Abstract
Background
Cortisol is a key stress hormone implicated in the pathogenesis of many age-related diseases. Longitudinal information on cortisol exposure has been restricted to animal models and a small number of human studies. The purpose of the present study was to quantify longitudinal change in cortisol across the adult life span.
Methods
We conducted a prospective longitudinal study of 24-hour urinary free cortisol excretion from ages 20 to 90 years and older. Participants were 1,814 men and women from the Baltimore Longitudinal Study of Aging who provided a total of 5,527 urine specimens for analysis. The average duration of longitudinal follow-up was 6.6 years. The primary outcome measure was 24-hour urinary free cortisol to creatinine ratio (UFC/Cr) as determined by liquid chromatography/mass spectrometry.
Results
UFC/Cr follows a U-shaped pattern across the life span with decreases in UFC/Cr in the 20s and 30s, relative stability in the 40s and 50s, and increases thereafter. This pattern of change was robust with respect to adjustment for several potential confounding factors.
Conclusions
Age-related changes in cortisol exposure raise important questions about the potential protective or exacerbating role of cortisol exposure in predicting medical, physiological, and behavioral outcomes.
Keywords: Cortisol, Aging, Glucocorticoid, Corticosteroid
Cortisol is a key stress hormone implicated in the pathogenesis of many age-related diseases and the development of aging phenotypes (1–3). Cross-sectional studies have generally reported that cortisol blood levels are maintained or increase slightly with aging, but there is limited corroboration of these findings by longitudinal observations (4,5).
The potential problems in extrapolating age trajectories from cross-sectional studies are pointed out by two studies in the literature. A cross-sectional analysis of 138 monkeys found a small positive correlation between cortisol and age (r = .20). However, 10-year longitudinal studies on a subset of 30 monkeys revealed vast interindividual differences in age trajectories, with some animals showing significant increases in cortisol levels, whereas others showed significant declines, and regression coefficients that ranged from +0.85 to −0.66. In a sample of over 1,700 men aged 40–70 years (baseline mean age, 55.2 years) in the Massachusetts Male Aging Study, Feldman and colleagues found no cross-sectional association between age and cortisol levels assessed in nonfasting blood samples collected 4 hours after waking (6). However, when they evaluated the same participants 7–10 years later, they found a significant decline of cortisol concentration of about 1% per year. These findings illustrate that cross-sectional studies may not capture the complexity of the effect of aging on cortisol and provide very little information about a given individual’s long-term exposure to cortisol.
There have been several additional studies in humans investigating cortisol changes over time, with varying methods and results. Seeman and colleagues (7) reported a 4.2%/year decline in 12-hour overnight urinary free cortisol (UFC) excretion (measured by cortisol:creatinine ratio [UFC/Cr]) in 194 participants aged 70–79 years. Rueggeberg and colleagues (8) measured plasma cortisol values in 157 participants (mean age, 72 years). Follow-up 4 years later revealed a significant increase in cortisol total daily exposure estimated by the area under the curve.
Lupien and colleagues evaluated longitudinal changes in plasma cortisol by performing hourly sampling over 24 hours in 17 women (mean age, 73 years) and 34 men (mean age, 74 years) followed for 3–6 years (9). They reported an average increase in cortisol of approximately 8.3%, with dramatic individual differences in average levels of cortisol and in age trajectories of change.
Given intense interest and an extensive literature linking cortisol to physiological, medical, and behavioral outcomes, it is surprising that there are currently so few longitudinal studies describing age-related trajectories in cortisol levels in human aging. The studies that do exist report conflicting results with some showing longitudinal increases (8,9) and others decreases (6). Existent studies, although well done, also have some weaknesses. Some are based on small sample sizes (7–9), whereas others have included only one baseline and one follow-up cortisol measure (6–8). Most notably, previous longitudinal studies have focused on populations of individuals who are middle-aged and older, which prevents the full characterization of cortisol changes from young adulthood through old age.
To better characterize longitudinal trajectories of cortisol excretion in human aging, we measured 24-hour urinary free cortisol and creatinine in 1,814 individuals from the Baltimore Longitudinal Study of Aging for a follow-up duration of up to 31 years. Repeated measures were used to describe the pattern/slope of cortisol levels across the full adult life span, from ages 20 to 90 and older. To our knowledge, this is the most comprehensive study to date investigating longitudinal age-related changes in 24-hour cortisol excretion in men and women.
Methods
Participants
Participants in the present investigation were volunteers participating in the Baltimore Longitudinal Study of Aging (BLSA), a study performed by the National Institute on Aging. Participants are generally highly educated (16.2 ± 2.6 years of education), community volunteers who travel to the NIA for comprehensive, medical, physiological, and neuropsychological evaluations.
At each visit, a 24-hour urine collection was performed using a highly standardized protocol. Overall, there were 966 men and 900 women who provided a total of 6,002 urine samples for UFC analysis. The sample was 74% White (N = 1,388), 20% African American (N = 370), and 6% other (N = 108). The mean age (SD) at baseline assessment was 59.4 (16.5) years, and the mean (SD) duration of follow-up was 6.8 (6.8) years with an average of 3.2 (range 0–12) repeated measures per participant. This protocol was approved by the local institutional review board, and all participants provided written informed consent to participate. Demographic information and medication usage and medical histories are presented in Table 1.
Table 1.
Demography and Medical History of Participants
| Mean (SD)/Frequency (%) | |||
|---|---|---|---|
| Number of participants (N) | 1,814 | ||
| % Men | 939 (51.8%) | ||
| Race | % White | 1,353 (74.6%) | |
| % African American | 358 (19.7%) | ||
| % Other | 103 (5.7%) | ||
| Age at baseline (y) | 59.7 (16.4) | ||
| Range 17.7–94.5 | |||
| Education (y) | 16.2 (2.6) | ||
| Range 8–21 | |||
| Total number of urine samples | 5,527 | ||
| Number of repeat measures | 3.0 (2.2) | ||
| Range 1–12 | |||
| Follow-up duration | 6.6 (6.9) | ||
| Range 0–31.2 years | |||
| Duration of storage (y) | 11.9 (6.4) | ||
| Range 2.0–48.1 | |||
| Medication | Frequency (%) by visita | Frequency (%) by participantb | |
| Diruetics | 902 (16.3%) | 443 (24.4%) | |
| Antidepressant | 407 (7.4%) | 237 (13.1%) | |
| Sedatives | 269 (4.9%) | 196 (10.8%) | |
| Thyroid hormone | 963 (17.4%) | 476 (26.2%) | |
| Diabetes | 334 (6.0%) | 148 (8.2%) | |
| Statins | 1,244 (22.5%) | 566 (31.2%) | |
| Corticosteroids | 523 (9.5%) | 363 (20.0%) | |
| Sex hormones | 876 (15.9%) | 411 (22.7%) | |
| Smoking status | 268 (4.9%) current | ||
| 2,832 (51.2%) former | |||
| 2,427 (43.9%) never | |||
| Medical condition | |||
| Congestive heart failure | 59 (1.1%) | 26 (1.4%) | |
| Myocardial infarction | 283 (5.1%) | 111 (6.1%) | |
| Hypertension | 2,027 (36.7%) | 694 (38.3%) | |
| Angina | 589 (10.7%) | 200 (11.0%) | |
| Diabetes | 369 (6.7%) | 137 (7.6%) | |
| Cancer | 748 (13.5%) | 281 (15.5%) |
aFrequency (%) of medication usage across all 5,527 visits.
bFrequency (%) of medication usage by participant (N = 1,814) at any visit.
Procedure
Urinary Free Cortisol Collection, Storage, and Assay
As part of their biennial assessments, volunteers provide 24-hour urine samples. All BLSA participants reside in the hospital unit for the duration of their visit (usually 2–3 days), and they are continuously monitored by nursing and hospital staff and reminded about compliance in providing full and complete urine samples. Participants were instructed to void upon waking at approximately 8 am and discard this specimen. Following initial voiding, all urine including the final specimen voided at the end of the 24-hour collection was collected by the participant in containers provided to them. The containers were then labeled with participant code, date, and time. The total urine volume and total duration of collection was measured and recorded on a participant record form. Subsequently, multiple 20-mL samples of urine were aliquoted from the total pool and frozen at −80°C and stored in the BLSA specimen bank. The mean duration of storage of the samples was (11.9 ± 6.4 years). Numerous studies have indicated that steroid hormones including cortisol are very stable in both plasma and urine even when frozen for durations longer than 10 years (10–13). In total, 6,002 study samples were available for analysis of UFC.
UFC was measured by Esoterix Inc. by liquid chromatography with mass spectrometry. Interassay coefficients of variation for mean values of 0.05, 2.84, 5.53, and 9.41 µg/dL were 13.4%, 6.6%, 4.4%, and 5.3%, respectively. Interassay coefficients of variation for creatinine mean values of 66.6 and 145.8 ng/mL were 1.5% and 1.1%, respectively.
Data Analytic Approach
Consistent with previous studies (7,14–16) and to account for age-related changes in kidney function (17), the primary outcome variable used for all analyses was the UFC/Cr. Previous studies have shown that creatinine-adjusted urinary metabolite concentrations correlate better with blood, serum, or plasma concentrations of the parent chemical than the unadjusted concentrations, suggesting that creatinine-adjusted analyte concentrations serve as good surrogates (14). As expected, urinary creatinine was negatively correlated with age (Spearman rho = −.15, p < .001).
Of the 6,002 samples collected, 319 samples were missing data for total urine volume collected. We eliminated from the analysis 93 participants with 24-hour urine volumes at the 99th (4,200 mL) and first (400 mL) percentile of the distribution. After calculating the UFC/Cr ratio, the distribution was positively skewed, so we excluded outliers which were more than 5 SD above the mean, where there was a break in the distribution of UFC/Cr values. Following exclusion of these outliers, the distribution was normal. We also excluded 6 data points collected between 1965 and 1975 because these dates of collection were much earlier than the majority of the samples. Another 20 cases were excluded because they were missing UFC data. In total, we excluded 475 (8%) of samples resulting in a final sample size for all analyses of 5,527.
Statistical Analyses
Analyses were conducted using SAS 9.3 (Cary, NC). We used linear mixed models to model the trajectories of cortisol concentrations over the life span while accounting for the longitudinal nature of the data. All the linear mixed models were estimated using the restricted maximum likelihood method.
The initial predictors included in the model were age, sex, date of visit, body mass index (BMI), low-density lipoprotein, high-density lipoprotein, smoking (current, former, never), alcohol use (absolute grams of alcohol per day), race (white and nonwhite), medication usage variables that included systemic corticosteroids (inhaled and topical excluded), diuretics, antidepressants, hypnosedatives, thyroid, sex hormones, diabetes medications and statins, and medical conditions that included myocardial infarction, cancer, diabetes, angina, congestive heart failure, and hypertension. All the medication and medical conditions were coded 0 as No and 1 as Yes. Age was centered at age 60 and divided by 10 (to allow interpretation by decade). Date of visit was centered at the mean 1999. All the other continuous predictors were standardized, and all predictors were time dependent.
To study the life-time trajectory of cortisol and the potential covariates that affect the trajectory, a “minimally adjusted” model was first performed to examine age-related patterns of change in UFC/Cr. Predictors included in this model were sex, date of visit, date of visit × date of visit, age, age × age, and sex interactions with all age terms. Sex × age interactions were not significant, so they were dropped from the model.
In the subsequent “fully adjusted” model, we added each of the additional covariates to the “minimally adjusted” model to test their individual effect on cortisol level and cortisol trajectory over time without adjusting for other covariates. The candidate predictors that had significant effects on cortisol (p < .1) were eligible for entering the “fully adjusted’ model. In the final step, we started with the “fully adjusted” model, where we added all the significant predictors and their interactions with age to the “minimally adjusted” model. We then used backward elimination to reduce the model until only significant predictors (p < .05) remained in the most parsimonious model.
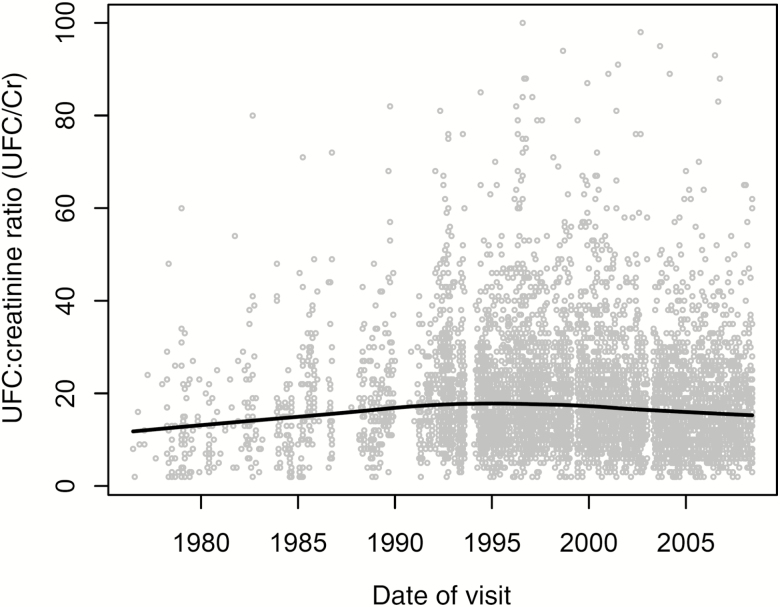
Initial exploratory analyses indicated a significant date of visit effect on UFC/Cr. As seen in Figure 1, the oldest and most recent samples tended toward the lowest values. This date effect was robust with respect to adjustments for age, sex, race, and BMI. Therefore, in subsequent adjusted models reported in this study, date of visit and date of visit × date of visit were used as covariates.
Figure 1.
Relationship between 24-hour urinary free cortisol to creatinine ratio (UFC/Cr) and date of visit (DOV). A significant nonlinear effect was found in which the oldest and newest samples tended to have lower UFC/Cr values than moderately aged samples. DOV effect was incorporated as a covariate in all models.
Results
Minimally Adjusted Model
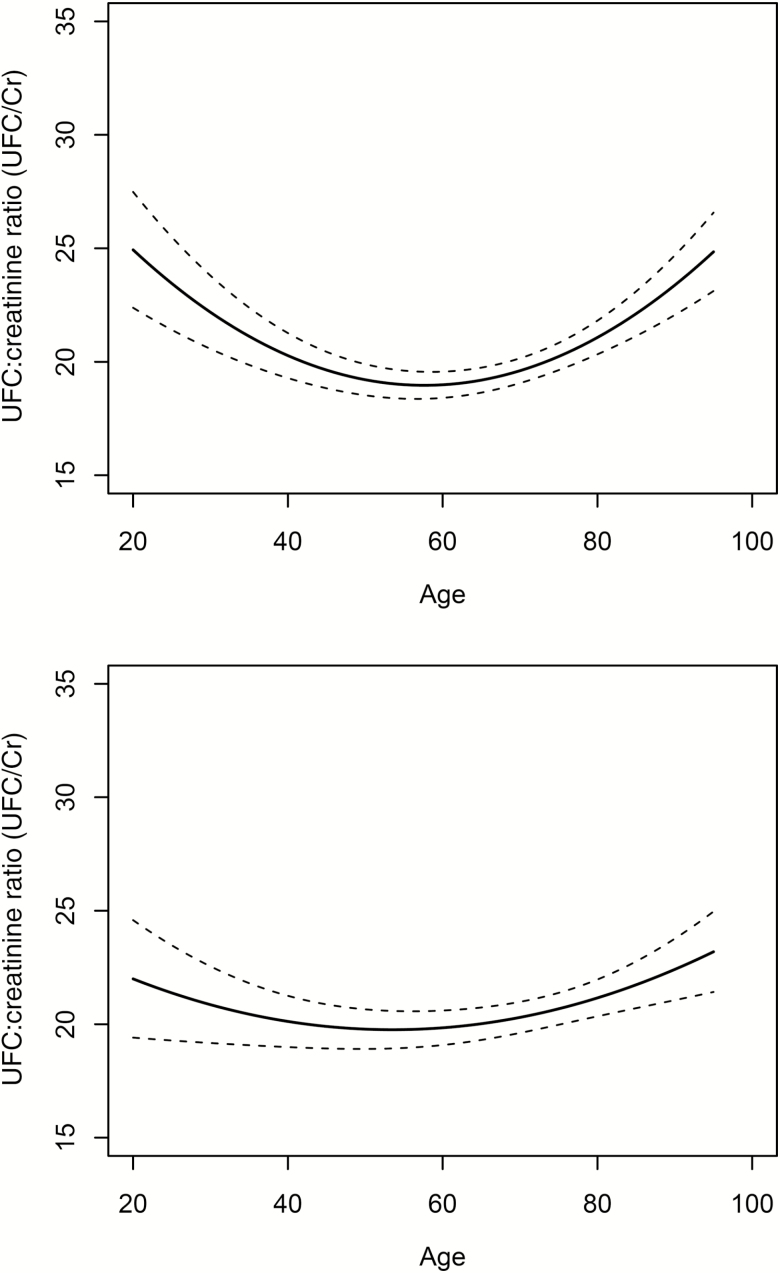
Table 2 provides mixed model regression results, and Figure 2 (left panel) shows the relationship between UFC/Cr and age from the minimally adjusted model. UFC/Cr across the life span was described by a nonlinear, U-shaped function (age × age effect. p < .0001). From approximately age 20, UFC/Cr declines until it reaches a minimum at approximately age 60. After age 60, the UFC/Cr increases progressively with older age. Sex was also a significant predictor with women having modestly higher UFC/Cr than men (men 1.20 [SE = 0.46] lower than female, p = .0098). There were no significant interactions between sex and age on UFC/Cr (p = .65). As described in Figure 1 and Table 2, there were significant linear (p < .0001) and nonlinear (p < .0001) effects of storage duration.
Table 2.
Association Between Age, Sex, and Date of Visit With Cortisol Levels: Results for the Minimally Adjusted Model
| Effect | Estimate | SE | P > |t| |
|---|---|---|---|
| Intercept | 18.99 | 0.29 | <.0001 |
| Sex | −1.20 | 0.46 | .0098 |
| Date of visit (y) (linear) | −0.18 | 0.030 | <.0001 |
| Date of visit (y) (quadratic) | −0.027 | 0.0025 | <.0001 |
| Age (decades) (linear effect) | 0.20 | 0.16 | .22 |
| Age (decades) (quadratic effect) | 0.42 | 0.070 | <.0001 |
Figure 2.
Relationship (solid line) between 24-hour urinary free cortisol to creatinine ratio (UFC/Cr) and age in minimally adjusted model (top panel) and in the fully adjusted model (bottom panel). A significant nonlinear effect was found in which UFC/Cr decreased from in early adulthood and was stable in middle adulthood and increased thereafter (dashed lines are 95% confidence intervals). The curvilinear effect was somewhat attenuated by inclusion of covariates in fully adjusted model.
Fully Adjusted Model
Table 3 shows results for the fully adjusted model, and Figure 2 (right panel) shows the corresponding fully adjusted relationship between UFC/Cr and age. There were significant linear and nonlinear effects of age which confirm the age trajectory of UFC/Cr found in the minimally adjusted model. Demographic variables associated with lower UFC/Cr included nonwhite race and higher BMI. We found no significant interaction of race and BMI with age in predicting UFC/Cr. Also, given in Table 3 are medical conditions and medications associated with UFC/Cr levels. The only variable to affect trajectories of change (ie, significant interaction with age) was high-density lipoprotein cholesterol levels. Higher high-density lipoprotein was associated with a more U-shaped UFC/Cr trajectory than lower high-density lipoprotein. (As sensitivity analysis, we repeated the variable selection process using Bayesian information criterion and assessed the resulting Bayesian model with the results from the mixed model approach. Bayesian information criterion is known to have a larger penalty for overfitting and generally results in a more parsimonious model. Using this approach, the final model was highly similar to the results achieved using the mixed model. The relation between UFC and age was a U-shaped, nonlinear function. As expected, this model produced a simpler solution in which some of the marginally significant terms presented in the final model dropped out, specifically, hypertension and statin medication use. These results are presented in Supplementary Figure 1 and Supplementary Table 1.)
Table 3.
Associations Between Age, Sex, and Date of Visit a Medical History With Cortisol Levels: Results From the Reduced Fully Adjusted Model
| Effect | Estimate | SE | P > |t| |
|---|---|---|---|
| Intercept | 22.99 | 0.45 | <.0001 |
| Date of visit (y) (linear) | −0.085 | 0.033 | .011 |
| Date of visit (quadratic) | −0.027 | 0.0025 | <.0001 |
| Age (decades) | 0.26 | 0.16 | .11 |
| Age (quadratic) | 0.20 | 0.072 | .0055 |
| Race (white/nonwhite) | −3.15 | 0.52 | <.0001 |
| High-density lipoprotein (linear) | 0.67 | 0.27 | .014 |
| High-density lipoprotein (quadratic) | −0.24 | 0.094 | .011 |
| Body mass index | −2.30 | 0.22 | <.0001 |
| Current smoker (Y/N) | −2.92 | 0.88 | .0009 |
| Former smoker (Y/N) | −0.99 | 0.44 | .023 |
| Hypertension (Y/N) | −0.87 | 0.42 | .038 |
| Corticosteroid usea (Y/N) | −1.27 | 0.49 | .010 |
| Diruetic use (Y/N) | −5.29 | 0.48 | <.0001 |
| Statin use (Y/N) | −0.93 | 0.41 | .025 |
| High-density lipoprotein × age (linear) | 0.11 | 0.14 | .43 |
| High-density lipoprotein × age (quadratic) | 0.16 | 0.062 | .0096 |
Note: aThe fully adjusted model was recomputed after excluding visits in which corticosteroid use was present. The exclusion of these visits did not substantially alter the findings.
Inter- and Intraindividual Differences in UFC/Cr Change With Age
We further investigated the UFC/Cr rates of change for each decade of life. The rate of change in each decade of life was calculated as the tangent of the fully adjusted model at each decadal midpoint (eg, slope for decade of 40s is tangent of the curve at age 45). Table 4 presents rates of change and percent rates of change across these decades. Significant linear decreases were found in the 20s, and the rate of the decline was less steep and only approached statistical significance in the 30s. Rates of change in UFC/Cr were nonsignificant in the 40s and 50s, and significant increases were observed in 60s, 70s, and 80s. To investigate the within-individual stability of UFC/Cr, intraclass correlation was performed. The within-individual correlation of UFC/Cr across all participants was r = .41, indicating moderate tracking and considerable fluctuation over time.
Table 4.
Mean Decadal (SEM) Rates of Change in UFC/Cr by Decades of Age
| Decade of Life | Number of Participant | UFC/Cr Rate of Change | p Value* | Percent Rate of Changea |
|---|---|---|---|---|
| 20s | 127 | −1.14 (0.53) | .033 | −5.96 |
| 30s | 114 | −0.74 (0.40) | .064 | −3.87 |
| 40s | 216 | −0.34 (0.27) | .21 | −1.78 |
| 50s | 518 | 0.060 (0.18) | .74 | 0.31 |
| 60s | 665 | 0.46 (0.17) | .0087 | 2.41 |
| 70s | 691 | 0.86 (0.26) | .0012 | 4.50 |
| 80s | 427 | 1.26 (0.39) | .0012 | 6.59 |
Note: aSample average UFC/Cr (19.12) was used as denominator to calculate % change.
*p Values represent means significantly different from zero.
Discussion
In the present study, we quantified UFC/Cr excretion across the adult life span in a sample of 1,866 men and women from the BLSA. Our analysis revealed that UFC/Cr follows a U-shaped pattern across the life span with decreases in UFC/Cr in the 20s and 30s, relative stability from the 40s and 50s, and increases thereafter. Both the minimally adjusted and fully adjusted models were consistent in revealing this U-shaped trajectory, although early adulthood decreases in UFC/Cr were reduced with full covariate adjustment. To our knowledge, this is the largest study to report longitudinal data on cortisol excretion across the entire adult life span.
Our data show both similarities and differences when compared with previous studies (6,7). In particular, older age in our study was associated with an upward trajectory of UFC/Cr from age 60 onward. This is consistent with investigations by Rueggeberg and colleagues (8) and Lupien and colleagues (9), both of which observed longitudinal increases in cortisol levels in participants whose mean age was in the 70s.
However, two studies have reported decreases in cortisol levels in human aging. Seeman and colleagues (7) reported decline of approximately 4.2%/year in UFC/Cr in participants aged 70–79 years. Compared with the present study, Seeman and colleagues (7) was based on a smaller sample size (N = 194), the participants were followed over a shorter duration (3 vs. 6.8 years) and 12 hours overnight rather than 24-hour urine collection was performed. Moreover, although medical comorbidities were assessed by Seeman and colleagues (7), baseline rates were not reported nor does it appear that they were taken into account in statistical models of cortisol change.
Feldman and colleagues investigated patterns of hormone change in a sample of over 1,700 men in the Massachusetts Male Aging Study (6). Within-individual follow-up 7–10 years later revealed annual declines of cortisol concentration of about 1%. However, men in this study (6) averaged 55.2 years of age at baseline and 62.7 years at follow-up, covering a much more restricted portion of the life span than in the present study. The upward inflection in UFC/Cr in our study occurred at approximately 60 years of age (see Figure 2). Consistent with Feldman and colleagues, our data did not show longitudinal increases in UFC/Cr within the 55- to 62-year age range.
A second important outcome of our study is the quantification of the large individual differences in UFC/Cr level and within-individual change over time. Table 4 reports mean rates of change within each decade of life. However, both across decades and within decades, there were individual differences around this mean. For example, among individuals in their 80s, within-individual slopes of UFC/Cr ranged from −75.5% to +43.3%. This finding is highly consistent with previous studies (6,7,9), which also observed highly variable patterns of within-individual trajectories. Moreover, the intraclass correlation in UFC/Cr was r = .41. This result is consistent with Rueggeberg and colleagues (8) who reported intraclass correlations of cortisol area under the curve of r = .30–.45 over a 4-year interval. Data from these studies indicate that cortisol levels are only moderately stable over time. This moderate stability of cortisol excretion is not surprising given the number of endogenous and exogenous factors that are known to affect cortisol production or excretion (eg, (18–23)).
There are several mechanisms that may potentially explain the increase in UFC/Cr among the older participants in this study. One possible mechanism is reduced negative feedback to endogenous cortisosteroid levels. In an animal model, Lee and colleagues (24) found reduced number of glucocorticoid receptors in the hippocampus, prefrontal cortex, and hypothalamus, and when dexamethasone was infused into these brain regions, the suppressive effect on endogenous cortisol levels was eliminated in aged animals. If a similar age-related loss in the number or sensitivity of central nervous system glucocorticoid receptors is present in humans, this could result in progressively increasing endogenous cortisol observed in the present study.
Because of the well-known role of the hypothalamic–pituitary–adrenal axis in the stress response, the possibility that there may be altered cortisol response to stress in aging has received much attention. Of particular interest is whether older adult participants may show a heightened cortisol response to an exogenous challenge. A meta-analysis of 48 studies investigating cortisol response to an external challenge as a function of age showed that compared with younger participants, older participants showed either increased cortisol secretion or reduced cortisol inhibition in response to a physiological or psychological challenge (25). The increase in cortisol response or reduction in cortisol inhibition to challenge may also manifest as the average increase in UFC observed in the present study.
Increased cortisol levels have also been associated with a number of medical conditions associated with aging (26). Proinflammatory cytokines which are secreted in a number of age-associated metabolic, somatic, and psychiatric conditions may act on multiple levels of the hypothalamic–pituitary–adrenal system ultimately increasing glucocorticoid secretion (26). Although we controlled statistically for numerous medical and health-related factors, the increasing disease burden with age, age-related increases in cytokines, or other endogenous factors may also play a role in increasing cortisol levels over time.
A second component of the life span changes in UFC/Cr observed in this study was the 5% decrease among young adults throughout the decade of the 20s. It is noteworthy that this effect was substantially dampened in our fully adjusted model when compared with the minimally adjusted model, suggesting that this effect in particular may have been influenced substantially by confounding factors. As previously discussed, younger adults have reduced hypothalamic–pituitary–adrenal response to an exogenous challenge (25). Self-perceived stress decreases and stress-coping mechanisms increase throughout the teenage years (27), so it is possible that the decade of the 20s is one in which young adults continue to develop better physiological or psychological stress-related coping mechanisms resulting in average decreases in UFC/Cr.
As well, cortisol changes over this time period could interact with changes in sex steroid levels during the prime of reproductive health (28,29) and could be related to reproductive status and strategies (30). In a sample of 630 Filipino men (aged 24–26), there was a positive correlation between endogenous cortisol and testosterone levels. Moreover, men in the study with a “mating orientation” had both higher cortisol and testosterone levels than men with a “parenting orientation” (30). The change from mating to parenting orientation is probably maximal during this decade of life and could contribute to cortisol declines.
There are several limitations to the present study. First, our UFC/Cr values showed systematic variation as a function of duration of storage (see Figure 1). Numerous studies have indicated that steroid hormones including cortisol are stable in both plasma and urine even when frozen for durations longer than 10 years (10–13). In a study similar to ours in the BLSA cohort, Harman and colleagues (31) found a duration-of-storage effect in plasma measures of testosterone. They reported a negative relationship between duration of storage and T levels in men. In the present study, the relationship between UFC/Cr and duration of storage was nonlinear; the oldest and the most recent samples had the lowest UFC/Cr levels. This pattern cannot be easily explained by factors such as evaporation or storage integrity (ie, the oldest and newest samples were most similar). A more likely explanation is that different people with different characteristics have been recruited into the BLSA over the span of this study. In view of this, we controlled for age, sex, race, and BMI on date of visit and found that these factors did not account for the duration of storage effect. Thus, consistent with the approach of Harman and colleagues, we adjusted for duration of storage in our models.
A second limitation of our study is that 24-hour UFC/Cr levels do not reveal information about potential age-related circadian changes in cortisol production or excretion. Several studies have shown significant age-related blunting of the circadian rhythm in cortisol levels (32–34). However, the assessment of 24-hour UFC/Cr also eliminates the potential confounds arising from the collection of a single daily or even multiple daily samples, which will be less reflective of cumulative exposure. Repeated sampling of cortisol levels throughout the day as well as 24-hour UFC collection can be considered complementary methodological approaches contributing to the knowledge of different aspects of age-related changes in patterns of cortisol exposure.
In summary, the present study described the pattern of longitudinal changes in cortisol across the adult life span. Early adulthood was associated with small decreases in cortisol with relative stability in middle age, followed by larger increases in older age. Moreover, our data suggest that several participant variables and medications may affect cortisol across the life span. Consistent with previous studies, we observed a high degree of inter- and intraindividual variability. This marked variability in cortisol levels and trajectories parallels the large age-related individual differences in health outcomes and in the function of various body systems. These data raise important questions about the potential protective or exacerbating role of cortisol exposure in predicting medical, physiological, and behavioral outcomes.
Funding
This work was funded by National Institute on Aging (NIH) (grant R01 AG028466) awarded to S.D.M. and in part by the Intramural Research Program, NIH.
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
All authors contributed substantially to the published work.
References
- 1. Maggio M, Cappola AR, Ceda GP, et al. The hormonal pathway to frailty in older men. J Endocrinol Invest. 2005;28(11 Suppl):15–19. [PubMed] [Google Scholar]
- 2. Vogelzangs N, Suthers K, Ferrucci L, et al. Hypercortisolemic depression is associated with the metabolic syndrome in late-life. Psychoneuroendocrinology. 2007;32:151–159. doi: 10.1016/j.psyneuen.2006.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gardner MP, Lightman S, Sayer AA, et al. ; Halcyon Study Team Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and physical performance at older ages: an individual participant meta-analysis. Psychoneuroendocrinology. 2013;38:40–49. doi: 10.1016/j.psyneuen.2012.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sherman B, Wysham C, Pfohl B. Age-related changes in the circadian rhythm of plasma cortisol in man. J Clin Endocrinol Metab. 1985;61:439–443. doi: 10.1210/jcem-61-3-439 [DOI] [PubMed] [Google Scholar]
- 5. Waltman C, Blackman MR, Chrousos GP, Riemann C, Harman SM. Spontaneous and glucocorticoid-inhibited adrenocorticotropic hormone and cortisol secretion are similar in healthy young and old men. J Clin Endocrinol Metab. 1991;73:495–502. doi: 10.1210/jcem-73-3-495 [DOI] [PubMed] [Google Scholar]
- 6. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201 [DOI] [PubMed] [Google Scholar]
- 7. Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J Clin Endocrinol Metab. 1997;82:2458–2465. doi: 10.1210/jcem.82.8.4173 [DOI] [PubMed] [Google Scholar]
- 8. Rueggeberg R, Wrosch C, Miller GE. Sleep duration buffers diurnal cortisol increases in older adulthood. Psychoneuroendocrinology. 2012;37:1029–1038. doi: 10.1016/j.psyneuen.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 9. Lupien S, Lecours AR, Schwartz G, et al. Longitudinal study of basal cortisol levels in healthy elderly subjects: evidence for subgroups. Neurobiol Aging. 1996;17:95–105. doi:10.1016/0197-4580(95)02005-5 [DOI] [PubMed] [Google Scholar]
- 10. Kley HK, Schlaghecke R, Krüskemper HL. Stability of steroids in plasma over a 10-year period. J Clin Chem Clin Biochem. 1985;23:875–878. [PubMed] [Google Scholar]
- 11. Miki K, Sudo A. Effect of urine pH, storage time, and temperature on stability of catecholamines, cortisol, and creatinine. Clin Chem. 1998;44(8 Pt 1):1759–1762. [PubMed] [Google Scholar]
- 12. Soliman SA, Abdel-Hay MH, Sulaiman MI, Tayeb OS. Stability of creatinine, urea and uric acid in urine stored under various conditions. Clin Chim Acta. 1986;160:319–326. doi:10.1016/0009-8981(86)90200-7 [DOI] [PubMed] [Google Scholar]
- 13. Bolelli G, Muti P, Micheli A, et al. Validity for epidemiological studies of long-term cryoconservation of steroid and protein hormones in serum and plasma. Cancer Epidemiol Biomarkers Prev. 1995;4:509–513. [PubMed] [Google Scholar]
- 14. Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiwa T, Oki K, Yamane K, Yoneda M, Awaya T, Nakanishi S, Kohno N. Significantly high level of late-night free cortisol to creatinine ratio in urine specimen in patients with subclinical Cushing’s syndrome. Clin Endocrinol. 2013;79:617–622. doi: 10.1111/cen.12197 [DOI] [PubMed] [Google Scholar]
- 16. de Kock A, Malan L, Hamer M, Cockeran M, Malan NT. Defensive coping and renovascular disease risk – adrenal fatigue in a cohort of Africans and Caucasians: the SABPA study. Physiol Behav. 2015;147:213–219. doi: 10.1016/j.physbeh.2015.04.033 [DOI] [PubMed] [Google Scholar]
- 17. Dowling TC, Wang ES, Ferrucci L, Sorkin JD. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: impact on renal drug dosing. Pharmacotherapy. 2013;33:912–921. doi: 10.1002/phar.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhathena SJ, Berlin E, Judd JT, et al. Effects of omega 3 fatty acids and vitamin E on hormones involved in carbohydrate and lipid metabolism in men. Am J Clin Nutr. 1991;54:684–688. doi: 10.1093/ajcn/54.4.684 [DOI] [PubMed] [Google Scholar]
- 19. Delarue J, Matzinger O, Binnert C, Schneiter P, Chioléro R, Tappy L. Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes Metab. 2003;29:289–295. doi:DM-06-2003 -29-3-1262-3636-101019-ART12 [DOI] [PubMed] [Google Scholar]
- 20. Field T, Hernandez-Reif M, Diego M, Schanberg S, Kuhn C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int J Neurosci. 2005;115:1397–1413. doi: 10.1080/00207450590956459 [DOI] [PubMed] [Google Scholar]
- 21. Starks MA, Starks SL, Kingsley M, Purpura M, Jäger R. The effects of phosphatidylserine on endocrine response to moderate intensity exercise. J Int Soc Sports Nutr. 2008;5:11. doi: 10.1186/1550-2783-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shalev I, Lerer E, Israel S, et al. BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology. 2009;34:382–388. doi: 10.1016/j.psyneuen.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 23. Brummett BH, Kuhn CM, Boyle SH, Babyak MA, Siegler IC, Williams RB. Cortisol responses to emotional stress in men: association with a functional polymorphism in the 5HTR2C gene. Biol Psychol. 2012;89:94–98. doi: 10.1016/j.biopsycho.2011.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee SY, Hwang YK, Yun HS, Han JS. Decreased levels of nuclear glucocorticoid receptor protein in the hippocampus of aged Long-Evans rats with cognitive impairment. Brain Res. 2012;1478:48–54. doi: 10.1016/j.brainres.2012.08.035 [DOI] [PubMed] [Google Scholar]
- 25. Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 26. Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seiffge-Krenke I, Aunola K, Nurmi JE. Changes in stress perception and coping during adolescence: the role of situational and personal factors. Child Dev. 2009;80:259–279. doi: 10.1111/j.1467-8624.2008.01258.x [DOI] [PubMed] [Google Scholar]
- 28. Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1111/j.1467-8624.2008.01258.x [DOI] [PubMed] [Google Scholar]
- 29. Brown GR, Spencer KA. Steroid hormones, stress and the adolescent brain: a comparative perspective. Neuroscience. 2013;249:115–128. doi: 10.1016/j.neuroscience.2012.12.016 [DOI] [PubMed] [Google Scholar]
- 30. Gettler LT, McDade TW, Kuzawa CW. Cortisol and testosterone in Filipino young adult men: evidence for co-regulation of both hormones by fatherhood and relationship status. Am J Hum Biol. 2011;23:609–620. doi: 10.1002/ajhb.21187 [DOI] [PubMed] [Google Scholar]
- 31. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219 [DOI] [PubMed] [Google Scholar]
- 32. Friedman M, Green MF, Sharland DE. Assessment of hypothalamic-pituitary-adrenal function in the geriatric age group. J Gerontol. 1969;24:292–297. [DOI] [PubMed] [Google Scholar]
- 33. Jensen J, Nilas L, Christiansen C. Cyclic changes in serum cholesterol and lipoproteins following different doses of combined postmenopausal hormone replacement therapy. Br J Obstet Gynaecol. 1986;93:613–618. doi:10.1111/j.1471-0528.1986.tb08035.x [DOI] [PubMed] [Google Scholar]
- 34. Touitou Y, Fèvre M, Lagoguey M, et al. Age- and mental health-related circadian rhythms of plasma levels of melatonin, prolactin, luteinizing hormone and follicle-stimulating hormone in man. J Endocrinol. 1981;91:467–475. doi:10.1677/joe.0.0910467 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.