Abstract
Objective
To investigate the safety and efficacy of escalating doses of the semi-synthetic triterpenoid omaveloxolone in patients with mitochondrial myopathy.
Methods
In cohorts of 8–13, 53 participants were randomized double-blind to 12 weeks of treatment with omaveloxolone 5, 10, 20, 40, 80, or 160 mg, or placebo. Outcome measures were change in peak cycling exercise workload (primary), in 6-minute walk test (6MWT) distance (secondary), and in submaximal exercise heart rate and plasma lactate (exploratory).
Results
No differences in peak workload or 6MWT were observed at week 12 with omaveloxolone treatment vs placebo for all omaveloxolone dose groups. In contrast, omaveloxolone 160 mg reduced heart rate at week 12 by 12.0 ± 4.6 bpm (SE) during submaximal exercise vs placebo, p = 0.01, and by 8.7 ± 3.5 bpm (SE) vs baseline, p = 0.02. Similarly, blood lactate was 1.4 ± 0.7 mM (SE) lower vs placebo, p = 0.04, and 1.6 ± 0.5 mM (SE) lower vs baseline at week 12, p = 0.003, with omaveloxolone 160 mg treatment. Adverse events were generally mild and infrequent.
Conclusions
Omaveloxolone 160 mg was well-tolerated, and did not lead to change in the primary outcome measure, but improved exploratory endpoints lowering heart rate and lactate production during submaximal exercise, consistent with improved mitochondrial function and submaximal exercise tolerance. Therefore, omaveloxolone potentially benefits patients with mitochondrial myopathy, which encourages further investigations of omaveloxolone in this patient group.
Clinicaltrials.gov identifier
Classification of evidence
This study provides Class II evidence that, for patients with mitochondrial myopathy, omaveloxolone compared to placebo did not significantly change peak exercise workload.
Mitochondrial diseases are heterogeneous, multisystemic syndromes primarily associated with respiratory chain dysfunction. Mutations in mitochondrial or nuclear genes encoding proteins involved in oxidative phosphorylation lead to reduced ATP generation, which preferentially affects organs with a high energy demand, such as skeletal muscle. Myopathy as expressed by symptoms of exercise intolerance with premature fatigue or muscle weakness is therefore common in mitochondrial diseases, for which there is no effective available treatment.1,2
Impaired respiratory chain function in mitochondrial diseases also results in excess production of reactive oxygen species (ROS), which damages mitochondria further and induces apoptosis.3
It is known that exercise training increases aerobic function, mitochondrial biogenesis, and mitochondrial density in patients with mitochondrial myopathy.4–7 Exercise training leads to activation of nuclear factor erythroid 2-like 2 (Nrf2), a transcription factor for a range of antioxidant and cytoprotective target genes.8–10 Furthermore, mouse and in vitro studies show that Nrf2 activation enhances mitochondrial metabolism and energy turnover and protects against ROS.10,11
Triterpenoids are small anti-inflammatory molecules derived from natural sources.12 Omaveloxolone is a semi-synthetic oleanolic triterpenoid, which is a potent activator of Nrf2.13,14 Omaveloxolone targets redox-sensitive cysteine residues on the regulatory molecule Keap1 and thereby rescues Nrf2 from degradation. Moreover, the blocking of Keap1 inhibits the NF-κB proinflammatory signaling pathway.14 These effects of omaveloxolone have the potential to improve muscle function, oxidative phosphorylation, antioxidant capacity, and mitochondrial biogenesis in patients with mitochondrial myopathy.
Methods
Classification of evidence
The objective of the present study was to establish if there is an effect of omaveloxolone on exercise capacity in patients with mitochondrial myopathy and if so, which omaveloxolone dose should be explored in a phase III trial. This study does not provide evidence that omaveloxolone has an effect on the primary outcome, peak oxidative capacity (Class II), or in the secondary outcome measure, 6-minute walk test (6MWT) distance (Class II), while it provides evidence that 160 mg omaveloxolone induces a reduction in heart rate by 12.0 ± 4.6 bpm (SE) (p < 0.05) and lactate production by 1.4 ± 0.7 mM (SE) (p < 0.05) compared to placebo at the end of 30 minutes of submaximal exercise, which were exploratory outcomes (Class II).
MOTOR study design
The study was conducted as an international, multicenter, double-blind, dose-ranging, randomized, placebo-controlled trial (figures 1 and 2). The study was registered on ClinicalTrials.gov (NCT02255422) on October 2, 2014, before participant enrollment. Fifty-three participants were randomized to 12 weeks of treatment with omaveloxolone capsules at escalating doses of 5, 10, 20, 40, 80, or 160 mg or placebo once daily. Cohorts of 8–13 participants were randomized to each dose level or placebo (3:1). The sample size was based on a dose-escalation scheme to evaluate initial safety and pharmacodynamic activity of omaveloxolone in this population. The small number of participants at each dose was not expected to fully characterize safety, efficacy, or pharmacodynamics, but rather identify the appropriate doses to select for evaluation in additional clinical trials. Safety was overseen by a data safety and monitoring board. Participants were enrolled from May 2015 to August 2017 at the University of Copenhagen, UT Southwestern Medical Center, Akron Children's Hospital, Children's Hospital of Philadelphia, Massachusetts General Hospital, Texas Children's Hospital, University of California Los Angeles, and University of Pittsburgh School of Medicine.
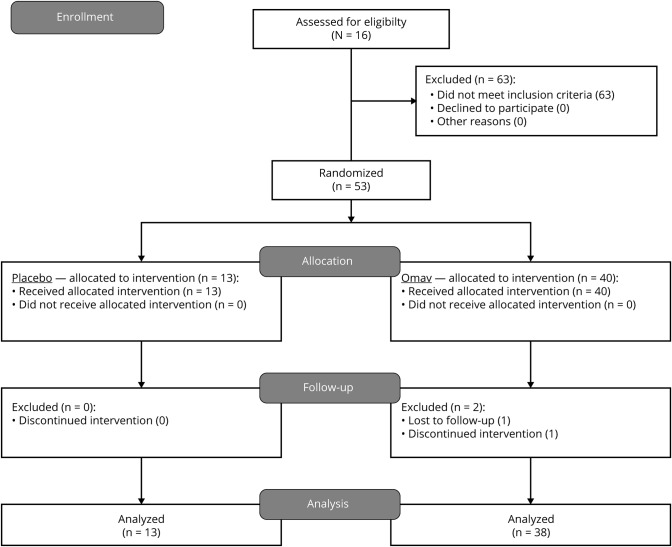
Figure 1. Diagram of the participant flow in the MOTOR study.
In the MOTOR study (Safety, Efficacy, and Pharmacodynamics of Omaveloxolone in Mitochondrial Myopathy Patients), 116 patients were screened. Fifty-three passed the criteria of inclusion/exclusion and were randomized to treatment with omaveloxolone (Omav) or placebo. Forty participants received treatment with omaveloxolone and 13 received placebo treatment. Six of the omaveloxolone-treated participants discontinued the intervention due to adverse events (unrelated or possibly related) or for personal reasons. Two of the participants were excluded from the final efficacy analysis as they had performed no postbaseline efficacy assessments. All 13 participants randomized to placebo treatment completed the study and were included in the final efficacy analysis. All participants were included in the safety analysis.
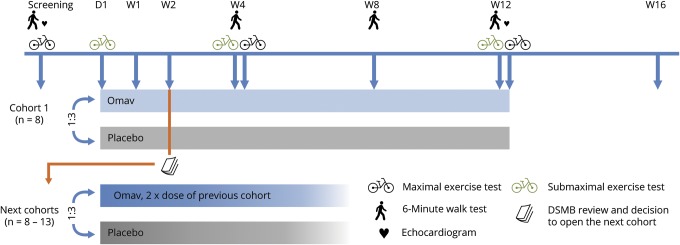
Figure 2. Design and visit schedule in a randomized double-blind dose-escalation study on omaveloxolone in mitochondrial myopathy.
Participants with mitochondrial myopathy were included in cohorts (8–13) and randomized 3:1 to omaveloxolone (Omav) or placebo treatment for 12 weeks. After 2 weeks of treatment, a data safety monitoring board (DSMB) reviewed the safety data and decided on the opening of the next dose level cohort. Six cohorts completed the protocol with 5, 10, 20, 40, 80, and 160 mg omaveloxolone, respectively. Study visits are indicated by vertical arrows and took place on day 1 (D1) and after the indicated number of weeks (W) of treatment. Laboratory measures were collected on all visits. Week 4 and 12 visits were conducted as a 2-day visit with a 6-minute walk test and a submaximal exercise test on the first and a maximal exercise test on the second day.
Eligibility criteria
Participants were required to have mitochondrial myopathy caused by a known nuclear or mitochondrial DNA mutation associated with reduced activity of at least 1 mitochondrially encoded respiratory chain complex. Second, they needed to have a history of exercise intolerance with or without weakness, typically in which modest exercise provokes heaviness, weakness, aching of active muscles, or tachycardia. Participants could be ≥18 and ≤75 years of age and should have the ability to complete maximal exercise testing, defined by being able to ride a cycle ergometer at 60 rpm against no added resistance for 3 minutes. Finally, participants had to have prominent muscle involvement defined as a peak work capacity of ≤1.5 Watts/kg evaluated by maximal cycle ergometry, corresponding to approximately 1/3 of that of a healthy individual.1 Participants were excluded if they had uncontrolled diabetes (HbA1c >11.0%), B-type natriuretic peptide level >200 pg/mL, or a history of clinically significant cardiac disease. They were required to discontinue all antioxidant supplements or medications that might interfere with omaveloxolone drug metabolism at least 14 days prior to randomization.
Randomization and masking
When participants met inclusion criteria, they were randomized by a computer-generated program at 3:1 (omaveloxolone:placebo) for each dose group. Once a data safety monitoring board had evaluated safety data from week 2 and 4 visits, investigators were informed if another cohort at the next dose level would open up. Participants and all study staff were blinded to participant assignment. In total, 40 participants were randomized to omaveloxolone at doses of 5–160 mg/d and 13 participants were randomized to placebo.
Outcome measures
The primary efficacy outcome measure of this study was change in peak workload during maximal exercise testing on a cycle ergometer. The secondary efficacy outcome measure was change in walked distance in a 6MWT. Exploratory endpoints included change in blood lactate and heart rate during submaximal exercise testing. With cycle ergometry testing, it has been shown multiple times that peak workload improves when oxidative capacity is improved with exercise training in patients with mitochondrial myopathy.5–7 The 6MWT has been shown to be sensitive to functional muscle improvements in this patient group.15
Pharmacodynamic markers included protein and enzyme levels in serum samples. Safety measures included weight, body mass index, vital sign measurements, physical examinations, laboratory test results (clinical chemistry, hematology, and urinalysis), concomitant medications, adverse events, and serious adverse events. For all outcome measures, the change after 12 weeks of treatment vs placebo was reported. Changes from baseline in the active treatment group were also described.
Assessment schedule
Maximal and submaximal exercise tests were performed at baseline and after 4 and 12 weeks of treatment. A 6MWT was performed at baseline and after 4, 8, and 12 weeks. After 2 weeks of treatment, the participants spent the day at the laboratory for measurements of pharmacokinetics. Four weeks after treatment termination (week 16), participants came for a follow-up visit. On all visits, blood and urine samples were collected (figure 2).
Assessment procedures
Maximal exercise test
All exercise tests were performed using a recumbent cycle ergometer (Lode, Groningen, Netherlands). The participants exercised against a stepwise increasing workload until exhaustion, targeting an 8- to 12-minute duration. The participants were instructed to keep the same pedaling speed throughout all tests and in repeat tests during the trial. Ventilation and O2 and CO2 gas exchanges were measured breath by breath using a metabolic cart or by collection of Douglas bags with gas analysis achieved by mass spectroscopy.
Submaximal exercise test
On the day after the maximal exercise test, participants performed a submaximal exercise test on the recumbent cycle ergometer for 30 minutes or until exhaustion. The workload was 40%–50% of the peak workload reached in the maximal exercise test at baseline. Gas exchanges, heart rate, and rates of perceived exertion were monitored continuously, similarly to the maximal exercise test, and were registered at rest and every 10 minutes during exercise. Blood was collected at rest and every 10 minutes during exercise. The exercise test was repeated at the same workload and pedaling speed on subsequent visits during the trial.
Six-minute walk test
This test followed a modified guideline for the 6MWT, the American Thoracic Society Statement,16 and was performed on the same day but prior to the submaximal exercise test. The test started at least 2 hours after any physical exertion.
Safety measures
To assess and ensure the safety and tolerability of the study treatment, we closely monitored changes in vital signs, body weight, ECGs, echocardiograms (baseline and week 12), concomitant medications, laboratory test results, and the reporting of adverse events.
Laboratory tests
Blood was analyzed for liver and kidney measures including alanine transaminase (ALT) and aspartate transaminase (AST), γ-glutamyl transferase (GGT), creatinine, and estimated glomerular filtration rate. Lipid panels, electrolytes, and hematology were measured, and creatine kinase and ferritin were measured as biomarkers of Nrf2 activity. Microscopic and macroscopic urinalysis and a urine pregnancy test was performed. For all analyses, standard clinical laboratory methods were used.
Statistical analysis
Peak work, change in blood lactate and heart rate during submaximal exercise testing, percent change from baseline in laboratory measures, and the 6MWT at multiple weeks were analyzed using a mixed model with repeated measures to account for the variability in multiple measurements collected over time (SAS version 9.3 or higher [SAS Institute, Cary, NC]). Analysis visits at baseline and weeks 4, 8, and 12 were used in the repeated measures analysis with an unstructured covariance structure (figure 2). The time of measurements collected during submaximal exercise testing (at baseline and weeks 4 and 12) was also used in the repeated measures model when analyzing heart rate and laboratory measures during submaximal exercise testing.
In the model, study week and the interaction between treatment and week were used as fixed effects, with individual patient as a random effect. The unstructured covariance structure used in the model was used to account for within-patient correlations and correct for the correlated measurements over time.
The pairwise dose group comparisons with placebo were estimated using the difference in adjusted means and 95% confidence intervals for the difference in changes from baseline to week 12. Significance of week 12 median change from baseline in creatine kinase was evaluated using a one-way analysis of variance.
Standard protocol approvals, registrations, and participant consents
All participants provided written informed consent. Study approval was obtained from the institutional review boards at all study sites and the ethics committee of the Capital Region of Denmark and the Danish Health and Medicines Agency (EUdract number 2014-003501-15).
Data availability
For this study, deidentified participant data, protocol, and statistical analysis plan will not be shared for external analysis.
Results
Demographics
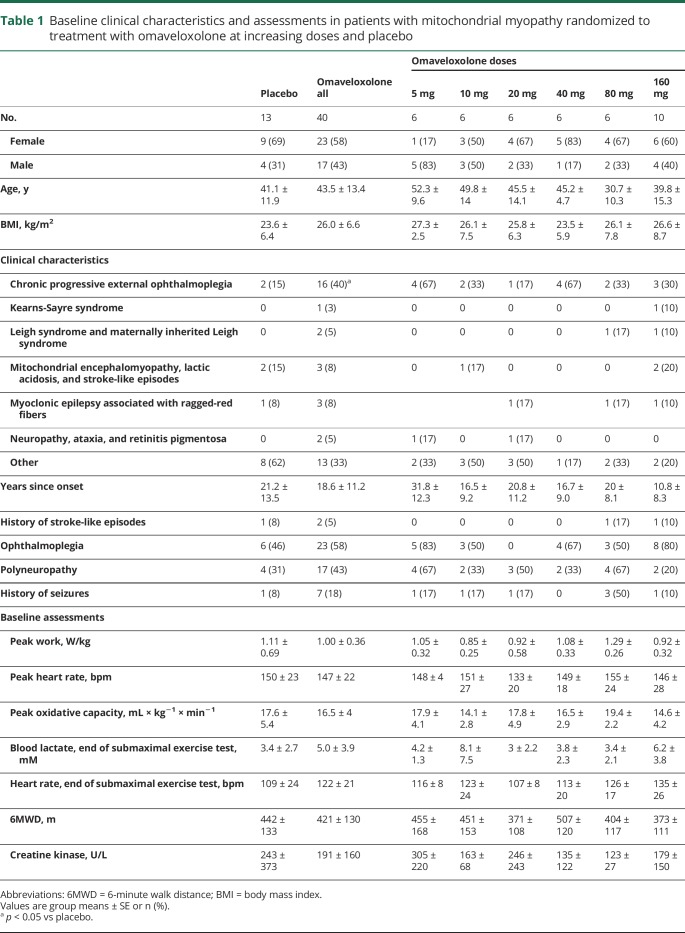
A total of 40 participants were allocated to omaveloxolone treatment and 13 to placebo treatment (figure 1). Among the omaveloxolone participants, 6 withdrew during the treatment period, 4 due to adverse events and 2 due to voluntary withdrawal (figure 1). Clinical presentations varied among the participants, though chronic progressive ophthalmoplegia was the dominating clinical syndrome, present in a larger proportion of the omaveloxolone group than the placebo group (table 1). The participants were evenly distributed among the different dose groups and the placebo group with regards to age, sex, peak work capacity, and medical history (table 1). Participants kept a stable body weight across all dosing groups.
Table 1.
Baseline clinical characteristics and assessments in patients with mitochondrial myopathy randomized to treatment with omaveloxolone at increasing doses and placebo
Pharmacodynamics
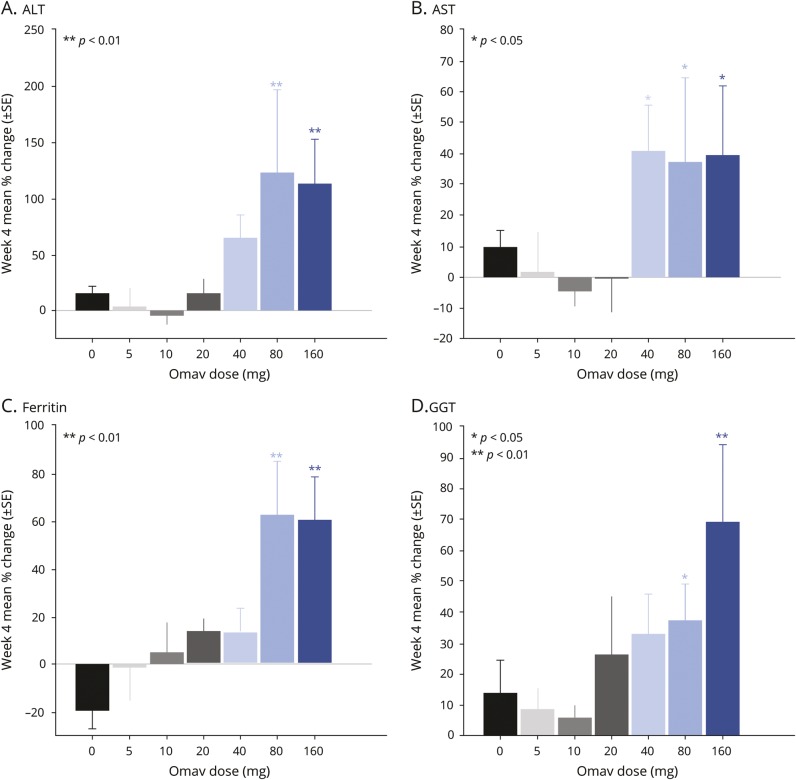
Omaveloxolone commonly affects several pharmacodynamic markers, such as ferritin and GGT, as demonstrated in vitro and in other human studies.14,16,17 As a sign of robust Nrf2 induction, plasma ferritin and GGT, ALT, and AST increased significantly after 4 weeks of treatment with omaveloxolone at 80 and 160 mg vs baseline (figure 3). One patient treated with omaveloxolone 40 mg and 3 participants treated with omaveloxolone 160 mg had increases in transaminases >3 times the upper limit of normal, but these subsequently dropped to normal while receiving omaveloxolone treatment. Changes in transaminases were not associated with signs or symptoms of liver injury, and concurrent increases in bilirubin were not observed.
Figure 3. Changes in plasma biomarkers of nuclear factor erythroid 2-like 2 (Nrf2) induction in patients with mitochondrial myopathy.
Changes in biomarkers of Nrf2 induction in patients with mitochondrial myopathy from baseline to after 4 weeks of treatment with a range of doses of omaveloxolone (Omav, 38 participants) and placebo (0 mg, 13 participants). (A) Alanine transaminase (ALT). (B) Aspartate transaminase (AST). (C) Ferritin. (D) γ-Glutamyl transferase (GGT). Presented are mean % change with SE bars after 4 weeks treatment vs baseline.
Assessments of efficacy
Maximal exercise
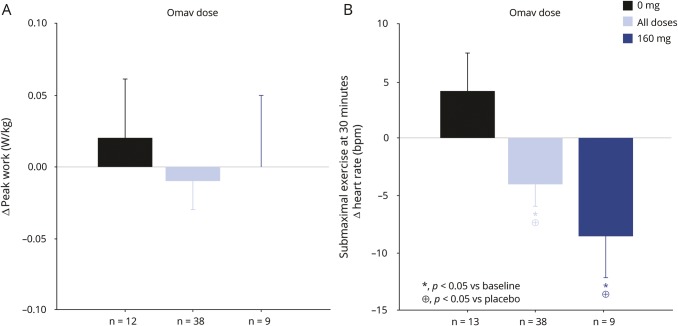
There was no difference in peak workload after 12 weeks of omaveloxolone treatment vs placebo (p = 0.73) or baseline (p = 0.77) (figure 4). Similarly, there was no difference vs placebo in maximal oxygen uptake (VO2max) (p = 0.68) or peak lactate production in all treatment groups vs placebo (p = 0.88). More than 57% of the participants in the active treatment group and more than 41% in the placebo group did not reach a maximal heart rate at week 12 that was within ±5 bpm of their baseline test, indicating they did not reach maximal work capacity.
Figure 4. Changes in peak work and submaximal exercise heart rate with omaveloxolone treatment.
Change in peak work in a maximal exercise test (A) and change in heart rate at the end of a 30-minute submaximal exercise test (B) on a recumbent ergometer in patients with mitochondrial myopathy after 12 weeks of treatment vs baseline. Presented are mean changes with SE bars in the placebo group (0 mg), the 160 mg omaveloxolone (Omav) group, and in all participants treated with omaveloxolone.
Submaximal exercise
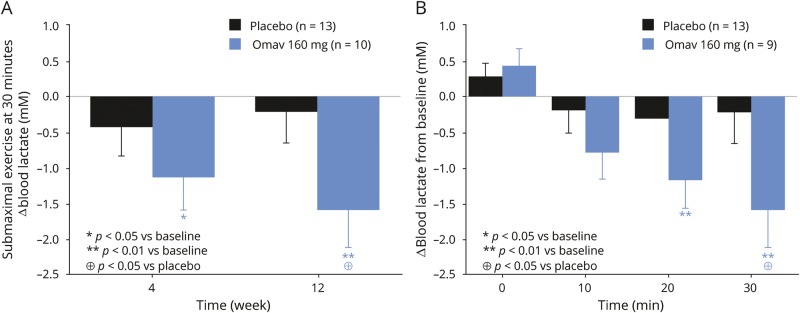
Blood lactate concentration at the end of the submaximal exercise test was significantly lower after 12 weeks of treatment in the omaveloxolone 160 mg–treated group compared to baseline and compared to the placebo group. The increase in blood lactate during submaximal exercise was lower in the participants treated with omaveloxolone 160 mg after 12 weeks compared to baseline (−1.6 ± 0.5 mM [SE], p = 0.003, 95% confidence interval [CI] −2.7 to −0.6) and vs placebo (−1.4 ± 0.7 mM [SE], p = 0.04, 95% CI −2.8 to −0.04). A decrease in blood lactate was observed at 4 weeks of treatment, but became significant after the full 12 weeks of treatment (figure 5). Furthermore, the heart rate at the end of submaximal exercise was lower in the participants treated with omaveloxolone 160 mg at 12 weeks compared to baseline (−8.7 ± 3.5 bpm [SE], p = 0.02, 95% CI −15.8 to −1.6) and vs placebo (−12.0 ± 4.6 bpm [SE], p = 0.01, 95% CI −21.2 to −2.7) (figure 4).
Figure 5. Change from baseline in blood lactate during submaximal exercise with 12 weeks omaveloxolone treatment.
Change in blood lactate (A) at the end of and (B) during 30 minutes of submaximal exercise on a recumbent cycle ergometer in patients with mitochondrial myopathy with 12 weeks of treatment with placebo and 160 mg omaveloxolone (Omav) vs baseline (BL). Presented are mean values with SE bars.
6-Minute walk test
There was large interparticipant and intraparticipant variation in the distance walked in the 6MWT. Indeed, 2 placebo-treated participants had week 12 improvements in the 6MWT that were greater than 130 meters, and the median intraparticipant variation in the 6MWT was 13.5 meters. There was no change vs baseline after 12 weeks of treatment in the omaveloxolone 160 mg group (p = 0.38).
Safety measures
There were no changes in vital signs, body weight, ECGs, or echocardiograms of clinical significance in the participants. There were no changes in clinical chemistry, hematology, urinalysis, or microscopy related to safety issues.
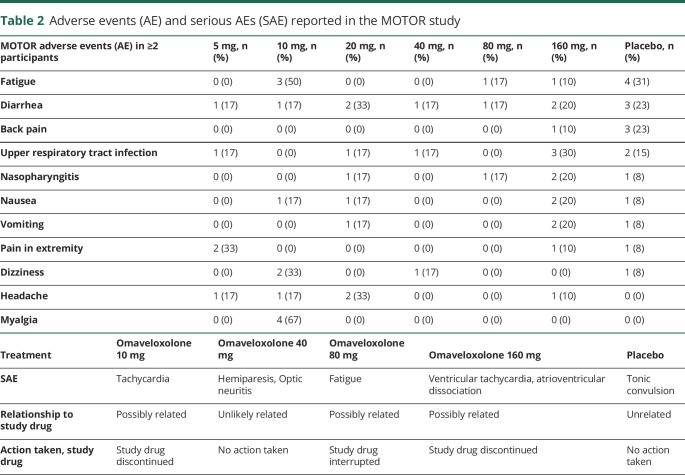
Adverse events are reported in table 2. Overall, adverse events were mild in severity, and most prominently included upper respiratory tract infections (table 2). Four omaveloxolone-treated participants discontinued due to adverse events but the frequency of discontinuation in the omaveloxolone-treated group (4/53) did not significantly differ from the placebo group (0/13) (p = 0.56, Fisher exact test). Therefore, we are unable to conclude if there is a higher tendency to adverse events among the omaveloxolone-treated patients, and this must be studied in a larger population. Two omaveloxolone-treated participants withdrew for personal reasons and no placebo-treated participants discontinued the treatment or the study.
Table 2.
Adverse events (AE) and serious AEs (SAE) reported in the MOTOR study
Seven serious adverse events were reported, of which 3 were evaluated as possibly related to the study drug and led to permanent discontinuation of the study drug. One omaveloxolone-treated participant discontinued due to nonserious myoclonus. This was considered related to his disease and not to omaveloxolone treatment (table 2).
Discussion
This dose-escalation study showed that treatment with 160 mg omaveloxolone led to a significant reduction in the exploratory endpoints: lactate production and heart rate during submaximal exercise. As most everyday activities are of a submaximal intensity, these results are potentially clinically meaningful and indicate that omaveloxolone may improve exercise tolerance in patients with mitochondrial myopathy. Submaximal work reflects mitochondrial metabolism and aerobic metabolism, while peak work proportionally relies more on anaerobic metabolism. Our findings of lower heart rate and reduced lactate during submaximal exercise reflect improved mitochondrial function,18 in line with preclinical evidence that Nrf2 activation by muscle-specific Keap1 knockout in mice increased the expression of fatty acid transport genes and walk distances,19 and that substrate use and oxygen consumption increased in overfed mice when they were treated with an analogue to omaveloxolone.20 Overall, omaveloxolone was well-tolerated. Three participants had serious adverse events of cardiac arrhythmias. These events were evaluated as unrelated to the study drug and are frequent in the studied patient group, which is the reason for carefully monitoring the heart with ECGs and echocardiograms throughout the study. Still, as 2 of these events occurred in the 160 mg group, a future trial should carefully monitor for cardiac arrhythmias.
No significant changes were observed in maximal exercise testing, as omaveloxolone did not improve peak work, which was the primary outcome measure in this trial. It is noteworthy that fewer than half of the participants reached the peak exercise heart rate that was achieved at baseline, and there was high variability in the peak heart rate in in all 3 maximal exercise tests. This poor test–retest agreement disqualified a large proportion of the maximal exercise tests and therefore limited the use of maximal oxidative capacity as a reliable endpoint. By contrast, the submaximal exercise tests were easily reproduced, and the results represent a reliable indicator of physiologic and metabolic responses to exactly the same conditions from test to test. This test is therefore more sensitive to changes in mitochondrial function and less affected by variations in the participants' ability to push themselves or different test approaches across the many study sites.
There was no change in the 6MWT, the secondary outcome measure, with omaveloxolone treatment at any dose. Similar to the maximal exercise test, the 6MWT is more sensitive to day-to-day variations related to motivation.21 In addition, the distance walked could have been influenced by neurologic symptoms of mitochondrial disease affecting balance and gait, which did not lead to exclusion in this study, contrary to another interventional study in patients with mitochondrial myopathies, in which the 6MWT changed significantly with treatment.15 Such deficits had less effect on the exercise tests where the participants were seated on the recumbent ergometer with a backrest, handlebars, and their feet strapped to the pedals.
There was a full induction of the Nrf2 signaling pathways after 4 weeks of treatment (figure 3). This was consistent with a significant treatment response. However, the clinical response of decreased blood lactate and heart rate during submaximal exercise testing became significant at the end of the 12-week treatment period (figures 4 and 5). Preclinical data show a physiologic response to omaveloxolone analogues within weeks in mice, but our results indicate longer response time in humans.20 It is likely that already damaged and dysfunctional mitochondria cannot be revived, but that the delayed response is due to the continuous but slow degradation of dysfunctional mitochondria and biogenesis of undamaged mitochondria that ultimately improve oxidative phosphorylation under the influence of omaveloxolone. Additional investigation is needed to determine if longer treatment period enhances the therapeutic response and improves work capacity.
As this was a dose-escalation study with relatively small groups per dose, a larger study population is necessary to determine whether the treatment effect correlates with muscle strength, specific mitochondrial clinical syndrome, or other factors. Indeed, heterogeneity in the various mitochondrial myopathies examined (myoclonic epilepsy associated with ragged-red fibers [MERRF], chronic progressive external ophthalmoplegia [CPEO], maternally inherited Leigh syndrome) could have influenced the study results with a relatively small number of participants per dose.
Baseline values for end submaximal heart rate and lactate tended to be higher in the 160 mg group compared to the placebo group. As a higher baseline value leaves a greater potential for improvement, we can speculate if the 2 groups were challenged equally in the exercise test, but the submaximal workload was set to 50% of maximal workload to ensure comparable conditions. Still, the trend towards lower baseline lactate and higher peak workload and oxygen uptake among the participants in the placebo group (table 2) could indicate that these participants have a less severe mitochondrial defect compared to the 160-mg group. The ratio of patients with CPEO, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes, and MERRF in the placebo vs the 160 mg group were comparable, while more participants in the placebo group had clinical syndromes classified as “other” compared to the 160 mg group. There are no significant differences or trends indicating that these “other” participants are less affected than the participants with well-defined syndromes. Overall there were too few patients per treatment group and per phenotype to yield firm conclusions about the superiority of the drug in one indication. Neither can we see any tendencies towards a correlation between treatment response and genotype in terms of mtDNA or nuclear DNA mutations, heteroplasmy, or deletion sizes.
Future investigators should carefully consider our findings when choosing outcome measures and how sensitive these measures are to extramuscular symptoms or consider excluding patients with, for example, significant gait or balance problems.
Antioxidant effects on mitochondrial function have been studied extensively but few studies have assessed its direct benefits in the treatment of mitochondrial diseases.2,22 Through Keap1 inhibition, omaveloxolone affects multiple signaling pathways that enhance mitochondrial function, biogenesis, and substrate turnover in addition to antioxidant effects.10,19,22 In concert, these actions potentially make omaveloxolone an effective treatment option, which is supported by the results of the present study. The reduction in submaximal heart rate is a sign of improved exercise tolerance and the reduction in blood lactate reflects increased aerobic work capacity, which can be of great value in the daily lives of the patients. These findings support further investigation of omaveloxolone for potential treatment of patients with primary mitochondrial myopathies.
Study funding
Reata Pharmaceuticals.
Disclosure
K. Madsen has received a research and travel grant from Reata. A. Buch has received research support from Reata Pharmaceuticals. B. Cohen receives research support from Reata Pharmaceuticals, BioElectron Technologies, and Horizon Pharmaceuticals; research support and consulting fees from Stealth Biotherapeutics; provides educational lecturing and CPT advising to the American Academy of Neurology; provides content editing services for Motive Medical Intelligence; and receives royalties from Elsevier. M. Falk reports no disclosures relevant to the manuscript. A. Goldsberry is employed by and has a financial interest in Reata Pharmaceuticals. A. Goldstein is on the Speakers Bureau and Scientific and Medical Advisory Board for the United Mitochondrial Disease Foundation. A. Karaa receives reimbursement for travel and consulting payments from Stealth BioTherapeutics, consulting payments from MitoBridge, is on the medical advisory board of MitoAction and scientific and medical advisory board of the United Mitochondrial Disease Foundation, is a board member of Rare New England and President of the Mitochondrial Medicine Society, and is an investigator in the North American Mitochondrial Disease Consortium. M. Koenig receives consultation, speaking, and clinical trial support from Novartis; consulting and clinical trial support from LAM Therapeutics; clinical trial support from Stealth, EryDel, Ultragenyx, Pfizer, and Reata Pharmaceuticals; and is on the speaker's bureau for Lundbeck. C. Muraresku reports no disclosures relevant to the manuscript. C. Meyer is employed by and has a financial interest in Reata Pharmaceuticals. M. O'Grady is employed by and has a financial interest in Reata Pharmaceuticals. F. Scaglia receives research support from Reata Pharmaceuticals, BioElectron Technologies, and Stealth Therapeutics and is an investigator in the North American Mitochondrial Disease Consortium. P. Shieh has served as an ad hoc consultant for Sarepta Therapeutics, Biogen, PTC Therapeutics, and Avexis; and serves on the speaker's bureau for Biogen, Grifols, Alexion, and CSL Behring. J. Vockley receives research funding from NIH, Ultragenyx Pharmaceuticals, Alexion Pharmaceuticals, Reata Pharmaceuticals, Mitobridge Pharmaceuticals, Biomarin Pharmaceuticals, Reneo Pharmaceuticals, Moderna Pharmaceuticals, and Horizon Pharmaceuticals and consulting fees from American Gene Technologies, Biomarin Pharmaceuticals, and Mitobridge Pharmaceuticals. Z. Zolkipli-Cunningham and R. Haller report no disclosures relevant to the manuscript. J. Vissing has received research and travel support and/or speaker honoraria from Sanofi/Genzyme, Sarepta Therapeutics, and Santhera Pharmaceuticals, and served as a consultant on advisory boards for Sanofi/Genzyme, Santhera Pharmaceuticals, Sarepta Therapeutics, Audentes Therapeutics, and Stealth Biotherapeutics within the last 3 years. Go to https://n.neurology.org/lookup/doi/10.1212/WNL.0000000000008861 for full disclosures.
Glossary
- 6MWT
6-minute walk test
- ALT
alanine transaminase
- AST
aspartate transaminase
- CI
confidence interval
- CPEO
chronic progressive external ophthalmoplegia
- GGT
γ-glutamyl transferase
- MERRF
myoclonic epilepsy associated with ragged-red fibers
- Nrf2
nuclear factor erythroid 2-like 2
- ROS
reactive oxygen species
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
References
- 1.Taivassalo T, Jensen TD, Kennaway N, DiMauro S, Vissing J, Haller RG. The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain 2003;126:413–423. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer G, Majamaa K, Turnbull DM, Thorburn D, Chinnery PF. Treatment for mitochondrial disorders. Cochrane Database Syst Rev 2012:CD004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Giovanni S, Mirabella M, Papacci M, Odoardi F, Silvestri G, Servidei S. Apoptosis and ROS detoxification enzymes correlate with cytochrome C oxidase deficiency in mitochondrial encephalomyopathies. Mol Cell Neurosci 2001;17:696–705. [DOI] [PubMed] [Google Scholar]
- 4.Taivassalo T, Gardner JL, Taylor RW, et al. Endurance training and detraining in mitochondrial myopathies due to single large-scale mtDNA deletions. Brain 2006;129:3391–3401. [DOI] [PubMed] [Google Scholar]
- 5.Jeppesen TD, Schwartz M, Olsen DB, et al. Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain 2006;129:3402–3412. [DOI] [PubMed] [Google Scholar]
- 6.Adhihetty PJ, Taivassalo T, Haller RG, Walkinshaw DR, Hood DA. The effect of training on the expression of mitochondrial biogenesis- and apoptosis-related proteins in skeletal muscle of patients with mtDNA defects. Am J Physiol Endocrinol Metab 2007;293:E672–E680. [DOI] [PubMed] [Google Scholar]
- 7.Cejudo P, Bautista J, Montemayor T, et al. Exercise training in mitochondrial myopathy: a randomized controlled trial. Muscle Nerve 2005;32:342–350. [DOI] [PubMed] [Google Scholar]
- 8.Muthusamy VR, Kannan S, Sadhaasivam K, et al. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic Biol Med 2012;52:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irving BA, Lanza IR, Henderson GC, Rao RR, Spiegelman BM, Nair KS. Combined training enhances skeletal muscle mitochondrial oxidative capacity independent of age. J Clin Endocrinol Metab 2015;100:1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludtmann MHR, Angelova PR, Zhang Y, Abramov AY, Dinkova-Kostova AT. Nrf2 affects the efficiency of mitochondrial fatty acid oxidation. Biochem J 2014;457:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmström KM, Baird L, Zhang Y, et al. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open 2013;2:761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman DJ. Natural products as sources of New drugs from 1981 to 2014. J Nat Prod 2016;79:629–661. [DOI] [PubMed] [Google Scholar]
- 13.Sporn MB, Liby KT, Yore MM, Fu L, Lopchuk JM, Gribble GW. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod 2011;74:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinkova-Kostova AT, Liby KT, Stephenson KK, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci 2005;102:4584–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaa A, Haas R, Goldstein A, Vockley J, Weaver WD, Cohen BH. Randomized dose-escalation trial of elamipretide in adults with primary mitochondrial myopathy. Neurology 2018;90:e1212–e1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Thoracic Society statement. Am J Respir Crit Care Med 2002;166:111–117.12091180 [Google Scholar]
- 17.Kerins MJ, Ooi A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxid Redox Signal 2018;29:1756–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeppesen TD, Orngreen MC, Van Hall G, Vissing J. Lactate metabolism during exercise in patients with mitochondrial myopathy. Neuromuscul Disord 2013;23:629–636. [DOI] [PubMed] [Google Scholar]
- 19.Uruno A, Furusawa Y, Yagishita Y, et al. The keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol 2013;33:2996–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha PK, Reddy VT, Konopleva M, Andreeff M, Chan L. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Lepr(db/db) mice. J Biol Chem 2010;285:40581–40592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alfano L, Lowes L, Berry K, Flanigan K, Cripe L, Mendell J. Role of motivation on performance of the 6-minute walk test in boys with Duchenne muscular dystrophy. Dev Med Child Neurol 2015;57:57–58. [Google Scholar]
- 22.Greco T, Fiskum G. Neuroprotection through stimulation of mitochondrial antioxidant protein expression. J Alzheimers Dis 2010;20:427–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For this study, deidentified participant data, protocol, and statistical analysis plan will not be shared for external analysis.