Abstract
We evaluated effects of calorie restriction (CR: consuming 60–65% of ad libitum [AL] intake) initiated late-in-life with or without acute exercise on insulin-stimulated glucose uptake (ISGU) of skeletal muscle by studying four groups of 26-month-old rats: sedentary-AL, sedentary-CR (8-week duration), 3 hours post-exercise (3hPEX)-AL and 3hPEX-CR. ISGU was determined in isolated epitrochlearis muscles incubated ± insulin. Muscles were assessed for signaling proteins (immunoblotting) and lipids (mass spectrometry). ISGU from sedentary-CR and 3hPEX-AL exceeded sedentary-AL; 3hPEX-CR exceeded all other groups. Akt (Ser473, Thr308) and Akt substrate of 160 kDa (AS160; Ser588, Thr642, Ser704) phosphorylation levels tracked with ISGU. Among the 477 lipids detected, 114 were altered by CR (including reductions in 15 of 25 acylcarnitines), and 27 were altered by exercise (including reductions in 18 of 22 lysophosphatidylcholines) with only six lipids overlapping between CR and exercise. ISGU significantly correlated with 23 lipids, including: acylcarnitine 20:1 (r = .683), lysophosphatidylethanolamine19:0 (r = −.662), acylcarnitine 24:0 (r = .611), and plasmenyl-phosphatidylethanolamine 37:5 (r = −.603). Muscle levels of ceramides (a lipid class previously linked to insulin resistance) were not altered by CR and/or exercise nor significantly correlated with ISGU, implicating other mechanisms (which potentially involve other lipids identified in this study) for greater ISGU and Akt and AS160 phosphorylation with these interventions.
Keywords: Calorie restriction, Exercise, Insulin, Lipids
Insulin resistance is a primary event in the progression to type 2 diabetes (1). In the absence of diabetes, insulin resistance is associated with many other age-related pathologies, including hypertension, coronary heart disease, stroke, and certain cancers (2–4). Because skeletal muscles account for the largest portion of insulin-mediated whole-body glucose uptake (GU) (5), they are an important target for strategies to enhance insulin sensitivity.
One exercise session or moderate calorie restriction (CR: consuming 60%–65% of ad libitum [AL] food intake) can independently enhance muscle insulin-stimulated glucose uptake (ISGU) (6–8). Furthermore, long-term CR (~27-month duration) combined with one exercise session induces a greater increase in ISGU than either long-term CR or exercise alone in 30-month-old rats (9). It would be valuable to know whether short-term CR initiated late-in-life combined with exercise is also more effective than either treatment alone. Our first aim was to examine independent and combined effects of short-term (8 weeks) CR and one exercise session on ISGU in muscles from ~26-month-old rats.
The second aim was to gain insights into mechanisms for the independent and combined effects of CR and exercise on ISGU. Earlier research evaluating long-term CR and acute exercise on ISGU found each treatment alone increased insulin-stimulated Akt phosphorylation, and CR plus exercise led to greater Akt phosphorylation than with either treatment alone (9). Therefore, we assessed Akt phosphorylation.
Akt substrate of 160 kDa (also called AS160 or TBC1D4) is a Rab GTPase activating protein, and AS160’s phosphorylation on two Akt phosphomotifs, Ser588 and Thr642, is crucial for insulin-stimulated GLUT4 glucose transporter translocation and ISGU (10). Because prior research indicated that CR or exercise can enhance AS160 phosphorylation on one or both sites (8,9), we assessed AS160 Ser588 and Thr642 phosphorylation. In addition, Kjobsted and colleagues reported AS160 phosphorylation on Ser704, an AMP-activated protein kinase (AMPK) phosphomotif, favors subsequent insulin-stimulated phosphorylation on Thr642 (11). Accordingly, we also evaluated AMPK Thr172 and AS160 Ser704 phosphorylation.
Earlier evidence implicates various lipids (eg, greater ceramides or diacylglycerols) in the development of insulin resistance (12). Little is known about CR and/or exercise effects on these lipids in muscles of old animals. Therefore, we used a lipidomics approach to discover independent and combined CR and exercise effects on numerous muscle lipids. We also determined abundance of GLUT4 and hexokinase II, which are essential for glucose transport and phosphorylation, respectively. In addition, we measured muscle glycogen because glycogen depletion has been proposed as potentially related to improved post-exercise insulin sensitivity (13).
Methods
Materials
Chemicals were from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Hanover Park, IL). Materials for sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting were from Bio-Rad Laboratories (Hercules, CA). Pierce MemCode Reversible Protein Stain Kit, bicinchoninic acid protein assay, and Tissue Protein Extraction Reagent were from Thermo Fisher Scientific (Waltham, MA). Anti-rabbit IgG horseradish peroxidase conjugate was from Cell Signaling Technology (Danvers, MA). Supplementary Table 1 summarizes the primary antibodies used. [3H]-2-Deoxy-D-glucose ([3H]-2-DG) and D-[14C]-mannitol were from PerkinElmer (Boston, MA).
Animal Treatment
Animal care procedures were approved by the University of Michigan Committee on Use and Care of Animals. Male Fischer 344×Brown Norway rats (n = 40) were from the National Institute of Aging Aged Rat Colony at ~24-months-old. Animals were individually housed at the University of Michigan under specific pathogen-free conditions (12–12 hour light–dark; lights out at 17:00 hours). After 1-week acclimation, CR was begun in half of the rats and maintained for 8-weeks. AL-fed rats were given NIH31 chow. CR rats were fed NIH31/NIA-fortified chow (65% of AL daily intake) between 15:30 and 16:30 hours daily. Food intake was determined daily. The terminal experiment was performed when animals were ~26 months old. Rats were fasted between 18:00 and 19:00 hours the night before the experiment. At ~07:00 hours on the next morning, exercised rats (3hPEX) swam in a barrel filled with water (35°C; 45 cm depth; 9 swim-bouts, 10-min/bout with 10-minute rest between bouts) (9). Sedentary control (SED) rats from each diet group remained in their cages during the exercise-period. Epitrochlearis muscles were dissected from anesthetized time-matched sedentary and exercised animals at 3 hours post-exercise. Because one rat in the CR group developed a tumor and was euthanized, final sample sizes were: SED-AL, n = 10; SED-CR, n = 9; 3hPEX-AL, n = 10; 3hPEX-CR, n = 10.
Muscle Dissection and Incubation
Rats were anesthetized (intraperitoneal sodium pentobarbital injection, 50 mg/kg). Epitrochlearis muscles were isolated and rinsed (Krebs–Henseleit buffer). One muscle was freeze-clamped using tongs cooled in liquid nitrogen and stored (−80°C) until analyzed. Contralateral muscles were longitudinally transected into two strips that were placed in vials including appropriate media and shaken while gassed (95% O2–5% CO2) in a heated (35°C) water bath. Krebs–Henseleit buffer (2 mL) supplemented with bovine serum albumin (BSA; 0.1%), 2 mM sodium pyruvate, 6 mM mannitol, and no insulin or insulin (1.2 nM) was included in vials during the initial incubation step (30 minutes). Muscles were transferred for 20 minutes to a vial containing 2 mL Krebs–Henseleit buffer/BSA, same insulin dose, 0.1 mM 2-DG (2.25 mCi/mmol [3H]-2-DG), and 5.9 mM mannitol (0.022 mCi/mmol [14C]-mannitol). Muscles were blotted, trimmed, freeze-clamped and stored (−80°C).
Muscle Processing for 2-Deoxyglucose Uptake and Immunoblotting
Frozen muscles were weighed, then homogenized in 1 mL ice-cold lysis buffer (Tissue Protein Extraction Reagent, 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 2.5 mM sodium pyrophosphate, 1 mM sodium vanadate, 1 mM β-glycerophosphate, 1 μg/mL leupeptin, 1 mM phenylmethylsulfonyl fluoride). Lysates were rotated (1 hour, 4°C) prior to centrifugation (15,000g, 15 minutes, 4°C). Supernatants were transferred to microfuge tubes and stored (−80°C). Protein concentration was determined (bicinchoninic acid procedure).
2-Deoxyglucose Uptake
Aliquots of lysate supernatants were pipetted into a vial with scintillation cocktail. A scintillation counter determined 3H and 14C disintegrations per minute. 2-DG uptake was calculated (14).
Immunoblotting
An equal amount of protein from lysates was combined with 6X Laemmli buffer, boiled, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (9% resolving gel), and transferred to polyvinyldifluoride membranes. Equal loading was confirmed using MemCode protein stain. BSA or non-fat milk (5% in TBST; Tris-buffered saline, pH 7.5, 0.1% Tween-20) was used for blocking (1 hour, RT). Membranes were washed (3 × 5 minutes in TBST), incubated with primary antibody (in TBST + 5% BSA) overnight (4°C) and washed (3 × 5 minutes in TBST). Next, membranes were incubated with secondary antibody (1 hour, room temperature, RT) and washed (3 × 5 minutes in TBST followed by 2 × 5 minutes in TBS). Enhanced chemiluminescence was used to visualize protein bands quantified via densitometry. Results were expressed relative to the normalized mean of all samples on the blot. Phosphoproteins were expressed relative to total protein.
Glycogen
Muscles frozen immediately post-dissection were weighed and homogenized (0.3 M perchloric acid) prior to glycogen determination (15).
Lipidomics
The University of Michigan Regional Comprehensive Metabolomics Resource Core performed sample preparation and lipidomics analysis (detailed description in Supplementary Materials). Briefly, muscles frozen immediately post-dissection were weighed before and after pulverization and homogenization. Protein precipitation was initiated with methanol addition, then equal volumes of water and chloroform were added. After equilibration for 5 minutes (RT), extract was centrifuged. Lipid extract collected from the organic phase was dried under nitrogen gas and reconstituted before mass spectrometry analysis. Quality Control samples prepared by pooling equal volumes of each sample were injected at the beginning and end of each analysis and after every 10 sample injections (16). Chromatographic separation used a Shimadzu CTO-20A Nexera X2 UHPLC. For lipid separation, lipid extract was injected onto a 1.8 µm particle diameter, 50 × 2.1 mm id Waters Acquity HSS T3 column. Mass spectrometry data were acquired in both positive and negative ionization modes using a TripleTOF 5600 equipped with a DuoSpray ion source coupled with automated calibration system (AB Sciex). Raw data were converted to mgf data format with proteoWizard software. The NIST MS PepSearch program was used to search the converted files against the LipidBlast libraries (http://fiehnlab.ucdavis.edu/projects/LipidBlast) for lipid identification, and lipid quantification used Multiquant software (AB-SCIEX) (17).
Statistical Analysis
Two-way analysis of variance (2-way analysis of variance [ANOVA]) was performed for each insulin dose. Independent and interaction effects of Diet and Exercise were determined. The Holm-Sidak test was used for post hoc analysis (SigmaPlot 13.0). Data lacking normal distribution and/or equal variance were transformed before performing 2-way ANOVA. Kruskal–Wallis 1-way ANOVA on ranks was used if transformation failed to normalize data or equalize variance. Post hoc analysis used Dunn’s method. Spearman Rank Order Correlation was used for associations between endpoints. Lipidomics analysis was performed using R-3.1.2 (18). Raw data were imputed using the K nearest-neighbor algorithm (19) and normalized to muscle mass. Data for two different modes were combined, and repeats were removed based on RSD% for Quality Control samples. Statistical analysis was done on data below 30% RSD for Quality Control samples. Two-way ANOVA was performed using Diet and Exercise as independent variables. p-values were adjusted with False Discovery Rate correction. Post hoc p-values were calculated using Tukey HSD post hoc analysis. Heatmaps for Z scores were created with Package gplots (20) using all data and for differential lipids in Diet and Exercise separately.
Results
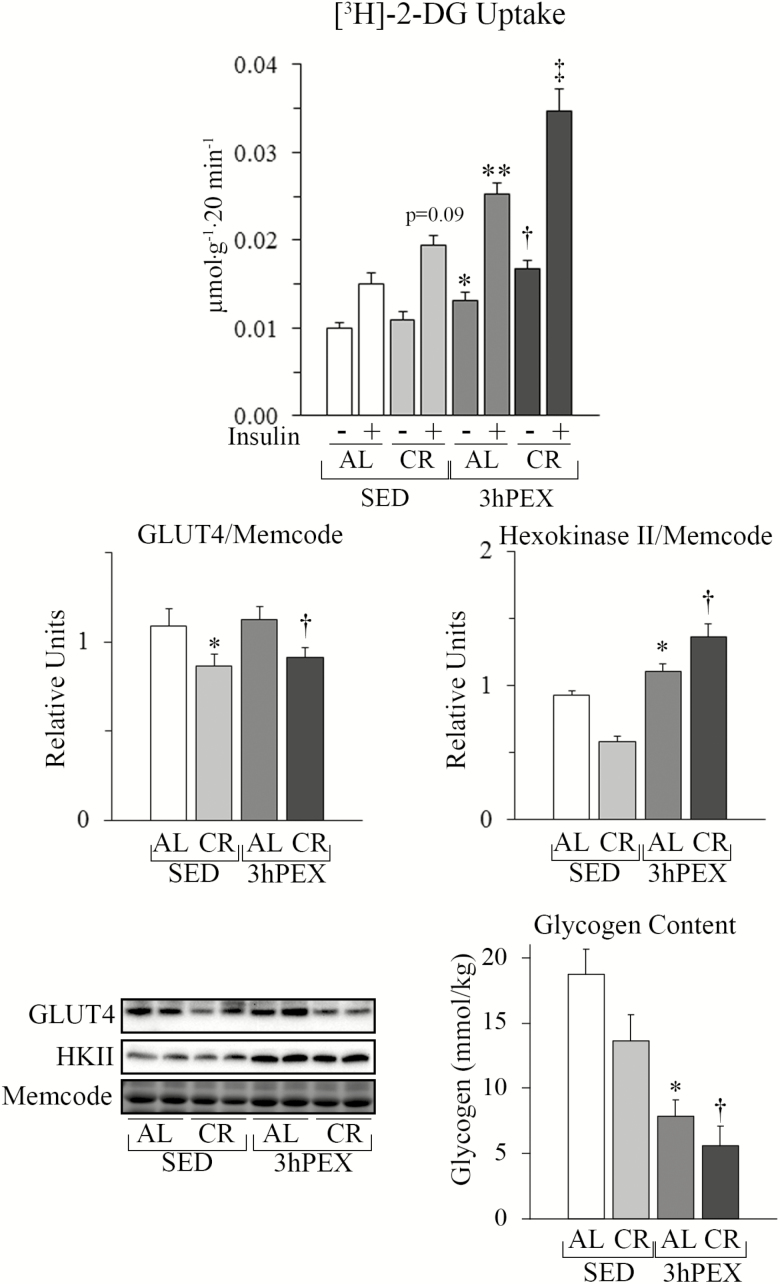
A hallmark of exercise is subsequently elevated ISGU by muscle (13). As expected, ISGU of 3hPEX-AL rats exceeded SED-AL controls (p < .001; Figure 1). Earlier studies reported CR can enhance ISGU in muscles from old rats (9), consistent with our observation that ISGU was greater for SED-CR versus SED-AL (ANOVA post hoc: p = .09; t-test: p < .05). Combined CR and exercise induced greater ISGU versus the independent effects of CR or exercise alone (p < .001). Exercise induced small increments in insulin-independent GU for 3hPEX-AL versus SED-AL (p < .05), and for combined CR and exercise versus either CR or exercise alone (p < .01).
Figure 1.
Glucose uptake, GLUT4, and hexokinase II protein abundance and glycogen in epitrochlearis muscle. Glucose uptake. For muscles incubated without insulin, statistical analysis indicated: main effects of Diet (p < .01) and Exercise (p < .001); *3hPEX-AL exceeded SED-AL (p < .05); †3hPEX-CR exceeded SED-CR (p < .001) and 3hPEX-AL (p < .01). For muscles incubated with insulin, post hoc analysis indicated: main effects of Diet (p < .001) and Exercise (p < .001); **3hPEX-AL exceeded SED-AL (p < .001); ‡3hPEX-CR exceeded SED-CR (p < .001) and 3hPEX-AL (p < .01). For muscles incubated with insulin, post hoc analysis indicated a non-significant trend (p = .09) for CR-SED exceeded AL-SED. CR-SED significantly exceeded AL-SED (p < .05) when compared using a t-test. GLUT4. There was a main effect of CR (p < .01) on GLUT4 protein abundance. Post hoc analysis revealed *SED AL exceeded SED CR (p < .05) and †3hPEX AL exceeded 3hPEX CR (p < .05). Hexokinase II. There was a main effect of Exercise (p < .001) and an interaction effect (p < .01) on Hexokinase II protein abundance. Post hoc analysis revealed *3hPEX AL exceeded SED AL (p < .001) and †3hPEX CR exceeded 3hPEX AL (p < .05). Glycogen. There were main effects of Diet (p < .05) and Exercise (p < .001) on epitrochlearis glycogen concentration. Post hoc analysis revealed that: *SED-AL exceeded 3hPEX-AL (p < .001); †SED-CR exceeded 3hPEX-CR (p < .001). Values shown as mean ± SEM (n = 9–10/group).
To probe mechanisms for improved ISGU, we evaluated GLUT4 and HKII abundance, proteins responsible for rate-controlling steps in glucose metabolism. GLUT4 abundance was unaltered by exercise and reduced ~20% by CR (p < .01; Figure 1). Previous research found greater GLUT4 translocation in insulin-stimulated epitrochlearis from 5.5-month-old CR versus AL rats (21). A similar effect may occur in older rats. HKII abundance was unaltered by CR, but increased ~20% in the exercised groups (p < .01; Figure 1), which would favor greater GU, but would not account for the ~90% increase in ISGU after exercise alone.
Vigorous exercise substantially reduces muscle glycogen, whereas CR modestly lowered glycogen in some, but not all studies (7,22,23). Combined effects of CR and exercise on muscle glycogen have not been reported for old rats. As expected, exercise caused marked glycogen depletion; SED-AL exceeded 3hPEX-AL (p < .001; Figure 1). There was a statistically non-significant trend for lower glycogen in SED-CR versus SED-AL. Finally, exercise combined with CR resulted in a further decline in glycogen; SED-CR exceeded 3hPEX-CR (p < .001). Glycogen was negatively correlated with ISGU (r = −.559; p < .001) and signaling proteins: pAktT308 (r = −.412; p < .05), pAktS473 (r = −.517; p < .001), pAMPKT172 (r = −.404; p < .05), pAS160S588 (r = −.613; p < .001), pAS160T642 (r = −.404; p < .05), and pAS160S704 (r = −.435; p < .01).
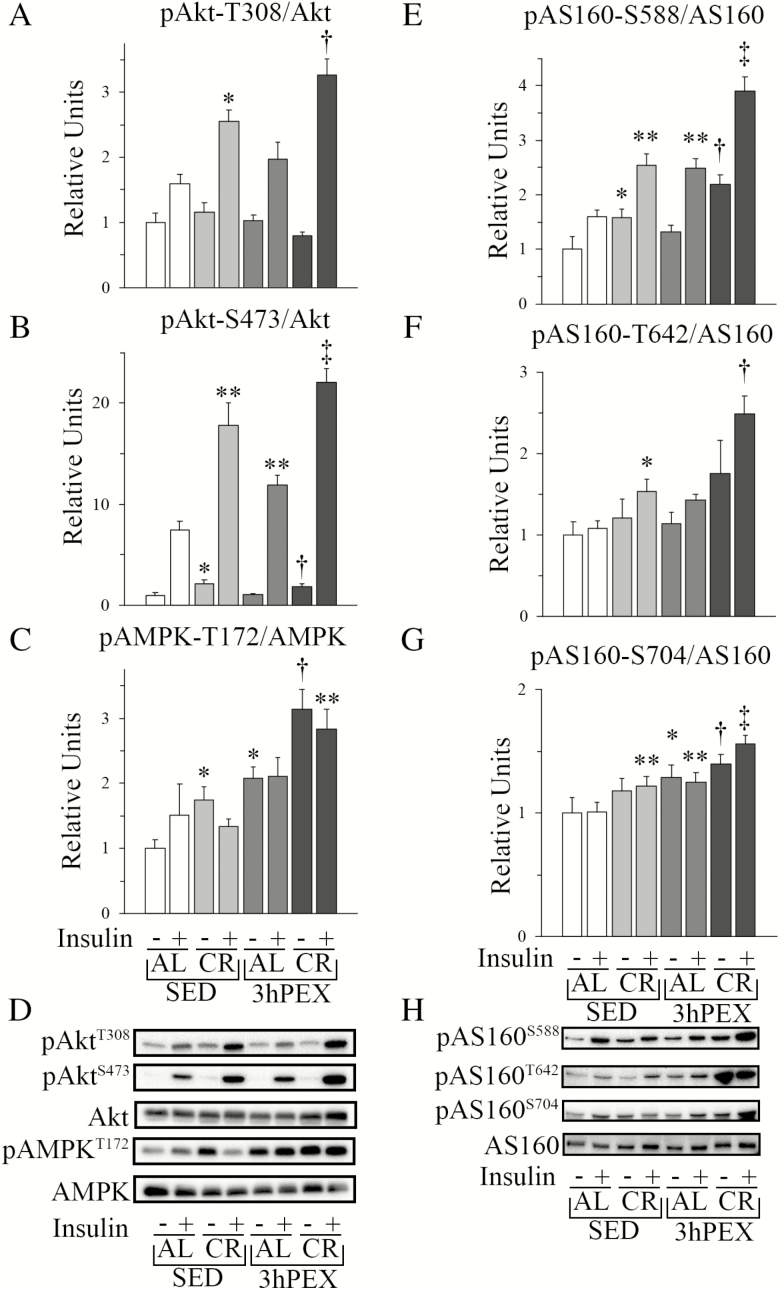
Akt phosphorylation on Ser473 and Thr308 is crucial for ISGU, and CR’s enhancement of insulin-mediated pAkt is essential for greater ISGU in muscles from CR versus AL animals (24,25). Consistent with earlier research, pAktT308 (p < .05) and pAktS473 (p < .01) were increased in insulin-stimulated muscles from CR rats (Figure 2). Combined CR and exercise, versus CR or exercise alone, led to further elevation of pAktT308 (p < .05) and pAktS473 (p < .01 versus SED-CR; p < .001 versus 3hPEX-AL) in insulin-stimulated muscles (Figure 2). Without insulin, there were small changes in pAktS473, with CR or exercise alone exceeding SED-AL, and 3hPEX-CR exceeding CR or exercise alone. In the context of the enhanced pAkt, it was important to assess CR and/or exercise effects on pAS160, an Akt substrate that regulates ISGU.
Figure 2.
Immunoblot results for Akt, AMPK, and AS160 in epitrochlearis muscle. (A) pAktT308. No differences were observed for muscles incubated without insulin. For muscles incubated with insulin statistical analysis indicated: main effects of Diet (p < .001) and Exercise (p < .05); *SED-CR exceeded SED-AL (p < .05); †3hPEX-CR exceeded SED-CR (p < .05) and 3hPEX-AL (p < .001). (B) pAktS473. For muscles incubated without insulin, statistical analysis indicated: main effect of Diet (p < .01); *SED-CR exceeded SED-AL (p < .01) and †3hPEX-CR exceeded 3hPEX-AL (p < .05). For muscles incubated with insulin, statistical analysis indicated: main effects of Diet (p < .001) and Exercise (p < .01); **SED-CR (p < .001) and 3hPEX-AL (p < .05) exceeded SED-AL; ‡3hPEX-CR exceeded SED-CR (p < .05) and 3hPEX-AL (p < .001). (C) pAMPKT172. For muscles incubated without insulin, statistical analysis indicated: main effects of Diet (p < .05) and Exercise (p < .001); *SED-CR (p < .05) and 3hPEX-AL (p < .01) exceeded SED-AL; †3hPEX-CR exceeded SED-CR (p < .001) and 3hPEX-AL (p < .01). For muscles incubated with insulin, post hoc analysis indicated: **3hPEX-CR exceeded SED-CR (p < .01). (D) Representative immunoblots for Akt and AMPK. (E) pAS160S588. For muscles incubated without insulin, post hoc analysis indicated: *SED-CR exceeded SED-AL (p < .01) and †3hPEX-CR exceeded 3hPEX-AL (p < .01). For muscles incubated with insulin, statistical analysis indicated: main effects of Diet (p < .001) and Exercise (p < .001) and interaction (p < .05); **SED-CR (p < .01) and 3hPEX-AL (p < .01) exceeded SED-AL; ‡3hPEX-CR exceeded SED-CR (p < .001) and 3hPEX-AL (p < .001). (F) pAS160T642. No effects of exercise or CR were observed for muscles incubated without insulin. For muscles incubated with insulin, statistical analysis indicated: main effects of Diet (p < .001) and Exercise (p < .001); *SED-CR exceeded SED-AL (p < .05); †3hPEX-CR exceeded SED-CR (p < .001) and 3hPEX-AL (p < .001). (G) pAS160S704. For muscles incubated without insulin, statistical analysis indicated: main effect of Exercise (p < .001); *3hPEX-AL exceeded SED-AL (p < .01) and †3hPEX-CR exceeded SED-CR (p < .05). For muscles incubated with insulin, statistical analysis indicated: main effects of Diet (p < .01) and Exercise (p < .01); **SED-CR and 3hPEX-AL exceeded SED-AL (p < .05); ‡3hPEX-CR exceeded SED-CR (p < .05) and 3hPEX-AL (p = .05). (H) Representative immunoblots for AS160. Values shown as mean ± SEM (n = 9–10/group).
AS160’s phosphorylation on Akt-phosphomotifs (Ser588 and Thr642) is necessary for insulin’s full-effect on GU (10). It was notable that CR alone and exercise alone induced greater insulin-stimulated pAS160 on each site (p-values from <.05 to <.001; Figure 2). Combined CR and exercise caused even greater phosphorylation on each site versus CR or exercise alone (p < .001; Figure 2). For muscles incubated without insulin, pAS160S588, but not pAS160T642, of the SED-CR group exceeded SED-AL (p < .01) and 3hPEX-CR exceeded 3hPEX-AL (p < .01). AS160 can also be phosphorylated on Ser704, an AMPK-phosphomotif, and greater pAS160S704 has been reported to favor greater pAS160T642 (11). Accordingly, we assessed pAS160S704 and discovered that it was increased by exercise alone versus SED-AL (p < .01), and for combined CR and exercise versus CR alone (p < .05) in muscles without insulin (Figure 2). Furthermore, in insulin-stimulated muscles, pAS160S704 was increased by either CR alone or exercise alone (p < .05), and combined CR and exercise induced greater pAS160S704 versus either treatment alone (p < .05; Figure 2). For muscles incubated without insulin, pAS160S704 in 3hPEX-AL exceeded SED-AL (p < .01) and 3hPEX-CR exceeded SED-CR (p < .05). The greater AS160 phosphorylation on all three phosphosites in insulin-stimulated muscles in response to CR alone, exercise alone, and combined CR and exercise supports the idea that greater phosphorylation on these sites contributes to independent and combined effects of CR and exercise on ISGU.
Because AMPK phosphorylates AS160 on Ser704, we assessed pAMPKT172. Without insulin, CR (p < .05) or exercise (p < .01) alone increased pAMPKT172 compared to SED-AL controls, and CR plus exercise further elevated pAMPKT172 above CR (p < .001) or exercise (p < .01) alone (Figure 2). In insulin-stimulated muscles, pAMPKT172 was greater (p < .01) for combined CR and exercise versus CR alone.
To gain insights into relationships between signaling proteins and ISGU, we performed correlation analyses. We found positive correlations between ISGU and insulin-stimulated pAkt, pAMPK, and pAS160: pAktT308 (r = .601; p < .001), pAktS473 (r = .510; p < .01), pAMPKT172 (r = .505; p < .05), pAS160S588 (r = .656; p < .001), pAS160T642 (r = .665; p < .001), and pAS160S704 (r = .538; p < .001).
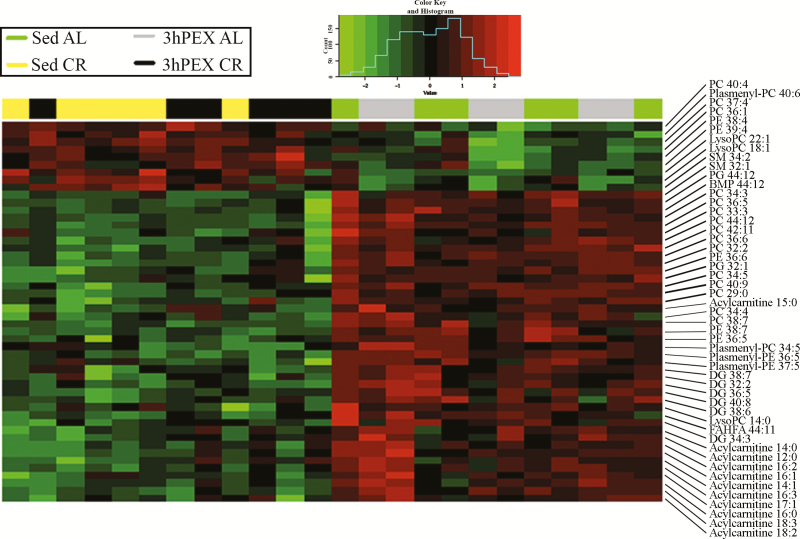
Accumulation of certain lipids has been linked to insulin resistance. Because higher ceramide levels have been linked to lower Akt phosphorylation, and CR enhanced Akt phosphorylation, we were especially interested in this class of lipids in response to CR. However, rather than assessing only a few lipids, we used a nontargeted lipidomics approach to cast a wider net. This strategy was successful, as lipidomics analysis identified 477 lipid species. Many of these lipids were responsive to the diet-treatments, as ANOVA revealed significant (p < .05) main effects of Diet on ~24% (n = 114) of these lipids (Supplementary Table 2). Figure 3 is a heatmap that represents a two-dimensional hierarchical clustering of data for the 50 lipids with the lowest p-values. Supplementary Table 2 identifies all 114 lipids with significant Diet main effects. Acylcarnitine is the lipid class with the most consistent CR-effect; the values for 16 acylcarnitine species (including 11 among the top 50) were greater for AL versus CR.
Figure 3.
Heatmap that depicts a two-dimensional hierarchical clustering of the data for the 50 lipid species in rat epitrochlearis muscle with the lowest p-values for main effects of Diet (n=6/group). Green denotes calorie restriction values lower than ad libitum, and red denotes calorie restriction values greater than ad libitum.
We performed correlation analysis of the 114 lipids with a significant Diet main effect to probe for potential relationships between the responsive lipids and ISGU. This analysis revealed that 18 were significantly (p < .05) correlated to ISGU (Table 1). The correlations were negative for 10 and positive for eight of these lipids. To gain further insights into possible mechanisms, we also probed associations between the lipids and the key signaling measurements. Five lipids were also significantly correlated with the insulin-stimulated pAktS473, pAktT308, pAS160S588, pAS160T642, and pAS160S704: negative correlations for diacylglycerol 36:5, plasmenyl-phosphatidylethanolamine 37:5, lysophosphatidylethanolamine 16:0 and phosphatidylglycerol 44:12; positive correlations for acylcarnitine 24:0. Three of these five lipids were also significantly correlated with pAMPKT172: plasmenyl-PE 37:5 and lysophosphatidylethantolamine 16:0 (negative correlations) and acylcarnitine 24:0 (positive correlation).
Table 1.
Correlations between Lipid Species and Insulin-Stimulated Glucose Uptake (ISGU) or Phosphorylated Signaling Proteins
| Lipid Species | Main Effect(s) | ISGU | pAktT308 | pAktS473 | pAMPKT172 | pAS160S588 | pAS160T642 | pAS160S704 |
|---|---|---|---|---|---|---|---|---|
| Acylcarnitine 18:0 | Exercise | r = .437* | NS | NS | NS | NS | NS | r = .413* |
| Acylcarnitine 20:1 | Exercise | r = .683**** | NS | NS | r = .436* | r = .480* | NS | r = .591** |
| Acylcarnitine 24:0 | Diet | r = .611*** | r = .540** | r = .533** | r = .583*** | r = .417* | r = .481* | r = .535** |
| Diacylglycerol 36:1 | Diet | r = .557*** | NS | NS | r = .413 | r = .494* | r = .433* | NS |
| Diacylglycerol 36:5 | Diet | r = −.408* | r = −.790**** | r = −.779**** | NS | r = −.546** | r = −.472* | r = −.562*** |
| Diacylglycerol 37:2 | Diet | r = .420* | r = .561*** | NS | NS | r = .459* | r = .431* | NS |
| Diacylglycerol 38:3 | Diet | r = .557*** | r = .489* | NS | r = .513* | r = .465* | r = .491* | NS |
| Diacylglycerol 38:4 | Diet | r = .457* | r = .555** | r = .428* | NS | r = .469* | r = .498* | NS |
| Diacylglycerol 40:4 | Diet | r = .548** | r = .513* | NS | NS | r = .550** | r = .524** | r = .470* |
| Plasmenyl-PC 36:5 | Diet | r = −.406* | r = −.621*** | r = −.600*** | NS | NS | NS | r = −.451* |
| Plasmenyl-PC 40:4 | Diet | r = .505* | r = .563*** | r = .566*** | NS | NS | r = .428* | NS |
| Plasmenyl-PE 37:5 | Diet | r = −.603*** | r = −.677**** | r = −.587*** | r = −.455* | r = −.587*** | r = −.547** | r = −.646**** |
| Lyso-PC 17:0 | Exercise | r = −.452* | NS | NS | r = −.424* | NS | NS | r = −.490* |
| Lyso-PC 18:0 | Diet and Exercise | r = −.434* | NS | NS | NS | NS | NS | NS |
| Unknown Lyso-PC 18:0 | Exercise | r = −.472* | NS | NS | NS | NS | NS | NS |
| Lyso-PC 19:0 | Exercise | r = −.662**** | NS | NS | r = −.404* | r = −.597*** | r = −.619*** | r = −.574*** |
| Lyso-PC 20:0 | Exercise | r = −.530** | NS | NS | NS | NS | NS | r = −.463* |
| Lyso-PE 16:0 | Diet and Exercise | r = −.447* | r = −.447* | r = −.550** | r = −.410* | r = −.523** | r = −.530** | r = −.602*** |
| PG 44:12 | Diet | r = −.492* | r = −.845**** | r = −.796**** | NS | r = −.651**** | r = −.516* | r = −.501* |
| PI 38:4 | Diet | r = −.497* | r = .452* | r = .419* | NS | r = .483* | r = .530** | NS |
| SM 42:3 | Exercise | r = −.509* | NS | NS | NS | NS | NS | r = −.427* |
| TG 40:0 | Exercise | r = −.422* | NS | NS | NS | NS | NS | NS |
Notes: The table summarizes detected lipid species with a significant main effect of Diet and/or Exercise that were significantly correlated with insulin-stimulated glucose uptake (ISGU). Correlation coefficients (r) are provided for each lipid versus ISGU and each of the phosphorylated proteins. Abbreviations are: AMP-activated protein kinase (AMPK); Akt substrate of 160 kDa (AS160); not significant (NS); Phosphatidylcholine (PC); Phosphatidylethanolamine (PE); Phosphatidylglycerol (PG); Phosphatidylinositol (PI); Sphingomyelin (SM); Triacylglycerol (TG).
p-values for Spearman Rank Correlations are indicated by: *<.05; **<.01; ***<.005; ****<.001.
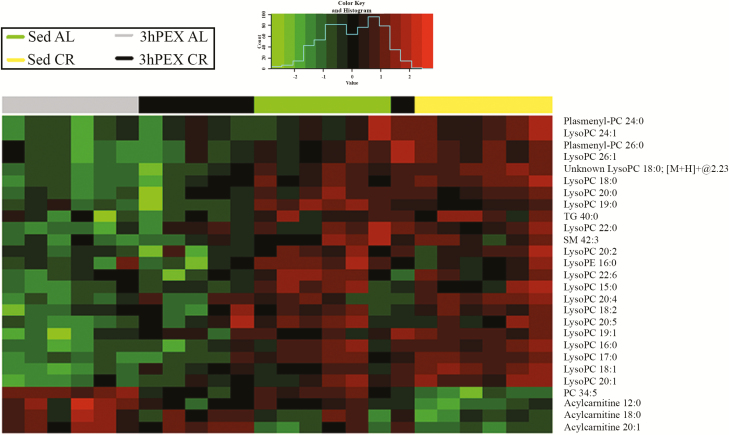
The exercise protocol was relatively brief (1.5 hours of exercise) compared to the 2-month-long CR protocol, but it represented a considerable metabolic challenge, as indicated by changes in glycogen and pAMPKT172. It seemed possible that it would also alter some muscle lipids. The results confirmed this possibility, but ANOVA revealed significant Exercise main effects (p < .05) on ~6% (n = 27) of the identified lipids (Supplementary Table 2). Figure 4 is a heatmap with two-dimensional hierarchical clustering of data for the 27 lipids with a significant main effect of Exercise. There were striking differences in the specific lipids that were responsive to CR and exercise. Lysophosphatidylcholine was the lipid class with the greatest representation for Exercise main effects (3hPEX less than SED; n = 18 of 27). Among the 27 lipids with significant Exercise main effects, correlation analysis revealed that 11 lipids were significantly correlated with ISGU (Table 1). Correlations were negative for nine of these lipids, and only lysophosphatidylethanolamine 16:0 was also significantly (p < .05) correlated with insulin-stimulated pAktS473, pAktT308, pAMPKT172, pAS160S588, pAS160T642, and pAS160S704.
Figure 4.
Heatmap that depicts a two-dimensional hierarchical clustering of the data for the 27 lipid species in rat epitrochlearis muscle with a significant main effect of Exercise (n=6/group). Green denotes exercise values lower than sedentary, and red denotes exercise values greater than sedentary.
Only a few lipids were responsive to both treatments. Significant main effects of both Diet and Exercise were found for six lipids (Supplementary Table 2: acylcarnitine 12:0, lysophosphatidylcholine 18:0, lysophosphatidylcholine 18:1, lysophosphatidylcholine 19:1, lysophosphatidylcholine 20:4, and phosphatidylcholine 34:5). Interestingly, CR- versus exercise-effects were in opposite directions for each of these lipids. Only lysophosphatidylcholine 18:0 was significantly correlated with ISGU (r = −.434). This lipid was not significantly correlated with any of the signaling proteins.
The magnitude of the combined effects of CR and exercise on ISGU was impressive, but the combined effects on lipids were quite limited. Post hoc analysis revealed combined CR and exercise resulted in significant differences for only five lipids versus both CR alone and exercise alone. Combined-treatment values were less than exercise alone and greater than CR alone for acylcarnitine 12:0, phosphatidylcholine 34:5 and phosphatidylcholine 40:9. Combined-treatment values for diacylglycerol 38:2 exceeded exercise or CR alone. Combined-treatment values for lysophosphatidylcholine 18:1 exceeded exercise alone and were less than CR alone. Combined-treatment values for plasmenyl-phosphatidylethanolamine 37:5 were less than exercise or CR alone. Only plasmenyl-phosphatidylethanolamine 37:5 significantly correlated with ISGU (r = −.603, p < .001). Plasmenyl-phosphatidylethanolamines have important structural functions in membranes, with higher concentrations favoring reduced membrane fluidity, and they may influence membrane trafficking and have antioxidant properties (26). The current results identify plasmenyl-phosphatidylethanolamine 37:5 as an intriguing candidate for future research to test if this lipid regulates ISGU.
Earlier research suggested that insulin sensitivity might be related to the sum of the phosphatidylcholine species, the sum of phosphatidylethanolamine species, or the ratio of these sums. We calculated these values and found no significant main effects or interactions for total phosphatidylcholines or total phosphatidylethanolamines (calculated by summing values for all species for each lipid class). There were also no significant main effects for PC:PE ratio, but there was a significant Diet × Exercise interaction (p < .001). Post hoc analysis revealed 3hPEX-CR was significantly lower (p < .05) than both SED-CR and 3hPEX-AL. PC:PE ratio was not significantly correlated with ISGU. These results did not support the idea that these lipids are crucial for changes in ISGU that we found with CR and/or exercise.
Discussion
The current study was the first to examine the independent and combined effects of CR initiated late-in-life and acute exercise on ISGU in skeletal muscles from old rats. Consistent with previous findings in rats that underwent 27-month CR and/or acute exercise (9), 8-week CR and acute exercise independently elevated ISGU in the current study. Furthermore, combining CR and exercise increased ISGU above values with either treatment independently.
Focusing on CR alone, greater ISGU in SED-CR versus SED-AL muscles was accompanied by enhanced insulin-stimulated pAkt. A similar relationship between ISGU and pAkt was previously observed in muscles from 24- to 30-month-old rats (9,24,27). Inhibition of the CR-increase in pAkt with a selective Akt inhibitor eliminated the CR-increase in ISGU (24,25), providing compelling evidence that greater pAkt is essential for CR-improvement in ISGU.
The mechanisms responsible for CR-induced enhancement of pAkt are uncertain. Insulin receptor tyrosine kinase phosphorylates insulin receptor substrate (IRS) proteins, and IRS1 is the predominant isoform in skeletal muscle (13). Tyrosine-phosphorylated IRS proteins engage phosphatidylinositol-3-kinase (PI3K), which is essential for insulin-stimulation of Akt and GU. Site-selective serine or threonine phosphorylation of IRS proteins has been linked to insulin resistance for: IRS tyrosine-phosphorylation, IRS-PI3K activity, pAkt, and ISGU. Greater IRS1-PI3K activity, pAkt, and insulin sensitivity were found in insulin-stimulated muscles from CR versus AL monkeys (28). However, insulin-stimulated muscles from CR versus AL rats had greater pAkt and ISGU without lower pIRS1S312 (29) or greater IRS1- or IRS2-PI3K activity (30). Furthermore, CR led to increased ISGU in muscles from IRS1-knockout mice (31). CR-effects on IRS proteins may contribute to enhanced pAkt and ISGU under some conditions, but other mechanisms are also likely important for these CR-induced outcomes.
CR alone induced greater pAS160 on Ser588 and Thr642 in insulin-stimulated muscles, similar to earlier results indicating that 8-weeks CR caused greater pAS160T642 and a trend for greater pAS160S588 in 24.5-month-old rats (24). Sharma and colleagues (9) reported that in insulin-stimulated muscles from 30-month-old rats, CR initiated at 3.5 months old caused increased pAS160S588 and pAS160T642. These data implicate greater pAS160 as part of the mechanism for CR effects on ISGU in muscle from older rats.
AS160S704 (equivalent to mouse Ser711) is located in a consensus AMPK-phosphomotif (32). However, pAMPKT172 was not increased by CR alone in insulin-stimulated muscles, suggesting more subtle aspects of AMPK regulation (eg, activity of specific AMPK heterotrimers) may cause greater pAS160S704 in muscles from CR rats. Ser704 phosphorylation is not essential for ISGU (32). Nonetheless, Kjøbsted and colleagues (11). found that preventing phosphorylation on Ser704 by mutating it to Ala704 attenuated Thr642 phosphorylation in muscle, suggesting pAS160S704 favors greater pAS160T642. The CR-increase in pAS160S704 may have played a role in CR’s enhancement of pAS160T642.
CR had major and complex effects on muscle lipids, with significant diet effects on ~24% of the 477 lipid species that were identified, including multiple classes of lipids. Interestingly, none of the 54 detected triacylglycerol species were altered by CR, consistent with earlier research indicating CR can increase ISGU without altered muscle triacylglycerol concentration (22).
Because higher ceramide levels have been associated with lower pAkt and ISGU (12), we anticipated that ceramides might be lowered by CR. Instead, none of the 27 ceramide species were reduced with CR, and none significantly correlated with ISGU. Similarly, Obanda and colleagues (33) reported 6 months of CR initiated at 12-months-old in rats increased levels of two of the six muscle ceramides they detected concomitant with increased whole-body insulin sensitivity and pAkt. Obese humans undergoing 16 weeks of CR attained improved whole-body insulin sensitivity with unaltered muscle ceramides (34). Reduced ceramide levels do not explain CR-effects on pAkt or insulin sensitivity.
Diacylglycerols have also been reported to be associated with insulin resistance (12). Unexpectedly, CR values exceeded AL values for six diacylglycerols, and ISGU was positively correlated with five diacylglycerols. ISGU was negatively correlated only with diacylglycerol 36:5, which was also negatively correlated with both pAkt and pAS160 on each of their sites. CR for 16 weeks by obese humans improved insulin sensitivity without altering muscle diacylglycerols (34). The available results argue against CR-reductions in diacylglycerols being required for improved insulin sensitivity.
Acylcarnitines were the lipid class most consistently altered by diet, with 15 of the 25 detected acylcarnitines significantly lower for CR versus AL treatment. The results suggest oxidation rate of these fatty acids by mitochondria outpaced their rate of appearance in muscles from CR versus AL rats. Acylcarnitine 24:0, which had greater levels in muscles from CR versus AL rats, was positively correlated with ISGU. Acylcarnitine 24:0 was also positively correlated to phosphorylation of Akt, AMPK, and AS160 on each of the measured sites.
There was a statistically nonsignificant trend for lower (27%) glycogen in SED-CR versus SED-AL animals. This result was comparable to the modest CR-related 24% reduction in epitrochlearis glycogen in 23-month-old rats with CR initiated at 14-weeks-old (7). Several studies have reported that CR can increase muscle GU even when there is no decrease in muscle glycogen (22,23). Because decrements in muscle glycogen have been absent or modest in studies that have demonstrated CR-induced increases in GU, reduced muscle glycogen seems unlikely to be the primary determinant of CR effects on insulin sensitivity.
We studied a single exercise session rather than chronic, regularly performed exercise because acute exercise can induce a substantial increase in ISGU. The magnitude of the increase in ISGU after acute exercise can exceed ~50%–70% of the increase obtained after chronic exercise training (35,36). Because chronic exercise is the regular performance of acute exercise, fully understanding chronic exercise requires a comprehension of the effects of acute exercise. In the current study, ISGU was increased by ~90% at 3hPEX, similar to the results of earlier studies in 24- to 30-month-old rats (6,8,9).
Previous research strongly suggests that enhanced ISGU after acute exercise is attributable to mechanisms localized in the contracting muscle. In humans, prior one-legged exercise results in greater ISGU in the exercised leg, but not the unexercised leg (37,38). Electrically stimulated muscle contraction by isolated rat muscles induced greater ISGU versus contralateral, unstimulated muscles (39).
The greater ISGU after exercise alone was accompanied by greater pAktS473 and a non-significant trend for elevated pAktT308. Earlier studies found that prior exercise by older rats led to greater pAktS473 and pAktT308 in insulin-stimulated epitrochlearis (8,9). In the current study, prior exercise caused increased pAS160S588, but not pAS160T642 in insulin-stimulated muscle. Previous research evaluating insulin-stimulated epitrochlearis found increased pAS160T642 and a trend for greater pAS160S588 in 24-month-old rats (8). Although pAS160S704 is believed to facilitate greater pAS160T642, the modest increase in pAS160S704 in the 3hPEX-AL group was not accompanied by a significant increase in pAS160T642 (11). These results suggest greater site-selective pAkt and pAS160 may play a role in the exercise-effect on ISGU in muscles from older rats.
The 58% reduction in muscle glycogen determined 3hPEX is similar to the ~48% decrease in muscles from 25-month-old rats immediately post-exercise (6). Results from earlier research in younger animals suggested that exercise-induced glycogen depletion may play a role in the enhanced insulin sensitivity by an uncertain mechanism (40).
Exercise and CR were characterized by very different lipidomic signatures. Exercise significantly altered ~6% (n = 27) of the 477 detected lipid species, versus ~24% (n = 114) for CR. Only six lipids were significantly altered by both exercise and CR, and each of these lipids was changed in opposite directions by the two conditions. What might account for the strikingly different effects of CR and acute exercise on muscle lipids? Each treatment resulted in an energy deficit achieved by fundamentally different processes. The CR-induced energy deficit was the direct result of lower dietary energy intake. CR-effects on muscle were an indirect consequence of this deficit. CR-effects on circulating factors (eg, plasma glucose, insulin, other hormones) may have influenced muscle lipids. It would be valuable for future research to use untargeted lipidomics to determine the plasma lipidome of older CR rats for comparison with the CR-effects on muscle lipids. In contrast to CR, the exercise-induced energy deficit was secondary to increased energy expenditure, and a direct consequence of contraction by the skeletal muscle. Muscle contraction involves marked shifts in subcellular calcium, tension development, increased ATP-turnover and the activation of enzymes that regulate the mobilization of energy stores. There are also major changes in muscle blood flow and delivery of extramuscular molecules (including glucose, hormones and lipids) to the muscle that likely modulate the direct effects of contraction on muscle lipids. Identifying the specific molecular processes responsible for CR and exercise effects on specific muscle lipids will be an interesting, but challenging endeavor.
In addition to the differences in the biological events that define CR and exercise, the protocols for CR and exercise differed dramatically in their duration: 8 weeks for CR compared to 3 hours for the exercise protocol (including 1.5 hours of exercise). Although apparently no study has used untargeted lipidomics to evaluate chronic exercise effects on muscle lipids in older rats, previous studies have documented exercise training effects on muscle lipids in young rodents. Levels of both phosphatidylcholines and phosphatidylethanolamines were greater in the extensor digitorum longus muscles of young mice after 5 weeks of wheel running exercise compared to sedentary controls (41). Levels of multiple phospholipid species (including phosphatidylcholines and phosphotidylethanolamines) changed, with some increasing and others decreasing, in white and red vastus lateralis muscles from young rats after 4 weeks of treadmill exercise training (42). Diacylglycerols and phosphatidylethanolamines, but not triacylglycerols or phosphatidylcholines, were greater in soleus muscles from young rats after 8 weeks of treadmill exercise training (up to 120 min/d) compared to sedentary controls (43). Apparently, no study has directly compared the effects of CR and chronic exercise on muscle lipids using an untargeted lipidomics approach in either young or older animals.
Neither diacylglycerols nor ceramides were significantly altered post-exercise, similar to earlier results after acute exercise by young rats (44). Furthermore, exercise alone significantly altered only 1 of 78 PC species detected, did not alter total PC levels, and did not alter total PE levels or the levels of any of its 32 detected species. Earlier research reported that acute exercise differentially influenced muscle PC and PE in muscle from type 2 diabetic humans (both classes increased), obese humans (both classes unaltered), and endurance-trained humans (both classes decreased). The PC:PE ratio was not different for the 3hPEX-AL versus the SED-AL group. Similarly, the muscle PC:PE ratio was unaltered 2 hours after acute exercise by type 2 diabetic, obese, and endurance-trained humans (45). The results of the current study do not implicate reduced diacylglycerols, ceramides or PC:PE ratio for the exercise-effect on ISGU.
Lysophosphatidylcholines have structural and signaling roles, and they primarily originate from membrane phosphatidylcholine hydrolysis via phospholipase A2 (46). More than 80% of the detected lysophosphatidylcholines (18 of 22) were lower post-exercise, including 6 that were negatively correlated with ISGU. Han and colleagues (47). reported that lysophosphatidylcholine levels were increased in muscle of insulin resistant db/db mice and provided evidence that lysophosphatidylcholine was a mediator of fatty acid-induced insulin resistance in L6 myotubes. The striking effect of exercise on muscle lysophosphatidylcholines appears to be a novel observation. In contrast to exercise, only 5 of 22 lysophosphatidylcholines responded to CR. To summarize, we discovered an inverse relationship between exercise and muscle lysophosphatidylcholines, a lipid species previously linked to insulin resistance in cultured myocytes.
Exercise and CR had strikingly different effects on acylcarnitines. Three of 25 acylcarnitines were significantly increased by exercise compared to the 15 acylcarnitines that were reduced with CR. Acylcarnitine 20:1, which was increased with exercise and unaltered by CR, was significantly, positively correlated with ISGU, pAMPKT172, pAS160S588, and pAS160T704, but not with pAktT308, pAktS473, or pAS160T642.
Focusing on the combined effects of CR and exercise, we observed that the ISGU in the combined treatment group exceeded CR alone by 88% and exercise alone by 37%. These results compare to earlier findings in 30-month-old rats in which CR initiated at ~3.5-months-old combined with exercise also caused greater ISGU than CR alone (53%) or exercise alone (77%) (9). In insulin-stimulated muscles, combined CR and exercise versus either CR alone or exercise alone was accompanied by greater phosphorylation of Akt and AS160 on all sites that were evaluated. The significant and positive correlations between ISGU and pAkt on Thr308 and Ser473 as well as pAS160 on Ser588, Thr642, and Ser704 provide evidence that these signaling processes play a role in enhanced insulin sensitivity with combined CR and exercise.
Combined CR and exercise resulted in significantly altered levels for only five lipids compared to both CR alone and exercise alone. It was striking that of these five lipids, only plasmenyl-phosphatidylethanolamine 37:5 was significantly correlated with ISGU. In addition to being inversely correlated with ISGU, this lipid was inversely correlated with the phosphorylation of Akt, AMPK, and AS160 on each of their key regulatory sites. These results are apparently the first evidence that plasmenyl-phosphatidylethanolamine 37:5 might be linked to insulin sensitivity. Future research that specifically alters the muscle levels of this molecule will be required to test if this lipid regulates ISGU.
A limitation of the study is that it did not include young control groups. However, we previously determined the independent effects of CR (7) and acute exercise (6) on ISGU by isolated epitrochlearis from both young adult (8–13 months old) and older (23–25 months old) rats. The relative increases in ISGU were not lower in older rats with either intervention. However, these earlier studies did not include combined CR and exercise groups.
Another limitation of the study is that it assessed only a single muscle that is comprised mainly of type II fibers. However, the fiber type profile of the rat epitrochlearis is similar to the average fiber type composition of a large portion of the rat’s musculature based on a study that reported the fiber type profile of 76 different rat skeletal muscles (48). We recently determined the effects of CR on ISGU and fiber type in single fibers from the epitrochlearis muscle of 23- to 26-month-old rats and found that CR led to increased ISGU in each fiber type (type I, IIA, IIB, IIBX, and IIX fibers) (49). We have not evaluated the effects of exercise alone or combined CR and exercise on ISGU in single fibers from old rats, but in single fibers from young (~2 months old), exercise alone increased ISGU in all fiber types except type IIX (50).
In conclusion, the additive effect of CR initiated late-in-life combined with exercise on ISGU in muscles from old rats was accompanied by greater Akt phosphorylation on key regulatory sites (Thr308 and Ser473), greater AS160 phosphorylation on Akt-dependent sites (Ser588 and Thr642), and greater AS160 phosphorylation on an AMPK-dependent site (Ser704). The CR-effects on numerous lipids contrasted with the much smaller number of lipids responsive to exercise, and there was very little overlap between the lipidomic fingerprints of CR versus acute exercise. It was also striking that the limited subset of lipids that were significantly correlated with ISGU did not include ceramides or diacylglycerides that are frequently cited as potential regulators of insulin sensitivity. Finally, ISGU was the primary focus of this experiment, but CR and exercise impact countless other biological processes. The discovery of more than 100 lipid species that were responsive to CR and/or exercise in the skeletal muscle from old rats provides valuable information with relevance to understanding the extensive functional consequences of these two interventions.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the National Institutes of Health (R01AG010026 and T32000114).
Supplementary Material
Acknowledgments
The authors thank the staff from the University of Michigan Regional Comprehensive Metabolomics Resource Core for the lipidomics analysis. The authors also thank Mark Pataky for technical assistance with the exercise protocol and muscle incubations.
Conflict of Interest
None declared.
References
- 1. Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990;113:909–915. doi:10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- 2. Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86:3574–3578. doi: 10.1210/jcem.86.8.7763 [DOI] [PubMed] [Google Scholar]
- 3. Haffner SM. Epidemiology of insulin resistance and its relation to coronary artery disease. Am J Cardiol. 1999;84:11J–14J. doi:10.1016/S0002-9149(99)00351-3. [DOI] [PubMed] [Google Scholar]
- 4. Kumari M, Brunner E, Fuhrer R. Minireview: mechanisms by which the metabolic syndrome and diabetes impair memory. J Gerontol A Biol Sci Med Sci. 2000;55:B228–B232. doi:10.1093/gerona/55.5.B228. [DOI] [PubMed] [Google Scholar]
- 5. DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi:10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 6. Cartee GD, Briggs-Tung C, Kietzke EW. Persistent effects of exercise on skeletal muscle glucose transport across the life-span of rats. J Appl Physiol (1985). 1993;75:972–978. doi: 10.1152/jappl.1993.75.2.972 [DOI] [PubMed] [Google Scholar]
- 7. Cartee GD, Kietzke EW, Briggs-Tung C. Adaptation of muscle glucose transport with caloric restriction in adult, middle-aged, and old rats. Am J Physiol. 1994;266:R1443–R1447. doi: 10.1152/ajpregu.1994.266.5.R1443 [DOI] [PubMed] [Google Scholar]
- 8. Xiao Y, Sharma N, Arias EB, Castorena CM, Cartee GD. A persistent increase in insulin-stimulated glucose uptake by both fast-twitch and slow-twitch skeletal muscles after a single exercise session by old rats. Age (Dordr). 2013;35:573–582. doi: 10.1007/s11357-012-9383-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma N, Wang H, Arias EB, Castorena CM, Cartee GD. Mechanisms for independent and combined effects of calorie restriction and acute exercise on insulin-stimulated glucose uptake by skeletal muscle of old rats. Am J Physiol Endocrinol Metab. 2015;308:E603–E612. doi: 10.1152/ajpendo.00618.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cartee GD. Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle. Diabetologia. 2015;58:19–30. doi: 10.1007/s00125-014-3395-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kjøbsted R, Treebak JT, Fentz J, et al. Prior AICAR stimulation increases insulin sensitivity in mouse skeletal muscle in an AMPK-dependent manner. Diabetes. 2015;64:2042–2055. doi: 10.2337/db14-1402 [DOI] [PubMed] [Google Scholar]
- 12. Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cartee GD. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am J Physiol Endocrinol Metab. 2015;309:E949–E959. doi: 10.1152/ajpendo.00416.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol (1985). 1994;76:979–985. doi: 10.1152/jappl.1994.76.2.979 [DOI] [PubMed] [Google Scholar]
- 15. Passonneau JV, Lauderdale VR. A comparison of three methods of glycogen measurement in tissues. Anal Biochem. 1974;60:405–412. doi:10.1016/0003-2697(74)90248-6. [DOI] [PubMed] [Google Scholar]
- 16. Gika HG, Macpherson E, Theodoridis GA, Wilson ID. Evaluation of the repeatability of ultra-performance liquid chromatography-TOF-MS for global metabolic profiling of human urine samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871:299–305. doi: 10.1016/j.jchromb.2008.05.048 [DOI] [PubMed] [Google Scholar]
- 17. Afshinnia F, Rajendiran TM, Karnovsky A, et al. Lipidomic signature of progression of chronic kidney disease in the chronic renal insufficiency cohort. Kidney Int Rep. 2016;1:256–268. doi: 10.1016/j.ekir.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Core Team R. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 19. Hastie T, Tibshirani R, Narasimhan B, Chu G.. pamr: Pam: prediction analysis for microarrays. R package version. 2014;1.55. http://CRAN.R-project.org/package=pamr [Google Scholar]
- 20. Warnes GR, Bolker B, Bonebakker L, et al. gplots: various R programming tools for plotting data. R package version. 2015;2.17.0. http://CRAN.R-project.org/package=gplots [Google Scholar]
- 21. Dean DJ, Brozinick JT Jr, Cushman SW, Cartee GD. Calorie restriction increases cell surface GLUT-4 in insulin-stimulated skeletal muscle. Am J Physiol. 1998;275:E957–E964. [DOI] [PubMed] [Google Scholar]
- 22. Gazdag AC, Wetter TJ, Davidson RT, et al. Lower calorie intake enhances muscle insulin action and reduces hexosamine levels. Am J Physiol Regul Integr Comp Physiol. 2000;278:R504–R512. doi: 10.1152/ajpregu.2000.278.2.R504 [DOI] [PubMed] [Google Scholar]
- 23. Wetter TJ, Gazdag AC, Dean DJ, Cartee GD. Effect of calorie restriction on in vivo glucose metabolism by individual tissues in rats. Am J Physiol. 1999;276:E728–E738. doi:10.1152/ajpendo.1999.276.4.E728. [DOI] [PubMed] [Google Scholar]
- 24. Wang H, Arias EB, Cartee GD. Calorie restriction leads to greater Akt2 activity and glucose uptake by insulin-stimulated skeletal muscle from old rats. Am J Physiol Regul Integr Comp Physiol. 2016;310:R449–R458. doi: 10.1152/ajpregu.00449.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharma N, Arias EB, Sequea DA, Cartee GD. Preventing the calorie restriction-induced increase in insulin-stimulated Akt2 phosphorylation eliminates calorie restriction’s effect on glucose uptake in skeletal muscle. Biochim Biophys Acta. 2012;1822:1735–1740. doi: 10.1016/j.bbadis.2012.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dean JM, Lodhi IJ. Structural and functional roles of ether lipids. Protein Cell. 2018;9:196–206. doi: 10.1007/s13238-017-0423-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sequea DA, Sharma N, Arias EB, Cartee GD. Calorie restriction enhances insulin-stimulated glucose uptake and Akt phosphorylation in both fast-twitch and slow-twitch skeletal muscle of 24-month-old rats. J Gerontol A Biol Sci Med Sci. 2012;67:1279–1285. doi: 10.1093/gerona/gls085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang ZQ, Floyd ZE, Qin J, et al. Modulation of skeletal muscle insulin signaling with chronic caloric restriction in cynomolgus monkeys. Diabetes. 2009;58:1488–1498. doi: 10.2337/db08-0977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharma N, Castorena CM, Cartee GD. Tissue-specific responses of IGF-1/insulin and mTOR signaling in calorie restricted rats. PLoS One. 2012;7:e38835. doi: 10.1371/journal.pone.0038835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davidson RT, Arias EB, Cartee GD. Calorie restriction increases muscle insulin action but not IRS-1-, IRS-2-, or phosphotyrosine-PI 3-kinase. Am J Physiol Endocrinol Metab. 2002;282:E270–E276. doi: 10.1152/ajpendo.00232.2001 [DOI] [PubMed] [Google Scholar]
- 31. Gazdag AC, Dumke CL, Kahn CR, Cartee GD. Calorie restriction increases insulin-stimulated glucose transport in skeletal muscle from IRS-1 knockout mice. Diabetes. 1999;48:1930–1936. doi:10.2337/diabetes.48.10.1930. [DOI] [PubMed] [Google Scholar]
- 32. Treebak JT, Taylor EB, Witczak CA, et al. Identification of a novel phosphorylation site on TBC1D4 regulated by AMP-activated protein kinase in skeletal muscle. Am J Physiol Cell Physiol. 2010;298:C377–C385. doi: 10.1152/ajpcell.00297.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Obanda DN, Yu Y, Wang ZQ, Cefalu WT. Modulation of sphingolipid metabolism with calorie restriction enhances insulin action in skeletal muscle. J Nutr Biochem. 2015;26:687–695. doi: 10.1016/j.jnutbio.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson ML, Distelmaier K, Lanza IR, et al. Mechanism by which caloric restriction improves insulin sensitivity in sedentary obese adults. Diabetes. 2016;65:74–84. doi: 10.2337/db15-0675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perseghin G, Price TB, Petersen KF, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804 [DOI] [PubMed] [Google Scholar]
- 36. Wojtaszewski JF, Richter EA. Effects of acute exercise and training on insulin action and sensitivity: focus on molecular mechanisms in muscle. Essays Biochem. 2006;42:31–46. doi: 10.1042/bse0420031 [DOI] [PubMed] [Google Scholar]
- 37. Wojtaszewski JF, Hansen BF, Gade, et al. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–331. doi:10.2337/diabetes.49.3.325. [DOI] [PubMed] [Google Scholar]
- 38. Wojtaszewski JF, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes. 1997;46:1775–1781. doi:10.2337/diab.46.11.1775. [DOI] [PubMed] [Google Scholar]
- 39. Funai K, Schweitzer GG, Castorena CM, Kanzaki M, Cartee GD. In vivo exercise followed by in vitro contraction additively elevates subsequent insulin-stimulated glucose transport by rat skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E999–1010. doi: 10.1152/ajpendo.00758.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Richter EA, Derave W, Wojtaszewski JF. Glucose, exercise and insulin: emerging concepts. J Physiol. 2001;535:313–322. doi:10.1111/j.1469-7793.2001.t01-2-00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Senoo N, Miyoshi N, Goto-Inoue N, et al. PGC-1α-mediated changes in phospholipid profiles of exercise-trained skeletal muscle. J Lipid Res. 2015;56:2286–2296. doi: 10.1194/jlr.M060533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mitchell TW, Turner N, Hulbert AJ, et al. Exercise alters the profile of phospholipid molecular species in rat skeletal muscle. J Appl Physiol (1985). 2004;97:1823–1829. doi: 10.1152/japplphysiol.00344.2004 [DOI] [PubMed] [Google Scholar]
- 43. Kawanishi N, Takagi K, Lee HC, et al. Endurance exercise training and high-fat diet differentially affect composition of diacylglycerol molecular species in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2018;314:R892–R901. doi: 10.1152/ajpregu.00371.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Castorena CM, Arias EB, Sharma N, Cartee GD. Postexercise improvement in insulin-stimulated glucose uptake occurs concomitant with greater AS160 phosphorylation in muscle from normal and insulin-resistant rats. Diabetes. 2014;63:2297–2308. doi: 10.2337/db13-1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Newsom SA, Brozinick JT, Kiseljak-Vassiliades K, et al. Skeletal muscle phosphatidylcholine and phosphatidylethanolamine are related to insulin sensitivity and respond to acute exercise in humans. J Appl Physiol (1985). 2016;120:1355–1363. doi: 10.1152/japplphysiol.00664.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Drzazga A, Sowińska A, Koziołkiewicz M. Lysophosphatidylcholine and lysophosphatidylinosiol–novel promissing signaling molecules and their possible therapeutic activity. Acta Pol Pharm. 2014;71:887–899. [PubMed] [Google Scholar]
- 47. Han MS, Lim YM, Quan W, et al. Lysophosphatidylcholine as an effector of fatty acid-induced insulin resistance. J Lipid Res. 2011;52:1234–1246. doi: 10.1194/jlr.M014787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol (1985). 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261 [DOI] [PubMed] [Google Scholar]
- 49. Wang H, Arias EB, Yu CS, Verkerke ARP, Cartee GD. Effects of calorie restriction and fiber type on glucose uptake and abundance of electron transport chain and oxidative phosphorylation proteins in single fibers from old rats. J Gerontol A Biol Sci Med Sci. 2017;72:1638–1646. doi: 10.1093/gerona/glx099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cartee GD, Arias EB, Yu CS, Pataky MW. Novel single skeletal muscle fiber analysis reveals a fiber type-selective effect of acute exercise on glucose uptake. Am J Physiol Endocrinol Metab. 2016;311:E818–E824. doi: 10.1152/ajpendo.00289.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.