Abstract
There is increasing concern that overlapping patents in the field of genetics will create a costly and legally complex situation known as a patent thicket, which, along with the associated issues of accumulating royalty payments, can act as a disincentive for innovation. One potential means of preventing this is for the patent holders to enter into a so-called patent pool, such as those established in the electronics and telecommunications industries. Precedents for these also exist in the field of genetics, notably with the patents pertaining to the SARS genome. In this review, we initially address the patent pool concept in general and its application in genetics. Following this, we will explore patent pools in the diagnostic field in more detail, and examine some existing and novel examples of patent pools in genetics.
Patent thickets and royalty stacking
The essence of innovation is cumulative investigation where each invention builds on many previous findings. However, if these previous findings are patented, each person who previously contributed must grant permission for their work to be used [1]. This leads to the emergence of a patent thicket (see Glossary), through which anyone who wishes to develop and eventually commercialize a new product must navigate his or her way [2].
Recent studies have reported on the licensing practices of the owners of patents for genetic inventions 3, 4, 5, 6, and concerns have been raised that patent thickets, resulting in royalty stacking (see Glossary), block access to patented technology through the accumulated license fees that a downstream inventor has to pay to upstream patent holders. Although the existence of an anticommons effect (see Glossary) of patents 7, 8 has not been validated by comprehensive empirical data, it is pertinent to reflect on ways to remedy this in the event that facts and cases arise that substantiate such an effect.
The patent pool model
Various mechanisms have been suggested to clear patent thickets [9], including patent pools, which are agreements between two or more patent owners to license one or more of their patents as a package to one another, and to third parties willing to pay the associated royalties (Figure 1 ). Agreements with third parties can be accomplished directly, between patentees and licensees, or indirectly, through the establishment of a body specifically set up to administer the pool 1, 9, 10, 11.
Figure 1.
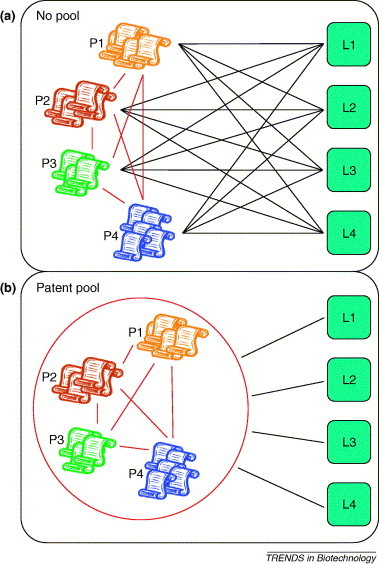
Comparative illustration of the different licenses needed in the absence (a) or presence (b) of a patent pool. P1–P4 represents the patent holders. L1–L4 represents the licensees. In the absence of a patent pool, licensees have to enter into negotiations with all the patent holders, which is a time consuming and expensive process. By contrast, in the presence of a patent pool licensees turn to the patent pool for acquiring the rights as one package, which results in simplification and a significant reduction of transaction costs.
Patent thickets have arisen in technical fields other than genetics, and patent pools have emerged previously to deal with overlapping patents 11, 12. For example, in 1917 an aircraft pool was formed that encompassed almost all aircraft manufacturers [13] and was crucial to the US entering World War I. In the late 1990s, several patent pools were formed in the electronics and telecommunications industries, starting with the moving picture experts group (MPEG)-2 pool in 1997 for inventions relating to the MPEG-2 standard (see Klein, J.I. (1997) Business Review Letter to Gerald R. Beeny), with others to follow (see Klein, J.I. Business Review Letter to Gerald R. Beeney regarding DVD (1998) and to Carey R. Ramos (1999) both regarding DVD–Video and DVD–ROM; and James, C.A. Business Review Letter to Ky P. Ewing regarding Third Generation Mobile Communication Systems (2002): available at http://www.usdoj.gov/atr/public/busreview/letters.htm).
In an attempt to deal with any potential anticompetitive effects of multiparty licensing agreements, such as patent pools, both the US antitrust agencies and the European Commission have established guidelines. The US antitrust agencies have developed the Antitrust Guidelines for the Licensing of Intellectual Property (IP Licensing Guidelines) [14]. In the European Union, the major competition laws (see Glossary) relating to technology licensing are laid down in the Commission Block Exemption Regulation (EC) No. 772/2004 on Technology Transfer Agreements [15] and the Guidelines on the Application of Article 81 of the EC Treaty to Technology Transfer Agreements [16]. Recently, the Japanese Fair Trade Commission issued its Guidelines on Standardization and Patent Pool Arrangements [17], which apply the same general principles. Close examination of foregoing guidelines, regulations and related decisions provides valuable information on the attitude of the US, European [18] and Japanese authorities towards patent pools. In short, patent pools should avoid creating anticompetitive restraints and will most probably be accepted if they meet the conditions set out in Box 1 .
Box 1. Checklist for a patent pool arrangement.
-
•
Validity of the patents: a patent is valid from the date of grant until the date of expiration, as defined by law, which is usually 20 years from the date of the filing provided the annual maintenance fees are paid.
-
•
Essentiality of the patents: a technology or patent is deemed to be essential if there are no substitutes for that technology inside or outside the pool and the technology in question constitutes a necessary part of the package of technologies for the purposes of producing the product(s) or carrying out the process(es) to which the pool relates.
-
•
Independent expert: an independent expert identifies and evaluates the essential patents related to the technology.
-
•
Non-exclusive licenses to the pool: a license is non-exclusive when one or more licensees are granted the right to use licensed technology covered by the patent(s) during the term of the license and when the licensor retains the right to use the licensed technology and the associated patent(s) as well.
-
•
Alternative technologies: licensees are free to develop and use alternative technologies.
-
•
Grantback provisions: a licensee should grant the licensor non-exclusive licenses for improvements on the licensed technology. This should be limited to essential patents and be settled on reasonable terms in order not to discourage further innovation.
-
•
Royalty allocation formula: royalties are distributed among the licensors according to an agreed allocation formula set forth in the patent pool agreement.
-
•
FRAND terms: royalties paid to the pool by the licensees should be fair, reasonable and non-discriminatory (the so-called FRAND terms), and licenses granted by the pool should be non-exclusive.
-
•
Safeguards for sensitive business information: competitively sensitive business information on the licensee is safeguarded in case auditing mechanisms for the management of royalties are established.
-
•
Mechanism for dispute resolution: an independent and, therefore, neutral dispute resolution mechanism in the agreements setting up the pool is desirable.
These are based on the guidelines laid down by the US IP Licensing Guidelines and Business Review Letters, the EU Transfer of Technology Guidelines and individual decisions, and the Japanese Guidelines on Standardization and Patent Pool Arrangements.
The establishment of a patent pool is a long, complex, multi-step process. In view of the varied issues and interests at stake, the expertise and joint collaboration of highly qualified patent attorneys, technical experts in the relevant field and legal advisors, both in the field of patent law and competition law, are required (Figure 2 ).
Figure 2.
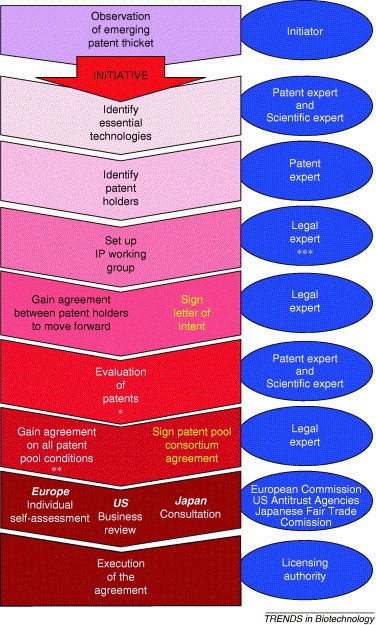
Overview of successive steps in the process of setting up a patent pool (block arrows on the left) and professional expertise needed at every step (associated balloons on the right). * Essential/non-essential character, ** Structure, technologies, royalties, dispute settlement system, etc., *** Legal expert: Attorneys and academic advisors This scheme is based on a document by James Simon.
Benefits and risks
The successful set-up of the electronics and telecommunications pools demonstrates that patent pools can have significant benefits, the first of which is the elimination of stacking licenses (see Glossary) [10]. A second benefit is the reduction of licensing transaction costs through the introduction of a system of ‘one-stop licensing’ for non-member licensees 10, 11, which provides an alternative to having to negotiate and acquire separate licenses directly from each of the patent owners (Figure 1). However, the initial cost of setting up and negotiating a pool agreement will often be high: all steps in the process involve costs [11] (Figure 2). A third benefit is a decrease in patent-related litigation 1, 10.
A patent pool also leads to the exchange of technical information that is not covered by patents, through a mechanism for sharing technical information relating to the patented technology that would otherwise be kept a trade secret [11]. Furthermore, patent pools can forestall government policy: it is better to encourage companies to establish patent pools than force them into a compulsory licensing scheme [11]. Such a suggestion, however, seems to ignore the fact that the major prerequisite for establishing patent pools is the voluntary participation of all patent holders, whereas the compulsory licensing mechanism is the last resort for patent holders who do not wish to enter into (reasonable) voluntary licensing negotiations.
Patent pools are, however, not without potential risks, for example, they might shield invalid patents [19] or entail the risk of inequitable remunerations (although expert valuation could settle disagreements on the value of the patents) [11]. The major criticism, however, is the danger of covering for a cartel and the subsequent anticompetitive effects this would have 1, 11, 19.
Patent pools for genetic inventions
To what extent the patent pool mechanism can be applied to genetic inventions, and whether such a scheme leads to the expected benefits are important questions. The Organization for Economic Co-operation and Development (OECD; www.oecd.org) considers the concept of a patent pool to be an interesting one for biotechnology but has some doubts as to whether the technologies and markets for genetic inventions are amenable to patent pools [20]. The medical biotechnology industry is perceived as fundamentally different from the electronics and telecommunications sectors, particularly as the generation of standards, as used in electronics and telecommunications, for the interoperability of electronic devices is seen as a strong incentive for setting up a patent pool. In the absence of this type of standard-driven incentive, dominant players in the biotech industry might be reluctant to join a pool because there is no apparent gain. Additionally, biotech companies rely heavily on their patent portfolio, and foster what has been called a bunker mentality: a defensive attitude focused on self-protection and secrecy [19]. In light of these considerations, the OECD recommends further study [20]. In the meantime, some valuable contributions to the debate, which focus on the importance of standard setting for diagnostic testing, have been reported 21, 22.
Golden rice
An instructive case on patterns of protection, and on negotiation through patent thickets, was published in the field of agricultural biotechnology 23, 24. In the Golden Rice case, Potrykus succeeded in genetically enriching rice grains with β-carotene, the precursor to vitamin A, which gives them a yellow hue: hence, they are called Golden Rice. Potrykus wanted to transfer the Golden Rice materials to developing countries for further breeding, and to introduce the trait into the local varieties consumed in developing countries. However, a freedom-to-operate survey initially uncovered 70 patents, belonging to 32 different companies and universities, embedded in Golden Rice. The six key-patent holders were approached, and an agreement was reached that allowed Potrykus to grant licenses, free of charge, to developing countries, with the right to sub-license (press releases 16 May 2000; 22 January 2001; and 14 October 2004; see www.syngentia.com). Consequently, a humanitarian board (HumBo; www.goldenrice.org) was established as a voluntary association to assist in the associated governance and decision making [25]. So far, approximately 20 master licenses have been granted to institutions in developing countries in Asia (Anatole F. Krattiger, personal communication).
The Golden Rice case is an example of how private and public organizations, in a combined effort, dealt with the patent thicket by creating a non-profit, humanitarian (and, therefore, probably atypical) patent pool in the form of a single licensing authority 26, 27, 28, 29.
SARS patent pool
A recent case in which overlapping patents are emerging, and in which laboratories try to remove the thicket by way of a pool, relates to the biomedical field, specifically to the severe acute respiratory syndrome (SARS) corona virus [30]. In response to the outbreak of SARS, the World Health Organization (WHO; www.who.int/en/) set up a network of laboratories to help control the disease, which led to the isolation of the causative virus and the sequencing of its genome. Two groups are credited with discovering the SARS genome, independently from each other 31, 32, and several of the contributing laboratories filed patent applications incorporating SARS genomic sequence data. Further research then led to the filing of additional patent applications by a multitude of public and private sector entities [30]. The WHO set up a SARS consultation group, who proposed ‘that a strategy be developed, in consultation with stakeholders, to address potential SARS corona virus related intellectual property issues and, thus, enhance development of intervention approaches’.
At present, the relevant parties have been identified, and principal agreement has been gained, officially, by the signing of a letter of intent. Highly qualified technical and legal experts have assisted the parties during the chain of negotiations. The resulting pool, should the parties conclude a full agreement, will be set-up in the USA, followed by attempts to set up pools elsewhere [30].
HNPCC patent pool: a test for diagnostic testing?
Genetic diseases are caused by mutations in genes. In some cases, such as hereditary non-polyposis colorectal cancer (HNPCC), the disease can be caused by a variety of mutations in one gene, or by one or more mutations in several genes. The diagnosis of HNPCC in a particular family is, in part, based on molecular genetic testing for germline mutations in one of the mismatch-repair (MMR) genes. Typically, patients are being tested for mutations in two or more out of four candidate genes (MLH1, MSH2, MSH6, and PMS2; see review of HNPCC on www.genetests.org). However, other genes involved in the MMR pathway have been reported to be associated with HNPCC (e.g. MLH2, MLH3, PMS1, MSH3, MSH5, MYH; see OMIM entries on http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM), and the number of genes identified as being involved in familial colorectal cancer is expected to grow. Some of these newly identified genes might soon be included on the shortlist for routine testing, and, as various patents have been filed, it is possible that overlapping patents might occur on the genetic data necessary to test for HNPCC. Should a patent thicket arise, an HNPCC patent pool, encompassing essential genomic patents, could help to eliminate the thicket and render proprietary genomic data more accessible for use. Additionally, such a patent pool should be considered a dynamic model with regard to both size and use, whereby the size and content of the pool will differ over time: competition law requires that additional essential patents, once granted, will enter the pool (e.g. relating to other genes with a role in the same pathology and on particular mutations in those genes) and others will disappear when no longer valid. Furthermore, the granting of licenses to a subset of patents should also be possible. Here, some genetic laboratories offering testing for the clinical condition as a whole might be interested in the entire pool, whereas other laboratories might only be interested in a license to a subset of patents in the pool, a subset of disease genes or mutations (which are of specific interest in view of the geographical heterogeneity of the distribution of mutations), a specific gene, or a particular mutation for the development of an antibody or another therapeutic or research tool. In addition, the licenses granted by the pool should be non-exclusive and non-discriminatory, thereby imposing fair and reasonable conditions and royalty rates.
Incentives
The initial impetus for patenting an invention is to award original research and recuperate investment through revenue from the royalties due on the commercialization of the invention; however, will the creation of a patent pool still provide the patentee with such significant gains?
Standards
Standards are technical specifications relating to a product or an operation, which are recognized by a large number of manufacturers and users [33]. Standards can be an important trigger to set up a pool, as illustrated in the electronics and telecommunications sectors, and this might also be true in the field of genetics 20, 21. A genetic standard should not necessarily be looked at in terms of a technical specification but could present itself as a set of mutations recognized by the international scientific community. Alternatively, it could reflect national or international best practice guidelines for genetic testing for a particular disease, such as the standards and guidelines issued by the American College of Medical Genetics (www.acmg.net) for cystic fibrosis [34] or Huntington's disease [35] – such guidelines could facilitate the establishment of corresponding patent pools. They could also be an important asset in the dissemination of knowledge of patent coverage for genetic inventions, and could promote the collection of licensing fees.
Potential revenue
The potential revenue from a patent will depend on the total number of patients eligible for a genetic test; however, the actual revenue will be determined by the amount of diagnostic kits sold by the manufacturers and the number of tests effectively carried out in diagnostic testing centers. At present, owners of genetic patents predominantly provide licenses to companies developing commercial kits and to large diagnostic laboratories. Patent pools might constitute the ideal means for raising the visibility and accessibility of smaller or public genetic laboratories and, thus, increase the actual amount of collected royalties, bridging the gap between potential and actual revenue. For example, some laboratories still use in-house methods to test for cystic fibrosis, although several appropriate kits are available commercially. For some genes, the diagnostic method for the detection of mutations is less amenable to the production of a commercial kit, which is presently the case for breast and ovarian cancer, tuberous sclerosis and neurofibromatosis. In such instances, litigation is difficult because data informing on the number of tests being performed are hard to find and legal action is costly; however, the introduction of one-stop licenses, through the establishment of patent pools, might promote a spontaneous registration by the users and simplify the collection of license fees.
For molecular diagnostic laboratories, a patent pool comprising the widely owned rights to diagnostic genes can help these institutions to adjust to the emerging phenomenon of patents in their practice, and facilitate the regularization of their service by creating clarity and legal certainty, in addition to lowering the barrier to entry into this field. For similar reasons, a patent pool can remove the reluctance to enter into research, and incite innovation and the development of new tests.
Some remaining hotspots
There are many remaining issues that must be considered when further exploring the patent pool model for genetic inventions. First, patent pools are designed to remove the stacking of multiple patents and multiple patent holders. Hence, the model is not applicable when a single patent holder controls all the patents relevant for the genetic testing for a particular disease, for example, one patent owner holds the different patents covering the diagnosis of hemochromatosis [36]. The three biotech cases discussed in this review – Golden Rice, SARS and the hypothetical example of HNPCC – involve multiple patents belonging to two or more patent holders.
Secondly, patent pools rely on the voluntary engagement of the patent owners; therefore, they do not offer a solution in cases where patent holders do not wish to grant reasonable licenses or refuse to license at all. In both the Golden Rice and the SARS cases, voluntary negotiations appear to have been successful and it can only be hoped that the same will be true in future cases. If not, a compulsory patent pool, in which the administering body would seek a compulsory license for essential technology from all patent holders that do not voluntarily engage in the pool, could be further investigated. However, it remains to be seen whether these measures will be permitted within the confines of intellectual property and competition law.
Finally, the major incentive for all parties is economic benefit. In order for a patent pool to be an effective solution, the right balance has to be achieved between the cost of creating a pool and the prospect of adequate revenue generated by royalties on the end-product. It remains to be seen whether a diagnostic-gene patent pool covering only one disease syndrome will reach such a balance, and to what extent small size pools will prove to be viable. Extending those pools to a wider range of, or to all, genetic disorders could prove to be more useful from an economic or a clearing point of view but might lead to some delicate problems from the perspective of competition law.
Conclusions
Given their specific features, and the potential for stacking licenses (see Glossary) in the genetics sector, setting up patent pools might prove to be helpful in the area of genetic testing by clearing patent thickets. Patent pools can be particularly useful for disorders caused by multiple defects in a single gene, diseases caused by one or more defects in multiple genes or for the more common multifactorial diseases, for which complex genetic associations are being discovered and, consequently, a larger thicket could emerge.
The emerging standards for good practice in medical and laboratory genetics can be helpful in setting up patent pools and, conversely, the thorough scientific evaluation of the patent portfolio in the framework of a patent pool could help to establish, or to adjust, those standards.
However, when setting up pools for the clearance of stacking licenses for diagnostic purposes, competition law has to be taken into account to avoid potential anticompetitive effects.
Various governmental and non-governmental institutions, such as WHO, OECD, HUGO and the National Institutes of Health (NIH; www.nih.gov), and professional societies, such as the American Society of Human Genetics (ASHG; www.faseb.org/genetics/ashg/ashgmenu.htm) and the European Society for Human Genetics (ESHG; http://www.eshg.org/), might act to promote the formation of patent pools in this area. Well-tailored pools could, indeed, serve economic and societal public-health goals. To prevent the establishment of such pools becoming prohibitively expensive as a result of the costly expertise required, funding from such organizations to aid setting up the pools will be more than welcome.
Acknowledgements
This research was supported by grant number G.0120.04 of the Fund for Scientific Research (FWO, Flanders, Belgium), the 6th Framework Programme of the European Union (Eurogentest) and the Vancraesbeeck Fund (KULeuven, Belgium). Special thanks go to Anatole F Krattiger and James Simon for their valuable information on the Golden rice and the SARS patent pool cases, respectively, and for interesting discussions.
Glossary
- Anticommons effect.
An effect arising from the situation where multiple owners each have the right to exclude others from the use of a resource and no one has an effective privilege of use: this results in under use of the resource [7,8].[7],[8]
- Antitrust or competition law.
Antitrust law is a term primarily used in the US, while in many other countries the term competition law is used. Most antitrust or competition laws have provisions dealing with mergers, abuse of a dominant position and anticompetitive practices.
- Patent thicket.
The intellectual property portfolios of several companies that form a dense web of overlapping intellectual property rights [1].[1]
- Royalty stacking.
The accumulation of royalties that have to be paid when confronted with a patent thicket [8].[8]
- Stacking licenses.
Give the owner of a patented invention used in upstream research rights in subsequent downstream innovations [7].[7]
References
- 1.Shapiro, C. (2001) Navigating the patent thicket: cross licenses, patent pools and standard setting. In Innovation Policy and the Economy (Vol. I) (Jaffe, E. et al., eds), pp. 119–150, MIT Press (Also available at http://haas.berkeley.edu/∼shapiro/thicket.pdf)
- 2.Scherer F.M. The economics of human gene patents. Acad. Med. 2002;77:1348–1367. doi: 10.1097/00001888-200212001-00006. [DOI] [PubMed] [Google Scholar]
- 3.Straus, J. et al. (2002) Genetic inventions and patent law, Max-Planck-Institüt für ausländisches und internationals Patent-, Urheber- und Wettbewerbsrecht & Bundesminsterium für Bilding und Forschung
- 4.Walsh, J. et al. (2003) Effects of research tool patents and licensing on biomedical innovation. In Patents in the Knowledge-Based Economy. (Cohen, W.M. and Merrill, S.A., eds), pp. 285–240, National Academic Press
- 5.Merz J.F. Diagnostic testing fails the test. Nature. 2002;415:577–579. doi: 10.1038/415577a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicol, D. and Nielsen, J. (2003) Patents and Medical Biotechnology: an Empirical Analysis of Issues Facing the Australian Industry, Centre for Law and Genetics, Occasional paper 6 (also available at http://www.law.unimelb.edu.au/ipria/publications/reports.html)
- 7.Heller M.A., Eisenberg R.S. Can patents deter innovation? The anticommons in biomedical research. Science. 1998;280:698–701. doi: 10.1126/science.280.5364.698. [DOI] [PubMed] [Google Scholar]
- 8.Heller M. The tragedy of the anticommons: property in the transition from Marx to markets. Harv. Law Rev. 1998;111:621–688. [Google Scholar]
- 9.Van Overwalle, G. et al. (2005) Models for facilitating access to patents on genetic inventions. Nat. Rev. Gen.DOI:10.1038/nrg1765 (http://www.nature.com/nrg/index.html) [DOI] [PMC free article] [PubMed]
- 10.Clark, J. (2000) Patent pools: a solution to the problem of access in biotechnology patents? White Paper commissioned by Q. Todd Dickinson, the Under Secretary of Commerce for Intellectual Property and Director of the United States Patent and Trademark Office (available at http://www.uspto.gov/web/offices/pac/dapp/opla/patentpool.pdf)
- 11.Merges R. Institutions for intellectual property transactions: the case of patent pools. In: Dreyfuss R., editor. Expanding the Boundaries of Intellectual Property. Oxford University Press; 2001. pp. 123–166. [Google Scholar]
- 12.Carlson S.C. Patent pools and the antitrust dilemma. Yale J. Regul. 1999;16:359–399. [Google Scholar]
- 13.Dykman H.T. Patent licensing within the manufacturer's aircraft association. Journal of the Patent Office Society. 1964;46:646. [Google Scholar]
- 14.US Department of Justice & Federal Trade Commission (1995) Antitrust Guidelines for the Licensing of Intellectual Property. (“IP Licensing Guidelines”), available at http://www.usDoJ.gov/atr/public/guidelines/ipguide.htm)
- 15.European Commission (2004) Regulation (EC) No. 772/2004 of 27 April 2004 on the application of Article 81 (3) of the Treaty to categories of technology transfer agreements [2004] O.J. L 123/11. This Regulation replaces Commission Regulation (EC) No. 240/96 of 31 January 1996 on the application of Article 85 (3) of the Treaty to certain categories of technology transfer agreements, [1996] O.J. L 31/2
- 16.European Commission (2004) Guidelines on the application of Article 81 of the EC Treaty to technology transfer agreements [2004] O.J. C 101/2
- 17.Japanese Fair Trade Commission (2005) Guidelines on Standardization and Patent Pool Arrangements (available at http://www.jftc.go.jp/e-page/legislation/ama/patentpool.pdf)
- 18.Goldstein J.A. Patent pools as a solution to the licensing problems of diagnostic genetics, United States and European perspectives. Drug Discovery World. 2005:86–91. [Google Scholar]
- 19.Carlson S.C. Patent pools and the antitrust dilemma. Yale Journal on Regulation. 1999;16:359–399. [Google Scholar]
- 20.Organization for Economic Co-Operation and Development (OECD) (2002) Genetic Inventions, Intellectual Property Rights and Licensing Practices, Report of a workshop organized by the OECD Working Party on Biotechnology (available at http://www.oecd.org/dataoecd/42/21/2491084.pdf)
- 21.Eversible T.J. Patent pools as a solution to the licensing problems of diagnostic genetics. Intellectual Property & Technology Law Journal. 2005;17:6–13. [Google Scholar]
- 22.Eversible T.J. Patent pools and standard setting in diagnostic genetics. Nat. Biotechnol. 2005;23:937–938. doi: 10.1038/nbt0805-937. [DOI] [PubMed] [Google Scholar]
- 23.Beyer P. Golden Rice: introducing the beta-carotene biosynthesis pathway into rice endosperm by genetic engineering to defeat vitamin A deficiency. J. Nut. 2002;132:506S–510S. doi: 10.1093/jn/132.3.506S. [DOI] [PubMed] [Google Scholar]
- 24.Ryder, R.D. et al. (2000) The intellectual and technical property components of pro-vitamin A rice (Golden Rice™): a preliminary freedom to operate review. In ISAAA Briefs No. 20, ISAAA: Ithaca (also available at www.isaaa.org)
- 25.Lubbock, A.C. (2003) Public goods and public policy for agricultural biotechnology. 7th ICABR International Conference, Ravalli (Italy), June 29 to July 3
- 26.Graff, G. and Silverman, D. (2001) Towards an intellectual property clearing house for Ag-biotechnology. An Issues Paper. IP Strategy Today Volume 3 (available at www.bioDevelopments.org)
- 27.Graff, G. et al. (2001) Towards an intellectual property clearing house for Ag-biotechnology. Summary of an Industry, Academia and International Development Round Table. IP Strategy Today Volume 3 (available at www.bioDevelopments.org)
- 28.Graff G.D. The public–private structure of intellectual property ownership in agricultural biotechnology. Nat. Biotechnol. 2003;21:989–995. doi: 10.1038/nbt0903-989. [DOI] [PubMed] [Google Scholar]
- 29.Parish R., Jargons R. Using the industry model to create physical science patent pools among academic institutions. Journal of the Association of University Technology Managers. 2003:65–79. [Google Scholar]
- 30.Simon J. Managing severe acute respiratory syndrome (SARS) intellectual property rights: the possible role of patent pooling. Bulletin of the World Health Organization. 2005;83:707–710. [PMC free article] [PubMed] [Google Scholar]
- 31.Rota P.A. Characterization of a novel corona virus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 32.Marra M.A. The genome sequence of the SARS-associated coronavirus. Characterization of a novel Corona virus associated with severe acute respiratory syndrome. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 33.European Commission (1992) Communication of 27 October 1992 on Intellectual Property Rights and Standardization, COM (92) 445 final
- 34.Richards C.S. Standards and guidelines for CFTR mutation testing. Genet. Med. 2002;4:379–391. doi: 10.1097/00125817-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Potter N.T. Technical standards and guidelines for Huntington's disease testing. Genet. Med. 2004;6:61–65. doi: 10.1097/01.gim.0000106165.74751.15. [DOI] [PubMed] [Google Scholar]
- 36.Mars J.F. Diagnostic testing fails the test. Nature. 2002;415:577–579. doi: 10.1038/415577a. [DOI] [PMC free article] [PubMed] [Google Scholar]


