. Considering how little we know about many skin commensals, it will be increasingly important to understand the mechanisms by which commensals prime skin immune defenses and to identify and characterize the inhibitory natural products they produce. With the dominance of S. aureus as a skin pathogen and the elevated S. aureus colonization levels during skin dysbiosis [75], understanding how to limit S. aureus colonization is an area in need of further investigation to prevent the transition from benign carriage to skin and soft tissue infections and to mitigate the burden on patients and healthcare systems [76].
Highlights:
Emerging evidence shows that cutaneous host defense relies upon extensive host/commensal cooperation. Resident commensals serve as the “true” first line of defense at the skin’s surface.
Skin commensals, including coagulase negative staphylococci (CoNS), fortify cutaneous immune competence by broadening the functional repertoire of the skin’s innate and adaptive immune cell networks in a manner that bolsters effector responses against pathogenic microbes, while sparring members of the normal flora.
CoNS can employ a diverse array of tactics that directly counter both the carriage and invasion of the common dermato-pathogen S. aureus.
The therapeutic efficacy of quorum-sensing inhibition has emerged as promising paradigm for both pharmacologic and probiotic interventions aimed at mitigating S. aureus-induced disease.
Acknowledgements
ARH was supported by a Merit Award (I01 BX002711) from the Department of Veteran Affairs and NIH public health service grant AI083211.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gallo RL (2017) Human Skin Is the Largest Epithelial Surface for Interaction with Microbes. J Invest Dermatol 137 (6), 1213–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen GJ and Bruggemann H (2014) Bacterial skin commensals and their role as host guardians. Benef Microbes 5 (2), 201–15. [DOI] [PubMed] [Google Scholar]
- 3.Grice EA and Segre JA (2011) The skin microbiome. Nat Rev Microbiol 9 (4), 244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd AL et al. (2018) The human skin microbiome. Nat Rev Microbiol 16 (3), 143–155. [DOI] [PubMed] [Google Scholar]
- 5.Oh J et al. (2016) Temporal Stability of the Human Skin Microbiome. Cell 165 (4), 854–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.K. B et al. (2014) Coagulase-Negative Staphylococci. Clinical Microbiology Reviews 27, 870–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon RJ and Lowy FD (2008) Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 46 Suppl 5, S350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowy FD (1998) Staphylococcus aureus infections. N. Engl. J. Med 339 (8), 520–32. [DOI] [PubMed] [Google Scholar]
- 9.Chambers HF and Deleo FR (2009) Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 7 (9), 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otto M (2010) Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol. 2010 (64), 143–162. [DOI] [PubMed] [Google Scholar]
- 11.DeLeo FR et al. (2010) Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 375 (9725), 1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham PL 3rd et al. (2006) A U.S. population-based survey of Staphylococcus aureus colonization. Ann Intern Med 144 (5), 318–25. [DOI] [PubMed] [Google Scholar]
- 13.Sollid JU et al. (2014) Staphylococcus aureus: determinants of human carriage. Infect Genet Evol 21, 531–41. [DOI] [PubMed] [Google Scholar]
- 14.Moran GJ et al. (2006) Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 355 (7), 666–674. [DOI] [PubMed] [Google Scholar]
- 15.Hersh AL et al. (2008) Arch Intern Med. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. 168 (14), 1585–1591. [DOI] [PubMed] [Google Scholar]
- 16.Chen YE et al. (2018) Skin microbiota-host interactions. Nature 553 (7689), 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belkaid Y and Tamoutounour S (2016) The influence of skin microorganisms on cutaneous immunity. Nat Rev Immunol 16 (6), 353–66. [DOI] [PubMed] [Google Scholar]
- 18.Byrd AL et al. (2018) The human skin microbiome. Nat Rev Microbiol. 16 (3), 143–155. [DOI] [PubMed] [Google Scholar]
- 19.Buffie CG and Pamer EG (2013) Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 13 (11), 790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grice EA and Segre JA (2011) The skin microbiome. Nat Rev Microbiol. 9 (4), 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallo RL (2017) Human Skin Is the Largest Epithelial Surface for Interaction with Microbes. J Invest Dermatol. 137 (6), 1213–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouchi T et al. (2011) Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J Exp Med 208 (13), 2607–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubo A et al. (2009) External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med. 206 (13), 2937–2946. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakatsuji T et al. (2013) The microbiome extends to subepidermal compartments of normal skin. Nat Commun. 4 (1431), doi: 10.1038/ncomms2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naik S et al. (2012) Compartmentalized control of skin immunity by resident commensals. Science 337 (6098), 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scharschmidt TC et al. (2015) A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity. 43 (5), 1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridaura VK et al. (2018) Contextual control of skin immunity and inflammation by Corynebacterium. J Exp Med. 15 (3), 785–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esaki H et al. (2016) Early-onset pediatric atopic dermatitis is Th2 but also Th17 polarized in skin Journal of Allergy and Clinical Immunology 138 (6), 1639–1651. [DOI] [PubMed] [Google Scholar]
- 29.Papp K et al. (2012) Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis New England Journal of Medicine 366 (13), 1181–1189. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa S et al. (2017) Staphylococcus aureus Virulent PSMα Peptides Induce Keratinocyte Alarmin Release to Orchestrate IL-17-Dependent Skin Inflammation. Cell Host Microbe 22 (5), 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linehan JL et al. (2018) Non-classical Immunity Controls Microbiota Impact on Skin Immunity and Tissue Repair. Cell. 172 (4), 784–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen W et al. (2014) Adaptive immunity to murine skin commensals. Proc Natl Acad Sci U S A. 111 (29), E2977–86. doi: 10.1073/pnas.1401820111.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai Y et al. (2010) Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 130 (9), 2211–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai Y et al. (2009) Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 15 (12), 1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kluytmans J et al. (1997) Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10 (3), 505–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krismer B et al. (2017) The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat Rev Microbiol 15 (11), 675–687. [DOI] [PubMed] [Google Scholar]
- 37.Uehara Y et al. (2000) Bacterial interference among nasal inhabitants: eradication of Staphylococcus aureus from nasal cavities by artificial implantation of Corynebacterium sp. J Hosp Infect 44 (2), 127–33. [DOI] [PubMed] [Google Scholar]
- 38.Yan M et al. (2013) Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe 14 (6), 631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park B et al. (2011) Intranasal application of S. epidermidis prevents colonization by methicillin-resistant Staphylococcus aureus in mice. PLoS One 6 (10), e25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwase T et al. (2010) Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465 (7296), 346–9. [DOI] [PubMed] [Google Scholar]
- 41.Sugimoto S et al. (2013) Staphylococcus epidermidis Esp degrades specific proteins associated with Staphylococcus aureus biofilm formation and host-pathogen interaction. J Bacteriol 195 (8), 1645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C et al. (2013) Secreted proteases control autolysin-mediated biofilm growth of Staphylococcus aureus. J Biol Chem 288 (41), 29440–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janek D et al. (2016) High Frequency and Diversity of Antimicrobial Activities Produced by Nasal Staphylococcus Strains against Bacterial Competitors. PLoS Pathog 12 (8), e1005812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zipperer A et al. (2016) Human commensals producing a novel antibiotic impair pathogen colonization. Nature. 535 (7613), 511–516. . [DOI] [PubMed] [Google Scholar]
- 45.Nakatsuji T et al. (2017) Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 9 (378), pii: eaah4680. doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cogen AL et al. (2010) Staphylococcus epidermidis antimicrobial delta-toxin (phenol-soluble modulin-gamma) cooperates with host antimicrobial peptides to kill group A Streptococcus. PLoS One 5 (1), e8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cogen AL et al. (2010) Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol 130 (1), 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakatsuji T et al. (2018) A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci Adv 4 (2), eaao4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghoul M and Mitri S (2016) The Ecology and Evolution of Microbial Competition. Trends Microbiol. 24, 833–845 [DOI] [PubMed] [Google Scholar]
- 50.Fleming V et al. (2006) Agr interference between clinical Staphylococcus aureus strains in an insect model of virulence. J Bacteriol 188 (21), 7686–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paharik AE et al. (2017) Coagulase-Negative Staphylococcal Strain Prevents Staphylococcus aureus Colonization and Skin Infection by Blocking Quorum Sensing. Cell Host Microbe. 22 (6), 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong YH et al. (2002) Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Appl Environ Microbiol 68 (4), 1754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novick RP and Geisinger E (2008) Quorum sensing in staphylococci. Annu Rev Genet. 2008 (42), 541–564. [DOI] [PubMed] [Google Scholar]
- 54.Thoendel M et al. (2011) Peptide signaling in the staphylococci. Chem Rev 111 (1), 117–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ji G et al. (1997) Bacterial interference caused by autoinducing peptide variants. Science 276 (5321), 2027–30. [DOI] [PubMed] [Google Scholar]
- 56.Wright JS et al. (2005) Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc Natl Acad Sci U S A. 102 (5), 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayville P et al. (1999) Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci U S A. 96 (4), 1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olson ME et al. (2014) Staphylococcus epidermidis agr quorum-sensing system: signal identification, cross talk, and importance in colonization. J Bacteriol 196 (19), 3482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Todd DA et al. (2017) Signal Biosynthesis Inhibition with Ambuic Acid as a Strategy To Target Antibiotic-Resistant Infections. Antimicrob Agents Chemother. 61 (8), pii: e00263–17. doi: 10.1128/AAC.00263-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muhs A et al. (2017) Virulence Inhibitors from Brazilian Peppertree Block Quorum Sensing and Abate Dermonecrosis in Skin Infection Models. Sci Rep 7, 42275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon CP et al. (2013) Attenuating Staphylococcus aureus Virulence Gene Regulation: A Medicinal Chemistry Perspective. J Med Chem. 56, 1389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gray B et al. (2013) Targeting agr- and agr-Like quorum sensing systems for development of common therapeutics to treat multiple gram-positive bacterial infections. Sensors (Basel) 13 (4), 5130–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cech NB and Horswill AR (2013) Small-molecule quorum quenchers to prevent Staphylococcus aureus infection. Future Microbiol 8, 1511–4. [DOI] [PubMed] [Google Scholar]
- 64.Otto M et al. (2001) Pheromone cross-inhibition between Staphylococcus aureus and Staphylococcus epidermidis. Infect Immun 69 (3), 1957–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otto M et al. (1999) Inhibition of virulence factor expression in Staphylococcus aureus by the Staphylococcus epidermidis agr pheromone and derivatives. FEBS Lett 450 (3), 257–62. [DOI] [PubMed] [Google Scholar]
- 66.Canovas J et al. (2016) Cross-Talk between Staphylococcus aureus and Other Staphylococcal Species via the agr Quorum Sensing System. Front Microbiol 7, 1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramsey MM et al. (2016) Staphylococcus aureus Shifts toward Commensalism in Response to Corynebacterium Species. Front Microbiol 7, 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Summerfield A et al. (2015) The immunology of the porcine skin and its value as a model for human skin. Molecular Immunology 66 (1), 14–21. [DOI] [PubMed] [Google Scholar]
- 69.Todd DA et al. (2016) Hybrid Quadrupole-Orbitrap mass spectrometry for quantitative measurement of quorum sensing inhibition. J Microbiol Methods 127, 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daly SM et al. (2015) omega-Hydroxyemodin limits Staphylococcus aureus quorum sensing-mediated pathogenesis and inflammation. Antimicrob Agents Chemother 59 (4), 2223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sully EK et al. (2014) Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog 10 (6), e1004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quave CL et al. (2015) Castanea sativa (European Chestnut) Leaf Extracts Rich in Ursene and Oleanene Derivatives Block Staphylococcus aureus Virulence and Pathogenesis without Detectable Resistance. PLoS One 10 (8), e0136486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wuster A and Babu MM (2008) Conservation and evolutionary dynamics of the agr cell-to-cell communication system across firmicutes. J Bacteriol 190 (2), 743–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kolar SL et al. (2013) Extracellular proteases are key mediators of Staphylococcus aureus virulence via the global modulation of virulence-determinant stability. Microbiologyopen 2 (1), 18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gallo RL and Nakatsuji T (2011) Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol 131 (10), 1974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee BY et al. (2013) The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Clin Microbiol Infect. 19 (6), 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]