Abstract
Telomeres progressively shorten with age, and it has been proposed that critically short and dysfunctional telomeres contribute to aging and aging-associated diseases in humans. For many years it was thought that telomere erosion was strictly a consequence of the “end replication problem,” or the inability of replicative polymerases to completely duplicate linear DNA ends. It is becoming increasingly evident, however, that telomere shortening of cultured human cells is also caused because of other replication defects in telomeric repeats, those that cause fragile telomeres and other aberrant telomeric structures that can be detected on metaphase chromosomes. Whether these replication defects contribute to telomere erosion also in human tissues is currently unknown. By analyzing peripheral blood mononuclear cells from a total of 35 healthy subjects ranging in age from 23 to 101 years, we demonstrated that telomeres increasingly display aberrant structures with advancing donor age. Although the percentages of fragile telomeres increased only until adulthood, the percentages of chromosomes displaying sister telomere loss and sister telomere chromatid fusions increased consistently throughout the entire human life span. Our data, therefore, suggest that telomeric replication defects other than the end replication problem contribute to aging-associated telomere erosion in humans.
Keywords: Telomeres, Human aging, Health
Aging is characterized by a gradual functional decline of organs and organ systems, as well as a failure to maintain tissue homeostasis, which progressively leads to an increased risk of diseases and death (1). Recent studies have provided strong evidence to the long-standing notion that a causal factor of age-related diseases and mortality is an aging-associated accumulation of senescent cells in mammalian tissues (2,3). Although it is currently unclear what drives cells into senescence in aging mammals, one cause of cellular senescence is shortening and dysfunction of telomeres, the ends of linear chromosomes (4).
Telomeres are dynamic nucleoprotein structures that protect chromosome ends from illegitimate DNA repair activities. Telomeres can function as “biological clocks” capable of counting cell division cycles as they progressively erode every time chromosomes are replicated (5). Once they are critically short or dysfunctional, telomeres activate a persistent DNA damage response that ultimately drives cells into senescence (6,7). As it is thought to be correlated with biological age, leukocyte telomere length (LTL) has been associated with human aging in the past (8). Although shorter telomeres are associated with older age in general, the reliability and consistency of LTL measurements as an indicator of biological age and age-associated diseases remain subject of investigation (9). As the rate of telomere attrition with age is highly heterogeneous and variable among individuals, very large cohorts are usually required to demonstrate a significant reduction of LTL. Even a recent study using a cohort of 465 subjects ranging in age from 21 to 88 years at the first visit, with an average of 13 years (7–19 years) follow-up, revealed a highly variable rate of change in LTL, with a relatively slow average rate of telomere shortening per year (10). Another shortcoming of LTL measurements is that it measures average lengths of millions of telomeres in a sample, thereby masking changes to individual chromosome ends that may have dramatic consequences for the functionality of a cell or the fitness of the organism.
Because of their repetitive G-rich nature, a high propensity to form secondary structures and hypersensitivity to oxidative DNA damage, telomeres are regions on chromosomes that are difficult to replicate (11). Following DNA replication stress, replication forks can stall and terminate prematurely in telomeres, leaving some chromosome ends incompletely replicated. This can cause cells to undergo senescence prematurely and before most telomeres in a cell are critically short. Dysfunctional telomeres cause cellular senescence regardless of whether they are short or long, highlighting the necessity to analyze the state of individual telomeres in a cell rather than merely measuring total telomere lengths (12,13).
Aberrant telomeric structures that are caused by DNA replication stress can be detected on metaphase chromosomes by telomere-fluorescence in situ hybridization (14,15). Telomeric signals are normally represented as a single dot with an intensity that is equal to the telomeric signal of its sister chromatid. Fragile telomeres (FT) are characterized by the presence of multiple or diffuse telomeric signals on a single chromatid arm. Although still unclear, these structural abnormalities potentially represent areas of de-condensed telomeric chromatin or single-stranded telomeric DNA that is not appropriately packaged due to stalled DNA replication forks (16). Other aberrant telomeric structures include different intensities of sister telomere signals and individual telomere signal-free ends, collectively called sister telomere loss (STL), indicating incomplete sister chromatid telomere replication during the preceding S-phase. Also, telomeric replication stress can result in telomere dysfunction, and sister telomere chromatid fusions (STCF) as the cell attempts to repair critically short telomeres. In this study, we demonstrate that such aberrant telomeric structures increasingly can be detected in cells from human donors in aging-associated manner, suggesting that telomeric replication stress contributes to progressive telomere erosion observed with advancing age.
Methods
Subjects
Fourteen milliliters of peripheral venous blood was obtained from 35 subjects (13 male and 22 female). Individuals were fit volunteers with clinical parameters into physiological ranges. Subjects with evidence of acute inflammatory, autoimmune or infectious diseases, cancer, and psychotic disorders were ruled out. After a clear explanation of the potential risks of the study, all subjects provided written informed consent to participate in the study approved by the institutional review board of University of Perugia (no. 3066/17).
Telomere Length Polymerase Chain Reaction
LTL was assessed in genomic DNA by using a quantitative PCR method as previously reported in a subset of 18 subjects. Briefly, we determined the relative ratio (T/S ratio) of telomere (T) repeat copy number to a single copy gene (S) copy number using a comparative quantitation approach. The adopted primer pairs, their final concentration, and the thermal cycling profiles were as described with minor modifications (17–19).
Metaphase Chromosome Preparation and Fluorescence In Situ Hybridization
This method for telomere abnormalities quantification as a marker of aging and age-related diseases has been patented (number 102017000135218). Peripheral blood mononuclear cells (PBMCs) were purified from heparinized venous blood using Lympholyte cell separation media (Cedarlane, Burlington, North Carolina) as specified by the manufacturer. PBMCs (106 cells/mL) were cultured for 48 hours at 37°C (5%CO2) in RPMI 1640 medium, supplemented with fetal bovine serum, l-glutamine, and phytohemagglutinin (PB-MAX Karyotyping Medium; Thermo Fisher Scientific, Waltham, Massachusetts). One hour before harvesting, KaryoMAX Colcemid Solution (Thermo Fisher Scientific) was added to cell cultures to obtain metaphase arrest. Cells collected by centrifugation were resuspended in a hypotonic KCl (0.075 M) solution for 10 minutes, fixed in acetic acid/methanol (1:3 vol/vol), and resuspended in 200 µL fixative. Metaphase spreading was accomplished by dropping 10 µL cell suspension onto cleaned slides. Air-dried preparations were kept at room temperature until use. Air-dried cells were washed three times for 10 minutes in phosphate-buffered saline and dehydrated by sequentially placing them in 70% ethanol, 90% ethanol, and 100% ethanol for 3 minutes each. After air-drying, DNA was denatured for 5 minutes at 80°C in hybridization buffer containing Cy3-conjugated telomere-specific peptide nucleic acid (TELC-CY3; Bio Inc) according to the manufacturer’s instruction. Slides were placed at room temperature overnight. Slides were washed sequentially with wash buffer (70% formamide/30% saline-sodium citrate buffer 2×; 15 minutes) and phosphate-buffered saline (15 minutes). The slides were rinsed with water, air–dried, and mounted in mounting medium containing 4′,6-diamidino-2-phenylindole. Metaphases were analyzed by immunofluorescence microscopy using an Axio Observer Z1 fluorescence microscope equipped with ApoTome 2 (Zeiss, Oberkochen, Germany) and the Zen Blue software (Zeiss).
Calculation and Statistical Analysis
Aberrant telomeric signals were scored on metaphase chromosomes (clearly detectable and not overlapping) and expressed as percentage of total telomeres. Image groups were blinded before quantification. Two biological replicates were carried out and processed separately (Experiment 1 and Experiment 2). Mean values from the two experiments were used for statistical analyses and shown as mean ± standard deviation (SD). An average of 1,231 ± 39 telomeres was analyzed from each subject and each experiment. Averages of metaphase chromosome scored were 293 ± 107 (Experiment 1) and 318 ± 104 (Experiment 2). Averages of telomeres analyzed were 1,204 ± 459 (Experiment 1) and 1,259 ± 434 (Experiment 2). To detect a simple correlation r (r = .700), using a two-sided test, 5% significance level test, with Type I error of 0.05 and 95% power, the required sample size is approximate 20 samples (GPower 3.1.7). A total of 35 subjects were enrolled, resulting in a 99% power at a significant level of 0.05. Data were normally distributed and presented as mean ± SD. Student’s unpaired t test was used to assess differences in all variables in the study, including gender differences. Pearson correlation coefficients were calculated to evaluate the relationship between variables as indicated. A segmented regression analysis allowed us to stratify the population into two groups: (a) 23–60 years old (including young, 20- to 40–year-old, and adult, 40- to 60–year-old subjects) defined as “younger” and (b) 40–110 years old (including adult, 40- to 60-year-old, and over 60, older subjects) defined as “older.” All p-values presented are 2-tailed, and a p < .05 was chosen for levels of significance. Statistical analyses were performed using SPSS 21 software package (SPSS, Inc., Chicago, Illinois) or PRISM 6 (GraphPad Software, La Jolla, California).
Data Availability
The data sets generated and/or analyzed during this study are available from the corresponding author upon reasonable request.
Results
Telomere Abnormalities During Aging
The abundance of aberrant telomeric structures was studied in PBMCs that were cultured ex vivo for a period of 48 hours from 35 individuals (22 women and 13 men; mean age, 54.9 ± 25.9 years) ranging in ages from 23 to 101 years. All subjects were fit, with physical and clinical parameters indicative of good health. Clinical characteristics of all study population and stratified by gender are reported in Table 1. No significant statistical difference was found between genders in all parameters examined with the exclusion of body mass index.
Table 1.
Characteristics of All Study Population and Stratified by Gender
| All (N = 35) | Male (n = 13) | Female (n = 22) | Gender Comparison | |
|---|---|---|---|---|
| Age, years (mean ± SD) | 54.9 ± 25.9 | 56.4 ± 26.6† | 54.1 ± 26.1‡ | p* = .8120 |
| Current smoker, n (%) | 4 (11.4) | 3 (25) | 1 (4.3) | p** = .1340 |
| BMI, kg/m2 (mean ± SD) | 24.4 ± 3.6 | 27.0 ± 2.5 | 23.4 ± 3.5 | p* = .0056 |
| SBP, mm Hg (mean ± SD) | 113.7 ± 11.7 | 116.7 ± 9.8 | 112.2 ± 12.6 | p* = .2908 |
| DBP, mm Hg (mean ± SD) | 69.2 ± 7.9 | 70.0 ± 7.4 | 68.9 ± 8.4 | p* = .7076 |
Notes: BMI = body mass index; DBP = diastolic blood pressure; SBP = systolic blood pressure.
†Normality test: KS distance = 0.2062, p > .1000.
‡Normality test: KS distance = 0.1413, p > .1000.
*Significance calculated using unpaired t test.
**Significance calculated using Chi-square test (χ2 = 2.772).
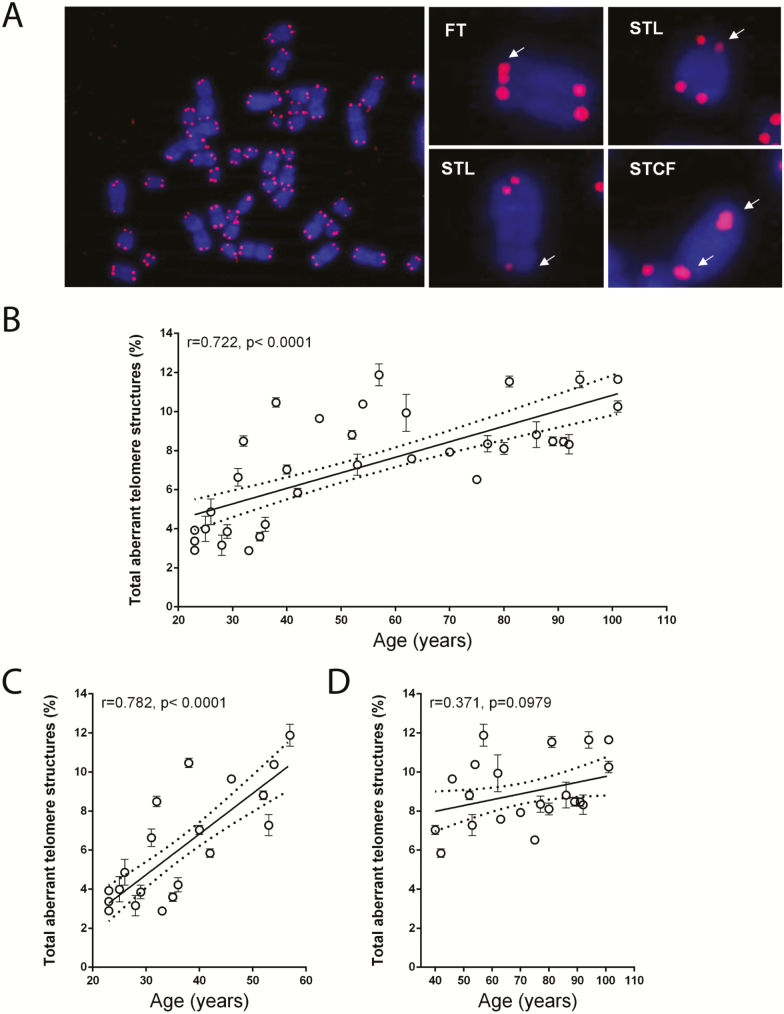
Representative images of aberrant telomeres structures such as FT, STL, and STCF are shown in Figure 1A. Linear correlation analysis revealed that total aberrant telomere structures increased progressively and significantly with advancing age (r = .722, p < .0001; Figure 1B). To better study changes of abnormalities along with aging, we looked at younger (20–60 years old) and older (40–110 years old) population groups. Although the percentages of total aberrant telomeric structures increased from 2.3% to 11.8% in the age range 20–60 years (r = .782, p < .0001; Figure 1C), these percentages did not significantly increase further in the age group 40–110 years (r = .371, p = .0979; Figure 1D). No significant differences in total aberrant telomeres were found between gender as revealed by unpaired t-test analysis (p = .2328). The mere modest increase in total aberrant telomeric structures after the age of 60 years suggests that either cells do not accumulate more than 12% aberrant telomeric structures or, alternatively, these structures become increasingly difficult to detect with advancing age because of telomere erosion.
Figure 1.
The abundance of aberrant telomeric structures in PBMCs increases with donor age. (A) PBMC metaphase chromosomes (blue) were processed by FISH using a peptide nucleic acid (PNA) complementary to telomeric repeats (red). A representative metaphase spread from a 31-year-old female subject as well as individual metaphase chromosomes displaying aberrant telomeric structures (right) are shown. White arrows point to aberrant telomeric structures. (B) Total aberrant telomere structures (percentage of the observed telomeres) in the PBMCs of each subject. (C) Total aberrant telomere structures (percentage of the observed telomeres) in the PBMCs of each subject belonging to the younger group. (D) Total aberrant telomere structures (percentage of the observed telomeres) in the PBMCs of each subject belonging to the older group. Data are expressed as mean (± SD) of counts from two experiments. Linear regression (continuous line) and 95% confidence band (dotted lines) are shown in B–D. FISH = fluorescence in situ hybridization; FT: fragile telomeres; PBMC = peripheral blood mononuclear cell; STCF = sister telomere chromatid fusions; STL = sister telomeres loss; r = Pearson correlation coefficient; p = statistical significance of Pearson correlation coefficient.
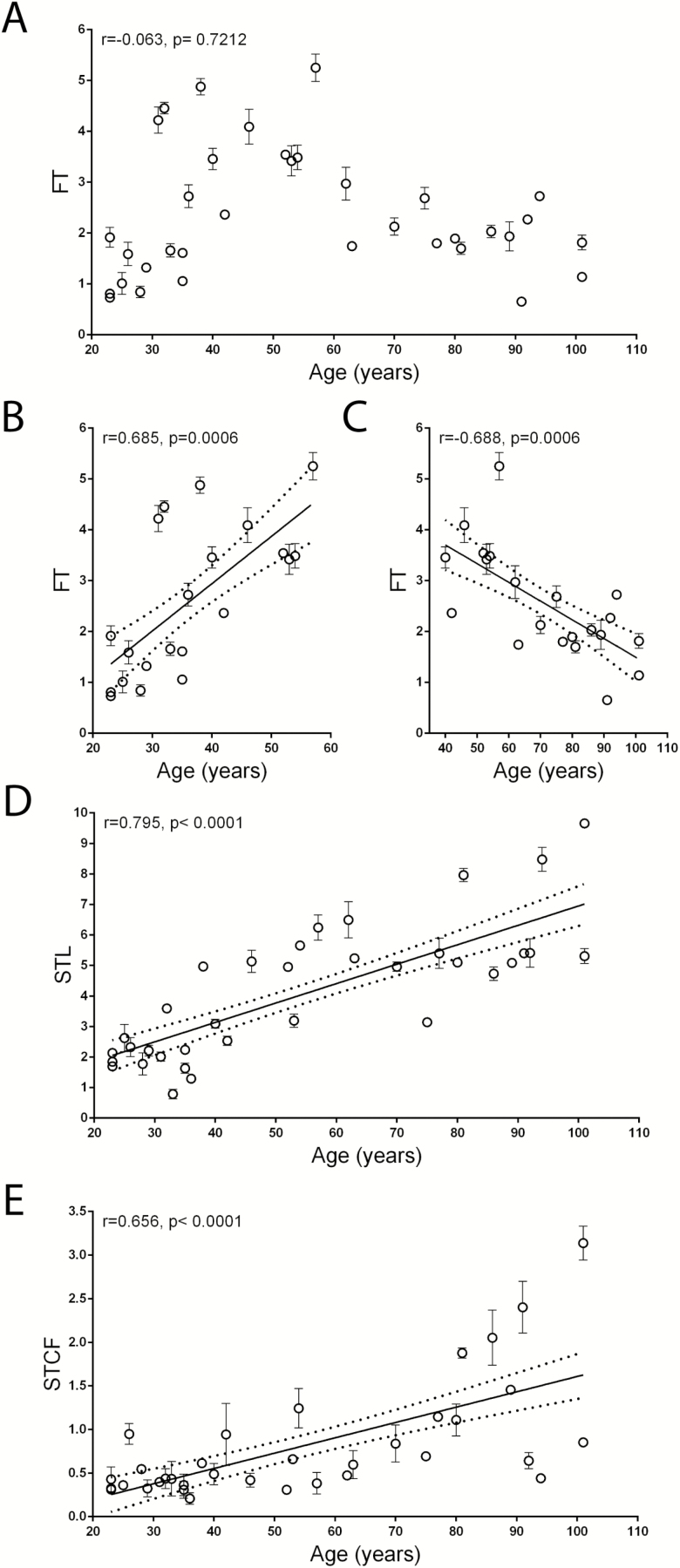
We next analyzed each telomere abnormality separately. Although no significant correlation between FT and age was found in the entire population (r = −.063, p = .7212; Figure 2A), a significant increase in FT within the age range of 20–60 years (r = .685, p = .0006) was observed, which was followed by a progressive decline until very old age (40–110 years, r = −.688 and p = .0009; Figure 2B and C). In contrast, the percentage of STL and STCF increased progressively and significantly throughout the entire age range (r = .795, p < .0001 and r = .656, p < .0001, respectively; Figure 2D and E), likely because these abnormalities can also be detected on critically short telomeres. Although a significant correlation was observed between STL and STCF (r = .384, p = .0229), no significant correlation was found between FT and STL (r = .175, p = .3134) and between FT and STCF (r = −.235, p = .1740). The correlation between total aberrant telomere structures, FT, STL, and STCF with age was further evaluated separately by gender. No difference was found in correlations between women and men as reported in Supplementary Figure 1.
Figure 2.
Correlation between aberrant telomeric structures (%) and age in the sample population. (A) Percentage of fragile telomeres (FT) in peripheral blood mononuclear cells (PBMCs) of all subjects. (B) Percentage of FT in PBMCs of subjects belonging to the younger group. (C) Percentage of FT in PBMCs of subjects belonging to the older group. (D) Percentage of telomeres displaying sister telomere loss (STL) in PBMCs of all subjects. (E) Percentage of sister telomere chromatid fusions (STCF) in PBMCs of all subjects. Data are expressed as the mean (± SD) from two experiments. Linear regression (continuous line) and 95% confidence band (dotted lines) are shown in B–E. r = Pearson correlation coefficient; p = statistical significance of Pearson correlation coefficient.
LTL and Telomere Abnormalities Subanalysis
To determine a possible correlation between telomeric abnormalities and LTL, we measured telomere length (LTL) in a subset of 18 subjects (12 women and 6 men; mean age, 41.6 ± 19.6 years) ranging in ages from 23 to 94 years. As anticipated, LTL shorting was observed with advancing age, although this trend did not reach statistical significance (r = −.375, p = .1252; Supplementary Figure 2A). In this subset, the correlation of FT, STL, and STCF with age was similar to that observed studying the whole set of data as shown in Supplementary Figures 2B–G. A significant positive correlation between FT and LTL (r = .602, p = .0082) was found (Supplementary Figure 2H). We hypothesize that such a finding does not reflect a true biological relationship between LTL and FT, but rather a technical artifact of FT measurement, specifically that the likelihood of detecting FT increases with telomere length. Considering the biphasic trend of FT during aging, we analyzed whether the correlation between FT and LTL was present both in younger and in the older groups. Indeed, we detected significant correlations in both groups (r = .676, p = .0057 and r = .856, p = .0141, respectively; Supplementary Figure 2I and L). Then we investigated whether the FT/LTL ratio is equal during aging and found that it significantly increases along with age (r = .779, p = .0001; Supplementary Figure 2M). However, when analyzing data in younger and older group, the FT/LTL ratio increased dramatically in the first part of life (20–60 years; r = .805, p = .0003; Supplementary Figure 2N) but did not increase significantly in the 40–110 years age group (r = .527, p = .2241; Supplementary Figure 2O). Our data thus demonstrate that FT and FT/LTL ratio increases rapidly during aging only in the first part of life and that the decrease of FT in the second part of the life (Figure 2C) depends on the shortening of telomere length. No significant correlation between STL and LTL (r = −.137, p = .5867; Supplementary Figure 2P) was found, whereas STCF and LTL (r = −.517, p = .0281; Supplementary Figure 2Q) were significantly correlated.
Discussion
Our data reveal an aging-associated increase of aberrant telomeric structures in human PBMCs, features that may indicate an increasing difficulty to replicate telomeres with advancing age. This findings therefore support the hypothesis that progressive telomere shortening that is observed with age may not simply be a consequence of the “end replication problem,” but instead, is likely due to a combination of factors that include incomplete telomere replication and telomeric replication stress.
Because of the inconsistency of LTL measurement, the reliability and usefulness of LTL as a biomarker of biological age remain controversial. Not only are the rates of aging-associated telomere attrition highly variable among different individuals, but LTL measurements also usually require very large cohorts in order to show trends that reach statistical significance (10,20,21). With this study we looked beyond LTL, with the direct quantification of telomere abnormalities as observed in metaphases. We demonstrate that such abnormal telomeric structures are much more closely correlated to biological age compared with the average LTL, suggesting that measurements of aberrant telomeric structures could serve as a novel and powerful biomarker for biological age. Our study reveals that even a small cohort of subjects (a total of 18 donors) was sufficient to generate statistically significant aging-associated increases in FT (in younger age), STL, and total aberrant telomeric structures, whereas LTL measurements did not. Although the percentages of FT increased in a linear manner in younger subjects, this increase was not as apparent in older subjects. As LTL progressively shorten with advancing age, it is likely that the decrease of FT in old subjects is caused by an increasing difficulty to detect FT on ever-shorter telomeres. Thus, measurements of FT might only reliably predict biological age up until a certain age limit. In contrast, FT levels normalized by LTL might prove to be accurate predictors of age throughout our entire life span. The same can be assessed for STC and STFC. Indeed, the percentages of chromosomes displaying STL and STCF increased consistently throughout the entire human life span. The change in the hematopoietic stem cells compartment, which seems not to be spared by the aging process, may also play a role. The lower detection of FT in the older age could be associated with the age-related decline of hematopoietic stem cells. However, further studies are necessary to test such a hypothesis.
The reasons for an aging-associated increase in telomeric replication stress, sporadic telomere loss, and other telomeric abnormalities observed here in PBMCs are currently unclear. Age and lifestyle factors that increase levels of inflammation and oxidative stress, such as smoking, obesity, pollution, and stress, are associated with shorter LTL (5), raising the possibility that aging-associated increases in levels of oxidative stress accelerate telomere erosion rates through mechanisms involving telomeric replication stress. Indeed, increasing level of oxidative stress in human cell cultures not only promotes the formation of fragile and aberrant telomeric structures (22,23), but it also increases telomere erosion rates significantly (23). One factor that is critical for efficient telomere replication, especially when oxidative stress levels are high, is a telomere-specific DNA replication protein called Stn1 (22). Significantly, genome-wide association studies identified Stn1 as one of the few loci involved in regulating LTL in humans (24), suggesting that aging-associated telomere shortening rates depend on how efficiently telomeres are replicated in humans cells.
In summary, our study reveals novel insights into the mechanisms that cause telomere shortening in aging humans and uncovers novel aging-associated features of telomeres that may prove useful for predicting biological age. Whether aberrant telomeric structures are also associated with other markers of cellular senescence awaits further investigations.
Funding
V.B. was supported by Fondazione Cassa di Risparmio di Perugia. U.H. was supported by the National Cancer Institute of the NIH (R01CA136533, R01CA184572).
Conflict of interest statement
None declared.
Supplementary Material
References
- 1. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker DJ, Childs BG, Durik M, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446 [DOI] [PubMed] [Google Scholar]
- 5. Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–1198. doi: 10.1126/science.aab3389 [DOI] [PubMed] [Google Scholar]
- 6. Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell. 2004;14:501–513. doi: 10.1016/S1097-2765(04)00256-4 [DOI] [PubMed] [Google Scholar]
- 7. D’Adda Di Fagagna F, Reaper PM, Clay-Farrace L, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118 [DOI] [PubMed] [Google Scholar]
- 8. Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol. 2006;35:1424–1429. doi: 10.1093/ije/dyl169. [DOI] [PubMed] [Google Scholar]
- 9. Aviv A, Shay JW. Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond B Biol Sci. 2018;373:20160436. doi: 10.1098/rstb.2016.0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lustig A, Liu HB, Metter EJ, et al. Telomere shortening, inflammatory cytokines, and anti-cytomegalovirus antibody follow distinct age-associated trajectories in humans. Front Immunol. 2017;8:1027. doi: 10.3389/fimmu.2017.01027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maestroni L, Matmati S, Coulon S. Solving the telomere replication problem. Genes (Basel). 2017;8:55. doi: 10.3390/genes8020055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hewitt G, Jurk D, Marques FD, et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708. doi: 10.1038/ncomms1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fumagalli M, Rossiello F, Clerici M, et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol. 2012;14:355–365. doi: 10.1038/ncb2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sfeir A, Kosiyatrakul ST, Hockemeyer D, et al. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009;138:90–103. doi: 10.1016/j.cell.2009.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martínez P, Thanasoula M, Muñoz P, et al. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009;23:2060–2075. doi: 10.1101/gad.543509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higa M, Fujita M, Yoshida K. DNA replication origins and fork progression at mammalian telomeres. Genes (Basel). 2017;8:112. doi: 10.3390/genes8040112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tedone E, Arosio B, Gussago C, et al. Leukocyte telomere length and prevalence of age-related diseases in semisupercentenarians, centenarians and centenarians’ offspring. Exp Gerontol. 2014;58:90–95. doi: 10.1016/j.exger.2014.06.018 [DOI] [PubMed] [Google Scholar]
- 18. Tedone E, Arosio B, Colombo F, et al. Leukocyte telomere length in Alzheimer’s disease patients with a different rate of progression. J Alzheimers Dis. 2015;46:761–769. doi: 10.3233/JAD-142808 [DOI] [PubMed] [Google Scholar]
- 19. Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin Y, Damjanovic A, Metter EJ, et al. Age-associated telomere attrition of lymphocytes in vivo is co-ordinated with changes in telomerase activity, composition of lymphocyte subsets and health conditions. Clin Sci (Lond). 2015;128:367–377. doi: 10.1042/CS20140481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen W, Kimura M, Kim S, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011;66:312–319. doi: 10.1093/gerona/glq223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boccardi V, Razdan N, Kaplunov J, et al. Stn1 is critical for telomere maintenance and long-term viability of somatic human cells. Aging Cell. 2015;14:372–381. doi: 10.1111/acel.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vallabhaneni H, O’Callaghan N, Sidorova J, Liu Y. Defective repair of oxidative base lesions by the DNA glycosylase Nth1 associates with multiple telomere defects. PLoS Genet. 2013;9:e1003639. doi: 10.1371/journal.pgen.1003639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levy D, Neuhausen SL, Hunt SC, et al. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci U S A. 2010;107:9293–9298. doi: 10.1073/pnas.0911494107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and/or analyzed during this study are available from the corresponding author upon reasonable request.