Critical care and other acute care providers on the frontline are no strangers to the problem of antibiotic resistance (AR). They strive daily at the bedside to predict the presence of resistant pathogens and preempt their detrimental effects in a highly vulnerable host population. However, what appears to represent frontline combat is in fact a complex interaction between the healthcare provider and their micro-adversaries with downstream implications that go far beyond the bedside and involve the local surroundings as well as the population at large. This cross-dimensional connectivity is probably best understood by conceptualizing the different milieux as concentric spheres (Fig. 1). The inner sphere represents a microcosm that encases the provider, the patient, and the pathogen. Here, management decisions prioritize improving patients’ outcomes and protecting them from acquiring resistant pathogens. The middle sphere pertains to the care setting and immediate surroundings. Here, institutional memory, guidelines, formularies, antibiograms, and feedback from antibiotic stewards inform providers’ predictions of resistance and selection of empiric therapy. The burden of resistance in the middle sphere is closely tied to provider behaviors around hand hygiene, isolation, decolonization, barrier protections, and procedural checklists. The outermost sphere pertains to the larger regional, national, or global environments. Indeed, this sphere is generally of lesser immediate relevance to the outcome of a severely ill patient. It is impacted by multiple external factors not limited to antibiotic overuse in food animals, agricultural contamination, international travel, and national and global policies as well as potential secondary infections and healthcare system collapses during viral pandemics such as coronavirus disease 2019. Busy clinicians siloed within their care settings often tend to place this outer sphere on the backburner.
Figure 1.
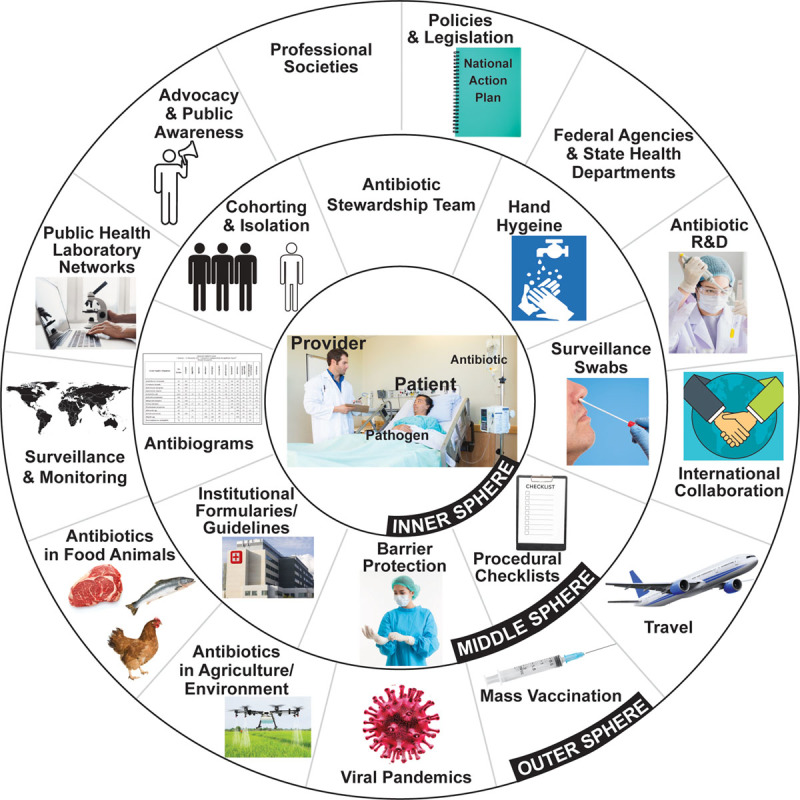
The different milieu of antibiotic resistance. Although symbolized by concentric spheres in the figure, these antibiotic resistance milieux are boundaryless in reality. This allows for cross-dimensional connectivity, such that an action taken at the patient bedside can impact the environment and vice versa.R & D = Research and Development.
However, in reality, these arbitrary spheres are rather fluid and an action in one can have far-reaching consequences in the other. Despite multiple, well-recognized drivers for the development of resistance (1), indiscriminate antibiotic prescribing in the inner sphere is undoubtedly the most important driver that has catapulted the resistance problem into the daunting global crisis as we know it today. By sheer virtue of the environments they work in and the populations they serve, the community of critical care and other frontline providers has the power and potential to tip the resistance scale in either direction even on a population scale. A paradigm change is in order. Providers must assume a greater stake in the greater good, i.e. curbing the resistance crisis. Maintaining a tighter grasp on the ever-changing epidemiology of resistance will enable them to frequently appraise their own practices in light of population-based observations.
The U.S. Centers for Disease Control and Prevention (CDC) recently released the 2019 AR Threats Report (2), a 140-page public document that provides an eye-catching landscape of the current state of the resistance crisis in the United States. This represents a tremendous body of work that was put together by a team of CDC scientists and a few external collaborators with collective expertise in infectious diseases, microbiology, epidemiology, data science, and policy. This is the second U.S. AR threats report of its kind. The previous report (3) from 2013 was based primarily on a point-prevalence survey and was thought to potentially underestimate the burden of deaths (4). The new 2019 report represents a major step forward; in addition to surveillance data, it leverages electronic health record and administrative data and relies on an American Hospital Association–weighted extrapolation to obtain national estimates. Importantly, this is an invaluable resource for critical care providers interested in obtaining a 30,000-foot view of the AR crisis in the United States—an account of that “outer sphere.”
In the 2019 CDC report, comparisons of pathogen-specific prevalence in the 2019 report are generally reported for 2012/2013 versus 2017 using data from same source for both periods.
The full report (2) is certainly worth a read. Below are some highlights from the report that focus on resistance phenotypes likely to be encountered in the critical care and other acute settings and may be of interest particularly to providers who work in these settings.
For those who prefer the bad news first…
U.S. BURDEN OF AR PATHOGENS AND ASSOCIATED DEATHS REMAINS UNACCEPTABLY HIGH
At 2.8 million infections due to AR pathogens and 35,900 attributable deaths each year (2), AR is still a very real problem in the United States. Given that sicker patients not only tend to acquire the more resistant variety of pathogens (5) but also suffer worse consequences from resultant inadequate empiric and targeted therapy (6, 7), critically ill patients represent a large proportion of this burden, particularly those who are worst hit. The following five resistant pathogen types are considered to pose an urgent threat: carbapenem-resistant Acinetobacter, carbapenem-resistant Enterobacteriaceae (CRE), Candida auris, drug-resistant Neisseria gonorrhoeae, and drug-resistant Clostridioides difficile (the latter more so as a consequence of resistant pathogens and antibiotic therapy).
INFECTIONS DUE TO EXTENDED-SPECTRUM β-LACTAMASE–PRODUCING ENTEROBACTERIACEAE ARE ON THE RISE
Presently, nearly 200,000 extended-spectrum β-lactamase (ESBL) Enterobacteriaceae infections occur among hospitalized patients in the United States, which represents an alarming 50% increase over the last half decade. This is particularly sobering given that in the late 1990s, less than 1% of Gram-negative pathogens were reported as being resistant to third-generation cephalosporins in the United States (8). The clonal spread that led to the global dissemination of ESBL among Enterobacteriaceae is predominantly the outcome of a notorious but successful long-term polyamorous relationship among an efficient carrier (plasmids), virulent strain (Escherichia coli ST131), and resilient resistance gene (blaCTX-M-15) (9). Today there are human gut, animal, and environmental reservoirs of ESBL, and it is not surprising that a large proportion of the Enterobacteriaceae ESBL infections reported by the CDC originated in the community. A recent randomized clinical trial (10) demonstrated a lack of noninferiority of piperacillin-tazobactam to meropenem in ESBL E. coli and Klebsiella pneumoniae bacteremia is likely to potentiate carbapenem use against these not uncommon infections and, which in turn, may further drive up carbapenem resistance rates in the future. Furthermore, the fear of not covering an ESBL-producing pathogen in patients presenting with sepsis is likely to drive empiric carbapenem therapy especially among those with ESBL-specific risk factors. However, a recent study demonstrated that the prevalence of ESBL Enterobacteriaceae in patients with culture-positive sepsis in U.S. hospitals is still in fact less than 1%, which may mitigate this fear to some extent (11). Furthermore, plasmids that carry the ESBL genes often carry other clinically important resistance genes as well such as those that encode carbapenemase production. This may result in Gram-negative pathogens displaying difficult-to-treat resistance (DTR) (6) or resistance to all high-efficacy, low-toxicity antibiotics that pose a management dilemma for providers and have been shown to portend a worse prognosis in multiple U.S. (6, 12, 13) and international (14, 15) studies.
CLINDAMYCIN-RESISTANT INVASIVE STREPTOCOCCAL INFECTIONS HAVE INCREASED DRASTICALLY OVER THE LAST 8 YEARS
By virtue of the serious illnesses caused by group-A streptococci (GAS), such as bloodstream infection, streptococcal toxic shock syndrome, and necrotizing soft-tissue infections, critical care providers in particular tend to encounter this pathogen and more frequently than before. The rate of clindamycin resistance in GAS has increased rather drastically over 2 years from 13% in 2015 to 22% in 2017, suggesting that more than one in every five GAS pathogens encountered in current practice is likely to be clindamycin resistant. Based on Infectious Diseases Society of America and Surgical Infection Society guideline recommendations, it is now standard practice to use clindamycin as an adjunct to β-lactams for its antitoxin property in serious invasive group-A streptococcal infections, such as necrotizing fasciitis and toxic shock syndrome (16). Although there is evidence from an animal study suggesting that clindamycin’s antitoxin activity may be preserved even against clindamycin-resistant GAS (17), the same is not definitely demonstrated in humans. Given the high case-fatality rates from serious invasive GAS infections and unclear benefit of other adjunctive therapies such as IV immunoglobulin in the absence of clindamycin (18), the ongoing loss of clindamycin activity in GAS nationally, and alarmingly high rates of clindamycin resistance elsewhere (19), this represents a sobering consequence of the AR crisis that critical care providers may continue to experience worldwide. Although candidate GAS vaccines are under evaluation (20), we are a long way from a vaccine being implemented in everyday practice.
Invasive disease due to non–group-A streptococci is not uncommon and may be clinically indistinguishable from GAS on presentation. Traditionally thought of as a pathogen impacting predominantly pregnant patients and neonates, group B streptococcal (GBS) infections are now being increasingly recognized as an important pathogen causing invasive disease in non–pregnant adults, especially in those with chronic conditions such as diabetes and obesity (21). Importantly, according to the 2019 AR threats report, around 40% of GBS remain resistant to clindamycin. There is nearly a one in two chances that clindamycin may be inactive if the pathogen is GBS, but its clinical effectiveness as an adjunct to β-lactams against this pathogen in vivo remains unclear. Other clinically important non–group-A streptococci such as group C and group G, which are also notorious for causing invasive disease, were not included in the report.
CANDIDA AURIS IS AN EMERGING THREAT, ESPECIALLY FOR ICUs
Drug-resistant Candida species are generally of the non-albicans variety and are responsible for 7% of Candida bloodstream infections in the United States, most of which are encountered and managed by critical care providers. Although the CDC reported a reassuring decline in overall drug-resistant Candida isolates between 2012 and 2017, they also alert us of 323 cases a year of a highly resistant fungus C. auris in the 2019 report. Although first isolated from the ear of a woman in Japan in 2009, this pathogen was only first reported to occur in the United States in 2013. However, compared with 2015–2017, the CDC has reported that the incidence more than tripled in 2018. This alarming rate of growth in incidence, the potential to display resistance to all routinely used antifungal agents, and an unacceptably high associated mortality rate earned C. auris “urgent” threat status. Most cases are clustered in New York, New Jersey, and Illinois, but several states reported at least one case. Critical care providers must keep a close eye out for this deadly pathogen that has resulted in outbreaks (22) with unmanageable spread even warranting ICUs to shut down.
Now, for the good news…
Critical care providers are often not privy to the outcome of their patients once they transfer out of the ICU. However, they are highly motivated by and responsive to outcomes data when available. One cannot stress enough the importance of demonstrating to critical care providers that their efforts at preventing infection are not going thankless and are in fact working, which will certainly bolster ongoing compliance with these efforts.
CARBAPENEM-RESISTANT ACINETOBACTER SPECIES AND MULTI-DRUG RESISTANCE PSEUDOMONAS AERUGINOSA ARE ON THE DECLINE AND CRE ARE NOT CONTINUING TO INCREASE
These highly resistant Gram-negative taxa generally affect debilitated patients and display a high propensity for causing ICU outbreaks, critical illness, and death. A decline or even stability in their incidence is certainly good news. Here is why:
The antibiotic armamentarium for carbapenem-resistant Acinetobacter baumannii remains very limited. Even though colistin is often active against this pathogen and generally the backbone for most treatment regimens, associated heteroresistance (23), suboptimal efficacy, and high toxicity make colistin a less than ideal option. Combining colistin with other agents such as carbapenems (24) and rifampin (25) has not shown to offer significant incremental benefit thus far; however, in light of very scarce options, combination regimens must continue to be explored. Although often active against carbapenem-resistant A. baumannii, tigecycline is also suboptimal primarily due to low blood levels, toxicity concerns, and suggestion of excess associated mortality (26). Sulbactam is effective but remains active in a small minority of carbapenem-resistant isolates (27). Of the several Gram-negative antibiotics approved over the last half decade, cefiderocol is the only drug that demonstrated in vitro activity against a sizable proportion of carbapenem-resistant A. baumannii isolates (28, 29). Although recently approved for complicated urinary tract infections (UTIs) due to Gram-negative pathogens lacking routine treatment options, a warning in the label (30) about higher all-cause mortality in patients with carbapenem-resistant Gram-negative infections places pause on its candidacy as the much anticipated go-to drug for carbapenem-resistant A. baumannii complex infections in critically ill patients until more favorable evidence becomes available. Non–antibiotic therapies, such as bacteriophages and monoclonal antibodies, are promising, but evidence is still evolving. For these reasons, it is safe to say that carbapenem-resistant A. baumannii complex infections remain a critical care provider’s worst nightmare.
Multidrug resistance in P. aeruginosa is usually the consequence of several potential intrinsic and adaptive resistance mechanisms at play. Although there is suggestion that resistance may diminish virulence potential, this is not universally true and virulent epidemic “high-risk clones” of multi-drug resistant (MDR)/extensively-drug resistant (XDR) this is not universally true and virulent epidemic “high-risk clones” of MDR/XDR P. aeruginosa have caused a number of outbreaks worldwide (31). From a therapeutic standpoint, the picture is less grim for carbapenem-resistant P. aeruginosa (the vast majority of which are in fact not DTR) (6) compared with carbapenem-resistant A. baumannii. Ceftolozane-tazobactam and ceftazidime-avibactam are Food and Drug Administration approved for complicated UTI, intra-abdominal infections, and hospital/ventilator-acquired pneumonia and display in vitro activity against many isolates of MDR and carbapenem-resistant P. aeruginosa (32) and have demonstrated moderate treatment success of 70–75% in observational studies (33–35). Despite the lack of dedicated trials of patients with MDR P. aeruginosa, pooled clinical trial data from ceftazidime-avibactam trials (36) suggest comparable efficacy to carbapenems when active. As such, we are likely to see these newer agents being used more often against MDR/XDR P. aeruginosa in lieu of older more toxic alternatives such as colistin and tigecycline.
Over the last decade, CRE have become a global menace (37). Carbapenem resistance in Enterobacteriaceae is predominantly conferred by a variety of traits (e.g., K. pneumoniae Carbapenemase, New Delhi metallo-β-lactamase [NMD]-1, oxacillinase-type, Verona integron-coated metallo-β-lactamase, and imipenemase) that lead to carbapenemase production. Before 2014, we only had access to toxic, less-efficacious antibiotics available against CRE. However, thanks to the collective efforts of governments, legislators, professional societies, industry, and federal and other international agencies; lesser toxic antibiotics have been recently approved and become available for use including ceftazidime-avibactam, meropenem-vaborbactam, eravacycline, and cefiderocol and demonstrate in vitro activity against a number of CRE. New antibiotics such as ceftazidime-avibactam with activity against CRE are now being used for these difficult-to-treat infections based on safety and efficacy against Gram-negative pathogens in trials that led to their approval and observational studies of patients specifically with CRE infections demonstrating superior effectiveness over colistin (38). Yet, colistin was found to be used more often than ceftazidime-avibactam throughout the first 2 years following the approval of ceftazidime-avibactam (39). This likely reflects a combination of provider reluctance due to unclear efficacy in sicker, septic patients, or lack of treatment experience, awareness, and in-house in vitro testing infrastructure, as well as higher costs. Furthermore, different CRE may display varying susceptibility to the new agents, depending on species and the trait that has resulted in resistance to carbapenems. For instance, treatment of metallo-β-lactamase producers (e.g., pathogens displaying NDM-1) often still necessitates reliance on older agents (such as colistin) or combinations of old and new agents, for example, aztreonam and ceftazidime-avibactam (for the avibactam component). A detailed review of therapeutic options for CRE is beyond the scope of this foreword and can be found elsewhere (40).
Declining incidence of infections due to highly resistant Gram-negative pathogens is certainly good news. Although it is still premature to make causal inferences between observed decreases and specific pathogen, host, behavioral, or societal factors, this observation speaks to the success of the currently implemented battery of infection control and other preventative strategies and strongly suggests that we keep up these efforts. Apart from universal precautions, more specific interventions such as increasing use of screening rectal swabs for active screening, use of molecular tests to cohort patients based on detection of highly transmissible carbapenemase genes, and continuation of contact precautions up to the end of hospitalization and on subsequent stays may have helped. However, implementation of these interventions is contingent on resources, personnel, and infrastructure and varies nationally. As such, impact of these interventions has been difficult to evaluate on a large scale.
METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS AND VANCOMYCIN-RESISTANT ENTEROCOCCUS RATES CONTINUE TO DECLINE
It is well recognized from previous studies that the rates of methicillin-resistant S. aureus (MRSA) among hospitalized patients have been on the decline over the last 1–2 decades in the United States (41, 42). The remarkable decrease in MRSA infections at 153 Veterans Health Administration hospitals was attributed to CDC recommendations of screening, tracking, contact isolation, hand washing, and heightening employee responsibility in preventing MRSA infections. Per the 2019 AR threats report, hospital-onset MRSA infections decreased by nearly a fifth between 2012 and 2017 in the United States. Although hospital-onset MRSA bloodstream infections decreased by 17% from 2005 to 2016, interestingly, no significant decrease was observed in the recent portion of that period (i.e., between 2013 and 2016). Have our preventative efforts been maximally saturated or are we beginning to slack in those efforts? Regardless, at 323,700 infections in 2017, MRSA still remains a major problem and one of the most common AR pathogens encountered in U.S. hospitals. Community-onset MRSA bloodstream infections decreased less vigorously between 2005 and 2016 at 7% per year, and its persistence in the community has been tied to the opioid crisis and associated injection drug use. Infections due to vancomycin-resistant Enterococcus (VRE) generally occur in patients from long-term care facilities as well as in the critically ill and immune compromised patients such as transplant recipients. VRE rates displayed a reassuring decline between 2012 and 2017. Although still relatively rare, there remains concern for ongoing emergence of treatment-limiting varieties of VRE that also display resistance to linezolid (43) and/or nonsusceptibility to daptomycin (44), two agents that currently represent the antibiotic bedrock for managing VRE infections.
C. DIFFICILE RATES ARE DECLINING
Although not directly a consequence of resistant pathogens, C. difficile, like AR, is closely tied to indiscriminate use of antibiotics. In the 2019 AR report, U.S. rates of C. difficile–associated disease decreased between 2012 and 2017. At least part of this decrease has been attributed to decreased use of fluoroquinolones, an antibiotic associated with a strain of C. difficile notorious for causing severe disease. Unfortunately, C. difficile remains a relatively common occurrence in ICUs in the United States today. This is especially true among those with greater healthcare contact such as long-term care residents and older, recently hospitalized patients and is associated with a high burden of morbidity and mortality. Hence, efforts to prevent C. difficile–associated disease including antibiotic stewardship, contact isolation, facility cleaning, hand washing, as well as its prompt detection and treatment must be emphasized among all healthcare providers.
DEATHS RELATING TO AR IN THE UNITED STATES HAVE DECREASED SINCE 2013
It is reassuring that the number of reported deaths attributable to AR decreased from around 44,000 in 2013 to 35,900 in 2019. The methodology applied for this estimation of AR-attributable deaths in the CDC 2019 AR report is set up in a way where decreased deaths are a direct reflection of decreased infection incidence. As such, decreased incidence of infections due to many high-mortality risk pathogens described above have translated into more lives saved.
CONCLUSIONS
In conclusion, 2019 AR threats report provides us with some data that we as a healthcare community should be proud of, but it should certainly not be a pretext for complacency. More efforts are necessary. The need of the hour is more practice guidelines for the management of AR pathogens, including guidance on the empiric use of newly approved agents when AR is strongly suspected on presentation. There is an urgent need for frontline providers to have a seat at the table in antibiotic policy and advocacy. The field is likely to benefit from physician investigators dually trained in infectious diseases and critical care medicine and pathways and curricula for these trainees should be better delineated (45, 46). We need to enhance our evidence base on the overlap between AR and sepsis; we need better guidance on how best to use and interpret rapid diagnostics along with wider implementation supported by outcome-based assessments of their utility. This way, our high-stakes treatment decisions on the frontline will be based on truth rather than on fear or empiricism. The field of AR is complex and at times overwhelming. Yet, every critical care provider must continue to play their part, familiarizing themselves with evolving nomenclature of pathogens and resistance, emerging trends and key genotypic resistance traits that have important therapeutic implications for their patients. I sincerely hope that recognition of the existence of the concentric spheres of AR among frontline providers will offer a constant reminder that our direct actions at the bedside are in fact not siloed and taken together can have far-reaching implications on the population.
ACKNOWLEDGMENTS
We thank Ms. Kelly Byrne for her assistance with preparing the figure.
Footnotes
The opinions expressed in this article are those of the author and do not represent any position or policy of the National Institutes of Health, the U.S. Department of Health and Human Services, or the U.S. government.
Supported, in part, by the intramural research program of the National Institutes of Health Clinical Center.
Dr. Kadri received support for article research from the National Institutes of Health, and he disclosed government work.
REFERENCES
- 1.Holmes AH, Moore LS, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387:176–187 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention: Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: Centers for Disease Control and Prevention; Available at: https://www.cdc.gov/drugresistance/pdf/threatsreport/2019-ar-threats-report-508.pdf. Accessed April 6, 2020 [Google Scholar]
- 3.Centers for Disease Control and Prevention: Antibiotic Resistance Threats in the United States. 2013Atlanta, GA: Centers for Disease Control and Prevention, [Google Scholar]
- 4.Burnham JP, Olsen MA, Kollef MH. Re-estimating annual deaths due to multidrug-resistant organism infections. Infect Control Hosp Epidemiol 2019; 40:112–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zilberberg MD, Shorr AF, Micek ST, et al. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: A retrospective cohort study. Crit Care 2014; 18:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadri SS, Adjemian J, Lai YL, et al. National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH–ARORI): Difficult-to-treat resistance in Gram-negative bacteremia at 173 US hospitals: Retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis 2018; 67:1803–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596 [DOI] [PubMed] [Google Scholar]
- 8.Diekema DJ, Pfaller MA, Jones RN. SENTRY Participants Group: Age-related trends in pathogen frequency and antimicrobial susceptibility of bloodstream isolates in North America: SENTRY antimicrobial surveillance program, 1997-2000. Int J Antimicrob Agents 2002; 20:412–418 [DOI] [PubMed] [Google Scholar]
- 9.Bevan ER, Jones AM, Hawkey PM. Global epidemiology of CTX-M β-lactamases: Temporal and geographical shifts in genotype. J Antimicrob Chemother 2017; 72:2145–2155 [DOI] [PubMed] [Google Scholar]
- 10.Harris PNA, Tambyah PA, Lye DC, et al. MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN): Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA 2018; 320:984–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee C, Kadri SS, Dekker JP, et al. Prevalence of antibiotic resistant pathogens in culture-positive sepsis and outcomes associated with inappropriate and unnecessarily broad empiric antibiotics. 2020, in press [DOI] [PMC free article] [PubMed]
- 12.Kadri SS, Lai YLE, Ricotta EE, et al. NIH Antimicrobial Resistance Outcomes Research Initiative (NIH-ARORI): External validation of difficult-to-treat resistance prevalence and mortality risk in Gram-negative bloodstream infection using electronic health record data from 140 US hospitals. Open Forum Infect Dis 2019; 6:ofz110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadri SS, Strich JR, Swihart BJ, et al. Attributable mortality from extensively drug-resistant Gram-negative infections using propensity-matched tracer antibiotic algorithms. Am J Infect Control 2019; 47:1040–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huh K, Chung DR, Ha YE, et al. Impact of difficult-to-treat resistance in Gram-negative bacteremia on mortality: Retrospective analysis of nationwide surveillance data. Clin Infect Dis. 2020 Jan 29 doi: 10.1093/cid/ciaa084. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Giannella M, Bussini L, Pascale R, et al. Prognostic utility of the new definition of difficult-to-treat resistance among patients with Gram-negative bloodstream infections. Open Forum Infect Dis 2019; 6:ofz505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America: Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–e52 [DOI] [PubMed] [Google Scholar]
- 17.Andreoni F, Zürcher C, Tarnutzer A, et al. Clindamycin affects group A Streptococcus virulence factors and improves clinical outcome. J Infect Dis 2017; 215:269–277 [DOI] [PubMed] [Google Scholar]
- 18.Parks T, Wilson C, Curtis N, et al. Polyspecific intravenous immunoglobulin in clindamycin-treated patients with streptococcal toxic shock syndrome: A systematic review and meta-analysis. Clin Infect Dis 2018; 67:1434–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu B, Fang Y, Fan Y, et al. High prevalence of macrolide-resistance and molecular characterization of Streptococcus pyogenes isolates circulating in China from 2009 to 2016. Front Microbiol 2017; 8:1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vekemans J, Gouvea-Reis F, Kim JH, et al. The path to group A streptococcus vaccines: World Health Organization Research and Development Technology Roadmap and Preferred Product Characteristics. Clin Infect Dis 2019; 69:877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francois Watkins LK, McGee L, Schrag SJ, et al. Epidemiology of invasive group B streptococcal infections among nonpregnant adults in the United States, 2008-2016. JAMA Intern Med 2019; 179:479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schelenz S, Hagen F, Rhodes JL, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control 2016; 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Rayner CR, Nation RL, et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2006; 50:2946–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect Dis 2018; 18:391–400 [DOI] [PubMed] [Google Scholar]
- 25.Durante-Mangoni E, Signoriello G, Andini R, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: A multicenter, randomized clinical trial. Clin Infect Dis 2013; 57:349–358 [DOI] [PubMed] [Google Scholar]
- 26.Prasad P, Sun J, Danner RL, et al. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Xu Q, Li T, et al. OXA-23 is a prevalent mechanism contributing to sulbactam resistance in diverse Acinetobacter baumannii clinical strains. Antimicrob Agents Chemother 2019; 63:e01676–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobias J, Dénervaud-Tendon V, Poirel L, et al. Activity of the novel siderophore cephalosporin cefiderocol against multidrug-resistant Gram-negative pathogens. Eur J Clin Microbiol Infect Dis 2017; 36:2319–2327 [DOI] [PubMed] [Google Scholar]
- 29.Kazmierczak KM, Tsuji M, Wise MG, et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int J Antimicrob Agents 2019; 53:177–184 [DOI] [PubMed] [Google Scholar]
- 30.Food and Drug Administration: Cefiderocol: Highlights of prescribing information. 2019
- 31.Oliver A, Mulet X, López-Causapé C, et al. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Updat 2015; 21–22:41–59 [DOI] [PubMed] [Google Scholar]
- 32.Buehrle DJ, Shields RK, Chen L, et al. Evaluation of the in vitro activity of ceftazidime-avibactam and ceftolozane-tazobactam against meropenem-resistant Pseudomonas aeruginosa isolates. Antimicrob Agents Chemother 2016; 60:3227–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haidar G, Philips NJ, Shields RK, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: Clinical effectiveness and evolution of resistance. Clin Infect Dis 2017; 65:110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munita JM, Aitken SL, Miller WR, et al. Multicenter evaluation of ceftolozane/tazobactam for serious infections caused by carbapenem-resistant Pseudomonas aeruginosa. Clin Infect Dis 2017; 65:158–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallagher JC, Satlin MJ, Elabor A, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: A multicenter study. Open Forum Infect Dis 2018; 5:ofy280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stone GG, Newell P, Gasink LB, et al. Clinical activity of ceftazidime/avibactam against MDR Enterobacteriaceae and Pseudomonas aeruginosa: Pooled data from the ceftazidime/avibactam phase III clinical trial programme. J Antimicrob Chemother 2018; 73:2519–2523 [DOI] [PubMed] [Google Scholar]
- 37.Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J Infect Dis 2017; 215:S28–S36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Duin D, Lok JJ, Earley M, et al. Antibacterial Resistance Leadership Group: Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2018; 66:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strich JR, Ricotta E, Warner S, et al. Pharmacoepidemiology of ceftazidime-avibactam use: A retrospective cohort analysis of 210 US hospitals. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa061. Feb 28. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheu CC, Chang YT, Lin SY, et al. Infections caused by carbapenem-resistant Enterobacteriaceae: An update on therapeutic options. Front Microbiol 2019; 10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kourtis AP, Hatfield K, Baggs J, et al. Emerging Infections Program MRSA Author Group: Vital signs: Epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections - United States. MMWR Morb Mortal Wkly Rep 2019; 68:214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones M, Jernigan JA, Evans ME, et al. Vital signs: Trends in Staphylococcus aureus infections in veterans affairs medical centers - United States, 2005-2017. MMWR Morb Mortal Wkly Rep 2019; 68:220–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yadav G, Thakuria B, Madan M, et al. Linezolid and vancomycin resistant enterococci: A therapeutic problem. J Clin Diagn Res 2017; 11:GC07–GC11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelesidis T, Humphries R, Uslan DZ, et al. De novo daptomycin-nonsusceptible enterococcal infections. Emerg Infect Dis 2012; 18:674–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kadri SS, Rhee C, Fortna GS, et al. Critical care medicine and infectious diseases: An emerging combined subspecialty in the United States. Clin Infect Dis 2015; 61:609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kadri SS, Rhee C, Magda G, et al. Synergy, salary, and satisfaction: Benefits of training in critical care medicine and infectious diseases gleaned from a national pilot survey of dually trained physicians. Clin Infect Dis 2016; 63:868–875 [DOI] [PMC free article] [PubMed] [Google Scholar]


