Abstract
This review summarizes the current view of amino acid transport by epithelial cells of vertebrates. A wide variety of transporter proteins are expressed in apical and basolateral membranes and collectively play complex interactive roles in controlling the entire organism’s overall metabolism of amino acids. Regulation of the transport systems can be manifested at many levels, including gene splicing and promoter regulation, interactions between requisite subunits of oligomers, thermodynamic electrochemical gradients contributed by ion exchangers, overlap of substrate specificity, selective tissue distribution, and specific spatial distribution of transporters leading to net vectorial flow of the amino acids. The next frontier for workers in this field is to uncover a comprehensive molecular understanding of the manner by which epithelial cells signal gene expression of transporters as triggered by substrates, hormones or other triggers, in order to further understand the trafficking and interactions among multimeric transport system proteins, to extend discoveries of novel small drug substrates for oral and ocular delivery, and to examine gene therapy or nanotherapy of diseases using small molecules delivered via amino acid transporters.
Keywords: Membrane Transporter, Amino acid, Epithelial, Epithelium
Text
Transporters of epithelial membranes arguably represent a vertebrate organism’s most significant collective array of regulatory mechanisms that control the intermediary metabolism of amino acids. Such transporters control metabolic reactions by regulating throughput of substrates and products, and creating steady state pools of metabolites. Epithelial membrane transporters modulate metabolic flows and pools of both the proteinogenic and the nonproteinogenic amino acids. Working in conjunction with one another, amino acid transporters maintain coordination and integrity of metabolic events within tissues and even between organs.
This chapter reviews the current understanding of amino acid transport systems and transporters in basolateral membranes and apical membranes of epithelial cells in humans and other vertebrates. The chapter content is focused on transporters and transport systems that serve amino acids which are either nonproteinogenic or proteinogenic nutrients and metabolites, although some transporter genes and polypeptides are technically subsets categorized within larger groupings that are structurally and mechanistically related to the neurotransmitter transporters [1]. Also, many apparently unrelated so-called transporter “orphan” genes have been historically clustered as a single branch off the SLC6 neurotransmitter superfamily, although they are now emerging as actually being epithelial amino acid transporters related in structure, function, and probably oligomeric association in the membrane [2, 3].
This chapter attempts to catalog the body of literature encompassing the epithelial barriers of the kidney, gastrointestinal tract, lung, cornea, liver, gallbladder, and other tissues. The reader is directed to several fine reviews [4–8] that delve into other details of renal and intestinal absorptive epithelial membranes in physiological processes or their involvement in absorptive pharmacokinetics [9] of monomer amino acids or natural dipeptides and drug analogues [10, 11].
The concept of membrane transport physiology involves physical entities of discrete transporter genes and expressed polypeptides, in addition to the interactions and functionality of these units. “Transporter” and “transport system” are colloquially – although incorrectly – thought of as one and the same entity. Although a transporter’s functional activity can indeed occur by the action of a single species of monomeric transporter protein, there are many instances of amino acid transport functional activity that are catalyzed only by the concerted interaction of multiple subunit proteins in the membrane. This latter scenario is termed a “transport system,” which is comprised of multimeric arrangement of transporter proteins, usually as heterodimers.
Monomeric Transporter Proteins, Genes, and Transport Systems
Table 1 summarizes the known monomeric transporter proteins involved in epithelial regulation of amino acid movement across apical and basolateral membranes. Although most work has been done for small intestinal and renal transport, representation occurs for colon, liver, lung, cornea, adipose, testis, stomach, choroid plexus, and placenta. There is evidence for some SLC6 family members to form oligomers/homodimers [12, 13] in nonepithelial membranes, but this has not been confirmed for epithelial cell membranes. Further studies in this arena may indeed uncover that all SLC6 members form oligomers.
Table 1.
Monomeric amino acid transporters and transport systems in epithelial membranes. Transporters are grouped by SLC (solute carrier) gene families [1], utilizing Human Genome Organization (HUGO) Nomenclature Committee classifications [14]. Functional transport systems are named using the Christensen naming plan [15]. Expression and localization derived from the Swedish Human Proteome Resource [69]. There is evidence for some SLC6 family members to form oligomers/homodimers [12, 13] in nonepithelial membranes, but this has not been established for epithelial cells
| Transport “system” functional name | Monomer protein common alias | Gene | Human gene locus | Sequence accession ID | Representative substrates | Ion dependency | Tissue epithelium | Membrane location |
|---|---|---|---|---|---|---|---|---|
| SLC1 family | ||||||||
| XAG − | EAAT3 | SLC1A1 | 9q24 | NM_004170 |
l-glutamate, d/l-aspartate, cystine (SS) |
H+, Na+, K+ | Kidney (K), Intestine (I), liver | Apical |
| XAG − | EAAT2 | SLC1A2 | 11p13–p12 | NM_004171 |
l-glutamate, d/l-aspartate, cystine (SS) |
H+, Na+, K+ | Liver | Apical |
| ASC | ASCT1 | SLC1A4 | 2p13−p15 | NM_003038 | Alanine, serine, threonine, cysteine, glutamine | Na+ | Lung, stomach, K, I, cornea | Apical |
| ASC | ASCT2 or ATB0 | SLC1A5 | 19q13.3 | NM_005628 | Alanine, serine, threonine, cysteine, glutamine, branched neutrals | Na+ | K, I, lung, colon | Apical |
| XAG − | EAAT5 | SLC1A7 | 1p32.3 | NM_006671 | l-Glutamate, d/l-Aspartate | H+, Na+, K+ | Retina | Apical |
| SLC6 family | ||||||||
| 5-HTT | SERT | SLC6A4 | 17q11.1–q12 | NM_001045 | Serotonin, carnitine, organic cations | Na+, Cl−, K+ | ||
| β (beta) | TAUT | SLC6A6 | 3p25–p24 | NM_003043 | Taruine, β-alanine, GABA | Na+, Cl− | K, retina, placenta | Basolateral |
| Creatine | CRTR | SLC6A8 | Xq28 | NM_005629 | Creatine | Na+, Cl− | K, I, retina | Apical |
| GLY | GLYT1 | SLC6A9 | 1p33 | NM_201649 | Glycine | Na+, Cl− | Lung, stomach retina | Basolateral |
| GAT2 | GAT2 | SLC6A13 | 12p13.3 | NM_016615 | GABA | Na+, Cl− | K, retina, choroid plexus | |
| B0,+ | ATB0,+ | SLC6A14 | Xq23–q24 | NM_007231 |
Neutrals and dibasics, arginine, d-serine |
Na+, Cl− | Colon | Apical |
| GLY | XTRP2 | SLC6A18 | 5p15.33 | NM_182632 | Glycine | Na+, Cl− | K | Apical |
| B0 (or B) | B0AT1 | SLC6A19 | 5p15.33 | NM_001003841 | Neutrals, glutamine | Na+ | K, I, placenta | Apical |
| IMINO | SIT1 | SLC6A20 | 3p21.6 | NM_020208 | Proline, sarcosine, pipecolate | Na+ | K, I, choroid plexus, stomach, colon | Apical |
| SLC7 family | ||||||||
| y+ | CAT-1 | SLC7A1 | 13q12–q14 | NM_003045 | Arginine, ornithine, lysine, histidine, dibasics | None | Universal | Basolateral |
| SLC15 family | ||||||||
| Pept1 | PEPT1 | SLC15A1 | 13q33–q34 | NM_005073 | Dipeptides and tripeptides; carnosine, β-lactam antibiotics, angiotensin converting enzyme inhibitors | H+ with NHE3 | I, K | Apical |
| Pept2 | PEPT2 | SLC15A2 | 3q13.3–q21 | NM_021082 | Dipeptides and tripeptides | H+ with NHE3 | I, K, lung, mammary gland | Apical |
| PTR4 | PHT1 | SLC15A4 | 12q24.32 | NM_145648 | Histidine, di-, tri-peptides | H+ | Retina | Apical |
| SLC16 family | ||||||||
| T | TAT1 | SLC16A10 | 6q21–q22 | NM_018593 | Aromatics, l-DOPA | − | K, I, placenta | Basolateral |
| SLC22 family | ||||||||
| OCTN2VT | OCTN2 | SLC22A5 | 5q23.3 | NM_003060 | l-carnitine, acetyl-l-carnitine | Na+ | I, K, lung, liver | Apical |
| SLC36 family | ||||||||
| Iminoacid | PAT1 | SLC36A1 | 5q33.1 | AF516142 | Proline, glycine, b-alanine, GABA, taurine, d-serine | H+ with NHE3 | K, I, liver, lung, colon | Apical |
| Iminoacid | PAT2 | SLC36A2 | 5q33.1 | AY162214 | Proline, glycine, l-alanine | H+ with NHE3 | K, Lung | Apical |
| SLC38 family | ||||||||
| A | SNAT1 | SLC38A1 | 12q12–q13.11 | NM_0030674 |
Glutamine alanine, asparagine, cysteine, histidine, serine |
Na+ | Placenta, retina | Apical |
| A | SNAT2 | SLC38A2 | 12q | NM_018976 | Alanine, asparagine, cysteine, glutamine, glycine, histidine, methionine, proline, serine | Na+ | I, K, placenta, lung, liver | Basolateral |
| N | SNAT3, SN1 | SLC38A3 | 3p21.3 | NM_006841 | Glutamine, histidine | Na+, H+ | K, retina | Basolateral |
| A | SNAT4 | SLC38A4 | 12q13 | NM_018018 | Alanine, asparagine, cysteine, glycine, threonine | Na+ | K, liver, placenta | Basolateral |
| N | SNAT5 | SLC38A5 | Xp11.23 | NM_033518 | Glutamine, histidine, serine, asparagine, alanine | Na+ , H+ | I | |
| SLC43 family | ||||||||
| LAT3 | POV1 | SLC43A1 | 11p11.2 | NM_003627 | Branched chain amino acids, phenylalanine | None | K | |
| LAT4 | LAT4 | SLC43A2 | 17p13.3 | NM_152346 | Branched chain amino acids, phenylalaninine | None | I, K, placenta | Basolateral |
The components of 1 are based on the Human Genome Organization (HUGO) Nomenclature Committee classification nomenclature [14] for transporters as SLC families, with gene family refinement by Hediger et al. [1] Transport “system” functional names are derived from the original nomenclature of Christensen [15] as extended by many laboratories over the past several decades. Note that SLC genes can be grouped by the function of their expression product. In Table 1 a given physiologically functional transporting system results from the activity of a single polypeptide expression product trafficked to the appropriate membrane.
So-called historically “orphan transporters” are now emerging as actually being clustered related amino acid transporters of the SLC6 family. The dendogram in Fig. 1 shows the mouse SLC6 series of transporter proteins. The human creatine transporter-2 (SLC6A10) apparently does not have an ortholog correlate in mouse.
Fig. 1.
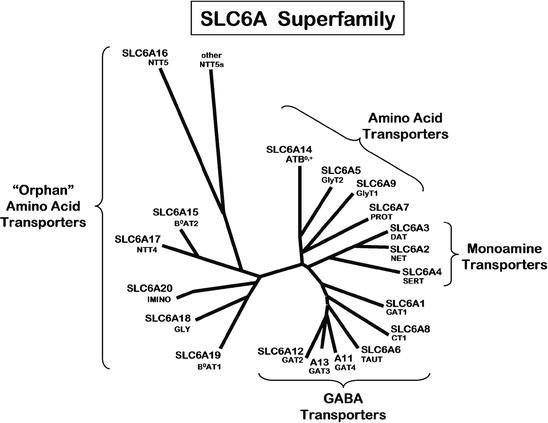
SLC6 family clustering of amino acid monomer transporters. Note that many apparently unrelated transporters have been historically clustered as a major branch of “orphans,” although their transporter polypeptide structures and possible oligomeric relationships are currently being uncovered as related. In this dendogram, the human gene SLC number is listed above the common name for each polypeptide expression product. Adapted from laboratories of Broer [2] and Verrey [3]
One of the “orphan” family genes, SLC6A20, encodes the SIT1 transporter serving proline and imino analogues via the sodium-dependent “IMINO” system in the intestine (IMINO has been characterized in [16]). In contrast, however, in the kidney and lung these substrates are served by the proton-activated “PAT/iminoacid” system which exhibits the activity profiles of two expressed transporter proteins PAT1 and PAT2, as encoded by the SLC36 family genes SLC36A1 and SLC36A2 (see Table 1). The latter genes reside on chromosome 5q33.1 in humans or 11B1.3 in mice, as shown in Fig. 2. The human and mouse open reading frames are each encoded by ten exons. This figure shows an example of the genomic exon/intron organization of the SLC36 genes responsible for tissue-dependent differential expression of the transporters.
Fig. 2.

Genomic exon/intron organization of the genes SLC36A1 (encoding PAT1) and SLC36A2 (encoding PAT2) on human chromosome 5q33.1. These genes are flanked by genes FAT2 and GM2A, shown for orientation. The mouse orthologs are located on chromosome 11B1.3. Putative exons are represented by vertical lines. Adapted from Boll et al. [66]
Heterodimer Regulation of Transport
For several transporting systems, a multimeric arrangement of polypeptides is required for physiological activity. Table 2 summarizes the well-established heterodimers required for these cases. Note that 4F2hc (alternative name CD98) is utilized as a subunit in many of these systems.
Table 2.
Heterodimeric amino acid transporters and transport systems in epithelial membranes. Transporter SLC (solute carrier) gene family names [1] are shown with Human Genome Organization (HUGO) Nomenclature Committee classifications [14]. Functional transport systems are named using the Christensen naming plan [15]. Expression and localization derived from the Swedish Human Proteome Resource [69]. The heavy chain 4F2hc subunit is synonymously called CD98
| Transport “System” functional unit name | Heterodimer subunits; protein common aliases | Subunit genes | Human gene locus | Sequence accession ID | Representative substrates | Ion dependency | Tissue epithelium | Membrane location |
|---|---|---|---|---|---|---|---|---|
| Asc |
asc1 plus 4F2hc |
SLC7A10 plus SLC3A2 |
19q12–13.1 11q13 |
Small neutral l- and d-amino acids, alanine, d-serine, cyst(e)ine, glycine | None | Basolateral | ||
| b0,+ |
b0,+AT plus rBAT |
SLC7A9 plus SLC3A1 |
19q13.1 2p16.3–p21 |
Dibasics, arginine, cystine, large neutrals (exchange extracellular dibasics with intracellular neutrals) | None | I, K, placenta | Apical | |
| L |
LAT1 plus 4F2hc |
SLC7A5 plus SLC3A2 |
16q24.3 11q13 |
Branched chain neutrals, l-DOPA | None | I, placenta | Basolateral | |
| L |
LAT2 plus 4F2hc |
SLC7A8 Plus SLC3A2 |
14q11.2 11q13 |
Branched chain neutrals (small and large) | None | I, K, placenta, stomach | Basolateral | |
| xc − |
xCT plus 4F2hc |
SLC7A11 plus SLC3A2 |
4q28–q32 11q13 |
Cystine/glutamate exchange | None | Choroid plexus, intestine, kidney | Basolateral (evidence in apical) | |
| y+L |
y+LAT1 plus 4F2hc |
SLC7A7 plus SLC3A2 |
14q11.2 11q13 |
Lysine, arginine, dibasics, neutrals | None for dibasics; Na+ for large neutrals | I, K, lung | Basolateral | |
| y+L |
y+LAT2 plus 4F2hc |
SLC7A6 plus SLC3A2 |
16q22.1–22.2 11q13 |
Lysine, arginine, dibasics, neutrals | None for dibasics; Na+ for large neutrals | I, K, lung | Basolateral |
Subunit polypeptides of amino acid transporters play multiple roles in nonepithelial cell types, and it is likely that in epithelial membranes they also serve multiple roles. For example, CD98/4F2hc at the basolateral membrane of intestinal epithelium contains a PDZ class II-binding domain [17] regulating integrin signaling, serving in such diverse roles as inflammation modulation, cell growth via mTOR [4, 18], cell adhesion, migration, and binding rotavirus enterotoxin [19]. 4F2hc (CD98) forms a heterodimer with amino acid transporter subunits in basolateral membranes – asc, LAT1, LAT-2, y+LAT1, y+LAT-2, and xCT – thereby resulting in six different functional transport systems (Table 2). Burdo et al. [20] reported evidence for xCT and 4F2hc in apical membranes of mouse and primate duodenum and renal tubules, as well as in choroid plexus although the particular membrane aspect was not specified for this brain tissue. In situ heterodimerization of y+LAT1 with 4F2hc has been visualized using acceptor photobleaching FRET microscopy [21]. Figure 3 shows the diverse ways by which the 4F2hc subunit plays a role in modulating amino acid transport at the basolateral membrane.
Fig. 3.
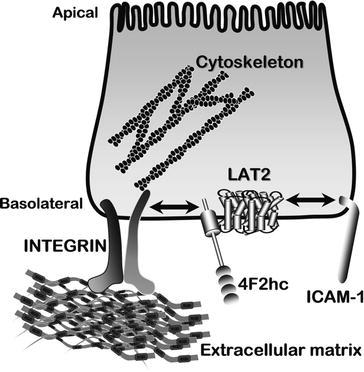

Interactions of subunit regulation of amino acid transport in intestinal basolateral membranes. Various example complexes are shown, including heterodimerization with LAT2, β1-integrins, and ICAM-1. 4F2hc (synonymous with CD98) modulates β1-integrin activation as well as activity of amino acid transport via LAT2. Furthermore, another polypeptide, ICAM-1, could independently modulate amino-acid transport activities via LAT2 at its extracellular C-terminal domain that contains a PDZ-binding domain. Adapted from Yan et al. [17, 67]
In addition to these 4F2hc basolateral systems, a separate dimerization coupling occurs at the apical surface which involves b0,+AT plus rBAT (Table 2). Extensive descriptions of models of the apical membrane heterodimer comprised of b0,+AT plus rBAT are presented elsewhere [5, 6, 22–26].
Mineralocorticoid receptors regulate at least some intestinal transporters. Work from Soares-da-Silva’s laboratory [27] demonstrates that chronic systemic aldosterone administration, acting through a spironolactone-sensitive mechanism, regulates rat intestinal LAT2 and 4F2hc subunit mRNA and protein expression as well as ASCT2 and LAT1 protein levels.
Dipeptide Transporters
Transepithelial movement of amino acids occurs by highly efficient transport of di- and tri-peptides across the apical membrane, with cytosolic hydrolysis by aminopeptidases then final basolateral transport of the constituent free amino acids. Proton-dependent secondary active transporters PEPT1 (SLC15A1) and PEPT2 (SLC15A2) are expressed in epithelia of small intestine, renal tubules, liver, and gallbladder. These transporters serve natural dipeptides, and are increasingly exploited for their ability to absorb certain oral pharmaceuticals as substrates [10]. Some examples of substrates accommodated by PEPT1 are shown in Table 3. Brandsch and colleagues [10] have extensively described peptidomimetic pharmaceutical substrates of PEPT1 and PEPT1.
Table 3.
Natural peptides and pharmaceuticals interacting with PEPT1. Affinity constants K i were obtained in Caco-2 cell uptake competition assays. Adapted from Brandsch et al. [10]
| PEPT1 substrate or inhibitor | K i(mM) | Affinity range (relative K i) |
|---|---|---|
| Lys[ZNO2)]–Val | 0.002 | High affinity (<0.5 mM) |
| Alafosfalin | 0.19 | |
| Ala-Lys | 0.21 | |
| Ceftibuten | 0.34 | |
| Valaciclovir | 0.49 | |
| Gly-Sar | 1.1 | Medium affinity (0.5–5 mM) |
| Pro–Pro | 1.2 | |
| δ-Aminolevulinic acid | 1.5 | |
| Cloxacillin | 3.0 | |
| Lys–Lys | 3.4 | |
| d-Ala-Lys | 7.0 | Low affinity (5–15 mM) |
| Cefadroxil | 7.2 | |
| Pro-Ala | 9.5 | |
| 4-Aminophenylacetic acid | 14 | |
| Cephalexin | 14 |
It has become increasingly apparent that natural biological systems, PEPT1 and PEPT2 play roles other than simply nitrogen absorption for the host organism. For example, although PEPT1 is not normally expressed in colon, its expression is induced at the transcriptional level in pathological inflamed states of colonic epithelial cells in inflammatory bowel disorders such as ulcerative colitis or Crohn's disease [28]. In this condition, bacterial metabolite dipeptides provide PEPT1 substrates absorbed intact into colonic cells and subsequently into the colonic lamina propria where immune cells reside [28]. Escherichia coli pathogenic peptide metabolites include Ac-muramyl-Ala-Glu and N-formylmethionyl-leucyl-phenylalanine. This triggers an inflammatory response by induction of MHC I molecules and AP1 and NFκB-related pathways leading to cytokines and macrophage infiltration. In the inflamed state, the sustained luminal acidic state further drives colonic transepithelial transport of the bacterial peptides. The distribution of PEPT1 in various regions along the gut is shown in Fig. 4.
Fig. 4.


Relative mRNA expression of PEPT1 (SLC15A1) in various regions of the gastrointestinal tract. Data points from individual patients. Means are shown as horizontal bars for each intestinal region. Adapted from data of Meier et al. [68]
Transepithelial Transport of Amino Acids
Transepithelial movement of amino acids requires communication between events at the apical and basolateral membrane of a given tissue epithelium (Fig. 5). The lion’s share of amino acid transport occurs in the intestine and the renal nephron. Apical membrane transporters of small intestine and large intestine handle the fundamental interface between the entire internal milieu and the external world of nutrient nitrogen, while the kidney rescues filtered monomer amino acids as well as di- and tri-peptides in the form of the peptides’ constituent free amino acids. Epithelial membrane systems of other tissues throughout the body provide a means of communication linking metabolic events among various organ systems.
Fig. 5.
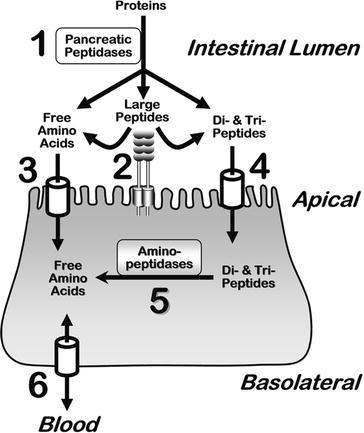

The fundamental concept of nutrient nitrogen digestion and absorption by small intestinal epithelium enterocytes. Luminal pancreatic peptidases (1) generate products of free amino acids, small di- and tri-peptide fragments, and residual large polypeptides. Enterocyte apical membrane-bound aminopeptidases (2) further hydrolyze large peptides to yield di- and tri-peptides. A variety of amino acid transport systems (3) move free amino acids across the apical membrane into the enterocyte cytosol. Certain di- and tri-peptides are absorbed intact into the cytosol via apical membrane transporters (4). Subsequently, cytosolic aminopeptidases (5) hydrolyze these peptides to generate free amino acids. The pool of free amino acids is then transported (6) across the basolateral membrane to the serosal side of the epithelium, and subsequently into the portal blood. In the interdigestive postprandial state, amino acids are taken up by the basolateral membrane from the blood. A variety of transporters are responsible for steps 3, 4, and 6, as detailed throughout the text and tables. Analogous events occur in the kidney lumen relating to degradation of peptide hormones, rescue of amino acids, and whole body nitrogen regulation
Thermodynamic Coupling
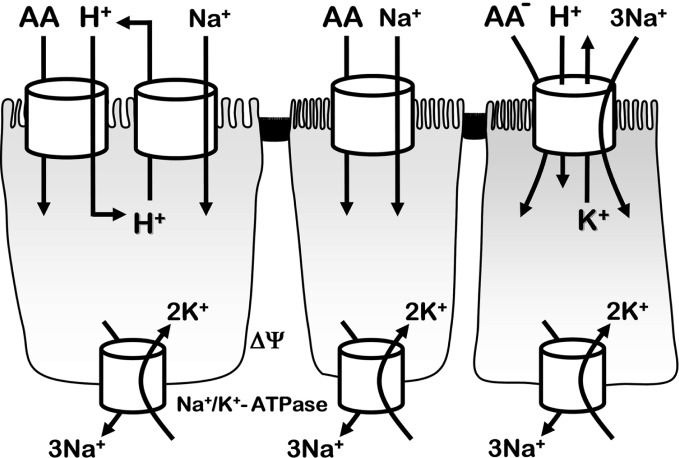
Secondary active transport of amino acids exploit epithelial transmembrane electrochemical gradients of Na+ and H+, and K+. As demonstrated by examples in Fig. 6, the activation can occur by Na+ alone, H+ alone, Na+ plus H+, or by coordinated coupling of Na+ and H+ gradient by two different transport systems. In the case of indirect H+ coupling, apical NHE sodium/proton exchangers are the predominant players. In all cases, the Na+/K+ ATPase regulates Na+, K+, and electrical gradients sensed by the apical and basolateral sides. Gerencser and Stevens [29, 30] provide a quantitative explanation of thermodynamic coupling regarding amino acid transport.
Fig. 6.

Schematic examples of ion-dependent coupling in amino acid transport at the apical membrane of an epithelium. Left cell shows a generic proton-dependent amino acid uptake coupled with Na+/H+ exchanger NHE3 (SLC9A3). The center cell shows a generic transporter coupling amino acid influx with Na+ influx, activated by the Na+ electrochemical potential across the apical membrane. The right cell shows active transport of anionic amino acids (e.g., glutamate or aspartate) via EAAT3 (SLC1A1) coupling to electrochemical gradients of Na+, H+, and K+. In all cases, transepithelial and epithelial cell membrane ion gradients and electrical potentials (ΔΨ) ultimately depend on the basolateral membrane Na+/K+-ATPase activity
Coordinated Thermodynamic and Metabolic Communication Among Amino Acid Transport Systems
Transport systems cooperate in a concerted manner to bring about net transepithelial transport, in addition to modulating the entire metabolism of amino acids. Membrane ion exchangers, primarily H+/Na+ electroneutral exchanger NHE3 and Na+/K+ ATPase, interject their important roles in modulating the activity of secondary active transporters of amino acids. Figure 7 demonstrates the coordination of selected transporter proteins and their functional transport systems in epithelial metabolism and movement of amino acids concerning the cytosol, and apical and basolateral membranes. In Fig. 7 note the coordination of TAT1 with LAT2/4F2hc which allows efflux of small neutral amino acids at the expense of cycling of aromatic amino acids [31]. Also note the roles of the many transporter subunits involved in coupling and regulating movements of cationic and neutral amino acids. Table 4 describes the manner by which the selectivities of all the cationic/dibasic amino acid transport systems are determined experimentally.
Fig. 7.
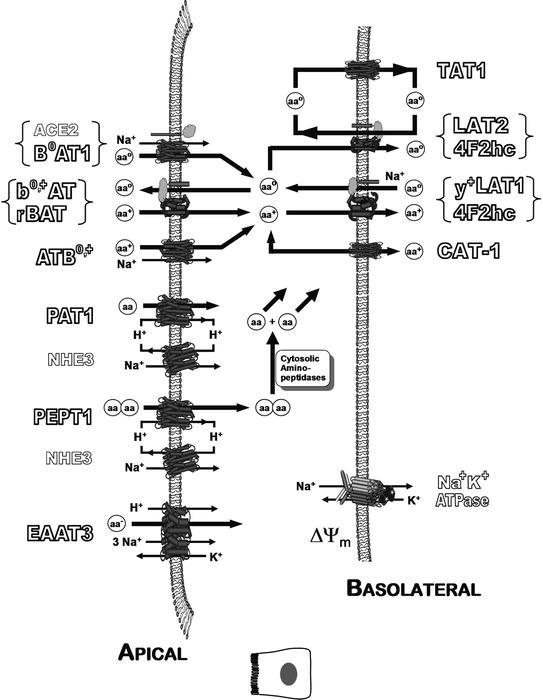
Coordinated thermodynamic and metabolic communication among selected amino acid transport systems in epithelial membranes. Amino acids are shown as cationic (AA+), zwitterion neutral (AAO), anionic (AA–), or generic (AA). Ion exchangers interject their important roles in modulating the secondary active transporters of amino acids. Various mechanisms maintain pH < 7 at the immediate apical membrane surface in various tissues. This figure is a generic representation of an epithelial cell, and not all shown transporters are in every tissue type. The role of ACE2 is described in the main text
Table 4.
Determination of epithelial membrane cationic/dibasic amino acid transport systems’ functional activities. Unlabeled amino acids in assay media are at concentrations >100X concentration of [3H]l-arginine. Modified from Bae et al. [70]
| Transport “system” functional activity name | Assay conditions; uptake of [3H]l-arginine | Parallel transport systems potentially involved with assay conditions | Calculating [3H]l-arginine uptake via single target transport system | Protein subunits giving target transport system activity in epithelium | Genes encoding subunits |
|---|---|---|---|---|---|
| B0,+ | (a) Na+ , Cl− | y+ ; y+L ; b0,+ ; B0,+ | B0,+ = (a) – (b) | ATB0,+ | SLC6A14 |
| b0,+ | (b) Na+ free | y+ ; y+L ; b0,+ | b0,+ = (b) – (c) |
b0,+AT plus rBAT |
SLC7A9 plus SLC3A1 |
| y+L | (c) Na+ free plus l-alanine | y+ ; y+L | y+L = (c) – (d) |
y+LAT1 plus 4F2hc; or y+LAT2 plus 4F2hc |
SLC7A6 plus SLC3A2;or SLC7A7 plus SLC3A2 |
| y+ | (d) Na+ plus l-leucine | y+ | y+ = (d) | CAT-1 | SLC7A1 |
Nonproteinogenic Creatine in the Intestinal Epithelium
Creatine is an important amino acid that serves several critical roles in intestinal epithelial transport physiology and intermediary metabolism. A metabolic interplay exists between creatine absorption, arginine and leucine absorption, and gross control of epithelial absorption of other nutrients in these tissues [32–35]. A putative model of creatine (Cr+) absorption in renal or intestinal absorptive epithelium is shown in Fig. 8. Na+ and Cl−-dependent creatine transport occurs across apical membranes and possibly basolateral membranes. In addition to transepithelial absorption, creatine enhances transepithelial absorption of other nutrients (glucose, other amino acids, etc) via villus contraction and pedundular motility through the actions of enterocyte actin-myosin as the result of entyerocyte creatine kinase
Fig. 8.


Participation of nonproteinogenic creatine in intestinal epithelium physiology. Putative model of creatine (Cr+) absorption in renal or intestinal absorptive epithelium. The branched chain amino acid l-leucine or its keto analogue α-ketoisocaproic acid (KIC), or l-arginine, can initiate mTOR-dependent SLC6A8 trafficking
phosphorylation. Intracelluar trafficking of SLC6A8 proteins sequested in cytosolic endosomal vesicles is intiated by mammalian targeting of the rapamycin (mTOR) signaling pathway [36]. The branched chain amino acid l-leucine or its keto analogue α-ketoisocaproic acid (KIC), or l-arginine, can initiate mTOR-dependent SLC6A8 trafficking.
A Major Transporter: SLC6A19 (B0AT1)
A major functional transport system serving the nutrient zwitterionic amino acids, initially called System NBB (Neutral Brush Border) was originally described by Stevens [37, 38] using rabbit small intestinal brush border membrane vesicles. Stevens then renamed NBB as B0 in order to be consistent with the pattern of competitive inhibition observed for Van Winkle’s “b” and “B” series of systems in blastocyte membranes [39]. However, H. Christensen interjected (personal communications), requesting that the “0” superscript be dropped, with the argument that it was redundant in naming a sodium-dependent transport system which served only zwitterion substrates. Thus, the designation “B” appeared briefly in the literature [40]. The mRNA was subsequently cloned as SLC6A19 by Kekuda and colleagues [41–43], and the literature now alternatively refers to the functional properties of expressed SLC6A19 as either B0 or B0AT1 (sodium-dependent cloned B0 Amino acid Transporter number 1).
B0AT1 is expressed prominently in apical membranes of renal proximal tubule and small intestine, as well as other nutrient absorptive epithelia (Table 3). Actually, B0AT1 is expressed all along the gastrointestinal tract intestine [44], with dominance in jejunum and ileum (Fig. 9).
Fig. 9.


Relative mRNA expression of system B0 (B0AT1 polypeptide monomer transporter from SLC6A19 gene) in various regions of the gastrointestinal tract: esophagus (E), stomach (S), duodenum (D), jejunum (J), ileum (I), colon (C), rectum (R), as well as pancreas (P). Real-time PCR forward primer was GTGTGGACAGGTTCAATAAGGACAT; reverse primer was CCACGTGACTTGCCAGAAGAT. Data points from individual patients redrawn from figure originally erroneously labeled in Terada et al. [44]. Means are shown as horizontal bars for each intestinal region
The B0AT1 functional transport system represents an apical membrane polypeptide monomer activated by a sodium-dependent Cl− independent mechanism. A putative structure-function relationship has been determined by Broer and colleagues [6, 45] , and a putative kinetic mechanism (see Fig. 10) has been worked out by the laboratories of Broer and Verrey [46–48].
Fig. 10.


Kinetic model of B0AT1 transporter. Apical membrane carrier (Ci intracellular; Co extracellular) first binds substrate amino acid AA then Na+ activator, as influenced by membrane potential ΔΨ. Preferred pathway shown with bold arrows. On the basis of models from Bröer’s group [46, 47] and Verrey’s group [48]
Regulation of Amino Acid Transport by ACE2 and Its Homolog Collectrin
Figure 7 above shows the requirement of ACE2 for apical membrane functioning of B0AT1. Therefore, ACE2 is also necessary for cooperative functioning of the other transporters that interact with B0AT1, such as the b0,+AT/rBAT heterodimer complex.
Many years ago our laboratory demonstrated that human intestinal apical membranes expressed angiotensin converting enzyme (ACE) [49–51], which is a peptidyl dipeptidase that removes a dipeptide from the C-terminus of peptides; we suggested that the function of this nonaminopeptidase was to hydrolyze peptides for subsequent absorption by transporters. However, ACE2, which is a carboxypeptidase, was subsequently discovered in apical membranes in kidney [52] and intestine [53–55]. ACE2 fortuitously is recognized as a binding receptor by a severe acute respiratory syndrome (SARS) coronavirus [56] (SARS-CoV), consistent with clinical observations that SARS-CoV causes severe gastrointestinal complications. A presumed intended biological function of ACE2 is proper trafficking and functioning of B0AT1, and then membrane removal of the C-terminal single neutral amino acid from nutrient peptides. These particular amino acid products of ACE2 hydrolysis are the very amino acids that are B0AT1 substrates [45]. A homologue of ACE2 found in kidney, collectrin, was shown to be a mediator of neutral amino acid transport activity and regulator of blood pressure [45, 57–59] via the transport interplay of arginine and neutral amino acids through cooperation of B0AT1 and the b0,+AT/rBAT transporters, as shown in Fig. 7. Collectrin knock out mice demonstrated reduced expression of apical membrane B0AT1, rBAT, and b0,+AT, as well as impaired intracellular trafficking of EAAC1, with the residual activity of B0AT1 attributable to ACE2. Broer’s laboratory demonstrated that the R240 moiety of B0AT1 interacts with intestinal ACE2 permitting proper apical membrane trafficking and expression of this sodium-dependent amino acid transporter [45].
SLC6A19 genomic DNA is composed of 19 exons and 18 introns on human chromosome 5 at location 5p15.33. Mutations in SLC6A19 on more and more alleles of patients with Hartnup disorder gives the aminoaciduria phenotype manifested in both intestine and kidney [3, 45, 60–63]. Dysfunctional SLC6A19 mutants of B0AT1 expressed in renal proximal tubule, arising from a variable number of tandem repeat minisatellite polymorphisms, are likely to be a risk factor for hypertension [64]. Figure 11 shows genomic intron/exon relationships concerning known SLC6A19 mutations, and a particular minisatellite repeat unit in intron 9 of SLC6A19 encodes a dysfunctional B0AT1 associated with essential hypertension in humans [64]. Interestingly, SLC6A18 encoding another “orphan” transporter, XTRP2, which serves glycine reabsorption in kidney, is adjacent to the SLC6A19 gene sequence, although SLC6A18 minisatellites are not involved in hypertension [65].
Fig. 11.


Allelic structure of human genomic DNA near the SLC6A19 gene. Monomeric transporter B0AT1 polypeptide is encoded by 19 exons of SLC6A19 (location 5p15.33) indicated by vertical bars. Minisatellite repeat (MS) positions are indicated by triangles, and introns are indicated by short horizontal bars under triangles. Minisatellite repeat unit MS7 in intron 9 of SLC6A19 encodes a dysfunctional B0AT1 associated with hypertension. The sequence of MS7 is shown. TERT (telomerase reverse transcriptase) and SLC6A18 appear adjacent upstream at 5p15.33. Adapted from Seol et al. [64]
Taken together, these elegant studies suggest that epithelial cell regulation of amino acid transport activity likely involves other undiscovered modulation factors that are affective either through trafficking or perhaps other mechanisms. One overall physiological relevance might be that collectrin in renal tubule membranes or allelic mutations could affect blood pressure [59, 64].
Summary
This review summarized the current view of amino acid transport by epithelial cells of vertebrates. A wide variety of transporter proteins are expressed in apical and basolateral membranes and collectively play complex interactive roles in controlling the entire organism’s overall metabolism of amino acids. Regulation of the transport systems can be manifested at many levels, including gene splicing and promoter regulation, interactions between requisite subunits of oligomers, thermodynamic electrochemical gradients contributed by ion exchangers, overlap of substrate specificity, selective tissue distribution, and specific spatial distribution of transporters leading to net vectorial flow of the amino acids. The next frontier for workers in this field is to uncover a comprehensive molecular understanding of the manner by which epithelial cells signal gene expression of transporters as triggered by substrates, hormones or other triggers, to further understand the trafficking and interactions among multimeric transport system proteins, to extend discoveries of novel small drug substrates for oral and ocular delivery, and to examine gene therapy or nanotherapy of diseases using small molecules delivered via amino acid transporters.
Contributor Information
George A. Gerencser, Phone: +1352392-4482, FAX: +1352846-0270, Email: gag@phys.med.ufl.edu
Bruce R. Stevens, Email: stevensb@ufl.edu
References
- 1.Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins: Introduction. Pflugers Arch. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- 2.Broer S. The SLC6 orphans are forming a family of amino acid transporters. Neurochem Int. 2006;48:559–567. doi: 10.1016/j.neuint.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Romeo E, Dave MH, Bacic D, et al. Luminal kidney and intestine SLC6 amino acid transporters of B0AT-cluster and their tissue distribution in Mus musculus. Am J Physiol Renal Physiol. 2006;290:F376–F383. doi: 10.1152/ajprenal.00286.2005. [DOI] [PubMed] [Google Scholar]
- 4.Boyd CA. Facts, fantasies and fun in epithelial physiology. Exp Physiol. 2008;93:303–314. doi: 10.1113/expphysiol.2007.037523. [DOI] [PubMed] [Google Scholar]
- 5.Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- 6.Broer S. Apical transporters for neutral amino acids: physiology and pathophysiology. Physiology (Bethesda) 2008;23:95–103. doi: 10.1152/physiol.00045.2007. [DOI] [PubMed] [Google Scholar]
- 7.Verrey F, Ristic Z, Romeo E, et al. Novel renal amino acid transporters. Annu Rev Physiol. 2005;67:557–572. doi: 10.1146/annurev.physiol.67.031103.153949. [DOI] [PubMed] [Google Scholar]
- 8.Cynober LA, ed. Metabolic & Therapeutic Aspects of Amino Acids in Clinical Nutrition, Second Edition: CRC Press; 2003.
- 9.Del Amo EM, Urtti A, Yliperttula M. Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci. 2008;35:161–174. doi: 10.1016/j.ejps.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Brandsch M, Knutter I, Bosse-Doenecke E. Pharmaceutical and pharmacological importance of peptide transporters. J Pharm Pharmacol. 2008;60:543–585. doi: 10.1211/jpp.60.5.0002. [DOI] [PubMed] [Google Scholar]
- 11.Brandsch M. Transport of L-proline, L-proline-containing peptides and related drugs at mammalian epithelial cell membranes. Amino Acids. 2006;31:119–136. doi: 10.1007/s00726-006-0307-0. [DOI] [PubMed] [Google Scholar]
- 12.Bartholomaus I, Milan-Lobo L, Nicke A, et al. Glycine transporter dimers: evidence for occurrence in the plasma membrane. J Biol Chem. 2008;283:10978–10991. doi: 10.1074/jbc.M800622200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hastrup H, Sen N, Javitch JA. The human dopamine transporter forms a tetramer in the plasma membrane: cross-linking of a cysteine in the fourth transmembrane segment is sensitive to cocaine analogs. J Biol Chem. 2003;278:45045–45048. doi: 10.1074/jbc.C300349200. [DOI] [PubMed] [Google Scholar]
- 14.Eyre TA, Ducluzeau F, Sneddon TP, Povey S, Bruford EA, Lush MJ. The HUGO gene nomenclature database, 2006 updates. Nucleic Acids Res. 2006;34:D319–D321. doi: 10.1093/nar/gkj147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen HN. Naming plan for membrane transport systems for amino acids. Neurochem Res. 1984;9:1757–1758. doi: 10.1007/BF00968086. [DOI] [PubMed] [Google Scholar]
- 16.Kowalczuk S, Broer A, Munzinger M, Tietze N, Klingel K, Broer S. Molecular cloning of the mouse IMINO system: an Na+- and Cl–-dependent proline transporter. Biochem J. 2005;386:417–422. doi: 10.1042/BJ20050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan Y, Vasudevan S, Nguyen H, Bork U, Sitaraman S, Merlin D. Extracellular interaction between hCD98 and the PDZ class II domain of hCASK in intestinal epithelia. J Membr Biol. 2007;215:15–26. doi: 10.1007/s00232-007-9001-8. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds B, Laynes R, Ogmundsdottir MH, Boyd CA, Goberdhan DC. Amino acid transporters and nutrient-sensing mechanisms: new targets for treating insulin-linked disorders? Biochem Soc Trans. 2007;35:1215–1217. doi: 10.1042/BST0351215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo NS, Zeng CQ, Hyser JM, et al. Inaugural Article: Integrins {alpha}1{beta}1 and {alpha}2{beta}1 are receptors for the rotavirus enterotoxin. Proc Natl Acad Sci USA 2008. [DOI] [PMC free article] [PubMed]
- 20.Burdo J, Dargusch R, Schubert D. Distribution of the cystine/glutamate antiporter system xc- in the brain, kidney, and duodenum. J Histochem Cytochem. 2006;54:549–557. doi: 10.1369/jhc.5A6840.2006. [DOI] [PubMed] [Google Scholar]
- 21.Kleemola M, Toivonen M, Mykkanen J, Simell O, Huoponen K, Heiskanen KM. Heterodimerization of y(+)LAT-1 and 4F2hc visualized by acceptor photobleaching FRET microscopy. Biochim Biophys Acta. 2007;1768:2345–2354. doi: 10.1016/j.bbamem.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Wagner CA, Lang F, Broer S. Function and structure of heterodimeric amino acid transporters. Am J Physiol Cell Physiol. 2001;281:C1077–C1093. doi: 10.1152/ajpcell.2001.281.4.C1077. [DOI] [PubMed] [Google Scholar]
- 23.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- 24.Fort J, de la Ballina LR, Burghardt HE, et al. The structure of human 4F2hc ectodomain provides a model for homodimerization and electrostatic interaction with plasma membrane. J Biol Chem. 2007;282:31444–31452. doi: 10.1074/jbc.M704524200. [DOI] [PubMed] [Google Scholar]
- 25.Palacin M, Kanai Y. The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflugers Arch. 2004;447:490–494. doi: 10.1007/s00424-003-1062-7. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez E, Jimenez-Vidal M, Calvo M, et al. The structural and functional units of heteromeric amino acid transporters. The heavy subunit rBAT dictates oligomerization of the heteromeric amino acid transporters. J Biol Chem. 2006;281:26552–26561. doi: 10.1074/jbc.M604049200. [DOI] [PubMed] [Google Scholar]
- 27.Amaral JS, Pinho MJ, Soares-da-Silva P. Genomic regulation of intestinal amino acid transporters by aldosterone. Mol Cell Biochem. 2008;313:1–10. doi: 10.1007/s11010-008-9735-3. [DOI] [PubMed] [Google Scholar]
- 28.Charrier L, Merlin D. The oligopeptide transporter hPepT1: gateway to the innate immune response. Lab Invest. 2006;86:538–546. doi: 10.1038/labinvest.3700413. [DOI] [PubMed] [Google Scholar]
- 29.Gerencser GA, Stevens BR. Thermodynamics of symport and antiport catalyzed by cloned or native transporters. J Exp Biol. 1994;196:59–75. doi: 10.1242/jeb.196.1.59. [DOI] [PubMed] [Google Scholar]
- 30.Gerencser GA, Stevens BR. Energetics of sodium-coupled active transport mechanisms in invertebrate epithelia. Am J Physiol. 1989;257:R461–R472. doi: 10.1152/ajpregu.1989.257.3.R461. [DOI] [PubMed] [Google Scholar]
- 31.Ramadan T, Camargo SM, Herzog B, Bordin M, Pos KM, Verrey F. Recycling of aromatic amino acids via TAT1 allows efflux of neutral amino acids via LAT2-4F2hc exchanger. Pflugers Arch. 2007;454:507–516. doi: 10.1007/s00424-007-0209-3. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Delgado M, Peral MJ, Cano M, Calonge ML, Ilundain AA. Creatine transport in brush-border membrane vesicles isolated from rat kidney cortex. J Am Soc Nephrol. 2001;12:1819–1825. doi: 10.1681/ASN.V1291819. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Delgado M, Garcia-Miranda P, Peral MJ, Calonge ML, Ilundain AA. Ontogeny up-regulates renal Na(+)/Cl(–)/creatine transporter in rat. Biochim Biophys Acta. 2007;1768:2841–2848. doi: 10.1016/j.bbamem.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Peral MJ, Garcia-Delgado M, Calonge ML, et al. Human, rat and chicken small intestinal Na+ – Cl– -creatine transporter: functional, molecular characterization and localization. J Physiol. 2002;545:133–144. doi: 10.1113/jphysiol.2002.026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tosco M, Faelli A, Sironi C, Gastaldi G, Orsenigo MN. A creatine transporter is operative at the brush border level of the rat jejunal enterocyte. J Membr Biol. 2004;202:85–95. doi: 10.1007/s00232-004-0721-8. [DOI] [PubMed] [Google Scholar]
- 36.Strutz-Seebohm N, Shojaiefard M, Christie D, Tavare J, Seebohm G, Lang F. PIKfyve in the SGK1 mediated regulation of the creatine transporter SLC6A8. Cell Physiol Biochem. 2007;20:729–734. doi: 10.1159/000110433. [DOI] [PubMed] [Google Scholar]
- 37.Stevens BR, Ross HJ, Wright EM. Multiple transport pathways for neutral amino acids in rabbit jejunal brush border vesicles. J Membr Biol. 1982;66:213–225. doi: 10.1007/BF01868496. [DOI] [PubMed] [Google Scholar]
- 38.Stevens BR, Kaunitz JD, Wright EM. Intestinal transport of amino acids and sugars: advances using membrane vesicles. Annu Rev Physiol. 1984;46:417–433. doi: 10.1146/annurev.ph.46.030184.002221. [DOI] [PubMed] [Google Scholar]
- 39.Van Winkle LJ. Amino acid transport in developing animal oocytes and early conceptuses. Biochim Biophys Acta. 1988;947:173–208. doi: 10.1016/0304-4157(88)90024-x. [DOI] [PubMed] [Google Scholar]
- 40.Stevens B. Amino acid transport in intestine. In: Kilberg M, Haussinger D, editors. Mammalian Amino Acid Transport. New York: Plenum Press; 1992. pp. 149–163. [Google Scholar]
- 41.Kekuda R, Prasad PD, Fei YJ, et al. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J Biol Chem. 1996;271:18657–18661. doi: 10.1074/jbc.271.31.18657. [DOI] [PubMed] [Google Scholar]
- 42.Kekuda R, Torres-Zamorano V, Fei YJ, et al. Molecular and functional characterization of intestinal Na(+)-dependent neutral amino acid transporter B0. Am J Physiol. 1997;272:G1463–G1472. doi: 10.1152/ajpgi.1997.272.6.G1463. [DOI] [PubMed] [Google Scholar]
- 43.Talukder JR, Kekuda R, Saha P, Arthur S, Sundaram U. Identification and characterization of rabbit small intestinal villus cell brush border membrane Na-glutamine cotransporter. Am J Physiol Gastrointest Liver Physiol. 2008;295:G7–G15. doi: 10.1152/ajpgi.00606.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terada T, Shimada Y, Pan X, et al. Expression profiles of various transporters for oligopeptides, amino acids and organic ions along the human digestive tract. Biochem Pharmacol. 2005;70:1756–1763. doi: 10.1016/j.bcp.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 45.Kowalczuk S, Broer A, Tietze N, Vanslambrouck JM, Rasko JE, Broer S. A protein complex in the brush-border membrane explains a Hartnup disorder allele. Faseb J. 2008;22:2880–2887. doi: 10.1096/fj.08-107300. [DOI] [PubMed] [Google Scholar]
- 46.Bohmer C, Broer A, Munzinger M, et al. Characterization of mouse amino acid transporter B0AT1 (slc6a19) Biochem J. 2005;389:745–751. doi: 10.1042/BJ20050083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Mara M, Oakley A, Broer S. Mechanism and putative structure of B(0)-like neutral amino acid transporters. J Membr Biol. 2006;213:111–118. doi: 10.1007/s00232-006-0879-3. [DOI] [PubMed] [Google Scholar]
- 48.Camargo SM, Makrides V, Virkki LV, Forster IC, Verrey F. Steady-state kinetic characterization of the mouse B(0)AT1 sodium-dependent neutral amino acid transporter. Pflugers Arch. 2005;451:338–348. doi: 10.1007/s00424-005-1455-x. [DOI] [PubMed] [Google Scholar]
- 49.Stevens BR, Fernandez A, Kneer C, Cerda JJ, Phillips MI, Woodward ER. Human intestinal brush border angiotensin-converting enzyme activity and its inhibition by antihypertensive Ramipril. Gastroenterology. 1988;94:942–947. doi: 10.1016/0016-5085(88)90551-3. [DOI] [PubMed] [Google Scholar]
- 50.Stevens BR, Fernandez A, Martinez del Rio C. Angiotensin converting enzyme in brush-border membranes of avian small intestine. J Exp Biol. 1988;135:1–8. doi: 10.1242/jeb.135.1.1. [DOI] [PubMed] [Google Scholar]
- 51.Stevens BR, Phillips MI, Fernandez A. Ramipril inhibition of rabbit (Oryctolagus cuniculus) small intestinal brush border membrane angiotensin converting enzyme. Comp Biochem Physiol C. 1988;91:493–497. doi: 10.1016/0742-8413(88)90066-7. [DOI] [PubMed] [Google Scholar]
- 52.Warner FJ, Lew RA, Smith AI, Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J Biol Chem. 2005;280:39353–39362. doi: 10.1074/jbc.M508914200. [DOI] [PubMed] [Google Scholar]
- 53.Chan PK, To KF, Lo AW, et al. Persistent infection of SARS coronavirus in colonic cells in vitro. J Med Virol. 2004;74:1–7. doi: 10.1002/jmv.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gembardt F, Sterner-Kock A, Imboden H, et al. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26:1270–1277. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malakauskas SM, Quan H, Fields TA, et al. Aminoaciduria and altered renal expression of luminal amino acid transporters in mice lacking novel gene collectrin. Am J Physiol Renal Physiol. 2007;292:F533–F544. doi: 10.1152/ajprenal.00325.2006. [DOI] [PubMed] [Google Scholar]
- 58.Danilczyk U, Sarao R, Remy C, et al. Essential role for collectrin in renal amino acid transport. Nature. 2006;444:1088–1091. doi: 10.1038/nature05475. [DOI] [PubMed] [Google Scholar]
- 59.Verrey F. Does kidney amino acid transport have something to do with blood pressure? Nephrol Dial Transplant. 2007;22:2449–2451. doi: 10.1093/ndt/gfm214. [DOI] [PubMed] [Google Scholar]
- 60.Seow HF, Broer S, Broer A, et al. Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nat Genet. 2004;36:1003–1007. doi: 10.1038/ng1406. [DOI] [PubMed] [Google Scholar]
- 61.Kleta R, Romeo E, Ristic Z, et al. Mutations in SLC6A19, encoding B0AT1, cause Hartnup disorder. Nat Genet. 2004;36:999–1002. doi: 10.1038/ng1405. [DOI] [PubMed] [Google Scholar]
- 62.Azmanov DN, Kowalczuk S, Rodgers H, et al. Further evidence for allelic heterogeneity in Hartnup disorder. Hum Mutat 2008. [DOI] [PubMed]
- 63.Ristic Z, Camargo SM, Romeo E, et al. Neutral amino acid transport mediated by ortholog of imino acid transporter SIT1/SLC6A20 in opossum kidney cells. Am J Physiol Renal Physiol. 2006;290:F880–F887. doi: 10.1152/ajprenal.00319.2005. [DOI] [PubMed] [Google Scholar]
- 64.Seol SY, Lee SY, Kim YD, et al. Minisatellite polymorphisms of the SLC6A19: susceptibility in hypertension. Biochem Biophys Res Commun. 2008;374:714–719. doi: 10.1016/j.bbrc.2008.07.094. [DOI] [PubMed] [Google Scholar]
- 65.Yoon YH, Seol SY, Heo J, Chung CN, Park IH, Leem SH. Analysis of VNTRs in the Solute Carrier Family 6, Member 18 (SLC6A18) and Lack of Association with Hypertension. DNA Cell Biol. 2008;27(10):559–567. doi: 10.1089/dna.2008.0755. [DOI] [PubMed] [Google Scholar]
- 66.Boll M, Foltz M, Rubio-Aliaga I, Daniel H. A cluster of proton/amino acid transporter genes in the human and mouse genomes. Genomics. 2003;82:47–56. doi: 10.1016/S0888-7543(03)00099-5. [DOI] [PubMed] [Google Scholar]
- 67.Yan Y, Vasudevan S, Nguyen HT, Merlin D. Intestinal epithelial CD98: An oligomeric and multifunctional protein. Biochim Biophys Acta. 2008;1780:1087–1092. doi: 10.1016/j.bbagen.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meier Y, Eloranta JJ, Darimont J, et al. Regional distribution of solute carrier mRNA expression along the human intestinal tract. Drug Metab Dispos. 2007;35:590–594. doi: 10.1124/dmd.106.013342. [DOI] [PubMed] [Google Scholar]
- 69.Resource SHP. Human Protein Atlas, Transporters, http://www.proteinatlas.org/search_advanced.php?proteinclass=Tr. In; 2008.
- 70.Bae SY, Xu Q, Hutchinson D, Colton CA. y+ and y+ L arginine transporters in neuronal cells expressing tyrosine hydroxylase. Biochim Biophys Acta. 2005;1745:65–73. doi: 10.1016/j.bbamcr.2004.12.006. [DOI] [PubMed] [Google Scholar]