Use of opioid analgesic medications for management of chronic nonmalignant pain has become increasingly common [16] yet there are presently no well-validated predictors of optimal opioid analgesic efficacy [17,50]. Elevated negative affect (NA; e.g., depression, anxiety) has been linked in clinical studies to possibly lower opioid analgesic responses as indicated by greater post-surgical opioid analgesic requirements [18,23,40]. The only two placebo-controlled studies of this issue found that elevated NA predicted smaller opioid analgesic responses in the context of laboratory-induced acute pain, in both chronic back pain patients [52] and healthy controls (with effects restricted to males only; 20]. Not only are additional well-controlled studies needed regarding links between NA and opioid analgesia, but effects of other previously unexamined psychosocial factors, such as function and pain-related cognitions, should be explored. Moreover, confidence in the validity of psychosocial factors as predictors of opioid analgesic responses would be enhanced if mechanisms underlying these effects were elucidated.
Results of our prior work suggest that endogenous opioid (EO) function may be one such mechanism. EO antinociceptive function can be assessed using opioid blockade methodology [7, 12,13,14,15], in which responses to standardized acute pain stimuli are compared between the intact state (placebo) and when opioid receptors are pharmacologically blocked. Opioid blockade effects thus provide a quantitative measure of endogenous opioid analgesic capacity. We found that elevated trait anger and expressive anger regulation (anger-out) are both associated with deficient EO function assessed via opioid blockade [7,8,12,13].
If individual differences in EO function serve as a mechanism linking psychosocial factors to reduced analgesic responses to opioid medications, then EO function should also be related to responses to opioid analgesics. Indirect evidence for this connection comes from both human studies [35] and non-human studies [41,49,51]. To our knowledge, only one program of research has directly tested these relationships in humans [9,10]. In this program, healthy controls and chronic low back pain patients underwent acute pain-induction under three (counterbalanced) conditions: 1) placebo, 2) intravenous naloxone, and 3) intravenous morphine. Comparison of placebo condition pain responses to those under naloxone and morphine provided quantitative indexes of EO function and morphine analgesic responses, respectively. We found that EO function was inversely correlated with morphine analgesia for outcomes reflecting both responses to laboratory pain tasks and low back pain intensity among subjects with chronic low back pain [9,10]. That is, greater EO function was related to less opioid analgesia and to both elevated acute and chronic pain responsiveness.
In the present study, we report additional analyses of an expanded dataset derived from the parent study [9]. We propose that EO function is a mechanism underlying associations between psychosocial factors (NA, perceived disability, pain catastrophizing) on the one hand, and morphine analgesic responses on the other. That is, we hypothesize that relationships between psychosocial factors and opioid analgesic efficacy are mediated by EO function. If hypotheses are confirmed, these mechanism-based predictive effects would increase confidence that psychosocial variables may have clinical utility in future precision pain medicine algorithms.
Method
Participants
Participants were 89 individuals with chronic low back pain who were recruited through on-line advertisements on the Vanderbilt University e-mail recruitment system, the Rush University Pain Clinic, advertisements in local print media, or posted flyers. The study was approved by the respective IRB committees at Vanderbilt University Medical Center and Rush University Medical Center, and all subjects signed written Consent Forms that were approved by the IRB committees. This sample size is nearly double that reported for low back pain participants in Bruehl et al. (n=45) [9], thereby providing the statistical power necessary for testing study hypotheses. Inclusion criteria were: 1) age between 18-55; 2) no self-reported history of cardiovascular disease, hypertension, liver, kidney disorders; diabetes, or seizure disorder; 3) no self-reported history of posttraumatic stress disorder, bipolar disorder, psychotic disorder, alcohol or drug dependence; 4) no use of anti-hypertensive medications; 5) no daily use of opioid analgesics; 6) chronic daily low back pain of at least 3 months duration, and 7) an average past month severity of at least 3 on a 0-10 verbal numeric pain intensity scale. Individuals with chronic pain related to malignancy, autoimmune disorders, or fibromyalgia were excluded. Potential subjects who were pregnant (determined by urine pregnancy screens) were excluded to avoid fetal exposure to naloxone and morphine. Seven subjects reported occasional use of as-needed opioid analgesics, but none in the preceding 3 days, with absence of recent use confirmed via urine opioid screen before each laboratory study session. Two subjects reported use of antidepressant medications. Subjects were compensated $375 for their time upon completion of the three study sessions. See Table 1 for sample characteristics.
Table 1. Sample Characteristics (N = 89).
| Characteristic | |
|---|---|
| Gender (% female) | 62.9% |
| Race: | |
| Caucasian | 60.7% |
| African-American | 32.6% |
| Ethnicity: | |
| Non-Hispanic | 95.5% |
| Age (years) | 36.9 ± 10.7 |
| Past Month VAS Chronic Pain Intensity (0-100) | 55.1 ± 22.8 |
| Mean Pain Duration (months) | 116.5 (104.3) |
Design
The study used a three session, double-blind, crossover design with administration of an opioid antagonist (naloxone), an opioid analgesic (morphine), or saline placebo in each session. Order of drug administration was randomized and counterbalanced. Identical data collection procedures and equipment were employed in a closely coordinated fashion at two sites (Vanderbilt University Medical Center and Rush University Medical Center).
Study Drugs
Blockade of opioid receptors was achieved by administration of naloxone, an opioid antagonist with a brief half-life (1.1 hours) [31]. As in our past work [8], an 8 mg dose in 20 ml normal saline was infused intravenously over a 10-minute period through an intravenous cannula placed in the non-dominant arm. At this dosage, naloxone provides effective blockade of all three major opioid receptor subtypes [30].
The opioid analgesic medication examined in this study was morphine sulfate, the prototypic mu opioid receptor agonist. As in similar laboratory pain studies with morphine [21], the current study employed a dosage of 0.08 mg/kg (in 20ml normal saline), which was infused in the same manner as naloxone. This dosage (approximately 6mg for a 165 lb individual) was selected because it was judged to be sufficient to produce analgesia, but low enough to avoid ceiling effects that might obscure key individual differences in morphine responding. Peak naloxone and morphine activity are both achieved within approximately 15 min [4]. Normal saline (20mL) was infused in an identical manner during the placebo condition.
Chronic Pain Measures
Chronic pain intensity was assessed pre-drug and again post-drug during each laboratory session using the McGill Pain Questionnaire-Short Form (MPQ) [34]. Instructions were modified to refer specifically to the low back pain being experienced “at this moment.” The MPQ is a well-validated measure that allows separate assessment of the sensory (MPQ-S) and affective (MPQ-A) qualities of pain [34]. The MPQ also includes a Present Pain Intensity (PPI) numeric scale of overall pain intensity. Ratings are made on a 0 (no pain) to 5 (excruciating) scale.
Measures of State Negative Affect
At the baseline pre-drug assessments for each session, subjects rated the extent to which they felt nervous, sad, irritated, hopeless, on edge, annoyed, uneasy, angry and discouraged. Responses were made on 10-point scales with anchors at 0 (not at all), 2 (somewhat), 4 (much), 6 (very much), and 9 (extremely). These items were summed to create a composite State Negative Affect scale. This scale was used in analyses to control for inter-session variability in negative affect.
Measures of Trait Negative and Positive Affect, Catastrophizing, and Perceived Disability
The Trait Anger Scale (TAS) [46] was used to tap the tendency to experience anger. Scores on the TAS reflect primarily the frequency and magnitude of angry episodes. High scorers describe themselves as “hot-headed”, quick-tempered, and dealing aggressively with frustration. The trait form of the State-Trait Anxiety Inventory (TAI) [45] was used to measure the tendency to experience anxiety symptoms. The Beck Depression Inventory (BDI) [3] was used to assess current depressive symptoms. It is a commonly used self-report measure which has well-established psychometric properties. Taken together, these scales tapped general negative affect (NA).
The Positive Affect (PA) scale from the Positive and Negative Affect Schedule (PANAS) [53] was used to tap positive affect. The PANAS is a reliable and valid instrument that contains a brief PA scale with 10 items. These descriptors are active, alert, attentive, determined, enthusiastic, excited, inspired, interested, proud, and strong. The PA scale has shown good psychometric characteristics [53]. We elected not to analyze the Negative Affect (NA) subscale because we analyzed three other scales that assessed NA-related constructs. Thus, we believed we had NA comprehensively assessed.
The Pain Catastrophizing Scale (PCS) [47] is a 13-item measure which asks respondents to rate, using a 5-point numeric scale ranging from 0 (not at all) to 4 (all the time), the degree to which they have negative thoughts and feelings when experiencing pain. The PCS has exhibited strong internal consistency (α=.93), concurrent and discriminant validity, and high test-retest reliability over a 6 week period (r =0.78) [38].
The Pain Disability Index (PDI) is a self-report instrument that has been used to assess the degree to which chronic pain interferes with various daily activities. For each aspect of daily life (family/home responsibilities, recreation, social activities, occupational activity, etc.), subjects rated their level of disability on a scale ranging from 0 (no disability) to 10 (total disability). A total score was computed by summing the various ratings. Psychometric qualities of the PDI have been reported to be good [48].
Procedure
All procedures were conducted at the Vanderbilt General Clinical Research Center or a dedicated research room at the Rush University Pain Center. All procedures were approved by the Institutional Review Boards at the respective institutions. After providing informed consent, subjects completed a packet of questionnaires, including information regarding demographics and chronic pain. Participants then provided urine samples to screen for pregnancy (females only) and recent opioid use. They then participated in three identical experimental sessions during which they received one of three study drugs -- placebo, naloxone, morphine – with the sessions scheduled approximately one week apart and at the same time of day to control for circadian rhythms.
Subjects remained seated upright in a comfortable chair throughout all laboratory procedures. During each session, subjects initially completed a 10-min seated rest period, after which an indwelling venous cannula was inserted into the dominant arm by a trained research nurse under physician supervision. After a 30-min rest period, subjects completed the MPQ to describe their current low back pain intensity and provided the state negative affect ratings described above. Subjects then received (via the cannula) saline placebo, naloxone, or morphine per the randomization protocol, with order of drug administration across the three sessions randomly determined and counterbalanced. The investigational pharmacy at each institution prepared and provided the study drugs in blinded fashion to the study nurses. After a 15-min rest period to allow peak drug activity to be achieved, subjects again described their current level of low back pain using the MPQ. Laboratory evoked pain tasks were conducted following these back pain ratings (procedures and results detailed fully in Bruehl et al. [9]). All subjects remained in the lab under observation for 2 hours after peak drug activity had been achieved to allow drug effects to remit, after which they were released to a responsible adult. We did not encounter adverse effects from any of the substances that required us to stop the procedures.
Statistical Analysis
All analyses were conducted using IBM SPSS for Windows Version 21 (SPSS Inc., Chicago, IL).
Preliminary Procedures
In preparation for conducting analyses, pre- to post-drug changes in back pain intensity within each drug condition were derived. That is, all the variables (described below) that reflect opioid blockade and morphine analgesia effects were computed using pre- to post-drug change scores for the respective pain intensity measures. Next, we computed variables that took into account the possibilities that: 1) there could be floor and/or ceiling effects of baseline pre-drug chronic pain level in each condition which could affect how much subjects' pain could change in response to the study drugs; 2) differences across sessions in subjects' state negative affect levels might confound results; and 3) variability in the common placebo condition values used to calculate both naloxone effects and morphine effects (see below) could bias derived indexes of EO function and morphine analgesia. To address these concerns in the most comprehensive manner possible, we regressed the pre- to post-drug MPQ-S, MPQ-A, and PPI change scores within each drug condition on baseline pre-drug levels of chronic pain and on the state Negative Affect scales. This procedure produced residualized variables for each drug condition reflecting pre to post-drug changes in pain indexes controlling simultaneously for baseline pain (i.e., potential floor or ceiling effects) and negative affect. We then generated correlations among these residualized variables and the trait measures (BDI, TAI, etc.) separately for each drug condition. These appear in Table 2. In 17 of the 18 bivariate relationships, baseline to post-drug changes in chronic pain indexes during placebo were not significantly related to the trait measures. These findings suggest that the trait measures were minimally related to pain changes with administration of placebo (i.e., placebo effects).
Table 2. Correlations Among Trait Psychosocial Variables and Residualized McGill Pain Questionnaire Scores for each Drug Condition (Placebo, Naltrexone, and Morphine).
| Trait Psychosocial Variables | ||||||
|---|---|---|---|---|---|---|
| BDI | TAI | TAS | PCS | PDI | PA | |
| Placebo | ||||||
| MPQ-Sensory | .15 | .03 | .09 | .05 | -.12 | -.03 |
| MPQ-Affective | -.17 | -.09 | .01 | -.30 | -.15 | .08 |
| MPQ-Present Pain | .01 | -.06 | .18 | -.04 | -.15 | .03 |
| Naltrexone | ||||||
| MPQ-Sensory | -.33 | -.24 | -.04 | -.42 | -.57 | .17 |
| MPQ-Affective | -.20 | -.10 | .09 | -.32 | -.24 | .06 |
| MPQ-Present Pain | -.37 | -.18 | -.07 | -.43 | -.46 | .26 |
| Morphine | ||||||
| MPQ-Sensory | -.29 | -.33 | -.15 | -.47 | -.50 | .23 |
| MPQ-Affective | -.53 | -.55 | -.17 | -.54 | -.54 | .46 |
| MPQ-Present Pain | -.51 | -.45 | -.13 | -.54 | -.62 | .44 |
Note: r = +/- .21, p < .05. BDI = Beck Depression Inventory; TAI = Trait Anxiety Inventory; TAS = Trait Anger Scale; PCS = Pain Catastrophizing Scale; PDI = Pain Disability Index; PA = Positive Affect Scale; MPQ = McGill Pain Questionnaire
We then generated partial correlations among the naloxone and morphine condition pre-post drug pain change indexes (described in the previous paragraph) and the trait measures, controlling for the corresponding placebo condition pre-post drug pain change indexes (also described in the previous paragraph). This procedure examined relationships between trait measures and changes in low back pain in response to naloxone and morphine administration that controlled for baseline pre-drug pain levels and negative affect, and which controlled for placebo effects. As expected given the near-null relationships between trait measures and pain changes under placebo, the magnitude of coefficients changed little from those shown in Table 2. For example, the relationships between BDI scores and naloxone effects and morphine analgesic responses changed from r = -.33 and r = -.29 in Table 2 to r = -.44 and r = -.38 when also controlling for placebo responses. These findings suggest that trait measures were related significantly to indexes of EO function and analgesic morphine responses even when placebo responses were statistically controlled, and further underscore the minimal effects placebo responses exerted on these relationships.
Given these twin sets of findings suggesting minimal placebo condition effects, we then proceeded to derive placebo-controlled EO function and morphine analgesic response variables. Specifically, we subtracted the variables reflecting placebo condition changes in pain intensity from baseline to post-drug (residualized for both baseline pain intensity and baseline state negative affect) from the corresponding naloxone and morphine condition variables. For EO function, larger positive blockade effect values indicated greater endogenous opioid analgesia. For morphine analgesic responses, larger negative scores indicated greater morphine analgesia.
Tests of Mediation
Pearson zero-order correlations were generated among trait measures and the derived opioid blockade effect and morphine effect variables described above in order to determine the degree to which psychosocial variables were related to EO function and morphine analgesic responses. Next, evaluation of mediation models was carried out. As described by Preacher and Hayes [42], two criteria were necessary to demonstrate mediation. Using BDI scores as an example, there needed to be: 1) a significant association between BDI scores and morphine analgesic responses, and 2) a significant indirect effect of BDI scores on morphine responses via EO function. Where psychosocial factors were related significantly to morphine responses, the approach of Preacher and Hayes [42] was used to test the significance of the indirect effect.
Direct and indirect effects were examined using bootstrapped mediation analysis [42] via custom SPSS dialogue (the Indirect Procedure; http://www.afhayes.com/spss-sas-and-mplus-macros-and-code.html#sobel). Bootstrapping is a nonparametric re-sampling procedure that does not assume normality for the indirect effect, or a*b path coefficient. This nonparametric feature differs from the ‘causal steps’ approach to indirect effects (mediation) testing [2]. Bootstrapping involves random and repeated sub-sampling of the data to derive an estimate of the a*b (indirect effect) path coefficient and a 95% confidence interval around the bootstrapped indirect effect parameter. If the 95% confidence interval for the indirect effect generated by the model does not include zero, then the hypothesized indirect (mediation) effect is significant at the p<.05 level. Following published recommendations [42], k = 1000 bootstraps were used. Bias-corrected and accelerated bootstraps were used to derive path coefficients and corresponding 95% confidence intervals for indirect effects.
Results
Zero-Order Correlations
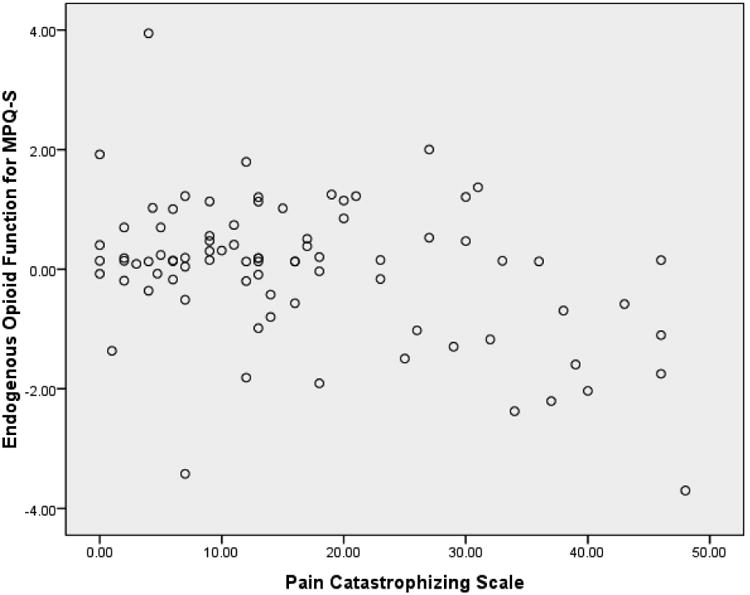
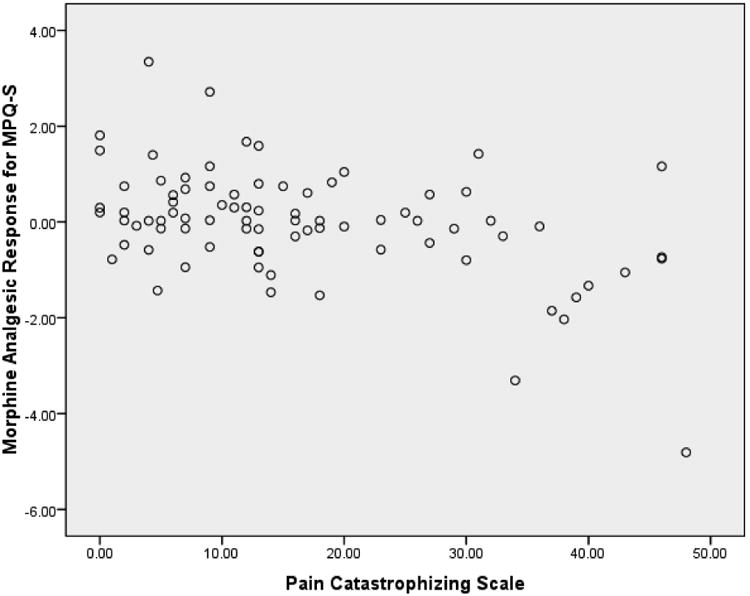
As shown in Table 3, all the psychosocial variables were related significantly to some morphine analgesic responses in directions indicating greater BDI, TAI, TAS, PCS and PDI scores were related to greater morphine analgesia, and that greater PA scores were related to less morphine analgesia. Results also showed that greater BDI, PCS and PDI scores were related to less EO function. See Figures 1 and 2 for example scatterplots between PCS scores and the blockade effect (Figure 1) and morphine analgesic response variables (Figure 2) for MPQ-S ratings of low back pain. Finally, findings indicated that the EO function variables for all three chronic pain indexes were related significantly to the corresponding morphine analgesic response variables in directions indicating that lower EO analgesic function was linked to greater morphine analgesic responses (r's = .42 to .77; p's < .01). For example, lower EO responses as reflected in MPQ-S scores were related to greater morphine analgesic responses for MPQ-S scores. Thus, the necessary relationships to test mediation effects were present for BDI, TAI, PCS, PDI and PA scores.
Table 3.
Correlations Between Trait Psychosocial Variables and Derived Endogenous Opioid Analgesia and Morphine Analgesia Variables.
| Trait Psychosocial Variables | ||||||
|---|---|---|---|---|---|---|
| BDI | TAI | TAS | PCS | PDI | PA | |
| Endogenous Opioid Analgesia | ||||||
| MPQ-Sensory | -.40 | -.22 | -.09 | -.39 | -.38 | .17 |
| MPQ-Affective | -.03 | .01 | .09 | -.32 | -.01 | -.09 |
| MPQ-Present Pain | -.31 | -.10 | -.21 | -.32 | -.26 | .18 |
| Morphine Analgesia | ||||||
| MPQ-Sensory | -.40 | -.32 | -.21 | -.45 | -.32 | .23 |
| MPQ-Affective | -.35 | -.44 | -.18 | -.22 | -.38 | .35 |
| MPQ-Present Pain | -.42 | -.31 | -.26 | -.38 | -.34 | .31 |
Note: r = +/- .21, p < .05.
BDI = Beck Depression Inventory; TAI = Trait Anxiety Inventory; TAS = Trait Anger Scale; PCS = Pain Catastrophizing Scale; PDI = Pain Disability Index; PA = Positive Affect scale; MPQ = McGill Pain Questionnaire
Figure 1.
Scatterplot of derived blockade effects for MPQ-S ratings of low back pain versus Pain Catastrophizing Scale scores. Blockade effects reflect the difference between placebo condition pre-post drug changes in pain (residualized for baseline pre-drug pain and state negative affect) and the corresponding residualized change in back pain in the naloxone condition.
Figure 2.
Scatterplot of derived morphine analgesic effects for MPQ-S ratings of low back pain versus Pain Catastrophizing Scale scores. Morphine analgesic effects reflect the difference between placebo condition pre-post drug changes in pain (residualized for baseline pre-drug pain and state negative affect) and the corresponding residualized change in back pain in the morphine condition..
Tests of Mediation
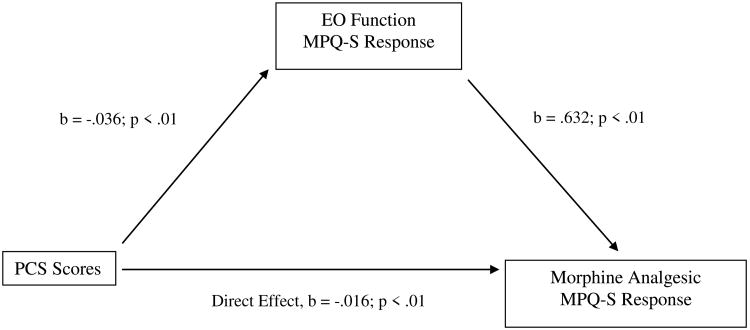
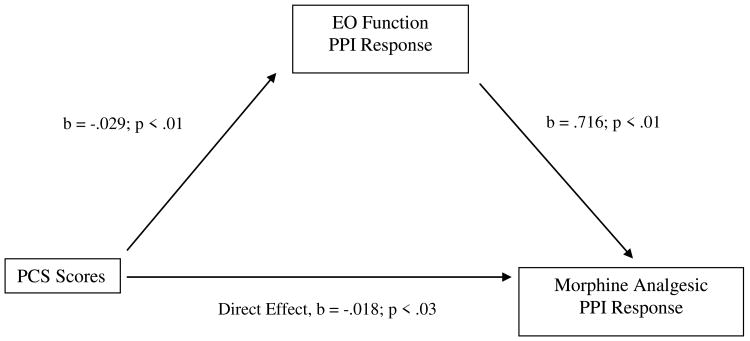
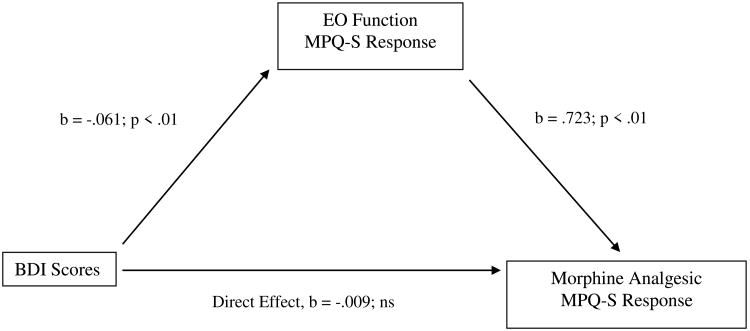
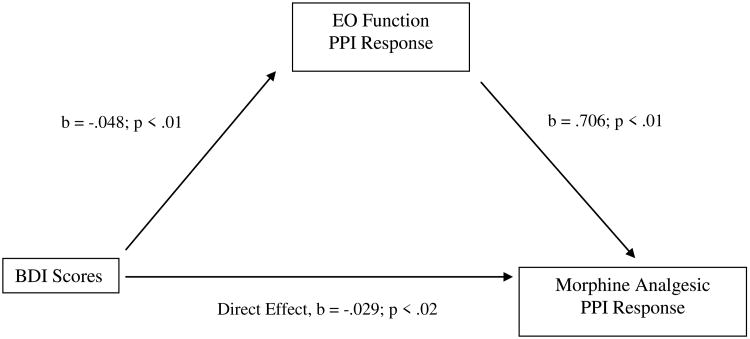
Table 4 displays the results of bootstrap tests of direct and indirect effects in the proposed mediation model for the three chronic pain measures. For BDI scores, the indirect effect in bootstrap tests was significant (i.e., the 95% confidence interval did not contain zero) for morphine analgesic responses on the MPQ-S, whereas the direct effect was not. See Figure 3. That is, the total effect of BDI scores on morphine analgesic responses was composed primarily of a significant indirect effect via EO responses on the MPQ-S. Also for BDI scores, the indirect effect in bootstrap tests was significant for morphine analgesic responses on the PPI, as was the direct effect. See Figure 4. That is, the total effect of BDI scores on morphine analgesic responses was composed of a significant indirect effect via EO responses on the PPI, as well as a direct, unmediated effect. These results suggest that greater BDI scores were related to lower EO function, which in turn was related to greater morphine analgesic responses.
Table 4. Summary of Significance of Direct and Indirect Effects for Model in which Association Between Psychosocial Factors and Morphine Analgesic Responsiveness is Mediated by Endogenous Opioid Function.
| IV- DV | Indirect Effect via Endogenous Opioid Function Bootstrap 95% Confidence Intervals | ||
|---|---|---|---|
| Direct Effect | Lower | Upper | |
| BDI – MPQ-Sensory | B = -.009 | -.083 | -.022 |
| BDI – MPQ-Present Pain | B = -.029* | -.087 | -.001 |
| TAI – MPQ-Sensory | B = -.013 | -.042 | -.002 |
| PCS – MPQ-Sensory | B = -.016* | -.048 | -.010 |
| PCS – MPQ-Present Pain | B = -.018* | -.047 | -.002 |
| PDI – MPQ-Sensory | B = -.003 | -.037 | -.009 |
| PDI – MPQ-Present Pain | B = -.016* | -.027 | .002 |
Note:
= p < .05.
Bootstrap 95% confidence intervals that do not contain zero are significant at p<.05. BDI = Beck Depression Inventory; TAI = Trait Anxiety Inventory; PCS = Pain Catastrophizing Scale; PDI = Pain Disability Index; PA = Positive Affect scale; MPQ = McGill Pain Questionnaire.
Figure 3.
Mediation pathway between Beck Depression Inventory (BDI) scores and morphine analgesia variable through EO function variable for McGill Pain Questionnaire-Sensory subscale (MPQ-S).
Figure 4.
Mediation pathway between Beck Depression Inventory (BDI) scores and morphine analgesia variable through EO function variable for McGill Pain Inventory-Present Pain Intensity (PPI).
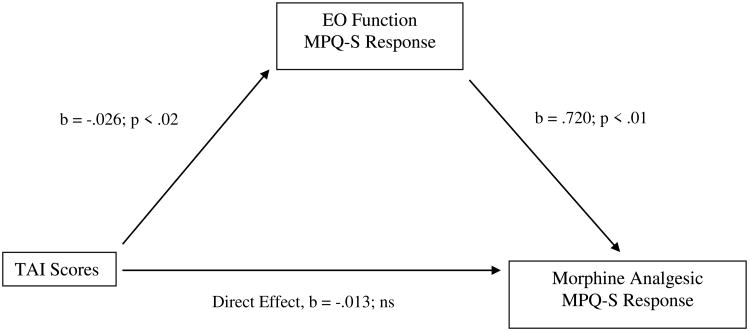
For TAI scores, the indirect effect in bootstrap tests was significant for morphine analgesic responses on the MPQ-S, while the direct effect was not. See Figure 5. Thus, the total effect of TAI scores on MPQ-S changes after morphine administration was composed mostly of a significant indirect effect via EO responses on the MPQ-S.
Figure 5.
Mediation pathway between Trait Anxiety Inventory (TAI) scores and morphine analgesia variable through EO function variable for McGill Pain Questionnaire-Sensory subscale (MPQ-S).
For PCS scores, the indirect effect in bootstrap tests was significant for morphine analgesic responses on the MPQ-S, while the direct effect was also significant. See Figure 6. This effect indicates that the total effect of PCS scores on MPQ-S changes after morphine administration was composed of a significant indirect effect via EO responses on the MPQ-S, as well as a direct effect that was unmediated by EO responses. Also for PCS scores, the indirect effect was significant for morphine analgesic responses on the PPI, while the direct effect was also significant. See Figure 7. These effects indicate that the total effect of PCS scores on PPI changes after morphine administration was composed of a significant indirect effect via EO responses on the PPI, as well as a direct, unmediated effect. These results suggest that greater PCS scores were related to lower EO function, which was in turn related to greater morphine analgesic responses.
Figure 6.
Mediation pathway between Pain Catastrophizing Scale (PCS) scores and morphine analgesia variable through EO function variable for McGill Pain Questionnaire-Sensory subscale (MPQ-S).
Figure 7.
Mediation pathway between Pain Catastrophizing Scale (PCS) scores and morphine analgesia variable through EO function variable for McGill Pain Questionnaire-Present Pain Intensity (PPI).
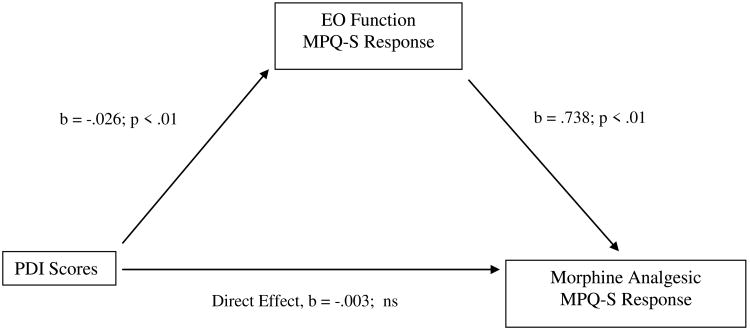
For PDI scores, the indirect effect in bootstrap tests was significant for morphine analgesic responses on the MPQ-S, while the direct effect was not. See Figure 8. Thus, the total effect of PDI scores on MPQ-S changes after morphine administration was composed of a significant indirect effect via EO responses on the MPQ-S. Also for PDI scores, the direct effect was significant for morphine analgesic responses on the PPI, but the indirect effect was not. These effects indicate that the total effect of PDI scores on PPI changes after morphine administration was composed of a significant direct effect on the PPI, whereas this effect was not significantly mediated by EO function.
Figure 8.
Mediation pathway between Pain Disability Index (PDI) scores and morphine analgesia variable through EO function variable for McGill Pain Questionnaire-Sensory subscale (MPQ-S).
Discussion
In the present study, we integrated previously separate literatures by proposing that EO function is a mechanism contributing to associations between psychosocial factors and responses to opioid analgesic medications. First, we found that psychosocial factors were related to both EO function and responses to opioid analgesics. Second, we found that links between opioid analgesic responsiveness and depressive symptoms, trait anxiety, pain catastrophizing and perceived disability were at least partially mediated by EO function. Results suggest that a range of psychosocial factors predict analgesic responses to opioid-based medications, and thus may serve as markers to help identify individuals who may benefit most from receiving opioid therapy. Results also suggest that people with elevated depressive symptoms, trait anxiety, tendency to catastrophize, and perceived disability are characterized by deficits in EO function; deficits that in turn predict enhanced responses to opioid analgesic,medications.
We found that all of the psychosocial factors assessed were related significantly to analgesic responses to an exogenous opioid (i.e., morphine). Higher depressive and anxious symptoms, trait anger, pain catastrophizing, and perceived disability were related to greater opioid analgesia, whereas greater positive affect was related to less opioid analgesia. Wasan and colleagues [52] used a similar method by computing within-subject difference scores in pain report between placebo and morphine conditions. Their findings were in a direction opposite to ours, suggesting that NA factors were related to diminished opioid analgesia. Similarly, Fillingim and colleagues [20] found NA factors to be related to diminished opioid analgesic responses in men but not in women. Although Fillingim and colleagues found sex differences and Wasan and colleagues did not, these prior results present a fairly consistent picture. However, an important methodological difference between these studies and the present study may help explain the divergent findings. Namely, in the Wasan and Fillingim studies, analgesic response outcomes involved changes in evoked pain stimulated during laboratory pain-induction procedures, whereas we assessed within-subject differences between placebo and morphine conditions with regard to spontaneous low back pain. Although it is not clear why opposing findings should emerge between acute and chronic pain, the difference may nonetheless signal important distinctions between psychosocial factor connections to opioid analgesia in the context of acute pain as opposed to chronic pain. More work is needed to explore these issues.
Bolstering our results that negative psychosocial factors are related to greater opioid analgesia for chronic pain are findings regarding the role of a plausible physiological mechanism. That is, the negative psychosocial factors we assessed were related negatively to EO function, meaning that patients high in negative affective (e.g., depressive) symptoms showed a deficit in EO analgesia. In our previous studies focused on anger-related factors, we hypothesized that trait anger and the trait tendency to express anger were related to high acute pain sensitivity because of deficient EO function [8,15]. This hypothesis was supported using an EO blockade procedure similar to that used in the present study. The present results extend these findings by identifying a wider range of psychosocial factors related to EO function. Results are also consistent with results of imaging work [28], in which induction of a sad mood was associated with decreased EO function in women with major depression disorder but not among healthy controls. Thus, well-documented links between negative psychosocial factors and elevated chronic pain intensity may be due to the EO deficits we report.
We previously reported on findings from the parent study – but based on half the current sample -- that lower EO function was related to greater responses to opioid analgesics [9]. Indeed, we showed that people with greater EO function achieved negligible analgesic benefit from morphine; an effect due, we speculated, to these individuals achieving sufficient analgesia via their EO systems. We speculated further that tonic elevated EO function might confer some degree of tolerance for opioid effects and thereby contribute to diminished responses to opioid analgesic medications. Pulling our present findings together, mediation analyses suggest that the greater morphine analgesic response of people with elevated negative psychosocial factors was due in part to their deficits in EO function. Put another way, deficits in EO function shown by patients with high levels of negative psychosocial factors make it more likely that opioid medications will augment their diminished endogenous analgesic capacity compared to patients with optimal EO function.
These findings may have important clinical implications. First, results imply that chronic pain patients with elevated levels of negative psychosocial factors may derive more analgesic benefit from opioid therapy for chronic pain than patients with low levels of negative psychosocial factors. Because the former exhibit relatively poor EO function, achieving optimal analgesia may be facilitated by augmentation with exogenous opioids. The latter patients, in contrast, may not achieve significant additional analgesia through opioid medications over and above the analgesia they derive from their relatively well-functioning EO systems. Hence, these patients may risk negative side effects of opioid therapy without the potential offsetting benefit of pain relief. An important caveat, however, is growing evidence that people characterized by depressive and anxiety symptoms in general [11], and pain catastrophizing in particular, may be at greatest risk for opioid misuse [1]. People with elevated NA may derive optimal analgesia from opioids for their chronic pain, but may also gain relief from emotional distress, perhaps elevating the chance they will use opioids for the latter purpose to their potential detriment. The possibility that opioid analgesics may work best for pain relief in exactly the same individuals who are also at greatest risk of misusing them presents a clinical conundrum for precision pain medicine [11]. Again, more work is needed to address these issues.
Second, rather than augment deficient EO function solely with opioid analgesics for patients with high levels of negative psychosocial factors, these patients may also benefit from non-pharmacological interventions that could boost EO function (e.g., acupuncture, aerobic exercise, relaxation training)[25,28,32,33]. Accumulating findings suggest that these approaches have the potential to provide a degree of analgesia similar to that achieved with opioid analgesic medications, and our findings further imply that these effects may be particularly potent for patients with deficient EO function. Even if, for instance, regular aerobic exercise cannot fully supply sufficient analgesia, it could at least help reduce the dose of opioid medication needed to achieve adequate analgesia in patients with high levels of negative psychosocial factors. Further work is needed to examine the degree to which a combination of non-pharmacological approaches and lower dose opioid therapy might maximize analgesic benefits while minimizing negative side effects and abuse risk related to higher dose opioid treatment [19,29,37].
Study limitations are noted. First, this sample was distinct from a typical chronic pain clinic sample in that none of the subjects were taking daily opioid analgesic medications. We needed to exclude daily opioid users to reduce risk because administration of naloxone to people taking daily opioid analgesics would trigger acute withdrawal symptoms. At the same time, we did not assess history of opioid use, and so cannot make distinctions in relationships among psychosocial factors, EO function and morphine responses based on whether opioids had been used regularly or not in the past. Second, only a single opioid analgesic agent was tested. It is unknown whether negative psychosocial factors are related differently to responses to opioid analgesic medications other than morphine, which is a conceivable outcome given that different opioid analgesic agents are known to activate distinct signaling pathways [43]. Third, present results were generated in the context of a single dose of morphine in individuals not taking daily opioids. The degree to which these findings may generalize to predicting long-term responses to daily opioids is unknown. Fourth, tests of mediation that do not involve true longitudinal effects, as reported here, can only suggest causal pathways. Although the pathway described here (i.e., negative affect to morphine analgesia through EO function) is potentially sound, it may be the case instead that individual differences in EO function affect emotion and cognitive variables, taking causal precedence. These competing causal pathways will need to be evaluated in future prospective studies.
In sum, we found that psychosocial factors were related to morphine analgesia but in a direction contrary to the only two existing placebo-controlled studies of this topic. Namely, we found that negative psychosocial factors (e.g., greater depressive symptoms) were related to greater opioid analgesic responses. Other study findings suggested that this relationship was partly due to an association between elevated negative psychosocial factors and deficient EO function. It may be that chronic pain patients with high levels of depressive and anxious symptoms, pain catastrophizing, and perceived disability exhibit relatively greater responses to opioid analgesic medications because morphine may augment their relatively weak EO systems. The opposite may be the case for people with low levels of negative psychosocial factors. If so, then the former group may experience a better balance between analgesia versus side effects than the latter group for whom analgesic effects may be minimal relative to the possibility of side effects. The results of this laboratory study may guide future work on finding markers for optimal opioid therapy in chronic pain patients undergoing daily opioid treatment.
Acknowledgments
This study described in this manuscript was supported by Grant # R01 DA031726 from the National Institute of Drug Abuse/NIH.
Footnotes
The authors report no conflicts of interest.
Contributor Information
John W. Burns PhD, Rush University Medical Center
Stephen Bruehl, Vanderbilt University Medical Center.
Christopher R. France, Ohio University.
Erik Schuster, Rush University Medical Center.
Daria Orlowska, Rush University Medical Center.
Asokumar Buvanendran, Rush University Medical Center.
Melissa Chont, Vanderbilt University Medical Center.
Rajnish K. Gupta, Vanderbilt University Medical Center.
References
- 1.Arteta J, Cobos B, Hu Y, Jordan K, Howard K. Evaluation of how depression and anxiety mediate the relationship between pain catastrophizing and prescription opioid misuse in a chronic pain population. Pain Med. doi: 10.1111/pme.12886. in press. [DOI] [PubMed] [Google Scholar]
- 2.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Personal Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 3.Beck AT, Ward CH, Mendelson M, Mock JE, Erbough JK. An inventory for measuring depression. Arch Gen Psychiatr. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 4.Berkowitz BA, Ngai SH, Hempstead J, Spector S. Disposition of naloxone: use of a new radioimmunoassay. J Pharmacol Exp Ther. 1975;195:499–504. [PubMed] [Google Scholar]
- 5.Blanco C1, Rafful C, Wall MM, Jin CJ, Kerridge B, Schwartz RP. The latent structure and predictors of non-medical prescription drug use and prescription drug use disorders: a national study. Drug Alcohol Depend. 2013;133:473–479. doi: 10.1016/j.drugalcdep.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brattberg G, Thorslund M, Wikman A. The prevalence of pain in a general population. The results of a postal survey in a county of Sweden. Pain. 1989;37:215–222. doi: 10.1016/0304-3959(89)90133-4. [DOI] [PubMed] [Google Scholar]
- 7.Bruehl S, Burns JW, Chung OY, Quartana P. Anger management style and emotional reactivity to noxious stimuli among chronic pain patients and healthy controls: the role of endogenous opioids. Health Psychol. 2008;27:204–214. doi: 10.1037/0278-6133.27.2.204. [DOI] [PubMed] [Google Scholar]
- 8.Bruehl S, Burns JW, Chung OY, Ward P, Johnson B. Anger and pain sensitivity in chronic low back pain patients and pain-free controls: The role of endogenous opioids. Pain. 2002;99:223–233. doi: 10.1016/s0304-3959(02)00104-5. [DOI] [PubMed] [Google Scholar]
- 9.Bruehl S, Burns JW, Gupta R, Buvanendran A, Chont M, Kinner E, Schuster E, Passik S, France CR. Endogenous opioid function mediates the association between laboratory evoked pain sensitivity and morphine analgesic responses. Pain. 2013;154:1856–1864. doi: 10.1016/j.pain.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruehl S, Burns JW, Gupta R, Buvanendran A, Chont M, Schuster EM, France CR. Endogenous opioid inhibition of chronic low back pain influences degree of back pain relief following morphine administration. Reg Anesthesia Pain Med. 2014;39:120–125. doi: 10.1097/AAP.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruehl S, Burns JW, Passik SD, Gupta R, Buvanendran A, Chont M, Schuster E, Orlowska D, France CR. The contribution of differential opioid responsiveness to identification of opioid risk in chronic pain patients. J Pain. 2015;16:666–675. doi: 10.1016/j.jpain.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruehl S, Chung OY, Burns JW. Trait anger and blood pressure recovery following acute pain: evidence for opioid-mediated effects. International J Behav Med. 2006;13:138–146. doi: 10.1207/s15327558ijbm1302_5. [DOI] [PubMed] [Google Scholar]
- 13.Bruehl S, Chung OY, Burns JW, Biridepalli S. The association between anger expression and chronic pain intensity: evidence for partial mediation by endogenous opioid dysfunction. Pain. 2003;106:317–324. doi: 10.1016/S0304-3959(03)00319-1. [DOI] [PubMed] [Google Scholar]
- 14.Bruehl S, Chung OY, Burns JW, Diedrich L. Trait anger expressiveness and pain-induced beta-endorphin release: support for the opioid dysfunction hypothesis. Pain. 2007;130:208–215. doi: 10.1016/j.pain.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns JW, Bruehl S, Chung OY, Magid E, Chont M, Goodlad JK, Gilliam W, Matsuura J, Somar K. Endogenous opioids may buffer effects of anger arousal on sensitivity to subsequent pain. Pain. 2009;146:276–282. doi: 10.1016/j.pain.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guidelines. J Pain. 2009;10:131–146. doi: 10.1016/j.jpain.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 18.De Cosmo G, Congedo E, Lai C, Primieri P, Dottarelli A, Aceto P. Preoperative psychologic and demographic predictors of pain perception and tramadol consumption using intravenous patient controlled analgesia. Clin J Pain. 2008;24:399–405. doi: 10.1097/AJP.0b013e3181671a08. [DOI] [PubMed] [Google Scholar]
- 19.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fillingim RB, Hastie BA, Ness TJ, Glover TL, Campbell CM, Staud R. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol. 2005;69:97–112. doi: 10.1016/j.biopsycho.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, Staud R. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug related behaviors? A structured evidence-based review Pain Med. 2008;9:444–459. doi: 10.1111/j.1526-4637.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- 23.Geha H, Nimeskern N, Beziat JL. Patient-controlled analgesia in orthognathic surgery: evaluation of the relationship to anxiety and anxiolytics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e33–36. doi: 10.1016/j.tripleo.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Harden RN, Bruehl S. Opioid prophylaxis of chronic pain: An examination of the ongoing controversy. J Back Musculoskel Rehab. 1997;9:155–180. doi: 10.3233/BMR-1997-9207. [DOI] [PubMed] [Google Scholar]
- 25.Harris RE, Zubieta JK, Scott DJ, Napadow V, Gracely RH, Clauw DJ. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs) Neuroimage. 2009;47:1077–1085. doi: 10.1016/j.neuroimage.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004 Dec;112(3):372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63:1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- 28.Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am. 2009;91:919–927. doi: 10.2106/JBJS.H.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koltyn KF. Analgesia following exercise: a review. Sports Med. 2000;29:85–98. doi: 10.2165/00007256-200029020-00002. [DOI] [PubMed] [Google Scholar]
- 30.Kuehn BM. Efforts aim to curb opioid deaths, injuries. JAMA. 2009;301:1213–1215. doi: 10.1001/jama.2009.367. [DOI] [PubMed] [Google Scholar]
- 31.Lewis J, Mansour A, Khachaturian H, Watson SJ, Akil H. Opioids and pain regulation. Pain Headache. 1987;9:129–159. [PubMed] [Google Scholar]
- 32.Martin WR. Drugs five years later: naloxone. Ann Intern Med. 1976;85:765–768. doi: 10.7326/0003-4819-85-6-765. [DOI] [PubMed] [Google Scholar]
- 33.McCubbin JA, Cheung R, Montgomery TB, Bulbulian R, Wilson JF. Aerobic fitness and opioidergic inhibition of cardiovascular stress reactivity. Psychophys. 1992;29:687–697. doi: 10.1111/j.1469-8986.1992.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 34.McCubbin JA, Wilson JF, Bruehl S, Ibarra P, Carlson CR, Norton JA, Colclough GW. Relaxation training and opioid inhibition of blood pressure response to stress. J Consult ClinPsychol. 1996;64:593–601. doi: 10.1037//0022-006x.64.3.593. [DOI] [PubMed] [Google Scholar]
- 35.Melzack R. The short form of the McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 36.Millan MJ. Descending control of pain. ProgNeurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 37.Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM. Cochrane Database Syst Rev. Vol. 20. ECRI Institute; 5200 Butler Pike, Plymouth Meeting, PA, USA, 19462: 2010. Long-term opioid management for chronic noncancer pain; p. CD006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 39.Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O'Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20:589–605. doi: 10.1023/a:1025570508954. [DOI] [PubMed] [Google Scholar]
- 40.Passik SD. Issues in long-term opioid therapy: unmet needs, risks, and solutions. Mayo Clin Proc. 2009;84:593–601. doi: 10.1016/S0025-6196(11)60748-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry F1, Parker RK, White PF, Clifford PA. Role of psychological factors in postoperative pain control and recovery with patient-controlled analgesia. Clin J Pain. 1994;10:57–63. doi: 10.1097/00002508-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Petraschka M, Li S, Gilbert TL, Westenbroek RE, Bruchas MR, Schreiber S, Lowe J, Low MJ, Pintar JE, Chavkin C. The absence of endogenous beta-endorphin selectively blocks phosphorylation and desensitization of mu opioid receptors following partial sciatic nerve ligation. Neuroscience. 2007;146:1795–1807. doi: 10.1016/j.neuroscience.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods InstrumComput. 2004;36:717–31. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 44.Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharm. 2011;60:58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith HS, Kirsh KL, Passik SD. Chronic opioid therapy issues associated with opioid abuse potential. J Opioid Manag. 2009;5:287–300. doi: 10.5055/jom.2009.0029. [DOI] [PubMed] [Google Scholar]
- 46.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 47.Spielberger CD, Jacobs G, Russell S, Crane R. Assessment of anger: The State-Trait Anger Scale. In: Butcher JN, Spielberger CD, editors. Advances in Personality Assessment. Vol. 2. Hillsdale, NJ: LEA; 1983. pp. 161–189. [Google Scholar]
- 48.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assessment. 1995;7:524–532. [Google Scholar]
- 49.Tait RC, Chibnall JT, Krause S. The Pain Disability Index: psychometric properties Pain. 1990;40:171–82. doi: 10.1016/0304-3959(90)90068-O. [DOI] [PubMed] [Google Scholar]
- 50.Tseng LF, Lin JJ, Collins KA. Partial antinociceptive cross-tolerance to intracerebroventricular [beta]-endorphin in mice tolerant to systemic morphine. Euro J Pharmac. 1993;241:63–70. doi: 10.1016/0014-2999(93)90933-9. [DOI] [PubMed] [Google Scholar]
- 51.Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- 52.Wardlaw SL, Kim J, Sobieszczyk S. Effect of morphine on proopiomelanocortin gene expression and peptide levels in the hypothalamus. Molecular Brain Res. 1996;41:140–147. doi: 10.1016/0169-328x(96)00084-8. [DOI] [PubMed] [Google Scholar]
- 53.Wasan AD, Davar G, Jamison R. The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain. 2005;117:450–461. doi: 10.1016/j.pain.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Personal Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. 1988. [DOI] [PubMed] [Google Scholar]